Submitted:
11 July 2024
Posted:
11 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Antibacterial Activity of Kopraphinol and Methylparaben
2.3. Antifungal Activity of Kopraphinol and Methylparaben
2.4. Thermal Stability
2.5. Contact Time (Killing Time)
2.6. Challenge Test
3. Results
3.1. Antimicrobial Activity
3.2. Thermal Stability
3.3. Killing Time
3.4. Challenge Test
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products; Official Journal of the European Union L342/59, Publications Office of the European Union: Luxembourg, 2009. [Google Scholar]
- Giorgio, A.; Miele, L.; De Bonis, S.; Conforti, I.; Palmiero, L.; Guida, M.; Libralato, G.; Aliberti, F. Microbiological stability of cosmetics by using Challenge Test procedure. J. Pure App. Microbiol. 2018, 12, 23–28. [Google Scholar] [CrossRef]
- SCCS (Scientific Committee on Consumer Safety). The SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and their Safety Evaluation, G: 2023. Appendix 9, 2023.
- Halla, N.; Fernandes, I.P.; Heleno, S.A.; Costa, P.; Boucherit-Otmani, Z.; Boucherit, K.; Rodrigues, A.E.; Ferreira, I.C.F.R.; Barreiro, M.F. Cosmetic preservation: A review on present strategies. Molecules 2018, 23, 1571. [Google Scholar] [CrossRef] [PubMed]
- Nowak, K; Jablónska, E; Ratajczak-Wrona, W. Controversy around parabens: Alternatives strategies for preservative use in cosmetics and personal care products. Environ. Res. 2021, 110488. [CrossRef]
- European Commission. Regulation (EU) N° 1004 (2014) of 18 September 2014 amending Annex V to Regulation (EC) N° 1223/2009 of the European Parliament and of the Council on cosmetic products; Official Journal of the European Union L282/5, Publications Office of the European Union: Luxembourg, 2009. [Google Scholar]
- Martins, A.M.; Marto, J.M. A sustainable life cycle for cosmetics: From design and development to post-use phase. Sustain. Chem. Pharm. 2023, 35, 101178. [Google Scholar] [CrossRef]
- Paramita, S.; Kusuma, I.W.; Sulistioadi, Y.B. ; Kiswanto (2024). Environmental and Safety Aspects of bio-based Cosmetics in Indonesia. In: Arung, E.T.; et al. Biomass-based Cosmetics 2024, pp.545-568, Springer, Singapore. [CrossRef]
- Poddebniak, P.; Kalinowska-Lis, U. A survey of preservatives used in cosmetic products. Appl. Sci. 2024, 14, 1581. [Google Scholar] [CrossRef]
- Kopraphinol (Radish Root Ferment Filtrate). Available online: https://www.kumarorganic.net/products/page.php?productcode=Prod-18-02-2019-HJIBQOWSP3&category=Preservative-Ingredients (accessed on 8 July 2024).
- Elshikh, M.; Ahmed, S.; Funston, S.; Dunlop, P.; McGaw, M.; Marchant, R.; Banat, I.M. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol. Lett. 2016, 38, 1015–1019. [Google Scholar] [CrossRef]
- Therese, K.L. , Bagyalakshmi R., Madhavan H.N., Deepa P. In-vitro susceptibility testing by agar dilution method to determine the minimum inhibitory concentrations of amphotericin B, fluconazole and ketoconazole against ocular fungal isolates. Ind. J. Med. Microbiol. 2006, 24, 273–279. [Google Scholar]
- Juliano, C.; Mattana, A.; Usai, M. Composition and in vitro Antimicrobial Activity of the Essential Oil of Thymus herba-barona Loisel growing wild in Sardinia. J. Essent. Oil Res. 2000, 12, 516–522. [Google Scholar] [CrossRef]
- Siegert, W. Comparison of microbial challenge testing methods for cosmetics. Household and Personal Care Today 2013, 8, 32–39. [Google Scholar]
- European Pharmacopeia 7th edition 2011. Chapter 5.1.3. Efficacy of antimicrobial preservation. Editor: Council of Europe: European Directorate for the Quality of Medicines and Healthcare, Strasbourg.
- Juliano, C.; Gavini, E.; Giunchedi, P.; Magrini, G.A. Evaluation of Manuka honey as an adjuvant antimicrobial preservative in a O/A emulsion. J. Appl. Cosmetol. 2016, 34, 87–98. [Google Scholar]
- Pérez-Rivero, C.; Lòpez-Gòmez, J.P. Unlocking the potential of fermentation in cosmetics: A review. Fermentation 2023, 9, 463. [Google Scholar] [CrossRef]
- Majchrzak, W.; Motyl, I.; Smigielski, K. Biological and cosmetical importance of fermented raw material: An overview. Molecules 2022, 27, 4845. [Google Scholar] [CrossRef] [PubMed]
- Romanowski, P.; Schueller, R. Definition and principles of multifunctional cosmetics. In: Multifunctional Cosmetics, 1st Ed., Schueller, R., Romanowski, P., Eds., Taylor & Francis, Boca Raton, FL, 2001; pp. 1-11.
- Cosmetic Ingredient Review. Safety assessment of Radish Root-derived ingredients as used in cosmetics, 2022. https://cir-safety.org/sites/default/files/Radish.pdf.
- Pinto, D.; Ciardiello, T.; Franzoni, M.; Pasini, F.; Giuliani, G.; Rinaldi, F. Effect of commonly used cosmetic preservatives on skin resident microflora dynamics. Sci. Rep. 2021, 11, 8695. [Google Scholar] [CrossRef]
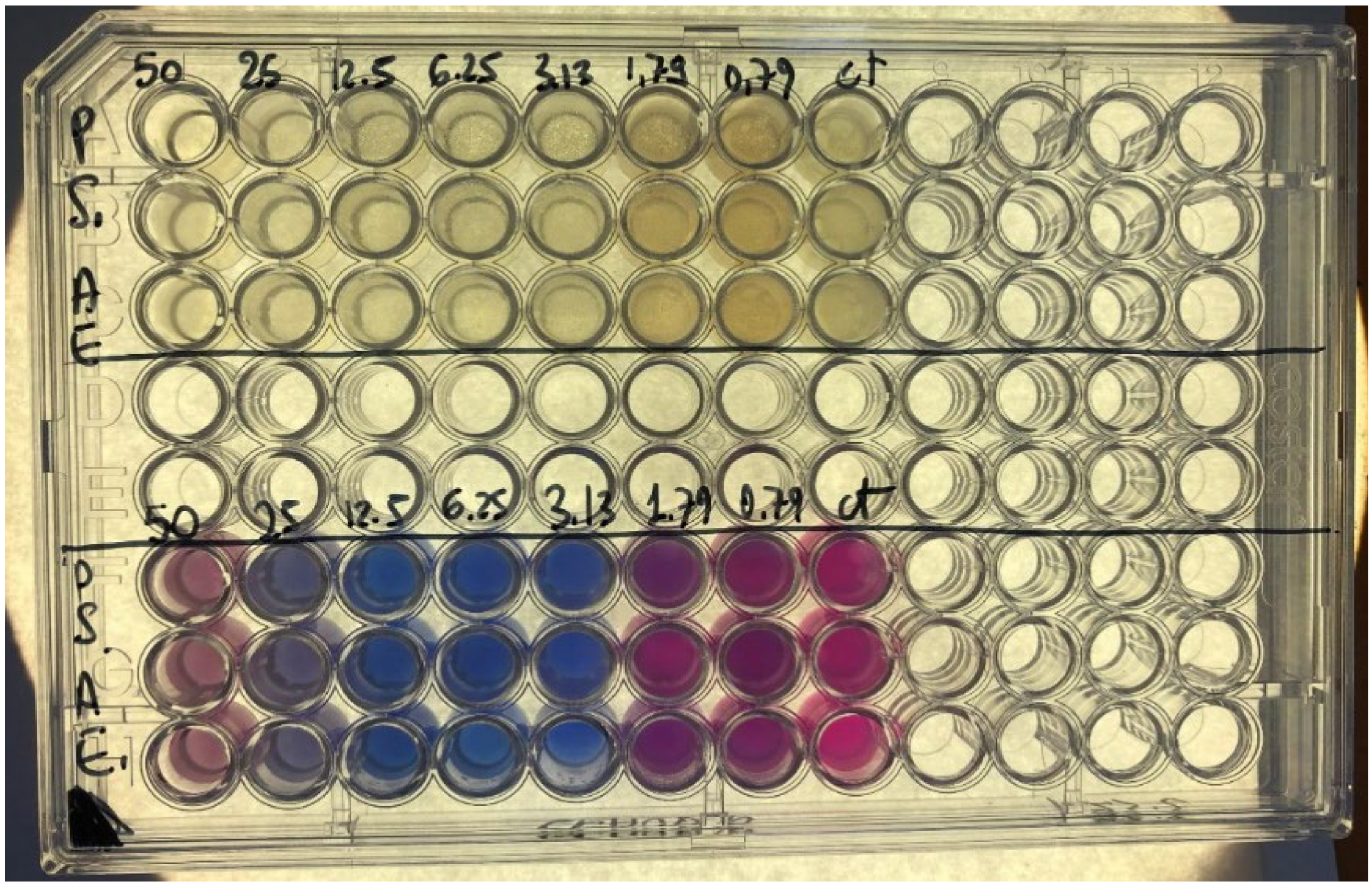
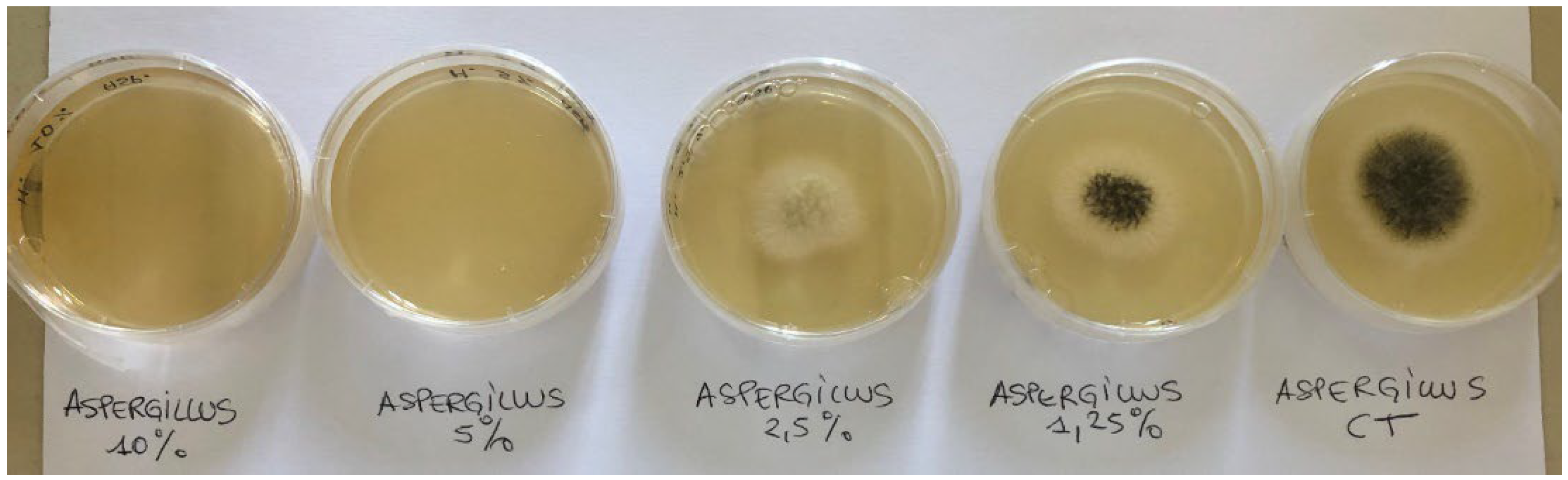
| Microorganisms | Kopraphinol (vol/vol) | Methyl paraben (wt/vol) |
|---|---|---|
| Escherichia coli (ATCC 8739) | 0.78±0.00% (6.25±0.00%) | 0.2±0.00% (0.2±0.00%) |
| Staphylococcus aureus (ATCC 6538) | 1.56±0.00% (12.5±0.00%) | 0.2±0.00% (0.2±0.00%) |
| Pseudomonas aeruginosa (ATCC 9027) | 6.25±0.00% (6.25±0.00%) | 0.2±0.00% (0.2±0.00%) |
| Candida albicans (ATCC 10231) | 1.56±0.00% (3.13±0.00%) | 0.05±0.00% (0.05±0.00%) |
| Candida spp. (from rectal swab) | 3.13±0.00% (6.25±0.00%) | n.d. |
| Candida spp. (from throat swab) | 1.56±0.00% (1.56±0.00%) | n.d |
| Candida spp. (from vaginal swab) | 1.56±0.00% (6.25±0.00%) | n.d. |
| Candida spp. (from mucosa of the cheek) | 1.56±0.00% (3.13±0.00%) | n.d. |
| Aspergillus brasiliensis (ATCC 16404) | 5.00±0.00% | 0.1±0.00% |
| Penicillium rubens (ATCC 9179) | 5.00±0.00% | n.d. |
| E. coli (ATCC 8739) | P. aeruginosa (ATCC 9027) | S. aureus (ATCC 6538) | C. albicans (ATCC 10231) | |
| MIC K | 0,78±0.00% | 6,25±0.00% | 1,56% | 1,56% |
| MIC K50 | 0,78±0.00% | 6,25% | 1,56% | 1,56% |
| MIC K100 | 0,78±0.00% | 6,25% | 1,56% | 1,56% |
| MIC K* | 0,78±0.00% | 6,25% | 1,56% | 1,56% |
| STRAIN | time 0 | 1h | 2h | 3h |
|---|---|---|---|---|
| E. coli (ATCC 8739) | 100% | 30% | 0,46% | 0,17% |
| P. aeruginosa (ATCC 9027) | 100% | 91,7% | 33,3% | 9,8% |
| S. aureus (ATCC 6538) | 100% | 120,8% | 141,7% | 83,3% |
| C. albicans (ATCC 10231) | 100% | 98,3% | 84,5% | 70,7% |
| TIME | Eur. Ph. criteria | Without preservative | Kopraphinol | Methylparaben |
|---|---|---|---|---|
| DAY 2 | 2 | 0.01 | >5 | >5 |
| DAY 7 | 3 | -0.138 | >5 | >5 |
| DAY 14 | - | - | - | - |
| DAY 28 | NI | >5 | >5 | >5 |
| TIME | Eur. Ph. criteria | Without preservative | Kopraphinol | Methylparaben |
|---|---|---|---|---|
| DAY 2 | 2 | 0.209 | >5 | 0.017 |
| DAY 7 | Kopra3 | >5 | >5 | >5 |
| DAY 14 | - | - | - | - |
| DAY 28 | NI | >5 | >5 | >5 |
| TIME | Eur. Ph. criteria | Without preservative | Kopraphinol | Methylparaben |
|---|---|---|---|---|
| DAY 2 | 2 | -1.438 | 4.828 | 1,216 |
| DAY 7 | 3 | -2.885 | >5 | -1,924 |
| DAY 14 | - | - | - | - |
| DAY 28 | NI | -2.793 | >5 | -0,711 |
| TIME | Eur. Ph. criteria | Without preservative | Kopraphinol | Methylparaben |
|---|---|---|---|---|
| DAY 2 | 2 | -3.117 | >5 | 0 |
| DAY 7 | 3 | -3.569 | >5 | 0.430 |
| DAY 14 | - | - | - | - |
| DAY 28 | NI | -3.944 | >5 | 2.964 |
| TIME | Eur. Ph. criteria | Without preservative | Kopraphinol | Methylparaben |
|---|---|---|---|---|
| DAY 2 | 2 | 0 | >5 | 0.405 |
| DAY 7 | 3 | 0 | >5 | 0.310 |
| DAY 14 | - | - | - | - |
| DAY 28 | NI | -0.109 | >5 | -0.182 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





