Submitted:
11 July 2024
Posted:
11 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
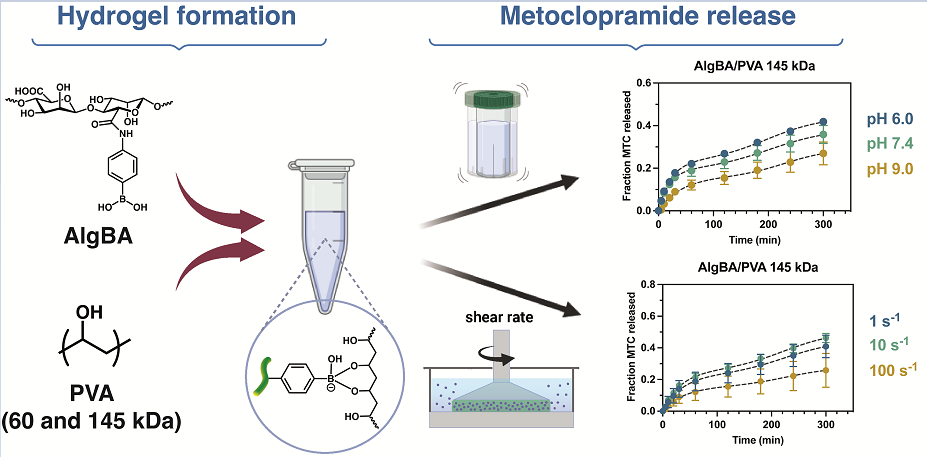
2.1. Synthesis of Modified Alginate with 4-Aminophenyl Boronic Acid (AlgBA)
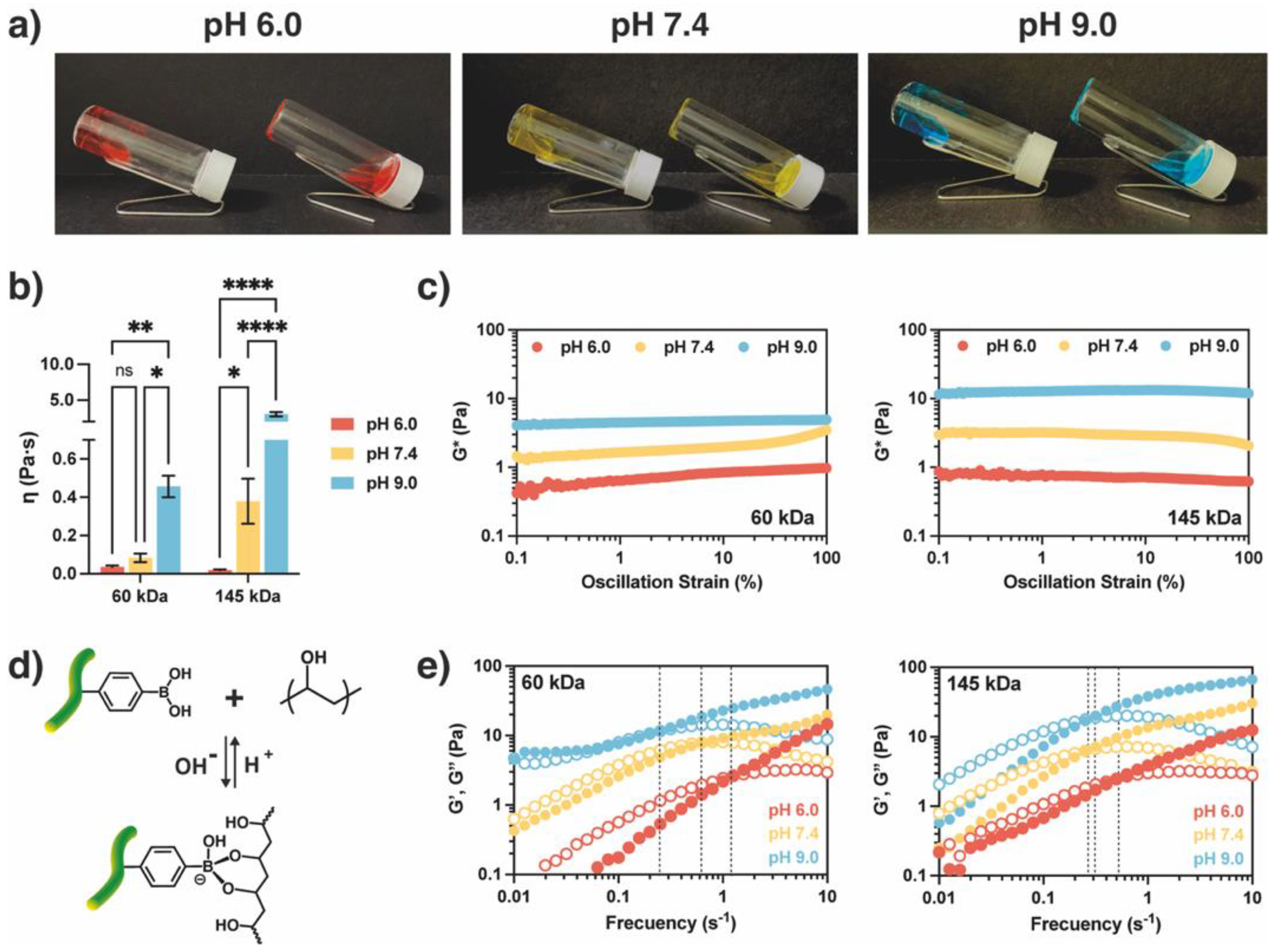
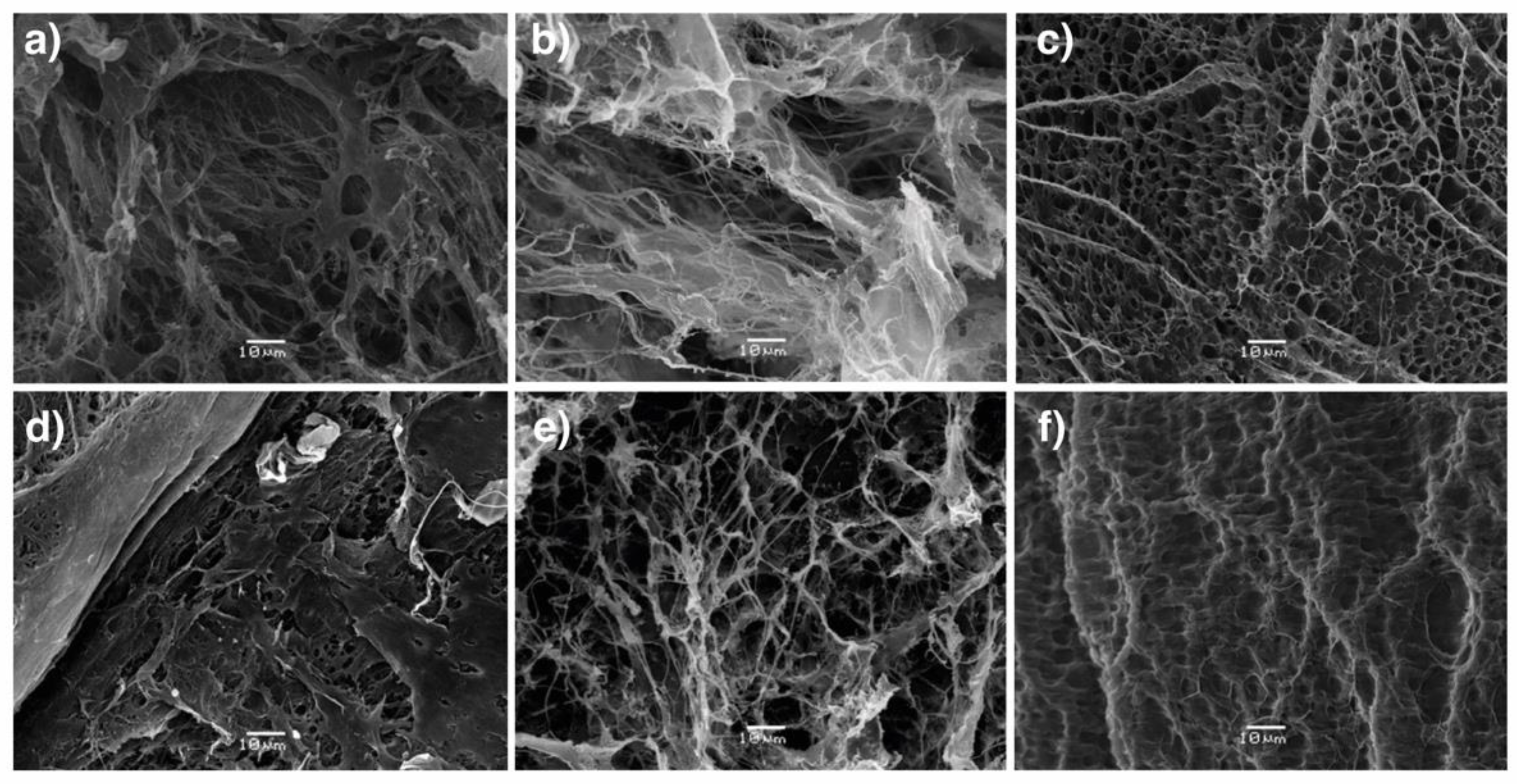
2.2. Preparation of AlgBA/PVA Hydrogels
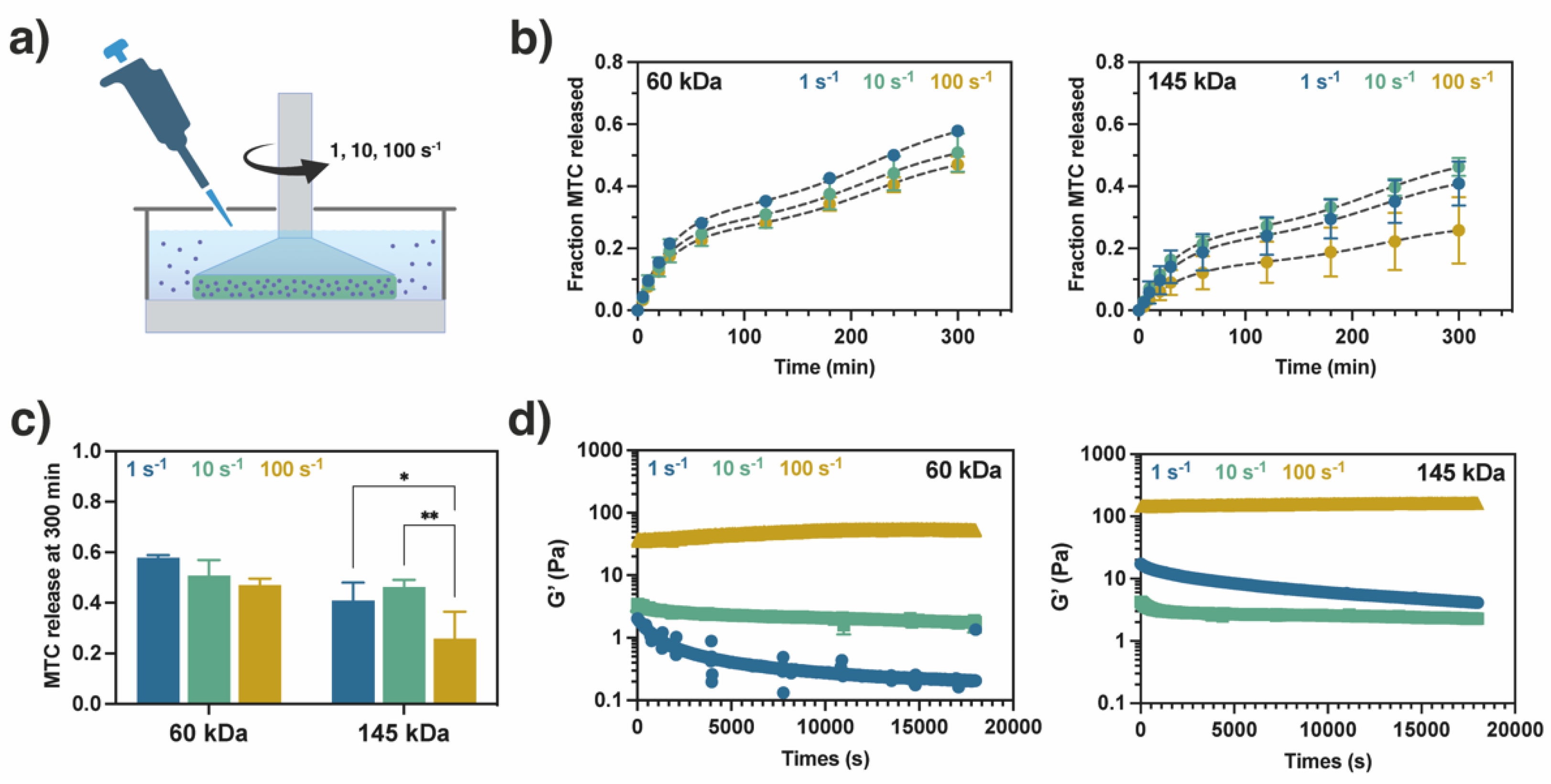
2.3. Study of Metoclopramide Drug Release
3. Conclusions
4. Materials and Methods
4.1. Reagents
4.2. Synthesis of Modified Alginate with 4-aminophenyl Boronic Acid (AlgBA)
4.3. Preparation of AlgBA/PVA Hydrogels
4.4. MTC Release Studies
4.5. Characterization Techniques
Author Contributions
Funding
Conflicts of Interest
References
- Guan, Y.; Zhang, Y. Boronic acid-containing hydrogels: synthesis and their applications. Chemical Society Reviews 2013, 42, 8106–8121. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.M.; Nakamura, C.V.; Auzély-Velty, R. Boronate-ester crosslinked hyaluronic acid hydrogels for dihydrocaffeic acid delivery and fibroblasts protection against UVB irradiation. Carbohydr Polym 2020, 247, 116845. [Google Scholar] [CrossRef] [PubMed]
- Pettignano, A.; Grijalvo, S.; Häring, M.; Eritja, R.; Tanchoux, N.; Quignard, F.; Díaz Díaz, D. Boronic acid-modified alginate enables direct formation of injectable, self-healing and multistimuli-responsive hydrogels. Chemical Communications 2017, 53, 3350–3353. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Choudhury, A.R. Stimuli-Responsive Polysaccharide-Based Smart Hydrogels and Their Emerging Applications. Industrial & Engineering Chemistry Research 2023, 62, 841–866. [Google Scholar] [CrossRef]
- Lin, X.; Wang, X.; Zeng, L.; Wu, Z.L.; Guo, H.; Hourdet, D. Stimuli-Responsive Toughening of Hydrogels. Chemistry of Materials 2021, 33, 7633–7656. [Google Scholar] [CrossRef]
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate Hydrogels as Biomaterials. Macromolecular Bioscience 2006, 6, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Bojorges, H.; López-Rubio, A.; Martínez-Abad, A.; Fabra, M.J. Overview of alginate extraction processes: Impact on alginate molecular structure and techno-functional properties. Trends in Food Science & Technology 2023, 140, 104142. [Google Scholar] [CrossRef]
- Azam, F.; Ahmad, F.; Ahmad, S.; Zafar, M.S.; Ulker, Z. Synthesis and characterization of natural fibers reinforced alginate hydrogel fibers loaded with diclofenac sodium for wound dressings. Int J Biol Macromol 2023, 241, 124623. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Raus, R.; Wan Nawawi, W.M.F.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian Journal of Pharmaceutical Sciences 2021, 16, 280–306. [Google Scholar] [CrossRef] [PubMed]
- Taemeh, M.A.; Shiravandi, A.; Korayem, M.A.; Daemi, H. Fabrication challenges and trends in biomedical applications of alginate electrospun nanofibers. Carbohydrate Polymers 2020, 228, 115419. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Shin, M.; Park, E.; Ryu, J.H.; Burdick, J.A.; Lee, H. Alginate-Boronic Acid: pH-Triggered Bioinspired Glue for Hydrogel Assembly. Advanced Functional Materials 2020, 30, 1908497. [Google Scholar] [CrossRef]
- Hong, S.H.; Kim, S.; Park, J.P.; Shin, M.; Kim, K.; Ryu, J.H.; Lee, H. Dynamic Bonds between Boronic Acid and Alginate: Hydrogels with Stretchable, Self-Healing, Stimuli-Responsive, Remoldable, and Adhesive Properties. Biomacromolecules 2018, 19, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Progress in Polymer Science (Oxford) 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Tønnesen, H.H.; Karlsen, J. Alginate in drug delivery systems. Drug Development and Industrial Pharmacy 2002, 28, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Bull, S.D.; Davidson, M.G.; van den Elsen, J.M.; Fossey, J.S.; Jenkins, A.T.; Jiang, Y.B.; Kubo, Y.; Marken, F.; Sakurai, K.; Zhao, J.; et al. Exploiting the reversible covalent bonding of boronic acids: recognition, sensing, and assembly. Acc Chem Res 2013, 46, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Brooks, W.L.A.; Deng, C.C.; Sumerlin, B.S. Structure–Reactivity Relationships in Boronic Acid–Diol Complexation. ACS Omega 2018, 3, 17863–17870. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Springsteen, G.; Deeter, S.; Wang, B. The relationship among pKa, pH, and binding constants in the interactions between boronic acids and diols—it is not as simple as it appears. Tetrahedron 2004, 60, 11205–11209. [Google Scholar] [CrossRef]
- Choi, Y.; Park, K.; Choi, H.; Son, D.; Shin, M. Self-Healing, Stretchable, Biocompatible, and Conductive Alginate Hydrogels through Dynamic Covalent Bonds for Implantable Electronics. Polymers 2021, 13, 1133. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, W.; Veiseh, O.; Appel, E.A.; Xue, K.; Webber, M.J.; Tang, B.C.; Yang, X.-W.; Weir, G.C.; Langer, R.; et al. Injectable and Glucose-Responsive Hydrogels Based on Boronic Acid–Glucose Complexation. Langmuir 2016, 32, 8743–8747. [Google Scholar] [CrossRef] [PubMed]
- Saravanou, S.F.; Tsitsilianis, C.; Pasparakis, G. Harnessing the Interplay of Triple Cross-Linked Hydrogels toward Multiresponsive Alginate-Based Injectable Gels for 3D Printing Bioapplications. ACS Macro Letters 2023, 12, 1614–1622. [Google Scholar] [CrossRef] [PubMed]
- Chai, Z.; Dong, H.; Sun, X.; Fan, Y.; Wang, Y.; Huang, F. Development of glucose oxidase-immobilized alginate nanoparticles for enhanced glucose-triggered insulin delivery in diabetic mice. Int J Biol Macromol 2020, 159, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Stubelius, A.; Lee, S.; Almutairi, A. The Chemistry of Boronic Acids in Nanomaterials for Drug Delivery. Accounts of Chemical Research 2019, 52, 3108–3119. [Google Scholar] [CrossRef] [PubMed]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nature Reviews Materials 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Song, C.; Wang, C.; Hu, Y.; Wu, J. Hydrogel-Based Controlled Drug Delivery for Cancer Treatment: A Review. Molecular Pharmaceutics 2020, 17, 373–391. [Google Scholar] [CrossRef] [PubMed]
- Sonker, M.; Bajpai, S.; Khan, M.A.; Yu, X.; Tiwary, S.K.; Shreyash, N. Review of Recent Advances and Their Improvement in the Effectiveness of Hydrogel-Based Targeted Drug Delivery: A Hope for Treating Cancer. ACS Applied Bio Materials 2021, 4, 8080–8109. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Lai, X.; Luo, Z.; Chen, Y.; Loh, X.J.; Ye, E.; Li, Z.; Wu, C.; Wu, Y.-L. Recent advances in mechanical force-responsive drug delivery systems. Nanoscale Advances 2022, 4, 3462–3478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, J.; Bomba, H.N.; Zhu, Y.; Gu, Z. Mechanical Force-Triggered Drug Delivery. Chemical Reviews 2016, 116, 12536–12563. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kaplan, J.A.; Colson, Y.L.; Grinstaff, M.W. Mechanoresponsive materials for drug delivery: Harnessing forces for controlled release. Advanced Drug Delivery Reviews 2017, 108, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pisapati, A.V.; Zhang, X.F.; Cheng, X. Recent Developments in Nanomaterial-Based Shear-Sensitive Drug Delivery Systems. Advanced Healthcare Materials 2021, 10, 2002196. [Google Scholar] [CrossRef] [PubMed]
- Zaki, N.M.; Awad, G.A.; Mortada, N.D.; Abd ElHady, S.S. Enhanced bioavailability of metoclopramide HCl by intranasal administration of a mucoadhesive in situ gel with modulated rheological and mucociliary transport properties. European Journal of Pharmaceutical Sciences 2007, 32, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Xiao, P.; Gu, J.; Wen, X.; Xu, J.; Zhao, C.; Zhang, J.; Chen, T. Self-healable macro-/microscopic shape memory hydrogels based on supramolecular interactions. Chemical Communications 2014, 50, 12277–12280. [Google Scholar] [CrossRef] [PubMed]
- Glynn, S.J.; Gaffney, K.J.; Sainz, M.A.; Louie, S.G.; Petasis, N.A. Molecular characterization of the boron adducts of the proteasome inhibitor bortezomib with epigallocatechin-3-gallate and related polyphenols. Organic & Biomolecular Chemistry 2015, 13, 3887–3899. [Google Scholar] [CrossRef]
- Vancoillie, G.; Hoogenboom, R. Synthesis and polymerization of boronic acid containing monomers. Polymer Chemistry 2016, 7, 5484–5495. [Google Scholar] [CrossRef]
- Piest, M.; Zhang, X.; Trinidad, J.; Engbersen, J.F.J. pH-responsive, dynamically restructuring hydrogels formed by reversible crosslinking of PVA with phenylboronic acid functionalised PPO–PEO–PPO spacers (Jeffamines®). Soft Matter 2011, 7, 11111–11118. [Google Scholar] [CrossRef]
- Marco-Dufort, B.; Iten, R.; Tibbitt, M.W. Linking Molecular Behavior to Macroscopic Properties in Ideal Dynamic Covalent Networks. Journal of the American Chemical Society 2020, 142, 15371–15385. [Google Scholar] [CrossRef] [PubMed]
- Zaki, N.M.; Awad, G.A.; Mortada, N.D.; Abd Elhady, S.S. Enhanced bioavailability of metoclopramide HCl by intranasal administration of a mucoadhesive in situ gel with modulated rheological and mucociliary transport properties. Eur J Pharm Sci 2007, 32, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.C.; Hanson, M.C.; Massey, A.P.; Karren, E.A.; Kiser, P.F. Dynamically Restructuring Hydrogel Networks Formed with Reversible Covalent Crosslinks. Advanced Materials 2007, 19, 2503–2507. [Google Scholar] [CrossRef]
- Gallagher, K.M.; Corrigan, O.I. Mechanistic aspects of the release of levamisole hydrochloride from biodegradable polymers. J. Controlled Release 2000, 69, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.I.V.; Tyler, P.; Borgognone, M.G.; Eriksen, B.M. Relationships between shear rheology and sensory attributes of hydrocolloid-thickened fluids designed to compensate for impairments in oral manipulation and swallowing. Journal of Food Engineering 2019, 263, 123–131. [Google Scholar] [CrossRef]

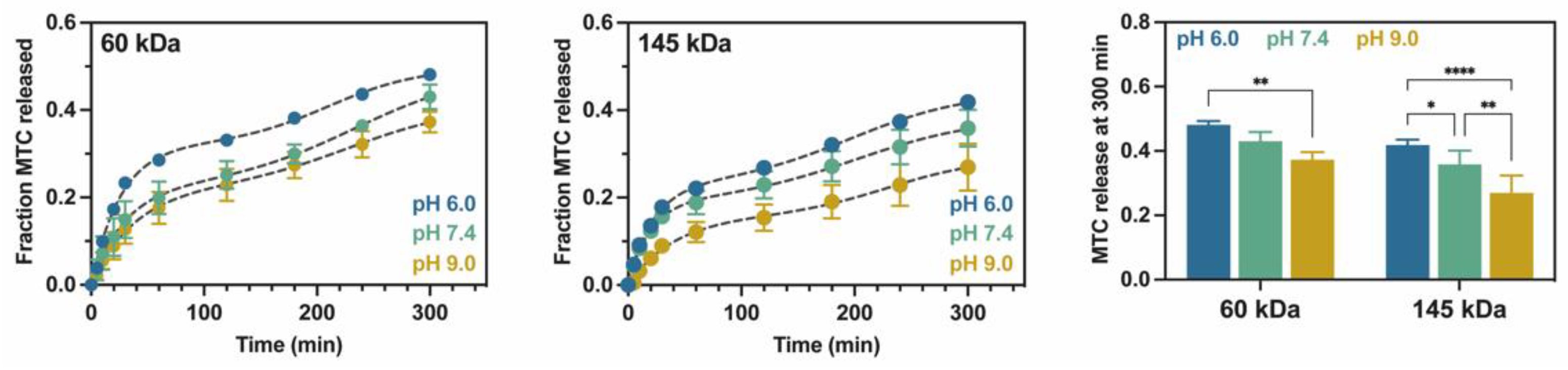
| 60 kDa | 145 kDa | |||||
| pH 6.0 | pH 7.4 | pH 9.0 | pH 6.0 | pH 7.4 | pH 9.0 | |
| Fmax | 0.318 ± 0.007 | 0.242 ± 0.026 | 0.226± 0.022 | 0.226 ± 0.010 | 0.190± 0.034 | 0.156 ± 0.401 |
| FB | 0.140 ± 0.024 | 0.030 ± 0.024 | 0.040± 0.031 | 0.009 ± 0.027 | 0.016± 0.016 | 0.018 ± 0.034 |
| k3 | 0.038 ± 0.002 | 0.032 ± 0.015 | 0.025± 0.007 | 0.044 ± 0.003 | 0.051± 0.014 | 0.025 ± 0.004 |
| k4 | 0.025 ± 0.003 | 0.021 ± 0.010 | 0.020± 0.001 | 0.019 ± 0.002 | 0.018± 0.001 | 0.022 ± 0.004 |
| tmax | 208.625 ± 7.364 | 231.450 ± 8.655 | 233.233± 18.551 | 196.850 ± 7.445 | 201.200± 6.969 | 233.800 ± 5.631 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





