1. Introduction
Dengue and Chikungunya are vector-borne diseases due to arbovirus belonging to
Flaviviridae and
Togaviridae families respectively. They are the most arbovirus identified in the world [
1,
2]. Mosquitoes are the most important arthropod disease vector in the world [
3]. Mosquitoes are estimated at 300 species capable of diseases transmission, however,
Aedes (A.) and
Culex mosquitoes are considered the most medically important mosquito vectors [
4].
Aedes aegypti and
Aedes albopictus have been reported to play major roles in the transmission of dengue virus (DENV) and chikungunya virus (CHIKV) to humans and animals [
5].In Africa, other species including
A. furcifer, A. vittatus, A. fulgens, A. luteocephalus, A. dalzieli, A. vigilax, A. camptorhynchites, Culex annulorostris and
Mansonia uniformis have also been implicated [
6]. Many factors influence the transmission of arboviruses, such as climate change and globalization directly impact the spread of mosquitoes [
7]. The tropical climate of Burkina Faso is suitable for mosquito breeding. Also, people largely depend on water storage devices, which creates most of the
A. Aegypti preferred larval water containers. In Burkina Faso, DENV is the mainly arbovirus responsible for outbreak with the highest morbidities and mortality [
8,
9,
10]. CHIKV seroprevalence was also reported [
11] and in December 2023 an outbreak of CHIKV occurred in Burkina Faso mainly in the region of Pouytenga with 311 cases confirmed by PCR [
12].When DENV serotypes co-circulate with CHIKV, this can lead to increase the risk for more severe dengue forms [
7].To date, no specific antiviral drug and no effective vaccine are available against DENV and CHIKV [
13]. However, until an effective vaccine become available, vector control will continue to be the primary approach for managing these arboviruses transmission. The best approach to predict when and where imminent DENV or CHIKV epidemics will occur is to conduct routine molecular monitoring by entomo-virologic surveillance of arbovirus circulation in mosquitoes and humans, for early detection of any serotype or genotype shift in a geographic area. Appropriate public health strategies can then be implemented to contain the cases and thereby control the infection [
14,
15,
16]. Mosquito-based arboviral surveillance is important to understand the dynamics of arbovirus disease transmission in a region [
3].
Due to the low arbovirus infection rates in mosquito populations, entomo-virologic surveillance need to maximize sample sizes during traps in order to increase detection probability [
17]. Generally, real time RT-PCR using TaqMan technology is used to detect arbovirus in mosquito. But this method is expensive if it is used for surveillance with lot of samples, principally in least developing countries, like Burkina Faso. Thus, we previously developed a low-cost new one-step multiplex Reverse Transcription Polymerase Chain Reaction (mRT-PCR) and a real-time, one-step, multiplex SYBR Green I RT-PCR (mRT-qPCR) for the rapid and simultaneous detection and serotyping of DENV and CHIKV for arbovirus diagnosis and surveillance in humans [
18] . The aim of this study is to show the potential performance of these two new molecular methods for detection of DENV serotypes and CHIKV circulating in mosquitoes by their screening, in field-collected mosquitoes (urban and sylvatic areas) from Hauts-Bassins region, Burkina Faso.
2. Materials and Methods
2.1. Study Area
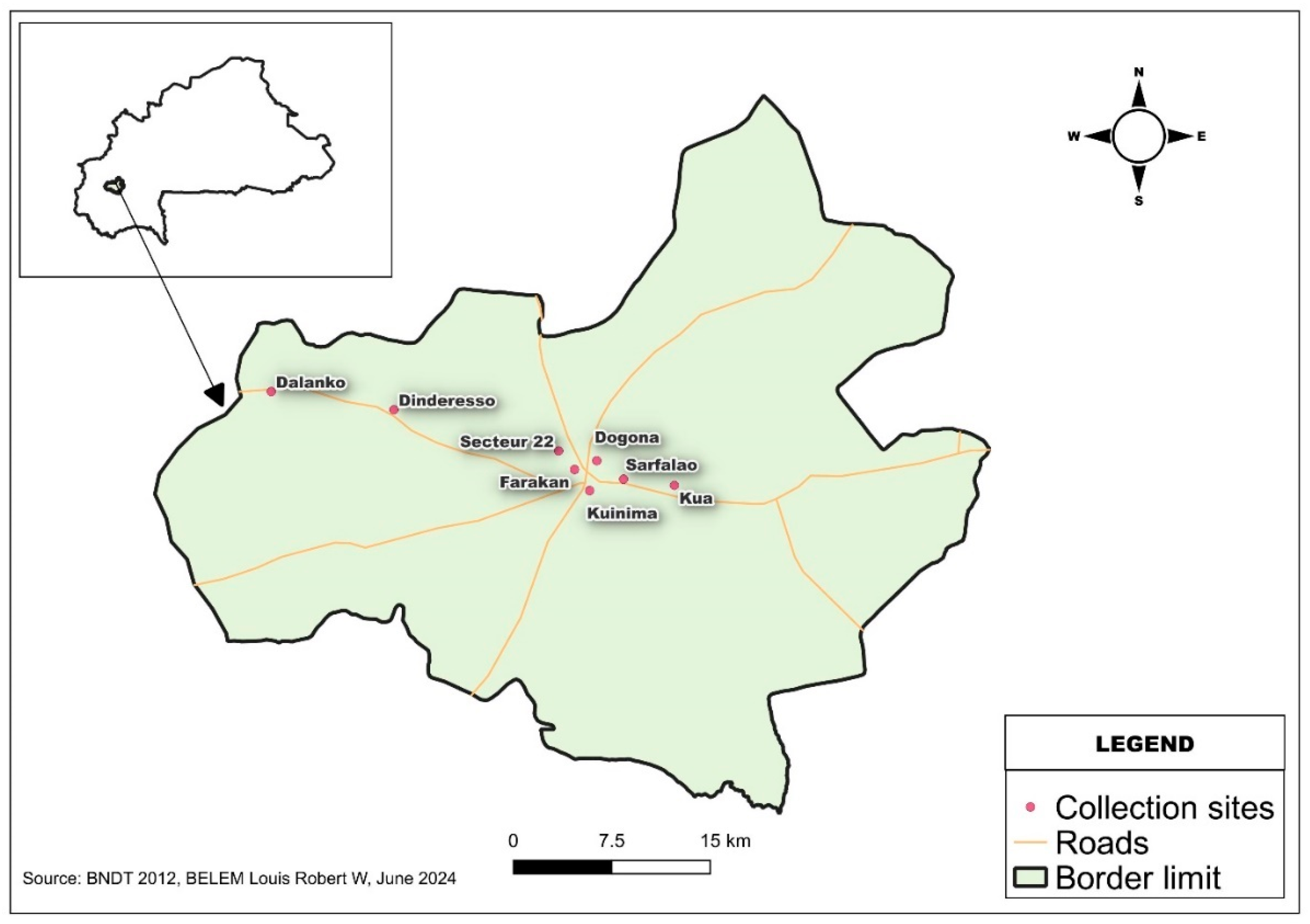
This study was conducted in Bobo-Dioulasso from July to August 2023. Bobo-Dioulasso is the economic capital of Burkina Faso and locate in the region of Hauts-Bassins, western of the country (11° 11′ 00″ North, 4° 17′ 00″ West) as indicated by the figure. 1. The study area, like the rest of the western part of the country have a South Sudanian climate characterized by two seasons: a rainy season from May to early October and a long dry season lasting around 6 months (October to April). The region of Hauts-Bassins vegetation is essentially dominated by wooded savannah and open forest and includes all subtypes from wooded savannah to grassy savannah. Mosquitoes were trapped in several any sectors (
Figure 1) of the urban area of Bobo-Dioulasso (Secteur 22, Farakan, Dogona, Kuinima, Sarfalao, and Kua) and the sylvatic area (Dindéresso and Dalanko).
2.2. Larval and Adult Mosquitoes Collection
Mosquitoes larvae were collected from mosquitoes breeding habitats such as terracotta jars, plastic container, metallic containers, and discarded tires. The collection was based on the predominance of Aedes larvae, distinguished from other larvae by their dark black color and curvilinear movements. Once the larvae had been identified, they were collected using a pipette and put in plastic cups containing water, the presence of pupae was notified. Mosquitoes larvae collected were transferred to the Centre Muraz and reared to adults.
Adult mosquitoes were collected using mechanical aspirators. The traps were performed in urban areas, mainly in households, and in forest areas, particularly near natural streams and rivers in order to determine the sylvatic circulation of DENV and CHIKV. Daytime collections were conducted to collect daytime-biting Aedes mosquitoes. Mosquitoes were put in cages and carried in the laboratory of entomology of Centre Muraz for identification. Collected adult mosquitoes and adult mosquitoes from larvae were anesthetized by incubation at -20°C for 5 min and identified using a microscope (Leica, S6E, Denaher) based on morphological characteristics. Only the genus Aedes was included in this study and stored in 1.5 mL micro-centrifuge tubes containing 200µL of RANlater (Thermo Fisher Scientific Baltics UAB, Lithuania) in pools of a maximum of 30 mosquitoes, according to genus, species, sex and collection sites. Samples were stored at -20°C prior to nucleic acid extraction and molecular screening of DENV and CHIKV at the Centre MURAZ molecular biology laboratory.
2.3. RNA extraction
All mosquitoes pools were first homogenized manually for nucleic acid extraction, by using 200µL of phosphate buffer saline (PBS) 1X (Sigma-Aldrich, Merck KGaA, Darmstadt, Allemagne). The homogenized mosquitoes were centrifuged for 5 min at 15,000× g, and 140µL of supernatant was removed from each subsample for Ribonucleic acid (RNA) extraction using QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions [
19] . A purified RNA was stored at -80 °C until further processing.
2.4. Molecular Screening of DENV1-4 and CHIKV
RNA extracted from mosquitoes samples was screened for DENV1-4 and CHIKV multiplex detection. Screening was carried out using the following master mix, PrimeScript One Step RT-PCR Kit (Takara bio-INC) for mRT-PCR and the one-step QuantiTect SYBR Green kit (Qiagen, Hilden, Germany) for mRT-q PCR. The primers used in this study and the protocol of these assays were previously described by Belem et al [
18] . mRT-PCR was performed using the SimpliAmp
TM thermal cycler (Applied Biosystems, …) and PCR product were then analyzed by gel electrophoresis. Real-Time RT-PCR was performed in the CFX96 Real-Time System (BIO-RAD, California, USA).
2.5. Data Managment
The real-time RT-PCR data were analyzed using the CFX manager software version 3.1 provided by Bio-Rad, a sample was positive if cycle threshold (C
t) value was equal to or less than 33 cycles for DENV and CHIKV[
18]. DENV serotyping as well as the differentiation between DENV and CHIKV was carried out by melting temperature (Tm) analysis. Arbovirus minimum infection rate (MIR) with 95% confidence interval was determined using the Excel 2016. The MIR uses the assumption that a positive pool contains only one infected mosquito. The map figure was done using QGIS version 3.26.3.
3. Results
3.1. Mosquitoes Collection and Identification
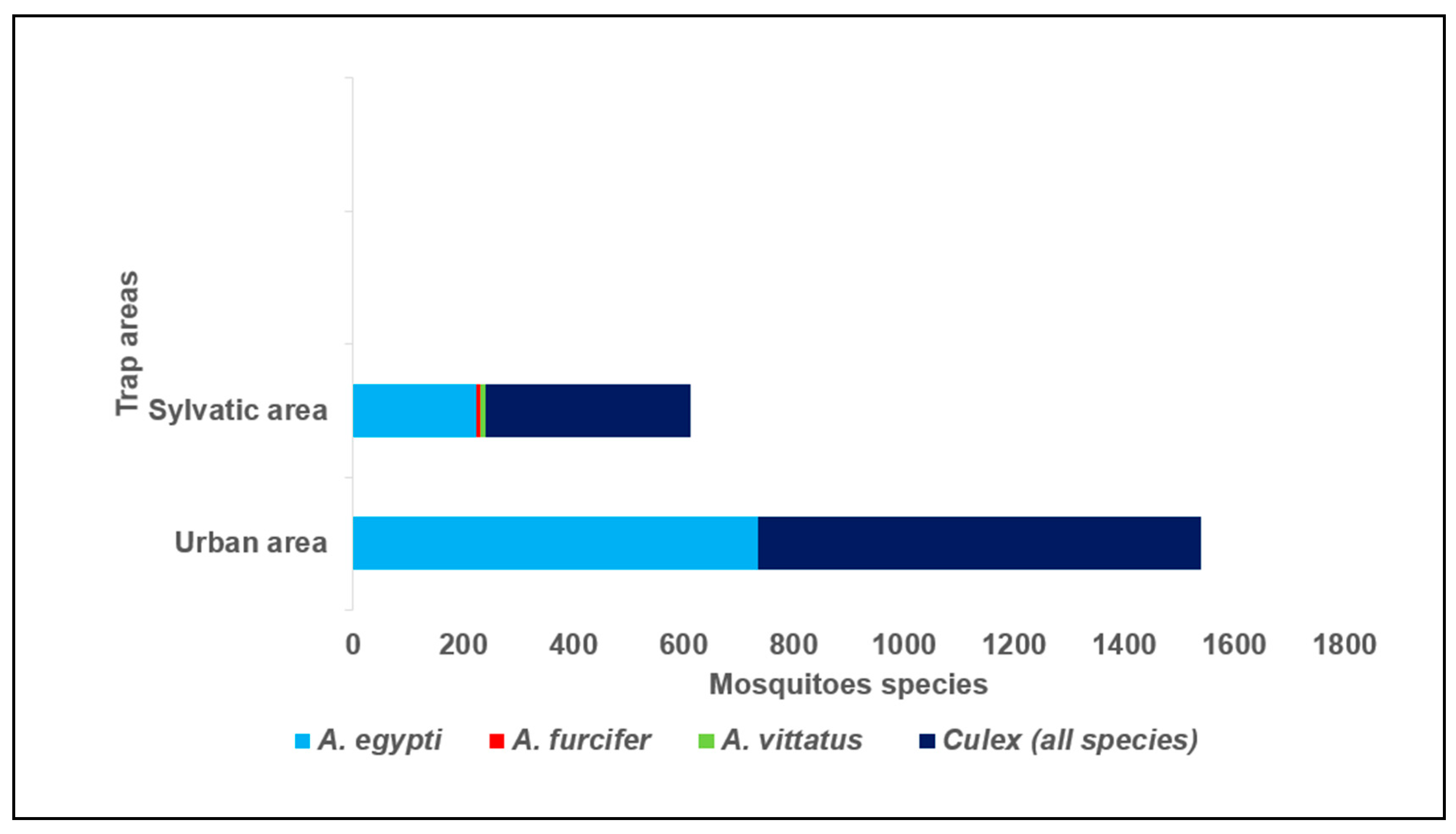
A total of 2150 mosquitoes were trapped at Bobo-Dioulasso from July to August 2023, including 976 (45.4%)
Aedes and 1174 (54.5%)
Culex. Only genus
Aedes has been included in for assays of this study. 69.0 % (1484/2150) of mosquitoes were collected in urban area against 31% (666/2150) in sylvatic areas (
Figure 2). 19.9 % (195/976) of
Aedes were larvae and became adult after rearing. Among
Aedes mosquitoes we have identified 959
A. egypti (476 males + 483 females), 6
A. furcifer (4 males +2 females) and 11
A. Vittatus (7 males +4 females). The repartition of mosquitoes traps was described in the figure 2. All
Aedes mosquitoes were divided in thirty-nine pools with a maximum of 30 mosquitoes/pool, according to species, sex and collection site
3.2. Molecular Detection of Arboviruses in Mosquitoes Pools
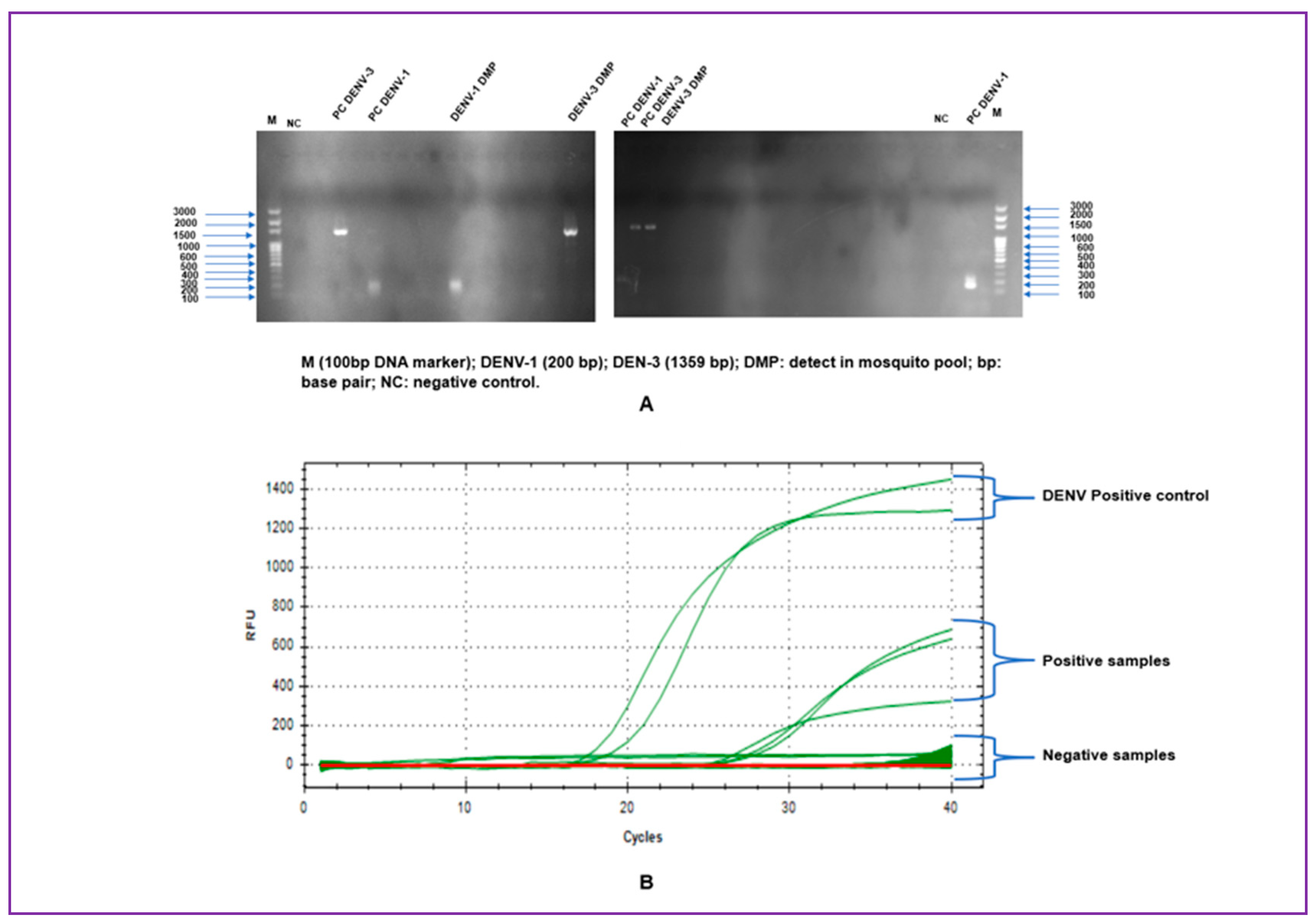
All RNA of 39 pools were screened by mRT-PCR and mRT-q PCR for DENV1-4 and CHIKV detection (
Figure 3). From the 39 pools tested, 7.7% (3/39) were positive for DENV. The concordance between the mRT-PCR and the mRT-qPCR were 100%. Therefore, all positive pools by mRT-PCR were also positive by mRT-qPCR. DENV has been detected only in
A. egypti female pools from adult mosquitoes traps and all positive were found in urban area of Bobo-Dioulasso (
Table 1). DENV has not been detected in mosquitoes from larvae. CHIKV has not been detected in mosquitoes pools. Of the three positive pools, DENV-1 was detected in one pool (1/3) and DENV-3 in two pools (2/3). DENV-2 and DENV-4 have been not detected. The MIR of DENV in urban area was 4.08 (CI 95% :3.01—25.91). Overall MIR of DENV in this study was 3.07 (95% CI: 2.24 —19.86) (
Table 1).
4. Discussion
In recent year, distribution of vector-borne viruses has increased exponentially, with climatic changes, migration and globalization. The distribution has taken place mainly in tropical climate countries like Burkina Faso. The entomo-virological surveillance plays an important role in arboviruses surveillance thanks to the efficacity to detected early arboviruses circulation and implement appropriate public health measures to contain the cases and thus control the infection[
16]. This study was performed to screen DENV1-4 and CHIKV circulation in mosquitoes with new molecular methods previously developed by Belem
et al, 2024[
18] and show their potential and usefulness in arboviruses surveillance. The study has taken place in the context of dengue epidemic in Bobo-Dioulasso, Burkina Faso. The main mosquitoes included in this study were genus
Aedes, among them we have identified 03 species. In urban area we have identified only
A. egypti, this can explain that it is the main dengue vector in Burkina urban areas [
20]. In Burkina Faso many people stored a long-time water in various container for routine household. This practice serves as permanent breeding sites and continuous source of
A. egypti [
21] . Another species like
A.
furcifer and
A. vittatus were also identified in forest area. Traps in this area were performed to detect DENV and CHIKV circulation in sylvatic cycle. In this study
A. Albopictus, second main vector of DENV and CHIKV, has been not identified. To date no study has identified this species in Burkina Faso [
22]
In this study our new molecular methods have detected DENV in three pools of female
A. egypti from traps in urban area, with MIR of 4.08 (IC95%:3.01—25.91). Serotyping has shown co-circulation of DENV-1 and DENV-3 in mosquitoes in Bobo-Dioulasso. These results demonstrate the efficacity of our new methods to detect arbovirus in mosquitoes. Virological surveillance strategies for
Aedes mosquitoes, constitutes a useful means for identifying high-risk areas for arboviruses transmission and an epidemic alert system [
16]. Our new molecular methods will be useful for entomo-virological surveillance, because they are sensitive, low-cost and easy to use. Detection of DENV in mosquitoes has been followed a dengue epidemic in Bobo-Dioulasso, declared by the Director of Medical and Technical Services of CHUSS/Bobo-Dioulasso the 11 August 2016 and reported in the weekly reports of epidemiologic situations [
12]. In this study we have not detect arbovirus in mosquitoes from collected larvae, but it is important to screen arboviruses in mosquitoes from larvae to determine vertical transmission. Vertical transmission can maintain the circulation of arbovirus in vector populations [
23]. This situation could be an origin of a re-emergence of dengue disease during raining season.
Traps of mosquitoes in sylvatic area were performed to determine sylvatic circulation of DENV and CHIKV. Major arboviruses (DENV, CHIKV, Zika virus and yellow fever virus) responsible of humans infection are originated in sylvatic cycles transmission, including vertebrate animals and wild mosquitoes [
24]. Also, major arboviruses may transfer from urban cycle to sylvatic cycle and could hinder arbovirus eradication. Moreover, arbovirus could re-emerge anytime from in a sylvatic cycle, creating outbreaks [
25]. Arbovirus emergence or re-emergence is an imminent threat of public health, consequently careful surveillance of arbovirus in the human population and the sylvatic environment is crucial to apply suitable public health measures to avoid outbreak[
16] . An important study involving large investigations on DENV serotypes and CHIKV circulation among wild mosquitoes, bats, monkeys in the several forest areas in Burkina Faso is needed to understand arbovirus sylvatic cycle and to control this. Our multiplex molecular methods will be useful for these large investigations.
5. Conclusions
This study shows the performance of mRT-PCR and mRT- q PCR in detection of arbovirus in mosquitoes. These results demonstrate the usefulness of our new molecular methods previously developed and validated for multiplex detection and serotyping of DENV and CHIKV in humans clinical samples. These methods are low-cost, simple and can be used for entomo-virological surveillance strategies for Aedes mosquitoes.
Author Contributions
Conceptualization, M.K.G., L.R.W.B., N.M., and I.S.; methodology, L.R.W.B., M.F.A., Z.L., A.L., and T.B.; validation, M.K.G., L.R.W.B., and I.S.; writing—original draft, L.R.W.B., M.K.G., E.B., K.G., R.K.Y., and I.S.; supervision, L.R.W.B., M.K.G. and I.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Fond National de la Recherche et de l’Innovation pour le Devéloppement of BURKINA FASO (Grant No. FONRID/AAP-Spécial-Jeunes/NCP/PCD/2022) and Centre d’Excellence Africain en Innovations Biotechnologiques pour l’Elimination des Maladies à Transmission Vectorielle of BURKINA FASO (Grant No.2020-000178/MESRSI/SG/UNB/P).
Data Availability Statement
Data are contained within the article and Supplementary data.
Acknowledgments
We sincerely thank Fond National de la Recherche et de l’Innovation pour le Devéloppement (FONRID) of Burkina Faso, Centre d’Excellence Africain en Innovations Biotechnologiques pour l’Elimination des Maladies à Transmission Vectorielle (CEA/ITECH-MTV) of BURKINA FASO and the World Academy of Science- International Centre for Genetic Engineering and Biotechnology (TWAS-ICGEB).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Konongoi, L.; Ofula, V.; Nyunja, A.; Owaka, S.; Koka, H.; Makio, A.; Koskei, E.; Eyase, F.; Langat, D.; Schoepp, R. J.; et al. Detection of Dengue Virus Serotypes 1, 2 and 3 in Selected Regions of Kenya: 2011-2014. Virol J 2016, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Chikungunya. Available online: https://www.who.int/health-topics/chikungunya#tab=tab_1 (accessed on 15 September 2023).
- Fang, Y.; Tambo, E.; Xue, J. B.; Zhang, Y.; Zhou, X. N.; Khater, E. I. M. Detection of DENV-2 and Insect-Specific Flaviviruses in Mosquitoes Collected from Jeddah, Saudi Arabia. Front. Cell Infect. Microbiol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Gao, X.; Gould, E. A. Factors Responsible for the Emergence of Arboviruses; Strategies, Challenges and Limitations for Their Control. Emerg. Microbes Infect. 2015, 4, e18. [Google Scholar] [CrossRef] [PubMed]
- Islam, M. A.; El Zowalaty, M. E.; Islam, S.; Sharif, M.; Rahman, M. R.; Amin, M. R.; Ali, M. M.; Rahman, M. T.; Morita, K.; Ashour, H. M. A Novel Multiplex RT-PCR Assay for Simultaneous Detection of Dengue and Chikungunya Viruses. Int J Mol Sci 2020, 21, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Olajiga, O. M.; Adesoye, O. E.; Emilolorun, A. P.; Adeyemi, A. J.; Adeyefa, E. O.; Aderibigbe, I. A.; Adejumo, S. A.; Adebimpe, W. O.; Opaleye, O. O.; Sule, W. F.; Oluwayelu, D. O. Chikungunya Virus Seroprevalence and Associated Factors among Hospital Attendees in Two States of Southwest Nigeria: A Preliminary Assessment. Immunol Invest 2017, 46, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Gould, E.; Pettersson, J.; Higgs, S.; Charrel, R.; de Lamballerie, X. Emerging Arboviruses: Why Today? One Health 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lim, J. K.; Seydou, Y.; Carabali, M.; Barro, A.; Dahourou, D. L.; Lee, K. S.; Nikiema, T.; Namkung, S.; Lee, J. S.; Shin, M. Y.; et al. Clinical and Epidemiologic Characteristics Associated with Dengue during and outside the 2016 Outbreak Identified in Health Facility-Based Surveillance in Ouagadougou, Burkina Faso. PLoS Negl Trop Dis 2019, 13. [Google Scholar] [CrossRef] [PubMed]
- Im, J.; Balasubramanian, R.; Ouedraogo, M.; Wandji Nana, L. R.; Mogeni, O. D.; Jeon, H. J.; van Pomeren, T.; Haselbeck, A.; Lim, J. K.; Prifti, K.; et al. The Epidemiology of Dengue Outbreaks in 2016 and 2017 in Ouagadougou, Burkina Faso. Heliyon 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Tarnagda, Z.; Cissé, A.; Bicaba, B. W.; Diagbouga, S.; Sagna, T.; Ilboudo, A. K.; Tialla, D.; Lingani, M.; Sondo, K. A.; Yougbaré, I.; et al. Dengue Fever in Burkina Faso, 2016. Emerg Infect Dis 2018, 24, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Lim, J. K.; Ridde, V.; Agnandji, S. T.; Lell, B.; Yaro, S.; Yang, J. S.; Hoinard, D.; Weaver, S. C.; Vanhomwegen, J.; Salje, H.; Yoon, I. K. Seroepidemiological Reconstruction of Long-Term Chikungunya Virus Circulation in Burkina Faso and Gabon. J Infect Dis 2022. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health and Public Hygiene of Burkina Faso. Weekly Report of Epidemiologic Situation, Week N°51, 2023.
- Pando-Robles, V.; Batista, C. V. Aedes-Borne Virus-Mosquito Interactions: Mass Spectrometry Strategies and Findings. Vector-Borne and Zoonotic Dis. 2017; 361–375. [Google Scholar] [CrossRef]
- Sarma, D. K.; Rathod, L.; Mishra, S.; Das, D.; Agarwal, A.; Sharma, G.; Singh, T. A.; Kumawat, M.; Singh, S.; Verma, V.; et al. Molecular Surveillance of Dengue Virus in Field-Collected Aedes Mosquitoes from Bhopal, Central India: Evidence of Circulation of a New Lineage of Serotype 2. Front Microbiol 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, A. L.; Van Den Hurk, A. F.; Meyer, D. B.; Ritchie, S. A. Searching for the Proverbial Needle in a Haystack: Advances in Mosquito-Borne Arbovirus Surveillance. Parasites and Vectors, BioMed Central Ltd. May 29, 2018. [CrossRef]
- dos Reis, I. C.; Gibson, G.; Ayllón, T.; de Medeiros Tavares, A.; de Araújo, J. M. G.; da Silva Monteiro, E.; Rodrigues Aguiar, A.; de Oliveira, J. V.; de Paiva, A. A. P.; Wana Bezerra Pereira, H.; et al. Entomo-Virological Surveillance Strategy for Dengue, Zika and Chikungunya Arboviruses in Field-Caught Aedes Mosquitoes in an Endemic Urban Area of the Northeast of Brazil. Acta Trop 2019, 197. [Google Scholar] [CrossRef] [PubMed]
- Batovska, J.; Mee, P. T.; Sawbridge, T. I.; Rodoni, B. C.; Lynch, S. E. Enhanced Arbovirus Surveillance with High-Throughput Metatranscriptomic Processing of Field-Collected Mosquitoes. Viruses 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Belem, L. R. W.; Ibemgbo, S. A.; Gomgnimbou, M. K.; Verma, D. K.; Kaboré, A.; Kumar, A.; Sangaré, I.; Sunil, S. Development of Multiplex Molecular Assays for Simultaneous Detection of Dengue Serotypes and Chikungunya Virus for Arbovirus Surveillance. Curr Issues Mol Biol 2024, 46, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- Miot, E. F.; Calvez, E.; Aubry, F.; Dabo, S.; Grandadam, M.; Marcombe, S.; Oke, C.; Logan, J. G.; Brey, P. T.; Lambrechts, L. Risk of Arbovirus Emergence via Bridge Vectors: Case Study of the Sylvatic Mosquito Aedes Malayensis in the Nakai District, Laos. Sci Rep 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Namountougou, M.; Soma, D. D.; Balboné, M.; Kaboré, D. A.; Kientega, M.; Hien, A.; Coulibaly, A.; Ouattara, P. E.; Meda, B. G.; Drabo, S.; et al. Monitoring Insecticide Susceptibility in Aedes Aegypti Populations from the Two Biggest Cities, Ouagadougou and Bobo-Dioulasso, in Burkina Faso: Implication of Metabolic Resistance. Trop Med Infect Dis 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Konongoi, S. L.; Nyunja, A.; Ofula, V.; Owaka, S.; Koka, H.; Koskei, E.; Eyase, F.; Langat, D.; Mancuso, J.; Lutomiah, J.; Sang, R. Human and Entomologic Investigations of Chikungunya Outbreak in Mandera, Northeastern Kenya, 2016. PLoS One 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Egid, B. R.; Coulibaly, M.; Dadzie, S. K.; Kamgang, B.; McCall, P. J.; Sedda, L.; Toe, K. H.; Wilson, A. L. Review of the Ecology and Behaviour of Aedes Aegypti and Aedes Albopictus in Western Africa and Implications for Vector Control. Curr Res Parasitol Vector Borne Dis 2022. [CrossRef] [PubMed]
- Honório, N. A.; Wiggins, K.; Eastmond, B.; Câmara, D. C. P.; Alto, B. W. Experimental Vertical Transmission of Chikungunya Virus by Brazilian and Florida Aedes Albopictus Populations. Viruses 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Vasilakis, N.; Cardosa, J.; Hanley, K. A.; Holmes, E. C.; Weaver, S. C. Fever from the Forest: Prospects for the Continued Emergence of Sylvatic Dengue Virus and Its Impact on Public Health. Nat Rev Microbiol 2011, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, L. T. M. Human Urban Arboviruses Can Infect Wild Animals and Jump to Sylvatic Maintenance Cycles in South America. Front Cell Infect Microbiol, 2019; 9, 259. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).