Submitted:
05 July 2024
Posted:
12 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Clinical Profiles and Demographics
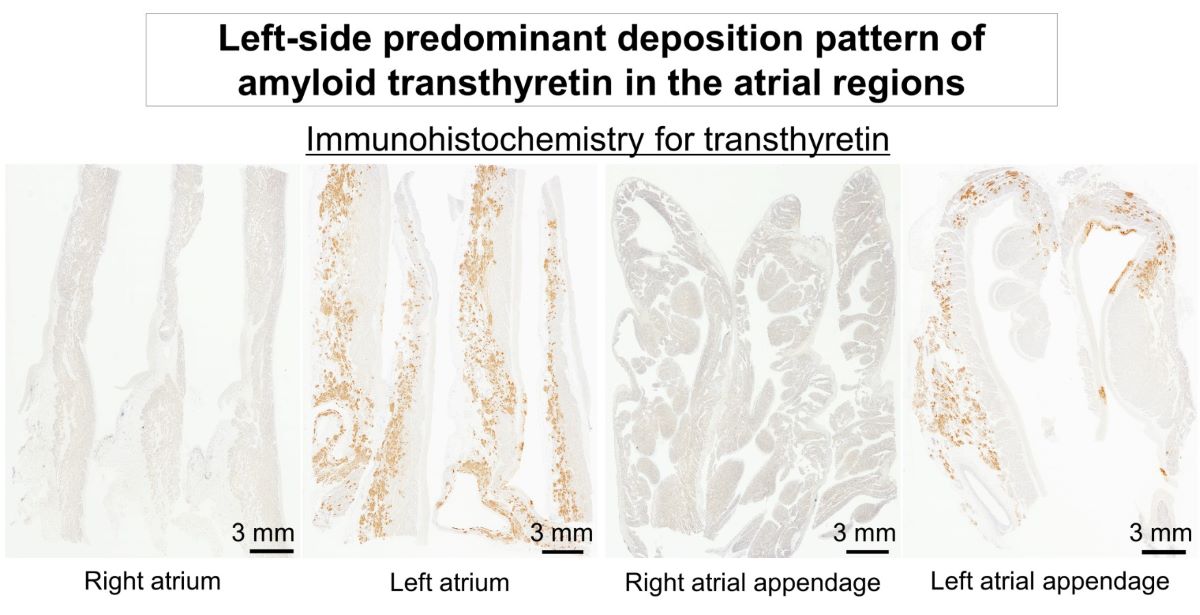
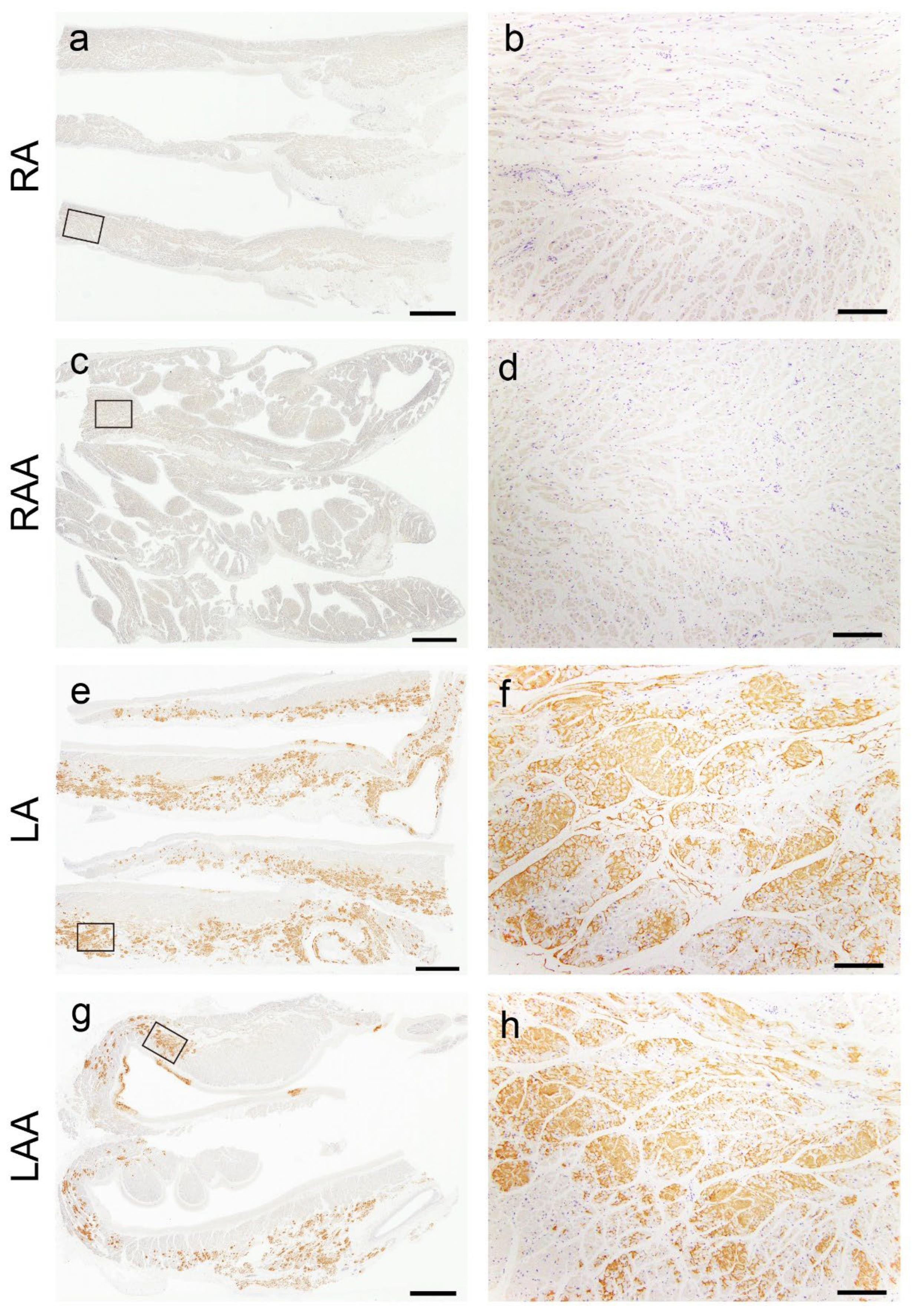
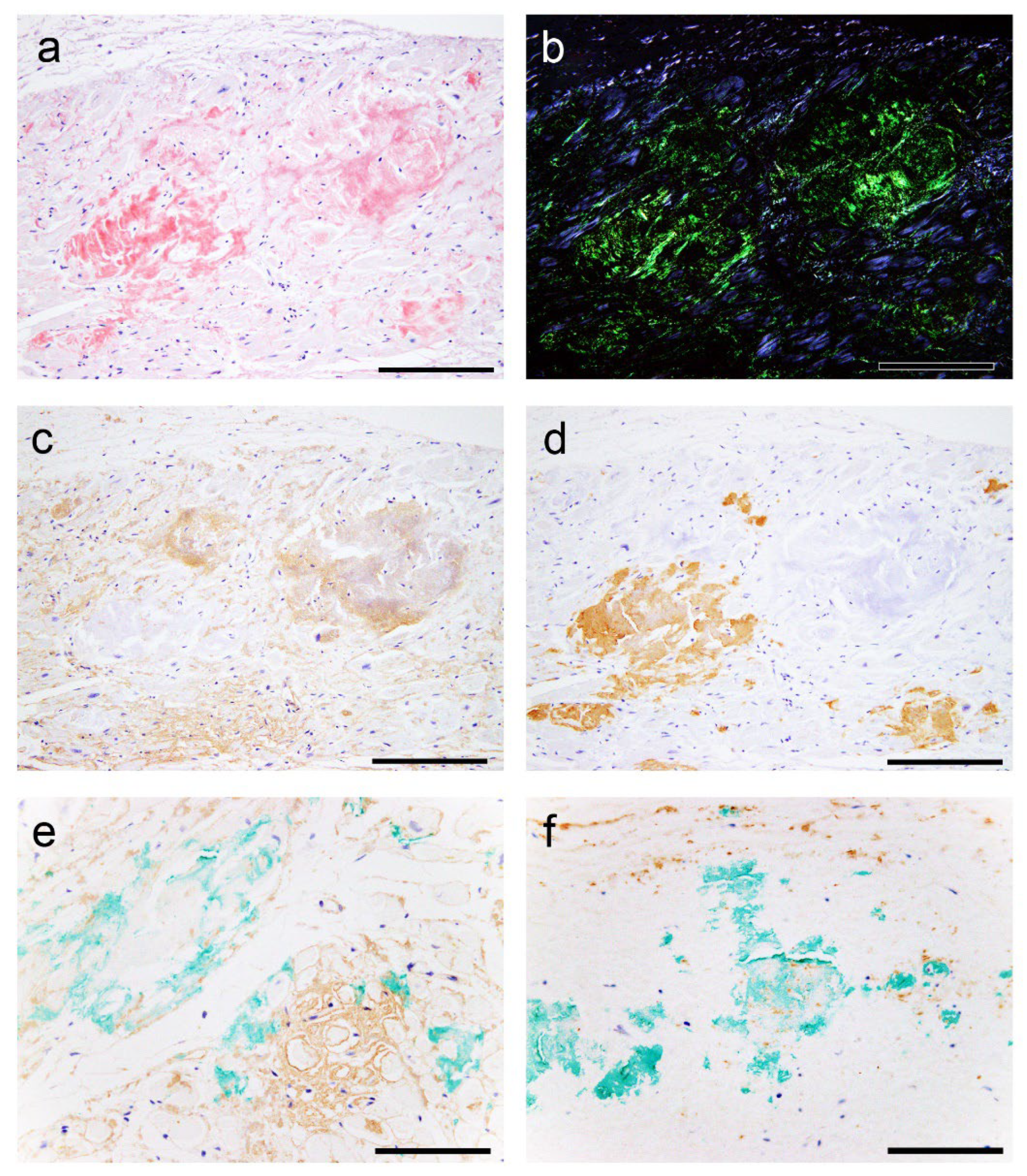
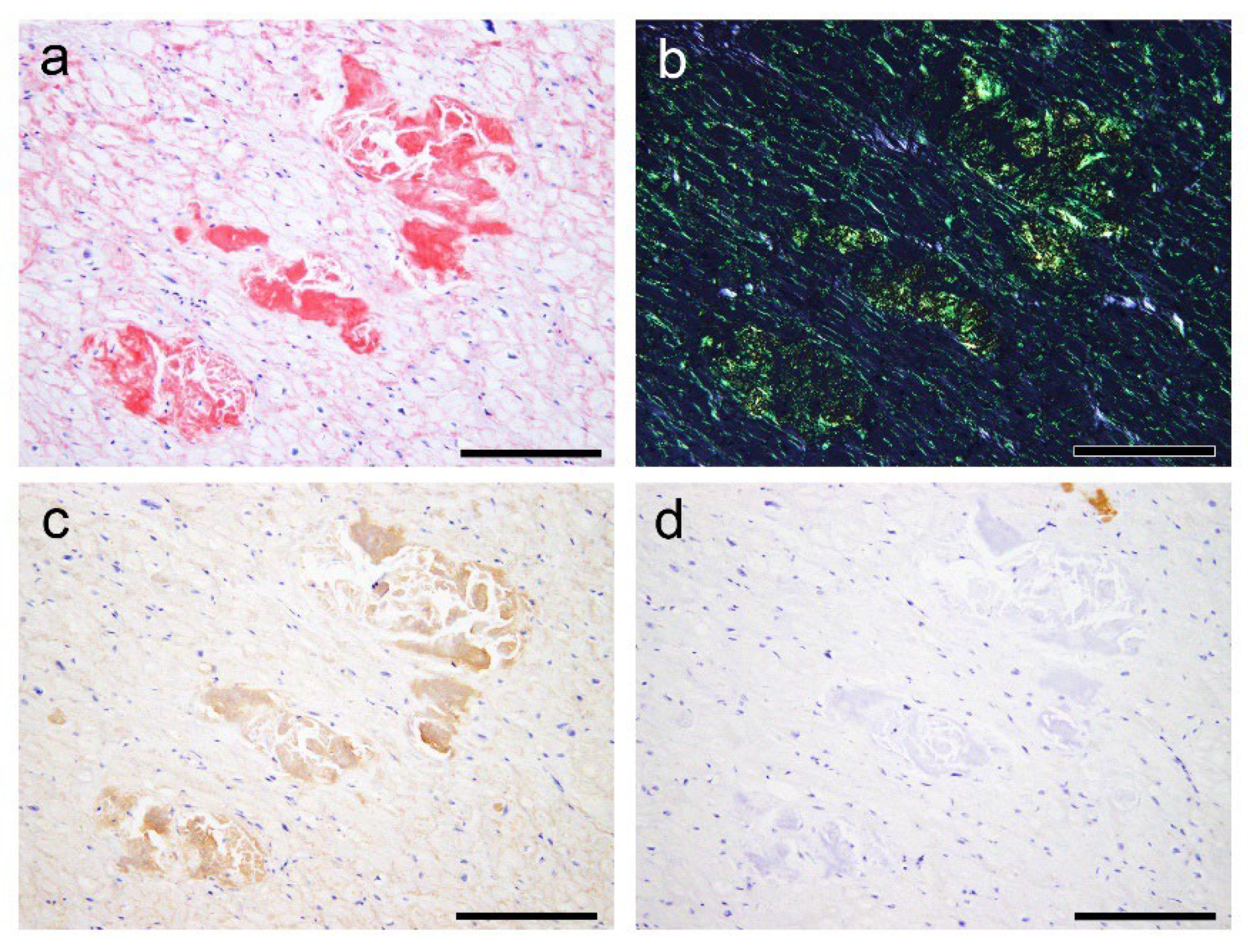
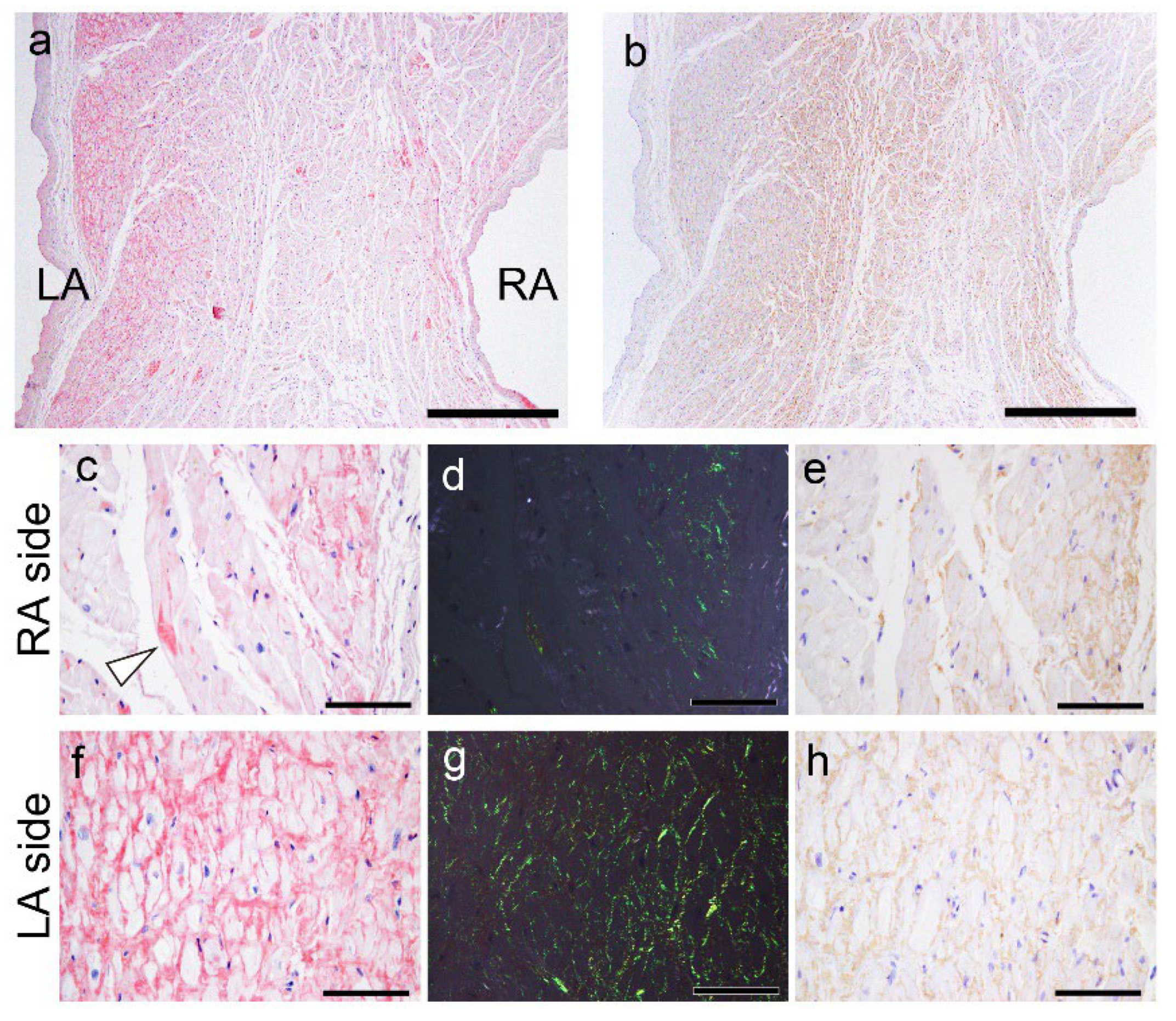
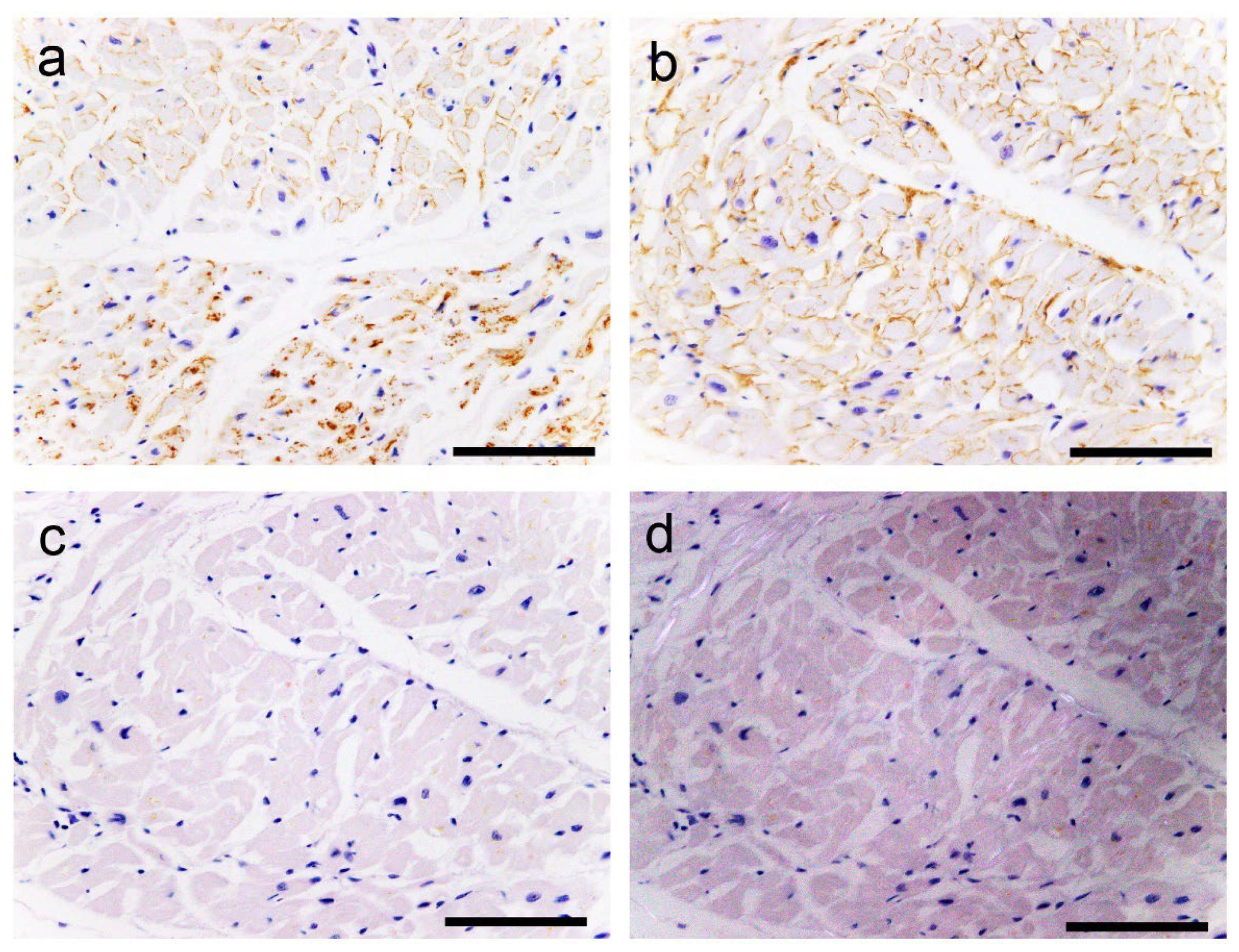
2.2. Histopathological Findings of ATTR and AANF in the Heart
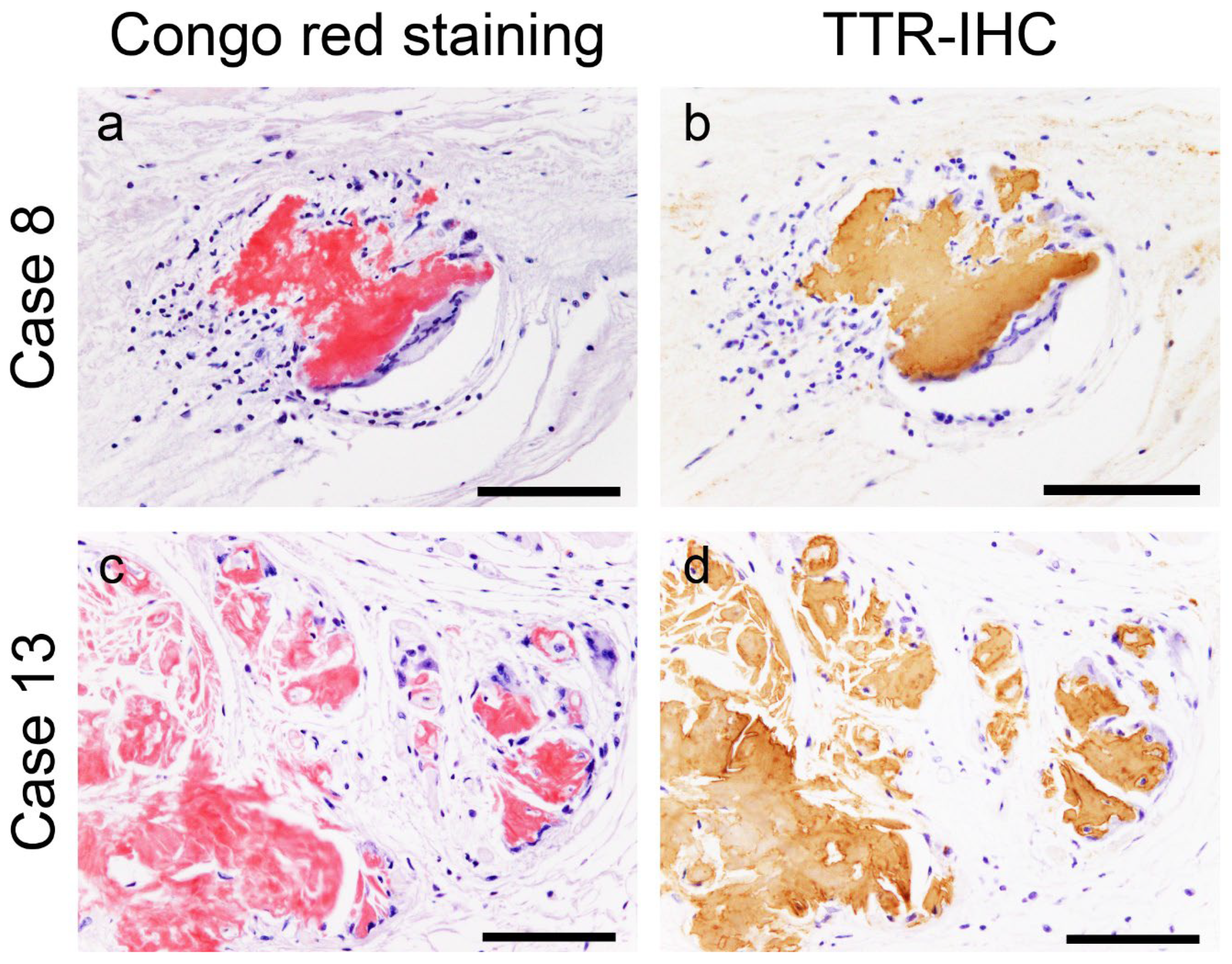
2.3. Evaluation of the Colocalisation of ATTR and AANF Using Single and Double IHC
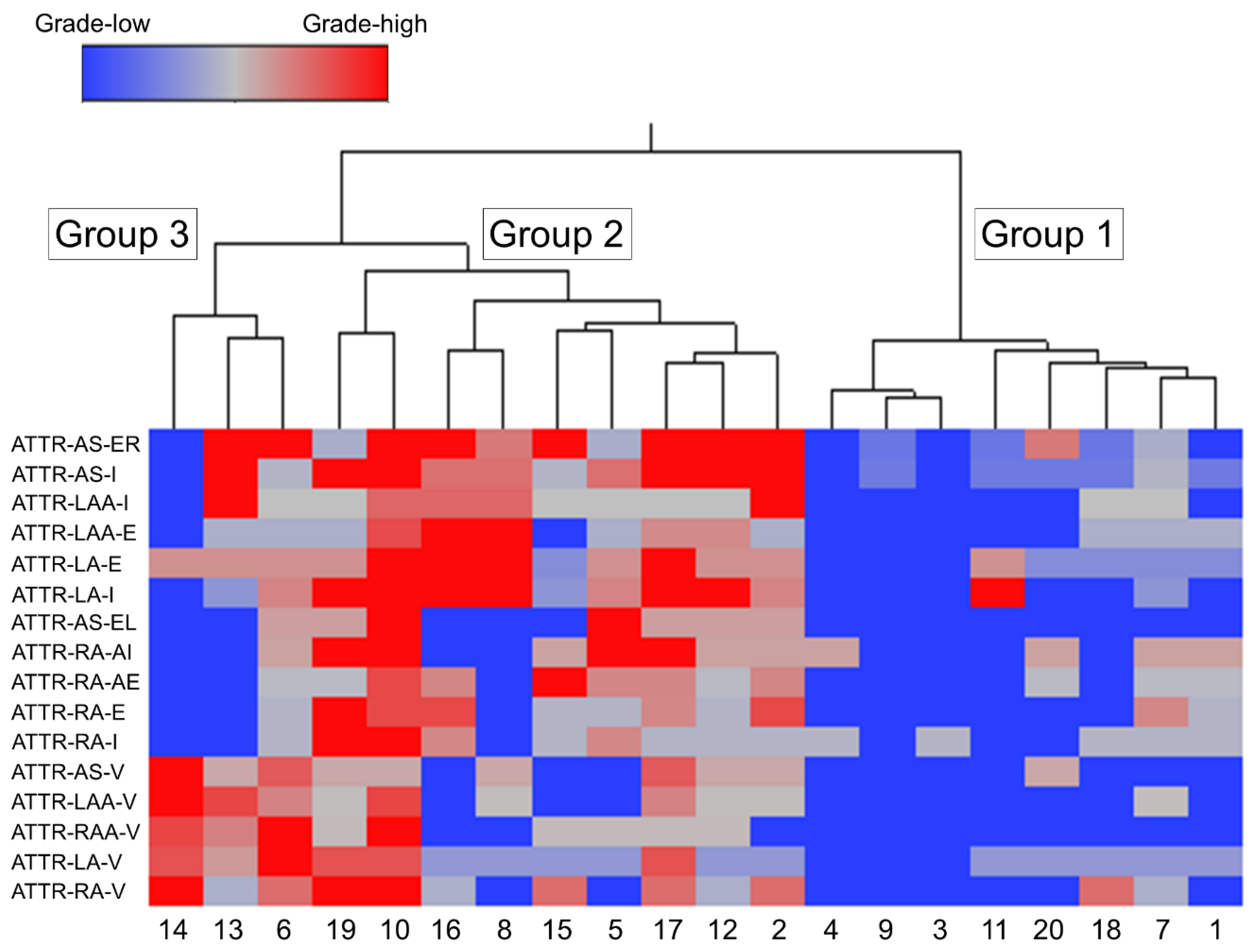
2.4. Subgroups Classified by Cluster Analysis
2.5. Comparison of ATTR and AANF Deposition Severity between Males and Females
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Tissue Samples
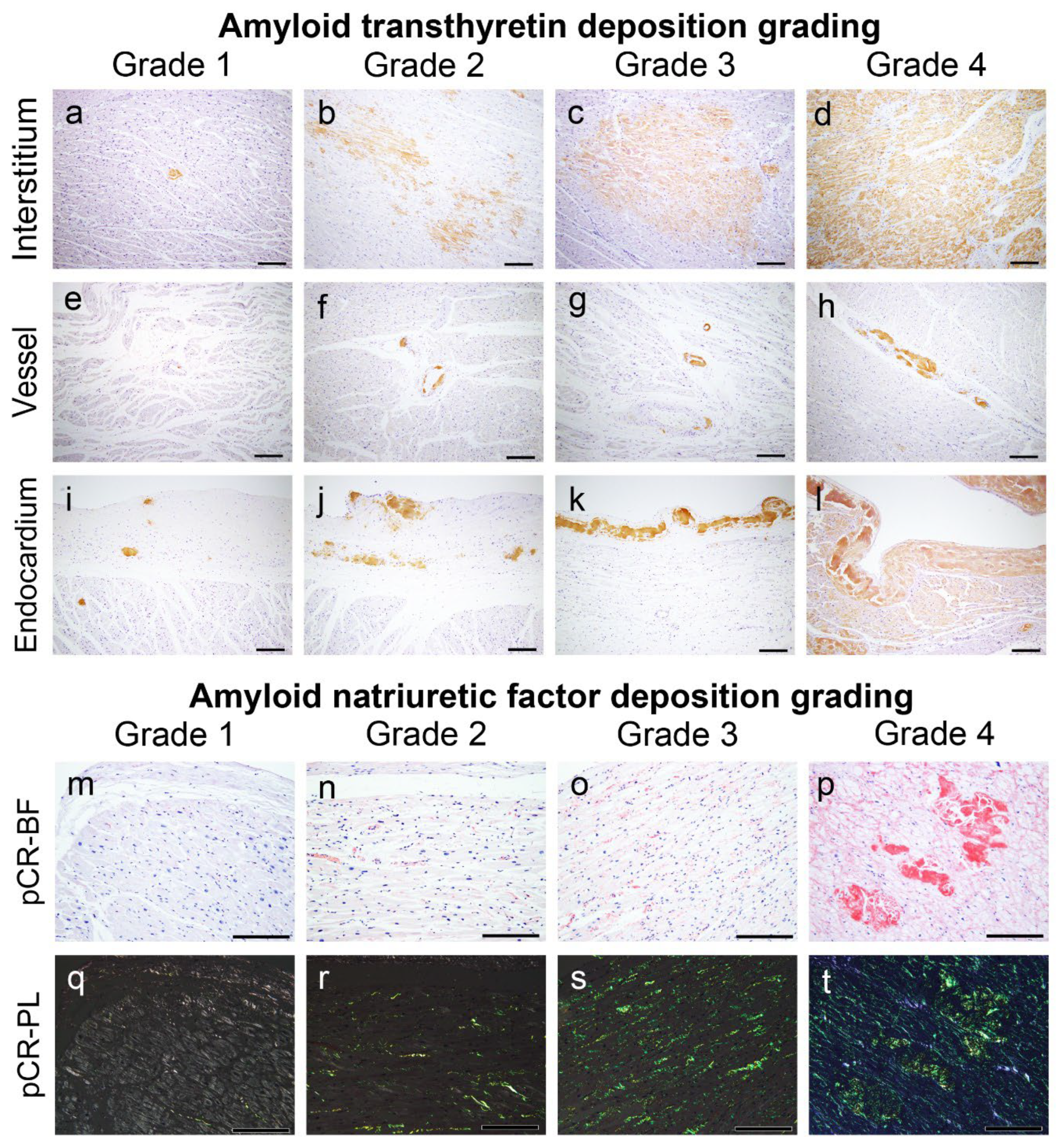
4.3. Semiquantitative Grading System for ATTR and AANF Deposition
4.4. Quantitative Analysis of ATTR Deposition Burden
4.5. Single and Double IHC
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Buxbaum, J. N.; Dispenzieri, A.; Eisenberg, D. S.; Fandrich, M.; Merlini, G.; Saraiva, M. J. M.; Sekijima, Y.; Westermark, P. , Amyloid nomenclature 2022: update, novel proteins, and recommendations by the International Society of Amyloidosis (ISA) Nomenclature Committee. Amyloid 2022, 29(4), 213–219. [Google Scholar] [CrossRef] [PubMed]
- Wechalekar, A. D.; Gillmore, J. D.; Hawkins, P. N. , Systemic amyloidosis. The Lancet 2016, 387(10038), 2641–2654. [Google Scholar] [CrossRef]
- Larsen, B. T.; Mereuta, O. M.; Dasari, S.; Fayyaz, A. U.; Theis, J. D.; Vrana, J. A.; Grogan, M.; Dogan, A.; Dispenzieri, A.; Edwards, W. D.; Kurtin, P. J.; Maleszewski, J. J. , Correlation of histomorphological pattern of cardiac amyloid deposition with amyloid type: a histological and proteomic analysis of 108 cases. Histopathology 2016, 68(5), 648–656. [Google Scholar] [CrossRef] [PubMed]
- Naiki, H.; Yamaguchi, A.; Sekijima, Y.; Ueda, M.; Ohashi, K.; Hatakeyama, K.; Ikeda, Y.; Hoshii, Y.; Shintani-Domoto, Y.; Miyagawa-Hayashino, A.; Tsujikawa, H.; Endo, J.; Arai, T.; Ando, Y. , Steep increase in the number of transthyretin-positive cardiac biopsy cases in Japan: evidence obtained by the nation-wide pathology consultation for the typing diagnosis of amyloidosis. Amyloid 2023, 30(3), 321–326. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, A.; Tasaki, M.; Ueda, M.; Ando, Y.; Naiki, H. , Epidemiological study of the subtype frequency of systemic amyloidosis listed in the Annual of the Pathological Autopsy Cases in Japan. Pathol. Int. 2024, 74(2), 68–76. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M. S.; Schwartz, J. H.; Gundapaneni, B.; Elliott, P. M.; Merlini, G.; Waddington-Cruz, M.; Kristen, A. V.; Grogan, M.; Witteles, R.; Damy, T.; Drachman, B. M.; Shah, S. J.; Hanna, M.; Judge, D. P.; Barsdorf, A. I.; Huber, P.; Patterson, T. A.; Riley, S.; Schumacher, J.; Stewart, M.; Sultan, M. B.; Rapezzi, C.; Investigators, A.-A. S. , Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N. Engl. J. Med. 2018, 379(11), 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Ruberg, F. L.; Maurer, M. S. , Cardiac amyloidosis due to transthyretin protein: a review. JAMA 2024, 331(9), 778–791. [Google Scholar] [CrossRef] [PubMed]
- Okada, A.; Kakuta, T.; Tadokoro, N.; Tateishi, E.; Morita, Y.; Kitai, T.; Amaki, M.; Kanzaki, H.; Ohta-Ogo, K.; Ikeda, Y.; Fukushima, S.; Fujita, T.; Kusano, K.; Noguchi, T.; Izumi, C. , Transthyretin derived amyloid deposits in the atrium and the aortic valve: insights from multimodality evaluations and mid-term follow up. BMC Cardiovasc. Disord. 2023, 23(1), 281. [Google Scholar] [CrossRef]
- Singulane, C. C.; Slivnick, J. A.; Addetia, K.; Asch, F. M.; Sarswat, N.; Soulat-Dufour, L.; Mor-Avi, V.; Lang, R. M. , Prevalence of right atrial impairment and association with outcomes in cardiac amyloidosis. J. Am. Soc. Echocardiogr. 2022, 35(8), 829–835. [Google Scholar] [CrossRef] [PubMed]
- Poterucha, T. J.; Maurer, M. S. , Too Stiff But Still Got Rhythm: Left atrial myopathy and transthyretin cardiac amyloidosis. JACC Cardiovasc. Imaging 2022, 15(1), 30–32. [Google Scholar] [CrossRef] [PubMed]
- Bandera, F.; Martone, R.; Chacko, L.; Ganesananthan, S.; Gilbertson, J. A.; Ponticos, M.; Lane, T.; Martinez-Naharro, A.; Whelan, C.; Quarta, C.; Rowczenio, D.; Patel, R.; Razvi, Y.; Lachmann, H.; Wechelakar, A.; Brown, J.; Knight, D.; Moon, J.; Petrie, A.; Cappelli, F.; Guazzi, M.; Potena, L.; Rapezzi, C.; Leone, O.; Hawkins, P. N.; Gillmore, J. D.; Fontana, M. , Clinical importance of left atrial infiltration in cardiac transthyretin amyloidosis. JACC Cardiovasc. Imaging 2022, 15(1), 17–29. [Google Scholar] [CrossRef] [PubMed]
- Ichimata, S.; Hata, Y.; Hirono, K.; Yamaguchi, Y.; Nishida, N. , Clinicopathological features of clinically undiagnosed sporadic transthyretin cardiac amyloidosis: a forensic autopsy-based series. Amyloid 2021, 28(2), 125–133. [Google Scholar] [CrossRef] [PubMed]
- Steiner, I.; Hajkova, P. , Patterns of isolated atrial amyloid: a study of 100 hearts on autopsy. Cardiovasc. Pathol. 2006, 15(5), 287–290. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, S.; Takahashi, M.; Ishihara, T.; Uchino, F. , Incidence and distribution of isolated atrial amyloid: histologic and immunohistochemical studies of 100 aging hearts. Pathol. Int. 1995, 45(5), 335–342. [Google Scholar] [CrossRef] [PubMed]
- Fayyaz, A. U.; Bois, M. C.; Dasari, S.; Padmanabhan, D.; Vrana, J. A.; Stulak, J. M.; Edwards, W. D.; Kurtin, P. J.; Asirvatham, S. J.; Grogan, M.; Maleszewski, J. J. , Amyloidosis in surgically resected atrial appendages: a study of 345 consecutive cases with clinical implications. Mod. Pathol. 2020, 33(5), 764–774. [Google Scholar] [CrossRef] [PubMed]
- Kuyama, N.; Takashio, S.; Nakamura, K.; Nishigawa, K.; Hanatani, S.; Usuku, H.; Yamamoto, E.; Ueda, M.; Fukui, T.; Tsujita, K. , Coexisting transthyretin and atrial natriuretic peptide amyloid on left atrium in transthyretin amyloid cardiomyopathy. Journal of Cardiology Cases 2024, 29(6), 261–264. [Google Scholar] [CrossRef] [PubMed]
- Gimelli, A.; Aimo, A.; Vergaro, G.; Genovesi, D.; Santonato, V.; Kusch, A.; Emdin, M.; Marzullo, P. , Cardiac sympathetic denervation in wild-type transthyretin amyloidosis. Amyloid 2020, 27(4), 237–243. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, M. C. A.; Cortez-Dias, N.; Cantinho, G.; Conceição, I.; Oliveira, A.; Bordalo e Sá, A.; Gonçalves, S.; Almeida, A. G.; de Carvalho, M.; Diogo, A. N. , Reduced myocardial 123-iodine metaiodobenzylguanidine uptake. Circ. Cardiovasc. Imaging 2013, 6(5), 627–636. [Google Scholar] [CrossRef] [PubMed]
- Javanshiri, K.; Drakenberg, T.; Haglund, M.; Englund, E. , Sudden cardiac death in synucleinopathies. J. Neuropathol. Exp. Neurol. 2023, 82(3), 242–249. [Google Scholar] [CrossRef] [PubMed]
- Hitosugi, M.; Takatsu, A.; Kinugasa, Y.; Takao, H. , Estimation of normal heart weight in Japanese subjects: development of a simplified normal heart weight scale. Leg. Med. (Tokyo) 1999, 1(2), 80–85. [Google Scholar] [CrossRef]
- Nishizawa, R. H.; Kawano, H.; Yoshimuta, T.; Eguchi, C.; Kojima, S.; Minami, T.; Sato, D.; Eguchi, M.; Okano, S.; Ikeda, S.; Ueda, M.; Maemura, K. , Effects of tafamidis on the left ventricular and left atrial strain in patients with wild-type transthyretin cardiac amyloidosis. Eur. Heart J. Cardiovasc. Imaging 2024, 25(5), 678–686. [Google Scholar] [CrossRef]
- Rausch, K.; Scalia, G. M.; Sato, K.; Edwards, N.; Lam, A. K.; Platts, D. G.; Chan, J. , Left atrial strain imaging differentiates cardiac amyloidosis and hypertensive heart disease. Int. J. Cardiovasc. Imaging 2021, 37(1), 81–90. [Google Scholar] [CrossRef] [PubMed]
- Brand, A.; Frumkin, D.; Hubscher, A.; Dreger, H.; Stangl, K.; Baldenhofer, G.; Knebel, F. , Phasic left atrial strain analysis to discriminate cardiac amyloidosis in patients with unclear thick heart pathology. Eur. Heart J. Cardiovasc. Imaging 2021, 22(6), 680–687. [Google Scholar] [CrossRef] [PubMed]
- Monte, I. P.; Faro, D. C.; Trimarchi, G.; de Gaetano, F.; Campisi, M.; Losi, V.; Teresi, L.; Di Bella, G.; Tamburino, C.; de Gregorio, C. , Left atrial strain imaging by speckle tracking echocardiography: The supportive diagnostic value in cardiac amyloidosis and hypertrophic cardiomyopathy. J Cardiovasc Dev Dis 2023, 10(6), 261. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.; Brayne, C. , Do anti-amyloid beta protein antibody cross reactivities confound Alzheimer disease research? J Negat Results Biomed 2017, 16(1), 1. [Google Scholar]
- Larsson, A.; Malmstrom, S.; Westermark, P. , Signs of cross-seeding: aortic medin amyloid as a trigger for protein AA deposition. Amyloid 2011, 18(4), 229–234. [Google Scholar] [CrossRef] [PubMed]
- Marcoux, J.; Mangione, P. P.; Porcari, R.; Degiacomi, M. T.; Verona, G.; Taylor, G. W.; Giorgetti, S.; Raimondi, S.; Sanglier-Cianferani, S.; Benesch, J. L.; Cecconi, C.; Naqvi, M. M.; Gillmore, J. D.; Hawkins, P. N.; Stoppini, M.; Robinson, C. V.; Pepys, M. B.; Bellotti, V. , A novel mechano-enzymatic cleavage mechanism underlies transthyretin amyloidogenesis. EMBO Mol. Med. 2015, 7(10), 1337–1349. [Google Scholar] [CrossRef] [PubMed]
- Milandri, A.; Farioli, A.; Gagliardi, C.; Longhi, S.; Salvi, F.; Curti, S.; Foffi, S.; Caponetti, A. G.; Lorenzini, M.; Ferlini, A.; Rimessi, P.; Mattioli, S.; Violante, F. S.; Rapezzi, C. , Carpal tunnel syndrome in cardiac amyloidosis: implications for early diagnosis and prognostic role across the spectrum of aetiologies. Eur. J. Heart Fail. 2020, 22(3), 507–515. [Google Scholar] [CrossRef] [PubMed]
- Ichimata, S.; Hata, Y.; Yoshida, K.; Nishida, N. , Autopsy of a multiple lobar hemorrhage case with amyloid-beta-related angiitis. Neuropathology 2020, 40(3), 280–286. [Google Scholar] [CrossRef] [PubMed]
- Ichimata, S.; Aikawa, A.; Sugishita, N.; Katoh, N.; Kametani, F.; Tagawa, H.; Handa, Y.; Yazaki, M.; Sekijima, Y.; Ehara, T.; Nishida, N.; Ishizawa, S. , Enterocolic granulomatous phlebitis associated with epidermal growth factor-containing fibulin-like extracellular matrix protein 1 deposition and focal amyloid properties: A case report. Pathol. Int. 2024, 74(3), 146–153. [Google Scholar] [CrossRef] [PubMed]
- Ichimata, S.; Hata, Y.; Nomoto, K.; Nishida, N. , Transthyretin-derived amyloid (ATTR) and sarcoidosis: Does ATTR deposition cause a granulomatous inflammatory response in older adults with sarcoidosis? Cardiovasc. Pathol. 2024, 107624. [Google Scholar] [CrossRef] [PubMed]
- Fontana, M.; Gilbertson, J.; Verona, G.; Riefolo, M.; Slamova, I.; Leone, O.; Rowczenio, D.; Botcher, N.; Ioannou, A.; Patel, R. K.; Razvi, Y.; Martinez-Naharro, A.; Whelan, C. J.; Venneri, L.; Duhlin, A.; Canetti, D.; Ellmerich, S.; Moon, J. C.; Kellman, P.; Al-Shawi, R.; McCoy, L.; Simons, J. P.; Hawkins, P. N.; Gillmore, J. D. , Antibody-associated reversal of ATTR amyloidosis-related cardiomyopathy. N. Engl. J. Med. 2023, 388(23), 2199–2201. [Google Scholar] [CrossRef] [PubMed]
- Rapezzi, C.; Riva, L.; Quarta, C. C.; Perugini, E.; Salvi, F.; Longhi, S.; Ciliberti, P.; Pastorelli, F.; Biagini, E.; Leone, O.; Cooke, R. M.; Bacchi-Reggiani, L.; Ferlini, A.; Cavo, M.; Merlini, G.; Perlini, S.; Pasquali, S.; Branzi, A. , Gender-related risk of myocardial involvement in systemic amyloidosis. Amyloid 2008, 15(1), 40–48. [Google Scholar] [CrossRef] [PubMed]
- Tanskanen, M.; Peuralinna, T.; Polvikoski, T.; Notkola, I. L.; Sulkava, R.; Hardy, J.; Singleton, A.; Kiuru-Enari, S.; Paetau, A.; Tienari, P. J.; Myllykangas, L. , Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2-macroglobulin and tau: a population-based autopsy study. Ann. Med. 2008, 40(3), 232–239. [Google Scholar] [CrossRef] [PubMed]
- Caponetti, A. G.; Rapezzi, C.; Gagliardi, C.; Milandri, A.; Dispenzieri, A.; Kristen, A. V.; Wixner, J.; Maurer, M. S.; Garcia-Pavia, P.; Tournev, I.; Plante-Bordeneuve, V.; Chapman, D.; Amass, L.; Investigators, T. , Sex-related risk of cardiac involvement in hereditary transthyretin amyloidosis: Insights from THAOS. JACC Heart Fail 2021, 9(10), 736–746. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C. M.; LoRusso, S.; Dispenzieri, A.; Kristen, A. V.; Maurer, M. S.; Rapezzi, C.; Lairez, O.; Drachman, B.; Garcia-Pavia, P.; Grogan, M.; Chapman, D.; Amass, L.; investigators, T. , Sex Differences in Wild-Type Transthyretin Amyloidosis: An Analysis from the Transthyretin Amyloidosis Outcomes Survey (THAOS). Cardiol Ther 2022, 11(3), 393–405. [Google Scholar] [CrossRef] [PubMed]
- Nebel, R. A.; Aggarwal, N. T.; Barnes, L. L.; Gallagher, A.; Goldstein, J. M.; Kantarci, K.; Mallampalli, M. P.; Mormino, E. C.; Scott, L.; Yu, W. H.; Maki, P. M.; Mielke, M. M. , Understanding the impact of sex and gender in Alzheimer’s disease: A call to action. Alzheimers Dement 2018, 14(9), 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Ichimata, S.; Yoshida, K.; Li, J.; Rogaeva, E.; Lang, A. E.; Kovacs, G. G. , The molecular spectrum of amyloid-beta (Abeta) in neurodegenerative diseases beyond Alzheimer’s disease. Brain Pathol. 2024, 34(1), e13210. [Google Scholar] [CrossRef] [PubMed]
- Tasaki, M.; Lavatelli, F.; Obici, L.; Obayashi, K.; Miyamoto, T.; Merlini, G.; Palladini, G.; Ando, Y.; Ueda, M. , Age-related amyloidosis outside the brain: A state-of-the-art review. Ageing Res Rev 2021, 70, 101388. [Google Scholar] [CrossRef] [PubMed]
- Coelho, T.; Maurer, M. S.; Suhr, O. B. , THAOS - The transthyretin amyloidosis outcomes survey: Initial report on clinical manifestations in patients with hereditary and wild-type transthyretin amyloidosis. Curr. Med. Res. Opin. 2013, 29(1), 63–76. [Google Scholar] [CrossRef]
- Lam, L.; Margeta, M.; Layzer, R. , Amyloid polyneuropathy caused by wild-type transthyretin. Muscle Nerve 2015, 52(1), 146–149. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Ueda, M.; Misumi, Y.; Masuda, T.; Nomura, T.; Tasaki, M.; Takamatsu, K.; Sasada, K.; Obayashi, K.; Matsui, H.; Ando, Y. , Genetic and clinical characteristics of hereditary transthyretin amyloidosis in endemic and non-endemic areas: experience from a single-referral center in Japan. J. Neurol. 2018, 265(1), 134–140. [Google Scholar] [CrossRef] [PubMed]
- Ishii, W.; Matsuda, M.; Nakamura, N.; Katsumata, S.; Toriumi, H.; Suzuki, A.; Ikeda, S.-i. , Phenol Congo Red Staining Enhances the Diagnostic Value of Abdominal Fat Aspiration Biopsy in Reactive AA Amyloidosis Secondary to Rheumatoid Arthritis. Intern. Med. 2003, 42(5), 400–405. [Google Scholar]
- Ueda, M.; Horibata, Y.; Shono, M.; Misumi, Y.; Oshima, T.; Su, Y.; Tasaki, M.; Shinriki, S.; Kawahara, S.; Jono, H.; Obayashi, K.; Ogawa, H.; Ando, Y. , Clinicopathological features of senile systemic amyloidosis: an ante- and post-mortem study. Mod. Pathol. 2011, 24(12), 1533–1544. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; Tinevez, J. Y.; White, D. J.; Hartenstein, V.; Eliceiri, K.; Tomancak, P.; Cardona, A. , Fiji: an open-source platform for biological-image analysis. Nat Methods 2012, 9(7), 676–682. [Google Scholar] [CrossRef] [PubMed]
- Ichimata, S.; Kim, A.; Nishida, N.; Kovacs, G. G. , Lack of difference between amyloid-beta burden at gyral crests and sulcal depths in diverse neurodegenerative diseases. Neuropathol. Appl. Neurobiol. 2023, 49(1), e12869. [Google Scholar] [CrossRef] [PubMed]
- Ichimata, S.; Martinez-Valbuena, I.; Lee, S.; Li, J.; Karakani, A. M.; Kovacs, G. G. , Distinct Molecular Signatures of Amyloid-Beta and Tau in Alzheimer’s Disease Associated with Down Syndrome. Int. J. Mol. Sci. 2023, 24(14), 11596. [Google Scholar]
- Milenkovic, I.; Jarc, J.; Dassler, E.; Aronica, E.; Iyer, A.; Adle-Biassette, H.; Scharrer, A.; Reischer, T.; Hainfellner, J. A.; Kovacs, G. G. , The physiological phosphorylation of tau is critically changed in fetal brains of individuals with Down syndrome. Neuropathol. Appl. Neurobiol. 2018, 44(3), 314–327. [Google Scholar]
| # | Age | Sex | BH | BW | BMI | HW | NHW* | Ratio** | C/TD | HT | HL | DM | ART | CHF | CAS |
| 1 | 81 | F | 147 | 43.6 | 20.2 | 346 | 242 | 1.4 | HyT/Sui | Neg | Pos | Neg | Neg | Neg | Rt |
| 2 | 87 | F | 147 | 36.3 | 16.8 | 321 | 218 | 1.5 | Dro/Sui | Pos | Neg | Neg | Neg | Neg | Neg |
| 3 | 80 | F | 146 | 45.9 | 21.5 | 387 | 248 | 1.6 | HyT/Acc | Pos | Neg | Pos | Neg | Neg | Neg |
| 4 | 79 | F | 164 | 72 | 26.8 | 584 | 361 | 1.6 | Dro/Acc | Pos | Neg | Neg | Af | Neg | Neg |
| 5 | 82 | M | 160 | 53.1 | 20.7 | 556 | 317 | 1.8 | HyT/Ill | Pos | Pos | Neg | Neg | Neg | Neg |
| 6 | 90 | F | 151 | 39.1 | 17.1 | 336 | 234 | 1.4 | HyT/Acc | Neg | Neg | Neg | Neg | Neg | Neg |
| 7 | 89 | M | 158 | 40.4 | 16.2 | 330 | 265 | 1.2 | Dro/Acc | Pos | Neg | Neg | Neg | Neg | Neg |
| 8 | 78 | M | 156 | 65.3 | 26.8 | 550 | 350 | 1.6 | CA/Ill | Pos | Neg | Pos | Neg | Neg | Neg |
| 9 | 90 | F | 148 | 44.7 | 20.4 | 306 | 247 | 1.2 | Dro/Acc | Pos | Neg | Neg | Neg | Neg | Neg |
| 10 | 97 | F | 153 | 51.5 | 22.0 | 356 | 278 | 1.3 | HS/Acc | Neg | Neg | Neg | Neg | Neg | Neg |
| 11 | 79 | F | 153 | 49.8 | 21.3 | 302 | 272 | 1.1 | Burn/Acc | Pos | Pos | Neg | Neg | Neg | Neg |
| 12 | 83 | M | 158 | 41.5 | 16.6 | 383 | 269 | 1.4 | Dro/Sui | Pos | Neg | Neg | Neg | Neg | Neg |
| 13 | 85 | F | 145 | 35.2 | 16.7 | 283 | 211 | 1.3 | Burn/Acc | NA | NA | NA | NA | NA | Lt |
| 14 | 94 | M | 159 | 29.4 | 11.6 | 379 | 219 | 1.7 | Dro/Acc | Neg | Neg | Neg | Neg | Neg | Neg |
| 15 | 81 | M | 161 | 58.5 | 22.6 | 450 | 339 | 1.3 | Dro/Acc | Pos | Pos | Neg | Neg | Neg | Neg |
| 16 | 85 | M | 157 | 51.8 | 21.0 | 364 | 306 | 1.2 | Dro/Sui | Pos | Neg | Neg | Neg | Neg | Neg |
| 17 | 89 | M | 158 | 44.2 | 17.7 | 304 | 280 | 1.1 | HyT/Acc | Pos | Neg | Neg | Neg | Neg | Lt |
| 18 | 80 | F | 152 | 69.6 | 30.1 | 408 | 329 | 1.2 | Dro/Acc | Pos | Neg | Neg | Neg | Neg | Neg |
| 19 | 89 | M | 159 | 37.4 | 14.8 | 435 | 254 | 1.7 | DOD/Sui | Pos | Neg | Pos | Neg | Neg | Neg |
| 20 | 93 | F | 144 | 41.8 | 20.2 | 365 | 232 | 1.6 | ACD/Ill | Pos | Pos | Neg | Neg | Neg | Rt, Lt |
| ATTR deposition rate (%) | Total # of cases = 20 |
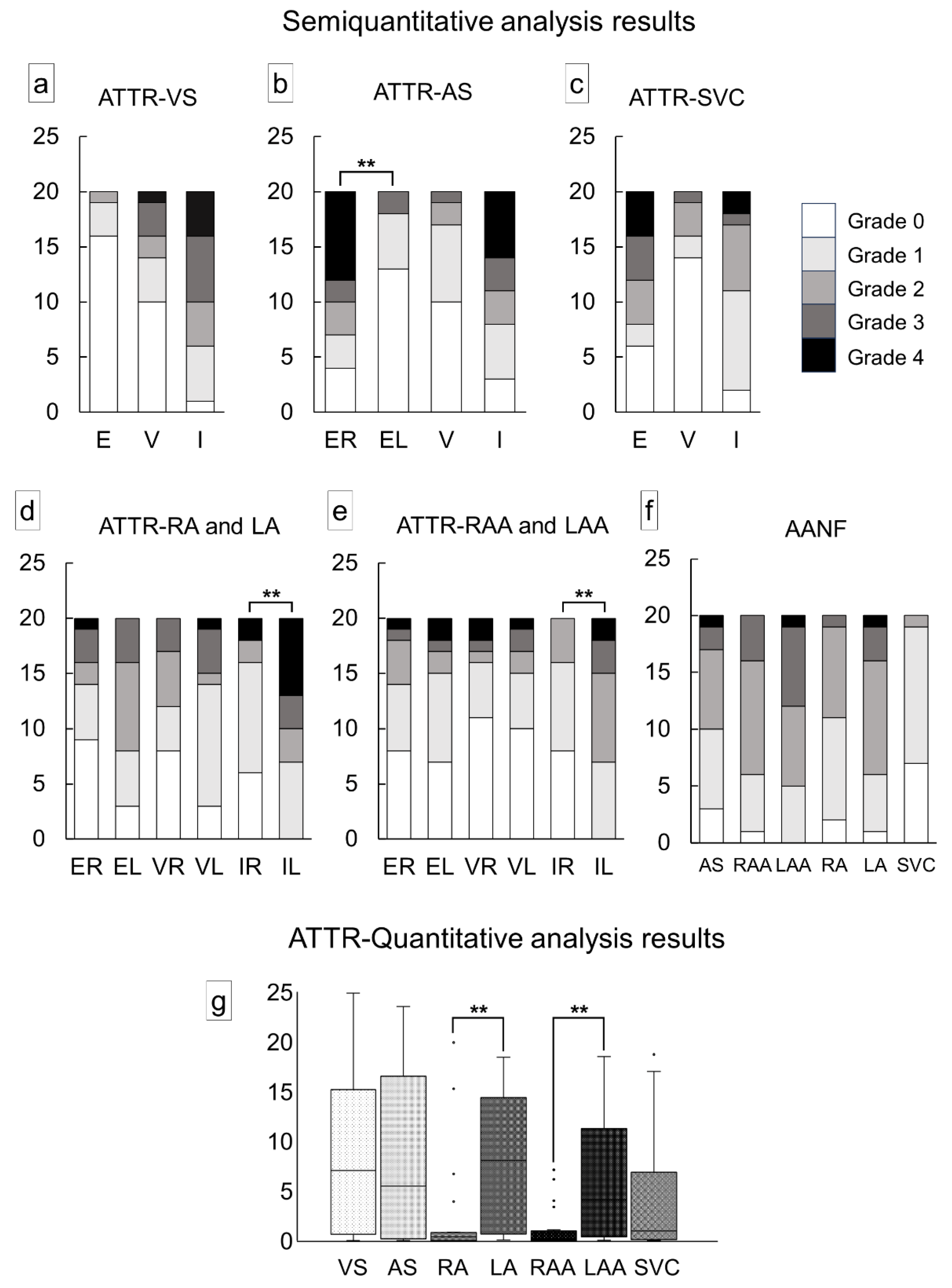
| VS (E/V/I) | 4 (20)/10 (50)/19 (95) |
| AS (ER/EL/V/I) | 16 (80)**/7 (35)/10 (50)/17 (85) |
| RA (E/V/I) | 11 (55)/12 (60)/14 (70) |
| RAA (E/V/I) | 12 (60)/9 (45)/12 (60) |
| LA (E/V/I) | 17 (85)/17 (85)/20 (100)* |
| LAA (E/V/I) | 14 (70)/10 (50)/20 (100)** |
| SVC (E/V/I) | 14 (70)/6 (30)/18 (90) |
| Sinoatrial node | 3 (15) |
| Atrioventricular node | 0 |
| Neural/perineural deposition | 2 (10)/6 (30) |
| AANF deposition rate (%) | Total # of cases = 20 |
| AS | 17 (85) |
| RA | 18 (90) |
| RAA | 19 (95) |
| LA | 19 (95) |
| LAA | 20 (100) |
| SVC | 13 (65) |
| Group 1 (N = 8) | Group 2 (N = 9) | Group 3 (N = 3) | p value* | ||
| Sex (F/M) | 7/1 | 2/7 | 2/1 | 0.02/0.49/0.24 | |
| Age (range) | 83.9 ± 5.4 (79–93) | 85.7 ± 5.3 (78–97) | 89.7 ± 3.7 (85–94) | 0.24 (1.00/0.28/0.67) | |
| N/PN involvement | 1 (13) | 4 (44) | 1 (33) | 0.29/0.49/1 | |
| ATTR deposition severity | |||||
| VS | E | 0 | 0.6 ± 0.7 (0–2) | 0 | 0.06 (0.08/1/0.32) |
| V | 0.3 ± 0.4 (0–1) | 1.1 ± 1.2 (0–3) | 3.0 ± 0.8 (2–4) | 0.02 (0.50/0.01/0.18) | |
| I-SQ | 1.6 ± 0.7 (1–3) | 3.3 ± 0.7 (2–4) | 1.3 ± 1.2 (0–3) | <0.01 (<0.01/1/0.06) | |
| I-Q | 2.9 ± 3.1 (0–9.2) | 15.6 ± 7.4 (2.3–24.9) | 3.2 ± 4.5 (0–9.6) | <0.01 (<0.01/1/0.03) | |
| AS | ER | 1.0 ± 1.0 (0–3) | 3.4 ± 0.8 (2–4) | 2.7 ± 1.9 (0–4) | <0.01 (<0.01/0.34/1) |
| EL | 0 | 1.1 ± 1.1 (0–3) | 0.3 ± 0.5 (0–1) | 0.02 (0.02/1/0.77) | |
| V | 0.1 ± 0.3 (0–1) | 0.8 ± 0.6 (0–2) | 2.0 ± 0.8 (1–3) | <0.01 (0.16/<0.01/0.32) | |
| I-SQ | 0.9 ± 0.6 (0–2) | 3.4 ± 0.7 (2–4) | 2.0 ± 1.6 (0–4) | <0.01 (<0.01/0.80/0.50) | |
| I-Q | 0.6 ± 0.8 (0–2.4) | 14.7 ± 5.2 (6.1–23.5) | 8.6 ± 8.9 (0–20.8) | <0.01 (<0.01/0.65/0.68) | |
| RA | E | 0.4 ± 0.7 (0–2) | 2.0 ± 1.2 (0–4) | 0.3 ± 0.5 (0–1) | 0.02 (0.02/1/0.16) |
| V | 0.4 ± 0.7 (0–2) | 1.6 ± 1.1 (0–3) | 2.0 ± 0.8 (1–3) | 0.03 (0.09/0.09/1) | |
| I-SQ | 0.6 ± 0.5 (0–1) | 1.8 ± 1.3 (0–4) | 0.3 ± 0.5 (0–1) | 0.047 (0.15/1/0.11) | |
| I-Q | 0.2 ± 0.3 (0–0.7) | 5.3 ± 6.9 (0–19.9) | 0.2 ± 0.3 (0–0.7) | 0.04 (0.07/1/0.20) | |
| RAA | E | 0.4 ± 0.5 (0–1) | 1.9 ± 1.1 (0–4) | 0.3 ± 0.5 (0–1) | 0.01 (0.02/1/0.11) |
| V | 0 | 1.0 ± 1.2 (0–4) | 3.0 ± 0.8 (2–4) | <0.01 (0.07/<0.01/0.24) | |
| I-SQ | 0.5 ± 0.5 (0–1) | 1.2 ± 0.8 (0–2) | 0.3 ± 0.5 (0–1) | 0.10 (0.20/1/0.27) | |
| I-Q | 0.1 ± 0.1 (0–0.2) | 2.5 ± 2.6 (0–7.1) | 0.0 ± 0.1 (0–0.1) | 0.03 (0.053/1/0.18) | |
| LA | E | 0.8 ± 0.7 (0–2) | 2.3 ± 0.7 (1–3) | 2.0 ± 0.0 (2) | <0.01 (<0.01/0.18/1) |
| V | 0.6 ± 0.5 (0–1) | 1.7 ± 0.9 (1–3) | 3.0 ± 0.8 (2–4) | <0.01 (0.11/<0.01/0.34) | |
| I-SQ | 1.5 ± 1.0 (1–4) | 3.6 ± 0.7 (2–4) | 2.0 ± 0.8 (1–3) | <0.01 (<0.01/1/0.23) | |
| I-Q | 2.4 ± 4.3 (0.1–13.8) | 11.9 ± 5.9 (1.7–18.4) | 5.4 ± 3.8 (0.1–9.0) | <0.01 (<0.01/1/0.27) | |
| LAA | E | 0.4 ± 0.5 (0–1) | 2.0 ± 1.3 (0–4) | 0.7 ± 0.5 (0–1) | 0.02 (0.02/1/0.47) |
| V | 0.1 ± 0.3 (0–1) | 1.0 ± 0.9 (0–3) | 3.0 ± 0.8 (2–4) | <0.01 (0.17/<0.01/0.19) | |
| I-SQ | 1.3 ± 0.4 (1–2) | 2.6 ± 0.7 (2–4) | 2.3 ± 1.2 (1–4) | <0.01 (<0.01/0.38/1) | |
| I-Q | 1.2 ± 1.4 (0–3.6) | 9.5 ± 5.8 (1.8–18.5) | 7.4 ± 6.8 (0.1–16.5) | <0.01 (<0.01/0.44/1) | |
| SVC | E | 0.9 ± 1.1 (0–3) | 2.7 ± 1.2 (0–4) | 2.3 ± 1.7 (0–4) | 0.06 (0.06/0.50/1) |
| V | 0 | 0.6 ± 0.8 (0–2) | 2.0 ± 0.8 (1–3) | <0.01 (0.50/<0.01/0.08) | |
| I-SQ | 0.9 ± 0.3 (0–1) | 2.1 ± 1.0 (1–4) | 2.3 ± 1.7 (0–4) | 0.045 (0.053/0.34/1) | |
| I-Q | 0.3 ± 0.5 (0–1.5) | 6.5 ± 5.4 (0.5–17.0) | 8.6 ± 7.7 (0–18.7) | 0.02 (0.02/0.30/1) | |
| AANF deposition severity | |||||
| AS | 1.4 ± 1.0 (0–3) | 1.8 ± 1.0 (1–4) | 1.3 ± 0.9 (0–2) | 0.86 (1/1/1) | |
| RA | 1.5 ± 0.9 (0–3) | 1.3 ± 0.7 (0–2) | 1.3 ± 0.5 (1–2) | 0.91 (1/1/1) | |
| RAA | 2.0 ± 1.0 (0–3) | 1.7 ± 0.5 (1–2) | 2.0 ± 0.8 (1–3) | 0.53 (0.84/1/1) | |
| LA | 1.9 ± 1.1 (0–4) | 1.7 ± 0.7 (1–3) | 2.7 ± 0.5 (2–3) | 0.16 (1/0.41/0.17) | |
| LAA | 2.3 ± 0.8 (1–3) | 2.1 ± 1.0 (1–4) | 2.3 ± 0.5 (2–3) | 0.87 (1/1/1) | |
| SVC | 0.6 ± 0.7 (0–2) | 0.7 ± 0.5 (0–1) | 1.0 ± 0.0 (1) | 0.52 (1/0.76/1) | |
| Semiquantitative ATTR deposition grading (range) | ||||
| Region | Female | Male | p value* | |
| Ventricular septum | Endocardium | 0.1 ± 0.3 (0–1) | 0.4 ± 0.7 (0–2) | 0.37 |
| Vessel | 0.9 ± 1.2 (0–3) | 1.2 ± 1.4 (0–4) | 0.66 | |
| Interstitium | 1.8 ± 1.1 (0–4) | 3.0 ± 0.9 (1–4) | 0.04 | |
| Atrial septum | Endocardium, right | 2.0 ± 1.7 (0–4) | 2.8 ± 1.3 (0–4) | 0.37 |
| Endocardium, left | 0.5 ± 0.9 (0–3) | 0.7 ± 0.9 (0–3) | 0.55 | |
| Vessel | 0.5 ± 0.7 (0–2) | 0.9 ± 1.0 (0–3) | 0.55 | |
| Interstitium | 1.7 ± 1.5 (0–4) | 2.8 ± 1.2 (0–4) | 0.15 | |
| Right atrium | Endocardium | 0.7 ± 1.1 (0–3) | 1.6 ± 1.3 (0–4) | 0.13 |
| Vessel | 0.9 ± 1.1 (0–3) | 1.4 ± 1.1 (0–3) | 0.30 | |
| Interstitium | 0.9 ± 1.1 (0–4) | 1.3 ± 1.2 (0–4) | 0.33 | |
| Right atrial appendage | Endocardium | 0.7 ± 1.0 (0–3) | 1.4 ± 1.2 (0–4) | 0.18 |
| Vessel | 0.9 ± 1.6 (0–4) | 0.9 ± 0.9 (0–3) | 0.41 | |
| Interstitium | 0.6 ± 0.6 (0–2) | 1.0 ± 0.8 (0–4) | 0.37 | |
| Left atrium | Endocardium | 1.3 ± 1.0 (0–3) | 2.1 ± 0.7 (1–3) | 0.08 |
| Vessel | 1.3 ± 1.2 (0–4) | 1.7 ± 0.9 (1–3) | 0.37 | |
| Interstitium | 2.0 ± 1.2 (1–4) | 3.1 ± 1.1 (1–4) | 0.07 | |
| Left atrial appendage | Endocardium | 0.7 ± 0.9 (0–3) | 1.7 ± 1.4 (0–4) | 0.13 |
| Vessel | 0.8 ± 1.2 (0–3) | 1.1 ± 1.2 (0–4) | 0.46 | |
| Interstitium | 1.9 ± 1.2 (1–4) | 2.1 ± 0.6 (1–3) | 0.37 | |
| Superior vena cava | Endocardium | 1.5 ± 1.5 (0–4) | 2.3 ± 1.4 (0–4) | 0.30 |
| Vessel | 0.5 ± 0.8 (0–2) | 0.7 ± 1.1 (0–3) | 0.77 | |
| Interstitium | 1.7 ± 1.2 (0–4) | 1.6 ± 1.1 (0–4) | 0.94 | |
| Semiquantitative AANF deposition grading (range) | ||||
| Region | Female | Male | p value | |
| Atrial septum | Interstitium | 1.5 ± 1.2 (0–4) | 1.7 ± 0.8 (1–3) | 0.71 |
| Right atrium | Interstitium | 1.5 ± 0.7 (0–2) | 1.3 ± 0.8 (0–3) | 0.30 |
| Right atrial appendage | Interstitium | 2.0 ± 0.9 (0–3) | 1.7 ± 0.7 (1–3) | 0.33 |
| Left atrium | Interstitium | 1.8 ± 0.7 (0–3) | 2.0 ± 1.1 (1–4) | 0.60 |
| Left atrial appendage | Interstitium | 2.4 ± 0.8 (1–3) | 2.0 ± 0.9 (1–4) | 1.00 |
| Superior vena cava | Interstitium | 0.6 ± 0.5 (0–1) | 0.8 ± 0.6 (0–2) | 0.71 |
| Quantitative ATTR deposition burden (%) | ||||
| Region | Female | Male | p value | |
| Ventricular septum | Interstitium | 5.3 ± 6.9 (0–24.0) | 12.8 ± 8.2 (0–24.9) | 0.056 |
| Atrial septum | Interstitium | 5.5 ± 7.7 (0–20.8) | 11.4 ± 7.5 (0–23.5) | 0.10 |
| Right atrium | Interstitium | 1.6 ± 4.3 (0–15.2) | 3.6 ± 6.1 (0–19.9) | 0.20 |
| Right atrial appendage | Interstitium | 0.4 ± 1.1 (0–4.0) | 2.1 ± 2.6 (0–7.1) | 0.20 |
| Left atrium | Interstitium | 4.3 ± 4.7 (0.1–13.8) | 10.6 ± 7.1 (0.1–18.4) | 0.04 |
| Left atrial appendage | Interstitium | 5.4 ± 6.8 (0–18.5) | 6.3 ± 4.3 (0.1–14.0) | 0.37 |
| Superior vena cava | Interstitium | 4.5 ± 6.3 (0–18.7) | 4.1 ± 5.0 (0–17.0) | 0.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





