Submitted:
12 July 2024
Posted:
12 July 2024
You are already at the latest version
Abstract
Keywords:
Introduction
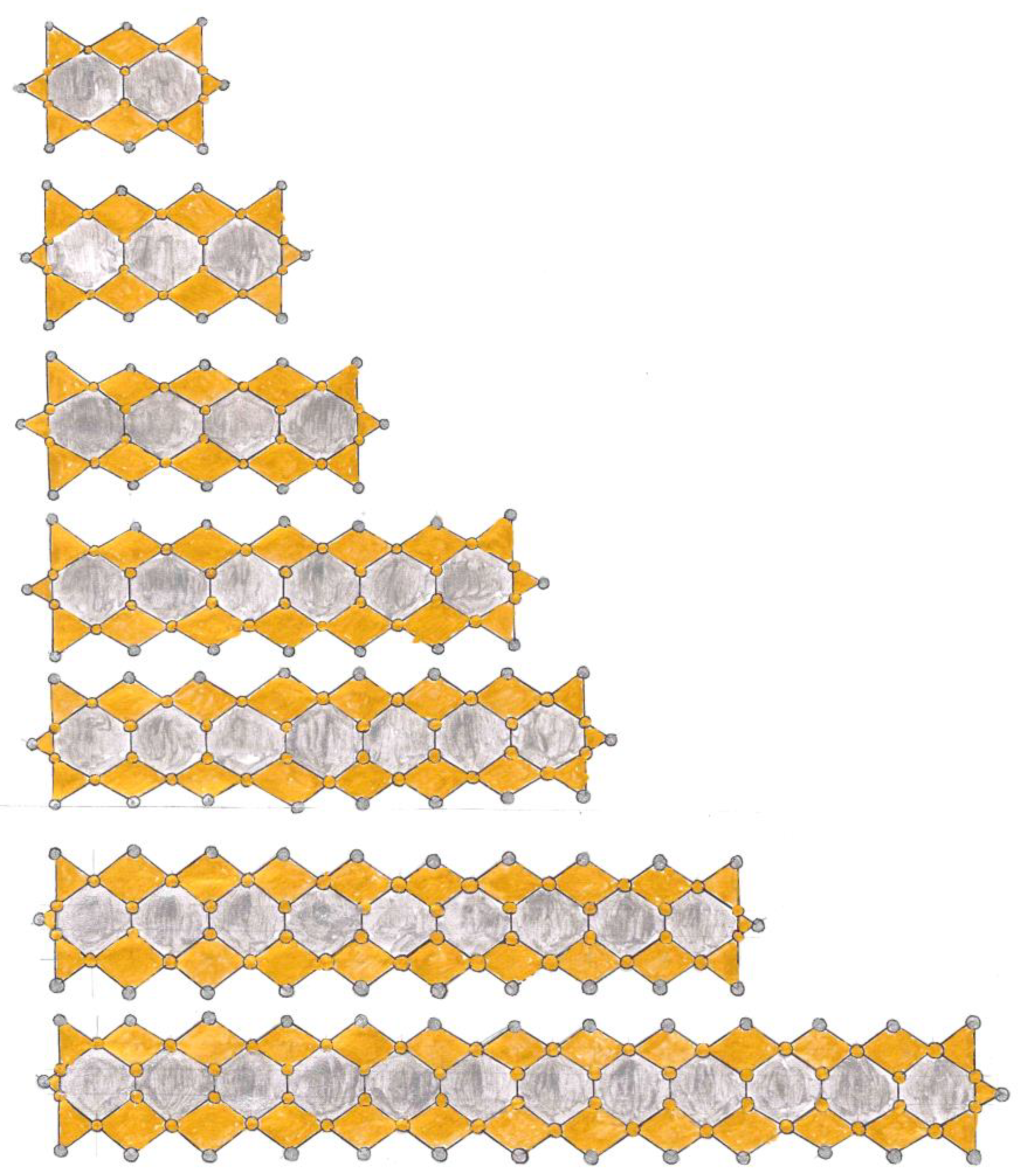
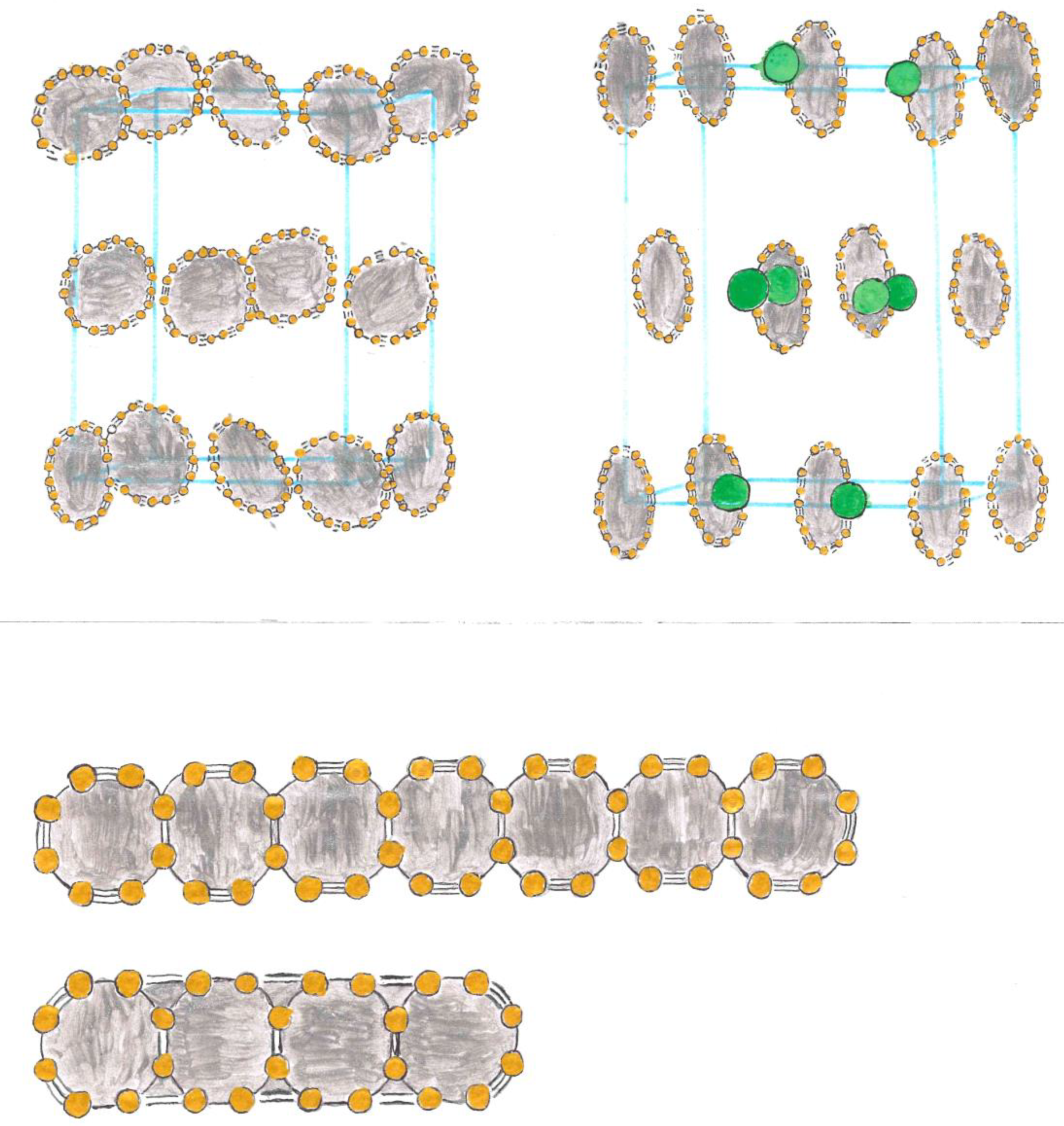
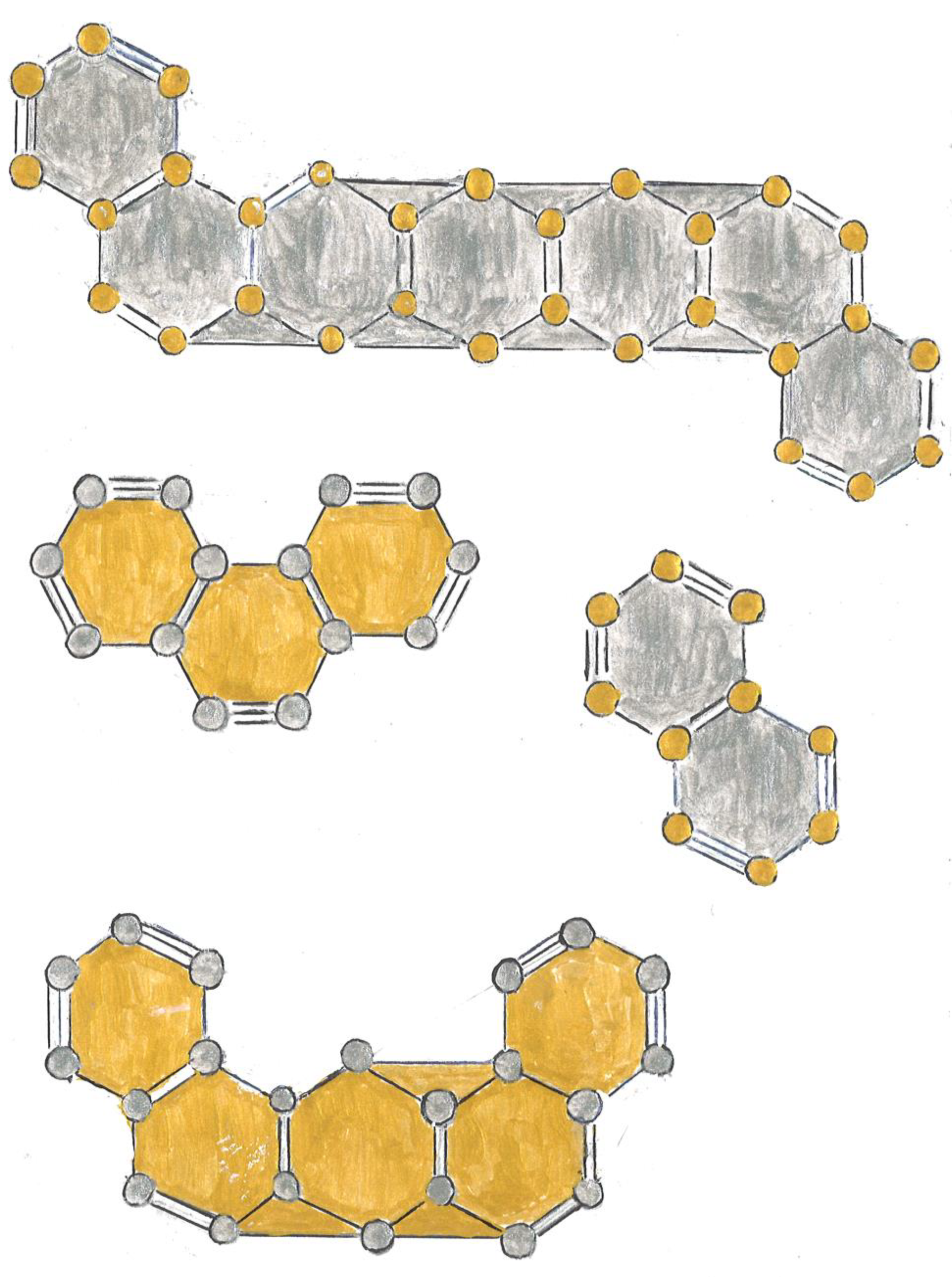
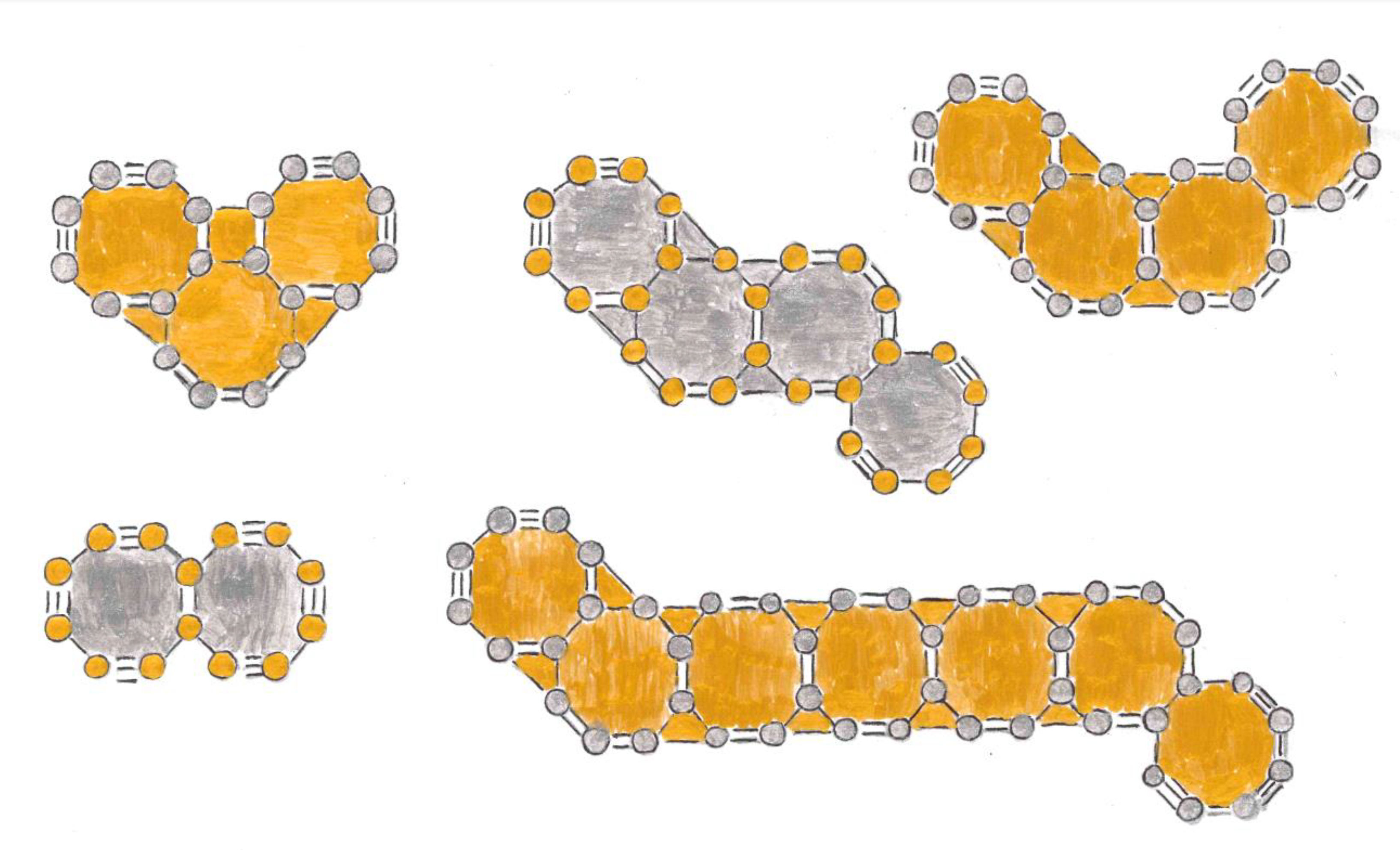
Exposition
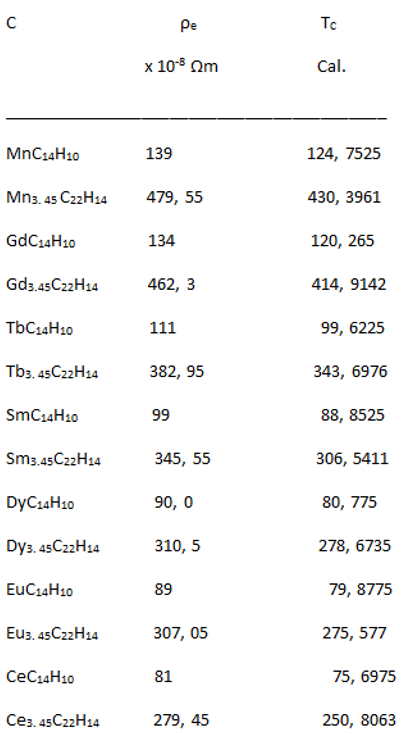
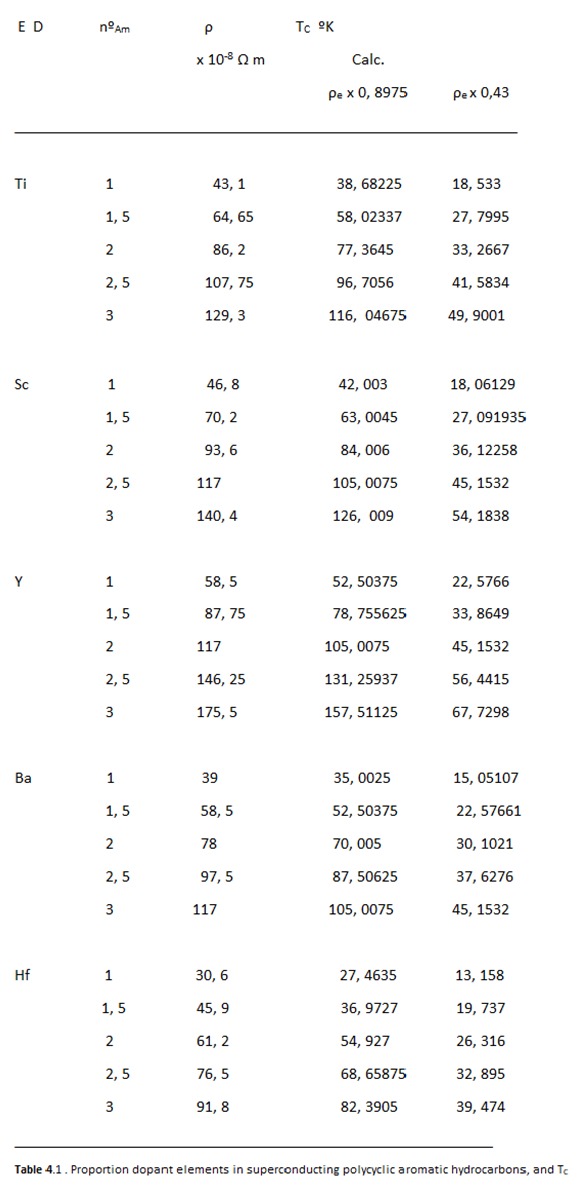
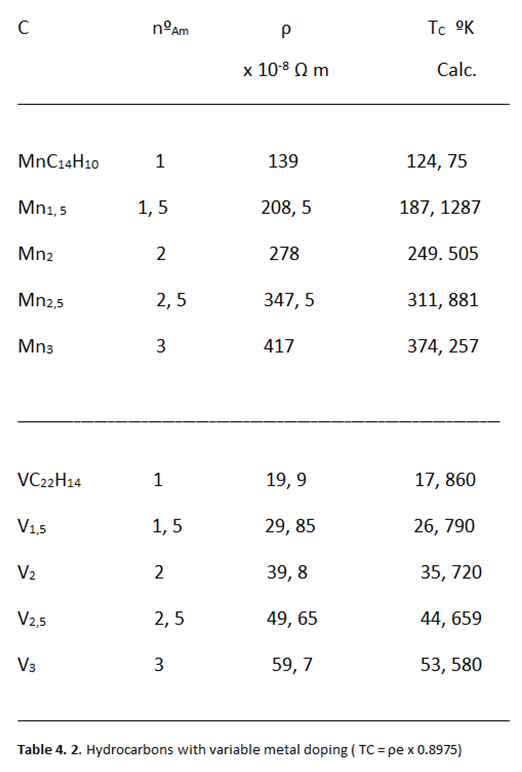
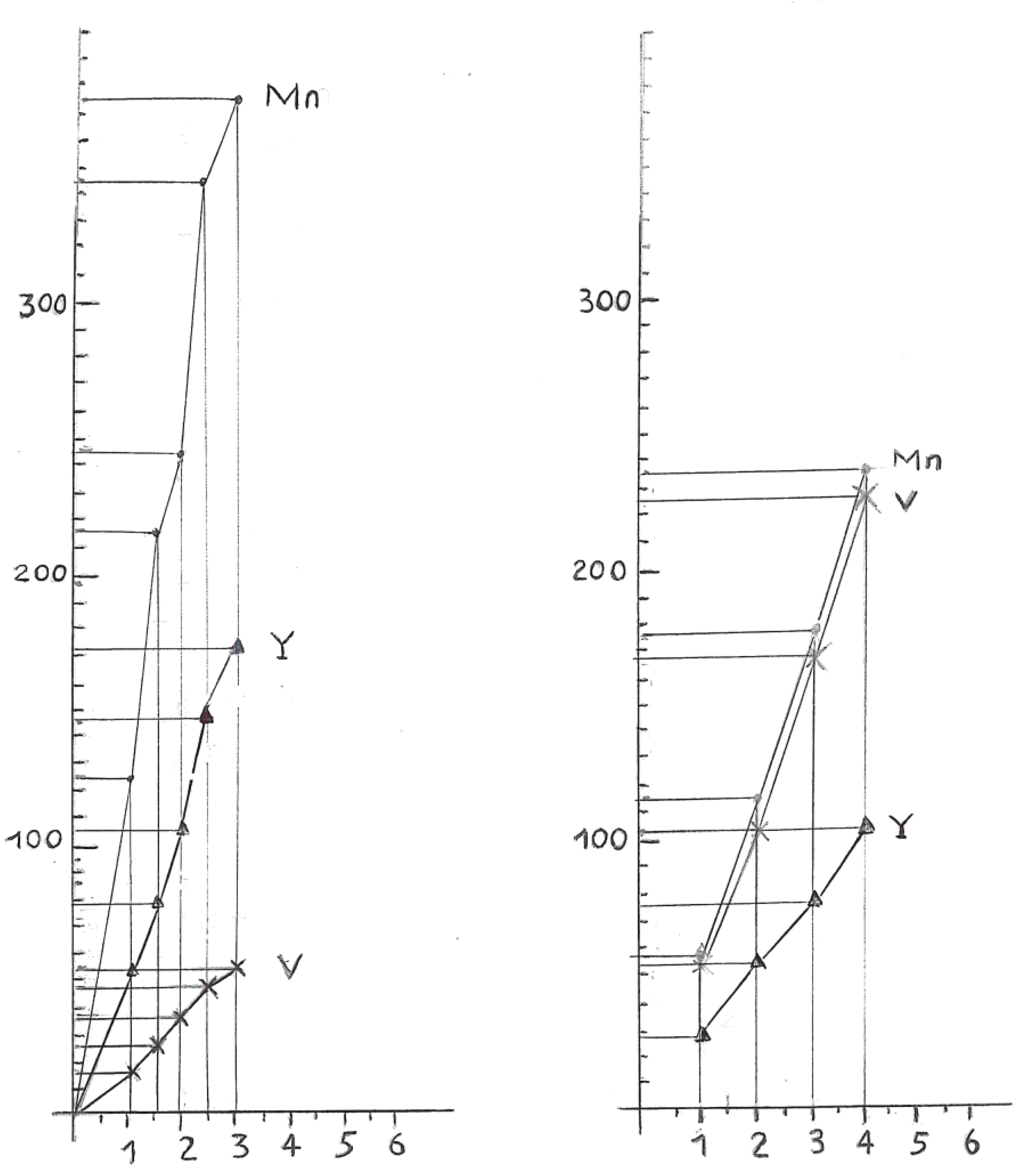
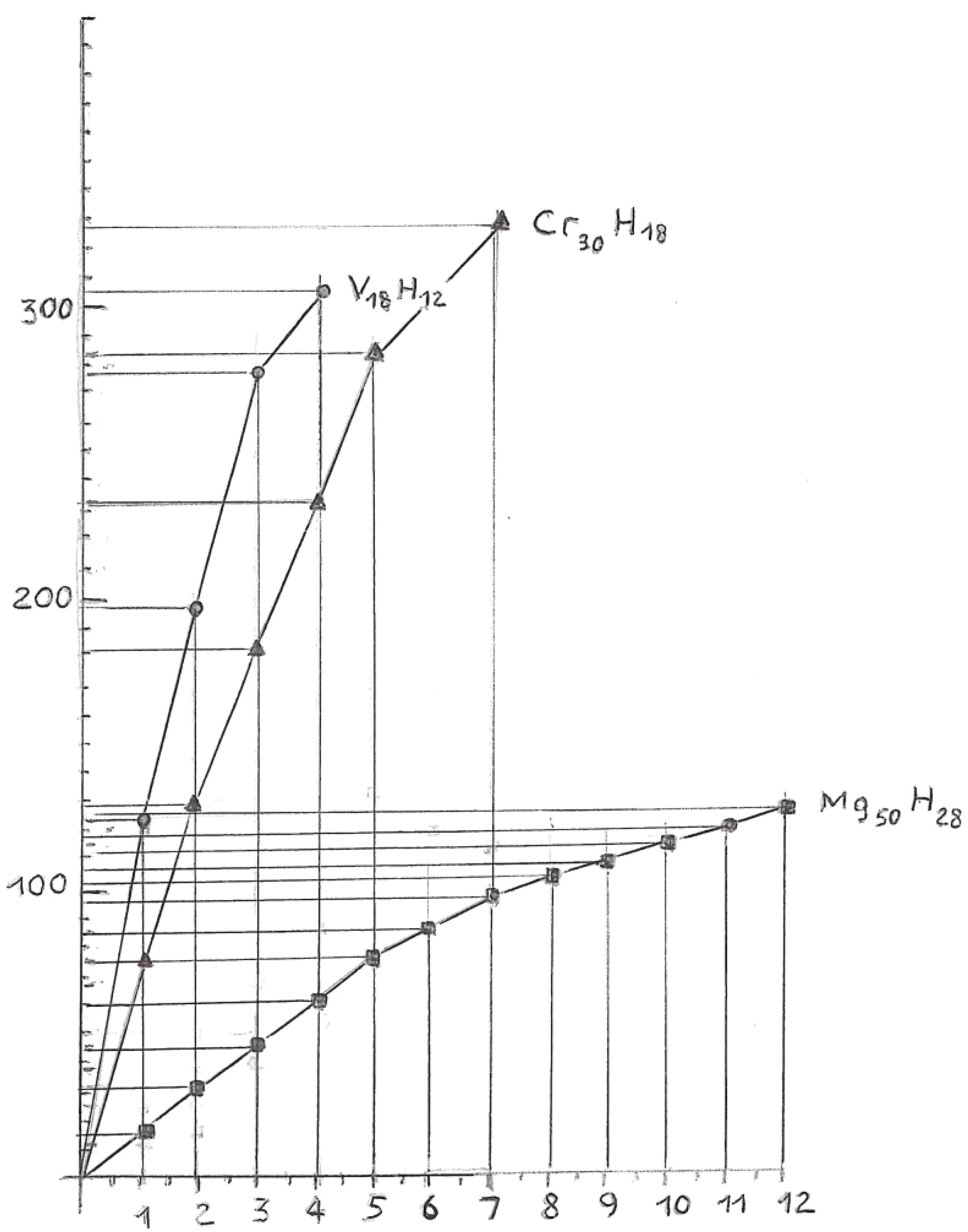
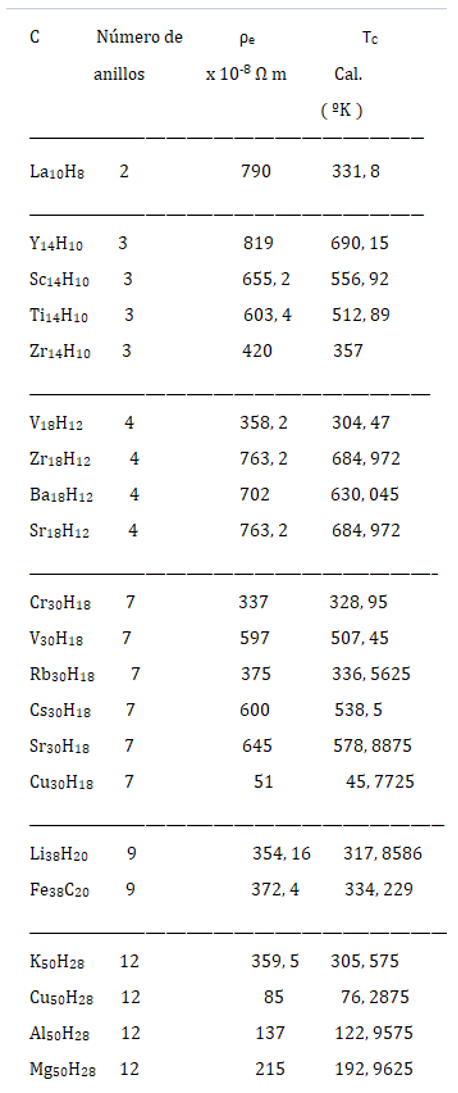
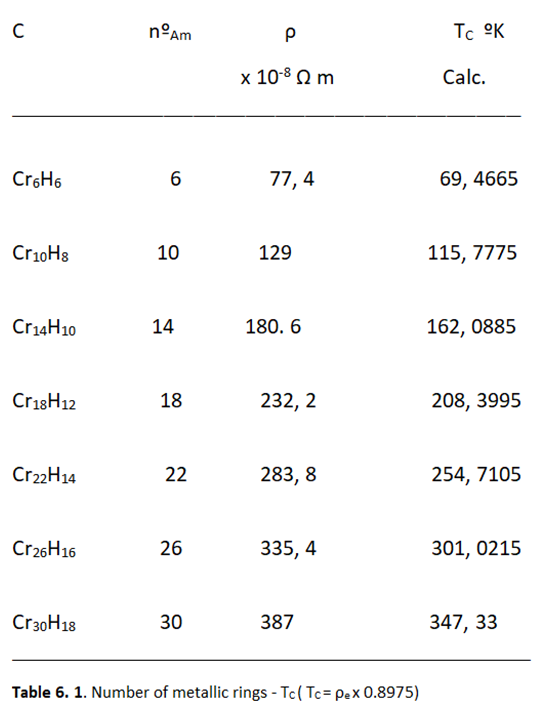
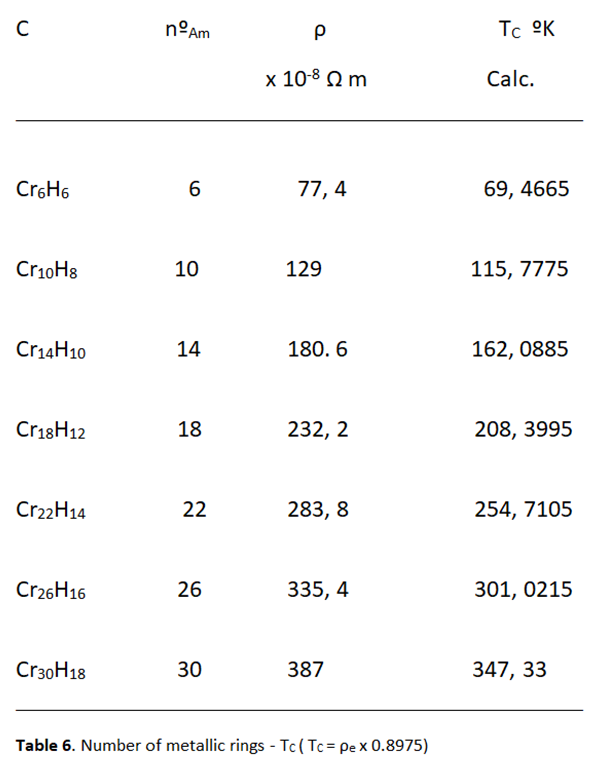
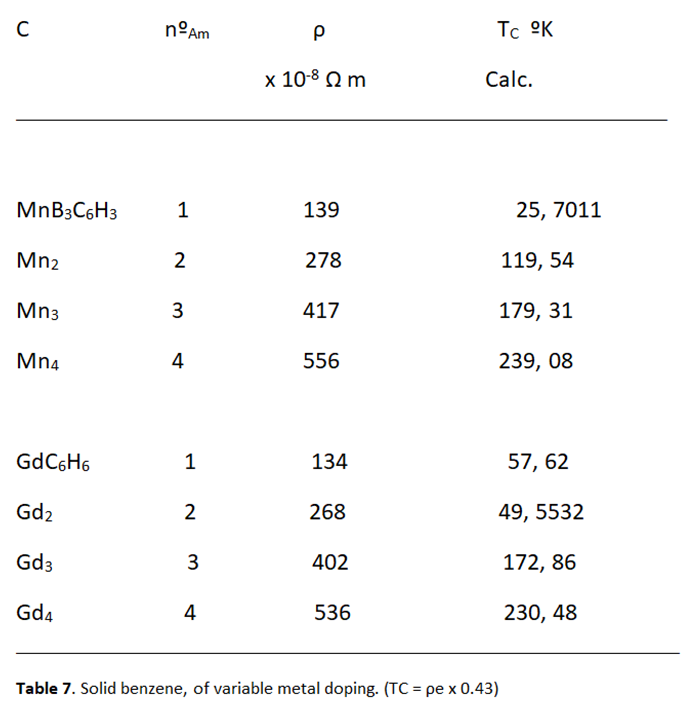
Relationship between Resistivity and Superconductivity






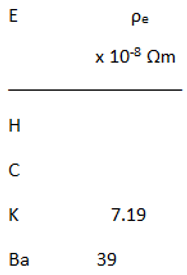 |

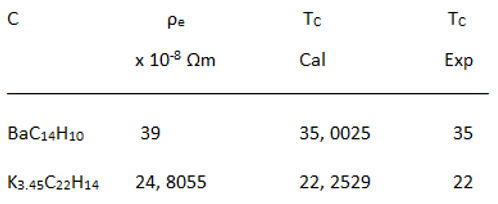 |
High Electrical Resistivity, among Metals
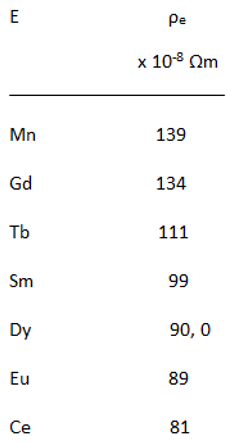 |
   |


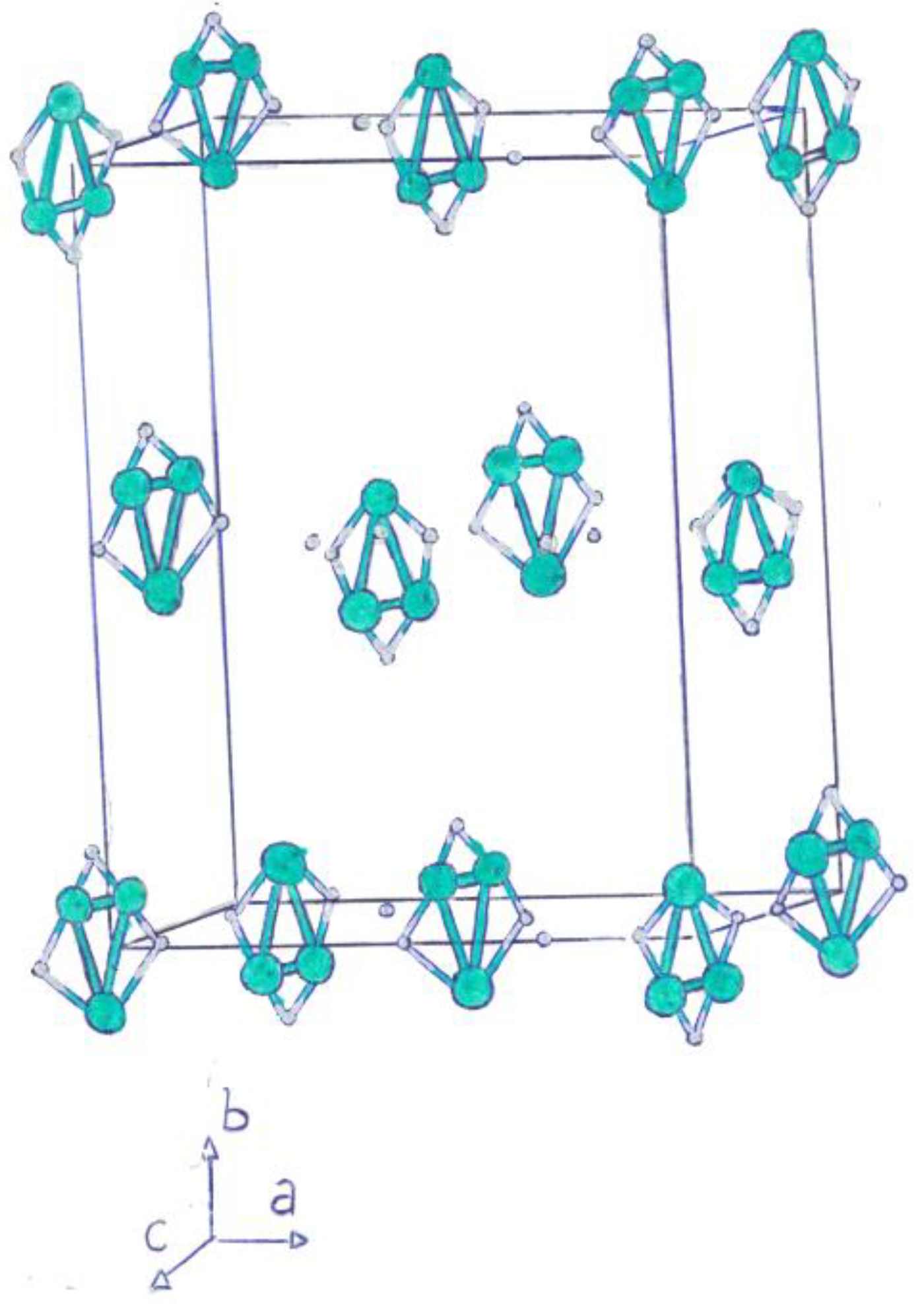
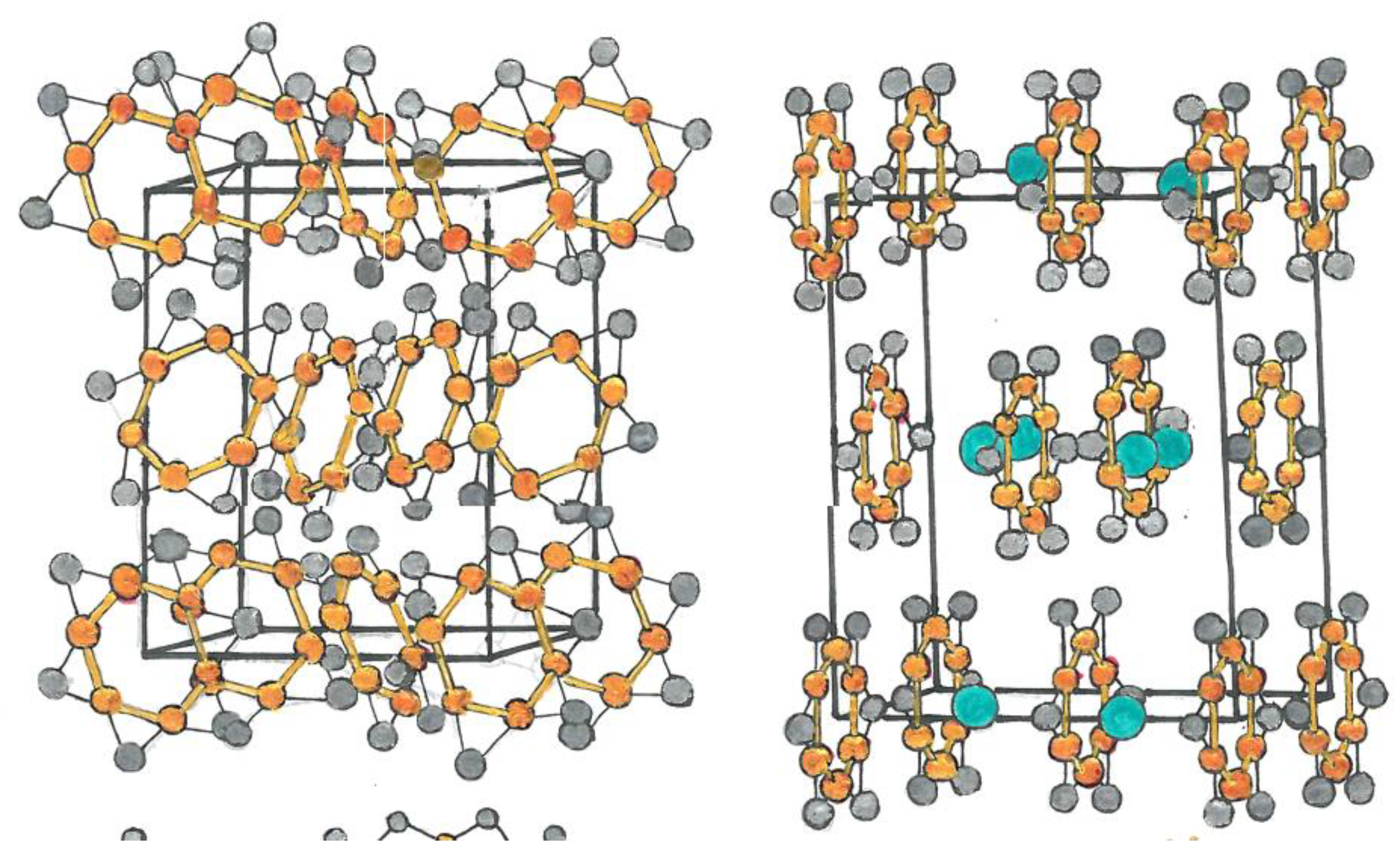
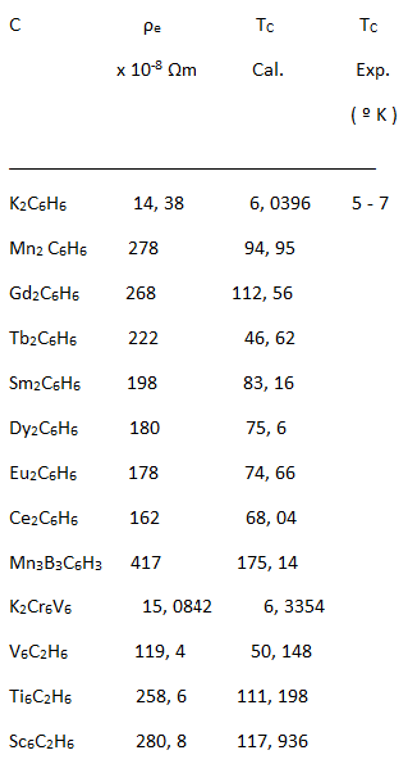 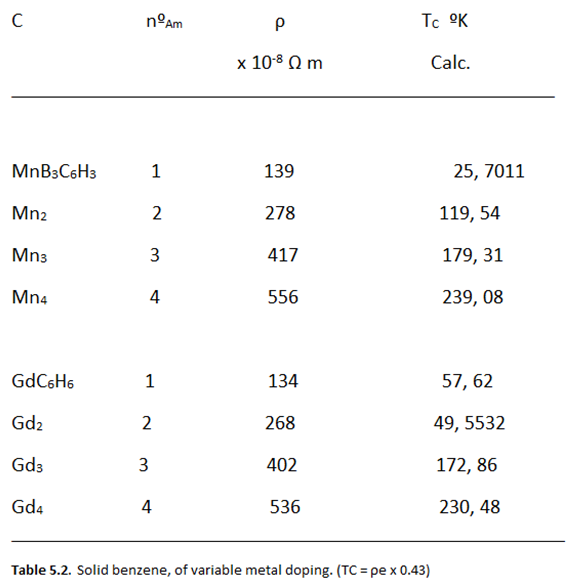 |
 |
 |
 |
 |
Conclusions
References
- Superconductivity in aromatic hydrocarbons. Yoshihiro Kubozono, Hidenori Goto, Taihei Jabuchi, Takayoshi Yokoya, Takashi Kambe, Yusuke Sakai, Masanari Izumi, Lu Zheng, Shino Hamao, Huyen L. T. Nguyen, Masafumi Sakata,Tomoko Kagayama and Katsuya Shimizu https://www.sciencedirect.com/science/article/am/pii/S0921453415000428.
- Materiales derivados de hidrocarbuuros aromáticos policíclicos intercalados con metales alcalinos. Manuel Carrera Fernández https://rua.ua.es/dspace/bitstream/10045/86012/1/tesis_manuel_carrera_fernandez.pdf.
- Synthesis and characterization of alkali-metal reduced polycyclic aromatic hydrocarbon based materials. A. Štefančič ( Table2-1, pag 35. ) http://etheses.dur.ac.uk/12339/1/Ales_Stefancic_PhD_Thesis.pdf?DDD7+.
- Superconductivity in transition metals. Daniel R. Slocombe, Vladimir L. Kuznetsov, Wojciech Grochala, Robert J. P. Williams and Peter P. Edwards https://royalsocietypublishing.org/doi/10.1098/rsta.2014.0476.
- D. Hillesheim, K. Gofryk, and A. S. Sefat. On the nature of filamentary superconductivity inmetal-doped hydrocarbon organic materials. DeGruyter Open Access https://www.degruyter.com/document/doi/10.1515/nsm-2015-0001/html.
- Correlated electronic structures and the phase diagram of hydrocarbon-based superconductors. https://arxiv.org/abs/1304.1613.
- vol-1-tipler-mosca-solucionary. Chapter 38.Solids and the Theory of Conduction. Page 2786.
- Combined theoretical and experimental characterizations of semiconductors for photoelectrocatalytic applications. Tangui Le Bahers and Kazuhiro Takanabe. ( Page 38 ).
- http://hyperphysics.phy-astr.gsu.edu/hbasees/Tables/elecon.html.
- Kittel, Introduction to Solid State Physics, 7th Ed. Chapter 6 Free Electron Fermi Gas.
- G. T. Meaden, Electrical resistance of metals, Plenum, 1965.
- INVESTIGATION OF EMPIRICAL LAWS FOR SUPERCONDUCTIVITY OF ALLOY AND COMPOUND SUPERCONDUCTORS. Liu Long-Jian and Zhang Zhuan-Jian. Chinese Physic, 2001.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





