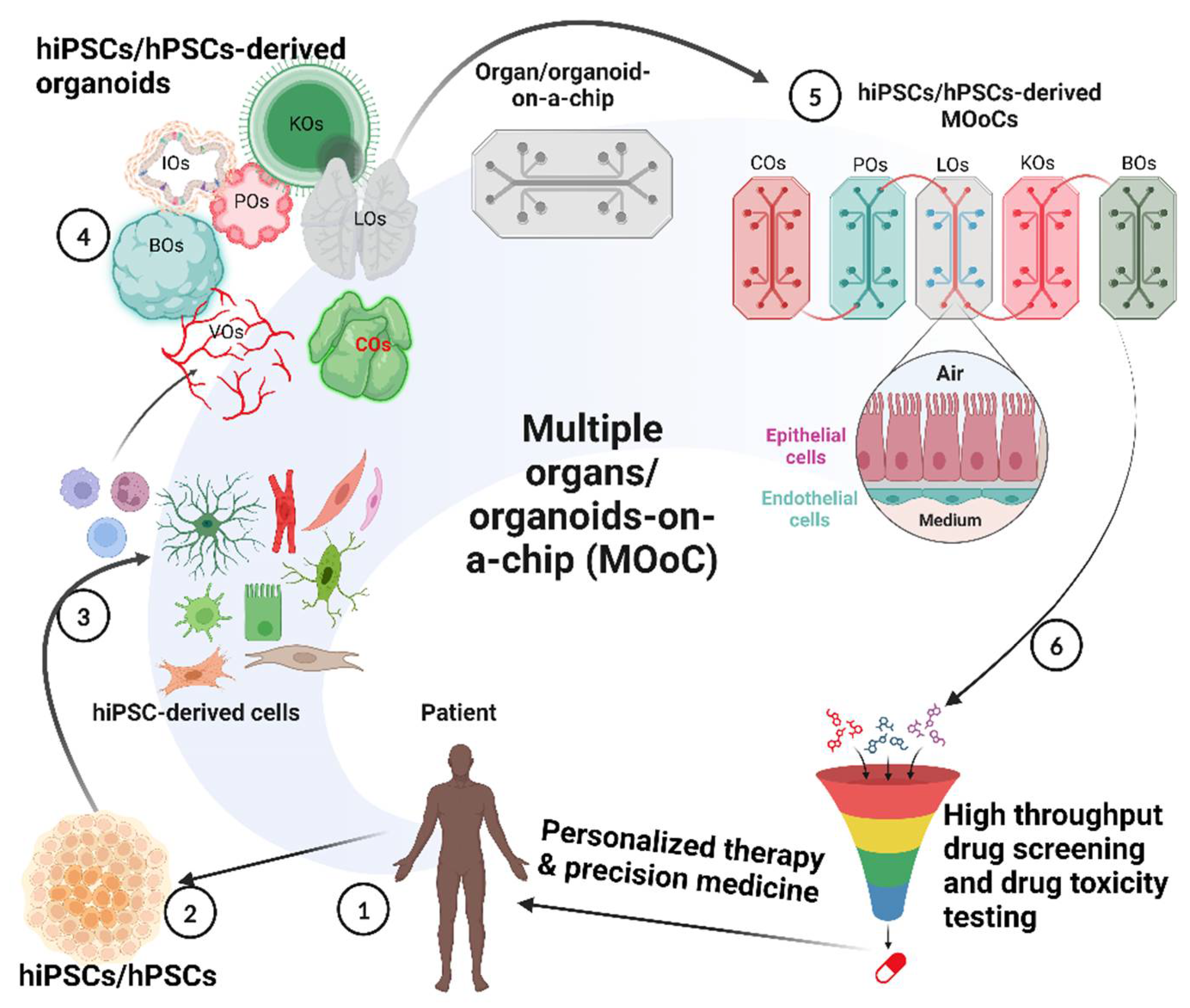
1. Introduction
Cardiovascular diseases (CVDs) pose a significant global health challenge, leading to over 4 million deaths annually in Europe alone, which constitutes 47% of all European mortality [
1], emphasizing the critical need for innovative research approaches to combat this pervasive health issue. The complexity of CVDs arises from their multifaceted nature, affecting both the heart and the vasculature. Atherosclerosis, a chronic vascular disease characterized by the gradual remodeling of blood vessel architecture, is often at the core of these conditions[
2]. This complex pathological process involves the interplay of various cell types, including dysfunctional endothelial cells (ECs), inflammatory leukocytes and macrophages, and dedifferentiated or apoptotic smooth muscle cells (SMCs) [
3]. The consequences of atherosclerosis can be severe, leading to life-threatening events such as myocardial infarction, ischemic stroke, and heart failure. Despite significant advances in understanding CVDs, traditional research models have shown limitations in fully capturing the intricacies of human cardiovascular pathophysiology. Animal models, while valuable, often fail to translate findings to clinical outcomes due to inherent physiological differences between species. Similarly, conventional two-dimensional (2D) cell cultures lack the structural and functional complexity of native tissues[
4], failing to replicate the dynamic microenvironment and multicellular interactions crucial for disease modeling. To address these challenges, recent years have witnessed remarkable progress in the development of advanced in vitro models that more closely mimic the human cardiovascular system. Among these innovations, vascular organoids (VOs) and vessel-on-chip (VoC) platforms have emerged as powerful tools for studying CVDs[
5,
6]. These three-dimensional (3D) models offer remarkable opportunities to investigate disease mechanisms, test therapeutic interventions, and conduct drug screening in a physiologically relevant context[
7]. VOs are self-organizing 3D structures derived from human pluripotent stem cells (hPSCs) that recapitulate key aspects of vascular development and function[
7]. By leveraging the intrinsic capacity of these cells to differentiate and organize into complex tissues, researchers can now generate miniature blood vessels that exhibit remarkable similarity to their in vivo counterparts. These organoids provide insights into vascular development, disease progression, and regeneration, offering a unique platform for personalized medicine approaches. Complementing organoid technology, VoC devices integrate microfluidic systems with human vascular cells to create functional blood vessel models[
8]. These platforms enable precise control over the cellular microenvironment, allowing to study the effects of hemodynamic forces, endothelial-blood cell interactions, and barrier function under both physiological and pathological conditions[
9]. The ability to manipulate individual parameters while maintaining overall system complexity makes VoC models invaluable for dissecting the molecular and cellular events underlying vascular diseases. The integration of these advanced models with cutting-edge technologies such as single-cell sequencing, high-resolution imaging, and computational modeling has further enhanced their utility. Researchers can now probe genetic variations[
10,
11,
12,
13,
14], cellular heterogeneity, and dynamic processes at unprecedented resolution, leading to new insights into disease mechanisms and potential therapeutic targets. Moreover, VO and VoC platforms are beginning to address the limitations of traditional drug discovery pipelines. By providing more accurate predictions of drug efficacy and toxicity in humans, these models have the potential to significantly reduce reliance on animal testing, accelerate the development of novel therapies, and improve the success rate of clinical trials[
5]. As we continue to unravel the complexities of CVDs, VO and VoC technologies stand at the forefront of a new era in cardiovascular research. Their ability to bridge the gap between simplified
in vitro systems and the intricacies of human physiology offers hope for developing more effective strategies to combat the global CVD burden. By espousing these innovative approaches, we move closer to realizing the goal of personalized, precise, and preventative cardiovascular medicine.
2. Vascular Organoids
VOs display intricate 3D structures that faithfully replicate the form and function of natural blood vessels, making them invaluable models for studying vascular biology and diseases. These organoids typically consist of various cellular elements, each playing a role in their growth and performance.
ECs play a crucial role in the formation of the innermost layer of VOs, serving to establish a barrier between the blood and surrounding tissues. These cells are derived from hPSCs including human embryonic stem cells (hESCs) and human induced pluripotent stem cell (hiPSC) through a highly intricate and carefully regulated differentiation process. In the course of development, vascular progenitor cells with the capacity to transform into endothelial and mural cells originate from the lateral and posterior mesoderm[
15]. Typically, protocols for inducing mesoderm from hPSCs entail activating the Wnt signalling pathway and stimulating BMP-4. Subsequent differentiation into ECs is accomplished by exposing the cells to VEGF-A, which promotes angiogenesis and the creation of new blood vessels [
16,
17].
Pericytes play a crucial role in stabilising ECs, providing structural support, and regulating blood flow. These specialised cells originate from the same progenitor cells as ECs but differentiate in response to factors like PDGF-BB and TGF-β signalling[
15]. The interaction between ECs and pericytes is essential for the maturation and proper functioning of the vascular network within VOs. In 3D cultures, these cells come together to form intricate endothelial/pericyte networks, closely resembling the
in vivo environment. Moreover, vascular SMCs play an important role in maintaining the structural integrity and contractility of blood vessels. These cells are derived from hPSCs through pathways involving mesoderm induction and exposure to specific growth factors. SMCs envelop the endothelial tubes, adding layers of support and contractile function that are essential for maintaining vessel tone and regulating blood pressure.
Fibroblasts play a key role as producers of the extracellular matrix (ECM), generating essential proteins like collagen and fibronectin. These proteins form a scaffold that supports cell attachment, migration, and differentiation. The ECM is crucial for maintaining the structural integrity of VOs and for facilitating cell signalling pathways that impact VO development. Understanding the cellular components and their interactions within VOs is crucial for optimising these models for research and therapeutic purposes, including drug testing and disease modelling. The ongoing refinement of these vascular models shows promise for substantial advancements in regenerative therapies.
2.1. Vascular Organoid Generation
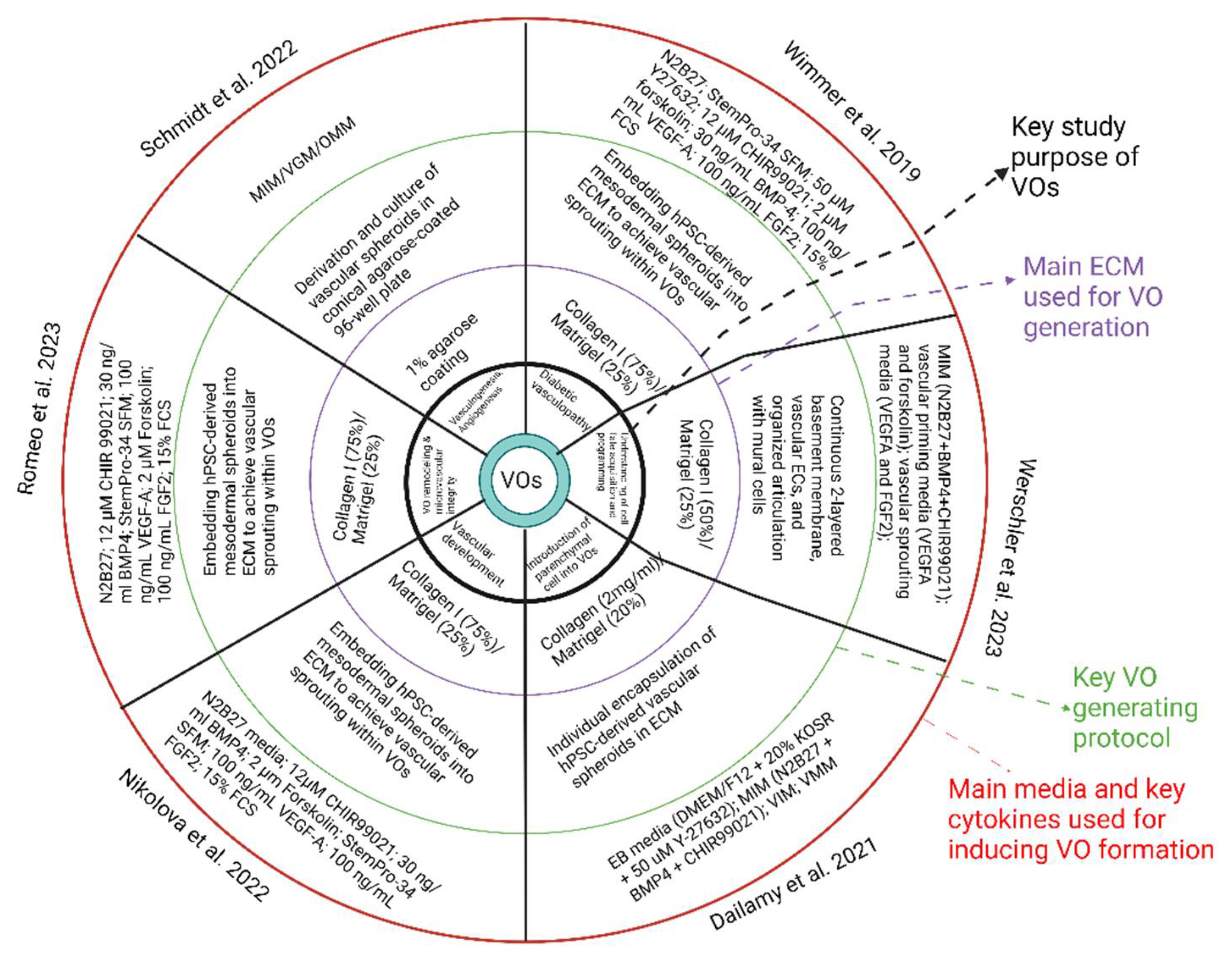
Several methods have been applied to successfully generate VOs from stem cells (
Figure 1). Among them, two primary methods are commonly utilised. One method involves differentiating and purifying various cell types and then merging them together, producing a multi-cellular organoid. A common, second method involves co-differentiation, which involves the simultaneous differentiation of various cell types from hPSCs.
The earliest protocol described for the development of human VOs was established by Wimmer et al. in 2019[
18]. This protocol leverages the differentiation of multiple vascular cell types from a pool of mesodermal progenitors, coupled with the innate self-organisation capabilities of these cells throughout development. The protocol begins with hPSCs aggregating into embryoid bodies, followed by mesodermal fate induction by activating Wnt signalling and BMP-4 stimulation. After then, vascular cells will be differentiated from these mesodermal progenitors through supplementation with VEGF-A and Forskolin. The developed aggregates of approximately 100 to 200 microns will be embedded within 3D, bi-layered collagen I-Matrigel gels to encourage vasculature sprouting. Importantly, collagen and Matrigel are temperature-sensitive and have the potential to undergo early polymerisation, resulting in irregular densities and uneven stiffness, which may inhibit sprouting [
19]. Regardless, the ECM serves as a critical buffer between the organoids and the plastic surface of the culture dish. Without this assembly, the increase in density of the organoids compared to the non-polymerised matrix would cause them to sink and come into contact with the bottom of the plastic dish, impeding uniform sprouting[
19]. Within a few hours of embedding, initial cellular will infiltration into the ECM, and the organoids will be cultured for an additional 4 days before being released from the Matrigel using fine syringe needles [
18]. Timing is a vital part of this process; releasing the organoids too late can cause fusion of the organoids, whilst removing them too early may result in insufficient differentiation, evidenced by dense cores. Despite being successful, when optimising protocols for VO development in the future, alternative approaches to this labour-intensive step, which also increases contamination risk, should be considered. This methodology has, for the first time, enabled the
in vitro assembly of a self-organising human capillary network.
More recent protocols for VOs heavily rely on Matrigel as an ECM to facilitate vessel sprouting. Matrigel is widely used due to its richness in ECM proteins such as collagen IV, laminin, and entactin, along with multiple growth factors. Despite this, the composition of Matrigel tends to fluctuate significantly between batches, with Matrigel lacking growth factors bearing inconsistency. In order to overcome this issue, Schmidt et al [
24] introduced a novel protocol for the development of VOs which eliminates the need for Matrigel. This approach utilised a conical agarose coating in 96-well plates to aggregate hiPSCs and support subsequent organoid culture. In a similar manner to earlier protocols, mesoderm induction was achieved using CHIR99021 and BMP4 over three days. However, vascular induction using this method entailed a single 48-hour dose of 100 ng/mL of VEGF, following which the organoids were maintained in N2B27 medium without additional vascular-specific growth factors[
24].
The lack of an ECM alternate and the modified culture conditions in the later protocol used by Schmidt et al [
24] have a considerable impact of organoid morphology. Early-stage organoids normally consist of loosely connected mesenchymal cells, with a vasculogenic region appearing by day 7. Cells within this region express CD31 which gradually infiltrate into other regions within the organoid. One limitation of the Schmidt et al. protocol is the absence of mural cells within
in vitro cultures. Although these cells, along with perfusion and the combining of SMA
+ mural cells with the vascular network were observed when the organoids were transplanted into a chick chorioallantois membrane, lacking mural cells within these VOs may impact the stability and functionality of the vascular networks. Despite this, Schmidt et al. demonstrated that 3D VOs can form without depending on a matrix and sustained exposure to pro-angiogenic factors, exploiting the cells’ innate self-organising capabilities within a suitable tissue context.
Both protocols for generating VOs from stem cells have contributed significantly to the field, each offering unique advantages and facing specific challenges. Protocol used by Wimmer et al[
18] was pioneering in its approach to create VOs. This method’s reliance on a 3D culture system using collagen I-Matrigel gels was innovative but also introduced complexities related to the temperature sensitivity and batch variability of Matrigel. The manual release of organoids from Matrigel, while effective, is a significant bottleneck and poses a risk of contamination, highlighting the need for either automation or alternative techniques in future optimisations. Conversely, protocol reported by Schmidt et al addresses some of these challenges by eliminating the need for Matrigel. Utilising a conical agarose coating in 96-well plates for cell aggregation, this protocol simplifies the culture conditions and reduced variability. The mesoderm induction using CHIR99021 and BMP4 is followed by a single dose of VEGF for vascular induction, streamlining the process and minimising the need for long-term exposure of angiogenic factors. This approach demonstrate that VOs can indeed form through the innate self-organising capabilities of the cells, however, the absence of mural cells within
in vitro cultures suggests that further refinement is required to ensure the stability and functionality of the vascular networks.
2.2. Functional Characteristics and Physiological Relevance of VOs
2.2.13. D Architecture
The benefits of 3D organoid systems surpass those of traditional 2D models in terms of architecture and physiology. Organoids developed from established differentiation protocols possess an inherent capacity for self-organization, leading to the formation of intricate 3D structures that closely mimic human organ morphology [
25,
26,
27]. Unlike 2D monotypic cellular models, 3D organoids undergo multilineage differentiation, resulting in a diverse cell population that forms complex, tissue-like structures. This self-organisation is facilitated by fundamental processes such as cellular migration, segregation, and spatially constrained lineage commitment, which are critical during organogenesis.
The use of 3D cell culture models has significantly advanced vascular biology research by providing more physiologically relevant representations of vessels and tissues. Traditional 2D models have been inadequate for accurately reproducing the spatial organisation of blood vessels, cell-cell adhesion, and cell-extracellular matrix interactions in vascular diseases. The emergence of 3D VOs has overcome these limitations by offering a model that closely simulates the intricate architecture of vascular tissues.
In addition, as researchers look for options to replace animal models, hPSC-derived 3D VOs not only offer a more precise portrayal of human diseases, but also allow for precise control of the microenvironment, including signalling pathways, and transcriptional and translational regulators, due to their cultivated nature. This ability is rather valuable for studying the spatial organisation and interactions within vascular tissues, furthering our understanding of vascular development and pathology. Moreover, the 3D architecture of VOs plays a crucial role in mimicking the spatial arrangement of natural blood vessels and adjacent tissues, providing a more precise and physiologically relevant model for conducting research and developing therapeutics.
2.2.2. Transplantation Studies
The integration of vascularised organoids into a suitable system holds promise for significant enhancement in organoid size and lifespan [
28]. Previous research has involved
in vivo implantation of pre-vascularized VOs, resulting in their integration with the host's vasculature and improved organoid survival. VOs have been utilised to study diabetic vascular complications, such as basement membrane thickening, by culturing them in a high-glucose medium with pro-inflammatory cytokines. They can also be transplanted into diabetic mice to generate chimeric humanised mouse models, enabling
in vivo modelling of diabetic vasculopathy [
18]. These models effectively mimic key diabetic vascular characteristics, including lumen narrowing and vessel regression, and facilitate the assessment of functional vessel parameters such as permeability, blood flow, and preclinical toxicology analysis of vasculopathy.
2.3. VO Applications
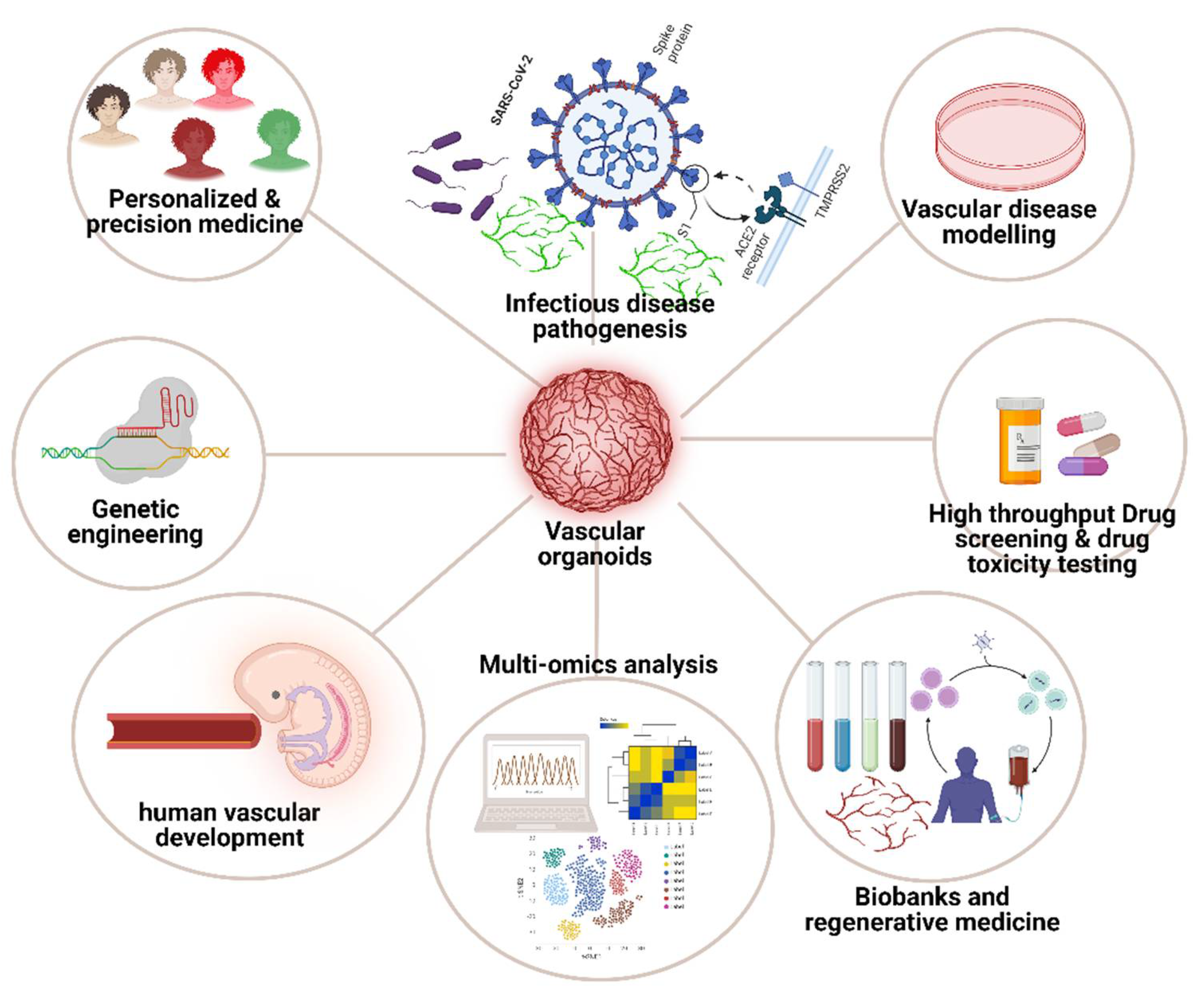
The emergence of VOs has provided physiologically relevant models for studying vascular development and diseases with multiple applications as illustrated in
Figure 2. In recent years, these organoids have significantly advanced tissue engineering techniques, largely due to their intrinsic capacity for self-organisation under
in vitro conditions. This self-organisation is critical for replicating the complex architecture and functionality of native vascular systems, which is essential for accurate disease modelling and therapeutic testing. In addition, the development of patient-derived, disease-specific VOs could enable researchers to investigate the underlying mechanisms of disease progression at a personalised level. This approach allows for the identification of disease-related genes and pathways, which can vary significantly between individuals. Additionally, these organoids provide a platform for assessing drug efficacy and toxicity in a patient-specific context, thereby facilitating the development of more targeted and effective treatments. A notable example is the study by Dang et al., [
29], which demonstrated the relationship between infectious disease and microcephaly using brain organoids, highlighting the potential of organoids in modelling complex disease interactions.
2.3.1. Infectious Disease Pathogenesis
In the realm of regenerative medicine, VOs have emerged as a pivotal tool, providing valuable insights into the complex interplay among blood vessels, pathogens, and immune cells[
30]. The use of VOs in various culture systems has significantly contributed to our understanding of these interactions. Recently, studies have leveraged the use of VOs to explore the mechanisms of SARS-CoV-2 infection and potential therapeutic interventions. Specifically, researchers have demonstrated the direct infection of blood vessels by SARS-CoV-2 and the subsequent use of soluble human ACE2 to hinder viral entry. This approach was particularly valuable given the multiple expression patterns of ACE2, the primary receptor for SARS-CoV-2, in several tissues.
In a study conducted by Monteil et al.,[
31], human VOs were utilised to model the vascular aspects of SARS-CoV-2 infection. The choice of VOs was driven by the need to understand the mechanisms by which the virus interacts with ECs, which are crucial components of the vascular system. This enabled the group to directly observe how SARS-CoV-2 affects these cells, contributing to the severe vascular complications seen in COVID-19 patients. It was observed that these VOs could be readily infected by SARS-CoV-2, with it also being demonstrated that infection could be significantly inhibited by the application of clinical-grade human recombinant soluble ACE2 during the initial stages of the infection[
31]. This ability to infect VOs and subsequently inhibit this infection with ACE2 provides a powerful platform for testing the efficacy of antiviral compounds in a controlled, physiologically relevant environment. Understanding the role of ACE2 in different tissues, as demonstrated by the infection patterns in the VOs, aids in explaining the multi-organ impact of SARS-CoV-2[
32,
33]. The use of VOs has provided pivotal insights into the vascular aspects of SARS-CoV-2 infection and highlighted the therapeutic potential of ACE2. These advancements highlight the importance of VO models in biomedical research, particularly for investigating intricate infectious diseases and developing targeted treatments.
Another similar, notable discovery involves the stimulation of VOs with SARS-CoV-2 antigens. In this study, Khan et al., [
34], aimed to determine the precise mechanisms by which SARS-CoV-2 induced endotheliitis, which remains unknown. The group investigated vascular permeability in the context of SARS-CoV-2-mediated endotheliitis using both patient samples and human 3D VOs composed of vascular endothelium, pericytes, and fibroblasts. By employing these VOs, it was revealed that ACE2 is predominately expressed in pericytes adjacent to vascular networks. Upon VOs being exposed to SARS-CoV-2 or its antigens, there was a significant reduction in the CD144 expression, which is essential for maintaining EC junctions of blood vessels.
The utilisation of VOs in these studies is crucial for multiple reasons. Firstly, these VOs replicate the intricate cellular architecture and function of the human vasculature, offering a more relevant model for investigating the interactions between SARS-CoV-2 and ECs than conventional 2D cell cultures. The VO model also enabled a detailed, comprehensive observation of ACE2 expression in pericytes and the subsequent effects of SARS-CoV-2 infection on endothelial permeability. This insight is crucial for understanding how the virus-induced vasculopathy and thrombotic complications. Additionally, the findings of these studies support the development of biomarker-guided therapies to mitigate thrombotic risks in COVID-19 patients by highlighting potential targets for therapeutic intervention.
2.3.2. Disease Modelling
The VOs developed by Wimmer et al., [
18] have also been employed to model diabetic vasculopathy. Analysis of dermal vasculature in patients with type 2 diabetes reveals a thick, multi-layered basement membrane. Similarly, VOs exposed to elevated levels of glucose, TNF (tumour necrosis factor) and IL-6 (interleukin 6)
in vitro exhibited an expanded basement membrane, mirroring the patient phenotype. Excessive ECM synthesis is predominantly mediated by pericytes, supporting previous findings that mural cells are responsible for basement membrane production and maintenance. Treatment with the γ-secretase inhibitor DAPT prevented basement membrane thickening and restored EC proliferation. In addition to this, pharmacological inhibition and genetic ablation studies identified DLL4 and NOTCH3 as key mediators and potential therapeutic targets in diabetic vasculopathy.
2.3.3. Drug Testing and Development
VOs derived from hiPSCs present significant promise as platforms for personalised drug testing. These organoids maintain the epigenetic characteristics of the donor patient, thus offering a highly physiologically relevant model for investigating vascular cell dysfunction that can result in CVDs. The retention of patient-specific epigenetic information enables a tailored approach to studying disease mechanisms and testing therapeutic interventions, enhancing the relevance and applicability of the findings to the specific patient condition. This advantageous characteristic makes iPSC-derived VOs particularly valuable for precision medicine, where understanding the nuances of each patient’s disease at the cellular level is crucial for developing effective treatments.
Although the current body of research utilising VOs for drug testing remains relatively limited, the promising results from studies by Monteil et al [
31] and Wimmer et al [
18] highlight their significant potential. These studies demonstrate that VOs can serve as robust platforms for personalised drug testing, offering a high degree of physiological relevance. Monteil et al. showcased how VOs could model SARS-CoV-2 infection and evaluate antiviral interventions, while Wimmer et al. utilised these organoids to replicate diabetic vasculopathy and test therapeutic compounds. Such findings indicate that VOs hold great promise for advancing precision medicine by enabling the tailored testing of drugs on patient-specific models.
2.4. Challenges and Limitations of Vascular Organoids
While vascular organoids mark a major breakthrough in regenerative medicine, they face several challenges and limitations that impede their full potential. These difficulties arise from their relatively small size and functional inconsistencies when compared to normal tissues, largely because they lack a fully mature vascular system. Additionally, the absence of certain microenvironmental cells, specifically immune cells and stromal cells[
35,
36], further restricts the potential of VOs.
A notable limitation of the protocol developed by Wimmer et al [
18] is the inability to generate vascular strictures
in vitro. Throughout the culture stage, the
in vitro VOs fail to develop the complexity required to form arteriole- or venule-like structures. As a result, these organoids lack the tunica media and adventitia, which complicates studies of atherosclerosis that affect these specific layers. While it remains possible to investigate certain disease characteristics, such as endothelial gene expression changes and angiogenic sprouting, the current organoid models are insufficient for investigating the excessive proliferation of VSMCs, macrophage infiltration, or plaque formation.
One strategy to overcome these limitations involves transplanting the organoids into the renal capsule of immunodeficient NOD-SCID (non-obese diabetic/severe combined immunodeficiency) mice. These organoids subsequently demonstrated integration with the host vasculature, forming a fully human endothelial and mural cell vasculature, which remained stable for over 6 months without the need for co-transplantation of mouse mesenchymal cells [
18]. This approach allows for the maturation and integration of the VOs within a living organism, perhaps enabling the study of more complex vascular structures and processes.
The lack of a consistent cellular microenvironment might compromise the physiological relevance and functional complexity of VOs. A well-defined cellular microenvironment is crucial for the normal functioning and development of organoids. The presence of immune cells is particularly important, as they play a crucial role in maintaining homeostasis, facilitating repair, and responding to pathogens. Similarly, stromal cells provide structural support and secrete essential growth factors that influence cellular behaviour. The absence of these cells leads to an inadequate representation of
in vivo conditions, thus limiting the usefulness of organoids in modelling complex biological processes and diseases. Despite this, Kim et al [
37] has suggested that the lack of a microenvironment in human VOs could also present certain advantages. However, it remains unclear whether this benefit extends specifically to VOs.
It has been suggested that a potential solution to circumvent such a limitation may be to co-culture VOs with mesenchymal and immune cell populations[
36,
38,
39,
40]. This approach aims to enhance the cellular complexity and functionality of VOs, thereby making them more akin to mature organs. For example, integrating ECs promotes vascularisation, effectively addressing the traditional challenge of nutrient and oxygen diffusion in larger tissue constructs. Moreover, the absence of a cellular microenvironment also provides certain benefits, as it allows for the focused study of specific cell types in isolation, ultimately simplifying the analysis of cell-specific behaviours and interactions. The development of VOs often faces limitations in generating larger organoids, which results in higher variability and complicates comparability between research studies. To mitigate these issues, it is advised to adhere to standardised design protocols[
41,
42].
Another major issue associated with VOs is their frequent irreproducibility which arises from inconsistencies in differentiating hPSCs into a specific type of organoid using organ-specific inducers. This often results in cellular composition that is heterogeneous in nature with an unidentified number of cells[
43]. When attempting to improve reproducibility, it has been proposed to use methods that determine organoid patterning, managing both the spatial and temporal aspects of organoid formation rather than relying on more speculative approaches[
44]. Additionally, optimising differentiation protocols for region-specific organoids and increasing standardisation using microwell-based techniques have also been suggested. This includes compartmentalisation to facilitate organoid-organoid communication while preventing uncontrolled fusion[
45,
46]. Moreover, it has also been demonstrated that using Matrigel or collagen to generate human VOs, both of which are widely available and frequently used, may inadvertently introduce heterogeneity and irreproducibility. To overcome this, researchers have started creating both mechanically and chemically defined synthetic ECMs for organoid culture[
47,
48].
Incorporating an ECM is crucial for the development of a fully mature and stable organoid structure. Despite this, the inclusion of an ECM is not without complications, particularly regarding cryopreservation. In this case, the ECM may hinder effective and rapid infiltration of the cryopreservation media, which introduces difficulties when attempting to recover and achieve optimal cell viability post freeze-thaw cycles. Therefore, developing, refining and implementing cryopreservation protocols tailored for VOs with ECMs is essential to ensure the widespread application VOs in research and clinical settings.
3. Vessel-on-Chip
VoC technology, an advanced subset of organ-on-chip (OoC) technology, is revolutionizing vascular research. This technology replicates the intricate structure and function of the human vascular system within a controlled, miniaturized, and biomimetic in vitro environment, bridging the gap between traditional in vitro models and in vivo studies. By offering a more physiologically relevant and ethically sound platform, VoC technology provides unprecedented opportunities to understand vascular biology and disease mechanisms.
VoC technology has emerged as a promising approach to recreate the intricate structure and function of blood vessels in a miniaturized and controlled microfluidic environment. This technology holds great potential for various applications, including disease modeling, drug testing, and investigating vascular physiology and pathology. One key aspect of VoC technology is the ability to fabricate biomimetic vascular structures. Marder et al. [
49] developed stem cell-derived VoC for CVD modeling, highlighting the potential of using patient-specific cells to create personalized vascular models. Additionally, Yan et al. [
50] introduced a rapid-patterning 3D VoC platform, enabling imaging and quantitative analysis of cell-cell junction phenotypes, which are crucial for understanding vascular processes.
Integrating physiologically relevant features into VoC models is another area of focus. Furthermore, de Graaf et al. [
51] presented a multiplexed fluidic circuit board for controlled perfusion of 3D VoC, enabling precise control over the microenvironment and fluid dynamics. Advanced microfabrication techniques are also being employed to create intricate vascular structures. Wu et al. [
52] demonstrated the use of acoustofluidic engineering to create functional VoC models, showcasing the potential of novel fabrication methods. Incorporating patient-derived cells is another key aspect of VoC technology. Bulut et al. [
53] developed a 3D VoC platform based on hiPSC-derived vascular ECs and SMCs, enabling the study of patient-specific vascular biology and disease mechanisms. Vascularized OoC models have also gained attention, as highlighted by Yin et al. [
54], who discussed advances in the model structure of
in vitro vascularized OoC systems. These models incorporate functional vascular networks, allowing for the investigation of vascular-tissue interactions and the role of the vascular system in various physiological and pathological processes. Imaging and monitoring capabilities are essential for studying dynamic processes in VoC models. Cuartas-Vélez et al. [
55] employed visible-light optical coherence tomography to track the dynamics of thrombus formation in a blood-VoC system, demonstrating the potential of advanced imaging techniques for investigating vascular pathologies.
Overall, VoC technology has made significant strides in replicating the complexity of blood vessels and their interactions with surrounding tissues. By combining advanced microfabrication techniques, patient-derived cells, physiologically relevant microenvironments, and advanced imaging modalities, these models are becoming increasingly valuable tools for basic research, disease modeling, and therapeutic development in the field of vascular biology and medicine (
Figure 3).
3.1. Microfluidic System in VoC Technology
Microfluidic technology has revolutionized the field of biomedical research, particularly in the study of vascular biology. Vascular cell biology is fundamental for understanding the mechanisms underlying major diseases such as atherosclerosis, diabetes, and cancer [
56]. The primary challenge in vascular research is replicating the dynamic, 3D microenvironment of blood vessels
in vitro. Traditional cell culture methods, which often involve static, two-dimensional conditions, fail to accurately mimic the complex interactions and mechanical forces experienced by vascular cells
in vivo [
57]. This gap in modelling physiological conditions has driven the adoption of microfluidic technology, which offers unparalleled precision in simulating the microenvironment of blood vessels.
Microfluidic devices manipulate small volumes of fluids within microscale channels, enabling precise control over the physical and chemical conditions to which cells are exposed. These devices are commonly fabricated from materials such as glass, polymers, and polydimethylsiloxane (PDMS), with PDMS being particularly favored due to its biocompatibility, optical transparency, and ease of fabrication [
56]. The fabrication process of microfluidic devices typically involves soft lithography, where a master mold is created using photolithography techniques. PDMS is then cast onto this mold to replicate the desired microstructures. The resulting PDMS chips can be bonded to glass or other PDMS layers to form enclosed microfluidic channels through which fluids can be precisely directed.
One of the significant advantages of microfluidic technology in vascular research is its ability to replicate the hemodynamic conditions of blood flow. ECs, which line the interior surface of blood vessels, are highly responsive to shear stress generated by blood flow. Microfluidic devices can generate controlled shear stress, enabling the study of EC responses under physiologically relevant conditions [
56]. This capability is crucial for understanding how mechanical forces influence vascular function and pathology.
Additionally, microfluidic platforms can establish gradients of growth factors, cytokines, and other signaling molecules, which are essential for studying processes such as angiogenesis and inflammation. Microfluidic devices also facilitate the co-culture of multiple cell types, which is critical for mimicking the complex cellular interactions within the vascular system. For instance, co-culturing ECs with SMCs or pericytes within a 3D ECM allows researchers to study the intricate signaling networks and structural organization of blood vessels [
52]. These multi-cellular models provide insights into the cellular dynamics and tissue architecture that are difficult to capture with traditional two-dimensional cultures.
Furthermore, the small scale of microfluidic devices reduces the consumption of cells and reagents, making experiments more efficient and cost-effective. The integration of microfluidic technology with OoC systems has further expanded the potential for vascular research. These advanced platforms aim to recreate the functional units of organs, incorporating features such as fluid flow, mechanical stretch, and complex cellular arrangements. VoC models, for instance, can simulate the permeability of blood vessels, the interaction of ECs with circulating immune cells, and the impact of pharmaceutical compounds on vascular integrity [
52,
53]. Such models are invaluable for drug screening, toxicology studies, and the investigation of disease mechanisms, providing a high-throughput and physiologically relevant alternative to animal models.
In summary, microfluidic technology has become an indispensable tool in vascular biology research. By offering a high degree of control over the experimental environment, microfluidic platforms enable the creation of more accurate and dynamic models of blood vessels. This advancement holds significant promise for enhancing our understanding of vascular biology and for the development of novel therapeutic strategies to combat vascular diseases.
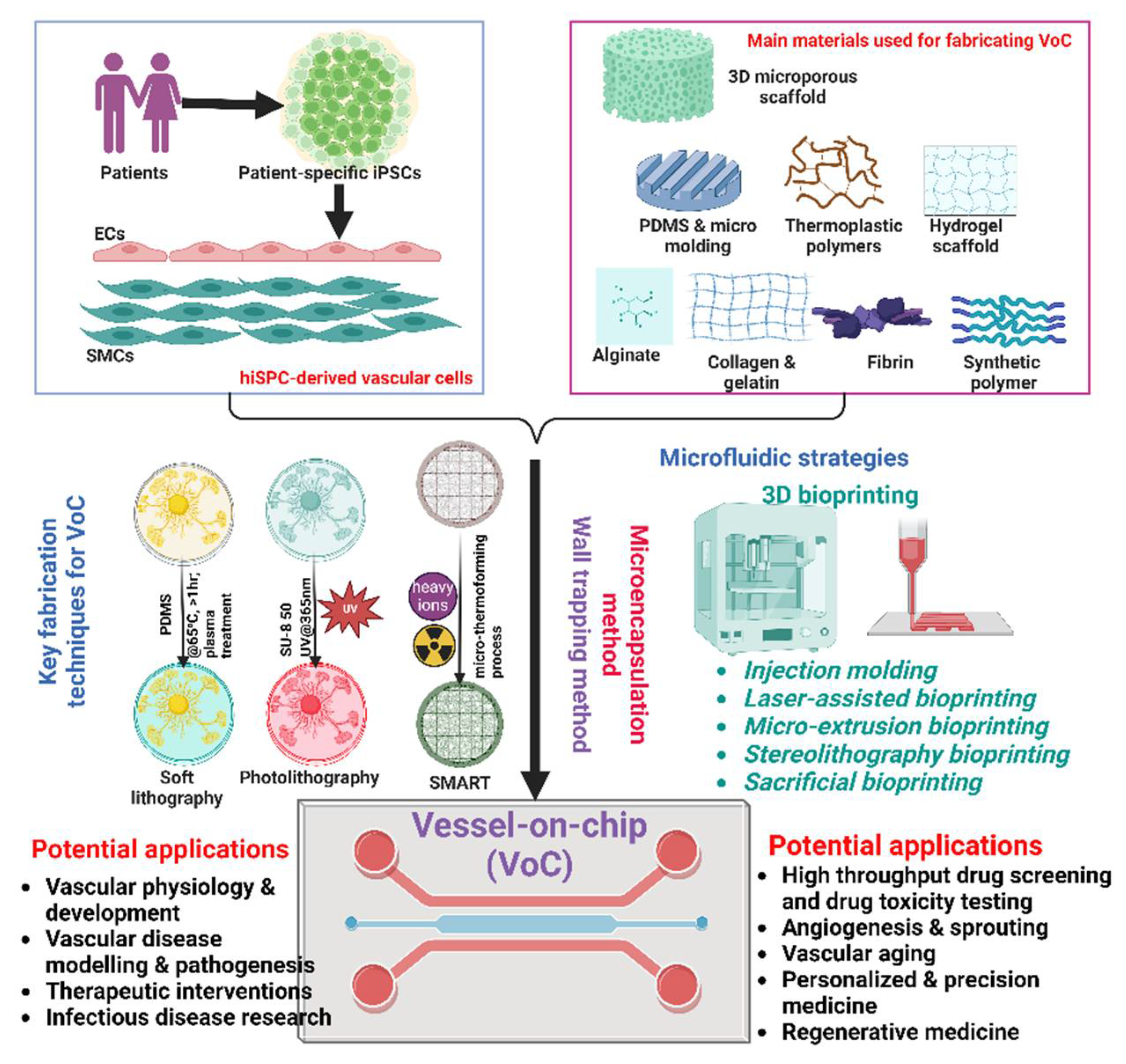
3.2. Main materials for fabricating VoC
3.2.1. Elatiomers and thermoplastics
Elastomers and thermoplastics constitute two fundamental classes of materials that have revolutionized the field of VoC platforms, offering distinct advantages and applications. Therefore, understanding the unique properties and applications of elastomers and thermoplastics is essential for optimizing VoC platforms for diverse biomedical research purposes.
3.2.1.1. Elatiomers
PDMS, as an elastomer with excellent optical, electrical and mechanical properties, has emerged as a cornerstone material in biomedical and microfluidic research, owing to its diverse applications and favorable physical properties. Widely utilized in fabricating biomedical devices, microfluidic systems, and biomodels, PDMS offers an array of advantages for such applications. Its chemical inertness, optical transparency, gas permeability, and thermal stability make it particularly attractive for use in microfluidic platforms and OoC devices [
58]. Additionally, PDMS exhibits low interfacial free energy, high physical toughness, and biocompatibility, rendering it suitable for cell culture and biomedical applications [
59]. In the context of microfluidics, PDMS enables the fabrication of intricate microstructures with high precision and cost-effectiveness through soft lithography techniques. Replica molding, microcontact printing, and micro-molding in microcapillaries are among the soft lithography methods commonly employed, with replica molding being the most prevalent [
60]. This method involves creating a master mold, usually from silicon, followed by replicating the desired structure in PDMS. PDMS's versatility extends to OoC devices, where it serves as a substrate for creating microenvironments that mimic physiological conditions of human organs [
61].
PDMS stands out as the most widely used elastomer in VoC applications [
58]. Its exceptional elasticity, transparency, and biocompatibility make it an ideal material for mimicking the mechanical properties of blood vessels. PDMS enables the fabrication of microfluidic devices with intricate geometries and precise control over channel dimensions, facilitating the replication of physiological flow conditions. Soft lithography techniques have revolutionized the rapid prototyping of PDMS-based microfluidic devices, allowing researchers to create customized vascular models with high fidelity [
62]. PDMS membranes serve as cell culture interfaces within these devices, supporting the adhesion, proliferation, and migration of ECs and other relevant cell types. However, despite its numerous advantages, PDMS has limitations, particularly in terms of small hydrophobic molecule absorption, leaching of uncrosslinked oligomers, and mechanical stiffness which can impact cellular behavior and experimental outcomes [
63]. The hydrophobic nature of PDMS can lead to the nonspecific adsorption of hydrophobic molecules, affecting drug screening assays and pharmacokinetic studies. Additionally, PDMS's relatively low Young's modulus may not accurately replicate the mechanical properties of native blood vessels, potentially influencing cellular responses and tissue development. To address these limitations, researchers are exploring alternative elastomers with enhanced mechanical properties and reduced small molecule absorption. Researchers are also actively exploring other strategies including surface modifications and the incorporation of alternative materials in combination with PDMS to mitigate these drawbacks. For instance, polyurethane-based elastomers and UV-curable polymers offer promising alternatives, providing greater control over material stiffness and surface properties [
64].
Overall, PDMS remains a material of choice in biomedical and microfluidic research, playing a pivotal role in advancing OoC technology and facilitating drug development studies through its unique combination of properties and ease of fabrication. By leveraging these novel elastomers, researchers aim to develop VoC platforms that better mimic the complex mechanical and biochemical cues present in native vascular environments.
3.2.1.2. Thermoplastics
Apart from elastomers, thermoplastic polymers, such as polycarbonate (PC) and cyclic olefin copolymers (COCs), have emerged as viable alternatives to elastomers in VoC applications [
65]. These materials offer structural stability, compatibility with high-throughput manufacturing processes, and reduced small molecule absorption, making them attractive for microfluidic platforms. Thermoplastics enable the fabrication of robust microfluidic systems with precise control over channel dimensions and geometries. They exhibit excellent biocompatibility and chemical inertness, minimizing the risk of adverse reactions with cultured cells or biological samples [
65]. Moreover, thermoplastics can withstand a wide range of temperatures and mechanical forces, making them suitable for long-term cell culture experiments. However, thermoplastics lack the elasticity of elastomers, which is essential for replicating dynamic vascular environments accurately. The significant difference in Young's modulus between thermoplastics and native ECM materials can affect cell adhesion, migration, and mechanotransduction processes [
59]. To address this limitation, researchers are exploring composite approaches that combine thermoplastics with hydrogels or elastomers to create hybrid devices with improved mechanical properties and cellular compatibility. By incorporating elastomeric components into thermoplastic-based microfluidic platforms, researchers aim to enhance the physiological relevance and functionality of VoC systems [
59].
3.2.1.3. Combining Elastomers and Thermoplastics
The integration of elastomers and thermoplastics represents a promising approach for optimizing VoC platforms. By leveraging the unique properties of both materials, researchers can overcome individual limitations and create hybrid devices with enhanced functionality and performance. For example, combining PDMS membranes with thermoplastic substrates allows researchers to capitalize on PDMS's elasticity for mimicking dynamic vascular environments while leveraging the structural stability and reduced small molecule absorption of thermoplastics [
59]. This hybrid approach enables the fabrication of microfluidic devices that accurately replicate physiological flow conditions while minimizing experimental artifacts associated with small molecule adsorption. Moreover, the development of composite materials that combine elastomeric and thermoplastic properties opens up new possibilities for creating advanced VoC platforms with tailored mechanical and biochemical properties [
59]. By engineering materials with controlled stiffness, porosity, and surface chemistry, researchers can design microfluidic devices that better mimic the complex microenvironment of native blood vessels, leading to more physiologically relevant experimental results.
In conclusion, elastomers and thermoplastics represent two distinct yet complementary classes of materials in VoC platforms. While elastomers offer exceptional elasticity and biocompatibility, thermoplastics provide structural stability and reduced small molecule absorption. By leveraging the unique properties of both materials and exploring hybrid approaches, researchers can optimize VoC platforms for a wide range of biomedical applications, advancing our understanding of vascular biology and disease mechanisms.
3.2.2. Hydrogels
Hydrogels are essential components in VoC technology due to their biocompatibility, tunable mechanical properties, and ability to mimic the natural ECM of blood vessels. Various hydrogels, both natural and synthetic, have been employed to replicate vascular environments accurately. Here, we explore different types of hydrogels used in VoC technology, detailing their properties and applications.
3.2.2.1. Alginate
Alginate is a naturally occurring polysaccharide derived from brown seaweed, renowned for its biocompatibility, non-toxicity, and ease of gelation. It forms hydrogels through ionic crosslinking with divalent cations like calcium. Alginate hydrogels provide a supportive 3D matrix that promotes cellular attachment and growth, making them suitable for vascular applications. However, alginate's mechanical properties and degradation rates are less than ideal. To enhance its functionality, alginate is often combined with other biopolymers such as gelatin or fibrin, which introduce cell-adhesion sites and improve biodegradability. These hybrid hydrogels have been used to create perfusable vascular channels and networks, demonstrating significant potential in replicating the complex architecture of blood vessels [
66].
3.2.2.2. Collagen and Gelatin
Collagen is the most abundant protein in the mammalian ECM and a critical component for tissue engineering. Its natural presence in the human body ensures high biocompatibility and the promotion of cellular activities such as adhesion, proliferation, and differentiation. Collagen hydrogels can be easily molded into various shapes, making them ideal for VoC applications. Despite its advantageous biological properties, collagen's mechanical strength is relatively low, and it is prone to rapid degradation. Enhancing collagen hydrogels' stability often involves crosslinking with agents like glutaraldehyde or genipin, or blending with more robust materials [
67]. Collagen-based VoC models have been instrumental in studying EC behavior and vascular permeability under physiological conditions [
68].
Gelatin, a form of hydrolyzed collagen, retains many of collagen's favorable biological properties but is more versatile in its applications. Gelatin hydrogels are particularly valued for their biocompatibility, low immunogenicity, and ease of processing. They can form hydrogels at physiological temperatures, which is advantageous for encapsulating living cells. However, like collagen, gelatin hydrogels suffer from weak mechanical properties and rapid degradation at body temperature. These limitations can be mitigated through chemical modifications, such as methacrylation, which allow for photo-crosslinking to create more stable structures. Gelatin methacrylate (GelMA) hydrogels are widely used in VoC systems to simulate the vascular environment and study endothelialization and cell-cell interactions under dynamic flow conditions [
69].
3.2.2.3. Fibrin
Fibrin, a protein involved in blood clotting, forms hydrogels through the polymerization of fibrinogen in the presence of thrombin. Fibrin hydrogels are highly biocompatible and support cell migration, proliferation, and differentiation, making them ideal for vascular tissue engineering. Their natural role in wound healing and angiogenesis further enhances their utility in VoC models. Fibrin hydrogels can mimic the dynamic ECM remodeling, providing a realistic environment for studying vascular biology [
70]. However, fibrin hydrogels are relatively weak and degrade quickly. To address these challenges, researchers often combine fibrin with other hydrogels or reinforce it with synthetic polymers, creating composite materials that offer better mechanical properties and controlled degradation rates.
3.2.2.4. Synthetic Polymer
Polyethylene Glycol (PEG) is a synthetic polymer known for its hydrophilicity, biocompatibility, and resistance to protein adsorption. PEG hydrogels are widely used in biomedical applications due to their tunable mechanical properties and chemical functionality. PEG hydrogels can be engineered to mimic various aspects of ECM by incorporating bioactive molecules and cell-adhesion peptides. In VoC applications, PEG-based hydrogels provide a stable and customizable platform for creating vascular networks. They offer precise control over porosity, stiffness, and degradation, enabling the study of cellular responses to different mechanical and biochemical cues [
71].
Polyvinyl Alcohol (PVA) is another synthetic polymer commonly used in hydrogel formation. PVA hydrogels are highly hydrophilic, biocompatible, and possess excellent mechanical properties. They can form stable and flexible gels through physical or chemical crosslinking. PVA hydrogels are particularly useful in VoC applications where mechanical stability and durability are crucial. Additionally, PVA can be modified with bioactive molecules to enhance cell adhesion and proliferation. PVA-based hydrogels have been employed to create robust vascular channels and networks, facilitating the study of fluid dynamics and EC behavior under physiological conditions [
72].
3.2.2.5. Hybrid Hydrogels
Combining natural and synthetic hydrogels results in hybrid materials that leverage the advantages of both. Hybrid hydrogels can offer the biological functionality of natural polymers and the mechanical robustness of synthetic ones. For instance, blending alginate with GelMA or collagen with PEG can produce hydrogels that are both biocompatible and mechanically stable. These hybrid hydrogels are particularly useful in VoC technology, where mimicking the complex structure and function of blood vessels requires materials with tailored properties. By adjusting the composition and crosslinking methods, researchers can fine-tune the physical and biochemical environment to study various aspects of vascular biology and pathology [
73]. In conclusion, hydrogels are integral to VoC technology, providing versatile platforms for simulating vascular environments. The ongoing development of natural, synthetic, and hybrid hydrogels continues to advance the field, enabling more accurate and functional models of blood vessels for research and therapeutic applications.
Table 1.
Main materials used for fabricating VoC.
Table 1.
Main materials used for fabricating VoC.
| Material |
Key Functional Traits |
Advantages |
Disadvantages |
Potential Solutions |
| PDMS (polydimethylsiloxane) |
Elasticity, Optical transparency, Gas permeability [50,59] |
Biocompatible, Enables intricate microstructure, Cost-effective fabrication [50,59] |
Absorbs small hydrophobic molecules, Leaching of uncrosslinked oligomers, Relatively low Young's modulus [62,63] |
Surface modifications, Incorporation of alternative materials [63,73] |
| Thermoplastics (e.g., PC, COCs) |
Structural stability, Chemical inertness [65,72] |
Reduced small molecule absorption, Compatible with high-throughput manufacturing, Wide temperature tolerance [65,72] |
Lack of elasticity, High Young's modulus compared to native ECM [59,65] |
Combining with hydrogels or elastomers to create hybrid devices [64,73] |
| Alginate |
Biocompatibility, Ease of gelation[66] |
Non-toxic, Provides 3D matrix for cell growth [66] |
Suboptimal mechanical properties, Non-ideal degradation rates [66,67] |
Combining with other biopolymers (e.g., gelatin, fibrin) [66,67] |
| Collagen |
High biocompatibility, Promotes cellular activities [67,68] |
Easily moldable, Natural presence in human body [67,68] |
Low mechanical strength, Rapid degradation [67,68] |
Crosslinking with agents like glutaraldehyde, Blending with more robust materials [67,68] |
| Gelatin |
Biocompatibility, Low immunogenicity [69] |
Retains collagen's biological properties, Forms hydrogels at physiological temperatures [69] |
Weak mechanical properties, Rapid degradation at body temperature[69] |
Chemical modifications (e.g., methacrylation), Photo-crosslinking [69] |
| Fibrin |
Supports cell migration and proliferation, Mimics dynamic ECM remodeling[67,70] |
- Highly biocompatible, Natural role in angiogenesis [67,70]
|
Relatively weak, Degrades quickly [67,70] |
Combining with other hydrogels, Reinforcing with synthetic polymers [67,70] |
| Polyethylene Glycol (PEG) |
Hydrophilicity, Tunable mechanical properties [71] |
- Resistant to protein adsorption, Customizable with bioactive molecules [71] |
Lacks inherent bioactivity [71] |
Incorporating cell-adhesion peptides and bioactive molecules [71] |
| Polyvinyl Alcohol (PVA) |
Highly hydrophilic, Excellent mechanical properties [71] |
Stable and flexible, Durable [71] |
Limited cell adhesion properties [71] |
Modification with bioactive molecules [71]
|
| Hybrid Hydrogels |
Combines properties of natural and synthetic materials [73] |
Tailored mechanical and biochemical properties, Enhanced functionality [73] |
Complexity in fabrication and characterization[73] |
Optimizing composition and crosslinking methods [73] |
3.3. Key Fabrication Techniques for VoC
3.3.1. Soft-Lithography
Soft lithography has emerged as a pivotal fabrication technique in the development of OoC systems, particularly for creating intricate microfluidic structures that mimic biological environments. This versatile approach leverages the unique properties of elastomeric materials, such as PDMS, enabling the construction of complex and biocompatible microchannels essential for cell culture and imaging applications.
The soft lithography process typically commences with the creation of a master mold using photolithography or micromilling techniques. Photolithography involves the use of a photosensitive material, such as a photoresist, which is patterned by exposure to ultraviolet light through a photomask, creating a 3D relief pattern. Alternatively, micromilling or 3D printing can be employed to directly machine the mold from thermoplastic materials like poly(methyl methacrylate) (PMMA) [
74]. Once the master mold is prepared, a PDMS pre-polymer mixture is poured over it, degassed to remove air bubbles, and cured, typically by heating, to solidify into an elastomeric form [
75]. The cured PDMS replica is then peeled away from the mold, revealing the negative of the original pattern as a network of microchannels. One of the key advantages of using PDMS in soft lithography is its gas permeability, which allows for the maintenance of a controlled microenvironment within the channels, crucial for cell culture applications [
75]. Additionally, PDMS microfluidic devices can be easily bonded to other surfaces, such as glass slides, through plasma treatment, creating enclosed microfluidic channels. Soft lithography also enables the fabrication of multilayered microfluidic devices, where multiple PDMS layers are aligned and bonded together. This capability facilitates the integration of complex channel networks, valves, and pumps within the microfluidic system, enabling the creation of dynamic microenvironments that simulate physiological conditions like pulsatile blood flow or peristaltic movements [
76].
While PDMS and soft lithography offer numerous advantages, there are some limitations to consider. PDMS is known for its high hydrophobicity and tendency to absorb small hydrophobic molecules, which can be a drawback in certain applications. Additionally, PDMS may swell or degrade when exposed to certain organic solvents, necessitating careful consideration of experimental conditions and potential surface modifications. Despite these challenges, soft lithography remains a cornerstone technique in the field of microfluidics and OoC systems, enabling the development of advanced in vitro models that closely mimic human physiological and pathological conditions. By facilitating precise control over the microenvironment, soft lithography contributes significantly to the advancement of biomedical research and the development of personalized medicine.
3.3.2. Photolithography
Photolithography has emerged as a powerful technique for fabricating microfluidic devices, including VoC systems, which aim to mimic the intricate structure and function of blood vessels. These devices have garnered significant interest in various fields, such as drug development, disease modeling, and fundamental vascular biology studies. Li et al. developed a PDMS-based microfluidic device with multi-height structures using a single-step photolithography process, where a printed circuit board served as the master mold [
77]. Revzin et al. utilized photolithography to create poly(ethylene glycol) hydrogel microstructures, demonstrating the versatility of this technique for various materials [
78]. Cokelet et al. pioneered the fabrication of
in vitro microvascular blood flow systems using photolithography, enabling the study of blood flow dynamics in microvasculature [
79]. Fenech et al. employed backside lithography to create microfluidic blood vasculature replicas, allowing for the investigation of blood cell behavior in physiologically relevant environments [
80]. Mathur et al. developed an innovative OoC platform using endothelial progenitor cells derived from blood to reconstitute vascular thromboinflammation in VoC, showcasing the potential of VoC systems for modeling complex vascular pathologies [
81]. Furthermore, de Graaf et al. reported on a scalable microphysiological system to model 3D blood vessels, demonstrating the integration of photolithography with advanced cell culture techniques [
82].
Photolithography offers several advantages in VoC fabrication, including precise control over geometrical features, the ability to create complex and intricate structures, and compatibility with a wide range of materials. However, it also presents challenges, such as the need for cleanroom facilities, limitations in feature resolution, and potential compatibility issues with certain materials. Despite these challenges, the combination of photolithography and microfluidics has enabled the creation of increasingly sophisticated VoC systems, allowing researchers to study vascular biology and pathologies in unprecedented detail. As the field continues to evolve, photolithography will likely remain a valuable tool in the fabrication of these biomimetic platforms, contributing to advancements in drug discovery, personalized medicine, and our fundamental understanding of vascular physiology.
3.3.3. Non-Lithographic Methods
While photolithography has been extensively utilized in the fabrication of VoC devices, researchers have also explored alternative non-lithographic methods to overcome some of the limitations associated with photolithography. One such approach is the substrate modification and replication by thermoforming (SMART) technology proposed by Kappings et al [
83]. This technique involves irradiating a polycarbonate film with heavy ions and subsequently employing a micro-thermoforming process to create a semicircular form, which is then bonded to form a porous microchannel.
The SMART technology addresses the challenge of recreating a rounded cross-section, which is often encountered in the microfabrication of in vitro vasculature models. By leveraging the tubular geometry and symmetry of real vasculature, this approach simplifies the process of creating a biomimetic vascular scaffold. However, despite its potential, the SMART technology has not yet been extensively demonstrated for fabricating multiscale and ubiquitous vasculature structures.
While non-lithographic methods like SMART technology offer alternative approaches to VoC fabrication, further research and development are necessary to fully exploit their capabilities and address the limitations of traditional photolithography-based techniques [
84]. The integration of these non-lithographic methods with existing fabrication strategies may pave the way for more accurate and physiologically relevant VoC models, enabling advancements in various fields, including drug development, disease modeling, and fundamental vascular biology research.
3.4. Microfluidic Strategies
Microfluidics, the science of manipulating and controlling fluids at microscale, has emerged as a game-changer in various fields, including biology, energy, and materials science [
85,
86]. This technology offers numerous advantages, such as precise fluid control, low sample and reagent consumption, high throughput, and the potential for integration and automation [
87]. Microfluidic devices, often fabricated using advanced materials and techniques [
85,
88], enable intricate manipulation of fluids, facilitating complex analyses, syntheses, and processes. The integration of nanomaterials into microfluidic platforms has further expanded their capabilities, enabling enhanced sensitivity, selectivity, and functionality. With continuous advancements, microfluidics holds immense potential for revolutionizing numerous applications across diverse domains [
86,
88].
3.4.1. Wall Trapping Method
Recreating vasculature in an OoC device is a crucial step towards developing physiologically relevant models for studying various biological processes and disease mechanisms. One promising approach to achieve this is the wall-trapping method, which involves seeding ECs onto the sidewalls of microfluidic channels to form an endothelial barrier [
82,
89].
The wall-trapping method can be realized using either a porous membrane or a hydrogel matrix. Porous membranes, often fabricated from PDMS, allow for co-culture of multiple cell types, enabling the study of cell-cell interactions [
89,
90,
91,
92]. However, the membrane's planar structure differs from the hollowed nature of
in vivo vasculature, posing a limitation. Alternatively, hydrogels, such as collagen or fibrin, can be utilized to create lumenized channels [
92]. This approach allows for full interaction between the trapped ECs and the surrounding cells without the need for an intermediate membrane.
One notable advantage of the hydrogel-based wall-trapping method is the ability to create hollowed structures, mimicking the tubular architecture of blood vessels. This can be achieved by utilizing a sacrificial material, such as a needle or a bioprinted structure, to generate a lumen within the hydrogel. Subsequently, the sacrificial material is removed, leaving behind a hollow channel lined with ECs. This approach has been successfully employed in angiogenesis models, enabling the study of vascular sprouting and the evaluation of antiangiogenic drugs [
92].
While the wall-trapping method offers several advantages, it is not without challenges. The fabrication of precise, hollowed structures within hydrogels can be technically demanding, often requiring specialized equipment or techniques [
92]. Additionally, the cell seeding process may subject the trapped cells to high shear stress, potentially compromising their viability and function. To overcome these limitations, ongoing research efforts are focused on refining the fabrication methods and exploring alternative materials and techniques. For instance, the integration of advanced bioprinting technologies holds promise for creating intricate, patient-specific vascular networks within hydrogel matrices [
93]. Moreover, the development of biocompatible and biomimetic materials that can better recapitulate the native ECM environment is an active area of research [
94].
Overall, the wall-trapping method represents a valuable tool in the field of VoC technology, enabling the recreation of physiologically relevant vasculature in vitro. By addressing the current limitations through innovative materials and fabrication strategies, this approach has the potential to unlock new avenues for studying vascular biology, disease modeling, and drug screening, ultimately contributing to the advancement of personalized medicine and regenerative therapies.
3.4.2. Microencapsulation Method
The microencapsulation method, also known as the self-assembling or self-morphogenesis method, has emerged as a promising approach for recreating vasculature in VoC technology. This method involves encapsulating ECs within microfluidic chambers or microchannels under precisely controlled microenvironmental conditions, allowing the spontaneous formation of vascular structures [
95].
A key advantage of the microencapsulation method is its ability to induce vasculogenesis and angiogenesis processes without subjecting the cells to high shear stress, which can compromise their viability and function. The encapsulated ECs self-organize and form vascular networks in response to specific morphogenetic cues, such as growth factors and ECM components. Vascular endothelial growth factor (VEGF) plays a pivotal role in promoting vascular sprouting and formation within these microencapsulated systems [
96]. Additionally, other factors like fibroblast growth factor (FGF) and angiopoietin (ANG) have been explored to modulate the formation and stabilization of the vascular structures [
97].
For instance, Campisi et al. successfully created a vascularized network by culturing hiPSC-derived ECs (hiPSC-ECs) in a microfluidic device supplemented with VEGF, enabling the tri-culture of iPSC-ECs, pericytes, and astrocytes to mimic the complex blood-brain barrier microenvironment [
98].
While the microencapsulation method enables the generation of intricate vascular networks, one limitation is the unpredictable sprouting patterns, which can be challenging when aiming to recapitulate precise tissue structures and functions. To address this issue, researchers have explored the application of microfluidic forces, such as shear stress, circumferential stress, and axial stress, to guide and shape the newly formed vasculature [
99]. These biomechanical forces, combined with other undefined factors influencing vasculogenesis and angiogenesis, offer the potential to exert greater control over the morphogenesis process.
However, further research is needed to fully understand and harness these factors for recreating accurate vascular architectures within VoC platforms. Overall, the microencapsulation method represents a valuable tool for studying vascular biology and developing physiologically relevant vascular models. By leveraging the self-organizing capabilities of ECs and the precise control of microenvironmental cues, this approach holds promise for advancing VoC technology and enabling applications in disease modeling, drug screening, and regenerative medicine [
94].
3.4.3. 3D Bioprinting
3D bioprinting has emerged as a promising technique for creating complex biomimetic structures, including blood vessels and vascular networks. This technology combines biomaterials, cells, and computer-aided design to fabricate 3D constructs with high precision and intricate geometries. The ability to engineer vascularized tissues has significant implications for regenerative medicine, drug testing, and disease modeling [
85,
100]
In this section, we will discuss the various approaches and advancements in 3D bioprinting for modeling vasculature. One of the key strategies employed in 3D bioprinting of vasculature involves the use of microfluidic chips. Salmon et al. [
87] demonstrated the engineering of neurovascular organoids using 3D printed microfluidic chips. They incorporated ECs and pericytes within a hydrogel matrix, enabling the formation of a perfusable vascular network. This approach allowed for the study of blood-brain barrier function and the interaction between neural and vascular components.
Another innovative approach is the development of continuously perfusable and customizable VoC platforms. This platform allows for real-time monitoring and manipulation of the vascular network, making it a valuable tool for studying vascular biology and disease modeling.
In addition to microfluidic systems, direct 3D bioprinting of vessel-like structures has also been explored. Gao et al. [
101] developed a method for 3D bioprinting of vessel-like structures with multilevel fluidic channels. By employing a coaxial nozzle system and a sacrificial material, they were able to create hierarchical vascular networks with interconnected channels. This approach holds promise for engineering complex vascularized tissues.
Xu et al. [
102] proposed a novel strategy for creating biomimetic blood vessels using 3D bioprinting technology. They employed a coaxial nozzle system to print a multi-layered construct, mimicking the structure of a blood vessel. The inner layer consisted of ECs, while the outer layer comprised SMCs, replicating the native architecture of blood vessels.
In addition to these approaches, researchers have explored various bioprinting methods for fabricating
in vitro tubular blood vessel models. Sasmal et al. [
85] provided a comprehensive review of 3D bioprinting techniques for modeling vasculature, including extrusion-based, inkjet-based, and laser-assisted bioprinting. They highlighted the advantages and limitations of each method and discussed the potential applications of these vascular models in drug testing and disease modeling. Kim et al. [
100] also reviewed bioprinting methods for fabricating
in vitro tubular blood vessel models. They discussed the importance of considering factors such as cell types, biomaterials, and printing parameters to achieve physiologically relevant and functional vascular constructs.
In summary, 3D bioprinting offers a versatile and powerful platform for engineering vascularized tissues and modeling vasculature. Researchers have developed various strategies, including microfluidic chips, continuously perfusable platforms, and direct bioprinting of vessel-like structures. These approaches have leveraged techniques such as coaxial nozzle systems, sacrificial materials, and multi-layered printing to create biomimetic vascular networks. The ability to precisely control the architecture, composition, and perfusion of these vascular constructs holds great potential for advancing regenerative medicine, drug testing, and disease modeling studies. Bioprinting has emerged as a promising technology for recreating vasculature in VoC models. This advanced fabrication technique allows for the precise deposition of biomaterials and living cells in a layer-by-layer manner, enabling the creation of intricate 3D structures that mimic the complexity of native vascular networks [
93,
103]. By leveraging different bioprinting strategies as discussed below, researchers can overcome the limitations of traditional microfabrication methods and achieve greater control over the architectural features, cell distribution, and biomimetic properties of the engineered vasculature [
98,
104]. However, challenges related to material selection, resolution, and scalability remain, and further developments are needed to fully realize the potential of bioprinting for VoC applications [
105].
3.4.3.1. Injection Molding
Injection molding has emerged as a significant fabrication technique for producing VoC devices, offering unparalleled precision, repeatability, and scalability [
94,
106]. This method involves injecting molten polymer into a meticulously designed mold cavity, where it cools and solidifies into intricate microvascular networks that mimic the complex physiology of blood vessels [
57,
69]. The injection molding process begins with the selection of an appropriate thermoplastic polymer, chosen for its ease of molding and stable properties upon cooling [
94]. The selected polymer is heated until it reaches a fluid state and then injected under high pressure into the mold cavity, which is designed to incorporate detailed features such as varying diameters and intricate geometries that accurately replicate natural blood vessels [
69,
93]. Once the polymer fills the mold, a carefully controlled cooling process takes place to prevent warping or deformation, which could compromise the functionality of the microchannels. After solidification, the molded part is ejected, revealing the desired vascular structures required for VoC applications [
85,
93].
One of the primary advantages of injection molding is the high precision and consistency it offers [
94]. The process can repeatedly produce microchannels with exact dimensions, ensuring the reliability and functionality of the VoC devices. This precision is particularly crucial when creating microvascular networks that need to closely mimic the natural physiological conditions of human blood vessels [
85]. Additionally, injection molding is highly efficient for mass production [
94]. Once the mold is created, it can produce a large number of identical devices with minimal variation, making it an attractive option for VoC technology, where multiple devices may be required for extensive testing and research [
85,
107]. This scalability can accelerate the development and application of VoC technologies in biomedical research and drug development.
The choice of polymer is critical in injection molding for VoC devices [
94]. PDMS is commonly used due to its biocompatibility, optical transparency, and flexibility, making it an excellent material for replicating the soft, elastic nature of blood vessels. However, PDMS can absorb small molecules, which may interfere with certain applications. In such cases, alternative materials like cyclic olefin copolymer or polystyrene may be used to provide chemical resistance or reduced absorption [
108].
Despite its numerous advantages, injection molding also presents some challenges. The initial cost of creating a mold can be high, requiring precise machining and robust materials to withstand the injection pressure and thermal cycling [
107]. While this investment is justified for large-scale production, it may pose a barrier for smaller research labs or early-stage development projects. Another challenge is the shrinkage and potential deformation of the polymer during cooling [
94]. Mold designers must account for these factors to ensure that the final product maintains the necessary dimensions and functionality. Additionally, while thermoplastics are versatile, not all polymers suitable for biological applications can be used in injection molding, potentially limiting material choices.
Despite these challenges, injection molding remains a powerful and versatile technique for fabricating VoC devices, providing the precision and scalability needed to replicate complex microvascular networks [
85,
93]. By carefully selecting appropriate materials and optimizing the molding process, researchers and manufacturers can produce high-quality, reliable devices that advance the capabilities of VoC technology in biomedical research.
3.4.3.2. Laser-Assisted Bioprinting
Laser-assisted bioprinting has emerged as a promising technique for creating intricate vascular structures within VoC platforms. This approach leverages the precision and versatility of laser technology to deposit biomaterials and living cells in predetermined patterns, enabling the fabrication of complex and biomimetic microvascular networks [
69,
85].
One of the key advantages of laser-assisted bioprinting is its ability to create on-demand patterns of various cell types and biomaterials with high spatial resolution [
108,
109]. This flexibility allows researchers to recreate the intricate architecture of blood vessels, including varying diameters, branching patterns, and the incorporation of supporting cell types, such as pericytes and SMCs [
110]. Moreover, laser-assisted bioprinting facilitates the integration of inorganic materials, such as hydroxyapatite, into the printed constructs, which can enhance the biomimetic properties and functionality of the engineered vasculature [
108]. This capability is particularly valuable for the development of bone-mimetic VoC models, enabling the study of vascular-bone interactions and the evaluation of bone regeneration strategies[
111] .
The bioprinting process can be performed with a wide range of biomaterials, including hydrogels, which provide a supportive and biomimetic environment for the encapsulated cells [
109,
110]. Alginate, a natural polysaccharide, has been successfully employed in laser-assisted bioprinting, allowing for the fabrication of intricate 3D cellular constructs with high viability and structural integrity [
109]. Despite its advantages, laser-assisted bioprinting also faces challenges that require further research and optimization. One crucial aspect is the selection of appropriate laser parameters, such as wavelength, pulse duration, and energy density, to ensure minimal damage to the deposited biomaterials and cells [
110,
112]. Additionally, the integration of vascularization strategies with other OoC components, such as microfluidic channels and porous membranes, remains an area that requires further exploration [
111].
Ongoing research efforts are focused on advancing the capabilities of laser-assisted bioprinting for VoC applications. This includes the development of novel bioinks with enhanced printability and biocompatibility, as well as the exploration of multi-material printing strategies to create heterogeneous and biomimetic vascular structures [
110,
112]. Furthermore, the integration of advanced imaging and feedback systems can facilitate real-time monitoring and optimization of the bioprinting process, ensuring accurate and reproducible results [
112].
Overall, laser-assisted bioprinting offers a powerful tool for recreating the intricate and biomimetic vasculature within VoC platforms, enabling the development of physiologically relevant models for various biomedical applications, including disease modeling, drug screening, and regenerative medicine [
69,
93,
112].
3.4.3.3. Micro-Extrusion Bioprinting
Micro-extrusion bioprinting has emerged as a versatile and promising technique for creating biomimetic vascular structures within VoC platforms. This technology involves the extrusion of bioinks, which are biomaterial-cell suspensions, through microscale nozzles to generate intricate 3D constructs [
113,
114]. One of the key advantages of micro-extrusion bioprinting is its ability to create complex and heterogeneous structures with high spatial control [
115]. This precision enables the fabrication of vascular networks with varying diameters, branching patterns, and the incorporation of different cell types, such as ECs, pericytes, and SMCs, to mimic the native architecture of blood vessels [
57,
116].
The selection of bioinks plays a crucial role in the success of micro-extrusion bioprinting for VoC applications. Various natural and synthetic biomaterials, including collagen, alginate, and hydrogels, have been explored as bioink components [
113,
117]. These materials provide a supportive and biocompatible environment for the encapsulated cells, facilitating their proliferation, differentiation, and organized assembly into vascular structures [
114,
118]. Micro-extrusion bioprinting also offers the ability to create multi-material constructs, enabling the integration of different cell types and biomaterials within a single printed structure [
119]. This capability is particularly valuable for recapitulating the heterogeneity and complexity of native vasculature, which often involves the interaction of multiple cell types and ECM components [
116,
117]. Despite its advantages, micro-extrusion bioprinting faces several challenges that require further research and optimization. One significant challenge is maintaining cell viability and function during the printing process, as the shear forces and extrusion pressures can potentially damage the cells [
114,
115]. Additionally, achieving precise control over the deposition and fusion of bioinks is crucial for creating seamless and continuous vascular structures [
115,
117].
Ongoing research efforts are focused on developing advanced bioinks with improved printability, biocompatibility, and mechanical properties [
113,
115]. Furthermore, the integration of micro-extrusion bioprinting with other fabrication techniques, such as sacrificial templating or stereolithography, holds promise for creating more complex and biomimetic vascular architectures within VoC platforms [
116,
120].
Overall, micro-extrusion bioprinting offers a powerful tool for recreating the intricate and heterogeneous vasculature within VoC models. By leveraging the versatility of bioink formulations and the precise deposition capabilities of this technology, researchers can develop physiologically relevant vascular models for studying various biological processes, disease mechanisms, and evaluating therapeutic interventions [
114,
117,
118].
3.4.3.4. Stereolithography Bioprinting
Stereolithography, a pioneering additive manufacturing technique, has revolutionized the field of 3D printing since its inception in the 1980s. Introduced by Chuck Hull through his groundbreaking patent [
121], this technology utilizes a photopolymerization process to selectively cure a liquid photopolymer resin, layer by layer, creating intricate 3D objects. The advent of stereolithography paved the way for numerous applications, including the fabrication of prototypes, molds, and end-use products across various industries. However, its true potential has been realized in the realm of biomedical engineering, where it has enabled the creation of complex biological constructs and OoC systems [
58].
Stereolithographic 3D bioprinting has emerged as a powerful tool for manufacturing intricate biomimetic structures with exceptional precision and resolution. By leveraging the principles of photopolymerization, researchers have been able to create highly detailed and biofunctionalized hydrogel constructs, capable of mimicking the intricate microarchitecture of native tissues [
122]. One notable application of this technology is the development of 3D culture chips with integrated perfusion networks, which provide a more physiologically relevant environment for cell culture studies. These advanced
in vitro models have the potential to improve our understanding of biological processes, disease mechanisms, and drug screening, reducing the need for animal testing [
123].
Moreover, the ability to fabricate multivascular networks and functional intravascular topologies within biocompatible hydrogels has opened up new avenues for tissue engineering and regenerative medicine. These vascularized constructs can facilitate the integration and survival of implanted cells or tissues, overcoming the longstanding challenge of maintaining adequate nutrient and oxygen supply [
118]. As the field of OoC technology continues to evolve, stereolithographic 3D bioprinting has emerged as a crucial manufacturing technique [
120]. By enabling the precise construction of microfluidic devices and biomimetic structures, researchers can create more sophisticated OoC systems that better recapitulate the complexity of human physiology. Furthermore, recent advancements in 3D bioprinting have facilitated the development of vascularized tumor-on-a-chip models, providing valuable insights into cancer biology and potentially accelerating the discovery of novel therapeutic strategies.
While stereolithography has already made significant contributions to the biomedical field, ongoing research and technological advancements promise to unlock even greater possibilities. With continued interdisciplinary collaboration among engineers, biologists, and clinicians, stereolithographic 3D bioprinting holds the potential to revolutionize personalized medicine, drug discovery, and tissue engineering, ultimately improving patient outcomes and advancing human health.
3.4.3.5. Sacrificial Bio-Printing
Sacrificial bioprinting has emerged as a powerful technique for creating intricate vascular networks within hydrogel constructs, enabling the development of advanced VoC systems. This approach involves the fabrication of sacrificial structures that are subsequently removed, leaving behind perfusable channels that mimic the intricate vasculature found in living tissues. One of the pioneering studies in this field [
114] demonstrated the feasibility of creating perfused functional vascular channels within 3D hydrogel constructs using a sacrificial bioprinting process. By printing a sacrificial material and encapsulating it within a cell-laden hydrogel, researchers were able to generate intricate vascular networks that could be perfused with desired fluids.
Building on this foundation, subsequent research [
116] has expanded the capabilities of sacrificial bioprinting to generate multi-scale vascular networks within 3D hydrogels. These hierarchical vascular architectures, consisting of interconnected channels of varying diameters, more accurately recapitulate the complexity of native vasculature, enabling the development of more physiologically relevant VoC models. To further enhance the biomimicry of these vascular constructs, researchers have explored the integration of complex channel geometries [
117,
118] and the incorporation of multiple fluidic channels within a single construct [
122]. These advancements have enabled the creation of more sophisticated VoC systems that better recapitulate the intricate branching patterns and hierarchical organization of native vasculature.
The application of sacrificial bioprinting in cancer research [
117] has also gained significant attention. By creating vascularized tumor-on-chip models, researchers can investigate the interplay between cancer cells and the vascular microenvironment, potentially leading to insights that could inform the development of novel therapeutic strategies. Furthermore, the integration of advanced fabrication techniques, such as two-photon 3D printing and scaffold molding [
121], has been explored to improve the fidelity and resolution of sacrificial bioprinting processes. These approaches have the potential to enhance the precision and complexity of the vascular networks created, further bridging the gap between engineered constructs and native tissues.
While sacrificial bioprinting has already made significant contributions to the field of VoC technology, ongoing research and technological advancements promise to unlock even greater possibilities. With continued interdisciplinary collaboration and the integration of complementary fabrication techniques, sacrificial bioprinting holds the potential to revolutionize the development of physiologically relevant VoC models, ultimately advancing our understanding of vascular biology and facilitating the translation of these technologies to clinical applications.
Table 2.
Key strategies for creating VoC.
Table 2.
Key strategies for creating VoC.
| Fabrication Technique |
Key Advantages |
Disadvantages |
Potential Solutions to Limitations |
| Soft-lithography |
Precise control over microstructures, Biocompatible (PDMS), Gas permeable, Enables multilayered devices [75] |
PDMS absorbs small hydrophobic molecules, PDMS may swell or degrade with organic solvents [75] |
Surface modifications, Use of alternative materials, Careful selection of experimental conditions [75] |
| 3D bioprinting |
Creates complex 3D structures, Enables vascularized tissues, High precision and customization [85,87,100,101] |
Technical complexity, Limited by printable materials, Challenges in maintaining cell viability [85,100] |
Development of advanced bioinks, Optimization of printing parameters, Integration with other fabrication methods [85,87,100,101] |
| Photolithography |
High precision for microfluidic structures, Compatible with various materials, Enables complex channel designs [77,78,79,80] |
Requires cleanroom facilities, Limited feature resolution, Material compatibility issues [77,78,79,80] |
Development of single-step processes [77], Exploration of alternative photo-sensitive materials [78], Integration with other fabrication techniques [80] |
| Non-lithographic methods (e.g., SMART) |
Creates rounded cross-sections, Simplifies biomimetic scaffold creation [89] |
Limited demonstration for multiscale structures, Less established than other methods [89] |
Further research and development, Integration with existing fabrication strategies [89] |
| Wall trapping method |
Enables co-culture of multiple cell types, Creates lumenized channels [95,96] |
Planar membrane structure (for porous membranes), High shear stress during cell seeding [95,96] |
Use of hydrogels instead of membranes, Optimization of cell seeding techniques, Integration with advanced bioprinting [95,96] |
| Microencapsulation method |
Induces spontaneous vascular formation, Low shear stress on cells, Mimics natural vasculogenesis [98,99,103] |
Unpredictable sprouting patterns, Challenges in controlling precise structures [98,99,103] |
Application of microfluidic forces to guide growth, Optimization of growth factor combinations, Integration with other fabrication methods [98,99,103] |
| Injection molding |
High precision and repeatability, Scalable for mass production, Efficient for complex microchannels [116] |
High initial mold cost, Polymer shrinkage and deformation, Limited material choices [116] |
Careful mold design to account for shrinkage, Development of new moldable biomaterials, Optimization of cooling processes [116] |
| Laser-assisted bioprinting |
High spatial resolution, On-demand patterning, Enables multi-material constructs [122,123] |
Potential cell damage from laser, Challenges in integrating with other components [122,123] |
Optimization of laser parameters, Development of protective bioinks, Integration of real-time monitoring systems [122,123] |
| Micro-extrusion bioprinting |
Creates complex, heterogeneous structures, Enables multi-material constructs, Wide range of printable materials [116,117] |
Shear stress may damage cells, Challenges in precise bioink deposition [116,117] |
Development of shear-thinning bioinks, Optimization of extrusion parameters, Integration with other fabrication techniques [116,117] |
| Stereolithography bioprinting |
High resolution and precision, Enables complex 3D structures, Rapid fabrication [119,120,121] |
Limited by photo-crosslinkable materials, Potential cytotoxicity of photoinitiators [120,121] |
Development of biocompatible photopolymers, Optimization of light exposure parameters, Integration with other bioprinting techniques [120,121] |
| Sacrificial bioprinting |
Creates perfusable channels, Enables complex vascular networks, Suitable for multi-scale structures |
Challenges in removing sacrificial material, Limited by properties of sacrificial materials |
Development of easily removable sacrificial materials, Integration with other fabrication techniques, Optimization of removal processes |
3.5. System Integration in Microfluidic Devices and VoC Technology
The design and integration of microchannels, oxygen supply systems, pumps, and valves are pivotal in the development of advanced microfluidic devices. These components collectively enhance the functionality, precision, and application range of microfluidic platforms, particularly in tissue engineering and disease modeling.
Microchannels serve as the foundational architecture of microfluidic devices. Their design and material composition significantly impact the overall performance and application scope of the system [
124]. For instance, cyclic olefin copolymer is frequently chosen for fabricating microchannels due to its robustness, suitability for mass production, favorable optical properties for imaging, and low chemical absorption. The typical configuration involves multiple microchannels motored via a multi-channel syringe pump, enabling precise control over fluid dynamics within the device. The serpentine-shaped microchannels are particularly advantageous for hydrodynamic trapping of organoids, ensuring accurate positioning within predefined locations. These channels can be adjusted in dimensions to accommodate different sizes of organoids, which is critical for experiments involving various cell types and sizes. This adaptability allows for the encapsulation of organoids within a fibrin hydrogel matrix without morphological alterations, maintaining their structural integrity and functionality.
Oxygen supply is a crucial consideration in microfluidic systems, especially for applications involving cell culture and tissue engineering [
70]. The microcirculation within these devices must mimic physiological conditions to ensure cell viability and function. In natural systems, oxygen is delivered to tissues primarily through diffusion, facilitated by the vascular architecture that ensures proximity between cells and capillaries. In microfluidic devices, oxygen delivery can be managed through the integration of gas-permeable materials or by embedding oxygen-generating elements within the system. Hemoglobin's role in oxygen transport
in vivo highlights the necessity for efficient diffusion and convection mechanisms in microfluidic models. Computational models and experimental setups often aim to replicate these conditions, ensuring that oxygen diffusion aligns with physiological parameters to support cellular metabolism and tissue health.
Pumps and valves are integral to the regulation of fluid flow within microfluidic devices [
125]. Syringe pumps, hydrostatic pressure systems, and pressure controllers are commonly employed to achieve precise flow control. Syringe pumps offer high precision and ease of use but are limited by the volume they can dispense. Hydrostatic pressure systems provide continuous flow and are straightforward to implement but may lack the precision needed for certain applications. Pressure controllers, on the other hand, offer stable and responsive flow control, making them ideal for experiments requiring fine adjustments. They can be integrated with feedback loops and sensors to maintain constant flow rates and pressures, essential for replicating physiological conditions within the microchannels.
Valves in microfluidic systems serve to control the direction and rate of fluid flow, enabling complex fluid manipulation and isolation of specific regions within the device. Various types of valves, including solenoid, pneumatic, and piezoelectric valves, can be integrated into microfluidic platforms. Solenoid valves are electrically controlled and provide rapid response times, making them suitable for high-throughput applications. Pneumatic valves use air pressure to actuate and are favored for their simplicity and reliability. Piezoelectric valves, which rely on piezoelectric materials that change shape under an electric field, offer precise control and are advantageous for applications requiring minimal mechanical disturbance.
Integration of sensors within microfluidic systems further enhances their capabilities [
126]. Pressure sensors, for instance, can monitor changes in fluid dynamics in real-time, providing critical data for assessing the system's performance and making necessary adjustments. In one approach, pressure sensors are integrated into the microfluidic chip using capillaries to track pressure changes by observing the movement of the liquid-gas boundary. This method allows for continuous monitoring and can be used to determine occlusion times and other dynamic parameters within the system. Additionally, advanced detection tools like surface acoustic wave lysis devices and multiplexed sensors can be incorporated to measure biochemical markers such as miRNAs, offering rapid and quantitative analysis essential for applications like ischemia-reperfusion injury assessment.
The integration of microchannels, oxygen supply systems, pumps, and valves into a cohesive microfluidic platform is fundamental to creating a reliable and versatile system for biological and medical research. The choice of materials and design configurations directly impacts the functionality and application range of these devices. For instance, the use of fibrin hydrogels in microchannels supports the encapsulation and growth of organoids, mimicking the ECM and providing a conducive environment for cellular interactions. The ability to adjust microchannel dimensions to fit specific organoid sizes ensures precise positioning and minimizes mechanical stress on the cells, maintaining their physiological relevance.
Oxygen delivery within these systems is managed through careful design and material selection, ensuring that the diffusion and convection of oxygen match physiological conditions. Computational models play a crucial role in optimizing these parameters, allowing researchers to simulate and adjust the microenvironment within the device. The integration of pumps and valves with feedback systems ensures stable and controlled fluid flow, replicating the dynamic conditions found in vivo. This is particularly important for applications involving the culture of delicate cell types or the formation of vascular networks, where precise control over flow rates and pressures is essential. The use of sensors within microfluidic devices provides real-time data on system performance, enabling dynamic adjustments and enhancing the reliability of the platform. This is particularly valuable in high-throughput screening applications, where rapid and accurate measurements are crucial. By integrating advanced detection tools, microfluidic platforms can perform complex biochemical analyses, broadening their application scope to include areas such as drug testing and disease modeling.
In summary, the integration of microchannels, oxygen supply systems, pumps, and valves within microfluidic devices creates a versatile and reliable platform for biological and medical research. The careful selection of materials and design configurations ensures that these systems can replicate physiological conditions, supporting the growth and analysis of various cell types and tissues. The incorporation of sensors and feedback systems further enhances the functionality and precision of these devices, making them invaluable tools for advancing our understanding of complex biological processes and developing new therapeutic approaches.
3.6. Key Advancements in VoC Technology
VoC technology has revolutionized the field of biomedical research by providing physiologically relevant in vitro models for studying vascular biology, disease mechanisms, and therapeutic interventions. Recent advancements in this domain have been driven by interdisciplinary contributions from bioengineering, microfluidics, stem cell research, and beyond. Herein, we explore the latest developments in VoC technology, highlighting their potential applications and future directions.
One of the most significant breakthroughs in this field has been the utilization of stem cell-derived vessels for CVD modeling. Researchers have successfully engineered VoC that mimic the physiological environment, enabling the study of disease mechanisms and drug responses in a more accurate and personalized manner. By leveraging stem cell technology, these platforms offer insights into individual-specific disease pathophysiology and therapeutic responses, paving the way for personalized medicine and drug testing [
109]. The development of continuously perfusable and customizable VoC platforms has addressed the limitations of traditional static culture systems [
88]. These innovative platforms allow precise control over vascular architecture and flow dynamics, incorporating matrix-free environments and microfluidic technologies. As a result, researchers can create biomimetic vascular networks with intricate geometries and physiologically relevant flow conditions, opening up new avenues for studying vascular development, angiogenesis, and disease progression in a more realistic setting [
127].
Bioprinting techniques have also made significant contributions to the advancement of VoC technology [
128]. Researchers have demonstrated the 3D bioprinting of vessel-like structures with multilevel fluidic channels, achieving biomimetic vascular networks [
128]. This approach offers spatial control over vascular architecture, enabling the creation of complex and intricate vascular structures that closely resemble their
in vivo counterparts. Bioprinting holds immense potential for tissue engineering and OoC applications, where vascularization is crucial for maintaining tissue viability and function. The integration of vascularized tumor-on-a-chip models has revolutionized cancer research by providing a more accurate representation of the tumor microenvironment [
129,
130]. These models incorporate vascularization to recapitulate the complex interplay between tumor cells, ECs, and the surrounding ECM. By incorporating vascularization, researchers can study tumor angiogenesis, drug efficacy, and metastasis in a physiologically relevant context, facilitating the development of personalized cancer therapies. Infectious disease research has also benefited from the advent of OoC models, including VoC platforms [
131]. These platforms offer a controlled environment to investigate host-pathogen interactions and evaluate antimicrobial agents. By recapitulating the vascular environment, researchers can gain insights into disease dynamics, such as pathogen dissemination, immune cell recruitment, and the effectiveness of therapeutic interventions.
Microfluidic technologies have played a pivotal role in advancing VoC platforms [
69,
70]. These microscale systems provide precise control over cellular microenvironments, enabling detailed mechanistic studies of vascular development, cell migration, and disease progression. Researchers have leveraged microfluidic technologies to create intricate vascular networks, manipulate flow conditions, and observe cellular responses in real-time. Advancements in stem cell differentiation have also contributed significantly to the field of VoC technology [
109]. Researchers have made progress in differentiating hPSCs into vascular cells, offering a scalable and reproducible approach for generating functional vascular networks. This innovation holds great potential for disease modeling, drug screening, and regenerative medicine applications, as it provides a continuous and renewable source of vascular cells for constructing physiologically relevant VoC platforms.
Furthermore, the integration of VoC platforms with other OoC systems has enabled the creation of more complex and physiologically relevant multi-organ models [
70,
132]. These integrated systems allow researchers to study the interconnected processes of various organ systems, including the vascular system's role in nutrient and oxygen delivery, waste removal, and immune cell trafficking.
Looking ahead, the field of VoC technology is poised for further advancements as researchers continue to push the boundaries of biomimicry and physiological relevance. The incorporation of advanced materials, such as hydrogels and scaffolds, will enable the creation of more intricate and biologically accurate vascular networks [
132]. Additionally, the integration of emerging technologies, such as machine learning and artificial intelligence, will facilitate the analysis of complex data sets generated by these platforms, providing insights into vascular biology and disease mechanisms [
133]. Moreover, the development of automated and high-throughput VoC platforms will enable more efficient and scalable drug screening and toxicity testing, accelerating the drug development process [
129]. These platforms will also facilitate personalized medicine approaches by allowing researchers to study patient-specific vascular responses and tailor therapies accordingly.
In conclusion, VoC technology has witnessed remarkable advancements across multiple fronts, including stem cell engineering, bioprinting, microfluidics, and disease modeling. These innovations have paved the way for more physiologically relevant in vitro models, enabling detailed studies of vascular biology, disease mechanisms, and drug responses. As the field continues to evolve, VoC platforms will play a pivotal role in advancing our understanding of vascular diseases, facilitating drug discovery, and ultimately contributing to the development of personalized and regenerative therapies.
3.7. Applications of VoC Technology
VoC technology has emerged as a promising approach in various biomedical research areas, offering unique opportunities to study vascular physiology, pathology, and potential therapeutic interventions (
Figure 3). This technology leverages microfluidic systems and advanced biomaterials to create miniaturized models that mimic the intricate structure and function of blood vessels [
134]. One significant application of VoC technology is the fabrication of biomimetic vascular structures for
in vitro modeling and investigation. In a study by Jia et al., microfluidic techniques were employed to fabricate helical hydrogel microfibers that mimic the natural helical structure of blood vessels. These biomimetic hydrogel microfibers can serve as valuable platforms for studying vascular physiology, pathology, and potential therapeutic interventions in a controlled and physiologically relevant environment [
135]. Another promising application lies in the realm of infectious disease research. Alonso-Roman et al. highlighted the potential of VoC for investigating infectious diseases. These models can provide insights into the interactions between pathogens and vascular cells, as well as the mechanisms underlying vascular dysfunction during infectious processes [
136]. Vascularized OoC models have also gained attention for their potential in studying various aspects of vascular biology and disease. Yin et al. discussed advances in the model structure of
in vitro vascularized OoC systems. These models incorporate functional vascular networks, enabling the investigation of vascular-tissue interactions, angiogenesis, and the role of the vascular system in various physiological and pathological processes [
54]. VoC technology has also shown promise in the field of tissue engineering and regenerative medicine. Gao et al. explored the 3D bioprinting of vessel-like structures with multilevel fluidic channels, paving the way for the development of personalized vascular grafts [
132]. The study of vascular aging has also benefited from the advent of VoC technology. Jiao et al. discussed advances in the differentiation of hPSCs into vascular cells, which can be utilized in VoC models to investigate the mechanisms underlying vascular aging [
137]. Furthermore, VoC systems have been employed to study the effects of fluid flow and shear stress on vascular cells and structures. Catros et al. utilized laser-assisted bioprinting to create on-demand patterns of human osteoprogenitor cells and nano-hydroxyapatite, demonstrating the potential of integrating bioprinting techniques with VoC technology [
138]. Bioprinting techniques have also been integrated with VoC technology, enabling the creation of complex vascular structures and the study of vascular-tissue interactions. Chliara et al. discussed the development and applications of bioprinting on OoC platforms, including VoC systems [
139]. This integration holds promise for advancing tissue engineering and regenerative medicine approaches, as well as providing insights into vascular development and morphogenesis.
3.8. Limitations and Challenges in VoC Technology
VoC models have emerged as powerful tools for studying vascular physiology, disease modeling, and drug testing. However, like any technology, they face several challenges and limitations that need to be addressed [
69]. One of the key challenges in VoC models is the accurate recapitulation of the complex microenvironmental cues present
in vivo. Factors such as the dynamic interplay between different cell types, the presence of biochemical gradients, and the influence of mechanical forces need to be carefully considered and integrated into these models [
87]. Another challenge lies in the fabrication and integration of functional vascular networks within these microfluidic devices. Rafiee et al. highlight the advances in coaxial additive manufacturing and their applications in creating physiologically relevant vascular structures [
140]. The integration of multiple tissue types and their interactions with the vascular component is also a significant challenge. Rothbauer et al. discuss the importance of recapitulating the biomimetic epithelium/endothelium interface in OoC models for studying physiological processes and disease mechanisms involving the crosstalk between these tissue types [
141]. Another limitation of VoC models is the difficulty in accurately modeling the dynamic processes involved in vascular pathologies. Dasgupta et al. highlight how microfluidic OoC technology is revolutionizing the study of mucosal tissues and vasculature, emphasizing the need for advanced imaging techniques to track dynamic processes [
142]. The incorporation of patient-specific cells and the ability to recapitulate individual variability is another challenge faced by VoC models. Marder et al. discuss the use of stem cell-derived VoC for CVD modeling, emphasizing the need for robust protocols to generate patient-specific vascular cells and integrate them into these platforms [
143]. Furthermore, the validation and correlation of data obtained from VoC models with
in vivo observations remain a critical step. Cuartas-Vélez et al. utilized a VoC platform to investigate endothelial COVID-19 fingerprints, highlighting the need for thorough validation and comparison with clinical data to ensure the relevance and translational potential of these models [
144]. Despite these challenges and limitations, VoC technology continues to evolve, with ongoing research efforts focused on addressing these issues. Multidisciplinary collaborations involving bioengineers, material scientists, biologists, and clinicians are crucial for overcoming these obstacles and advancing the development of more physiologically relevant and predictive VoC models [
145].
4. Vascularized Organoids
Unsurprisingly, the central cells localized in larger tissue organoids often struggle with acquiring nutrients and removing waste, which limits their growth and functionality. In recent years, both
in vitro and
in vivo vascularisation approaches have been explored to address these issues (
Figure 4). The process of
in vivo vascularisation involves the transplantation of organoids into suitable animal models, thereby encouraging organoid integration with the host's vascular system. This approach has shown potential in generating functional VOs. For instance, W. Van den Berg et al. successfully transplanted hPSC-derived renal organoids into the renal capsule of immunodeficient mice, leading to the formation of a vascular network between the transplanted organoid and host blood vessels[
146]. Microscopy imaging confirmed the presence of a vascular system within the organoids. Functional study also demonstrated the functionality of these vascularised renal organoids, showing their ability for selective permeability and re-uptake, similar to human kidney function.
Various studies have employed similar strategies for different types of organoids. For instance, it has been demonstrated that brain organoids transplanted into the mouse brain established blood circulation, showing potential for modelling neurological diseases[
147]. However, challenges persist in
in vivo vascularisation. The main concern is the integration of human organoids with animal vasculature, which could result in the gradual replacement of organoid cells, extracellular matrix, and structure by host animal cells, compromising the human-specific properties of the organoids. This approach is inherently limited by the use of conventional animal models and thus requires further research to fully comprehend and address these issues.
The concept of
in vitro vascularisation involves creating a vascular system within organoids using gene editing, mixed cell cultures, and microfluidic platforms. The goal is to establish an independent vascular network capable of sustaining the growth, development, and function of the organoid without relying on animal models. This has been achieved by Cakir et al [
148] who employed genetic engineering techniques to induce the expression of EVT2, a gene involved in regulating angiogenesis, in human cortical organs, ultimately generating vascular brain organoids with characteristics akin to the blood-brain barrier. Another technique often proposed is the introduction of pro-angiogenic factors that promote the co-culturing of ECs with other cell types to facilitate the natural development of vascular structures. This was achieved by Shi et al [
149], who co-cultured hESCs with human umbilical vein endothelial cells (HUVECs) in vitro, ultimately giving rise to cerebral organoids that had an optimal, well-developed tube-like vascular system. In contrast to the co-culturing technique, gene editing techniques will depend on the overexpression of various cytokines and as a result, these techniques are accompanied by some shortcomings, namely an increase in the occurrence of gene mutations, the requirement for technical expertise, and the relatively high cost.
Although it is acceptable to develop these organoids with intricate vascular characteristics using these co-culturing and gene editing techniques, the organoids themselves will therefore be selective with regard to the delivery of oxygen and nutrients, an attribute which conflicts with innate perfusion vessels. In the following section, we will dicuss different type of vascularized tissue organoids (
Figure 4).
4.1. Vascularized Cardiac Organoids (COs)
Vascularized cardiac OoCs are advanced
in vitro models that integrate hPSC-derived COs with microfluidic system [
150,
151]. These sophisticated platforms mimic the structural and functional properties of the human heart by incorporating a functional vascular network within the 3D COs [
152]. The development of these models involves several key steps. First, hPSCs are differentiated into cardiomyocytes, ECs, and other supporting cell types using specific signaling molecules and growth factors [
104,
137]. These differentiated cells are then assembled into 3D COs using techniques such as self-organization or bioprinting [
153,
154]. To achieve vascularization, ECs are co-cultured with the cardiac cells, and angiogenic factors are introduced to promote the formation of a functional vascular network [
155,
156]. The vascularized COs are subsequently integrated into microfluidic devices that can precisely recreate physiological conditions of the heart, including fluid flow, shear stress, and nutrient gradients [
157]. Vascularized COs on a chip offer significant advantages over traditional
in vitro models and animal models for studying cardiac development, disease modeling, and drug testing [
63,
158]. By recapitulating the complex cellular interactions and physiological processes of the human heart, these organoids provide a more accurate representation of cardiac physiology and pathophysiology compared to 2D cell cultures [
159]. Additionally, they enable personalized medicine by utilizing patient-derived iPSCs, allowing for the study of individual genetic variations and their impact on disease progression [
49]. Furthermore, these advanced models serve as valuable tools for drug screening and toxicity testing, facilitating the evaluation of potential therapeutic compounds on human-relevant tissue models, which could potentially reduce the need for animal testing [
150,
160]. Despite the significant progress made in the development of vascularized COs on a chip, several challenges remain. One major challenge is achieving long-term organoid viability and maturation, which is hindered by the limited diffusion of nutrients and oxygen within COs [
161]. Strategies to address this challenge include improving the efficiency of vascular network formation and integrating perfusion systems to facilitate nutrient and waste exchange [
150,
151]. Another challenge is the accurate recapitulation of the complex architecture and physiological functions of the human heart, including the formation of well-organized ventricular and atrial chambers, as well as the integration of various cell types, such as cardiac conduction system cells [
63]. Future directions in this field may involve the incorporation of advanced bioengineering techniques, such as 3D bioprinting, to precisely control the spatial organization of cells and the integration of vascular networks [
118]. Additionally, the development of multi-organ systems, combining vascularized COs with other organ models, could enable the study of inter-organ interactions and systemic effects [
162].
4.2. Vascular Brain Organoids (BOs)
BOs derived from hPSCs have emerged as powerful
in vitro models for studying brain development, function, and disease mechanisms. However, a major limitation of these BOs is the lack of a functional vascular system, which hinders their long-term viability, maturation, and physiological relevance. To address this challenge, researchers have developed vascularized BOs, which incorporate a functional vascular network within the 3D brain-like structures. The development of vascularized BOs involves several key steps. First, hPSCs are differentiated into neural progenitor cells, which are then guided to form organized brain-like structures through techniques such as spinning bioreactors or microfluidic devices [
54,
163]. These BOs are subsequently co-cultured with ECs, either derived from hPSCs or obtained from other sources, to facilitate the formation of a vascular network within the organoid [
145,
164]. Bioengineering techniques, such as 3D bioprinting, have been employed to precisely control the spatial organization of different cell types and the integration of pre-formed vascular structures [
165]. Additionally, OoC systems provide a controlled microenvironment that can mimic
in vivo conditions and facilitate the formation and perfusion of vascular networks within the organoid [
145]. Vascularized BOs offer several advantages over traditional
in vitro models and animal models for studying brain development, disease modeling, and drug testing [
55]. By recapitulating the complex cellular interactions, tissue architecture, and physiological processes present in the human brain, these BOs provide a more accurate representation of brain physiology and pathophysiology compared to 2D cell cultures or non-vascularized BOs. Additionally, vascularized BOs enable the study of neurovascular interactions, which play crucial roles in various neurological disorders, such as stroke, Alzheimer's disease, and brain tumors [
145]. These organoids can serve as valuable tools for investigating the mechanisms underlying these diseases and for testing potential therapeutic compounds in a human-relevant model system. Despite the significant progress in the development of vascularized BOs, several challenges remain. One major challenge is achieving long-term organoid viability and maturation, as the limited diffusion of nutrients and oxygen within the organoid can hinder proper development and functionality [
123]. Strategies to address this challenge include improving the efficiency of vascular network formation and integrating perfusion systems to facilitate nutrient and waste exchange [
145]. Another challenge is the accurate recapitulation of the complex architecture and physiological functions of the human brain, including the formation of distinct brain regions, the integration of various cell types (e.g., neurons, astrocytes, oligodendrocytes), and the establishment of proper connectivity and neural circuitry [
55,
87]. Future directions in the field of vascularized BOs may involve the incorporation of advanced bioengineering techniques, such as 3D bioprinting and microfluidic devices, to precisely control the spatial organization of cells and the integration of vascular networks [
145,
165]. Additionally, the development of more sophisticated OoC systems that can mimic the dynamic microenvironment of the brain, including mechanical and electrical stimuli, could further enhance the physiological relevance of these organoids [
145]. Overall, vascularized BOs represent a promising avenue for advancing our understanding of brain biology, disease mechanisms, and drug development, while also offering the potential for personalized medicine and reducing the reliance on animal models.
4.3. Vascular Kidney Organoids (KOs)
KOs are 3D cellular structures that mimic the architecture, physiology, and functionality of the human kidney. However, one of the major challenges in developing functional KOs is the lack of a functional vascular system, which is crucial for nutrient and oxygen supply, as well as waste removal. Vascularized KOs aim to address this challenge by incorporating a vascular network, enhancing the organoids' maturation, survival, and physiological relevance [
166,
167]. One widely adopted approach is the co-culture of KOs with ECs, which can self-organize and form primitive vascular structures within the organoid [
168,
169]. Various types of ECs, such as HUVECs or hPSC-derived ECs, have been employed in these co-culture systems. Another strategy involves the incorporation of decellularized ECM derived from kidney tissue as a scaffold for vascular network formation and organoid maturation [
169,
170]. The ECM provides a natural, biocompatible environment that retains the structural and biochemical cues necessary for cellular organization and vascularization. Bioprinting techniques, which involve the precise deposition of cells, biomaterials, and growth factors, have emerged as a powerful tool for engineering vascularized KOs [
171,
172]. These techniques allow for the creation of intricate vascular architectures within the organoid, mimicking the complex hierarchical structure of the kidney vasculature.
Microfluidic OoC systems have also been employed to provide a controlled microenvironment and perfusion of nutrients, enabling the development and maintenance of vascularized KOs [
173]. These systems consist of micrometer-scale channels and chambers that mimic the physiological conditions of the body, including fluid flow, shear stress, and nutrient gradients. Researchers have combined multiple strategies, such as co-culture systems, ECM scaffolds, and microfluidic platforms, to enhance the vascularization and maturation of KOs [
174,
175]. Additionally, the incorporation of other cell types, such as pericytes and SMCs, has been explored to further stabilize and mature the vascular networks within the organoids [
170,
176]. Despite significant progress, challenges remain in achieving long-term stability, functional maturation, and adequate perfusion throughout the organoid [
177,
178].
Ongoing research efforts focus on optimizing engineering strategies, exploring novel biomaterials, and integrating advanced technologies to create more physiologically relevant and predictive vascularized KOs [
174,
178]. Vascularized KOs offer numerous applications in biomedical research and clinical settings. They can serve as powerful tools for studying kidney development, disease modeling, and drug testing, providing a more physiologically relevant and predictive platform compared to traditional cell culture models [
179,
180]. Additionally, together with hiPSCs, vascularized KOs may pave the way for personalized medicine by enabling the development of patient-specific disease models and the testing of potential therapeutic interventions [
175]. Vascularized KOs offer several advantages over traditional
in vitro models, such as 2D cell cultures and animal models. They more closely recapitulate the complex 3D architecture, cellular composition, and microenvironment of the human kidney, providing a more accurate representation of physiological processes and disease mechanisms [
170,
174]. Furthermore, vascularized KOs reduce the need for animal experiments, aligning with the principles of ethical and humane research practices [
167].
4.4. Vascular Lung Organoids (LOs)
LOs are 3D biomimetic models derived from hPSCs or adult stem cells that recapitulate key structural and functional features of the human lung [
181,
182]. These self-organized, multicellular constructs mimic the cellular complexity, architecture, and physiological processes of the native lung, providing a powerful
in vitro platform for studying lung development, modeling respiratory diseases, and testing potential therapeutic interventions [
180,
183]. The generation of LOs typically involves co-culturing lung epithelial cells with mesenchymal cells and, in some cases, ECs to mimic the complex cellular interactions found in the native lung [
184]. This co-culture system facilitates the self-organization and differentiation of the various cell types into organoid structures that resemble the intricate branching patterns and cellular composition of the lung [
181]. Advanced techniques, such as microfluidic systems and bioprinting, have been employed to create preformed vascular networks within LOs, enhancing their physiological relevance and longevity [
166].
Recently, significant progress has been made in improving the complexity and maturity of LOs. One notable advancement is the incorporation of immune cells, enabling the study of host-pathogen interactions and the modeling of viral infections, such as SARS-CoV-2 [
145,
182]. This has facilitated the development of potential therapeutic strategies and a deeper understanding of disease mechanisms. Additionally, the integration of biomaterials and scaffolds has facilitated the creation of organoid constructs with enhanced structural integrity and vascularization, further improving their physiological relevance [
167,
183]. Furthermore, advances in bioengineering and stem cell technology have led to the generation of more mature and functional LOs. For instance, researchers have developed bioengineered niches that promote the
in vivo engraftment and maturation of hPSC-derived LOs, enabling better recapitulation of lung development and function [
167].
Despite the remarkable progress, several challenges remain in the development and application of LOs. One of the major limitations is the incomplete recapitulation of the complex cellular heterogeneity and architectural features of the human lung [
185]. While LOs can capture key aspects of lung biology, they may not fully represent the diverse cell types and intricate organization found in the native organ. Additionally, achieving long-term viability, functional maturation, and proper vascularization of LOs remains a significant challenge [
186]. Maintaining the organoids in a physiologically relevant state over extended periods is crucial for their use in disease modeling, drug testing, and regenerative medicine applications. Another key challenge is the lack of standardized protocols and scalable manufacturing processes, which can hinder reproducibility and high-throughput applications [
180]. Variations in KOs generation techniques and culture conditions can lead to inconsistencies in KOs quality and behavior, hampering their widespread adoption in research and clinical settings. Furthermore, the ethical considerations surrounding the use of hPSCs need to be carefully addressed, particularly in the context of potential clinical applications [
180].
LOs hold significant potential for various applications in biomedical research and clinical translation. They can serve as valuable tools for studying lung development, elucidating disease mechanisms, and testing the efficacy of potential therapeutic interventions in a physiologically relevant context [
180,
184]. For instance, LOs have been employed to investigate respiratory diseases such as lung cancer, chronic obstructive pulmonary disease (COPD), and pulmonary fibrosis [
166]. Moreover, LOs offer a promising platform for personalized medicine approaches. Patient-derived LOs can be used to develop personalized treatment strategies and test the efficacy of potential therapies, paving the way for precision medicine [
187]. This is particularly valuable for conditions with high inter-individual variability, where tailored treatments may be more effective. Additionally, LOs have been explored for their potential in regenerative medicine applications, such as tissue engineering and organ transplantation. By providing a physiologically relevant microenvironment and supporting vascular networks, these organoid constructs may facilitate the integration and functionality of implanted tissues or organs [
167]. Importantly, LOs have proven valuable in the study of viral infections, particularly in the context of the COVID-19 pandemic. Researchers have utilized LOs to model SARS-CoV-2 infection, investigate host-pathogen interactions, and develop potential therapeutic strategies [
182,
187]. This has contributed to a better understanding of the disease pathogenesis and the identification of potential treatment targets. In summary, LOs represent a powerful tool for biomedical research and hold great promise for various applications, ranging from disease modeling and drug discovery to personalized medicine and regenerative therapies. While challenges remain, ongoing efforts in bioengineering, stem cell technology, and manufacturing processes are paving the way for the widespread adoption of these biomimetic systems in both research and clinical settings.
4.5. Vascular Pancreatic Organoids (POs)
POs are 3D
in vitro models that recapitulate the cellular composition, architecture, and functionality of the pancreas [
188,
189]. These self-organizing structures, derived from hPSCs or adult stem cells, have emerged as powerful tools for studying pancreatic development, disease modeling, and regenerative medicine applications [
190,
191]. The generation of POs typically involves culturing pancreatic stem cells or progenitor cells in a specialized medium that promotes their self-organization into 3D structures [
192]. This process aims to mimic the intricate cellular interactions and signaling pathways involved in pancreatic organogenesis [
189,
193]. Advanced techniques, such as co-culturing with supporting cell types like ECs or mesenchymal cells, have been employed to enhance the maturity and functionality of these organoids [
191,
194].
One of the significant advancements in the field of POs is their ability to model pancreatic diseases, including pancreatic cancer and diabetes [
195,
196]. POs derived from patient samples can recapitulate the genetic and molecular features of the disease, providing a valuable platform for studying disease mechanisms and testing potential therapeutic interventions [[
195]. Additionally, pancreatic cancer organoids have been used for personalized drug screening, enabling the identification of effective treatment strategies tailored to individual patients [
195]. Furthermore, recent studies have demonstrated the potential of POs for regenerative medicine applications, particularly in the context of diabetes [
197]. By providing a physiologically relevant microenvironment and supporting cellular interactions, these POs can facilitate the differentiation and maturation of insulin-producing beta cells, paving the way for the generation of functional islet cells for transplantation [
189,
197].
Despite the remarkable progress, several challenges remain in the development and application of POs. One of the major hurdles is achieving the complete recapitulation of the complex cellular heterogeneity and spatial organization found in the native pancreas [
190,
197]. While current techniques can generate organoids with multiple pancreatic cell types, ensuring proper ratios, distribution, and functional integration of these cells remains a significant challenge. Another challenge is the limited vascularization and nutrient supply within POs, which can impact their long-term viability and functionality [
194]. Strategies such as incorporating vascular networks or implementing microfluidic systems are being explored to address this issue [
168,
194]. Furthermore, the scalability and reproducibility of POs production are essential for their widespread adoption in research and clinical applications. Efforts are underway to develop standardized protocols and automated manufacturing processes to ensure consistent and scalable POs generation [
190,
198].
POs have demonstrated their potential in various applications, including disease modeling, drug discovery, and regenerative medicine [
188,
190,
198]. In the realm of disease modeling, POs have been employed to study host-pathogen interactions, such as viral infections or the interplay between pancreatic cells and the gut microbiome [
199]. Additionally, they have facilitated the investigation of developmental processes, including pancreatic organogenesis and the differentiation of endocrine and exocrine cell types [
189,
193]. In the context of drug discovery, pancreatic cancer organoids have shown promise for personalized drug screening [
195]. By recapitulating the genetic and molecular features of a patient's tumor, these organoids can be used to test the efficacy of various therapeutic agents, enabling the identification of tailored treatment strategies [
195]. Moreover, POs hold significant promise for regenerative medicine applications, particularly in the treatment of diabetes [
197]. By mimicking the complex cellular interactions and microenvironmental cues found in the native pancreas, these POs may facilitate the generation of functional islet cells for transplantation, potentially offering a more effective and long-lasting treatment for diabetic patients [
197]. Furthermore, POs have emerged as valuable tools for studying pancreatic cancer biology and developing novel therapeutic approaches [
188,
196]. They provide a physiologically relevant platform for investigating the molecular mechanisms underlying pancreatic cancer progression, metastasis, and drug resistance, ultimately contributing to the development of more effective treatments [
196]. As research in this field progresses, interdisciplinary collaborations among bioengineers, cell biologists, clinicians, and regulatory bodies will be crucial in addressing the remaining challenges and translating the promising potential of POs into tangible benefits for patients and the biomedical community [
200].
Taken together, POs have emerged as a powerful tool for studying pancreatic development, disease modeling, and therapeutic applications. With their ability to recapitulate the cellular composition and functionality of the pancreas, these organoids provide a physiologically relevant platform for investigating various aspects of pancreatic biology [
188,
190,
198]. However, further research is needed to overcome the challenges related to vascularization, scalability, and reproducibility to fully harness the potential of POs in clinical settings [
168,
194,
198]. Interdisciplinary collaborations and continued efforts in developing standardized protocols and advanced techniques will be essential to drive the field forward and ultimately translate the promising findings into patient care [
200].
4.6. Multiorgans-on-Chip (MOoC) Systems with Vascular Components
MOoC systems are advanced
in vitro models that aim to recapitulate the complex physiological environments and interactions of multiple organ systems within a microfluidic device [
201]. These systems have emerged as powerful tools for studying human physiology, disease mechanisms, and drug testing, offering a more accurate representation of
in vivo conditions compared to traditional cell culture models [
8,
202]. By integrating multiple organ models within a single platform, researchers can investigate the intricate crosstalk and systemic responses that occur between different organ systems, enabling a more comprehensive understanding of physiological processes and disease pathways [
203] (
Figure 5). The development of MOoC systems has its roots in the convergence of various disciplines, including microfluidics, tissue engineering, and microfabrication techniques [
201]. Early efforts focused on creating single OoC models, such as lung-on-a-chip [
8], which paved the way for the integration of multiple organ models within a single platform [
201,
202]. These initial developments demonstrated the potential of microfluidic systems to recreate organ-specific microenvironments and physiological functions, providing valuable insights into disease mechanisms and drug responses [
202]. As the field progressed, researchers recognized the importance of incorporating vascular components to better simulate the interconnectivity and crosstalk between different organ systems [
204]. The inclusion of vascular networks not only enables the transport of nutrients, oxygen, and waste products but also facilitates communication between different organ models through signaling molecules and circulating factors [
201]. This realization led to the development of more sophisticated MOoC systems that integrate vascular components, allowing for a more accurate representation of
in vivo conditions [
203].
The incorporation of vascular components is a crucial aspect of MOoC systems, as it enables the simulation of blood flow, nutrient transport, and communication between different organ models [
203]. These vascular components can take various forms, ranging from simple microfluidic channels lined with ECs to mimic blood vessels to more complex vascular networks created using advanced techniques like bioprinting or microfabrication [
201]. One approach to creating vascular components involves patterning microfluidic channels and seeding them with ECs, which can form a continuous endothelial layer mimicking the structure and function of blood vessels [
202]. These endothelialized channels can be integrated with organ compartments, allowing for the exchange of nutrients, signaling molecules, and waste products between the organ models and the vascular network [
201].
Another strategy involves the use of bioprinting techniques to create more intricate vascular networks within the MOoC system [
204]. By using biocompatible hydrogels and specialized bioprinting methods, researchers can fabricate complex 3D vascular structures that better mimic the intricate architecture of
in vivo vasculature [
203]. These bioprinted vascular networks can be perfused with culture media or blood-mimicking fluids, enabling the study of organ-specific vascular interactions and responses [
203]. Specifically, the design and fabrication of MOoC systems with vascular components involve interdisciplinary approaches, leveraging expertise in fields such as microfluidics, biomaterials, and microfabrication [
201]. Commonly used materials include PDMS, glass, and biocompatible polymers such as polyethylene glycol or poly(lactic-co-glycolic acid) [
204]. Advanced techniques like soft lithography, 3D printing, and microfluidic patterning are employed to create intricate microchannel networks and tissue compartments [
201].
Soft lithography, a widely used technique in microfluidics, involves the fabrication of microstructures using elastomeric materials like PDMS [
201]. This technique allows for the creation of precisely defined microchannels and chambers, which can be used to construct organ compartments and vascular networks within the MOoC system [
204]. 3D printing technologies, such as stereolithography and two-photon polymerization, have also been employed in the fabrication of MOoC systems [
201]. These techniques enable the creation of complex 3D structures with high resolution, facilitating the integration of intricate vascular networks and organ compartments within a single device [
205].
MOoC systems with vascular components have numerous applications in biomedical research and drug development. They enable the study of organ-organ interactions, systemic responses to drugs or toxins, and the investigation of disease mechanisms involving multiple organ systems [
202]. For instance, these systems can be used to evaluate the effect of a potential therapeutic compound on various organ models simultaneously, providing insights into its systemic effects and potential off-target toxicities [
201]. Additionally, MOoC systems with vascular components can be utilized for personalized medicine applications by incorporating patient-derived cells or tissues [
204]. This approach allows for the study of individual patient responses to drugs or treatments, potentially leading to more personalized and effective therapeutic strategies [
205]. Furthermore, these systems can be employed in the study of vascular diseases, such as atherosclerosis, thrombosis, and vascular inflammation [
201]. By incorporating vascular components and mimicking blood flow conditions, researchers can investigate the mechanisms underlying vascular pathologies and evaluate potential therapeutic interventions [
202].
While MOoC systems with vascular components offer significant advantages, they also present several technological challenges. These challenges include achieving physiologically relevant flow rates, maintaining long-term culture conditions, integrating complex vascular networks, and ensuring scalability and reproducibility [
201]. Achieving physiologically relevant flow rates within the vascular networks of the MOoC system stands out as one of the key challenges facing by this system. Improper flow rates can lead to disturbances in nutrient transport, shear stress patterns, and overall organ functionality [
203]. Researchers have explored various solutions, such as incorporating on-chip pumps or using external pumping systems to precisely control flow rates within the vascular channels [
201]. Maintaining long-term culture conditions is another challenge, as the viability and functionality of the organ models and vascular components need to be sustained over extended periods to accurately represent physiological processes and disease progression [
205]. Strategies like incorporating perfusion systems, optimizing culture media formulations, and implementing advanced monitoring systems have been explored to address this challenge [
204]. Integrating complex vascular networks that accurately mimic
in vivo vasculature is also a significant hurdle. Advanced techniques like bioprinting and microfabrication have been employed to create intricate 3D vascular structures, but challenges related to perfusion, cell seeding, and integration with organ compartments remain [
201]. Ensuring scalability and reproducibility is crucial for the widespread adoption and translation of MOoC systems into clinical applications. Researchers have explored the use of automated fluidic handling systems and standardized protocols to improve the reproducibility and scalability of these systems [
204]. Furthermore, the integration of vascularized BOs with other organ models, such as COs, KOs, LOs, POs, and/or other tissue organoids, could lead to the development of human systems on chip, enabling the study of inter-organ interactions and systemic effects of whole human body [
87].
5. Future Prospects and Innovations as Well as Potential Challenges
The field of VOs, VoC, OoC, and MOoC systems with vascular components is rapidly evolving, with ongoing efforts to enhance their complexity, functionality, and clinical relevance. Future prospects include the integration of advanced biosensing technologies, the development of self-organizing vascular networks, and the incorporation of immune system components [
201]. Advanced biosensing technologies, such as electrochemical sensors, optical sensors, and microelectrode arrays, can be integrated into these systems to enable real-time monitoring of various physiological parameters, including pH, oxygen levels, and metabolite concentrations [
205]. This continuous monitoring capability will provide valuable insights into organ-organ interactions and facilitate the study of dynamic physiological processes.
The development of self-organizing vascular networks within these systems is another exciting prospect. By leveraging principles from developmental biology and tissue engineering, researchers aim to create vascular networks that can self-assemble and remodel in response to environmental cues and organ-specific demands [
204]. This approach could lead to more physiologically relevant models and enable the study of processes like angiogenesis and vascular remodeling [
201]. Additionally, the incorporation of immune system components, such as immune cells and lymphoid tissues, into VOs, VoC, OoC, or MOoC systems is an area of active research [
202]. These advancements will enable the investigation of immune-related processes, such as inflammation, immune responses to pathogens, and immune-mediated diseases, providing a more comprehensive understanding of human physiology and disease mechanisms [
205]. Furthermore, the use of machine learning and artificial intelligence for data analysis and predictive modeling holds promise for further optimizing these functional systems and accelerating biomedical discoveries [
204]. By analyzing the vast amounts of data generated from these systems, machine learning algorithms can identify patterns, predict outcomes, and optimize experimental conditions, leading to more efficient and accurate research outcomes.
VOs and VoC systems have emerged as promising tools for studying vascular biology, disease modeling, and drug screening. However, these advanced
in vitro models face several biological challenges that need to be addressed to improve their physiological relevance and reliability [
36,
206].
One of the primary challenges is achieving the full spectrum of cellular complexity found in native vasculature. While many models incorporate ECs and some supporting cell types like pericytes or SMCs, they often lack the complete array of cells present
in vivo. This includes various immune cells, progenitor cells, and specialized stromal cells that contribute to vascular function and homeostasis [
207,
208]. Another significant challenge is recreating the hierarchical structure of the vascular system. Native vasculature consists of a complex network of vessels ranging from large arteries and veins to small capillaries. Current VOs and VoC models often struggle to generate this hierarchical organization, which is crucial for proper blood flow dynamics and oxygen/nutrient distribution [
209,
210]. The complexities of ECM composition and structure found in native blood vessels present another challenge for VOs and VoC systems. The vascular ECM plays a critical role in regulating cell behavior, vessel stability, and mechanotransduction. Many current models rely on simplified ECM components or animal-derived materials like Matrigel, which may not accurately represent the complex and tissue-specific ECM found in human vasculature [
47,
48].
Perfusion and fluid dynamics pose significant challenges in both VOs and VoC systems. While chip-based models often incorporate flow, achieving physiologically relevant shear stress levels and flow patterns throughout complex vascular networks remains difficult. For VOs, the lack of perfusion can lead to limitations in nutrient and oxygen availability, particularly in larger constructs. This can result in necrotic cores and altered cellular behavior, limiting the long-term viability and functionality of the models [
211,
212]. The maturation of vascular structures is another critical challenge. Many current models produce vessels that resemble early developmental stages rather than fully mature, functional vasculature. This includes limitations in barrier function, proper vessel wall organization, and functional specialization of different vessel types [
213,
214]. Innervation of vascular models presents an additional challenge. The autonomic nervous system plays a crucial role in regulating vascular tone and function, yet most current models lack this neural component. Incorporating functional innervation into VOs and VoC systems could significantly enhance their physiological relevance and ability to model neurovascular interactions [
171].
The integration of vascular systems with other tissue types poses both opportunities and challenges. While vascularization can enhance the function and viability of other organoid types, achieving proper integration and cross-talk between vascular and parenchymal components remains difficult. This includes challenges in coordinating the developmental timing of different tissue types and ensuring appropriate interactions between vascular and tissue-specific cells [
215,
216]. Modeling disease states in VOs and VoC systems presents its own set of challenges. While these models offer the potential to recapitulate aspects of vascular pathologies, accurately representing complex, multifactorial diseases like atherosclerosis or diabetic vasculopathy remains challenging. This includes difficulties in recreating the long-term progression of these diseases and incorporating systemic factors that influence vascular health [
208,
217]. The immune component of vascular biology presents another significant challenge. The vasculature plays a crucial role in immune cell trafficking and inflammatory responses, yet many current models lack a robust immune component. Incorporating functional immune cells and modeling their interactions with the vascular system is crucial for studying inflammation-related vascular diseases and immune-mediated drug responses [
218].
Standardization and reproducibility remain ongoing challenges in the field. The complexity of these models, combined with variations in cell sources, materials, and protocols, can lead to significant variability among research laboratories and even between different experiments. Developing standardized protocols and quality control measures is crucial for the widespread adoption and reliability of these models [
219]. Long-term stability and functionality of vascular models is another area of concern. Many current models show decreased viability or altered function over extended culture periods, limiting their utility for studying chronic conditions or long-term drug effects. Improving the longevity of these models while maintaining their physiological relevance is an important goal [
220]. Finally, scaling and high-throughput applications present challenges, particularly for more complex, perfused systems. While VOs and VoC systems offer some advantages in terms of scalability, integrating advanced vascular models into high-throughput screening platforms for drug discovery and toxicity testing remains difficult [
221].
In conclusion, while VOs and VoC systems offer exciting opportunities for advancing vascular biology research and drug development, they face numerous biological challenges. Addressing these challenges will require interdisciplinary approaches, combining advances in stem cell biology, tissue engineering, microfluidics, and materials science. As these models continue to evolve, they hold the potential to provide increasingly accurate and physiologically relevant platforms for studying vascular health and disease [
36,
206].
In a similar vein, OoC technology has emerged as a revolutionary approach to study various physiological processes and disease mechanisms, aiming to recapitulate the complex microenvironment of living organs by combining microfluidics, bioengineering, and cell biology techniques [
139,
158]. OoCs are miniaturized devices that mimic the structural, functional, and biochemical characteristics of specific organs, enabling researchers to investigate cellular responses, drug interactions, and disease progression in a controlled and physiologically relevant setting [
64,
182]. While single OoCs have provided valuable insights into organ-specific processes, there is a growing interest in developing multi-organ-on-a-chip (MOoC) platforms. These platforms integrate multiple organ models on a single chip, allowing for the study of inter-organ communication, systemic responses, and the potential impact of one organ's dysfunction on others [
158,
182]. MOoCs offer a more comprehensive and realistic representation of the human body, bridging the gap between traditional cell culture models and animal studies [
183,
222]. The development of OoCs and MOoCs has been facilitated by advancements in microfabrication techniques, such as 3D printing and bioprinting [
139,
183]. These techniques enable the creation of intricate microfluidic channels, biomimetic structures, and the precise patterning of cells and biomaterials. Consequently, researchers can precisely control the cellular microenvironment, mimicking
in vivo conditions more accurately [
64,
182].
Moreover, multi-organ chip systems with vascular components represent a transformative approach to studying human physiology, disease mechanisms, and drug responses. The integration of vascular networks enhances the physiological relevance of these systems, enabling more accurate modeling of organ-organ interactions and systemic responses [
201]. Despite the technological challenges, ongoing advancements and interdisciplinary collaborations are driving the field forward, with promising prospects for future innovations and applications [
205]. As these systems continue to evolve, they will become even more powerful tools for understanding complex biological interactions and developing new therapeutic strategies [
204].
6. Conclusion
The advent of VOs and VoC models revolutionised the study of vascular biology and pathology. These advanced in vitro systems offer unprecedented opportunities for modelling human vascular diseases, drug testing, and personalised medicine, addressing significant gaps left by traditional 2D cell cultures and animal models. The development of VOs has provided a robust platform for mimicking the complex architecture and cellular interactions of human vasculature. Studies utilising these organoids have shed light on critical mechanisms of diseases such as SARS-CoV-2-induced endotheliitis and diabetic vasculopathy. By offering a more physiologically relevant model, VOs enable detailed investigations into endothelial permeability, vessel maturation, and the interactions between ECs and other cells including mural cells, SMCs, fibroblasts and immune cells. The potential to use patient-derived iPSCs for creating personalised VOs further enhances their value in precision medicine, allowing for the study of individual-specific disease mechanisms and drug responses.
Complementing the utilisation of VOs, VoC models excel in providing controlled microenvironments to study specific aspects of vascular physiology, such as shear stress effects and blood-brain barrier dynamics. These models have been instrumental in exploring the impact of mechanical forces on EC behaviour and the functionality of tissue-tissue interfaces, such as the alveolar-capillary interface and the renal vascular-tubule unit. The precise control over experimental conditions in VoC systems makes them ideal for detailed mechanistic studies and for testing drug efficacy and toxicity in a patient-specific context.
However, the adoption of VoC technology is accompanied by several technical challenges that require innovative solutions. This may include optimising physiological flow rates within vascular networks, maintaining long-term viability and functionality of integrated organotypic models, and ensuring scalability and reproducibility across various experimental setups.
Advances in microfluidic design, biomaterial development, and tissue engineering are actively addressing these challenges, aiming to enhance the reliability and applicability of VoC systems. Moving forward, the continued evolution of VoC technology holds promise for transformative advancements in vascular biology and therapeutic innovation. Future developments may focus on integrating advanced biosensing technologies for real-time monitoring of physiological parameters, enhancing the complexity of vascular networks through bioprinting and microfabrication techniques, and incorporating immune and stromal components to better mimic in vivo vascular microenvironments.
UltimatelyVoC models represent a paradigm shift in vascular research, offering more refined tools to study vascular biology, disease mechanisms, and therapeutic interventions with unprecedented precision and relevance. The complementary strengths of VOs and VoC models suggest that integrated approaches could offer even greater insights into vascular biology and disease. Hybrid models combining the detailed mechanistic control of VoC systems with the complex tissue-like environment of organoids could lead to the development of more physiologically relevant vascularised tissue constructs. Such advancements could significantly, enhance our understanding about organ-specific vascular diseases and improve the predictive accuracy of therapeutic responses.
Despite their potential, both VOs and VoC models face challenges that need to be addressed. Issues such as the lack of a fully mature vascular system in organoids, functional inconsistencies, and the absence of certain microenvironmental cells limit their physiological relevance. Standardisation of protocols and the incorporation of immune and stromal cells could enhance the functionality and reproducibility of these models. Additionally, advancements in cryopreservation techniques and the development of synthetic ECMs could further improve the stability and applicability of VOs. As these technologies mature and become more accessible, they are poised to drive significant progress in understanding and treating vascular diseases, ultimately benefiting patients and advancing human health.
Figure 1.
Diagram showing the main methods to generate vascular organoids (VOs), Created with BioRender.com. The basic protocol for VO generation from hPSCs or hiPSCs is based on a stepwise differentiation of hPSC (or hiPSC) aggregates with sequential changing of culture media and further differentiation into vascular networks in a 3D matrix, with a variety of modification as reported by Wimmer, et al[
18], Schmidt, et al[
19], Romeo, et al[
20], Nikolova, et al[
21], Dailamy, et al[
22], and Werschler, et al[
23]
. MIM, mesoderm induction medium (DMEM-F12, 2 mM, Ascorbic acid, 355 μM CHIR 99021, 10 μM BMP4; VGM, vascular growth medium (Neurobasal medium/DMEM-F12, N2B27, 2 mM Ascorbic acid, 100ng/ml VEGF-A); OMM, organoid maintenance medium (Neurobasal medium/DMEM-F12, N2B27, 2 mM Ascorbic acid); VIM, vascular induction media (N2/B27 + VEGF + FSK); VMM, vascular maturation media (StemPro + 15%FBS + VEGF + bFGF).
Figure 1.
Diagram showing the main methods to generate vascular organoids (VOs), Created with BioRender.com. The basic protocol for VO generation from hPSCs or hiPSCs is based on a stepwise differentiation of hPSC (or hiPSC) aggregates with sequential changing of culture media and further differentiation into vascular networks in a 3D matrix, with a variety of modification as reported by Wimmer, et al[
18], Schmidt, et al[
19], Romeo, et al[
20], Nikolova, et al[
21], Dailamy, et al[
22], and Werschler, et al[
23]
. MIM, mesoderm induction medium (DMEM-F12, 2 mM, Ascorbic acid, 355 μM CHIR 99021, 10 μM BMP4; VGM, vascular growth medium (Neurobasal medium/DMEM-F12, N2B27, 2 mM Ascorbic acid, 100ng/ml VEGF-A); OMM, organoid maintenance medium (Neurobasal medium/DMEM-F12, N2B27, 2 mM Ascorbic acid); VIM, vascular induction media (N2/B27 + VEGF + FSK); VMM, vascular maturation media (StemPro + 15%FBS + VEGF + bFGF).
Figure 2.
Schematic illustration of the key applications for vascular organoids (VOs). Increasing numbers of studies demonstrate a variety of promising application for hPSC-derived VOs including infectious disease pathogenesis (such as SARS-CoV2 and pathogenic bacteria), in vitro vascular disease modelling, high throughput drug screening and drug toxicity testing, creating human VO biobanks for regenerative medicine, multi-omics analysis to probe novel insights into signaling pathway underlying vascular development and disease aetiology, exploring key developmental events in human vascular systems, genetic engineering and editing for inherited disorders, and personalized & precision medicine.
Figure 2.
Schematic illustration of the key applications for vascular organoids (VOs). Increasing numbers of studies demonstrate a variety of promising application for hPSC-derived VOs including infectious disease pathogenesis (such as SARS-CoV2 and pathogenic bacteria), in vitro vascular disease modelling, high throughput drug screening and drug toxicity testing, creating human VO biobanks for regenerative medicine, multi-omics analysis to probe novel insights into signaling pathway underlying vascular development and disease aetiology, exploring key developmental events in human vascular systems, genetic engineering and editing for inherited disorders, and personalized & precision medicine.
Figure 3.
Schematic diagram illustrating the main strategies to generate vessel-on-chip (VoC) and potential applications of VoCs. Different functional vascular cells can be derived from human induced pluripotent stem cells (hiPSCs), which are incorporated into various 3D microporous scaffolds to create functionals VoCs using a variety of fabrication techniques and microfluidic strategies. These human VoCs offer a wide range of potential applications, encompassing various fields such as studying vascular physiology, pathology, and potential therapeutic interventions, exploring infectious disease pathogenesis, using for in vitro vascular disease modelling as well as high throughput drug screening and drug toxicity testing, providing novel insights into vascular aging and angiogenesis, and creating human VoC biobanks for regenerative medicine. EC, endothelial cell; SMC, smooth muscle cells; SMART, substrate modification and replication by thermoforming.
Figure 3.
Schematic diagram illustrating the main strategies to generate vessel-on-chip (VoC) and potential applications of VoCs. Different functional vascular cells can be derived from human induced pluripotent stem cells (hiPSCs), which are incorporated into various 3D microporous scaffolds to create functionals VoCs using a variety of fabrication techniques and microfluidic strategies. These human VoCs offer a wide range of potential applications, encompassing various fields such as studying vascular physiology, pathology, and potential therapeutic interventions, exploring infectious disease pathogenesis, using for in vitro vascular disease modelling as well as high throughput drug screening and drug toxicity testing, providing novel insights into vascular aging and angiogenesis, and creating human VoC biobanks for regenerative medicine. EC, endothelial cell; SMC, smooth muscle cells; SMART, substrate modification and replication by thermoforming.
Figure 4.
Schematic diagram illustrating the main strategies to generate vascularized organoids. Potentially, multiple methods could be applied to generate vascularized tissue organoids such as in vivo transplantation of tissues-specific organoids into mice which allows for host vasculature integration, co-culturing either hiPSCs/hPSCs with mature endothelial cells or hPSC-derived tissues-specific cells with hPSC-derived endothelial cells, genetic engineering vascular-inducing transcription factors (TFs, such as ETV2) into tissue-specific organoids, including vascular induction factors (such as VEGF-A, WNTs, BMPs) into tissue-specific organoids, and different fusion strategies (co-incubating tissue-specific spheroids/EBs (embryonic body) with vascular spheroids/EBs with our without ECM scaffolds, or co-culturing tissue-specific organoids with vascular organoids (Vos)).
Figure 4.
Schematic diagram illustrating the main strategies to generate vascularized organoids. Potentially, multiple methods could be applied to generate vascularized tissue organoids such as in vivo transplantation of tissues-specific organoids into mice which allows for host vasculature integration, co-culturing either hiPSCs/hPSCs with mature endothelial cells or hPSC-derived tissues-specific cells with hPSC-derived endothelial cells, genetic engineering vascular-inducing transcription factors (TFs, such as ETV2) into tissue-specific organoids, including vascular induction factors (such as VEGF-A, WNTs, BMPs) into tissue-specific organoids, and different fusion strategies (co-incubating tissue-specific spheroids/EBs (embryonic body) with vascular spheroids/EBs with our without ECM scaffolds, or co-culturing tissue-specific organoids with vascular organoids (Vos)).
Figure 5.
Schematic diagram illustrating human multiple organs-on-chip (MOoC). HiPSCs/hPSCs-derived tissue-specific cells or organoids could be integrated into human MOoC system by using sophisticated microfluidic devices/system. These MOoC system recapitulate the complex physiological environments and interactions of multiple organ systems and better mimic human, allowing for the study of complex physiological system-system interactions and diseases in high-throughput controlled manner. BOs, brain organoids; COs, cardiac organoids; IOs, intestinal organoids; KOs, kidney organoids; LOs, lung organoids, POs, pancreatic organoids; VOs, vascular organoids. .
Figure 5.
Schematic diagram illustrating human multiple organs-on-chip (MOoC). HiPSCs/hPSCs-derived tissue-specific cells or organoids could be integrated into human MOoC system by using sophisticated microfluidic devices/system. These MOoC system recapitulate the complex physiological environments and interactions of multiple organ systems and better mimic human, allowing for the study of complex physiological system-system interactions and diseases in high-throughput controlled manner. BOs, brain organoids; COs, cardiac organoids; IOs, intestinal organoids; KOs, kidney organoids; LOs, lung organoids, POs, pancreatic organoids; VOs, vascular organoids. .