1. Introduction
As demands for the advancement of medical and personal health care, human machine interfaces (HMI) and internet of things (IoT) technologies grow and novel sensors are being developed, the all-in-one integration of the characteristics such as reliability, low cost and user-friendliness has remained challenging. During recent years, various types of wearable sensors have been developed, while one of their main sensing capabilities is electrophysiological recording including electromyography (EMG), electrocardiography (ECG), electroencephalography (EEG), and electrooculography (EOG) [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11]. In the manufacturing of these wearable sensors, different materials such as metal, semiconductor particles and films, conductive polymer and composites have been utilized [
12,
13,
14,
15,
16,
17,
18]. Among these materials, conductive polymer composites have shown promise for the commercialization due to the low-cost and scalability of the manufacturing process, tunable mechanical and electrical properties, biocompatibility, reusability, and robustness of the wearable sensors made of conductive polymer campsites. However, most conductive polymers and composites are mechanically stiff in comparison with human skin. Mechanical characteristics of the sensors not only play a role in making sensors comfortable to wear but more importantly significantly influences the performance of the sensor by affecting motion artifacts, impacting the sensor-skin interface impedance and consequently signal-to-noise ratio (SNR). Between various types of conductive polymers and polymer composites that are widely used for biophysiological sensing such as poly(3,4-ethylenedioxythiophene):polystyrene sulfonate (PEDOT:PSS), polypyrrole (PPy), hydrogel composites with different types of polymer matrix and conductive fillers like carbon nanotubes, carbon black, silver nanoparticles, graphene flakes, reduced graphene oxide, etc [
19,
20], hydrogels are conductive polymers that can be made extremely soft.
Hydrogels with characteristics such as outstanding softness and stretchability, biocompatibility, tunable conductivity, and simple synthesis have attracted interests for the application in the wearable sensors. Hydrogels contain high amounts of water and therefore, are ionically conductive [
21,
22]. Also, they can be self-adhesive which helps in forming stable contact at their interface with the skin. High conductivity and softness of the hydrogel-based sensors assist in achieving lower skin-sensor interface impedance and consequently better signal quality and larger SNR [
23,
24,
25]. Further, unlike commercial and medical grade wet gel Ag/AgCl electrodes and many other commercial wearable sensors which cause irritation and allergic reaction because of the use of aggressive adhesives to hold them in place, hydrogels are self-adhesive and usually do not cause irritation [
24,
25]. Hydrogels are also generally biocompatible and have been used for the wound dressing, drug delivery, and tissue engineering [
5,
28,
29]. To improve the conductivity of the hydrogels, various salts, intrinsically conductive polymers like PEDOT:PSS, PPy, and metal and carbon fillers such as metal particles/wires, carbon nanotube, carbon black, and graphene are incorporated within the hydrogel networks [
30,
31,
32]. But adding mechanically stiff fillers to ultrasoft hydrogel results in significant increase in the overall stiffness of the hydrogel. Further, since hydrogel contains considerable amounts of water, it dries out quickly and that results in dropping of the electrical conductivity of the hydrogel and also degrading its mechanical properties.
Various methods are used to address this issue such as adding water-retention agents like salts and glycerol and encapsulation of hydrogel [
31,
33,
34,
35]. Despite improvements that are made in fabricating hydrogel sensors for electrophysiological measurements, developing cost-efficient, long lasting, reusable, and robust hydrogel sensors with high electrical conductivity, softness, and mechanical and electrical stability require further studies and advancements.
Here, we report the development of a soft, highly conductive, long lasting, reusable, low-cost, self-adhesive hydrogel-based sensor for long-term electromyography and electrocardiography recording with high quality and low motion artifacts. The sensor shows very good quality of signal recording, high SNR, low motion artifact and long lifetime. The sensor can be stored for over two months and be reused later after rehydrating it. The method of fabrication of this sensor is simple, time- and cost-effective. Due to softness of the hydrogel-based sensor, it forms conformal contact to the skin and as the results allows achieving low sensor-skin interface impedance, high quality of signal recording with higher SNR than gold standard medical grade Ag/AgCl wet gel electrodes and minimized motion artifacts.
2. Results and discussion
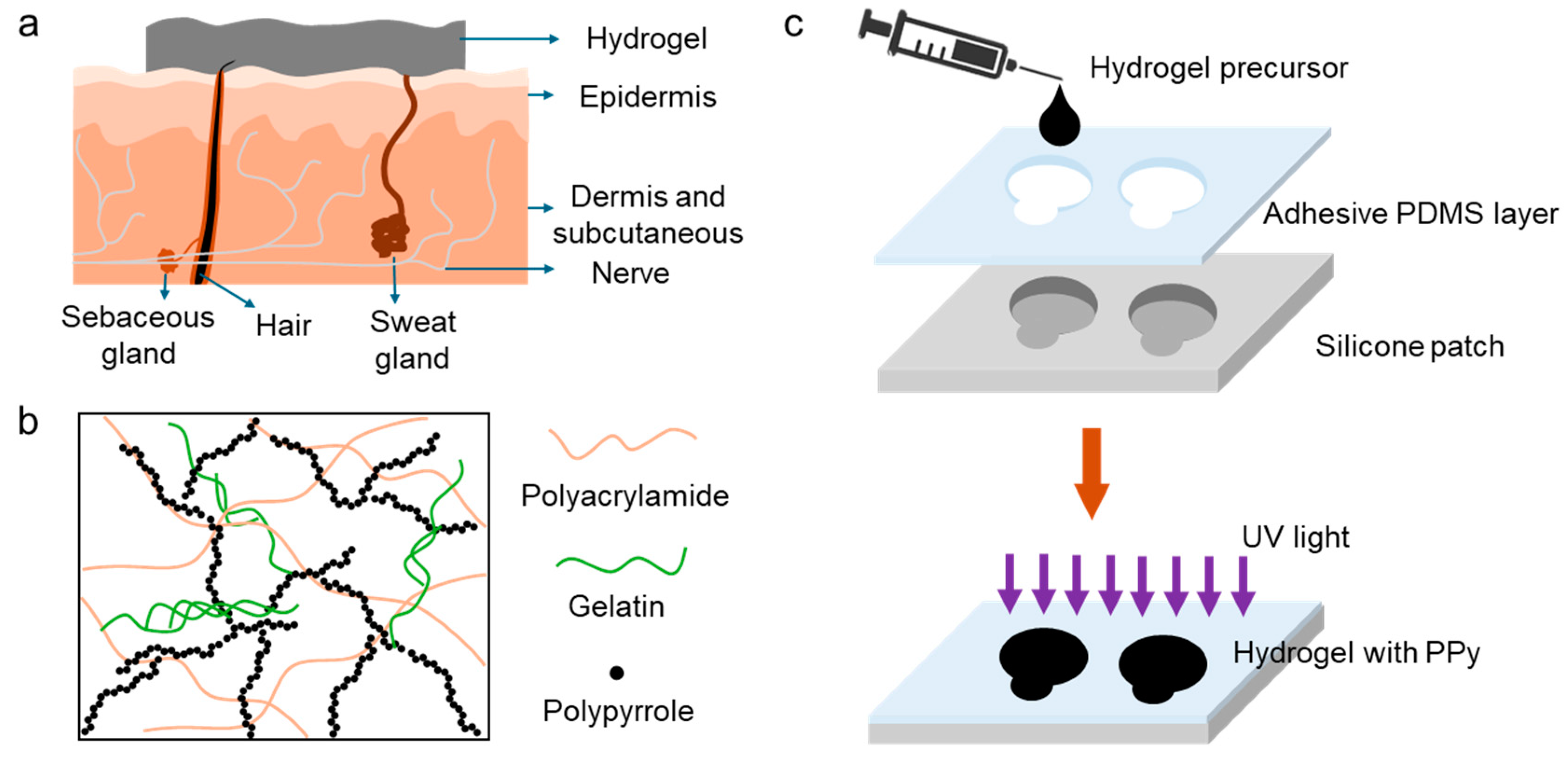
The fabrication of sensor starts with making hydrogel precursor. The hydrogel precursor consists of 10 wt% of gelatine, 10 wt% of acrylamide, 0.8 wt% of poly (ethylene glycol) diacrylate, 0.0002 wt% of 2,2-Diethoxy acetophenone as ultraviolet (UV) initiator, and approximately 79.2% wt% of deionized (DI) water. This precursor is mixed at 50 ℃ for 15 min to ensure homogenous consistency of all the components. The hydrogel is obtained after photopolymerization under 365 nm UV light. The resultant hydrogel is crosslinked both physically and chemically. The gelatine is self-crosslinked by forming triple helix structures through interchain hydrogen bonds, and the polyacrylamide is crosslinked by the chemical crosslinker, poly (ethylene glycol) diacrylate, resulting in the 3D network of the hydrogels. Besides, the reversible hydrogen bonding interactions formed between the hydroxyl, amino, and carbonyl groups of gelatine and polyacrylamide, which contribute to the self-adhesive property of the hydrogel.
Figure 1a is the schematic of a hydrogel sensor with conformal contact to the skin which results in the reducing sensor-skin interface impedance and consequently improving signal-to-noise ratio. In addition to the sensor-skin interface, the conductivity of the sensor plays an important role in the sensing performance of the wearable devices where high conductivity can enhance the signal quality. To achieve high conductivity, a biocompatible conductive polymer, polypyrrole (PPy) was introduced into the hydrogel networks.
Figure 1b shows the schematic of the crosslinked hydrogel containing PPy, where the connected PPy forms a conducting path for the electrons. The developed sensor is composed of a soft Ecoflex frame, a thin layer of adhesive polydimethylsiloxane (PDMS), and conductive hydrogels. The adhesive PDMS was cast on the surface of the matrix and used to ensure a stable sensor-skin interface. As shown in
Figure 1c, the sensor was fabricated through a low cost, simple, and scalable process of molding and casting. The hydrogel precursor was poured in the designated spaces in a fabricated Ecoflex frame followed by its polymerization and incorporation of PPy.
To perform ECG and EEG, at least three sensors/electrodes are required, reference, working and ground electrodes, from which reference and working electrodes must be at least 2 cm away and ground electrode must be placed somewhere on the body that is physically distant from the origin of the signal of interest. To make hydrogel-based sensors, a frame composed of the Ecoflex with the reference and working electrodes being embedded in it, was made. The ground electrode was a separate stand-alone electrode.
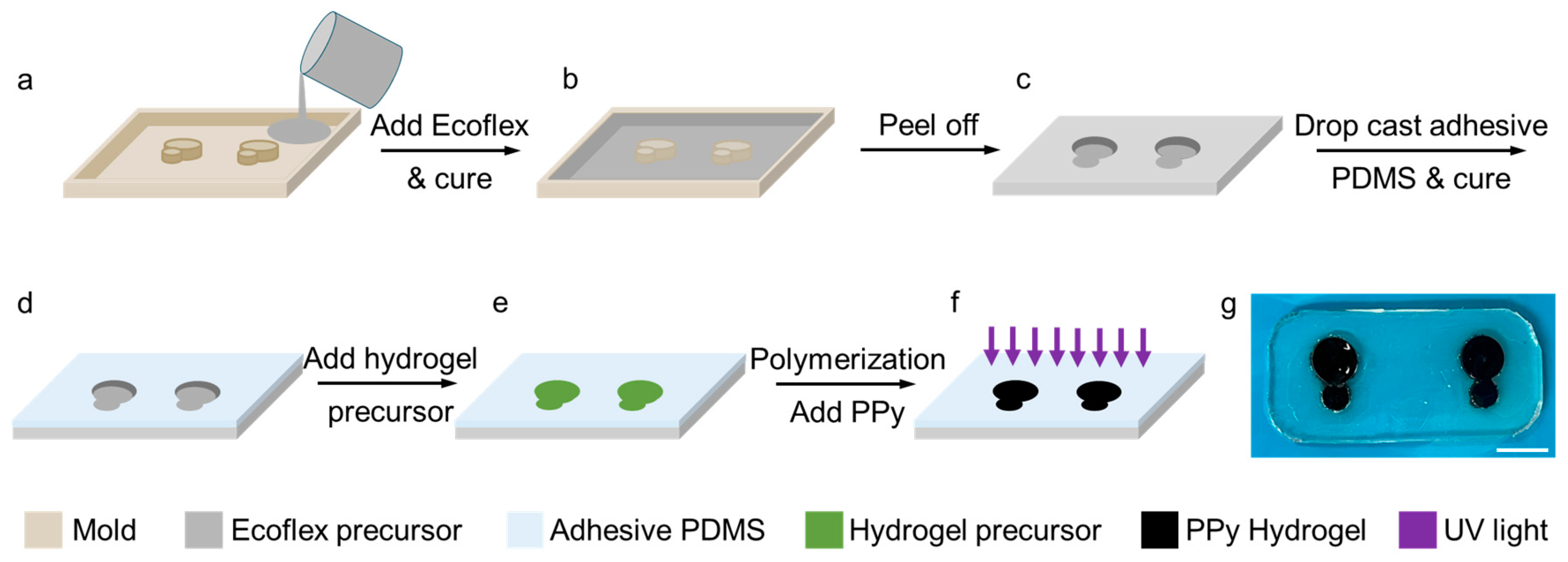
Figure 2 shows the process of the fabrication of the sensor. A mold was printed using a three-dimensional (3D) printer (Statasys printer) as shown in
Figure 2a. The total surface area of each sensor node was made to be 0.79 cm
2 which is only 45% of the commercial medical grade wet gel electrode (1.77 cm
2). In the next step, the Ecoflex precursor was poured in the printed mold, which was prepared by mixing two Ecoflex agents with a ratio of 1:1 followed by the de-bubbling using a vacuum chamber. A frame was obtained after curing the Ecoflex precursor at 50 ℃ for 30 mins (
Figure 2b, c) to host the working and reference electrodes. After demolding, the Ecoflex surface was covered with a thin layer of adhesive PDMS precursor, and it was cured at 50 ℃ for 20 min to allow self-adhesion of the sensor patch to the skin (
Figure 2d). Then the hydrogel precursor was filled in the cavities formed in the Ecoflex frame and it was polymerized under 365 nm UV light at 25 W for 1 hour (
Figure 2e, f). After polymerization, the hydrogel was soaked in 8 wt% pyrrole solution for 1 hour and then in 5 wt% potassium persulfate solution for 30 minutes, and finally was dipped in DI water for three times, each time for 15 minutes, to remove the unreacted chemicals. The optical image in
Figure 2g shows the fabricated sensor with the transparent and colorless patch and black hydrogel electrodes.
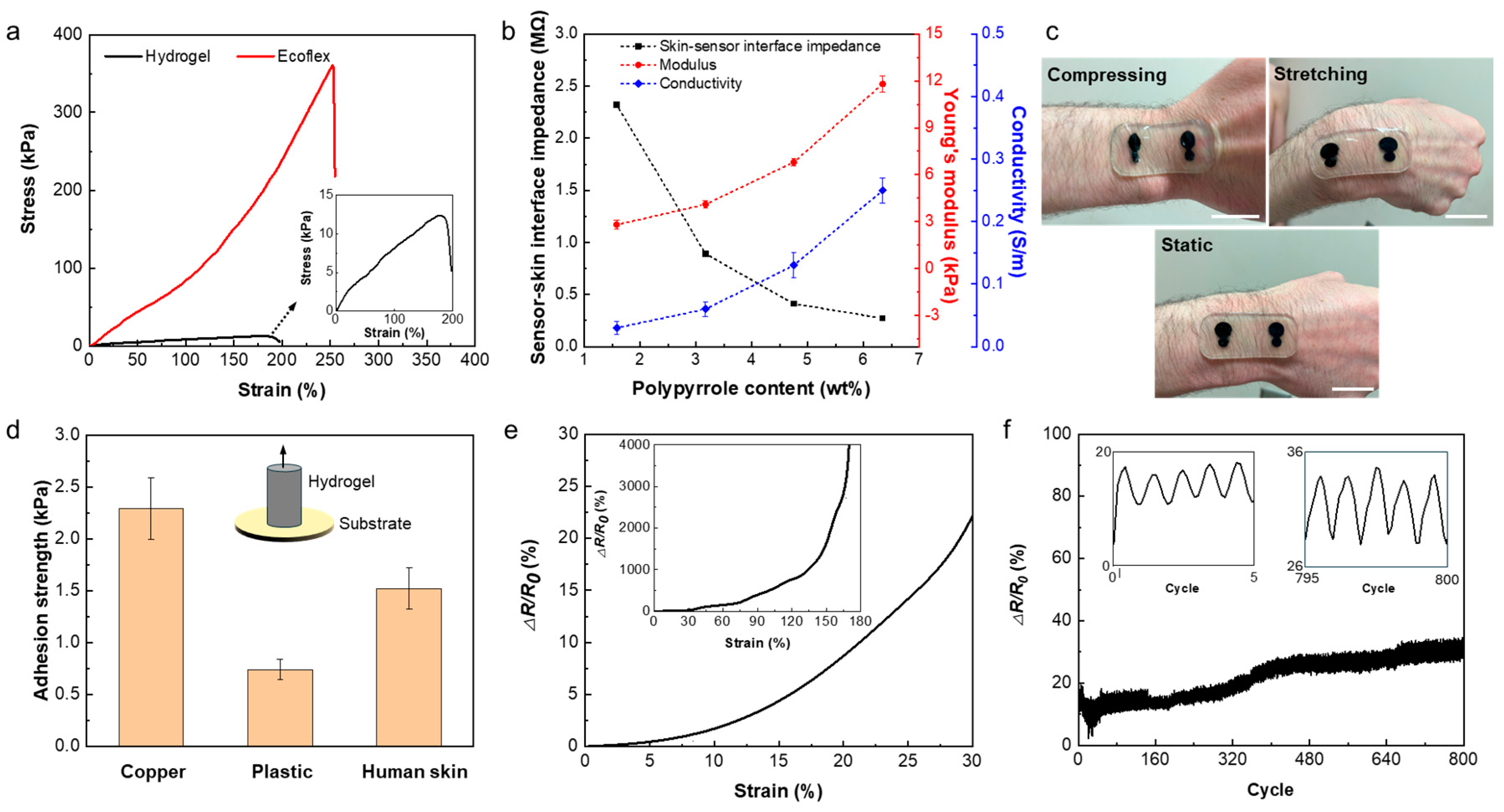
In order to study the mechanical characteristics of the sensor, the strain-stress behaviour of both Ecoflex and hydrogel was studied using a Univert CellScale tensile machine with 50 N and 10 N load cells, respectively. The strain-stress curves of the sensor patch and hydrogel are shown in
Figure 3a. The plots indicate the hyper-elasticity of the sensor patch and the ultra softness and high stretchability of the hydrogel. The Young’s moduli of the Ecoflex and hydrogel were measured to be 89.1 kPa and 12.9 kPa, and the elongation to break was 255 % and 190 % for the patch and the hydrogel electrodes, respectively.
Figure S1 shows the deformability of the sensor placed on the wrist of the subject with strain applied to it by flexing the wrist. Because of the mechanical softness and self-adhesion of the hydrogel-based sensor, lamination of it on different part of the body with different topology including chest, arm, forearm, leg, ankle and wrist is feasible as it can be observed in
Figure S2. These results indicate that the hydrogel-based sensor forms a stable and robust interface with the skin which could decrease the susceptibility of the sensors to motion.
The sensing quality of the wearable electrophysiological sensors is mainly impacted by the electrical conductivity of the sensor and conformability of the sensor to the skin, which both affect the sensor-skin interface impedance and consequently SNR. Despite the hydrogel’s limited ionic conductivity due to the existence of the ions and water, further improvement in the conductivity of such hydrogel is required for high performance in electrophysiological signal recordings. To increase the conductivity of the hydrogel, we used PPy as a conductive polymer within the hydrogel network. Adding PPy increases the conductivity of the hydrogel but also increases its stiffness. Therefore, there is a tradeoff between increasing the conductivity of the hydrogel by adding PPy and maintaining the mechanical softness and conformability of the sensor to the skin.
Figure 3b shows the conductivity of hydrogels versus the polypyrrole contents. The PPy contents within the hydrogel range from 1.58 wt% to 6.34 wt% resulting from soaking the hydrogel in pyrrole aqueous solutions containing 2-8 wt% of pyrrole. As it can be observed, the conductivity of the hydrogel increases by increasing the PPy content from 0.03
0.01 S m
-1 at 1.58 wt% of PPy to 0.25
0.02 S m
-1 at 6.34 wt% of PPy. It should be noted that no tests were performed beyond 6.34 wt%, due to the limited solubility of the pyrrole in DI water as seen in
Figure S3. Meanwhile, Young’s modulus of hydrogel increases from 3 kPa to 12.9 kPa and sensor-skin interface impedance decreases from 2.32 MΩ to 140 KΩ when the PPy contents increase from 1.58 wt% to 6.34 wt% (
Figure 3b and
Figure S4). The sensor-skin interface impedance was measured by placing the hydrogel-based sensor on the forearm. The elongation to break of the hydrogel was also affected by the PPy content which decreases from 500% to 180% by increasing PPy contents from 1.58 wt% to 6.34 wt% as it can be seen in
Figure S5.
Based on the obtained results, the hydrogel with 6.34 wt% of PPy (6-PPy/hydrogel) was used for the fabrication of the sensors. Hydrogel has self-adhesive properties that help in forming a more stable interface with the skin and minimizing the motion artifacts during mobile sensing.
Figure 3c demonstrates the effective adhesion of the hydrogel sensor to the skin, where no delamination is observed when the hydrogel sensor goes through various deformations like stretching and compressing. Such robust sensor-skin interface can be ascribed to the self-adhesion property of the hydrogel and the adhesive PDMS with reported adhesion force of 1.1 N/cm
2 [
36]. Our results show that the 6-PPy/hydrogel can adhere to different materials including plastics, metal, and human skin.
Figure 3d represents the measured adhesive strength of 6-PPy/hydrogel to different surfaces such as metal, plastic, and the skin. The adhesion strength between 6-PPy/hydrogel and copper are higher than that between the hydrogel and plastic and the skin. The adhesion strength between the skin and 6-PPy/hydrogel is 1.52
0.2 kPa which is sufficient for the adhering sensor to the skin stably. Self-adhesion of hydrogel can be ascribed to the dynamic hydrogen bonding interactions between the hydrogel and the substrates.
The change in the electrical conductivity of 6-PPy/hydrogel due to applied tensile strain was measured and it is presented in
Figure 3e. The 6-PPy/hydrogel shows 22.5% increase in the electrical resistance at the applied strain of 30%. The electrical stability of the sensors is important for long-term measurements, which is affected by the anti-fatigue property of the used electrode materials. To study fatigue behavior of 6-PPy/hydrogel, strain cycling test was performed (
Figure 3f). The changes in the electrical resistance of the 6-PPy/hydrogel for 800 cycles of 30% tensile strain suggest that the resistance change of the hydrogel is stable. The resistance drift of less than 40% was observed before reaching a plateau after 400 cycles, which could be ascribed to the gradual drying out of the high-water content hydrogels. We addressed this issue by casting 6-PPy/hydrogel in Ecoflex to help water retention and increase the lifetime of the sensor.
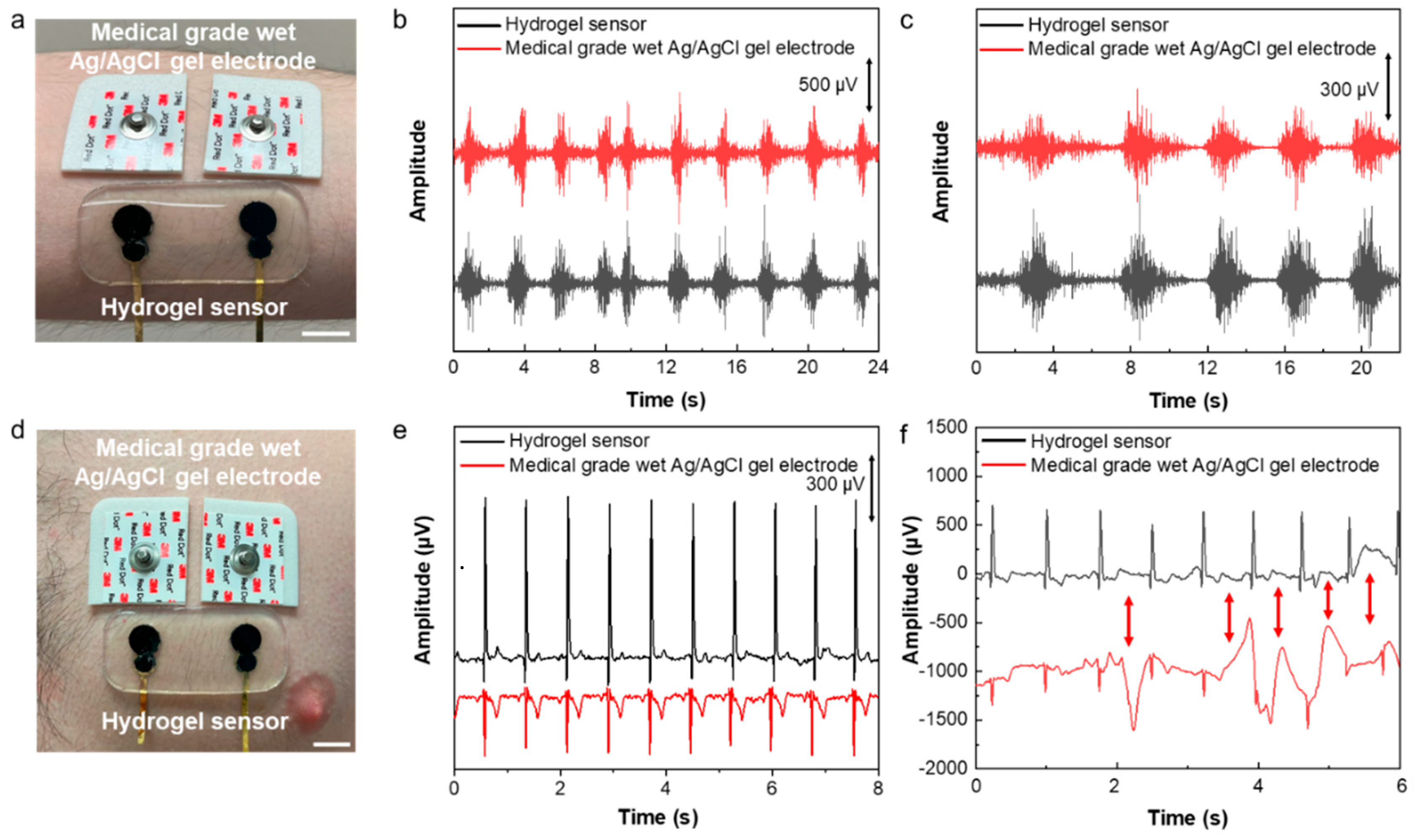
The developed sensor was used for electrophysiological signal recording. Electromyography (EMG) and electrocardiography (ECG) were performed by placing the sensor on the forearm flexor muscle and chest, respectively. As it can be observed in
Figure 4, in both cases a set of medical grade silver/silver chloride (Ag/AgCl) wet gel electrodes were also placed next to our developed sensor for the concurrent signal recording. The EMG signals were recorded while the subject was squeezing a hand grip. The result shows a clear EMG signal with the SNR of 26.92 dB recorded by our developed hydrogel sensor in comparison to 22.97 dB recorded by the medical grade electrodes (
Figure 4b).
To study the performance of the sensor for EMG sensing during daily activity like physical exercises, sensor-skin interface impedance was measured before and after exercise and sweating. As shown in
Figure S6, there is no big difference between the sensor-skin interface impedances tested before exercise and after running for 20 minutes, indicating the robustness and high stability of the hydrogel-based sensor-skin interface. This high-quality interface and low sensor-skin impedance contribute to the high performance of the hydrogel sensor in EMG recording as shown in
Figure 4c.
The developed sensor’s application for performing ECG was also demonstrated. The hydrogel sensor was placed on the chest next to a pair of medical grade wet gel Ag/AgCl electrodes as gold standard for recording ECG signals simultaneously (
Figure 4d). The signals with essential ECG characteristic peaks of P, Q, R, S and T were recorded using both sets of sensor and electrodes, the results in
Figure 4e demonstrate significantly higher signal amplitude measured by the hydrogel sensor than the medical grade electrodes. As a result, the SNR of the ECG signals recorded using our sensor was 11.39 dB higher than that measured by the medical grade wet electrodes (33.55dB compared to 22.16 dB). The motion artifact study was performed while recording ECG concurrently using our sensor and medical grade wet gel electrodes. The motions were induced by poking the skin using a glass rod in the vicinity of our sensor and medical grade electrodes. The results presented in
Figure 4f show that our sensor is significantly less susceptible to motion, while the medical grade electrodes have shown significant artifacts in the ECG signals. The lower motion artifact of the hydrogel-based sensor is due to its high deformability and softness, as well as efficient self-adhesion and conformability to the skin microscopic features which ensures a stable sensor-skin interface and high sensing performance.
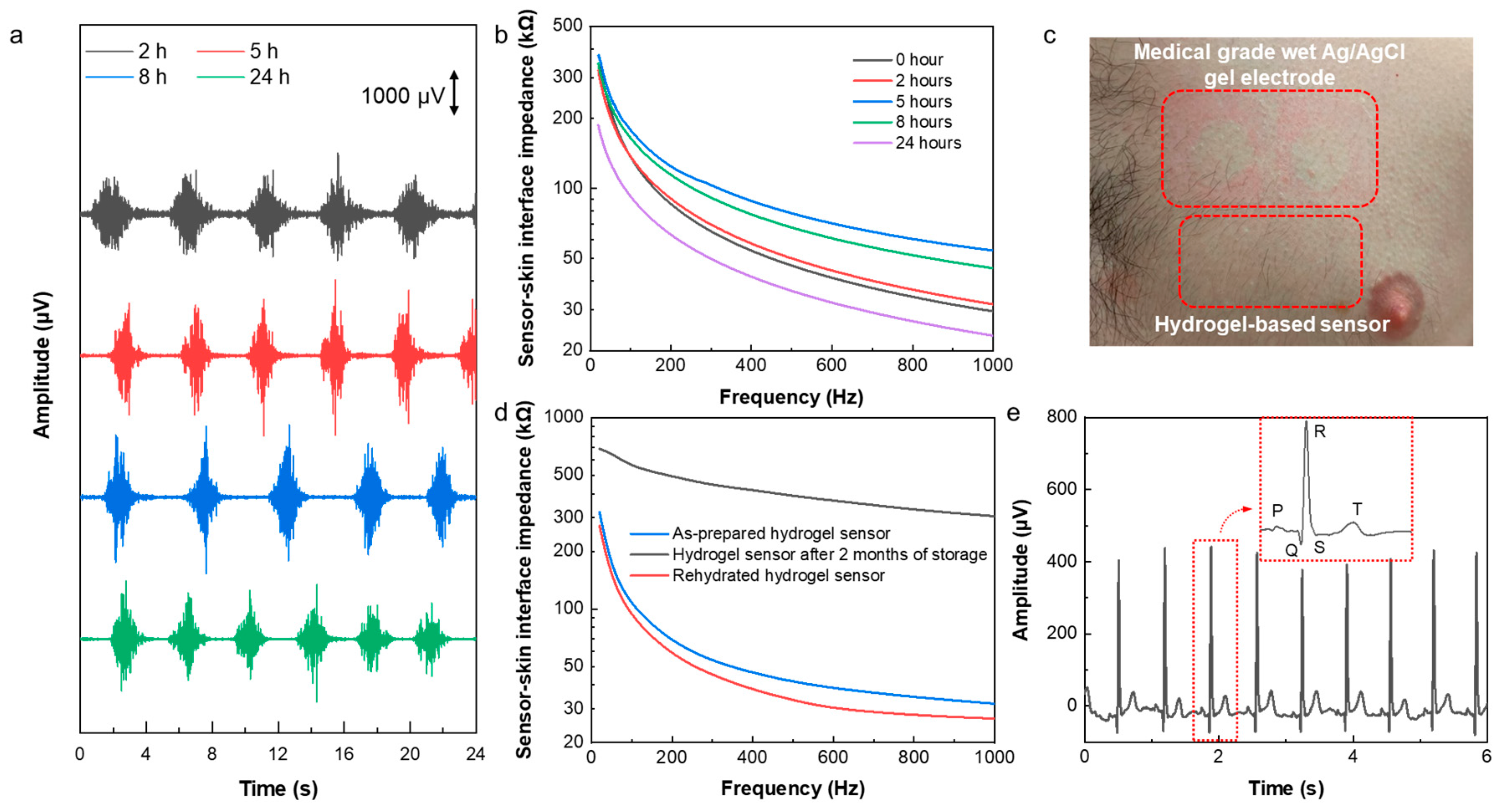
Wearable sensors have successfully been applied for long-term health monitoring during daily life. Signal quality, comfort, and skin reaction are the most important aspects for long-term electrophysiological sensing. To study the capability of the hydrogel-based sensor for the long-term monitoring, it was worn for 24 hours, and EMG was recorded after wearing it on the forearm for 2, 5, 8, and 24 hours. As it can be seen in
Figure 5a, the signal quality of the recorded EMG signals did not degrade over time but even slightly improved. That is because of the self-adhesion and the enhanced conductivity of the hydrogel sensors, the high stability of the sensor-skin interface due to the existence of the Ecoflex frame that acts as an encapsulation. Thus, the sensor demonstrated its capability of being used for the long-term sensing.
Figure 5b shows the changes in the sensor-skin interface impedance in the frequency range of 20 Hz to 1 kHz by time. This result shows the interface impedance decreases over time in the first 24 hours after wearing the sensor, which can be attributed to sweating and increasing the water and salt contents at the interface of the sensor with the skin. After removing the sensor, as it is shown in
Figure 5c, there is no sign of irritation and/or allergic reaction to the sensor. However, in the case of medical grade Ag/AgCl wet gel electrodes (3M), clear indication of skin irritation was observed.
Finally, the long-lasting property and extended lifetime of the sensor was investigated. In order to study the reusability and lifetime of the hydrogel sensor, the sensor-skin interface impedance and ECG signals were measured after storing a used sensor for a period of 2 months in an ambient environment. The hydrogel sensors dry out inevitably even being stored in a sealed container, which limits the lifetime and reusability of the hydrogel-based sensors. However, our developed sensor can be used and stored for extended period of time and restore its full electrical performance after rehydration process. As shown in
Figure S7, a used sensor that was stored for 2 months is fully rehydrated in 15 minutes by adding a drop of water on each electrode in the sensor to re-hydrate it.
Figure 5d shows the sensor-skin interface impedance of the rehydrated hydrogel sensor which decreased significantly from 304.9 kΩ before the rehydration to 26.7 kΩ after rehydration at 1 kHz and it is very similar to that of as-prepared hydrogel sensor indicating a full restoration of the used and stored sensors. As a result, the ECG signals recorded by the reused and rehydrated sensor demonstrates high amplitude and clear characteristic P, Q, R, S, and T peaks (
Figure 5e). These results show the extended lifetime and high reusability of the hydrogel sensor, making it a good candidate for long-term health monitoring.