1. Wastewater
Clean and portable water plays an essential role in the preservation and maintenance of livelihoods in aquatic and terrestrial habitats. Nevertheless, the ongoing problem is clean water provision at an adequate rate relative to the growing human population demand which in turn promotes industrialization based effluents to the air, soils, and available surface and groundwater resources resulting in water pollution. The wastewater may contain chemical, physical, and biological pollutants. Amongst these contaminants, the toxic metals are regarded to be highly resistant to conventional treatment due to their solubility, and non-biodegradability in water and as a result they stand a chance to form partake in secondary pollution thus leading to non-suitability of the treated wastewater for human and animal consumption. The metals are mostly sourced from industrial works such as manufacturing, paper, pharmaceutical, smelting, and mining operations. Consequently, the toxic metal wastewater generation may lead to clean water supply challenges, scarcity, and expense to affected communities.
1.2. Impact of Toxic Metal Presence in Wastewater and Challenges of Their Removal
Although metal abundance has been naturally experienced on Earth for several years since approximately 75% of the gross elements in the chemical periodic table are ranked as metals, most of the environmental depositions are sourced from daily anthropogenic activities (industrial production, smelting, mining operations, agricultural, batteries, and galvanizing works) [
1]. Consequently, toxic metals participate in most of the trace inorganic contaminants as outlined by the US Environmental Protection Agency [
2]. Toxic metals are explained to be elements with a density greater than 5 g/cm
3, have high atomic mass, have persistence, pose toxicity to the water source, and thus cause wastewater. Moreover, toxic metal water contamination may also occur via sediment resuspension, metal ion soil erosion, leaching, atmospheric discharge, and metal from water resources to groundwater [
1]. Among all the pollutants, the toxic metals participate in the pollutants pool and most of them are readily soluble in water especially in sulphates and nitrates forms, thus posing a challenge in wastewater treatment due to their long life span, forming oxidative species, and are persistent in an environment. Unfortunately, the toxic metals tend to accumulate without being seen in the host environment, unlike the municipal waste and petroleum hydrocarbons, thus making the challenge in the remediation process which may result in the consumption of polluted water for use in house, agricultural, and industrial application with subsequent health and production impairments.
1.2.1. Lead (Pb)
Lead is described as an element with greater density and atomic mass in comparison with water molecules. In fact, lead possesses a density of 11.34 g/cm3 and a weight of 207.2 g/mol, thus considered one of the heavy metals in water. Lead has been used in several industrial applications such as plumbing, explosives, batteries, gasoline, and cosmetics manufacturing.
Gradual lead intake through drinkable water can cause abdominal cramps, diarrhoea, vomiting, nausea, reproductive, renal, central nervous system malfunctions, depreciated seedling growth, abnormal root length, mitosis cell division and photosynthesis in humans, animals and plants [
3,
4]. In patients, the unmonitored iron intake may destructively damage the gastrointestinal mucosa and hypovolemia sourced from blood loss too. These could result in the mortality of affected patients (humans and animals) [
5]. Consequently, the elimination of lead ions in drinkable water and wastewater is essential for adequate water and source conservation.
1.2.2. Cadmium (Cd)
Cadmium is an element with a density of 8.65 g/cm
3 and an atomic mass of 112.41 g/mol greater than water. Its uses include anti-corrosion electroplating, plastic stabilization, batteries, pigmentations, and solar cells. On the other hand, the unmonitored accumulation of cadmium and its compounds renders cadmium a toxic element prevalent in the industry that discharges effluents that get into the waterways, resulting in environmental pollution, human and animal wellbeing defects. Consequently, the use of cadmium is decreasing due to its toxicity trait. The International Agency for Research on Cancer and the National Toxicology Program has declared cadmium as a carcinogen (prostate, lung, urinary, kidney, and breast cancer) in humans and animals [
6]. Additionally, the carcinogenicity mechanism is reported to be multifactorial. Cadmium toxicity causes water and nutrients translocation, metabolism and photosynthetic disruptions, and uplifts reactive oxygen species generation which initiates both the cell biomolecule destruction and plant membrane damage [
7]. Commonly, cadmium may destruct the uptake and mass transport of nutritional elements such as magnesium, calcium, and potassium in a plant.
Conclusively, this constitutes the essentiality of cadmium removal in wastewater for safer use in residences, industries, and agricultural purposes.
1.3. Cadmium and Lead Wastewater Treatment
Toxic metal emissions and related effluents are regarded as prominent health challenges to both terrestrial and aquatic organism and their habitat. Several traditional methods such as chemical precipitation, ion exchange, solvent extraction, coagulation, and flocculation have been explored for use in metal treatment. Among the aforementioned techniques, the adsorption method offers a variety of applications in comparison with other methods relative to their drawbacks, for example, large production of sludge, expensive disposal, low efficiency, and sensitive operating conditions [
8]. Although they have a track record of removing the metals, adsorption is more practical, efficient and feasible due to operation simplicity, design simplicity, removing contaminant concentration under 100 ppm unlike other methods, less or no sludge generation, environmental friendliness and fairly supports recycle and reuse [
9]. The adsorption method is a method that includes the deposition of gas or liquid (adsorbate) on a solid surface (adsorbent), resulting in molecular film formation.
The process is partitioned into two types which are chemisorption and physisorption. Chemisorption is described to be adsorption through chemical bonding (which includes the generation of covalent or ionic bonds via chemical reactions), whereas physisorption occurs through physical interaction between the adsorbent and adsorbate, and is considered non-specific since it supports random and multilayer adsorption. As a result, precipitation, redox reactions, precipitation, simplified diffusion, hydrogen bonding, complexation, and electrostatic interaction are feasible mechanisms to adsorb toxic metal ions onto the adsorbent surface for water treatment.
Furthermore, the adsorption method may be monitored by variations in process parameters such as pH, temperature, contact time, and initial concentration [
10]. Additionally, the process’s efficiency is also attributed to the adsorbent’s nature – active sites/surface area, kinetics, and selectivity [
11]. As a result, several toxic metals including Cd and Pb have been successfully removed via adsorption with high adsorption amounts, capacity, and removal efficiencies [
12,
13]. Moreover, the regulated maximum contaminant or permissible limits assigned by the World Health Organization for Cr (chromium), Ni (nickel), Pb (lead), Cd (cadmium), As (arsenic), Cu (copper), Hg (mercury), zinc (Zn) and cobalt (Co) in drinkable water are 0.05, 2.0, 0.05, 0.003, 0.01, 2.5, 0.001, 5.0, 0.1 mg/L and there is a need for monitoring and treating their amounts in water and wastewater [
14].
1.3.1. Nanotechnology and Nanomaterials
Nanotechnology is defined as technology at the nanoscale level in which systems, devices, or materials are fabricated through controlling matter at the nanoscale length to enhance the unique material properties at the nano-level [
15]. Moreover, nanotechnology includes several nanomaterials - described as materials having at least one dimension in the nanoscale range of 1-100 nanometers (nm). Their versatility allows them to be applied not only in wastewater treatment but also in sensors, pharmaceuticals, and electronics. In contrast with conventional materials, nanomaterials have improved surface area, catalytic activity, porosity, shape, lighter weight, electrical properties, high reactivity, adsorption, and catalytic nature [
16].
These materials and associated synthesis techniques, removal principles, and application routes have shown a promising affinity towards toxic metal removal in wastewater as compared to the bulk conventional adsorbents [
17].
1.3.2. Reduced Graphene Oxide/Metal Oxide (rGO/MO) Nanocomposite
Multiple studied adsorptive materials capable of removing metals from aqueous solutions are clays, biological and agricultural waste, fly ash, chitosan, zeolites, natural oxides, peat moss and activated carbon [
18,
19,
20,
21]. However, the reported limitations of these adsorbents are their relatively small metal-binding constants, small selectivity, low removal capacities, unstable at low or high pH values, and intolerance for high toxic metal ion concentrations [
22,
23].
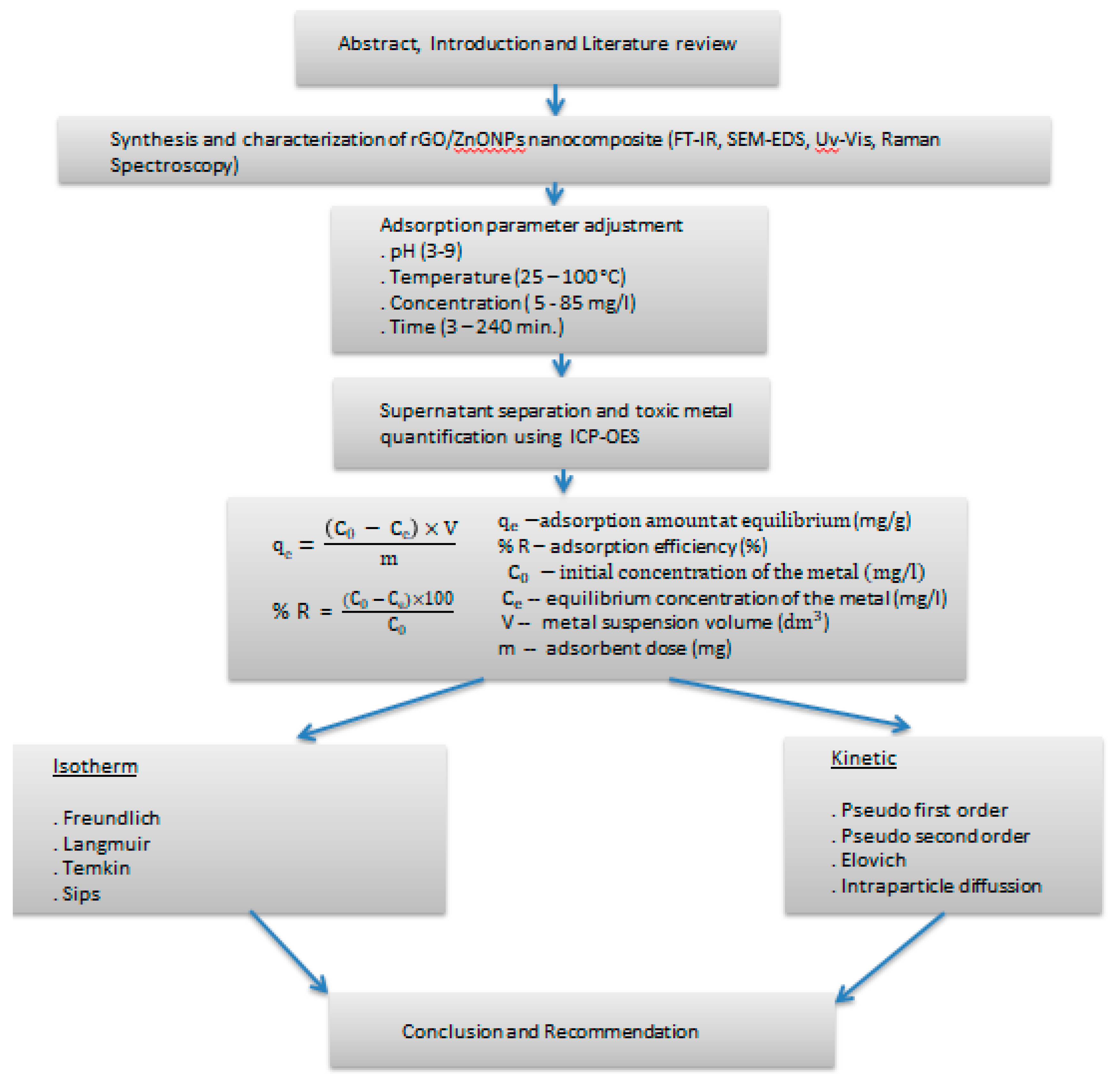
Figure 1.
Research roadmap in the current study.
Figure 1.
Research roadmap in the current study.
2. Review
2.1. Challenges with Various Nanoparticles for the Treatment of Toxic Metals
Recent research projects have shown that metal oxide nanoparticles exhibit capabilities for toxic metal ion removal in wastewater [
24,
25]. Nanotechnology has gained prominent interest in the environmental application field sourced from the associated controllable physicochemical properties, magnetism, and larger surface area [
26]. However, the common challenge in their toxic metal removal application has been separation or instability in agglomeration [
27,
28]. It has been evidenced that the single metal nanoparticles suffer good separation after the wastewater treatment thus gradually combatting their recycle and reuse phase. This could cause the generation of secondary pollutants and if they are treated by biological and photocatalytic adsorbent regeneration, these methods could be unsuitable and unsafe for treating spent adsorbent since the methods rely on contaminants degradation and combustion [
29,
30].
However, among various materials used for toxic metal water treatment, metal oxide nanoparticles such as cerium oxide (CeO
2), iron oxide (Fe
3O
4 and Fe
2O
3), titanium oxide (TiO
2) and zinc oxide (ZnO) exhibit improved tunable functionalization, surface reactivity, selectivity, photocatalytic nature and act as exceptional adsorbent for wastewater remediation due to their affinitive surface functional groups towards various toxic metals including Pb(II) and Cd (II) [
31,
32,
33,
34]. Among metal nanoparticles, zinc oxide nanoparticles have received pronounced attention for wastewater treatment since the material may act as a precipitant, photocatalytic, and adsorbent agent for selected toxic metals [
35,
36,
37,
38]. The possible property drawbacks associated with the ZnO nanoparticles are agglomeration, instability, leaching, and depreciated thermal characteristics for efficient application during toxic metal uptake in wastewater [
39,
40,
41]. Therefore, the synthesis of surfacing the nanoparticles onto the supporting material with a high surface area has been a feasible and ongoing research interest resulting in good nanocomposite material and thus it was investigated in this study.
2.2. Nanocomposite
The graphene material has drawn attention due to its leading optical, mechanical, thermal, and flexibility properties [
42]. Reduced graphene oxide as a graphene derivative is majorly synthesized by the generation of graphite oxidation to graphene oxide and the reduction of oxygen content on the graphene sheets by thermal and chemical treatment leading to multifunctional surface generation [
43]. The functionalisable surface of the reduced graphene oxide allows for the impregnation of the nanoparticle materials on graphene layers to produce a smooth sorption surface [
44]. Moreover, the material may be macro clusters while the sorption surface may become regular. Although there are some limitations with chemical modification, this method gives a control on tuning the functional groups for subsequent improved adsorption efficiency and selectivity for wastewater treatment [
44,
45].
Reduced graphene oxide/ metal oxide nanocomposites have been synthesized in various ways aiming at achieving chemical (advanced chemical stability), physical (high thermal and electrical conductivity, mechanical strength, large surface-to-volume ratio, porosity, capacitance) and biological (antiviral, antibacterial, antioxidant) properties which served as promising and compatible characteristics for supercapacitors, fuel and solar cells, drug delivery, tissue engineering, cancer treatment, biosensors [
46,
47,
48].
The graphene/ZnO electrochemical sensor for para-nitrophenol was successfully developed and recorded a low detection limit of 8.8 × 10–9 M using square wave voltammetry [
49]
. The sensor proved to exhibit high sensitivity and specificity for para-nitrophenol against interfering compounds which are ortho-nitrophenol, 2,4-di-nitrophenol, and meta-nitrophenol in groundwater and river samples. These also signify that the composite resulted in an improvement of the electrochemical performance of the modified glassy carbon electrode which led to improved and fast recoveries [
49]
. In another study, the graphene/ZnO nanocomposite was tested for a supercapacitor project where the material performed well with 122.4 F/g in comparison with rGO (102.5 F/g) and graphene oxide (2.13 F/g) [
50]. The good performance was attributed to the well-dispersed electronegative ZnO particle (with a size between 30-70 nm which qualifies large surface area and high electron mobility) on the graphene sheet and the synergistic effect sourced between the nanocomposite structure which was confirmed by EDS, HRTEM, XRD, UV-vis and galvanostatic charge/discharge techniques
and cyclic voltammetry, thus resulting to a potential electromaterial for advanced supercapacitor purpose . The graphene/ZnONPs nanocomposite exhibits good carriage and pH sensitive ejection of conjugated and aromatic anticancer drugs [
51]. Furthermore, the composite possesses the capability for endosomal escape post intracellular use for genetic therapy and transportation, hence the material are promising candidate for cancer
therapeutics, drug delivery, and co-delivery [
51,
52,
53].
ZnO is considered as a wide band gap semiconductive material consisting of large charge carriers recombination leading to its possible instability and decreased photocatalytic efficiency [
53]. In order to accommodate the drawbacks, the material has been modified with other metal oxide, polymers, zeolite, and nanocarbon based material for photocatalytic behavior improvement [
53].
Majorly, the graphene material has proved to efficiently hybridize with ZnO for catalytic characteristics. The zinc oxide nanorods were hydrothermally deposited on bilayered reduced graphene oxide for photodegradation of methyl orange in water. The degradation efficiency increased by 15 % when the composite was tested as compared to the unmodified zinc oxide nanorods. Furthermore, the composite reaction rate (1.4 × 10
−2 min
−1) was higher than the rGO (1 × 10
−3 min
−1 ) and ZnO (7.2 × 10
−3 min
−1), proofing the fast kinetic reaction due to synergistic interactions sourced from the composite’s rGO and ZnO [
54]. In another study, the
rGO/ZnO nanocomposite was applied in gas to liquid conversion through the photoreduction of carbon dioxide (CO
2) to methanol (CH
3OH). The CH
3OH yields for
rGO/ZnO nanocomposite and ZnO nanorods were 263.17 and 52.36 μmol/gcat [
55]
. This indicated the five times increase in CH
3OH yield
from a pure ZnO to rGO/ZnO nanocomposite which could be sourced from an improved charge separation and catalytic activity by nanocomposite photocalyst. Furthermore, the nanocomposite proved to support improved photoreductive stability, cycling, and reusability properties with a minimal decrease of 5% in CH
3OH yield [
55]
.
As a result, these cases serve as proof of graphene/ZnONPs nanocomposites being multifunctional materials compatible with various applications. Therefore, the current study is aligned with water treatment and the review will majorly be on toxic metal removal from wastewater using the selected nanocomposite through the adsorption method.
Recently, to overcome these limitations, nanolayered rGO (reduced graphene oxide) functionalized with MO (metal oxide) nanoparticles allowing for available adsorbate binding sites, with improved selectivity and surface area, no/less particle-particle aggregation, improved adsorption capacities, and efficiencies have been studied for wastewater treatment [
56,
57]. A study recorded high adsorption capacities and efficiencies of selected metals in the range of 100-600 mg/g and greater than 85 % have been obtained with rGO/ZnONPs nanocomposites [
14,
58,
59]. Several studies have been conducted on cadmium and lead removal using GO/ZnONPs composites as compared to rGO/ZnONPs nanocomposites [
60,
61,
62,
63]. As a result, this study focuses on rGO/ZnONPs nanocomposite synthesis and adsorptive application in the remediation of cadmium and lead in wastewater.
2.3. Adsorption Limitations
Although adsorption is regarded as a simple and efficient method for toxic metal treatment, the generation of suitable adsorbing materials may not be cost-effective and some adsorbents like commercialized activated carbons may not allow for recycling post the water treatment and this could lead to potential unsustainable large scale toxic metal wastewater remediation [
14,
64]. As a result, the drawbacks may be fairly accommodated with moderate to low-cost, recyclable, and reusable nanocomposite adsorbent which outputs efficient metal uptake. In this context, this study investigated the fabrication of rGO/ZnONPs nanocomposite as a promising adsorbent for uptaking Cd and Pb with the aid of reuse, interfering species and monitoring the adsorption through various process parameters, equilibrium, and kinetic models.
2.4. rGO/ZnONPs Nanocomposite for Treatment of Pb and Cd in Wastewater
2.4.1. Cadmium (Cd) Wastewater Treatment
The zinc oxide nanoparticles were prepared by co-precipitation. The achieved nanoparticles were confirmed by XRD to be having hexagonal phase structure. Furthermore, the graphene oxide layers were fabricated via the Hummers method and the Aloe vera leaf extract was used for conversion of graphene oxide to reduced graphene oxide [
65]. Although the ZnO nanoparticles seemed to have swallowed the graphene material peak, the overall composite’s crystallinity, surface functionalization, and morphology were evidenced by XRD, TEM, and AFM. The composite's average crystallite size was 70 nm, with the surface wrinkled and roughness showing the composite generation. The uptake of the cadmium by the ZnO/rGO was investigated based on time (0-120 min.) and pH (2-8). The results showed that the adsorption efficiency increased relative to pH increase and this could be due to competition for available and reactive sites for H
+ and Cd
2+ adsorption. Although removal efficiency increases, the Cd at pH 8 could form a precipitate that depreciates the adsorption and possible desorption for reuse. Therefore pH 6 was preferred with efficiency greater than 80 % [
65]. The sonochemical method was used for PANI/ZnO/r-GO synthesis, which was found to be an amorphous, semi-agglomerated surface [
62]. The material was applied for Cd adsorption which was optimally achieved at the time (60 min.: 60.9%), adsorbent quantity (0.2 g: 63%), temperature (60 °C: 57%), pH (6: 98%) and concentration (10 mg/l: 24%). The adsorption was well described by Freundlich (R
2 = 0.99) and pseudo second order (R
2 = 0.95) as compared to Langmuir (R
2 = 0.93), Jonavoic (R
2 = 0.97) and pseudo first order (R
2 = 0.94). This implied that the adsorption was a non-specific uptake and proceeded through multi layered configuration [
62]. In conclusion, multiple studies have been conducted on the effect of ZnO/rGO composites for Cd removal with uplifted efficiencies [
61,
66,
67].
2.4.2. Lead (Pb) Wastewater Treatment
In another study, the ZnO/rGO nanocomposite was sono-chemically synthesized for Pb(II) adsorption. The composite was found to be semi crystalline, photoactive, sphere shaped nanoparticles dispersed on rGO sheets with some agglomeration [
68]. The adsorption process was optimized pH (2 - 6), contact time (0 – 70 min), concentration (20 – 100 mg/l), temperature (5 – 80 °C) and adsorbent dosage (20 – 100 mg). The optimum parameters were 60 min., 100 mg, pH 6, 20 mg/l, and 65 °C with maximum removal efficiencies of 68, 68, 98, 17 and 57 %, respectively. The adsorption data well fitted Langmuir isotherm and pseudo first order with the highest regression coefficients of 0.993 and 0.973. The composite sustained three recycling phases (with 0.1M HCl) for reuse with the following descending efficiencies: 78, 63 and 41 % [
68]. The removal deficiency could have been due to unsuccessful full desorption and significant surface compositional change when exposed to the Pb as evidenced by irregular and agglomerates presence from SEM images after the adsorption [
68]. The ZnO/rGO nanocomposite was hydrothermally synthesized in an autoclaved reactor at 180 °C for 6 hours [
66]. The nanoparticle's TEM image showed spherical shapes deposited on the graphene sheet. The adsorption of Pb(II) was then investigated from 1 to 50 mg/l (initial concentration), 10 to 50 mg (adsorbent quantity), and the maximum capacity was achieved under room temperature for five minutes. As a result, 50 mg of the adsorbent dose, pH 6, resulted in the highest removal efficiency of 75.4 %. The adsorption improvement by functionalizing the graphene’s surface with ZnO nanoparticles has been evidenced in other studies too [
63,
69,
70].
Table 1.
Adsorption of Pb(II) and Cd(II) in wastewater using graphene oxide based composite materials.
Table 1.
Adsorption of Pb(II) and Cd(II) in wastewater using graphene oxide based composite materials.
| Adsorbent |
Adsorbent Nature |
Equilibrium Time (min.) |
Toxic Metal Treated |
Adsorption Efficiency (%) |
Kinetic and Thermodynamic Model |
Recycle Reaction |
Ref. |
| ZnFe2O4/ rGO |
Crystalline, rough and wrinkled surface, weak magnetism |
60 |
Pb (II) |
98 |
Langmuir and pseudo second order (chemisorption) |
5 |
[71] |
| ZnO/GO |
Weak crystallinity, hollow shaped spaces, and flake shaped morphology |
120 |
Pb (II);
Cd (II) |
97; 96 |
Langmuir and pseudo second order (chemisorption) |
- |
[72] |
| ZnO/G |
Well surfaced spherical ZnO nanoparticles on graphene, |
25 |
Pb (II) |
92 |
Endothermic, spontaneous, Langmuir, pseudo second order, |
3 |
[70] |
| Fe3O4/rGO |
Homogeneously dispersed nanoparticles on rGO sheet, magnetic and thermally stable composite, |
45 |
Pb (II) |
96 |
Exothermic and spontaneous reaction, Temkin, chemisorption, liquid film diffusion |
- |
[73] |
| CuO/rGO |
Homogeneously dispersed nanoparticles on an rGO sheet with minimal sheet agglomeration |
175 |
Cd (II) |
91 |
Langmuir |
- |
[74] |
| TiO2/rGO |
Irregular and mesoporous surface |
150 |
Pb (II) |
89 |
Langmuir, pseudo second order |
- |
[75] |
| ZnO/rGO |
Good distribution of ZnO nanoparticles on rGO sheet, wrinkled surface |
90 |
Cd (II) |
90 |
Langmuir, pseudo second order, chemisorption |
- |
[65] |
| PTP-SiO2/rGO |
Sphere shaped nanoparticles distributed moderately on rGO surface, amorphous nature |
60 |
Cd (II), Pb (II) |
62, 66 |
Jovanovic isotherm, Spontaneous adsorption, pseudo second order |
3 |
[76] |
| WO3/rGO |
Increased average crystallite size upon surfacing on rGO, strong bonding existing between WO3 and rGO signaled by weakening and broadened WO3 Raman spectroscopy peak, mesoporous surface |
200 |
Pb (II), Cd (II) |
93.35, 89.95 |
Electrostatic attraction |
- |
[77] |
| FA/GO |
Well covered graphene oxide surface by folic acid with uplifted roughness, irregularly shaped composite surface |
90 |
Cd (II) |
82 |
Freundlich isotherm, pseudo second order, endothermic, multilayered, and spontaneous adsorption |
2 |
[78] |
| Mn–Fe3O4/G |
Weak magnetism, well dispersed particle on a graphene sheet |
58 |
Cd (II) |
94 |
Langmuir-Redlich Peterson isotherm, spontaneous removal, chemisorption, pseudo-first and pseudo-second order |
2 |
[79] |
| ZnO/rGO |
Slight agglomerated surface, sheet like structure, electronegative and photo-active surface |
10, 90 |
Cd (II), Pb (II) |
~ 100 |
Freundlich, Sips, pseudo first oder |
3, 7 |
This study |
2.5. Experimental Procedure
2.5.1. Zinc Oxide Nanoparticles (ZnONPs)
0.5 M ZnSO4.7H2O (100 ml) was precipitated with 1 M NaOH (100 ml) at room temperature for 3 hours. The obtained white precipitate was washed with 0.5 M MeOH (250 ml) and 200 ml of deionized water. Lastly, the product was oven dried at 100 °C for 35 minutes and calcined at 200 °C for 2 hours.
2.5.2. Reduced Graphene Oxide (rGO)
H2SO4 (50 ml) was pre-cooled for 30 minutes in an ice bath. Thereafter, the graphite (3 g) and NaNO3 (1.5 g) were added to H2SO4 (50 ml) with continuous stirring for 30 minutes in an ice bath. Moreover, KMnO4 (3 g) was moderately added for an hour while stirring. Afterwards, the mixture was stirred at 40 °C and added H2O (100 ml). Furthermore, the composition was stirred for an additional 2 hours at 90 °C and added H2O (50 ml) with H2O2 (6 ml) for an additional 2 hours. Then, the graphene oxide (GO) was filtered and washed with H2O (~ 500 ml) and 0.5 M MeOH(50 ml). The resultant black precipitate was dried at ~ 100 °C for overnight.
For reduction, the dried GO (1 g) was dissolved into H2O (100 ml) with NaBH4 (0.2 g) for 4 hours at 100 °C in a reflux setup followed by bath ultrasonication (2 hrs) for sheet exfoliation. Lastly, the graphene was washed with H2O (200 ml), dried at ~ 100 °C for overnight and thermally treated in the tube furnace at 350 °C (10 °C/min) for 2 hours under nitrogen flow.
2.5.3. rGO/ZnONPs Nanocomposite
A weight ratio of 1:3(w/w) (rGO/ZnONPs) was used for composite synthesis. The rGO/ZnONPs nanocomposite was prepared by dispersion of reduced graphene oxide in ethanol (99 %) and mixing with ZnONPs powder while continuously stirring in an inert environment for 1 to 6 hours. Thereafter, the resultant black-white precipitate was washed with 1 M ethanol (50 ml) and deionized water (100 ml) to remove excess solvent and dried in a vacuum oven at 100 °C for 30 minutes.
2.6. Characterization of Adsorbents
The adsorbent was analyzed for its material characteristics. FT-IR, Uv-vis, SEM-EDS, and Raman spectroscopy were used to investigate functional groups present, optical nature, surface morphology, elemental composition, and defects density in the adsorbent.
The functionalization was studied using attenuated total reflectance FT-IR from 400 to 4000 cm-1 in five replicate scans, spectral resolution of 4 cm-1 using Perkin–Elmer-Spectrum100 FTIR spectrometer, coupled with universal ATR top plate and diamond crystal. Furthermore, Uv-vis analysis was done by dissolving 0.0015 g of the synthesized material in 20 ml of deionized water and using a quartz cuvette of light path 1 cm. The Uv-vis measurements were carried out on Wirsam Scientific (Uv/vis 920) GBC instrument with processing parameters: step size (0.133 nm), speed (300 nm/min), slit width (2 nm), and results read by Cintral v 2.4 software. The morphology was monitored by LEO 1450 scanning electron microscope (at 10 kV) and corresponding microimages were generated by using SMARTSEM v 5.03.06 software. The sample preparation was done by evenly dispersing fine powdered samples over carbon tape attached to copper stubs. Thereafter, the samples were gold capped using the sputtering tool for clear scanning electron microscope observations. Raman spectra were done using the Jobin–Yvon LabRAM HR Raman spectrometer.
3. Adsorption Study
Batch tests were done to study the impacts of initial solution pH values, dosage, contact time, initial concentration, and temperature on the adsorption capacity of Pb (II) and Cd (II) ions using rGO/ZnONPs. Separately, 100 mL of Pb (II) and Cd (II) synthetic wastewater was prepared from Pb(NO3)2.4H2O and CdC4H6O4.2H2O while magnetically stirring at 180 rpm with the adsorbent. After the equilibrium, the aqueous phase was separated from solids by centrifugation (3000 rpm, 15 min) and subsequently filtered through 0.22 µm membranes to quantify Pb (II) and Cd (II) residual concentration. The triplicate equilibrium concentrations of Pb (II) and Cd (II) were measured via the ICP-OES instrument. Moreover, the resultant adsorbent was exposed to desorption by using 0.01 M H2SO4 (50 ml) solution for 45 minutes under magnetic stirring (80 rpm) and resulted in adsorbent recycling and reuse.
The interference study was carried out by one pot method made up of copper, iron, chromium, zinc, cadmium, and lead. 90 mg, pH 5, 25 °C, 10 ppm (for each metal), 200 rpm, 4 hours were applied during the testing.
The equilibrium removal capacity and efficiency were determined using eq. 1 and 2:
where V is the suspension volume (dm
3), and m is the adsorbent weight (g), C
0 is the initial concentration (mg/L), C
e is the aqueous-phase equilibrium metal concentration (mg/L).
3.1. Adsorption Isotherms
The batch experimental data was fitted by using Langmuir, Freundlich, Tempkin, and Sips model in the determination of sorption capacity via eq. 3 – 6.
where,
q = amount adsorbed (mg/g)
= adsorption capacity related to a monolayer surface coverage;
= Langmuir constant corresponding to the adsorption energy;
=Freundlich constant related to the adsorption capacity;
= Equilibrium binding constant related to Tempkin isotherm;
n = adsorption intensity constant;
= metallic equilibrium concentration in aqueous phase (mg/l);
B = abbreviation of RT/, R – gas constant (8.314 J/mol K), K – absolute temperature, = Tempkin isotherm constant
qm = maximum amount adsorbed
K = Sips isotherm model constant
3.2. Adsorption Kinetics
For investigation of kinetics, 0.015 and 0.050 g of rGO/ZnONPs were added to 100 ml of Pb
2+ solution (3 mg/L) and Cd
2+ (10 mg/L). The suspensions were stirred at 180 rpm under room temperature. The samples were withdrawn at 3 to 240 minutes. Afterward, the data was fitted by pseudo-first (eq. 7) and pseudo-second order equations (eq. 8), intraparticle diffusion (eq. 9), and elovich (eq. 10).
where,
A (mg/g/min) = initial rate of adsorption
B (g/mg) = term related to the extent of surface coverage and activation energy for chemisorption
b = rate constant
t (min.) = adsorption time
y & qt (mg/g) = adsorption capacity at time (t)
qe & a (mg/g) = adsorption uptake at equilibrium
k1 (min-1) = rate constant related to pseudo-first order
k2 (g/(mg min)) = rate constant corresponding to pseudo-second order kinetic model
Kd = intraparticle diffusion rate constant
C = plot intercept implying boundary layer effect or surface adsorption
4. Results and Discussion
4.1. Characterization
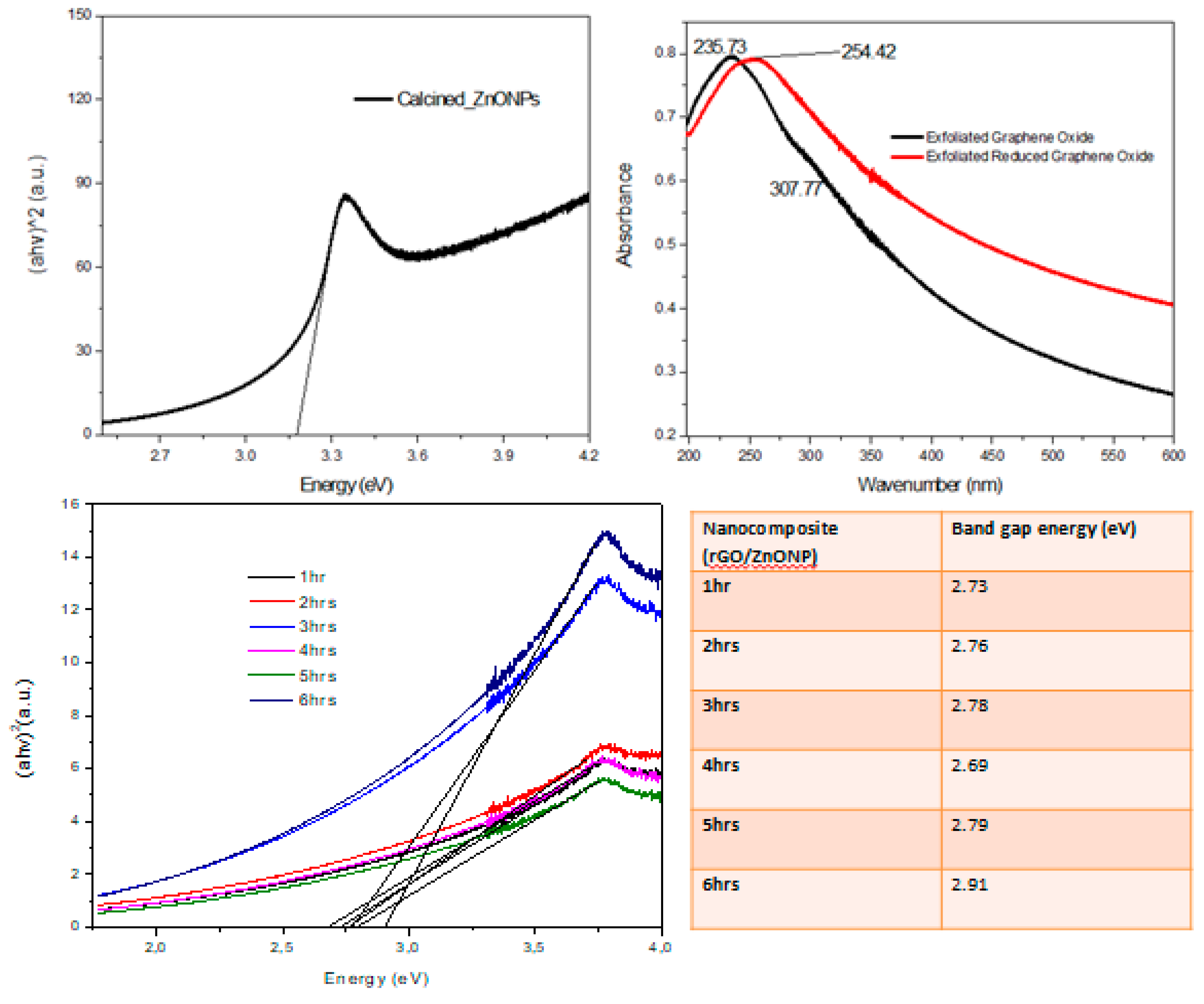
4.1.1. UV-vis
The determination of band gap energy was performed via direct extrapolation (DE) which is normally achieved by best-fitting straight line curve to the linear portion of the adsorption spectrum (where (αhν)
2 = 0). The determined ZnONPs band gap energy was 3.172 eV (
Figure 2) which is near the reported band gap energy of ~ 3.37 eV [
80,
81].
Furthermore, the optical nature of rGO and GO was also monitored via uv-vis spectroscopy (
Figure 2). In GO, a peak (235.73 nm) and a shoulder (307.77 nm) were observed while only a single peak (254.42 nm) was detected in rGO spectrum. Consequently, the adsorption peak at 235.73 nm and a shoulder at 307.77 nm constitute the aromatic C-C bonds’ π-π* transition and C=O bonds’ n-π* transition implying the oxidation of the graphene sheet. In rGO, the shifting of the absorption peak is due to the aromatic C-C bonds' π-π* transition, evidence of oxygen content elimination and the disappearance of the C=O bonds' n-π* transition [
82]. The optimized nanocomposite’s band gap energy was the lowest when grown at 4 hours (2.69 eV), this value showed that the composite may also be treated as a potential photocatalyst besides the adsorption behavior which was studied in this work.
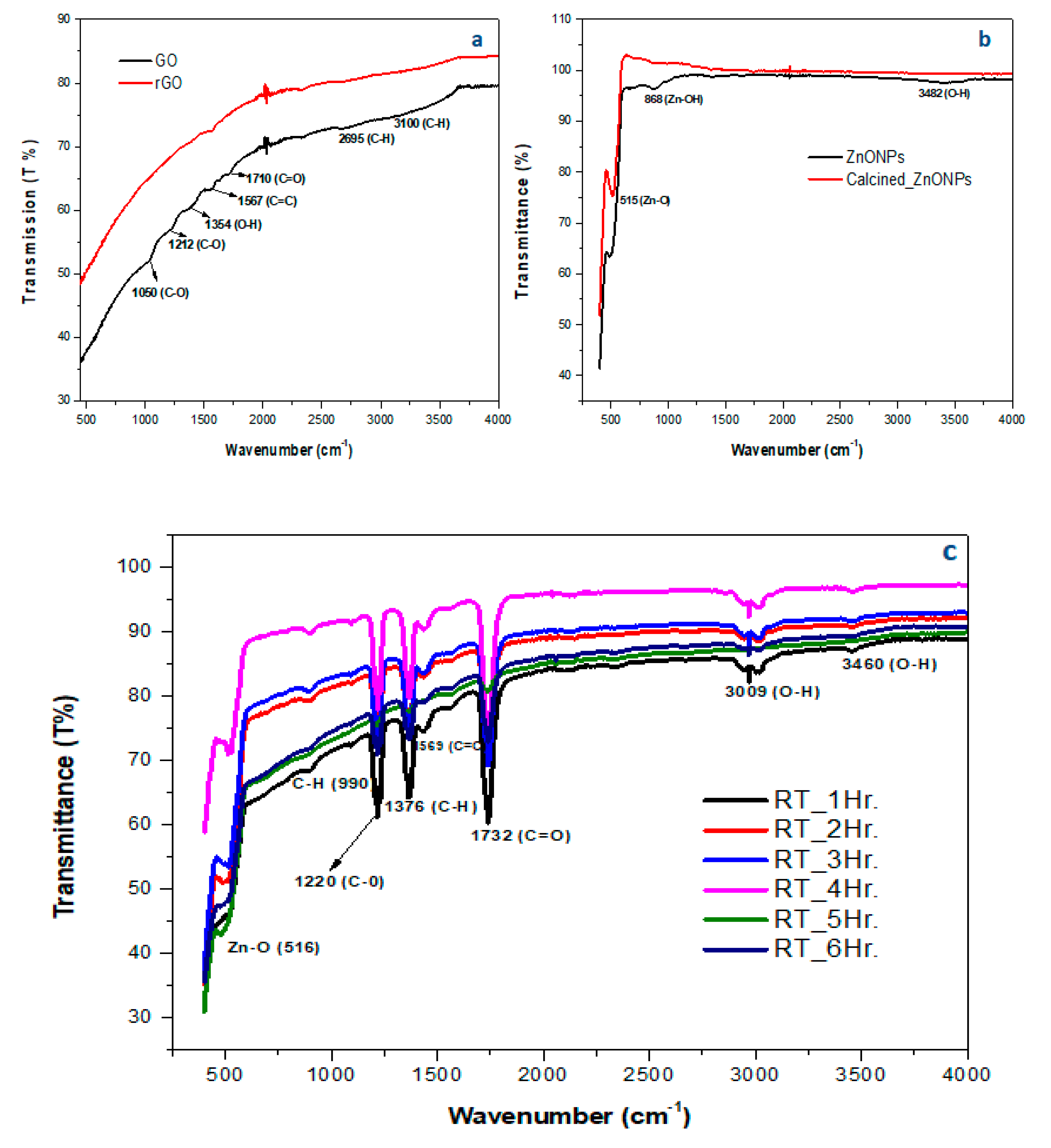
4.1.2. FTIR
Figure 3(a) shows the spectrum of graphene oxide with the presence of oxygen functional group stretches and vibrations at 1050, 1212, 1354, and 1710 cm
-1 for C-O, O-H, and C=O confirming oxidation of graphene surface [
83]. During the conversion to rGO, the carbon groups (C-H, C=C) were present at 1567, 2695, and 3100 cm
-1 revealing the restoration of sp
2 hybridized graphene structure with noticeable elimination of oxygen functional groups (1050 – 1710 cm
-1)
Figure 2(a).
In
Figure 3(b), the FT-IR spectrum of the calcined ZnONPs showed an intense and sharp peak at 515 cm
−1, indicating the existence of Zn-O vibrations [
84]. Moreover, the hydroxyl group present in room temperature ZnONPs at 3482 and 868 cm
-1 was fairly eliminated, confirming the successful synthesis of ZnONPs rather than Zn-OHNPs [
85].
In the case of rGO/ZnONPs nanocomposite (
Figure 3(c)), the peak at 516 cm
-1 indicated the stretching vibration of the ZnO [
86]. The graphene sheet structural peaks were signalled by the C-H, and C=C at 1376, and 1569 cm
-1. Furthermore, the binding of the ZnONPs onto rGO sheets was attributed to C-O, and C=O at 1220, and 1732 cm
-1 [
87]. The broad and medium stretching vibration mode at 3009, and 3460 cm
-1 constitutes the hydroxyl group from water molecules that are absorbed on the composite surface [
88]. Thus, these results have shown the formation of ZnONPs on the rGO matrix. Moreover, the optimized composite at room temperature for 4 hours was selected for further characterization and subsequent metal ion removal tests. The composite showed a pronounced peak for Zn-O signaling the successful deposition as compared to other composite synthesized at room temperature (1,2,3,5 and 6 hours) (
Figure 3(c)).
4.1.3. Morphology of ZnONPs and rGO/ZnONPs
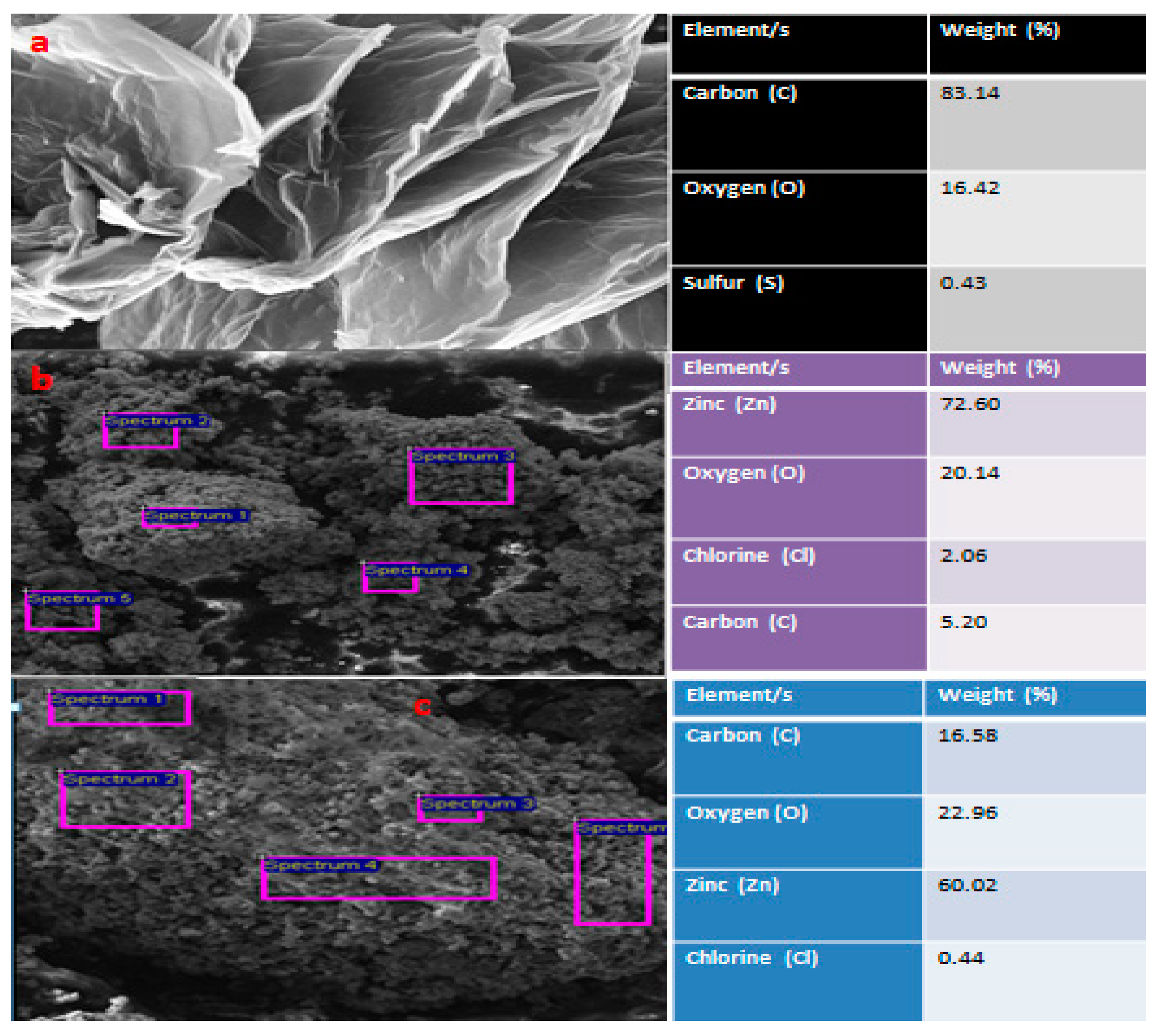
Figure 4(a) showed fairly separated rGO sheets with high carbon content (83.14 %). The surface revealed that the spherical ZnONPs were achieved with almost fair distribution accompanied by agglomeration which may be sourced from a lack of thorough purification and moisture exposure (
Figure 4 (b)). The round shaped particles signaled modification of ZnONPs on the rGO sheet. This was supported by the appearance of a new peak of Zn–O stretch on FTIR spectra of the synthesized rGO/ZnONPs nanocomposite (
Figure 3(c)). The corresponding EDS showed the high presence of ZnONPs’ participants (Zn and O) due to the used weight ratio in this study. Similar results have been documented by authors [
89,
90,
91].
4.1.4. Defect Density Study
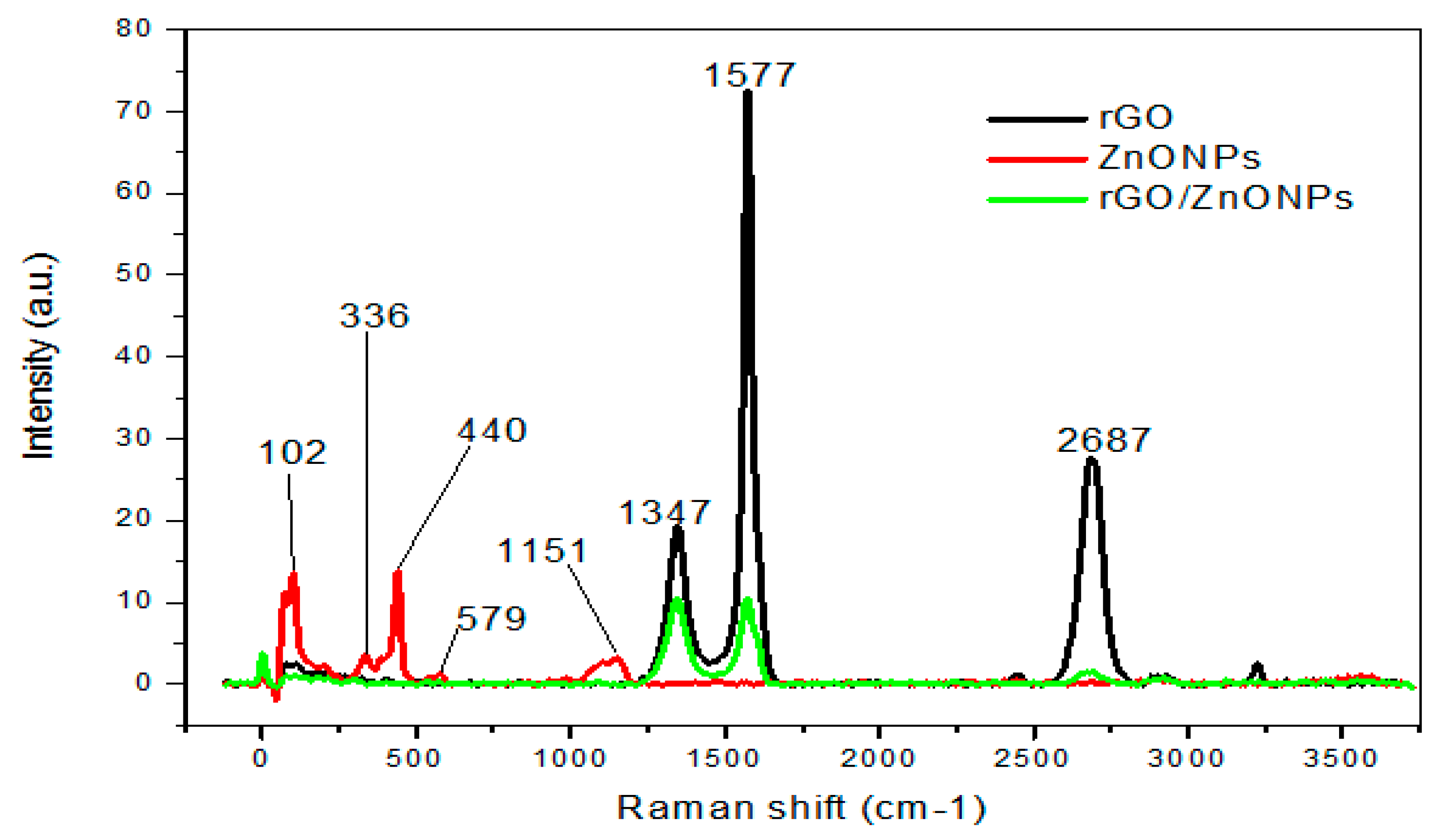
The spectrum of rGO contained three prominent peaks at 1347, 1577, and 2687 cm
-1 assigned to D, G, and 2D [
92]. The D and G bands are sourced from sp
2 carbon with information on carbon pairs in a ring, the aromatic ring breathing mode, and defects. Amongst these bands, the G band (for sp
2 carbon structure) appeared to be sharp and with the highest intensity (~ 4 folds of the D band). The observed D and 2D are associated with disordered crystalline carbon structural defects (oxygen, sulphur content) and a set of the graphene sheet, respectively [
93]. Five peaks were present in ZnONPs, which are positioned at 102, 336, 440, 579, and 1151 cm
-1. The peak at 336 cm
-1 was assigned to the second order structure of ZnONPs sourced from zone boundary phonon, and 440 cm
-1 was attributed to the E2 mode of wurtzite ZnO [
94]. The weak broadband at 579 cm
-1 was attributed to E1(L) and implying marginal defects on ZnONPs surface, the sharp peak for optical phonon E2 mode was recorded at 102 cm
-1, and the localized acoustic phonon mode was positioned at 50 cm
-1 [
95].
The Raman spectrum of rGO/ZnONPs is shown in
Figure 5 with noticeable four bands at 10.76, 1347, 1577, and 2687 cm
-1. Furthermore, the reduced graphene oxide spectrum and rGO/ZnONPs showed D, G, and 2D bands at relatively same frequency implying that the graphene structure is fairly maintained in the composite. In observation, the intensity of D, G, and 2D bands decreased due to ZnONPs modified rGO surface suggesting successful functionalization [
96]. The optical mode at 10.76 cm
-1 is assigned to the E2 mode of the zinc and oxygen sub lattice [
97].
4.2. Removal Study
4.2.1. Adsorption Performance
For the determination of adsorption efficiency, the initial pH is an essential aspect because it includes the principal interaction between the adsorbent and adsorbate species. At various pH numbers, the activity and concentration of hydronium (H
+) and hydroxyl group (OH
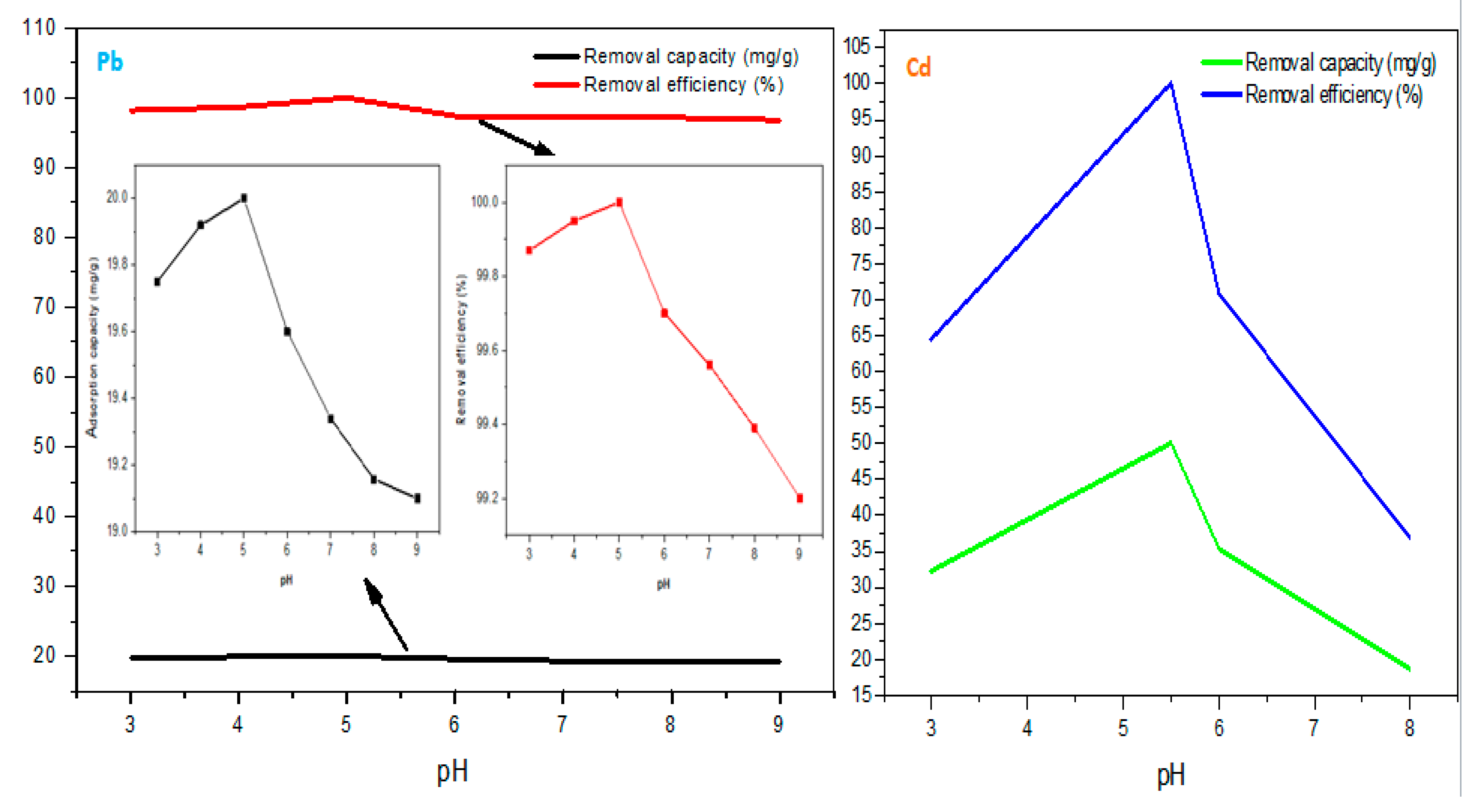
-) tend to be different and this may have an impact on electrostatic interaction between the adsorbent surface and adsorbates, being one of the adsorption mechanisms. Consequently, the effect of pH on the adsorption capacity of Pb(II) and Cd(II) using rGO/ZnONPs was investigated from pH 3 to 9 (
Figure 6). For Pb adsorption, at pH 3 (19.75 mg/g) and 4(19.91 mg/g), the adsorption capacities are lower than at pH 5 (20.00 mg/g); this could be due to enhanced adsorbent surface protonation (more acidic) which possibly lead to hindrance of oxygenated functional group protonation, less Pb
2+ adsorption sites and thus leading to repulsive interaction between the H
+ and Pb
2+ at pH 3 and 4. Moreover, at pH 6 and 7, the adsorption performance decreased drastically due to the gradual insolubility of Pb resulting in possible hydroxide precipitation which may be Pb(OH)
2(s), Pb
4(OH
4)
2+(s), Pb
4(OH
4)
4+(s) and Pb
6(OH
8)
4+(s) [
98].
Cd adsorption was favoured by pH 5.5, implying that the adsorbates’ transport within the adsorbent surface outperformed the presence of hydronium (H+) ion in the acidic phase and hydroxyl group (OH-) in the alkaline level. This resulted in maximum removal capacity and efficiency of 49.99 mg/g and 99.99 %. The implication was that the alkaline pH may be avoided because of the inactivity of adsorption and enhanced precipitation which may result in secondary pollution (sludge formation). Thus, the removal of Pb2+ and Cd2+ is the least efficient at a more basic pH = 9 (19.09 mg/g) and 8 (18.51 mg/g). Conclusively, the optimum adsorption pH was found to be 5 and 5.5 for Pb2+ and Cd2+.
Furthermore,
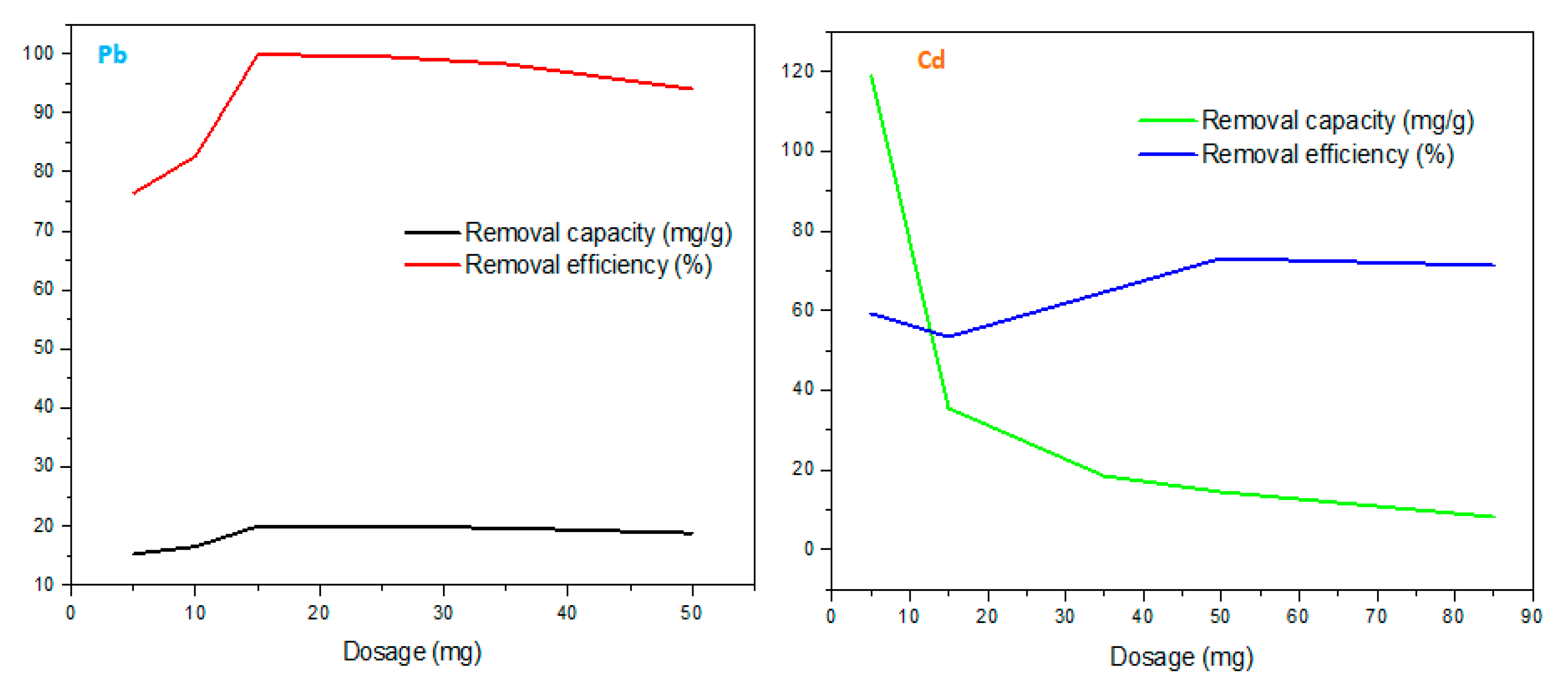
Figure 7 illustrates Pb
2+ and Cd
2+ metal uptake using various adsorbent dosages (5-50 mg). During the Pb
2+ adsorption, it can be seen that rapid adsorption took place from 5 to 15 mg due to occupation of available surface sites. When 15 mg of rGO/ZnONPs was utilized for sorption, the Pb
2+ removal capacity reached a maximum of 19.98 mg/g. From 15 to 25 mg dosage, the process had equilibrium characteristics thus indicating the excessive site state for the studied 3 ppm. Hence, the 15 mg seemed to be the optimum rGO/ZnONPs dosage for Pb(II) adsorption. However, at 25 to 50 mg, there was a depreciation of removal efficiency on the adsorbent surface and this may be sourced from slight desorption (physisorption characteristic), stirring effect, and competition of sites amongst the H
+ and Pb
2+.
From 5 to 15 mg, there was a decrease in removal efficiency from 59.37 to 53.55 mg/g. This could be sourced from the unavailability of uptake sites related to H+ and Cd2+ ion concentration. However, the efficiency increased from 15 mg with the maximum removal efficiency related to Cd2+ recorded as 73.13 % using 50 mg. Afterwards, there was a slight decrease in efficiency from 50 to 85 mg and the source may be excess site preoccupied by H+ ion and Cd2+ site competition.
An increase in adsorbent dosage resulted in a decrease in adsorption capacity, this could be due to net active sites being available at lower doses whereas only active sites fraction was exposed at larger doses. Therefore, the high adsorbent dose usage could result in sudden material aggregation and thus decrease material net surface area, pore volumes, and sizes which will result in a decrease in adsorption performance [
99].
The adsorbent’s available site to adsorbates concentration ratio is key for adsorption, especially in signaling the saturation/unsaturated site relations and equilibrium state. Consequently,
Figure 8 shows the study on the concentration of Pb
2+ and Cd
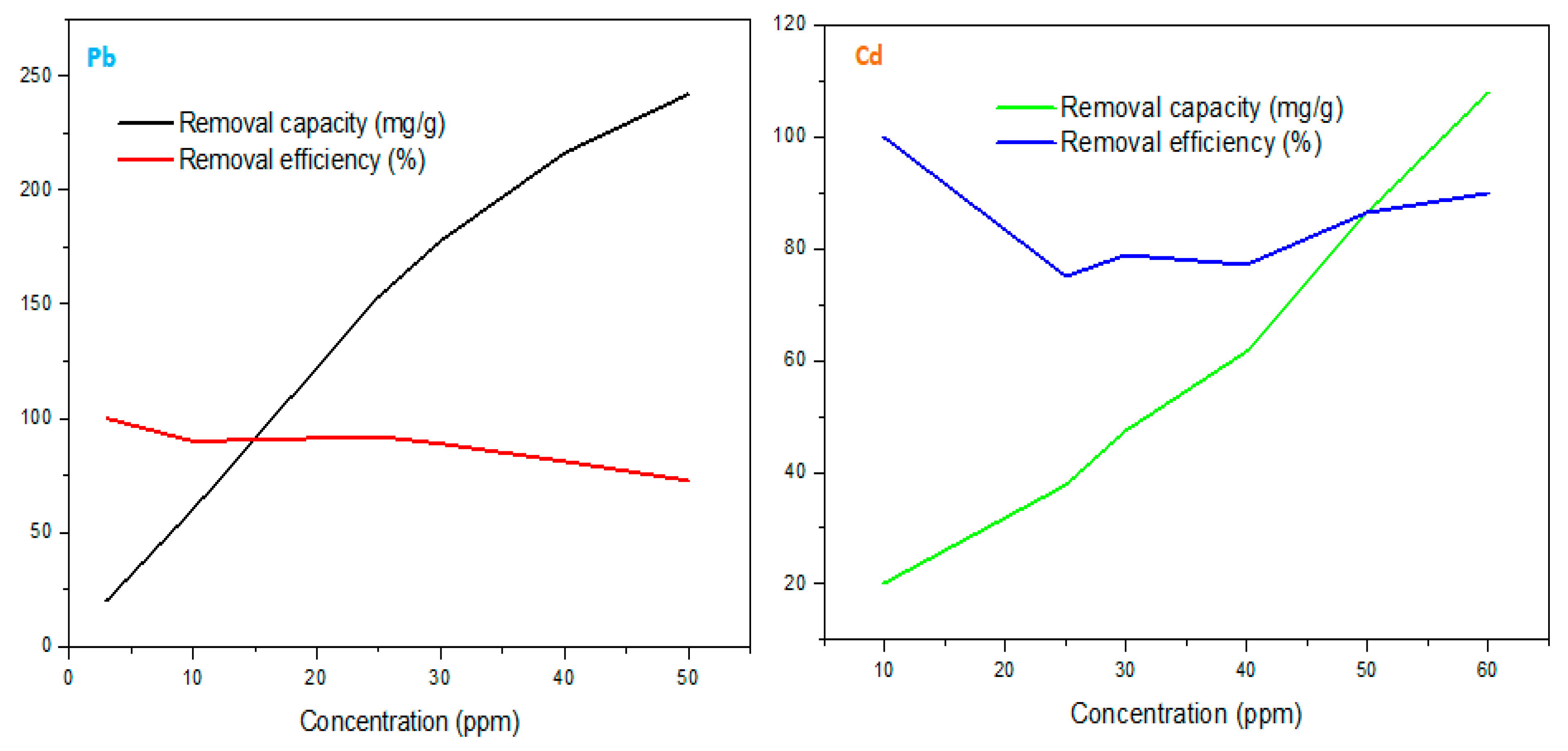
2+ (3 – 60 ppm). For Pb
2+ removal, the trend implied a proportional increment of both the initial concentration and corresponding removal capacity even at a maximum of 50 ppm with 242.013 mg/g. However, the removal efficiency moderately decreased with increased concentrations since the surface occupation was gradually reaching the maximum uptake level with the order: 50<40<30<25<10<3 ppm = 72.60<81.080<88.87<91.92<89.85<99.99 %.
During Cd
2+ removal, the adsorption capacity increased with an increase in concentration, implying that the adsorbent had active binding sites for Cd
2+. However, the removal efficiency decreased from 99.79 to 89.84 % possibly because of desorption and the unavailability of sufficient adsorption sites [
100].
Various treatment environment deals with different pollutants’ specific temperatures. Therefore, the temperature effect on adsorption performance towards incoming adsorbate species was investigated.
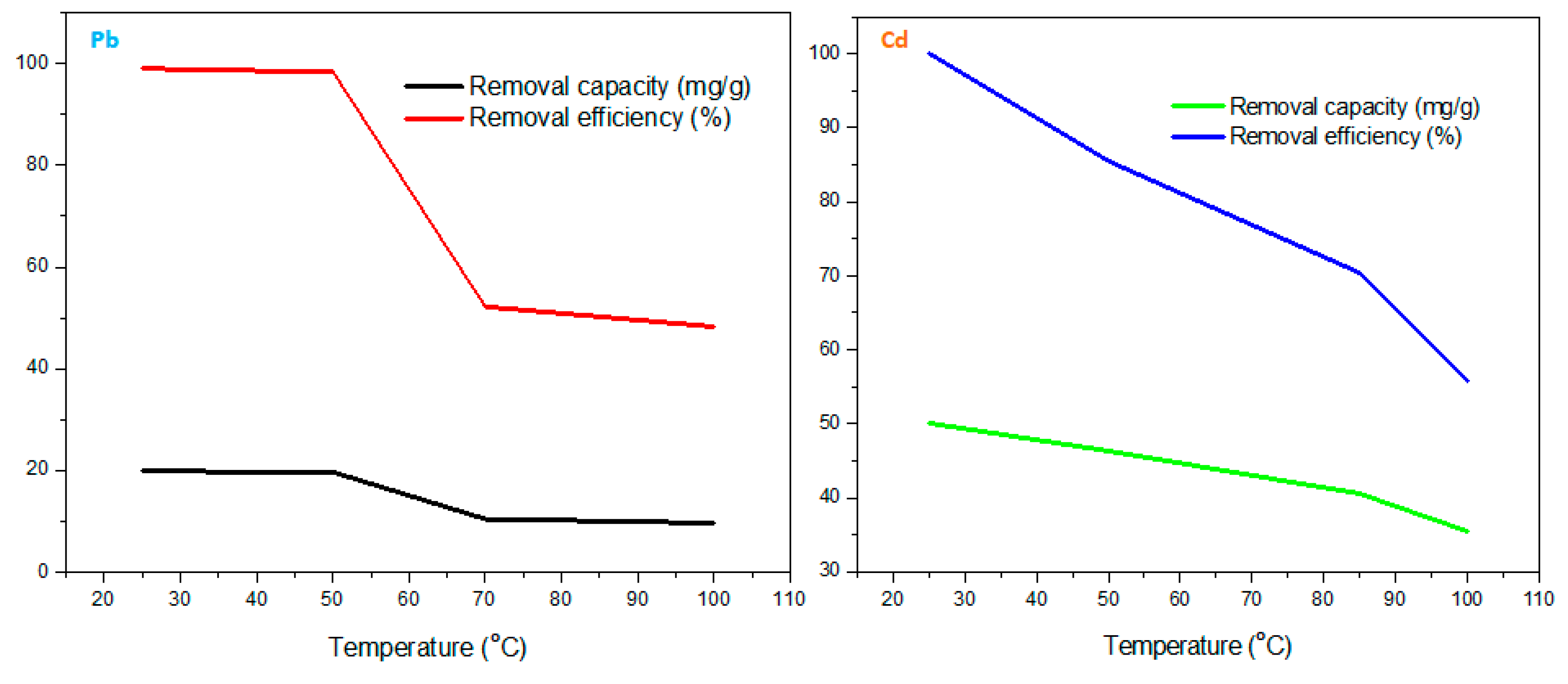
Figure 9 shows the temperature study on the adsorption of Pb
2+ and Cd
2+. An inverse proportional relationship between the temperature (25-100 °C) and adsorption performance was observed. Evidently, there were maximum capacity (19.82 and 49.93 mg/g) and efficiency (99.073 and 99.86 %) at 25 °C as compared to lowered levels until minimum capacity (9.66 and 35.60 mg/g) and efficiency (48.28 and 55.79 %) at 100 °C for Pb
2+ and Cd
2+. This observation could be due to an increased temperature (heat deposition) disturbing the surface adsorption mechanism between the adsorbent and adsorbate ions. Therefore, the optimum temperature was found to be 25 °C.
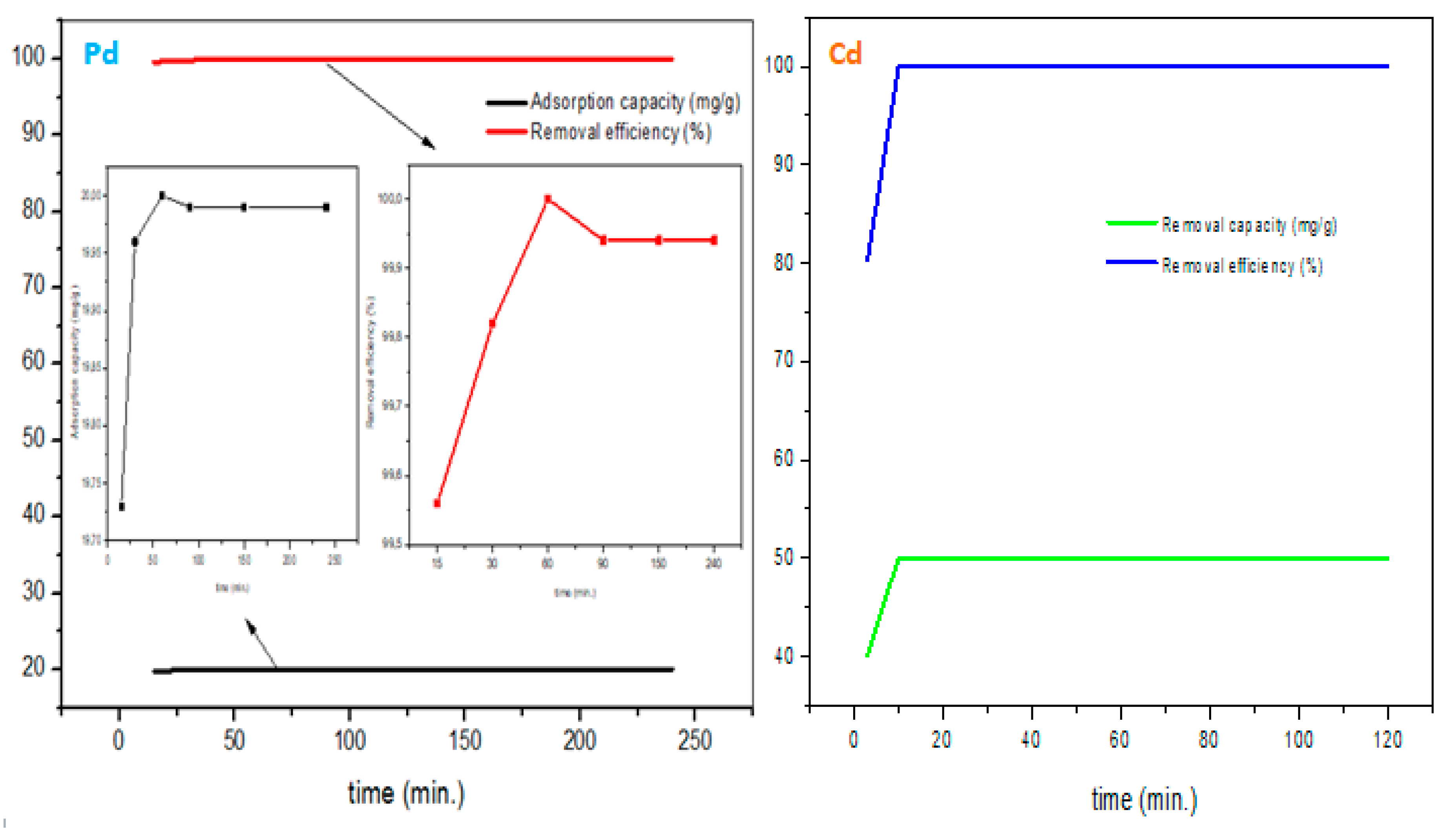
Additionally, since the adsorption is partitioned into two phases (rapid and equilibrium), the contact time study was conducted to determine the generation of each phase. As a result,
Figure 10 shows the investigated Pb
2+ and Cd
2+ removal from 0 – 240 minutes. Rapid adsorption was seen from 0-90 minutes (due to active sites abundance) and equilibrium plateau clocked at 90 – 240 minutes showing the maximum adsorption (19.99 mg/g; 99.96 %) for Pb
2+ uptake. On the other hand, there was a rapid Cd
2+ removal in a short period of 10 minutes and equilibrium was quickly reached too, thus fast kinetic rates characteristic.
The life cycle assessment of adsorbent is dependent on adsorbent synthesis, method costs, and secondary waste management to meet consistent desorption-adsorption trials [
101].
As a result,
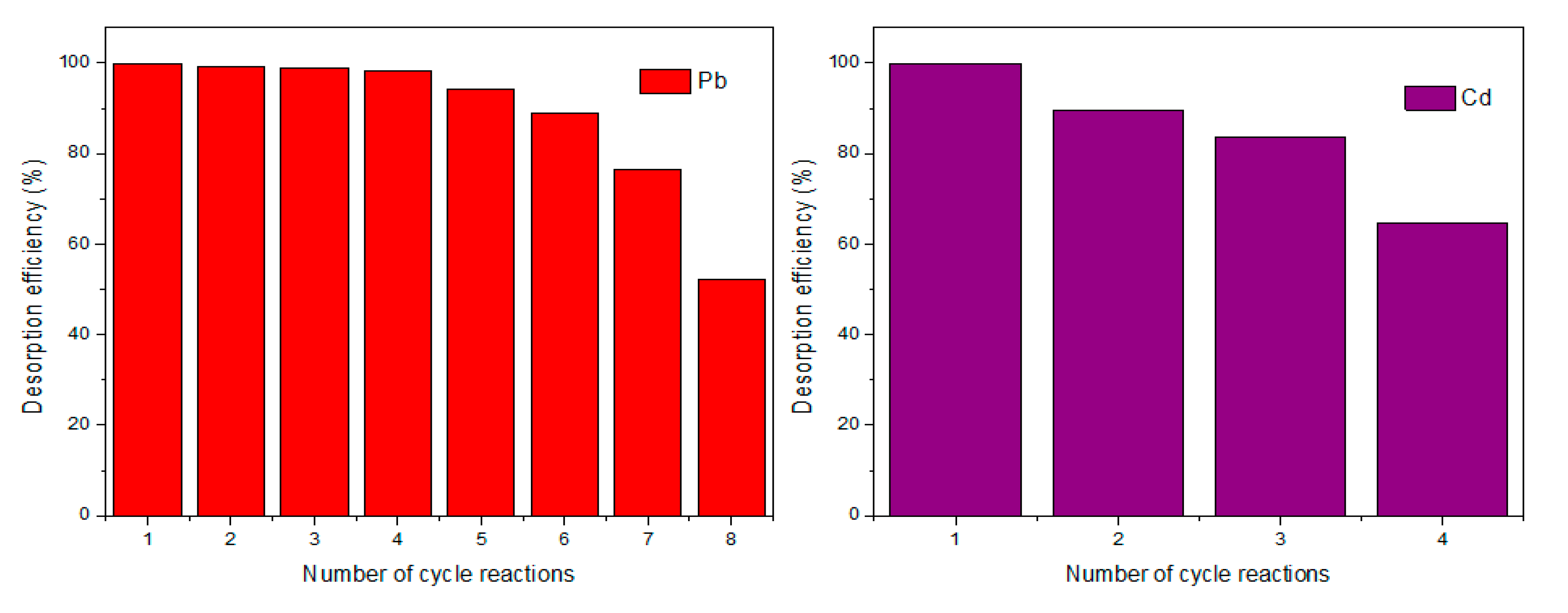
Figure 11 shows the uptake efficiency of the Pb
2+ and Cd
2+ in multiple cycles using rGO/ZnONPs. The use of H
2SO
4 solution allowed for successful desorption, recovery and reuse of rGO/ZnONPs and the material may reach half of its removal efficiency after 7 desorption cycles. The maximum efficiency achieved at cycle 1 was 99.96 % until the lowered efficiency of 52.21 % for Pb
2+.
In the case of Cd
2+ removal, the material supported 3 successive desorption efficiencies towards almost half the performance of the uptake. This could be attributed to the unavailability of sites, enhanced population of Cd
2+, lack of magnetism, low acid strength for desorption, and loss of structural material formation (surface area and porosity) [
102,
103].
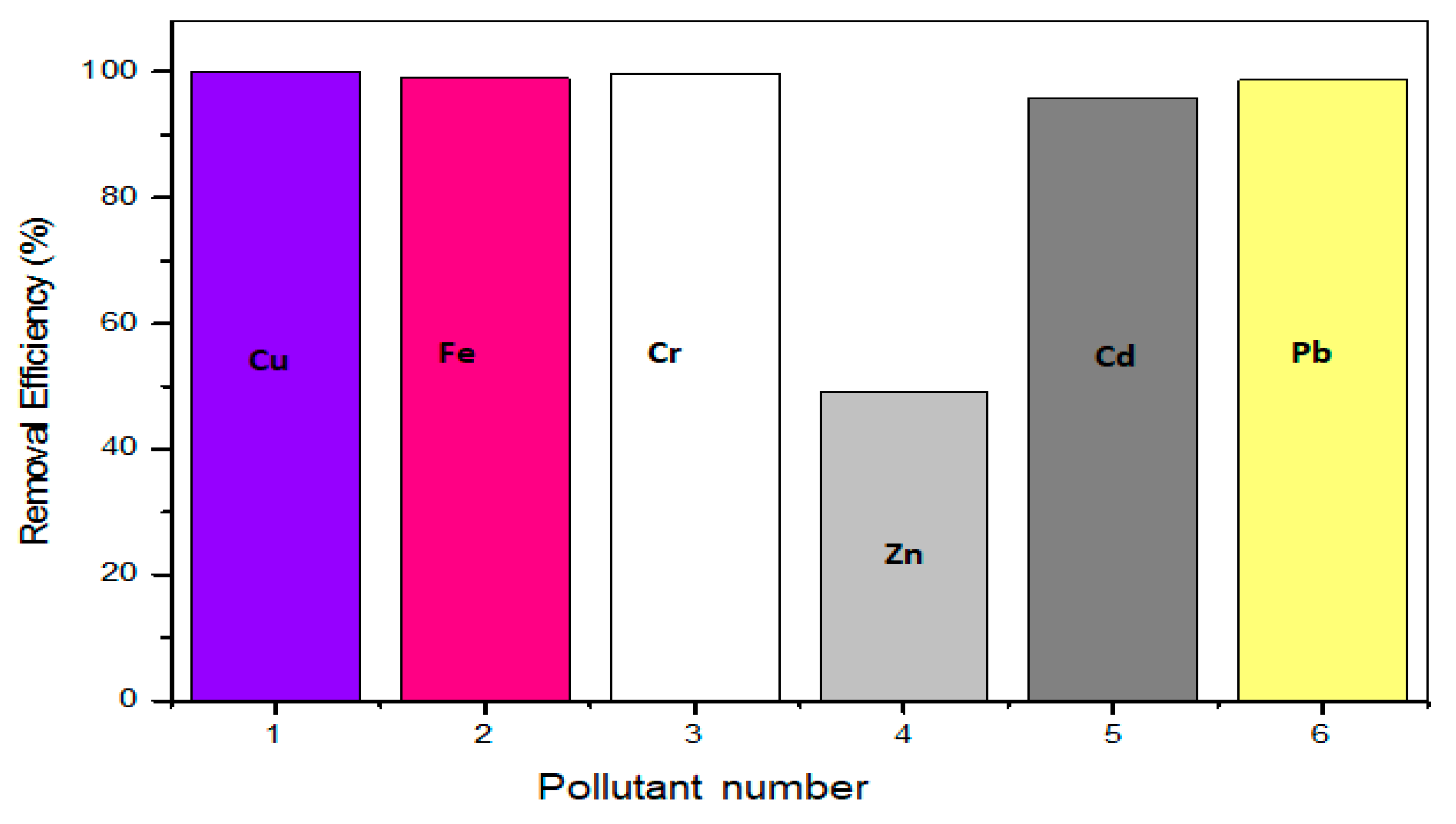
The observed removal efficiency trend was Cu > Cr > Fe > Pb > Cd > Zn. The rgo/ZnONPs nanocomposite showed an affinity towards Cu, Fe, Cr, Cd, and Pb, unlike Zn removal efficiency. This constitutes selectivity and specificity towards the five metal ions [
104]. The rgo/ZnONPs nanocomposite’s lowest adsorption performance towards Zn metal adsorption may be attributed to undesirable used contact time, concentration, temperature, agitation speed and pH, thus leading to poor transport to and within the adsorbent active surface sites or quick and spontaneous desorption after a possible physisorption phase [
105,
106].
4.2.2. Adsorption Mechanism
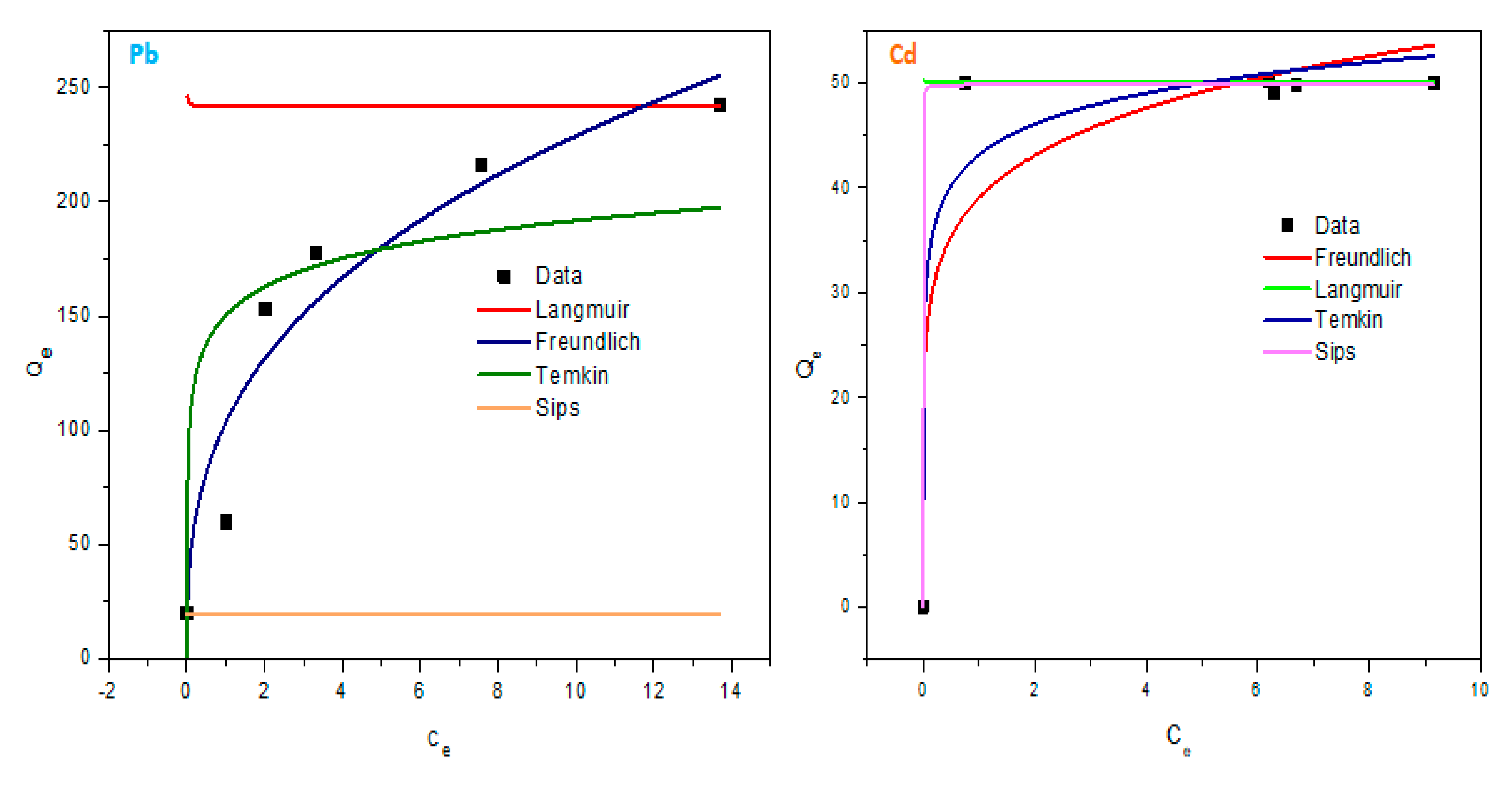
The adsorption experimental data was fitted using Langmuir, Freundlich, Temkin and Sips isotherm models, and the corresponding parameters are summarized in
Table 1. After an investigation of adsorption factors, the results showed a best fit by using the Freundlich model (R
2 ~ 1) with an unfavourable fit related to Sips, Langmuir, and Temkin isotherm models for Pb(II) adsorption. Freundlich model assumes multilayer adsorption, that is, the adsorbed layer is only multiple molecules in thickness, so adsorption can only occur at an infinite number of sites and the adsorption occurs at the heterogeneous adsorbent surface (physisorption characteristic). Multiple studies have been conducted with the use of graphene-zinc oxide composites for Pb (II) uptake, where Freundlich was dominant over the other isotherm models.
During the Cd(II) adsorption, it can be observed that the isotherm data was well fitted by the Sips adsorption model (R2 = 0.999) as compared to others. Since the model is a hybrid of Langmuir and Freundlich, this implies that the adsorption can fairly be described by Freundlich behaviour (multilayer adsorption with physical interaction) at low concentrations and be favourable to Langmuir (monolayer adsorption with chemical interaction) model at adsorbate high concentration.
Table 1.
Parameters of the Freundlich, Sips, Temkin, and Langmuir isotherms for Pb(II) and Cd(II) adsorption.
Table 1.
Parameters of the Freundlich, Sips, Temkin, and Langmuir isotherms for Pb(II) and Cd(II) adsorption.
| Pollutant |
Isotherm Model |
Parameter |
|
| Pb (II) |
Langmuir |
qmax |
242.0133 |
| KL
|
-4424.779 |
| R2
|
-1.319 |
| Freundlich |
n |
0.345 |
| Kf
|
0.479 |
| R2
|
0.915 |
| Temkin |
B |
18.00530 |
| At
|
427.121 |
| R2
|
0.698 |
| Sips |
qm
|
19.552 |
| K |
1.368 |
| n |
0.687 |
| R2
|
-2.447 |
| Cd (II) |
Langmuir |
qmax |
50.003 |
| KL
|
-2.510 |
| R2
|
0.350 |
| Freundlich |
n |
0.143 |
| Kf
|
39.0222 |
| R2
|
0.838 |
| Temkin |
B |
4.255 |
| At
|
20.357 |
| R2
|
0.952 |
| Sips |
qm
|
49.999 |
| K |
0.7 |
| n |
50.734 |
| R2
|
0.999 |
Figure 13.
Isotherm plot of Langmuir, Freundlich, Temkin and Sips for Pb(II) and Cd(II) adsorption.
Figure 13.
Isotherm plot of Langmuir, Freundlich, Temkin and Sips for Pb(II) and Cd(II) adsorption.
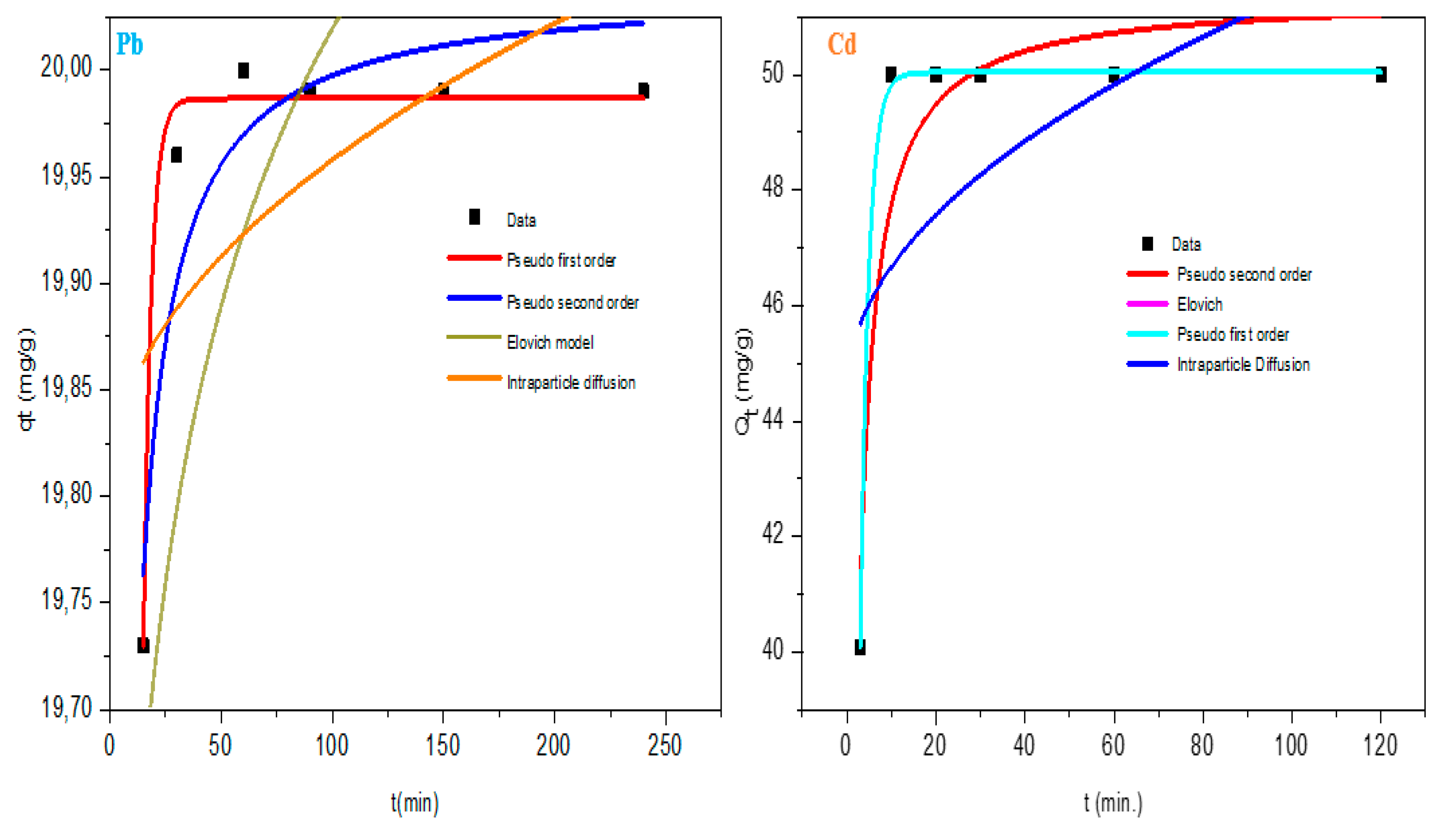
4.2.3. Kinetic Models
Figure 14 showed the best fit with the correlation coefficient of pseudo first order being favoured (R
2 = 0.99) and followed by descending order of elovich < intraparticle diffusion < pseudo second order. From the models, the adsorbate’s constant uptake rate and interaction with the contact time at the interface of solid and solution could be quantified [
107]. In terms of the pseudo-first order model, the assumption is that the adsorption site occupying rate is correlated to the number of unoccupied sites [
108]. The (R
2) of this model is ~ 1, it depicted that the adsorption had occurred through physisorption which is in correlation with the Freundlich isotherm of Pb(II) adsorption [
109]. In the case of Cd(II) adsorption, the kinetic data was favoured by pseudo first order with R
2 = 0.999 and b = 0.538 as compared to pseudo second order (R
2 = 0.905), elovich (R
2 = 0.322) and intraparticle diffusion (R
2 = 0.323). The alignment with pseudo first order constitutes a fast kinetic rate and affinitive surface for Cd(II). The lowest correlation coefficient related to Elovich also constitutes that chemisorption (partial characteristic of the Sips model) was involved in the metal uptake [
110].
5. Conclusion
This study has shown a possible optimized development route for rGO/ZnONPs nanocomposite. The method was achieved under room temperature, inert environment, and for 4 hours. It was observed that the synthesized nanocomposite has the potential to be treated as a multifunctional material (photoactive and adsorptive capability). Furthermore, pronounced peaks in FTIR and Raman spectra have shown the successful functionalization of reduced graphene oxide surface with minimal defects while fairly retaining the sp2 carbon of the support material. Afterward, the nanocomposite was tested for adsorption of Pb(II) and Cd(II) in wastewater. The observation showed that the optimized equilibrium contact times (10; 90 min.), pH (5; 5.5), temperature (25 °C), adsorbent dose (15; 50 mg), initial concentrations (3; 10 mg/l) were achieved for Pb(II) and Cd(II) uptake with corresponding adsorption capacity (19.99; 49.99 mg/g) and removal efficiency (~ 100 %) for Pb(II) and Cd (II) removal.
Furthermore, the adsorption behaviour was monitored by isotherm and kinetic study. Both studies revealed favouritism of pseudo first order with Pb and Cd isotherm related to the Freundlich and Sips adsorption model, respectively. As a result, the possible adsorption mechanisms are due to the presence of strong ionic bonding and electrostatic interactions involving dipole-dipole moment between the oxygen group of the supported ZnO nanoparticle and the adsorbate (Cd (II) and Pb (II)). Furthermore, the electronegativity of the nanocomposites proved to be accommodating co-existing metals (with efficiency > 90 %), thus can potentially act as multifunctional cation adsorbate remover. The rGO/ZnONPs nanocomposite also proved to support the desorption and reuse mode with considerable removal efficiencies, especially during Pb(II) adsorption (7 successive cycles towards half material efficiency) as compared to Cd (II) adsorption (3 successive cycles towards half material efficiency) and this could be improved by combatting leaching of the material by further functionalization with a capping agent. Conclusively, the functionalized rGO/ZnONPs nanocomposite could act as a potential material for contaminants (not necessarily toxic metals) removal in wastewater. Therefore, this nanocomposite presents the simple route of surface tuning, less energy consumption, is efficient, reusable, and possesses technical feasibility in affinitive adsorbent production for wastewater treatment.
6. Recommendations/Future Perspectives
Although there are multiple prospects for nanocomposites and nanomaterials, such as high adsorption capacity and efficiency, tunable surface for various functionalities, porosity, challenges like durability, harsh chemical use for pH adjustment, specificity, selectivity, real world practical application, scaling up, long term stability, regeneration, and reusability are supposed to be deeply researched. Additionally, thorough concentration should be channelled into understanding the effect of shape, particle size, surface pore diameter and volume, surface area, adsorbent type and material's stability on the adsorption thermodynamics and kinetics model, engineering efficient and sustainable adsorbents and various adsorption phases for removal of toxic metal ion in wastewater. Although treatment of real wastewater from industries may require large amounts of adsorbing material, the focus should be on up scaled production of adsorbents while maintaining low cost, low energy consumption, reproducibility, and stable compatibility in wastewater treatment plants.
References
- P. B. Tchounwou, C. G. Yedjou, A. K. Patlolla, and D. J. Sutton, “Heavy metal toxicity and the environment,” EXS, vol. 101. Springer, Basel, pp. 133–164, 2012. [CrossRef]
- D. Türkmen, M. Bakhshpour, S. Akgönüllü, S. Aşır, and A. Denizli, “Heavy Metal Ions Removal From Wastewater Using Cryogels: A Review,” Front. Sustain., vol. 3, p. 765592, Mar. 2022. [CrossRef]
- M. A. Assi, M. N. M. Hezmee, A. W. Haron, M. Y. M. Sabri, and M. A. Rajion, “The detrimental effects of lead on human and animal health,” Vet. World, vol. 9, no. 6, pp. 660–671, Jun. 2016. [CrossRef]
- S. Collin et al., “Bioaccumulation of lead (Pb) and its effects in plants: A review,” J. Hazard. Mater. Lett., vol. 3, p. 100064, Nov. 2022. [CrossRef]
- S. Ahmed, M. F. Uddin, M. S. Hossain, A. Jubair, M. N. Islam, and M. Rahman, “Heavy metals contamination in shrimp and crab from southwest regions in Bangladesh: Possible health risk assessment,” Toxicol. Reports, vol. 10, p. 580, Jan. 2023. [CrossRef]
- J. Huff, R. M. Lunn, M. P. Waalkes, L. Tomatis, and P. F. Infante, “Cadmium-induced cancers in animals and in humans,” Int. J. Occup. Environ. Health, vol. 13, no. 2, pp. 202–212, 2007. [CrossRef]
- F. U. Haider et al., “Cadmium toxicity in plants: Impacts and remediation strategies,” Ecotoxicol. Environ. Saf., vol. 211, Mar. 2021. [CrossRef]
- A. E. Burakov et al., “Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review,” Ecotoxicology and Environmental Safety, vol. 148. Academic Press, pp. 702–712, Feb. 01, 2018. [CrossRef]
- M. T. Bankole et al., “Selected Heavy Metals Removal From Electroplating Wastewater by Purified and Polyhydroxylbutyrate Functionalized Carbon Nanotubes Adsorbents,” Sci. Rep., vol. 9, no. 1, pp. 1–19, Dec. 2019. [CrossRef]
- N. S. Topare and V. S. Wadgaonkar, “A review on application of low-cost adsorbents for heavy metals removal from wastewater,” Mater. Today Proc., vol. 77, pp. 8–18, Jan. 2023. [CrossRef]
- A. Heidari, H. Younesi, Z. Mehraban, and H. Heikkinen, “Selective adsorption of Pb(II), Cd(II), and Ni(II) ions from aqueous solution using chitosan-MAA nanoparticles,” Int. J. Biol. Macromol., vol. 61, pp. 251–263, Oct. 2013. [CrossRef]
- H. A. Alalwan, M. A. Kadhom, and A. H. Alminshid, “Removal of heavy metals from wastewater using agricultural byproducts,” J. Water Supply Res. Technol. - AQUA, vol. 69, no. 2, pp. 99–112, Mar. 2020. [CrossRef]
- H. Zhang et al., “Efficient removal of heavy metal ions from wastewater and fixation of heavy metals in soil by manganese dioxide nanosorbents with tailored hollow mesoporous structure,” Chem. Eng. J., vol. 459, p. 141583, Mar. 2023. [CrossRef]
- M. Albqmi, Z. Frontistis, Z. Raji, A. Karim, A. Karam, and S. Khalloufi, “Adsorption of Heavy Metals: Mechanisms, Kinetics, and Applications of Various Adsorbents in Wastewater Remediation—A Review,” Waste , vol. 1, no. 3, pp. 775–805, Sep. 2023. [CrossRef]
- W. G. Kreyling, M. Semmler-Behnke, and Q. Chaudhry, “A complementary definition of nanomaterial,” Nano Today, vol. 5, no. 3, pp. 165–168, Jun. 2010. [CrossRef]
- I. Khan, K. Saeed, and I. Khan, “Nanoparticles: Properties, applications and toxicities,” Arabian Journal of Chemistry, vol. 12, no. 7. Elsevier B.V., pp. 908–931, Nov. 01, 2019. [CrossRef]
- M. N. Subramaniam, P. S. Goh, W. J. Lau, and A. F. Ismail, “The roles of nanomaterials in conventional and emerging technologies for heavy metal removal: A state-of-the-art review,” Nanomaterials, vol. 9, no. 4, Apr. 2019. [CrossRef]
- S. Babel and T. A. Kurniawan, “Low-cost adsorbents for heavy metals uptake from contaminated water: A review,” Journal of Hazardous Materials, vol. 97, no. 1–3. Elsevier, pp. 219–243, Feb. 28, 2003. [CrossRef]
- E. Pehlivan, S. Cetin, and B. H. Yanik, “Equilibrium studies for the sorption of zinc and copper from aqueous solutions using sugar beet pulp and fly ash,” J. Hazard. Mater., vol. 135, no. 1–3, pp. 193–199, Jul. 2006. [CrossRef]
- D. L. Guerra, C. Airoldi, and K. S. de Sousa, “Adsorption and thermodynamic studies of Cu(II) and Zn(II) on organofunctionalized-kaolinite,” Appl. Surf. Sci., vol. 254, no. 16, pp. 5157–5163, Jun. 2008. [CrossRef]
- M. Irannajad and H. Kamran Haghighi, “Removal of Heavy Metals from Polluted Solutions by Zeolitic Adsorbents: a Review,” Environmental Processes, vol. 8, no. 1. Springer Science and Business Media Deutschland GmbH, pp. 7–35, Mar. 01, 2021. [CrossRef]
- B. G. Fouda-Mbanga, E. Prabakaran, and K. Pillay, “Carbohydrate biopolymers, lignin based adsorbents for removal of heavy metals (Cd2+, Pb2+, Zn2+) from wastewater, regeneration and reuse for spent adsorbents including latent fingerprint detection: A review,” Biotechnol. Reports, vol. 30, p. e00609, Jun. 2021. [CrossRef]
- A. T. Smith, A. M. LaChance, S. Zeng, B. Liu, and L. Sun, “Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites,” Nano Mater. Sci., vol. 1, no. 1, pp. 31–47, Mar. 2019. [CrossRef]
- W. A. Khoso, N. Haleem, M. A. Baig, and Y. Jamal, “Synthesis, characterization and heavy metal removal efficiency of nickel ferrite nanoparticles (NFN’s),” Sci. Reports , vol. 11, no. 1, pp. 1–10, Feb. 2021. [CrossRef]
- S. Panahi et al., “A study on effective adsorption of lead from an aqueous solution using Copper Oxide nanoparticles,” IOP Conf. Ser. Mater. Sci. Eng., vol. 1058, no. 1, p. 012074, Feb. 2021. [CrossRef]
- M. L. Del Prado-Audelo, I. García Kerdan, L. Escutia-Guadarrama, J. M. Reyna-González, J. J. Magaña, and G. Leyva-Gómez, “Nanoremediation: Nanomaterials and Nanotechnologies for Environmental Cleanup,” Front. Environ. Sci., vol. 9, p. 793765, Dec. 2021. [CrossRef]
- S. W. Bian, I. A. Mudunkotuwa, T. Rupasinghe, and V. H. Grassian, “Aggregation and dissolution of 4 nm ZnO nanoparticles in aqueous environments: Influence of pH, ionic strength, size, and adsorption of humic acid,” Langmuir, vol. 27, no. 10, pp. 6059–6068, Jun. 2011. [CrossRef]
- H. T. Phan and A. J. Haes, “What Does Nanoparticle Stability Mean?,” J. Phys. Chem. C, vol. 123, no. 27, pp. 16495–16507, Jul. 2019. [CrossRef]
- T. Velempini, M. E. H. Ahamed, and K. Pillay, “Heavy-metal spent adsorbents reuse in catalytic, energy and forensic applications- a new approach in reducing secondary pollution associated with adsorption,” Results Chem., vol. 5, p. 100901, Jan. 2023. [CrossRef]
- J. Kim, K. Lee, B. K. Seo, and J. H. Hyun, “Effective removal of radioactive cesium from contaminated water by synthesized composite adsorbent and its thermal treatment for enhanced storage stability,” Environ. Res., vol. 191, p. 110099, Dec. 2020. [CrossRef]
- A. R. Contreras, E. Casals, V. Puntes, D. Komilis, A. Sánchez, and X. Font, “Use of cerium oxide (CeO2) nanoparticles for the adsorption of dissolved cadmium (II), lead (II) and chromium (VI) at two different pHs in single and multi-component systems.,” Glob. Nest J., vol. 17, no. 3, pp. 536–543, 2015.
- P. N. Dave and L. V. Chopda, “Application of iron oxide nanomaterials for the removal of heavy metals,” J. Nanotechnol., vol. 2014, 2014. [CrossRef]
- V. Dhiman and N. Kondal, “ZnO Nanoadsorbents: A potent material for removal of heavy metal ions from wastewater,” Colloids and Interface Science Communications, vol. 41. Elsevier B.V., p. 100380, Mar. 01, 2021. [CrossRef]
- Sosun, A. Ali, A. Mannan, U. Ali Shah, and M. Zia, “Removal of of toxic metal ions (Ni2+ and Cd2+) from wastewater by using TOPO decorated iron oxide nanoparticles,” Appl. Water Sci., vol. 12, no. 5, pp. 1–15, May 2022. [CrossRef]
- B. A. Al-Mur, “Green Zinc Oxide (ZnO) Nanoparticle Synthesis Using Mangrove Leaf Extract from Avicenna marina: Properties and Application for the Removal of Toxic Metal Ions (Cd2+ and Pb2+),” Water, vol. 15, no. 3, p. 455, Jan. 2023. [CrossRef]
- E. Y. Shaba, J. O. Jacob, J. O. Tijani, and M. A. T. Suleiman, “A critical review of synthesis parameters affecting the properties of zinc oxide nanoparticle and its application in wastewater treatment,” Appl. Water Sci. 2021 112, vol. 11, no. 2, pp. 1–41, Feb. 2021. [CrossRef]
- A. Thi Le, S.-Y. Pung, S. Sreekantan, A. Matsuda, D. Phu Huynh, and D. Phu Huynh Mechanisms, “Mechanisms of removal of heavy metal ions by ZnO particles,” Heliyon, vol. 5, p. 1440, 2019. [CrossRef]
- D. Zhang, X. L. Ma, Y. Gu, H. Huang, and G. W. Zhang, “Green Synthesis of Metallic Nanoparticles and Their Potential Applications to Treat Cancer,” Front. Chem., vol. 8, p. 799, Oct. 2020. [CrossRef]
- E. F. C. Chaüque, J. N. Zvimba, J. C. Ngila, and N. Musee, “Fate, behaviour, and implications of ZnO nanoparticles in a simulated wastewater treatment plant,” Water SA, vol. 42, no. 1, pp. 72–81, Jan. 2016. [CrossRef]
- A. U. H. Khan, Y. Liu, R. Naidu, C. Fang, R. Dharmarajan, and H. Shon, “Interactions between zinc oxide nanoparticles and hexabromocyclododecane in simulated waters,” Environ. Technol. Innov., vol. 24, p. 102078, Nov. 2021. [CrossRef]
- R. Khan, M. A. Inam, S. Z. Zam, D. R. Park, and I. T. Yeom, “Assessment of Key Environmental Factors Influencing the Sedimentation and Aggregation Behavior of Zinc Oxide Nanoparticles in Aquatic Environment,” Water , vol. 10, no. 5, p. 660, May 2018. [CrossRef]
- M. Sang, J. Shin, K. Kim, and K. J. Yu, “Electronic and Thermal Properties of Graphene and Recent Advances in Graphene Based Electronics Applications,” Nanomater. , vol. 9, no. 3, p. 374, Mar. 2019. [CrossRef]
- F. Akbar, M. Kolahdouz, S. Larimian, B. Radfar, and H. H. Radamson, “Graphene synthesis, characterization and its applications in nanophotonics, nanoelectronics, and nanosensing,” J. Mater. Sci. Mater. Electron., vol. 26, no. 7, pp. 4347–4379, Jul. 2015. [CrossRef]
- R. Vidu et al., “Removal of Heavy Metals from Wastewaters: A Challenge from Current Treatment Methods to Nanotechnology Applications,” Toxics, vol. 8, no. 4, pp. 1–37, Dec. 2020. [CrossRef]
- Q. Kong et al., “Strategies to improve the adsorption properties of graphene-based adsorbent towards heavy metal ions and their compound pollutants: A review,” J. Hazard. Mater., vol. 415, p. 125690, Aug. 2021. [CrossRef]
- C. Kavitha, “A review on reduced Graphene oxide hybrid nano composites and their prominent applications,” Mater. Today Proc., vol. 49, pp. 811–816, Jan. 2022. [CrossRef]
- V. V. Khedekar, S. Mohammed Zaeem, and S. Das, “Graphene-metal oxide nanocomposites for supercapacitors: A perspective review,” Adv. Mater. Lett., vol. 9, no. 1, pp. 2–19, Jan. 2018. [CrossRef]
- A. D. Sontakke, S. Tiwari, and M. K. Purkait, “A comprehensive review on graphene oxide-based nanocarriers: Synthesis, functionalization and biomedical applications,” FlatChem, vol. 38, p. 100484, Mar. 2023. [CrossRef]
- R. A. Dar et al., “Performance of graphene-zinc oxide nanocomposite coated-glassy carbon electrode in the sensitive determination of para-nitrophenol,” Sci. Reports , vol. 12, no. 1, pp. 1–14, Jan. 2022. [CrossRef]
- M. Saranya, R. Ramachandran, and F. Wang, “Graphene-zinc oxide (G-ZnO) nanocomposite for electrochemical supercapacitor applications,” J. Sci. Adv. Mater. Devices, vol. 1, no. 4, pp. 454–460, Dec. 2016. [CrossRef]
- B. S. Dash, G. Jose, Y. J. Lu, and J. P. Chen, “Functionalized Reduced Graphene Oxide as a Versatile Tool for Cancer Therapy,” Int. J. Mol. Sci., vol. 22, no. 6, pp. 1–24, Mar. 2021. [CrossRef]
- H. Afzal et al., “Enhanced drug efficiency of doped ZnO–GO (graphene oxide) nanocomposites, a new gateway in drug delivery systems (DDSs),” Mater. Res. Express, vol. 7, no. 1, p. 015405, Jan. 2020. [CrossRef]
- E. Nagaraj, P. Shanmugam, K. Karuppannan, T. Chinnasamy, and S. Venugopal, “The biosynthesis of a graphene oxide-based zinc oxide nanocomposite using Dalbergia latifolia leaf extract and its biological applications,” New J. Chem., vol. 44, no. 5, pp. 2166–2179, Feb. 2020. [CrossRef]
- L. Toporovska et al., “Zinc oxide: reduced graphene oxide nanocomposite film for heterogeneous photocatalysis,” Opt. Quantum Electron., vol. 52, no. 1, pp. 1–12, Jan. 2020. [CrossRef]
- L. Zhang, N. Li, H. Jiu, G. Qi, and Y. Huang, “ZnO-reduced graphene oxide nanocomposites as efficient photocatalysts for photocatalytic reduction of CO2,” Ceram. Int., vol. 41, no. 5, pp. 6256–6262, Jun. 2015. [CrossRef]
- M. Ensafi Avval, P. N. Moghadam, and M. M. Baradarani, “Synthesis of a new nanocomposite based-on graphene-oxide for selective removal of Pb2+ ions from aqueous solutions,” Polym. Compos., vol. 40, no. 2, pp. 730–737, Feb. 2019. [CrossRef]
- S. K. Sahoo and G. Hota, “Functionalization of graphene oxide with metal oxide nanomaterials: synthesis and applications for the removal of inorganic, toxic, environmental pollutants from water,” in Handbook of Functionalized Nanomaterials for Industrial Applications, Elsevier, 2020, pp. 299–326.
- K. S. Ranjith, P. Manivel, R. T. Rajendrakumar, and T. Uyar, “Multifunctional ZnO nanorod-reduced graphene oxide hybrids nanocomposites for effective water remediation: Effective sunlight driven degradation of organic dyes and rapid heavy metal adsorption,” Chem. Eng. J., vol. 325, pp. 588–600, Oct. 2017. [CrossRef]
- I. Ali, C. Peng, I. Naz, and M. A. Amjed, “Water Purification Using Magnetic Nanomaterials: An Overview,” in Nanotechnology in the Life Sciences, Springer, Cham, 2019, pp. 161–179.
- A. I. Abd-Elhamid, E. M. A. Elgoud, and H. F. Aly, “Alginate modified graphene oxide for rapid and effective sorption of some heavy metal ions from an aqueous solution,” Cellulose, vol. 29, no. 11, pp. 6231–6245, Jul. 2022. [CrossRef]
- P. Bharadwaj, G. R. Kiran, and S. G. Acharyya, “Remarkable performance of GO/ZnO nanocomposites under optimized parameters for remediation of Cd (II) from water,” Appl. Surf. Sci., vol. 626, p. 157238, Jul. 2023. [CrossRef]
- N. C. Joshi and P. Gururani, “Advances of graphene oxide based nanocomposite materials in the treatment of wastewater containing heavy metal ions and dyes,” Curr. Res. Green Sustain. Chem., vol. 5, p. 100306, Jan. 2022. [CrossRef]
- A. Maqbool et al., “Development of ZnO-GO-NiO membrane for removal of lead and cadmium heavy metal ions from wastewater,” Chemosphere, vol. 338, p. 139622, Oct. 2023. [CrossRef]
- Y. El Maguana, N. Elhadiri, M. Benchanaa, and R. Chikri, “Activated Carbon for Dyes Removal: Modeling and Understanding the Adsorption Process,” J. Chem., vol. 2020, p. 1, 2020. [CrossRef]
- R. Ramadan and S. K. Abdel-Aal, “Facile synthesis of nanostructured ZnO–rGO based graphene and its application in wastewater treatment,” J. Mater. Sci. Mater. Electron., vol. 32, no. 14, pp. 19667–19675, Jul. 2021. [CrossRef]
- A. Ghozatloo and A. Enayatollahi, “Enhancing the effect of zinc oxide on the absorption of heavy metals from wastewater by using silica in graphene bed,” Anal. Methods Environ. Chem. J., vol. 2, no. 04, pp. 27–38, Dec. 2019. [CrossRef]
- Nik-Abdul-Ghani, Jami, and Alam, “The role of nanoadsorbents and nanocomposite adsorbents in the removal of heavy metals from wastewater: A review and prospect,” Pollution, vol. 7, no. 1, pp. 153–179, 2021. [CrossRef]
- N. C. Joshi et al., “Sustainable synthetic approach and applications of ZnO/r-GO in the adsorption of toxic Pb2+ and Cr6+ ions,” Inorg. Chem. Commun., vol. 145, p. 110040, Nov. 2022. [CrossRef]
- T. Naseem et al., “Reduced Graphene Oxide/Zinc Oxide Nanocomposite: From Synthesis to its Application for Wastewater Purification and Antibacterial Activity,” J. Inorg. Organomet. Polym. Mater., vol. 30, no. 10, pp. 3907–3919, Oct. 2020. [CrossRef]
- X. Zhao, B. Hu, J. Ye, and Q. Jia, “Preparation, Characterization, and Application of Graphene–Zinc Oxide Composites (G–ZnO) for the Adsorption of Cu(II), Pb(II), and Cr(III),” J. Chem. Eng. Data, vol. 58, no. 9, pp. 2395–2401, Sep. 2013. [CrossRef]
- M. A. Ahmed, M. A. Ahmed, and A. A. Mohamed, “Facile adsorptive removal of dyes and heavy metals from wastewaters using magnetic nanocomposite of zinc ferrite@reduced graphene oxide,” Inorg. Chem. Commun., vol. 144, p. 109912, Oct. 2022. [CrossRef]
- O. Hosseinkhani, A. Hamzehlouy, S. Dan, N. Sanchouli, M. Tavakkoli, and H. Hashemipour, “Graphene oxide/ZnO nanocomposites for efficient removal of heavy metal and organic contaminants from water,” Arab. J. Chem., vol. 16, no. 10, p. 105176, Oct. 2023. [CrossRef]
- T. Guo et al., “Efficient removal of aqueous Pb(II) using partially reduced graphene oxide-Fe3O4,” Adsorpt. Sci. Technol., vol. 36, no. 3–4, pp. 1031–1048, May 2018. [CrossRef]
- V. Kumari, S. Kaushal, and P. P. Singh, “Green synthesis of a CuO/rGO nanocomposite using a Terminalia arjuna bark extract and its catalytic activity for the purification of water,” Mater. Adv., vol. 3, no. 4, pp. 2170–2184, Feb. 2022. [CrossRef]
- K. M. Al-Qahtani, M. H. H. Ali, and A. G. Al-Afify, “Synthesis and use of TiO2@rGo nanocomposites in photocatalytic removal of chromium and lead ions from wastewater,” J. Elem., vol. 25, no. 1, pp. 315–322, 2020. [CrossRef]
- N. C. Joshi, B. S. Rawat, P. Semwal, and N. Kumar, “Effective removal of highly toxic Pb2+ and Cd2+ ions using reduced graphene oxide, polythiophene, and silica-based nanocomposite,” J. Dispers. Sci. Technol., vol. 2022, pp. 1–2, 2022. [CrossRef]
- F. Mashkoor et al., “Synergistic effects of tungstate trioxide hemihydrate decorated reduced graphene oxide for the adsorption of heavy metals and dyes and postliminary application in supercapacitor device,” J. Clean. Prod., vol. 418, p. 138067, Sep. 2023. [CrossRef]
- M. Eftekhari, M. Akrami, M. Gheibi, H. Azizi-Toupkanloo, A. M. Fathollahi-Fard, and G. Tian, “Cadmium and copper heavy metal treatment from water resources by high-performance folic acid-graphene oxide nanocomposite adsorbent and evaluation of adsorptive mechanism using computational intelligence, isotherm, kinetic, and thermodynamic analyses,” Environ. Sci. Pollut. Res., vol. 27, no. 35, pp. 43999–44021, Dec. 2020. [CrossRef]
- D. Nandi, T. Basu, S. Debnath, A. K. Ghosh, A. De, and U. C. Ghosh, “Mechanistic insight for the sorption of Cd(II) and Cu(II) from aqueous solution on magnetic mn-doped Fe(III) oxide nanoparticle implanted graphene,” J. Chem. Eng. Data, vol. 58, no. 10, pp. 2809–2818, Oct. 2013. [CrossRef]
- K. Davis, R. Yarbrough, M. Froeschle, J. White, and H. Rathnayake, “Band gap engineered zinc oxide nanostructures via a sol–gel synthesis of solvent driven shape-controlled crystal growth,” RSC Adv., vol. 9, no. 26, pp. 14638–14648, May 2019. [CrossRef]
- C. Zhang, Q. Tu, L. F. Francis, and U. R. Kortshagen, “Band Gap Tuning of Films of Undoped ZnO Nanocrystals by Removal of Surface Groups,” Nanomater. , vol. 12, no. 3, p. 565, Feb. 2022. [CrossRef]
- M. K. Rabchinskii et al., “Nanoscale perforation of graphene oxide during photoreduction process in the argon atmosphere,” J. Phys. Chem. C, vol. 120, no. 49, pp. 28261–28269, Dec. 2016. [CrossRef]
- I. O. Faniyi et al., “The comparative analyses of reduced graphene oxide (RGO) prepared via green, mild and chemical approaches,” SN Appl. Sci., vol. 1, no. 10, pp. 1–7, Oct. 2019. [CrossRef]
- S. Alamdari et al., “Preparation and Characterization of Zinc Oxide Nanoparticles Using Leaf Extract of Sambucus ebulus,” Appl. Sci. , vol. 10, no. 10, p. 3620, May 2020. [CrossRef]
- S. Wilson Balogun et al., “Green synthesis and characterization of zinc oxide nanoparticles using bashful (Mimosa pudica), leaf extract: a precursor for organic electronics applications,” 123AD. [CrossRef]
- P. Ramesh, K. Saravanan, P. Manogar, J. Johnson, E. Vinoth, and M. Mayakannan, “Green synthesis and characterization of biocompatible zinc oxide nanoparticles and evaluation of its antibacterial potential,” Sens. Bio-Sensing Res., vol. 31, p. 100399, Feb. 2021. [CrossRef]
- A. S. Yasin et al., “Design of zinc oxide nanoparticles and graphene hydrogel co-incorporated activated carbon for efficient capacitive deionization,” Sep. Purif. Technol., vol. 277, p. 119428, Dec. 2021. [CrossRef]
- B. Bhuyan, B. Paul, D. D. Purkayastha, S. S. Dhar, and S. Behera, “Facile synthesis and characterization of zinc oxide nanoparticles and studies of their catalytic activity towards ultrasound-assisted degradation of metronidazole,” Mater. Lett., vol. 168, pp. 158–162, Apr. 2016. [CrossRef]
- T. Chitradevi, A. Jestin Lenus, and N. Victor Jaya, “Structure, morphology and luminescence properties of sol-gel method synthesized pure and Ag-doped ZnO nanoparticles,” Mater. Res. Express, vol. 7, no. 1, p. 015011, Dec. 2019. [CrossRef]
- Q. A. Drmosh et al., “A novel approach to fabricating a ternary rGO/ZnO/Pt system for high-performance hydrogen sensor at low operating temperatures,” Appl. Surf. Sci., vol. 464, pp. 616–626, Jan. 2019. [CrossRef]
- C. Rodwihok, D. Wongratanaphisan, Y. L. T. Ngo, M. Khandelwal, S. H. Hur, and J. S. Chung, “Effect of GO Additive in ZnO/rGO Nanocomposites with Enhanced Photosensitivity and Photocatalytic Activity,” Nanomater. , vol. 9, no. 10, p. 1441, Oct. 2019. [CrossRef]
- V. Sharma, Y. Jain, M. Kumari, R. Gupta, S. K. Sharma, and K. Sachdev, “Synthesis and Characterization of Graphene Oxide (GO) and Reduced Graphene Oxide (rGO) for Gas Sensing Application,” Macromol. Symp., vol. 376, no. 1, p. 1700006, Dec. 2017. [CrossRef]
- G. Surekha, K. V. Krishnaiah, N. Ravi, and R. Padma Suvarna, “FTIR, Raman and XRD analysis of graphene oxide films prepared by modified Hummers method,” J. Phys. Conf. Ser., vol. 1495, no. 1, p. 012012, Mar. 2020. [CrossRef]
- V. I. Korepanov, S. Y. Chan, H. C. Hsu, and H. o. Hamaguchi, “Phonon confinement and size effect in Raman spectra of ZnO nanoparticles,” Heliyon, vol. 5, no. 2, p. e01222, Feb. 2019. [CrossRef]
- S. Thakur and S. K. Mandal, “Precursor-and Time-Dependent Morphological Evolution of ZnO Nanostructures for Comparative Photocatalytic Activity and Adsorption Dynamics with Methylene Blue Dye,” ACS Omega, vol. 5, no. 27, pp. 16670–16680, Jul. 2020. [CrossRef]
- T. Xu, L. Zhang, H. Cheng, and Y. Zhu, “Significantly enhanced photocatalytic performance of ZnO via graphene hybridization and the mechanism study,” Appl. Catal. B Environ., vol. 101, no. 3–4, pp. 382–387, Jan. 2011. [CrossRef]
- C. Cobianu et al., “Sonochemically synthetized ZnO-graphene nanohybrids and its characterization,” Rev. Adv. Mater. Sci., vol. 59, no. 1, pp. 176–187, Jan. 2020. [CrossRef]
- P. A. Nikolaychuk, “ The revised potential – pH diagram for Pb – H 2 O system ,” Ovidius Univ. Ann. Chem., vol. 29, no. 2, pp. 55–67, Jul. 2018. [CrossRef]
- A. A. Alghamdi, A. B. Al-Odayni, W. S. Saeed, A. Al-Kahtani, F. A. Alharthi, and T. Aouak, “Efficient Adsorption of Lead (II) from Aqueous Phase Solutions Using Polypyrrole-Based Activated Carbon,” Mater. , vol. 12, no. 12, p. 2020, Jun. 2019. [CrossRef]
- M. Z. A. Zaimee, M. S. Sarjadi, and M. L. Rahman, “Heavy Metals Removal from Water by Efficient Adsorbents,” Water, vol. 13, no. 19, p. 2659, Sep. 2021. [CrossRef]
- K. G. Akpomie et al., “Adsorption mechanism and modeling of radionuclides and heavy metals onto ZnO nanoparticles: a review,” Appl. Water Sci. , vol. 13, no. 1, pp. 1–24, Nov. 2022. [CrossRef]
- S. Moosavi, C. W. Lai, S. Gan, G. Zamiri, O. Akbarzadeh Pivehzhani, and M. R. Johan, “Application of efficient magnetic particles and activated carbon for dye removal from wastewater,” ACS Omega, vol. 5, no. 33, pp. 20684–20697, Aug. 2020. [CrossRef]
- S. M. Prabhu et al., “Magnetic nanostructured adsorbents for water treatment: Structure-property relationships, chemistry of interactions, and lab-to-industry integration,” Chem. Eng. J., vol. 468, p. 143474, 2023. [CrossRef]
- W. Liao et al., “Simultaneous removal of cadmium, lead, chromate by biochar modified with layered double hydroxide with sulfide intercalation,” Bioresour. Technol., vol. 360, p. 127630, Sep. 2022. [CrossRef]
- A. Gupta et al., “A Review of Adsorbents for Heavy Metal Decontamination: Growing Approach to Wastewater Treatment,” Materials (Basel)., vol. 14, no. 16, p. 4702, Aug. 2021. [CrossRef]
- M. Verma, I. Lee, Y. Hong, V. Kumar, and H. Kim, “Multifunctional β-Cyclodextrin-EDTA-Chitosan polymer adsorbent synthesis for simultaneous removal of heavy metals and organic dyes from wastewater,” Environ. Pollut., vol. 292, p. 118447, Jan. 2022. [CrossRef]
- S. Z. N. Ahmad et al., “Pb(II) removal and its adsorption from aqueous solution using zinc oxide/graphene oxide composite,” Chem. Eng. Commun., vol. 208, no. 5, pp. 646–660, 2020. [CrossRef]
- H. Huang, Y. Wang, Y. Zhang, Z. Niu, and X. Li, “Amino-functionalized graphene oxide for Cr(VI), Cu(II), Pb(II) and Cd(II) removal from industrial wastewater,” Open Chem., vol. 18, no. 1, pp. 97–107, Jan. 2020. [CrossRef]
- S. Z. N. Ahmad et al., “Pb(II) removal and its adsorption from aqueous solution using zinc oxide/graphene oxide composite,” Chem. Eng. Commun., vol. 208, no. 5, pp. 646–660, 2021. [CrossRef]
- F. Cherono, N. Mburu, and B. Kakoi, “Adsorption of lead, copper and zinc in a multi-metal aqueous solution by waste rubber tires for the design of single batch adsorber,” Heliyon, vol. 7, no. 11, p. e08254, Nov. 2021. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).