1. Introduction
Sacubitril/valsartan, a first-in-class neprilysin inhibitor plus angiotensin receptor antagonist combination (ARNI), was approved in 2015 for the treatment of heart failure with reduced ejection fraction (HFrEF) [
1]. Sacubitril, a neprilysin inhibitor, increases natriuretic peptide (NP) levels, which has a protective effect on the heart [
2]. Valsartan, an angiotensin type 1 receptor blocker (ARB), prevents the long-term adverse effects of renin-angiotensin-aldosterone system (RAAS) activation on cardiac function [
3]. Treatment with sacubitril/valsartan has been shown to be superior to enalapril in reducing heart failure-related hospitalization and cardiovascular and all-cause mortality in patients with HFrEF [
4]. A meta-analysis showed that sacubitril/valsartan reversed cardiac remodeling in patients with HFrEF compared with ARBs or angiotensin-converting enzyme inhibitors (ACEIs) alone [
5]. There are ongoing clinical studies investigating the efficacy, safety, and tolerability of the sacubitril/valsartan combination in heart failure and other diseases including diabetes [
6].
Diabetes is a chronic, endocrine disease that affects hundreds of millions of people worldwide. People with diabetes are 2-3 times more likely to develop cardiovascular disease than non-diabetics [
7]. Cardiovascular complications include a major risk factor for mortality in individuals with both type 1 and type 2 diabetes [
8]. Diabetic cardiomyopathy (DCM) is a dysfunction of the cardiac muscle which occurs independently of hypertension or coronary artery disease in diabetes. DCM can lead to heart failure in the late stages of the disease [
9]. Increased activity of RAAS contributes to the pathogenesis of the diabetic heart [
10]. Treatment approaches for heart failure are similar in patients with or without diabetes and drugs targeting the RAAS (ARBs and ACEIs) are considered first-line treatment agents in patients with HF and concomitant diabetes [
11]. However, the recent European Society of Cardiology guideline recommends SGLT2 inhibitors for the management of patients with heart failure and type 2 diabetes [
12]. Decreased levels of NPs are associated with obesity [
13], insulin resistance [
14] and the development of diabetes [
15]. Therefore, it was proposed that therapeutic agents that increase NPs may be beneficial in the treatment of diabetes and its complications [
16,
17].
Alterations in β-adrenergic receptor (β-AR) signaling contribute to the cardiac dysfunction in diabetes [
18,
19]. Sympathetic overdrive in diabetes [
20] leads to blunted β
1- and β
2–AR mediated cardiac contractile responses [
21,
22], which may occur in part due to reduced expression of β
1- and β
2–ARs [
23,
24]. On the other hand, β
3–AR mediated cardiac relaxation [
25,
26] as well as receptor expression is increased in diabetes [
21,
23,
24]. Elevated β
3–AR expression plays a protective role against the deleterious effects of catecholamines in cardiac tissue [
27]. Similarly, sympathetic overdrive results in reduced β
1- and β
2-AR mediated responsiveness in heart failure [
28]. Impaired β–AR signaling in pathological conditions can affect the function of several downstream components such as sarcoplasmic reticulum calcium ATPase 2a (SERCA2a), phospholamban (PLN), and phosphorylated phospholamban (p-PLN).
Considering the role of the RAAS and NPs in diabetes and the beneficial effects of these systems on the heart, the sacubitril/valsartan combination may also be a promising therapeutic approach for diabetic cardiac dysfunction. The sacubitril/valsartan combination improves glucose homeostasis in patients with concomitant HFrEF and diabetes [
29]. Moreover, sacubitril/valsartan has been shown to reduce sympathetic nervous system activity in patients with HFrEF [
30]. There are few preclinical studies investigating the effect of sacubitril/valsartan on the diabetic heart. Based on the results of these studies, reduced fibrosis [
31,
32], inflammation and apoptosis [
33,
34], oxidative stress [
33] and endoplasmic reticulum stress [
34] have been suggested as possible mechanisms underlying this effect. It is therefore plausible that the sacubitril/valsartan combination affects β–AR mediated responses in the diabetic heart.
Based on these findings, the current study was conducted to investigate the effect of sacubitril/valsartan on cardiac contraction/relaxation in comparison with valsartan using β–AR agonists and, the effect of treatments on diastolic downstream components in a rat model of type 2 diabetes induced by high fat diet (HFD) plus low dose streptozotocin (STZ).
2. Results
2.1. General Characteristics of Animals
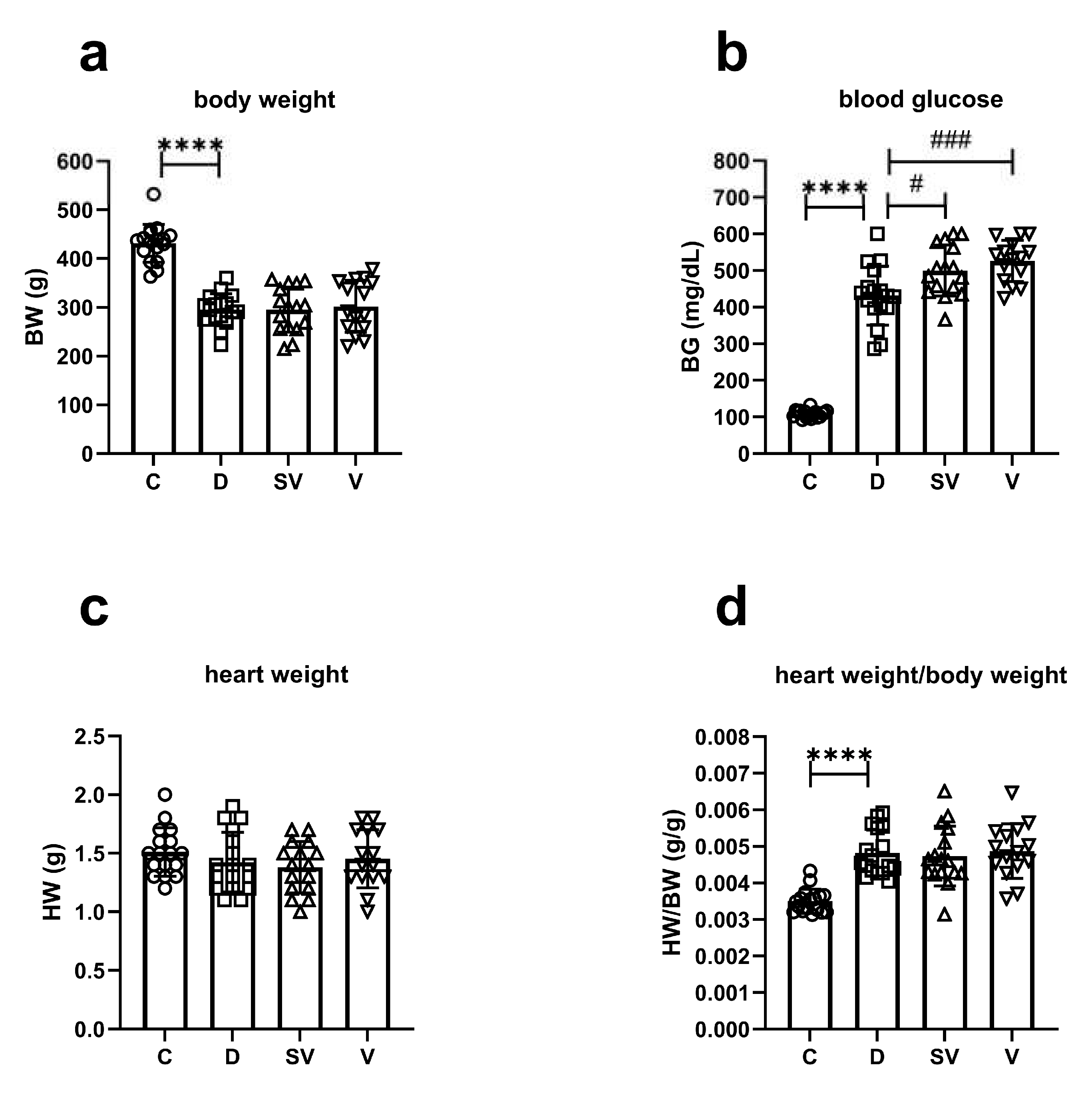
The body weight of diabetic rats at study end was reduced compared to control rats as typically observed in this model. Neither sacubitril/valsartan nor valsartan treatment reversed the loss of body weight in diabetes (
Figure 1a). Blood glucose at study end was higher in diabetic rats as expected. However, blood glucose levels in rats treated with either sacubitril/valsartan or valsartan were markedly higher than in diabetic rats (
Figure 1b). In addition, sacubitril/valsartan caused a greater increase in blood glucose levels than valsartan throughout the treatment period (
Supplementary Figure S1). Heart weight was comparable between groups (
Figure 1c). The ratio of heart weight to body weight (HW/BW), an indicator of cardiac hypertrophy, was higher in diabetic rats compared to the control group. This ratio was not improved by either treatment (
Figure 1d). Blood glucose levels of diabetic and treated diabetic rats were higher than those of control rats at each time point of the oral glucose tolerance test (OGTT) experiments (
Supplementary Figure S2 and
Supplementary Table S1). There was no difference in fasting insulin levels between the experimental groups, but the Homeostatic Model Assessment-Insulin Resistance (HOMA-IR) index was increased in diabetic rats and was not attenuated in treated diabetic rats (
Supplementary Table S2). Plasma cholesterol and triglyceride levels were substantially increased in diabetic rats compared with the control group. Neither treatment reversed the increase in plasma cholesterol and triglyceride levels (
Supplementary Figure S3).
2.2. In Vivo Cardiac Parameters
2.2.1. In Vivo Pressure-Volume (PV) Loop Analysis
Basal blood pressure level was measured in rats under anesthesia using a pressure-volume catheter placed in the carotid artery. Systolic blood pressure, diastolic blood pressure, and mean arterial pressure were comparable between the groups. Heart rate (HR) was decreased in the diabetic group and was not normalized with either treatment approach. End systolic and end diastolic volumes (ESV and EDV) were slightly increased in the diabetic group and not normalized by treatment. The indices of these parameters were significantly higher in diabetic rats and not reversed by treatment. While stroke volume (SV) was comparable between groups, surprisingly, the SV index was markedly increased only in diabetic rats compared to the control group (
Table 1).
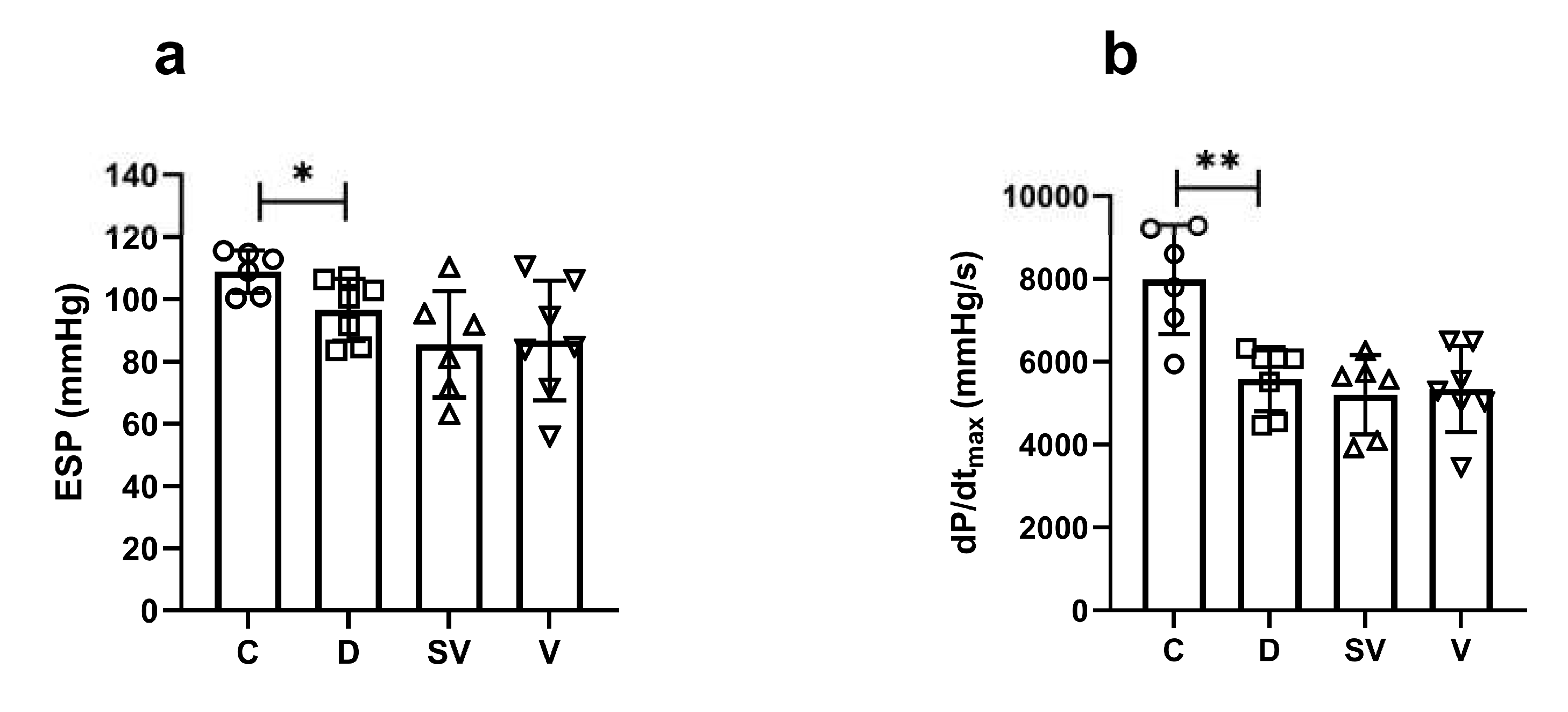
End systolic pressure (ESP) which indicates the systolic function of the heart, decreased in the diabetic group and both treatments had no effect on this parameter (
Figure 2a). The rate of contraction, which is another indicator of systolic cardiac function, was significantly reduced in the diabetic group and was not affected by the treatments (
Figure 2b).
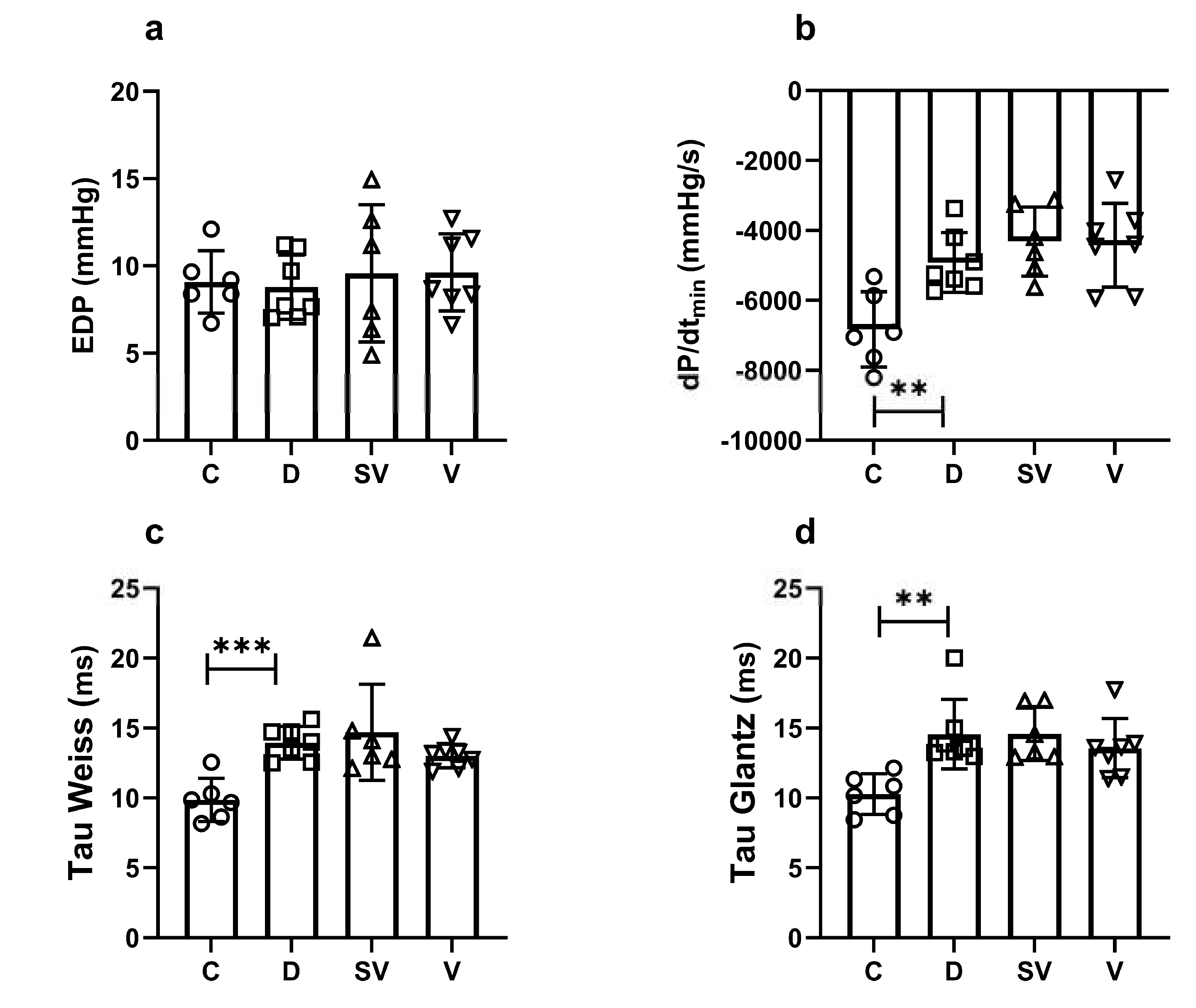
End diastolic pressure (EDP), one of the diastolic function parameters of the heart, was similar between groups (
Figure 3a). Another indicator of cardiac diastolic function, the rate of relaxation (dP/dt
min) was decreased in diabetic rats compared to control and both treatment approaches did not normalize this parameter (
Figure 3b). Tau Weiss and Tau Glantz values, the time constants for isovolumic relaxation, were significantly increased in diabetic rats and neither sacubitril/valsartan nor valsartan treatment had ameliorated Tau values (
Figure 3c and 3d respectively).
Preload independent cardiac parameters; the slope of end systolic pressure-volume relationship (ESPVR), end diastolic pressure-volume relationship (EDPVR) and preload recruitable stroke work (PRSW) were comparable between groups (
Table 2).
2.2.2. In Vivo Echocardiography Analysis
In vivo echocardiographic analysis showed that ejection fraction (EF) (%) and fractional shortening (FS) (%) were markedly reduced in diabetic rats. This indicates impaired systolic function due to diabetes. These parameters were improved by both sacubitril/valsartan and valsartan treatment without superiority to each other (
Figure 4 and
Table 3). Interventricular septum thickness at end diastole index (IVSId), left ventricular internal dimension at end diastole index (LVIDId), left ventricular internal dimension at end systole (LVIDs), left ventricular internal dimension at end systole index (LVIDIs) and left ventricular posterior wall thickness at end systole index (LVPWIs) values were significantly higher in diabetic group. Both treatments improved IVSId and only valsartan improved LVIDs values. Cardiac output (CO) was markedly reduced in diabetic group and was not reversed by treatments. Other echocardiographic parameters were comparable between groups (
Table 3).
2.3. β-AR Mediated Responsiveness
In the papillary muscle preparation, the contractile response induced by isoprenaline, a non-selective β-AR agonist, was reduced in the diabetic group as expected. Both sacubitril/valsartan and valsartan treatment slightly improved the contractile response compared with the diabetic group, without being superior to each other (
Figure 5a and 5b). Despite a noticeable effect of the treatment approaches on isoprenaline induced contraction, the effect was not statistically significant between groups due to the high standard deviation. In the papillary muscle, forskolin mediated contractile response was partially higher in sacubitril/valsartan treated diabetic rats compared to other groups (
Figure 5c).
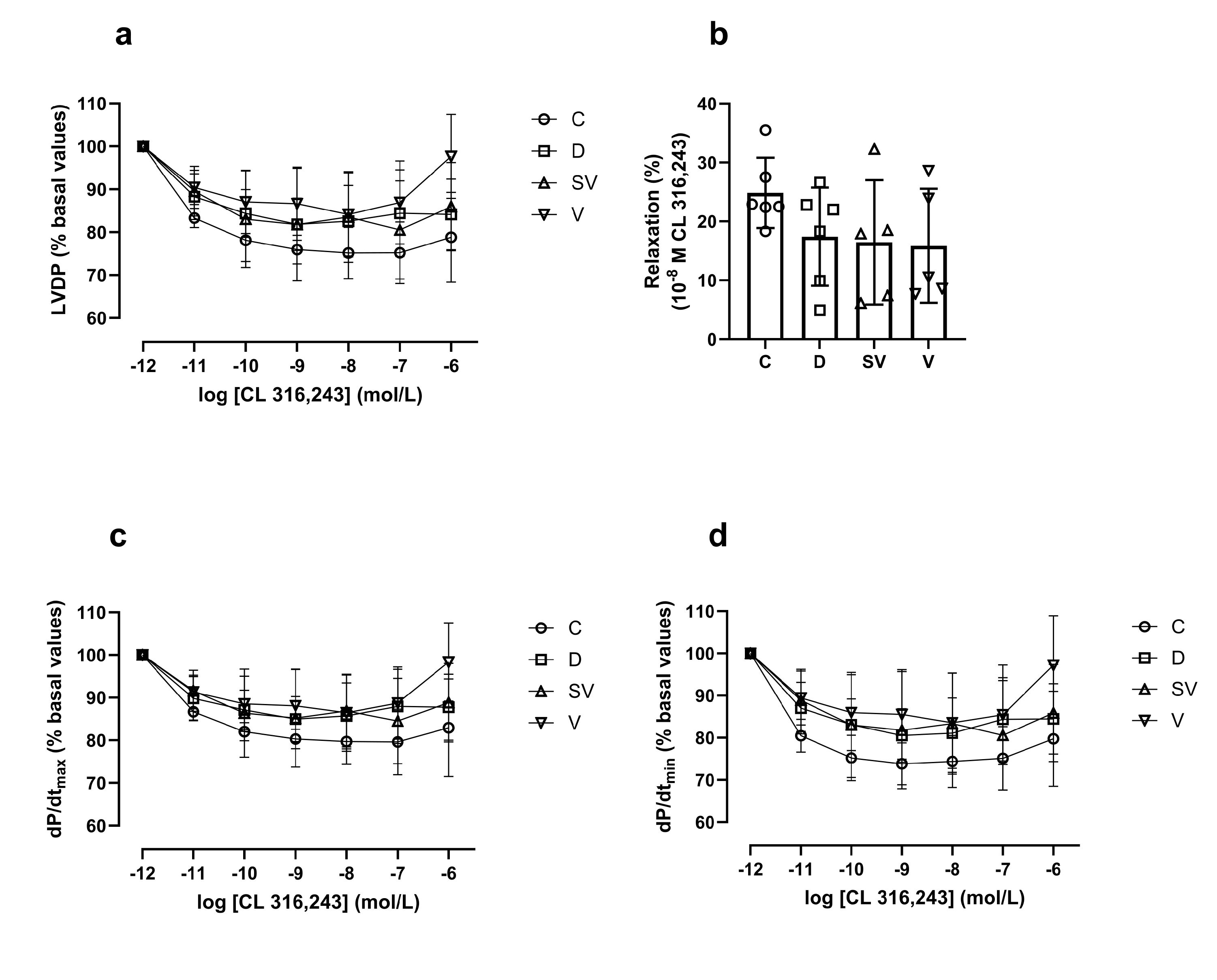
In the Langendorff heart preparation, the % change in left ventricular developed pressure (LVDP) induced by CL 316,243, a selective β
3-AR agonist, did not differ between groups (
Figure 6a). Moreover, the rate of contraction (dP/dt
max) and relaxation (% basal value) (
Figure 6c and 6d respectively) and the relaxation response induced by 10
-8 M CL 316,243 (considered as E
max value) (
Figure 6b) were comparable in all groups.
2.4. Protein Expression of Diastolic Components
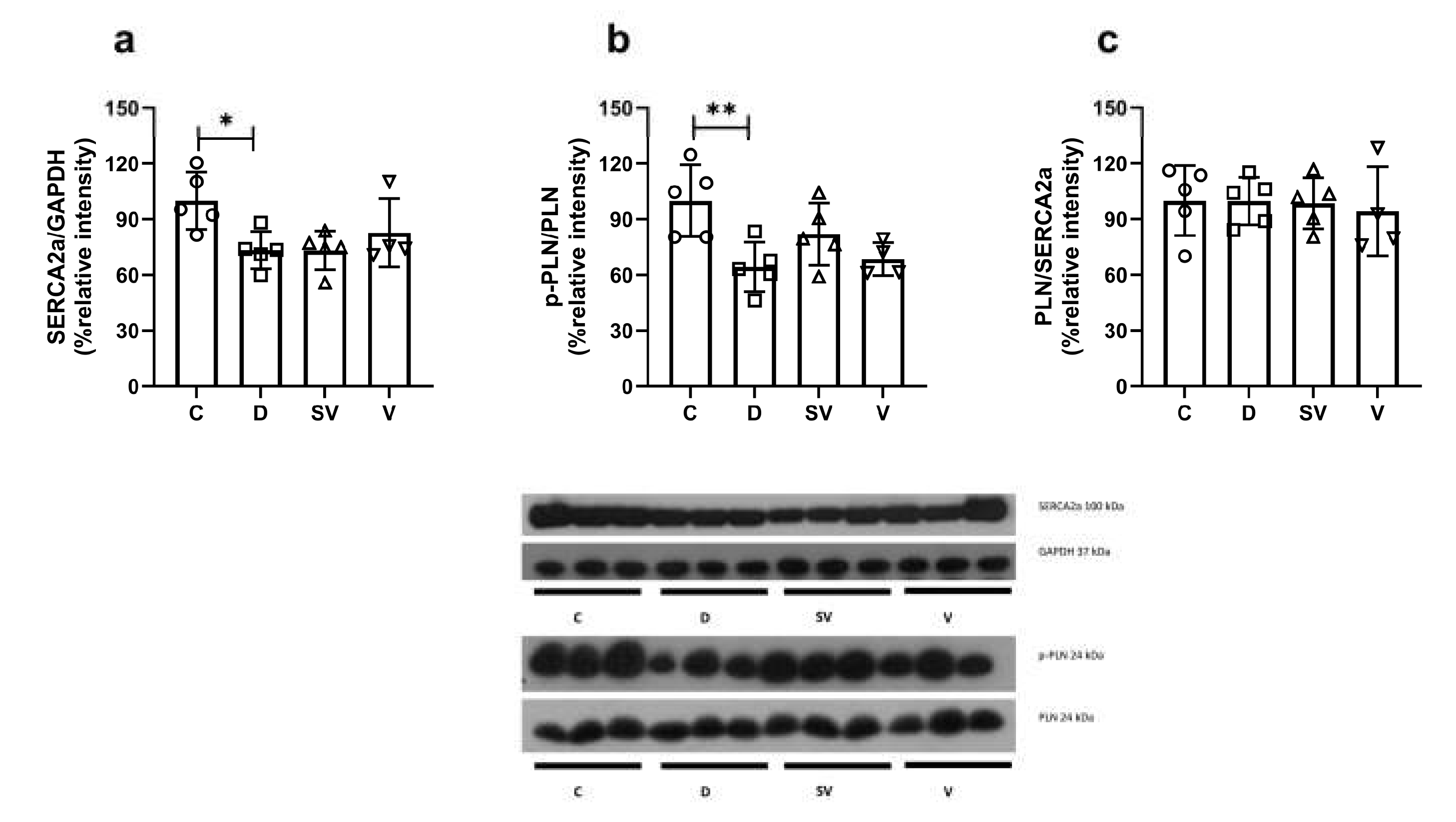
SERCA2a expression was reduced in the diabetic group and was not reversed by either treatment approach (
Figure 7a). The p-PLN to PLN ratio, which indicates the reduced inhibitory activity of PLN on SERCA2a function, was markedly reduced in diabetic rats and was not normalized with treatments (
Figure 7b). In addition, the PLN to SERCA2a ratio was comparable between groups (
Figure 7c).
3. Discussion
To the best of our knowledge, the present study is the first to investigate the effect of the sacubitril/valsartan combination on cardiac contraction and relaxation by β-AR agonists and on expression of proteins related to diastolic function. Our results indicate:
Sacubitril/valsartan and valsartan were comparable and slightly increased β1- and β2-AR mediated contractile responses, which were impaired in diabetic rats.
In vivo diastolic parameters and the protein expression of the diastolic components were not affected by the sacubitril/valsartan and valsartan treatments.
3.1. Critique of Study Design and Experimental Model
The type 2 diabetes model induced by HFD plus a low dose of STZ was chosen for this study because this experimental model accurately reflects hyperglycemia, hyperlipidemia and insulin resistance observed in type 2 diabetic patients [
35,
36]. Furthermore, the HFD plus low dose STZ diabetes model resembles the late stage of type 2 diabetes [
37]. Considering the increasing prevalence of type 2 diabetes worldwide and its cardiovascular complications, the HFD and low dose STZ induced diabetes model is a suitable experimental model for the current study. Based on the findings such as elevated fasting insulin levels, high HOMA-IR index and increased triglyceride levels, we can conclude that the experimental model has been established. On the other hand, the weight loss in the diabetic groups does not fit with the model, as it is a common feature of type 1 diabetes. Nevertheless, there are other studies reporting weight loss in this diabetic model [37-40].
The randomization for group allocation was performed after the acclimatization period, as prespecified. The blood glucose levels of both sacubitril/valsartan and valsartan treated diabetic rats were numerically higher than those of untreated diabetic rats at the end of the study. However, interpretation of these data is hampered by the fact that rats with higher blood glucose levels were randomly assigned to the treated groups. Furthermore, sacubitril/valsartan treatment resulted in a greater increase in blood glucose levels than valsartan treatment alone in the time course analysis (
Supplementary Figure S1). Unlike our findings, sacubitril/valsartan combination improved glucose homeostasis in heart failure patients with concomitant diabetes [
29] and in high fat and high fructose fed rats [
41]. In addition, studies have shown that valsartan and other ARBs have little or no effect on blood glucose levels [
3]. We found markedly increased cholesterol and triglyceride levels in diabetic rats, and both treatments failed to reduce this increase (
Supplementary Figure S3). However, sacubitril/valsartan has been shown to reduce triglyceride levels in patients with HFpEF [
42]. On the other hand, preclinical studies have reported conflicting results on the effect of sacubitril/valsartan combination on blood glucose and lipid levels. For example, sacubitril/valsartan treatment improved blood glucose and lipid parameters in type 1 diabetic rats [
43] and ameliorated blood lipid levels but not glucose levels in Zucker obese rats [
44]. However, it did not affect either blood glucose or lipid levels in the genetic model of type 2 diabetic mice [
45]. The different effects of sacubitril/valsartan may be explained by the different animal models used in these studies.
3.2. Cardiac Hypertrophy
Hypertrophy is a common feature of the diabetic heart [
46,
47] and the HW/BW ratio is an indicator of cardiac hypertrophy. In the current study, the HW/BW ratio was greater in diabetic rats. However, neither sacubitril/valsartan nor valsartan treatment improved this ratio. Consistent with this finding, neither treatment approach had a beneficial effect on increased LVIDId, LVIDIs and LVPWIs based on echocardiographic analysis. Sacubitril/valsartan improved only IVSId and valsartan improved both IVSId and LVIDs.
Improved cardiac hypertrophy with sacubitril/valsartan treatment has been shown in diabetes [
33,
34] and in other disease models [48-50]. However, supporting our data, some studies revealed that the combination did not show an improvement in cardiac hypertrophy. For instance, Miyoshi et al. reported that sacubitril/valsartan attenuated cardiac fibrosis but had no effect on cardiac hypertrophy [
51]. Similarly, both treatments failed to attenuate cardiac hypertrophy in Zucker obese rats [
52]. While most studies suggest a beneficial effect of sacubitril/valsartan on cardiac remodeling, further studies are needed to reach a definitive conclusion in diabetic models.
3.3. Cardiac Hemodynamic Parameters
Diastolic and/or systolic dysfunction are important features of the diabetic heart [
53,
54]. Diastolic dysfunction is an early finding of diabetes whereas systolic dysfunction is observed in the late stage of the pathology [
9]. Based on our echocardiographic analysis, EF (%) and FS (%) were markedly reduced in diabetic rats, indicating systolic dysfunction. Furthermore, these parameters were improved with each treatment. Twelve-month of treatment with sacubitril/valsartan resulted in a greater increase in EF (%) compared with valsartan alone (10.0% vs. 4.6%, respectively) in diabetic patients with acute myocardial infarction [
55]. Preclinical studies also support the superiority of sacubitril/valsartan over valsartan alone in improving systolic function in diabetes [
31,
34] or in the myocardial infarction model [
56]. In contrast to these studies, we found that sacubitril/valsartan combination had a similar effect to valsartan on systolic function. In line with the reduction in EF and FS, dP/dt
max was also diminished in the diabetic group, further confirming systolic dysfunction due to diabetes. On the other hand, neither treatment did not correct this parameter.
In patients with HFrEF, sacubitril/valsartan treatment ameliorated diastolic and systolic echocardiographic parameters, which is associated with clinical improvement [
57]. In addition, switching from ACEi/ARB to sacubitril/valsartan improved both diastolic and systolic cardiac function in patients with HFrEF [
58,
59]. In the current study, the diastolic dysfunction was also confirmed by reduced dP/dt
min and increased Tau values. Furthermore, increased ESV and EDV indices support the impairment of diastolic function. None of these parameters improved in the treatment groups. Unlike our findings, Tau constant decreased and some hemodynamic parameters improved in rats with myocardial infarction treated with sacubitril/valsartan [
60]. Despite the same drug dose and duration of treatment, the discrepancy between the study by von Lueder et al. and ours could be partly explained by the differences in the animal models. In another study, sacubitril/valsartan treatment improved diastolic function parameters (i.e. E’/A’ ratio, diastolic stiffness, myocardial performance index) better than valsartan alone in Zucker obese rats [
52]. However, diastolic function was preserved in rats treated with sacubitril/valsartan, but not in rats treated with valsartan in the HFpEF model [
61]. Although the doses of the drugs are the same, the different results between these studies and ours could be due to the duration of the treatment (10-week [
52] and 8-week [
61]) and/or the different animal models.
3.4. β-Adrenergic Responsiveness
β
1- and β
2-AR mediated contractile response to isoprenaline was markedly reduced in diabetic rats. Blunted β
1- and β
2-AR mediated contractility in diabetic rat heart has also been reported in previous studies [
62,
63]. Impairment of cardiac contractile response in diabetic heart has been attributed to reduced β
1-AR expression levels [
62,
64,
65] and hyperinsulinemia induced desensitization of β
2-AR [
66]. Although both treatments improved the decline in contractile performance, the results were not statistically significant due to the high standard deviation. Nevertheless, considering the E
max values, it could be assumed that sacubitril/valsartan is more effective than valsartan alone on the contractile response. This effect may be related to the sympatho-inhibitory effect of sacubitril/valsartan [
30] and/or improved Ca
2+ homeostasis [
56,
67]. As β
1- and β
2-ARs mediate the cardiac contraction through the adenylyl cyclase (AC)-cyclic adenosine monophosphate (cAMP)-cAMP-dependent protein kinase A (PKA) downstream pathway [
68], we also assessed forskolin induced contractility. Despite slightly higher values in the sacubitril/valsartan treated group, the contractile response was comparable between the groups.
CL 316,243, a β
3-AR agonist, induced relaxation in a concentration dependent manner in the Langendorff perfused heart preparation. Although the relaxation response was slightly lower in diabetic rats, there was no statistically significant difference between groups. Interestingly, we found in previous studies that β
3-AR mediated relaxation was enhanced in the diabetic heart [
25,
69,
70]. Of note, those studies have been performed in an STZ diabetic model that mimics type 1 diabetes. On the other hand, the model we used in the current study mimics type 2 diabetes. Consistent with our findings, Derkach et al. reported that cardiac β
3-AR mediated relaxation response is preserved in HFD and low dose STZ induced diabetic rats [
71]. Another explanation for the different results may be that HFD and low dose STZ induced diabetes present a milder cardiac phenotype, which contributes to preserved β
3-AR mediated relaxation.
3.5. Protein Expression of Diastolic Components
During systole, Ca
2+ influx from L-type Ca
2+ channels into cardiomyocytes triggers Ca
2+ induced Ca
2+ release which enables cardiac contraction. Reuptake of cytosolic Ca
2+ into the sarcoplasmic reticulum (SR) by SERCA2a is a critical step in cardiac relaxation and subsequent contraction [
72]. Reduced SERCA2a protein density is a common feature in the pathogenesis of diabetic cardiac dysfunction [
62,
73] and heart failure [
74]. The decrease in SERCA2a protein expression may be due to reduced phosphorylation of PLN, which has an inhibitory effect on SERCA2a [
75,
76].
Changes in SERCA2a expression are associated with diastolic parameters such as relaxation rate and Tau constant [
77]. Cardiac SERCA2a expression was decreased in 6-week STZ-induced diabetic rats and this finding was attributed to impaired diastolic function [
73]. Similar results have been reported by other researchers in 8- and 12-week diabetic rats [
62,
78,
79]. In parallel with these studies, we found that SERCA2a was downregulated in diabetic rats, which may have contributed to the impaired relaxation rate and Tau constant values. However, both treatment approaches failed to normalize the reduced SERCA2a protein expression.
PLN regulates SERCA2a activity through its inhibitory effect. p-PLN to PLN ratio is an indicator of PLN activity. A decreased ratio of p-PLN to PLN indicates inadequate phosphorylation of PLN during diastole, leading to impaired cardiac relaxation. A decrease in the ratio of p-PLN to PLN, due to or independent of adrenergic stimulation, has been reported in type 2 diabetic animals [
19]. In our study, p-PLN to PLN ratio was decreased in the diabetic group which correlates with impaired in vivo diastolic parameters. Treatment approaches failed to normalize this ratio. Changes in PLN to SERCA2a ratio has been suggested to involve diastolic dysfunction in type 2 diabetic heart [
80]. However, we found similar PLN to SERCA2a ratio in our study.
4. Materials and Methods
4.1. Animals and the Study Protocol
The study protocol had been approved by the animal welfare committee of Ankara University (permit 2016-23-198) and was in line with NIH Guidelines for Care and Use of Laboratory Animals. Four-week-old male Sprague Dawley rats were obtained from the Department of Molecular Biology and Genetics, Laboratory Animals Unit, Bilkent University and housed under 12:12-hour light:dark cycle with chow and water ad libitum. After a 2-week acclimatization period, the rats were randomized into groups (control (C), diabetic (D), sacubitril/valsartan treated diabetic (SV), valsartan treated diabetic (V)). These rats were given either a HFD (45% kcal fat) or a control diet (10% kcal fat). After 4-week, the rats on the HFD received an intraperitoneal injection of STZ (30 mg/kg, in citrate buffer, pH 4.5), while the rats on the control diet received an injection of vehicle (citrate buffer, pH 4.5). One-week after STZ injection, the rats with blood glucose levels above 200 mg/dl were considered diabetic. Some rats received a second (37 rats), third (15 rats) or fourth (11 rats) STZ injection if necessary. The need for multiple doses of STZ injection to establish the diabetes model caused a delay in the experimental protocol. Following 10-week of diabetes, the rats were treated with either sacubitril/valsartan (68 mg/kg/day, oral gavage) or valsartan treatment (31 mg/kg/day, oral gavage) for 4-week. Sacubitril/valsartan and valsartan treatment dosage, and treatment period were applied according to ‘Guidance to investigators for formulating and administering LCZ696-ABA and valsartan to rats’ by Novartis. Body weight and plasma glucose levels were measured weekly. Regarding a delay of diabetes establishment, the rats reached the point for in vitro experiments at 25-29-week of age instead of the planned time point (24-week of age). Rats were anesthetized under isoflurane (2% infusion) and the hearts were excised. Tissues and plasma samples were stored at -80 °C for further experiments. Plasma cholesterol and triglyceride levels were measured at the time of sacrifice using enzymatic colorimetric kits. Western blot experiments were performed on a new set of animals, not on the tissues of the animals in which in vivo and in vitro functional studies were performed, due to breakdown of the -80 °C freezer during the study.
4.2. Oral Glucose Tolerance Test (OGTT)
OGTT was performed during the third week of the sacubitril/valsartan and valsartan treatment. The rats were fasted for 12-hour, and 2 g/kg glucose was administered by oral gavage. Blood glucose levels were measured at 0, 30, 60, 90 and 120 min using a glucometer via the tail vein. Blood samples were collected in EDTA tubes, and plasma was separated. Plasma samples were stored at -80 °C for insulin level measurements. The HOMA-IR index was calculated as previously described [(fasting blood glucose (mmol/L) x fasting insulin level (µIU/mL))/22.5] [
81]. Fasting plasma insulin levels during the OGTT analysis were measured by using ELISA kits.
4.3. In Vivo PV Loop Analysis
Rats were injected with 3000 U/kg intraperitoneal heparin 10 min before anesthesia and then anesthetized with isoflurane (2% infusion) at the end of 4-week sacubitril/valsartan and valsartan treatment. Body temperature was maintained at 37°C. The right carotid artery was dissected, and a small incision was made on the surface of the carotid artery. After the catheter was inserted into the carotid artery, the blood pressure was recorded. The catheter was then placed into the left ventricle. After a 10 min stabilization period, PV loops were recorded at constant preload. The following hemodynamic parameters were measured and analyzed: ESP, EDP, ESV, EDV, SV, CO, HR, dP/dtmax, dP/dtmin, Tau. Volume dependent hemodynamic parameters were calculated as body weight normalized index values to eliminate the effect of body weight changes due to diabetes. Preload independent cardiac parameters (PRSW, ESPVR and EDPVR) were then measured and analyzed by occlusion of the inferior vena cava for 5-second.
4.4. In Vivo Echocardiography Experiment
Due to logistic reasons, the echocardiography experiment was carried out 2 to 3-week after the start of the sacubitril/valsartan and valsartan treatment period. The rats were anesthetized by 2% isoflurane inhalation, placed in the left lateral recumbent position and scanned using the Arietta V60 ultrasound cardiovascular system echo scanner (Hitachi Healthcare Inc.) with a pediatric S31 (9-2 MHz) phased array transducer and a high temporal and spatial resolution cardiac application. The transmission frequency was 10 MHz, the depth was 2.5 cm, and the frame rate was 225 frames per second. The conventional two-dimensional (2D) short-axis images were acquired at the level of the papillary muscles. They were then digitally stored for further offline analysis. Left ventricular dimensions were measured using M-mode echocardiography through the short-axis view at the mid-papillary level and left ventricular EF was calculated using the Teicholz method. DAS-RS1echoLAB (Hitachi Healthcare Inc) was used for the analysis of radial strain (Srad), strain rate (SRrad), and circumferential strain rate (SRcirc). This 2D strain program tracks the movement of speckle patterns due to reflection, scattering, and interference between tissue and ultrasound beams in echocardiographic images. They can be tracked from frame to frame throughout the cardiac cycle. This method allows easy assessment of rotation, torsion, and synchronous disturbance for the left ventricle, as well as strain and strain rate. The endocardial boundary was marked, while the outer boundary was adjusted to fit the epicardial contour. The software automatically tracked and calculated strain and strain rate in the radial and circumferential directions throughout the six cardiac cycles. Peak systolic Srad, SRrad and SRcirc were obtained from 6 segments of the papillary muscle levels. Data from at least three different cardiac cycles were averaged. Volume parameters, wall thickness and internal dimension values were given both as raw data and as body weight ratio index as previously described [
82].
4.5. In Vitro Papillary Muscle Experiment
Hearts were excised under terminal 2% isoflurane inhalation anesthesia. Immediately after exsanguination, the hearts were placed in the dissection dish containing heparinized Krebs-Henseleit solution (120 mM NaCl, 4.8 mM KCl, 1.25 mM CaCl2.2H2O, 1.25 mM MgSO4.7H2O, 1.2 mM KH2PO4, 25 mM NaHCO3, 10 mM C6H12O6.H2O) gassed with 95% O2 and 5% CO2 mixture to maintain pH 7.4 at 30 °C. Papillary muscles were carefully dissected from the left ventricular wall, avoiding any mechanical tension and mounted on the force transducer (Commat Pharmacology & Physiology Instruments, Ankara, Türkiye) in the horizontal organ bath chamber. Papillary muscles were perfused continuously with Krebs-Henseleit solution through a peristaltic pump at 5 ml/min. Papillary muscles were stimulated with electric field pulses at 0.6 Hz, 2 ms, twice the threshold voltage value. After a 60 min stabilization period, the papillary muscles were gradually stretched and Lmax (maximal contraction value) was recorded. Tension was adjusted to 90% of Lmax value and experiments were performed at adjusted tension individually for each preparation. A cumulative concentration-response curve of isoprenaline (0.1 nM-10 µM) was generated. After a washout period, the papillary muscles were challenged with 3 µM forskolin (adenylyl cyclase activator).
4.6. In Vitro Langendorff Heart Preparation Experiment
Hearts excised from the rats under terminal 2% isoflurane anesthesia were rapidly cannulated through the aorta and perfused retrogradely with the Krebs-Henseleit solution (120 mM NaCl, 4.8 mM KCl, 1.25 mM CaCl2.2H2O, 1.25 mM MgSO4.7H2O, 1.2 mM KH2PO4, 25 mM NaHCO3, 10 mM C6H12O6.H2O). Krebs-Henseleit solution was continuously gassed with a mixture of 95% O2 and 5% CO2 to maintain pH 7.4 at 37°C. Surrounding adjacent tissue was removed and the hearts were allowed to beat spontaneously to record the flow rate for each preparation. The hearts were then freed from the atria and paced with an electrode placed in the right ventricle connected to a stimulator (Grass S44 Stimulator, Quincy, MA, USA) to maintain a heart rate of 300 beat/min. An elastic cling film balloon which is connected to a pressure transducer (Commat Pharmacology & Physiology Instruments, Ankara, Türkiye) via a polyethylene tube was inserted into the left ventricle to measure left ventricular function. The left ventricular end diastolic pressure (LVEDP) value was set individually for each preparation according to the Frank-Starling curve. Hearts were perfused at constant flow for 30 min to reach a steady state and a concentration response curve of CL 316,243, a selective β3-AR agonist, (10 pM- 1 µM) was generated. Left ventricular function was assessed by the following parameters: LVDP, LVEDP, dP/dtmax and dP/dtmin.
4.7. Western Blot Experiments
Frozen left ventricular tissue was powdered in liquid nitrogen and homogenized with a mixture of RIPA buffer, sodium orthovanadate and protease inhibitor cocktail. After sonication, samples were agitated at +4°C for 2-hour and centrifuged at 12000 rpm at +4°C to obtain the supernatant fraction. The protein concentration of the samples was measured by bicinchoninic acid assay (BCA). Samples containing equal amounts of protein (10 or 45 or 100 µg) were loaded onto 4% acrylamide SDS-PAGE stacking gel and separated by 10% acrylamide SDS-PAGE separation gel. Proteins were transferred to either polyvinylidene difluoride or nitrocellulose membranes at 100 V for 2-hour (10 µg), 3-hour (45 µg) or 4-hour (100 µg). The membranes were blocked with Tris-buffered saline containing 0.1% Tween 20 (TBST) and 5% bovine serum albumin for 1-hour at room temperature to prevent non-specific protein binding. The membranes were then incubated with primary antibodies (SERCA2a (1/2000), PLN (1/2000), p-PLN (PLN
ser16/thr17) (1/1000), GAPDH (1/5000)) at +4°C for overnight. The membranes were then washed with TBST and incubated with horseradish peroxidase (HRP) conjugated antirabbit secondary antibodies (SERCA2a (1/5000), PLN (1/2000), p-PLN (PLN
ser16/thr17) (1/2000), GAPDH (1/5000)) at +4°C for 1.5-hour. The membranes were washed with TBST and incubated with enhanced chemiluminescence (ECL) mixture for 1 min, and then the blots were exposed to film. Films were scanned and protein bands were analyzed by using Image J. GAPDH was used as a housekeeping gene to normalize densitometric values of protein bands; quantitative GAPDH signals in each group are shown in
Supplementary Figure S4.
4.8. Data Analysis
Descriptive statistics were performed for each parameter and data are expressed as mean ± SD. Prism-GraphPad was used for all analyses and generated graphs (version 9.1.2, La Jolla, CA, USA). The efficacy of isoprenaline was determined by fitting a 3-parameter model of the concentration-response curve to obtain estimated E
max values. When the same model was applied to derive estimated E
max values of CL 316,243, ambiguous values were observed. Therefore, the E
max values were considered as those obtained at 10 nM CL 316,243, where the highest relaxation was observed. All data were assumed to be normally distributed. Unpaired
t-test was used to compare C and D groups for model validation. The unpaired
t-test was used as a hypothesis test to compare D and SV groups and D and V groups. p-values < 0.05 were considered statistically significant. Data from two Western blots was pooled and analyzed for GAPDH validation using one-way ANOVA followed by post-hoc Bonferroni test (
Supplementary Figure S4).
4.9. Chemicals
HFD and the control diet were purchased from Altromin Spezialfutter GmbH&Co. KG (Lage, Germany) and Arden Research & Experiment (Ankara, Türkiye). Sacubitril/valsartan and valsartan were kindly gifted by Novartis AG (Basel, Switzerland). Isoflurane was purchased from Adeka (Samsun, Türkiye). Chemicals for Krebs-Henseleit solution, STZ, CL 316,243, isoprenaline and forskolin were from Sigma-Aldrich (Ankara, Türkiye). GAPDH (14C10) primary antibody (CST2118S) (RRID: AB_561053), PLN primary antibody (CST8495) (RRID: AB_10949105), p-PLN (PLNser16/thr17) primary antibody (CST8496) (RRID: AB_10949102), SERCA2a primary antibody (CST4388) (RRID: AB_2227684) and antirabbit seconder antibody (CST7074) (RRID: AB_2099233) were obtained from Cell Signaling (USA). Triglyceride (GPO-PAP) and cholesterol (CHOD-PAP) enzymatic colorimetric kits were purchased from ADS analytic diagnostic systems (Istanbul, Türkiye). The rat insulin ELISA kit (201-11-0708) was purchased from SunRed Biotechnology Company (China).
5. Conclusions
Our data show that the ameliorative effect of sacubitril/valsartan on EF and FS was similar to that of valsartan alone in HFD and low dose STZ induced diabetes. Neither sacubitril/valsartan nor valsartan treatment has a beneficial effect on blood glucose, triglyceride, and cholesterol levels. In addition, neither treatment reversed cardiac hypertrophy or systolic or diastolic dysfunction in vivo. On the other hand, β1- and β2-AR mediated responsiveness appear to have improved in both sacubitril/valsartan and valsartan treated groups, but high standard deviations make it difficult to make a definitive statement. Sacubitril/valsartan combination slightly ameliorated cAMP induced contraction. In terms of β3-AR mediated responsiveness there was no statistically significant difference between the groups. Based on these results in our study, the beneficial effect of sacubitril/valsartan treatment on EF and FS may be attributed to improved β1- and β2-AR mediated responses. However, further studies are needed to make a definitive statement.
Limitations of this study:
The age of the animals was considered as a baseline characteristic rather than blood glucose levels at the time of randomized group allocation. Therefore, it was not possible to interpret whether sacubitril/valsartan or valsartan affected glycemic control in the present study.
Interpretation of the statistical significance data in some experiments was difficult with large standard deviations. In addition, we were not able to increase the sample size due to compliance with ethical constraints and the limited number of animals in the study.
Cardiac β-AR subtype mRNA and/or protein expression levels could not be measured due to the non-specific binding capacity of the antibodies [
83] and for economic reasons.
The possible involvement of components contributing to the β-AR signaling pathways could not be investigated in the present study.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: Blood glucose levels during treatment; Figure S2: Time course blood glucose levels during OGTT; Figure S3: Plasma lipid levels; Figure S4: GAPDH validation as a housekeeping gene. C; Table S1: Blood glucose levels at each time point during OGTT; Table S2: Fasting blood glucose and insulin levels prior to OGTT and HOMA-IR values.
Author Contributions
Conceptualization, B.R.E. and E.A.I.; methodology, B.R.E., E.A.I., Z.E.Y.D., I.K., A.E.M., and K.S.; formal analysis, B.R.E; investigation, B.R.E., E.A.I., Z.E.Y.D., I.K., A.E.M., and K.S.; writing—original draft preparation, B.R.E., E.A.I., and M.C.M.; writing—review and editing, B.R.E., E.A.I., M.C.M., and Z.E.Y.D.; supervision, E.A.I and M.C.M; project administration, B.R.E. and E.A.I.; funding acquisition, B.R.E. and E.A.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Scientific and Technological Research Council of Türkiye (TUBITAK SBAG-117S936 and SBAG-115S564) and Ankara University Scientific Research Projects Coordination Unit (19H0237004). B.R.E. is a researcher supported by TUBITAK (2211/A) during her PhD.
Institutional Review Board Statement
The study protocol had been approved by the animal welfare committee of Ankara University (permit no: 2016-23-198, date of approval: 23.11.2016) and was in line with NIH Guidelines for Care and Use of Laboratory Animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We would like to thank Novartis AG (Basel, Switzerland) for providing sacubitril/valsartan and valsartan compounds as a gift.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FDA. Center for Drug Evaluation and Research. 2015. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/207620Orig1s000OtherR.pdf.
- Mangiafico, S.; Costello-Boerrigter, L.C.; Andersen, I.A.; Cataliotti, A.; Burnett Jr, J.C. Neutral endopeptidase inhibition and the natriuretic peptide system: an evolving strategy in cardiovascular therapeutics. European heart journal 2013, 34, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.C.; Brunner, H.R.; Foster, C.; Huo, Y. Angiotensin II type 1 receptor antagonists in animal models of vascular, cardiac, metabolic and renal disease. Pharmacology & therapeutics 2016, 164, 1–81. [Google Scholar] [CrossRef]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014, 371, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, R.; Lu, C.; Chen, Q.; Xu, T.; Li, D. Effects of the angiotensin-receptor neprilysin inhibitor on cardiac reverse remodeling: meta-analysis. Journal of the American Heart Association 2019, 8, e012272. [Google Scholar] [CrossRef] [PubMed]
- Badreldin, H.A.; Aldosari, N.; Alnashwan, L.; Almutairi, T.; Yousif, N.; Alsulaiman, K.; Aljuhani, O.; Hafiz, A.; Alshaya, O. What the near Future Holds for Sacubitril/Valsartan: A Summary of Major Ongoing Studies. Journal of Cardiovascular Development and Disease 2022, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- IDF. International Diabetes Federation. IDF Diabetes Atlas 2019, 9. [Google Scholar]
- Haas, A.V.; McDonnell, M.E. Pathogenesis of cardiovascular disease in diabetes. Endocrinology and Metabolism Clinics 2018, 47, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Palomer, X.; Pizarro-Delgado, J.; Vazquez-Carrera, M. Emerging Actors in Diabetic Cardiomyopathy: Heartbreaker Biomarkers or Therapeutic Targets? Trends Pharmacol Sci 2018, 39, 452–467. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; DeMarco, V.G.; Sowers, J.R. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nature Reviews Endocrinology 2016, 12, 144–153. [Google Scholar] [CrossRef]
- Kenny, H.C.; Abel, E.D. Heart failure in type 2 diabetes mellitus: impact of glucose-lowering agents, heart failure therapies, and novel therapeutic strategies. Circulation research 2019, 124, 121–141. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. European heart journal 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Larson, M.G.; Levy, D.; Benjamin, E.J.; Leip, E.P.; Wilson, P.W.; Vasan, R.S. Impact of obesity on plasma natriuretic peptide levels. Circulation 2004, 109, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Plante, E.; Menaouar, A.; Danalache, B.A.; Broderick, T.L.; Jankowski, M.; Gutkowska, J. Treatment with brain natriuretic peptide prevents the development of cardiac dysfunction in obese diabetic db/db mice. Diabetologia 2014, 57, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Lazo, M.; Young, J.H.; Brancati, F.L.; Coresh, J.; Whelton, S.; Ndumele, C.E.; Hoogeveen, R.; Ballantyne, C.M.; Selvin, E. NH2-terminal pro–brain natriuretic peptide and risk of diabetes. Diabetes 2013, 62, 3189–3193. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, D.; Bi, H.; Zhang, H. The role of natriuretic peptides in diabetes and its complications. Biomedicine & Pharmacotherapy 2016, 84, 1826–1832. [Google Scholar] [CrossRef]
- Moro, C. Targeting cardiac natriuretic peptides in the therapy of diabetes and obesity. Expert opinion on therapeutic targets 2016, 20, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Varma, U.; Koutsifeli, P.; Benson, V.; Mellor, K.; Delbridge, L. Molecular mechanisms of cardiac pathology in diabetes–Experimental insights. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 2018, 1864, 1949–1959. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, B.R.; Michel, M.C.; Arioglu-Inan, E. Expression and signaling of β-adrenoceptor subtypes in the diabetic heart. Cells 2020, 9, 2548. [Google Scholar] [CrossRef]
- Iyngkaran, P.; Anavekar, N.; Majoni, W.; Thomas, M.C. The role and management of sympathetic overactivity in cardiovascular and renal complications of diabetes. Diabetes & metabolism 2013, 39, 290–298. [Google Scholar] [CrossRef]
- Amour, J.; Loyer, X.; Le Guen, M.; Mabrouk, N.; David, J.-S.; Camors, E.; Carusio, N.; Vivien, B.; Andriantsitohaina, R.; Heymes, C. Altered Contractile Response due to Increased β3-Adrenoceptor Stimulation in Diabetic CardiomyopathyThe Role of Nitric Oxide Synthase 1–derived Nitric Oxide. Anesthesiology: The Journal of the American Society of Anesthesiologists 2007, 107, 452–460. [Google Scholar] [CrossRef]
- Carillion, A.; Feldman, S.; Na, N.; Biais, M.; Carpentier, W.; Birenbaum, A.; Cagnard, N.; Loyer, X.; Bonnefont-Rousselot, D.; Hatem, S. Atorvastatin reduces β-Adrenergic dysfunction in rats with diabetic cardiomyopathy. PLoS One 2017, 12, e0180103. [Google Scholar] [CrossRef] [PubMed]
- Haley, J.M.; Thackeray, J.T.; Thorn, S.L.; DaSilva, J.N. Cardiac β-adrenoceptor expression is reduced in Zucker diabetic fatty rats as type-2 diabetes progresses. Plos one 2015, 10, e0127581. [Google Scholar] [CrossRef] [PubMed]
- Bidasee, K.R.; Zheng, H.; Shao, C.-H.; Parbhu, S.K.; Rozanski, G.J.; Patel, K.P. Exercise training initiated after the onset of diabetes preserves myocardial function: effects on expression of β-adrenoceptors. Journal of applied physiology 2008, 105, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Kayki-Mutlu, G.; Arioglu-Inan, E.; Ozakca, I.; Ozcelikay, A.T.; Altan, V.M. beta3-Adrenoceptor-mediated responses in diabetic rat heart. Gen Physiol Biophys 2014, 33, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Okatan, E.N.; Tuncay, E.; Hafez, G.; Turan, B. Profiling of cardiac β-adrenoceptor subtypes in the cardiac left ventricle of rats with metabolic syndrome: Comparison with streptozotocin-induced diabetic rats. Canadian Journal of Physiology and Pharmacology 2015, 93, 517–525. [Google Scholar] [CrossRef]
- Gauthier, C.; Langin, D.; Balligand, J.L. Beta3-adrenoceptors in the cardiovascular system. Trends Pharmacol Sci 2000, 21, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Hartupee, J.; Mann, D.L. Neurohormonal activation in heart failure with reduced ejection fraction. Nature Reviews Cardiology 2017, 14, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Seferovic, J.P.; Claggett, B.; Seidelmann, S.B.; Seely, E.W.; Packer, M.; Zile, M.R.; Rouleau, J.L.; Swedberg, K.; Lefkowitz, M.; Shi, V.C. Effect of sacubitril/valsartan versus enalapril on glycaemic control in patients with heart failure and diabetes: a post-hoc analysis from the PARADIGM-HF trial. The lancet Diabetes & endocrinology 2017, 5, 333–340. [Google Scholar] [CrossRef]
- Bunsawat, K.; Ratchford, S.M.; Alpenglow, J.K.; Stehlik, J.; Smith, A.S.; Richardson, R.S.; Wray, D.W. Sympathoinhibitory effect of sacubitril-valsartan in heart failure with reduced ejection fraction: A pilot study. Autonomic Neuroscience 2021, 235, 102834. [Google Scholar] [CrossRef]
- Suematsu, Y.; Miura, S.i.; Goto, M.; Matsuo, Y.; Arimura, T.; Kuwano, T.; Imaizumi, S.; Iwata, A.; Yahiro, E.; Saku, K. LCZ696, an angiotensin receptor–neprilysin inhibitor, improves cardiac function with the attenuation of fibrosis in heart failure with reduced ejection fraction in streptozotocin-induced diabetic mice. European journal of heart failure 2016, 18, 386–393. [Google Scholar] [CrossRef]
- Ai, J.; Shuai, Z.; Tang, K.; Li, Z.; Zou, L.; Liu, M. Sacubitril/valsartan alleviates myocardial fibrosis in diabetic cardiomyopathy rats. Hellenic journal of cardiology: HJC= Hellenike kardiologike epitheorese 2021, 62, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Zhao, L.; Ren, X.-M.; Ye, P.; Hu, Z.-Y. LCZ696, an angiotensin receptor-neprilysin inhibitor, ameliorates diabetic cardiomyopathy by inhibiting inflammation, oxidative stress and apoptosis. Experimental Biology and Medicine 2019, 244, 1028–1039. [Google Scholar] [CrossRef] [PubMed]
- Belali, O.M.; Ahmed, M.M.; Mohany, M.; Belali, T.M.; Alotaibi, M.M.; Al-Hoshani, A.; Al-Rejaie, S.S. LCZ696 Protects against Diabetic Cardiomyopathy-Induced Myocardial Inflammation, ER Stress, and Apoptosis through Inhibiting AGEs/NF-κB and PERK/CHOP Signaling Pathways. International Journal of Molecular Sciences 2022, 23, 1288. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tu, H.; Zheng, H.; Zhang, L.; Tran, T.P.; Muelleman, R.L.; Li, Y.-L. Alterations of calcium channels and cell excitability in intracardiac ganglion neurons from type 2 diabetic rats. American Journal of Physiology-Cell Physiology 2012, 302, C1119–C1127. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Liu, F.-C.; Deng, C.-Y.; Zhang, M.-Z.; Yang, M.; Xiao, D.-Z.; Lin, Q.-X.; Cai, S.-T.; Kuang, S.-J.; Chen, J. Left ventricular deformation associated with cardiomyocyte Ca 2+ transients delay in early stage of low-dose of STZ and high-fat diet induced type 2 diabetic rats. BMC cardiovascular disorders 2016, 16, 1–12. [Google Scholar] [CrossRef]
- Davidson, E.P.; Coppey, L.J.; Shevalye, H.; Obrosov, A.; Yorek, M.A. Vascular and neural complications in type 2 diabetic rats: improvement by sacubitril/valsartan greater than valsartan alone. Diabetes 2018, 67, 1616–1626. [Google Scholar] [CrossRef]
- Liu, H.-j.; Zhang, C.-y.; Song, F.; Xiao, T.; Meng, J.; Zhang, Q.; Liang, C.-l.; Li, S.; Wang, J.; Zhang, B. A novel partial agonist of peroxisome proliferator-activated receptor γ with excellent effect on insulin resistance and type 2 diabetes. Journal of Pharmacology and Experimental Therapeutics 2015, 353, 573–581. [Google Scholar] [CrossRef]
- Sahin, K.; Tuzcu, M.; Orhan, C.; Sahin, N.; Kucuk, O.; Ozercan, I.H.; Juturu, V.; Komorowski, J.R. Anti-diabetic activity of chromium picolinate and biotin in rats with type 2 diabetes induced by high-fat diet and streptozotocin. British Journal of Nutrition 2013, 110, 197–205. [Google Scholar] [CrossRef]
- Yesilyurt, Z.E.; Erdogan, B.R.; Karaomerlioglu, I.; Muderrisoglu, A.E.; Michel, M.C.; Arioglu-Inan, E. Urinary bladder weight and function in a rat model of mild hyperglycemia and its treatment with dapagliflozin. Frontiers in pharmacology 2019, 10, 911. [Google Scholar] [CrossRef]
- Abo-Khookh, A.M.; Ghoneim, H.A.; Abdelaziz, R.R.; Nader, M.A.; Shawky, N.M. The dual inhibitor Sacubitril-valsartan ameliorate high-fat high-fructose-induced metabolic disorders in rats superiorly compared to valsartan only. Journal of Pharmacy and Pharmacology 2023, 75, 846–858. [Google Scholar] [CrossRef]
- Selvaraj, S.; Claggett, B.L.; Packer, M.; Zannad, F.; Anand, I.S.; Pieske, B.; Zhao, Z.; Shi, V.C.; Lefkowitz, M.P.; McMurray, J.J. Effects of sacubitril/valsartan on serum lipids in heart failure with preserved ejection fraction. Journal of the American Heart Association 2021, 10, e022069. [Google Scholar] [CrossRef]
- Alqahtani, F.; Mohany, M.; Alasmari, A.F.; Alanazi, A.Z.; Belali, O.M.; Ahmed, M.M.; Al-Rejaie, S.S. Angiotensin II receptor Neprilysin inhibitor (LCZ696) compared to Valsartan attenuates Hepatotoxicity in STZ-induced hyperglycemic rats. International Journal of Medical Sciences 2020, 17, 3098. [Google Scholar] [CrossRef] [PubMed]
- Habibi, J.; Aroor, A.R.; Das, N.A.; Manrique-Acevedo, C.M.; Johnson, M.S.; Hayden, M.R.; Nistala, R.; Wiedmeyer, C.; Chandrasekar, B.; DeMarco, V.G. The combination of a neprilysin inhibitor (sacubitril) and angiotensin-II receptor blocker (valsartan) attenuates glomerular and tubular injury in the Zucker Obese rat. Cardiovascular diabetology 2019, 18, 1–17. [Google Scholar] [CrossRef]
- Myakala, K.; Jones, B.A.; Wang, X.X.; Levi, M. Sacubitril/valsartan treatment has differential effects in modulating diabetic kidney disease in db/db mice and KKAy mice compared with valsartan treatment. American Journal of Physiology-Renal Physiology 2021, 320, F1133–F1151. [Google Scholar] [CrossRef] [PubMed]
- Negishi, K. Echocardiographic feature of diabetic cardiomyopathy: where are we now? Cardiovascular diagnosis and therapy 2018, 8, 47. [Google Scholar] [CrossRef]
- Rubler, S.; Dlugash, J.; Yuceoglu, Y.Z.; Kumral, T.; Branwood, A.W.; Grishman, A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. The American journal of cardiology 1972, 30, 595–602. [Google Scholar] [CrossRef]
- Ge, Q.; Zhao, L.; Liu, C.; Ren, X.; Yu, Y.-h.; Pan, C.; Hu, Z. LCZ696, an Angiotensin Receptor-Neprilysin Inhibitor, Improves Cardiac Hypertrophy and Fibrosis and Cardiac Lymphatic Remodeling in Transverse Aortic Constriction Model Mice. BioMed Research International 2020, 2020. [Google Scholar] [CrossRef]
- Kusaka, H.; Sueta, D.; Koibuchi, N.; Hasegawa, Y.; Nakagawa, T.; Lin, B.; Ogawa, H.; Kim-Mitsuyama, S. LCZ696, angiotensin II receptor-neprilysin inhibitor, ameliorates high-salt-induced hypertension and cardiovascular injury more than valsartan alone. American journal of hypertension 2015, 28, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Suematsu, Y.; Jing, W.; Nunes, A.; Kashyap, M.L.; Khazaeli, M.; Vaziri, N.D.; Moradi, H. LCZ696 (sacubitril/valsartan), an angiotensin-receptor neprilysin inhibitor, attenuates cardiac hypertrophy, fibrosis, and vasculopathy in a rat model of chronic kidney disease. Journal of cardiac failure 2018, 24, 266–275. [Google Scholar] [CrossRef]
- Miyoshi, T.; Nakamura, K.; Miura, D.; Yoshida, M.; Saito, Y.; Akagi, S.; Ohno, Y.; Kondo, M.; Ito, H. Effect of LCZ696, a dual angiotensin receptor neprilysin inhibitor, on isoproterenol-induced cardiac hypertrophy, fibrosis, and hemodynamic change in rats. Cardiology journal 2019, 26, 575–583. [Google Scholar] [CrossRef]
- Aroor, A.R.; Mummidi, S.; Lopez-Alvarenga, J.C.; Das, N.; Habibi, J.; Jia, G.; Lastra, G.; Chandrasekar, B.; DeMarco, V.G. Sacubitril/valsartan inhibits obesity-associated diastolic dysfunction through suppression of ventricular-vascular stiffness. Cardiovascular Diabetology 2021, 20, 1–18. [Google Scholar] [CrossRef]
- Werner, R.A.; Eissler, C.; Hayakawa, N.; Arias-Loza, P.; Wakabayashi, H.; Javadi, M.S.; Chen, X.; Shinaji, T.; Lapa, C.; Pelzer, T. Left Ventricular Diastolic Dysfunction in a Rat Model of Diabetic Cardiomyopathy using ECG-gated 18 F-FDG PET. Scientific reports 2018, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, K.; Wang, W.; Wen, Z.; Wang, P.; Liu, L.; Wang, D.W. Glucagon-like peptide-1 ameliorates cardiac lipotoxicity in diabetic cardiomyopathy via the PPAR α pathway. Aging Cell 2018, 17, e12763. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Li, H.; Chen, X.; Wang, Y.; Lin, W.; Chen, H.; Huang, S.; Han, S.; Guan, F.; Huang, Z. Efficacy and safety of sacubitril valsartan in treating heart failure with midrange ejection fraction after acute myocardial infarction in diabetic patients. Medicine 2022, 101. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.-C.; Wo, H.-T.; Lee, H.-L.; Lin, S.-F.; Chu, Y.; Wen, M.-S.; Chou, C.-C. Sacubitril/valsartan therapy ameliorates ventricular tachyarrhythmia inducibility in a rabbit myocardial infarction model. Journal of cardiac failure 2020, 26, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Pericas, P.; Mas-Lladó, C.; Ramis-Barceló, M.F.; Valadrón, I.; Noris Mora, M.; Pasamar Márquez, L.; González Colino, R.; Forteza Albertí, J.F.; Peral Disdier, V.; Rossello, X. Impact of Sacubitril–Valsartan Treatment on Diastolic Function in Patients with Heart Failure and Reduced Ejection Fraction. High Blood Pressure & Cardiovascular Prevention 2021, 28, 167–175. [Google Scholar] [CrossRef]
- Ganesananthan, S.; Shah, N.; Shah, P.; Elsayed, H.; Phillips, J.; Parkes, A.; Morgan, A.; Yousef, Z. Real-world treatment switching to sacubitril/valsartan in patients with heart failure with reduced ejection fraction: A cohort study. Open Heart 2020, 7, e001305. [Google Scholar] [CrossRef] [PubMed]
- Martens, P.; Beliën, H.; Dupont, M.; Vandervoort, P.; Mullens, W. The reverse remodeling response to sacubitril/valsartan therapy in heart failure with reduced ejection fraction. Cardiovascular therapeutics 2018, 36, e12435. [Google Scholar] [CrossRef] [PubMed]
- von Lueder, T.G.; Wang, B.H.; Kompa, A.R.; Huang, L.; Webb, R.; Jordaan, P.; Atar, D.; Krum, H. Angiotensin receptor neprilysin inhibitor LCZ696 attenuates cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophy. Circulation: Heart Failure 2015, 8, 71–78. [Google Scholar] [CrossRef]
- Nordén, E.S.; Bendiksen, B.A.; Andresen, H.; Bergo, K.K.; Espe, E.K.; Hasic, A.; Hauge-Iversen, I.M.; Veras, I.; Hussain, R.I.; Sjaastad, I. Sacubitril/valsartan ameliorates cardiac hypertrophy and preserves diastolic function in cardiac pressure overload. ESC heart failure 2021, 8, 918–927. [Google Scholar] [CrossRef]
- Arioglu-Inan, E.; Ozakca, I.; Kayki-Mutlu, G.; Sepici-Dincel, A.; Altan, V.M. The role of insulin–thyroid hormone interaction on β-adrenoceptor-mediated cardiac responses. European journal of pharmacology 2013, 718, 533–543. [Google Scholar] [CrossRef]
- Dinçer, Ü.D.; Onay, A.; Arı, N.; Özçelikay, A.T.; Altan, V.M. The effects of diabetes on β-adrenoceptor mediated responsiveness of human and rat atria. Diabetes research and clinical practice 1998, 40, 113–122. [Google Scholar] [CrossRef]
- Dinçer, Ü.D.; Bidasee, K.R.; Güner, Ş.; Tay, A.; Özçelikay, A.T.; Altan, V.M. The effect of diabetes on expression of β1-, β2-, and β3-adrenoreceptors in rat hearts. Diabetes 2001, 50, 455–461. [Google Scholar] [CrossRef]
- Matsuda, N.; Hattori, Y.; Gando, S.; Akaishi, Y.; Kemmotsu, O.; Kanno, M. Diabetes-induced down-regulation of β1-adrenoceptor mRNA expression in rat heart. Biochemical pharmacology 1999, 58, 881–885. [Google Scholar] [CrossRef]
- Fu, Q.; Shi, Q.; West, T.M.; Xiang, Y.K. Cross-talk between insulin signaling and GPCRs. Journal of cardiovascular pharmacology 2017, 70, 74. [Google Scholar] [CrossRef]
- Eiringhaus, J.; Wünsche, C.M.; Tirilomis, P.; Herting, J.; Bork, N.; Nikolaev, V.O.; Hasenfuss, G.; Sossalla, S.; Fischer, T.H. Sacubitrilat reduces pro-arrhythmogenic sarcoplasmic reticulum Ca2+ leak in human ventricular cardiomyocytes of patients with end-stage heart failure. ESC Heart Failure 2020, 7, 2992–3002. [Google Scholar] [CrossRef]
- George, M.S.; Pitt, G.S. The real estate of cardiac signaling: location, location, location. Proceedings of the National Academy of Sciences 2006, 103, 7535–7536. [Google Scholar] [CrossRef]
- Arioglu-Inan; G Kayki-Mutlu; BR Erdogan; AE Muderrisoglu; I Karaomerlioglu; ZE Yesilyurt; S Degirmenci; B Turan; Altan, V. The effects of leptin on cardiac function in streptozotocin diabetic rats. BPS Pharmacology 2017 Abstract Book 2017. [Google Scholar]
- Uyar-Boztas, C.; Arioglu-Inan, E.; Muderrisoglu, A.; Kayki-Mutlu, G.; Erdogan, B.; Yesilyurt, Z.; Karaomerlioglu, I.; Altan, V. The effect of sitagliptin on beta-adrenoceptor-mediated cardiac responses in streptozotocin induced diabetic rats. Diabetes Stoffwech H 2017, 26, 15. [Google Scholar]
- Derkach, K.; Bondareva, V.; Moyseyuk, I.; Shpakov, A. The influence of two-month treatment with bromocryptine on activity of the adenylyl cyclase signaling system in the myocardium and testes of rats with type 2 diabetes mellitus. Tsitologiia 2014, 56, 907–918. [Google Scholar]
- Bers, D.M. Cardiac excitation–contraction coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Connelly, K.; Kelly, D.J.; Zhang, Y.; Prior, D.L.; Martin, J.; Cox, A.; Thai, K.; Feneley, M.P.; Tsoporis, J.; White, K. Functional, structural and molecular aspects of diastolic heart failure in the diabetic (mRen-2) 27 rat. Cardiovascular research 2007, 76, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Kawase, Y.; Hajjar, R.J. The cardiac sarcoplasmic/endoplasmic reticulum calcium ATPase: a potent target for cardiovascular diseases. Nature Clinical Practice Cardiovascular Medicine 2008, 5, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Netticadan, T.; Temsah, R.M.; Kent, A.; Elimban, V.; Dhalla, N.S. Depressed levels of Ca2+-cycling proteins may underlie sarcoplasmic reticulum dysfunction in the diabetic heart. Diabetes 2001, 50, 2133–2138. [Google Scholar] [CrossRef] [PubMed]
- Watanuki, S.; Matsuda, N.; Sakuraya, F.; Jesmin, S.; Hattori, Y. Protein kinase C modulation of the regulation of sarcoplasmic reticular function by protein kinase A-mediated phospholamban phosphorylation in diabetic rats. British journal of pharmacology 2004, 141, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Müller, O.J.; Lange, M.; Rattunde, H.; Lorenzen, H.-P.; Müller, M.; Frey, N.; Bittner, C.; Simonides, W.; Katus, H.A.; Franz, W.-M. Transgenic rat hearts overexpressing SERCA2a show improved contractility under baseline conditions and pressure overload. Cardiovascular research 2003, 59, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Teshima, Y.; Takahashi, N.; Saikawa, T.; Hara, M.; Yasunaga, S.; Hidaka, S.; Sakata, T. Diminished expression of sarcoplasmic reticulum Ca2+-ATPase and ryanodine sensitive Ca2+ channel mRNA in streptozotocin-induced diabetic rat heart. Journal of molecular and cellular cardiology 2000, 32, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cannell, M.B.; Phillips, A.R.; Cooper, G.J.; Ward, M.-L. Altered calcium homeostasis does not explain the contractile deficit of diabetic cardiomyopathy. Diabetes 2008, 57, 2158–2166. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.; Nikolaev, V.O.; De Jong, K.A. Understanding the role of SERCA2a microdomain remodeling in heart failure induced by obesity and type 2 diabetes. Journal of Cardiovascular Development and Disease 2022, 9, 163. [Google Scholar] [CrossRef]
- Bowe, J.E.; Franklin, Z.J.; Hauge-Evans, A.C.; King, A.J.; Persaud, S.J.; Jones, P.M. Metabolic phenotyping guidelines: assessing glucose homeostasis in rodent models. Journal of endocrinology 2014, 222, G13–G25. [Google Scholar] [CrossRef]
- Mátyás, C.; Kovács, A.; Németh, B.T.; Oláh, A.; Braun, S.; Tokodi, M.; Barta, B.A.; Benke, K.; Ruppert, M.; Lakatos, B.K. Comparison of speckle-tracking echocardiography with invasive hemodynamics for the detection of characteristic cardiac dysfunction in type-1 and type-2 diabetic rat models. Cardiovascular diabetology 2018, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hamdani, N.; van der Velden, J. Lack of specificity of antibodies directed against human beta-adrenergic receptors. Naunyn-Schmiedeberg's archives of pharmacology 2009, 379, 403–407. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
General characteristics at study end. (a) Body weight (BW); (b) Blood glucose (BG); (c) Heart weight (HW); (d) The ratio of heart weight to body weight (HW/BW). C, Control (n=17); D, Diabetic (n=17); SV, Sacubitril/valsartan treated diabetic (n=16); V, Valsartan treated diabetic (n=15). ****, p<0.0001 compared to control. #, p<0.05; ###, p<0.001 compared to diabetic.
Figure 1.
General characteristics at study end. (a) Body weight (BW); (b) Blood glucose (BG); (c) Heart weight (HW); (d) The ratio of heart weight to body weight (HW/BW). C, Control (n=17); D, Diabetic (n=17); SV, Sacubitril/valsartan treated diabetic (n=16); V, Valsartan treated diabetic (n=15). ****, p<0.0001 compared to control. #, p<0.05; ###, p<0.001 compared to diabetic.
Figure 2.
In vivo basal cardiac parameters of systolic function. (a) ESP, end systolic pressure; (b) dp/dtmax, rate of contraction. C, Control (n=6); D, Diabetic (n=7); SV, Sacubitril/valsartan treated diabetic (n=6); V, Valsartan treated diabetic (n=7). *, p<0.05; **, p<0.01 compared to control.
Figure 2.
In vivo basal cardiac parameters of systolic function. (a) ESP, end systolic pressure; (b) dp/dtmax, rate of contraction. C, Control (n=6); D, Diabetic (n=7); SV, Sacubitril/valsartan treated diabetic (n=6); V, Valsartan treated diabetic (n=7). *, p<0.05; **, p<0.01 compared to control.
Figure 3.
In vivo basal cardiac parameters of diastolic function. (a) EDP, end diastolic pressure; (b) dP/dtmin, rate of relaxation; (c) and (d) Tau, isovolumic relaxation constant. C, Control (n=6); D, Diabetic (n=7); SV, Sacubitril/valsartan treated diabetic (n=6); V, Valsartan treated diabetic (n=7). **, p<0.01; ***, p<0.001 compared to control.
Figure 3.
In vivo basal cardiac parameters of diastolic function. (a) EDP, end diastolic pressure; (b) dP/dtmin, rate of relaxation; (c) and (d) Tau, isovolumic relaxation constant. C, Control (n=6); D, Diabetic (n=7); SV, Sacubitril/valsartan treated diabetic (n=6); V, Valsartan treated diabetic (n=7). **, p<0.01; ***, p<0.001 compared to control.
Figure 4.
In vivo echocardiography parameters of systolic function. (a) % EF, ejection fraction; (b) % FS, fractional shortening. C, Control (n=8); D, Diabetic (n=7); SV, Sacubitril/valsartan treated diabetic (n=7); V, Valsartan treated diabetic (n=9). ****, p<0.0001 compared to control. ##, p<0.01; ####, p<0.0001 compared to diabetic.
Figure 4.
In vivo echocardiography parameters of systolic function. (a) % EF, ejection fraction; (b) % FS, fractional shortening. C, Control (n=8); D, Diabetic (n=7); SV, Sacubitril/valsartan treated diabetic (n=7); V, Valsartan treated diabetic (n=9). ****, p<0.0001 compared to control. ##, p<0.01; ####, p<0.0001 compared to diabetic.
Figure 5.
Isoprenaline and forskolin mediated contractile responses. (a) Cumulative concentration-response curve. (b) Emax, efficacy C, Control (n=8); D, Diabetic (n=7); SV, Sacubitril/valsartan treated diabetic (n=8); V; Valsartan treated diabetic (n=6). *, p<0.05 compared to control. (c) Contraction response at 3 µM forskolin (% of control). C, Control (n=7); D, Diabetic (n=7); SV, Sacubitril/valsartan treated diabetic (n=5); V; Valsartan treated diabetic (n=5).
Figure 5.
Isoprenaline and forskolin mediated contractile responses. (a) Cumulative concentration-response curve. (b) Emax, efficacy C, Control (n=8); D, Diabetic (n=7); SV, Sacubitril/valsartan treated diabetic (n=8); V; Valsartan treated diabetic (n=6). *, p<0.05 compared to control. (c) Contraction response at 3 µM forskolin (% of control). C, Control (n=7); D, Diabetic (n=7); SV, Sacubitril/valsartan treated diabetic (n=5); V; Valsartan treated diabetic (n=5).
Figure 6.
CL 316,243 mediated relaxation responses. (a) LVDP, left ventricle developed pressure; (b) % relaxation response at 10-8 M CL 316,243; (c) dP/dtmax (% basal values), rate of contraction; (d) dP/dtmin (%basal values), rate of relaxation. C, Control (n=6); D, Diabetic (n=6); SV, Sacubitril/valsartan treated diabetic (n=5); V; Valsartan treated diabetic (n=5).
Figure 6.
CL 316,243 mediated relaxation responses. (a) LVDP, left ventricle developed pressure; (b) % relaxation response at 10-8 M CL 316,243; (c) dP/dtmax (% basal values), rate of contraction; (d) dP/dtmin (%basal values), rate of relaxation. C, Control (n=6); D, Diabetic (n=6); SV, Sacubitril/valsartan treated diabetic (n=5); V; Valsartan treated diabetic (n=5).
Figure 7.
Protein expression levels and representative images. (a) SERCA2a protein expression level normalized to GAPDH; (b) The ratio of p-PLN to PLN; (c) The ratio of PLN to SERCA2a. C, Control (n=5); D, Diabetic (n=5); SV, Sacubitril/valsartan treated diabetic (n=5); V; Valsartan treated diabetic (n=4). *, p<0.05; **, p<0.01 compared to control.
Figure 7.
Protein expression levels and representative images. (a) SERCA2a protein expression level normalized to GAPDH; (b) The ratio of p-PLN to PLN; (c) The ratio of PLN to SERCA2a. C, Control (n=5); D, Diabetic (n=5); SV, Sacubitril/valsartan treated diabetic (n=5); V; Valsartan treated diabetic (n=4). *, p<0.05; **, p<0.01 compared to control.
Table 1.
In vivo basal cardiac hemodynamic parameters.
Table 1.
In vivo basal cardiac hemodynamic parameters.
| |
C (n=6) |
D (n=7) |
SV (n=6) |
V (n=7) |
| SBP (mmHg) |
110.89 ± 18.41 |
103.40 ± 11.69 |
93.54 ± 19.72 |
91.52 ± 20.41 |
| DBP (mmHg) |
79.29 ± 22.43 |
71.94 ± 9.23 |
67.69 ± 16.18 |
65.99 ± 20.57 |
| MAP (mmHg) |
100.36 ± 19.55 |
92.91 ± 10.77 |
84.92 ± 18.24 |
83.01 ± 20.13 |
| HR (beat/min) |
311.13 ± 39.09 |
247.17 ± 20.27** |
256.29 ± 18.74 |
251.35 ± 21.76 |
| EDV (µL) |
370.64 ± 69.67 |
436.66 ± 46.89 |
436.09 ± 76.22 |
411.52 ± 74.01 |
| EDVI (µL/g) |
0.92 ± 0.16 |
1.56 ± 0.29*** |
1.59 ± 0.31 |
1.43 ± 0.29 |
| ESV (µL) |
162.06 ± 37.83 |
200.17 ± 26.07 |
209.34 ± 26.93 |
191.61 ± 22.42 |
| ESVI (µL/g) |
0.40 ± 0.08 |
0.71 ± 0.13*** |
0.77 ± 0.18 |
0.67 ± 0.11 |
| SV (µL) |
208.58 ± 47.93 |
236.49 ± 36.31 |
226.75 ± 60.39 |
219.91 ± 60.81 |
| SVI (µL/g) |
0.52 ± 0.12 |
0.85 ± 0.19** |
0.82 ± 0.18 |
0.76 ± 0.21 |
Table 2.
Preload independent in vivo cardiac parameters.
Table 2.
Preload independent in vivo cardiac parameters.
| |
C (n=5) |
D (n=6) |
SV (n=6) |
V (n=6) |
| ESPVR |
0.390 ± 0.156 |
0.449 ± 0.166 |
0.334 ± 0.106 |
0.317 ± 0.132 |
| EDPVR |
0.010 ± 0.002 |
0.007 ± 0.003 |
0.006 ± 0.003 |
0.006 ± 0.003 |
| PRSW |
62.58 ± 9.44 |
53.69 ± 6.83 |
56.86 ± 7.73 |
53.31 ± 3.79 |
Table 3.
In vivo echocardiography parameters.
Table 3.
In vivo echocardiography parameters.
| |
C (n=8) |
D (n=7) |
SV (n=7) |
V (n=9) |
| IVSd (mm) |
1.91 ± 0.58 |
2.31 ± 0.38 |
1.83 ± 0.25#
|
1.78 ± 0.25##
|
| IVSId (mm/kg) |
4.30 ± 1.37 |
6.94 ± 1.04** |
5.64 ± 0.65#
|
5.73 ± 1.13#
|
| LVIDd (mm) |
5.49 ± 0.67 |
5.06 ± 0.91 |
5.21 ± 1.12 |
4.92 ± 0.50 |
| LVIDId (mm/kg) |
12.26 ± 1.55 |
15.33 ± 3.35* |
16.46 ± 5.39 |
15.84 ± 2.52 |
| LVPWd (mm) |
2.35 ± 1.90 |
2.20 ± 0.72 |
1.89 ± 0.29 |
2.01 ± 0.66 |
| LVPWId (mm/kg) |
5.05 ± 3.47 |
6.51 ± 1.65 |
5.82 ± 0.87 |
6.47 ± 2.30 |
| IVSs (mm) |
2.93 ± 0.83 |
2.76 ± 0.51 |
2.17 ± 0.45# |
2.17 ± 0.35#
|
| IVSIs (mm/kg) |
6.50 ± 1.69 |
8.31 ± 1.65 |
6.76 ± 1.65 |
6.96 ± 1.37 |
| LVIDs (mm) |
3.01 ± 0.21 |
3.71 ± 0.51** |
3.43 ± 0.78 |
3.10 ± 0.21##
|
| LVIDIs (mm/kg) |
6.75 ± 0.72 |
11.27 ± 2.20**** |
10.86 ± 3.80 |
9.96 ± 1.16 |
| LVPWs (mm) |
2.93 ± 0.49 |
2.80 ± 0.66 |
2.56 ± 0.54 |
2.79 ± 0.60 |
| LVPWIs (mm/kg) |
6.57 ± 1.36 |
8.33 ± 1.43* |
7.80 ± 1.09 |
9.00 ± 2.29 |
| CO (mL/dk) |
101.25 ± 39.07 |
52.86 ± 29.84* |
60.14 ± 28.64 |
51.11 ± 13.64 |
| CI (mL/dk.g) |
0.23 ± 0.09 |
0.16 ± 0.09 |
0.19 ± 0.11 |
0.16 ± 0.05 |
| EF (%) |
81.60 ± 4.20 |
57.27 ± 7.21**** |
69.56 ± 5.84##
|
72.69 ± 3.72####
|
| FS (%) |
45.01 ± 4.54 |
26.04 ± 4.60**** |
34.34 ± 4.31##
|
36.63 ± 3.11####
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).