Submitted:
13 July 2024
Posted:
15 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
- Oligo- or anovulation
- Clinical and/or biochemical signs of hyperandrogenism,
- Polycystic ovaries
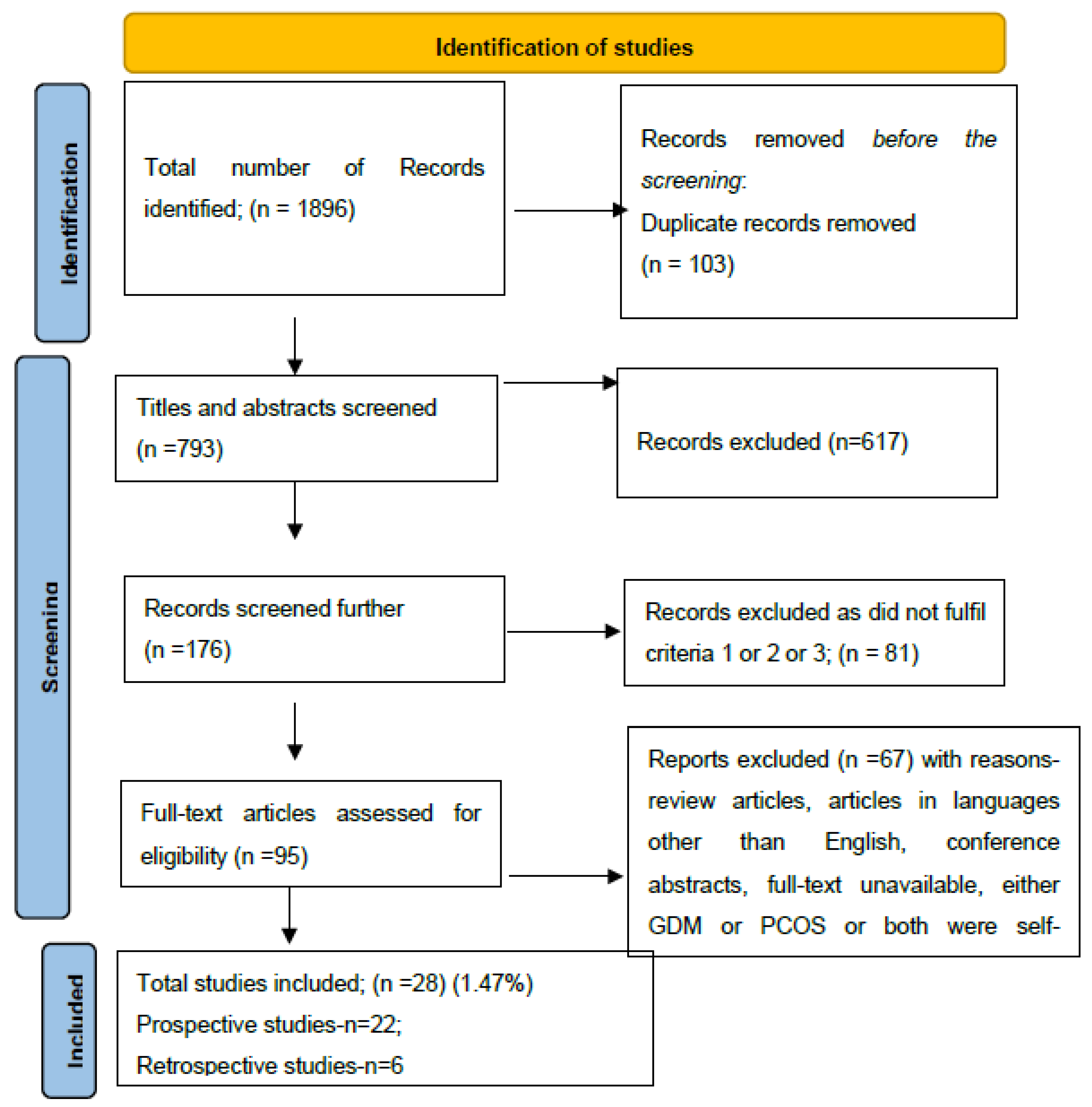
2. Materials and Methods
- Studies reporting pregnant women with pre-pregnancy confirmed PCOS by Rotterdam criteria.
- Studies using either 100 grams or 75 grams Oral Glucose Tolerance Test (OGTT) for screening GDM.
- Studies including both PCOS and GDM.
3. Results
3.1. Study Characteristics
3.2. Prevalence of PCOS in GDM (Retrospective Studies)
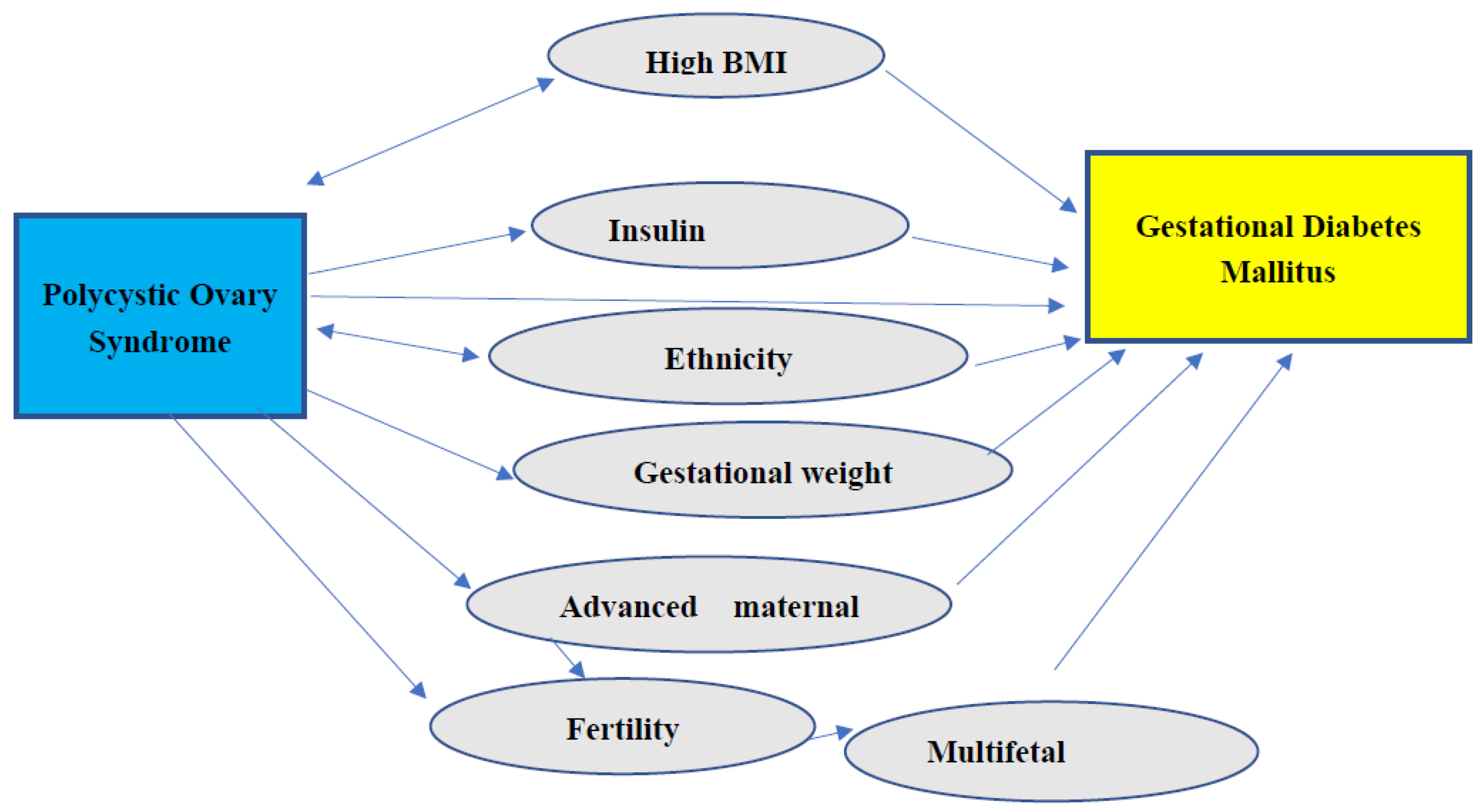
3.3. Other Risk Factors in PCOS Contributing to the Development of GDM
4. Discussion
4.1. Risk of GDM in PCOS
4.1.1. Family History of Diabetes, Ethnicity, and Occurrence of GDM
4.1.2. PCOS and Insulin Resistance
4.1.3. GDM and Obesity
4.1.4. GDM and Gestational Weight Gain
4.1.5. GDM and ART
4.2. Prevalence of PCOS in GDM
4.3. Predictors of GDM in PCOS Patients
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ehrmann, D.A. Polycystic ovary syndrome. N Engl J Med. 2005, 352, 1223–1236. [Google Scholar] [CrossRef] [PubMed]
- Sirmans, S.M.; Pate, K.A. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol 2013, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.; Deeks, A.; Moran, L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010, 8, 41–41. [Google Scholar] [CrossRef] [PubMed]
- Azziz, R.; Carmina, E.; Chen, Z.; Dunaif, A.; Laven, J.S.; Legro, R.S.; Lizneva, D.; Natterson-Horowtiz, B.; Teede, H.J.; Yildiz, B.O. Polycystic ovary syndrome. Nat Rev Dis Primers 2016, 2, 16057. [Google Scholar] [CrossRef] [PubMed]
- Asghari, K.M.; Nejadghaderi, S.A.; Alizadeh, M.; Sanaie, S.; Sullman, M.J.M.; Kolahi, A.-A.; Avery, J.; Safiri, S. Burden of polycystic ovary syndrome in the Middle East and North Africa region, 1990–2019. Sci. Rep. 2022, 12, 1–11. [Google Scholar] [CrossRef]
- United Nations Children’s Fund (UNICEF). Progress for children: a report card on adolescents; UNICEF: NewYork, 2012. [Google Scholar]
- Hahn, S.; E Janssen, O.; Tan, S.; Pleger, K.; Mann, K.; Schedlowski, M.; Kimmig, R.; Benson, S.; Balamitsa, E.; Elsenbruch, S. Clinical and psychological correlates of quality-of-life in polycystic ovary syndrome. Eur. J. Endocrinol. 2005, 153, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Strowitzki, T. Advanced diagnosis of polycystic ovary syndrome—new prediction models with standard parameters. Fertil. Steril. 2021, 115, 92–93. [Google Scholar] [CrossRef]
- The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [CrossRef] [PubMed]
- Koric, A.; Singh, B.; VanDerslice, J.A.; Stanford, J.B.; Rogers, C.R.; Egan, D.T.; Agyemang, D.O.; Schliep, K. Polycystic ovary syndrome and postpartum depression symptoms: a population-based cohort study. Am. J. Obstet. Gynecol. 2021, 224, 591–e1. [Google Scholar] [CrossRef]
- D’alterio, M.N.; Sigilli, M.; Succu, A.G.; Ghisu, V.; Laganà, A.S.; Sorrentino, F.; Nappi, L.; Tinelli, R.; Angioni, S. Pregnancy outcomes in women with polycystic ovarian syndrome. Minerva Obstet. Gynecol. 2022, 74, 45–59. [Google Scholar] [CrossRef]
- Eroglu, D.; Zeyneloglu, H.B. Metabolic disorders in patients with recent gestational diabetes mellitus. J. Obstet. Gynaecol. Res. 2006, 32, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, F.G.; Leveno, K.J.; Bloom, S.L.; Hauth, J.C.; Gilstrap, L.C.I.I.I.; Wenstrom, K.D. Diabetes. In: Cunningham FG, editors. Williams Obstetrics. New York: McGraw-Hill; 2005;1172–1173.
- Zhu, Y.; Zhang, C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: a Global Perspective. Curr. Diabetes Rep. 2016, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, P.; A Magee, L.; Banerjee, A.; Coleman, M.A.; Von Dadelszen, P.; Denison, F.; Farmer, A.; Finer, S.; Fox-Rushby, J.; Holt, R.; et al. Gestational diabetes: opportunities for improving maternal and child health. Lancet Diabetes Endocrinol. 2020, 8, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Rajab, K.E.; Issa, A.A.; Hasan, Z.A.; Rajab, E.; Jaradat, A.A. Incidence of gestational diabetes mellitus in Bahrain from 2002 to 2010. Int. J. Gynecol. Obstet. 2012, 117, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.M.; Dhatt, G.S.; Punnose, J.; Koster, G. Gestational diabetes: dilemma caused by multiple international diagnostic criteria. Diabet. Med. 2005, 22, 1731–1736. [Google Scholar] [CrossRef] [PubMed]
- Leng, J.; Shao, P.; Zhang, C.; Tian, H.; Zhang, F.; Zhang, S.; Dong, L.; Li, L.; Yu, Z.; Chan, J.C.N.; et al. Prevalence of Gestational Diabetes Mellitus and Its Risk Factors in Chinese Pregnant Women: A Prospective Population-Based Study in Tianjin, China. PLOS ONE 2015, 10, e0121029–e0121029. [Google Scholar] [PubMed]
- Eades, C.E.; Cameron, D.M.; Evans, J.M. Prevalence of gestational diabetes mellitus in Europe: A meta-analysis. Diabetes Res. Clin. Pr. 2017, 129, 173–181. [Google Scholar] [CrossRef]
- Bashir, M.M.; Ahmed, L.A.; Elbarazi, I.; Loney, T.; Al-Rifai, R.H.; Alkaabi, J.M.; Al-Maskari, F. Incidence of gestational diabetes mellitus in the United Arab Emirates; comparison of six diagnostic criteria: The Mutaba’ah Study. Front. Endocrinol. 2022, 13, 1069477. [Google Scholar] [CrossRef]
- Agarwal, M.M.; Dhatt, G.S.; Othman, Y. Gestational diabetes in a tertiary care hospital: implications of applying the IADPSG criteria. Arch. Gynecol. Obstet. 2012, 286, 373–378. [Google Scholar] [CrossRef]
- Hashim, M.; Radwan, H.; Hasan, H.; Obaid, R.S.; Al Ghazal, H.; Al Hilali, M.; Rayess, R.; Chehayber, N.; Mohamed, H.J.J.; Naja, F. Gestational weight gain and gestational diabetes among Emirati and Arab women in the United Arab Emirates: results from the MISC cohort. BMC Pregnancy Childbirth 2019, 19, 1–11. [Google Scholar] [CrossRef]
- Feig, D.S.; Berger, H.; Donovan, L.; Godbout, A.; Kader, T.; et al. Diabetes Canada Clinical Practice Guidelines Expert Committee Diabetes Pregnancy Can, J. Diabetes. 2018;42(Suppl. S1):S255–82.
- Vitacolonna, E.; Succurro, E.; Lapolla, A.; Scavini, M.; Bonomo, M.; Di Cianni, G.; Di Benedetto, A.; Napoli, A.; Tumminia, A.; Festa, C.; et al. Guidelines for the screening and diagnosis of gestational diabetes in Italy from 2010 to 2019: critical issues and the potential for improvement. Acta Diabetol. 2019, 56, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- HAPO Study Cooperative Research Group; Metzger, B. E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hod, M.; et al. Hyperglycemia and Adverse Pregnancy Outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [CrossRef]
- Behboudi-Gandevani, S.; Amiri, M.; Bidhendi Yarandi, R.; Ramezani Tehrani, F. The impact of diagnostic criteria for gestational diabetes on its prevalence: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Rani, P.R. Screening and Diagnosis of Gestational Diabetes Mellitus, Where Do We Stand. J. Clin. Diagn. Res. 2016, 10, QE01–4. [Google Scholar] [CrossRef] [PubMed]
- Cortel, M.R.B.P.; Manalo, M.E.M.; Canivel, R.R.C.; Matias, R.S.; Dizon, A.J.B.; Bacani, M.N.S.; Dalmacio, J.S.B. Screening and Diagnosis of Gestational Diabetes Mellitus Using 75-g Oral Glucose Tolerance Test Following the WHO, ADA, and IADPSG Criteria. J. Diabetes Metab. 2018, 09, 1–4. [Google Scholar] [CrossRef]
- Yang, H.; Wei, Y.; Gao, X.; Xu, X.; Fan, L.; He, J.; Hu, Y.; Liu, X.; Chen, X.; Yang, Z.; et al. Risk factors for gestational diabetes mellitus in Chinese women—a prospective study of 16 286 pregnant women in China. Diabet. Med. 2009, 26, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, U.J.; Latif, L. RISK FACTORS ASSOCIATED WITH GESTATIONAL DIABETES MELLITUS IN FEMALES PRESENTED AT A TERTIARY CARE HOSPITAL OF LAHORE. Biomedica 2015, 31, 315–317. [Google Scholar]
- Brennan, L.; Teede, H.; Skouteris, H.; Linardon, J.; Hill, B.; Moran, L. Lifestyle and Behavioral Management of Polycystic Ovary Syndrome. J. Women's Heal. 2017, 26, 836–848. [Google Scholar] [CrossRef]
- Boomsma, C.; Eijkemans, M.; Hughes, E.; Visser, G.; Fauser, B.; Macklon, N. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum. Reprod. Updat. 2006, 12, 673–683. [Google Scholar] [CrossRef]
- Kjerulff, L.E.; Sanchez-Ramos, L.; Duffy, D. Pregnancy outcomes in women with polycystic ovary syndrome: a meta-analysis. Am J Obstet Gynecol. 2011;204:558.e551-556.
- Qin, J.Z.; Pang, L.H.; Li, M.J.; Fan, X.J.; Huang, R.D.; Chen, H.Y. Obstetric complications in women with polycystic ovary syndrome: a systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2013, 11, 1–56. [Google Scholar] [CrossRef]
- Yu, H.F.; Chen, H.S.; Rao, D.P.; Gong, J. Association between polycystic ovary syndrome and the risk of pregnancy complications: a PRISMA-compliant systematic review and meta-analysis. Medicine. 2016;95:e4863.
- NICE guideline [NG3]. Diabetes in pregnancy: management from preconception to the postnatal period. Available online: https://www.nice.org.uk (accessed on 11 January 2024).
- de Wilde, M.A.; Veltman-Verhulst, S.M.; Goverde, A.J.; Lambalk, C.B.; Laven, J.S.E.; Franx, A.; Koster, M.P.H.; Eijkemans, M.J.C.; Fauser, B.C.J.M. Preconception predictors of gestational diabetes: a multicentre prospective cohort study on the predominant complication of pregnancy in polycystic ovary syndrome. Hum. Reprod. 2014, 29, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.; Rasul, R.A. The Prevalence of Prenatal Diabetes Mellitus in Rania City: Women with Polycystic Ovaries. kufa J. Nurs. Sci. 2023, 13. [Google Scholar] [CrossRef]
- The Joanna Briggs Institute Checklist for Randomized Controlled Trials. Available online: http://joannabriggs.org/research/critical-appraisal-tools.html (accessed on 3 January 2022).
- Altieri, P.; Gambineri, A.; Prontera, O.; Cionci, G.; Franchina, M.; Pasquali, R. Maternal polycystic ovary syndrome may be associated with adverse pregnancy outcomes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 149, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Veltman-Verhulst, S.M.; Van Haeften, T.W.; Eijkemans, M.J.C.; De Valk, H.W.; Fauser, B.C.J.M.; Goverde, A.J. Sex hormone-binding globulin concentrations before conception as a predictor for gestational diabetes in women with polycystic ovary syndrome. Hum. Reprod. 2010, 25, 3123–3128. [Google Scholar] [CrossRef] [PubMed]
- Han, A.R.; Kim, H.O.; Cha, S.W.; Park, C.W.; Kim, J.Y.; Yang, K.M.; Song, I.O.; Koong, M.K.; Kang, I.S. Adverse pregnancy outcomes with assisted reproductive technology in non-obese women with polycystic ovary syndrome: a case-control study. Clin. Exp. Reprod. Med. 2011, 38, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Roos, N.; Kieler, H.; Sahlin, L.; Ekman-Ordeberg, G.; Falconer, H.; Stephansson, O. Risk of adverse pregnancy outcomes in women with polycystic ovary syndrome: population based cohort study. BMJ 2011, 343, d6309–d6309. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Muñoz, E.; Castellanos-Barroso, G.; Ramírez-Eugenio, B.Y.; Ortega-González, C.; Parra, A.; Castillo-Mora, A.; De la Jara-Díaz, J.F. The risk of gestational diabetes mellitus among Mexican women with a history of infertility and polycystic ovary syndrome. Fertil. Steril. 2012, 97, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, M.; Sheikhan, F.; Arabipoor, A.; Hosseini, R.; Nourbakhsh, F.; Zolfaghari, Z. Gestational diabetes mellitus risk factors in women with polycystic ovary syndrome (PCOS). Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 181, 195–199. [Google Scholar] [CrossRef] [PubMed]
- de Wilde, M.A.; Goverde, A.J.; Veltman-Verhulst, S.M.; Eijkemans, M.J.C.; Franx, A.; Fauser, B.C.J.M.; Koster, M.P.H. Insulin action in women with polycystic ovary syndrome and its relation to gestational diabetes. Hum. Reprod. 2015, 30, 1447–1453. [Google Scholar] [CrossRef]
- Sawada, M.; Masuyama, H.; Hayata, K.; Kamada, Y.; Nakamura, K.; Hiramatsu, Y. Pregnancy complications and glucose intolerance in women with polycystic ovary syndrome. Endocr. J. 2015, 62, 1017–1023. [Google Scholar] [CrossRef]
- Jin, J.; Lu, Z.; Li, Y.; Cowart, L.A.; Lopes-Virella, M.F.; Huang, Y. Docosahexaenoic acid antagonizes the boosting effect of palmitic acid on LPS inflammatory signaling by inhibiting gene transcription and ceramide synthesis. PLoS One. 2018, 13: e0193343.
- Xiao, Q.; Cui, Y.-Y.; Lu, J.; Zhang, G.-Z.; Zeng, F.-L. Risk for Gestational Diabetes Mellitus and Adverse Birth Outcomes in Chinese Women with Polycystic Ovary Syndrome. Int. J. Endocrinol. 2016, 2016, 1–6. [Google Scholar] [CrossRef]
- Xia, H.; Zhang, R.; Sun, X.; Wang, L.; Zhang, W. Valuable predictors of gestational diabetes mellitus in infertile Chinese women with polycystic ovary syndrome: a prospective cohort study. Gynecol. Endocrinol. 2017, 33, 448–451. [Google Scholar] [CrossRef]
- Zheng, W.; Huang, W.; Zhang, L.; Tian, Z.; Yan, Q.; Wang, T.; Zhang, L.; Li, G. Early pregnancy metabolic factors associated with gestational diabetes mellitus in normal-weight women with polycystic ovary syndrome: a two-phase cohort study. Diabetol. Metab. Syndr. 2019, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fougner, S.L.; Vanky, E.; Løvvik, T.S.; Carlsen, S.M. No impact of gestational diabetes mellitus on pregnancy complications in women with PCOS, regardless of GDM criteria used. PLOS ONE 2021, 16, e0254895. [Google Scholar] [PubMed]
- Li, X.; Liu, X.; Zuo, Y.; Gao, J.; Liu, Y.; Zheng, W. The risk factors of gestational diabetes mellitus in patients with polycystic ovary syndrome. Medicine 2021, 100, e26521. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, L.; A Naaz, S.; Das, B.; Dash, P.; Pattanaik, M. Adverse Pregnancy Outcome in Polycystic Ovarian Syndrome: A Comparative Study. Cureus 2022, 14, e25790. [Google Scholar] [CrossRef]
- Ouyang, P.; Duan, S.; You, Y.; Jia, X.; Yang, L. Risk prediction of gestational diabetes mellitus in women with polycystic ovary syndrome based on a nomogram model. BMC Pregnancy Childbirth 2023, 23, 1–8. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Zhao, H.; Ding, H.; Tan, J.; Chen, J.; Zhang, R.; Azziz, R.; Yang, D. Risks for Gestational Diabetes Mellitus and Pregnancy-Induced Hypertension Are Increased in Polycystic Ovary Syndrome. BioMed Res. Int. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Sterling, L.; Liu, J.; Okun, N.; Sakhuja, A.; Sierra, S.; Greenblatt, E. Pregnancy outcomes in women with polycystic ovary syndrome undergoing in vitro fertilization. Fertil. Steril. 2016, 105, 791–797. [Google Scholar] [CrossRef]
- Liu, S.; Mo, M.; Xiao, S.; Li, L.; Hu, X.; Hong, L.; Wang, L.; Lian, R.; Huang, C.; Zeng, Y.; et al. Pregnancy Outcomes of Women With Polycystic Ovary Syndrome for the FirstIn VitroFertilization Treatment: A Retrospective Cohort Study With 7678 Patients. Front. Endocrinol. 2020, 11. [Google Scholar] [CrossRef]
- Qiu, M.; Qu, J.; Tian, Y.; Wang, Y. The influence of polycystic ovarian syndrome on obstetric and neonatal outcomes after frozen-thawed embryo transfer. Reprod. Biomed. Online 2022, 45, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, T.; Albogami, A.; Allhuaidan, A.; Alfawaz, S.; Murad, S.; Kofi, M. Prevalence of Gestational Diabetes Mellitus and Associated Risk Factors Among Pregnant Women Attending Antenatal Care in Primary Health Care Centers in Riyadh, Saudi Arabia. Fam. Med. Prim. Care: Open Access 2021, 5. [Google Scholar] [CrossRef]
- Mustaniemi, S.; Vääräsmäki, M.; Eriksson, J.G.; Gissler, M.; Laivuori, H.; Ijäs, H.; Bloigu, A.; Kajantie, E.; Morin-Papunen, L. Polycystic ovary syndrome and risk factors for gestational diabetes. Endocr. Connect. 2018, 7, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Bond, R.; Pace, R.; Rahme, E.; Dasgupta, K. Diabetes risk in women with gestational diabetes mellitus and a history of polycystic ovary syndrome: a retrospective cohort study. Diabet. Med. 2017, 34, 1684–1695. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, A.; Hanley, A.; Ni, A.; Tomlinson, G.; Feig, D.S. Does the presence of polycystic ovary syndrome increase the risk of obstetrical complications in women with gestational diabetes? J. Matern. Neonatal Med. 2010, 23, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Foroozanfard, F.; Moosavi, S.G.A.; Mansouri, F.; Bazarganipour, F. Obstetric and Neonatal Outcome in PCOS with Gestational Diabetes Mellitus. 2014, 8, 7–12.
- Kashanian, M.; Fazy, Z.; Pirak, A. Evaluation of the relationship between gestational diabetes and a history of polycystic ovarian syndrome. Diabetes Res. Clin. Pr. 2008, 80, 289–292. [Google Scholar] [CrossRef]
- Kakoly, N.S.; Earnest, A.; Moran, L.J.; Teede, H.J.; Joham, A.E. Group-based developmental BMI trajectories, polycystic ovary syndrome, and gestational diabetes: a community-based longitudinal study. BMC Med. 2017, 15, 1–9. [Google Scholar] [CrossRef]
- Turhan, N.; Seçkin, N.; Aybar, F.; Inegöl, I. Assessment of glucose tolerance and pregnancy outcome of polycystic ovary patients. Int. J. Gynecol. Obstet. 2003, 81, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care. 2007;30 Suppl 2:S141–S6.
- Callesen, N.F.; Ringholm, L.; Stage, E.; Damm, P.; Mathiesen, E.R. Insulin requirements in type 1 diabetic pregnancy: do twin pregnant women require twice as much insulin as singleton pregnant women? Diabetes Care. 2012;35:1246–8.
- Elkholi, D.G.E.Y.; Nagy, H.M. The effects of adipocytokines on the endocrino-metabolic features and obstetric outcome in pregnant obese women with polycystic ovary syndrome. Middle East Fertil. Soc. J. 2014, 19, 293–302. [Google Scholar] [CrossRef]
- Haakova, L.; Cibula, D.; Rezabek, K.; Hill, M.; Fanta, M.; Zivny, J. Pregnancy outcome in women with PCOS and in controls matched by age and weight. Hum. Reprod. 2003, 18, 1438–1441. [Google Scholar] [CrossRef]
- Bahri Khomami M, JohamAE, Boyle JA, et al. The role of maternal obesity in infant outcomes in polycystic ovary syndrome—a systematic review, meta-analysis, and meta-regression. Obes Rev 2019, 20, 842–858. [CrossRef] [PubMed]
- Khomami, M.B.; Boyle, J.A.; Tay, C.T.; Vanky, E.; Teede, H.J.; Joham, A.E.; Moran, L.J. Polycystic ovary syndrome and adverse pregnancy outcomes: Current state of knowledge, challenges and potential implications for practice. Clin. Endocrinol. 2018, 88, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Bian, C.; Zhao, X. Association of polycystic ovary syndrome with metabolic syndrome and gestational diabetes: Aggravated complication of pregnancy. Exp. Ther. Med. 2017, 14, 1271–1276. [Google Scholar] [CrossRef]
- Esmaeilzadeh, S.; Tahmasbpour, E.; Gholinezhad-Chari, M. Hyperhomocysteinemia, insulin resistance and body mass index in Iranian young women with polycystic ovary syndrome. Middle East Fertil. Soc. J. 2017, 22, 149–155. [Google Scholar] [CrossRef]
- Stepto, N.K.; Cassar, S.; Joham, A.E.; Hutchison, S.K.; Harrison, C.L.; Goldstein, R.F.; Teede, H.J. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum. Reprod. 2013, 28, 777–784. [Google Scholar] [CrossRef]
- VrbÍková, J.; Cibula, D.; Dvor̆áková, K.; Stanická, S.; Šindelka, G.; Hill, M.; Fanta, M.; Vondra, K.; Škrha, J. Insulin Sensitivity in Women with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2004, 89, 2942–2945. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Dunaif, A. Insulin Resistance and the Polycystic Ovary Syndrome Revisited: An Update on Mechanisms and Implications. Endocr. Rev. 2012, 33, 981–1030. [Google Scholar] [CrossRef]
- Afandi, B.; Hassanein, M.M.; Majd, L.M.; Nagelkerke, N.J.D. Impact of Ramadan fasting on glucose levels in women with gestational diabetes mellitus treated with diet alone or diet plus metformin: a continuous glucose monitoring study. BMJ Open Diabetes Res. Care 2017, 5, e000470. [Google Scholar] [CrossRef]
- Dube, R. Using Metformin in Pregnancy for Different Indications: Are We Any Wiser now? Applied Clinical Research, Clinical Trials & Regulatory Affairs.2016;3:3-19.
- Dumesic, D.A.; Oberfield, S.E.; Stener-Victorin, E.; Marshall, J.C.; Laven, J.S.; Legro, R.S. Scientific Statement on the Diagnostic Criteria, Epidemiology, Pathophysiology, and Molecular Genetics of Polycystic Ovary Syndrome. Endocr. Rev. 2015, 36, 487–525. [Google Scholar] [CrossRef]
- Dube, R. Does endothelial dysfunction correlate with endocrinal abnormalities in patients with polycystic ovary syndrome? Avicenna J. Med. 2016, 06, 91–102. [Google Scholar] [CrossRef]
- Chowdhury, F.; Dube, R.; Riyaz, R.; Khan, K.; Al-Zuheiri, S.T.S.; Rangraze, I.R. Title-Efficacy of metformin as monotherapy in gestational and pre-gestational diabetic pregnant women. J. Adv. Pharm. Educ. Res. 2024, 14, 84–90. [Google Scholar] [CrossRef]
- Joham, A.E.; Ranasinha, S.; Zoungas, S.; Moran, L.; Teede, H.J. Gestational Diabetes and Type 2 Diabetes in Reproductive-Aged Women With Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2014, 99, E447–E452. [Google Scholar] [CrossRef] [PubMed]
- Kent, J.; Dodson, W.C.; Kunselman, A.; Pauli, J.; Stone, A.; Diamond, M.P.; Coutifaris, C.; Schlaff, W.D.; Alvero, R.; Casson, P.; et al. Gestational Weight Gain in Women With Polycystic Ovary Syndrome: A Controlled Study. J. Clin. Endocrinol. Metab. 2018, 103, 4315–4323. [Google Scholar] [CrossRef] [PubMed]
- Palomba, S.; de Wilde, M.A.; Falbo, A.; Koster, M.P.; La Sala, G.B.; Fauser, B.C. Pregnancy complications in women with polycystic ovary syndrome. Hum. Reprod. Updat. 2015, 21, 575–592. [Google Scholar] [CrossRef]
- Durie, D.E.; Thornburg, L.L.; Glantz, J.C. Effect of Second-Trimester and Third-Trimester Rate of Gestational Weight Gain on Maternal and Neonatal Outcomes. Obstet. Gynecol. 2011, 118, 569–575. [Google Scholar] [CrossRef]
- Siega-Riz, A.M.; Viswanathan, M.; Moos, M.-K.; Deierlein, A.; Mumford, S.; Knaack, J.; Thieda, P.; Lux, L.J.; Lohr, K.N. A systematic review of outcomes of maternal weight gain according to the Institute of Medicine recommendations: birthweight, fetal growth, and postpartum weight retention. Am. J. Obstet. Gynecol. 2009, 201, 339–e1. [Google Scholar] [CrossRef]
- Rees, D.A.; Jenkins-Jones, S.; Morgan, C.L. Contemporary Reproductive Outcomes for Patients With Polycystic Ovary Syndrome: A Retrospective Observational Study. J. Clin. Endocrinol. Metab. 2016, 101, 1664–1672. [Google Scholar] [CrossRef]
- Ali, A.D.; Mehrass, A.A.-K.; Al-Adhroey, A.H.; Al-Shammakh, A.; Amran, A. Prevalence and risk factors of gestational diabetes mellitus in Yemen. Int. J. Women's Heal. 2016, 8, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Read, S.; Berger, H.; Feig, D.; Fleming, K.; Ray, J.G.; Shah, B.R.; Lipscombe, L. Influence of Ethnicity on the Association between Body Mass Index and Prevalence of Gestational Diabetes. Diabetes 2020, 69. [Google Scholar] [CrossRef]
- Vollenhoven, B.; Kovacs, S.G.; Burge, H.; Healy, D. Prevalence of gestational diabetes rnellitus in polycystic ovarian syndrome (PCOS) patients pregnant after ovulation induction with gonadotrophins. Aust N Z J Obstet Gynaecol 2000; 40:1:54-8.
- Ashrafi, M.; Gosili, R.; Hosseini, R.; Arabipoor, A.; Ahmadi, J.; Chehrazi, M. Risk of gestational diabetes mellitus in patients undergoing assisted reproductive techniques. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 176, 149–152. [Google Scholar] [CrossRef]
- Wang, Y.A.; Nikravan, R.; Smith, H.C.; Sullivan, E.A. Higher prevalence of gestational diabetes mellitus following assisted reproduction technology treatment. Hum. Reprod. 2013, 28, 2554–2561. [Google Scholar] [CrossRef] [PubMed]
- Moosazadeh, M.; Asemi, Z.; Lankarani, K.B.; Tabrizi, R.; Maharlouei, N.; Naghibzadeh-Tahami, A.; Yousefzadeh, G.; Sadeghi, R.; Khatibi, S.R.; Afshari, M.; et al. Family history of diabetes and the risk of gestational diabetes mellitus in Iran: A systematic review and meta-analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, S99–S104. [Google Scholar] [CrossRef] [PubMed]
- Ban, M.; Sun, Y.; Chen, X.; Zhou, X.; Zhang, Y.; Cui, L. Association between maternal polycystic ovarian syndrome undergoing assisted reproductive technology and pregnancy complications and neonatal outcomes: a systematic review and meta-analysis. J. Ovarian Res. 2024, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Farland, L.V.; E Stern, J.; Liu, C.-L.; Cabral, H.J.; Coddington, C.C.; Diop, H.; Dukhovny, D.; Hwang, S.; A Missmer, S. Polycystic ovary syndrome and risk of adverse pregnancy outcomes: a registry linkage study from Massachusetts. Hum. Reprod. 2022, 37, 2690–2699. [Google Scholar] [CrossRef] [PubMed]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef] [PubMed]
- Popova, P.V.; Klyushina, A.A.; Vasilyeva, L.B.; Tkachuk, A.S.; Bolotko, Y.A.; Gerasimov, A.S.; Pustozerov, E.A.; Kravchuk, E.N.; Predeus, A.; Kostareva, A.A.; et al. Effect of gene-lifestyle interaction on gestational diabetes risk. Oncotarget 2017, 8, 112024–112035. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Hu, Y.; Zeng, J.; Zheng, L.; Ye, P.; Wei, D.; Chen, D. Analysis of risk factors related to gestational diabetes mellitus. Taiwan. J. Obstet. Gynecol. 2020, 59, 718–722. [Google Scholar] [CrossRef] [PubMed]
- West, S.; Ollila, M.; Franks, S.; Piltonen, T.; Jokelainen, J.; Nevalainen, J.; Puukka, K.; Ruokonen, A.; Järvelin, M.; Auvinen, J.; et al. Overweight, obesity and hyperandrogenemia are associated with gestational diabetes mellitus: A follow-up cohort study. Acta Obstet. et Gynecol. Scand. 2020, 99, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Alkaabi, J.; Almazrouei, R.; Zoubeidi, T.; Alkaabi, F.M.; Alkendi, F.R.; Almiri, A.E.; Sharma, C.; Souid, A.-K.; Ali, N.; Ahmed, L.A. Burden, associated risk factors and adverse outcomes of gestational diabetes mellitus in twin pregnancies in Al Ain, UAE. BMC Pregnancy Childbirth 2020, 20, 1–8. [Google Scholar] [CrossRef]
- Juber, N.F.; Abdulle, A.; AlJunaibi, A.; AlNaeemi, A.; Ahmad, A.; Leinberger-Jabari, A.; Al Dhaheri, A.S.; AlZaabi, E.; Mezhal, F.; Al-Maskari, F.; et al. Maternal Early-Life Risk Factors and Later Gestational Diabetes Mellitus: A Cross-Sectional Analysis of the UAE Healthy Future Study (UAEHFS). Int. J. Environ. Res. Public Heal. 2022, 19, 10339. [Google Scholar] [CrossRef]
- Hedderson, M.M.; Darbinian, J.A.; Ferrara, A. Disparities in the risk of gestational diabetes by race-ethnicity and country of birth. Paediatr. Périnat. Epidemiology 2010, 24, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, A.; Giveon, S.; Rubin, C.; Novikov, I.; Ziv, A.; Kalter-Leibovici, O. Gestational diabetes risk in a multi-ethnic population. Acta Diabetol. 2019, 57, 263–269. [Google Scholar] [CrossRef]
- Manoharan, V.; Wong, V.W. Impact of comorbid polycystic ovarian syndrome and gestational diabetes mellitus on pregnancy outcomes: a retrospective cohort study. BMC Pregnancy Childbirth 2020, 20, 1–7. [Google Scholar] [CrossRef]
- Thomann, R.; Rossinelli, N.; Keller, U.; Tirri, B.F.; De Geyter, C.; Ruiz, J.; Kränzlin, M.; Puder, J.J. Differences in low-grade chronic inflammation and insulin resistance in women with previous gestational diabetes mellitus and women with polycystic ovary syndrome. Gynecol. Endocrinol. 2008, 24, 199–206. [Google Scholar] [CrossRef]
- Barnes RA, Wong T, Ross GP, et al. Excessive weight gain before and during gestational diabetes mellitus management: What is the impact? Diabetes Care 2020, 43, 74–81. [CrossRef]
- Gilmore, L.A.; Klempel-Donchenko, M.; Redman, L.M. Pregnancy as a window to future health: Excessive gestational weight gain and obesity. Semin. Perinatol. 2015, 39, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Oken, E.; Kleinman, K.P.; Belfort, M.B.; Hammitt, J.K.; Gillman, M.W. Associations of Gestational Weight Gain with Short- and Longer-term Maternal and Child Health Outcomes. Am. J. Epidemiology 2009, 170, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Rf, G.; Sk, A.; S, R.; M, M.; Ja, B.; Mh, B.; N, L.; G, H.; F, C.; L, R.; et al. Association of Gestational Weight Gain with Maternal and Infant Outcomes: A Systematic Review and Meta-analysis. Yearb. Paediatr. Endocrinol. 2018. [Google Scholar] [CrossRef]
- Alejandro, E.U.; Mamerto, T.P.; Chung, G.; Villavieja, A.; Gaus, N.L.; Morgan, E.; Pineda-Cortel, M.R.B. Gestational Diabetes Mellitus: A Harbinger of the Vicious Cycle of Diabetes. Int. J. Mol. Sci. 2020, 21, 5003. [Google Scholar] [CrossRef]


| Threshold for GDM diagnosis | Fasting [mmol/L (mg/dL)] |
1HPG [mmol/L (mg/dL)] |
2HPG [mmol/L (mg/dL)] |
|---|---|---|---|
| 75-g OGTT | |||
| ADA | 5.1 (92.0) | 10.0 (180.0) | 8.5 (152.0) |
| IADPSG | 5.1 (≥92.5) | 10.0 (≥180.0) | 8.5 (≥153.0) |
| WHO | 5.1–6.9 (92.0–125.0) | ≥10.0 (180.0) | 8.5–11.0 (153.0–199.0) |
| Author [Reference] |
Sample size | Prevalence of GDM n (%) |
Prevalence of PCOS | Serial number |
|---|---|---|---|---|
| Altieri et al. [40] | 516 | 3 (20%) | 15 | |
| Veltman-Verhulst et al. [41] | 50 | 21 (42%) | 50 | |
| Han A R et al. [42] | 336 | 10 (2.9%) | 336 | |
| Roos N et al. [43] | 1,195,123 | 125 (3.3%) | 3787 | |
| Reyes-Munoz et al. [44] | 104 | 14 (26.9%) | 52 | |
| de Wilde et al. [37] | 326 | 41 (21.6%) | 189 | |
| Ashrafi et al. [45] | 702 | 104 (44.4%) | 234 | |
| de Wilde et al. [46] | 72 | 22 (30.5%) | 72 | |
| Sawada et al. [47] | 113 | 12 (24.5%) | 64 | |
| Pan et al. [48] | 7,629 | 636 (20.46%) | 3,109 | |
| Xiao et al. [49] | 2389 | 64 (18.1%) | 352 | |
| Rees et al. [87] | 27,204 | 253 (4.4%) | 9068 | |
| Xia et al. [50] | 94 | 31 (32.9%) | 94 | |
| Zheng et al. -1[51] | 566 | 39 (26.5%) | 242 | 14 A. |
| Zheng et al. -2[51] | 18,106 | 135 (22.09%) | 877 | 14 B. |
| Fougner et al. [52] | 791 | 297 (41.1%) | 722 | |
| Li et al. [53] | 196 | 47 (23.98%) | 196 | |
| Patnaik et al. [54] | 102 | 9 (17.6%) | 51 | |
| Ouyang et al. [55] | 434 | 104 (24%) | 434 | |
| Wang et al. [56] | 814 | 79 (54.9 %) | 144 | |
| Sterling et al. [57] | 394 | 11 (15.5%) | 71 | |
| Liu et al. [58] | 7678 | 37 (9.7%) | 381 | |
| Qiu et al. [59] | 16,506 | 272 (14.49%) | 1876 | |
| Total | 1,280,245 | 2, 366 (10.55%) | 22,416 |
| Serial no. | Reference | GDM (n) | PCOS (n) |
|---|---|---|---|
| 1. | 60 | 125 | 15 |
| 2. | 61 | 1014 | 174 |
| 3. | 62 | 34,686 | 520 |
| 4. | 63 | 171 | 44 |
| 5. | 64 | 261 | 131 |
| 6. | 65 | 94 | 15 |
| Total | 36,351 | 899 |
| Serial no. | Factor [ reference] | Evidence [reference] |
|---|---|---|
| 1. | High BMI [41,46,51,61,66,67,68,69,70,71] |
High BMI increases PCOS [41] High BMI causes GDM but not PCOS [46,60,61,66,67,68,69,70,71] Normal BMI with PCOS had a higher risk of GDM than obese [51] |
| 2. | IR [72,73,74,75,76,77,78,79,80,81,82,83] |
IR was higher in Obese PCOS only [77] IR was higher even with a normal BMI [78] Use of Insulin sensitizers in patients with PCOS [79,80,81,82,83] |
| 3. | Gestational weight gain (GWG) [72,84,85,86,87,88] | GWG is higher in certain ethnicities [72] Overweight women have higher GWG [84,85,86] |
| 4. | Ethnicity and family history [55,72,89,90,91] | GWG is higher in certain ethnicities [72] GDM and PCOS both have a higher prevalence in certain ethnicities [81,82,83,84,85,86,87,88,89] |
| 5. | Multifetal gestation, ART [92,93,94] |
GDM is not higher in PCOS conceived with ART if age and BMI matched [90] GDM is higher with ART compared to spontaneous conception and in Multifetal gestation following ART [93,94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





