1. Introduction
Various anthropogenic activities, escalating urbanization, industrialization, and economic growth are leading to the production of huge quantities of solid waste around the globe. The management of this solid waste has now become an ecological and technical problem for all [
1]. Compost is an organic fertilizer that can be safely used in agriculture after assessing its stability and maturity [
2]. Stability is usually defined in terms of the bioavailability of organic matter and refers exclusively to the resistance of compost organic matter to further degradation [
3]. Stability refers to a particular phase or decomposition or state of organic composting substances, which is related to the type of organic compounds present and the resulting biological activity in the materials [
4,
5]. Maturity is a term used to determine the level of phytotoxic substances in compost samples and the suitability of compost for plant growth. Also, maturity can be easily defined as a measure of composting completion [
1,
6]. Also, maturity is defined as the suitability of the material for plant growth and is often associated with the degree of compost humification [
7]. Maturity is not described by a single method that is universally applied to all types of compost due to variation in feedstock composition and composting procedures, so maturity is best assessed by measuring two or more tests such as physical, chemical, plant, and microbiological tests [
8,
9].
The total amount of soluble nitrogen in the organic mass decreases during composting and represents mineralization [
10]. During maturation, ammonium nitrogen levels decrease, while nitrate levels rise. The increased N-NO
3-/N-NH
4+ ratio is an indicator of compost maturity [
11]. When immature compost is introduced into the soil, there is a serious lack of nitrogen in crops, and rapid decomposition of immature compost causes a decrease in oxygen concentration in the root system, inhibits plant growth by producing phytotoxic substances, based on organic acids, ethylene oxide and ammonia [
12,
13].
Garglio et al. (2002) used garden cress as an indicator, while Fauci et al. (2002) used pinto beans and tomatoes in a biological study of plant growth [
14,
15]. Smith and Hughes (2001) compared the germination of garden cress and cellulolytic activity[
16]. Degradation of cellulose using filter paper as a substrate had a negative correlation with the fresh mass of cress roots. Although garden cress is very often used as an indicator plant worldwide, there is no universal plant species or universal germination test procedure. Also, there is little data on which plant species is more sensitive to toxic substances in compost than garden cress. Warman (1999) compared the germination of garden cress, radish, and cabbage in compost, compost, and soil mixtures, and in compost extracts and concluded that such tests are not sensitive enough to determine the differences between mature and immature composts[
17].
Germination index (GI) is the best way to test the phytotoxicity of compost for plant growth because the results are quite simple and reliable [
18]. The biological germination test is widely used to examine salinity, soil pathogens, toxic substances, and some other physical and chemical properties of compost [
19,
20], which could be the main potential causes of phytotoxicity. Several researchers state that phytotoxic compounds are gradually removed during the composting process, which could explain the increase in GI with composting time. The germination index (GI), which combines measures of relative seed germination (G%) and relative root elongation (L%), was used to assess compost toxicity [
21,
22,
23]. It has been observed that a GI value of 80% indicates the disappearance of phytotoxins in composts [
24]. Tiquia et al. (1996) used this value not only as an indicator of phytotoxicity disappearance but also as an indicator of compost maturity [
22]. The germination index is a maturity test based on seed germination and initial plant growth using a liquid compost extract [
24]. Compost is considered mature when the germination index is higher than 60% compared to the control with distilled water [
25]. The germination index is the most sensitive parameter used to assess the toxicity of compost to seedlings and to test whether the compost is mature [
23,
26,
27]. Tang et al. (2006) state that the extract ratio is a very important factor influencing GI [
28]. They showed that an extract ratio of 10: 1 was suitable for estimating GI changes during compost maturation. They also stated that different extraction ratios give different forms of GI change during the maturation process. The most popular germination test used by researchers is the garden cress experiment [
26,
29]. According to them, compost is non-toxic when the germination is higher than 85% or the weight of plant seedlings is higher than 90%. In addition, the authors found that the GI at each time of composting did not show significant changes by diluting the extract, nor when the extract was diluted to 75% with distilled water. Increased GI indicates reduced phytotoxicity and thus a more mature product [
22,
23,
30,
31].
Phytotoxicity is one of the most important criteria for assessing the maturity and suitability of compost for use in agriculture to avoid technological and environmental risks by introducing compost into the soil [
3,
7,
22]. Immature compost also contains phytotoxic compounds such as heavy metals [
21], phenolic compounds [
32], ethylene and ammonia [
21], and increased salt accumulation [
31], and organic acids [
33] that could slow seed germination and plant growth. Phytotoxicity is best assessed by germination or growth testing [
3,
14], but plant indicators must be carefully selected [
34].
The study aimed to compare the maturity estimates of compost based on the results of the germination test conducted with seeds of different species with the assessment of maturity based on chemical methods of substrate analysis. Also, the study aims to compare the suitability of monocotyledonous and dicotyledonous plant species for conducting a germination test.
2. Materials and Methods
2.1. Compost Sampling
The initial raw material for the production of compost was the waste of green and woody parts of plants collected during the maintenance of public green areas. The compost was prepared on a concrete dry surface, and then plant waste was placed in windrow with a width of 3 m, height of 2 m and length of 20 m. The compost was sampled [
35] after 3 months of windrow composting with occasional turning of the compost mass.
2.2. Analysis of Physical Properties of Compost
As part of the research on the physical properties of compost, compact density, percentage of water, percentage of dry matter, ash content, and organic matter content were analyzed.
The compact density of compost was determined by laboratory measurements following EN 13040 using cylinders of known volume and mass. Compact density values are expressed in g/L or g/dm
3 [
36].
The proportion of water and dry matter in compost samples was determined by drying 100 g of fresh compost matter at 103 ± 2 ° C to constant weight according to EN 13040 [
36]. The proportion of water and dry matter in composts is expressed as a percentage.
The laboratory procedure for determining the content of organic matter and ash in compost samples is prescribed by standard EN 13039 and is carried out by drying for at least 4 hours, at 103 ± 2 °C and 6 hours by successive annealing at 450 ± 10 °C in the annealing furnace and by weighing each additional hour of annealing to a constant mass [
37]. The content of organic matter and ash in composts is expressed as a percentage (percentage of dry matter of compost).
2.3. Analysis of Chemical Properties of Compost
The conducted research of chemical properties of compost included the following properties: pH value, electrical conductivity (EC), the content of total carbon and nitrogen, C/N ratio, the ratio of ammonium and nitrate form of nitrogen (NH4-N/NO3-N ratio), the content of total P and K.
The compost reaction, i.e. the pH value, was determined in a suspension of fresh compost in deionized water in a volume ratio of 1: 5 (60 ml of fresh sample and 300 ml of deionized water) after shaking for 60 minutes on a shaker. The pH value of compost was measured electrometrically (ie with pH meters that measure the difference in electrical potential) according to the European standard EN 13037: 2011 [
38]. Electrical conductivity was also measured (with a conductometer) in a suspension of fresh compost in deionized water in a volume ratio of 1: 5 (60 ml of fresh sample and 300 ml of deionized water) after shaking for 60 minutes on a shaker, according to European standard EN 13038: 2011 [
39].
The organic carbon content was determined by wet composting [
40]: 50 mg of dry sample was weighed into destruction cuvettes, filled with 5 ml of 0.27 M K
2Cr
2O
7 and 7.5 ml of concentrated H
2SO
4, destroyed for 30 minutes on a destruction block on 135 ° C, quantitatively transferred to volumetric flasks and diluted with deionized water to a total volume of 100 ml. After 10 minutes of centrifugation at 2000 rpm, the organic carbon concentration was measured indirectly by spectrophotometric (spectrophotometric absorption measurement at 585 nm with calibration with standard glucose solutions). For laboratory determination of total nitrogen concentration, the Kjeldahl digestion method after the destruction of the sample with a mixture of acids with heating. The total nitrogen content in compost is expressed as a percentage. The C/N ratio was calculated using data on total organic carbon and total nitrogen content.
Concentrations of two mineral forms of nitrogen (NH
4-N and NO
3-N) were determined in the causes of compost according to the standard EN 13652: 2001 [
41]. In the analysis, 10 g of fresh sample was used to determine the mineral forms of nitrogen. The results are expressed as g/kg NH
4-N and g/kg NO
3-N in a dry matter or in mg/L NH
4-N and NO
3-N in a fresh matter.
The concentration of total phosphorus was determined by the phosphorus-molybdenum method in a compost sample solution prepared by destroying the dry sample by digestion with nitric and hydrochloric acid. Total potassium concentrations were measured in-stock solution after digestion with nitric and hydrochloric acid using inductively coupled plasma optical emission spectrometry (ICP-OES). The measured concentrations P and K are expressed in g/kg dry matter of compost.
2.4. Analysis of Biological Properties of Compos
Within the biological properties of compost, laboratory measurement of compost respiration intensity and germination test were performed.
The respiration intensity of the compost was measured as the rate of CO
2 release from a fresh sample weighing 50 g after two days of incubation at room temperature. The emitted CO
2 was determined based on the neutralization of part of the template with NaOH according to the TMECC 05.08-B method [
44]. The results are presented in mg CO
2/g DM/day.
2.5. Germination Test
A modified Zucconi et al. (1981) germination test method using compost extracts. The method combines measuring shoot length and measuring root growth [
26]. Testing was performed on 4 plant species. The germination test determined the influence of different solutions (extracts of compost and ammonium carbonate solution) on germination, shoot growth, and elongation of roots of 4 plant species: garden cress (
Lepidium sativum L.), cucumber (
Cucumis sativus L.), barley (
Hordeum vulgare L.). and triticale (×
Triticosecale Wittmack).
The following solutions were used in the germination test, i.e. compost extracts: deionized water (control treatment), compost extract 1:2.5 (1:2.5 compost: deionized water v/v) - mark CE2.5, compost extract 1:10 (1:10 compost: deionized water v/v) - mark CE10, ammonium carbonate solution 200 mg/L (200 mg/L NH4-N) - mark SOL-1, ammonium carbonate solution 400 mg/L (400 mg/L NH4- N) - mark SOL-2 and ammonium carbonate solution 600 mg/L (600 mg/L NH4-N) – mark SOL-3.
Compost extracts were prepared by weighing the required mass of compost to prepare an extract in a ratio of 1:10 (15.06 g in 200 mL) and an extract in a ratio of 1:2.5 (60.24 g in 200 mL). The weighed masses depended on the specific density of the compost (0.753 g/cm3), and the planned ratios of 1:2.5 and 1:10 represent the ratios of compost volume and water. To the weighed mass of compost was added 200 mL of deionized water, the resulting suspension was stirred and after 30 minutes the compost extract was separated.
Ammonium carbonate solutions were prepared by weighing a certain mass of ammonium carbonate and dissolving in deionized water to a volume of 1,000 mL. Solutions of 200 mg/L NH4-N, 400 mg/L NH4-N and 600 mg/L NH4-N were prepared. pH values of 7.81, 7.90 and 7.91 were measured in the prepared solutions; and conductivities 1.438 µS/cm, 2.83 µS/cm, and 4.15 µS/cm.
For the germination test, seeds of four plant species were prepared and selected for the test (cucumber, garden cress, barley, and triticale). The seeds were washed according to the prescribed procedure and 200 seeds of each plant species were prepared.
Petri dishes were prepared and filter paper was placed on the bottom of each Petri dish. Pipette 5 mL of solution (water, 2 compost extracts, or 3 ammonium carbonate solution) onto the filter paper and place 10 seeds of a certain plant species. A total of 24 Petri dishes (6 different solutions × 4 replicates) were placed for each species, meaning that each treatment was set in four replicates.
The mass of each Petri dish was weighed and recorded, after which the Petri dishes were transferred to controlled conditions at 25 °C. After the allotted time for germination (3 days, ie 72 hours), the number of germinated seeds in each Petri dish was recorded and the root and shoot length of each plant was measured.
Based on the above data, the following parameters were statistically processed: germinated rate (GR) as percentage of germinated seeds, root length per plant (RLP), root length index (RI), germination index (GI), shoot rate (SR) as percentage of visible shoots, shoot length per plant (SLP), shoot length index (SI), and vitality shoot index (MLSV).
The germinated rate (GR) was determined by counting all seeds on which germination is visible, ie the beginning of radicle development is visible (the percentage of germinated seeds out of a total of 10).
The root length per plant (RLP) includes measured length of each root in a single treatment and replication, i.e. the Petri dish. It is expressed as Root Length per Plant (RLP) = ∑RL/NGS (NGS = number of germinated seeds) in cm.
The root length index (RI) is percentage difference of the root length of germinated seeds on the material under investigation compared to the root length of the control. RI is expressed as percentage: RI (%) = [(RLs1/RLc + RLs2/RLc + RLs3/RLc + RLs4/RLc)/4] x 100.
The germination index (GI) was calculated as a product of two ratios: the ratio of the number of germinated seeds of each treatment and control and the ratio of root length per plant of each treatment and control. GI = (Germinated seeds/Germinated seeds in control) × (Root Length per Plant/Root Length per Plant in control) [
42].
The shoot rate (SR) was determined by counting the seeds on which the beginning of the development of coleoptile is visible, ie the shoot is visible (the percentage of visible shoots out of a total of 10).
The shoot length per plant (SLP) includes measured length of each shoot in a particular treatment and replication, i.e., the Petri dish. It is expressed as Shoot Length per Plant (SLP) = ∑SL/NS (NS = number of shoots visible) in cm.
The shoot length index (SI) is percentage difference of the shoot length of visible shoots on the material under investigation compared to the shoot length of the control. SI is expressed as percentage: SI (%) = [(SLs1/SLc + SLs2/SLc + SLs3/SLc + SLs4/SLc)/4] x 100.
Vitality shoot index or Munoo – Liisa vitality index (MLSV) is index calculated from the shoot rate and the shoot length (modified formula according to EN 16086-2 [
41]. MLSV is expressed as percentage: MLSV (%) = [[(SRs1×SLs1) + (SRs2×SLs2) + (SRs3×SLs3) + (SRs4×SLs4)]/[4 × (SRc×SLc)]] x 100.
2.6. Statistical Data Processing
Statistical data processing was performed with software packages MS Excel and SAS for Windows 9.1.3. (SAS Institute Inc., Cary, NC, USA). These parameters were statistically processed by analysis of variance (ANOVA) with the least significant difference (LSD) test.
4. Discussion
In this research, the results of laboratory analysis of compost and germination test were combined and compared as indicators of compost maturity and potential phytotoxicity on four test plant species (cucumber, garden cress, triticale and barley). Also, with the aim of measuring the phytotoxic or phytostimulating effect, solutions of different concentrations of ammonium nitrogen were used.
pH as very important factor [
46] at the end of composting should be in an acceptable range for plants. In this study, an average pH value of 8.66 was found, which means that the pH is in an acceptable range although very close to the upper limit values. Stable and mature compost should have a pH value within the acceptable range of 5.5-9.0 [
47].
Electrical conductivity (EC) can serve as a measure of soluble nutrients, cations, and anions, and a lower EC can be result of lower content of cations in the soil [
1]. But, when the salinity of the soil (EC) is ≥ 4 mS/cm, the soil is considered saline with potential salt stress, especially for glycophytes [
48]. Composts obtained from municipal waste have high salt concentrations which, in addition to inhibiting plant growth, negatively affect soil structure [
49], but EC of 5 mS/cm is the upper limit for the substrate in container production [
50]. In this study, a conductivity of 2.37 mS/cm was determined, which according to other results [
50,
51] should not cause phytotoxicity.
As most of the nitrogen is found in organic form, bound in the structure of proteins and simple peptides, intensive decomposition of organic matter in the first weeks of composting leads to ammonification and later to nitrification, but in conditions of good aeration and after lowering the temperature of the compost pile below 40
°C [
52]. The optimal C/N ratio in compote is considered to be 25/1 to 35/1 [
2,
53]. However, there is a possibility of producing quality compost at lower C/N ratios, and good examples are pig manure with wood sawdust with C/N 15 [
54], waste from green area and waste of the food industry with C/N 19 [
55], pig manure with rice straw [
56] and chicken manure with wood sawdust [
56] with C/N ratio 20. Since the average C/N ratio for the analyzed compost is 9.93, the compost can be evaluated as a mature organic fertilizer.
Nitrogen transformation is a rather complicated process that depends simultaneously on many aspects such as pH, temperature, C/N ratio, and starting materials [
2,
58]. The maturity threshold of organic fertilizer according to NH
4-N/NO
3-N ratio is <0.16 [
59], which means that in mature organic fertilizer there should be 6.25 times more nitrate than ammonium form of nitrogen. The average NH
4-N/NO
3-N ratio in tested compost was 0.044 and 23 times more nitrate (93.9 mg/L) than ammonia (4.04 mg/L) nitrogen was found. Thus, the evaluation of compost maturity according to the NH
4-N/NO
3-N ratio is that compost is a mature organic fertilizer.
The stability of organic fertilizer is measured by respiration rate (CO
2 release in mg/g of fertilizer/day). Intensity of respiration is used to assess the stability of compost [
60]. The respiration intensity of compost was determined to average 0.267 mg CO
2/g DM/day. Since the respiration intensity is <1, the assessment is that the compost is very stable, i.e. mature finished compost, without continuing decomposition, without odor, and potential phytotoxicity.
The germination test and germination index were used to investigate possible phytotoxic effect of the compost as substrate [
25,
60]. Immature compost could content phytotoxic components (toxic to plants) that inhibit seed germination, especially in highly sensitive seeds. The phytotoxicity of organic fertilizer, substrate, or other medium or solution, is interpreted from high phytotoxic to phytostimulant activity based on germination index values [
18,
25,
61,
62]: GI < 0.50 high phytotoxicity, GI 0.50 - 0.80 moderate phytotoxicity, GI 0.80 - 1.00 no phytotoxicity and GI > 1.00 phytostimulative effect. However, the results obtained by GI index research should be interpreted carefully because they are influenced by seed type and compost source [
20,
63]. The application of immature compost causes negative effects on seed germination, growth, and development of plants since immature compost, among other effects, could cause high microbiological activity that reduces the oxygen concentration in the soil, and blocks (microbiologically fixes) the existing available nitrogen [
64,
65].
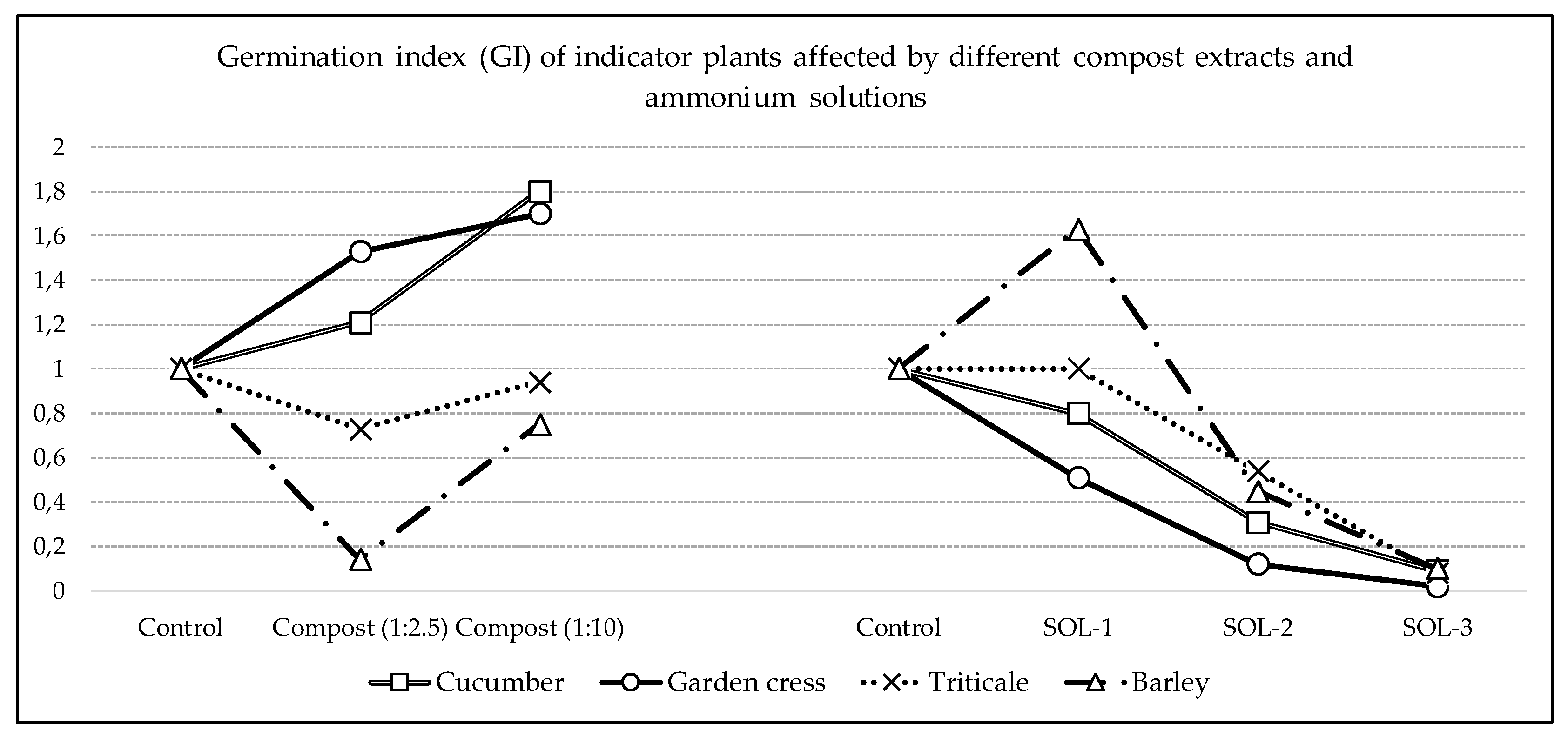
Among four tested plant species as an average for all tested treatments in the germination test experiment (compost extracts and ammonium solutions), barley has the lowest germination index 0.62, which means that moderate phytotoxicity to barley was present. Also, moderate phytotoxicity in average was present for triticale (GI = 0.72) and garden cress (GI 0.78). The average germination index for cucumber was 0.84, which means no phytotoxicity was determined. The final evaluation is that in three out of four tested plant species there was a phytotoxic effect as the average for all treated treatments.
But it is the average of all treatments that hides the significantly different effect of compost extract and ammonia solutions. Thus, the highest average GI for two different compost extracts was determined for garden cress (1.62) and cucumber (1.51), which detects a phytostimulating effect, while a high phytotoxic effect was determined for barley (GI average 0.45), and no phytotoxic effect was determined for triticale (average GI 0.84).
On the other hand, the effect of ammonia solutions is the opposite because, on average, moderate phytotoxicity was found for all solutions for barley (0.73) and triticale (0.54), and high phytotoxicity for cucumber (0.40) and garden cress (0.22), which means that we can expect differences in the effects of compost extracts and ammonia solutions on the tested plant species, but perhaps also a different reaction of the tested plant species.
In cucumber, neither the compost extract in the ratio 1:2.5 (less diluted compost) nor the ammonium solution of the lowest concentration (200 mg/L NH4-N) had a significant effect on GI, although the compost extract acted as a phytostimulator (1.21), and the ammonia solution in phytotoxic (0.80) direction. However, undoubtedly the compost extract in a ratio of 1:10 (more diluted compost) resulted in a strong phytostimulating effect (GI = 1.80), and the more concentrated ammonium nitrogen solution in a high phytotoxic (GI = 0.31 and 0.09) effect. Here we can conclude that the tested compost has a pronounced phytostimulative effect on cucumber, and that the phytotoxicity of the ammonium solution for cucumber is significantly lower than 400 mg/L NH4-N, probably much closer to 200 than 400 mg/L.
We can conclude similarly on the basis of RI, RLP and GR, while the phytostimulative and phytotoxic effects are even more pronounced on the cucumber shoots. Namely, a pronounced phytostimulative effect of both compost extracts on the length, index and vitality of shoots was determined, while in the treatments with 400 and 600 mg/L NH4-N there were no shoots at all.
A very similar finding was also found for garden cress, both compost extracts had a phytostimulating effect because the GI was 1.53 and 1.70 (although statistical significance was not proven due to variability), while the GI was already at the limit of high phytotoxicity in the treatment of the lowest concentration ammonia solution (GI = 0.51).
The difference between garden cress and cucumber is visible in the comparison of shoot growth indicators. Namely, the compost extracts did not stimulate the growth of garden cress as much as they did with cucumber. Also, the SOL-1 solution had a stimulating effect on the length, index and vitality of garden cress shoots, in contrast to cucumber. Although SOL-2 and SOL-3 (solutions with higher concentrations of ammonia) had an extremely phytotoxic effect on garden cress shoots, there were still garden cress shoots unlike cucumber shoots.
The reaction of triticale and barley in the germination test was significantly different from that of garden cress and cucumber. First, the compost extract at a ratio of 1:2.5 was moderately phytotoxic (0.73) to triticale and highly phytotoxic (0.14) to barley, reducing GR, RLP and RI, especially in barley. However, shoot length, shoot index and vitality were higher in triticale and lower in barley than in the control treatment. By diluting the compost extract (ratio 1:10), this phytotoxic effect completely disappeared in triticale (GI = 0.94) and was significantly mitigated in barley (GI = 0.75) with a neutral to mild stimulatory effect on triticale and barley shoots.
At the same time, SOL-1 had no significant effect on triticale, but had a strong phytostimulating effect on GI (1.63), length, index and vitality of barley roots and an even more pronounced stimulating effect on barley shoot. As expected, SOL-2 and SOL-3 solutions had an inhibitory effect on the root and shoot of triticale, and on the root of barley (to a lesser extent). However, the SOL-2 solution had a stimulating effect on the barley shoot, and neither did the SOL-3 solution have a phytotoxic effect on the barley shoot.
Multiple regression analysis showed that NH
4+-N content is an important factor influencing seed germination and root growth of selected plant species [
66]. In a study by Cheung et al. (1989) Chinese cabbage was the most sensitive species to metal toxicity and was recommended as a test species to assess the toxicity of heavy metals[
67]. We can conclude that garden cress and cucumber are the most suitable species and barley the less suitable species for determining the phytotoxicity of the ammonium form of nitrogen. However, barley as a test plant is very suitable for determining some other phytotoxicity because it reacted very sensitively to the compost extract in the ratio 1:2.5, which was almost absent in the ratio 1:10. On the other hand, garden crass and cucumber did not react so sensitively to the compost extract ratio, but there are other compounds that contribute to the phytotoxic effect such as the ammonium form of nitrogen [
68].
Regarding the influence of ammonium carbonate solutions on the germination index, we can conclude that we obtained the expected results. Namely, the maturity threshold of organic fertilizer is considered to be <400 mg/kg NH
4-N [
5], which is the concentration of NH
4-N in solution 2. Consequently, solution 2 has an average high phytotoxic effect (GI = 0.36), and solution 3 also (GI = 0.07). Similar to compost extract, solution 1 (with 200 mg/kg NH
4-N) in average for all four tested species has no phytotoxic effect (GI = 0.99), which can also be explained by the possible different reactions of the analyzed plant species to the ammonium form of nitrogen as a phytotoxic component. Also, it is important to conclude that according to these results, the limit value of 400 mg/kg NH
4-N is not a good indicator of phytotoxicity for all species, as shown by the example of garden cress. This may be related to research of Cheung et al. (1989) who reported that seeds of root crops, cereals, and legumes, which contain large amounts of food supplies, will have less sensitivity to toxicity than seeds of deciduous plants with less food supply [
67]. Chinese cabbage and Chinese spinach seeds were the most sensitive species probably because their seeds are very small.
5. Conclusions
The interpretation of the maturity of the tested compost based on chemical analyzes is in accordance with the results of the germination test with cucumber and garden cress, but the compost can still be phytotoxic to other plant species, especially monocotyledons. By evaluating the chemical properties of the compost, primarily the C/N and NH4-N/NO3-N ratios, as well as the NH4-N concentration, we can conclude that the tested compost is mature and that we do not expect a phytotoxic effect. However, the pH value of the compost is above 8.5, which, in addition to the established conductivity, still leaves the possibility of a phytotoxic effect. At the same time, the very low intensity of microbiological respiration indicates that the tested compost is stable.
The choice of the plant for the germination test is very significant because the germination test with compost extract shows an undoubted phytostimulating effect on garden cress and cucumber, but with a more pronounced phytostimulating effect of compost extract in the ratio 1:10 than 1:2.5. However, no such effect was found on the monocotyledonous test plants triticale and barley, and very important was ratio of compost extract, because the 1:10 extract had no significant effect, and the 1:2.5 extract had a phytotoxic effect, moderate on triticale and high on barley.
On the other side, the phytotoxicity of the ammonium solution for cucumber and garden cress was already at significantly lower concentrations than 400 mg/L NH4-N, probably much closer to 200 mg/L, which is not toxic for triticale and is even phytostimulant for barley.
The general conclusion is that garden cress and cucumber are suitable test plants for determining phytostimulative effect of compost with low concentrations of NH4-N and NH4-N/NO3-N ratios, but they are not suitable for determining phytotoxicity for monocotyledonous plants, especially if the cause of phytotoxicity is some non-ammonium component, despite the maturity and stability of the compost according to the usual analyses.
Barley is the most suitable species for determining non-ammonium phytotoxic components in compost and phytostimulative or phytotoxic effect of ammonium form of nitrogen.
It would be very useful to conduct a comparative germination test with compost extracts in the ratio 1:2.5 and 1:10, whereby the 1:2.5 extract is used as a test for phytotoxicity, and the 1:10 extract for the phytostimulating effect.