Submitted:
14 July 2024
Posted:
15 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics Statement
2.2. Sample Size Calculation
2.3. Assessment of the Participants’ Demographic Data
2.4. Assessment of Attitude Towards RSV Vaccination
2.5. Statistical Analysis
3. Results
3.1. Description of the Study Sample
3.2. Attitude Towards RSV Vaccination in the Study Sample
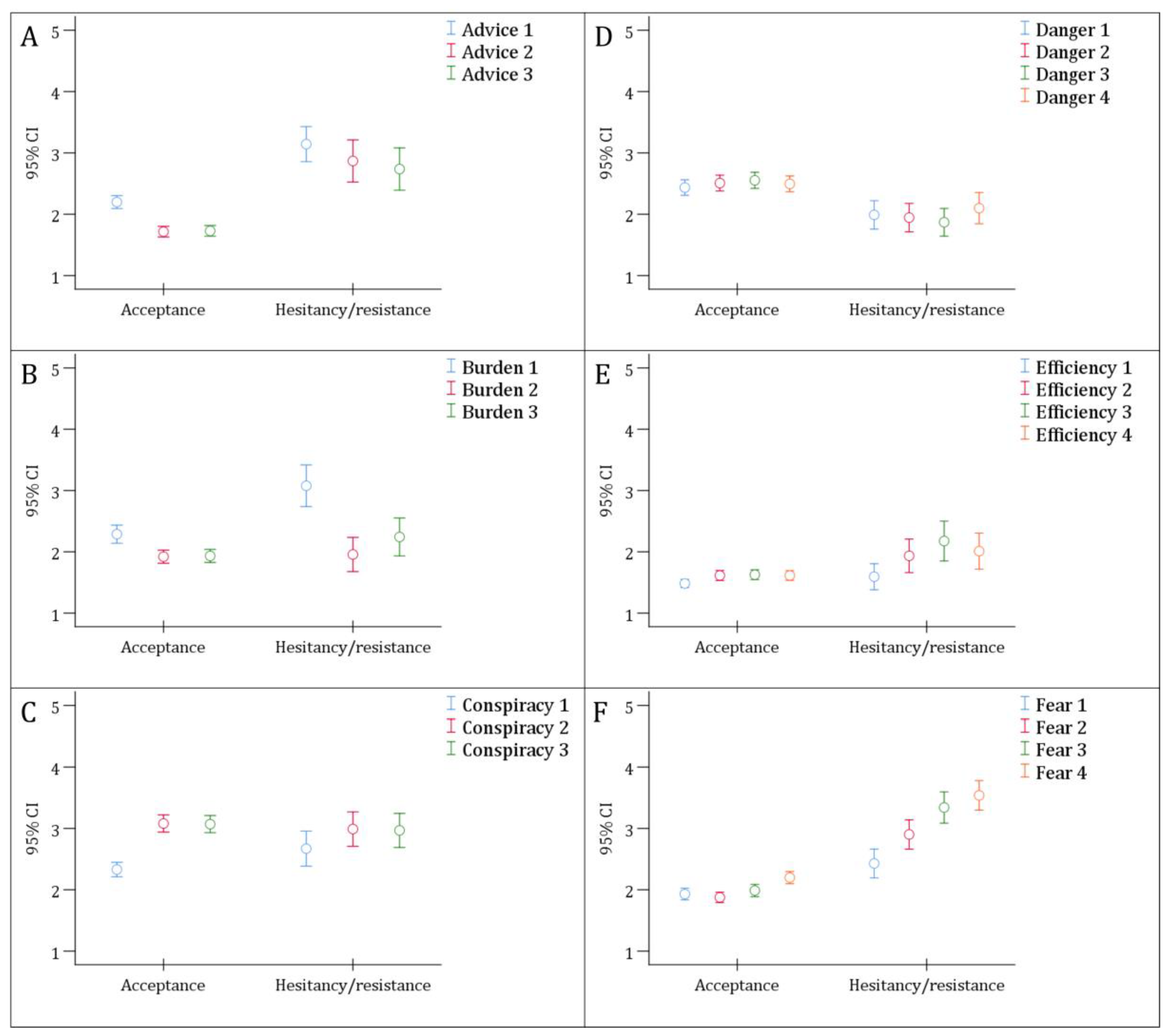
3.3. Correlation of the ABCDEF Constructs with RSV Vaccine Attitude
3.4. Multivariate Analysis for the Factors Associated with RSV Vaccine Acceptance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABCDEF scale | Advice, Burden, Conspiracy, Dangers, Efficiency, and Fear scale |
| aOR | Adjusted odds ratio |
| CI | Confidence interval |
| COVID-19 | Coronavirus disease 2019 |
| FDA | The United States Food and Drug Administration |
| HCW | Healthcare worker |
| IQR | Interquartile range |
| JOD | Jordanian dinar |
| LRTIs | Lower respiratory tract infections |
| RSV | Respiratory syncytial virus |
| VBS | Vaccine behavior score |
| WHO | World Health Organization |
| GBS | Group B Streptococcus |
References
- Munro, A.P.S.; Martinón-Torres, F.; Drysdale, S.B.; Faust, S.N. The disease burden of respiratory syncytial virus in Infants. Curr Opin Infect Dis 2023, 36, 379–384. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Blau, D.M.; Caballero, M.T.; Feikin, D.R.; Gill, C.J.; Madhi, S.A.; Omer, S.B.; Simões, E.A.F.; Campbell, H.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet 2022, 399, 2047–2064. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; McAllister, D.A.; O'Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Shi, T.; Bont, L.J.; Chu, H.Y.; Zar, H.J.; Wahi-Singh, B.; Ma, Y.; Cong, B.; Sharland, E.; et al. Global disease burden of and risk factors for acute lower respiratory infections caused by respiratory syncytial virus in preterm infants and young children in 2019: a systematic review and meta-analysis of aggregated and individual participant data. Lancet 2024, 403, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Johnson, E.K.; Shi, T.; Campbell, H.; Chaves, S.S.; Commaille-Chapus, C.; Dighero, I.; James, S.L.; Mahé, C.; Ooi, Y.; et al. National burden estimates of hospitalisations for acute lower respiratory infections due to respiratory syncytial virus in young children in 2019 among 58 countries: a modelling study. Lancet Respir Med 2021, 9, 175–185. [Google Scholar] [CrossRef]
- Baraldi, E.; Checcucci Lisi, G.; Costantino, C.; Heinrichs, J.H.; Manzoni, P.; Riccò, M.; Roberts, M.; Vassilouthis, N. RSV disease in infants and young children: Can we see a brighter future? Hum Vaccin Immunother 2022, 18, 2079322. [Google Scholar] [CrossRef] [PubMed]
- Simões, E.A.F.; Bont, L.; Manzoni, P.; Fauroux, B.; Paes, B.; Figueras-Aloy, J.; Checchia, P.A.; Carbonell-Estrany, X. Past, Present and Future Approaches to the Prevention and Treatment of Respiratory Syncytial Virus Infection in Children. Infectious Diseases and Therapy 2018, 7, 87–120. [Google Scholar] [CrossRef] [PubMed]
- Cromer, D.; van Hoek, A.J.; Newall, A.T.; Pollard, A.J.; Jit, M. Burden of paediatric respiratory syncytial virus disease and potential effect of different immunisation strategies: a modelling and cost-effectiveness analysis for England. Lancet Public Health 2017, 2, e367–e374. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Luo, E.; Fan, L.; Zhang, W.; Yang, Y.; Du, Y.; Yang, X.; Xing, S. Clinical research on RSV prevention in children and pregnant women: progress and perspectives. Front Immunol 2023, 14, 1329426. [Google Scholar] [CrossRef] [PubMed]
- Kampmann, B.; Madhi, S.A.; Munjal, I.; Simões, E.A.F.; Pahud, B.A.; Llapur, C.; Baker, J.; Pérez Marc, G.; Radley, D.; Shittu, E.; et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N Engl J Med 2023, 388, 1451–1464. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Pérez Marc, G.; Zareba, A.M.; Falsey, A.R.; Jiang, Q.; Patton, M.; Polack, F.P.; Llapur, C.; Doreski, P.A.; Ilangovan, K.; et al. Efficacy and Safety of a Bivalent RSV Prefusion F Vaccine in Older Adults. N Engl J Med 2023, 388, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Syed, Y.Y. Respiratory Syncytial Virus Prefusion F Subunit Vaccine: First Approval of a Maternal Vaccine to Protect Infants. Paediatr Drugs 2023, 25, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, D.; Wilkins, N.; van Leeuwen, E.; Watson, C.H.; Crofts, J.; Flasche, S.; Jit, M.; Atkins, K.E. Protecting infants against RSV disease: an impact and cost-effectiveness comparison of long-acting monoclonal antibodies and maternal vaccination. Lancet Reg Health Eur 2024, 38, 100829. [Google Scholar] [CrossRef] [PubMed]
- Koltai, M.; Moyes, J.; Nyawanda, B.; Nyiro, J.; Munywoki, P.K.; Tempia, S.; Li, X.; Antillon, M.; Bilcke, J.; Flasche, S.; et al. Estimating the cost-effectiveness of maternal vaccination and monoclonal antibodies for respiratory syncytial virus in Kenya and South Africa. BMC Med 2023, 21, 120. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Abu Raya, B.; Baraldi, E.; Flanagan, K.; Martinon Torres, F.; Tsolia, M.; Zielen, S. RSV Prevention in All Infants: Which Is the Most Preferable Strategy? Front Immunol 2022, 13, 880368. [Google Scholar] [CrossRef] [PubMed]
- Kherfan, T.; Sallam, M. Prospective Attitudes towards Respiratory Syncytial Virus (RSV) Vaccination: Validation of a Survey Instrument among Young Females in Jordan Pending Vaccine Authorization. Vaccines (Basel) 2023, 11, 1386. [Google Scholar] [CrossRef] [PubMed]
- Chawanpaiboon, S.; Anuwutnavin, S.; Kanjanapongporn, A.; Pooliam, J.; Titapant, V. A qualitative study of pregnant women’s perceptions and decision-making regarding COVID-19 vaccination in Thailand. Scientific Reports 2024, 14, 5128. [Google Scholar] [CrossRef] [PubMed]
- Kola-Palmer, S.; Keely, A.; Walsh, J. 'It has been the hardest decision of my life': a mixed-methods study of pregnant women's COVID-19 vaccination hesitancy. Psychol Health 2023, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.L.; Schulkin, J.; Power, M.L. Vaccine hesitancy in pregnant Women: A narrative review. Vaccine 2023, 41, 4220–4227. [Google Scholar] [CrossRef]
- MacDonald, N.E. Vaccine hesitancy: Definition, scope and determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef]
- Peretti-Watel, P.; Larson, H.J.; Ward, J.K.; Schulz, W.S.; Verger, P. Vaccine hesitancy: clarifying a theoretical framework for an ambiguous notion. PLoS Curr 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Dubé, E.; Laberge, C.; Guay, M.; Bramadat, P.; Roy, R.; Bettinger, J. Vaccine hesitancy: an overview. Hum Vaccin Immunother 2013, 9, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Larson, H.J.; Jarrett, C.; Eckersberger, E.; Smith, D.M.; Paterson, P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007-2012. Vaccine 2014, 32, 2150–2159. [Google Scholar] [CrossRef] [PubMed]
- Betsch, C.; Schmid, P.; Heinemeier, D.; Korn, L.; Holtmann, C.; Böhm, R. Beyond confidence: Development of a measure assessing the 5C psychological antecedents of vaccination. PLoS One 2018, 13, e0208601. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.; Robinson, K.; Vallée-Tourangeau, G. The 5As: A practical taxonomy for the determinants of vaccine uptake. Vaccine 2016, 34, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Adeyanju, G.C.; Engel, E.; Koch, L.; Ranzinger, T.; Shahid, I.B.M.; Head, M.G.; Eitze, S.; Betsch, C. Determinants of influenza vaccine hesitancy among pregnant women in Europe: a systematic review. Eur J Med Res 2021, 26, 116. [Google Scholar] [CrossRef] [PubMed]
- Simsekoglu, N.; Akyuz, E.; Guven, R.; Pasin, O. Attitudes toward COVID-19 vaccines during pregnancy and breastfeeding. Front Public Health 2024, 12, 1286891. [Google Scholar] [CrossRef] [PubMed]
- Rand, C.M.; Olson-Chen, C. Maternal Vaccination and Vaccine Hesitancy. Pediatr Clin North Am 2023, 70, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Bauer-Maison, N.; Guarna, G.; RD, D.S. Social media misinformation about pregnancy and COVID-19 vaccines: A systematic review. Med Princ Pract 2024. [Google Scholar] [CrossRef]
- Rosso, A.; Massimi, A.; Pitini, E.; Nardi, A.; Baccolini, V.; Marzuillo, C.; De Vito, C.; Villari, P. Factors affecting the vaccination choices of pregnant women for their children: a systematic review of the literature. Hum Vaccin Immunother 2020, 16, 1969–1980. [Google Scholar] [CrossRef]
- Patterson, L.; Berry, E.; Parsons, C.; Clarke, B.; Little, A.; Beggs, J.; Chuter, A.; Jackson, T.; Hsia, Y.; McGrath, H.; et al. Using the COM-B framework to elucidate facilitators and barriers to COVID-19 vaccine uptake in pregnant women: a qualitative study. BMC Pregnancy Childbirth 2023, 23, 640. [Google Scholar] [CrossRef]
- Spina, C.I.; Brewer, S.E.; Ellingson, M.K.; Chamberlain, A.T.; Limaye, R.J.; Orenstein, W.A.; Salmon, D.A.; Omer, S.B.; O'Leary, S.T. Adapting Center for Disease Control and Prevention's immunization quality improvement program to improve maternal vaccination uptake in obstetrics. Vaccine 2020, 38, 7963–7969. [Google Scholar] [CrossRef] [PubMed]
- Sallam, M.; Al-Sanafi, M.; Sallam, M. A Global Map of COVID-19 Vaccine Acceptance Rates per Country: An Updated Concise Narrative Review. J Multidiscip Healthc 2022, 15, 21–45. [Google Scholar] [CrossRef] [PubMed]
- Epitools - Epidemiological Calculators. Sample size to estimate a proportion or apparent prevalence with specified precision. Available online: https://epitools.ausvet.com.au/oneproportion (accessed on 15 January 2024).
- The Jordan Department of Statistics. Jordan 2021 Statistical Yearbook. Available online: http://dosweb.dos.gov.jo/databank/yearbook/YearBook2021.pdf (accessed on 15 January 2024).
- The U.S. Food and Drug Administration (FDA). FDA Approves First Vaccine for Pregnant Individuals to Prevent RSV in Infants. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-vaccine-pregnant-individuals-prevent-rsv-infants (accessed on 12 April 2024).
- McCormack, S.; Thompson, C.; Nolan, M.; Imcha, M.; Dee, A.; Saunders, J.; Philip, R.K. Maternal awareness, acceptability and willingness towards respiratory syncytial virus (RSV) vaccination during pregnancy in Ireland. Immunity, Inflammation and Disease 2024, 12, e1257. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xiu, S.; Yang, L.; Li, L.; Yang, M.; Wang, X.; Shen, Y.; Wang, W.; Lin, L. Perceptions about respiratory syncytial virus (RSV) and attitudes toward the RSV vaccine among the general public in China: A cross-sectional survey. Hum Vaccin Immunother 2024, 20, 2310916. [Google Scholar] [CrossRef]
- Wilson, R.J.; Paterson, P.; Jarrett, C.; Larson, H.J. Understanding factors influencing vaccination acceptance during pregnancy globally: A literature review. Vaccine 2015, 33, 6420–6429. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, A.; Takaku, M.; Saitoh, A. High rates of vaccine hesitancy among pregnant women during the coronavirus disease 2019 (COVID-19) pandemic in Japan. Human Vaccines & Immunotherapeutics 2022, 18, 2064686. [Google Scholar] [CrossRef]
- Kilada, S.; French, N.; Perkins, E.; Hungerford, D. Pregnant women's attitudes and behaviours towards antenatal vaccination against Influenza and COVID-19 in the Liverpool City Region, United Kingdom: Cross-sectional survey. Vaccine X 2023, 15, 100387. [Google Scholar] [CrossRef] [PubMed]
- Azami, M.; Nasirkandy, M.P.; Esmaeili Gouvarchin Ghaleh, H.; Ranjbar, R. COVID-19 vaccine acceptance among pregnant women worldwide: A systematic review and meta-analysis. PLoS One 2022, 17, e0272273. [Google Scholar] [CrossRef] [PubMed]
- Januszek, S.M.; Faryniak-Zuzak, A.; Barnaś, E.; Łoziński, T.; Góra, T.; Siwiec, N.; Szczerba, P.; Januszek, R.; Kluz, T. The Approach of Pregnant Women to Vaccination Based on a COVID-19 Systematic Review. Medicina (Kaunas) 2021, 57, 977. [Google Scholar] [CrossRef]
- Daşıkan, Z.; Ekrem, E.C.; Kıratlı, D. COVID-19 Vaccine Acceptance Among Pregnant, Lactating, and Nonpregnant Women of Reproductive Age in Turkey: A Cross-Sectional Analytical Study. Disaster Med Public Health Prep 2023, 17, e505. [Google Scholar] [CrossRef] [PubMed]
- AbuAlrub, S.; AlShekh, H.B.; Hani, S.B.; Abu Baker, M. The COVID-19 Vaccination Acceptance among Jordanian Pregnant Women: A Cross-sectional Descriptive Study. The Open Nursing Journal 2023, 17. [Google Scholar]
- Masa'deh, R.; Momani, A.; Rayan, A.; Hamaideh, S.H.; Masadeh, O.M.; Al-Yateem, N. COVID-19 vaccine hesitancy among women planning for pregnancy, pregnant or breastfeeding mothers in Jordan: A cross-sectional study. PLoS One 2023, 18, e0286289. [Google Scholar] [CrossRef] [PubMed]
- Yuen, C.Y.; Tarrant, M. Determinants of uptake of influenza vaccination among pregnant women - a systematic review. Vaccine 2014, 32, 4602–4613. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, Y.; Liang, H.; Chen, Y. Seasonal Influenza Vaccine Acceptance among Pregnant Women in Zhejiang Province, China: Evidence Based on Health Belief Model. Int J Environ Res Public Health 2017, 14, 1551. [Google Scholar] [CrossRef] [PubMed]
- Yuet Sheung Yuen, C.; Yee Tak Fong, D.; Lai Yin Lee, I.; Chu, S.; Sau-mei Siu, E.; Tarrant, M. Prevalence and predictors of maternal seasonal influenza vaccination in Hong Kong. Vaccine 2013, 31, 5281–5288. [Google Scholar] [CrossRef] [PubMed]
- Horsfall, M.; Eikelenboom, M.; Draisma, S.; Smit, J.H. The Effect of Rapport on Data Quality in Face-to-Face Interviews: Beneficial or Detrimental? Int J Environ Res Public Health 2021, 18, 10858. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, P. First RSV vaccine approvals. The Lancet Microbe 2023, 4, e577. [Google Scholar] [CrossRef] [PubMed]
- Topalidou, X.; Kalergis, A.M.; Papazisis, G. Respiratory Syncytial Virus Vaccines: A Review of the Candidates and the Approved Vaccines. Pathogens 2023, 12, 1259. [Google Scholar] [CrossRef] [PubMed]
- Fleming-Dutra, K.E.; Jones, J.M.; Roper, L.E.; Prill, M.M.; Ortega-Sanchez, I.R.; Moulia, D.L.; Wallace, M.; Godfrey, M.; Broder, K.R.; Tepper, N.K.; et al. Use of the Pfizer Respiratory Syncytial Virus Vaccine During Pregnancy for the Prevention of Respiratory Syncytial Virus-Associated Lower Respiratory Tract Disease in Infants: Recommendations of the Advisory Committee on Immunization Practices - United States, 2023. MMWR Morb Mortal Wkly Rep 2023, 72, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Verwey, C.; Dangor, Z.; Madhi, S.A. Approaches to the Prevention and Treatment of Respiratory Syncytial Virus Infection in Children: Rationale and Progress to Date. Paediatr Drugs 2024, 26, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Balsells, E.; Wastnedge, E.; Singleton, R.; Rasmussen, Z.A.; Zar, H.J.; Rath, B.A.; Madhi, S.A.; Campbell, S.; Vaccari, L.C.; et al. Risk factors for respiratory syncytial virus associated with acute lower respiratory infection in children under five years: Systematic review and meta-analysis. J Glob Health 2015, 5, 020416. [Google Scholar] [CrossRef] [PubMed]
- Limaye, R.J.; Sauer, M.; Njogu, R.; Singh, P.; Fesshaye, B.; Karron, R.A. Characterizing Attitudes Toward Maternal RSV Vaccines Among Pregnant and Lactating Persons in Kenya: Key Considerations for Demand Generation Efforts for Vaccine Acceptance. J Pediatric Infect Dis Soc 2023, 12, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Simas, C.; Larson, H.J.; Paterson, P. ''Those who do not vaccinate don't love themselves, or anyone else'': a qualitative study of views and attitudes of urban pregnant women towards maternal immunisation in Panama. BMJ Open 2021, 11, e044903. [Google Scholar] [CrossRef] [PubMed]
- Patterson, L.; Berry, E.; Parsons, C.; Clarke, B.; Little, A.; Beggs, J.; Chuter, A.; Jackson, T.; Hsia, Y.; McGrath, H.; et al. Using the COM-B framework to elucidate facilitators and barriers to COVID-19 vaccine uptake in pregnant women: a qualitative study. BMC Pregnancy and Childbirth 2023, 23, 640. [Google Scholar] [CrossRef] [PubMed]
- Badur, S.; Ota, M.; Öztürk, S.; Adegbola, R.; Dutta, A. Vaccine confidence: the keys to restoring trust. Hum Vaccin Immunother 2020, 16, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Kilich, E.; Dada, S.; Kummervold, P.E.; Denny, C.; Paterson, P.; Larson, H.J. “Vaccines for pregnant women…?! Absurd” – Mapping maternal vaccination discourse and stance on social media over six months. Vaccine 2020, 38, 6627–6637. [Google Scholar] [CrossRef] [PubMed]
- Simas, C.; Larson, H.J.; Paterson, P. "Saint Google, now we have information!": a qualitative study on narratives of trust and attitudes towards maternal vaccination in Mexico City and Toluca. BMC Public Health 2021, 21, 1170. [Google Scholar] [CrossRef]
- Correa-de-Araujo, R.; Yoon, S.S.S. Clinical Outcomes in High-Risk Pregnancies Due to Advanced Maternal Age. J Womens Health (Larchmt) 2021, 30, 160–167. [Google Scholar] [CrossRef]
- Li, J.; Yan, J.; Jiang, W. The role of maternal age on adverse pregnancy outcomes among primiparous women with singleton birth: a retrospective cohort study in urban areas of China. J Matern Fetal Neonatal Med 2023, 36, 2250894. [Google Scholar] [CrossRef] [PubMed]
- Bayrampour, H.; Heaman, M.; Duncan, K.A.; Tough, S. Advanced maternal age and risk perception: A qualitative study. BMC Pregnancy and Childbirth 2012, 12, 100. [Google Scholar] [CrossRef]
- Mahameed, H.; Al-Mahzoum, K.; AlRaie, L.A.; Aburumman, R.; Al-Naimat, H.; Alhiary, S.; Barakat, M.; Al-Tammemi, A.B.; Salim, N.A.; Sallam, M. Previous Vaccination History and Psychological Factors as Significant Predictors of Willingness to Receive Mpox Vaccination and a Favorable Attitude towards Compulsory Vaccination. Vaccines (Basel) 2023, 11, 897. [Google Scholar] [CrossRef] [PubMed]
- Limaye, R.J.; Fesshaye, B.; Singh, P.; Karron, R.A. RSV awareness, risk perception, causes, and terms: Perspectives of pregnant and lactating women in Kenya to inform demand generation efforts for maternal RSV vaccines. Hum Vaccin Immunother 2023, 19, 2258580. [Google Scholar] [CrossRef] [PubMed]
- Limaye, R.J.; Malik, F.; Frew, P.M.; Randall, L.A.; Ellingson, M.K.; O’Leary, S.T.; Bednarczyk, R.A.; Oloko, O.; Salmon, D.A.; Omer, S.B. Patient Decision Making Related to Maternal and Childhood Vaccines: Exploring the Role of Trust in Providers Through a Relational Theory of Power Approach. Health Education & Behavior 2020, 47, 449–456. [Google Scholar] [CrossRef]
- Berendes, S.; Mounier-Jack, S.; Ojo-Aromokudu, O.; Ivory, A.; Tucker, J.D.; Larson, H.J.; Free, C. "Figuring stuff out myself" - a qualitative study on maternal vaccination in socially and ethnically diverse areas in England. BMC Public Health 2023, 23, 1408. [Google Scholar] [CrossRef] [PubMed]
- Fesshaye, B.; Wade, S.A.; Lee, C.; Singh, P.; Zavala, E.; Ali, H.; Rahman, H.; Siddiqua, T.J.; Atker, S.; Karron, R.A.; et al. Sources of COVID-19 Vaccine Promotion for Pregnant and Lactating Women in Bangladesh. Vaccines (Basel) 2023, 11, 1387. [Google Scholar] [CrossRef] [PubMed]
- Limaye, R.J.; Singh, P.; Paul, A.; Fesshaye, B.; Lee, C.; Zavala, E.; Wade, S.; Ali, H.; Rahman, H.; Akter, S.; et al. COVID-19 vaccine decision-making among pregnant and lactating women in Bangladesh. Vaccine 2023, 41, 3885–3890. [Google Scholar] [CrossRef] [PubMed]
- Zavala, E.; Fesshaye, B.; Lee, C.; Mutwiwa, S.; Njagi, W.; Munyao, P.; Njogu, R.; Gur-Arie, R.; Paul, A.M.; Holroyd, T.A.; et al. Lack of clear national policy guidance on COVID-19 vaccines influences behaviors in pregnant and lactating women in Kenya. Hum Vaccin Immunother 2022, 18, 2127561. [Google Scholar] [CrossRef] [PubMed]
- Limaye, R.J.; Singh, P.; Fesshaye, B.; Karron, R.A. Lessons learned from COVID-19 vaccine acceptance among pregnant and lactating women from two districts in Kenya to inform demand generation efforts for future maternal RSV vaccines. BMC Pregnancy and Childbirth 2024, 24, 221. [Google Scholar] [CrossRef] [PubMed]
- Limaye, R.J.; Singh, P.; Fesshaye, B.; Lee, C.; Schue, J.; Karron, R.A. "Why has this new vaccine come and for what reasons?" key antecedents and questions for acceptance of a future maternal GBS vaccine: Perspectives of pregnant women, lactating women, and community members in Kenya. Hum Vaccin Immunother 2024, 20, 2314826. [Google Scholar] [CrossRef] [PubMed]
- Omer, S.B.; O'Leary, S.T.; Bednarczyk, R.A.; Ellingson, M.K.; Spina, C.I.; Dudley, M.Z.; Chamberlain, A.T.; Limaye, R.J.; Brewer, S.E.; Frew, P.M.; et al. Multi-tiered intervention to increase maternal immunization coverage: A randomized, controlled trial. Vaccine 2022, 40, 4955–4963. [Google Scholar] [CrossRef]
| Variable | Category | Count (%) |
|---|---|---|
| Age | < 30 years | 200 (49.5) |
| ≥ 30 years | 204 (50.5) | |
| Pregnancy stage | First trimester | 189 (46.8) |
| Second trimester | 107 (26.5) | |
| Third trimester | 108 (26.7) | |
| Number of children | None | 103 (25.5) |
| One | 109 (27.0) | |
| Two or more | 192 (47.5) | |
| Educational level | High school or less | 81 (20.0) |
| Undergraduate | 262 (64.9) | |
| Postgraduate | 61 (15.1) | |
| Occupation | Unemployed | 229 (56.7) |
| Employed (non-HCW3) | 105 (26.0) | |
| HCW | 70 (17.3) | |
| Monthly income of household | ≤ 1000 JOD4 | 263 (65.1) |
| > 1000 JOD | 141 (34.9) | |
| Residence | Amman | 282 (69.8) |
| Outside the Capital | 122 (30.2) | |
| Nationality | Jordanian | 377 (93.3) |
| Non-Jordanian | 27 (6.7) | |
| Vaccine behavior score1 | < 3 | 304 (75.2) |
| ≥ 3 | 100 (24.8) | |
| Have you heard of RSV2 before this study? | Yes | 219 (54.2) |
| No | 185 (45.8) |
| Variable | Category | RSV4 vaccine attitude | p value, χ2 | |
|---|---|---|---|---|
| Acceptance | Hesitancy/resistance | |||
| Count (%) | Count (%) | |||
| Age | < 30 years | 168 (84.0) | 32 (16.0) | 0.002, 9.662 |
| ≥ 30 years | 145 (71.1) | 59 (28.9) | ||
| Pregnancy stage | First trimester | 142 (75.1) | 47 (24.9) | 0.255, 2.737 |
| Second trimester | 89 (83.2) | 18 (16.8) | ||
| Third trimester | 82 (75.9) | 26 (24.1) | ||
| Number of children | None | 82 (79.6) | 21 (20.4) | 0.159, 3.684 |
| One | 90 (82.6) | 19 (17.4) | ||
| Two or more | 141 (73.4) | 51 (26.6) | ||
| Educational level | High school or less | 53 (65.4) | 28 (34.6) | 0.011, 8.932 |
| Undergraduate | 213 (81.3) | 49 (18.7) | ||
| Postgraduate | 47 (77.0) | 14 (23.0) | ||
| Occupation | Unemployed | 172 (75.1) | 57 (24.9) | <0.001, 17.026 |
| Employed (non-HCW2) | 74 (70.5) | 31 (29.5) | ||
| HCW | 67 (95.7) | 3 (4.3) | ||
| Monthly income of household | ≤ 1000 JOD3 | 189 (71.9) | 74 (28.1) | <0.001, 13.600 |
| > 1000 JOD | 124 (87.9) | 17 (12.1) | ||
| Residence | Amman | 226 (80.1) | 56 (19.9) | 0.051, 3.805 |
| Outside the Capital | 87 (71.3) | 35 (28.7) | ||
| Nationality | Jordanian | 294 (78.0) | 83 (22.0) | 0.360, 0.837 |
| Non-Jordanian | 19 (70.4) | 8 (29.6) | ||
| Vaccine behavior score1 | < 3 | 226 (74.3) | 78 (25.7) | 0.009, 6.909 |
| ≥ 3 | 87 (87.0) | 13 (13.0) | ||
| Construct | Category | RSV1 vaccine attitude | p value χ2 | |
|---|---|---|---|---|
| Acceptance | Hesitancy/resistance | |||
| Count (%) | Count (%) | |||
| Advice | Agree | 259 (82.7) | 32 (35.2) | <0.001, 101.666 |
| Neutral | 47 (15.0) | 31 (34.1) | ||
| Disagree | 7 (2.2) | 28 (30.8) | ||
| Burden | Agree | 228 (72.8) | 37 (40.7) | <0.001, 32.865 |
| Neutral | 74 (23.6) | 45 (49.5) | ||
| Disagree | 11 (3.5) | 9 (9.9) | ||
| Conspiracy | Agree | 144 (46.0) | 28 (30.8) | 0.026, 7.282 |
| Neutral | 104 (33.2) | 42 (46.2) | ||
| Disagree | 65 (20.8) | 21 (23.1) | ||
| Danger | Agree | 192 (61.3) | 59 (64.8) | 0.009, 9.523 |
| Neutral | 70 (22.4) | 28 (30.8) | ||
| Disagree | 51 (16.3) | 4 (4.4) | ||
| Efficiency | Agree | 298 (95.2) | 67 (73.6) | <0.001, 38.749 |
| Neutral | 12 (3.8) | 16 (17.6) | ||
| Disagree | 3 (1.0) | 8 (8.8) | ||
| Fear | Agree | 237 (75.7) | 20 (22.0) | <0.001, 107.051 |
| Neutral | 71 (22.7) | 50 (54.9) | ||
| Disagree | 5 (1.6) | 21 (23.1) | ||
| RSV1 vaccine acceptance vs. hesitancy/resistance; Nagelkerke R2=0.529 | aOR5 (95% CI6) | p value |
|---|---|---|
| Age | ||
| < 30 years | 2.454 (1.242–4.851) | 0.010 |
| ≥ 30 years | Ref. | |
| Educational level | ||
| High school or less | 0.978 (0.284–3.361) | 0.971 |
| Undergraduate | 3.266 (1.150–9.270) | 0.026 |
| Postgraduate | Ref. | |
| Occupation | ||
| Unemployed | 0.307 (0.074–1.281) | 0.105 |
| Employed (non-HCW2) | 0.222 (0.054–0.906) | 0.036 |
| HCW | Ref. | |
| Monthly income of household | ||
| ≤ 1000 JOD3 | 1.425 (0.582–3.488) | 0.438 |
| > 1000 JOD | Ref. | |
| Residence | ||
| Amman | 1.675 (0.84–3.339) | 0.143 |
| Outside the Capital | Ref. | |
| Vaccine behavior score4 | ||
| < 3 | 0.405 (0.167–0.978) | 0.045 |
| ≥ 3 | Ref. | |
| Advice construct | ||
| Agree | 10.379 (3.195–33.719) | <0.001 |
| Neutral | 3.172 (0.933–10.784) | 0.065 |
| Disagree | Ref. | |
| Burden construct | ||
| Agree | 0.852 (0.150–4.840) | 0.857 |
| Neutral | 0.877 (0.155–4.946) | 0.882 |
| Disagree | Ref. | |
| Conspiracy construct | ||
| Agree | 2.408 (0.867–6.687) | 0.092 |
| Neutral | 1.431 (0.592–3.464) | 0.426 |
| Disagree | Ref. | |
| Danger construct | ||
| Agree | 0.258 (0.043–1.553) | 0.139 |
| Neutral | 0.224 (0.036–1.388) | 0.108 |
| Disagree | Ref. | |
| Efficiency construct | ||
| Agree | 0.866 (0.098–7.622) | 0.897 |
| Neutral | 0.563 (0.052–6.049) | 0.636 |
| Disagree | Ref. | |
| Fear construct | ||
| Agree | 21.489 (4.995–92.446) | <0.001 |
| Neutral | 3.696 (0.953–14.331) | 0.059 |
| Disagree | Ref. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





