Submitted:
15 July 2024
Posted:
16 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Methods
2.1. Cells
2.2. QPCR Analysis
2.3. Cell Lysis and Immunoblotting
2.4. Statistical Analysis
3. Results
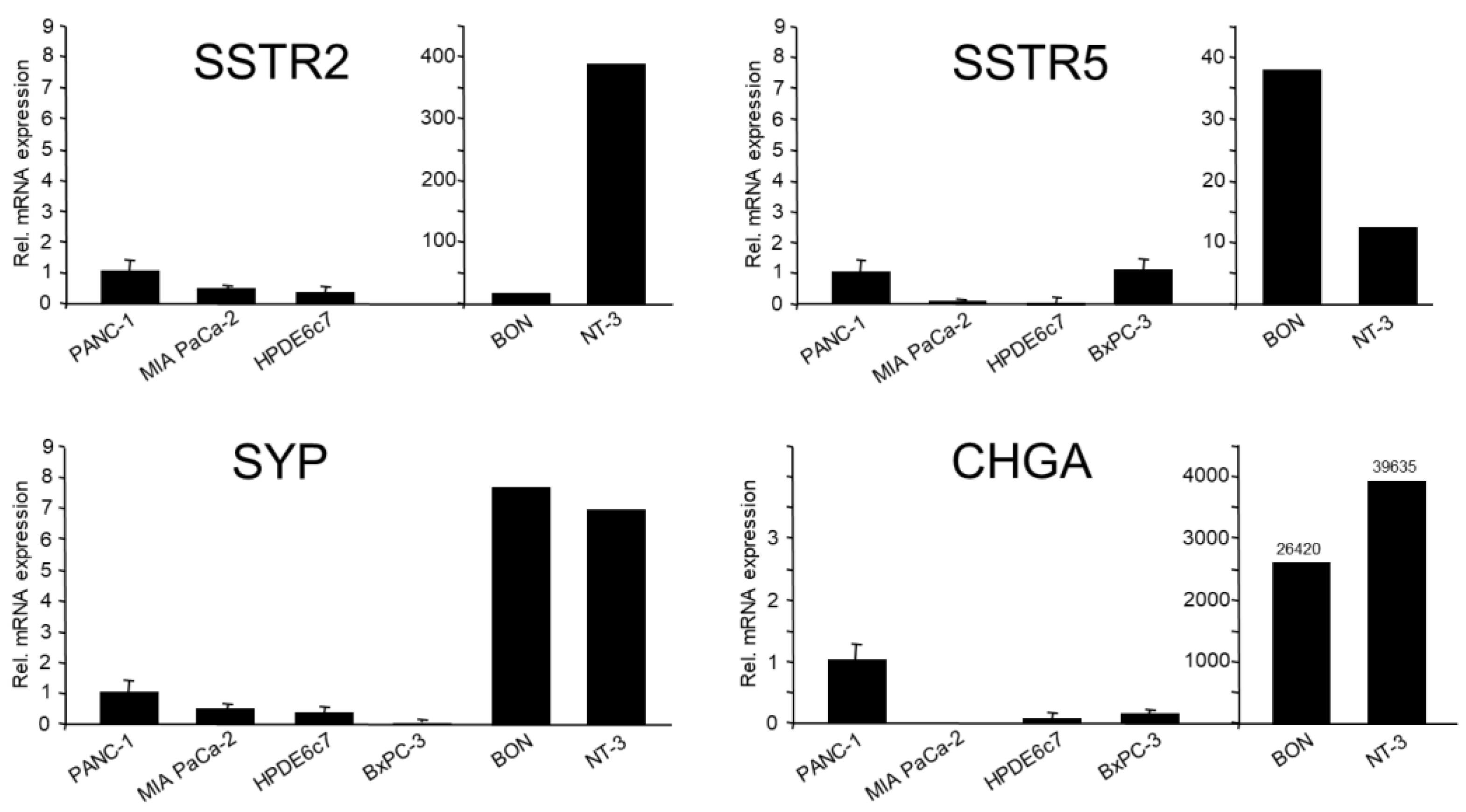
3.1. PANC-1 and MIA PaCa-2 Cells, but Not Normal Pancreatic Duct Epithelial Cells or Epithelial-Subtype Pancreatic Tumor Cells, Constitutively Express Various NED Markers
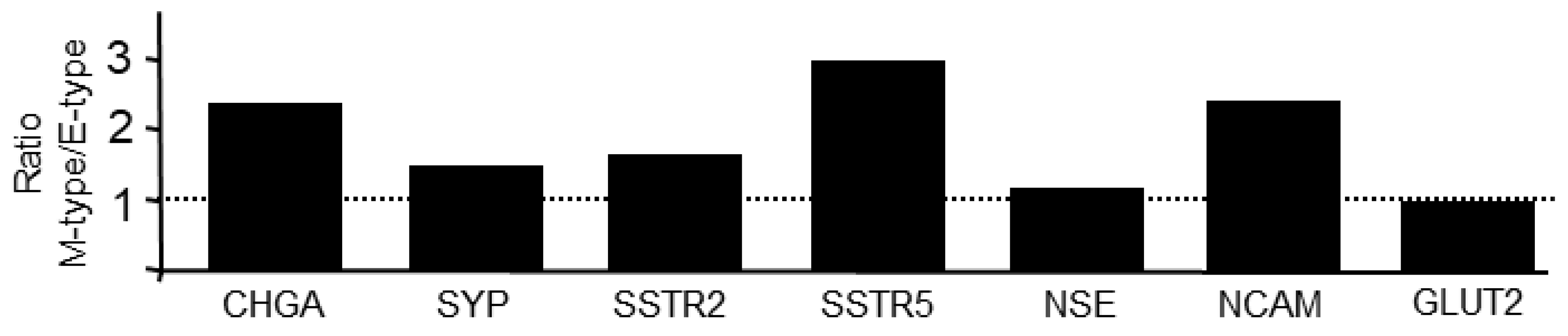
3.2. NED Marker Expression Varies in Single Cell-Derived Clones of PANC-1 Cells with Different EMT Phenotypes
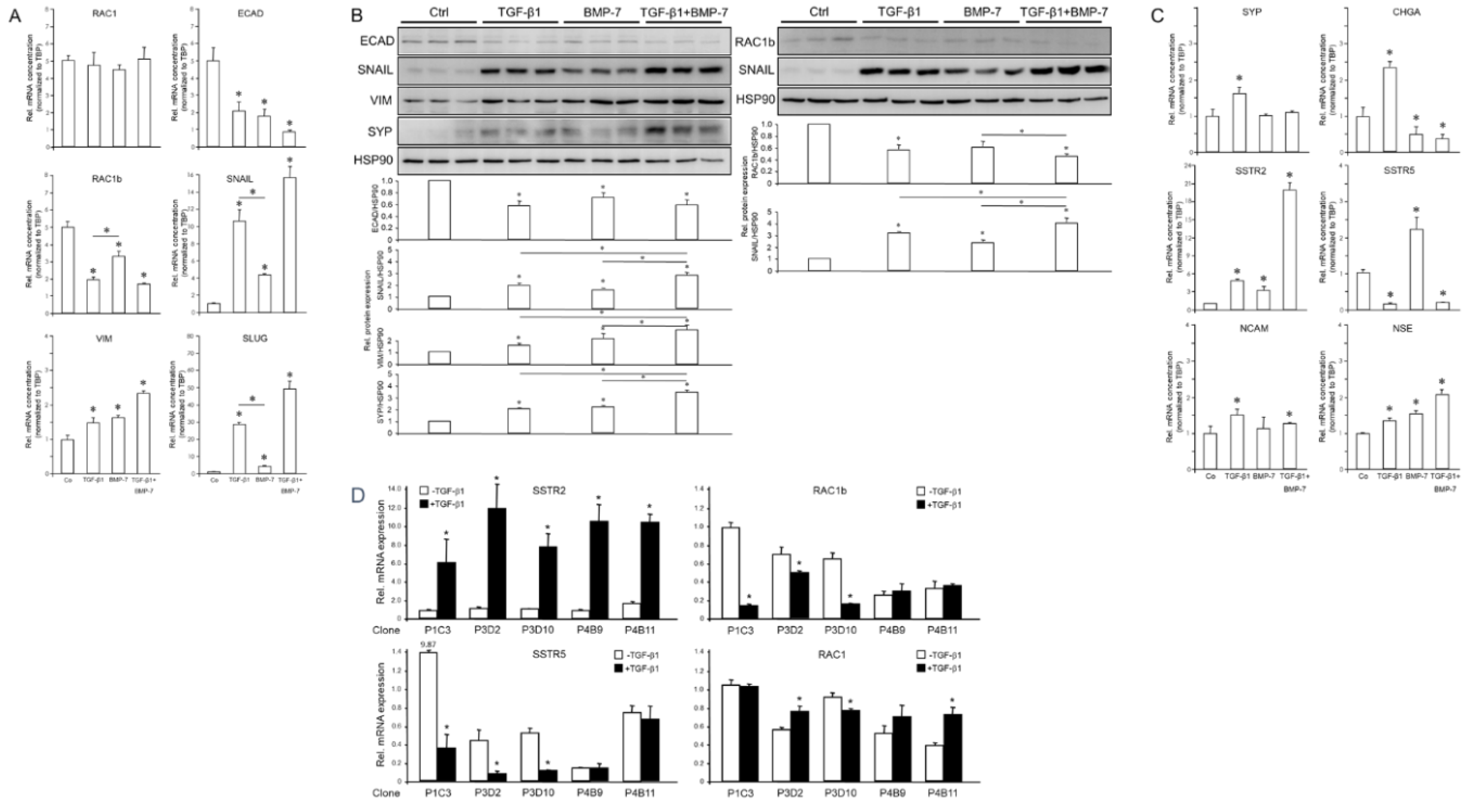
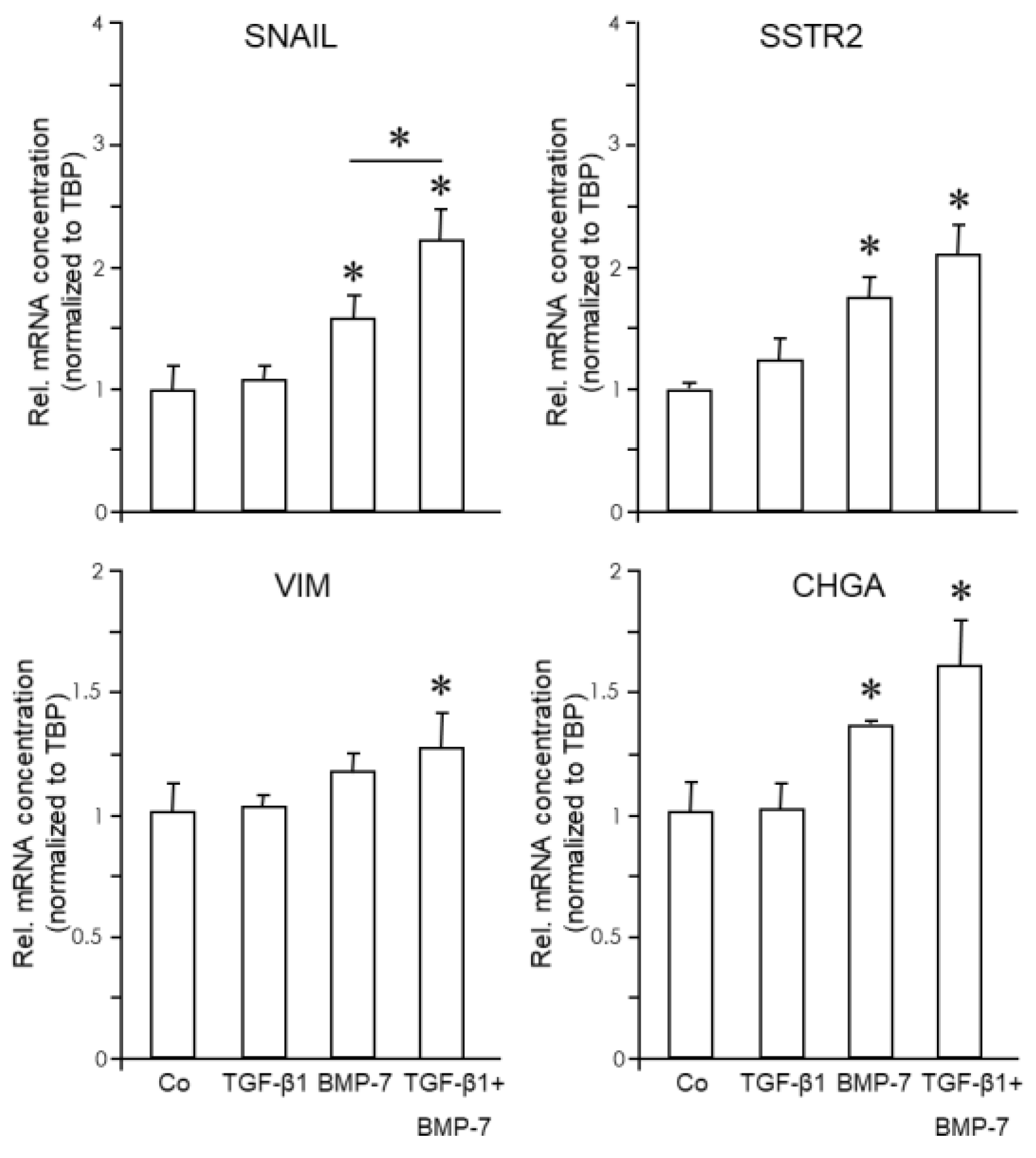
3.3. Treatment of PANC-1 Cells with TGF-β1 or BMP-7 Alters EMT and NED-Associated Gene Expression
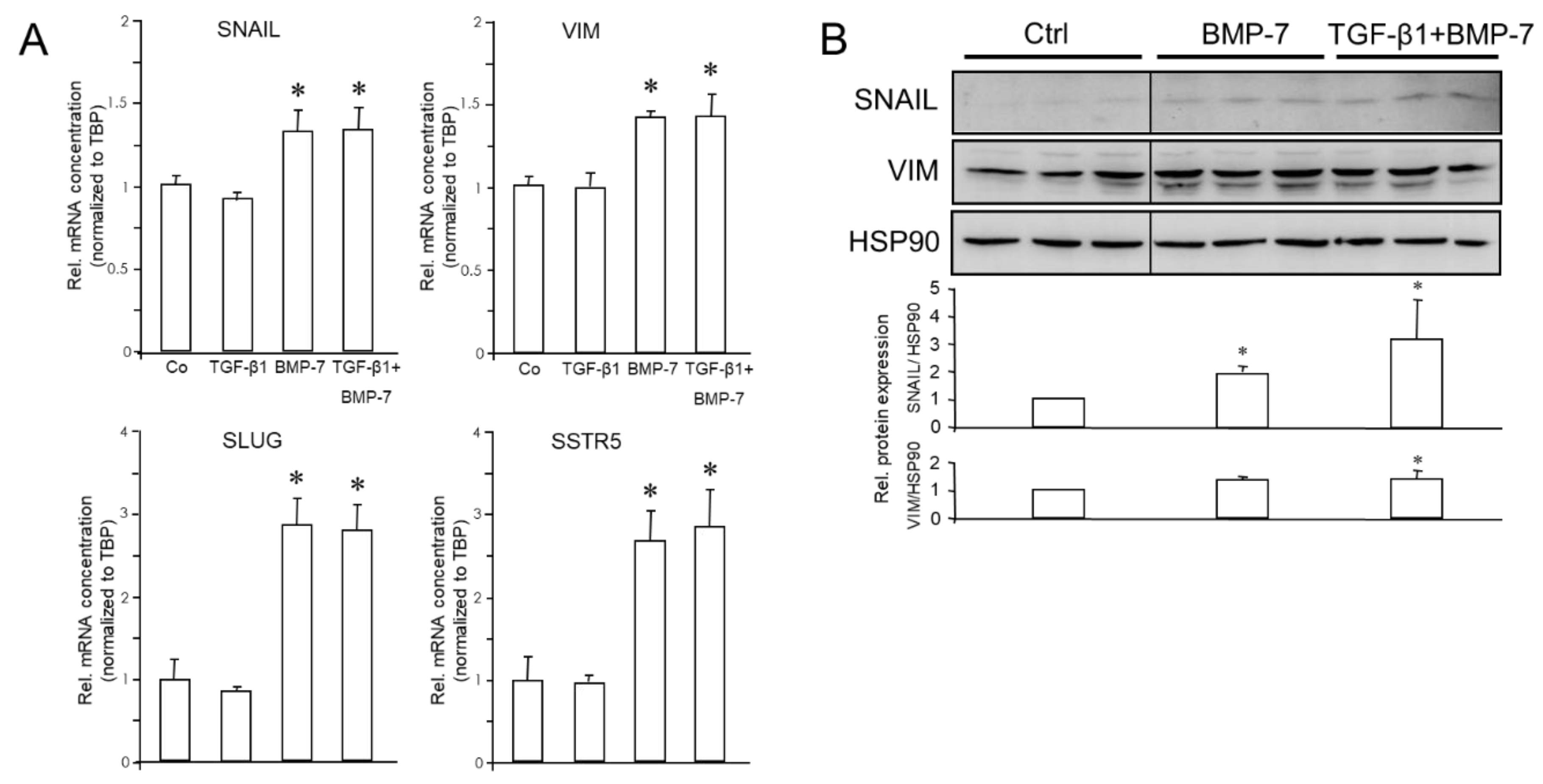
3.4. Treatment of MIA PaCa-2 Cells with BMP-7 but Not TGF-β1 Alters EMT and NED-Associated Gene Expression
3.5. The Expression of NED Markers is Altered During MET and Positively Correlates with that of Mesenchymal Markers
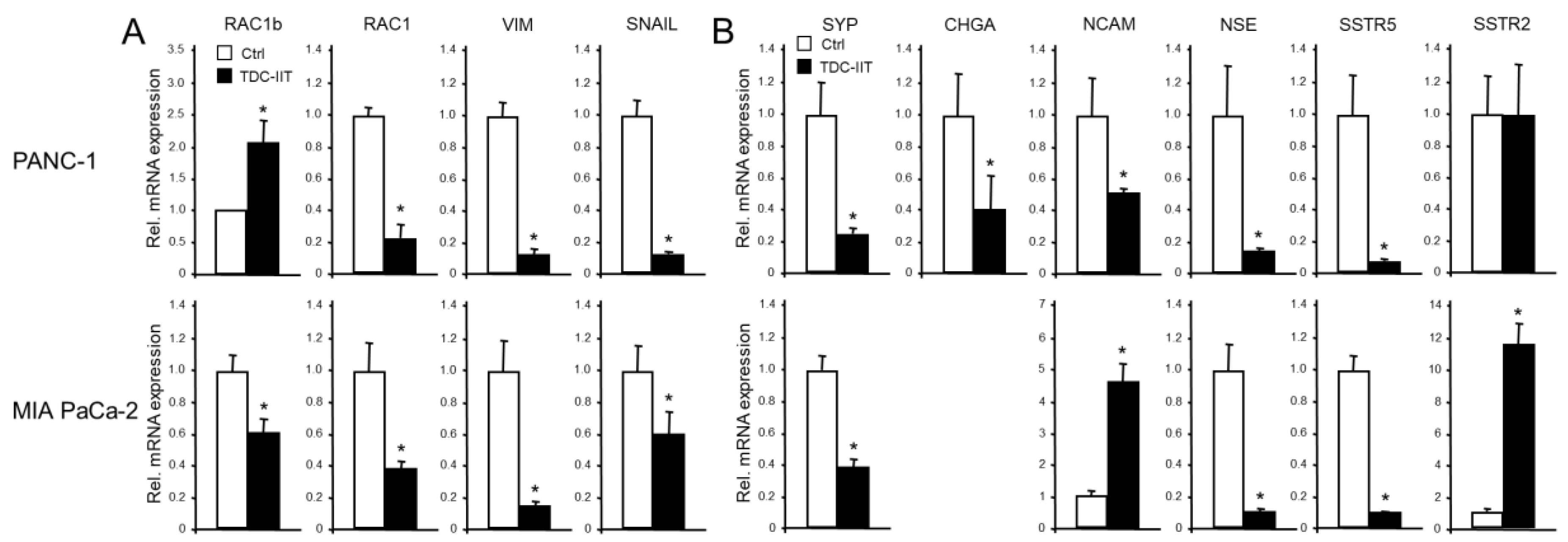
3.6. Cell Lines Derived from SCCOHT Differentially Respond to TGF-β1 or BMP-7 Treatment with Upregulation of EMT and NED Markers
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflict of Interest
References
- Gao, H.L.; Wang, W.Q.; Yu, X.J.; Liu, L. Molecular drivers and cells of origin in pancreatic ductal adenocarcinoma and pancreatic neuroendocrine carcinoma. Exp. Hematol. Oncol. 2020, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Luchini, C.; Scarpa, A. Neoplastic Progression in Neuroendocrine Neoplasms of the Pancreas. Arch. Pathol. Lab. Med. 2023, Mar 7. [CrossRef]
- Gradiz, R.; Silva, H.C.; Carvalho, L.; Botelho, M.F.; Mota-Pinto, A. MIA PaCa-2 and PANC-1 - pancreas ductal adenocarcinoma cell lines with neuroendocrine differentiation and somatostatin receptors. Sci. Rep. 2016, 6, 21648. [Google Scholar] [CrossRef] [PubMed]
- Schmidtlein, P.M.; Volz, C.; Braun, R.; Thürling, I.; Lapshyna, O.; Wellner, U.F.; Konukiewitz, B.; Lehnert, H.; Marquardt, J.U.; Ungefroren, H. A Comparative Endocrine Trans-Differentiation Approach to Pancreatic Ductal Adenocarcinoma Cells with Different EMT Phenotypes Identifies Quasi-Mesenchymal Tumor Cells as Those with Highest Plasticity. Cancers (Basel). 2021, 13, 4663. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.D.; Stone, B.; Thibodeau, B.J.; Baschnagel, A.M.; Galoforo, S.; Fortier, L.E.; Ketelsen, B.; Ahmed, S.; Kelley, Z.; Hana, A.; et al. The significance of Trk receptors in pancreatic cancer. Tumour Biol. 2017, 39, 1010428317692256. [Google Scholar] [CrossRef] [PubMed]
- Ungefroren, H.; Thürling, I.; Färber, B.; Kowalke, T.; Fischer, T.; De Assis, L.V.M.; Braun, R.; Castven, D.; Oster, H.; Konukiewitz, B.; et al. The Quasimesenchymal Pancreatic Ductal Epithelial Cell Line PANC-1-A Useful Model to Study Clonal Heterogeneity and EMT Subtype Shifting. Cancers (Basel) 2022, 14, 2057. [Google Scholar] [CrossRef] [PubMed]
- Dickersin, G.R.; Kline, I.; Scully, R.E. Small cell carcinoma of the ovary with hypercalcemia: a report of eleven cases. Cancer. 1982, 49, 188–197. [Google Scholar] [CrossRef]
- Young, R.H.; Oliva, E.; Scully, R.E. Small cell carcinoma of the hypercalcemic type in the ovary. Gynecol. Oncol. 1995, 57, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Otte, A.; Göhring, G.; Steinemann, D.; Schlegelberger, B.; Groos, S.; Länger, F.; Kreipe, H.H.; Schambach, A.; Neumann, T.; Hillemanns, P.; et al. A tumor-derived population (SCCOHT-1) as cellular model for a small cell ovarian carcinoma of the hypercalcemic type. Int. J. Oncol. 2012, 41, 765–775. [Google Scholar] [CrossRef]
- Upchurch, K.S.; Parker, L.M.; Scully, R.E.; Krane, S.M. Differential cyclic AMP responses to calcitonen among human ovarian carcinoma cell lines: a clacitonin-responsive line derived from a rare tumor type. J. Bone Miner. Res. 1986, 1, 299–304. [Google Scholar] [CrossRef]
- Gamwell, L.F.; Gambaro, K.; Merziotis, M. , Crane, C.; Arcand, S.L.; Bourada, V.; Davis, C.; Squire, J.A.; Huntsman, D.G.; Tonin, P.N.; et al. Small cell ovarian carcinoma: genomic stability and responsiveness to therapeutics. Orphanet J. Rare Dis. 2013, 8, 33. [Google Scholar] [CrossRef]
- Otte, A.; Rauprich, F.; Hillemanns, P.; Park-Simon, T.W.; von der Ohe, J.; Hass, R. In vitro and in vivo therapeutic approach for a small cell carcinoma of the ovary hypercalcaemic type using a SCCOHT-1 cellular model. Orphanet J. Rare Dis. 2014, 9, 126. [Google Scholar] [CrossRef] [PubMed]
- Hass, R. von der Ohe, J. Ungefroren, H. The intimate relationship among EMT, MET and TME: A T(ransdifferentiation) E(nhancing) M(ix) to be exploited for therapeutic purposes. Cancers (Basel). 2020, 12, 3674. [Google Scholar] [CrossRef] [PubMed]
- Nouri, M.; Ratther, E.; Stylianou, N.; Nelson, C.C.; Hollier, B.G.; Williams, E.D. Androgen-targeted therapy-induced epithelial mesenchymal plasticity and neuroendocrine transdifferentiation in prostate cancer: an opportunity for intervention. Front. Oncol. 2014, 4, 370. [Google Scholar] [CrossRef] [PubMed]
- Soundararajan, R.; Paranjape, A.N.; Maity, S.; Aparicio, A.; Mani, S.A. EMT, stemness and tumor plasticity in aggressive variant neuroendocrine prostate cancers. Biochim- Biophys. Acta Rev. Cancer. 2018, 1870, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Conteduca, V.; Aieta, M.; Amadori, D.; De Giorgi, U. Neuroendocrine differentiation in prostate cancer: current and emerging therapy strategies. Crit. Rev. Oncol. Hematol. 2014, 92, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.D.; Choo, R.; Huang, J. Neuroendocrine differentiation in prostate cancer: a mechanism of radioresistance and treatment failure. Front. Oncol. 2015, 5, 90. [Google Scholar] [CrossRef] [PubMed]
- Gravemeyer, J.; Lange, A.; Ritter, C.; Spassova, I.; Song, L.; Picard, D.; Remke, M.; Horny, K.; Sriram, A.; Gambichler, T.; et al. Classical and Variant Merkel Cell Carcinoma Cell Lines Display Different Degrees of Neuroendocrine Differentiation and Epithelial-Mesenchymal Transition. J. Invest. Dermatol. 2021, 141, 1675–1686. [Google Scholar] [CrossRef] [PubMed]
- Groves, S.M.; Panchy, N.; Tyson, D.R.; Harris, L.A.; Quaranta, V.; Hong, T. Involvement of Epithelial-Mesenchymal Transition Genes in Small Cell Lung Cancer Phenotypic Plasticity. Cancers (Basel). 2023, 15, 1477. [Google Scholar] [CrossRef] [PubMed]
- Luley, K.B.; Biedermann, S.B.; Künstner, A.; Busch, H.; Franzenburg, S.; Schrader, J.; Grabowski, P.; Wellner, U.F.; Keck, T.; Brabant, G.; et al. A Comprehensive Molecular Characterization of the Pancreatic Neuroendocrine Tumor Cell Lines BON-1 and QGP-1. Cancers (Basel). 2020, 12, 691. [Google Scholar] [CrossRef]
- Leu, F.P.; Nandi, M.; Niu, C. The effect of transforming growth factor beta on human neuroendocrine tumor BON cell proliferation and differentiation is mediated through somatostatin signaling. Mol. Cancer Res. 2008, 6, 1029–1042. [Google Scholar] [CrossRef]
- Ungefroren, H.; Künstner, A.; Busch, H.; Franzenburg, S.; Luley, K.; Viol, F.; Schrader, J.; Konukiewitz, B.; Wellner, U.F.; Meyhöfer, S.M.; et al. Differential Effects of Somatostatin, Octreotide, and Lanreotide on Neuroendocrine Differentiation and Proliferation in Established and Primary NET Cell Lines: Possible Crosstalk with TGF-β Signaling. Int. J. Mol. Sci. 2022, 23, 15868. [Google Scholar] [CrossRef] [PubMed]
- Dicken, H.; Hensley, P.J.; Kyprianou, N. Prostate tumor neuroendocrine differentiation via EMT: The road less traveled. Asian J. Urol. 2019, 6, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Li, R. X.; Yiu, W.H.; Tang, S.C. Role of bone morphogenetic protein-7 in renal fibrosis. Front. Physiol. 2015, 6, 114. [Google Scholar] [CrossRef]
- Pan, Q.; Wang, Y.Q.; Li, G.M.; Duan, X.Y.; Fan, J.G. Fuzheng Huayu Recipe Ameliorates Liver Fibrosis by Restoring Balance between Epithelial-to-Mesenchymal Transition and Mesenchymal-to-Epithelial Transition in Hepatic Stellate Cells. Biomed. Res. Int. 2015, 2015, 935903. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.R.; Seok, S.H.; Kim, D.J.; Han, J.H.; Kim, T.H.; Jung, H.; Lee, B.H.; Park, J.H. Bone morphogenetic protein 7 induces mesenchymal-to-epithelial transition in melanoma cells, leading to inhibition of metastasis. Cancer Sci. 2009, 100, 2218–2225. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, M.; Shah, A.A.; Kalluri, R. Bone morphogenic protein-7 induces mesenchymal to epithelial transition in adult renal fibroblasts and facilitates regeneration of injured kidney. J. Biol. Chem. 2005, 280, 8094–8100. [Google Scholar] [CrossRef]
- Bi, W.R.; Jin, C.X.; Xu, G.T.; Yang, C.Q. Bone morphogenetic protein-7 regulates Snail signaling in carbon tetrachloride-induced fibrosis in the rat liver. Exp. Ther. Med. 2012, 4, 1022–1026. [Google Scholar] [CrossRef]
- Kim, S.; Jeong, C.H.; Song, S.H.; Um, J.E.; Kim, H.S.; Yun, J.S.; Han, D.; Cho, E.S.; Nam, B.Y.; Yook, J. I,.; et al. Micellized Protein Transduction Domain-Bone Morphogenetic Protein-7 Efficiently Blocks Renal Fibrosis Via Inhibition of Transforming Growth Factor-Beta-Mediated Epithelial-Mesenchymal Transition. Front. Pharmacol. 2020, 11, 591275. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, D.; Zhu, Z.; Li, X.; An, G.; Niu, P.; Chen, L.; Tian, L. Bone morphogenetic protein-7 prevented epithelial-mesenchymal transition in RLE-6TN cells. Toxicol. Res. (Camb). 2016, 5, 931–937. [Google Scholar] [CrossRef]
- Cho, K.; Kim, N.H.; Seo, S.H.; Song, S.H.; Jeong, C.H.; Kim, H.S.; Um, J.E.; Ku, M.; Yang, J.; Park, J.Y.; et al. A micellized bone morphogenetic protein-7 prodrug ameliorates liver fibrosis by suppressing transforming growth factor-β signaling. Am. J. Cancer Res. 2022, 12, 763–778. eCollection 2022. [Google Scholar]
- Benten D, Behrang Y, Unrau L, Weissmann V, Wolters-Eisfeld G, Burdak-Rothkamm S, Stahl FR, Anlauf M, Grabowski P, Möbs M. ; et al. Establishment of the First Well-differentiated Human Pancreatic Neuroendocrine Tumor Model. Mol. Cancer Res. 2018, 16, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Zinn, R.; Otterbein, H.; Lehnert, H.; Ungefroren, H. RAC1B: A Guardian of the Epithelial Phenotype and Protector Against Epithelial-Mesenchymal Transition. Cells. 2019, 8, 1569. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.W.; Mattingly, C.A.; Strodel, W.E. Increased tumorigenicity in the human pancreatic cell line MIA PaCa-2 is associated with an aberrant regulation of an IGF-1 autocrine loop and lack of expression of the TGF-beta type RII receptor. J. Cell. Physiol. 1995, 165, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Puente, E.; Saint-Laurent, N.; Torrisani, J.; Furet, C.; Schally, A.V.; Vaysse, N.; Buscail, L.; Susini, C. Transcriptional activation of mouse sst2 somatostatin receptor promoter by transforming growth factor-beta. Involvement of Smad4. J. Biol. Chem. 2001, 276, 13461–13468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sang, X.; Zhang, R.; Chi, J.; Bai, W. CD105 expression is associated with invasive capacity in ovarian cancer and promotes invasiveness by inhibiting NDRG1 and regulating the epithelial-mesenchymal transition. Am. J. Transl. Res. 2021, 13, 12461–12479. eCollection 2021. [Google Scholar] [PubMed]
- Jorand, R.; Biswas, S.; Wakefield, D.L.; Tobin, S.J.; Golfetto, O.; Hilton, K.; Ko, M.; Ramos, J.W.; Small, A.R.; Chu, P.; et al. Molecular signatures of mu opioid receptor and somatostatin receptor 2 in pancreatic cancer. Mol. Biol. Cell. 2016, 27, 3659–3672. [Google Scholar] [CrossRef] [PubMed]
- Gil, J.; Marques-Pamies, M.; Valassi, E.; Serra, G.; Salinas, I.; Xifra, G.; Casano-Sancho, P.; Carrato, C.; Biagetti, B.; Sesmilo, G.; et al. Molecular characterization of epithelial-mesenchymal transition and medical treatment related-genes in non-functioning pituitary neuroendocrine tumors. Front. Endocrinol. (Lausanne). 2023, 14, 1129213. [Google Scholar] [CrossRef] [PubMed]
- Sruthi, K.K.; Natani, S.; Ummanni, R. Tumor protein D52 (isoform 3) induces NF-κB - STAT3 mediated EMT driving neuroendocrine differentiation of prostate cancer cells. Int. J. Biochem. Cell Biol. 2024, 166, 106493. [Google Scholar] [CrossRef] [PubMed]
- Natani, S.; Ramakrishna, M.; Nallavolu, T.; Ummanni, R. MicroRNA-147b induces neuroendocrine differentiation of prostate cancer cells by targeting ribosomal protein RPS15A. Prostate. 2023, 83, 936–949. [Google Scholar] [CrossRef] [PubMed]
- McKeithen, D.; Graham, T.; Chung, L.W.; Odero-Marah, V. Snail transcription factor regulates neuroendocrine differentiation in LNCaP prostate cancer cells. Prostate. 2010, 70, 982–992. [Google Scholar] [CrossRef]
- Bery, F.; Cancel, M.; Guéguinou, M.; Potier-Cartereau, M.; Vandier, C.; Chantôme, A.; Guibon, R.; Bruyère, F.; Fromont, G.; Mahéo, K. Zeb1 and SK3 Channel Are Up-Regulated in Castration-Resistant Prostate Cancer and Promote Neuroendocrine Differentiation. Cancers (Basel)., 2021, 13, 2947. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.N.; Chen, W.Y.; Yeh, H.L.; Chen, W.H.; Jiang, K.C.; Li, H.R.; Dung, P.V.T.; Chen, Z.Q.; Lee, W.J.; Hsiao, M.; et al. MCTP1 increases the malignancy of androgen-deprived prostate cancer cells by inducing neuroendocrine differentiation and EMT. Sci. Signal. 2024, 17, eadc9142. [Google Scholar] [CrossRef]
- Natani, S.; Dhople, V.M.; Parveen, A.; Sruthi, K.K.; Khilar, P.; Bhukya, S.; Ummanni, R. AMPK/SIRT1 signaling through p38MAPK mediates Interleukin-6 induced neuroendocrine differentiation of LNCaP prostate cancer cells. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 119085. [Google Scholar] [CrossRef]
- Bosutti, A.; Zanconati, F.; Grassi, G.; Dapas, B.; Passamonti, S.; Scaggiante, B. Epigenetic and miRNAs Dysregulation in Prostate Cancer: The role of Nutraceuticals. Anticancer Agents Med. Chem. 2016, 16, 1385–1402. [Google Scholar] [CrossRef]
- Ellenrieder, V.; Hendler, S.F.; Boeck, W.; Seufferlein, T.; Menke, A.; Ruhland, C.; Adler, G.; Gress, T.M. Transforming growth factor beta1 treatment leads to an epithelial-mesenchymal transdifferentiation of pancreatic cancer cells requiring extracellular signal-regulated kinase 2 activation. Cancer Res. 2001, 61, 4222–4228. [Google Scholar]
- Ungefroren, H.; Konukiewitz, B.; Braun, R.; Wellner, U.F.; Lehnert, H.; Marquardt, J.U. TAp73 Inhibits EMT and Cell Migration in Pancreatic Cancer Cells through Promoting SMAD4 Expression and SMAD4-Dependent Inhibition of ERK Activation. Cancers (Basel). 2023, 15, 3791. [Google Scholar] [CrossRef]
- Thakur, A.K.; Nigri, J.; Lac, S.; Leca, J.; Bressy, C.; Berthezene, P.; Bartholin, L.; Chan, P.; Calvo, E.; Iovanna, J.L.; et al. TAp73 loss favors Smad-independent TGF-β signaling that drives EMT in pancreatic ductal adenocarcinoma. Cell Death Differ. 2016, 23, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Katiyar, S.K. Grape seed proanthocyanidins inhibit migration potential of pancreatic cancer cells by promoting mesenchymal-to-epithelial transition and targeting NF-κB. Cancer Lett. 2013, 334, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Meehan, B.; Macdonald, E.; Venneti, S.; Wang, X.Q.D.; Witkowski, L.; Jelinic, P.; Kong, T.; Martinez, D.; Morin, G.; et al. CDK4/6 inhibitors target SMARCA4-determined cyclin D1 deficiency in hypercalcemic small cell carcinoma of the ovary. Nat. Commun. 2019, 10, 558. [Google Scholar] [CrossRef]
- Otte, A.; Rauprich, F.; von der Ohe, J.; Yang, Y.; Kommoss, F.; Feuerhake, F.; Hillemanns, P.; Hass, R. c-Met inhibitors attenuate tumor growth of small cell hypercalcemic ovarian carcinoma (SCCOHT) populations. Oncotarget. 2015, 6, 31640–31658. [Google Scholar] [CrossRef]
- Takada, M.; Hirata, K.; Ajiki, T.; Suzuki, Y.; Kuroda, Y. Expression of receptor for advanced glycation end products (RAGE) and MMP-9 in human pancreatic cancer cells. Hepatogastroenterology. 2004, 51, 928–930. [Google Scholar] [PubMed]
- Carl, C.; Flindt, A.; Hartmann, J.; Dahlke, M.; Rades, D.; Dunst, J.; Lehnert, H.; Gieseler, F.; Ungefroren, H. Ionizing radiation induces a motile phenotype in human carcinoma cells in vitro through hyperactivation of the TGF-beta signaling pathway. Cell. Mol. Life Sci. 2016, 73, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Neuzillet, C.; de Gramont, A.; Tijeras-Raballand, A.; de Mestier, L.; Cros, J.; Faivre, S.; Raymond, E. Perspectives of TGF-beta inhibition in pancreatic and hepatocellular carcinomas. Oncotarget. 2014, 5, 78–94. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




