Submitted:
13 July 2024
Posted:
15 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
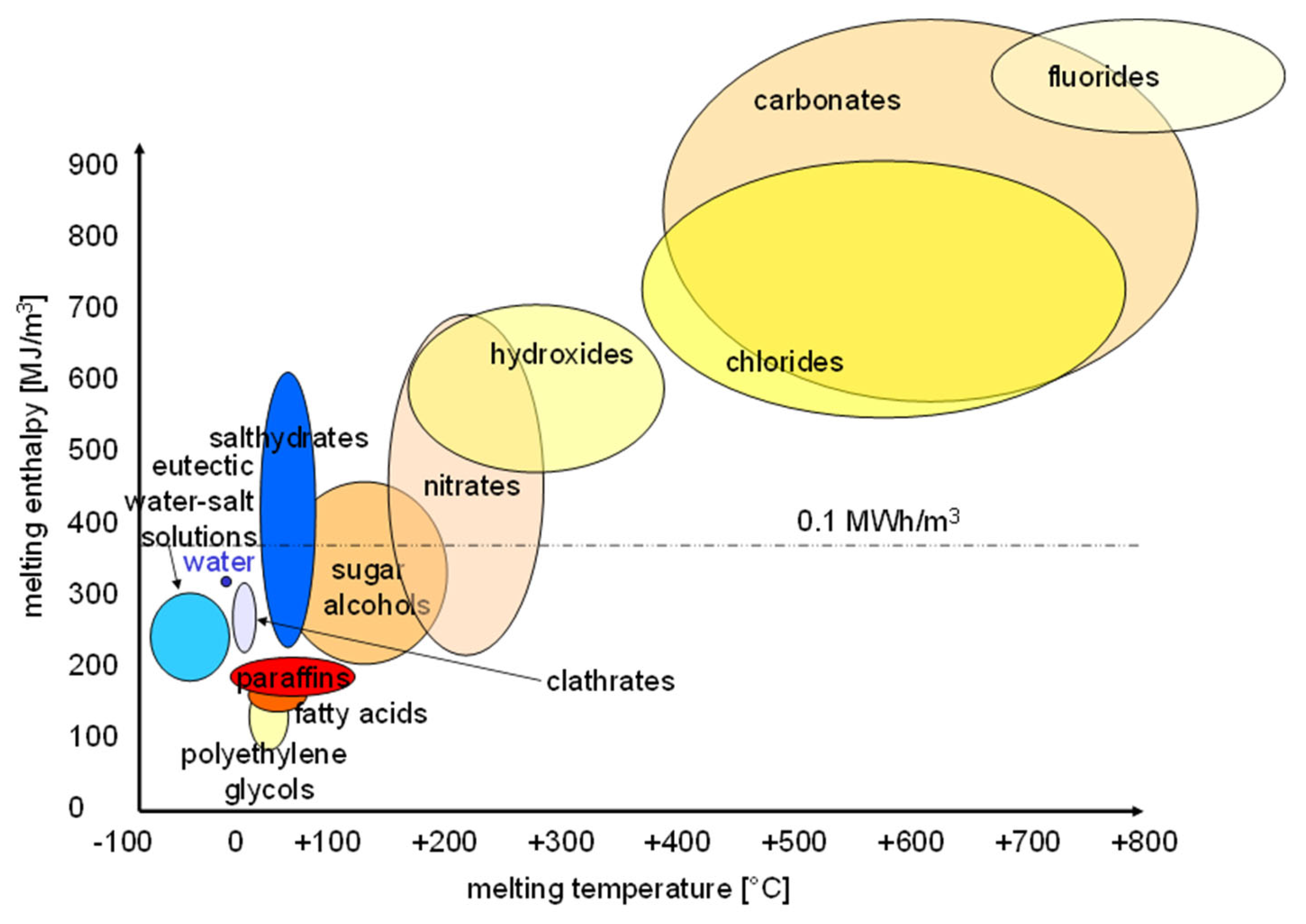
1.1. Background on PCMs
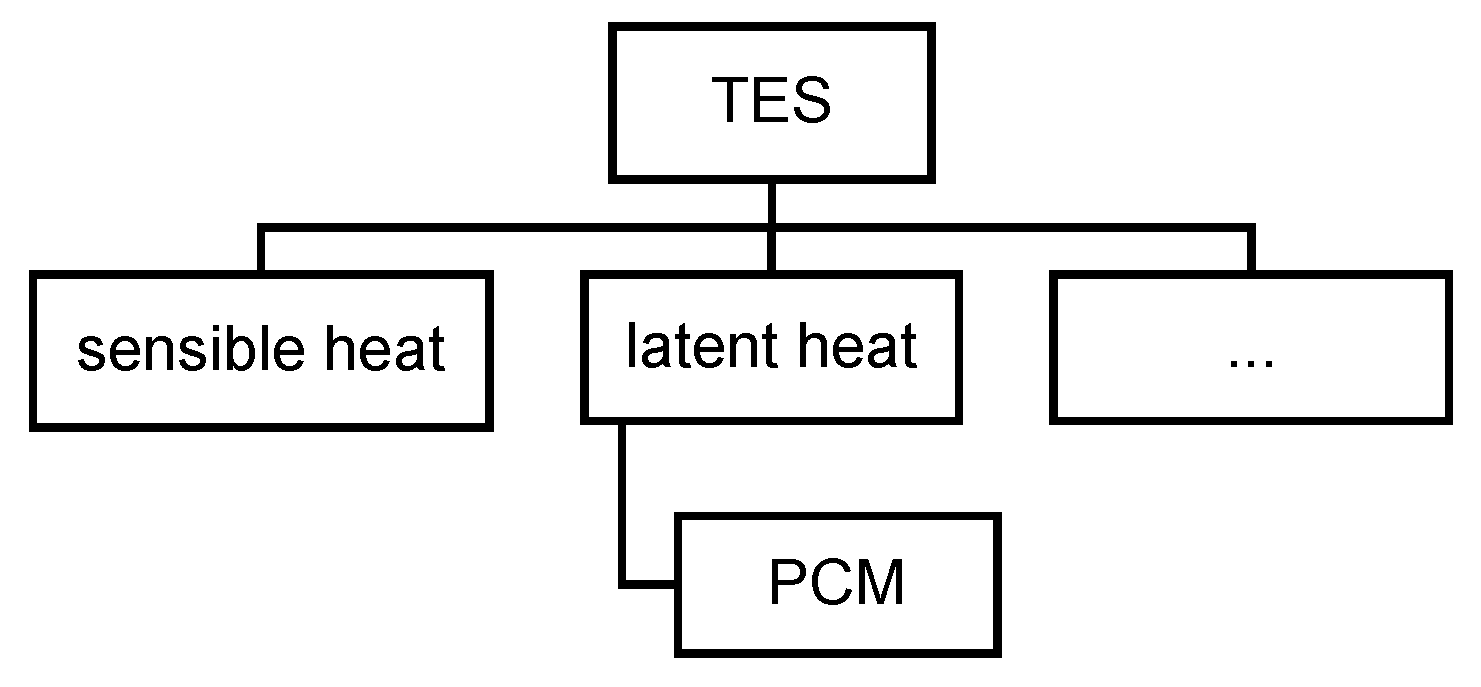
1.2. Review of Classification of PCMs as Used in the Early Years
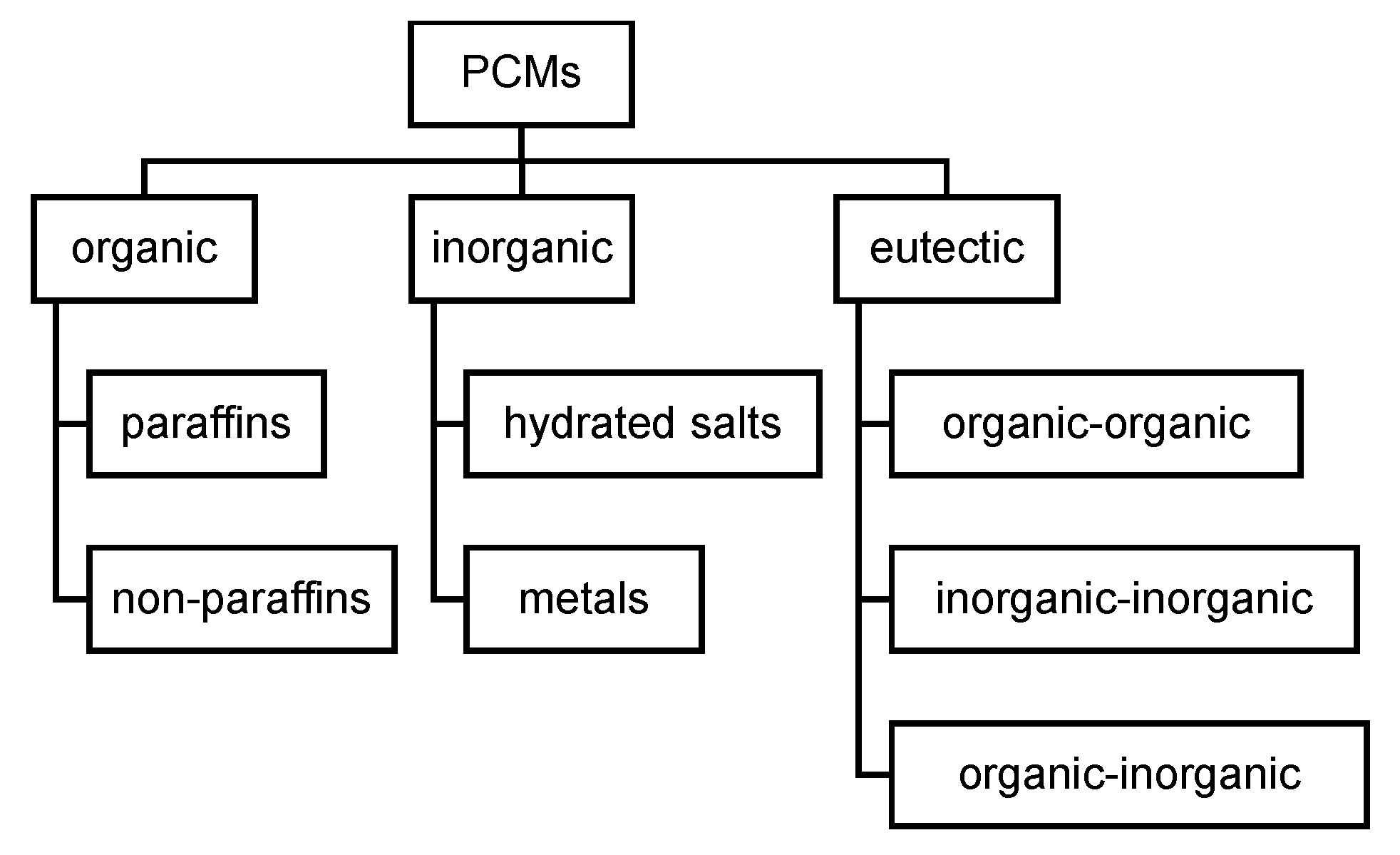
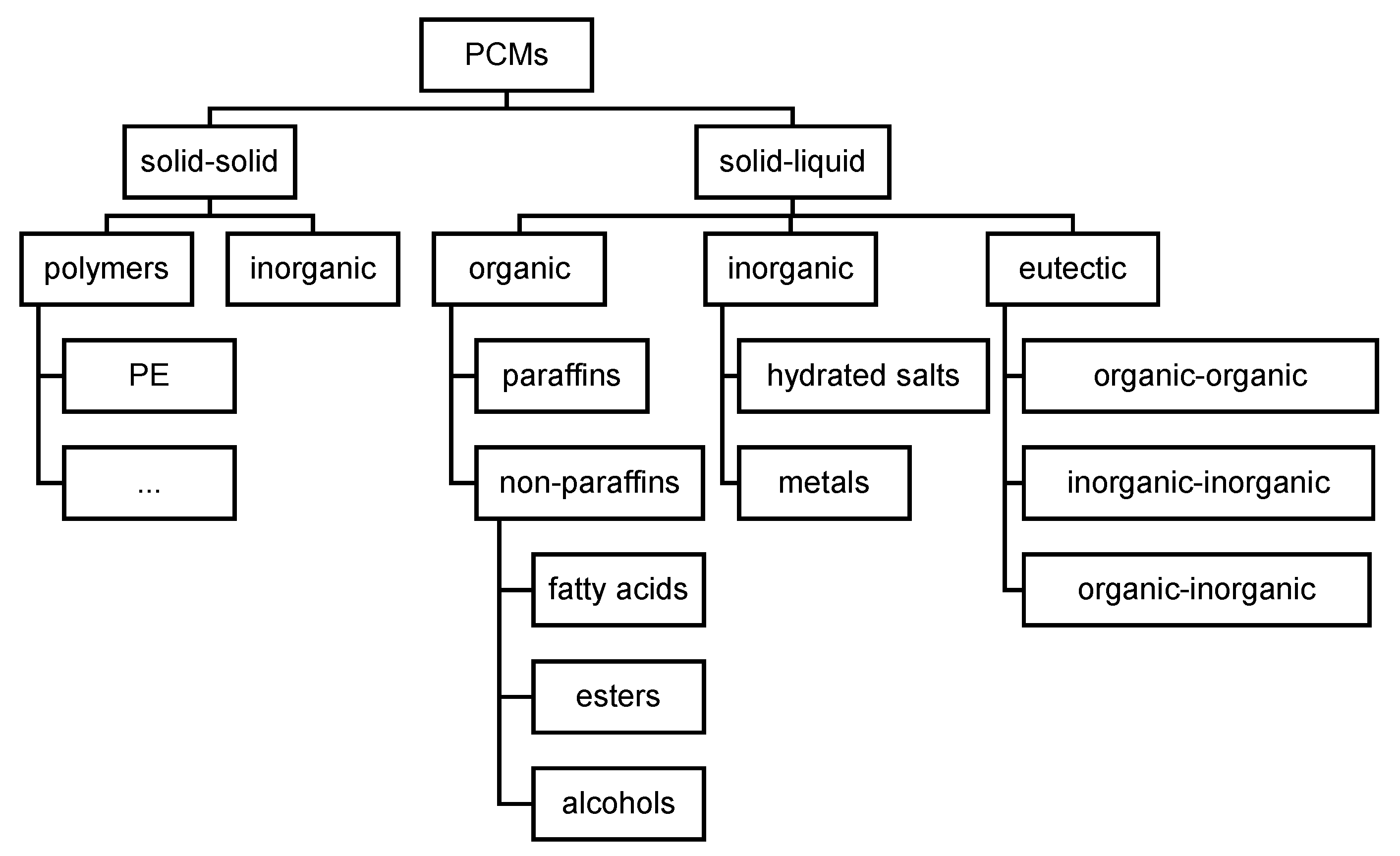
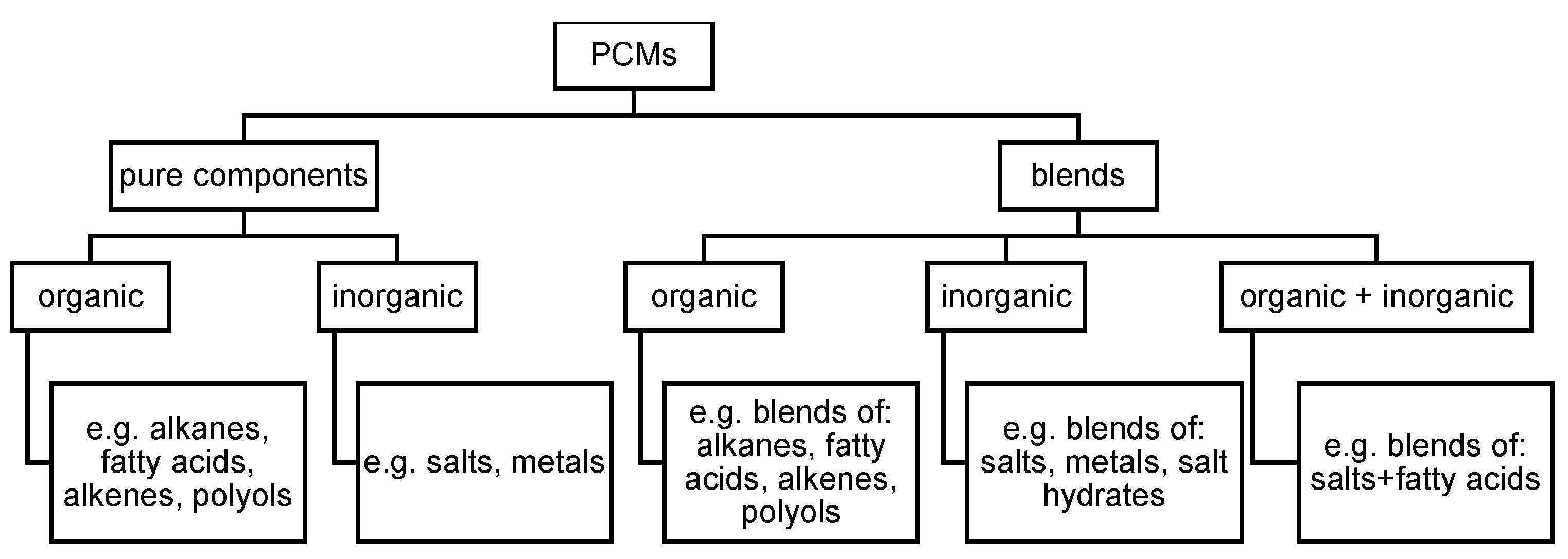
1.3. Review of Classification of PCM as Commonly Used Nowadays
2. Analysis of weaknesses
3. Development of Consistent, Useful Classification
3.1. Review of Options for Classification Criteria
3.2. Selection of Classification Criteria with Regard to Goal and Focus
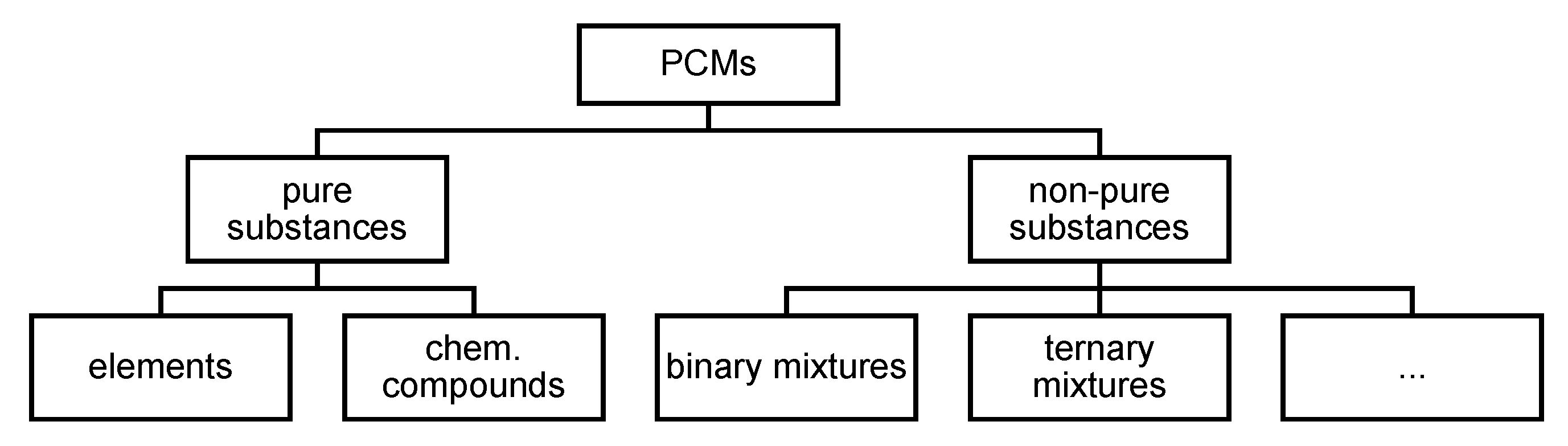
3.3. Suggestion of a New Classification of PCMs
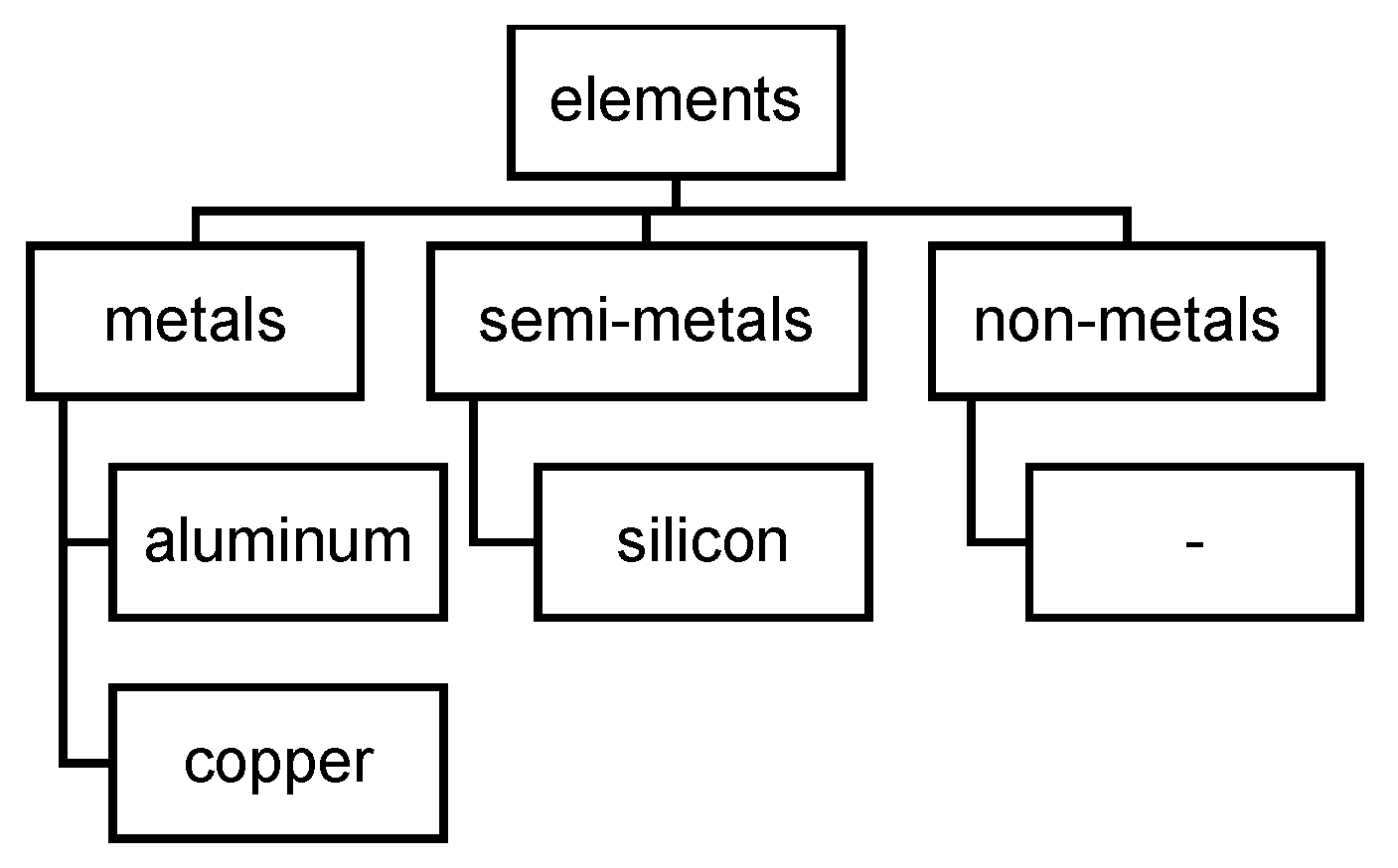
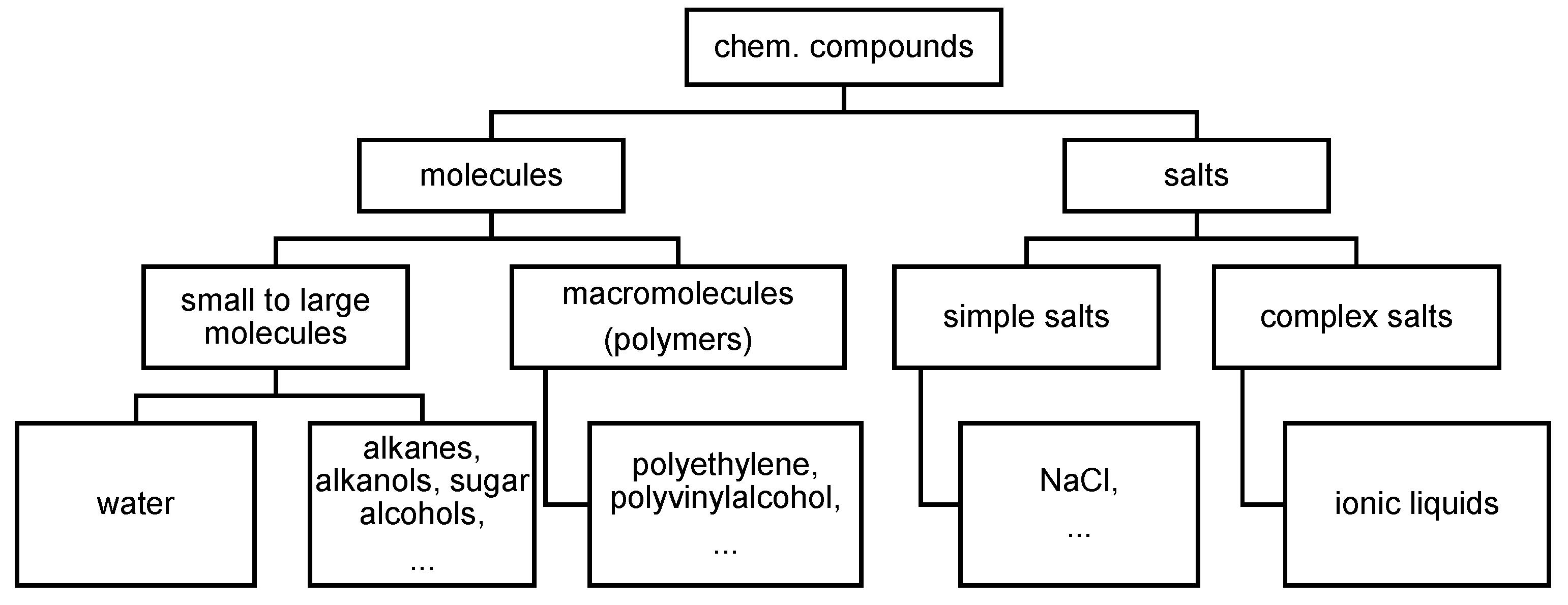
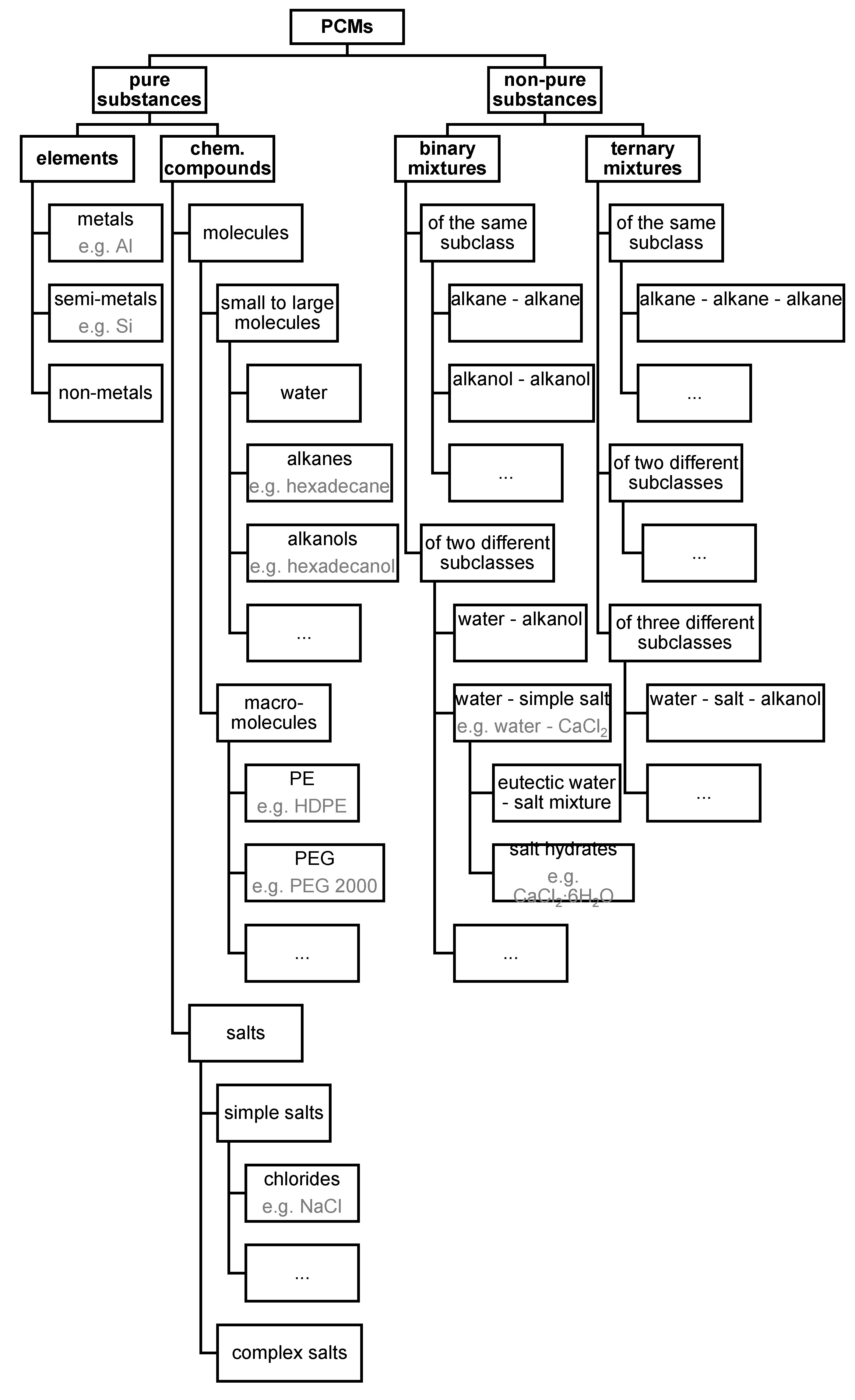
3.3.1. Pure Substances: Elements and Chem. Compounds
- saturated or unsaturated (due to double or triple bonds),
- branched or linear,
- cyclic or open chain.
- Further on, organic compounds differ by functional groups (attached to the rest R), e.g.
- alcohols R-OH
- carboxylic acids R-COOH
- amines R-NH2
- amides R-CON-R´R´´
- esters R-COO-R´
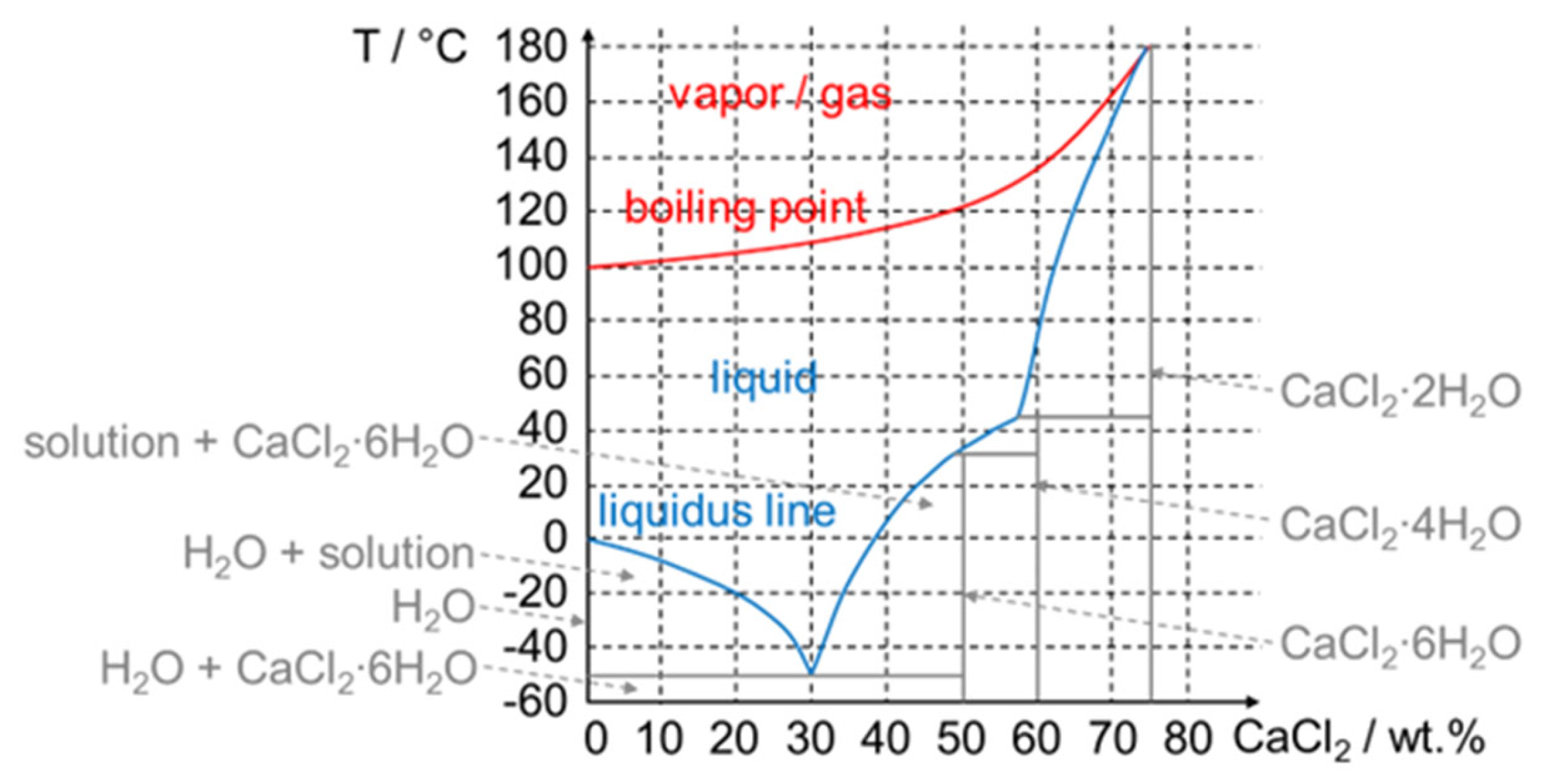
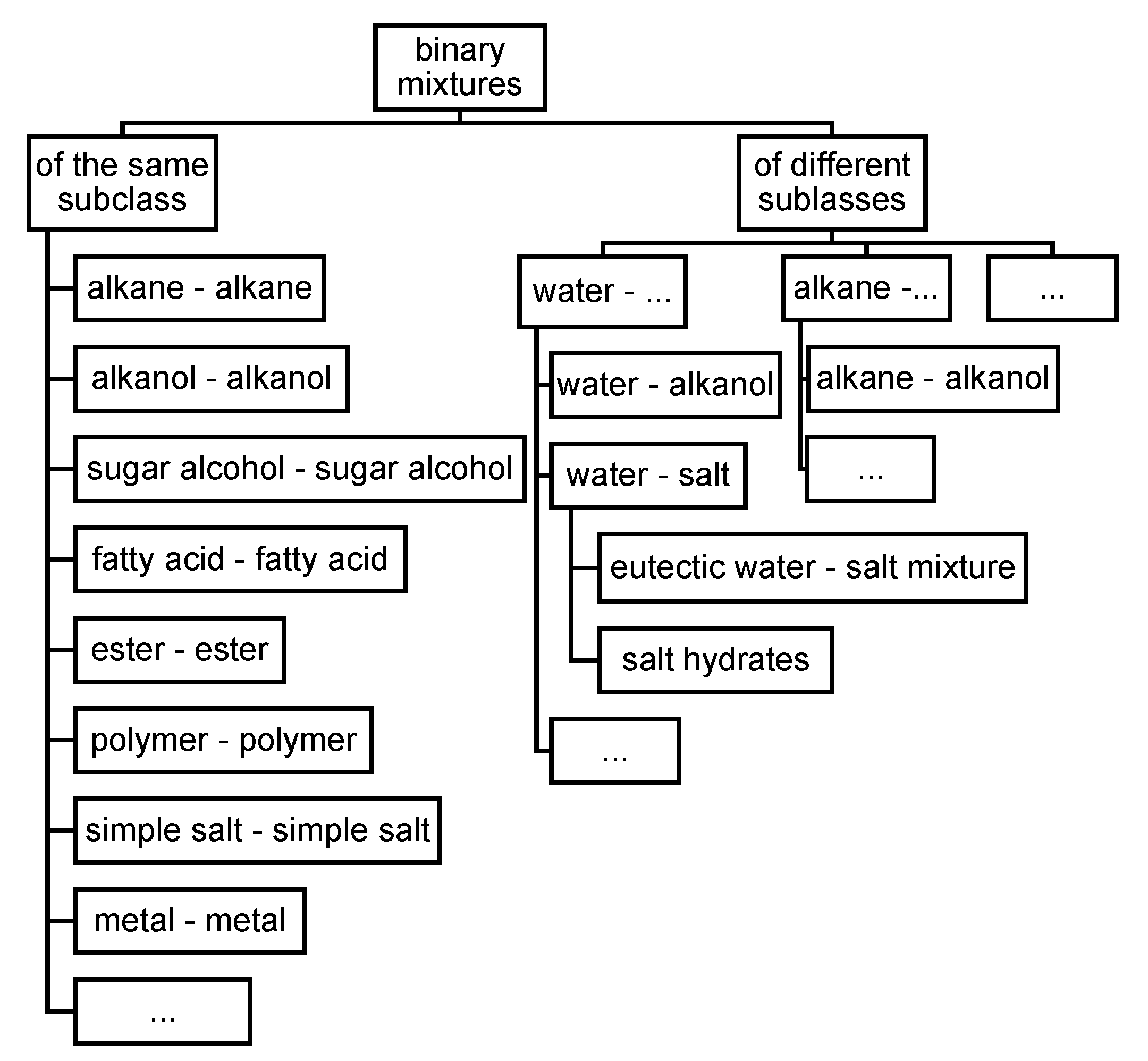
3.3.2. Non-Pure Substances, Meaning Mixtures
4. Summary and Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mehling, H.; Cabeza, L.F. Heat and cold storage with PCM; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-540-68556-2. [Google Scholar]
- Mehling, H.; Brütting, M.; Haussmann, T. PCM products and their fields of application - An overview of the state in 2020/2021. J. Energy Storage 2022, 51, 104354. [Google Scholar] [CrossRef]
- Mehling, H. Use of Phase Change Materials for Food Applications - State of the Art in 2022. Appl. Sci. 2023, 13, 3354. [Google Scholar] [CrossRef]
- Mehling, H. Analysis of Existing Approaches to Investigate Property Degradation of Phase Change Materials and Development of a New Systematic Approach. Appl. Sci. 2023, 13, 8682. [Google Scholar] [CrossRef]
- Podara, C.V.; Kartsonakis, I.A.; Charitidis, C.A. Towards Phase Change Materials for Thermal Energy Storage: Classification, Improvements and Applications in the Building Sector. Appl. Sci. 2021, 11, 1490. [Google Scholar] [CrossRef]
- Kushwah, A; Gaur, M.K.; Pandit, R.K. The Role of Phase Change Materials for Lifetime Heating of Buildings in Cold Climatic Conditions. IJBES, 2020; 7, 81–96. [CrossRef]
- Kavya, V.S.; Ramana, A.S. Recent Trends in PCM-Integrated Solar Dryers. Eng. Proc. 2024, 61, 6. [Google Scholar] [CrossRef]
- Jouhara, H.; Żabnieńska-Góra, A.; Khordehgaa, N.; Ahmad, D.; Lipinski, T. Latent thermal energy storage technologies and applications: A review. Int. J. Thermofluids 2020, 5–6, 100039. [Google Scholar] [CrossRef]
- Gunasekara, S.N.; Barreneche, C.; Inés Fernández, A.; Calderón, A.; Ravotti, R.; Ristić, A.; Weinberger, P.; Ömur Paksoy, H.; Koçak, B.; Rathgeber, C.; Ningwei Chiu, J. and Stamatiou, A. Thermal Energy Storage Materials (TESMs) - What Does It Take to Make Them Fly? Crystals 2021, 11, 1276. [Google Scholar] [CrossRef]
- Atkins, P.W. Physical Chemistry, 4th ed; Oxford University Press: Oxford, UK, 1990; ISBN 0-19-855284-X. [Google Scholar]
- Brown, T.L.; LeMay, H.E.; Bursten, B.E.; Murphy, C.J.; Woodward, P.M.; Stoltzfus, M.W. Chemistry: The Central Science. Global Edition. 13th ed. Pearson Education Limited, 2015. ISBN 978-1-292-05771-2.
- Quant, L.; Diarce, G.; Bouzas, L.; García-Romero, A. A comprehensive study of the phase segregation of a urea-based phase change material tested under thermal cycling conditions. J. Energy Storage 2023, 60, 106621. [Google Scholar] [CrossRef]
- Lane, G.A. Solar Heat Storage: Latent Heat Material - Volume II: Technology, 1st ed.; CRC Press: Boca Raton, FL, USA, 1986; eBook published 2017. [Google Scholar] [CrossRef]
- Mehling, H. Enthalpy and temperature of the phase change solid–liquid – An analysis of data of compounds employing entropy. Solar Energy 2013, 95, 290–299. [Google Scholar] [CrossRef]
- Mehling, H.; White, M.A. Analysis of trends in phase change enthalpy, entropy and temperature for alkanes, alcohols and fatty acids. Chem. Phys. Impact 2023, 6, 100222. [Google Scholar] [CrossRef]
- Matuszek, K.; Vijayaraghavan, R.; Forsyth, C.M.; Mahadevan, M.; Kar, M.; MacFarlane, D.R. Pyrazolium Phase-Change Materials for Solar-Thermal Energy Storage. ChemSusChem 2020, 13, 159–164. [Google Scholar] [CrossRef]
- Mondieig, D.; Rajabalee, F.; Metivaud, V. n-Alkane Binary Molecular Alloys. Chem. Mater. 2004, 16, 786–798. [Google Scholar] [CrossRef]
- Gunasekara, S.N. Phase Equilibrium-aided Design of Phase Change Materials from Blends. Doctoral Thesis, KTH Royal Institute of Technology, Sweden, 2017. [Google Scholar]
- Palomo Del Barrio, E.; Cadoret, R.; Daranlot, J.; Achchaq, F. New sugar alcohols mixtures for long term thermal energy storage applications at temperatures between 70 °C and 100 °C. Solar Energy Materials & Solar Cells 2016, 155, 454–468. [Google Scholar] [CrossRef]
- Bidiyasar, R.; Kumar, R.; Jakhar, N. State-of-the-Art Review of Organic Phase Change Materials for Low-Temperature Thermal. Energy Storage Technology Preprint 2022. [Google Scholar] [CrossRef]
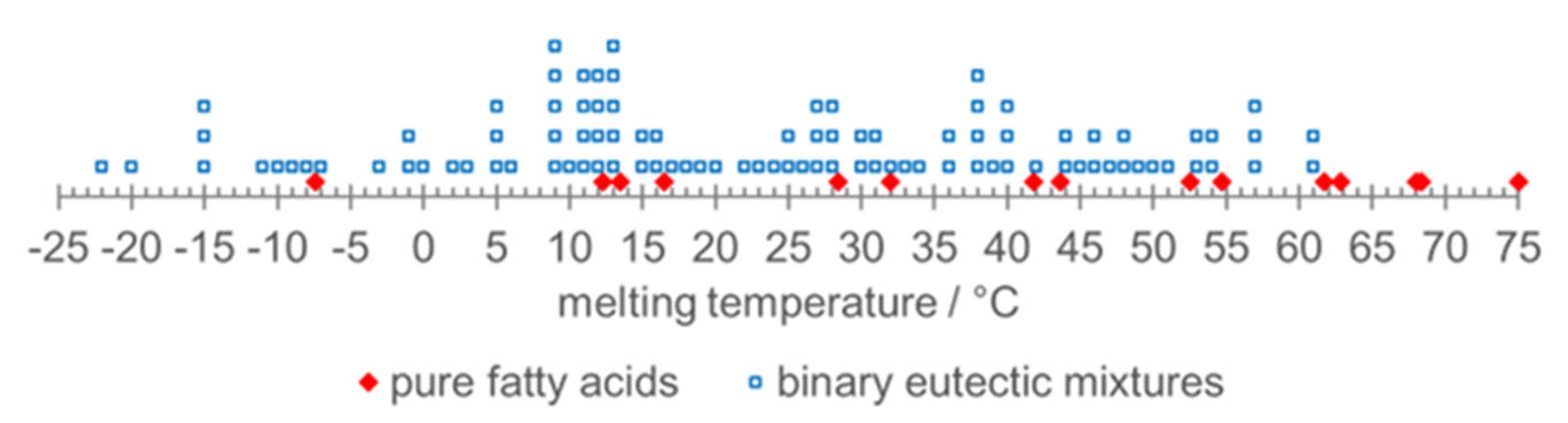
- Zhou, D.; Xiao, S.; Xiao, X.; Liu, Y. Preparation, Phase Diagrams and Characterization of Fatty Acids Binary Eutectic Mixtures for Latent Heat Thermal Energy Storage. Separations 2023, 10, 49. [Google Scholar] [CrossRef]
- Rubio-Pérez, G.; Muñoz-Rujas, N.; Aguilar, F.; Ravotti, R.; Müller, L.; Montero, E. Evolution of the Study of Phase Diagram of Binary and Ternary Mixtures Involving Fatty Acid Esters. Materials 2021, 14, 369. [Google Scholar] [CrossRef]
- Stewart, K.M.E.; Stonecipher, E.; Ning, H.; Pillay, S.B. Mixing rules for high density polyethylene-polypropylene blends. Can J Chem Eng. 2023, 101, 5395–5407. [Google Scholar] [CrossRef]
- Maldonado, J.M.; Fullana-Puig, M.; Martín, M.; Solé, A.; Fernández, Á.G.; de Gracia, A.; Cabeza, L.F. Phase Change Material Selection for Thermal Energy Storage at High Temperature Range between 210 °C and 270 °C. Energies 2018, 11, 861. [Google Scholar] [CrossRef]
- Rathgeber, C.; Schmit, H.; Hennemann, P.; Hiebler, S. Investigation of pinacone hexahydrate as phase change material for thermal energy storage around 45 °C. Applied Energy 2014, 136, 7–13. [Google Scholar] [CrossRef]
- Yang, L.; Villalobos, U.; Akhmetov, B.; Gil, A.; Onn Khor, J.; Palacios, A.; Li, Y.; Ding, Y.; Cabeza, L.F.; Tan, W.L.; Romagnoli, A. A comprehensive review on sub-zero temperature cold thermal energy storage materials, technologies, and applications: State of the art and recent developments. Applied Energy 2021, 288, 116555. [Google Scholar] [CrossRef]
- Rathgeber, C.; Schmit, H.; Hiebler, S. Mixtures of alkanes, fatty acids and alcohols as novel phase change materials: preparation and characterization with DSC and T-history. In Proceedings of the 2nd International Conference on Sustainable Energy Storage, Trinity College Dublin, Ireland, 19–21 June 2013. [Google Scholar]
- Solar Energy Research lnstitute. Phase-Change Thermal Energy Storage. Final Subcontract Report On the Symposium Held in Helendale, California, 19–20 October 1988.
- Kahwaji, S.; White, M.A. Prediction of the properties of eutectic fatty acid phase change materials. Thermochimica Acta 2018, 660, 94–100. [Google Scholar] [CrossRef]
- Kahwaji, S.; White, M.A. Data supporting the prediction of the properties of eutectic organic phase change materials. Data inBrief 2018, 17, 724–730. [Google Scholar] [CrossRef]
- Soodoo, N.; Poopalam, K.D.; Bouzidi, L.; Narine, S.S. Fundamental structure-function relationships in vegetable oil based phase change materials: A critical review. Journal of Energy Storage 2022, 51, 104355. [Google Scholar] [CrossRef]
- Gallart-Sirvent, P.; Martín, M.; Villorbina, G.; Balcells, M.; Solé, A.; Barrenche, C.; Cabeza, L.F.; Canela-Garayoa, R. Fatty acid eutectic mixtures and derivatives from non-edible animal fat as phase change materials. RSC Adv. 2017, 7, 24133. [Google Scholar] [CrossRef]
| Thermal properties (for heat storage and heat transfer …) | |
| phase change enthalpy, more precise enthalpy change on phase change ∆pch | |
| phase change temperature Tpc (e.g. low, medium, and high temperature PCM) | |
| thermal conductivity | |
| Chemical properties (with regard to safety, compatibility, sensitivity to change of composition) | |
| safety, specifically combustibility, fire hazard … | |
| compatibility, specifically corrosivity … | |
| sensitivity to loosing matter to the ambient or taking up matter from it | |
| Phase change properties | |
| type of phase change (solid-solid, solid-liquid …; the one that is used, thus not a material property) | |
| type of phase transition (peritectic, eutectic, …) | |
| formation of phases with different composition (congruent, incongruent, or semicongruent melting) | |
| Composition (chemical / physical) | |
| organic, inorganic, or a combination | |
| pure substance (element, chem. compound) or non-pure substance (homogeneous, heterogeneous mixture) | |
| material class (for elements e.g. metal, for chem. compounds e.g. alkane, alcohol, fatty acid, salt, and for mixtures e.g. salt hydrate, gas hydrate, etc. thus giving information on the components as well as how the components interact) | |
| molecule structure (specifically referring to chem. compounds alone as well as in mixtures: linear, branched, grafted, crosslinked … mono-, oligo-, polymer) | |
| Production and life cycle | |
| source (bio-based, fossil-based, food-based, waste-based, extracted or modified by a chem. process) | |
| availability (commercial product, lab scale produced, R&D …) | |
| sink (bio-degradable, landfill deposition, waste incineration, …) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





