Submitted:
15 July 2024
Posted:
15 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Stability of Nicotinamide Riboside
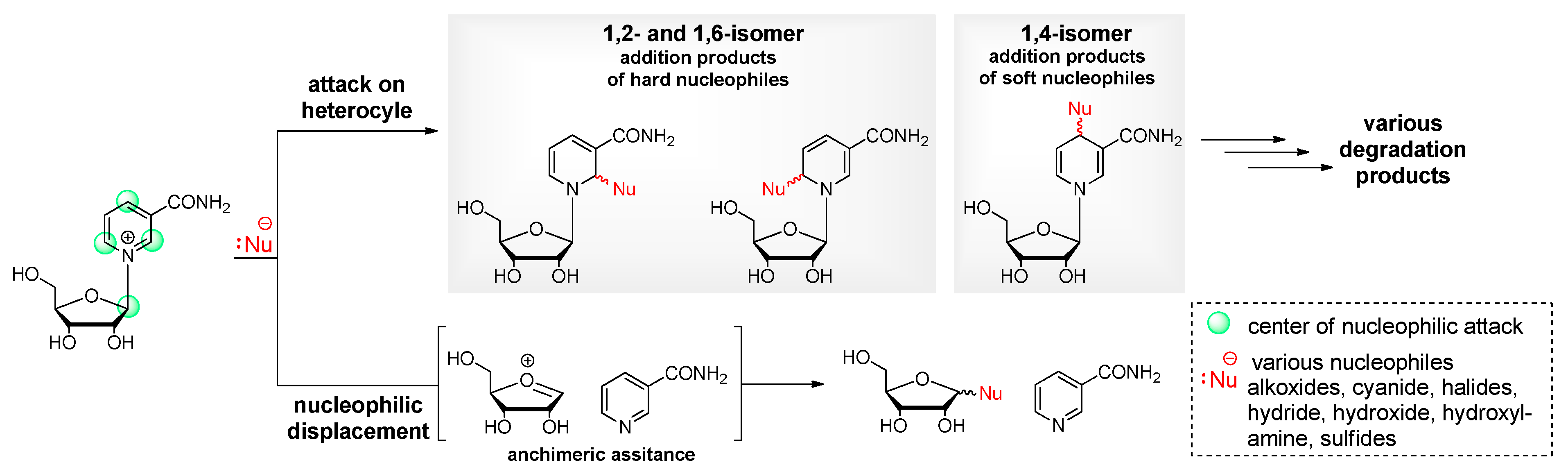
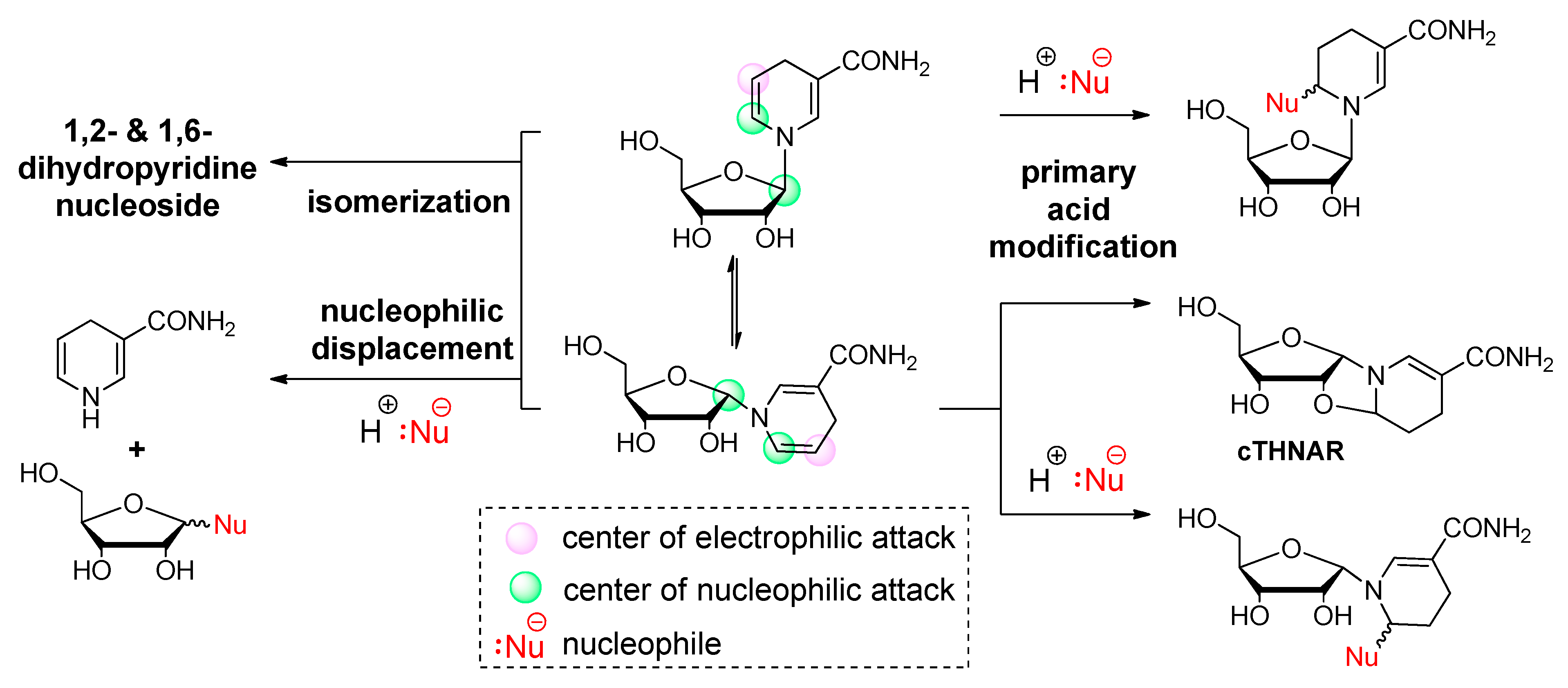
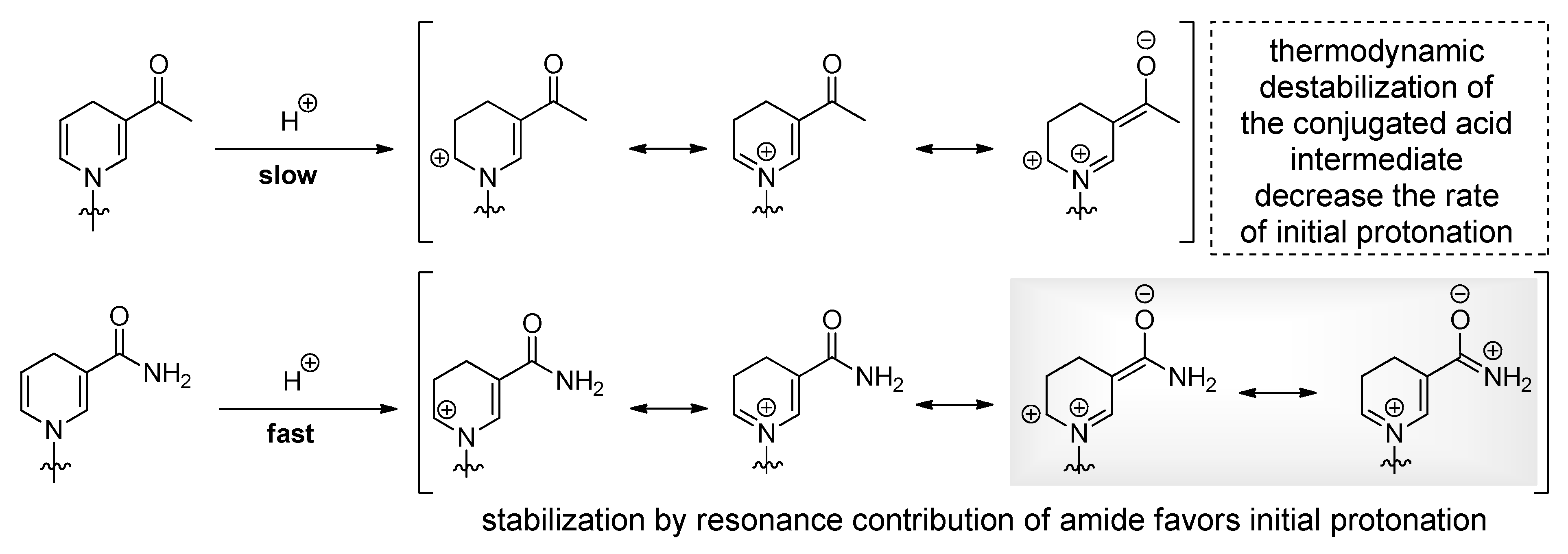
2.1. Nucleophilic Attack On The Anomeric Carbon And The Pyridinium Moiety Leads To Decomposition Of Nicotinamide Riboside Under Alkaline Conditions
3. Synthesis of Nicotinamide Riboside
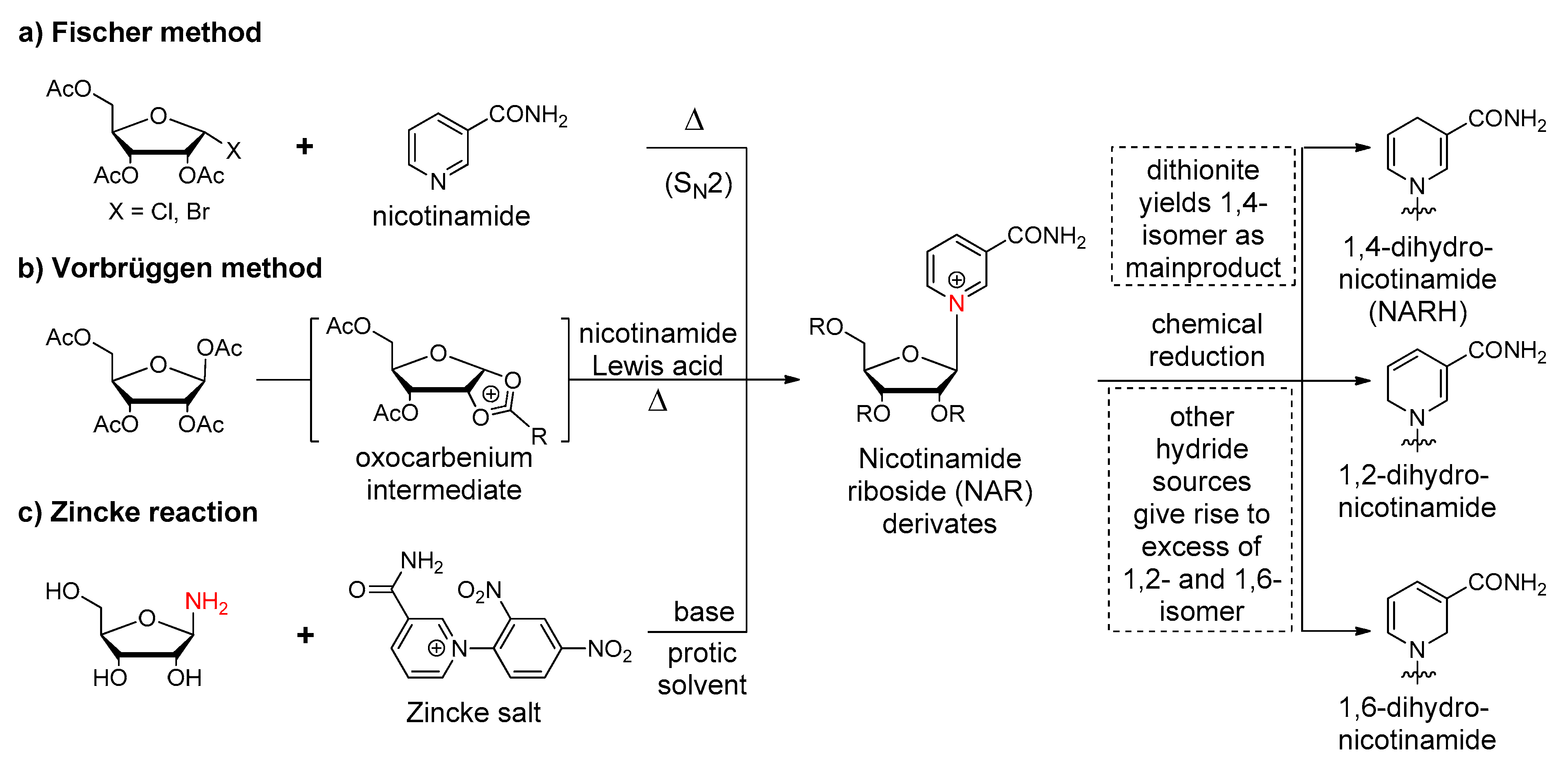
3.1. Synthetic Paths To Nicotinamide Riboside
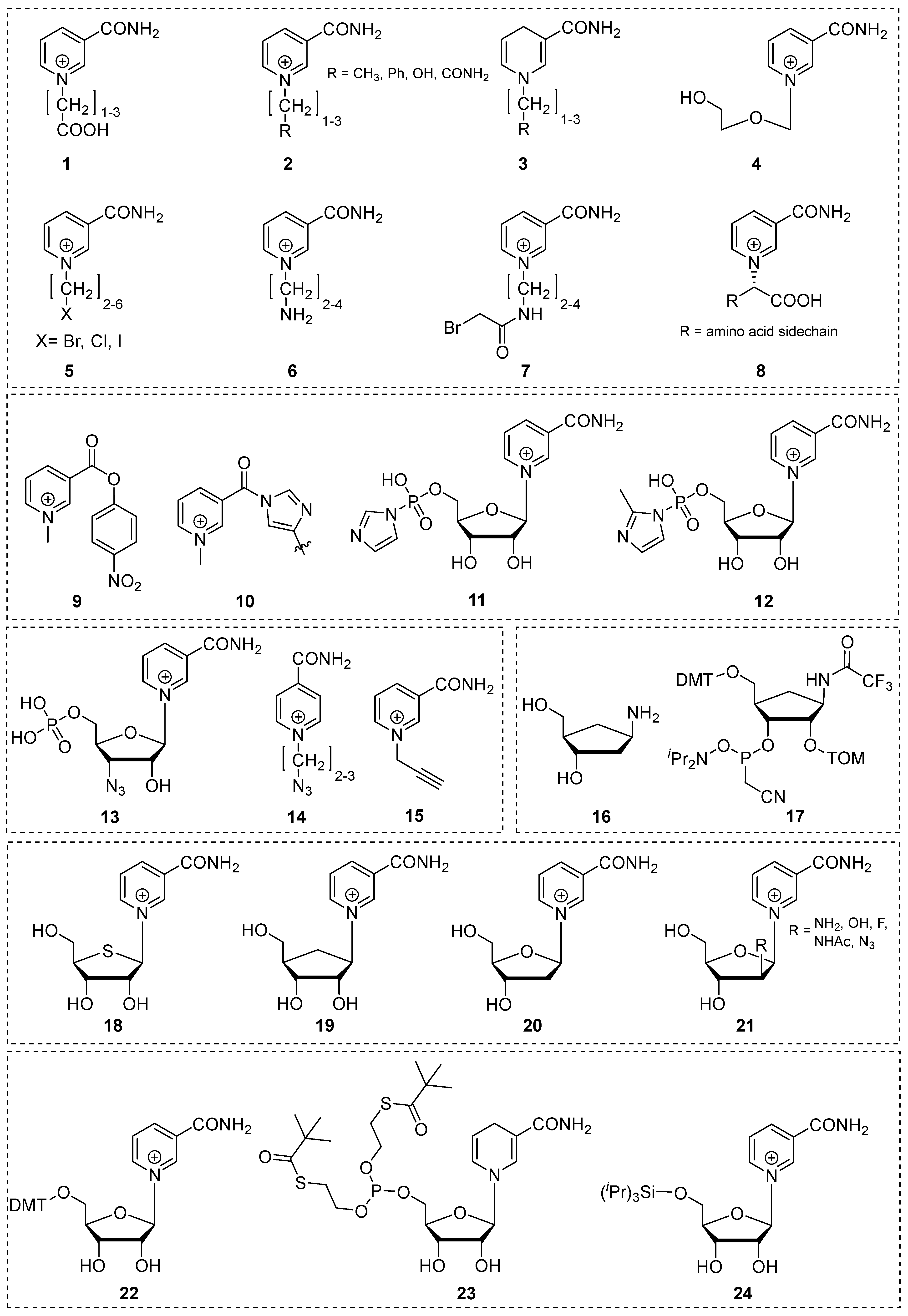
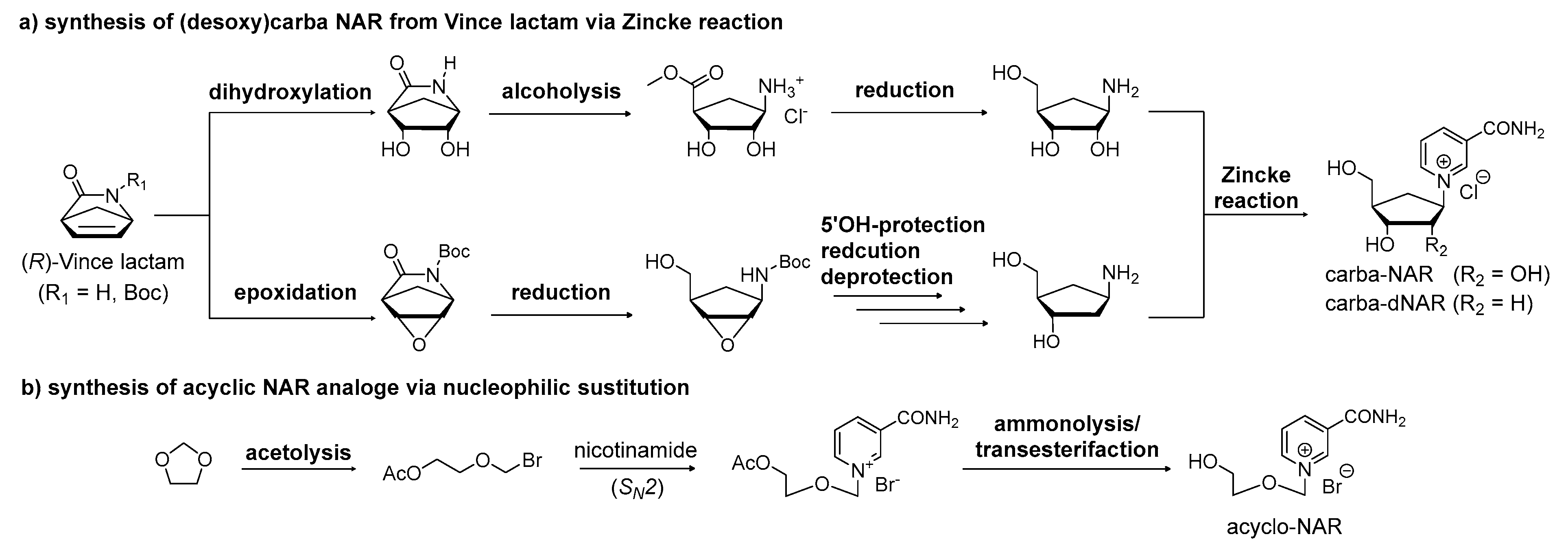
3.2. Synthetic Modification of the Structure of the Ribose Moiety
3.3. Synthetic Modifications Of The Nicotinamide Base
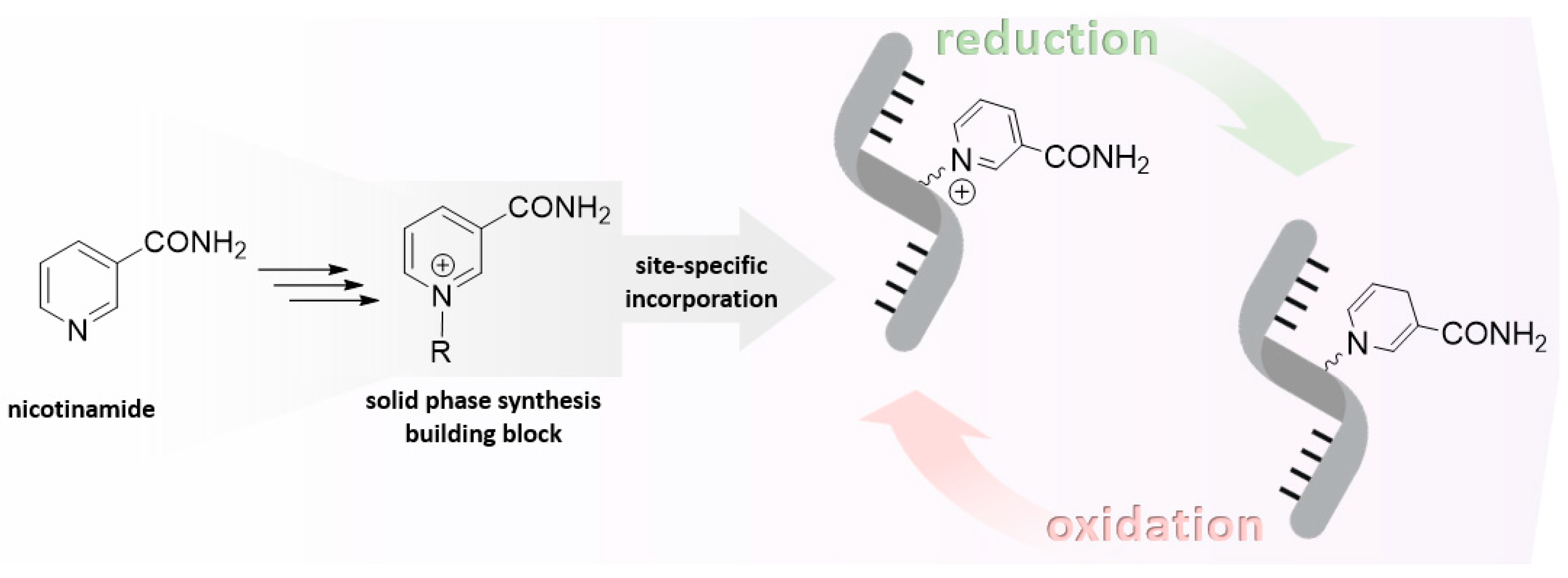
4. Attempts to Incorporate Nicotinamide Riboside in RNA
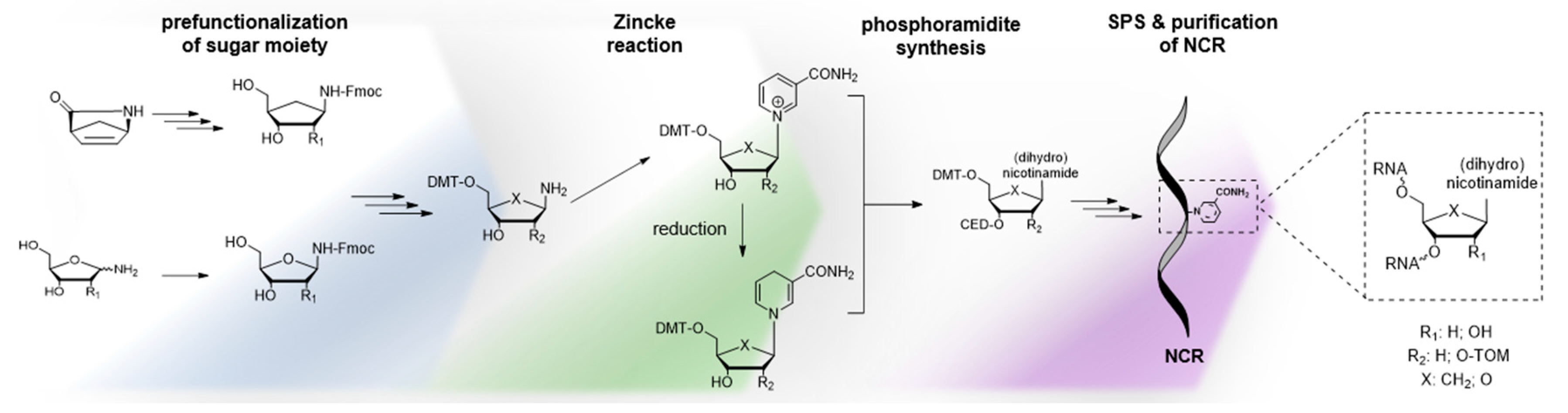
4.1. Synthesis of NAR/NARH Building Blocks
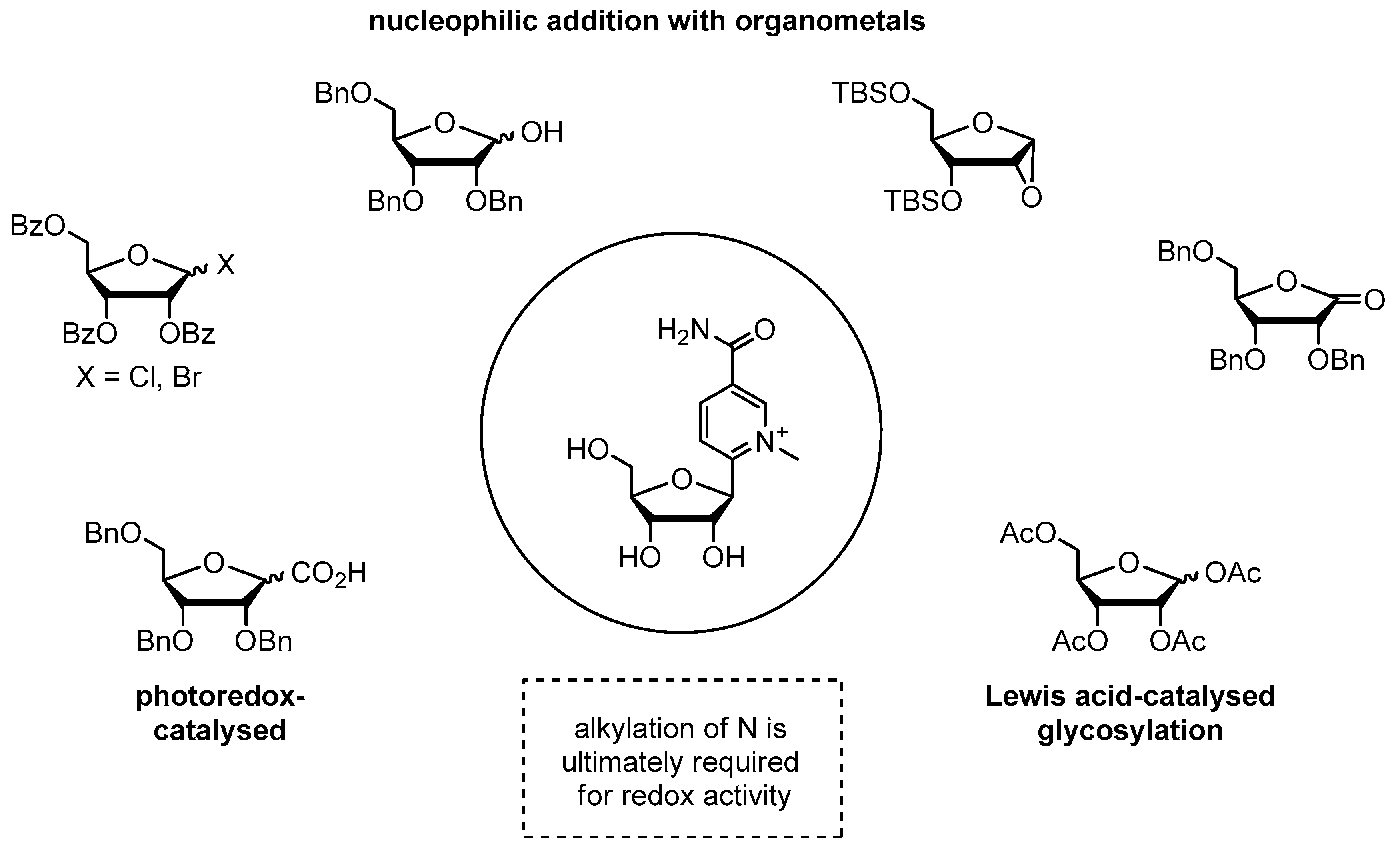
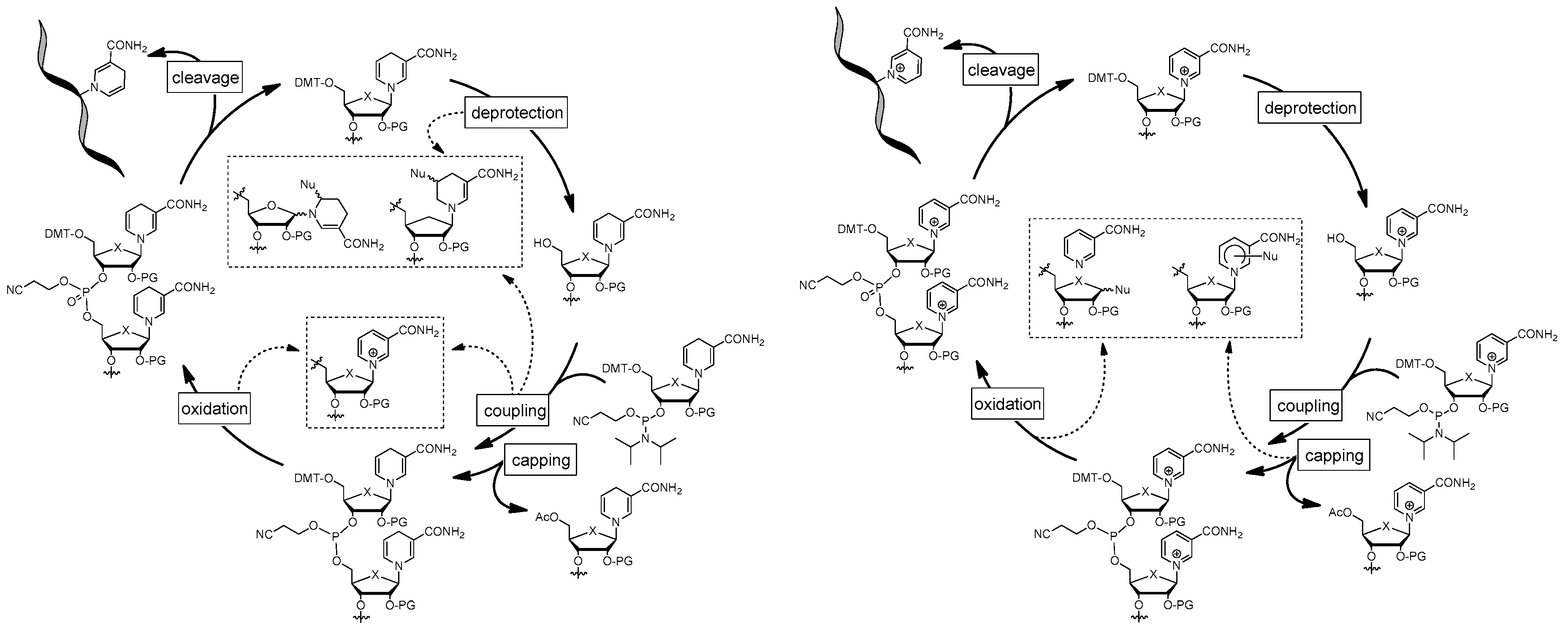
4.2. Application of a NAR/NARH-Phosphoramidite
5. Conclusions
References
- Panchapakesan, Shanker S. S., and Ronald R. Breaker. "The Case of the Missing Allosteric Ribozymes." Nat Chem Biol 17, no. 4 (2021): 375-382. [CrossRef]
- Robertson, M. P., and G. F. Joyce. "The Origins of the RNA World." Cold Spring Harbor Perspectives in Biology 4, no. 5 (2012). [CrossRef]
- Vitreschak, A. "Riboswitches: The Oldest Mechanism for the Regulation of Gene Expression?" Trends Genet. 20, no. 1 (2004): 44-50. [CrossRef]
- Breaker, R. R. "Riboswitches and the RNA World." Cold Spring Harbor Perspectives in Biology 4, no. 2 (2012). [CrossRef]
- Breaker, Ronald R. "Riboswitches: From Ancient Gene-Control Systems to Modern Drug Targets." Future Microbiol 4, no. 7 (2009): 771-773. [CrossRef]
- Goldman, Aaron D., and Betul Kacar. "Cofactors Are Remnants of Life’s Origin and Early Evolution." J Mol Evol 89, no. 3 (2021): 127-133. [CrossRef]
- Huang, Faqing, Charles Walter Bugg, and Michael Yarus. "RNA-Catalyzed Coa, NAD, and FAD Synthesis from Phosphopantetheine, NMN, and FMN." Biochemistry 39, no. 50 (2000): 15548-15555. [CrossRef]
- Kirschning, Andreas. "Coenzymes and Their Role in the Evolution of Life." Angew Chem Int Ed 60, no. 12 (2021): 6242-6269. [CrossRef]
- Breaker, Ronald R., and Gerald F. Joyce. "Self-Incorporation of Coenzymes by Ribozymes." J Mol Evol 40, no. 6 (1995): 551-558. [CrossRef]
- Fujita, Yuki, Hiroyuki Furuta, and Yoshiya Ikawa. "Construction of an Artificial Ribozyme Which Ligates an RNA Fragment Activated by Nicotinamide Mononucleotide." Nucleic Acids Symp Ser 50, no. 1 (2006): 231-232. [CrossRef]
- Fujita, Yuki, Hiroyuki Furuta, and Yoshiya Ikawa. "Tailoring RNA Modular Units on a Common Scaffold: A Modular Ribozyme with a Catalytic Unit for -Nicotinamide Mononucleotide-Activated RNA Ligation." RNA 15, no. 5 (2009): 877-888. [CrossRef]
- Cleaves, H. James, and Stanley L. Miller. "The Nicotinamide Biosynthetic Pathway Is a By-Product of the RNA World." J Mol Evol 52, no. 1 (2001): 73-77. [CrossRef]
- Kim, Hyo-Joong, and Steven A. Benner. "A Direct Prebiotic Synthesis of Nicotinamide Nucleotide." Chem Eur J 24, no. 3 (2018): 581-584. [CrossRef]
- Griffiths, Hollie B. S., Courtney Williams, Sarah J. King, and Simon J. Allison. "Nicotinamide Adenine Dinucleotide (NAD+): Essential Redox Metabolite, Co-Substrate and an Anti-Cancer and Anti-Ageing Therapeutic Target." Biochem Soc Trans 48, no. 3 (2020): 733-744. [CrossRef]
- Emahi, Ismaila, Paige R. Gruenke, and Dana A. Baum. "Effect of Aptamer Binding on the Electron-Transfer Properties of Redox Cofactors." J Mol Evol 81, no. 5-6 (2015): 186-193. [CrossRef]
- Tsukiji, S., K. Ramaswamy, and H. Suga. "Ribozymes That Use Redox Cofactors." Pure Appl Chem 76, no. 7-8 (2004): 1525-1536. [CrossRef]
- Tsukiji, Shinya, Swetansu B. Pattnaik, and Hiroaki Suga. "An Alcohol Dehydrogenase Ribozyme." Nat Struct Mol Biol 10, no. 9 (2003): 713-717. [CrossRef]
- Campbell, M. T. D., D. S. Jones, G. P. Andrews, and S. Li. "Understanding the Physicochemical Properties and Degradation Kinetics of Nicotinamide Riboside, a Promising Vitamin B(3)Nutritional Supplement." Food Nutr Res 63 (2019). [CrossRef]
- Oppenheimer, N. J. "NAD Hydrolysis: Chemical and Enzymatic Mechanisms." Mol Cell Biochem 138, no. 1-2 (1994): 245-251. [CrossRef]
- Handlon, Anthony L., and Norman J. Oppenheimer. "Substituent Effects on the pH-Independent Hydrolysis of 2'-Substituted Nicotinamide Arabinosides." J Org Chem 56, no. 17 (1991): 5009-5010. [CrossRef]
- Minato, H., E. Yamazaki, and M. Kobayashi. "Reactions of Several Nucleophiles with Quaternary Salts of Nicotinamide." Chem. Lett. 5, no. 5 (1976): 525-530. [CrossRef]
- Engbersen, J. F. J., A. Koudijs, H. M. Sleiderink, and M. C. R. Franssen. "Addition of Cyanide Ion to Nicotinamide Cations in Acetonitrile. Formation of Non-Productive Charge-Transfer Complexes." J. Chem. Soc., Perkin Trans. 2, no. 1 (1990): 79-83. [CrossRef]
- Makarov, M. V., F. Hayat, B. Graves, M. Sonavane, E. A. Salter, A. Wierzbicki, N. R. Gassman, and M. E. Migaud. "Chemical and Biochemical Reactivity of the Reduced Forms of Nicotinamide Riboside." ACS Chem Biol 16, no. 4 (2021): 604-614. [CrossRef]
- You, W., K. M. Hugar, R. C. Selhorst, M. Treichel, C. R. Peltier, K. J. T. Noonan, and G. W. Coates. "Degradation of Organic Cations under Alkaline Conditions." J Org Chem 86, no. 1 (2021): 254-263. [CrossRef]
- Johnson, S. L., and D. L. Morrison. "The Alkaline Reaction of Nicotinamide Adenine Dinucleotide, a New Transient Intermediate." J Biol Chem 245, no. 17 (1970): 4519-4524. [CrossRef]
- Guilbert, C. C., and S. L. Johnson. "Isolation and Characterization of the Fluorescent Alkali Product from Diphosphopyridine Nucleotide." Biochemistry 10, no. 12 (1971): 2313-2316. [CrossRef]
- Liu, R., and L. E. Orgel. "Enzymatic Synthesis of Polymers Containing Nicotinamide Mononucleotide." Nucleic Acids Res 23, no. 18 (1995): 3742-3749. [CrossRef]
- Zeynizadeh, Behzad, Karim Akbari Dilmaghani, and Asli Roozijoy. "Aromatization of Hantzsch Ester 1,4-Dihydropyridines with Iodine under Normal Conditions and Ultrasound Irradiation." J Chin Chem Soc 52, no. 5 (2005): 1001-1004. [CrossRef]
- Cai, Xiao-hua, Hai-jun Yang, and Guo-lin Zhang. "Aromatization of 1,4-Dihydropyridines with Selenium Dioxide." Can J Chem 83, no. 3 (2005): 273-275. [CrossRef]
- Nakamichi, Natsuki, Yuka Kawashita, and Masahiko Hayashi. "Activated Carbon-Promoted Oxidative Aromatization of Hantzsch 1,4-Dihydropyridines and 1,3,5-Trisubstituted Pyrazolines Using Molecular Oxygen." Synthesis, no. 7 (2004): 1015-1020. [CrossRef]
- Abdoli-Senejani, M., and K. Karami. "Ultrasound-Assisted Heterogeneous Oxidation of 1,4-Dihydropyridines." Org Prep Proced Int 52, no. 4 (2020): 274-281. [CrossRef]
- Boecker, Ronald H., and F. Peter Guengerich. "Oxidation of 4-Aryl- and 4-Alkyl-Substituted 2,6-Dimethyl-3,5-Bis(Alkoxycarbonyl)-1,4-Dihydropyridines by Human Liver Microsomes and Immunochemical Evidence for the Involvement of a Form of Cytochrome P-450." J Med Chem 29, no. 9 (1986): 1596-1603. [CrossRef]
- Zarei, A., L. Khazdooz, M. Enayati, S. Madarshahian, T. J. Wooster, G. Ufheil, and A. Abbaspourrad. "Dihydronicotinamide Riboside: Synthesis from Nicotinamide Riboside Chloride, Purification and Stability Studies." RSC Adv 11, no. 34 (2021): 21036-21047. [CrossRef]
- Sollenberger, P. Y., and R. B. Martin. "Mechanism of Enamine Hydrolysis." J. Am. Chem. Soc. 92, no. 14 (1970): 4261–4270. [CrossRef]
- Maas, W., M. J. Janssen, E. J. Stamhuis, and H. Wynberg. "Mechanism of Enamine Reactions. IV. The Hydrolysis of Tertiary Enamines in Acidic Medium." J. Org. Chem. 32, no. 4 (1967): 1111–1115. [CrossRef]
- Johnston, C. C., D. E. Metzler, and J. L. Gardner. "Acid-Catalyzed Addition of Water to 1 4-Dihydronicotinamide Derivatives." Biochemistry 2, no. 4 (1963): 689-696. [CrossRef]
- Oppenheimer, N. J., and N. O. Kaplan. "The Alpha Beta Epimerization of Reduced Nicotinamide Adenine Dinucleotide." Arch Biochem Biophys 166, no. 2 (1975): 526-535. [CrossRef]
- Johnson, S. L., and P. T. Tuazon. "Acid-Catalyzed Hydration of Reduced Nicotinamide Adenine Dinucleotide and Its Analogues." Biochemistry 16, no. 6 (1977): 1175-1183. [CrossRef]
- Branlant, G., B. Eiler, and J. F. Biellmann. "A Word of Caution: 1,4,5,6-Tetrahydronicotinamide Adenine Dinucleotide (Phosphate) Should Be Used with Care in Acidic and Neutral Media." Anal Biochem 125, no. 2 (1982): 264-268. [CrossRef]
- Ammon, H. L., and L. H. Jensen. "The "Dimeric Acid Product" of 1-Methyl-1,4-Dihydronicotinamide." J Am Chem Soc 88, no. 3 (1966): 613-614. [CrossRef]
- Ilic, S., U. Pandey Kadel, Y. Basdogan, J. A. Keith, and K. D. Glusac. "Thermodynamic Hydricities of Biomimetic Organic Hydride Donors." J Am Chem Soc 140, no. 13 (2018): 4569-4579. [CrossRef]
- Zhu, X. Q., Y. Tan, and C. T. Cao. "Thermodynamic Diagnosis of the Properties and Mechanism of Dihydropyridine-Type Compounds as Hydride Source in Acetonitrile with "Molecule ID Card"." J Phys Chem B 114, no. 5 (2010): 2058-2075. [CrossRef]
- Anderson Jr., A. G., and G. Berkelhammer. "A Study of the Primary Acid Reaction on Model Compounds of Reduced Diphosphopyridine Nucleotide." J. Am. Chem. Soc. 80, no. 4 (1958): 992–999. [CrossRef]
- Nowak, C., A. Pick, L. I. Csepei, and V. Sieber. "Characterization of Biomimetic Cofactors According to Stability, Redox Potentials, and Enzymatic Conversion by Nadh Oxidase from Lactobacillus Pentosus." ChemBioChem 18, no. 19 (2017): 1944-1949. [CrossRef]
- Zachos, I., M. Doring, G. Tafertshofer, R. C. Simon, and V. Sieber. "Carba Nicotinamide Adenine Dinucleotide Phosphate: Robust Cofactor for Redox Biocatalysis." Angew Chem Int Ed 60, no. 26 (2021): 14701-14706. [CrossRef]
- Haynes, L. J., and A. R. Todd. "66. Codehydrogenases. Part I. The Synthesis of Dihydronicotinamide-D-Ribofuranoside [N-D-Ribofuranosidyl-1 : 2(or 6)-Dihydronicotinamide]." J Chem Soc (Resumed) (1950): 303. [CrossRef]
- Haykes, L. J., N. A. Hughes, G. W. Kenner, and Alexander Todd. "734. Codehydrogenases. Part II. A Synthesis of Nicotinamide Nucleotide." J Chem Soc (Resumed) (1957): 3727. [CrossRef]
- Mikhailopulo, I. A., and A. I. Miroshnikov. "New Trends in Nucleoside Biotechnology." Acta Naturae 2, no. 2 (2010): 36-58. [CrossRef]
- Lee, Jaemoon, Hywyn Churchil, Woo-Baeg Choi, Joseph E. Lynch, F. E. Roberts, R. P. Volante, and Paul J. Reider. "A Chemical Synthesis of Nicotinamide Adenine Dinucleotide (NAD+)." Chem Commun, no. 8 (1999): 729-730. [CrossRef]
- Jarman, M., and W. C. J. Ross. "4-Substituted Nicotinic Acids and Nicotinamides. Part II. The Preparation of 4-Methylnicotinamide Riboside." J Chem Soc C: Organic, no. 2 (1969): 199. [CrossRef]
- Tanimori, Shinji, Takeshi Ohta, and Mitsunori Kirihata. "An Efficient Chemical Synthesis of Nicotinamide Riboside (NAR) and Analogues." Bioorg Med Chem Lett 12, no. 8 (2002): 1135-1137. [CrossRef]
- Franchetti, Palmarisa, Michela Pasqualini, Riccardo Petrelli, Massimo Ricciutelli, Patrizia Vita, and Loredana Cappellacci. "Stereoselective Synthesis of Nicotinamide β-Riboside and Nucleoside Analogs." Bioorg Med Chem Lett 14, no. 18 (2004): 4655-4658. [CrossRef]
- Yang, Tianle, Noel Yan-Ki Chan, and Anthony A. Sauve. "Syntheses of Nicotinamide Riboside and Derivatives: Effective Agents for Increasing Nicotinamide Adenine Dinucleotide Concentrations in Mammalian Cells." J Med Chem 50, no. 26 (2007): 6458-6461. [CrossRef]
- Zhang, Ning, and Anthony A. Sauve. "Synthesis of β-Nicotinamide Riboside Using an Efficient Two-Step Methodology." Curr Prot Nucleic Acid Chem 71, no. 1 (2017). [CrossRef]
- Atkinson, M. R., R. K. Morton, and R. Naylor. "98. Synthesis of Glycosylpyridinium Compounds from Glycosylamines and from Glycosyl Halides." J Chem Soc (Resumed) (1965): 610. [CrossRef]
- Makarov, M. V., N. W. Harris, M. Rodrigues, and M. E. Migaud. "Scalable Syntheses of Traceable Ribosylated NAD+ Precursors." Org Biomol Chem 17, no. 38 (2019): 8716-8720. [CrossRef]
- Makarov, Mikhail V., Faisal Hayat, Briley Graves, Manoj Sonavane, Edward A. Salter, Andrzej Wierzbicki, Natalie R. Gassman, and Marie E. Migaud. "Chemical and Biochemical Reactivity of the Reduced Forms of Nicotinamide Riboside." ACS Chem Biol16, no. 4 (2021): 604-614. [CrossRef]
- Carelli, Vincenzo, Felice Liberatore, Luigi Scipione, Barbara Di Rienzo, and Silvano Tortorella. "Dithionite Adducts of Pyridinium Salts: Regioselectivity of Formation and Mechanisms of Decomposition." Tetrahedron 61, no. 43 (2005): 10331-10337. [CrossRef]
- Paul, Caroline E., Isabel W. C. E. Arends, and Frank Hollmann. "Is Simpler Better? Synthetic Nicotinamide Cofactor Analogues for Redox Chemistry." ACS Catalysis 4, no. 3 (2014): 788-797. [CrossRef]
- Szczepankiewicz, Bruce G., Han Dai, Karsten J. Koppetsch, Dongming Qian, Fan Jiang, Cheney Mao, and Robert B. Perni. "Synthesis of Carba-NAD and the Structures of Its Ternary Complexes with Sirt3 and Sirt5." J Org Chem 77, no. 17 (2012): 7319-7329. [CrossRef]
- Akabane-Nakata, Masaaki, Tyler Chickering, Joel M. Harp, Mark K. Schlegel, Shigeo Matsuda, Martin Egli, and Muthiah Manoharan. "RNAs Containing Carbocyclic Ribonucleotides." Org Lett 24, no. 2 (2022): 525-530. [CrossRef]
- Largy, E., W. Liu, A. Hasan, and D. M. Perrin. "Base-Pairing Behavior of a Carbocyclic Janus-AT Nucleoside Analogue Capable of Recognizing A and T within a DNA Duplex." ChemBioChem 14, no. 16 (2013): 2199-2208. [CrossRef]
- Rydzik, Anna M., Regina Balk, Martin Koegler, Tobias Steinle, Doris Riether, and Dirk Gottschling. "Access to 1′-Amino Carbocyclic Phosphoramidite to Enable Postsynthetic Functionalization of Oligonucleotides." Org Lett 23, no. 17 (2021): 6735-6739. [CrossRef]
- Dai, Zhefu, Xiao-Nan Zhang, Fariborz Nasertorabi, Qinqin Cheng, Hua Pei, Stan G. Louie, Raymond C. Stevens, and Yong Zhang. "Facile Chemoenzymatic Synthesis of a Novel Stable Mimic of NAD+." Chem Sci 9, no. 44 (2018): 8337-8342. [CrossRef]
- Zang, Hongjun, Jing Lou, Shuolei Jiao, Huanxin Li, Yannan Du, and Jiao Wang. "Valorization of Chitin Derived N-Acetyl-D-Glucosamine into High Valuable N-Containing 3-Acetamido-5-Acetylfuran Using Pyridinium-Based Ionic Liquids." J Mol Liq 330 (2021): 115667. [CrossRef]
- Craig, John H., Ping C. Huang, T. Gordon Scott, and Nelson J. Leonard. "Synthetic Spectroscopic Models Related to Coenzymes and Base Pairs. X. Quaternized Bisnicotinamides." J Am Chem Soc 94, no. 16 (1972): 5872-5879. [CrossRef]
- Knox, Richard J., Terence C. Jenkins, Stephen M. Hobbs, Shiuan Chen, Roger G. Melton, and Philip J. Burke. "Bioactivation of 5-(Aziridin-1-Yl)-2,4-Dinitrobenzamide (CB 1954) by Human NAD(P)H Quinone Oxidoreductase 2: A Novel Co-Substrate-Mediated Antitumor Prodrug Therapy1." Canc Res 60, no. 15 (2000): 4179-4186.
- Malver, Olaf, Mina J. Sebastian, and Norman J. Oppenheimer. "Alteration in Substrate Specificity of Horse Liver Alcohol Dehydrogenase by an Acyclic Nicotinamide Analog of NAD+." DNA Repair 23 (2014): 95-100. [CrossRef]
- Bušić, Valentina, Karolina Vrandečić, Tamara Siber, Sunčica Roca, Vice Tomičić, and Dajana Gašo Sokač. "Novel Synthetic Routes to Quaternary Pyridinium Salts and Their Antifungal Activity." Croat Chem Acta 95, no. 1 (2022): 31-38. [CrossRef]
- Chennamaneni, Srinivas Rao, Venkateswarlu Vobalaboina, and Achaiah Garlapati. "Quaternary Salts of 4,3′ and 4,4′ Bis-Pyridinium Monooximes: Synthesis and Biological Activity." Bioorg Med Chem Lett 15, no. 12 (2005): 3076-3080. [CrossRef]
- Friedman, Orrie M., Kurt Pollak, and Ezra Khedouri. "1-(β-Chloroethyl)-3-Carbamylpyridinium Chloride. Prototype of a New Class of Latently Cytotoxic Potential Antitumor Agents." J Med Chem 6, no. 4 (1963): 462-463. [CrossRef]
- Plapp, Bryce V., Christoph Woenckhaus, and Gerhard Pfleiderer. "Evaluation of N1-(Ω-Bromoacetamidoalkyl)Nicotinamides as Inhibitors of Dehydrogenases." Arch Biochem Biophys 128, no. 2 (1968): 360-368. [CrossRef]
- Robins, Morris J., and Peter W. Hatfield. "Nucleic Acid Related Compounds. 37. Convenient and High-Yield Syntheses of N-[(2-Hydroxyethoxy)Methyl] Heterocycles as "Acyclic Nucleoside" Analogues." Can J Chem 60, no. 5 (1982): 547-553. [CrossRef]
- Štefko, Martin, Lenka Slavětínská, Blanka Klepetářová, and Michal Hocek. "General and Modular Synthesis of Isomeric 5-Substituted Pyridin-2-yl and 6-Substituted Pyridin-3-yl C-Ribonucleosides Bearing Diverse Alkyl, Aryl, Hetaryl, Amino, Carbamoyl, and Hydroxy Groups." J Org Chem 76, no. 16 (2011): 6619-6635. [CrossRef]
- Ma, Y., S. Liu, Y. Xi, H. Li, K. Yang, Z. Cheng, W. Wang, and Y. Zhang. "Highly Stereoselective Synthesis of Aryl/Heteroaryl-C-Nucleosides Via the Merger of Photoredox and Nickel Catalysis." Chem Commun 55, no. 97 (2019): 14657-14660. [CrossRef]
- Maeba, Isamu, Katsumi Iwata, Fumitaka Usami, and Hiroshi Furukawa. "C-Nucleosides. 1. Synthesis of 3-(β-D-Ribofuranosyl)Pyridazines." J Org Chem 48, no. 18 (1983): 2998-3002. [CrossRef]
- Singh, Ishwar, and Oliver Seitz. "Diastereoselective Synthesis of -Aryl-C-Nucleosides from 1,2-Anhydrosugars." Org Lett 8, no. 19 (2006): 4319-4322. [CrossRef]
- Brotschi, Christine, Adrian Häberli, and Christian J. Leumann. "A Stable DNA Duplex Containing a Non-Hydrogen-Bonding and Non-Shape-Complementary Base Couple: Interstrand Stacking as the Stability Determining Factor." Angew Chem Int Ed 40, no. 16 (2001): 3012-3014. [CrossRef]
- Franchetti, Palmarisa, Loredana Cappellacci, Mario Grifantini, Anna Barzi, Giuseppe Nocentini, Hongyoan Yang, Ayrn O'Connor, Hiremagalur N. Jayaram, Christopher Carrell, and Barry M. Goldstein. "Furanfurin and Thiophenfurin: Two Novel Tiazofurin Analogs. Synthesis, Structure, Antitumor Activity, and Interactions with Inosine Monophosphate Dehydrogenase." J Med Chem 38, no. 19 (1995): 3829-3837. [CrossRef]
- Kabat, Marek M., Krzysztof W. Pankiewicz, and Kyoichi A. Watanabe. "Nucleosides. 141. Synthesis of 5-β-D-Ribofuranosylnicotinamide and Its N-Methyl Derivative. The Isosteric and Isoelectronic Analogs of Nicotinamide Nucleoside." J Med Chem 30, no. 5 (1987): 924-927. [CrossRef]
- Pankiewicz, Krzysztof, Kyoichi Watanabe, Krystyna Lesiak-Watanabe, Barry Goldstein, and Hiremagalur Jayaram. "The Chemistry of Nicotinamide Adenine Dinucleotide (NAD) Analogues Containing C-Nucleosides Related to Nicotinamide Riboside." Curr Med Chem 9, no. 7 (2002): 733-741. [CrossRef]
- Höfer, Katharina, Florian Abele, Jasmin Schlotthauer, and Andres Jäschke. "Synthesis of 5′-NAD-Capped RNA." Bioconjugate Chem 27, no. 4 (2016): 874-877. [CrossRef]
- Göckel, A., and C. Richert. "Synthesis of an Oligonucleotide with a Nicotinamide Mononucleotide Residue and Its Molecular Recognition in DNA Helices." Org Biomol Chem 13, no. 41 (2015): 10303-10309. [CrossRef]
- Kjellstrand, Martin, Klas Broo, Linda Andersson, Cecilia Farre, Åke Nilsson, and Lars Baltzer. "The Site-Selective Incorporation of a NAD+ Cofactor Mimic into a Folded Helix–Loop–Helix Polypeptide Motif." J Chem Soc, Perkin Trans 2, no. 12 (1997): 2745-2750. [CrossRef]
- Zhang, B., X. Q. Zhu, J. Y. Lu, J. He, P. G. Wang, and J. P. Cheng. "Polysiloxane-Supported NAD(P)H Model 1-Benzyl-1,4-Dihydronicotinamide: Synthesis and Application in the Reduction of Activated Olefins." J Org Chem 68, no. 8 (2003): 3295-3298. [CrossRef]
- Chen, Zhe, Anna Ka Yee Kwong, Zhenjun Yang, Liangren Zhang, Hon Cheung Lee, and Lihe Zhang. "Cheminform Abstract: Studies of the Synthesis of Nicotinamide Nucleoside and Nucleotide Analogues and Their Inhibitions Towards CD38 NADase." ChemInform 43, no. 15 (2012). [CrossRef]
- Kovarik, Zrinka, Jarosław Kalisiak, Nikolina Maček Hrvat, Maja Katalinić, Tamara Zorbaz, Suzana Žunec, Carol Green, Zoran Radić, Valery V. Fokin, K. Barry Sharpless, and Palmer Taylor. "Reversal of Tabun Toxicity Enabled by a Triazole-Annulated Oxime Library—Reactivators of Acetylcholinesterase." Chem Eur J 25, no. 16 (2019): 4100-4114. [CrossRef]
- Brunner, Heiko, Stefanie Ackermann, Sandra Lucks, Jun Wu, and James Adolf. "Aqueous Composition for Depositing a Cobalt Deposit and Method for Electrolytically Depositing Such a Deposit." Atotech Deutschland Gmbh. WO Patent 2019/013762 A1, January 17, 2019.
- Mutschler, Hannes, and Philipp Holliger. "Non-Canonical 3′-5′ Extension of RNA with Prebiotically Plausible Ribonucleoside 2′,3′-Cyclic Phosphates." J Am Chem Soc 136, no. 14 (2014): 5193-5196. [CrossRef]
- Tereshko, Valentina, Sergei Gryaznov, and Martin Egli. "Consequences of Replacing the DNA 3‘-Oxygen by an Amino Group: High-Resolution Crystal Structure of a Fully Modified N3‘ → P5‘ Phosphoramidate DNA Dodecamer Duplex." J Am Chem Soc 120, no. 2 (1998): 269-283. [CrossRef]
- Liu, Rihe, and Leslie E. Orgel. "Enzymatic Synthesis of Polymers Containing Nicotinamide Mononucleotide." Nucleic Acids Res 23, no. 18 (1995): 3742-3749. [CrossRef]
- Kam, Bernard L., and Norman J. Oppenheimer. "Synthesis of a New Class of D-Aldopentofuranosylamines, the 5-O-Trityl-D-Aldopentofuranosylamines." Carbohyd Res 77, no. 1 (1979): 275-280. [CrossRef]
- Livingston, D., J. M. McKearin, B. Szczepankiewicz, and J. N. Kremsky. 2017. "Nicotinamide Mononucleotide Derivatives and Their Uses for Treating Diseases and Improving Cell and Tissue Survival." WO Patent 2017/024255 A1, February 09, 2017.
- Hayat, F., and M. E. Migaud. "Nicotinamide Riboside-Amino Acid Conjugates That Are Stable to Purine Nucleoside Phosphorylase." Org Biomol Chem 18, no. 15 (2020): 2877-2885. [CrossRef]
- Xu, Hui, and Fangjun Chen. "Method for Preparing Beta-Nicotinamide Mononucleotide." Changsha Innovative Drug Industry Tech Research Institute Limited Company. CN Patent 114685582 A, January 07, 2022.
- Matteucci, M. D., and M. H. Caruthers. "The Use of Zinc Bromide for Removal of Dimethoxytrityl Ethers from Deoxynucleosides." Tetrahedron Lett 21, no. 34 (1980): 3243-3246. [CrossRef]
- Berridge, M. V., and A. S. Tan. "Characterization of the Cellular Reduction of 3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyltetrazolium Bromide (MTT): Subcellular Localization, Substrate Dependence, and Involvement of Mitochondrial Electron Transport in MTT Reduction." Arch Biochem Biophys 303, no. 2 (1993): 474-482. [CrossRef]
- Araki, M., Y. Okuno, Y. Hara, and Y. Sugiura. "Allosteric Regulation of a Ribozyme Activity through Ligand-Induced Conformational Change." Nucleic Acids Res 26, no. 14 (1998): 3379-3384. [CrossRef]
- Strohbach, D., N. Novak, and S. Müller. "Redox-Active Riboswitching: Allosteric Regulation of Ribozyme Activity by Ligand-Shape Control." Angew Chem Int Ed 45, no. 13 (2006): 2127-2129. [CrossRef]
- Chen, A. G., N. Sudarsan, and R. R. Breaker. "Mechanism for Gene Control by a Natural Allosteric Group I Ribozyme." RNA 17, no. 11 (2011): 1967-1972. [CrossRef]
- Strohbach, Denise, Nina Novak, and Sabine Müller. "Redox-Active Riboswitching: Allosteric Regulation of Ribozyme Activity by Ligand-Shape Control." Angew Chem Int Ed 45, no. 13 (2006): 2127-2129. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



