1. Introduction
MXenes are two-dimensional nanosheets produced from transition metal carbides, nitrides, and carbonitrides which rejuvenated the current research activities in the material science. MXenes were first etched by a renowned scientist Yuri Gogotsi in 2011 from MAX phase precursor [
1,
2]. MXenes have attracted significant research interest worldwide and have shown promising potential in energy storage applications [
3,
4,
5,
6,
7,
8] due to their layered structure, superior hydrophilicity, metallic properties, high charge carrier mobility, tunable band gap, and rich surface chemistry [
9,
10,
11]. The layered structure of MXene is joined by weak Van der Waals interactions [
12]. MXene possess unique electrical, optical, and mechanical attributes which ultimately opened the new doors for researchers and scientists to conduct deep analysis in assorted fields for more than a decade [
13,
14]. The combination of d-block elements could possibly increase the notable MXene characteristics. However, these d-block metals possess the ability to establish ordered layered structure in the lattice as seen in (Mo
2/3Y
1/3)
2CT
x or as layered atomic formations like Mo
2TiC
2T
x, converting into single layer MXene with ideal configurations. The orderly arranged MXene was first invented in 2014 and later revised the following year.
Epoxies, on the other hand, are commonly utilized in cutting-edge engineering activities because they exhibit prominent strength, low shrinkage during curing, a low level of residual stresses, and resistance to various chemicals [
15,
16,
17,
18]. Due to their molecular structure and having low viscosity, a high level of adhesion and processability can be achieved, so epoxies are regularly integrated as matrix to impregnate the fibers during the manufacturing of fiber reinforced plastic (FRP) composites. Researchers face challenges utilizing epoxy due to their unfavorable characteristics. The highly cross-linked networks results in quite low fracture toughness, which limits their use in high end applications.
Many researchers have wrapped up the discussion that internal stresses are developed following curing reveals a highly entangled cross-linked polymeric chains of native epoxy triggers low fracture toughness. As epoxies are highly cross linked, the crack grows without any hinderance and its advancement from the plastic deformation is constrained [
19,
20]. One way to improve the toughness of these brittle epoxies is by adding thermoplastics or elastomeric blends, while incorporation of nanomaterials could also promote the key properties improvement [
21,
22]. In epoxy/MXene nanocomposites, MXenes can significantly improve the physical as well as chemical properties of the matrix at minimal loadings [
23,
24]. However, this improvement is directly linked with the MXenes nanoparticles uniform dispersion throughout the polymeric matrix. MXenes, when achieve uniform dispersion, can contribute to sharing the external stresses to avoid stress concentration and inhibit crack growth, resulting in improved mechanical properties [
25,
26,
27,
28]. Out of all solution processed MXenes, Ti
3C
2T
x presents highest metallic conductivity, approaching 10,000S/cm for films, along with Youngs modulus of 330 ± 30 GPa for single Ti
3C
2T
x flake [
29,
30,
31]. These properties transform MXene into a viable candidate to use as discontinuous fibers within epoxy nanocomposites. Previous research has documented that MXenes could be added as a filler material into a variety of polymers such as polyvinyl alcohol, poly-pyrrole, and sodium alginate with enhanced electrochemical properties and improved Electromagnetic interference shielding (EMI) [
11].
The research into improved properties of MXene/epoxy nanocomposites is progressing swiftly. However, in practice, simple dispersion of MXene in epoxy polymer is not effective [
32,
33,
34], as the MXene nanomaterials tend to reaggregate within polymeric matrix due to presence of strong Van der Waals interactions, even after homogenization [
35]. A high level of dispersion of MXene within epoxy resin with relatively high viscosity is generally difficult. Therefore, using solvents as dispersion media is broadly accepted and considered as a straightforward technique to isolate the single flakes of MXene within nanocomposites.
Gogotsi investigated the dispersion of MXene in various organic solvents and concluded that N,N-dimethylformamide(DMF), N-methyl-2-pyrrolidone (NMP) and DMSO provided high quality dispersions [
1]. Inspired from the positive outcome of MXene dispersions in organic solvents show potential for researching and expanding its application horizon. MXenes could possibly be blended with various nanomaterials and polymers in organic solvents to fabricate nanocomposites, which can be used for water sensitive applications. To achieve a higher dispersion of larger MXene concentrations, two methods are reported in the literature. In solvent exchange (SE) processing route, it demands various solvent transfers to gain stable dispersions of MXene in organic solvents [
36]. As MXene dispersions are quite stable, transfer of solution via solvent exchange method imposes restrictions. Moreover, a series of solvent exchanges are needed to drop down the water content, which potentially lead towards wastage of MXene solution. A substitute technique to achieve a high level of stable dispersions is surface modification of MXene in any type of solvent. These methods involve complicated and laborious procedures, it’s a demanding time to explore a suitable organic solvent that could possibly stabilize the MXene dispersions for longer times with ease of processing. Therefore, there is an urgent need to develop an efficient methodology for preparing larger dispersions of MXene in organic solvents. Nevertheless, previous studies reported that, the concentration of MXene stayed below 0.5mg/mL. This showcase the current challenge of achieving a high level of MXene dispersions within organic solvents, which is mandatory for higher production. Hence, a more reliable method should be built up which has capability to achieve high MXene concentrations in organic solvents to ensure larger productions while preventing agglomeration. No previous literature has investigated the effect of DMF solvent volume on the properties of MXene/epoxy nanocomposites with increasing concentrations of MXene. This research aims to study in depth the use of this solvent in the fabrication of nanocomposites.
2. Materials and Methods
Epoxy was used as matrix material in current research which was fabricated utilizing EPOPHEN EL5 - a bis-phenol A liquid resin system, EPOPHEN EHA57 – a diamine catalyst, both were acquired from Polyfibre UK Ltd. This resin system provides balanced properties with epoxy content ranging from 4.76-5.25 mol/kg. EL5 is mostly liquid at room temperature has a viscosity of 12000-15000 cp while hardener (catalyst) is only 45 cp viscous. The mixing ratio for epoxy and hardener is 2:1 according to the guidelines from the company. This epoxy formulation was chosen because it performs quite well as a thermosetting polymeric matrix. MXenes-Ti
3C
2T
x, a combination of few layer nanoflakes were procured from NanoPlexus UK with product name: MXNTi
3C
2T
x-FLN (
Figure 1). According to the manufacturer’s provided datasheet, the nanoflakes possess surface area of 25 m
2/g, an average lateral size of 1µm to 4 µm, and thickness of 1nm to 3nm. Dimethylformamide (DMF) was purchased from Sigma-Aldrich (Sigma-Aldrich Company Ltd., Gillingham, UK) with 99.9% purity.
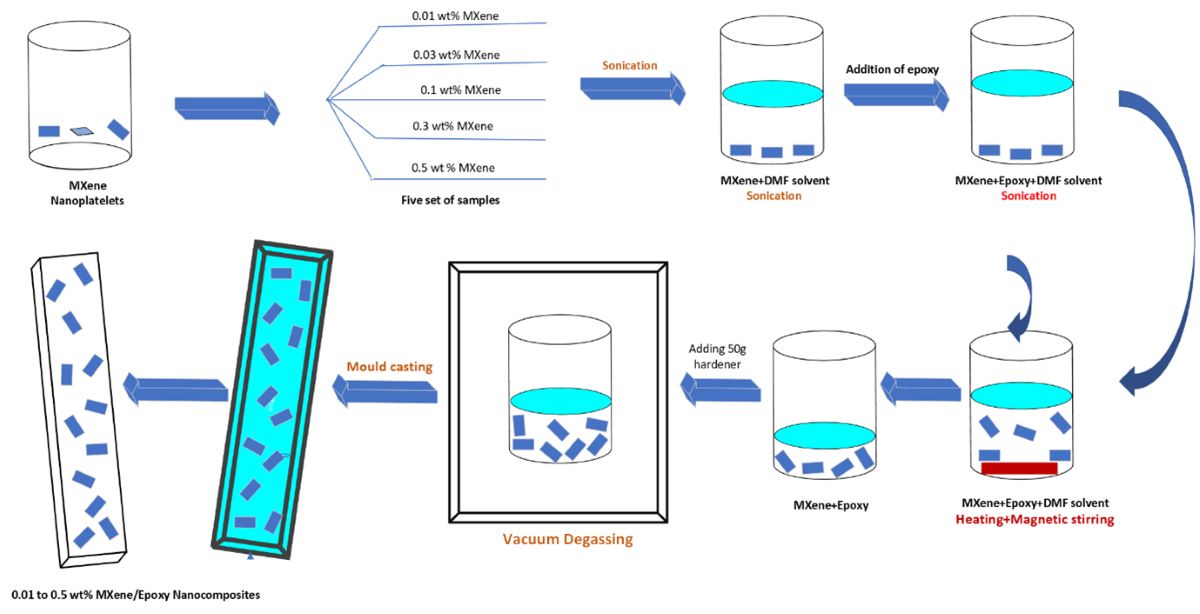
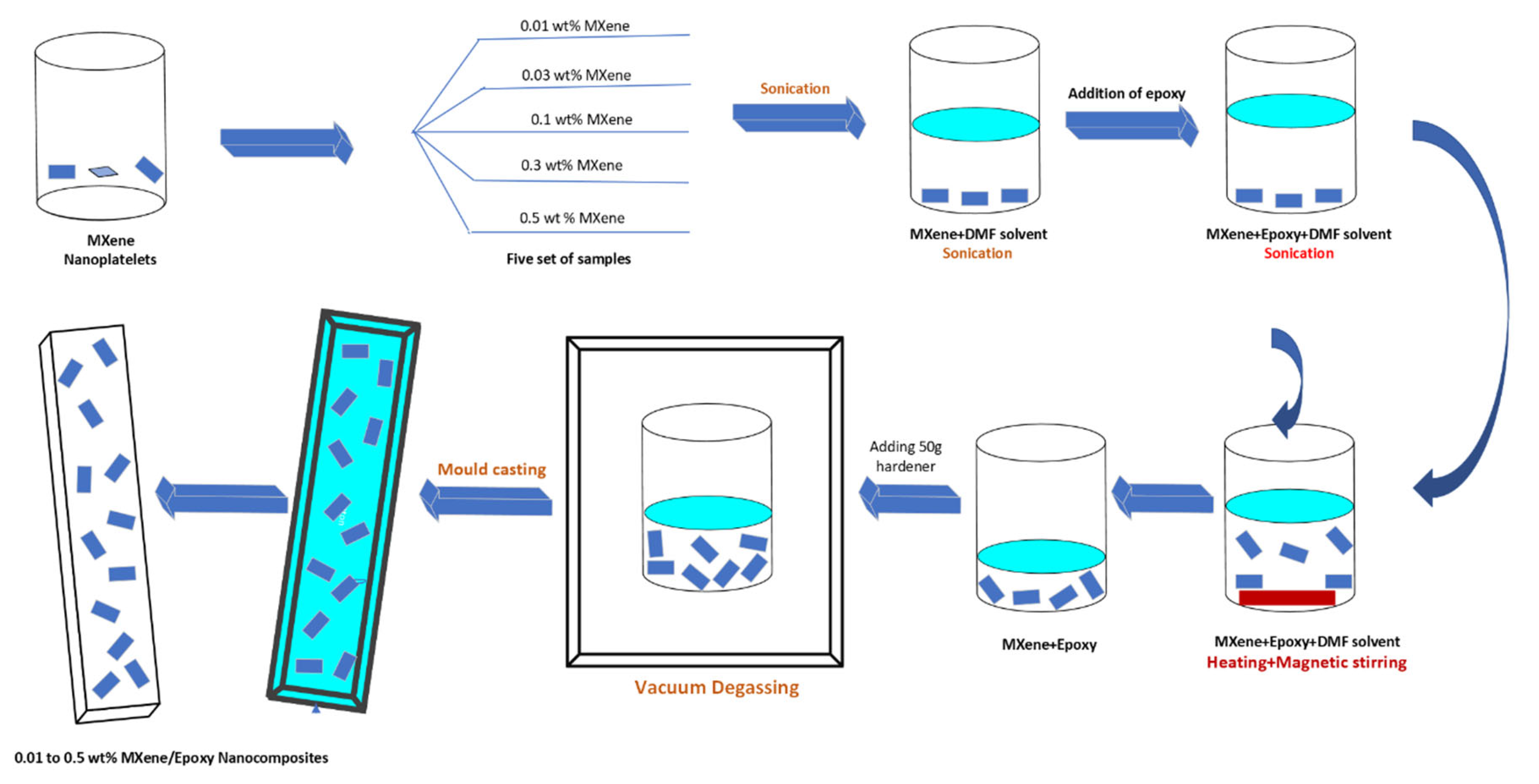
Standard epoxy was used a reference material, and one set of samples were fabricated. Another 5 set of samples with varying MXene compositions ranging from 0.1 wt. % to 0.5 wt. % were prepared. The MXene nanoflakes were first dispersed in fixed 100ml DMF dosage following sonication. To understand the relationship between the quantity of DMF, nanoflakes dispersion rate, and the properties of nanocomposites, 0.1 wt.% to 0.5 wt. % MXene/epoxy nanocomposites were fabricated at fixed DMF dosage. Weighted MXene nanoflakes from 0.1-0.5 wt. % were first dispersed in DMF say 100ml, respectively, marked as D-10, D-30, D-100, D-300, and D-500, accordingly, followed by bath sonication for 60 minutes. Epoxy monomer was then added to the dispersion and again sonicated for another 30 minutes. DMF solvent was removed by heating the mixture at hot plate while maintaining the temperature at 150 °C with constant stirring. At standard DMF dosages, the mixture was heated for 4 hours to complete evaporation of solvent. All mixtures were weighted to ensure the complete evaporation of DMF. The mixtures were cooled down to room temperature then hardener was added followed by hand stirring for two minutes. The created air bubbles were removed by placing the mixture under vacuum for few minutes. The patterns were printed using 3D printer strictly following respective ASTM standards, which were then utilized to fabricate the rubber silicone molds. Lastly, the molds were filed with degassed mixture. Pre curing of the nanocomposites took place at room temperature for 24hrs while the post curing took place at 120°C for 8hrs. The nanocomposites fabrication process is shown in
Figure 2.
Universal testing machine (Instron 3382, Instron corporation, Norwood, MA, USA) was used to conduct flexural testing, and the crosshead speed was maintained at 2mm/min for the testing samples. Three-point flexural testing was performed following ASTM D790 with samples dimensions of 3mm×12.7mm×48mm. The fractured surfaces of the nanocomposites were investigated using Scanning electron microscopy (SEM) and analysis was conducted by FEI Quanta 200 microscope (FEI corporation, Hillsboro, OR, USA). The fractured surfaces were cut off from the samples and a Gold thin coating was applied using a Emscope sputter coater, model SC500A, (Quorum technologies Ltd., Laughton, UK). Five specimens were tested for flexural testing for each type of nanocomposites and their mean values were reported. The nanoindentation testing of samples was performed using Vickers hardness tester with applied load of 2N. Three samples of each specimen type were tested with load and unload time was approximately 2 minutes.
Thermal properties of the nanocomposites were investigated by conducting Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) using TA Instruments Q500 thermal analyzer (TA Instruments, New Castle, DE, USA). A rise in temperature from room temperature to 600°C for DSC, while, from room temperature to 800°C for TGA analysis with a ramp rate of 10°C/min. The electromagnetic Interference shielding (EMI) was carried out using Multifield EMF Meter, 3.5GHz (Seeit UK). It simultaneously measures magnetic field LF with triple axis, electric field LF, and electromagnetic field RF. The surface roughness of the samples was conducted using Alicona Infinite Focus (Alicona Imaging GmbH). It measures in 3D the dimensions, shape, and roughness with one optical sensor with high precision and fast detection.
3. Results and discussion
3.1. MXene Nanoflakes dispersion
10mg MXene nanoflakes were pre-weighted and added into 100g of each epoxy resin, catalyst, and DMF solvent. The mixtures were sonicated using bath Sonicator and results were compared at different parameters. For each sample the sonication time was increased from 7 min to 60 minutes. One series of tests were completed at 20°C while the second was completed at 40°C. The ultrasonic wave strength (amplitude) was set at 20KHz. After finishing sonication of samples at fixed parameters these are straight poured into standard cuvettes to conduct UV-Vis spectroscopy. After sonication, spontaneous UV-Vis testing is mandatory as the MXene nanoflakes tend to agglomerate just after few minutes of sonication due to strong van der Waals interactions.
3.1.1. Evaluation of UV light Transmittance at Fixed Temp of 20°C
All the samples were sonicated at fixed temperature of 20°C while other parameters were altered. To confirm the light transmission through each sample UV-Vis spectroscopy was performed. Within the visible range the wavelength of the passing light was set at 450nm.
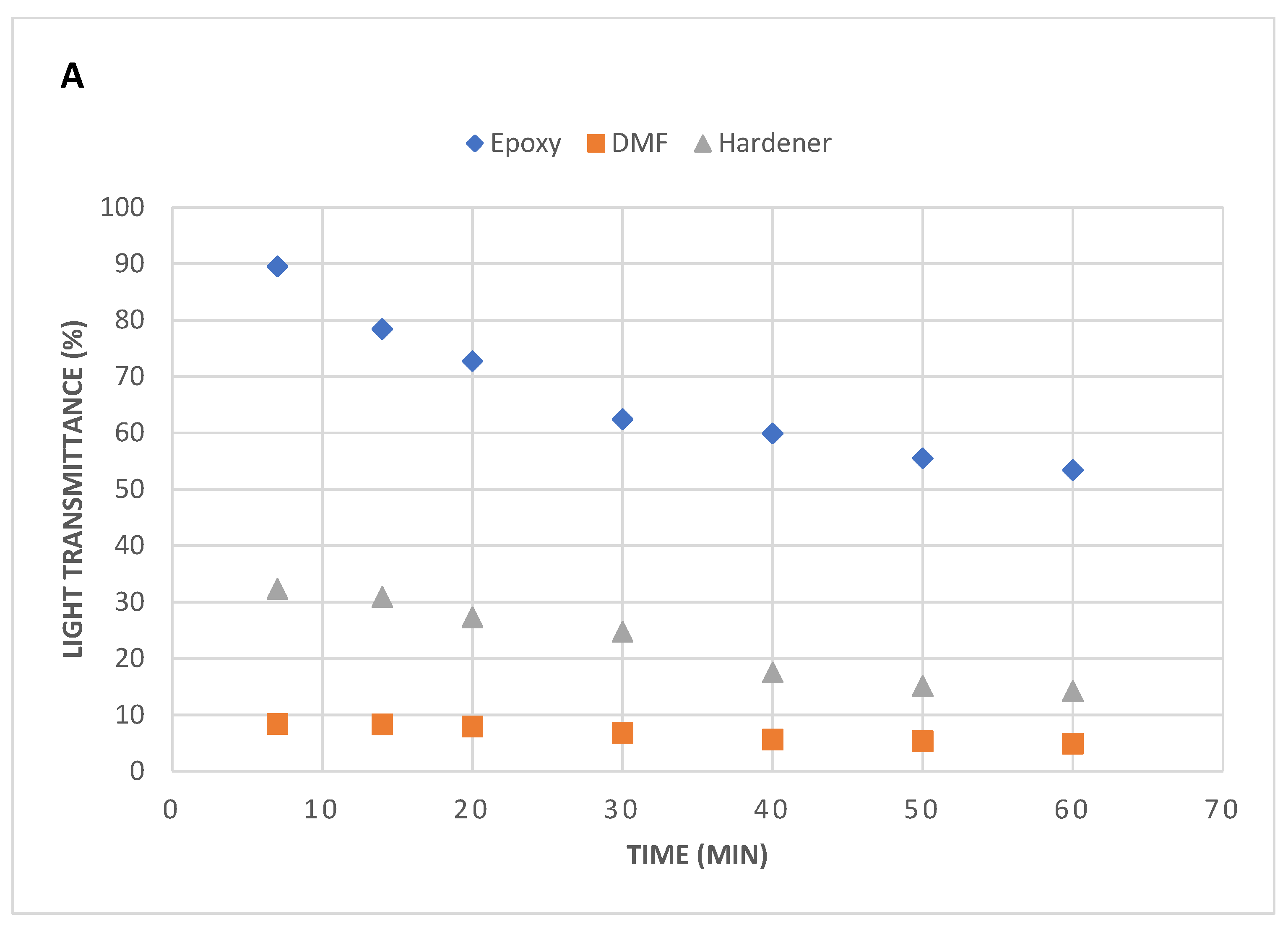
A huge variation in the light transmission rate was observed in different samples (
Figure 3). The standard epoxy sample exhibited a high light transmittance rate after being sonicated for 7 minutes say 91%. A lowering trend in the light transmittance was observed when the sonication time reached up to 60 minutes. The larger molecular chains of epoxy are densely entangled, which make it very difficult for the nanoflakes to disperse easily. So, the higher sonication times reduces the resins viscosity with increasing temperature and results in lowering the light transmittance values.
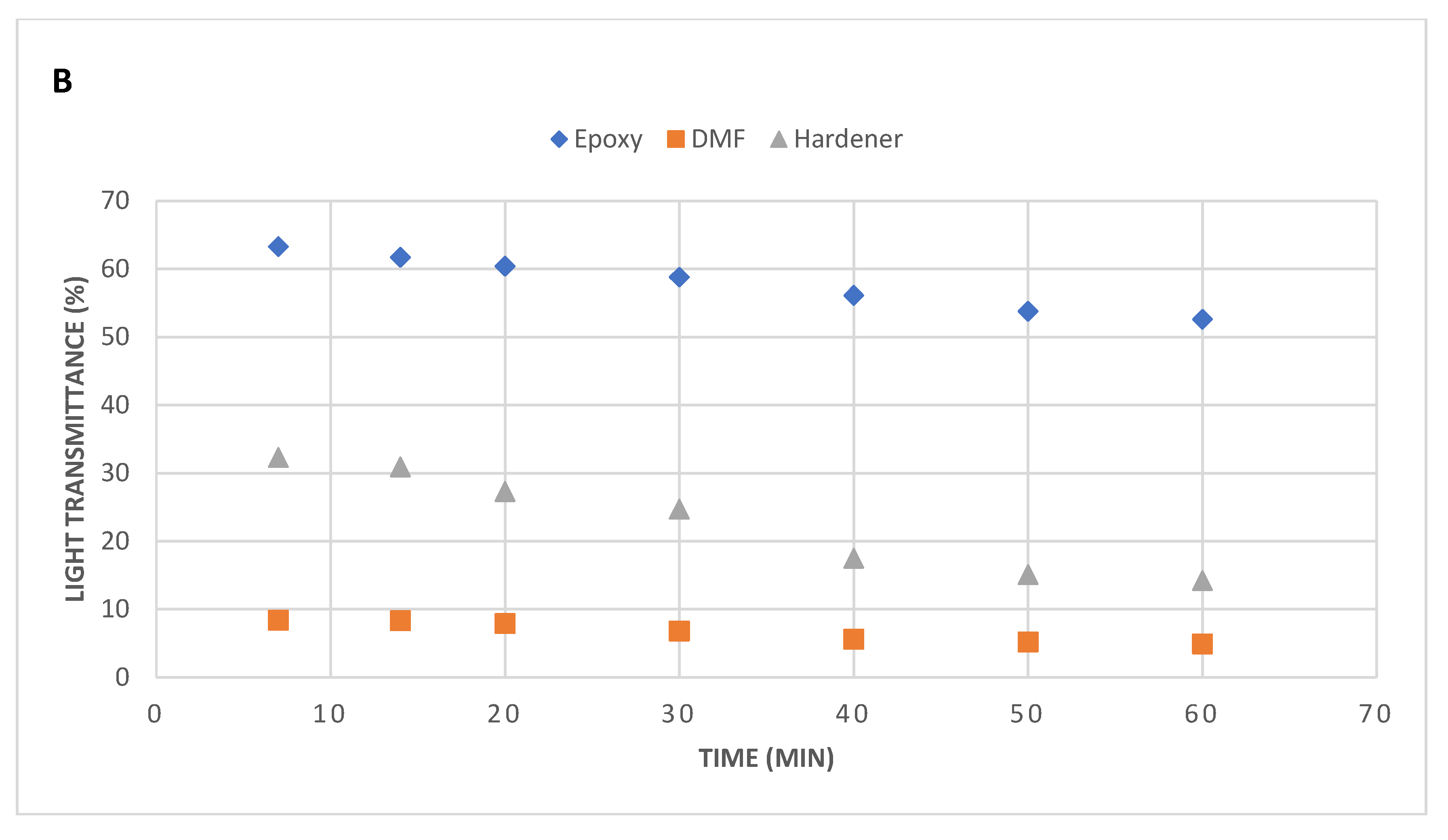
On contrary, compared to epoxy, the lights transmittance behaviour through hardener (catalyst) and DMF solvent was incredible. Point to note is a high level of dispersion state of nanoflakes were attained at less sonication times. Both hardener and DMF holds lower viscosity compared to epoxy which helped in very low rates of light transmission. At lower sonication times, the hardener revealed higher light transmittance value compared with DMF, but with increased sonication time, the transmittance declined. The results obtained from DMF samples were quite astonishing and their transmittance remained consistent and under 10% over the time of 60 minutes. These results reveal that DMF solvent possesses even lower viscosity compared to hardener (catalyst).
Even though both hardener and DMF solvent samples revealed a high level of uniform dispersions, but serious issues were observed during their processing. Once the sonication is over, the nanoflakes tend to agglomerate and settle at the bottom of the flask due to their lower viscosities, which results in further reduction of transmittance. To tackle this issue, we performed UV Vis spectroscopy to attain the accurate light transmission values from these low viscous materials.
3.1.2. Evaluation of UV Light Transmittance at Fixed Temp of 40°C
All the samples were sonicated at fixed temperature of 40°C wile their time to sonicate increased gradually from 7 to 60 minutes. Light transmittance and absorbance values were measured by performing UV Vis spectroscopy. MXene nanoflakes were added separately into epoxy, hardener, and DMF to prepare dispersions for testing. The epoxy samples showed a drop in light transmittance from 63% to 52.6% when sonicated from 7 to 60 minutes (
Figure 4). However, the hardener (catalyst) again revealed intermediate values. Its light transmission values dropped from 32.3% to 14.2% when sonication time increased from 7 to 60 minutes. On contrary, DMF solvent samples again depicted extraordinary below 10% light transmittance values at any sonication time.
When epoxy samples were sonicated at 20°C for 7 minutes it depicted a high value of light transmission of 89.5% while at 40°C it dropped to 63.3%. At 40°C the epoxy long molecular chains were free to move which helped the nano flakes to disperse even more uniformly which ultimately resulted in decline of transmission values. Again, lesser agglomerations were noticed in the samples sonicated at 40°C compared with 20°C.
Due to lower viscosities possessed by both hardener and DMF, no visible change in light transmission was observed at 40°C. The MXene nanoflakes dispersed uniformly resulted in an absorbance rate of 1.252, which peaked among all the experiments conducted.
3.1.3. Evaluation of UV Light Transmittance though Epoxy
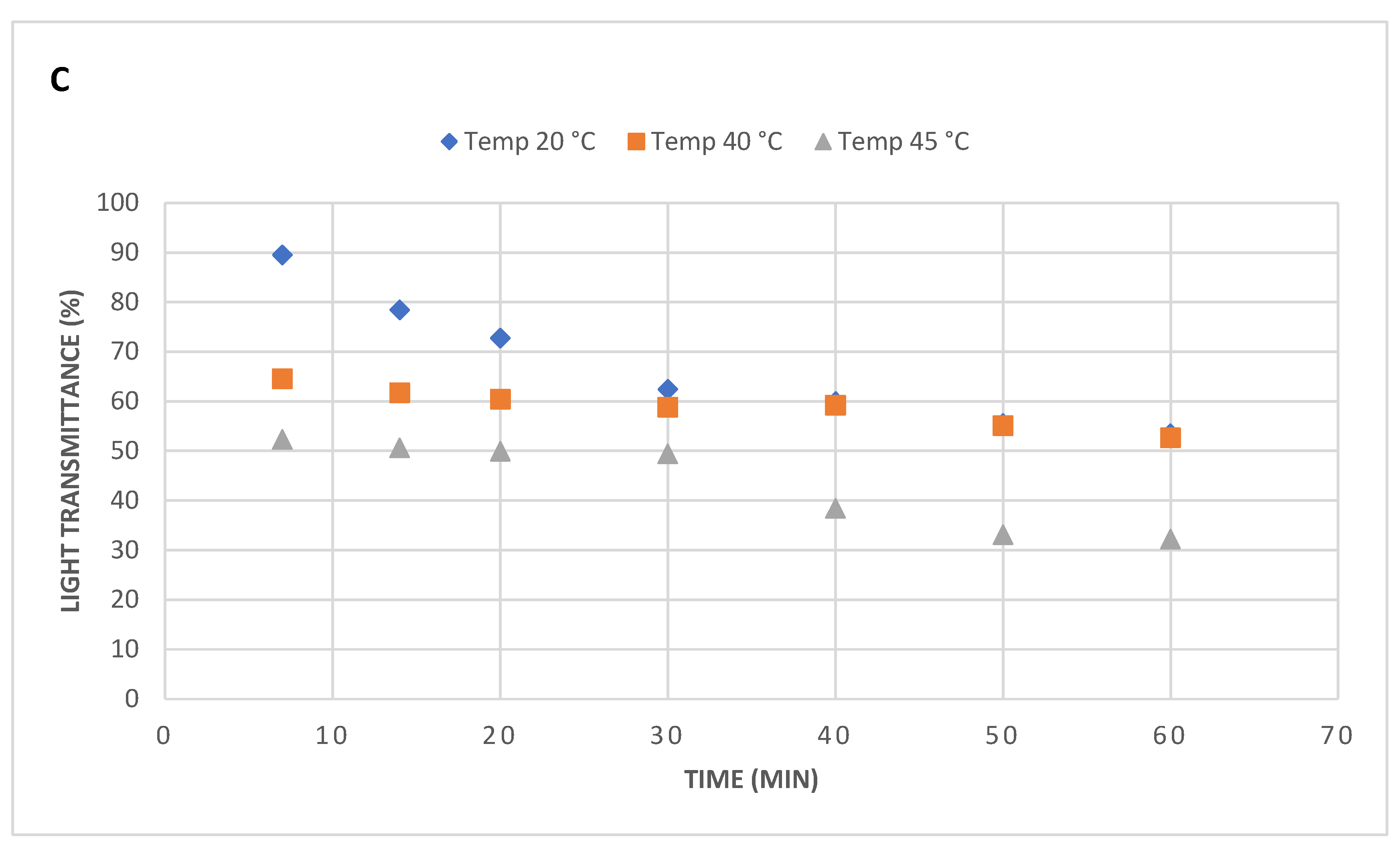
In this series of experiments, only epoxy/MXene samples were sonicated at three different temperatures say 20,40, and 45°C along with time variation, and their light transmittance was measured using UV vis spectroscopy. When samples were sonicated for only 7 minutes, at 20°C the sample depicted very high light transmittance of 89.5% while at 40°C and 45 °C its further dropped from 64.5% to 52.3% (
Figure 5). However, as the sonication time is increased to 60 minutes, samples treated at 20°C and 40°C exhibited almost same transmittance of 52.6%, while at 45°C, it even dropped to very low value of 32.2%. Nevertheless, samples treated at 20°C, showed a consistent drop in light transmittance up to 30minutes of sonication time and later no appreciable drop was observed. On contrary, at 40°C, the transmittance went down gradually from 64.5% to 52.6%. Interestingly, the samples treated at 45°C, depicted lower transmittance values at any time in the graph. These results clearly state that higher temperatures trigger in lowering the polymers viscosity-which ultimately enhances the chains motion and helps the nanomaterials to dissipate evenly.
3.1.4. Storage Life of Epoxy/MXene Dispersions
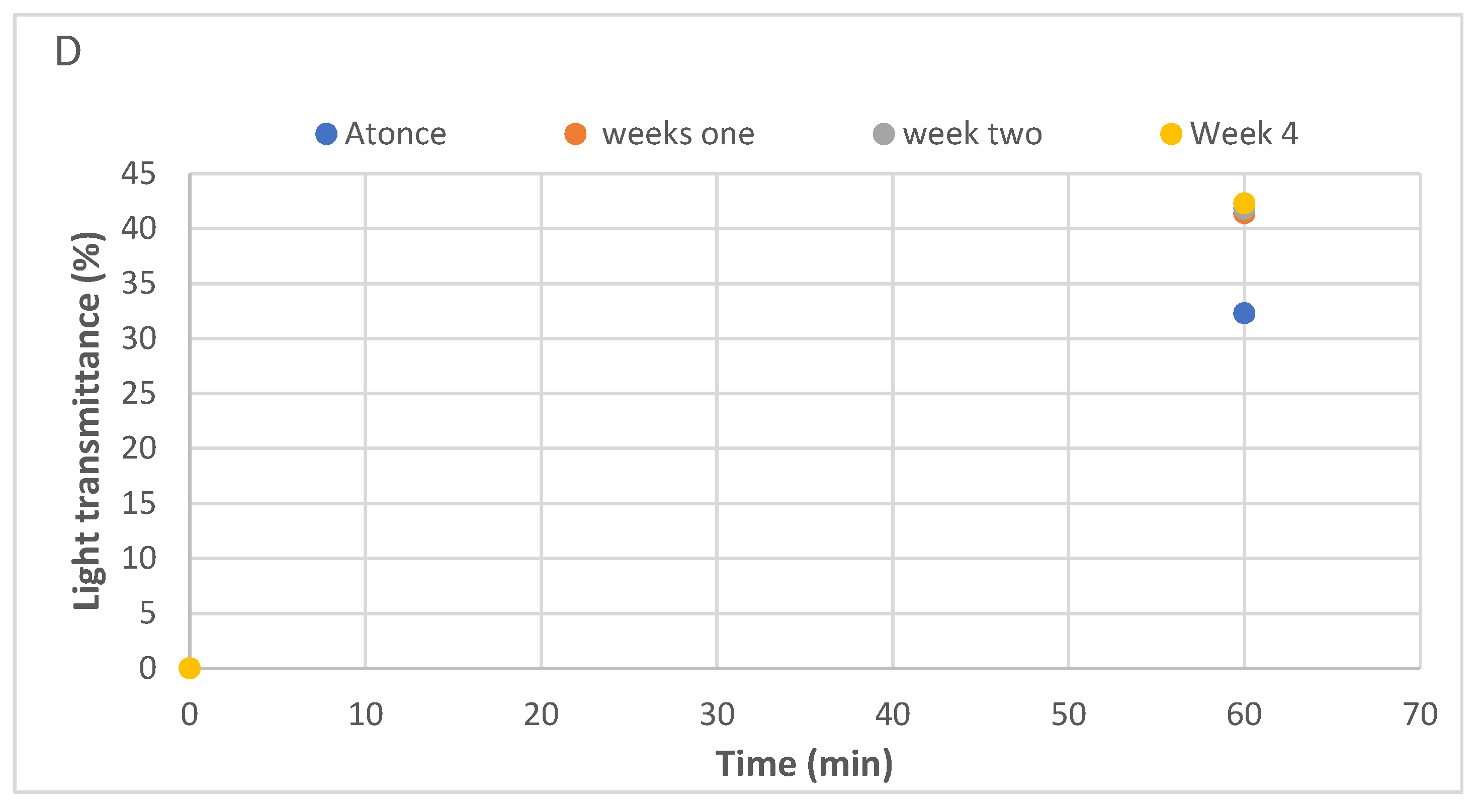
Only epoxy/MXene samples were sonicated at 45°C for 60 minutes following the parameters set in previous findings.In the first instance, the samples were sonicated at set parameters and their UV Vis spectroscopy was performed instantly to investigate the light transmission data. The samples revealed 32.4% of light transmittance after sonication (
Figure 6). This value aligns with my earlier observations. The sample was stored for a week in a glass bottle having a lid. After a week, UV Vis spectroscopy was again performed on the stored sample, revealing a light transmittance value of 41.3%, which was higher compared to the sonicated sample. Similar values were found after two and four weeks. The results depict the reliability and repeatability of MXene dispersions in epoxy resin which were stable even after weeks and months of sonication.
3.2. Mechanical Characteristics of Nanocomposites
Previous research on MXene/epoxy nanocomposites largely focused to enhance the mechanical attributes. However, the dispersion state of MXene nanosheets within polymeric matrix have a significant impact on all these properties as well as it alters the behaviour of polymeric chains. The mechanical properties of the nanocomposites are summarized in
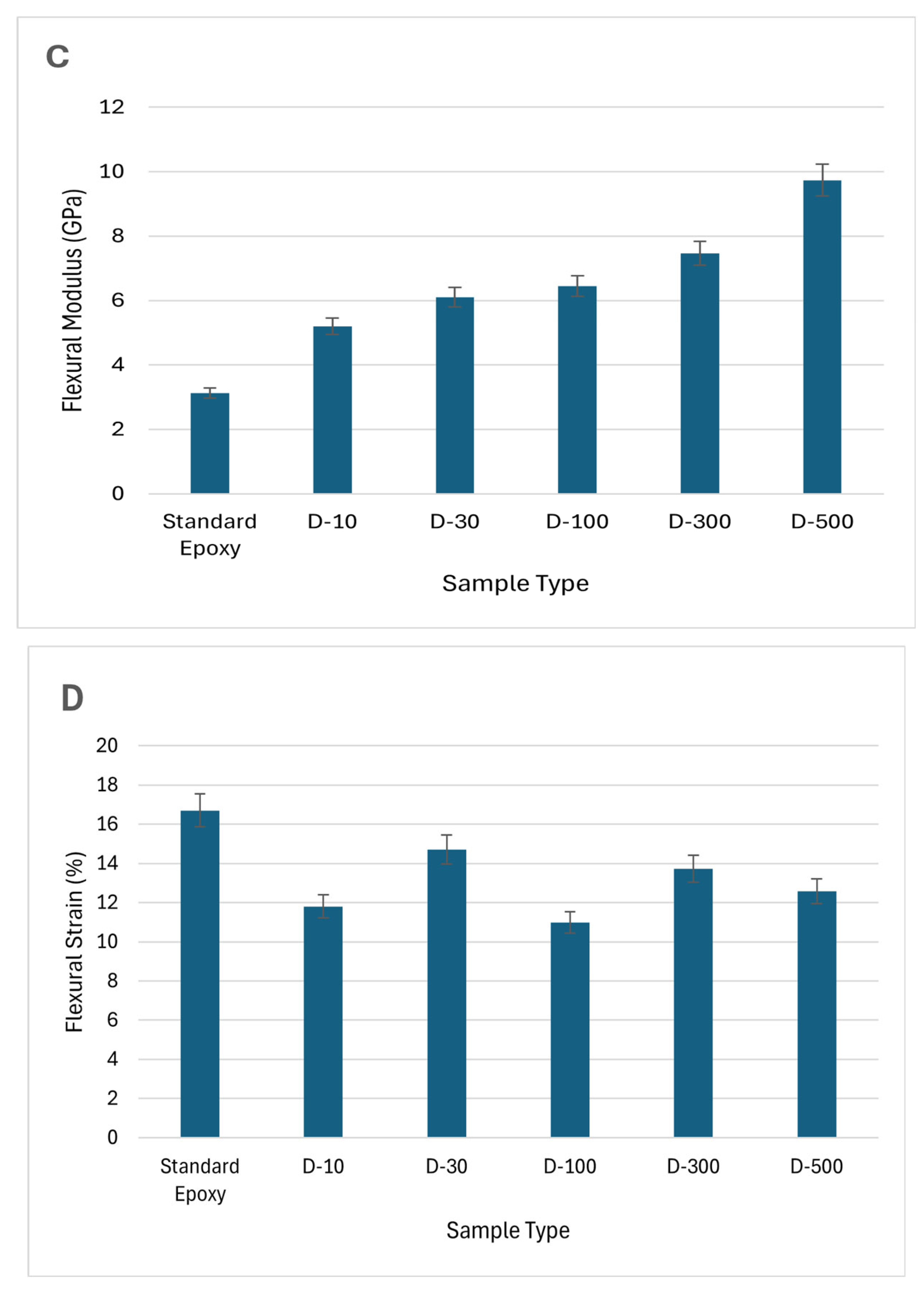
Figure 7.
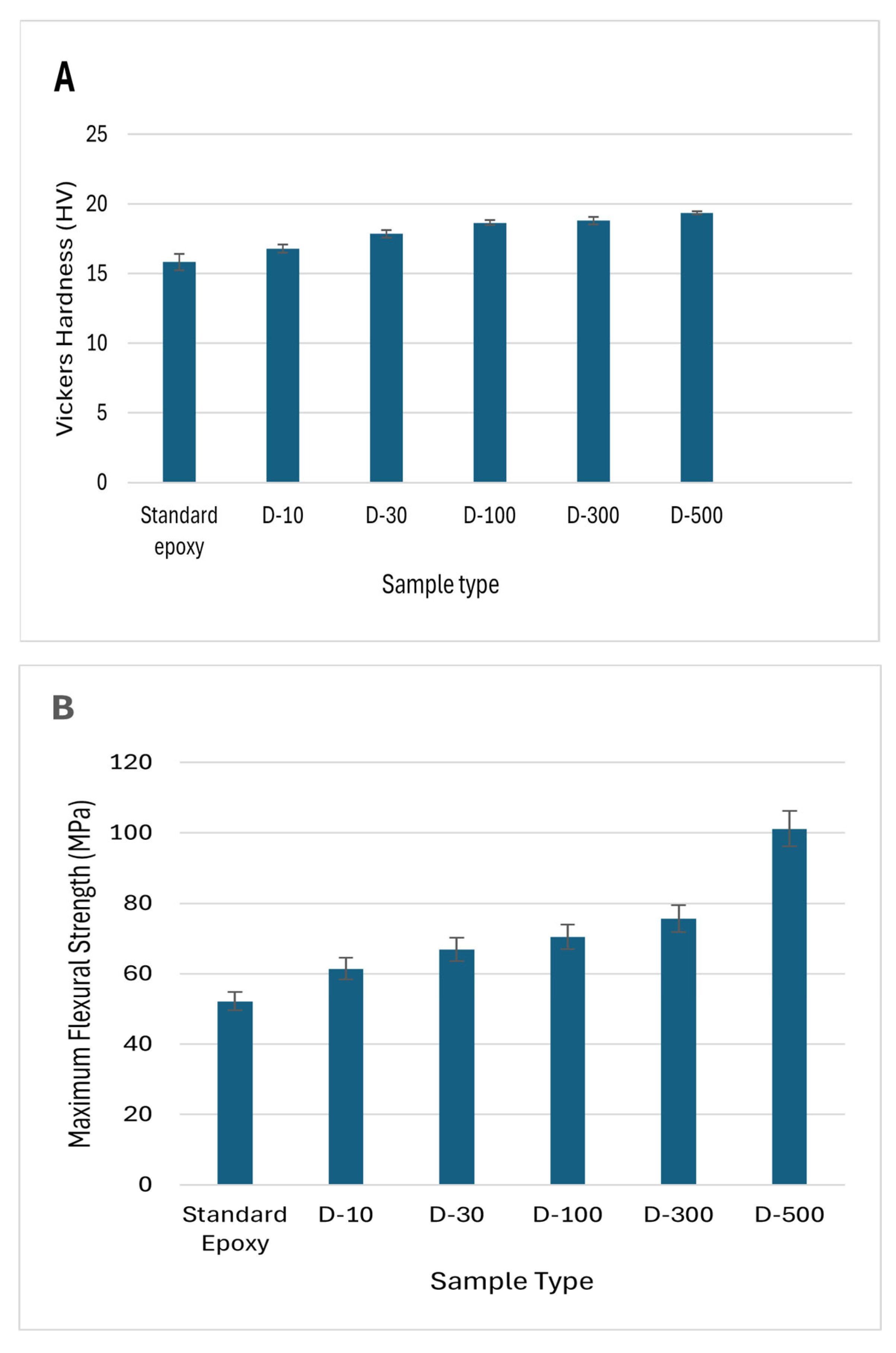
Figure 8 clearly illustrates that standard epoxy samples possess the lowest mechanical attributes. Overall, the usage of DMF solvent significantly improved the mechanical properties of MXene/epoxy nanocomposites. For instance, D-500 samples showed the highest Vickers hardness values of 19.35HV, a high Flexural strength of 100MPa, and flexural modulus of 9.7GPa. A high dispersion level of MXene nanosheets within DMF played a major role. When MXene nanosheets are uniformly dispersed, it improves the materials energy absorption and dissipation capacity, blocks the polymeric chains movement, reduces the interface area, thereby leading to enhanced mechanical properties.
On contrary, huge variations were observed in flexural strains in different sample types. Standard epoxy samples exhibited the highest flexural strain values while D-100 samples showed the lowest. The highest flexural stain values shown by standard epoxy is due to the aging of polymer resulted in plasticity, while DMF treated samples showed lower plasticity before rupture. This decline in flexural strain values could be due to the reaggregation of the MXene nanosheets which occurred during the nanocomposites manufacturing.
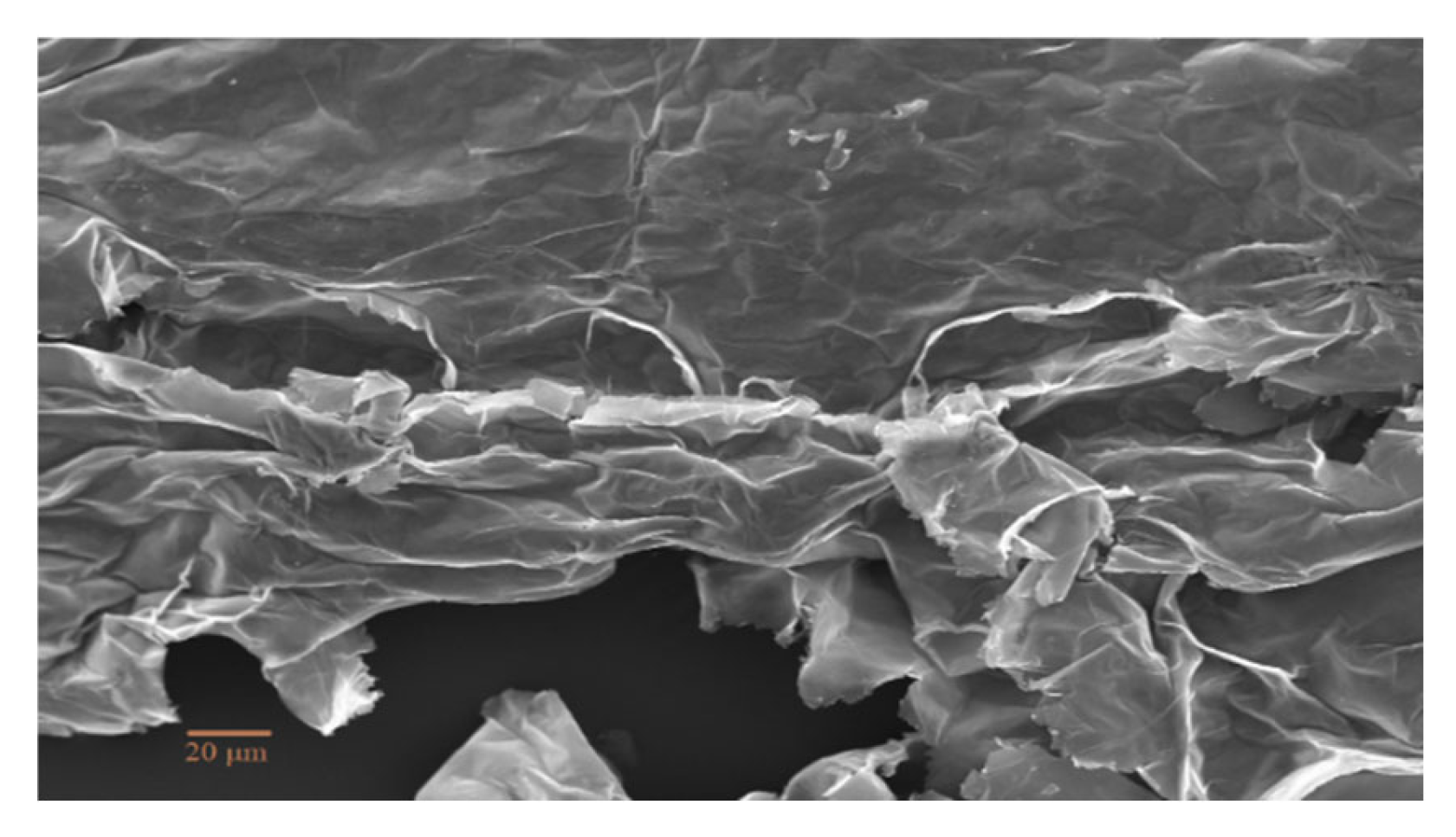
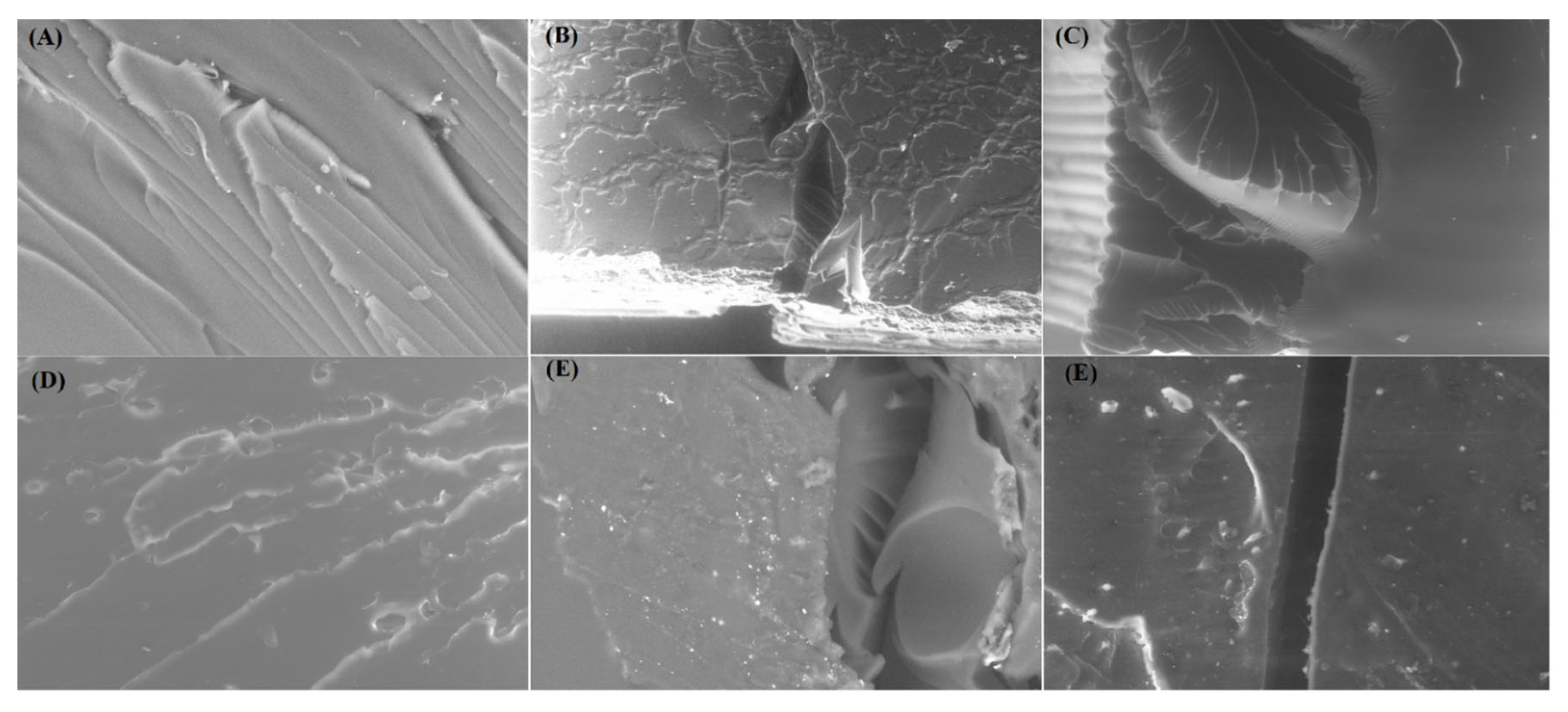
The morphology of fractured surfaces is observed under the SEM microscope following flexural tests. A very smooth fracture surface can be observed in
Figure 8 (A) indicating a brittle failure with apparent signs of plastic deformation. This depicts the epoxy rich regions which possibly leads towards brittle failures due to highly crossed-linked density. For standard epoxy samples however, the fracture morphology took a drastic change from brittle to plastic deformation. Due to the aging of the standard epoxy composite it showed plasticity. While rest of nanocomposite samples showed plastic deformation before rupture. The morphological surfaces showing the advancing transverse cracks which are arrested due the presence of MXene nanosheets. The bridging of the nanosheets can be clearly seen in the images. These images demonstrate that the nanosheets are uniformly dispersed with the epoxy matrix resulted in strong interface area. MXene nanosheets showing their effectiveness in carrying as well as disseminating the external loads which resulted in higher mechanical strength can be seen in
Figure 8 (A-E).
3.3. Thermogravimetric Analysis (TGA) of Nanocomposites
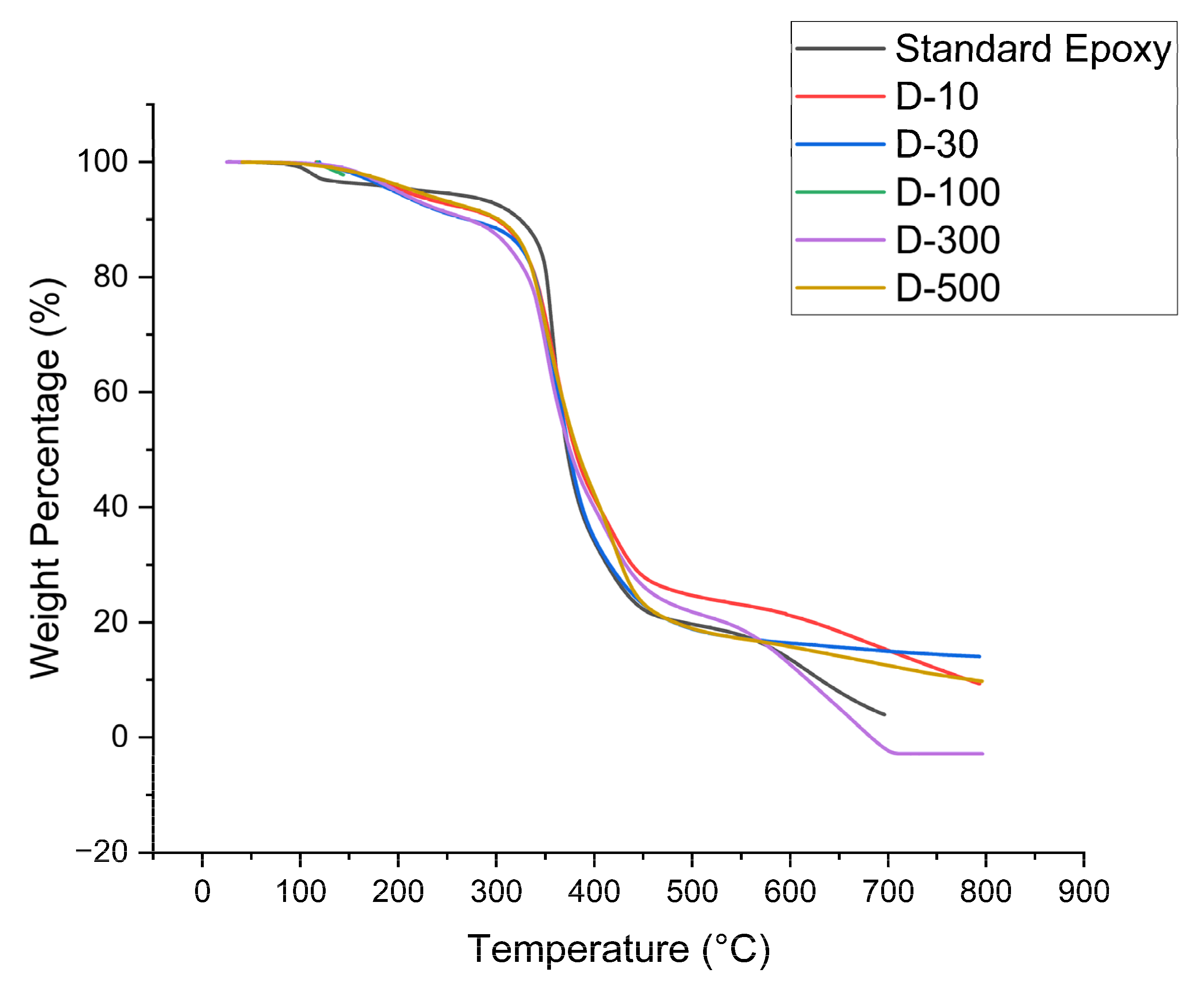
Major thermal characteristics of the nanocomposites, mandatory for different engineering applications, requires their thermal decomposition at high temperatures. Interestingly, the TGA curves depicted a two-stage weight loss pattern, pointing towards consistent decomposition process. The first weight loss step occurred between 50
°C to 350
°C, involved in the evaporation of moisture and other thermally unstable components physically attached to the main polymer chains. The second weight loss step occurred between 350
°C to 800
°C, where depolymerization and breakdown of long epoxy chains are responsible for separation of primary polymeric chains. A high level of thermal stability was observed in all DMF treated nanocomposite samples shown in
Figure 9 as they decomposed less.
The uniform dispersion of MXenes nanoplatelets have developed a strong interfacial adhesion between polymer resin and MXenes, effectively restricted the resin’s chain movement. Remarkably, the MXene nanoplatelets, within epoxy resin promote cross-linking, improved the heat stability as well as densely crosslinked networks of nanocomposites. However, this study revealed that DMF treated MXene nanocomposites could be utilized in applications requiring moderate temperature range as the infusion of nanoplatelets notably improved the initial heat stability and char formation, making these nanocomposites optimum choice for thermal management.
3.4. Differential Scanning Calorimetry (DSC)
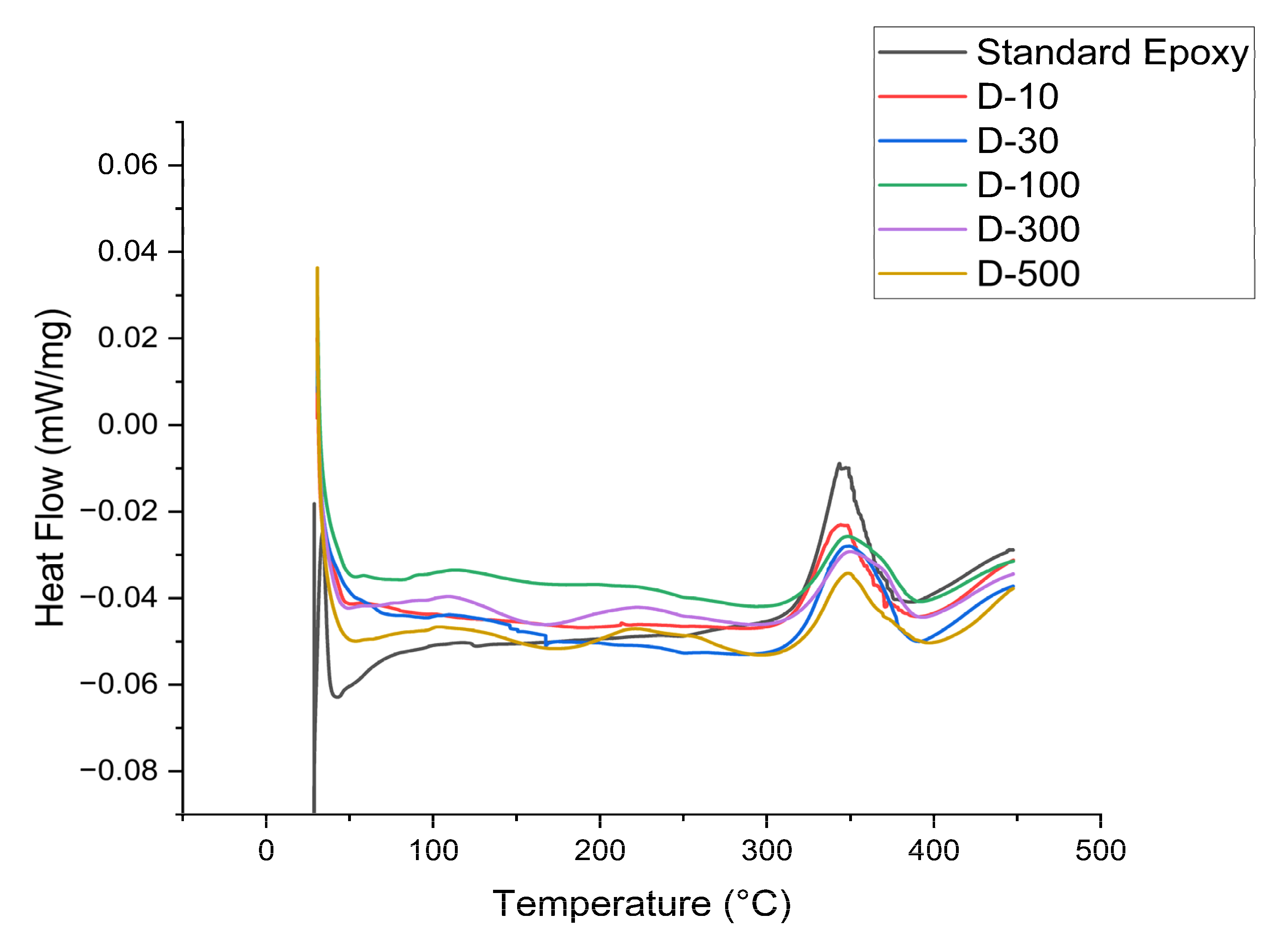
The DSC curves in the
Figure 10 displayed endothermic peaks in the earlier test phase for all nnaocomposite samples, substantiating the glass transition (Tg) phenomenon. The standard epoxy samples exhibited a lower Tg of 42°C. However, all DMF treated nanocomposite samples depicted a higher Tg value compared to standard epoxy. The highest Tg value of 52°C is shown by D-10 sample. This appreciable increase in the Tg value emphasize that the incorporation of MXene nanoplatelets at various weight percentages may restrict the polymeric chain motion, thus enhancing the glass transition temperature. A region of cold crystallization can be seen from 100°C to 300°C temperature range. In this region some of the polymer chains starts melting with temperature rise and once they distribute heat, they become crystals again as less heat cannot retain them in liquid state. Nevertheless, the exothermic peaks were shown on the right side of the figure illustrating the melting point (M.P) of the nanocomposites. Standard epoxy sample possessed a M.P of 340°C while all DMF treated samples exhibited a higher M.P and a high of 350°C is shown by 0.5 wt. % DMF treated MXene nanocomposite. All the DMF treated nanocomposites exhibited an increase in M.P due to the high level of nanoplatelets dispersion achieved. The terminal groups of the MXene made strong chemical connections with the polymeric chains while their tunneling effect helped in the dissipation of heat, resulted in enhanced M.P. Also, this increase in the M.P demonstrated that nanoplatelets might behave as nucleating agents that could promote a perfect crystalline ordered arrangement during curing.
3.5. EMI Shielding of MXene/Epoxy Nanocomposites
To protect the electronic devices and humans from effect of electromagnetic radiations EMI shielding is key phenomenon. The most important property possessed by EMI shielding materials is their high metallic conductivity. The minimum conductivity value required by the Emi shielding materials is 1S/cm, however, different MXene compositions have conductivity in the range of 5S/cm to 20,000S/cm. A high electric conductivity of MXenes places them at top suited EMI shielding materials. MXene films are composed of layered two dimensional MXene sheets which drastically enhance the electric conductivity. Their innovative design features differentiate from conventional EMI shielding materials but also commences the absorption of incoming electromagnetic waves. The integration of conductive filler in Conductive polymeric composites (CPC) are generally considered as the new generation of materials for EMI shielding because they are lightweight, corrosion resistant and have easy processability.
Six types of nanocoating’s were prepared at varying MXene concentrations including standard epoxy as reference. EF80 flexible epoxy resin and EF80 hardener was utilized in the fabrication of nanocoating’s both procured from easy composites, UK. Firstly, pre-weighted MXene nanosheets were added to flexible epoxy resin followed by sonication for the disintegration and uniform dispersion of MXene sheets. The resin to hardener ratio was 100:145. Now the hardener is added, and mixture is manually stirred for 2 minutes and put under vacuum to remove any entrapped air bubbles. Polyester peel ply fabric was coated with the mixture following dip coating. The fabric was completely soaked in solution and allowed the formation of uniform nanocoatings. After five minutes, the cloth was removed at slow speed, so that coating remains uniform all over. The pre-curing took place at room temperature for 24hrs followed by post curing at 120C for 6hrs. The purpose of utilizing flexible epoxy resin is that they behave plastically and was able to completely cover the mobile phone with two to three wraps for subsequent EMI shielding tests.
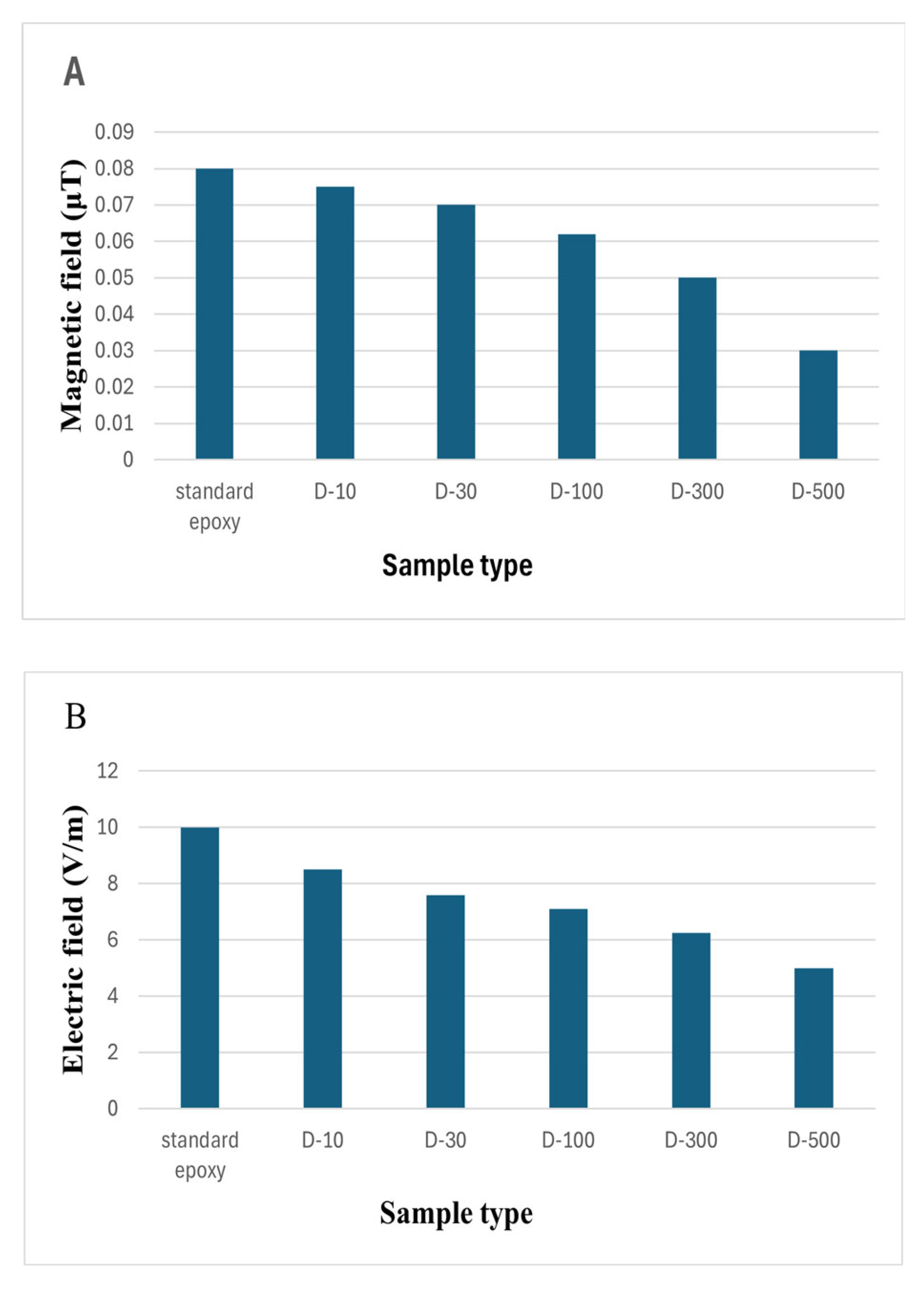
The coatings were wrapped around a cellular phone and their EMI properties were investigated using Seeit Multifield EMF meter, 3.5GHz.
Figure 11 (A) illustrates that the magnetic field properties of the nanocoating’s is decline with the increasing concentration of MXene nanosheets. Likewise,
Figure 11 (B) is showing results for electric field with same declining trend. The lowest magnetic and electric field values are shown by 0.5 wt.% MXene nanocoating’s. This downfall trend clearly states that the nanosheets were uniformly dispersed and helped in absorbing more radiations. These findings suggest that increase in MXene concentrations will render these values to zero.
3.6. Surface Roughness Tests
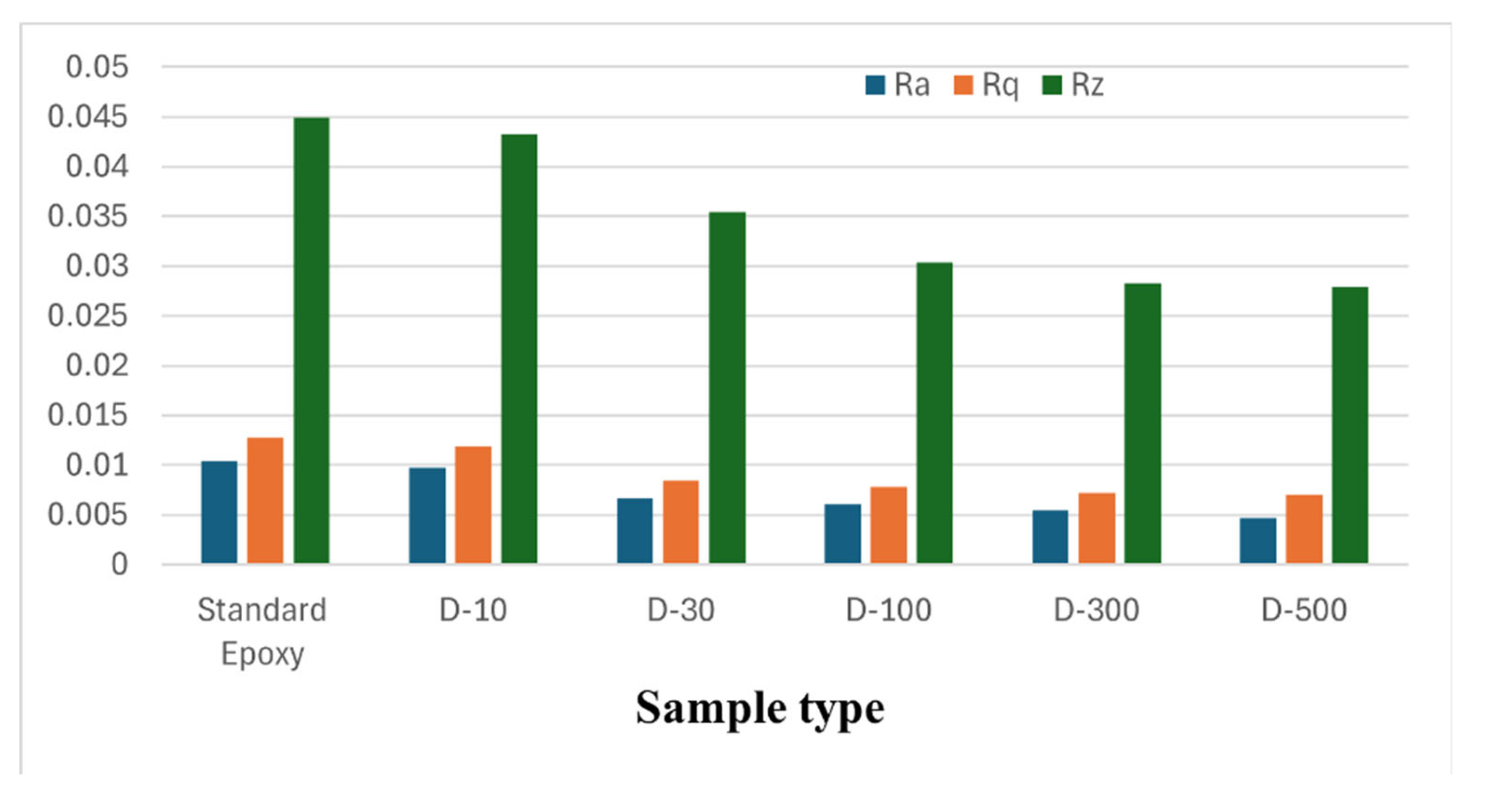
The surface roughness tests of the fractured nanocomposite surfaces were performed using Alicona Infinite Focus.
Figure 12 illustrates the Ra, Rq, and Rz values of sample types and results indicate that with increasing the MXene concentration, these values are gradually falling off. A decline in the roughness parameters at higher MXene concentrations reveal that nanosheets are well dispersed and created strong chemical interactions with polymeric matrix.
The Rq values are a bit higher compared to Ra values for the same surface, as Rq values are more sensitive to peaks and valleys. While Rz indicate the maximum peak to valley height, it provides in depth information about the outer surface features. All declining values indicate that the surfaces are becoming less rough, but some important features still persist.
However, increasing the MXene nanosheets concentration, improved the mechanical, thermal, and EMI shielding properties. Well, 0.5 wt.% MXene concentration performed exceptionally well in enhancing different properties of the nanocomposite. This suggest that the terminal groups present on MXene nanosheets created good structure property relationship with epoxy matrix.
4. Discussion
The two-dimensional MXenes have the potential to emerge as ideal materials for fabricating the next generation of energy storage devices because they possess a unique atomic and layered structure.mThe MXene nanosheets are ideal candidates for energy storage devices such as supercapacitors and different type of batteries due to their higher surface area, high electrical conduction, and quick ion diffusion rates. Supercapacitors is one the most thrilling application of MXene nanosheets in energy storage devices. These devices could charge and discharge quickly and are perfect to be used in high end power applications. Owing to their large surface areas at nanoscale and high conductivity MXenes have shown high capacitance and quick charge and discharge cycles. Moreover, the terminal groups of MXene can be tailored to improve their electrochemical performance by increasing their specific capacitance or decline in impedance. MXene could also be used in the manufacturing of energy storage batteries. These nanomaterials might be used as anodes, cathodes, or both in Li-ion or Na-ion batteries. These are the ideal candidates for future energy storage applications as they hold high specific capacities, enhanced cycling stability, and quick charge/discharge cycles. The unique attributes associated with MXene including their higher surface area, enhanced metallic conduction, and very fast diffusion rates due to the tunneling effect document them as ideal materials in energy storage devices. Further research and development in energy storage applications is mandatory, including supercapacitors and various batteries as promising use. These MXene could also be utilized in the fabrication of type 4 composite pressure vessels for hydrogen storage. Type 4 pressure vessels have a polymer liner inside which has no capability to stop the leakage of hydrogen gas owing to its small size. The integration of MXene in these polymer liners provide two-way benefits; in terms of mechanical integrity as well as due their small size they possess larger surface area which would aid in stopping the leakage.
5. Conclusions
In this research, DMF a polar solvent, was used to study its effects on the dispersion of MXenes, the formation and different characteristics of Mxene/epoxy nanocomposites. This comprehensive study provides enough details about the introduction of DMF solvent in reducing the Van der Waals interaction between MXene nanosheets and their uniform dispersion and could also be used as a reference for dispersion of various type of nanomaterials. In this study, MXenes dispersion level, mechanical properties, SEM analysis, TGA, DSC, EMI shielding effectiveness, and surface roughness were analyzed. All the results demonstrated that increasing the concentration of MXene enhanced the properties of the nanocomposites. The usage of DMF helped in overcoming the strong Van der Waals interactions between the MXene nanosheets and dispersed them uniformly, but they possess long processing times and higher temperatures. No noticeable agglomeration of nanosheets was found even when 0.5 wt. % of MXene was added. This uniform dispersion of nanosheets accounts for the improved properties of MXene/epoxy nanocomposites. For small concentration of nanosheets, we could reduce the processing time by utilizing lower volume of solvents and benefiting both economically and from health perspectives. These results would aid in optimization of processing parameters for nanocomposites fabrication. However, this research documented relationship between the solvent dosage, MXene concentration, and processing of MXene/epoxy nanocomposites for the first time, and it ahs identified the optimum dispersion parameters in various media. Therefore, further study is required to better understand the solvent behaviour at higher concentrations of MXene.
Author Contributions
Conceptualization, M.S.S, M.Y, F.I, N.H.F and A.A.J.; methodology, M.S.S and M.Y.; validation, F.I., A.A.J. and R.A.I.; formal analysis, A.A.J.; investigation, A.AJ. and M.S.S.; resources, I.S. and M.S.S.; data curation, A.A.I.; writing—original draft preparation, A.A.J; writing—review and editing, I.S. and N.F.H; visualization, A.A.J.; supervision, M.S.S; project administration, M.S.S.; funding acquisition, M.S.S. and N.H.F. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
We would like to thank the Carnegie Trust for the research funding and Edinburgh Napier University for providing the Alicona Infinite Focus equipment for surface roughness measurement; without their contributions, this research would not have been possible.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Naguib, M.; Barsoum, M.W.; Gogotsi, Y. Ten Years of Progress in the Synthesis and Development of MXenes. Advanced Materials 2021, 33. [Google Scholar] [CrossRef]
- Hatter, C.B.; Shah, J.; Anasori, B.; Gogotsi, Y. Micromechanical Response of Two-Dimensional Transition Metal Carbonitride (MXene) Reinforced Epoxy Composites. Compos B Eng 2020, 182. [Google Scholar] [CrossRef]
- Lin, L.; Ning, H.; Song, S.; Xu, C.; Hu, N. Flexible Electrochemical Energy Storage: The Role of Composite Materials. Compos Sci Technol 2020, 192. [Google Scholar] [CrossRef]
- Jamil, F.; Ali, H.M.; Janjua, M.M. MXene Based Advanced Materials for Thermal Energy Storage: A Recent Review. J Energy Storage 2021, 35. [Google Scholar] [CrossRef]
- Liu, C.; Li, F.; Lai-Peng, M.; Cheng, H.M. Advanced Materials for Energy Storage. Advanced Materials 2010, 22. [Google Scholar] [CrossRef]
- Ezika, A.C.; Sadiku, E.R.; Idumah, C.I.; Ray, S.S.; Hamam, Y. On Energy Storage Capacity of Conductive MXene Hybrid Nanoarchitectures. J Energy Storage 2022, 45. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Q. Ferroelectric Polymer Composites for Capacitive Energy Storage. In Organic Ferroelectric Materials and Applications; Elsevier, 2022; pp. 477–502. [Google Scholar]
- Liu, Y.; Yu, J.; Guo, D.; Li, Z.; Su, Y. Ti3C2Tx MXene/Graphene Nanocomposites: Synthesis and Application in Electrochemical Energy Storage. J Alloys Compd 2020, 815. [Google Scholar] [CrossRef]
- Ezika, A.C.; Sadiku, E.R.; Idumah, C.I.; Ray, S.S.; Hamam, Y. On Energy Storage Capacity of Conductive MXene Hybrid Nanoarchitectures. J Energy Storage 2022, 45. [Google Scholar] [CrossRef]
- Jamil, F.; Ali, H.M.; Janjua, M.M. MXene Based Advanced Materials for Thermal Energy Storage: A Recent Review. J Energy Storage 2021, 35. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, J.; Guo, D.; Li, Z.; Su, Y. Ti3C2Tx MXene/Graphene Nanocomposites: Synthesis and Application in Electrochemical Energy Storage. J Alloys Compd 2020, 815. [Google Scholar] [CrossRef]
- Wei, J.; Saharudin, M.S.; Vo, T.; Inam, F. N,N-Dimethylformamide (DMF) Usage in Epoxy/Graphene Nanocomposites: Problems Associated with Reaggregation. Polymers (Basel) 2017, 9. [Google Scholar] [CrossRef]
- Riazi, H.; Nemani, S.K.; Grady, M.C.; Anasori, B.; Soroush, M. Ti3C2MXene-Polymer Nanocomposites and Their Applications. J Mater Chem A Mater 2021, 9. [Google Scholar] [CrossRef]
- Saharudin, M.S.; Ilyas, R.A.; Awang, N.; Hasbi, S.; Shyha, I.; Inam, F. Advances in Sustainable Nanocomposites. Sustainability (Switzerland) 2023, 15. [Google Scholar] [CrossRef]
- Che Nasir, N.A.; Saharudin, M.S.; Wan Jusoh, W.N.; Kooi, O.S. Effect of Nanofillers on the Mechanical Properties of Epoxy Nanocomposites. In Advanced Structured Materials; 2022; Volume 167. [Google Scholar]
- Saharudin, M.S.; Atif, R.; Shyha, I.; Inam, F. The Degradation of Mechanical Properties in Polymer Nano-Composites Exposed to Liquid Media - A Review. RSC Adv 2016, 6. [Google Scholar] [CrossRef]
- Wei, J.; Saharudin, M.S.; Vo, T.; Inam, F. Effects of Surfactants on the Properties of Epoxy/Graphene Nanocomposites. Journal of Reinforced Plastics and Composites 2018, 37. [Google Scholar] [CrossRef]
- Saharudin, M.S.; Che Nasir, N.A.; Hasbi, S. Tensile and Corrosion Resistance Studies of MXenes/Nanocomposites: A Review. In Advanced Structured Materials; Springer Science and Business Media Deutschland GmbH, 2022; Volume 167, pp. 189–198. [Google Scholar]
- Zukiene, K.; Monastyreckis, G.; Kilikevicius, S.; Procházka, M.; Micusik, M.; Omastová, M.; Aniskevich, A.; Zeleniakiene, D. Wettability of MXene and Its Interfacial Adhesion with Epoxy Resin. Mater Chem Phys 2021, 257. [Google Scholar] [CrossRef]
- Li, C.; Xu, J.; Xu, Q.; Xue, G.; Yu, H.; Wang, X.; Lu, J.; Cui, G.; Gu, G. Synthesis of Ti3C2 MXene@PANI Composites for Excellent Anticorrosion Performance of Waterborne Epoxy Coating. Prog Org Coat 2022, 165. [Google Scholar] [CrossRef]
- Wei, J.; Saharudin, M.S.; Vo, T.; Inam, F. N,N-Dimethylformamide (DMF) Usage in Epoxy/Graphene Nanocomposites: Problems Associated with Reaggregation. Polymers (Basel) 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Saharudin, M.S.; Wei, J.; Shyha, I.; Inam, F. Biodegradation of Halloysite Nanotubes-Polyester Nanocomposites Exposed to Short Term Seawater Immersion. Polymers (Basel) 2017, 9. [Google Scholar] [CrossRef]
- Giménez, R.; Serrano, B.; San-Miguel, V.; Cabanelas, J.C. Recent Advances in MXene/Epoxy Composites: Trends and Prospects. Polymers (Basel) 2022, 14. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Y.; Li, L.; Wang, Y.; Wang, J.; Xiong, J.; Du, S.; Zhang, P.; Shi, X.; Yu, J. MXene/Polymer Nanocomposites: Preparation, Properties, and Applications. Polymer Reviews 2021, 61. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J. Incorporating MXene into Boron Nitride/Poly(Vinyl Alcohol) Composite Films to Enhance Thermal Andmechanical Properties. Polymers (Basel) 2021, 13. [Google Scholar] [CrossRef]
- Qian, Y.; Zhong, J.; Ou, J. Superdurable Fiber-Reinforced Composite Enabled by Synergistic Bridging Effects of MXene and Carbon Nanotubes. Carbon N Y 2022, 190, 104–114. [Google Scholar] [CrossRef]
- Ding, R.; Sun, Y.; Lee, J.; Nam, J. Do; Suhr, J. Enhancing Interfacial Properties of Carbon Fiber Reinforced Epoxy Composites by Grafting MXene Sheets (Ti2C). Compos B Eng 2021, 207. [Google Scholar] [CrossRef]
- Feng, A.; Hou, T.; Jia, Z.; Zhang, Y.; Zhang, F.; Wu, G. Preparation and Characterization of Epoxy Resin Filled with Ti3C2Tx MXene Nanosheets with Excellent Electric Conductivity. Nanomaterials 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Li, J.; Simon, P.; Xu, B. Two-Dimensional MXenes for Electrochemical Capacitor Applications: Progress, Challenges and Perspectives. Energy Storage Mater 2021, 35, 630–660. [Google Scholar] [CrossRef]
- Iqbal, A.; Hong, J.; Ko, T.Y.; Koo, C.M. Improving Oxidation Stability of 2D MXenes: Synthesis, Storage Media, and Conditions. Nano Converg 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Jia, F.; Zhuo, L.; Lu, Z.; Si, L.; Huang, J.; Zhang, M.; Ma, Q. Ultrathin MXene/Aramid Nanofiber Composite Paper with Excellent Mechanical Properties for Efficient Electromagnetic Interference Shielding. Nanoscale 2019, 11, 23382–23391. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Zhou, K.; Qian, X.; Shi, C.; Yu, B. MXene as Emerging Nanofillers for High-Performance Polymer Composites: A Review. Compos B Eng 2021, 217. [Google Scholar] [CrossRef]
- Wei, J.; Vo, T.; Inam, F. Epoxy/Graphene Nanocomposites - Processing and Properties: A Review. RSC Adv 2015, 5. [Google Scholar] [CrossRef]
- Wei, J.; Atif, R.; Vo, T.; Inam, F. Graphene Nanoplatelets in Epoxy System: Dispersion, Reaggregation, and Mechanical Properties of Nanocomposites. J Nanomater 2015, 2015. [Google Scholar] [CrossRef]
- Ronchi, R.M.; Arantes, J.T.; Santos, S.F. Synthesis, Structure, Properties and Applications of MXenes: Current Status and Perspectives. Ceram Int 2019, 45, 18167–18188. [Google Scholar] [CrossRef]
- Wei, J.; Saharudin, M.S.; Vo, T.; Inam, F. Dichlorobenzene: An Effective Solvent for Epoxy/Graphene Nanocomposites Preparation. R Soc Open Sci 2017, 4. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).