Submitted:
15 July 2024
Posted:
16 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
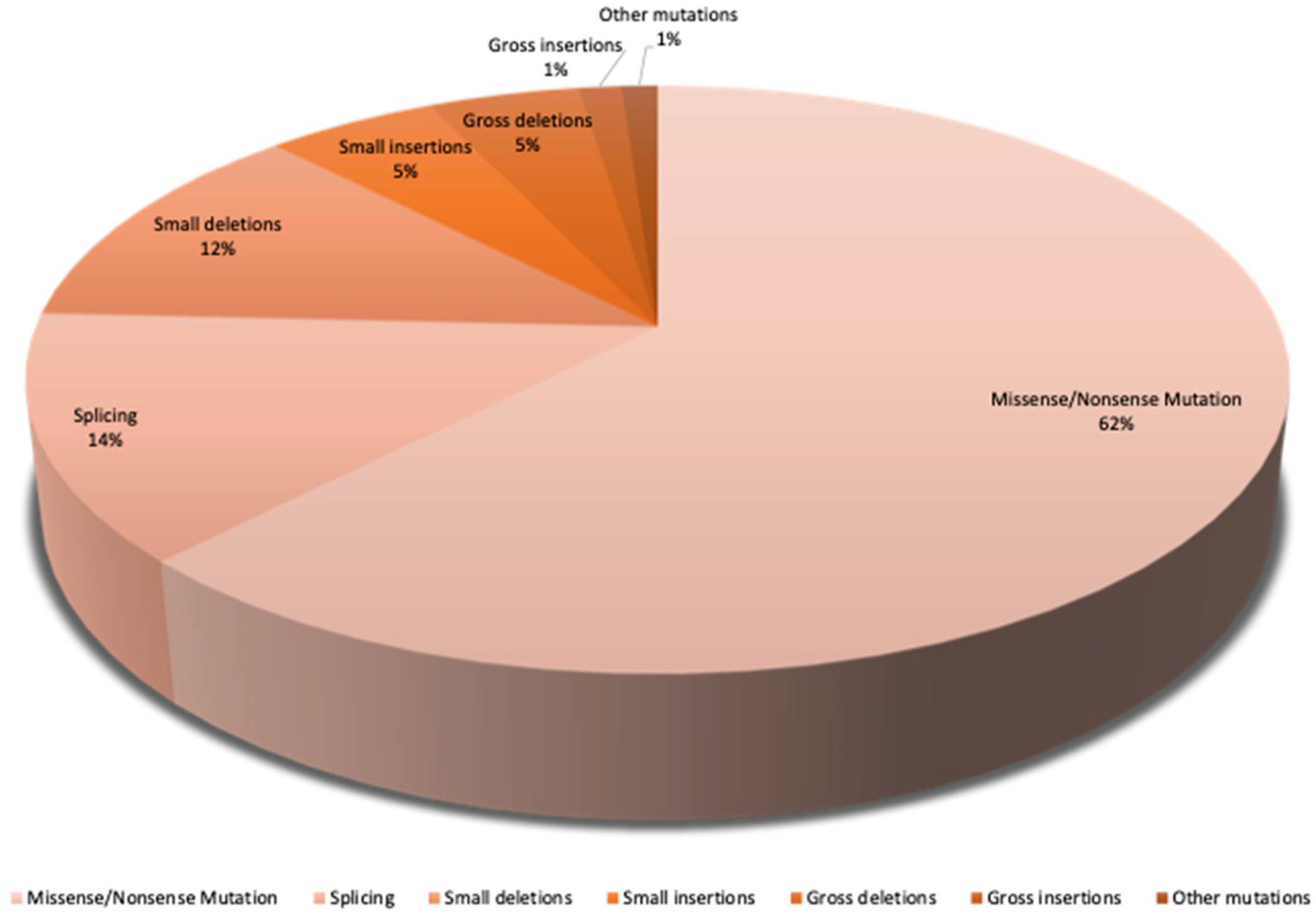
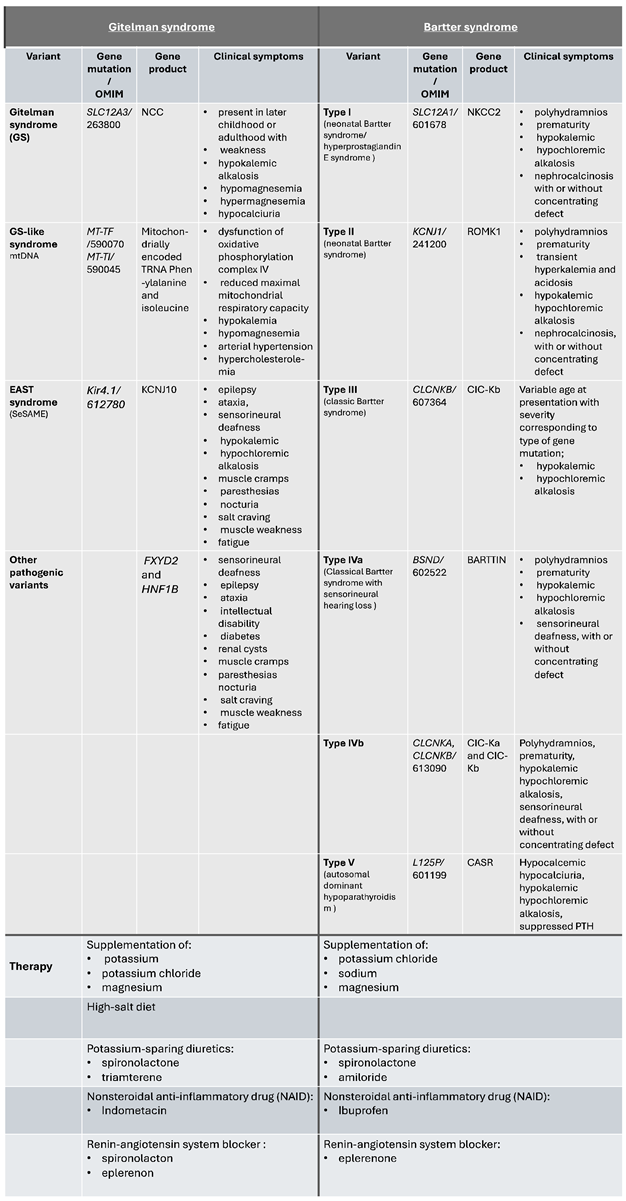
2. Gitelman Syndrome
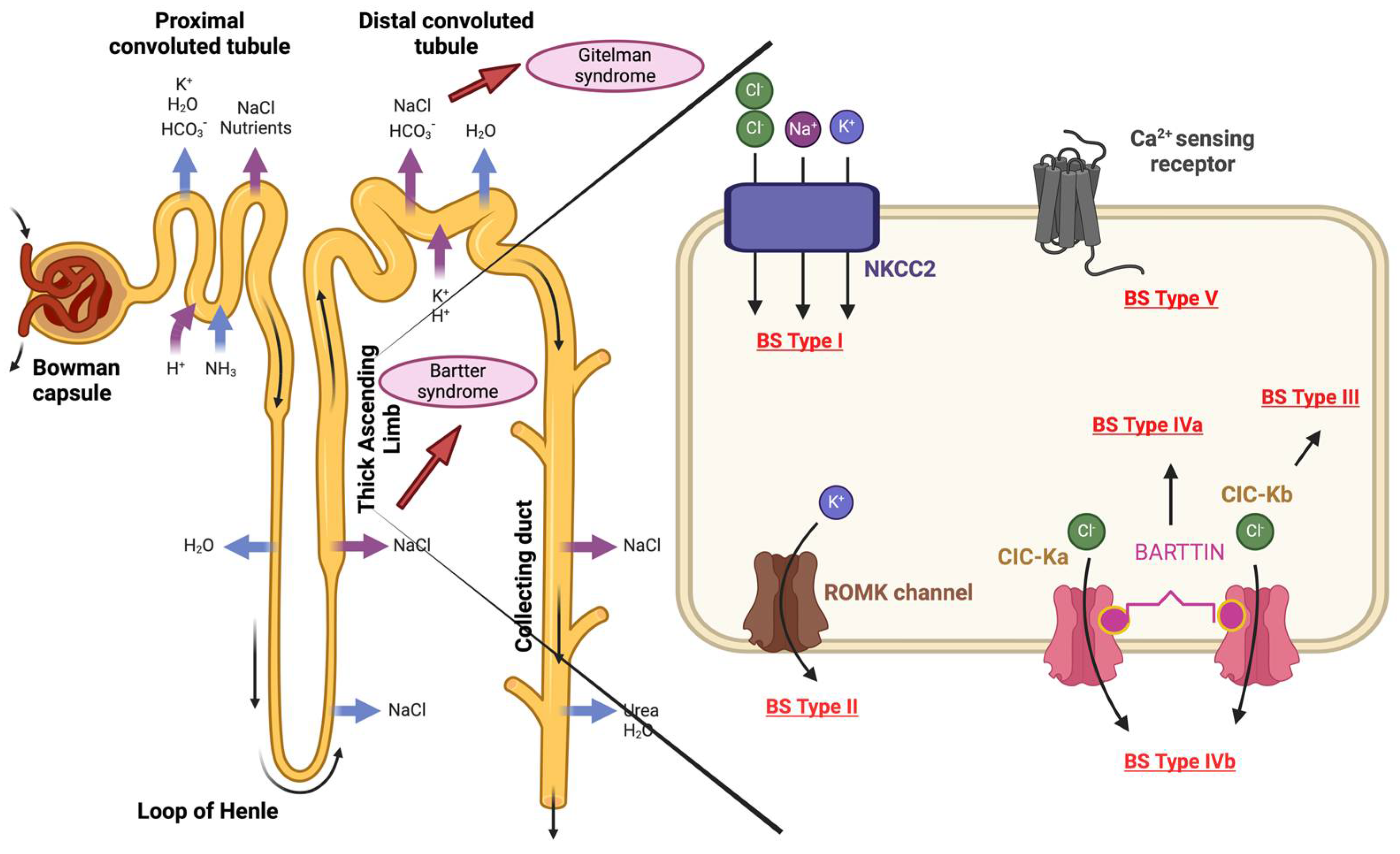
3. Bartter Syndrome
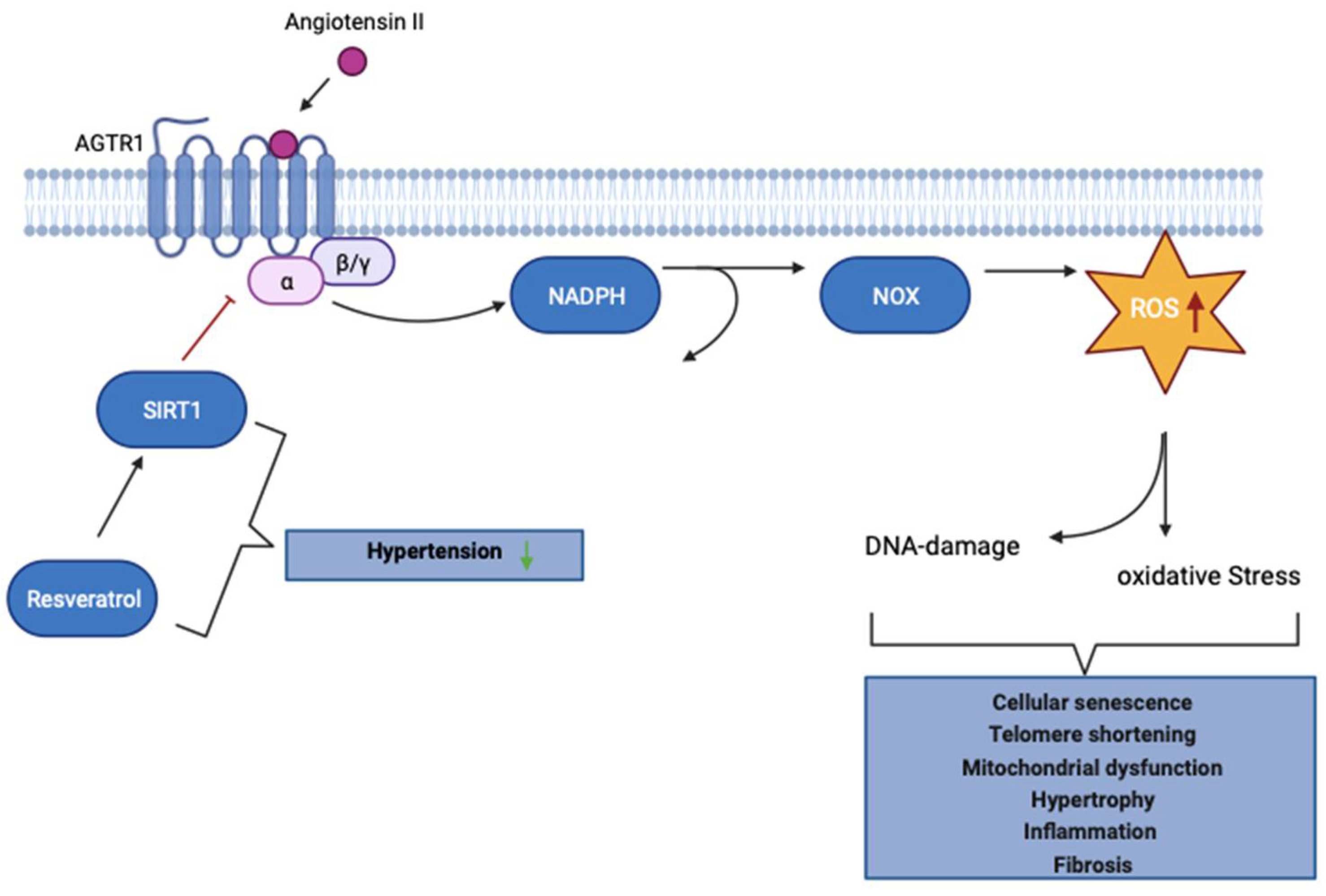
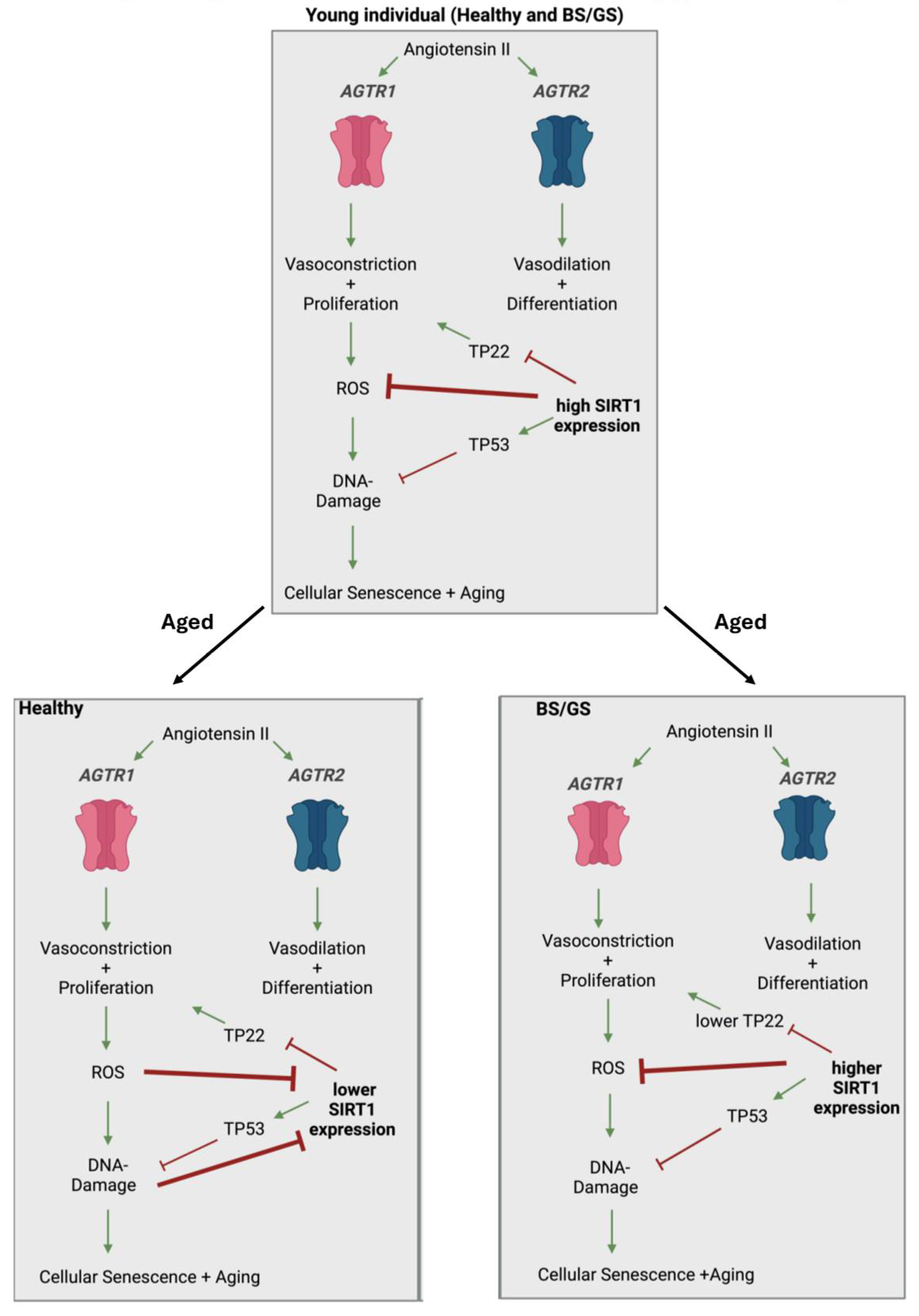
4. Aging and RAAS
5. Conclusions
6. The Future Direction of Research
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Fountain, J.H.; Kaur, J.; Lappin, S.L. Physiology, Renin Angiotensin System. StatPearls Publishing LLC.; 2023.
- Walsh, S.B.; Unwin, R.J. Renal tubular disorders. Clin Med (Lond). 2012, 12, 476–9. [Google Scholar] [CrossRef] [PubMed]
- Kopp, J.B.; Anders, H.-J.; Susztak, K.; Podestà, M.A.; Remuzzi, G.; Hildebrandt, F.; Romagnani, P. Podocytopathies. Nat Rev Dis Primers. 2020, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Remuzzi, G.; Benigni, A.; Remuzzi, A. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. JCI. 2006, 288–96. [Google Scholar] [CrossRef] [PubMed]
- Currie, G.; Delles, C. Proteinuria and its relation to cardiovascular disease. Int J Nephrol Renovasc Dis. 2014, 13–24. [Google Scholar]
- da Silva Cunha, T.; Pfefermann Heilberg, I. Bartter syndrome: causes, diagnosis, and treatment. Int J Nephrol Renovasc Dis. 2018, 11, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Cruz, D.N.; Shaer, A.J.; Bia, M.J.; Lifton, R.P.; Simon, D.B. Gitelman’s syndrome revisited: an evaluation of symptoms and health-related quality of life. Kidney International. 2001, 59, 710–7. [Google Scholar] [CrossRef] [PubMed]
- Gitelman, H.J.; Graham, J.B.; Welt, L.G. A new familial disorder characterized by hypokalemia and hypomagnesemia. Trans Assoc Am Physicians. 1966, 79, 221–35. [Google Scholar] [PubMed]
- Simon, D.B.; Nelson-Williams, C.; Bia, M.J.; Ellison, D.; Karet, F.E.; Molina, A.M.; Vaara, I.; Iwata, F.; Cushner, H.M.; Koolen, M.; Gainza, F.J.; Gitleman, H.J.; Lifton, R.P. Gitelman’s variant of Bartter’s syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nature Genetics. 1996, 12, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Kondo, A.; Nagano, C.; Ishiko, S.; Omori, T.; Aoto, Y.; Rossanti, R.; Sakakibara, N.; Horinouchi, T.; Yamamura, T.; Nagai, S.; Okada, E.; Shima, Y.; Nakanishi, K.; et al. Examination of the predicted prevalence of Gitelman syndrome by ethnicity based on genome databases. Scientific Reports. 2021, 11. [Google Scholar]
- San-Cristobal, P.; de los Heros, P.; Ponce-Coria, J.; Moreno, E.; Gamba, G. WNK Kinases, Renal Ion Transport and Hypertension. Am J Nephrol. 2008, 28, 860–70. [Google Scholar] [CrossRef]
- Gamba, G. Role of WNK kinases in regulating tubular salt and potassium transport and in the development of hypertension. American Journal of Physiology - Renal Physiology. 2005, 288, 245–52. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Mishima, E.; Shima, H.; Akiyama, Y.; Suzuki, C.; Suzuki, T.; Kobayashi, T.; Suzuki, Y.; Nakayama, T.; Takeshima, Y.; Vazquez, N.; Ito, S.; Gamba, G.; et al. Exonic Mutations in the SLC12A3 Gene Cause Exon Skipping and Premature Termination in Gitelman Syndrome. J Am Soc Nephrol. 2015, 26, 271–9. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Poussou, R.; Dahan, K.; Kahila, D.; Venisse, A.; Riveira-Munoz, E.; Debaix, H.; Grisart, B.; Bridoux, F.; Unwin, R.; Moulin, B.; Haymann, J.-P.; Vantyghem, M.-C.; Rigothier, C.; et al. Spectrum of mutations in Gitelman syndrome. J Am Soc Nephrol. 2011, 22, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Stenson, P.D.; Ball, E.V.; Mort, M.; Phillips, A.D.; Shiel, J.A.; Thomas, N.S.T.; Abeysinghe, S.; Krawczak, M.; Cooper, D.N. Human Gene Mutation Database (HGMD): 2003 update. Hum Mutat. 2003, 21, 577–81. [Google Scholar] [CrossRef]
- Hsu, Y.-J.; Hoenderop, J.G.; Bindels, R.J.M. TRP channels in kidney disease. Biochim Biophys Acta. 2007, 1772, 928–36. [Google Scholar] [CrossRef] [PubMed]
- Knoers, N.V.A. Inherited forms of renal hypomagnesemia: an update. Pediatr Nephrol. 2009, 24, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Foo, J.N.; O’Roak, B.J.; Zhao, H.; Larson, M.G.; Simon, D.B.; Newton-Chen, C.; State, M.W.; Levy, D.; Lifton, R.P. Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nature Genetics volume. 2008, 40, 592–9. [Google Scholar] [CrossRef]
- Herrero-Morín, J.D.; Rodríguez, J.; Coto, E.; Gil-Peña, H.; Alvarez, V.; Espinosa, L.; Loris, C.; Gil-Calvo, M.; Santos, F. Gitelman syndrome in Gypsy paediatric patients carrying the same intron 9 + 1 G>T mutation. Clinical features and impact on quality of life. Nephrol Dial Transplant. 2011, 26, 151–5. [Google Scholar] [CrossRef]
- Tseng, M.-H.; Yang, S.-S.; Hsu, Y.-J.; Fang, Y.-W.; Wu, C.-J.; Tsai, J.-D.; Hwang, D.-Y.; Lin, S.-H. Genotype, phenotype, and follow-up in Taiwanese patients with salt-losing tubulopathy associated with SLC12A3 mutation. J Clin Endocrinol Metab. 2012, 97, 1478–82. [Google Scholar] [CrossRef]
- Wang, L.; Dong, C.; Xi, Y.-G.; Su, X. Thiazide-sensitive Na+-Cl- cotransporter: genetic polymorphisms and human diseases. Acta Biochim Biophys Sin (Shanghai). 2015, 47, 325–34. [Google Scholar] [CrossRef]
- Loffing, J.; Loffing-Cueni, D.; Valderrabano, V.; Kläusli, L.; Hebert, S.C.; Rossier, B.C.; Hoenderop, J.G.; Bindels, R.J.; Kaissling, B. Distribution of transcellular calcium and sodium transport pathways along mouse distal nephron. American Journal of Physiology - Renal Physiology. 2001, 281, F1021–7. [Google Scholar] [CrossRef] [PubMed]
- Knoers, N.V.A.M.; Levtchenko, E. Gitelman syndrome. Orphanet J Rare Dis. 2008, 3, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Graziani, G.; Fedeli, C.; Moroni, L.; Cosmai, L.; Badalamenti, S.; Ponticelli, C. Gitelman syndrome: pathophysiological and clinical aspects*. QJM: An International Journal of Medicine. 2010, 103, 741–8. [Google Scholar] [CrossRef] [PubMed]
- Bettinelli, A.; Bianchetti, M.G.; Girardin, E.; Caringella, A.; Cecconi, M.; Appiani, A.C.; Pavanello, L.; Gastaldi, R.; Isimbaldi, C.; Lama, G.; Marchesoni, C.; Matteucci, C.; Patriarca, P.; et al. Use of calcium excretion values to distinguish two forms of primary renal tubular hypokalemic alkalosis: Bartter and Gitelman syndromes. 1992, 120, 38–43.
- Jeck, N.; Schlingmann, K.P.; Reinalter, S.C.; Kömhoff, M.; Peters, M.; Waldegger, S.; Seyberth, H.W. Salt handling in the distal nephron: lessons learned from inherited human disorders. Am J Physiol Regul Integr Comp Physiol. 2005, 288, 782–95. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, A.; Bockenhauer, D.; Bolignano, D.; Calò, L.A.; Cosyns, E.; Devuyst, O.; Ellison, D.H.; Karet Frankl, F.E.; Knoers, N.V.A.; Konrad, M.; Lin, S.-H.; Vargas-Poussou, R. Gitelman syndrome: consensus and guidance from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney International. 2017, 91, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Colussi, G.; Macaluso, M.; Brunati, C.; Minetti, L. Calcium metabolism and calciotropic hormone levels in Gitelman’s syndrome. Miner Electrolyte Metab. 1994, 20, 294–301. [Google Scholar]
- Rudin, A.; Aurell, M.; Wilske, J. Low urinary calcium excretion in Bartter’s syndrome. Scand J Urol Nephrol. 1988, 22, 35–9. [Google Scholar] [CrossRef] [PubMed]
- Agus, Z.S. Hypomagnesemia. J Am Soc Nephrol. 1999, 10, 1616–22. [Google Scholar] [CrossRef]
- Elisaf, M.; Panteli, K.; Theodorou, J.; Siamopoulos, K.C. Fractional excretion of magnesium in normal subjects and in patients with hypomagnesemia. Magnes Res 1. 1997, 10, 315–20. [Google Scholar]
- Ravarotto, V.; Bertoldi, G.; Stefanelli, L.F.; Nalesso, F.; Calò, L.A. Gitelman’s and Bartter’s Syndromes: From Genetics to the Molecular Basis of Hypertension and More. Kidney Blood Press Res. 2022, 47, 556–64. [Google Scholar] [CrossRef]
- Fuhimura, J.; Nozu, K.; Yamamura, T.; Minamikawa, S.; Nakanishi, K.; Horinouchi, T.; Nagano, C.; Sakakibara, N.; Nakanishi, K.; Shima, Y.; Miyako, K.; Nozu, Y.; Morisada, N.; et al. Clinical and Genetic Characteristics in Patients With Gitelman Syndrome. Kidney Int Rep. 2019, 4, 119–25. [Google Scholar] [CrossRef]
- Adalat, S.; Hayes, W.N.; Bryant, W.; Booth, J.; Woolf, A.S.; Kleta, R.; Subtil, S.; Clissold, R.; Colclough, K.; Ellard, S.; Bockenhauer, D. HNF1B Mutations Are Associated With a Gitelman-like Tubulopathy That Develops During Childhood. Kidney Int Rep. 2019, 4, 1304–11. [Google Scholar] [CrossRef] [PubMed]
- Bockenhauer, D.; Feather, S.; Stanescu, H.C.; Bandulik, S.; Zdebik, A.A.; Reichold, M.; Tobin, J.; Lieberer, E.; Sterner, C.; Landoure, G.; Arora, R.; Sirimanna, T.; Thompson, D.; et al. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med. 2009, 360, 1960–70. [Google Scholar] [CrossRef] [PubMed]
- Scholl, U.I.; Choi, M.; Liu, T.; Ramaekers, V.T.; Häusler, M.G.; Grimmer, J.; Tobe, S.W.; Farhi, A.; Nelson-Williams, C.; Lifton, R.P. Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc Natl Acad Sci U S A. 2009, 106, 5842–7. [Google Scholar] [CrossRef] [PubMed]
- Mayan, H.; Farfel, Z.; Karlish, S.J.D. Renal Mg handling, FXYD2 and the central role of the Na,K-ATPase. Physiol Rep. 2018, 6, e13843. [Google Scholar] [CrossRef] [PubMed]
- Suzumoto, Y.; Columbano, V.; Gervasi, L.; Giunta, R.; Mattina, T.; Trimarchi, G.; Capolongo, G.; Simeoni, M.; Perna, A.F.; Zacchia, M.; Toriello, G.; Pollastro, R.M.; Rapisarda, F.; et al. A case series of adult patients affected by EAST/SeSAME syndrome suggests more severe disease in subjects bearing KCNJ10 truncating mutations. Intractable Rare Dis Res. 2021, 10, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Deng, C.-X.; Mostoslavsky, R. Recent progress in the biology and physiology of sirtuins. Nature. 2009, 460, 587–91. [Google Scholar] [CrossRef] [PubMed]
- Viering, D.; Schlingmann, K.P.; Hureaux, M.; Nijenhuis, T.; Mallett, A.; Chan, M.M.Y.; van Beek, A.; van Eerde, A.; Coulibaly, J.-M.; Vallet, M. ; et. al. Gitelman-Like Syndrome Caused by Pathogenic Variants in mtDNA. J Am Soc Nephrol. 2022, 33, 305–25. [Google Scholar]
- Yang, L.; Fan, J.; Liu, Y.; Ren, Y.; Liu, Z.; Fu, H.; Qi, H.; Yang, J. Case report: Gitelman syndrome with diabetes: Confirmed by both hydrochlorothiazide test and genetic testing. Medicine (Baltimore). 2023, 102, e33959. [Google Scholar] [CrossRef]
- Nuñez-Gonzale, L.; Carrera, N.; Garcia-Gonzale, M.A. Molecular Basis, Diagnostic Challenges and Therapeutic Approaches of Bartter and Gitelman Syndromes: A Primer for Clinicians. Int J Mol Sci. 2021, 22, 11414. [Google Scholar] [CrossRef]
- Liaw, L.C.; Banerjee, K.; Coulthard, M.G. Dose related growth response to indometacin in Gitelman syndrome. Arch Dis Child. 1999, 81, 508–10. [Google Scholar] [CrossRef] [PubMed]
- Bartter, F.C.; Pronove, P.; Gill, J.R.; Maccardle, R.C. Hyperplasia of the juxtaglomerular complex with hyperaldosteronism and hypokalemic alkalosis. A new syndrome. Am J Med. 1962, 33, 811–28. [Google Scholar] [CrossRef]
- Lee, B.H.; Cho, H.Y.; Lee, H.; Han, K.H.; Kang, H.G.; Ha, I.S.; Lee, J.H.; Park, Y.S.; Shin, J.I.; Lee, D.-Y.; Kim, S.-Y.; Choi, Y.; Cheong, H.I. Genetic basis of Bartter syndrome in Korea. ndt. 2012, 27, 1516–21. [Google Scholar] [CrossRef]
- Konrad, M.; Vollmer, M.; Lemmink, H.H.; Van Den Heuvel, L.P.W.J.; Jeck, N.; Vargas-Poussou, R.; Lakings, A.; Ruf, R.; Deschênes, G.; Antignac, C.; Guay-Woodford, L.; Knoers, N.V.A.M.; Seyberth, H.W.; et al. Mutations in the chloride channel gene CLCNKB as a cause of classic Bartter syndrome. J Am Soc Nephrol. 2000, 11, 1449–59. [Google Scholar] [CrossRef]
- Kurtz, I. Molecular pathogenesis of Bartter’s and Gitelman’s syndromes. Kidney Int. 1998, 54, 1396–410. [Google Scholar] [CrossRef] [PubMed]
- Bokhari, S.R.A.; Zulfiqar, H.; Mansur, A. Bartter Syndrome. StatPearls Publishing LLC.; 2023.
- Colussi, G.; Bettinelli, A.; Tedeschi, S.; De Ferrari, M.E.; Syrèn, M.L.; Borsa, N.; Mattiello, C.; Casari, G.; Bianchetti, M.G. A thiazide test for the diagnosis of renal tubular hypokalemic disorders. Clin J Am Soc Nephrol. 2007, 2, 454–60. [Google Scholar] [CrossRef] [PubMed]
- Verberckmoes, R.; van Damme, B.; Clement, J.; Amery, A.; Michielsen, P. Bartter’s syndrome with hyperplasia of renomedullary cells: successful treatment with indomethacin. Kidney Int. 1976, 9, 302–7. [Google Scholar] [CrossRef]
- Nascimento, C.L.P.; Garcia, C.L.; Schvartsman, B.G.S.; Vaisbich, M.H. Treatment of Bartter syndrome. Unsolved issue. J Pediatr (Rio J). 2014, 90, 512–7. [Google Scholar] [CrossRef]
- Morales, J.M.; Ruilope, L.M.; Coto, A.; Alcazar, J.M.; Prieto, C.; Nieto, J.; Rodicio, J.L. Long-term enalapril therapy in Bartter’s syndrome. Nephron. 1988, 48, 327. [Google Scholar] [CrossRef]
- Hené, R.J.; Koomans, H.A.; Dorhout Mees, E.J.; vd Stolpe, A.; Verhoef, G.E.; Boer, P. Correction of Hypokalemia in Bartter’s Syndrome by Enalapril. Am J Kidney Dis. 1987, 9, 200–5. [Google Scholar] [CrossRef]
- Fulchiero, R.; Seo-Mayer, P. Bartter Syndrome and Gitelman Syndrome. Pediatr Clin North Am. 2019, 66, 121–34. [Google Scholar] [CrossRef] [PubMed]
- Armanios, M.; de Cabo, R.; Mannick, J.; Partridge, L.; van Deursen, J.; Villeda, S. Translational strategies in aging and age-related disease. Nat Med. 2015, 21, 1395–9. [Google Scholar] [CrossRef] [PubMed]
- Lòpez-Otìn, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell. 2013, 153, 1194–217. [Google Scholar] [CrossRef] [PubMed]
- Erichsen, L.; Adjaye, J. Crosstalk between age accumulated DNA-damage and the SIRT1-AKT-GSK3ß axis in urine derived renal progenitor cells. Aging. 2022, 14, 8179–204. [Google Scholar] [CrossRef] [PubMed]
- López-Otìn, C.; Blasco, M.A.; Patridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell. 2023, 186, 243–78. [Google Scholar] [CrossRef] [PubMed]
- Sastre, J.; Pallardò, F.V.; Viña, J. Mitochondrial oxidative stress plays a key role in aging and apoptosis. IUBMB Life. 2000, 49, 427–35. [Google Scholar] [CrossRef] [PubMed]
- Erichsen, L.; Thimm, C.; Bohndorf, M.; Rahman, M.S.; Wruck, W.; Adjaye, J. Activation of the Renin–Angiotensin System Disrupts the Cytoskeletal Architecture of Human Urine-Derived Podocytes. Cells. 2022, 11, 1095. [Google Scholar] [CrossRef] [PubMed]
- Thimm, C.; Erichsen, L.; Wruck, W.; Adjaye, J. Unveiling Angiotensin II and Losartan-Induced Gene Regulatory Networks Using Human Urine-Derived Podocytes. Int J Mol Sci. 2023, 24, 10551. [Google Scholar] [CrossRef]
- Erichsen, L.; Kloss, L.D.F.; Thimm, C.; Bohndorf, M.; Schichel, K.; Wruck, W.; Adjaye, J. Derivation of the immortalized cell line-UM51-PrePodo-hTERT and its responsiveness to Angiotensin II and activation of RAAS. Cells. 2023, 12. [Google Scholar] [CrossRef]
- Barreto-Chaves, M.L.; Mello-Aires, M. Effect of luminal angiotensin II and ANP on early and late cortical distal tubule HCO3- reabsorption. American Journal of Physiology. 1996, 271, F977–84. [Google Scholar] [CrossRef]
- Kwon, T.-H.; Nielsen, J.; Kim, Y.-H.; Knepper, M.A.; Frøkiaer, J.; Nielsen, S. Regulation of sodium transporters in the thick ascending limb of rat kidney: response to angiotensin, I. I. American Journal of Physiology. 2003, 285, F152–65. [Google Scholar] [CrossRef] [PubMed]
- Rocque, B.L.; Babayeva, S.; Li, J.; Leung, V.; Nezvitsky, L.; Cybulsky, A.V.; Gros, P.; Torban, E. Deficiency of the Planar Cell Polarity Protein Vangl2 in Podocytes Affects Glomerular Morphogenesis and Increases Susceptibility to Injury. Journal of the American Society of Nephrology. 2015, 26, 576–86. [Google Scholar] [CrossRef] [PubMed]
- Tojo, A.; Tisher, C.C.; Madsen, K.M. Angiotensin II regulates H(+)-ATPase activity in rat cortical collecting duct. American Journal of Physiology. 1994, 267, F1045–51. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Xu, C.; Zhang, M.; Wang, M.; Min, L.; Qian, C.; Wang, Q.; Ni, Z.; Mou, S.; Dai, H.; Pang, H.; Gu, L. Yes-associated protein regulates podocyte cell cycle re-entry and dedifferentiation in adriamycin-induced nephropathy. Cell Death & Disease. 2019, 10, 915. [Google Scholar]
- Wang, T.; Giebisch, G. Effects of angiotensin II on electrolyte transport in the early and late distal tubule in rat kidney. American Journal of Physiology. 1996, 271, F143–9. [Google Scholar] [CrossRef]
- Zhuo, J.; Maric, C.; Harris, P.J.; Alcorn, D.; Mendelsohn, F. Localization and Functional Properties of Angiotensin II AT1 Receptors in the Kidney: Focus on Renomedullary Interstitial Cells. Hypertension Research. 1997, 20, 233–50. [Google Scholar] [CrossRef] [PubMed]
- Gasparo, M.; Unger, T. International Union of Pharmacology. XXIII. The angiotensin II receptors. Pharmacological Reviews [Internet]. 2000 [cited 2021 Aug 22]. Available from: https://www.researchgate. 1234. [Google Scholar]
- Siragy, H.M. AT1 and AT2 Receptor in the Kidney: Role in Health and Disease. Seminars in Nephrology. 2004, 24, 93–100. [Google Scholar] [CrossRef]
- Ames, M.; Atkins, C.; Pitt, B. The renin-angiotensin-aldosterone system and its suppression. Journal of Veterinary Internal Medicine. 2019, 33, 363–82. [Google Scholar] [CrossRef]
- Berry, C.; Touyz, A.F.; Dominiczak, R.; Webb, C.; Johns, D.G. Angiotensin receptors: signaling, vascular pathophysiology, and interactions with ceramide. AM J Physiol Heart Circ Physiol. 2001, 2337–65. [Google Scholar] [CrossRef]
- Widdop, R.; Jones, E.; Hannan, R.; Gaspari, T. Angiotensin AT2 receptors: cardiovascular hope or hype? British Journal of Pharmacology. 2003, 809–24. [Google Scholar] [CrossRef]
- Nouet, S.; Nahmias, C. Signal Transduction from the Angiotensin II AT2 Receptor. 2000, 1–6.
- Gilliam-Davis, S.; Payne, V.S.; Kasper, S.O.; Tommasi, E.N.; Robbins, M.E.; Diz, D.I. Long-term AT1 receptor blockade improves metabolic function and provides renoprotection in Fischer-344 rats. Am J Physiol Heart Circ Physiol. 2007, 293, H1327–33. [Google Scholar] [CrossRef] [PubMed]
- Herbert, K.E.; Mistry, Y.; Hastings, R.; Poolman, T.; Niklason, L.; Williams, B. Angiotensin II-mediated oxidative DNA damage accelerates cellular senescence in cultured human vascular smooth muscle cells via telomere-dependent and independent pathways. Circ Res. 2008, 102, 201–8. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, L.; Li, Y. Change of telomere length in angiotensin II-induced human glomerular mesangial cell senescence and the protective role of losartan. Mol Med Rep. 2011, 4, 255–60. [Google Scholar] [PubMed]
- Brooks, C.; Gu, W. How does SIRT1 affect metabolism, senescence and cancer? Nat Rev Cancer. 2009, 9, 123–8. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.-F.; Zhai, C.; Yan, X.-L.; Zhao, D.-D.; Wang, J.-X.; Zeng, Q.; Chen, L.; Nan, X.; He, L.-J.; Li, S.-T.; Yue, W.; Pei, X.-T. SIRT1 is required for long-term growth of human mesenchymal stem cells. J Mol Med (Berl). 2012, 90, 389–400. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, M.; Ge, Y.; Wang, X. SIRT1 and aging related signaling pathways. Mech Ageing Dev. 2020, 187, 111215. [Google Scholar] [CrossRef] [PubMed]
- Satoh, A.; Brace, C.S.; Rensing, N.; Clifton, P.; Wozniak, D.; Herzog, E.D.; Yamada, K.A.; Imai, S. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and, L. H. Cell Metab. 2013, 18, 416–30. [Google Scholar] [CrossRef] [PubMed]
- Jun, P.; Chang-ping, H.; Yuan-Jian, L. Reply to ‘Number and function of circulating endothelial progenitor cells and calcitonin gene-related peptide in hypertension: support from and opportunities in Bartter’s and Gitelman’s syndromes patients. ’ Journal of Hypertension. 2010, 28, 2171. [Google Scholar]
- Liu, T.; Yang, Q.; Zhang, X.; Qin, R.; Shan, W.; Zhang, H.; Chen, X. Quercetin alleviates kidney fibrosis by reducing renal tubular epithelial cell senescence through the SIRT1/PINK1/mitophagy axis. 2020, 257, 118116.
- Miyazaki, R.; Ichiki, T.; Hashimoto, T.; Inanaga, K.; Imayama, I.; Sadoshima, J.; Sunagawa, K. SIRT1, a Longevity Gene, Downregulates Angiotensin II Type 1 Receptor Expression in Vascular Smooth Muscle Cells. Arterioscler Thromb Vasc Biol. 2008, 28, 1263–9. [Google Scholar] [CrossRef]
- Jang, I.-A.; Kim, E.N.; Lim, J.H.; Kim, M.Y.; Ban, T.H.; Yoon, H.E.; Park, C.W.; Chang, Y.S.; Choi, B.S. Effects of Resveratrol on the Renin-Angiotensin System in the Aging Kidney. Nutrients. 2018, 10, 1741. [Google Scholar] [CrossRef]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; Moon, R.C.; Pezzuto, J.M. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997, 275, 218–20. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, J.; Rottinghaus, G.E.; Simonyi, A.; Lubahn, D.; Sun, G.Y.; Sun, A.Y. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res. 2002, 958, 439–47. [Google Scholar] [CrossRef] [PubMed]
- Dryden, S.C.; Nahhas, F.; Nowak, J.E.; Goustin, A.-S.; Tainsky, M.A. Role for human SIRT2 NAD-dependent deacetylase activity in control of mitotic exit in the cell cycle. Mol Cell Biol. 2003, 23, 3173–85. [Google Scholar] [CrossRef] [PubMed]
- Blander, G.; Guarente, L. The Sir2 family of protein deacetylases. Annu Rev Biochem. 2004, 73, 417–35. [Google Scholar] [CrossRef] [PubMed]
- Nishikori, S.; Yasuda, J.; Murata, K.; Takegaki, J.; Harada, Y.; Shirai, Y.; Fujita, S. Resistance training rejuvenates aging skin by reducing circulating inflammatory factors and enhancing dermal extracellular matrices. Sci Rep. 2023, 13, 10214. [Google Scholar] [CrossRef] [PubMed]
- Aversa, Z.; White, T.A.; Heeren, A.A.; Hulshizer, C.A.; Saul, D.; Zhang, X.; Molina, A.J.A.; Redman, L.M.; Martin, C.K. ; et. al. Calorie restriction reduces biomarkers of cellular senescence in humans. Aging Cell. 2023, 23. [Google Scholar]
- Dorling, J.L.; Martin, C.K.; Redman, L.M. Calorie restriction for enhanced longevity: The role of novel dietary strategies in the present obesogenic environment. Ageing Res Rev. 2020, 64, 101038. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, E.W.; Most, J.; Mey, J.T.; Redman, L.M. Calorie Restriction and Aging in Humans. Annu Rev Nutr. 2020, 40, 105–33. [Google Scholar] [CrossRef] [PubMed]
- Hurley, B.F.; Hanson, E.D.; Sheaff, A.K. Strength Training as a Countermeasure to Aging Muscle and Chronic Disease. Sports Med. 2012, 41, 289–306. [Google Scholar] [CrossRef]
- Vermeji, W.P.; Dollé, M.E.T.; Reiling, E.; Jaarsma, D.; Payan-Gomez, C.; Bombardieri, C.R.; Wu, H.; Roks, A.J.M.; Botter, S.M.; van der Eerden, B.C.; Youssef, S.A.; Kuiper, R.V.; Nagarajah, B.; et al. Restricted diet delays accelerated ageing and genomic stress in DNA-repair-deficient mice. Nature. 2016, 537, 427–31. [Google Scholar] [CrossRef]
- Neuhauser, H.; Kuhnert, R.; Born, S. 12-Monats-Prävalenz von Bluthochdruck in Deutschland. Journal of Health Monitoring. 2017, 2, 57–63. [Google Scholar]
- Higashi, Y.; Kihara, Y.; Noma, K. Endothelial dysfunction and hypertension in aging. Hypertension Research volume. 2012, 35, 1039–47. [Google Scholar] [CrossRef] [PubMed]
- Buford, T.W. Hypertension and aging. Ageing Research Reviews. 2016, 26, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Zhongjie, S. Aging, Arterial Stiffness, and Hypertensio. Hypertension. 2015, 65, 252–6. [Google Scholar]
- Genestra, M. Oxyl radicals, redox-sensitive signalling cascades and antioxidants. Cell Signal. 2007, 19, 1807–19. [Google Scholar] [CrossRef] [PubMed]
- Salisbury, D.; Bronas, U. Reactive oxygen and nitrogen species: impact on endothelial dysfunction. Nurs Res. 2015, 64, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Rastogi, R.; Geng, X.; Ding, Y. Nicotinamide adenine dinucleotide phosphate oxidase activation and neuronal death after ischemic stroke. Neural Regen Res. 2019, 14, 948–53. [Google Scholar]
- Guo, D.; Liang, S.; Wang, S.; Tang, C.; Yao, B.; Wan, W.; Zhang, H.; Jiang, H.; Ahmed, A.; Zhang, Z.; Gu, Y. Role of epithelial Na+ channels in endothelial function. J Cell Sci. 2016, 129, 290–7. [Google Scholar] [PubMed]
- Bhargava, P.; Schnellmann, R.G. Mitochondrial energetics in the kidney. Nat Rev Nephrol. 2017, 13, 629–46. [Google Scholar] [CrossRef]
- Wang, Z.; Ying, Z.; Bosy-Westphal, A.; Zhang, J.; Schautz, B.; Later, W.; Heymsfield, S.B.; Müller, M.J. Specific metabolic rates of major organs and tissues across adulthood: evaluation by mechanistic model of resting energy expenditure. Am J Clin Nutr. 2010, 92, 1369–77. [Google Scholar] [CrossRef]
- Bhatia, D.; Capili, A.; Choi, M.E. Mitochondrial dysfunction in kidney injury, inflammation, and disease: potential therapeutic approaches. Kidney Res Clin Pract. 2020, 39, 244–58. [Google Scholar] [CrossRef]
- Emma, F.; Montini, G.; Parikh, S.; Salviati, L. Mitochondrial dysfunction in inherited renal disease and acute kidney injury. Nat Rev Nephrol. 2016, 12, 267–80. [Google Scholar] [CrossRef]
- Galvan, D.L.; Green, N.H.; Danesh, F.R. The hallmarks of mitochondrial dysfunction in chronic kidney disease. Kidney International. 2017, 92, 1051–7. [Google Scholar] [CrossRef] [PubMed]
- Honda, T.; Hirakawa, Y.; Nangaku, M. The role of oxidative stress and hypoxia in renal disease. Kidney Res Clin Pract. 2019, 38, 414–26. [Google Scholar] [CrossRef]
- Zhan, M.; Brooks, C.; Liu, F.; Dong, Z. Mitochondrial dynamics: regulatory mechanisms and emerging role in renal pathophysiology. Kidney Int. 2013, 83, 568–81. [Google Scholar] [CrossRef]
- Goto, Y.; Itami, N.; Kajii, N.; Tochimaru, H.; Endo, M.; Horai, s. Renal tubular involvement mimicking Bartter syndrome in a patient with Kearns-Sayre syndrome. J Pediatr. 1990, 116, 904–10. [Google Scholar] [CrossRef] [PubMed]
- Emma, F.; Pizzini, C.; Tessa, A.; Di Giandomenico, S.; Onetti-Muda, A.; Santorelli, F.M.; Bertini, E.; Rizzoni, G. “Bartter-like” phenotype in Kearns-Sayre syndrome. Pediatr Nephrol. 2006, 21, 355–60. [Google Scholar] [CrossRef]
- Gonzalez-Vicente, A.; Hong, N.; Garvin, J.L. Effects of reactive oxygen species on renal tubular transport. Am J Physiol Renal Physiol. 2019, 317, F444–55. [Google Scholar] [CrossRef] [PubMed]
- Kwakernaak, A.J.; Waanders, F.; Slagman, M.C.J.; Dokter, M.M.; Laverman, G.D.; de Boer, R.A.; Navis, G. Sodium restriction on top of renin–angiotensin–aldosterone system blockade increases circulating levels of N-acetyl-seryl-aspartyl-lysyl-proline in chronic kidney disease patients. J Hypertens. 2013, 31, 2425–32. [Google Scholar] [CrossRef]
- Kobori, H.; Nishiyama, A.; Abe, Y.; Navar, L.G. Enhancement of Intrarenal Angiotensinogen in Dahl Salt-Sensitive Rats on High Salt Diet. Hypertension. 2003, 41, 592–7. [Google Scholar] [CrossRef]
- Kocks, M.; Buikema, H.; Gschwend, S.; Boomsma, F.; de Zeeuw, D.; Navis, G. High Dietary Sodium Blunts Effects of Angiotensin-converting Enzyme Inhibition on Vascular Angiotensin I–to–Angiotensin II Conversion in Rats. Journal of Cardiovascular Pharmacology. 2003, 42, 601–6. [Google Scholar] [CrossRef] [PubMed]
- Felder, R.A.; Gildea, J.J.; Xu, P.; Yue, W.; Armando, I.; Carey, R.M.; Jose, P.A. Inverse Salt Sensitivity of Blood Pressure: Mechanisms and Potential Relevance for Prevention of Cardiovascular Disease. Curr Hypertens Rep. 2022, 24, 361–74. [Google Scholar] [CrossRef] [PubMed]
- Virgilio, D.; Calò, L.A.; Favaro, S.; Borsatti, A. Resting and stimulated cytosolic free calcium levels in neutrophils from patients with Bartter’s syndrome. Clin Sci (Lond). 1987, 72, 483–8. [Google Scholar] [CrossRef] [PubMed]
- Verploegen, M.; Vargas-Poussou, R.; Walsh, S.B.; Alpay, H.; Amouzegar, A.; Ariceta, G.; Atmis, B.; Francesco, E.; Nijenhuis, T. Parathyroid hormone and phosphate homeostasis in patients with Bartter and Gitelman syndrome: an international cross-sectional study. Nephrol Dial Transplant. 2022, 37, 2474–86. [Google Scholar] [CrossRef] [PubMed]
- Calò, L.A.; Ceolotto, G.; Milani, M.; Pagnin, E.; van den Heuvel, L.P.; Sartori, M.; Davis, P.A.; Costa, R.; Semplicini, A. Abnormalities of Gq-mediated cell signaling in Bartter and Gitelman syndromes. Kidney Int. 2001, 60, 882–9. [Google Scholar] [CrossRef] [PubMed]
- Calò, L.A.; D’Angelo, A.; Cantaro, S.; Rizzolo, M.; Favaro, S.; Antonello, A.; Borsatti, A. Intracellular calcium signaling and vascular reactivity in Bartter’s syndrome. Nephron. 1996, 72, 570–3. [Google Scholar] [CrossRef]
- Calò, L.A.; Puato, M.; Schiavo, S.; Zanardo, M.; Tirrito, C.; Pagnin, E.; Balbi, G.; Davis, P.A.; Palatini, P.; Pauletto, P. Absence of vascular remodelling in a high angiotensin-II state (Bartter’s and Gitelman’s syndromes): implications for angiotensin II signalling pathways. Nephrology Dialysis Transplantation. 2008, 23, 2804–9. [Google Scholar] [CrossRef]
- Tura, O.; Mills, N.L.; Hadoke, P.W. Does Bartter’s syndrome/Gitelman’s syndrome provide a clinical model for investigating the association between calcitonin gene-related peptide and angiotensin II-mediated senescence of endothelial progenitor cells? J Hypertens. 2010, 28, 2170–1. [Google Scholar] [CrossRef]
- Ravarotto, V.; Simioni, F.; Sabbadin, C.; Pagnin, E.; Maiolino, G.; Armanini, D.; Caló, L.A. Proinflammatory/profibrotic effects of aldosterone in Gitelman’s syndrome, a human model opposite to hypertension. J Endocrinol Invest. 2019, 42, 521–6. [Google Scholar] [CrossRef]
- Atlas, S. The Renin-Angiotensin Aldosterone System: Pathophysiological Role and Pharmacologic Inhibition. J Manag Care Pharm. 2007, 9–20. [Google Scholar] [CrossRef]
- Calò, L.A.; Davis, P.A. Number and function of circulating endothelial progenitor cells and calcitonin gene-related peptide in hypertension: support from and opportunities in Bartter’s and Gitelman’s syndromes patients. J Hypertens. 2012, 28, 2169–70. [Google Scholar] [CrossRef] [PubMed]
- Ben-Porath, I.; Weinberg, R.A. When cells get stressed: an integrative view of cellular senescence. J Clin Invest. 2004, 113, 8–13. [Google Scholar] [CrossRef]
- Sherman, M.H.; Bassing, C.H.; Teitell, M.A. Regulation of cell differentiation by the DNA damage response. Trends Cell Biol. 2011, 21, 312–9. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, H.; Trauzold, A.; Sebens, T.; Deppert, W.; Fölsch, U.R.; Schmidt, W.E. The proliferation-associated early response gene p22/PRG1 is a novel p53 target gene. oncogene. 1998, 16, 2479–87. [Google Scholar] [CrossRef] [PubMed]
- Calò, L.A.; Pagnin, E.; Davis, P.A.; Sartori, M.; Semplicini, A. Oxidative stress-related factors in Bartter’s and Gitelman’s syndromes: relevance for angiotensin II signalling. 2003, 18, 1518–25.
- Pagnin, E.; Paul, D.; Sartori, M.; Semplicini, A.; Pessina, A.C.; Calò, L.A. Rho kinase and PAI-1 in Bartter’s/Gitelman’s syndromes relationship to angiotensin II signaling. Journal of Hypertension. 2004, 22, 1963–9. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.A.; Pagnin, E.; Dal Maso, L.; Caielli, P.; Maiolino, G.; Fusaro, M.; Rossi, G.P.; Calò, L.A. SIRT1, heme oxygenase-1 and NO-mediated vasodilation in a human model of endogenous angiotensin II type 1 receptor antagonism: implications for hypertension. Hypertension Research. 2013, 36, 873–8. [Google Scholar] [CrossRef]
- Morimoto, A.; Uzu, T.; Fujii, T.; Nishimura, M.; Kuroda, S.; Nakamura, S.; Inenaga, T.; Kimura, G. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet. 1997, 350, 1734–7. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, M.H.; Fineberg, N.S.; Fineberg, S.E.; Weinberger, M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001, 37, 429–32. [Google Scholar] [CrossRef]
- Joe, B.; Shapiro, J.I. Molecular mechanisms of experimental salt-sensitive hypertension. J Am Heart Assoc. 2012, 1, e002121. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Kurz, S.; Münzel, T.; Tarpey, T.; Freeman, B.A.; Griendling, K.K.; Harrison, D.G. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996, 97, 1916–23. [Google Scholar]
- Sartori, M.; Parotto, E.; Bonso, E.; Semplicini, A.; Palatini, P.; Pessina, A.C.; Calò, L.A. Autonomic nervous system function in chronic hypotension associated with Bartter and Gitelman syndromes. Am J Kidney Dis. 49: 330–5.
- Harris, P.J.; Young, J.A. Dose-dependent stimulation and inhibition of proximal tubular sodium reabsorption by angiotensin II in the rat kidney. Pflugers Arch. 1977, 367, 295–7. [Google Scholar] [CrossRef] [PubMed]
- Calò, L.A.; Davis, P.A.; Rossi, G.P. Understanding the mechanisms of angiotensin II signaling involved in hypertension and its long-term sequelae: insights from Bartter’s and Gitelman’s syndromes, human models of endogenous angiotensin II signaling antagonism. J Hypertens. 2014, 32, 2109–19. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.D.R.; Antonelou, M.; Sathiananthamoorthy, S.; Rega, M.; Henderson, S.; Ceron-Gutierrez, L.; Barcenas-Morales, G.; Müller, C.A.; Doffinger, R.; Walsh, S.B.; Salama, A.D. Inherited salt-losing tubulopathies are associated with immunodeficiency due to impaired IL-17 responses. Nat Commun. 2020, 11, 4368. [Google Scholar] [CrossRef]
- Rahman, M.S.; Wruck, W.; Spitzhorn, L.-S.; Nguyen, L.; Bohndorf, M.; Martins, S.; Asar, F.; Ncube, A.; Erichsen, L.; Graffmann, N.; Adjaye, J. The FGF, TGFβ and WNT axis Modulate Self-renewal of Human SIX2 + Urine Derived Renal Progenitor Cells. Scientific Reports. 2020, 10, 739. [Google Scholar] [CrossRef] [PubMed]
- Bohndorf, M.; Ncube, A.; Spitzhorn, L.-S.; Enczmann, J.; Wruck, W.; Adjaye, J. Derivation and characterization of integration-free iPSC line ISRM-UM51 derived from SIX2-positive renal cells isolated from urine of an African male expressing the CYP2D6 *4/*17 variant which confers intermediate drug metabolizing activity. Stem Cell Res. 2017, 25, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Qian, R.; Shan, X.; Yang, L.; Chen, H.; Ding, Y.; Chen, C.; Chu, M.; Lin, J.; Wang, D. Generation of a human induced pluripotent stem cell line (WMUi021-A) from a Gitelman syndrome patient carrying a SLC12A3 gene mutation (c.179C > T). Stem Cell Research. 2021, 53.
- Lim, S.W.; Shin, Y.J.; Cui, S.; Ko, E.J.; Lee, K.I.; Lee, J.Y.; Chung, B.H.; Yang, C.W. Generation of a human induced pluripotent stem cell line (CMCi002-A) from a patient with Gitelman’s syndrome. Stem Cell Research. 2020, 49. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-S.; Lo, Y.-F.; Wu, C.-C.; Lin, S.-W.; Yeh, C.-J.; Chu, P.; Sytwu, H.-K.; Uchida, S.; Sasaki, S.; Lin, S.-H. SPAK-Knockout Mice Manifest Gitelman Syndrome and Impaired Vasoconstriction. J Am Soc Nephrol. 2010, 21, 1868–77. [Google Scholar] [CrossRef] [PubMed]
- Nicolet-Barousse, L.; Blanchard, A.; Roux, C.; Pietri, L.; Bloch-Faure, M.; Kolta, S.; Chappard, C.; Geoffroy, V.; Morieux, C.; Jeunemaitre, X.; Shull, G.E.; Meneton, P.; Paillard, M.; et al. Inactivation of the Na-Cl Co-Transporter ( NCC ) Gene Is Associated With High BMD Through Both Renal and Bone Mechanisms: Analysis of Patients With Gitelman Syndrome and Ncc Null Mice. J Bone Miner Res. 2005, 20, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Nomura, N.; Tajima, M.; Sugawara, N.; Morimoto, T.; Kondo, Y.; Ohno, M.; Uchida, K.; Mutig, K.; Bachmann, S.; Soleimani, M.; Ohta, E.; Ohta, A.; Sohara, E.; et al. Generation and analyses of R8L barttin knockin mouse. Am J Physiol-Ren Physiol. 2011, 301, F297–307. [Google Scholar] [CrossRef]
- Matsumura, Y.; Uchida, S.; Kondo, Y.; Miyazaki, H.; Ko, S.B.H.; Hayama, A.; Morimoto, T.; Liu, W.; Arisawa, M.; Sasaki, S.; Marumo, F. Overt nephrogenic diabetes insipidus in mice lacking the CLC-K1 chloride channel. Nat Genet. 1999, 21, 95–8. [Google Scholar] [CrossRef]
- Dong, K.; Yan, Q.; Lu, M.; Wan, L.; Hu, H.; Guo, J.; Boulpaep, E.; Wang, W.; Giebisch, G.; Hebert, S.C.; Wang, T. Romk1 Knockout Mice Do Not Produce Bartter Phenotype but Exhibit Impaired K Excretion. J Biol Chem. 2016, 291, 5259–69. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-H.; Yu, I.-S.; Jiang, S.-T.; Lin, S.-W.; Chu, P.; Chen, A.; Sytwu, H.-K.; Sohara, E.; Uchida, S.; Sasaki, S.; Yang, S.-S. Impaired phosphorylation of Na + -K + -2Cl − cotransporter by oxidative stress-responsive kinase-1 deficiency manifests hypotension and Bartter-like syndrome. Proc Natl Acad Sci. 2011, 108, 17538–43. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





