1. Introduction
The hypoxic environment produces a series of physiological changes in the inhabitants of high-altitude regions due to decreased oxygen availability. In the Andean region, one of the most conspicuous changes is an increase in red blood cell count, accompanied by elevated levels of hemoglobin concentration ([Hb]) and hematocrit (Hct), and subsequent arterial pulmonary hypertension (PHT) [
1], thus preventing those inhabitants from carrying out intense physical activity [
2,
3]. These changes are especially prominent in permanent residents of areas located at more than 5000 m above sea level [
4]. Many physiological and performance evaluations, such as the six-minute walk test (6MWT), do not yield normal values depending on the altitude of residence, especially in very high locations such as La Rinconada, currently considered the highest inhabited city in the world (5100 m). In addition, when applying this test to those high-altitude living patients, it is unknown whether disproportionate increases in [Hb] and Hct values could affect the results. However, despite their prolonged stay under these conditions, several physiological adjustments allow them to fit their physical capacity to this environment. High [Hb] and Hct values typical of this high-altitude acclimatization process could alter the results and generate an erroneous interpretation of the 6MWT results, because of the increased blood viscosity and, as a consequence, a potential decrease in tissue perfusion [
5,
6].
The increase in the number of red blood cells stimulated by hypoxia occurs intending to improve oxygen transport to tissues [
7], however when it exceeds certain values it could have negative effects on tissue oxygenation [
5,
6,
8,
9,
10,
11,
12], especially when they reach high values as is the case of excessive erythrocytosis (EE). When [Hb] levels are above 21 g/dl in men and 19 g/dL in women, and this sign is accompanied by other respiratory, neurological, or cardiovascular symptoms, it constitutes a pathological entity known as chronic mountain sickness (CMS) [
13]. We hypothesize that these hematological changes could interfere with the execution of the 6MWT altering the normal range results and leading to an inappropriate interpretation. Furthermore, they could have a significant impact on daily activities and the development of submaximal physical activity in these individuals.
The main objective of this study is to determine the differences in the 6MWT performance and the hemodynamic response during this test on subjects who permanently reside at more than 5,000 meters of altitude with and without EE. The 6MWT is a simple, low-cost, sensitive test, mainly used in the evaluation and follow-up of the therapeutic response of patients with respiratory and cardiovascular diseases [
12,
14]. We applied this test to evaluate apparently healthy patients with and without EE to determine whether the high increase in [Hb] and Hct interferes with 6MWT performance in a severe hypoxic environment.
2. Materials and Methods
2.1. Participants
The study was carried out in La Rinconada located at more than 5100 meters above sea level in the Puno Region within southern Peru, considered the highest city in the world with more than 60,000 inhabitants [
15]. Seventy-six people, all male, apparently healthy and meeting the inclusion criteria were evaluated.
Information on age, sex, weight, height, length of residence in La Rinconada, and their place of origin was obtained during a collective medical consultation organized by the local Miners’ Association.
For blood sampling, aseptic measures were taken by using an alcohol-moistened cotton swab on the tip of the patient's middle or ring fingers, followed by capillary puncture using a sterile lancet. After removing the lancet, a waiting period allowed for the spontaneous formation of a blood drop. The initial two drops were removed with a cotton swab to prevent errors, ensuring the third drop was of sufficient volume to fill a micro cuvette for [Hb] measurement and a capillary tube for Hct determination. The microcuvette with the blood sample was positioned for analysis using a HEMOCUE HB 201+ portable hemoglobinometer, employing the azide methemoglobin method within a measurement range of 0 to 25.6 g/dL. Hematocrit measurements were conducted using a Hemata Stat II microcentrifuge from EKF Diagnostics, involving the placement of the tube for centrifugation and subsequent measurements. The current international consensus on chronic mountain sickness [
13] was used to consider a case of excessive erythrocytosis (EE).
2.2. Six-Minute Walking Test
The 6MWT was performed according to the protocol established by the American Thoracic Society [
16]. The individuals responsible for implementing the test were trained to avoid errors during the examination. The test was carried out in a large environment consisting of a 60-meter-long course without any obstacles or conditions that could alter the examination. Vital sign measurements included the following: heart rate (HR), oxygen saturation (SpO
2), systolic blood pressure (SBP), and diastolic blood pressure (DBP). For the initial measurements, the subjects rested for 10 minutes in the sitting position. After, the subjects immediately performed the 6MWT. Recordings were made just at the end of the test and after 3 and 5 minutes of recovery.
The Borg scale was used to evaluate the perception of dyspnea and fatigue. A Nellcor® Oximax® N-65 pulse oximeter with a resolution of 1% saturation and a heart rate range of 30–235 beats per minute was placed on the index finger. A Riester brand Ri-Champion automated adult digital blood pressure monitor was used to measure blood pressure with an arm sphygmomanometer able to measure from 30 to 280 mm and a heart rate from 40 to 200 beats per minute with accuracy for blood pressure within ±3 mmHg and pulse ±5%. A Camry model EB9068-59 digital scale and a measuring rod were used to estimate the weight and height of the subjects evaluated and subsequently calculate the body mass index (BMI). The measuring instruments were previously calibrated, and the respective verification was made by comparing the measurements with similar instruments [
12]. At the end of the test, the distance traveled in 6 minutes was recorded.
2.3. Statistical Analysis
Descriptive data are reported as mean ± standard deviation. The Student’s t-test was used to determine the significance of the difference between the two groups. The effect size was estimated by Cohen’s d, which is determined by calculating the mean difference between your two groups, and then dividing the result by the pooled standard deviation. An α level of 0.05 was established for testing null hypotheses. Data analysis was performed using the IBM SPSS v26 statistical package.
3. Results
Seventy-six young healthy subjects agreed to participate in this study, but finally, 71 subjects completed the protocol. All of the subjects were male: 36 had no EE while 35 presented with EE. The subjects without EE had a BMI of 26.71 ± 3.45 kg/m
2 which was similar to subjects with EE (BMI of 26.79 ± 3.34 kg/m
2). The control group's average [Hb] and Hct levels were 18.7 ± 1.2 g/dL and 60.4 ± 7.1%, respectively. Major differences were expectedly observed with [Hb] and Hct in subjects with EE, showing an average [Hb] level of 23.4 ± 1.6 g/dL and a Hct level of 73.6 ± 5.9% were observed (p = 0.009). No significant differences were observed in the other variables studied (
Table 1).
Blood pressure and heart rate in all of the subjects were also evaluated at rest and before testing their sub-maximal physical capacity. Resting SBP values were higher in patients with EE by approximately 10 mmHg (
Table 2); however, they did not exceed the values considered normal (120/80 mmHg). Statistical significance was marginal between individuals with EE and the control groups (p = 0.052). Likewise, DBP values remained within normal limits with no statistical difference in both groups (p = 0.395). The baseline vital signs showed similarities in some variables measured between subjects with and without EE (
Table 2). The SpO
2 variance was also similar in both groups; however, subjects with EE maintained lower SpO
2 levels as compared to control subjects (p = 0.001).
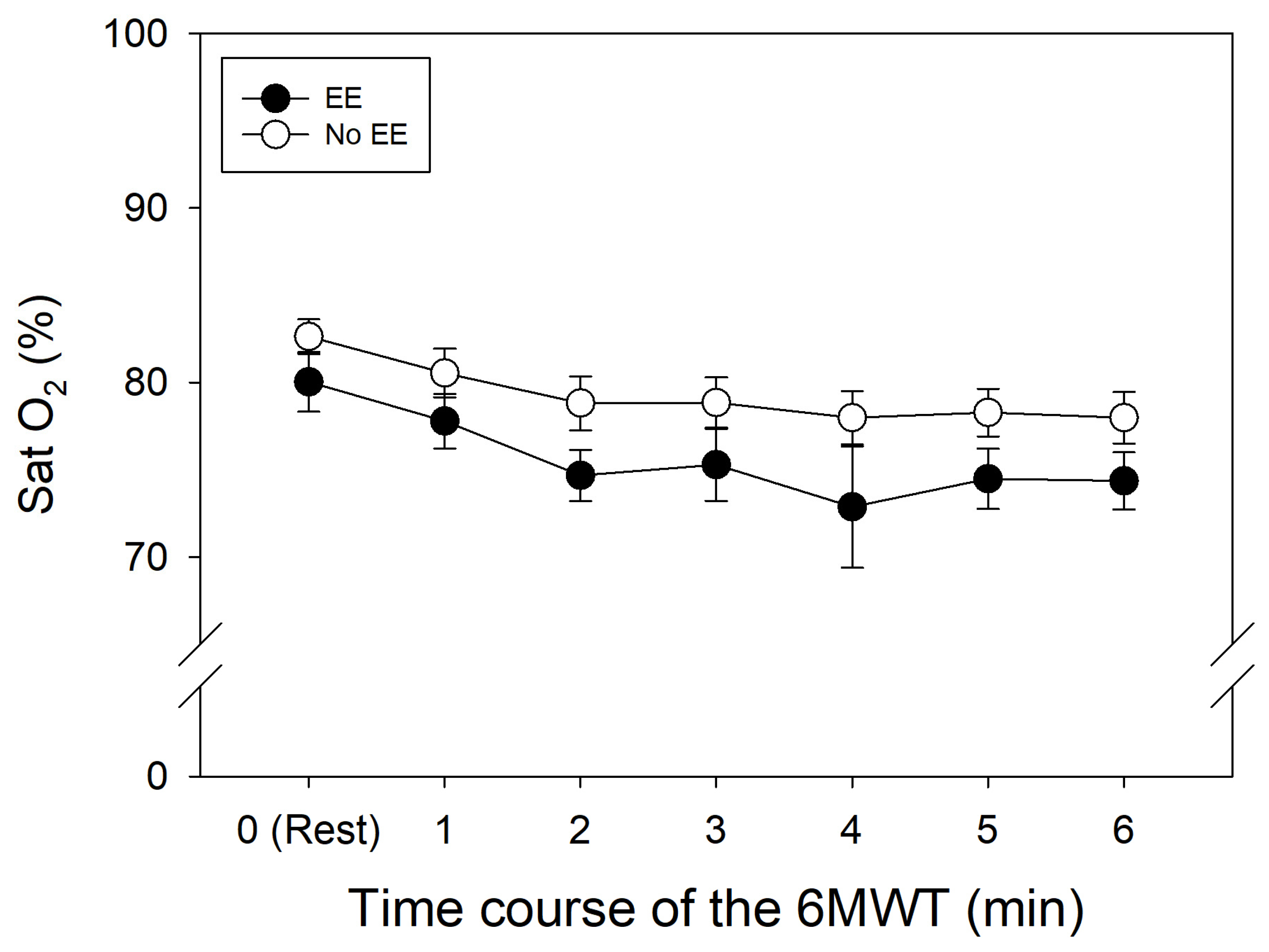
SpO
2 levels in the control and EE groups decreased along the test, especially in the first two minutes, and showed similar drops (
Figure 1).
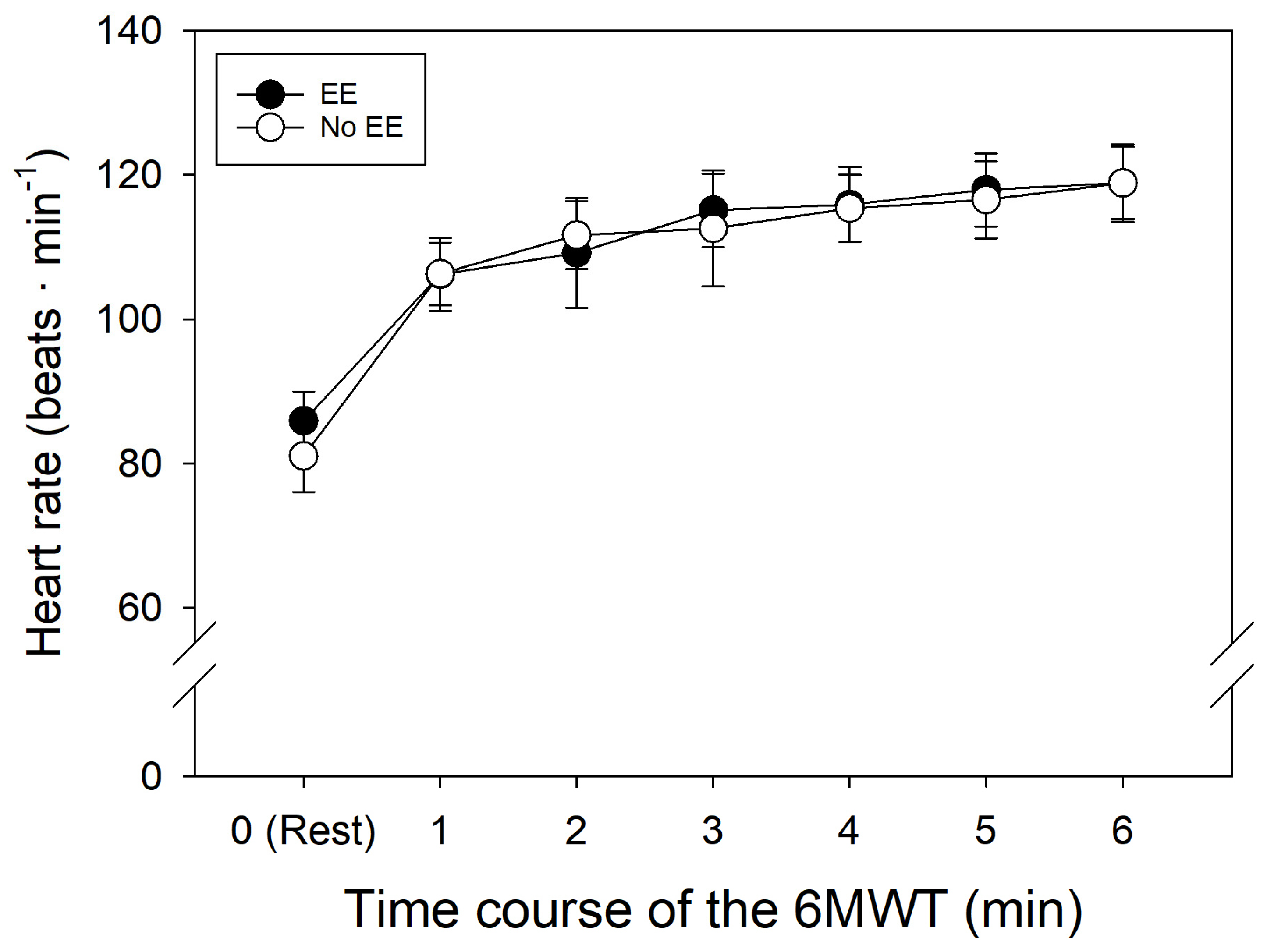
On the other hand, as expected, HR progressively increased in both of the groups during the 6MWT (
Figure 2).
Table 6. MWT did not offer relevant differences. The total distance traveled was 503 ± 68 m in subjects without EE and 494.6 ± 66 m in subjects with EE (
Table 3), which represents only a 1.58% improvement in the control group relative to the subjects with EE. This slight difference did not reach statistical significance (p = 0.433). These results are slightly worse than the reference parameters near sea level (~600 m), [
17]. The sensation of dyspnea and fatigue was similar in both groups and the Borg scale showed that subjects with EE may have a lower sensation of dyspnea than subjects without EE (
Table 3). Although these parameters never reached statistical significance, maybe a higher sample size may provide further insight into the differences observed for dyspnea and fatigue perception.
4. Discussion
One of the procedures used very frequently for the evaluation and monitoring of patients with cardiac and respiratory diseases, as well as the evaluation of their response to treatment, is the 6MWT [
18], whose reference values have been developed for low altitudes, however, despite their wide usefulness, there is no information about values in severe hypoxic environments such as the case of La Rinconada, located at more than 5,000 meters of altitude. In the present work, we have carried out this procedure to determine possible changes in performance when this procedure is carried out in conditions of severe hypoxia and on the other hand to evaluate if the excessive increase in [Hb] and Hct, as in the case of EE, could alter the results of this test.
La Rinconada is a city located above 5,100 m with many of the residents working in the existing gold mines in the area. Here, the prevalence of EE is 44.1% and 13.9% for CMS [
19]. The residents with EE are chiefly characterized by the increase in [Hb] and Hct levels, which could limit the work performance of these individuals.
The findings in the present study indicate that in subjects with and without EE, the performance in 6MWT was similar and that the high-altitude environment may not have a major role in altering the response to 6MWT. These findings are in line with previous reports that hypobaric hypoxia does not alter the hemodynamic response during physical effort [
20,
21] probably due to the acclimatization process in these subjects since they remain in these places for long periods. The results in this study indicate that DBP and SBP values were slightly increased in the subjects with EE relative to the control group and that the 6MWT did not impact these parameters likely due to adaptive responses to the high-altitude environment [
22]. These modest differences may allow the residents to lead a normal daily life potentially through physiological changes inherent to the altitude adaptation process, probably including a reduction in peripheral resistance that allows blood perfusion to be maintained in the organs where a high level of oxygenation is required during physical activity [
23].
Despite similar basal HR and final HR during the 6MWT in both groups (p = 0.228), the increased levels of [Hb] and Hct in subjects with EE do not alter their response to submaximal physical activity, which allows them to carry out their daily tasks adequately in a hypoxic environment [
23]. However, oxygen saturation was slightly lower in the subjects with EE (
Figure 1). This could be due to reduced capillary transit time in lung vessels, due to the usual pulmonary hypertension associated with the EE condition [
13].
While no statistically significant differences were observed between the two groups, the dyspnea score evaluated through the Borg scale tended to be lower in subjects with EE relative to the control group (Table 4) (p = 0.27). Fatigue was also similarly reported in both groups (p = 0.33), suggesting that subjects with EE have a similar effort perception to subjects without EE, during the 6MWT.
The overall findings in this study allow us to illustrate how the human body can adapt to severe environmental conditions. In high-altitude regions, such as here presented in La Rinconada, low barometric pressure limits the uptake of oxygen and decreases the alveolar-arterial oxygen gradient. However, compensation mechanisms are activated to favor oxygen uptake at the cellular level, allowing an adequate hemodynamic response during physical activity [
24]. Despite the excessive increase in [Hb] and Hct and a lower level of SpO
2, the residents can carry out their daily activities without major complications [
18,
25]. This is not surprising because excessive erythrocytosis as identified via direct RBCV determination was found to not be a clinical sign of CMS among the residents of La Rinconada [
26]. Although the 6MWT test is generally used for the follow-up and the prognosis of cardio-respiratory diseases, this test allowed us to evaluate the potential impact of excessive increases in [Hb] and Hct on the physical capacity of these high-altitude residents. We conclude that subjects with EE do not present marked limitations in carrying out their daily activities compared to subjects with normal [Hb] and Hct values. Our findings agree with previous studies which show that there is a slight decrease in physical capacity in subjects with CMS at 4300 m [
27]. The present study has been carried out in a population located at more than 5100 m. However, we observe only a slight decrease in performance during the 6MWT, as compared to the results that are considered normal in regions at lower altitudes.
Our study is not free of limitations, the 6MWT is a simple, reproducible, and easy-to-execute method to evaluate patients with cardiac or respiratory diseases and indirectly measures the submaximal physical capacity. However, further studies measuring the maximum oxygen consumption could allow us to better understand the physical capacity of these individuals living in a severely hypoxic environment. We have not been able to evaluate female subjects since they did not agree to participate in the study or because their [Hb] and Hct values are within normal values and cannot be classified as EE for women of reproductive age. We also highlight that there is a difference of approximately 4 years in age between the control group and the group of patients with EE (47 vs. 43 years respectively), which could affect the final results of the 6MWT. However, no major difference has been observed in those variables that could be altered by age. Finally, the participants in the present study did not have cardiac or respiratory diseases, which has allowed us to adequately evaluate whether the increase in [Hb] and Hct values, occurring in patients with EE, whether or not altered the results of the 6MWT.
5. Conclusions
The results of this study indicate that the reference values used at sea level in the 6MWT can be valid and indicative for the evaluation of people who live permanently at high altitudes independently of their Hct and [Hb] values. EE does not significantly alter the results of 6MWT of subjects who live permanently in a severe hypoxic environment, without relevant hemodynamic changes during the test.
Author Contributions
Conceptualization: K.M.V.C, M.Y., G.V., and I.H.; methodology: K.M.V.C., R.A.R.C, J.T.F., and V.V.; formal analysis: K.M.V.C., M.Y., J.T.F., and I.H.; investigation: K.M.V.C, R.A.R.C, J.T.F, H.O.T.R., M.M.Q.T., S.A.Q.H., Y.M.P.V., A.G.C.H., and I.H.; resources: M.Y., G.F.P.V., A.A.S.G., and I.H.; data curation: G.V., K.M.V.C, R.A.R.C, J.T.F, H.O.T.R., M.Y. and I.H.; writing—original draft preparation: K.M.V.C., J.T.F., and I.H..; writing—review and editing: G.V, M.Y., K.M.V.C., J.T.F., M.Y., and I.H.; visualization: K.M.V.C., J.T.F., and I.H.; supervision: J.T.F. and I.H.; project administration: I.H.; funding acquisition: M.Y., G.F.P.V., A.A.S.G., and I.H. All authors have read, reviewed, and agreed to the published version of the article.
Funding
The study was self-financed. M.Y. is supported by the National Institute of Health National Heart Lung and Blood grant K99 HL164888, the American Society of Hematology Scholar Award, the Eleanor and Miles Shore Faculty Development Award, and a research award from the Foundation for Women’s Wellness.
Institutional Review Board Statement
The study was conducted following the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the corresponding authors, upon reasonable request.
Acknowledgments
We thank the authorities of the La Rinconada Minor Population Center, the Miners Association, and all the institutions that promote permanent health control in this place. We also thank the study participants for their time and for their great willingness to collaborate.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sydykov, A.; Mamazhakypov, A.; Maripov, A.; Kosanovic, D.; Weissmann, N.; Ghofrani, H.A.; Sarybaev, A.S.; Schermuly, R.T. Pulmonary Hypertension in Acute and Chronic High Altitude Maladaptation Disorders. Int J Environ Res Public Health 2021, 18(4)1692. [CrossRef] [PubMed]
- Beidleman, B.A.; Fulco, C.S.; Cadarette, B.S.; Cymerman, A.; Buller, M.J.; Salgado, R.M.; Posch, A.M.; Staab, J.E.; Sils, I.V.; Yurkevicius, B.R.; Luippold, A.J.; Welles, A.P.; Muza, S.R. Is normobaric hypoxia an effective treatment for sustaining previously acquired altitude acclimatization? J Appl Physiol 2017, 123(5):1214-27. [CrossRef] [PubMed]
- Siebenmann, C.; Robach, P.; Lundby, C. Regulation of blood volume in lowlanders exposed to high altitude. J Appl Physiol 2017, 123(4):957-66. [CrossRef] [PubMed]
- Farias, J.G.; Jimenez, D.; Osorio, J.; Zepeda, A.B.; Figueroa, C.A.; Pulgar, V.M. Acclimatization to chronic intermittent hypoxia in mine workers: a challenge to mountain medicine in Chile. Biol Res 2013 46(1):59-67. [CrossRef]
- Solway, S.; Brooks, D.; Lacasse, Y.; Thomas, S. A Qualitative Systematic Overview of the Measurement Properties of Functional Walk Tests Used in the Cardiorespiratory Domain. Chest 2001, 119(1):256-70. [CrossRef] [PubMed]
- Enright, P.L.; McBurnie, M.A.; Bittner, V.; Tracy, R.P.; McNamara, R.; Arnold, A.; Newman, A.B.; Cardiovascular Health Study. The 6-min walk test: a quick measure of functional status in elderly adults. Chest 2003, 123(2):387-98.
- West, J.B. Oxygen Conditioning: A New Technique for Improving Living and Working at High Altitude. Physiology 2016, 31(3):216-22. [CrossRef] [PubMed]
- Vona, M.; Mazzuero, G.; Lupi, A.; Vettorato, C.; Bosso, P.; Cohen-Solal, A. Effects of altitude on effort tolerance in non-acclimatized patients with ischemic left ventricular dysfunction. Eur J Cardiovasc Prev Rehabil 2006, 13(4):617-24. [CrossRef] [PubMed]
- Lazio, M.P.; Van Roo, J.D.; Pesce, C.; Malik, S.; Courtney, D.M. Postexercise peripheral oxygen saturation after completion of the 6-minute walk test predicts successfully reaching the summit of Aconcagua. Wilderness Environ Med 2010, 21(4):309-17. [CrossRef] [PubMed]
- Beatty, A.L.; Schiller, N.B.; Whooley, M.A. Six-Minute Walk Test as a Prognostic Tool in Stable Coronary Heart Disease: Data from the Heart and Soul Study. Arch Intern Med 2012,172(14):1096-102. [CrossRef] [PubMed]
- Polkey, M.I.; Spruit, M.A.; Edwards L.D.; Watkins, M.L.; Pinto-Plata, V.; Vestbo, J.; Calverley, P.M.; Tal-Singer, R.; Agustí, A.; Bakke, P.S.; Coxson, H.O.; Lomas, D.A,; MacNee, W.; Rennard, S.; Silverman, E.K.; Miller, B.E.; Crim, C.; Yates, J.; Wouters, E.F.; Celli, B; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Study Investigators. Six-Minute-Walk Test in Chronic Obstructive Pulmonary Disease: Minimal Clinically Important Difference for Death or Hospitalization. Am J Respir Crit Care Med 2013, 187(4):382-6.
- Lang, M.; Faini, A.; Caravita, S.; Bilo, G.; Anza-Ramìrez, C.; Villafuerte, F.C.; Macarlupu, J.L.; Salvioni, E.; Agostoni, P. Parati, G. Blood pressure response to six-minute walk test in hypertensive subjects exposed to high altitude: effects of antihypertensive combination treatment. Int J Cardiol 2016, 219:27-32.
- León-Velarde, F.; Maggiorini, M.; Reeves, J.T.; Aldashev, A.; Asmus, I.; Bernardi, L.; Ge, R.L.; Hackett, P.; Kobayashi, T.; Moore, L.G.; Penaloza, D.; Richalet, J.P.; Roach, R.; Wu, T.; Vargas, E.; Zubieta-Castillo, G.; Zubieta-Calleja, G. Consensus statement on chronic and subacute high altitude diseases. High Alt Med Biol 2005, 6(2):147-57. [CrossRef] [PubMed]
- Deboeck, G.; Nisset, G.; Vachiery, J.L.; Moraine, J.J.; Naejie, R. Physiological response to the six-minute walk test in pulmonary arterial hypertension. Eur Respir J 2005, 26(4):667-72. [CrossRef] [PubMed]
- Enserink, M. Hypoxia city. Science 2019 365(6458):1098-103. [CrossRef]
- American Thoracic Society Statement. Guidelines for the Six-Minute Walk Test. Am J Respir Crit Care Med 2002, 166(1):111-7.
- Gochicoa-Rangel, L.; Mora-Romero, U.; Guerrero-Zúñiga, S.; Silva-Cerón, M.; Cid-Juárez, S.; Velázquez-Uncal, M.; Durán-Cuéllar, A.; Salas-Escamilla, I.; Mejía-Alfaro, R.; Torre-Bouscoulet, L. Prueba de caminata de 6 minutos: recomendaciones y procedimientos. Neumol Cir Torax 2015, 74(2):127-136. [CrossRef]
- Bilo, G.; Caldara, G.; Styczkiewicz, K.; Revera, M.; Lombardi, C.; Giglio, A.; Zambon, A.; Corrao, G.; Faini, A.; Valentini, M.; Mancia, G.; Parati, G. Effects of selective and nonselective beta-blockade on 24-h ambulatory blood pressure under hypobaric hypoxia at altitude. J Hypertens 2011, 29(2):380-7. [CrossRef] [PubMed]
- Hancco, I.; Bailly, S.; Baillieul, S.; Doutreleau, S.; Germain, M.; Pépin, J.L.; Verges, S. Excessive Erythrocytosis and Chronic Mountain Sickness in Dwellers of the Highest City in the World. Front Physiol 2020, 11:773. [CrossRef] [PubMed]
- Bilo, G.; Villafuerte, F.C.; Faini, A.; Anza-Ramírez, C.; Revera, M.; Giuliano, A.; Caravita, S.; Gregorini, F.; Lombardi, C.; Salvioni, E.; Macarlupu, J.L.; Ossoli, D.; Landaveri, L.; Lang, M.; Agostoni, P.; Sosa, J.M.; Mancia, G.; Parati, G. Ambulatory Blood Pressure in Untreated and Treated Hypertensive Patients at High Altitude: The High Altitude Cardiovascular Research–Andes Study. Hypertension 2015, 65(6):1266-72. [CrossRef] [PubMed]
- Theunissen, S.; Balestra, C.; Bolognési, S.; Borgers, G.; Vissenaeken, D.; Obeid, G.; Germonpré, P.; Honoré, P.M.; De Bels, D. Effects of Acute Hypobaric Hypoxia Exposure on Cardiovascular Function in Unacclimatized Healthy Subjects: A “Rapid Ascent” Hypobaric Chamber Study. Int J Environ Res Public Health 2022, 19(9):5394. [CrossRef] [PubMed]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; Galderisi, M.; Grobbee, D.E.; Jaarsma, T.; Kirchhof, P.; Kjeldsen, S.E.; Laurent, S.; Manolis, A.J.; Nilsson, P.M.; Ruilope, L.M.; Schmieder, R.E.; Sirnes, P.A.; Sleight, P.; Viigimaa, M.; Waeber, B.; Zannad, F.; Redon, J.; Dominiczak, A.; Narkiewicz, K.; Nilsson, P.M.; Burnier, M.; Viigimaa, M.; Ambrosioni, E.; Caufield, M.; Coca, A.; Olsen, M.H.; Schmieder, R.E.; Tsioufis, C.; van de Borne, P.; Zamorano, J.L.; Achenbach, S.; Baumgartner, H.; Bax, J.J.; Bueno, H.; Dean, V.; Deaton, C.; Erol, C.; Fagard, R.; Ferrari, R.; Hasdai, D.; Hoes, A.W.; Kirchhof, P.; Knuuti, J.; Kolh, P.; Lancellotti, P.; Linhart, A.; Nihoyannopoulos, P.; Piepoli, M.F.; Ponikowski, P.; Sirnes, P.A.; Tamargo, J.L.; Tendera, M.; Torbicki, A.; Wijns, W.; Windecker, S.; Clement, D.L.; Coca, A.; Gillebert, T.C.; Tendera, M.; Rosei, E.A.; Ambrosioni, E.; Anker, S.D.; Bauersachs, J.; Hitij, J.B.; Caulfield, M.; De Buyzere, M.; De Geest, S.; Derumeaux, G.A.; Erdine, S.; Farsang, C.; Funck-Brentano, C.; Gerc, V.; Germano, G.; Gielen, S.; Haller, H.; Hoes, A.W.; Jordan, J.; Kahan, T.; Komajda, M.; Lovic, D.; Mahrholdt, H.; Olsen, M.H.; Ostergren, J.; Parati, G.; Perk, J.; Polonia, J.; Popescu, B.A.; Reiner, Z.; Rydén, L.; Sirenko, Y.; Stanton, A.; Struijker-Boudier, H.; Tsioufis, C.; van de Borne, P.; Vlachopoulos, C.; Volpe, M.; Wood, D.A. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013, 34(28):2159-219. [PubMed]
- Savonitto, S.; Cardellino, G.; Doveri, G.; Pernpruner, S.; Bronzini, R.; Milloz, N.; Colombo, M.D.; Sardina, M.¸Nassi, G.; Marraccini P. Effects of acute exposure to altitude (3,460 m) on blood pressure response to dynamic and isometric exercise in men with systemic hypertension. Am J Cardiol 1992, 70(18):1493-7.
- West, J.B. Physiological Effects of Chronic Hypoxia. N Engl J Med 2017, 376(20):1965-71. [CrossRef] [PubMed]
- Valentini, M.; Revera, M.; Bilo, G., Caldara, G.; Savia, G.; Styczkiewicz, K.; Parati, S.; Gregorini, F.; Faini, A.; Branzi, G.; Malfatto, G., Magrì, D.; Agostoni, P.; Parati, G. Effects of Beta-Blockade on Exercise Performance at High Altitude: A Randomized, Placebo-Controlled Trial Comparing the Efficacy of Nebivolol versus Carvedilol in Healthy Subjects. Cardiovasc Ther 2012, 30(4):240-8.
- Oberholzer, L.; Lundby, C.; Stauffer, E.; Ulliel-Roche, M.; Hancco, I.; Pichon, A.; Lundby, A.M.; Villafuerte, F.C.; Verges, S.; Robach, P. Reevaluation of excessive erythrocytosis in diagnosing chronic mountain sickness in men from the world's highest city Blood 2020, 136(16):1884-1888.
- Groepenhoff, H.; Overbeek, M.J., Mulè, M.; van der Plas, M.; Argiento, P.; Villafuerte, F.C.; Beloka, S.; Faoro, V.; Macarlupu, J.L.; Guenard, H.; De Bisschop, C.; Martinot, J.B.; Vanderpool, R.; Penaloza, D.; Naeije, R. Exercise Pathophysiology in Patients with Chronic Mountain Sickness. Chest 2012, 142(4):877-884.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).