1. Introduction
1.1. Organic Photovoltaics (OPV)
Since few years ago, research in Organic Photovoltaic Solar Cells (OPV) is increasing because of the importance of renewable energy sources nowadays. This technology is interesting because, comparing with the traditional materials used for solar cells, OPV can be fabricated on flexible substrates, making them lightweight and potentially suitable for applications where traditional rigid solar panels are not practical [
1]. Also, they can be produced using low-cost printing techniques, as an inkjet or roll-to-roll printing [
2,
3]. This allows for large-scale and potentially cost-effective manufacturing processes compared to the more complex and expensive methods used for silicon and perovskite solar cells. The materials used in OPV are generally more abundant and less toxic than some of the materials used in silicon and perovskite solar cells [
4,
5]. This can have positive implications for both environmental impact and resource availability. Organic solar cells offer greater versatility in terms of design. Organic solar cells offer greater versatility in terms of design [
6,
7]. The molecular structures of organic materials can be modified to optimize properties such as absorption spectra and charge transport. OPV can be used in tandem with other types of solar cells, including silicon and perovskite cells, to create tandem solar cells [
8]. Tandem structures have the potential to improve overall efficiency by capturing a broader spectrum of sunlight [
9]. However, it’s crucial to acknowledge some challenges associated with organic solar cells, including lower efficiency compared to silicon and perovskite cells, stability issues, and a shorter lifespan. Research is ongoing to address these challenges and improve the performance of organic solar cells. In this paper is discussed one method to stabilize and improve the efficiency of a classic inverted organic solar cell. The Bulk-Heterojunction active layer (BHJ) used for this work is poly(3-hexylthiophene)/ [6,6]-phenyl-C61-butyric acid methyl ester (P3HT/PC
60BM). This blend is commonly used in the field of organic solar cells due to its favourable properties, such as good charge transport and efficient light absorption [
10]. This model system is often used for studying the effects of various parameters, including additives [
11], on morphology optimization [
12], and the control of recombination dynamics [
13].
The devices have been fabricated according to the structure of inverted OSCs. In the recent years, compared to the direct configuration, the inverted architecture has drawn academic interest in the field of organic solar cells, due to the overcoming of some issues, such as device stability, affected by the acidic and hygroscopic properties of PEDOT:PSS when in direct contact with ITO, and the use of low-work function metals as top electrodes, with the tendency to oxidate when exposed to air and water vapor [
14]. Moreover, the increase of vertical phase separation [
15], improved lifetime and compatibility with R2R fabrication processes [
16] make the inverted design extremely promising regarding the commercialization of OSCs [
17]. All these features have led to choose, in the present work, the use of the above-mentioned architecture.
1.2. Mechanism of Generation of Photocurrent in Organic Solar Cells (OSC)
The photocurrent generation in OPV is based on dissociation into free charges of Frenkel- type excitons [
18]. Initially, the excited electron on LUMO and the corresponding hole on HOMO, resulting from the absorption of an incident photon, are strongly attracted each other, due to Coulomb force [
19]. In order to generate photocurrent, it is mandatory that the exciton dissociates into free charges. For this reason, a critical aspect of the organic solar cells regards the design and architecture of the active layer [
18], typically a bulk-heterojunction constituted by electron-donor (D) material and electron-acceptor (A) material [
20]. Once the exciton is photogenerated in the donor material, it diffuses until its electron finds an energy level lower than the LUMO it belongs to, i.e the LUMO of the acceptor material. Due to the energy gap between the two LUMOs, the exciton dissociates and ideally the electron separates from the hole it has left behind in the HOMO of the donor material [
21]. The two charges are free to move and can be transported and collected at the electrodes [
22]. Nevertheless, the generation of photocurrent is not assured upon the absorption of a photon within the photoactive layer of an organic solar cell. Excitons are neutral systems, not influenced by an electrical field and moving randomly. They need to reach the D/A interface and separate fast, before the so-called geminate charge recombination (CR) occurs [
18]. For this reason, the phase separation of donor/acceptor semiconductors in the BHJ has to be comparable to the exciton diffusion length L = (Dτ)
1/2, where D is the coefficient of diffusion and τ is the lifetime of the exciton [
23]. A trade-off is required between an efficient collection of photogenerated carriers without recombination (thin layers) and an efficient absorption of the light (thick layer) [
24].
1.3. Effect of Singlet and Triplet States in Organic Solar Cells
As mentioned before, the interpenetrating system (BHJ) consisting of donor and acceptor materials, mixed together, assures a level of morphology that facilitates the photogenerated excitons to dissociate to free charge carriers at the donor/acceptor (D/A) interfaces [
25].
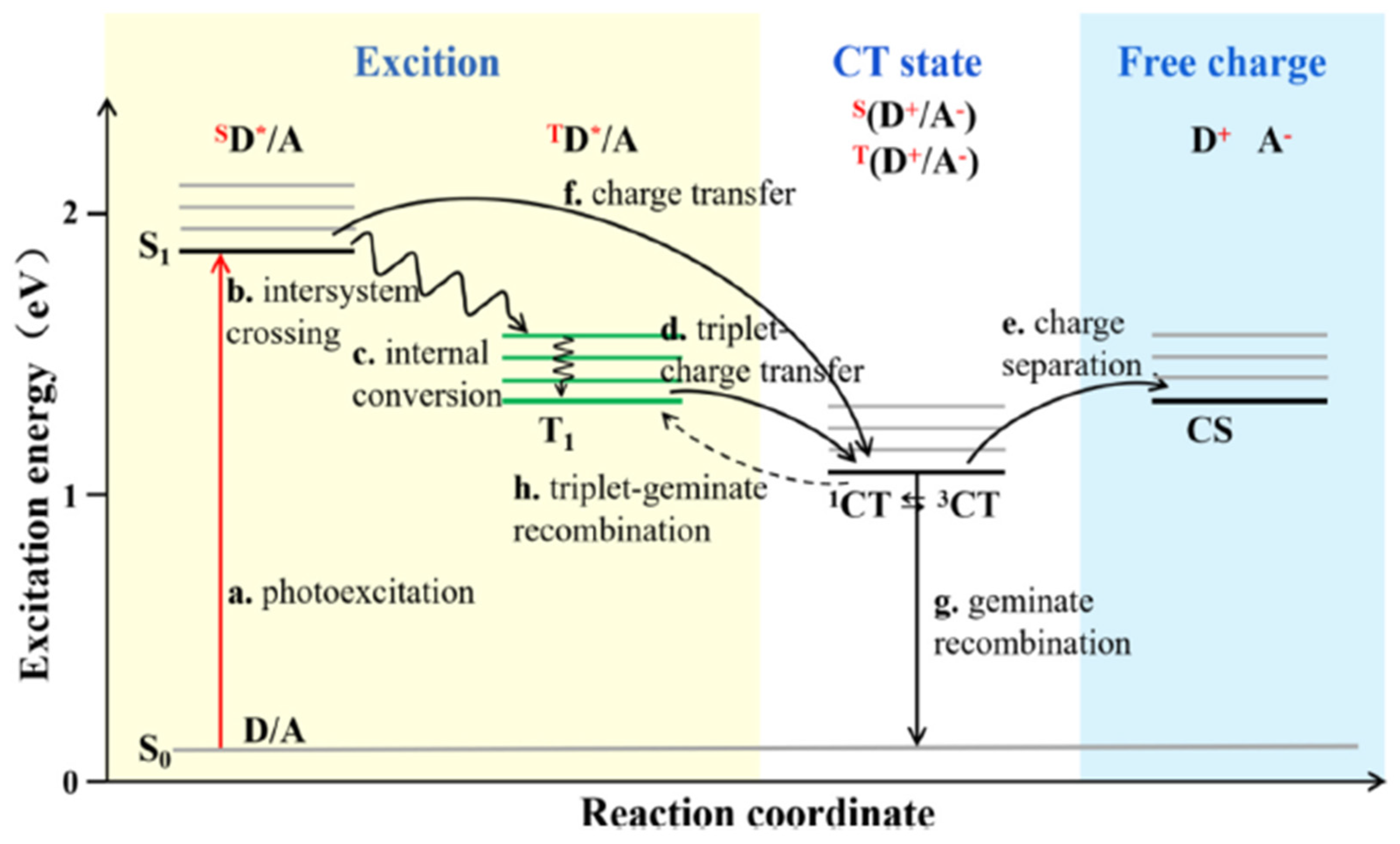
If the process does not occur rapidly, the borderline transient system (charge transfer state - CT) created by the electron on the LUMO of the acceptor material and the hole on the HOMO of the donor material annihilate, producing CR [
26]. The geminate recombination and the exciton dissociation compete each other and the prevalence of one or other determine the performance of the organic solar cells. For instance, it is expected a decrease of photogenerated current and so efficiency decrease of OSCs if the rate of CR exceeds the rate of generation of free charges [
27]. It must be clarified that, generally, due to the selection rule in the electronic dipole transition process, excitons directly formed by absorbing photons are singlets (spin character S = 0) [
28] For singlets the diffusion length is around 10 nm [
29]. This value, compared to the reasonable thickness of bulk hetero-junction for efficient absorption of light, can limit the probability of exciton dissociation process and PCE of organic solar cells [
30]. A consolidated strategy to prevent geminate recombination from CT, related to a single state, is the introduction of triplet charge transfer (3CT) states, related to triplet states (S =1) [
25]. Triplet state excitons have more extended lifetimes than singlet excitons [
21]. Accordingly, thanks to higher diffusion lengths, it occurs sufficient time for CT state to dissociate the bound charge pair, reducing recombination. Moreover, the charge recombination from the triplet charge transfer state (3CT) to the primary triplet excited state (T1) of donor materials (shown in
Figure 1), can be avoided if the energy level of 3CT is lower than that of T1 [
25].
1.4. Organometallic Complexes in OSC
Recently, a series of studies have been reported the electronic contribution of organometallic molecules in OPV [
31]. Several studies have reported the beneficial effect of organometallic complexes in triplet state in solar cells [
32]. Triplet states do not participate in the charge separation of low-gap polymers; it can indirectly affect the efficiency of charge separation by providing direct charge recombination to the low triplet state.[
33] The emission state is a triplet state, but the accurate expression of the character of the triplet state has long been a hot issue under investigation [
34,
35]. First, the states dominated by singlets and triplets are coupled through fast intersystem crossing (50 fs) through their metal-to-ligand charge transfer components [
36,
37]. Second, the triplet state decay is collected, improving the conversion efficiency [
38].
N-heterocyclic carbenes (NHCs) are very versatile ligands in transition-metal chemistry [
39]. Their electronic and steric properties are unique to stabilize a large variety of transition-metal complexes with notable applications in catalysis and materials science that have been extensively studied in the last three decades. Although some NHC metal complexes have emissive properties [
40], palladium(II) emitters have typically low radiative recombination ratesIn contrast, Zysman-Colman and co-workers reported highly luminescent
d10 palladium(0) complexes of formula [Pd(NHC)
2]. [
41] Nonetheless, practical applications of these palladium(0) complexes are limited by their easy oxidation under aerobic conditions [
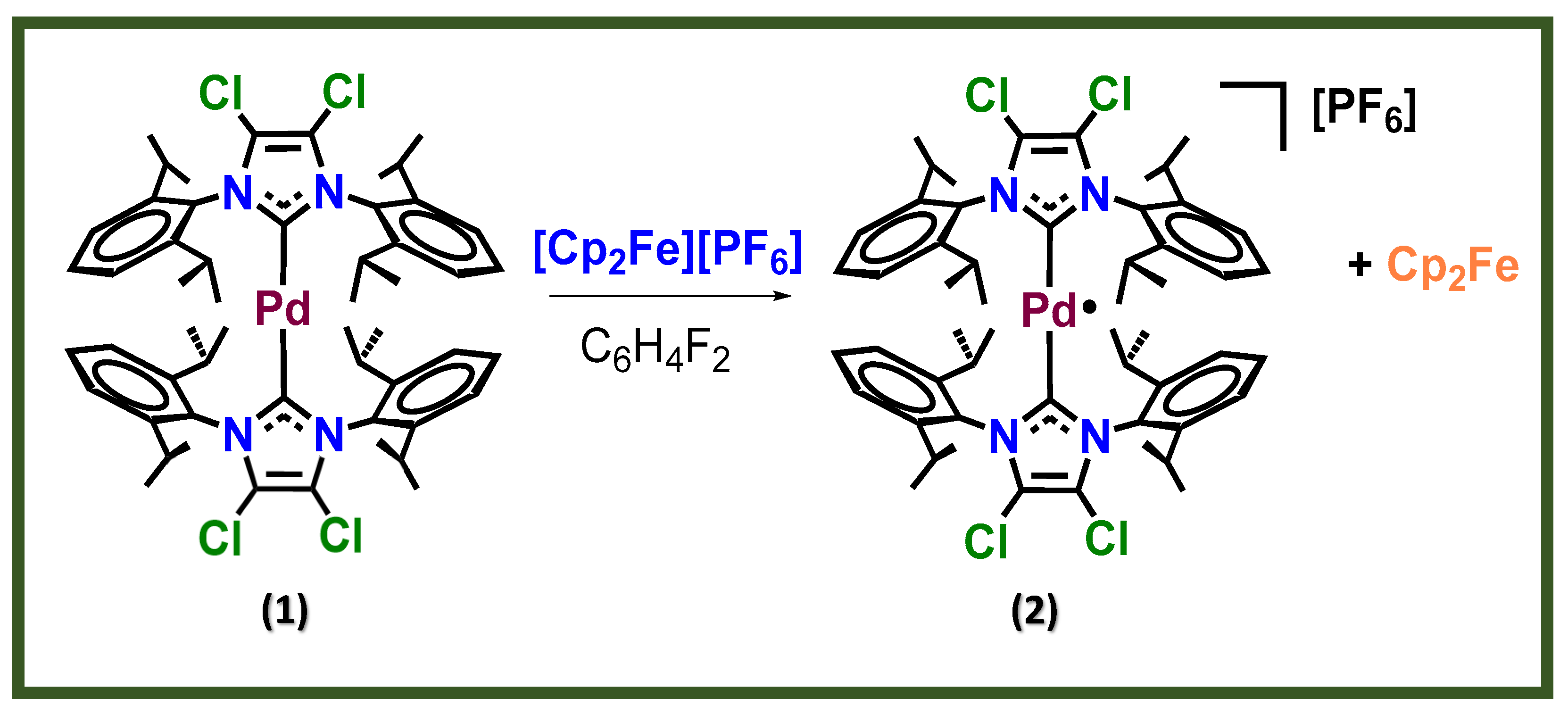
42]. In a previous work [
43], some of us have shown that starting form a Pd(0) complex (1) Bis-[1,3-Bis-(2,6-diisopropylphenyl)-4,5-dichloroimidazol-2-ylidene]palladium(0), but with a different formulation Bis-[1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene]palladium(I) hexafluoridophosphate, [Pd(IPr)
2][PF
6], can be synthetised as complex 2 by a chemical oxidation, as shown in
Figure 2, to generate [Pd
I(NHC)
2]
+ complexes [
43]. Complex 2 is a rare case of a paramagnetic palladium complex in oxidation state I and, unlike the corresponding Pd
0 complex, is indefinitely stable in the solid state in air and for several days in solution. The most important characteristic of this complex related to this investigation is the role of one unpaired electron of a singly occupied molecular orbital (orbital SOMO) inside the P3HT:PC
60BM Bulk-Heterojunction of an OPV solar cell.
1.5. Target of This Work
To enhance the photovoltaic performance, in terms of Voc and Jsc, it holds great importance to design and synthesize photovoltaic materials featuring an increased number of 3CT states [
44]. Our aim is to explore if the introduction of doublet state molecules as additive in the OPV BHJ can be helpful in controlling recombination dynamics and lifetimes of photogenerated carriers during the charge transfer mechanism as shown in
Figure 1 for Singlet-Triplet dynamics.
The motivation of this work is, therefore, to investigate the effect of Pd(I) metalloradical doublet state complex on the performance of a classic organic solar cell with inverted structure (
Figure 3).
We have been decided to use a P3HT:PC
60BM system because the energy levels fit well with the Homo and Lumo levels of the palladium molecule, favouring the cascade effect that occurs in ternary systems.[
45]
It is known that non-fullerene acceptors improve the photostability of solar cells [
46,
47]. In this work we demonstrate that photochemical degradation can be reduced also in fullerene based OPV thanks to the role of suitable Pd-based complexes.
2. Materials and Methods
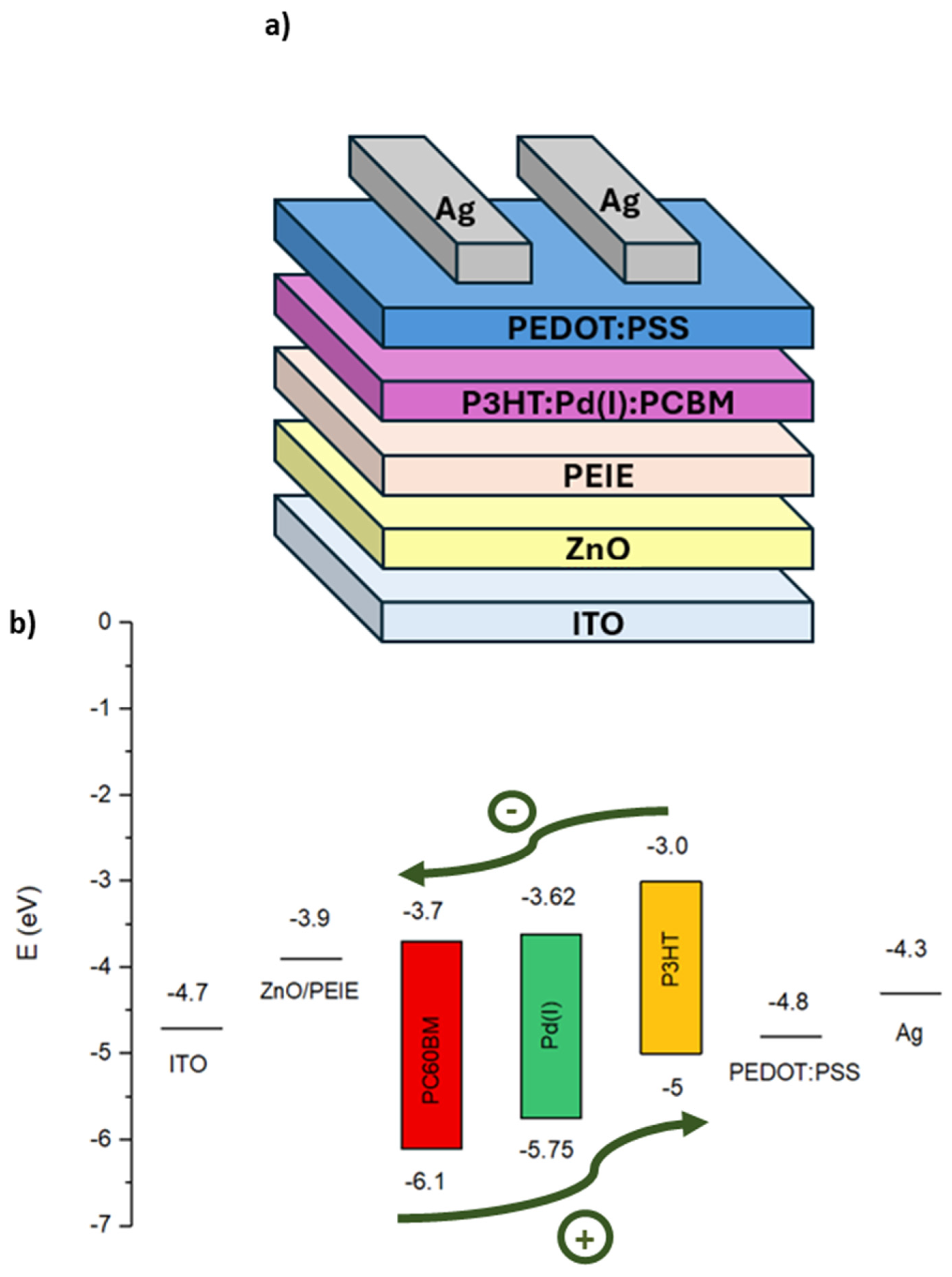
Commercial Glass/ITO substrates is used for the cell fabrication glass substrate of 2.5 x 2.5 cm
2 coated with indium tin oxide (ITO) with a sheet resistance of 10 W/sq as the transparent conducting electrode. The ITO substrates were patterned by a ps UV laser. The investigation is focused on the standard inverted structure with the composition ITO/ZnO/PEIE/P3HT:PC
60BM/PEDOT:PSS/Ag (
Figure 4a). The patterned ITO/glass substrates were cleaned according to the following methodology: a manual cleaning process with a diluted soap solution, LABWASH
® PREMIUM extra 1% (VWR International Srl, Milan, Italy) in aqueous solution, was performed. In the following step, the soap residue is removed via rinsing with purified water. Afterwards, the substrates were placed inside a beaker filled with D.I. water and were placed inside an ultrasonic bath for 15 min. The substrates were then dried with a N
2 air gun and sonicated in a beaker filled with acetone for 15 min, dried with a N
2 air gun and sonicated in a beaker filled with 2-propanol for 15 min. The substrates were finally exposed for 15 min inside the UV Ozone 218 Cleaner L2002A3 (Ossila, Leiden, Netherlands) to clean organic residues and to improve the wettability and the performance of the first layer.
After the cleaning steps, every deposition is carried on under inert conditions, inside a MBRAUN Glovebox with controlled N
2 atmosphere. ZnO ink was filtered by 0.45 PTFE filter, stirred during 20 min in an ultrasonic bath and subsequentially deposited above the ITO substrates by spin coating at 2000 rpm for 45 s and annealed at 110°C during 10 min. Then, PEIE 0.1% interlayer solution in EtOH was stirred during 20 min and deposited by spin coating at 5000 rpm for 45 s and annealed at 110 °C for 10 min. After these steps, different ratios of the active layer with [1,3-Bis-(2,6-diisopropylphenyl)-4,5-dichloroimidazol-2-ylidene]palladium(I) hexafluoridophosphate, [Pd(IPr
Cl)
2][PF
6] (2), dopant were deposited by spin coater with 800 rpm for 45 s and annealed at 110°C during 10 min. The active layer was prepared blending poly(3-hexylthiophene, P3HT and [6,6]-phenyl-C61-butyric acid methyl ester, PC
60BM, with the 1:1 ratio and 20 mg/mL of concentration, solved in 1,2-chlorobenzene and stirred 20 h at room temperature. Before using it, the solution is stirred at 50 °C during 1 h.[
48,
49,
50] The Pd(I) complex is dissolved in chlorobenzene with the proportion 20 mg/mL and stirred at room temperature 1 h and subsequentially filtered by 0.45 PTFE filter before mixing it with the BHJ (see the different mass of Pd complex inside the active layer in
Table 1). PEDOT:PSS HTL Solar (Ossila) solution was diluted with 2-propanol with the ratio 1:5 and stirred 10 min depositing it. Then, the deposition is spin-casted at 3000 rpm during 45 s with 20 s of previous casting; then annealed at 110 °C during 10 min. The last step of this cell fabrication is evaporating 100 nm of silver electrode with a metal evaporator at 10
-6 mbar through shadow mask. Four devices with 0.09 cm
2 area each were fabricated on each substrate.
Device performance was characterized outside the glovebox under a Sun Simulator. For the measurements, we used a shadow mask (9 mm2) to illuminate only one cell at a time. Before each measurement the irradiation level is verified at the quote and position where the solar cell is placed by means of a calibrated Pyranometer (Skye model SKS1110).
J−V measurements of the OSCs were performed with a Class-A Sun Simulator (ABET 2000) equipped with an AM1.5G filter (ABET). The calibration of the Sun Simulator was made by using a Si-based reference cell (RR-226-O, RERA Solutions) to obtain a 1 Sun Illumination Condition. Arkeo platform (Cicci Research S.R.L.) was used for J−V characterization and for MPPT. A voltage step of 20 mV/s and a scan rate of 200 mV/s were set. EQE characterization was performed with an Arkeo system (Cicci Research s.r.l.) with a 150 W xenon lamp and a double grating (300 to 1400 nm). A Si photodiode was used for incident light calibration prior to the EQE measurement. An UV−vis spectrophotometer (Shimadzu UV-2550) equipped with an integrated sphere was used for the acquisition of absorbance spectra. The spectra from 200 nm to the NIR region were acquired with an UV−vis-NIR Jasco V-630 Double Beam Spectrophotometer equipped with a single monochromator. Micro Raman and Photoluminescence measurements were performed by a Horiba Jobin Yvonne LabRAM ARAMIS spectrophotometer equipped with a 457 nm laser source and a 2400 lines/cm edge filter. All the spectra were acquired setting the laser power to 10 %, with an acquisition time of 2 s per point and 50 acquisitions for each pattern. The thicknesses were measured with a Bruker DEKTAK XT profilometer. Photovoltage decay and photostability studies were performed with dedicated Arkeo platforms (Cicci Research S.R.L.)
DFT calculations: the complexes have been optimized in 2-hexanone solvent with the TPSSh functional, using the SDD+f basis set for Pd (SDD ECP for internal electrons) and 6-311G(d,p) basis set for all the non-metal atoms (see the details in the Supplementary Information).
SEM microscopy images, quantities and spectrums were acquired by HitachiFE–SEMS4000 instrument.
Syntheses and characterization of the precursor of the additive molecule Bis-(2,6-diisopropylphenyl)-4,5-dichloroimidazol-2-ylidene]palladium(0), [Pd(IPrCl)2] (1), and the additive, Bis-(2,6-diisopropylphenyl)-4,5-dichloroimidazol-2-ylidene]palladium(I) hexafluoridophosphate, [Pd(IPrCl)2][PF6] (2), are described in the Supplementary Information of this paper (S3-S6).
3. Results and Discussion
3.1. Device Optoelectronic Properties
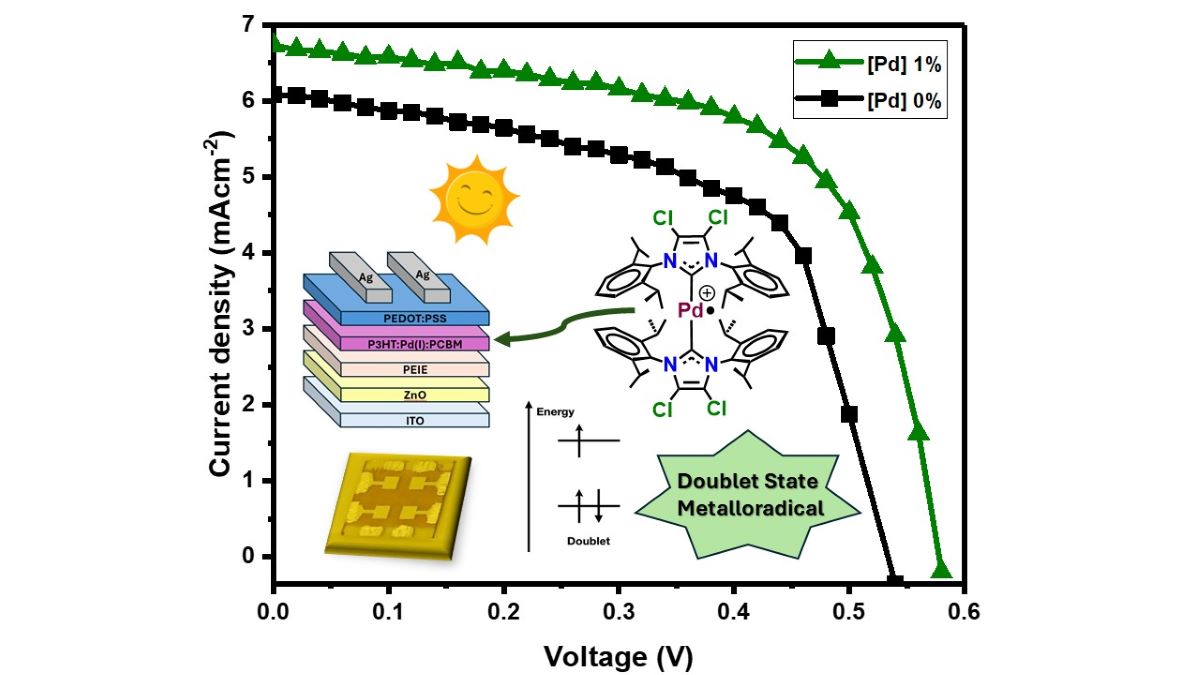
In this paper we investigate the optimal quantity of Pd(I) metalloradical complex inside the blend, as an additive doping molecule, to improve the electrical properties of a classic inverted organic solar cell (
Figure 4,a). The choice of this additive molecule, apart from its doublet state and its metalloradical character, has been evaluated by its HOMO and LUMO values (
Table 2), since it fits perfectly with its use in the active layer as a semiconductor material (
Figure 4,b).
In addition, it is a stable Pd(I) molecule that is easy to handle and synthesize through Pd(0) NHC-biscarbene precursors that were already well known for their use in OLED devices (
Figure 2).[
41]
The behaviour of P3HT:PC
60BM BHJ under the effect of the small quantities of Pd(I) complex additive (2) is explored. As shown
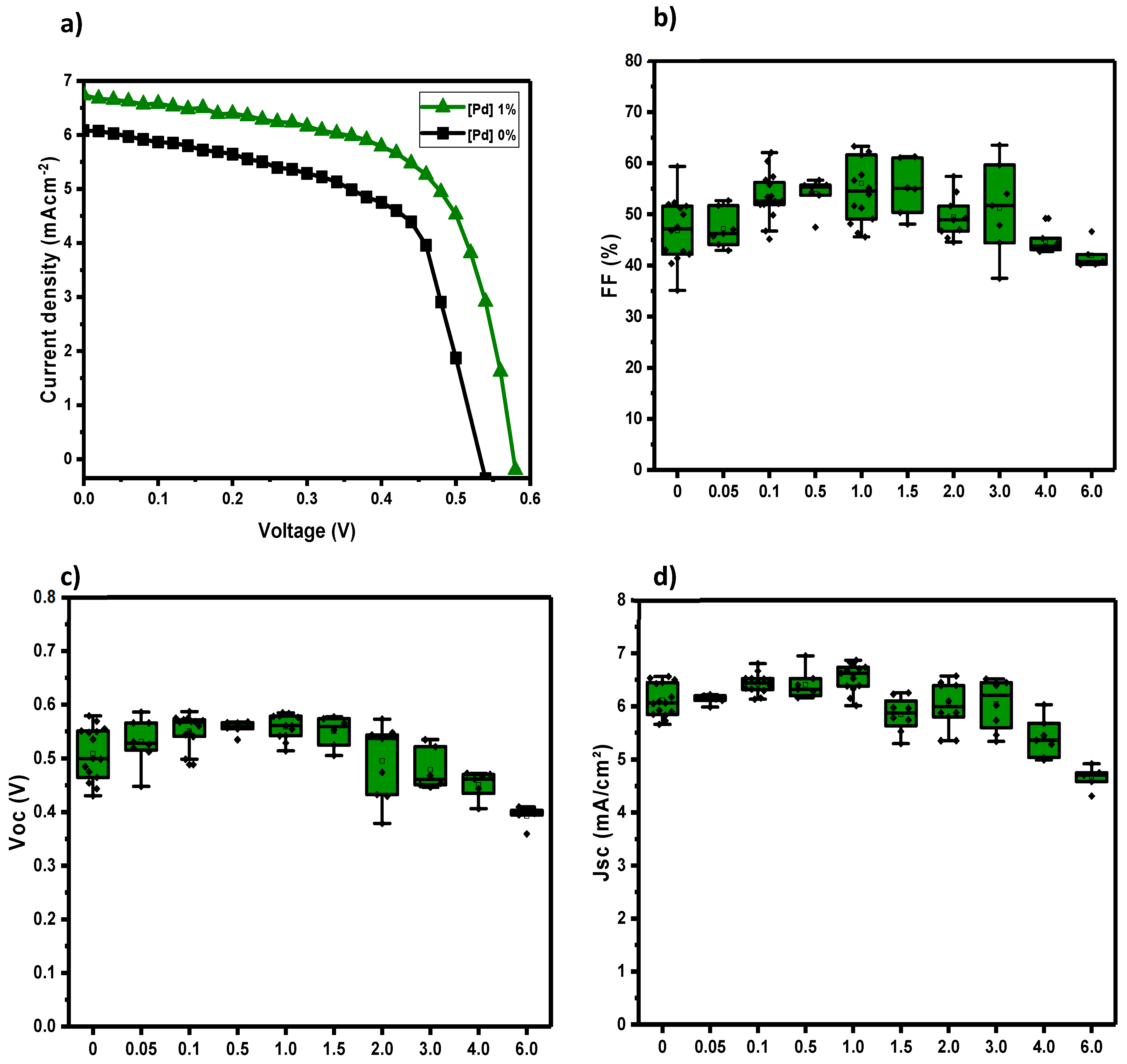
Figure 5, we observed that small quantities of Pd(I) complex improve the IV characteristics. The efficiencies are in the separate
Figure 6. In
Figure 5a we can show the difference between the IV of the best case (1 wt%) vs the reference cell, without Pd additive. Adding 1 wt% of Pd additive also improves the performance of the solar cell in terms of fill factor (FF) (
Figure 5b), open-circuit voltage (Voc) (
Figure 5c), and short circuit current (Jsc) (
Figure 5d). The improvements start with 0.05 wt%. These improvements were increasing until the 1.0 wt% of the additive. Consequently, the optimal concentration of Pd(I) complex was found at 1.0 wt%. In
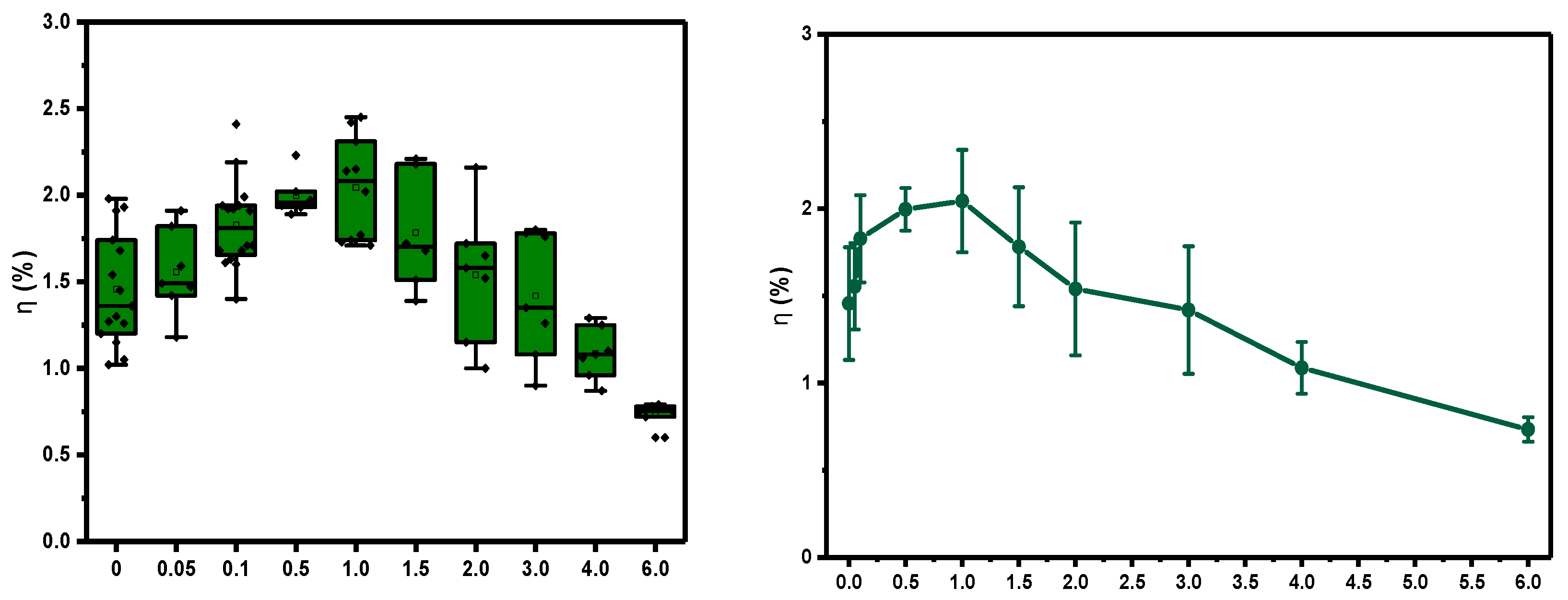
Figure 6, we show how this complex significantly increases the efficiency of the solar cell with low amounts of the additive (2). We have associated this behaviour due to the doublet state property of this organometallic molecule that we assume is favourable for the charge transfer mechanism at the donor/acceptor interface. However, the further increase of quantities of this molecule, from 1.5 wt% and so on, cannot upgrade further the performance of the solar cell. Moreover, at the highest amounts of the additive, from 3 wt% onwards, the electrical performance of the solar cell does not improve, and the situation begins to worsen, giving performances even lower than the reference cell.
We believe that this behaviour is associated to a worsening in the bulk-heterojunction morphology, as discussed in section 2.2 regarding SEM images of the active layer. In
Table 3, Comparing the optimal quantity of the additive (2) inside the blend (1.0 wt%) with the reference (without additive), we calculated that the improvements are: 40% for the efficiency (Eff), 20% for the fill factor (FF), 7% for the short circuit current (Jsc) and 10% for the open-circuit voltage (Voc). Therefore, the effect of the optimal quantity of this additive (2) is positive in all the main photovoltaic parameters.
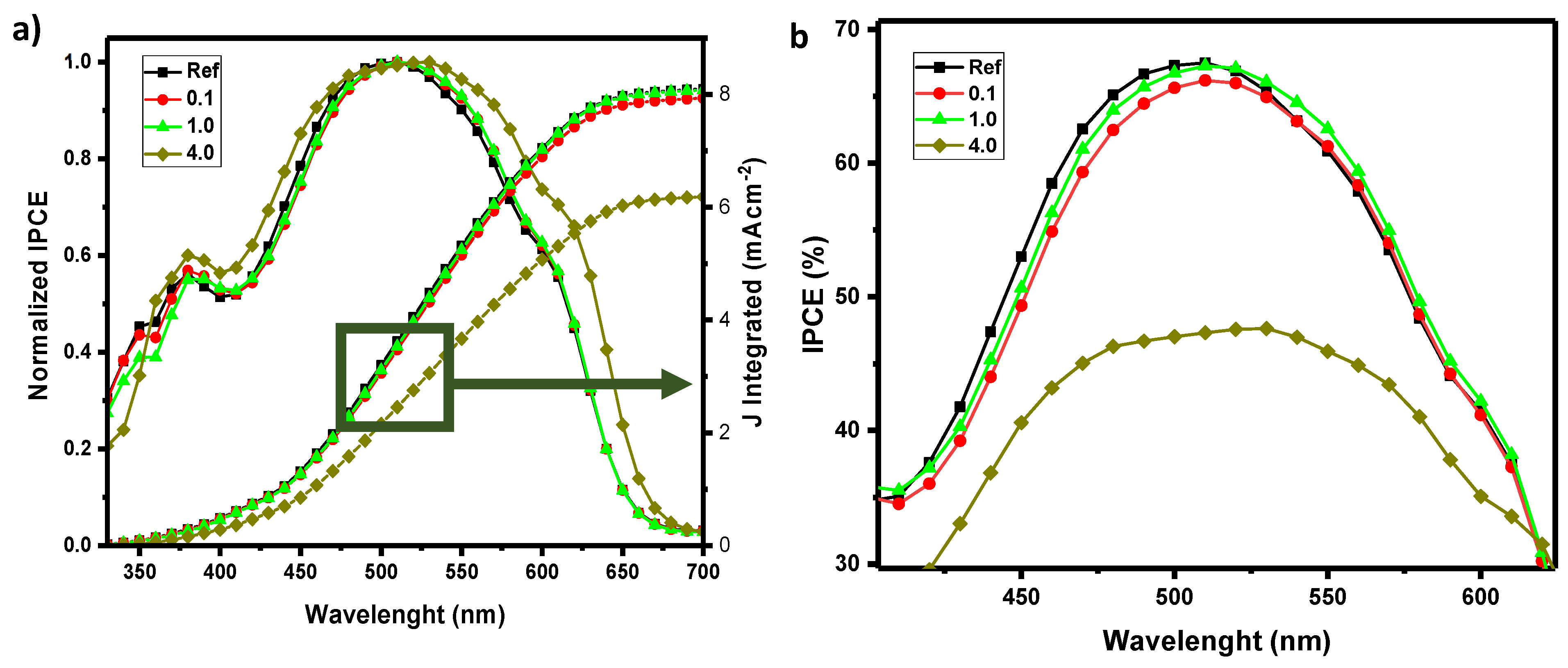
In addition, IPCE and Photoluminescence spectrums are suggesting a change in morphology of the bulk heterojunction (
Figure 7 and
Figure 8). When the additive (2) is added, IPCE trends show a peak shift towards high wavelengths (red shift), for increasing concentrations of Pd as dopant (
Figure 7). This can be related to the higher rate of doublet state excitons in the mechanism of photocurrent generation, introduced by the adding of an organometallic complex to the bulk heterojunction. We observed a gradual decrease of the quantum efficiency for higher quantity of Pd (4 wt%), due to the fact that heavily doping the photoactive layer of the studied devices can affect negatively the morphology of the BHJ and therefore the process of conversion of incident photons as photogenerated carriers. These trends can explain the morphology changes of the bulk hetero-junction, observed also by the SEM analysis in the following section. The deviation of J
SC values from JV curve and integrated IPCEs spectra are summarized in
Table 3.[
51,
52]
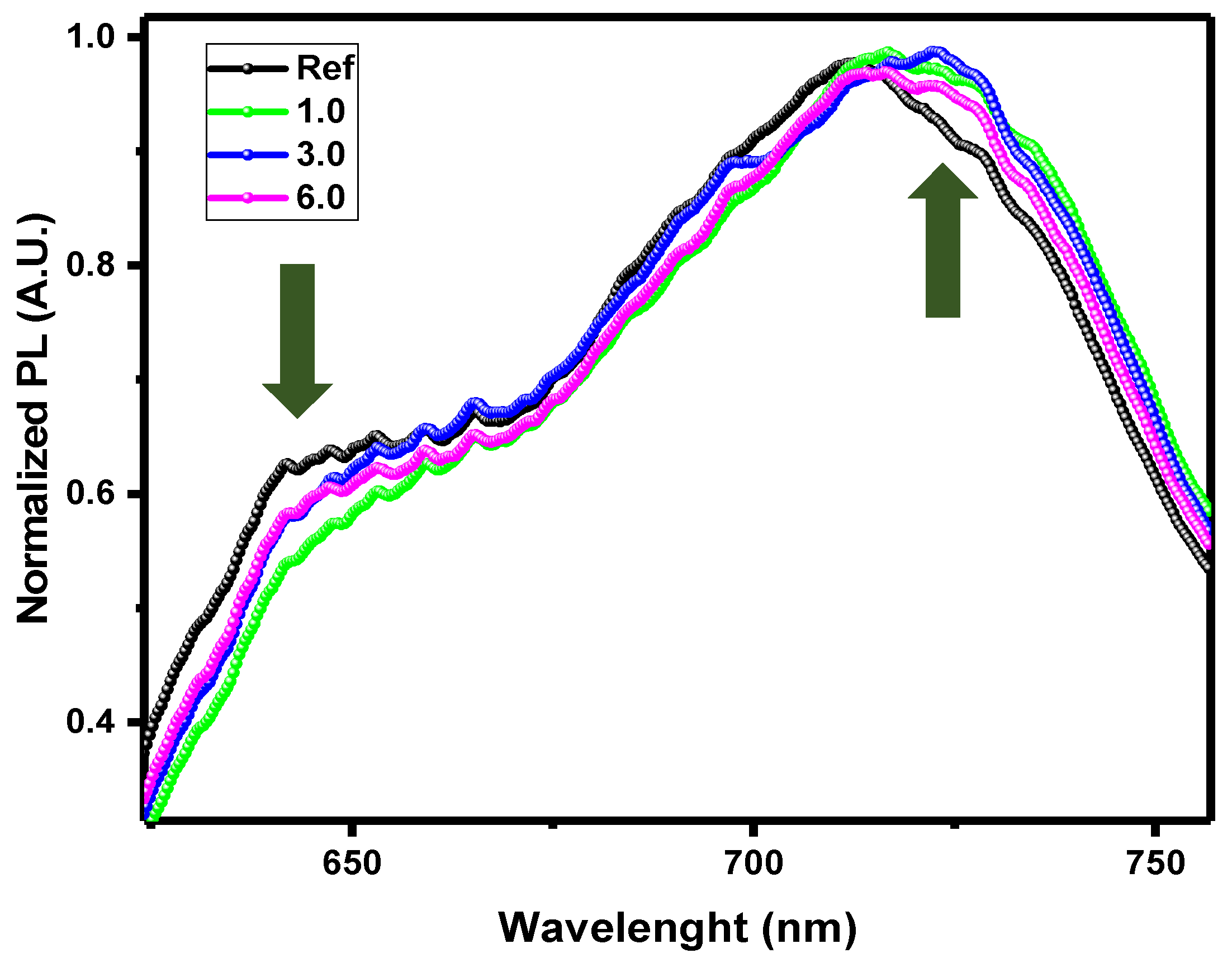
Photoluminescence spectra show a slight red shift for increasing doping concentration of Pd dopant (
Figure 8). This is because the effect of the organometallic complex, allows the generation of reasonable rate of doublet state excitons with longer lifetimes and potential for ‘delayed’ photon emission as observed in triplet states charge transfer mechanism.[
25]
3.2. Morphological Studies of the Active Layer
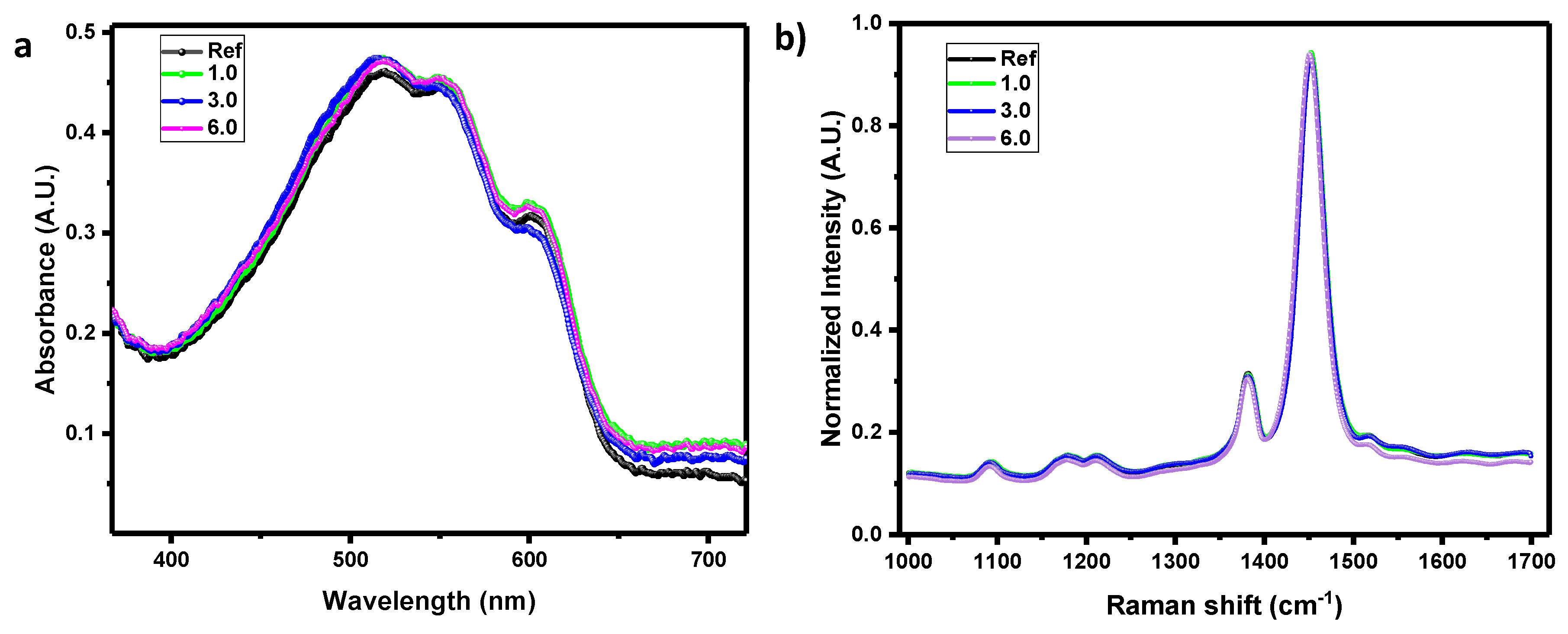
Morphological analysis has been done performing Raman, Absorbance, SEM, and thickness studies. The palladium molecule has an unpair electron with a radical character [
43], therefore we decided to study the possible radical reaction with the fullerene PC
60BM by Raman technique. However, we did not observe any chemical modification associated with the radical reaction (
Figure 9b). Accordingly, Raman spectra confirm that additive metalloradical complex (2) is not making any chemical reaction with the different layers deposited in the solar cells (
Figure 9b). Since Raman spectra are not affected by %Pd content in the BHJ, this behaviour suggest that Pd complex can be only associated to a reorganization of the morphology without changes of donor/acceptor spectral fingerprints. Thickness studies at the profilometer demonstrate that the improvement of the electrical characteristics is not associated to an effect of the palladium complex on the thickness of the bulk heterojunction film (
Table 5). A confirmation that the thickness of the solar cell does not vary when adding the additive (2) is provided by the fact that there are no changes of the absolute values in the absorbance spectra (
Figure 8).
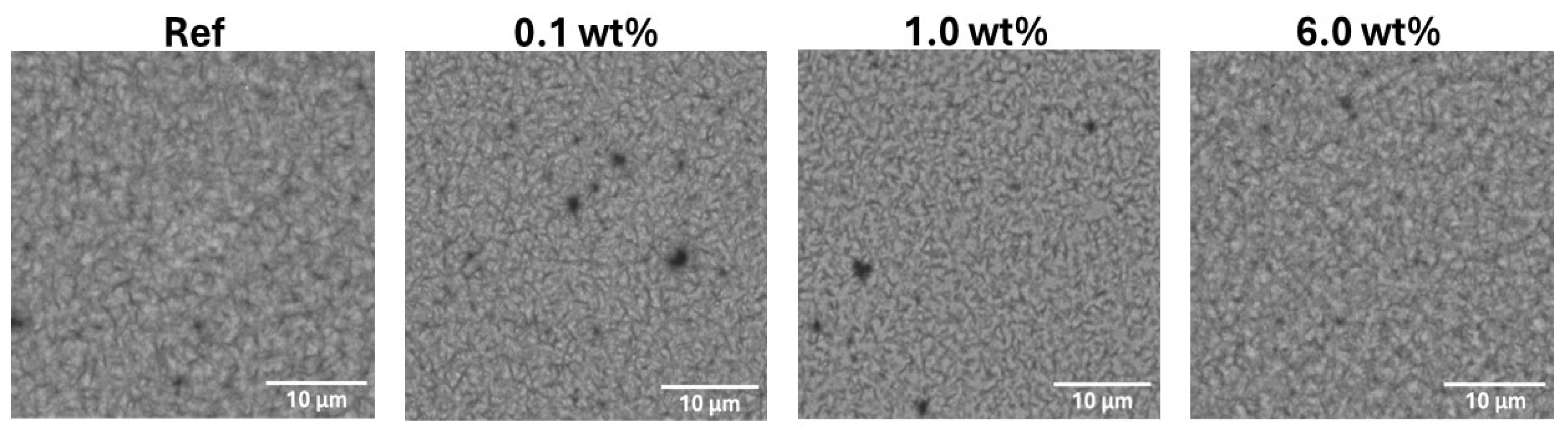
The morphological studies by SEM technique shows the difference of the thin film surfaces with different concentration of the additive (
Figure 10). Increasing the Pd quantities, the films appearances are more homogeneous, and the crystalline grains are smaller up to 1 wt% of Pd case. However, increasing the concentration up to 6 wt% reveal that the grain size increases again. This result is confirming that the morphological changes are directly related to the electrical behaviour.
3.3. Recombination Analysis
Dark J-V plots (
Figure 11) reveal that the 1 wt% optimal quantities of Pd complex (2), is optimizing the recombination dynamics. This trend is associated to the doublet state property, reducing the recombinations inside the bulk-heterojunction and, therefore, lowering the reverse saturation current. The diode reverse saturation current are directly proportional to the recombinations, and therefore improved diode rectification is observed upon adding the Pd complex, reaching a maximum of about 1 wt%. From 3.0 wt% and so on, the rectification decrease, but still better than the reference case.
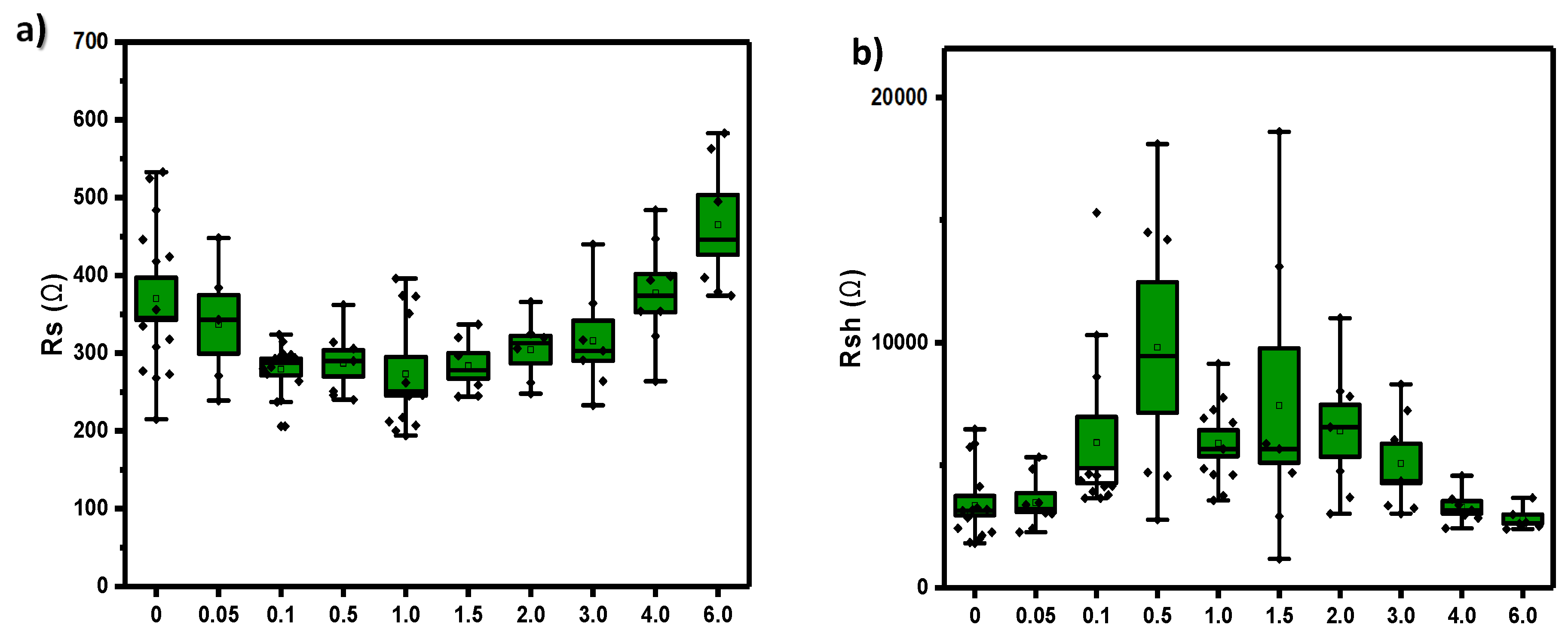
Furthermore, the shunt resistance and series resistance (
Figure 12) are consistent with this diode rectification. The series resistances (
Figure 12a) decrease with increasing amounts of Pd additive, reaching the minimum at 1 wt% but increases from 3 wt% onwards. Shunt resistances exhibit the opposite behaviour, as expected (
Figure 12b).
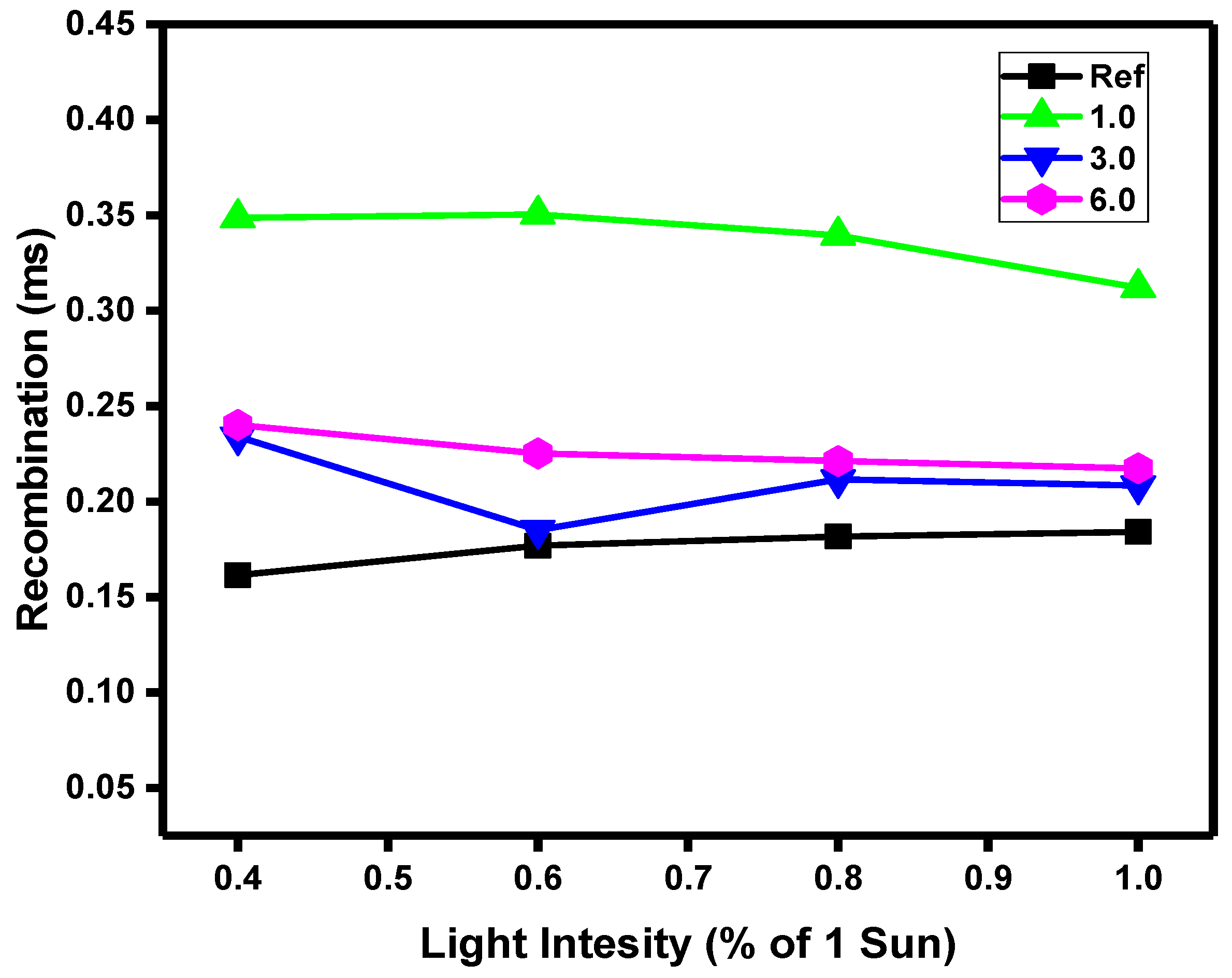
We also performed the photovoltage decay studies of the solar cells. These studies reveal that Pd(I) additive inside the BHJ, decreases the recombinations rate in the active layer (
Figure 13). That is because a doublet state complex, with an unpaired electron, helps to decrease the recombination inside the active layer, increasing of photocurrent generation, due to promoted mechanisms of charge separation at the interfaces donor/acceptor, and reducing the recombination times as expected.
3.4. Photostability
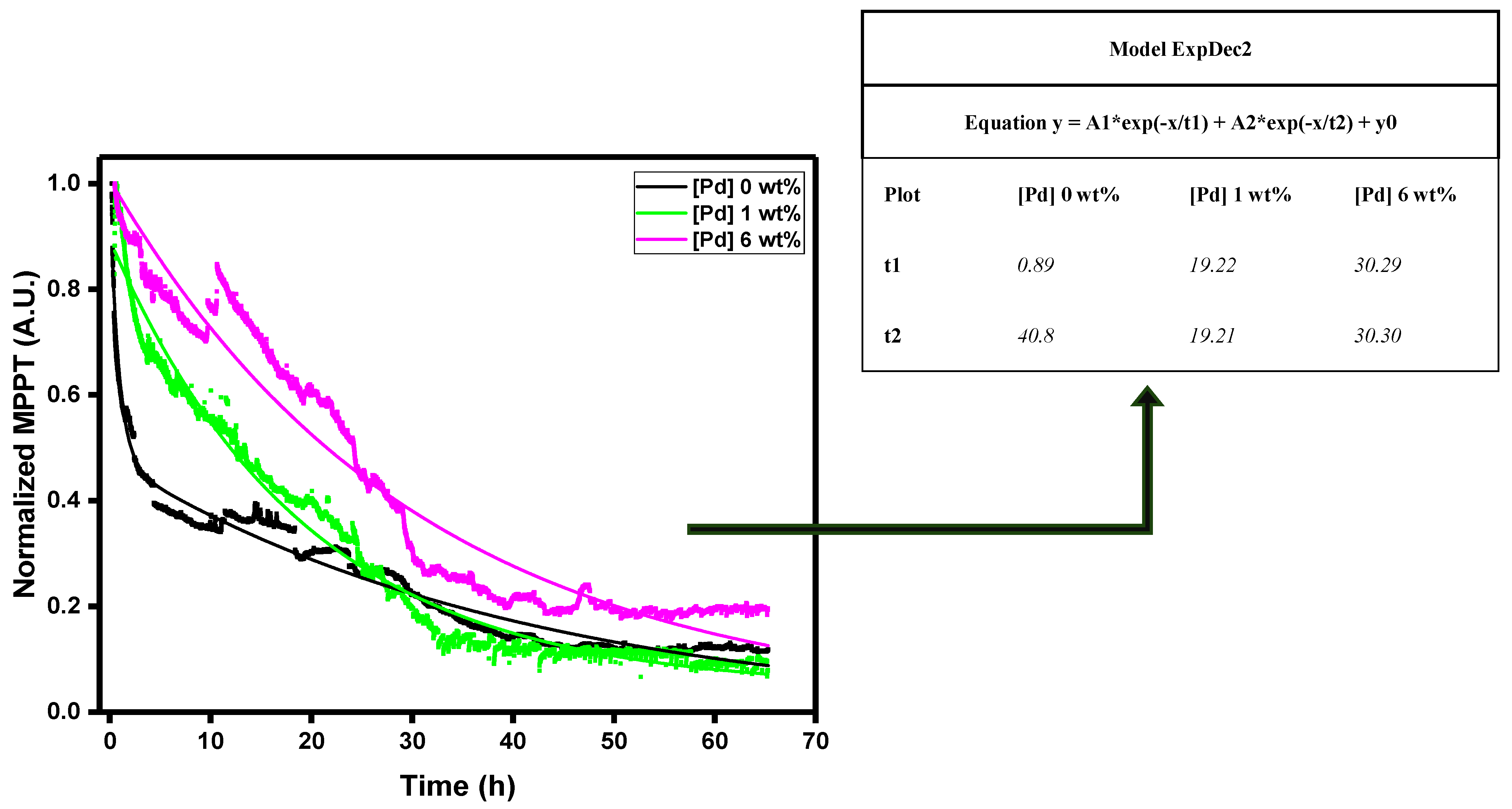
The photochemical degradation is tested on devices with different concentrations of the donor material inside the BHJ. The stability is measured under 1 Sun (
Figure 14). By adding the additive, the stability of the device improves significantly. Since the photostability is associated to the overall Pd(I) complex concentration, the best result in terms of stability is achieved at the maximum concentration tested in this work ,6 wt%.
In the
Figure 14 we used two exponential decay curves to understand the trend of decay due to the degradation of unencapsulated devices. We observed that the fast initial decay rate, t1, is strongly depending on Pd(I) complex. The reference case, without any additive, present a very fast decay rate t1= 0.89 h. Increasing the additive content, we observed t1= 19.22 h, for 1.0 wt%, and t1= 30.29 h, for 6.0 wt%. This trend clearly shows a great improvement in the stabilization of the solar cell due to the presence of Pd(I) complex.
4. Conclusions
Doping at low concentration P3HT:PC
60BM with Pd(I) complex improves the photovoltaic performances of the solar cells. The optimal concentration to improve the performance is 1 wt% of the additive inside the BHJ. This behavior is due to the fact that Pd(I) is a doublet state organometallic molecule with an unpair electron that has a positive role on the recombination mechanisms, increasing notably the photocurrent generation, inside the active layer. The specificity of this Pd molecule on fullerene acceptors, due to its radical character, could be evaluated.[
53] However, in this work we observed that there is no chemical reaction between the BHJ and Pd(I). In this work is also clearly demonstrated that Pd(I) molecule has a clear effect on the BHJ morphology, with a reduced roughness at the optimal Pd(I) concentration. We also demonstrated that the doublet state Pd(I) can increase strongly the photostability of the unencapsulated solar cells against the photochemical degradation.
However, in the future development of this work, further studies will be also focused in more efficient blends, either based on FA or NFA acceptors, compared to the simple and well-studied model system P3HT:PC60BM, that has been selected also due to the good fitting of the Homo and Lumo values of the Pd molecule in the P3HT:PC60BM system.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org. 1. Synthesis of palladium complexes S3-S6; 1.1. General procedures S3; 1.2. Synthesis and characterization of bis[1,3-bis-(2,6-diisopropylphenyl)-4,5-dichloroimidazol-2-ylidene]palladium(0), [Pd(IPr
Cl)
2] (
1) S3; 1.3. Synthesis and characterization of bis-[1,3-bis-(2,6-diisopropylphenyl)-4,5-dichloroimidazol-2-ylidene]palladium(I) hexafluoridophosphate, [Pd(IPr
Cl)
2][PF
6] (2) S4-S6; 2. DFT calculations of the energies of the highest occupied and lowest unoccupied molecular orbitals (MOs) of [Pd(IPr
Cl)
2][PF
6] (2) S7-S10; 3. SEM data S11-S15; 3.1. Spectra S11-S12; 3.2. EDX analysis S13-S15; 4. References S16.
Author Contributions
Conceptualization, discussion of the results, writing, review and editing A.E.A.-Q, V.C., C.G.Y., E.D.J and A.R.; device fabrication A.E.A.-Q, V.C. and A.U.R.; SEM characterization F.Z.; DFT characterization A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been partially supported by the ITA-NTN “Integrated Terrestrial And Non-Terrestrial Networks” project under the RESTART “RESearch and innovation on future Telecommunications systems and networks, to make Italy more smart” initiative of the PNRR. This work was also supported by the Spanish MCIN/AEI/10.13039/501100011033 and the European Union “NextGenerationEU”/PRTR with projects PID2020-114637GB-I00 and TED2021-129634B-I00.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We would like to thank Prof. Claudia Barolo for the discussions that initially helped to develop the idea of this paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ylikunnari, M.; Välimäki, M.; Väisänen, K.-L.; Kraft, T. M.; Sliz, R.; Corso, G.; Po, R.; Barbieri, R.; Carbonera, C.; Gorni, G.; Vilkman, M., Flexible OPV modules for highly efficient indoor applications. Flexible and Printed Electronics 2020, 5, (1), 014008. [CrossRef]
- Sampaio, P.; González, M.; Ferreira, P.; Vidal, P.; Pereira, J.; Ferreira, H.; Oprime, P., Overview of printing and coating techniques in the production of organic photovoltaic cells. International Journal of Energy Research 2020, 44. [CrossRef]
- Koidis, C.; Logothetidis, S.; Ioakeimidis, A.; Laskarakis, A.; Kapnopoulos, C., Key factors to improve the efficiency of roll-to-roll printed organic photovoltaics. Organic Electronics 2013, 14, 1744-1748. [CrossRef]
- Lee, S.; Jeong, D.; Kim, C.; Lee, C.; Kang, H.; Woo, H. Y.; Kim, B. J., Eco-Friendly Polymer Solar Cells: Advances in Green-Solvent Processing and Material Design. ACS Nano 2020, 14, (11), 14493-14527. [CrossRef] [PubMed]
- McDowell, C.; Bazan, G., Organic solar cells processed from green solvents. Current Opinion in Green and Sustainable Chemistry 2017, 5, 49-54. [CrossRef]
- Xu, T.; Yu, L., How to design low bandgap polymers for highly efficient organic solar cells. Materials Today 2014, 17, (1), 11-15. [CrossRef]
- Krebs, F. C., Polymeric Solar Cells: Materials, Design, Manufacture. Destech Publications: 2010.
- Chen, B.; Zheng, X.; Bai, Y.; Padture, N.; Huang, J., Progress in Tandem Solar Cells Based on Hybrid Organic-Inorganic Perovskites. Advanced Energy Materials 2017, 7, 1602400. [CrossRef]
- Zhang, Z.; Li, Z.; Meng, L.; Lien, S.-Y.; Gao, P., Perovskite-Based Tandem Solar Cells: Get the Most Out of the Sun. Advanced Functional Materials 2020, 30. [CrossRef]
- Mauer, R.; Kastler, M.; Laquai, F., The Impact of Polymer Regioregularity on Charge Transport and Efficiency of P3HT:PCBM Photovoltaic Devices. Advanced Functional Materials, v.20, 2085-2092 (2010) 2010, 20.
- Yang, C.-M.; Wu, C.-H.; Liao, H.-H.; Lai, K.-Y.; Cheng, H.-P.; Horng, S.-f.; Meng, H.-F.; Shy, J. T., Enhanced photovoltaic response of organic solar cell by singlet-to-triplet exciton conversion. Applied Physics Letters 2007, 90, 133509-133509. [CrossRef]
- Çaldıran, Z.; Erkem, Ü.; Baltakesmez, A.; Biber, M., Effects of the PENTACENE as doping material on the power conversion efficiency of P3HT:PCBM based ternary organic solar cells. Physica B: Condensed Matter 2021, 607, 412859.
- Barbour, L. W.; Hegadorn, M.; Asbury, J. B., Watching Electrons Move in Real Time: Ultrafast Infrared Spectroscopy of a Polymer Blend Photovoltaic Material. Journal of the American Chemical Society 2007, 129, (51), 15884-15894. [CrossRef]
- Abbas, Z.; Ryu, S. U.; Haris, M.; Song, C. E.; Lee, H. K.; Lee, S. K.; Shin, W. S.; Park, T.; Lee, J.-C., Optimized vertical phase separation via systematic Y6 inner side-chain modulation for non-halogen solvent processed inverted organic solar cells. Nano Energy 2022, 101, 107574. [CrossRef]
- Zhang, L.; Hu, L.; Wang, X.; Mao, H.; Zeng, L.; Tan, L.; Zhuang, X.; Chen, Y., Regulation of Crystallinity and Vertical Phase Separation Enables High-Efficiency Thick Organic Solar Cells. Advanced Functional Materials 2022, 32. [CrossRef]
- Griffith, M.; Cooling, N.; Vaughan, B.; Elkington, D.; Hart, A.; Lyons, A.; Qureshi, S.; Belcher, W.; Dastoor, P., Combining Printing, Coating and Vacuum Deposition on the Roll-to-Roll Scale: A Hybrid Organic Photovoltaics Fabrication. IEEE Journal of Selected Topics in Quantum Electronics 2015, 22, 1-1. [CrossRef]
- Xue, R.; Zhang, J.; Li, Y.; Li, Y., Organic Solar Cell Materials toward Commercialization. Small 2018, 14, 1801793. [CrossRef] [PubMed]
- Brédas, J.-L.; Norton, J.; Cornil, J.; Coropceanu, V., Molecular Understanding of Organic Solar Cells: The Challenges. Accounts of chemical research 2009, 42, 1691-9. [CrossRef] [PubMed]
- Scharber, M. C.; Sariciftci, N. S., Efficiency of bulk-heterojunction organic solar cells. Prog Polym Sci 2013, 38, (12), 1929-1940. [CrossRef] [PubMed]
- Wadsworth, A.; Hamid, Z.; Kosco, J.; Gasparini, N.; McCulloch, I., The Bulk Heterojunction in Organic Photovoltaic, Photodetector, and Photocatalytic Applications. Advanced Materials 2020, 32, 2001763. [CrossRef] [PubMed]
- Yamanaka, T.; Nakanotani, H.; Adachi, C., Significant role of spin-triplet state for exciton dissociation in organic solids. Sci Adv 2022, 8, (9), eabj9188. [CrossRef] [PubMed]
- Proctor, C.; Kuik, M.; Nguyen, T.-Q., Charge carrier recombination in organic solar cells. Progress in Polymer Science 2013, 38, 1941–1960. [CrossRef]
- Zhang, K.-N.; Jiang, Z.-N.; Wang, T.; Qiao, J.-W.; Feng, L.; Qin, C.-C.; Yin, H.; So, S.-K.; Hao, X.-T., Exploring the mechanisms of exciton diffusion improvement in ternary polymer solar cells: From ultrafast to ultraslow temporal scale. Nano Energy (Print) 2021, v. [CrossRef]
- Tang, Z.; Tress, W.; Inganas, O., Light trapping in thin film organic solar cells. Materials Today 2014. [CrossRef]
- Li, S.; Chen, Y.; Wang, Z.; Chen, J.; Zhang, J.; Nie, J.; Duan, Y.; Geng, Y.; Su, Z., Theoretical studies of new iridium-based terpolymer donors for high-efficiency triplet-material-based organic photovoltaics: Incorporation of different iridium(III) complexes. Materials Chemistry and Physics 2023, 302, 127780. [CrossRef]
- Su, Y.-W.; Lan, S.-C.; Wei, K.-H., Organic photovoltaics. Materials Today 2012, 15, 554–562. [CrossRef]
- Jungbluth, A.; Kaienburg, P.; Riede, M., Charge transfer state characterization and voltage losses of organic solar cells. Journal of Physics: Materials 2022, 5.
- Jin, Y.; Zhang, Y.; Liu, Y.; Xue, J.; Li, W.; Qiao, J.; Zhang, F., Limitations and Perspectives on Triplet-Material-Based Organic Photovoltaic Devices. Advanced Materials 2019, 31, 1900690. [CrossRef] [PubMed]
- Clarke, T. M.; Durrant, J. R., Charge photogeneration in organic solar cells. Chem Rev 2010, 110, (11), 6736-67. [CrossRef]
- Winroth, G.; Podobinski, D.; Cacialli, F., Dopant optimization for triplet harvesting in polymer photovoltaics. Journal of Applied Physics 2011, 110, (12).
- Gao, H.; Yu, R.; Ma, Z.; Gong, Y.; Zhao, B.; Lv, Q.; Tan, Z. a., Recent advances of organometallic complexes in emerging photovoltaics. Journal of Polymer Science 2021, 60, (6), 865-916. [CrossRef]
- Baranoff, E.; Yum, J.-H.; Graetzel, M.; Nazeeruddin, M., Cyclometallated iridium complexes for conversion of light into electricity and electricity into light. Journal of Organometallic Chemistry 2009, 694. [CrossRef]
- Marin-Beloqui, J. M.; Congrave, D. G.; Toolan, D. T. W.; Montanaro, S.; Guo, J.; Wright, I. A.; Clarke, T. M.; Bronstein, H.; Dimitrov, S. D., Generating Long-Lived Triplet Excited States in Narrow Bandgap Conjugated Polymers. Journal of the American Chemical Society 2023, 145, (6), 3507-3514. [CrossRef]
- Kober, E. M.; Meyer, T. J., Concerning the absorption spectra of the ions M(bpy)32+ (M = Fe, Ru, Os; bpy = 2,2’-bipyridine). Inorganic Chemistry 1982, 21, (11), 3967-3977. [CrossRef]
- Heindl, M.; Hongyan, J.; Hua, S. A.; Oelschlegel, M.; Meyer, F.; Schwarzer, D.; González, L., Excited-State Dynamics of [Ru((S-S)bpy)(bpy)(2)](2+) to Form Long-Lived Localized Triplet States. Inorg Chem 2021, 60, (3), 1672-1682. [CrossRef]
- Lo, S.-C.; Burn, P. L., Development of Dendrimers: Macromolecules for Use in Organic Light-Emitting Diodes and Solar Cells. Chemical Reviews 2007, 107, (4), 1097-1116. [CrossRef] [PubMed]
- Sato, T.; Nozawa, S.; Tomita, A.; Hoshino, M.; Koshihara, S.-y.; Fujii, H.; Adachi, S.-i., Coordination and Electronic Structure of Ruthenium(II)-tris-2,2′-bipyridine in the Triplet Metal-to-Ligand Charge-Transfer Excited State Observed by Picosecond Time-Resolved Ru K-Edge XAFS. The Journal of Physical Chemistry C 2012, 116, (27), 14232-14236. [CrossRef]
- Sun, Y.; Giebink, N. C.; Kanno, H.; Ma, B.; Thompson, M. E.; Forrest, S. R., Management of singlet and triplet excitons for efficient white organic light-emitting devices. Nature 2006, 440, (7086), 908-912. [CrossRef] [PubMed]
- Hopkinson, M. N.; Richter, C.; Schedler, M.; Glorius, F., An overview of N-heterocyclic carbenes. Nature 2014, 510, (7506), 485-96. [CrossRef] [PubMed]
- Visbal, R.; Gimeno, M. C., N-heterocyclic carbene metal complexes: photoluminescence and applications. Chemical Society Reviews 2014, 43, (10), 3551-3574. [CrossRef] [PubMed]
- Henwood, A. F.; Lesieur, M.; Bansal, A. K.; Lemaur, V.; Beljonne, D.; Thompson, D. G.; Graham, D.; Slawin, A. M. Z.; Samuel, I. D. W.; Cazin, C. S. J.; Zysman-Colman, E., Palladium(0) NHC complexes: a new avenue to highly efficient phosphorescence. Chem Sci 2015, 6, (5), 3248-3261. [CrossRef]
- Cai, X.; Majumdar, S.; Fortman, G. C.; Cazin, C. S. J.; Slawin, A. M. Z.; Lhermitte, C.; Prabhakar, R.; Germain, M. E.; Palluccio, T.; Nolan, S. P.; Rybak-Akimova, E. V.; Temprado, M.; Captain, B.; Hoff, C. D., Oxygen Binding to [Pd(L)(L′)] (L= NHC, L′ = NHC or PR3, NHC = N-Heterocyclic Carbene). Synthesis and Structure of a Paramagnetic trans-[Pd(NHC)2(η1-O2)2] Complex. Journal of the American Chemical Society 2011, 133, (5), 1290-1293.
- Maties, G.; Gómez-Sal, P.; Yebra, C. G.; Andrés, R.; de Jesús, E., Reversible Single-Electron-Transfer to Oxygen in a Stable N-Heterocyclic Carbene Palladium(I) Metalloradical. Inorg Chem 2023, 62, (49), 19838-19842. [CrossRef] [PubMed]
- Maiti, S.; Siebbeles, L. D. A., Developments and Challenges Involving Triplet Transfer across Organic/Inorganic Heterojunctions for Singlet Fission and Photon Upconversion. J Phys Chem Lett 2023, 14, (49), 11168-11176. [CrossRef]
- Adnan, M.; Irshad, Z.; Hussain, R.; Lee, W.; Kim, M.; Lim, J., Efficient ternary active layer materials for organic photovoltaics. Solar Energy 2023, 257, 324-343. [CrossRef]
- Balasubramanian, S.; León-Luna, M. Á.; Romero, B.; Madsen, M.; Turkovic, V., Vitamin C for Photo-Stable Non-fullerene-acceptor-Based Organic Solar Cells. ACS Applied Materials & Interfaces 2023, 15, (33), 39647-39656.
- Turkovic, V.; Prete, M.; Bregnhøj, M.; Inasaridze, L.; Volyniuk, D.; Obrezkov, F. A.; Grazulevicius, J. V.; Engmann, S.; Rubahn, H.-G.; Troshin, P. A.; Ogilby, P. R.; Madsen, M., Biomimetic Approach to Inhibition of Photooxidation in Organic Solar Cells Using Beta-Carotene as an Additive. ACS Applied Materials & Interfaces 2019, 11, (44), 41570-41579.
- Abdallaoui, M.; Sengouga, N.; Chala, A.; Meftah, A. F.; Meftah, A. M., Comparative study of conventional and inverted P3HT: PCBM organic solar cell. Optical Materials 2020, 105, 109916. [CrossRef]
- Baek, W.-H.; Yang, H.; Yoon, T.-S.; Kang, C. J.; Lee, H. H.; Kim, Y.-S., Effect of P3HT:PCBM concentration in solvent on performances of organic solar cells. Solar Energy Materials and Solar Cells 2009, 93, (8), 1263-1267. [CrossRef]
- Zhao, D. W.; Kyaw, A. K. K.; Sun, X. W., Organic Solar Cells with Inverted and Tandem Structures. In Energy Efficiency and Renewable Energy Through Nanotechnology, 2011; pp 115-170.
- Saliba, M.; Etgar, L., Current Density Mismatch in Perovskite Solar Cells. ACS Energy Letters 2020, 5, (9), 2886-2888. [CrossRef]
- Wang, L. M.; Li, Q.; Liu, S.; Cao, Z.; Cai, Y. P.; Jiao, X.; Lai, H.; Xie, W.; Zhan, X.; Zhu, T., Quantitative Determination of the Vertical Segregation and Molecular Ordering of PBDB-T/ITIC Blend Films with Solvent Additives. ACS Appl Mater Interfaces 2020, 12, (21), 24165-24173. [CrossRef]
- Inasaridze, L. N.; Shames, A. I.; Martynov, I. V.; Li, B.; Mumyatov, A. V.; Susarova, D. K.; Katz, E. A.; Troshin, P. A., Light-induced generation of free radicals by fullerene derivatives: an important degradation pathway in organic photovoltaics? Journal of Materials Chemistry A 2017, 5, (17), 8044-8050. [CrossRef]
Figure 1.
Schematic illustration of photoinduced energy and charge transfer mechanisms. Reproduced from Li, S. et al.l [
26].
Figure 1.
Schematic illustration of photoinduced energy and charge transfer mechanisms. Reproduced from Li, S. et al.l [
26].
Figure 2.
Reaction of the oxidation of Pd(0) organometallic complex precursor to obtain the Pd(I) additive (2).
Figure 2.
Reaction of the oxidation of Pd(0) organometallic complex precursor to obtain the Pd(I) additive (2).
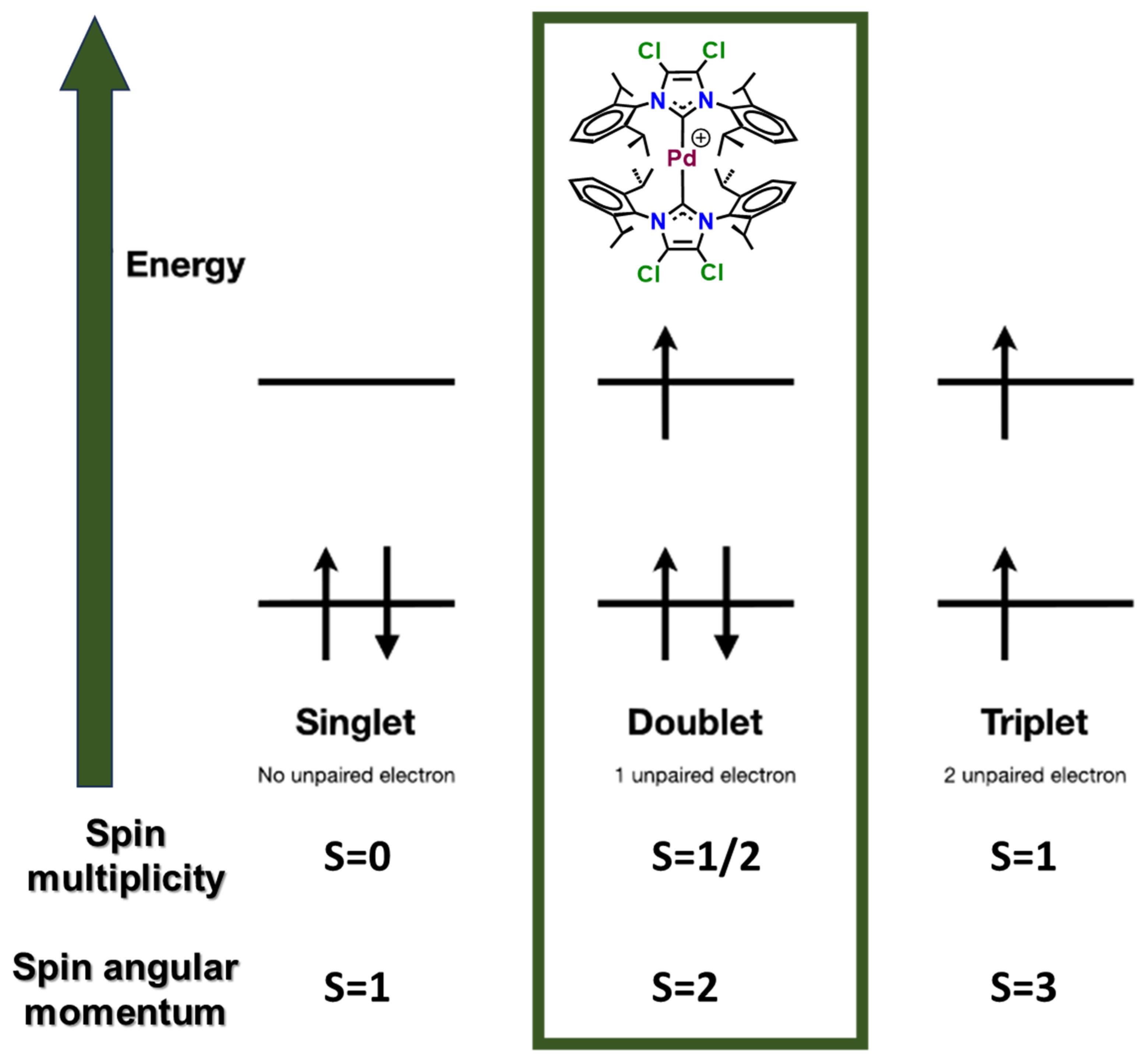
Figure 3.
Spin multiplicity and spin angular momentum of the different state configurations.
Figure 3.
Spin multiplicity and spin angular momentum of the different state configurations.
Figure 4.
a) Representation of the inverted architecture of OPV devices investigated in the work b) Diagram of energy levels for the solar cell stack.
Figure 4.
a) Representation of the inverted architecture of OPV devices investigated in the work b) Diagram of energy levels for the solar cell stack.
Figure 5.
a) J-V curves of reference (0 wt% Pd) and 1 wt% of Pd complex (2) b) FF box charts of different %wt Pd complex (2) c) Open-circuit voltage box charts of different %wt Pd complex (2) d) Short circuit current box charts of different %wt Pd complex (2).
Figure 5.
a) J-V curves of reference (0 wt% Pd) and 1 wt% of Pd complex (2) b) FF box charts of different %wt Pd complex (2) c) Open-circuit voltage box charts of different %wt Pd complex (2) d) Short circuit current box charts of different %wt Pd complex (2).
Figure 6.
Efficiency vs %wt of Pd complex (2) as additive inside the active layer. Box charts (left) and bar error (right).
Figure 6.
Efficiency vs %wt of Pd complex (2) as additive inside the active layer. Box charts (left) and bar error (right).
Figure 7.
Normalized IPCE and Integrated current density of different proportions of Pd inside the bulk heterojunction of the solar cell (a); IPCE (%) (b).
Figure 7.
Normalized IPCE and Integrated current density of different proportions of Pd inside the bulk heterojunction of the solar cell (a); IPCE (%) (b).
Figure 8.
Normalized Photoluminescence spectrum at different wavelengths of different proportions of Pd inside the bulk heterojunction of the solar cell.
Figure 8.
Normalized Photoluminescence spectrum at different wavelengths of different proportions of Pd inside the bulk heterojunction of the solar cell.
Figure 9.
Absorbance spectrum of different concentration of complex (2) inside the bulk heterojunction of the solar cell (a); Normalized Raman spectrum of different concentration of complex (2) (b).
Figure 9.
Absorbance spectrum of different concentration of complex (2) inside the bulk heterojunction of the solar cell (a); Normalized Raman spectrum of different concentration of complex (2) (b).
Figure 10.
SEM images of 10 µm of the active layer above the solar cell stack, with different concentrations of Pd(I) additive, with BSE detector; 5 keV.
Figure 10.
SEM images of 10 µm of the active layer above the solar cell stack, with different concentrations of Pd(I) additive, with BSE detector; 5 keV.
Figure 11.
Dark JV measurements of different proportions additive (2) in wt%
Figure 11.
Dark JV measurements of different proportions additive (2) in wt%
Figure 12.
a) Series resistance b) shunt resistance. Box charts of different wt% Pd complex.
Figure 12.
a) Series resistance b) shunt resistance. Box charts of different wt% Pd complex.
Figure 13.
Transient Photovoltage Decay of different concentrations of Pd dopant complex . Photo-voltage time decay as a function of pulse light intensity.
Figure 13.
Transient Photovoltage Decay of different concentrations of Pd dopant complex . Photo-voltage time decay as a function of pulse light intensity.
Figure 14.
- Photostability studies with light soaking instrument with an exponential decay model equation of the different trends. Normalized MPPT trends.
Figure 14.
- Photostability studies with light soaking instrument with an exponential decay model equation of the different trends. Normalized MPPT trends.
Table 1.
Different proportions of the Pd(I) complex additive in wt% and mg/L with the active layer P3HT:PC60BM.
Table 1.
Different proportions of the Pd(I) complex additive in wt% and mg/L with the active layer P3HT:PC60BM.
| [Pd]/[P3HT:PC60BM] wt% |
[Pd] in the active layer solution (µg/mL) |
| 0 (Ref) |
0 |
| 0.05 |
10 |
| 0.10 |
20 |
| 0.50 |
100 |
| 1.00 |
200 |
| 1.50 |
300 |
| 2.00 |
400 |
| 3.00 |
600 |
| 4.00 |
800 |
| 6.00 |
1200 |
Table 2.
Energies (in eV) of the highest occupied and lowest unoccupied molecular orbitals (MOs) of [Pd(IPrCl2)2]+ complex. The highest occupied alpha MO is the SOMO.
Table 2.
Energies (in eV) of the highest occupied and lowest unoccupied molecular orbitals (MOs) of [Pd(IPrCl2)2]+ complex. The highest occupied alpha MO is the SOMO.
| Highest occupied |
Lowest unoccupied |
| alpha |
beta |
alpha |
beta |
| -5.75 |
-5.94 |
-1.38 |
-3.62 |
Table 3.
Detailed photovoltaic parameters of the OPV cells with different concentration of Pd complex (2).
Table 3.
Detailed photovoltaic parameters of the OPV cells with different concentration of Pd complex (2).
| wt% [Pd] |
Voc (V) |
Jsc (mA cm-2) |
FF (%) |
Eff (%) |
| 0.00 (Ref) |
0.509 ± 0.049 |
6,11 ± 0,31 |
46,8 ± 6,4 |
1,46 ± 0,32 |
| 0.05 |
0.532 ± 0.043 |
6,13 ± 0,08 |
47,2 ± 3,7 |
1,55 ± 0,25 |
| 0.10 |
0.550 ± 0.033 |
6,43 ± 0,19 |
53,4 ± 4,4 |
1,83 ± 0,25 |
| 0.50 |
0.558 ± 0.011 |
6,40 ± 0,27 |
54,1 ± 3,1 |
2,00 ± 0,12 |
| 1.00 |
0.559 ± 0.023 |
6,54 ± 0,28 |
56,1 ± 9,5 |
2,04 ± 0,29 |
| 1.50 |
0.550 ± 0.029 |
5,84 ± 0,33 |
55,1 ± 5,4 |
1,78 ± 0,34 |
| 2.00 |
0.495 ± 0.068 |
6,01 ± 0,44 |
49,5 ± 4,3 |
1,54 ± 0,38 |
| 3.00 |
0.479 ± 0.039 |
6,04 ± 0,48 |
51,2 ± 8,9 |
1,42 ± 0,37 |
| 4.00 |
0.451 ± 0.024 |
5,40 ± 0,36 |
44,5 ± 2,2 |
1,09 ± 0,15 |
| 6.00 |
0.393 ± 0.020 |
4,65 ± 0,23 |
42,0 ± 2,7 |
0,73 ± 0,07 |
Table 4.
Comparison of Jsc from Sun Simulator and JV Integrated from IPCE curve.
Table 4.
Comparison of Jsc from Sun Simulator and JV Integrated from IPCE curve.
| wt% [Pd] |
Jsc (mA/cm-2) - JV Curve |
J Integrated (mA/cm-2) - IPCE |
Deviation value (%) |
| 0.0 (Ref) |
6,11 ± 0,31 |
8.10 |
33 |
| 0.1 |
6,43 ± 0,19 |
7.93 |
23 |
| 1.0 |
6,54 ± 0,28 |
8.08 |
24 |
| 4.0 |
5,40 ± 0,36 |
6.19 |
15 |
Table 5.
Different thicknesses considering Pd(I) dopant.
Table 5.
Different thicknesses considering Pd(I) dopant.
| [Pd]/[P3HT:PC60BM] (wt%) |
ITO/ZnO/PEIE/ PBDB-T:ITIC:Pd (nm) |
ITO/ZnO/PEIE/ PBDB-T:ITIC :Pd/PEDOT:PSS (nm) |
| 0 (Ref) |
107 ± 3 |
126 ± 1 |
| 0.1 |
103 ± 4 |
119 ± 1 |
| 1.0 |
104 ± 4 |
124 ± 3 |
| 3.0 |
106 ± 2 |
117 ± 2 |
| 6.0 |
106 ± 2 |
116 ± 1 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).