1. Introduction
Inflammation has been shown to be one of the key factors for disease pathogenesis or progression in several common, complex diseases such as Alzheimer’s disease (AD) and type 1 diabetes (T1D). Viral-induced inflammation due to the double-stranded DNA herpes simplex virus 1 (HSV-1) has been associated with AD risk [
1,
2,
3,
4], whereas viral-induced inflammation due to the single-stranded RNA coxsackie B virus (CVB) has been associated with T1D risk [
5,
6]. In parallel, embryonic stem cell or induced stem cell-derived models such as 3D cerebral organoids and stem cell-derived islets (sc-islets) have been shown to be promising in vitro human cell systems for studying the molecular and cellular effects of viral-induced inflammation that are relevant for common, complex diseases, especially when primary tissue from living human donors may not be easily accessible [
4,
7,
8,
9].
Previously, we used dissociated cells from 3D cerebral organoids (termed as dcOrgs) and found that HSV-1 infection in dcOrgs led to several molecular and cellular phenotypes associated with AD neuropathology [
4]. However, viral infections in dcOrgs by using the single-stranded RNA virus influenza A (IAV) did not result in these AD-associated phenotypes. This suggest that AD pathology can be activated by only some viruses, thus implicating specific host responses toward these viruses that are important for disease pathology. Interestingly, we also observed that dcOrg samples that were infected with HSV-1 and treated with the anti-viral drug acyclovir (ACV), as well as dcOrg samples with UV-inactivated HSV-1, had differential expression in transcripts associated with autoimmune diseases such as T1D and rheumatoid arthritis (RA). This indicates that the presence of HSV-1 viral constructs is sufficient to elucidate host inflammatory programs that are shared amongst different autoimmune diseases. As such, we sought to explore the global transcriptomic profile of HSV-1-induced inflammation in sc-islets and compared bulk RNA sequence (RNA-seq) data from HSV-1 infection (with and without ACV treatment) of sc-islets and dcOrgs to understand the perturbed gene networks and pathways that are in common or specific to each stem cell-derived system.
2. Materials and Methods
2.1. Cerebral organoid differentiation and dissociation
We used a single donor induced pluripotent stem cell (iPSC) line for differentiating into dcOrgs. The donor line was obtained from the Harvard Personal Genome Project (HUID: hu43860C, PGP#: 1), which is a unique resource for sharing genomes, phenotypes and cell-lines for research [
4,
7,
10,
11,
12,
13,
14,
15,
16]. 3D cerebral organoids were differentiated using a previously published protocol by Lancaster
et al. [
4,
7,
17]. Embryoid bodies comprising of ~9,000 cells each, were formed in 96-well ultra-low attachment plates and transferred to 24-well ultra-low attachment plates with 500 μL of neural induction media on Day 6. Subsequently, the organoids were embedded in 40 μL of Matrigel four days later and placed in 2 mL of differentiation media on an orbital shaker at 90 rpm for differentiation. After 2-4 months of differentiation, we dissociated the 3D cerebral organoids using our previously reported protocol [
4,
16]. Briefly, the organoids were washed in cold 1×DPBS for 10 minutes and then incubated in 500 μL of 0.25% Trypsin-EDTA for 15 minutes to dissociate the organoids. Dissociated cells were passed through a 30 μm cell strainer prior to replating on Matrigel-coated plates. Following dissociation, cells were passaged for at least a month to allow recovery.
2.2. sc-islet Differentiation
The luciferase expressing ES cell line HVRDe008-A-1 (GAPluc) [
18] were maintained and differentiated as described, protocol v8 [
19]. The glucose-stimulated insulin secretion index, calculated by dividing the amount of insulin secreted following incubation in high glucose (20 mM) by that following incubation with basal glucose (2.8 mM), was 1.5 on Stage 6 Day 15.
2.3. HSV-1 Viral Infection and ACV Treatment of dcOrgs
In our work, we used the HSV-1 K26GFP KOS strain that was generously provided by David Knipe and Prashant Desai [
20,
21,
22]. The virus has a green fluorescent protein (GFP) fused in frame with the viral UL35 ORF such that it will express a VP26-GFP fusion protein during late infection. Dissociated cells dcOrgs were infected according to our previously reported protocol [
4]. Briefly, 1×10
6 dcOrgs per well were plated onto Matrigel-coated 6-well plates and we performed HSV-1 inoculation for 1 hour using a multiplicity of infection (MOI) of 2. 200 μM of ACV was added to some of the HSV-1-infected wells immediately. After an hour, inoculation media was removed and the dcOrgs were rinsed with 1×DPBS, and differentiation media was added to the dcOrgs for 23 hours. For the ACV-treated dcOrgs, 200 μM of ACV was added to the differentiation media for 23 hours. We confirmed that the dcOrgs were infected using a fluorescence microscope the next day.
2.4. HSV-1 Viral Infection and ACV Treatment of sc-islets
We used the same HSV-1 K26GFP KOS strain for viral infections with the sc-islets. 500,000 cells per well were plated in low attachment 24-well plates and inoculated with HSV-1 for 1 hour using a MOI of 5 in incomplete CMRL 1066 media. 200 μM of ACV was added to some of the HSV-1-infected cells immediately. After an hour, inoculation media was removed and replaced with CMRL media supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin solution, and 1% GlutaMAX. 200μM of ACV was added to the cells that were infected with HSV-1 and treated with ACV. The cells were harvested after 48 hours.
2.5. Immunofluorescence Staining and Imaging of sc-islets
Infected sc-islets were immediately washed once with 500 μL phosphate-buffered saline (PBS), centrifuged at 300×
g for 3 minutes, then were resuspended in 500 μL 10% buffered formalin for 30 minutes, then embedded in paraffin and sectioned in the UMass Chan Morphology Core laboratory (
www.umassmed.edu/morphology/protocols). Sections were deparaffinized with xylene, rehydrated with ethanol, and heated in antigen retrieval buffer (#S2367, Dako). Slides were then blocked with 1% bovine serum albumin (BSA) in PBS, washed with PBS, and incubated with chicken anti-GFP (#ab13970, Abcam) and rabbit anti-insulin (#C27C9, Cell Signaling Technology) conjugated to Alexa Fluor 647 (#9008S, Cell Signaling Technology) in 1% BSA in PBS at 4
oC overnight. After washing with PBS, slides were incubated with anti-chicken Alexa Fluor 488 secondary antibody (#A11039, Invitrogen) at 1:500 dilution and 1 μg/mL of DAPI (#62248, ThermoFisher Scientific) for 1 hour in the dark and washed with PBS. Stained slides were then washed with PBS, mounted on microscope slip covers with Prolong Diamond Antifade Mountant (#P36961, Invitrogen) and cured at room temperature in the dark overnight. Imaging was performed using a Nikon Eclipse Ti series microscope (10× objective) and a Leica DMi8 THUNDER Imager microscope (40× objective).
2.6. RNA Extraction and Library Preparation
We fixed the cells with 4% paraformaldehyde and extracted RNA using the PureLinkTM FFPE RNA Isolation Kit, followed by DNase I treatment. We shipped all total RNA samples overnight on dry ice to Psomagen for quantification, ribosomal RNA depletion and library preparation, followed by 151bp paired-end sequencing with a total of 40 million reads on a NovaSeq6000.
2.7. RNA-seq alignment and analyses
As previously described [
4], we performed pre-processing using FastQC [
23], Trimmomatic v0.39 [
24], HISAT2 v2.2.1 [
25] and SAMtools v1.9 [
26]. For reference alignment, we used a concatenated GRCh37 human reference genome and human alphaherpesvirus 1 Kos strain reference genome (GenBank: JQ673480.1). We performed expression quantification using StringTie2 v1.3.6 [
27]. Pearson’s correlations
were calculated using the fragments per kilobase of exon per million mapped fragments (FPKM) values between sample pairs. Schematic figures were created using BioRender. All other figures were created using R or Python.
For the rescued analyses, we performed conditional analyses by identifying the DEGs between HSV-1-infected versus HSV-1-infected and ACV-treated samples. Next, DEGs between HSV-1-infected versus uninfected samples that were significant (adjusted P≤0.05) and had the same fold change direction (up-regulated or down-regulated), were classified as rescued.
Pathway analysis on DEG lists were performed using g:Profiler with g:SCS multiple testing correction and a significance threshold of 0.05 [
28]. DEGs were queried against the Gene Ontology knowledgebase [
29]. We excluded pathways with electronic GO annotations and only included annotated genes in the domain scope. Pathways surpassing the significance threshold were filtered to only include pathways with a term size of less than 500 and more than 15 genes.
3. Results
3.1. High Correlations Were Observed across RNA-seq Data from sc-islets
Previously, we detected high correlations in RNA-seq data from different dcOrg replicates using our approach [
4], and sought to similarly evaluate the correlations in RNA-seq data from different sc-islet replicates. We observed that using a MOI of 5, ~40% of islets were infected after 24 hours of infection (not shown) and ~80% of sc-islets, including insulin-positive cells, were infected after 48 hours of infection (
Figure 1A,B). Subsequently, we harvested sc-islets after 48 hours of infection for RNA extraction and sequencing. From the RNA-seq data on the sc-islets, we found that 70 ± 11% of all transcripts in the triplicate HSV-1-infected sc-islets were viral transcripts, and 1.5 ± 0.4% of all transcripts in the triplicate ACV-treated (HSV-1-infected and ACV-treated) sc-islets were viral transcripts. Previously, we observed that using a MOI of 2 resulted in >50% of infected dcOrgs after 24 hours of infection, and 60-81% of all transcripts in HSV-1-infected dcOrgs were viral transcripts, whereas 0.15-3% of all transcripts in treated dcOrgs were viral transcripts [
4].
Principal components analyses (PCA) showed that 87.8% of the variation was captured by the first principal component (PC1) and that the infection status of the sc-islets correlated with PC1 (
Figure 1C). Pearson’s correlations (
) were high between replicate samples with the same condition (
=0.85-0.99), whereas correlations were lower between uninfected versus infected samples (
=0.36-0.59), treated versus infected samples (
=0.47-0.87) and treated versus uninfected samples (
=0.72-0.96), as shown in
Table S1.
3.2. Differentially Expressed Genes in HSV-1-Infected versus Uninfected sc-islets Were Enriched for Autoimmune Disease-Associated Genes.
We computed the list of differentially expressed genes (DEGs) from HSV-1-infected versus uninfected sc-islets (
Table S2). We tested if DEGs were enriched for any lists of genes associated with 21 common diseases and traits from the GWAS Catalog (
Figure 1D,
Table S3). We found enrichment of the DEGs for several autoimmune disease-associated genes, including for T1D, P=5.6×10
-3; RA, P=0.02; psoriasis (PSO), P=0.026; Crohn’s disease (CD), P=0.045; as well as a neurodegenerative and autoimmune disease multiple sclerosis (MS), P=0.022. Unlike our prior results from dcOrgs, we did not observe enrichment of the DEGs from HSV-1-infected versus uninfected sc-islets for AD-associated genes (P=0.69).
There were six DEGs in common across T1D, RA, PSO, CD, and MS-associated genes: IRF1, CD226, RUNX3, KEAP1, ZMIZ1, and ATXN2. These 6 genes are involved in host response to viral infection, such as cytokine production and oxidative stress response, leading to cell death. However, the GSEA results did not change significantly after removing these six DEGs (T1D P=7×10
-3; RA P=0.039; PSO P=0.025; CD P=0.062; MS P=0.034), indicating that the enrichment of DEGs was likely to be driven by several genes that were exclusively associated with each autoimmune disease. The T1D-specific genes include the insulin gene (INS), which was significantly decreased in HSV-1 infected sc-islets (log
2 fold change = -2.7, adjusted P=4.1×10
-3). Additional T1D-specific genes are shown in
Table S4.
3.3. ACV Treatment of HSV-1-Infected sc-islets Did Not Rescue Human Transcript Differential Expression.
If ACV treatment was effective in rescuing human transcript expression associated with these autoimmune diseases in HSV-1-infected sc-islets, we would expect DEGs from HSV-1-infected versus HSV-1-infected and ACV-treated sc-islets (Islet_Inf-vs-ACV) to be similarly enriched in these autoimmune disease associated genes. However, when we conducted Gene Set Enrichment Analysis (GSEA) using the Islet_Inf-vs-ACV DEGs, we observed no enrichment for any of these six autoimmune disease-associated genes (T1D P=0.17; RA P=0.75; PSO P=0.45; CD P=0.37; MS P=0.67).
Alternatively, if ACV treatment was not effective in rescuing human transcript expression associated with these autoimmune diseases, we would expect to observe an enrichment of DEGs from ACV-treated versus uninfected sc-islets (Islet_ACV-vs-Ctrl). For this analysis, GSEA showed no enrichment of the DEGs for these select autoimmune disease-associated genes (T1D P=0.095; RA P=0.74; PSO P=0.24; CD P=0.25; MS P=0.052). These results suggest that 200 μM ACV treatment to inhibit HSV-1 DNA replication in sc-islets may not be sufficient to rescue human transcript expression in genes associated with T1D and potentially other autoimmune diseases.
These results are in contrast to our prior results with ACV treatment on HSV-1-infected dcOrgs where we observed a dosage-dependent effect of ACV-treatment on rescuing perturbations in AD-associated transcripts [
4]. We previously found that a set of ACV-treated HSV-infected dcOrgs with low abundance of viral transcripts (0.15-0.17% of all transcripts mapped to viral transcripts) showed an enrichment of DEGs associated with AD when compared to HSV-1-infected dcOrgs, which indicated a rescue of human transcript expression associated with AD. A second set of ACV-treated HSV-infected dcOrgs with higher abundance of viral transcripts detected (2.4-3% of all transcripts mapped to viral transcripts) did not show an enrichment of DEGs associated with AD when compared to HSV-1-infected dcOrgs, which indicated a lack of rescue. However, we observed an enrichment of DEGs associated with AD when we compared the second set of ACV-treated dcOrgs with uninfected dcOrgs, which indicated that there was a dosage-dependent effect of ACV treatment on rescuing transcript perturbations in AD-associated genes. These results also suggest that 200 μM ACV treatment to inhibit HSV-1 DNA replication in sc-islets may not be sufficient to rescue human transcript expression in genes associated with T1D and potentially other autoimmune diseases.
3.4. Analyses of DEGs That Are in Common between HSV-1-Infected dcOrgs and sc-islets Revealed Genes Involved in Amyloid-Beta Clearance, RNA Metabolic, Processing and Stability, as Well as Mitochondria Function
We further compared the RNA-seq data from the islets to our previously generated dcOrgs RNA-seq data [
4]. In our dcOrgs data, we had two sets of replication experiments for each comparison (dcOrgs1 and dcOrgs2) and we compared the DEGs in sc-islets with dcOrgs1 or sc-islets with dcOrgs2 that were significant (adjusted P-value ≤ 0.05) and had log
2 fold changes in the same direction (up-regulated or down-regulated).
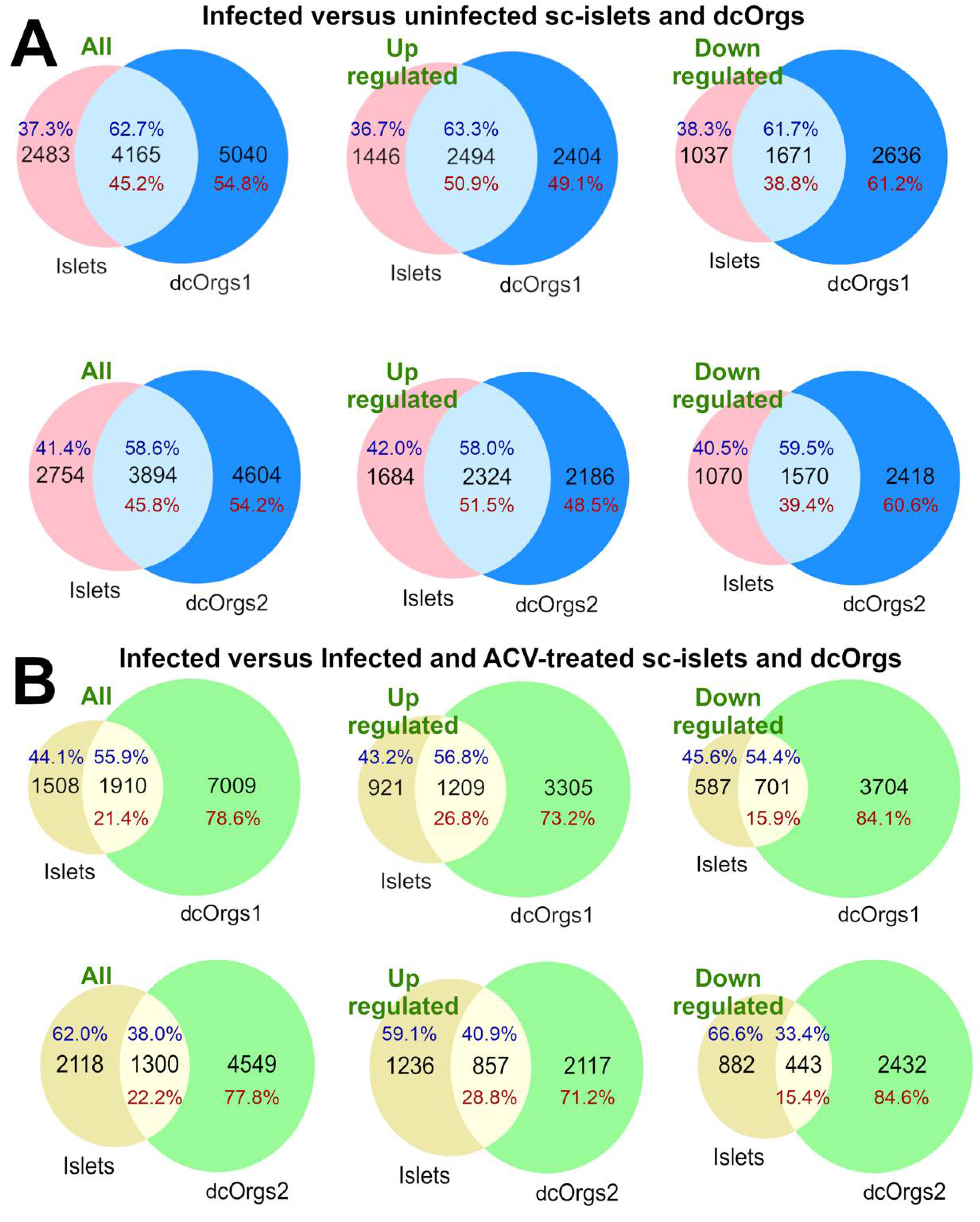
We found that 45.2% and 45.8% of the genes that were DEGs in HSV-1-infected versus uninfected dcOrgs were also DEGs in HSV-1-infected versus uninfected sc-islets, as shown in
Figure 2A. More up-regulated DEGs were common to both dcOrgs and sc-islets compared to down-regulated DEGs (50.9% versus 38.8% for dcOrgs1, and 51.5% versus 39.4% for dcOrgs2; odds ratio (OR)=1.6; Fisher’s Exact Test P<1×10
-10 for both replicates;
Table S5).
3.5. Proportionally Fewer Common Human DEGs Are Rescued with ACV Treatment of HSV-1-Infected sc-islets Compared to dcOrgs
Next, we wondered about the effects of ACV treatment on HSV-1-infected sc-islets versus dcOrgs. We used the DEGs identified from HSV-1-infected and ACV-treated sc-islets or dcOrgs, versus HSV-1-infected sc-islets or dcOrgs for these comparisons. We observed that fewer DEGs were common to both the sc-islets and dcOrgs (21.4% for dcOrgs1 and 22.2% for dcOrgs2,
Figure 2B), compared to DEGs in common between HSV-1-infected sc-islets and dcOrgs (
Figure 2A). These results indicated that ACV treatment of HSV-1-infected dcOrgs had more tissue-specific effects and therefore, fewer DEGs were attributed to ACV treatment that were in common between dcOrgs and sc-islets.
However, there were still proportionally more up-regulated DEGs than down-regulated DEGs that were in common between treated sc-islets and dcOrgs (26.8% versus 15.9% for dcOrgs1, and 28.8% versus 15.4% for dcOrgs2; OR=1.9 and 2.2; Fisher’s Exact Test P<1×10
-10 for both replicates,
Table S5).
3.6. ACV Treatment of HSV-1-Infected sc-islets Did Not Completely Inhibit True Late Viral Gene Expression
Previously, we found that ACV treatment on our second set of dcOrgs (dcOrgs2) was not as effective as ACV treatment on our first set of dcOrgs (dcOrgs1) [
4]. Since ACV primarily inhibits transcript expression of HSV-1 true late viral genes, we observed significant differences in the ranked expression of true late viral genes between both sets of dcOrgs but did not observe significant differences in the ranked expression of leaky late viral genes between both sets of dcOrgs [
4]. We compared the ranked expression of leaky late or true late viral genes between the sc-islets and both sets of dcOrgs (
Table S6), and found that there was a strong correlation in the expression of leaky late viral genes between sc-islets and dcOrgs1 or dcOrgs2 (P=3.4×10
-4 and 4.1×10
-4 respectively). However, the ranked expression of true late viral genes in the sc-islets were more similar to those in dcOrgs2 (P=7.7×10
-3) than dcOrgs1 (P=0.11). These results indicate that ACV treatment at the dosage and duration used in our study was not as effective on inhibiting true late viral gene expression in the sc-islets.
3.7. Gene Ontology Analyses of DEGs in HSV-1-Infected sc-islets and dcOrgs Identified Common Functional Categories Involved in the Transferase Complex, Mitochondrial and Autophagy Function
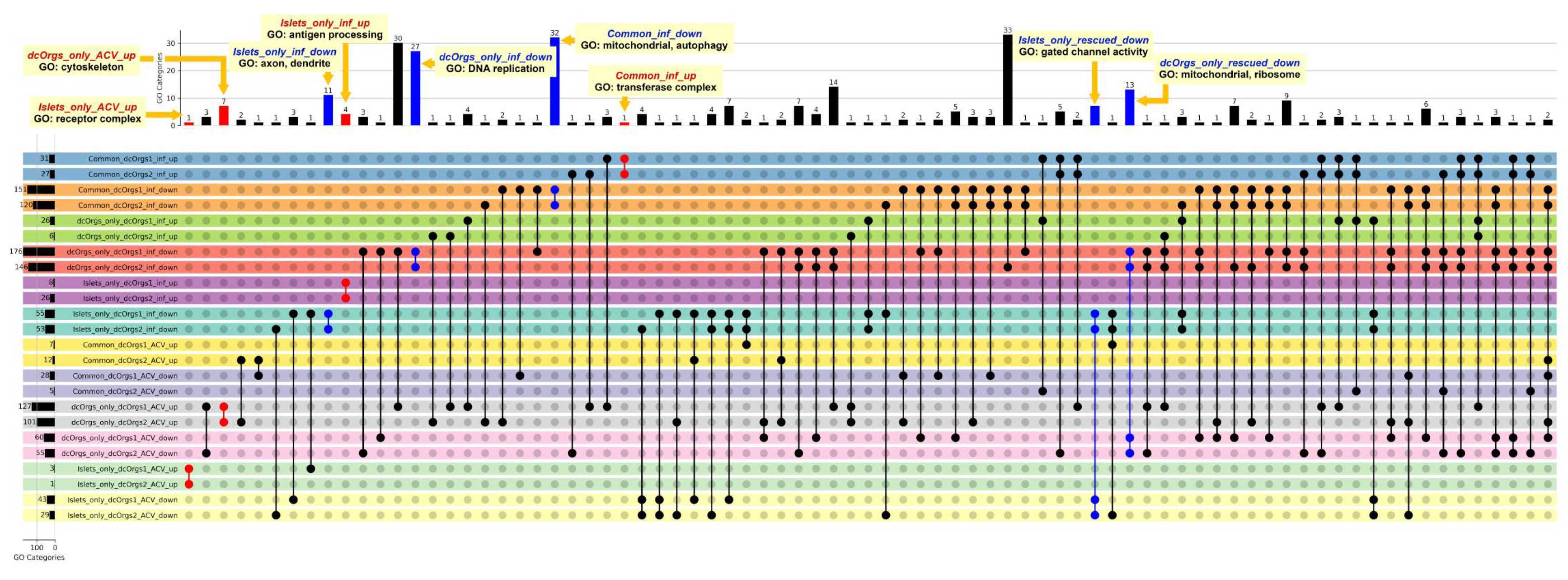
Next, we performed gene ontology (GO) enrichment analyses to identify the distinct GO categories that were enriched among the various groups of DEGs (
Figure 3). The GO categories that were up-regulated in common between HSV-1-infected sc-islets and HSV-1-infected dcOrgs were in the transferase complex involved in transferring phosphorus-containing groups (Common_inf_up;
Table S7). Phosphatases are necessary for viral entry into cells or cell-to-cell spread and it was reported that phosphorylation can modify regulatory protein activity in infected cells [
30,
31]. The GO categories that were down-regulated in common between sc-islets and dcOrgs included mitochondrial and autophagy function (Common_inf_down), which are key processes that are critical for innate immune activation, but are also reported to be hijacked by viruses to suppress innate immunity. [
32,
33,
34].
The bar graph at the top shows the numbers of shared GO categories across the DEG results, and the UpSet plot shows the DEG results that share these distinct GO categories. Shaded circles indicate the DEG result that share the GO category; the red shaded circles highlight the up-regulated DEG results with GO categories that were mentioned in the main text; the blue shaded circles highlight the down-regulated DEG results with GO categories that were mentioned in the main text. The first four rows in the UpSet plot show the shared GO categories for the up-regulated genes in common due to HSV-1 infection in sc-islets and both sets of dcOrg replicates (Common_dcOrgs1_inf_up and Common_dcOrgs2_inf_up), down-regulated genes in common in HSV-1-infected sc-islets and both dcOrg replicates (Common_dcOrgs1_inf_down and Common_dcOrgs2_inf_down). The next eight rows in the UpSet plot show the shared GO categories for up-regulated or down-regulated genes exclusively in dcOrgs only, or exclusively in islets only. The results for the HSV-1-infected and ACV-treated datasets are shown in the next 12 rows.
The GO categories that were up-regulated only in sc-islets included genes that were involved in antigen processing and presentation of peptide antigen, as well as response to bacterium (Islets_only_inf_up), which is consistent with prior observations in islets from T1D patients [
35,
36]. The GO categories that were down-regulated only in sc-islets included axon and dendrite development (Islets_only_inf_down), which is consistent with prior reports that impaired insulin signaling can affect dendrite and synaptic regeneration [
37,
38]. The GO categories that were down-regulated only in dcOrgs included DNA replication, recombination, helicase and conformation change (dcOrgs_only_inf_down).
We explored the direct effects of ACV treatment on sc-islets and dcOrgs, by comparing the GO categories that were enriched in HSV-1-infection and ACV treatment versus HSV-1-infection on sc-islets and dcOrgs (
Figure 3). The DEGs in these categories are comprised of human genes where the expression had been perturbed by ACV treatment (up-regulated or down-regulated). We observed that the categories that were up-regulated only in dcOrgs were involved in cytoskeleton and organelle organization (dcOrgs_only_ACV_up), whereas the categories that were up-regulated only in sc-islets were in receptor complexes (Islets_only_ACV_up), including cytokine receptors (IL23R, IL31RA, IL5RA) and toll-like receptors (TLR1, TLR7).
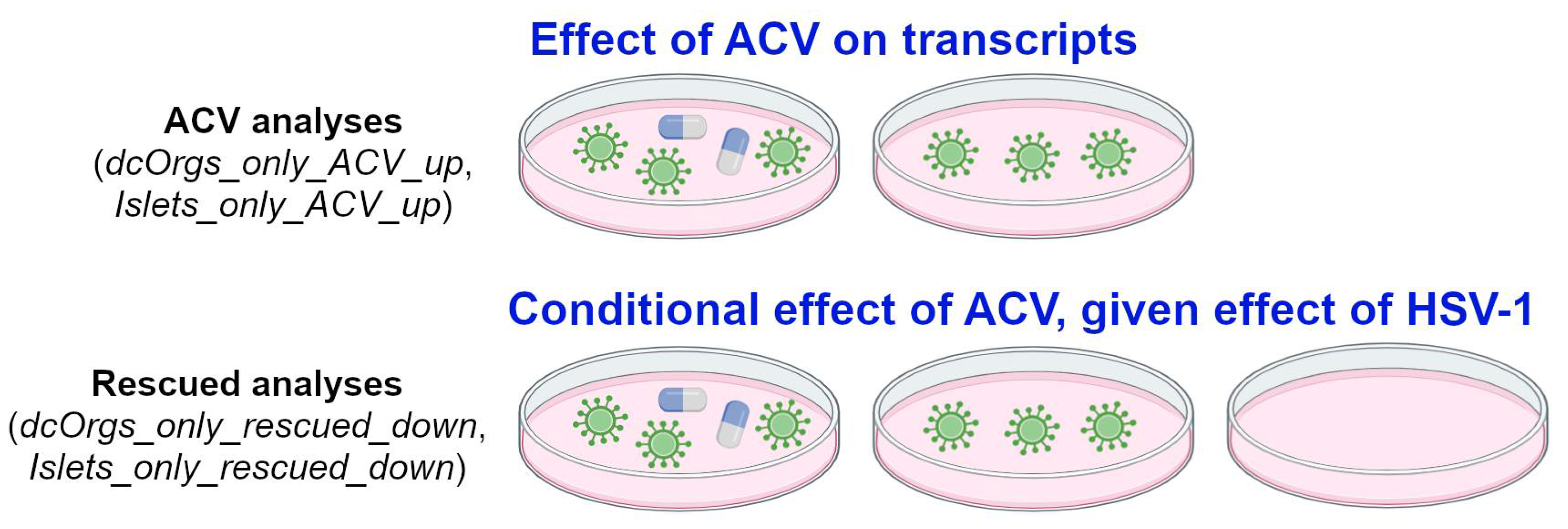
Finally, we explored the conditional effects of ACV treatment on sc-islets and dcOrgs (called “rescued analyses”), illustrated in
Figure 4. We compared the GO categories that were (1) enriched in HSV-1-infection and ACV treatment versus HSV-1-infection and (2) also enriched in HSV-1-infection versus uninfected in sc-islets and dcOrgs. The GO categories that were down-regulated due to HSV-1 infection and rescued by ACV treatment only in sc-islets included gated channel activity and monoatomic ion channel complex (Islets_only_rescued_down). On the other hand, the GO categories that were down-regulated due to HSV-1 infection and rescued only in dcOrgs included mitochondrial and ribosome subunits (dcOrgs_only_rescued_down).
For the ACV analyses e.g. dcOrgs_only_ACV_up and Islets_only_ACV_up, the comparisons aim to evaluate the effect of ACV treatment on human transcripts. For instance, if the expression of a gene is up-regulated due to ACV treatment, by comparing HSV-1-infected and ACV-treated samples with HSV-1-infected samples. For the rescued analyses, e.g. dcOrgs_only_rescued_down and Islets_only_rescued_down, the comparisons aim to evaluate the conditional effect of ACV treatment, given the effect of HSV-1-infection on human transcripts. For instance, if the expression of a gene is up-regulated due to HSV-1 infection, and the expression of the same gene is down-regulated by ACV treatment, then we would term the gene as a rescued gene.
3.8. Comparisons of Transcriptomic Perturbations by RNA and DNA viruses in sc-islets and dcOrgs Revealed Similarities in Gene Expression
HSV-1 is a double-stranded DNA virus, whereas CVB is a single-stranded RNA virus that had been associated with T1D pathogenesis. Previously, Nyalwidhe et al. infected primary or sc-islets with CVB and performed RNA-seq [
8,
9]. Similarly, we had infected dcOrgs with the RNA virus influenza A virus (IAV) and performed RNA-seq [
4]. We asked if the global human transcriptomic profiles of RNA viral infections in sc-islets and dcOrgs were more similar to the transcriptomic profiles of an RNA viral infection (CVB) in sc-islets versus a DNA viral infection (HSV-1) in dcOrgs. We found that human transcripts that were up-regulated by CVB infection in sc-islets were more enriched for up-regulated transcripts by IAV infection in dcOrgs versus HSV-1 infection in dcOrgs (odds ratios, OR=3.92 and 3.25, P=1.39×10
-5 and 1.96×10
-4 across two dcOrg replicates;
Table S8). On the other hand, human transcripts that were down-regulated by CVB infection in sc-islets remained proportionally unchanged for down-regulated transcripts by IAV infection in dcOrgs versus HSV-1 infection in dcOrgs (OR=1.17 and 1.15, P=0.57 and 0.67). These results indicated that there were more up-regulated human transcripts that were in common between the two RNA viruses (CVB and IAV) than between the RNA virus CVB and the DNA virus HSV-1.
Down-regulated transcripts due to CVB infection in sc-islets were enriched for down-regulated transcripts by HSV-1 infection in sc-islets versus HSV-1 infection in dcOrgs (OR=2.51 and 2.47, P=2.13×10-11 and 1.14×10-10 across two dcOrg replicates). On the other hand, there were modest enrichment of up-regulated transcripts due to CVB infection in sc-islets that were in common with HSV-1 infection in sc-islets versus HSV-1 infection in dcOrgs (OR=1.91 and 1.58, P=3.07×10-4 and 0.013 across two dcOrg replicates). These results indicated that more down-regulated transcripts were in common between viral infections in the same cell culture system (sc-islets) by CVB and HSV-1.
Up-regulated transcripts by HSV-1 infection in dcOrgs had more transcripts in common with HSV-1 infection in sc-islets than CVB infection in sc-islets (OR=6.67 and 5.41, P<2.2×10-16 for both dcOrg replicates). Down-regulated transcripts by HSV-1 infection in dcOrgs did not have a significant enrichment of transcripts that were in common with HSV-1 infection in sc-islets versus CVB infection in sc-islets (OR=0.82 and 0.87, P=0.046 and 0.19 for both dcOrg replicates). These results indicated that more up-regulated transcripts were in common between HSV-1 infection across both cell culture systems (islets and dcOrgs).
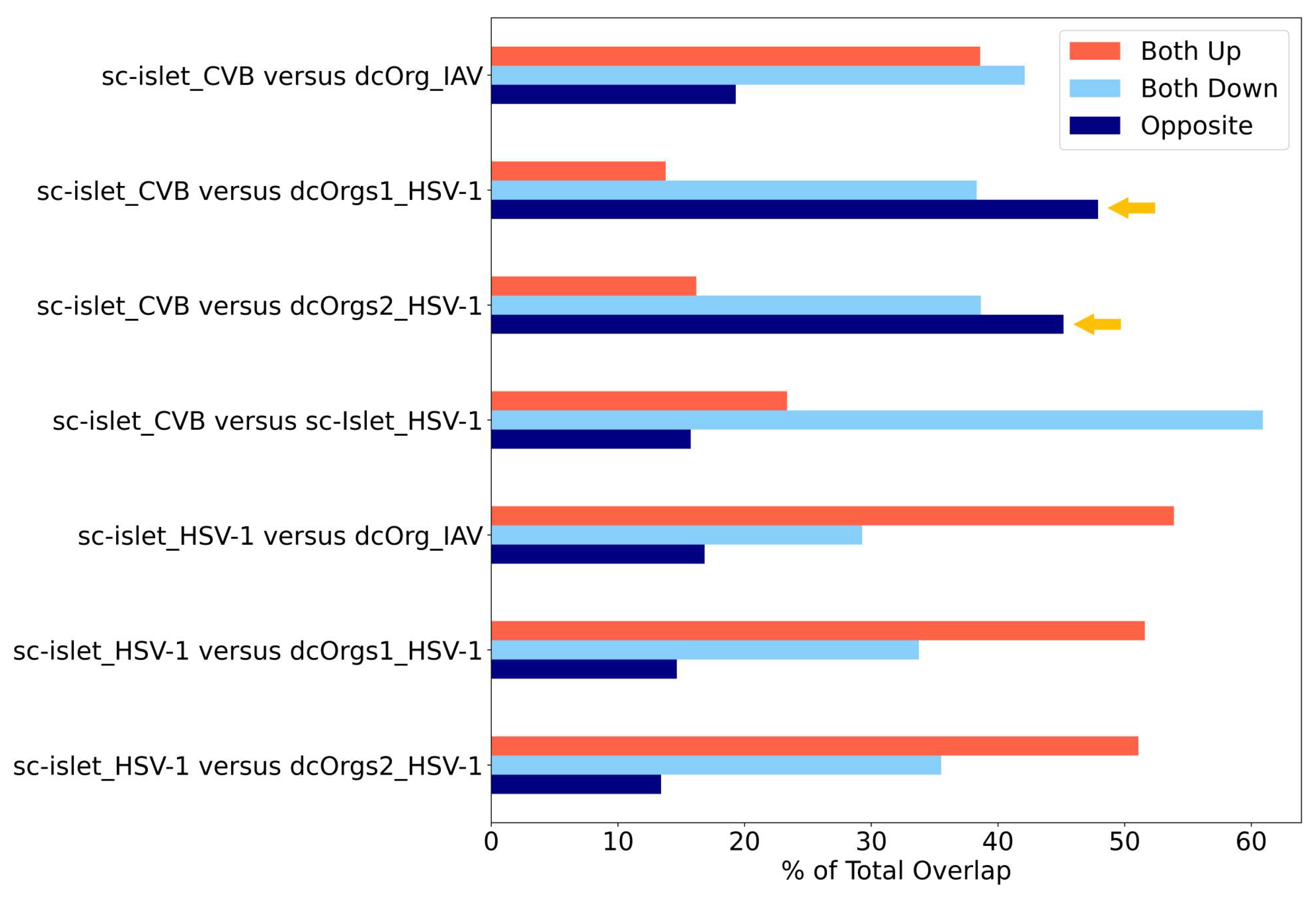
By using the percentage of transcripts that showed opposite directions as another measure for identifying conditions that are specific to each cell culture system, we observed that CVB-infected sc-islets and HSV-1-infected dcOrgs had the highest percentages of transcripts with discordant fold changes (
Figure 5). Collectively, these results showed that CVB-infected sc-islets may be a superior model for studying transcriptomic perturbations that are specific to the system and thus, may be more relevant for T1D-associated processes. On the other hand, HSV-1-infected dcOrgs may be great models for studying transcriptomic perturbations that are specific to the system and may be more relevant for AD-associated processes.
There are also several interesting results identified from our analyses. We can use HSV-1-infected sc-islets versus HSV-1-infected dcOrgs or IAV-infected dcOrgs to study up-regulated human transcripts that were in common (
Figure 5). These transcripts may be relevant to inflammation across multiple tissues and organs. Similarly, we can use CVB-infected sc-islets versus HSV-1-infected sc-islets to study down-regulated human transcripts that were in common, and these transcripts may be specifically relevant to inflammation in sc-islets.
The figure shows the percentages of DEGs that are up-regulated, down-regulated, or have fold change differences in opposite directions in sc-islet infected by CVB or HSV-1 or dcOrgs infected by IAV or HSV-1, normalized by the total number of DEGs. The systems with the highest discordance (dark blue bars that represent opposite direction), indicated by the yellow arrows, were CVB-infected sc-islets and HSV-1-infected dcOrgs.
4. Discussion
Virus-induced inflammation models using human embryonic stem cell or iPSC derived dcOrgs and sc-islets have shown to be valuable tools for studying molecular transcriptomic signatures and processes that had been associated with AD and T1D. Although AD and T1D are distinct diseases, there are interesting molecular similarities between AD and T1D. For instance, it has been reported consistently that innate immune activation in AD leading to neuronal loss is a key feature found in post-mortem brains from AD patients [
39]. Similarly, inflammation leading to pancreatic beta-cell loss associated with T1D had been reported [
40,
41]. Moreover, beta-amyloid (Aβ) buildup associated with AD can occur in both the brain and pancreas [
42].
We previously found that HSV-1 infected of dcOrgs led to transcriptomic perturbations associated with AD, and ACV treatment was sufficient to rescue these transcriptomic perturbations associated with AD in HSV-1-infected dcOrgs [
4]. In this study, we found that HSV-1-infection of sc-islets led to transcriptomic perturbations associated with T1D and other autoimmune diseases. ACV treatment was insufficient to rescue these transcriptomic perturbations associated with T1D in HSV-1-infected sc-islets.
CVB-infected sc-islets and HSV-1-infected dcOrgs had the highest percentages of transcripts with discordant fold changes, indicating that we may be able to study specific inflammation-induced transcriptomic processes that are unique to each system, and that these processes may inform us about disease-specific biology associated with T1D or AD. At the same time, we can use different viruses such as RNA viruses (CVB, IAV) or DNA viruses (HSV-1) across both cell culture systems, to perturb transcripts that may inform us about the underlying common biology of inflammation-induced changes across multiple tissues or diseases.
There are several future directions for our work, including direct comparisons of different DNA or RNA viruses (CVB, IAV, human cytomegalovirus, Zika virus, Sendai virus, HIV-1, SARS-CoV-2) in both cell culture systems. In addition, we can expand our work to exploring the use of cytokines or DNA/RNA mimetics in inducing autoimmune transcripts. In our current work, we differentiated dcOrgs and sc-islets from individual donors. In the future, we can explore these neuroinflammation-induced effects using organoids from multiple donors, in order to identify common processes across multiple donors. Single-cell RNA sequencing studies can also provide further delineation of cell types in which interesting transcripts are up-regulated or down-regulated in each system, to enable insights into the initiation of autoimmunity involved in disease pathogenesis and progression.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1: Correlations within or across conditions for the sc-islet RNA-seq data. Table S2: DESeq2 results for the sc-islet datasets. Table S3: GSEA nominal P-values from the sc-islet differential expression comparisons. Table S4: Genes contributing to the GSEA enrichment that are specific to T1D. Table S5: Combined DESeq2 results comparing sc-islets and dcOrgs. Table S6: Correlations in leaky late versus true late HSV-1 viral transcript expression between sc-islets and dcOrgs. Table S7: Gene ontology categories across comparison groups. Table S8: Comparisons of HSV-1, CVB and IAV infections in sc-islets and dcOrgs.
Author Contributions
Conceptualization, Jennifer Wang, Yingleong Chan and Elaine Lim; Formal analysis, Jonathan Sundstrom, Nathaniel Barton, Pepper Dawes, Benjamin Readhead, Jennifer Wang, Yingleong Chan and Elaine Lim; Funding acquisition, George Church, Benjamin Readhead, David Harlan, David Knipe, Jennifer Wang, Yingleong Chan and Elaine Lim; Investigation, Jonathan Sundstrom, Emma Vanderleeden, Liam Murray, Meagan Olson, Khanh Tran, Samantha Chigas, Adrian Orszulak, Jennifer Wang, Yingleong Chan and Elaine Lim; Methodology, Jonathan Sundstrom, Emma Vanderleeden, Nathaniel Barton, Sambra Redick, Pepper Dawes, Liam Murray, David Knipe, Jennifer Wang, Yingleong Chan and Elaine Lim; Resources, Sambra Redick, George Church, Benjamin Readhead, HyungSuk Oh, David Harlan and David Knipe; Supervision, George Church, David Harlan, David Knipe, Jennifer Wang, Yingleong Chan and Elaine Lim; Writing – original draft, Jonathan Sundstrom, Emma Vanderleeden, Jennifer Wang, Yingleong Chan and Elaine Lim; Writing – review & editing, Jonathan Sundstrom, Emma Vanderleeden, Nathaniel Barton, Sambra Redick, Pepper Dawes, Liam Murray, Meagan Olson, Khanh Tran, Samantha Chigas, Adrian Orszulak, HyungSuk Oh, Jennifer Wang, Yingleong Chan and Elaine Lim.
Funding
This research was funded by the National Institutes of Health (NIH) AMP-AD grant U01AG061835 (PI: Benjamin Readhead; Sub-contract PIs: George Church, Yingleong Chan, Elaine Lim), NIH P01 grant AI09681 (PI: David Knipe), JDRF COE-2020-967-M-N (PI: David Harlan; Sub-contract PI: Jennifer Wang) and UMass Chan Medical School startup funds (PIs: Elaine Lim, Yingleong Chan).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board and Institutional Biosafety Committee (IBC) of UMass Chan Medical School. HSV-1 is a Biosafety Level (BSL) 2 pathogen.
Data Availability Statement
The RNA sequence data for the HSV-1-infected sc-islets will be deposited into GEO and our scripts will be uploaded to GitHub.
Acknowledgments
We thank the Church lab (Harvard University) for generously providing the PGP1 iPSCs and the Melton lab (Harvard University) for generously providing the GAP luc.ES cell line.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Itzhaki, R.F.; Lin, W.R.; Shang, D.; Wilcock, G.K.; Faragher, B.; Jamieson, G.A. Herpes simplex virus type 1 in brain and risk of Alzheimer's disease. Lancet 1997, 349, 241–244. [Google Scholar] [CrossRef]
- Cairns, D.M.; Rouleau, N.; Parker, R.N.; Walsh, K.G.; Gehrke, L.; Kaplan, D.L. A 3D human brain-like tissue model of herpes-induced Alzheimer's disease. Sci Adv 2020, 6, eaay8828. [Google Scholar] [CrossRef]
- Eimer, W.A.; Vijaya Kumar, D.K.; Navalpur Shanmugam, N.K.; Rodriguez, A.S.; Mitchell, T.; Washicosky, K.J.; Gyorgy, B.; Breakefield, X.O.; Tanzi, R.E.; Moir, R.D. Alzheimer's Disease-Associated beta-Amyloid Is Rapidly Seeded by Herpesviridae to Protect against Brain Infection. Neuron 2018, 99, 56–63 e3. [Google Scholar] [CrossRef]
- Olson, M.N.; Dawes, P.; Murray, L.F.; Barton, N.J.; Sundstrom, J.; Orszulak, A.R.; Chigas, S.M.; Tran, K.; Aylward, A.J.; Caliandro, M.F.; et al. Development of a high-throughput, quantitative platform using human cerebral organoids to study virus-induced neuroinflammation in Alzheimer’s disease. bioRxiv, 2024. [Google Scholar] [CrossRef]
- Cinek, O.; Stene, L.C.; Kramna, L.; Tapia, G.; Oikarinen, S.; Witso, E.; Rasmussen, T.; Torjesen, P.A.; Hyoty, H.; Ronningen, K.S. Enterovirus RNA in longitudinal blood samples and risk of islet autoimmunity in children with a high genetic risk of type 1 diabetes: the MIDIA study. Diabetologia 2014, 57, 2193–2200. [Google Scholar] [CrossRef]
- Oikarinen, S.; Martiskainen, M.; Tauriainen, S.; Huhtala, H.; Ilonen, J.; Veijola, R.; Simell, O.; Knip, M.; Hyoty, H. Enterovirus RNA in blood is linked to the development of type 1 diabetes. Diabetes 2011, 60, 276–279. [Google Scholar] [CrossRef]
- Lim, E.T.; Chan, Y.; Dawes, P.; Guo, X.; Erdin, S.; Tai, D.J.C.; Liu, S.; Reichert, J.M.; Burns, M.J.; Chan, Y.K.; et al. Orgo-Seq integrates single-cell and bulk transcriptomic data to identify cell type specific-driver genes associated with autism spectrum disorder. Nature Communications 2022, 13, 3243. [Google Scholar] [CrossRef]
- Nyalwidhe, J.O.; Gallagher, G.R.; Glenn, L.M.; Morris, M.A.; Vangala, P.; Jurczyk, A.; Bortell, R.; Harlan, D.M.; Wang, J.P.; Nadler, J.L. Coxsackievirus-Induced Proteomic Alterations in Primary Human Islets Provide Insights for the Etiology of Diabetes. J Endocr Soc 2017, 1, 1272–1286. [Google Scholar] [CrossRef]
- Nyalwidhe, J.O.; Jurczyk, A.; Satish, B.; Redick, S.; Qaisar, N.; Trombly, M.I.; Vangala, P.; Racicot, R.; Bortell, R.; Harlan, D.M.; et al. Proteomic and Transcriptional Profiles of Human Stem Cell-Derived beta Cells Following Enteroviral Challenge. Microorganisms 2020, 8. [Google Scholar] [CrossRef]
- Smullen, M.; Reichert, J.M.; Dawes, P.; Wang, Q.; Readhead, B.; Church, G.M.; Lim, E.T.; Chan, Y. Reliable multiplex generation of pooled induced pluripotent stem cells for genetic testing. bioRxiv, 2022. [Google Scholar] [CrossRef]
- Ball, M.P.; Thakuria, J.V.; Zaranek, A.W.; Clegg, T.; Rosenbaum, A.M.; Wu, X.; Angrist, M.; Bhak, J.; Bobe, J.; Callow, M.J.; et al. A public resource facilitating clinical use of genomes. Proc Natl Acad Sci U S A 2012, 109, 11920–11927. [Google Scholar] [CrossRef]
- Ball, M.P.; Bobe, J.R.; Chou, M.F.; Clegg, T.; Estep, P.W.; Lunshof, J.E.; Vandewege, W.; Zaranek, A.; Church, G.M. Harvard Personal Genome Project: lessons from participatory public research. Genome Med 2014, 6, 10. [Google Scholar] [CrossRef]
- Mao, Q.; Ciotlos, S.; Zhang, R.Y.; Ball, M.P.; Chin, R.; Carnevali, P.; Barua, N.; Nguyen, S.; Agarwal, M.R.; Clegg, T.; et al. The whole genome sequences and experimentally phased haplotypes of over 100 personal genomes. Gigascience 2016, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.; Tung, M.; Garruss, A.S.; Zaranek, S.W.; Chan, Y.K.; Lunshof, J.E.; Zaranek, A.W.; Ball, M.P.; Chou, M.F.; Lim, E.T.; et al. An unbiased index to quantify participant's phenotypic contribution to an open-access cohort. Sci Rep 2017, 7, 46148. [Google Scholar] [CrossRef]
- Chan, Y.; Chan, Y.K.; Goodman, D.B.; Guo, X.; Chavez, A.; Lim, E.T.; Church, G.M. Enabling multiplexed testing of pooled donor cells through whole-genome sequencing. Genome Med 2018, 10, 31. [Google Scholar] [CrossRef]
- Dawes, P.; Murray, L.F.; Olson, M.N.; Barton, N.J.; Smullen, M.; Suresh, M.; Yan, G.; Zhang, Y.; Fernandez-Fontaine, A.; English, J.; et al. oFlowSeq: a quantitative approach to identify protein coding mutations affecting cell type enrichment using mosaic CRISPR-Cas9 edited cerebral organoids. Hum Genet, 2023. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc 2014, 9, 2329–2340. [Google Scholar] [CrossRef]
- Gerace, D.; Boulanger, K.R.; Hyoje-Ryu Kenty, J.; Melton, D.A. Generation of a heterozygous GAPDH-Luciferase human ESC line (HVRDe008-A-1) for in vivo monitoring of stem cells and their differentiated progeny. Stem Cell Res 2021, 53, 102371. [Google Scholar] [CrossRef]
- Veres, A.; Faust, A.L.; Bushnell, H.L.; Engquist, E.N.; Kenty, J.H.; Harb, G.; Poh, Y.C.; Sintov, E.; Gurtler, M.; Pagliuca, F.W.; et al. Charting cellular identity during human in vitro beta-cell differentiation. Nature 2019, 569, 368–373. [Google Scholar] [CrossRef]
- Macdonald, S.J.; Mostafa, H.H.; Morrison, L.A.; Davido, D.J. Genome sequence of herpes simplex virus 1 strain KOS. J Virol 2012, 86, 6371–6372. [Google Scholar] [CrossRef]
- Desai, P.; Person, S. Incorporation of the green fluorescent protein into the herpes simplex virus type 1 capsid. J Virol 1998, 72, 7563–7568. [Google Scholar] [CrossRef]
- Colgrove, R.C.; Liu, X.; Griffiths, A.; Raja, P.; Deluca, N.A.; Newman, R.M.; Coen, D.M.; Knipe, D.M. History and genomic sequence analysis of the herpes simplex virus 1 KOS and KOS1.1 sub-strains. Virology 2016, 487, 215–221. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: a quality control tool for high throughput sequence data. 2020. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc.
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; Genome Project Data Processing, S. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Kovaka, S.; Zimin, A.V.; Pertea, G.M.; Razaghi, R.; Salzberg, S.L.; Pertea, M. Transcriptome assembly from long-read RNA-seq alignments with StringTie2. Genome Biol 2019, 20, 278. [Google Scholar] [CrossRef] [PubMed]
- Kolberg, L.; Raudvere, U.; Kuzmin, I.; Adler, P.; Vilo, J.; Peterson, H. g:Profiler-interoperable web service for functional enrichment analysis and gene identifier mapping (2023 update). Nucleic Acids Res 2023, 51, W207–W212. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, K.W.; Kohn, A.; Sklyanskaya, E.; Roizman, B. Herpes simplex virus phosphoproteins. I. Phosphate cycles on and off some viral polypeptides and can alter their affinity for DNA. J Virol 1980, 33, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, J.C.; Yokota, H.; Craven, R.C.; Schmitt, A.; Wills, J.W. The HSV-1 mechanisms of cell-to-cell spread and fusion are critically dependent on host PTP1B. PLoS Pathog 2018, 14, e1007054. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Bowman, J.W.; Jung, J.U. Autophagy during viral infection - a double-edged sword. Nat Rev Microbiol 2018, 16, 341–354. [Google Scholar] [CrossRef]
- Foo, J.; Bellot, G.; Pervaiz, S.; Alonso, S. Mitochondria-mediated oxidative stress during viral infection. Trends Microbiol 2022, 30, 679–692. [Google Scholar] [CrossRef]
- Goswami, R.; Majumdar, T.; Dhar, J.; Chattopadhyay, S.; Bandyopadhyay, S.K.; Verbovetskaya, V.; Sen, G.C.; Barik, S. Viral degradasome hijacks mitochondria to suppress innate immunity. Cell Res 2013, 23, 1025–1042. [Google Scholar] [CrossRef]
- Li, Y.; Sun, F.; Yue, T.T.; Wang, F.X.; Yang, C.L.; Luo, J.H.; Rong, S.J.; Xiong, F.; Zhang, S.; Wang, C.Y. Revisiting the Antigen-Presenting Function of beta Cells in T1D Pathogenesis. Front Immunol 2021, 12, 690783. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.J.; Rodriguez-Calvo, T.; Gerling, I.C.; Mathews, C.E.; Kaddis, J.S.; Russell, M.A.; Zeissler, M.; Leete, P.; Krogvold, L.; Dahl-Jorgensen, K.; et al. Islet cell hyperexpression of HLA class I antigens: a defining feature in type 1 diabetes. Diabetologia 2016, 59, 2448–2458. [Google Scholar] [CrossRef]
- Agostinone, J.; Alarcon-Martinez, L.; Gamlin, C.; Yu, W.Q.; Wong, R.O.L.; Di Polo, A. Insulin signalling promotes dendrite and synapse regeneration and restores circuit function after axonal injury. Brain 2018, 141, 1963–1980. [Google Scholar] [CrossRef] [PubMed]
- Campbell-Thompson, M.; Butterworth, E.A.; Boatwright, J.L.; Nair, M.A.; Nasif, L.H.; Nasif, K.; Revell, A.Y.; Riva, A.; Mathews, C.E.; Gerling, I.C.; et al. Islet sympathetic innervation and islet neuropathology in patients with type 1 diabetes. Sci Rep 2021, 11, 6562. [Google Scholar] [CrossRef] [PubMed]
- Ising, C.; Heneka, M.T. Functional and structural damage of neurons by innate immune mechanisms during neurodegeneration. Cell Death Dis 2018, 9, 120. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, T.M.; Dror, E.; Schulze, F.; Traub, S.; Berishvili, E.; Barbieux, C.; Boni-Schnetzler, M.; Donath, M.Y. The Role of Inflammation in beta-cell Dedifferentiation. Sci Rep 2017, 7, 6285. [Google Scholar] [CrossRef] [PubMed]
- Roep, B.O.; Thomaidou, S.; van Tienhoven, R.; Zaldumbide, A. Type 1 diabetes mellitus as a disease of the beta-cell (do not blame the immune system?). Nat Rev Endocrinol 2021, 17, 150–161. [Google Scholar] [CrossRef]
- Verma, N.; Velmurugan, G.V.; Winford, E.; Coburn, H.; Kotiya, D.; Leibold, N.; Radulescu, L.; Despa, S.; Chen, K.C.; Van Eldik, L.J.; et al. Abeta efflux impairment and inflammation linked to cerebrovascular accumulation of amyloid-forming amylin secreted from pancreas. Commun Biol 2023, 6, 2. [Google Scholar] [CrossRef]
Figure 1.
Analyses of RNA-seq data from sc-islets showed enrichment of autoimmune disease genes. (A) Images of unfixed HSV-1-infected sc-islets, HSV-1-infected and ACV-treated sc-islets, and uninfected sc-islets. (B) Immunofluorescence staining of HSV-1-infected, HSV-1-infected and ACV-treated sc-islets, and uninfected sc-islets. (C) PCA plot of the sc-islet RNA-seq data to show clustering of uninfected (blue), infected (red) and HSV-1-infected and ACV-treated (orange) samples along PC1 (x-axis). (D) Plot to show GSEA results from comparisons of the sc-islet RNA-seq datasets across 21 common diseases. The y-axis shows the -log10(Nominal P-value from the GSEA result) and the x-axis shows each disease: AD for Alzheimer’s disease, ALS for amyotrophic lateral sclerosis, MS for multiple sclerosis, PD for Parkinson’s disease, ADHD for attention deficit hyperactivity disorder, ASD for autism spectrum disorder, BD for bipolar disorder, MDD for major depressive disorder, OCD for obsessive compulsive disorder, SCZ for schizophrenia, TS for Tourette’s syndrome, CD for Crohn’s disease, IBD for inflammatory bowel disease, PSO for psoriasis, RA for rheumatoid arthritis, T1D for type 1 diabetes, T2D for type 2 diabetes, BMI for body mass index, FBRAIN for FMRI brain measurements, HEIGHT for height, WAISTHIP for waist-to-hip ratio. The comparison groups are: HSV-1-infected and ACV-treated versus uninfected sc-islets (ACV-vs-Ctrl, in red), HSV-1-infected versus HSV-1-infected and ACV-treated sc-islets (Inf-vs-ACV), and HSV-1-infected versus uninfected sc-islets (Inf-vs-Ctrl, in blue).
Figure 1.
Analyses of RNA-seq data from sc-islets showed enrichment of autoimmune disease genes. (A) Images of unfixed HSV-1-infected sc-islets, HSV-1-infected and ACV-treated sc-islets, and uninfected sc-islets. (B) Immunofluorescence staining of HSV-1-infected, HSV-1-infected and ACV-treated sc-islets, and uninfected sc-islets. (C) PCA plot of the sc-islet RNA-seq data to show clustering of uninfected (blue), infected (red) and HSV-1-infected and ACV-treated (orange) samples along PC1 (x-axis). (D) Plot to show GSEA results from comparisons of the sc-islet RNA-seq datasets across 21 common diseases. The y-axis shows the -log10(Nominal P-value from the GSEA result) and the x-axis shows each disease: AD for Alzheimer’s disease, ALS for amyotrophic lateral sclerosis, MS for multiple sclerosis, PD for Parkinson’s disease, ADHD for attention deficit hyperactivity disorder, ASD for autism spectrum disorder, BD for bipolar disorder, MDD for major depressive disorder, OCD for obsessive compulsive disorder, SCZ for schizophrenia, TS for Tourette’s syndrome, CD for Crohn’s disease, IBD for inflammatory bowel disease, PSO for psoriasis, RA for rheumatoid arthritis, T1D for type 1 diabetes, T2D for type 2 diabetes, BMI for body mass index, FBRAIN for FMRI brain measurements, HEIGHT for height, WAISTHIP for waist-to-hip ratio. The comparison groups are: HSV-1-infected and ACV-treated versus uninfected sc-islets (ACV-vs-Ctrl, in red), HSV-1-infected versus HSV-1-infected and ACV-treated sc-islets (Inf-vs-ACV), and HSV-1-infected versus uninfected sc-islets (Inf-vs-Ctrl, in blue).
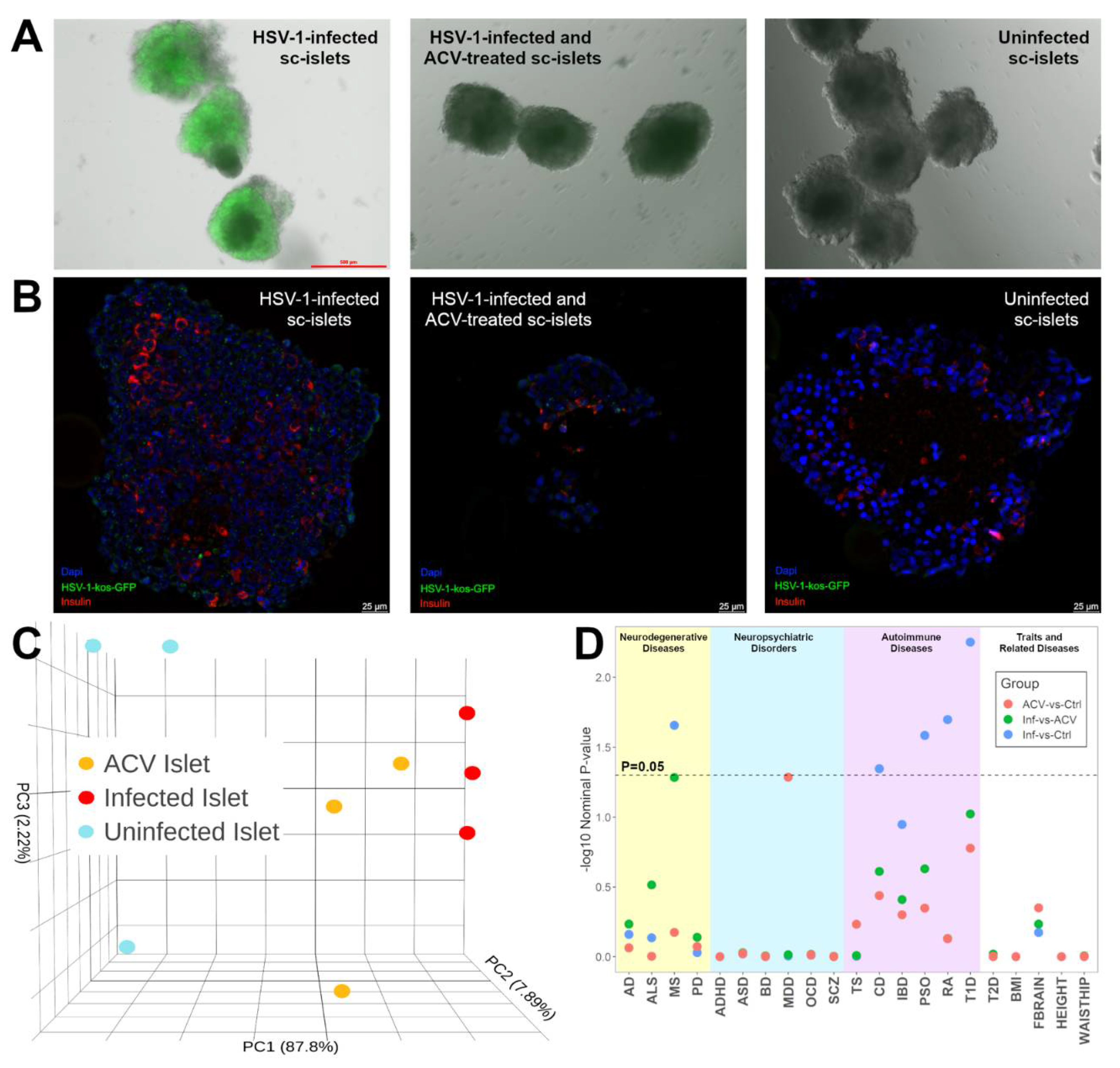
Figure 2.
Comparisons of DEGs from HSV-1 infection in sc-islets versus 2 dcOrg replicates (dcOrgs1 and dcOrgs2). (A) Venn diagrams showing the numbers of HSV-1-infected versus uninfected DEGs that are in common between sc-islets and dcOrgs1, as well as sc-islets and dcOrgs2. The percentages in blue text are calculated using the sc-islet DEGs and the percentages in red text are calculated using the dcOrg DEGs. (B) Venn diagrams showing the numbers of HSV-1-infected versus HSV-1-infected and ACV-treated DEGs that are in common between sc-islets and dcOrgs1, as well as sc-islets and dcOrgs2. The percentages in blue text are calculated using the sc-islet DEGs and the percentages in red text are calculated using the dcOrg DEGs.
Figure 2.
Comparisons of DEGs from HSV-1 infection in sc-islets versus 2 dcOrg replicates (dcOrgs1 and dcOrgs2). (A) Venn diagrams showing the numbers of HSV-1-infected versus uninfected DEGs that are in common between sc-islets and dcOrgs1, as well as sc-islets and dcOrgs2. The percentages in blue text are calculated using the sc-islet DEGs and the percentages in red text are calculated using the dcOrg DEGs. (B) Venn diagrams showing the numbers of HSV-1-infected versus HSV-1-infected and ACV-treated DEGs that are in common between sc-islets and dcOrgs1, as well as sc-islets and dcOrgs2. The percentages in blue text are calculated using the sc-islet DEGs and the percentages in red text are calculated using the dcOrg DEGs.
Figure 3.
Shared GO categories across HSV-1-infected, or HSV-1-infected and ACV-treated sc-islets and dcOrgs.
Figure 3.
Shared GO categories across HSV-1-infected, or HSV-1-infected and ACV-treated sc-islets and dcOrgs.
Figure 4.
Schematic of our ACV and rescued analyses.
Figure 4.
Schematic of our ACV and rescued analyses.
Figure 5.
Comparisons of DEGs from CVB, IAV, and HSV-1 infection in sc-islets and dcOrgs revealed highest discordance between CVB-infected sc-islets and HSV-1-infected dcOrgs.
Figure 5.
Comparisons of DEGs from CVB, IAV, and HSV-1 infection in sc-islets and dcOrgs revealed highest discordance between CVB-infected sc-islets and HSV-1-infected dcOrgs.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).