Submitted:
15 July 2024
Posted:
16 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Standard Protocols, Approval, Data Availability
2.2. Patients’ Clinical Data Analysis
2.3. Management
2.4. Statistical Analysis
3. Results
3.1. Patients Characteristics
3.2. Surgery
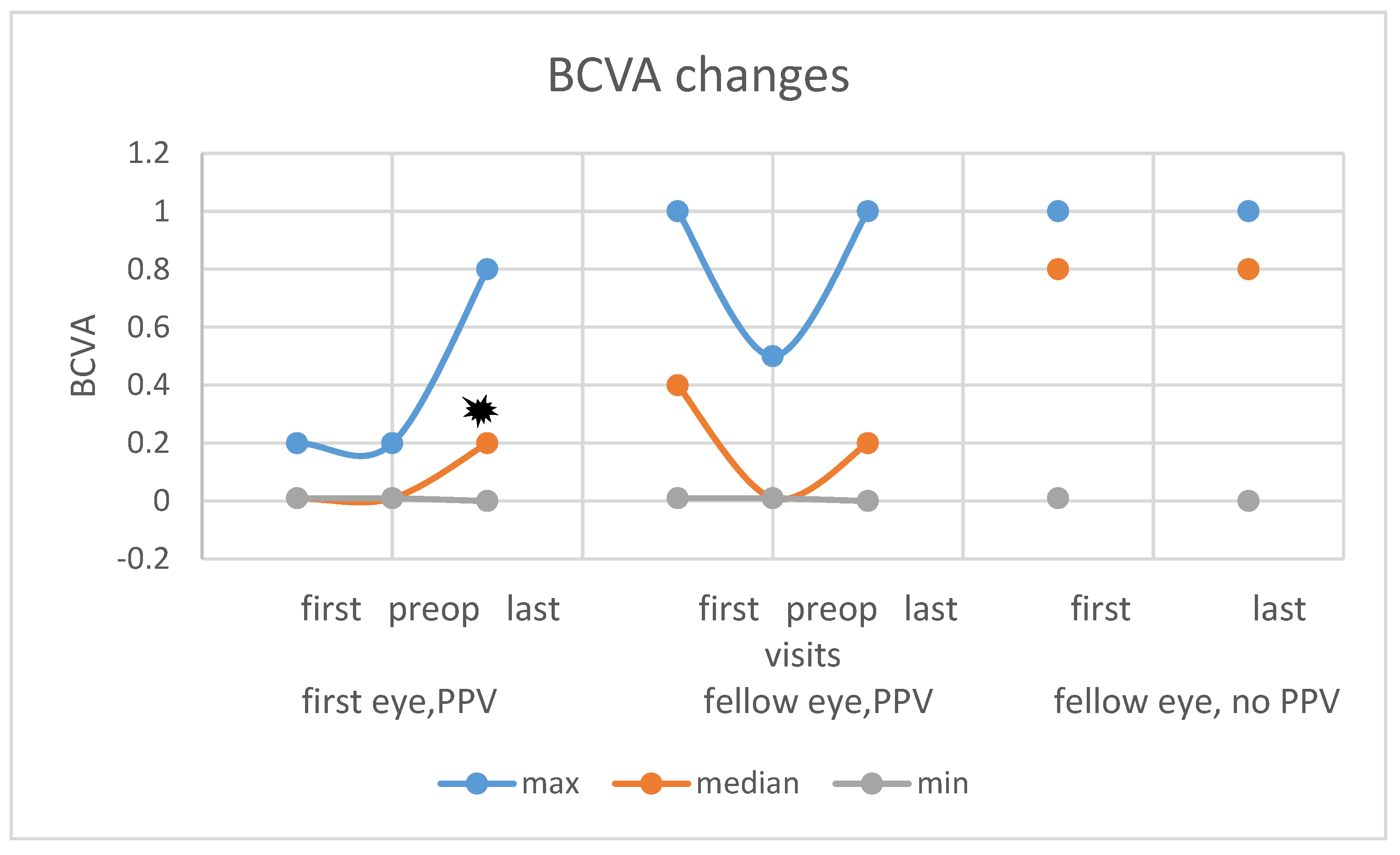
3.3. Functional Results
3.4. Factors Affecting final BCVA
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, H., et al., IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract, 2022. 183: p. 109119. [CrossRef]
- Klein, R., et al., The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XXII the twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology, 2008. 115(11): p. 1859-68.
- Flynn, H.W., Jr., et al., Pars plana vitrectomy in the Early Treatment Diabetic Retinopathy Study. ETDRS report number 17. The Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology, 1992. 99(9): p. 1351-7. [CrossRef]
- Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. The Diabetic Retinopathy Study Research Group. Ophthalmology, 1981. 88(7): p. 583-600.
- Antoszyk, A.N., et al., Effect of Intravitreous Aflibercept vs Vitrectomy With Panretinal Photocoagulation on Visual Acuity in Patients With Vitreous Hemorrhage From Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. Jama, 2020. 324(23): p. 2383-2395.
- Khan, R., et al., Need for Vitreous Surgeries in Proliferative Diabetic Retinopathy in 10-Year Follow-Up: India Retinal Disease Study Group Report No. 2. Ophthalmic Research, 2021. 64(3): p. 432-439. [CrossRef]
- Figueira, J., et al., Ranibizumab Plus Panretinal Photocoagulation versus Panretinal Photocoagulation Alone for High-Risk Proliferative Diabetic Retinopathy (PROTEUS Study). Ophthalmology, 2018. 125(5): p. 691-700. [CrossRef]
- Tonello, M., et al., Panretinal photocoagulation versus PRP plus intravitreal bevacizumab for high-risk proliferative diabetic retinopathy (IBeHi study). Acta Ophthalmol, 2008. 86(4): p. 385-9. [CrossRef]
- Gross, J.G., et al., Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. Jama, 2015. 314(20): p. 2137-2146.
- Gross, J.G., et al., Five-Year Outcomes of Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA Ophthalmol, 2018. 136(10): p. 1138-1148.
- Sivaprasad, S., et al., Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet, 2017. 389(10085): p. 2193-2203. [CrossRef]
- Nicholson, L., et al., Mechanistic Evaluation of Panretinal Photocoagulation Versus Aflibercept in Proliferative Diabetic Retinopathy: CLARITY Substudy. Invest Ophthalmol Vis Sci, 2018. 59(10): p. 4277-4284. [CrossRef]
- Nicholson, L., et al., Retinal Nonperfusion Characteristics on Ultra-Widefield Angiography in Eyes With Severe Nonproliferative Diabetic Retinopathy and Proliferative Diabetic Retinopathy. JAMA Ophthalmol, 2019. 137(6): p. 626-631. [CrossRef]
- Maturi, R.K., et al., Effect of Intravitreous Anti-Vascular Endothelial Growth Factor vs Sham Treatment for Prevention of Vision-Threatening Complications of Diabetic Retinopathy: The Protocol W Randomized Clinical Trial. JAMA Ophthalmol, 2021. 139(7): p. 701-712.
- Gella, L., et al., Prevalence of posterior vitreous detachment in the population with type II diabetes mellitus and its effect on diabetic retinopathy: Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetic Study SN-DREAMS report no. 23. Jpn J Ophthalmol, 2012. 56(3): p. 262-7. [CrossRef]
- Ono, R., et al., Prospective assessment of proliferative diabetic retinopathy with observations of posterior vitreous detachment. Int Ophthalmol, 2005. 26(1-2): p. 15-9. [CrossRef]
- Early vitrectomy for severe vitreous hemorrhage in diabetic retinopathy. Two-year results of a randomized trial. Diabetic Retinopathy Vitrectomy Study report 2. The Diabetic Retinopathy Vitrectomy Study Research Group. Arch Ophthalmol, 1985. 103(11): p. 1644-52.
- Berrocal, M.H. and L. Acaba-Berrocal, Early pars plana vitrectomy for proliferative diabetic retinopathy: update and review of current literature. Curr Opin Ophthalmol, 2021. 32(3): p. 203-208. [CrossRef]
- Glassman, A.R., et al., Visual Acuity, Vitreous Hemorrhage, and Other Ocular Outcomes After Vitrectomy vs Aflibercept for Vitreous Hemorrhage Due to Diabetic Retinopathy: A Secondary Analysis of a Randomized Clinical Trial. JAMA Ophthalmol, 2021. 139(7): p. 725-733.
- Simunovic, M.P. and D.A. Maberley, ANTI-VASCULAR ENDOTHELIAL GROWTH FACTOR THERAPY FOR PROLIFERATIVE DIABETIC RETINOPATHY: A Systematic Review and Meta-Analysis. Retina, 2015. 35(10): p. 1931-42.
- Zhang, Z.H., et al., Vitrectomy with or without preoperative intravitreal bevacizumab for proliferative diabetic retinopathy: a meta-analysis of randomized controlled trials. Am J Ophthalmol, 2013. 156(1): p. 106-115.e2. [CrossRef]
- Zhao, X.Y., S. Xia, and Y.X. Chen, Antivascular endothelial growth factor agents pretreatment before vitrectomy for complicated proliferative diabetic retinopathy: a meta-analysis of randomised controlled trials. Br J Ophthalmol, 2018. 102(8): p. 1077-1085. [CrossRef]
- Lee, B.J. and H.G. Yu, Vitreous hemorrhage after the 25-gauge transconjunctival sutureless vitrectomy for proliferative diabetic retinopathy. Retina, 2010. 30(10): p. 1671-7. [CrossRef]
- Oshima, Y., et al., Microincision vitrectomy surgery and intravitreal bevacizumab as a surgical adjunct to treat diabetic traction retinal detachment. Ophthalmology, 2009. 116(5): p. 927-38. [CrossRef]
- Motoda, S., et al., Predictors of postoperative bleeding after vitrectomy for vitreous hemorrhage in patients with diabetic retinopathy. J Diabetes Investig, 2018. 9(4): p. 940-945. [CrossRef]
- Hershberger, V.S., et al., Fibrovascular ingrowth at sclerotomy sites in vitrectomized diabetic eyes with recurrent vitreous hemorrhage: ultrasound biomicroscopy findings. Ophthalmology, 2004. 111(6): p. 1215-21. [CrossRef]
- Yang, C.M., P.T. Yeh, and C.H. Yang, Intravitreal long-acting gas in the prevention of early postoperative vitreous hemorrhage in diabetic vitrectomy. Ophthalmology, 2007. 114(4): p. 710-5.
- Yoshida, S., et al., Increased vitreous concentrations of MCP-1 and IL-6 after vitrectomy in patients with proliferative diabetic retinopathy: possible association with postoperative macular oedema. Br J Ophthalmol, 2015. 99(7): p. 960-6. [CrossRef]
- Naithani, P., et al., Role of topical nepafenac in prevention and treatment of macular edema after vitreoretinal surgery. Retina, 2012. 32(2): p. 250-5. [CrossRef]
- Jung, Y.H. and Y. Lee, Efficacy of vitrectomy combined with an intraoperative dexamethasone implant in refractory diabetic macular edema. Acta Diabetol, 2019. 56(6): p. 691-696. [CrossRef]
- Elman, M.J., et al., Intravitreal Ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results. Ophthalmology, 2015. 122(2): p. 375-81. [CrossRef]
- Elman, M.J., et al., Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology, 2011. 118(4): p. 609-14. [CrossRef]
- Diabetic Retinopathy Clinical Research, N., et al., Randomized trial of peribulbar triamcinolone acetonide with and without focal photocoagulation for mild diabetic macular edema: a pilot study. Ophthalmology, 2007. 114(6): p. 1190-1196.
- Jampol, L.M., A.R. Glassman, and N.M. Bressler, Comparative Effectiveness Trial for Diabetic Macular Edema: Three Comparisons for the Price of 1 Study From the Diabetic Retinopathy Clinical Research Network. JAMA Ophthalmol, 2015. 133(9): p. 983-4.
- Ross, E.L., et al., Cost-effectiveness of Aflibercept, Bevacizumab, and Ranibizumab for Diabetic Macular Edema Treatment: Analysis From the Diabetic Retinopathy Clinical Research Network Comparative Effectiveness Trial. JAMA Ophthalmol, 2016. 134(8): p. 888-96.
- Wells, J.A., et al., Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med, 2015. 372(13): p. 1193-203.
- Maturi, R.K., et al., Effect of Adding Dexamethasone to Continued Ranibizumab Treatment in Patients With Persistent Diabetic Macular Edema: A DRCR Network Phase 2 Randomized Clinical Trial. JAMA Ophthalmol, 2018. 136(1): p. 29-38.
- Lauermann, P., et al., Risk Factors for Severe Bleeding Complications in Vitreoretinal Surgery and the Role of Antiplatelet or Anticoagulant Agents. Ophthalmol Retina, 2021. 5(8): p. e23-e29. [CrossRef]
- Randomized clinical trial evaluating intravitreal ranibizumab or saline for vitreous hemorrhage from proliferative diabetic retinopathy. JAMA Ophthalmol, 2013. 131(3): p. 283-93.
- Berrocal, M.H., L. Acaba-Berrocal, and A.M. Acaba, Long-Term Outcomes of Same Patient Eyes Treated with Pars Plana Vitrectomy in One Eye and Conventional Treatment in the Other for Complications of Proliferative Diabetic Retinopathy. J Clin Med, 2022. 11(18). [CrossRef]
- Patel, V., et al., Outcomes of Pars Plana Vitrectomy with Panretinal Photocoagulation for Treatment of Proliferative Diabetic Retinopathy Without Retinal Detachment: A Seven-Year Retrospective Study. Clin Ophthalmol, 2023. 17: p. 471-478. [CrossRef]
- Schreur, V., et al., Long-term outcomes of vitrectomy for proliferative diabetic retinopathy. 2021. 99(1): p. 83-89. [CrossRef]
- Gupta, B., et al., Visual and anatomical outcomes following vitrectomy for complications of diabetic retinopathy: The DRIVE UK Study. Eye, 2012. 26(4): p. 510-516. [CrossRef]
- Liao, M., et al., Characteristics and outcomes of vitrectomy for proliferative diabetic retinopathy in young versus senior patients. BMC Ophthalmol, 2020. 20(1): p. 416. [CrossRef]
- Sato, T., et al., Characteristics of cases with postoperative vitreous hemorrhage after 25-gauge vitrectomy for repair of proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol, 2017. 255(4): p. 665-671. [CrossRef]
- Yorston, D., et al., Predictive clinical features and outcomes of vitrectomy for proliferative diabetic retinopathy. Br J Ophthalmol, 2008. 92(3): p. 365-8. [CrossRef]
- Tandias, R., et al., Posterior vitreous detachment status as a predictive factor for outcomes of vitrectomy for diabetic vitreous hemorrhage. Retina, 2022. 42(6): p. 1103-1110. [CrossRef]
- McCullough P, Mohite A, Virgili G, Lois N. Outcomes and Complications of Pars Plana Vitrectomy for Tractional Retinal Detachment in People With Diabetes A Systematic Review and Meta-analysis JAMA Ophthalmol, 2023. 141(2):186-195. [CrossRef]
| Overall | Type 1 | Type 2 | p-value | |
| General characteristics | ||||
| Number (female/male) |
66 (22/45) | 40 (15/25) | 26 (19/7) | ns |
| Age, years | 52 (21-80) | 45 (21-78) | 58 (45-80) | 0.000* |
| follow up after PPV on first eye, months (mean min-max) | 38 (9-125) | 32.5 (9-125) | 38.5 (9-102) | ns |
| Duration of DM, years | 20 (0-57) | 24 (0-50) | 15 (2-57) | 0.000* |
| Systemic characteristics | ||||
| HbA1c (%) at the time of first PPV (mean min-max) | 7.3 (5.5-13.5) | 7.8 (5.7-12.4) | 7 (5.5-11.6) | 0.029* |
| HbA1c (%) at the end of follow-up (mean min-max) | 7.3 (5.1-17.0) | 7.6 (5.1-17) | 6.9 (5.4-9.2) | 0.004* |
| Mean HbA1c (%) (mean min-max) |
7.5 (5.4-13.1) | 7.7 (5.4-13.1) | 7.2 (5.6-10.4) | 0.002* |
| Renal insufficiency, (patients) | 9 | 7 | 2 | ns |
| Severe CV complications (patients) | 17 | 10 | 7 | ns |
| Unstable blood pressure (patients) | 18 | 12 | 6 | ns |
| Visual acuity | ||||
| BCVA at first attendance (mean min-max) | 0.05 (0.001-1) | 0.1 (0.005-1.0) | 0.04 (0.01-1.0) | ns |
| Good BCVA at first attendance (eyes) | 38 | 27 | 11 | ns |
| Legally blind at first attendance (eyes) | 86 | 46 | 40 | ns |
| Preoperative ocular factors | ||||
| Iris rubeosis at first attendance (eyes) | 9 | 7 | 2 | ns |
| Glaucoma at first attendance (eyes) | 11 | 5 | 6 | ns |
| MRD before ppv (eyes) | 44 | 29 | 15 | ns |
| TRD before ppv (eyes) | 46 | 28 | 18 | ns |
| Vitreous hemorrhage (eyes) | 85 | 49 | 36 | ns |
| Preoperative anti-VEGF injections (eyes) | 41 | 29 | 12 | ns |
| Preoperative laser (eyes) | 96 | !! | !! | ns |
| parameters at first/at final (No. of eyes); | all eyes (n=132) | First eyes (n=66) | Fellow eyes with PPV (n=31) | Fellow eyes without PPV (n=35) |
| good BCVA | 38/53 | 1/21 | 13/10 | 24/24 |
| legally blind eyes | 80/61 | 58/38 | 13/17 | 13/10 |
| tractional retinal detachment | 47/9 | 27/4 | 18/3 | 3/3 |
| macular tractional detachment | 44/2 | 28/9 | 14/0 | 2/2 |
| posterior hyaloid detachment | 29/- | 24/- | 5/- | - |
| silicone oil usage | -/58 | -/38 | -/20 | - |
| intraoperative complication | -/2 | -/2 | -/0 | - |
| postop. vitreous hemorrhage | -/21 | -/17 | -/4 | - |
| neovascularization on iris | 9/10 | 5/6 | 4/4 | - |
| glaucoma | 11/39 | 7/26 | 4/13 | - |
| refractory stage glaucoma | 0/10 | 0/5 | 0/5 | - |
| good BCVA at final | ||
| parameter | correlation coefficient | p value |
| age | ,246** | 0,004 |
| type of DM | -0,028 | 0,752 |
| duration of DM | ,181* | 0,037 |
| HbA1c at baseline | -0,144 | 0,100 |
| HbA1c at final | -0,162 | 0,063 |
| HbA1c, average | -,173* | 0,047 |
| renal failure | 0,035 | 0,692 |
| severe CV complication | -0,129 | 0,140 |
| unstable blood pressure | -,189* | 0,030 |
| follow-up | 0,039 | 0,657 |
| fellow eye in severe condition | ,201* | 0,021 |
| legal blindness at start | -,406** | 0,000 |
| good BCVA at START | ,469** | 0,000 |
| first BCVA | ,404** | 0,000 |
| rubeosis | -,222* | 0,011 |
| preoperative secondary glaucoma. | -,247** | 0,004 |
| vitreous hemorrhage | 0,023 | 0,824 |
| tractional macular detachment | -0,448 | 0,000 |
| posterior hyloid detachment | ,355** | 0,018 |
| vitrectomy must be performed | -,313** | 0,000 |
| phaco-combined vitrectomy | -,236** | 0,006 |
| silocon oil was used | -,417** | 0,000 |
| number of ppv | -,279** | 0,001 |
| preoperative use of anti-VEGF | 0,120 | 0,245 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





