1. Introduction
1.1. Pre-Proposal Context
Enteroviruses (EV) are a highly diverse genus of small, icosahedral viruses with single-stranded, positive-sense RNA genomes [
1]. They cause a wide range of human diseases occurring most frequently in infants and young children, such as non-specific febrile illness, exanthemas, respiratory tract infections, and ocular infections. Although infections caused by non-polio enteroviruses (NPEV) are usually benign, they can be severe and have deleterious outcomes in some patients, particularly in children and immunocompromised patients.
Enteroviruses genus presents 7 human pathogen species (EV-A, EV-B, EV-C, EV-D and RV-A, RV-B, RV-C) divided into 11 subgroups: polioviruses (PV), coxsackieviruses groups (CV-A, CV-B), echoviruses (E), four enterovirus subgroups, named from A to D, and rhinovirus (RV) classified into RV-A, RV-B and RV-C subgroups. The whole enterovirus RNA strands are always composed of a coding region divided into three parts [
2]. The first part is composed of structural viral proteins 1, 2, 3, and 4 (VP1-4); the second part contains 2A protease, 2B protein, and 2C protein, and the last part comprises 3A protease, 3B protein, 3C protease, and RNA-dependent RNA polymerase or 3D protein. Viruses of the Enterovirus genus share several conserved protein motifs in the RNA-dependent RNA polymerase, capsid proteins, viral proteases, and other non-structural proteins.
The emergence of new viral variants, such as enterovirus-A71 (EV-A71) and enterovirus-D68 (EV-D68), have been associated with more severe disease manifestations than previously described, including sepsis, myopericarditis, and central nervous system infections [
3,
4,
5]. The worldwide prevalence and species distribution of enteroviruses varies among continents. For example, Enterovirus D68 is most prevalent in North America, while Enterovirus B is the predominant variant in Africa, Europe, and Southern Asia. However, there is a more variable distribution of variants among other continents, where a mixture of Enterovirus A71, Coxsackievirus A, and Enterovirus B are the most represented [
6].
1.2. The Need for Therapies
The large number of RV/EV strains and their antigenic diversity are significant obstacles in broad-spectrum vaccine development. Several clinical trials evaluating the efficacy of small molecules have been carried out in recent years, but there are no approved therapies targeting these viruses. Some of the reasons for these failures include viral resistance, single-targeted approaches, and lack of clinical efficiency, which pose significant obstacles in drug development [
7,
8]. Moreover, the importance of combination therapies involving different therapeutic mechanisms to control a disease is well known in clinical practice. To address these challenges, several combinations of molecules were studied.
2. Materials and Methods
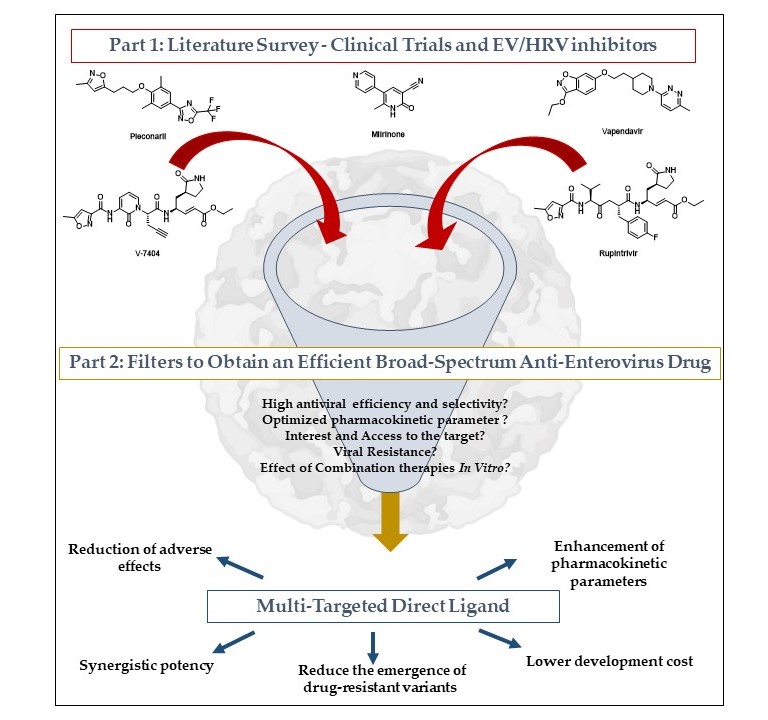
The main goal of this work is to identify promising compounds and to propose multi-target directed ligand (MTDL) systems, from combination therapy to chimera design. The workflow is organized into two distinct parts. The first is a literature survey highlighting previous clinical trials involving NPEV inhibitors, and the second highlights selection criteria for potentially synthesized MTDL.
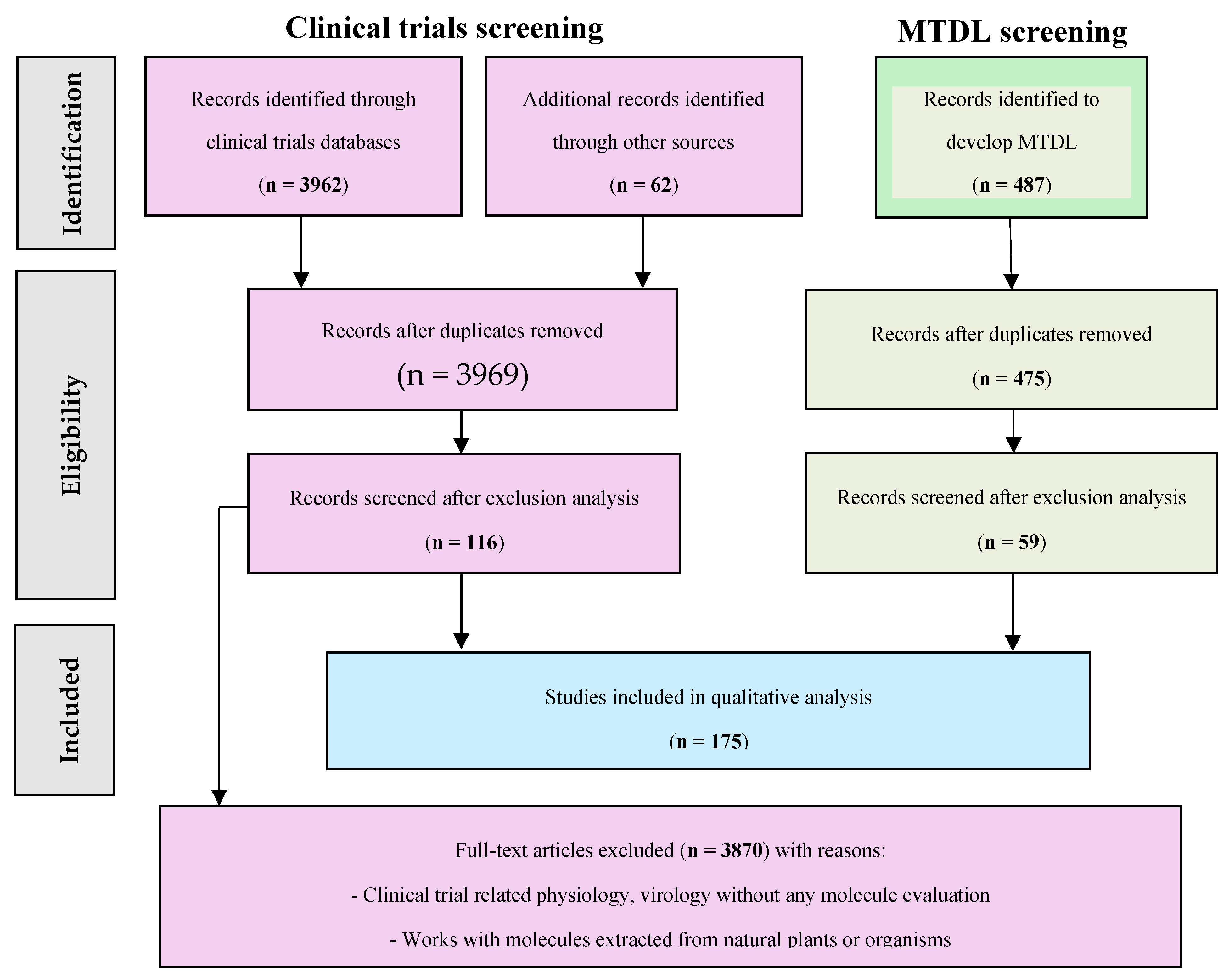
The Prisma flowchart has been adapted to classify the data and identify studies to be included in our analysis. The details of the items are presented with the main results of our research in the results section,
Scheme 1 [
9].
2.1. Literature Survey
The literature survey was conducted using the website Clinicaltrials.gov (CT), the European Union Drug Regulating Authorities Clinical Trials Database (EudraCT), the International Clinical Trials Registry Platform (ICTRP), and PubMed. The data collection period occurred in March 2024. The search parameters included every clinical trial written in English. The keywords used for study inclusion were enterovirus (namely, “Coxsackievirus”, “Echovirus”, “Enterovirus”, “Enterovirus Infections”, or “Rhinovirus). The exclusion criteria were clinical trials on vaccines, devices, or biological drugs. For the combination part, the literature survey was conducted on PubMed only, using keywords “combination” and “Enterovirus”.
The type and name of the inhibitor, its chemical structure, studied type of EV infection or disease, concentration, doses, and pharmaceutical form for drug delivery, parameters of cohorts (age, gender), and conclusions of studies were used for reporting.
2.2. MTDL Candidate Proposal
The two data types used to discuss an MTDL strategy were compound parameters (broad-spectrum activity, target selectivity, effect during in vitro combination, identified mechanism of action) and virus characteristics (interest in virus, viral resistance, target’s interest, and knowledge of the target).
We discuss the interest of each association and analyze molecule properties to propose an optimized MTDL with higher potency and the most favorable PK/PD parameters.
MTDL are commonly designed according to three categories. The least invasive method is to link several inhibitors, which have been studied in combination therapy. Another pathway consists of fusing similar structures presented in two compounds to synthesize only one compound. Finally, some inhibitors with the same pharmacophoric elements can be merged into one inhibitor. To optimize MTDL, initial compounds must have similar activity and PK/PD or physicochemical parameters to keep the same ADME profile and have similar levels of doses to be administered.
3. Results
3962. records were identified through the database (3270 from CT, 25 from EudraCT, 167 from ICTRP, and 500 from PubMed). 54 full clinical trials were accessed for analysis (55 duplicates removed and 3853 excluded). 487 records were identified through Pubmed. 59 articles were accessed for analysis (12 duplicates removed and 416 excluded). 62 references were added to describe the context, combinations, and biological details needed for the perspective. Results are presented in the
Scheme 1.
Four main types of viral targets were highlighted: structural proteins (involved in cellular entry and uncoating to release RNA), non-structural proteins (polymerase or proteases which are active during replication and translation of RNA), human targets (inhibiting viral proliferation by action on cell metabolism), and non-specified targets (unknown inhibitor mechanism of action) [
10].
This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
Part 1: NPEV-inhibitors evaluated in clinical trials.
3.1. Structural Proteins
Virus entry is the first step in the viral replication cycle and offers opportunities to develop inhibitors targeting viral structural capsid proteins [
10]. Therefore, several molecules have been developed to target capsid proteins of enteroviruses.
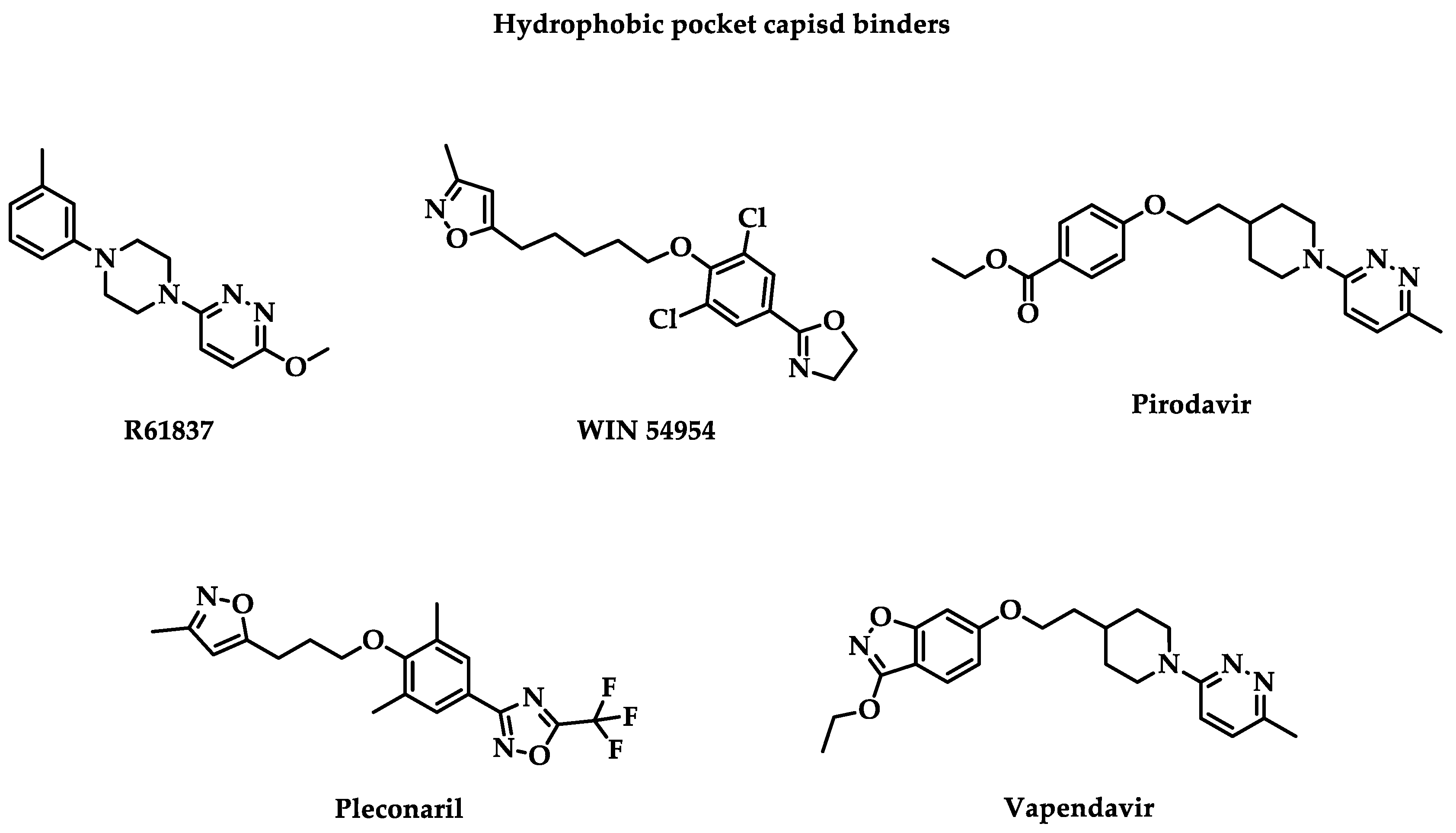
The most studied target is the hydrophobic pocket VP1 capsid protein constituted with two essential structures: “canyon” or “hydrophobic pocket” and “pocket factor.” Canyon is the first identified druggable surface pocket. The hydrophobic pocket is characterized by an open side, allowing accessibility for inhibitors, and a closed side, the end of the pocket. There are different binding sites in the viral capsid. When molecules bind with the capsid canyon, they stabilize the viral capsid and prevent the virus recognition by its receptors [
11]. Likewise, it blocks the viral entry and thus the uncoating to release RNA and VP4 inside the cell by a higher affinity for the drug than for the receptor. In fact, other cavities have been described, like the R209 cavity. The following classical hydrophobic pocket capsid binders are presented in
Figure 1. Associated clinical trial parameters are presented in
Table 1.
R61837
The first clinical trial studying capsid binder as an inhibitor of enterovirus was published in 1989 for a double-blind placebo-controlled phase 2 clinical trial with an evaluation of
R61837 on 105 volunteers between 18 and 50 years old to treat HRV-9 infections [
12].
R61837 was prepared for the nasal route. Each patient received a 25- or 36 mg total dose of
R61837 divided during six days. This trial concluded that
R61837 doesn’t prevent HRV-9 infections but associated colds. A decrease in nasal secretion and an improvement in the clinical score were observed during the treatment but stopped when patients no longer received the drug. Patients had well-tolerated
R61837. The trial conclusion was preconized to increase the drug dose to observe higher efficiency against HRV-9.
WIN 54954
The most famous capsid binder family is derived from drug candidates developed by Winthrop company (WIN compounds). They possess three aromatic cores with one alkyl linker.
In 1990, a second randomized, double-blind, placebo-controlled phase 2 clinical trial was performed to evaluate the efficiency of
WIN 54954 against HRV-39 infections [
13].
WIN 54954 possessed an in vitro broad spectrum activity with nanomolar potency against several HRV and CV [
14]. Two cohorts were recruited in Virginia and South Carolina in January and November 1990. A total of 69 volunteers between 18 and 50 years old were recruited.
WIN 54954 was administrated by oral capsules containing 100 or 400 mg of active ingredient for six days for a total dose of 1.20 or 2.40 g per day. The trials concluded that there is no significant efficiency in preventing HRV-39 colds. The main proposed limitations of this study were inadequate daily dose to have sufficient experimental power, lack of performance of measuring instruments, or development of drug resistance during the trial.
Pirodavir
The same year, an R61837 derivative named
Pirodavir (R77975) was evaluated for a randomized, double-blind, placebo-controlled phase 2 clinical trial against all HRV colds [
15].
Pirodavir is an anti-enteroviral with a nanomolar in vitro potency against a broad spectrum of rhinoviruses and several coxsackieviruses, echoviruses, and enteroviruses [
16]. In the first trial,
pirodavir was administrated by nasal route. Patients recruited for this study ranged in age from 18 to 64 years. Patients used the nasal spray twice per nostril for six days. Each intranasal spray released 500 µg of drug or placebo diluted at a concentration of 5 g/L. Overall, they received 30 doses of
pirodavir. The clinical trial concluded with a non-significant benefit to use the drug with efficiency and some mild adverse effects versus placebo. Indeed, there is a significantly lower frequency of virus isolation on day 3 (P < 0.001) and on day 5 (P = 0.002). However, the active cohort suffered of blood in mucus (P < 0.01), unpleasant taste, and nasal dryness (P ≤ 0.05).
A second randomized double-blind placebo-controlled phase 2 clinical trial was published in 1992 to evaluate the efficiency of a nasal spray containing
pirodavir against HRV-39 infections [
17]. Patients between 18 and 64 years old received two sprays for each nostril. They received a total of 2 mg of
pirodavir per day. The trial concluded that there is no significant difference of seroconversion’s rate. However, there is a lower overall number of days of virus shedding on days 2 (P < 0.001), on day 3 (P = 0.005) and day 4 (P = 0.04) for the first study. During the second and third studies, there was no significant difference between the placebo and virus-shedding cohorts. Eventually, even if the drug seems to irritate the respiratory mucosa and cause nasal dryness, there is no serious adverse effect, as blood in mucus case reported on the active cohort.
Pleconaril
In 1996, two phase 2 clinical trials for the compound
WIN 63843, also named
pleconaril or VP 63843, were performed to evaluate its efficiency against CV-A21 infections and EV infections, respectively [
18,
19].
Pleconaril is a broad-spectrum inhibitor with micro or nanomolar in vitro potency against coxsackieviruses and enteroviruses, except for CV-B3 [
20]. It was also used to cure echovirus-infected neonates [
21]. Respectively, 33 and 38 adults were enrolled to receive a 400 mg total daily dose for seven days, or 200 mg or 400 mg three times, by oral route. The first study concluded that
pleconaril reduces viral shedding in nasal secretion (P < 0.001), nasal mucus production (P = 0.004), and total respiratory illness symptom scores (P = 0.013) compared with placebo. However, it also causes some adverse effects, like nausea, abdominal pain, dysmenorrhea, and an increase in the frequency of urine. For the second trial, children in the active cohort received three doses of 5.0 mg/kg. Because of the rarity of EV infections, it was challenging to design a placebo-controlled trial.
In 1999, three randomized, double-blind, placebo-controlled studies were performed to evaluate
pleconaril efficiency against suspected EV meningitis or sepsis [
22,
23,
24]. These studies respectively recruited 20 patients under 12 months, 250 patients over 14 years old, and 61 neonates (with 43 confirmed EV sepsis) patients.
Pleconaril was administrated by liquid or suspended oral formulation with respective concentrations of 5 mg/kg/dose (3 times a day), 200 or 400 mg (3 times a day), and 8.5 mg/kg for 7 days. Conclusions of studies on infants were encouraging with a virus culture which turned to negative quicker for the active cohort (P = 0.08) and a higher survival probability after the treatment (P = 0.26) and over 18 months (P = 0.07, P = 0.23 for validated enteroviruses infections neonates) even if the duration of hospitalization was similar (P = 0.18). No adverse effects were attributed to the treatment despite the drug’s tendency to accumulate. The trial on neonates finished during the study due to the expiration of the study drug. The last study performed on children and adults is more mitigated about its conclusion. Indeed, there are some limitations, such as the low number of patients with validated EV meningitis and the difficulty of oral admission for patients with nausea. The headache resolution is quicker for the active cohort with moderate or severe nausea (P = 0.009) but not for very severe nausea (P = 0.05) or without nausea (P = 0.15). However, there is a light low treatment-related adverse effect (P = 0.14) or treatment-emergent adverse effect (P = 0.71). Analysis is exploratory, not for licensure, due to a failure to define the clinical benefit by the Food Drug Administration (FDA).
In 2000, a randomized, double-blind, placebo-controlled phase 3 of clinical trials was performed on 2096 patients to evaluate
pleconaril efficiency against HRV common colds [
25]. Patients received 200 mg of oral tablets three times per day for five days. By day 3, there is no culturable virus for
pleconaril-treated patients (P < 0.0001). By day 6, the number of samples is significantly lower for the active cohort (P = 0.07). The study used wild-type viruses and resistant-induced viruses. Symptom resolution of the
pleconaril cohort was quicker than the placebo one if they were exposed to the wild type (P < 0.0001) but not favorable with an exposition to the resistance strain. Some resistance issues can explain this result.
In the early 2000s, three studies were conducted to evaluate
pleconaril efficiency against enteroviral sepsis, CV-B infections, both in newborns, and the common cold due to rhinovirus infections in children and adults [
26,
27,
28]. They are either randomized, double-blind, placebo-controlled phase 2 of clinical trials or clinical application studies. Respectively, 61, 4, and 311 patients were recruited to receive drug or placebo by oral route for newborns and nasal route for children and adults. For these studies, few significant results were identified. Any result was associated or published after the end of the first trial. Even if it suggests a beneficial effect in combination with earlier treatment during the clinical application, no clear proof was identified that only
pleconaril decreases CV-B symptoms. Finally, both drug and placebo cohorts had the same levels of RV PCR-positive colds (P = 0.973) in the third study. As a result, no positive effect was identified in this case.
Vapendavir
The last promising capsid binder, evaluated against enteroviruses, particularly rhinoviruses, was the compound
BTA798, also named
vapendavir [
29]. In 2008, a randomized double-blind placebo-controlled phase 2 clinical trial on 240 male patients to evaluate its efficiency against the common cold [
30].
Vapendavir was administrated by oral route with 25, 100, or 200 mg of the drug. The result of this study identified a dose-dependent decrease in the incidence of HRV-39 infections. However, most of these infections were asymptomatic, which does not enable an evaluation of the protection against upper respiratory tract illness. The drug was well-tolerated even if one patient was excluded from the study due to neutropenic sepsis, which can be caused by the drug. This study was prematurely ended.
In 2010, a second randomized double-blind placebo-controlled phase 2 clinical trial was conducted to evaluate the efficiency of vapendavir against rhinoviral infections [
31]. Patients were recruited among adults, ranging from 18 to 70 years. The drug-treated cohort had lower cold symptoms and impact scores than the placebo cohort (P = 0.020).
In 2015,
vapendavir was evaluated in two randomized, double-blind, placebo-controlled phase 2 clinical trials against asthma due to RV infections [
32,
33]. For the first study, it was administered as oral capsules to 480 patients, including 24 patients over 65 years old. Each capsule contains 132 mg of the drug. Two cohorts were formed, receiving 264 (2 doses) or 528 mg (4 doses). These two cohorts had better clinical scores than placebo one (P = 0.319), individually and combined. Likewise, the 268 mg cohort had less moderate or severe asthma exacerbation (P = 0.563), as well as the 528 mg cohort (P = 0.632), or both combined (P = 0.942). For the second study, 455 patients aged 18 to 75 years received 264 or 528 mg of drugs. No results were associated or published after the end of the trials.
In 2017, a randomized, double-blind, placebo-controlled phase 2 clinical trial began to evaluate the efficiency of
vapendavir against upper respiratory tract infection due to HRV [
34]. 65 patients, ranging from 12 to 75 years, should be recruited.
Vapendavir should be administered as tablets containing 264 mg of drugs. Unfortunately, the trial was withdrawn due to a company decision.
Eventually, a randomized placebo-controlled phase 2 clinical trial was conducted to evaluate the effect of
vapendavir against COPD associated with enterovirus and rhinovirus infections [
35].
Vapendavir will be administered twice daily as tablets containing 1000 mg (4 from 40 to 75 years, for seven days.
3.2. Viral Non-Structural Protein
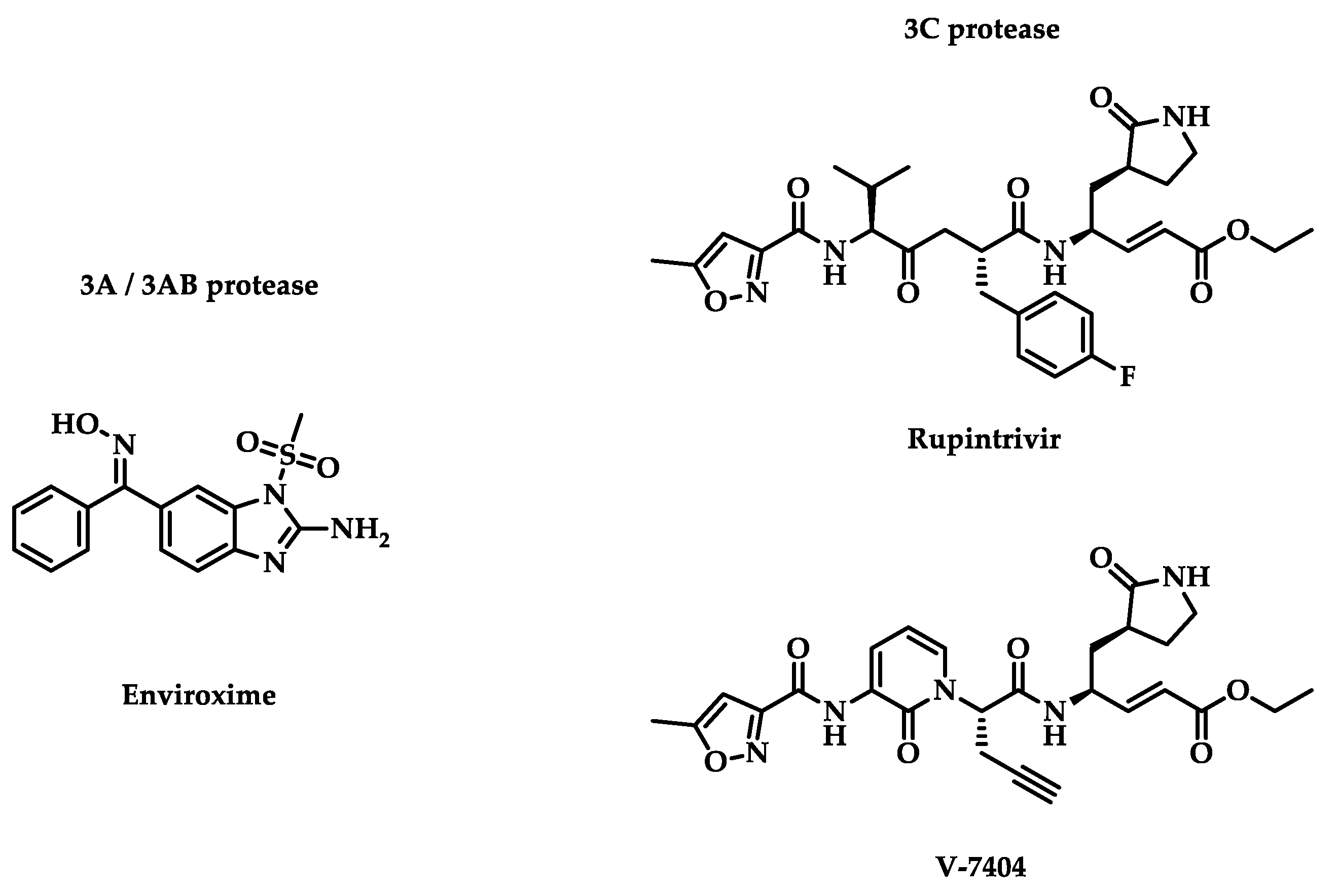
The viral capsid is the first accessible target from the viral replication cycle. However, other targets were identified during viral replication or translation. In addition to structural proteins, enteroviruses possess non-structural proteins crucial to the replication cycle. Several inhibitors were evaluated in clinical trials, and their chemical structures are represented in
Figure 2.
3.2.1. 3A/3AB Proteases
Enteroviral 3A protease was described as a promotor of binding between EV and the host cell phosphatidylinositol 4-kinase III β (PI4K β) by its interaction with host factor ACBD3 [
36]. Indeed, the replication of EV-A71 may depend on PI4K β even if the mechanism is not defined to date [
37,
38,
39]. Thus, 3A protease seems to play a key role in the replication of EV-A71 and EV-D68, but not for HRV-A16. 3A protease comes from 3AB protease, which is cleaved by 3C protease [
40]. The role of 3AB protease in EV replication was not well known. However, some chaperone activity was identified for EV-A71 [
41]. Both 3A protease and its precursor, 3AB protease, have membrane-binding properties.
Enviroxime
Enviroxime is an inhibitor of EV and RV 3A /3AB protease [
42]. It was one of the first anti-enteroviral compounds with a determined mechanism, which was studied in phase 2 clinical trials. In 1981, a placebo-controlled and a randomized, double-blind placebo-controlled study was published evaluating the efficiency against HRV-9 infections and HRV-39 infections, respectively [
43,
44].
Enviroxime was administrated by nasal route. 48 and 40 adults received 102 mg, four times daily for six days and 1 or 3 times daily for eight days, respectively. The first study concluded that a decrease in mean daily nasal secretion weights was observed in the active cohort (P = 0.02). However, there is no significant difference in clinical score between cohorts (P = 0.28) or viral quantity in nasal washings (P = 0.16). A slightly substantial difference from the third day for rhinorrhoea was observed. There is a negative correlation between quantitative indices of HRV infections and drug quantity.
Enviroxime was well tolerated except for one patient who presented nausea due to the active ingredient. The second study measured no significant protection against HRV-39 infections versus placebo.
In 1982, two randomized, double-blind, placebo-controlled, phase 3 and phase 2 clinical trials were performed against HRV natural common cold and general rhinovirus infection, respectively [
45]. The two studies gathered 1020 and 99 patients to receive 42 mg and 16 mg weekly of
enviroxime by nasal route. The first study concludes that there is limited benefit due to the lack of clear reproducibility of the administration or the little practical importance of delivering media. Unlike these first conclusions, most of the results of the second study were identified as negative for the drug administration.
Finally, a last double-blind placebo-controlled phase 2 clinical study evaluating
enviroxime against HRV-9 infection was published in 1983 [
46]. 41 patients were recruited, ranging from 18 to 64 years, to receive 284 µg of drug per nostril, six times a day, for five days a nasal spray solution. The mean clinical score of enviroxime-treated patients was significantly lower than the placebo cohort on day 5 (P = 0.04). However, no differences were identified in nasal secretion in both cohorts.
3.2.2. 3C Protease
As described previously, the 3C protease cleaves the viral polyprotein at eight different sites, including those to release 3AB and 3A proteases [
40]. Due to the role the 3C protease plays in EV processing, it makes the protease an essential target against EV. The 3C-protease inhibitors tend to be more efficient than
enviroxime and
pleconaril, which in turn were more effective than
vapendavir and
pirodavir [47]. 3C protease is a protease that is found in picornaviruses and performs several functions. In coxsackieviruses, 3C protease activates apoptosis via several caspases (caspase-3, caspase-8, and caspase-9) [
48]. Moreover, in rhinoviruses, 3C protease targets OCT-1 transcription factor for proteolytic cleavage [
49,
50]. These aspects make this a target to be favored. Promising molecules have been evaluated in clinical trials since discovering the role of 3C protease inhibitors.
Rupintrivir
The historic 3C inhibitor of enterovirus is
rupintrivir, first synthesized in 1999 [
51]. Three years later, a randomized, double-blind, placebo-controlled phase 1 clinical trial was published to evaluate the pharmacokinetic parameter of the HRV inhibitor [
52]. The drug was delivered to 36 male patients, ranging from 18 to 50 years, as a nasal spray solution containing 4 or 8 mg, 1 or 6 times, for seven days.
Rupintrivir was measured with low concentration (< 0.52 ng/mL in plasma and < 2% in nasal wash) at the end of treatment. Few metabolite concentrations were also observed. However,
rupintrivir was identified as safe and well tolerated over time, with substantial drug detection for 9 hours after administration.
In 2003, a randomized, double-blind, placebo-controlled phase 2 clinical trial was published to evaluate the efficiency of
rupintrivir against HRV-39 and HRV-Hanks infections [
53]. 8 mg of the drug was delivered 2 or 5 times a day for seven days to 202 patients (83 males and 119 females), ranged in age from 18 to 60 years, as a nasal spray solution. The active cohort had lower mean total daily symptoms than the placebo cohort (P = 0.014) and a higher incidence reduction on viral culture (P = 0.04 – 2 doses per day group / P = 0.03 – 5 doses per day group). It also has a decrease in viral titers (P < 0.05) but no significant difference in cold cases. The mean cumulative nasal discharge was lower for drug-treated patients (P = 0.05 – 2 doses per day group / P = 0.12 – 5 doses per day group). Finally, no significant local nasal adverse effects were declared.
V-7404
In 2005, a phase 1 clinical trial was published to evaluate the pharmacokinetic parameters of anti-rhinoviral
V-7404, a
rupintrivir derivative, administered orally for fasted or fed patients [
54,
55]. The oral route has more risk of adverse effects than the nasal route but can access other HRV replication sites for diseases localized elsewhere than in the respiratory tract. 14 male patients, ranging from 18 to 50 years, received 200, 500 (fasted or fed), 1000, 2000 mg of drug. The study concludes that for 500 mg doses, the free maximum serum concentration (C
max) was superior to the compound’s half maximal effective concentration (EC50), inhibiting 80% of HRV serotypes.
In 2018, a randomized double-blind placebo-controlled phase 1 clinical trial was conducted to evaluate the
V-7404 safety and pharmacokinetic parameters against EV infections [
56]. For this study, 77 patients (33 males and 44 females) aged from 18 to 45 years were recruited to receive an oral solution. Different weights (200, 500, 1000, and 2000 mg) were tested in one single dose or one or two doses per day for two weeks.
V-7404 was well tolerated, even with multiple doses, and with acceptable safety. Only mild or moderate treatment-emerged adverse effects were observed. The drug was adsorbed in less than an hour, and its half-life took between 0.3 and 2.3 hours. The plasma concentration of the drug was quickly under detection, which was the limit of measurement tools. This is why an exponential decrease in its plasma concentration was observed. Pharmacokinetic parameter quality increases with a dose-proportional factor. At last, fed patients have a longer time to reach maximum serum concentrations than fasted ones.
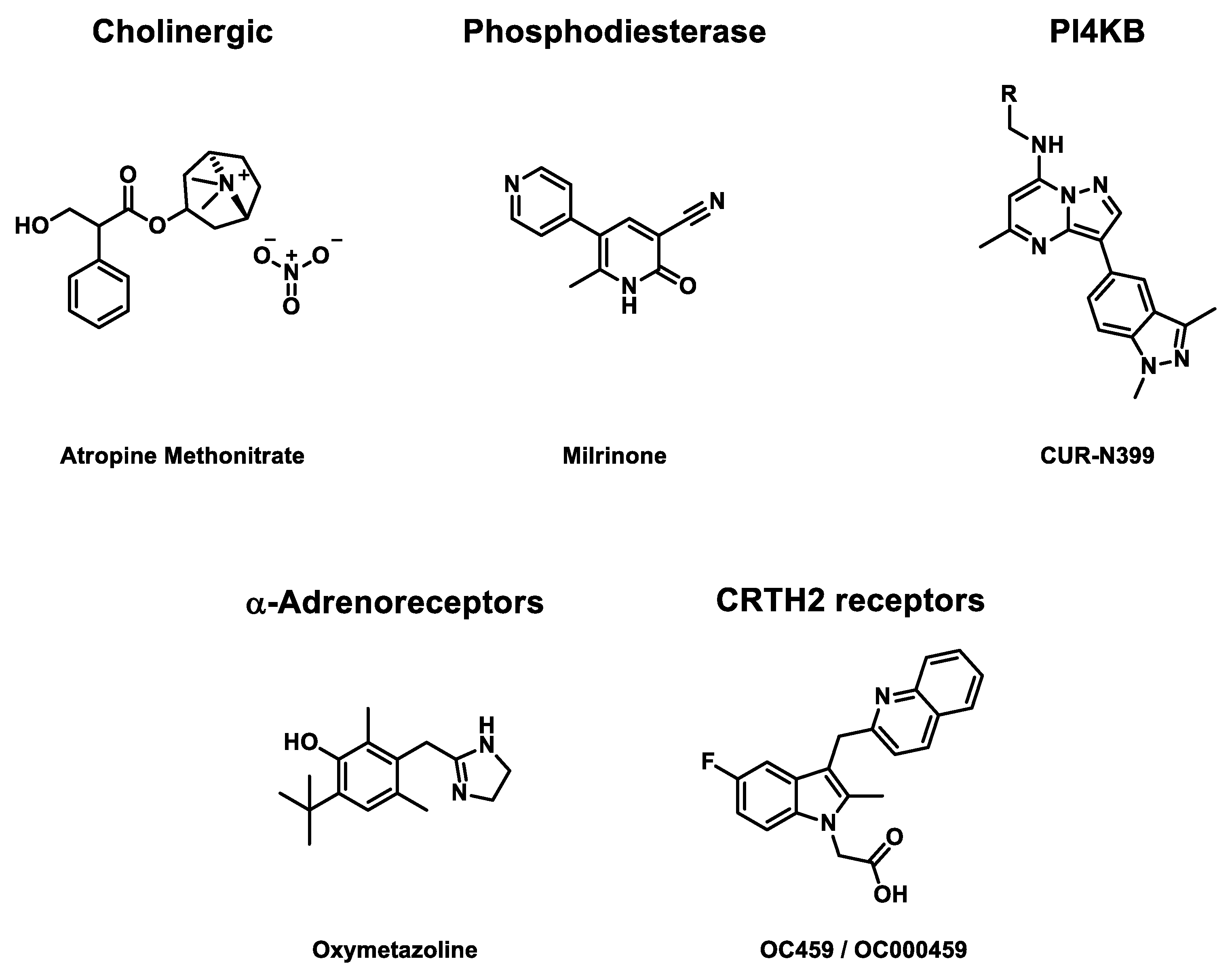
3.3. Targeting Cell Metabolism to Inhibit Enteroviruses
Some molecules also target human enzymes, which imply a reduction of viral charge and try to avoid any major side effects. They target cell metabolism functions that enteroviruses use to replicate. Inhibiting these steps needs to balance the viral activity reduction and the cell’s vital task. Some compounds, represented in
Figure 3, were evaluated in several clinical trials (
Table 3).
3.3.1. Cholinergic Receptors
Cholinergic receptors play a significant role in signal transduction in the nervous system [
57]. In 1987, randomized double-blind placebo-controlled phase 2 clinical trials were published to evaluate the efficiency of
atropine methonitrate, a known cholinergic antagonist, against HRV-39 infections [
58,
59]. The study recruited 30 adults to receive two injections of nasal spray solution per nostril containing 250 or 500 µg of the drug. Patients had to repeat the treatment four times per day for five days. No significant effects on nasal symptoms in the active cohort were observed, but nasal mucus production was reduced for 250 µg-treated patients with a four-daily prescription. However, for this concentration assay, the placebo weight of nasal mucus in the placebo cohort was higher than for other concentrations. At last, for high doses of drugs, some nasal adverse effects were detected.
3.3.2. Phosphodiesterase 3
Milrinone is a second-generation phosphodiesterase 3 PDE3 inhibitor [
60]. PDE3 preferably hydrolyses cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) [
61]. Milrinone has been proven to be efficient against asthma [
62,
63]. In 1998, a placebo-controlled phase 2 clinical trial evaluated its efficiency against EV-A71 pulmonary edema [
64]. Drug is injected intravenously at a flow between 0.35 and 0.55 µg/kg/min for 72 hours. For this study, 24 patients (16 males and eight females), ranging from 5 to 18 years, were recruited. Due to the novelty of
milrinone at the time of the clinical trial, it was not randomized. The study concludes with lower mortality (P = 0.005), with a lower concentration of white blood cells (P = 0.009) and platelets (P = 0.001) in the active cohort. Likewise, a lower concentration of interleukin-13 (P = 0.001), a mark of EV-A71 infection, was observed in drug-treated patients.
In 2007, a second randomized placebo-controlled phase 2 clinical trial was performed to evaluate the efficiency of milrinone against brainstem encephalitis in children under 5 [
65]. 41 children (35 males and six females) were recruited to receive 50 µg/kg intravenously. Then, a flow of 0.5 µg/kg/min for 72 hours. The active cohort had significantly lower mortality after one week of treatment (P = 0.012). There were also longer ventilator-free days (P = 0.01), undoubtedly due to the higher survival probability. No drug-related adverse effects were observed.
3.3.3. Phosphatidylinositol 4-Kinase Beta (PI4K β)
As previously described, PI4K β plays a crucial role in EV replication, including EV-A, EV-D, and CV-B, but not HRV-A16 [
66]. EV binding with PI4K β leads to change of cellular endomembranes to build replication organelles, which protect EV RNA during the genome replication. In 2021, a randomized double-blind placebo-controlled phase 1 clinical trial was begun to assess the efficiency of
CUR-N399 against chronic obstructive pulmonary disease (COPD) due to enterovirus or rhinovirus infections [
67].
CUR-N399 is a PI4K β inhibitor, especially against EV-A71 infections [
68]. Oral capsules were distributed to 74 adults having either a drug or placebo inside. Two types of study were decided. The first delivered one single dose to patients containing 2.5, 7.5, 17.5, 35 or 50 mg. The other is a multiple-dose study. For seven days, patients received 10, 25, or 50 mg per day. Currently, the results have not been published.
3.3.4. α-Adrenoceptor
α-Adrenoceptors are divided into two subtypes (α1-adrenoceptor and α2-adrenoceptor) with a large panel of effects on human cell behavior. Contrary to β-adrenoceptor, they interact globally for smooth muscle contraction, glycogenosis, adenylyl cyclase inhibition, intracellular cAMP reduction, neurotransmitter release, or central vasodilation reduction [
69,
70]. In 2007,
oxymetazoline, an α-adrenoceptor agonist, was used in a randomized double-blind placebo-controlled phase 2 clinical trial against HRV-39 infections [
71,
72]. The drug is administrated as an intranasal liquid solution containing 22.5 µg of drug diluted in 45 µL of citrate buffer three times a day for 5 days. 94 patients (45 males and 49 females), ranging from 18 to 64 years, were recruited to assess the study. On the second day of treatment, a lower virus titer was observed in the active cohort (P = 0.04). No medication-caused severe adverse effects were identified except 2, one for the active cohort and one for the placebo one.
3.3.5. Prostaglandin D2 Receptor 2 (CRTH2 receptor)
Prostaglandin D
2 receptor 2 (CRTH2 receptor) mainly mediates inflammation. It is located on several cell membranes [
73].
OC459 / OC000459 is a CRTH2 receptor antagonist, already studied in combination treatment [
74]. In 2015, a randomized double-blind placebo-controlled phase 2 clinical trial was conducted to evaluate its efficiency against asthma and the common cold due to rhinovirus infections [
75]. Tablets were prepared to deliver 50 mg of the drug. 44 patients aged from 18 to 55 years received one tablet once daily for five days. The active cohort has a lower respiratory symptom score (P = 0.78) and changes in asthma score (P = 0.49) compared to the placebo cohort. Also, airway hyperresponsiveness was higher for drug-treated patients (P = 0.12), while viral load was lower for them (P = 0.75).
3.4. Unknown or Other Mechanisms of Action
In addition, some inhibitors of enterovirus were developed without identification of the intended target or because they were similar to other pathogens (viruses, bacteria…, etc.) and reduced symptoms from close diseases.
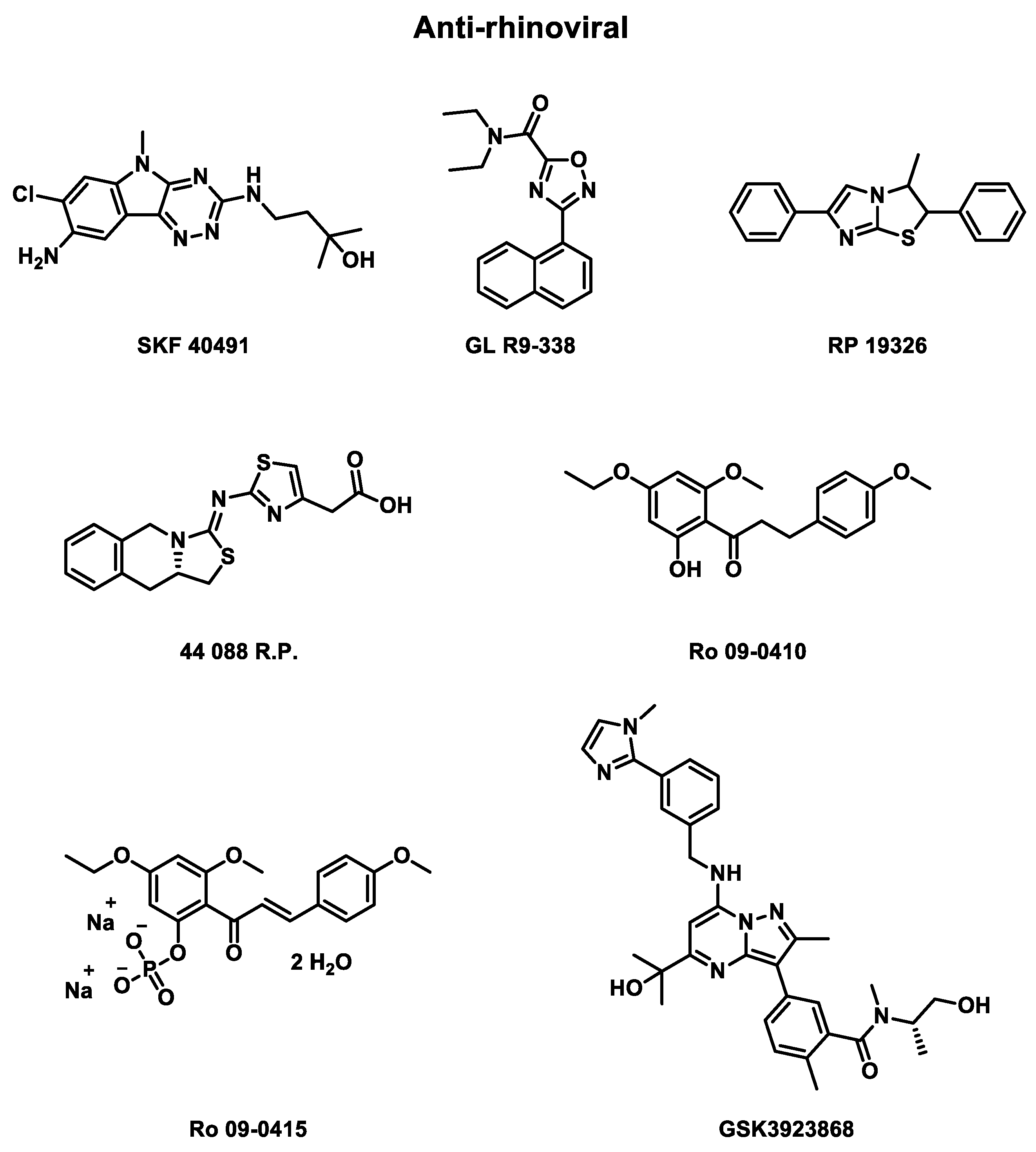
3.4.1. Anti-Rhinovirus
During 1950’s, some antirhinovirals were developed without identifying the viral part targeted and evaluated in clinical trials (
Table 4) to assess their efficiency, as represented in
Figure 4.
Rhône Poulenc was one of the first laboratories to synthesize some antirhinoviral candidates that were evaluated in clinical trials. In 1976, a randomized double-blind placebo-controlled phase 2 clinical trial was published to evaluate the efficiency of
SKF 40491, from Smith Kline and French Laboratories,
GL R9-338, from Glaxo Laboratories, and
RP 19326 (Rhône Poulenc) against HRV infections [
76]. As a solution spray or a suspension drop, these compounds were delivered to 89 patients. Unfortunately, the in vivo half-time of these drugs is too short to observe striking clinical improvement.
Rhône Poulenc conducted another randomized placebo-controlled phase 2 clinical trial in 1987 to evaluate the effect of
44 081 R.P. against HRV-EL and HRV-1B infections [
77]. 58 patients were recruited to receive 300µg of drug or placebo 6 times daily for 6 days. Again, no significant efficiency were observed against HRV infections.
Roche also performed three phase 2 clinical trials to measure the efficiency of one different compound each time against HRV infections. In 1984, the first randomized double-blind placebo-controlled study was published to evaluate the effect of
Ro 09-0415 against HRV-9 infections [
78]. The drug was delivered as an oral capsule to a part of 57 patients. The study concludes with no significant prevention or decrease for the severity of the infection. This can be explained by the compound’s too-low concentration reaching nasal mucosa. Moreover, the treatment formulation seems to be irritant for intranasal use.
In 1987, a
Ro 09-0410 dephosphorylated
Ro 09-0415 against HRV-2 infections report study was published [
79].
Ro 09-0410 inhibit HRV uncoating [
80]. 50 mg of the drug was sprayed in 0.05 mL of nasal solution, three times per nostril and three times daily, for five days. This double-blind placebo-controlled study recruited 50 adults. No prevention of viral infections was observed, but a tendency to lower the virus shedding and the antibody response as well as increase the nasal secretion (0.01 < P < 0.05).
Finally, in 1990, another phase 2 clinical trial was published evaluating the effect of HRV-2-resistant at the same drug (
Ro 09-0410) delivered this time in an oral solution [
81]. 42 patients were recruited. The main goal of this study was to evaluate the effect of wild-type viruses versus resistant-induced viruses. Thus, no in vivo evaluation of the drug was performed.
Eventually, GlaxoSmithKline laboratory is actually conducting two randomized, double-blind placebo-controlled phase 1 clinical trial, which began in 2022, for its compounds GSK3923868 against asthma and chronic obstructive pulmonary disease (COPD) during rhinovirus infections [
82,
83]. Both studies recruited 68 patients, ranging from 18 to 64 years, divided into two cohorts, where the active cohort received the drug as an inhalation powder.
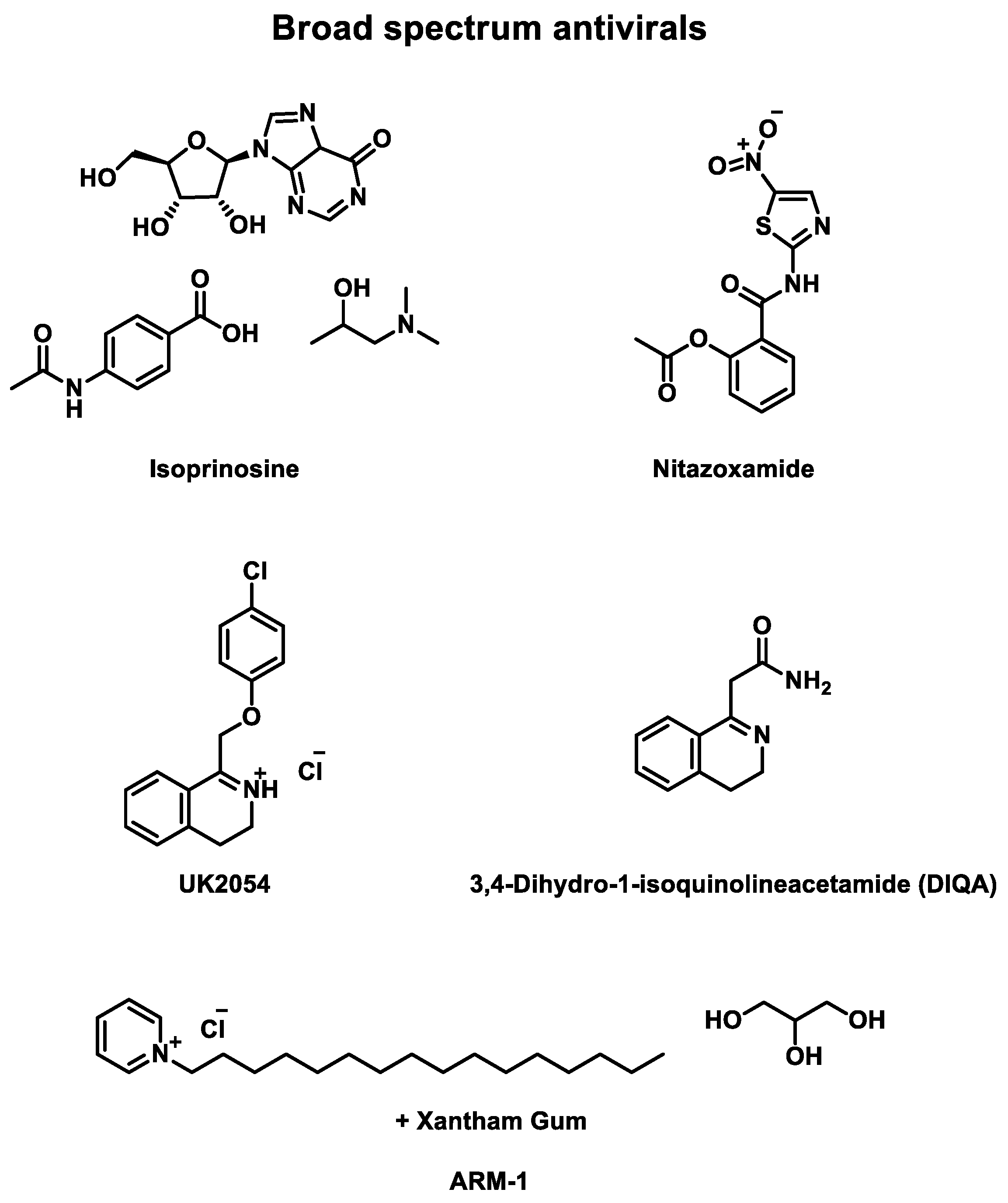
3.4.2. Broad Spectrum Antiviral
Numerous rhinovirus inhibitors were developed and studied in clinical trials, although many other broad-spectrum antivirals have also been evaluated against EV infections (
Table 5) and other upper respiratory tract infections caused by viruses. They are represented in
Figure 5.
For instance,
isoprinosine, a combination of inosine, acetamidobenzoic acid, and dimethylaminoisopropanol, has a broad spectrum against several viruses [
84]. Three double-blind placebo-controlled phase 2 clinical trials were published between 1973 and 1977 to evaluate the effect of
isoprinosine delivered as an oral tablet. The first study targeted HRV-9 and 55 patients were recruited. HRV-13 [
85]. They received 6 g daily for seven days. No significant differences were identified between placebo or drug treatment [
86].
In 1974, another clinical trial focused on HRV-30 and HRV-44 infections [
87]. 37 males received four times 1.5 g of the drug or placebo. A slight clinical improvement was observed for drug-treated patients, but not significant to prove the efficiency of the drug on this HRV serotypes [
86].
Finally, a third randomized study was published to evaluate the effect against HRV-21 [
88]. 39 patients were recruited to receive oral tablets. In the active cohort, patients received 4 g of the drug for 5 or 7 days. This time, significant reductions of clinical illness and viral shedding were observed [
86]. Indeed, the active cohort had a lower mean symptom score (P = 0.04) or cumulative symptoms score (P < 0.001). However, no significant difference was identified in mean symptom durations (0.05 < P < 0.1).
Consequently, isoprinosine is active against HRV-21 and slightly efficient against HRV-30 and HRV-44 but not against HRV-9 and HRV-13. No adverse effects caused by the drug were identified. Clinical trials with more patients in cohorts could clarify the efficiency of this drug against HRV infections.
Another actor developed a compound series of broad-spectrum antiviral. In 2018, a randomized double-blind placebo-controlled phase 3 clinical trial was performed to evaluate the efficiency of
nitazoxanide against EV and RV infections [
89]. 1756 adults were recruited to receive 300 mg of the drug twice daily for five days. The drug is administered by oral tablets. A slight improvement in infection management was observed in the active cohort. For instance, it takes a quicker time from the first dose to symptom response (P = 0.4009) or time to regain the ability to perform normal activities (P = 0.1923) than the placebo cohort. Likewise, less complications were observed for drug-treated patients (P = 0.2057). Eventually, they also had a lower proportion of positive for EV/RV RT-PCR for day 7 (P = 0.0132). In 2020, a second phase 3 clinical trial began under the same conditions as the previous study, adding a vitamin super B-complex administered to 800 adults with either drug or placebo. Currently, this study is still recruiting patients.
Some other laboratories submitted several antivirals to evaluate their efficiency against upper respiratory or HRV infections. In 1963, a randomized double-blind placebo-controlled phase 2 clinical trial was performed to measure the effect of UK2054 against HRV-9 infections [
90]. 60 patients were divided into two equal cohorts. No antiviral activity was found in this trial.
In 1973, a double-blind placebo-controlled phase 2 clinical trial was published to assess the effect of
3,4-Dihydro-1-isoquinolineacetamide (DIQA) against HRV-24 infection [
91]. Among 21 male volunteers, ranging in age from 21 to 42, 10 received 500 mg of the drug in an oral capsule. No significant difference in cold reduction was observed even if the active cohort’s symptoms were milder (rhinorrhea).
Finally, in 2013, a randomized double-blind placebo-controlled phase 2 clinical trial was conducted to evaluate the efficiency of
ARM-1 against upper respiratory, including rhinovirus, infections [
92].
ARM-1 is a formulation of
cetylpyridinium chloride, mixed with glycerin and xanthan gum, already identified as antiviral [
93]. The study recruited 94 patients (48 males and 46 females) aged from 18 to 43 years to receive oral spray solutions. This spray contained either a placebo or a drug. Patients sprayed three doses, three times daily, for 75 days. A lower detected upper respiratory infection (P = 0.41) and cough symptom (P = 0.012) were compared to the placebo cohort in the active cohort. Between days 5 and 9, drug-treated patients had a lower duration of non-fever symptoms (P = 0,019). The drug seemed to be safe and well-tolerated, with a reduction of symptoms and no drug-related adverse effects. However, some limitations were identified, such as the slight power of the drug in URI incidence and the lack of patients with symptoms. Future studies may be performed on multiple sites and seasons for more significant results.
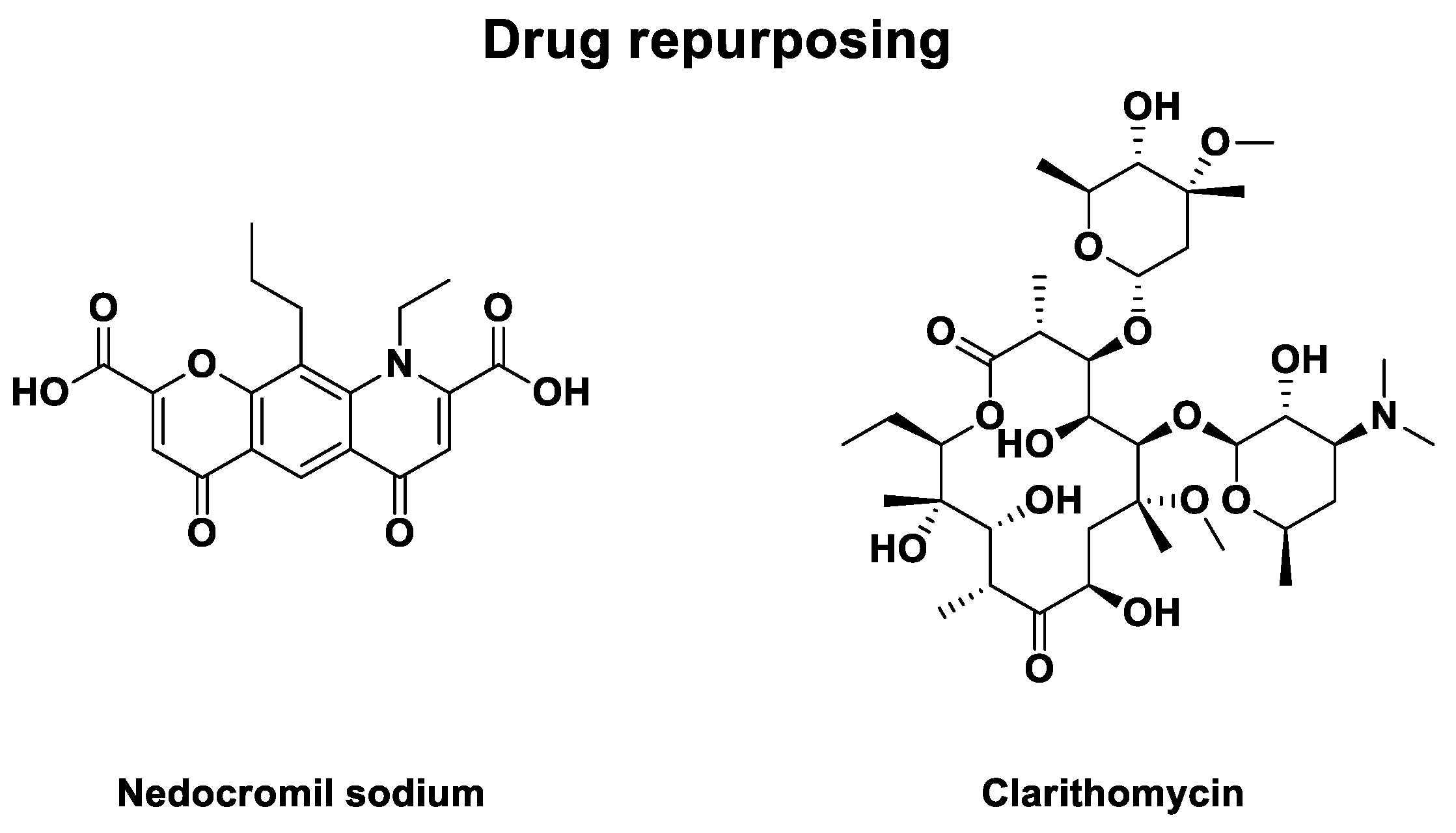
3.4.3. Other Drug Repurposing
Eventually, some drug repurposing led to phase 2 clinical trials against HRV infections. They are described in
Table 6. In 1990, a randomized double-blind placebo-controlled study to evaluate the effect of
nedocromil sodium, an anti-asthmatic compound represented in
Figure 6, against HRV-9 and HRV-14 infections was published [
94,
95]. 49 patients were recruited to receive a placebo or drug as nasal spray solution. The active cohort was exposed to 1.3 mg of the drug in 0.13 mL per nostril, four times daily, for one week. The study concludes with a lower nasal secretion for drug-treated patients (P < 0.05). Assays of drug tolerance identified no risk for
nedocromil sodium with no significant local irritation. However, no difference was observed in the frequency of viral shedding efficiency with no activity
in vitro.
In 2000, a second drug repurposing led to a randomized double-blind placebo-controlled study evaluating the efficiency of
clarithromycin against HRV-16 infections [
96].
Clarithomycin is an antibiotic for treating pulmonary diseases or skin and ORL infections [
97]. Its structure is represented in
Figure 6. Treatment was delivered as two daily oral capsules, each containing 500 mg of the drug or 160 / 800 mg of control (trimethoprim-sulfamethoxazole), for 8 days. 24 adults were recruited and divided into two cohorts. The drug-treated patients had no clinical effects compared to the control antibiotic cohort.
Part 2: Filters to obtain an efficient broad-spectrum anti-enterovirus drug
3.5. Candidates’ Selection for MTDL Strategy
Several criteria must be evaluated to select candidates for MTDL design. One of the first is the number of enteroviruses inhibited by the compound. For instance, some capsid binders are not efficient anymore against HRV-C, due to modified conformation of the VP1 capsid protein compared to other EVs [
98,
99,
100]. However, combining different compounds in an MTDL can be complementary to target all the enterovirus of interest. Another main criteria to consider is the inhibitor’s potency against viruses, either in vitro or
in vivo. This parameter is not sufficient to select candidates. Indeed, additive, antagonist, or synergistic effects must be considered between pharmacophores included in MTDL. These effects are evaluated in combination assays.
3.5.1. Combination of 2 Compounds
The compound combination leads to an additive, an antagonist, or a synergistic effect of all drugs involved. Additive effects can be produced by equivalent or overlapping actions (retinoic acid and trichostatin A) or independent actions (doxorubicin and trabectedin) [
101]. Synergetic effect can be created with several interactions: anti-counteractive actions (cisplatin and topotecan) [
102], complementary actions (celecoxib and emodin) [
81] or facilitating actions (gentamycin and vancomycin) [
103]. Eventually, some drug-drug interactions may modify the efficacy of a mono target by interacting with human pharmacodynamics with additive or antagonistic combinations [
10].
To date, no combinations of drugs that could be included in our study have been evaluated in clinical trials. This is why we only consider the additive or synergistic effect of targets, introducing other compounds tested in in vitro assays.
Capsid binders targeting the hydrophobic pocket were tested in combination, with only an additive effect observed. Indeed, once one capsid binder is in the hydrophobic pocket, the other can no longer act simultaneously due to steric obstruction. In contrast,
VP1-1 targeting the R209 cavity has a strong synergistic effect in combination with the usual capsid binder,
pleconaril [105]. This facilitates the hypothesis of a different mechanism of
VP1-1 with that of pleconaril.
However, the most interesting combinations could target the outer capsid and viral RNA by targeting different stages of viral replication. Strong synergistic effects (> 100 µM²% or ZIP synergy code > 15) have been identified for capsid binder combinations with 3C protease and BRAF inhibitors [
106]. The combination of a capsid binder with PI4KIIIβ, 2C protease, or 3A protein inhibitors leads to moderate, weak, or additive synergistic effects [
107,
108,
109,
110]. Combinations of 2C protease inhibitors with 3A protein, viral 37S RNA, or uncoating inhibitors lead to a strong synergistic effect. Similarly, 3A protein inhibitors are highly synergistic with viral 37S RNA or uncoating inhibitors [
107,
108,
109,
110,
111]. The combination of 3C protease inhibitors with capsid binders, BRAF, OSBP1, RNA-dependent RNA polymerase, and other protein inhibitors leads to a strong synergistic effect [
106,
107,
108,
109,
110,
111,
112]. However, BRAF and the other protein inhibitors tested individually have too many side effects to pursue the combination study. Finally, PI4KIIIβ inhibitors targeting various parts of phosphokinases have a synergistic effect, whereas, combined with 2C protease inhibitors, they produce a moderate to weak synergy [
113,
114]. Additionally, combining capsid binders can lead to an additive effect and an antagonistic effect with RNA-dependent RNA polymerases (RdRp). However, RdRp inhibitors has synergistic effect with compounds exacerbating autophagy [
115]. Eventually, some synergistic effect is observed with high concentration, while low concentration induced antagonistic effect [
116].
All results, synergistic effects, associated scores or combined indexes are presented in
Table 7.
3.5.2. Combination of 3 Compounds
Some assays were performed by combining 3 compounds in vitro to improve the efficiency of compound combinations and avoid drug resistance.
Some triple consecutive alternating administration (CAA) combination assays were performed by the Galabov Bulgarian team, namely one drug per day in 3-day cycles. They combined disoxaril, guanidine hydrochloride, and oxoglaucine (DGO) against CV-B1 and CV-B3 [
117,
118,
119,
120]. Other CAA combinations studies were led by the same team substituting disoxaril by pleconaril (PGO combination) or guanidine-hydrochloride by MDL-860 (PMO combination) against same viruses [
117,
118,
119,
120,
121,
122,
123]. These first assays were initially performed to decrease the resistance established with a combination of disoxaril and enviroxime [
124]. In this type of experiment, order of administration seems essential: first, a capsid binder, then a 2C protease inhibitor, and finally, an active compound in the early stage of viral replication. Other orders of administration showed less or no inhibition of viral proliferation. Likewise, adding PTU-23 in the combination to make a 4-day cycle suppresses the antiviral potency. DGO combinations are more efficient than PGO combinations. Substituting guanidine-hydrochloride (45 mg/kg) by MDL-860 (75 mg/kg) increases the anti-enteroviral effect. The concentration of MDL-860 should be compulsory to maintain the potency without developing toxicity due to higher doses. CAA combinations keep the potency of the most efficient drugs among the three inhibitors. Moreover, combination with CAA should reduce the risk of resistance apparition but also increase drug sensitivity in comparison with every drug treatment used alone or administrated simultaneously. This is an exciting approach, but it remains to be seen whether it is effective and can be implemented in humans, where the time of diagnosis does not necessarily coincide with the onset of enteroviral infection.
Recently, a new study combined remdesivir (RNA-depedent-RNA-polymerase inhibitor), rupintrivir (3C protease), and pleconaril (capsid binder) with a higher in vitro efficiency than monotherapy or two-drug cocktails [
125]. This combination was successfully evaluated on a broad spectrum of enterovirus, including five coxsackieviruses, three echoviruses, encephalomyocarditis virus, EV-A71, EV-D68, foot-and-mouth viruses, three polioviruses, nine rhinoviruses. Moreover, this 3-drug cocktail also delayed the emergence of antiviral drug resistance for any compound in the combination.
Even if clinical trials of capsid binders were stopped, the hydrophobic pocket inside VP1 capsid canyon remains the easiest target with conserved domains between species (except for HRV-C) [
98,
99,
100]. However, non-structural proteins are also prime targets for the weak fluctuation of their constitution between species, even if double-membrane vesicles protect them.
An MTDL strategy is based on combination therapies, which result in a synergistic effect with only one compound, not a cocktail of drugs. According to the most recent combination therapies tested, capsid binders have a strong synergistic effect with 3C protease inhibitors and a moderate or weak effect with 3A protease inhibitors. PI4KIIIβ inhibitors also have an additive synergy with capsid binders.
3.5.3. Other Criteria
The degree of resistance can be a determinant for the selection of candidates for broad-spectrum enterovirus inhibitors. For instance,
pleconaril treatment confronts some drug resistance in some clinical trials, with a part of natural resistance [
126,
127]. Eventually, the selectivity of viral targets by inhibitors is also important to reduce drug-emergent adverse effects due to binding with unexpected human proteins.
3.6. Biological Parameters for MTDL Strategy
3.6.1. Enteroviruses of Interest
Determining which EVs should be included in the MTDL concept strategy requires analysis of pathogenesis, epidemiology and evolution, surveillance program, and already-market therapeutic options [
128]. Considering all these points, we have selected non-polio enteroviruses (NPEVs), including important human pathogens, such as coxsackieviruses, echoviruses, numbered enteroviruses, and rhinoviruses [
10].
Infections related to these viruses have been described frequently over the last decade. We first noted clusters of infection, followed by a global emergence for EV species, specifically, EV-A-71 in the Asia-Pacific region and EV-D-68 in North America and in Europe. Rhinoviruses (RV) epidemiologic studies classified these viruses as the most frequent infectious agents in humans worldwide [
129,
130,
131].
NPEVs can cause a wide range of health disorders with varying presentation and severity, most often in pediatric and immunocompromised patients. NPEV neonatal diseases are often related to nonspecific symptoms but can lead to severe or fatal clinical situations [
132]. When considering epidemiological data, the most common pathogens with the highest incidence are due to EV-A, principally with hand, foot, and mouth disease (HFMD), whose etiologic agents are EV-A71, CV-A6, CV-A10, and CV-A16. EV-A infections have been linked with other pathologies. For example, EV-A-71 or Echovirus E11 may be the causative agents of severe and life-threatening neurological symptoms — often involving long-term sequelae and cardiopulmonary complications [
133]. Recently, EV-A71 has been reported to cause serious epidemics in Asia that are accompanied by severe neurological complications similar to poliomyelitis [
134]. Moreover, acute hemorrhagic conjunctivitis (AHC) was signaled during EV-A70 or CV-A24 infections [
135]. The second most represented are EV-B, which includes Echoviruses E30, E6, E11, and Coxsackievirus B3 [
6]. Severe EV-B infections have been linked mainly to acute flaccid paralysis (AFP). Meningitis or encephalitis can also be linked to EV-B infections, especially with Echovirus 30. Likewise, myocarditis is mainly provoked by the CV-B subgroup (from CV-B1 to CV-B6), and Echovirus 11 [
136]. CV-B1 can also cause severe infections in infants that may lead to sepsis [
137]. Moreover, in 2023, at least 7 European countries experienced an outbreak of Echovirus 11, leading to serious conditions in newborns [
137]. Eventually, EV-D species cause mainly pneumonia, bronchiolitis, or acute flaccid myelitis (AFM) [
3]. For instance, EV-D comprises the re-emergent EV-D68, recently responsible for epidemics of respiratory diseases and polio-like paralysis AFM leading to death in some cases [
138]. Interestingly, European countries have observed a re-emergence of EV-D68 post-COVID-19 lockdown [
139]. Rhinoviruses have been neglected for decades because they were only related to mild common cold with less virulence. Recently, they were recognized as critical causative agents of lower respiratory tract infections (LRTIs) and severe respiratory disease [
140,
141,
142].
Finally, considering more than one virus species in a large-scale program of medicinal chemistry is pertinent if these pathogens are detected in the same type of disease or isolated from the same clinical samples. Moreover, the drug candidate must exert a broad-spectrum activity, which, to our knowledge, is the case for several viruses of this genus.
3.6.2. Biological Access to the Target
Enteroviruses are obligatory intracellular parasites that need host cells to replicate. Externally accessible targets are mainly situated on viral capsid. Likewise, capsid binders targeting hydrophobic pockets can interact with their target in the extracellular viral cycle sequence. Sometimes, the pocket is empty, like in HVR-B14, but mostly, the pocket is filled with a fatty acid or a fatty acid derivative (lipped fatty acid or sphingosine), called a pocket factor [
143].
For inhibitors targeting viral proteins or proteases, the first main barrier remains the cellular membrane after entering the cell before inhibiting viral replication. Moreover, it is known that ssRNA (+RNA) viruses remodel intracellular membranes to support viral replication. Enteroviruses make closed single-membrane tubules and then double-membrane vesicles to escape the surveillance of the cell’s immune system [
144,
145,
146,
147].
Consequently, inhibitors targeting viral non-structural proteins may have more difficulty accessing their targets than inhibitors of viral structural proteins. However, any target will be privileged to meet our objective of proposing an MTDL candidate. Thus, the pharmacokinetic parameters of MTDL candidates are critical and may need refinement in the function of the target.
3.6.3. Conserved Residues of Targets - Resistances
Some features within the capsid are highly conserved for most HRV species. For example, the hydrophobic pocket sequence has a high degree of amino acid identity for both HRV-A and HRV-B species [
148]. Most recently, this remaining identity has also been found in EV-A71 and the whole serotype of the enterovirus genus [
149]. These results are due to the proximity between the hydrophobic pocket and the VP1-VP3 interface. Amino acids near the interface between viral proteins are the most conserved from one serotype to another.
Differences between protein or protease coding sequences are rare for the rest of the viral genome, like in species EV-A [
150]. Indeed, mutations of amino acids in these scaffolds should lead to irreversible changes in the recognition of viral substrates, even if some were detected after in vitro mutation assays [
151,
160].
One of the risks of using antivirals is the emergence of resistance, which results in reduced or loss of activity. This phenomenon has been observed for capsid-binding inhibitors after numerous in vitro expositions [
5,
8,
152].
4. Discussion
As any anti-enteroviral was refused to be commercialized due to several clinical trials failing of evaluated compounds, can their lack of efficiency and adverse effects be addressed with multi-target directed ligands (MTDLs)?
MTDL combined pharmacophores from different inhibitors to target several enterovirus (EV) proteins with one compound. They are drugs containing two or more pharmacophores structurally overlapping or separated by a spacer in a single molecule. Thus, the efficiency of the drug can be multiplied as an MTDL rather than a single molecule. Indeed, MTDL mimics drug combinations, including additive or synergistic effects. With well-associated combinations, synergy can increase benefit-risk balance and favor the success of clinical trial studies. Research on this topic spans various disease applications, including Alzheimer’s [
161,
162], cancer, and infectious diseases. Understanding drug resistance in cancer supports the development of MTDLs, coupled with clinical experience, which has revealed a multi-target strategy to enhance anticancer activity [
163]. This approach holds significant potential in fighting infectious diseases [
164,
165,
166,
167,
168,
169,
170]. In virology, multi-target compounds have been developed for pulmonary diseases targeting both F508del-CFTR and PI4KIIIβ [
171]. This approach is fascinating as it simultaneously targets the virus and disease pathogenesis [
172]. Virtual screening was recently done in SARS-CoV-2, CV-B1, CV-B3 and EV1 treatment research with the goal to design multi-target compounds [
106,
115,
173]. Many projects described above were designated for in silico evaluation of the interest of linked, merged, or fused compounds to produce an efficient compound library [
174,
175].
Several combinations can increase the potency of enterovirus inhibitors to achieve a broad-spectrum effect against non-polio enteroviruses (NPEVs) or enhance antiviral potency. A broad-spectrum approach is interesting because most NPEVs have similar mild clinical manifestations with widely different complications. Having a single compound for every NPEV eliminates the need for virus identification, leading to more efficient patient management. This advantage is crucial for the patient, as therapy initiation can be prompt without the time-consuming process of viral typing, which requires specialized equipment. Fusing, merging, or linking several inhibitors in one MTDL leads to a need for a balance between the affinity of the MTDL with its different targets and its pharmacokinetic parameters.
The single-chemical approach utilized by MTDLs can reduce adverse effects and enhance pharmacokinetic/pharmacodynamics parameters compared to combinations of individual single-target drugs [
161,
162]. However, MTDLs based on a linker to bind selected candidates can have a high molecular weight. Formulation or transport studies must be performed to evaluate the bioavailability of synthesized new MTDLs.
This approach may also lead to a more effective treatment, having additive or synergistic effects by targeting several viral proteins. Some bindings can be modified for fused or merged MTDLs. Indeed, including partial structure or pharmacophore of initial inhibitor in one compound could lead to adverse interactions or steric hindrance with the targeted viral proteins. These effects could decrease the absolute efficiency of MTDL without considering synergistic effects. Thus, MTDL should modify only the structure without affecting the binding mode of the initial inhibitor. MTDLs, gathering several initial inhibitors, generally have a higher molecule weight that implies a better specificity to target considered viral proteins. MTDLs try to maintain the same binding mode with the target but do not have the same interactions with unintentional cell metabolism steps. Consequently, it automatically decreases adverse effects linked with these steps [
161,
162].
Moreover, the MTDL strategy delayed the emergence of drug-related resistance. Viruses can develop lower sensitivity due to multiple contacts with the drug in monotherapy or due to lower doses of inhibitors in combinations [
125]. Having one merged or fused compound targeting several parts of the virus, the plurality of targets, enables delaying the onset of resistance due to acquired mutations, as well as maintaining higher concentrations deleting drug-drug interactions present in classic combinations. Even with enzyme-cleavable linked drugs, MTDL release can be controlled to avoid these issues.
MTDL design is also easier to conduct than combinations due to a reduced number of parameters to evaluate, even in comparison with single-target drug development [
161,
162]. Thus, MTDL design should have reduced cost development with superior antiviral activity.
Eventually, MTDL pharmacokinetic parameters are easier to manage than a combination strategy [
161,
162]. Different initial drugs gathered in MTDL don’t need the same bioavailability, pharmacokinetics, or metabolism to form an MTDL. Contrary to MTDL, combination therapy must apply this criterion to be efficient, with the same pharmaceutical form and pharmacodynamics, and avoid drug-drug interactions.
5. Conclusions
In this study, we reviewed clinical trials that evaluated inhibitors of enterovirus and classified them according to their target. Viral structural protein inhibitors only target VP1 capsid protein. 3A / 3AB and 3C proteases are viral non-structural proteins. Several human proteins (phosphodiesterase 3 PED3, phosphatidylinositol 4-kinase β PI4KB or prostaglandin D2 receptor 2 CRTH2 receptor) were studied as potential anti-enteroviral targets as well as some broad-spectrum antiviral, anti-rhinovirus inhibitors without defined mechanism of actions, and other class of drugs (anti-asthmatic and antibiotics). Non-structural proteins have a low mutation rate and are conserved between each enterovirus serotype. However, synthesizing double-membrane vesicles, protecting viral genetic material during translation or replication, makes them more challenging to access. On the other hand, capsid seems more straightforward to access. Moreover, the capsid hydrophobic pocket also has a conserved sequence, except for HRV-C serotypes. According to combination assays described in the literature, targets to select to have the best efficiency are capsid VP1, 3C protease, and a host target, like PI4KIIIβ. However, even if enterovirus inhibitors were well studied, none were commercialized due to a lack of benefit in the efficiency-risk-cost balance. This perspective proposes MTDL as a solution to overcome these obstacles. They enable enhanced efficiency with additive or synergistic effects. Likewise, it allows the reduction of opportunities for resistance to appear and limits drug-emerged adverse effects. Moreover, with fewer parameters to evaluate, MTDL assays are more accessible to develop rather than cocktail combination clinical trials. However, using linked, fused, or merged MTDL, the pharmacokinetic parameters, and binding modifications must be considered to obtain an optimized broad-spectrum enterovirus inhibitor.
Author Contributions
Conceptualization, M.R. and H.R.; formal analysis, H.R.; investigation, H.R.; data curation, M.R. and H.R.; writing—original draft preparation, H.R.; writing—review and editing, H.R, M.R, F.T., P.R. and P.V.; supervision, M.R. and P.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding OR This research was funded by Ministère de l’Enseignement supérieur, de la Recherche et de l’Innovation, Ecole doctorale 250 grant.
Data Availability Statement
Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Peigue-Lafeuille, H. Picornaviridae. In Traité de virologie médicale, 1st ed.; Estem Edition,Eds.; 2003; pp 389–405.
- Palmer, P; Lebon, P. Entérovirus. In Les virus transmissibles de la mère à l’enfant, 1st ed.; Médecine Sciences Edition, Eds.; 1999; pp.305–318.
- Pons-Salort, M.; Parker, E.P.; Grassly, N.C. The Epidemiology of non-polio enteroviruses: recent advances and outstanding questions. Curr. Opin. Infect. Dis. 2015, 28, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, H.; Poelman, R.; Knoester, M.; Van Leer-Buter, C.C.; Niesters, H.G.M. Enterovirus D68 - The New polio? Front. Microbiol. 2018, 9, 2677. [Google Scholar] [CrossRef] [PubMed]
- Morens, D.M.; Folkers, G.K.; Fauci, A.S. Acute flaccid myelitis: Something old and something new. mBio. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Zhang, Y.; Scheuermann, R.H. Epidemiology and sequence-based evolutionary analysis of circulating non-polio enteroviruses. Microorganisms. 2020, 8, 1856. [Google Scholar] [CrossRef] [PubMed]
- Grozdanov, P.; Joffret, M.L.; Stoyanova, A.; Polston, P.; Achouri, E.; Nikolova, I.; Delpeyroux, F.; Galabov, A.S. Genome analysis of coxsackievirus B1 isolates during the consecutive alternating administration course of triple antiviral combination in newborn mice. Antivir. Chem. Chemother. 2020, 28. [Google Scholar] [CrossRef] [PubMed]
- Lanko, K.; Shi, C.; Patil, S.; Delang, L.; Matthijnssens, J.; Mirabelli, C.; Neyts, J. Assessing in vitro resistance development in enterovirus A71 in the context of combination antiviral treatment. ACS. Infect. Dis. 2021, 7, 2801–2806. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; Chou, R.; Glanville, J.; Grimshaw, J.M.; Hróbjartsson, A.; Lalu, M.M.; Li, T.; Loder, E.W.; Mayo-Wilson, E.; McDonald, S.; McGuinness, L.A.; Stewart, L.A.; Thomas, J.; Tricco, A.C.; Welch, V.A.; Whiting, P.; Moher, D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. B.M.J. 2021, 372. [Google Scholar] [CrossRef]
- Baggen, J.; Thibaut, H.J.; Strating, J.R.P.M.; van Kuppeveld, F.J.M. The life cycle of non-polio enteroviruses and how to target it. Nat. Rev. Microbiol. 2018, 16, 391. [Google Scholar] [CrossRef] [PubMed]
- Anasir, M.I.; Zarif, F.; Poh, C.L. Antivirals blocking entry of enteroviruses and therapeutic potential. J. Biomed. Sci. 2021, 28, 10. [Google Scholar] [CrossRef]
- l-Nakib, W.; Higgins, P.G. , Barrow, G.I.; Tyrrell, D.A.; Andries, K.; Vanden Bussche, G.; Taylor, N.; Janssen, P.A. Suppression of colds in human volunteers challenged with rhinovirus by a new synthetic drug (R61837). Antimicrob. Agents Chemother. 1989, 33, 522–525. [Google Scholar] [CrossRef]
- Turner, R.B.; Dutko, F.J.; Goldstein, N.H.; Lockwood, G.; Hayden, F.G. Efficacy of oral WIN 54954 for prophylaxis of experimental rhinovirus infection. Antimicrob. Agents Chemother. 1993, 37, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Woods, M.G.; Diana, G.D.; Rogge, M.C.; Otto, M.J.; Dutko, F.J.; McKinlay, M.A. In vitro and in vivo activities of WIN 54954, a new broad-spectrum antipicornavirus drug. Antimicrob. Agents Chemother. 1989, 33, 2069–2074. [Google Scholar] [CrossRef] [PubMed]
- Hayden, F.G.; Hipskind, G.J.; Woerner, D.H.; Eisen, G.F.; Janssens, M.; Janssen, P.A.; Andries, K. Intranasal pirodavir (R77,975) treatment of rhinovirus colds. Antimicrob. Agents Chemother. 1995, 39, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Andries, K.; Dewindt, B.; Snoeks, J.; Willebrords, R.; van Eemeren, K.; Stokbroekx, R.; Janssen, P.A. In vitro activity of pirodavir (R 77975), a substituted phenoxy-pyridazinamine with broad-spectrum antipicornaviral activity. Antimicrob. Agents Chemother. 1992, 36, 100–107. [Google Scholar] [CrossRef]
- Hayden, F.G.; Andries, K.; Janssen, P.A. Safety and efficacy of intranasal pirodavir (R77975) in experimental rhinovirus infection. Antimicrob. Agents Chemother. 1992, 36, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Schiff, G.M.; Sherwood, J.R. Clinical activity of pleconaril in an experimentally induced coxsackievirus A21 respiratory infection. J. Infect. Dis. 2000, 181, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Rotbart, H.A.; Webster, A.D.; Pleconaril Treatment Registry Group. Treatment of potentially life-threatening enterovirus infections with pleconaril. Clin. Infect. Dis. 2001, 32, 228–235. [Google Scholar] [CrossRef]
- Pevear, D.C.; Tull, T.M.; Seipel, M.E.; Groarke, J.M. Activity of pleconaril against enteroviruses. Antimicrob. Agents Chemother. 1999, 43, 2109–2115. [Google Scholar] [CrossRef]
- Aradottir, E.; Alonso, E.M.; Shulman, S.T. Severe neonatal enteroviral hepatitis treated with pleconaril. Pediatr. Infect. Dis. J. 2001, 20, 457–459. [Google Scholar] [CrossRef]
- Abzug, M.J.; Cloud, G.; Bradley, J.; Sánchez, P.J.; Romero, J.; Powell, D.; Lepow, M.; Mani, C.; Capparelli, E.V.; Blount, S.; Lakeman, F.; Whitley, R.J.; Kimberlin, D.W.; National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Double blind placebo-controlled trial of pleconaril in infants with enterovirus meningitis. Pediatr. Infect. Dis. J. 2003, 22, 335–341. [Google Scholar] [CrossRef]
- Desmond, R.A.; Accortt, N.A.; Talley, L.; Villano, S.A.; Soong, S.J.; Whitley, R.J. Enteroviral meningitis: natural history and outcome of pleconaril therapy. Antimicrob. Agents Chemother. 2006, 50, 2409–2414. [Google Scholar] [CrossRef] [PubMed]
- Abzug, M.J.; Michaels, M.G.; Wald, E.; Jacobs, R.F.; Romero, J.R.; Sánchez, P.J.; Wilson, G.; Krogstad, P.; Storch, G.A.; Lawrence, R.; Shelton, M.; Palmer, A.; Robinson, J.; Dennehy, P.; Sood, S.K.; Cloud, G.; Jester, P.; Acosta, E.P.; Whitley, R.; Kimberlin, D.; National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. A Randomized, double-blind, placebo-controlled trial of pleconaril for the treatment of neonates with enterovirus sepsis. J. Pediatric. Infect. Dis. Soc. 2016, 5, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Pevear, D.C.; Hayden, F.G.; Demenczuk, T.M.; Barone, L.R.; McKinlay, M.A.; Collett, M.S. Relationship of pleconaril susceptibility and clinical outcomes in treatment of common colds caused by rhinoviruses. Antimicrob. Agents Chemother. 2005, 49, 4492–4499. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Allergy and Infectious Diseases (NIAID). Pleconaril enteroviral sepsis syndrome. A Double-blind, placebo-controlled, virologic efficacy trial of pleconaril in the treatment of neonates with enteroviral sepsis syndrome. National Library of Medecine, National Center for Biotechnology Information. https://clinicaltrials.gov/study/NCT00031512 (accessed 04-Jul-2024).
- Bauer, S.; Gottesman, G.; Sirota, L.; Litmanovitz, I.; Ashkenazi, S.; Levi, I. Severe coxsackie virus B infection in preterm newborns treated with pleconaril. Eur. J. Pediatr. 2002, 161, 491–493. [Google Scholar] [CrossRef] [PubMed]
- Merck Sharp & Dohme LLC. Effects of pleconaril nasal spray on common cold symptoms and asthma exacerbations following rhinovirus exposure (study P04295). A Placebo-controlled study of the effects of pleconaril nasal spray on common cold symptoms and asthma exacerbations following rhinovirus exposure. National Library of Medecine, National Center for Biotechnology Information. https://ClinicalTrials.gov/show/NCT00394914 (accessed 04-Jul-2024).
- Watson, K.G.; Brown, R.N.; Cameron, R.; Chalmers, D.K.; Hamilton, S.; Jin, B.; Krippner, G.Y.; Luttick, A.; McConnell, DB.; Reece, P.A.; Ryan, J.; Stanislawski, P.C.; Tucker, S.P.; Wu, W.Y.; Barnard, D.L.; Sidwell, R.W. An orally bioavailable oxime ether capsid binder with potent activity against human rhinovirus. J. Med. Chem. 2003, 46, 3181–3184. [Google Scholar] [CrossRef] [PubMed]
- Biota Scientific Management Pty Ltd. A Phase II, double-blind placebo-controlled study to determine the prophylactic efficacy of oral BTA798 in an experimental rhinovirus challenge model. EU Clinical Trials Register. https://www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2008-001714-24 (accessed 04-Jul-2024).
- Biota Scientific Management Pty Ltd. A Phase 2 study of BTA798 in asthmatic adults with symptomatic human rhinovirus infection (RHINO). A Phase 2 multicenter, randomized, double-blind, placebo-controlled study of BTA798 in asthmatic adults with symptomatic human rhinovirus infection. National Library of Medecine, National Center for Biotechnology Information. https://ClinicalTrials.gov/show/NCT01175226 (accessed 04-Jul-2024).
- Biota Pharmaceuticals, Inc. A Phase 2, multicenter, randomized, double-blind, placebo-controlled dose-ranging study of vapendavir in moderate to severe asthmatic adults with symptomatic human rhinovirus infection. EU Clinical Trials Register. https://www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2014-001785-95 (accessed 04-Jul-2024).
- Biota Pharmaceuticals, Inc. A Phase 2, multicenter, randomized, double-blind, placebo-controlled dose-ranging study of vapendavir in moderate to severe asthmatic adults with symptomatic human rhinovirus infection. World Health Organisation, International Clinical Trias Registery Platform. https://trialsearch.who.int/Trial2.aspx?TrialID=NCT02367313 (accessed 04-Jul-2024).
- Vaxart, H. A Study of vapendavir treatment of hematopoietic stem cell transplant subjects with symptomatic rhinovirus infection. A Phase 2, multicenter, randomized, double-blind, placebo-controlled study of vapendavir treatment of hematopoietic stem cell transplant subjects with symptomatic rhinovirus infection. National Library of Medecine, National Center for Biotechnology Information. https://ClinicalTrials.gov/show/NCT03024177. (accessed on 4 July 2024).
- Altesa Biosciences, Inc. RCT of vapendavir in patients with COPD and human rhinovirus/enterovirus upper respiratory infection. A Trial in participants with chronic obstructive pulmonary disease (COPD) to evaluate the impact of vapendavir on the development of lower respiratory tract symptoms following rhinovirus challenge. National Library of Medecine, National Center for Biotechnology Information, 2017. https://ClinicalTrials.gov/show/NCT06149494 (accessed 04-Jul-2024).
- Xiao, X.; Lei, X.; Zhang, Z.; Ma, Y.; Qi, J.; Wu, C.; Xiao, Y.; Li, L.; He, B.; Wang, J. Enterovirus 3A facilitates viral replication by promoting phosphatidylinositol 4-kinase IIIβ-ACBD3 Interaction. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Hsu, N.Y.; Ilnytska, O.; Belov, G.; Santiana, M.; Chen, Y.H.; Takvorian, P.M.; Pau, C.; van der Schaar, H.; Kaushik-Basu, N.; Balla, T.; Cameron, C.E.; Ehrenfeld, E.; van Kuppeveld, F.J.; Altan-Bonnet, N. Viral reorganization of the secretory pathway generates distinct organelles for RNA replication. Cell. 2010, 141, 799–811. [Google Scholar] [CrossRef]
- Arita, M.; Kojima, H.; Nagano, T.; Okabe, T.; Wakita, T.; Shimizu, H. Phosphatidylinositol 4-kinase III beta is a target of enviroxime-like compounds for antipoliovirus activity. J. Virol. 2011, 85, 2364–2372. [Google Scholar] [CrossRef]
- Qin, Y.; Lin, L.; Chen, Y.; Wu, S.; Si, X.; Wu, H.; Zhai, X.; Wang, Y.; Tong, L.; Pan, B.; Zhong, X.; Wang, T.; Zhao, W.; Zhong, Z. Curcumin inhibits the replication of enterovirus 71 in vitro. Acta. Pharm. Sin. B. 2014, 4, 284–294. [Google Scholar] [CrossRef]
- Lu, G.; Qi, J.; Gao, G.F.; et al. Enterovirus 71 and Coxsackievirus A16 3C Proteases: Binding to Rupintrivir and Their Substrates and Anti-Hand, Foot, and Mouth Disease Virus Drug Design. J. Virol. 2011, 85, 10319–10331. [Google Scholar] [CrossRef]
- Tang, F.; Xia, H.; Wang, P.; Yang, J.; Zhao, T.; Zhang, Q.; Hu, Y.; Zhou, X. The identification and characterization of nucleic acid chaperone activity of human enterovirus 71 nonstructural protein 3AB. Virology. 2014, 464–465, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Enviroxime PubChem Page. https://pubchem.ncbi.nlm.nih.gov/compound/enviroxime. (accessed on 4 July 2024).
- Phillpotts, R.J.; Jones, R.W.; Delong, D.C.; Reed, S.E.; Wallace, J.; Tyrrell, D.A. The activity of enviroxime against rhinovirus infection in man. Lancet. 1981, 1, 1342–1344. [Google Scholar] [CrossRef] [PubMed]
- Hayden, F.G.; Gwaltney, J.M. Jr. Prophylactic activity of intranasal enviroxime against experimentally induced rhinovirus type 39 infection. Antimicrob. Agents Chemother. 1982, 21, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.D.; Monto, A.S.; DeLong, D.C.; Exelby, A.; Bryan, E.R.; Srivastava, S. Controlled trial of enviroxime against natural rhinovirus infections in a community. Antimicrob. Agents Chemother. 1985, 27, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Levandowski, R.A.; Pachucki, C.T.; Rubenis, M.; Jackson, G.G. Topical enviroxime against rhinovirus infection. Antimicrob. Agents Chemother. 1982, 22, 1004–1007. [Google Scholar] [CrossRef] [PubMed]
- Phillpotts, R.J.; Wallace, J.; Tyrrell, D.A.; Tagart, V.B. Therapeutic activity of enviroxime against rhinovirus infection in volunteers. Antimicrob. Agents Chemother. 1983, 23, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Meijer, A.; Delang, L.; et al. Antiviral activity of broad-spectrum and enterovirus-specific inhibitors against clinical isolates of enterovirus D68. Antimicrob. Agents Chemother. 2015, 59, 7782–7785. [Google Scholar] [CrossRef] [PubMed]
- Chau, D.H.; Yuan, J.; Yang, D.; et al. Coxsackievirus B3 proteases 2A and 3C induce apoptotic cell death through mitochondrial injury and cleavage of eIF4GI but not DAP5/p97/NAT1. Apoptosis. 2007, 12, 513–524. [Google Scholar] [CrossRef]
- Amineva, S.P.; Aminev, A.G.; Gern, J.E.; et al. Rhinovirus 3C protease precursors 3CD and 3CD’ localize to the nuclei of infected cells. J. Gen. Virol. 2004, 85 (Pt 10) Pt 10, 2969–2979. [Google Scholar] [CrossRef]
- Dragovich, P.S.; Prins, T.J.; Zhou, R.; Webber, S.E.; Marakovits, J.T.; Fuhrman, S.A.; Patick, A.K.; Matthews, D.A.; Lee, C.A.; Ford, C.E.; Burke, B.J.; Rejto, P.A.; Hendrickson, T.F.; Tuntland, T.; Brown, E.L.; Meador, J.W. 3rd; Ferre, R.A.; Harr, J.E.; Kosa, M.B.; Worland, S.T. Structure-based design, synthesis, and biological evaluation of irreversible human rhinovirus 3C protease inhibitors. 4. Incorporation of P1 lactam moieties as L-glutamine replacements. J. Med. Chem. 1999, 42, 1213–1224. [Google Scholar] [CrossRef]
- Hsyu, P.H.; Pithavala, Y.K.; Gersten, M.; Penning, C.A.; Kerr, B.M. Pharmacokinetics and safety of an antirhinoviral agent, ruprintrivir, in healthy volunteers. Antimicrob. Agents Chemother. 2002, 46, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Hayden, F.G.; Turner, R.B.; Gwaltney, J.M.; Chi-Burris, K.; Gersten, M.; Hsyu, P.; Patick, A.K.; Smith, G.J. 3rd; Zalman, L.S. Phase II, randomized, double-blind, placebo-controlled studies of ruprintrivir nasal spray 2-percent suspension for prevention and treatment of experimentally induced rhinovirus colds in healthy volunteers. Antimicrob. Agents Chemother. 2003, 47, 3907–3916. [Google Scholar] [CrossRef] [PubMed]
- Patick, A.K.; Brothers, M.A.; Maldonado, F.; Binford, S.; Maldonado, O.; Fuhrman, S.; Petersen, A.; Smith, G.J. 3rd; Zalman, L.S.; Burns-Naas, L.A.; Tran, J.Q. In vitro antiviral activity and single-dose pharmacokinetics in humans of a novel, orally bioavailable inhibitor of human rhinovirus 3C protease. Antimicrob. Agents Chemother. 2005, 49, 2267–2275. [Google Scholar] [CrossRef] [PubMed]
- Dragovich, P.S.; Prins, T.J.; Zhou, R.; Johnson, T.O.; Hua, Y.; Luu, H.T.; Sakata, S.K.; Brown, E.L.; Maldonado, F.C.; Tuntland, T.; Lee, C.A.; Fuhrman, S.A.; Zalman, L.S.; Patick, A.K.; Matthews, D.A.; Wu, E.Y.; Guo, M.; Borer, B.C.; Nayyar, N.K.; Moran, T.; Chen, L.; Rejto, P.A.; Rose, P.W.; Guzman, M.C.; Dovalsantos, E.Z.; Lee, S.; McGee, K.; Mohajeri, M.; Liese, A.; Tao, J.; Kosa, M.B.; Liu, B.; Batugo, M.R.; Gleeson, J.P.; Wu, Z.P.; Liu, J.; Meador, J.W. 3rd.; Ferre, R.A. Structure-based design, synthesis, and biological evaluation of irreversible human rhinovirus 3C protease inhibitors. 8. Pharmacological optimization of orally bioavailable 2-pyridone-containing peptidomimetics. J. Med. Chem. 2003, 46, 4572–4585. [Google Scholar] [CrossRef] [PubMed]
- Kankam, M.K.; Burns, J.M.; Collett, M.S.; Corrado, M.L.; Hincks, J.R. A Phase 1 study of the safety, tolerability, and pharmacokinetics of single and multiple oral doses of V-7404 in healthy adult volunteers. Antimicrob. Agents Chemother. 2021, 65. [Google Scholar] [CrossRef] [PubMed]
- Carlson, A.B.; Kraus, G.P. Physiology, Cholinergic Receptors. In StatPearls, 1st ed.; 2024.
- Gaffey, M.J.; Gwaltney, J.M. Jr.; Dressler, W.E.; Sorrentino, J.V.; Hayden, F.G. Intranasally administered atropine methonitrate treatment of experimental rhinovirus colds. Am. Rev. Respir. Dis. 1987, 135, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Pierce, R.J.; Allen, C.J.; Campbell, A.H. A comparative study of atropine methonitrate, salbutamol, and their combination in airways obstruction. Thorax. 1979, 34, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Shipley, J.B.; Tolman, D.; Hastillo, A.; Hess, M.L. Milrinone: basic and clinical pharmacology and acute and chronic management. Am. J. Med. Sci. 1996, 311, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Mika, D.; Leroy, J.; Fischmeister, R.; Vandecasteele, G. Rôle des phospho-diestérases des nucléotides cycliques de types 3 et 4 dans le couplage excitation-contraction et les arythmies cardiaques. PhD Dissertation, INSERM UMR-S 769, Université Paris-Sud, Châtenay-Malabry, France, 2013. [Google Scholar]
- Beute J, Lukkes M, Koekoek EP, Nastiti H, Ganesh K, de Bruijn MJ, Hockman S, van Nimwegen M, Braunstahl GJ, Boon L, Lambrecht BN, Manganiello VC, Hendriks RW, KleinJan A. A pathophysiological role of PDE3 in allergic airway inflammation. JCI Insight. 2018, 3. [CrossRef]
- Sobhy, A.; Eldin, D.M.K.; Zaki, H.V. The Use of Milrinone Versus Conventional treatment for the management of life-threatening bronchial asthma. Open Anesthesiology Journal. 2019, 13, 12–17. [Google Scholar] [CrossRef]
- Wang, S.M.; Lei, H.Y.; Huang, M.C.; Wu, J.M.; Chen, C.T.; Wang, J.N.; Wang, J.R.; Liu, C.C. Therapeutic efficacy of milrinone in the management of enterovirus 71-induced pulmonary edema. Pediatr. Pulmonol. 2005, 39, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.Y.; Khanh, T.H.; Thoa le, P.K.; Tseng, F.C.; Wang, S.M.; Thinh le, Q.; Lin, C.C.; Wu, H.C.; Wang, J.R.; Hung, N.T.; Thuong, T.C.; Chang, C.M.; Su, I.J.; Liu, C.C. Milrinone therapy for enterovirus 71-induced pulmonary edema and/or neurogenic shock in children: a randomized controlled trial. Crit. Care Med. 2013, 41, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Melia, C.E.; van der Schaar, H.M.; Lyoo, H.; Limpens, R.W.A.L.; Feng, Q.; Wahedi, M.; Overheul, G.J.; van Rij, R.P.; Snijder, E.J.; Koster, A.J.; Bárcena, M.; van Kuppeveld, F.J.M. Escaping Host Factor PI4KB Inhibition: Enterovirus Genomic RNA Replication in the Absence of Replication Organelles. Cell Rep. 2017, 21, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Curovir, AB. A Randomised, double-blind, single-centre, placebo-controlled, first-in-human clinical trial evaluating the safety, tolerability and pharmacokinetics of single and multiple ascending doses of CUR-N399 in healthy volunteers. National Library of Medecine, National Center for Biotechnology Information. https://ClinicalTrials.gov/show/NCT05016687 (accessed 04-Jul-2024).
- Cheong, D.H.J.; Yogarajah, T.; Wong, Y.H.; Arbrandt, G.; Westman, J.; Chu, J.J.H. CUR-N399, a PI4KB inhibitor, for the treatment of Enterovirus A71 infection. Antiviral Res. 2023, 218. [Google Scholar] [CrossRef] [PubMed]
- Biazi,G. R.; Frasson, I.G.; Miksza, D.R.; de Morais, H.; de Fatima Silva, F.; Bertolini, G.L.; de Souza, H.M. Decreased hepatic response to glucagon, adrenergic agonists, and cAMP in glycogenolysis, gluconeogenesis, and glycolysis in tumor-bearing rats. J. Cell Biochem. 2018, 119, 7300–7309. [Google Scholar] [CrossRef] [PubMed]
- Durkee, C.A.; Covelo, A.; Lines, J.; Kofuji, P.; Aguilar, J.; Araque, A. Gi/o protein-coupled receptors inhibit neurons but activate astrocytes and stimulate gliotransmission. Glia. 2019, 67, 1076–1093. [Google Scholar] [CrossRef] [PubMed]
- Winther, B.; Buchert, D.; Turner, R.B.; Hendley, J.O.; Tschaikin, M. Decreased rhinovirus shedding after intranasal oxymetazoline application in adults with induced colds compared with intranasal saline. Am. J. Rhinol. Allergy. 2010, 24, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Haenisch, B.; Walstab, J.; Herberhold, S.; Bootz, F.; Tschaikin, M.; Ramseger, R.; Bönisch, H. Alpha-adrenoceptor agonistic activity of oxymetazoline and xylometazoline. Fundam. Clin. Pharmacol. 2010, 24, 729–739. [Google Scholar] [CrossRef]
- Brightling, C.; Kulkarni, S.; Lambrecht, B.N.; Sandham, D.; Weiss, M.; Altman, P. The pharmacology of the prostaglandin D2 receptor 2 (DP2) receptor antagonist, fevipiprant. Pulm. Pharmacol. Ther. 2021, 68. [Google Scholar] [CrossRef]
- Liu, W.; Min, J.; Jiang, H.; Mao, B. Chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2) antagonists in asthma: a systematic review and meta-analysis protocol. BMJ Open. 2018, 8. [Google Scholar] [CrossRef]
- Imperial College London. Effect of OC459 on the response to rhinovirus challenge in asthma. Effect of the CRTH2 antagonist OC459 on the response to rhinovirus challenge in asthma. National Library of Medecine, National Center for Biotechnology Information. https://ClinicalTrials.gov/show/NCT02660489 (accessed 04-Jul-2024).
- Reed, S.E.; Craig, J.W.; Tyrrell, D.A. Four compounds active against rhinovirus: comparison in vitro and in volunteers. J. Infect. Dis. 1976, 133 Suppl, A128–A1235. [Google Scholar] [CrossRef]
- Zerial, A.; Werner, G.H.; Phillpotts, R.J.; Willmann, J.S.; Higgins, P.G.; Tyrrell, D.A. Studies on 44 081 R.P., a new antirhinovirus compound, in cell cultures and in volunteers. Antimicrob. Agents Chemother 1985, 27, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Phillpotts, R.J; Higgins, P.G.; Willman, J.S.; Tyrrell, D.A.; Lenox-Smith, I. Evaluation of the antirhinovirus chalcone Ro 09-0415 given orally to volunteers. J. Antimicrob. Chemother. 1984, 14, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Al-Nakib, W.; Higgins, P.G.; Barrow, I.; Tyrrell, D.A.; Lenox-Smith, I.; Ishitsuka, H. Intranasal chalcone, Ro 09-0410, as prophylaxis against rhinovirus infection in human volunteers. J. Antimicrob. Chemother. 1987, 20, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Ishitsuka, H.; Ninomiya, Y.T.; Ohsawa, C.; Fujiu, M.; Suhara, Y. Direct and specific inactivation of rhinovirus by chalcone Ro 09-0410. Antimicrob. Agents Chemother. 1982, 22, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Yasin, S.R.; al-Nakib, W.; Tyrrell, D.A. Pathogenicity for humans of human rhinovirus type 2 mutants resistant to or dependent on chalcone Ro 09-0410. Antimicrob. Agents Chemother. 1990, 34, 963–966. [Google Scholar] [CrossRef] [PubMed]
- GlaxoSmithKline Research & Development Limited. A Randomised, double-blind, placebo controlled, repeat dose phase 1b study to assess the efficacy, safety, tolerability, pharmacokinetics and pharmacodynamics of inhaled GSK3923868 during experimental human rhinovirus infection in participants with mild asthma. World Health Organisation, International Clinical Trias Registery Platform. https://trialsearch.who.int/Trial2.aspx?TrialID=ISRCTN15115094 (accessed 04-Jul-2024).
- GlaxoSmithKline. A Randomized, double-blind, placebo controlled, repeat dose phase 1b study to assess the efficacy, safety, tolerability, pharmacokinetics and pharmacodynamics of inhaled GSK3923868 during experimental human rhinovirus infection in participants with mild asthma. World Health Organisation, International Clinical Trias Registery Platform. https://trialsearch.who.int/Trial2.aspx?TrialID=NCT05398198 (accessed 04-Jul-2024).
- Chang, T.W.; Weinstein, L. Antiviral activity of isoprinosine in vitro and in vivo. Am. J. Med. Sci. 1973, 265, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Soto, A.J.; Hall, T.S.; Reed, S.E. Trial of the antiviral action of isoprinosine against rhinovirus infection of volunteers. Antimicrob. Agents Chemother. 1973, 3, 332–334. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.W.; Heel, R.C. Ribavirin and inosiplex: a review of their present status in viral diseases. Drugs. 1981, 22, 111–128. [Google Scholar] [CrossRef]
- Pachuta, D.M.; Togo, Y.; Hornick, R.B.; Schwartz, A.R.; Tominaga, S. Evaluation of isoprinosine in experimental human rhinovirus infection. Antimicrob. Agents Chemother. 1974, 5, 403–408. [Google Scholar] [CrossRef]
- Waldman, R.H.; Gangulyj, R. Therapeutic efficacy of inosiplex (Isoprinosine) in rhinovirus infection. Ann. N. Y. Acad. Sci. 1977, 284, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Romark Laboratories, L.C. A Phase III, randomized, double-blind, placebo controlled trial to evaluate the efficacy and safety of nitazoxanide in the treatment of colds due to enterovirus/rhinovirus infection. National Library of Medecine, National Center for Biotechnology Information. https://ClinicalTrials.gov/show/NCT03605862 (accessed 04-Jul-2024).
- Reed, S.E.; Bynoe, M.L. The antiviral activity of isoquinoline drugs for rhinoviruses in vitro and in vivo. J. Med. Microbiol. 1970, 3, 346–52. [Google Scholar] [CrossRef]
- Togo, Y.; Schwartz, A.R.; Hornick, R.B. Antiviral effect of 3, 4-dihydro-1-isoquinolineacetamide hydrochloride in experimental human rhinovirus infection. Antimicrob. Agents Chemother. 1973, 4, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Esper, F.; Buchheit, K.; Arters, K.; Adkins, I.; Ghannoum, M.A.; Salata, R.A. Randomized, double-blind, placebo-controlled clinical trial to assess the safety and effectiveness of a novel dual-action oral topical formulation against upper respiratory infections. BMC Infect. Dis. 2017, 17, 74. [Google Scholar] [CrossRef]
- Cetylpyridinuium chloride page in Adis Insight website. https://adisinsight.springer.com/drugs/800048807 (accessed 04-Jul-2024).
- Barrow, G.I.; Higgins, P.G.; al-Nakib, W.; Smith, A.P.; Wenham, R.B.; Tyrrell, D.A. The effect of intranasal nedocromil sodium on viral upper respiratory tract infections in human volunteers. Clin. Exp. Allergy. 1990, 20, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Gazdik, F.; Jahnova, E.; Horvathova, M.; Pijak, M.R.; Gazdikova, K. Antiasthmatic effects of nedocromil sodium. Bratisl. Lek. Listy. 2003, 104, 222–226. [Google Scholar]
- Abisheganaden, J.A.; Avila, P.C.; Kishiyama, J.L.; Liu, J.; Yagi, S.; Schnurr, D.; Boushey, H.A. Effect of clarithromycin on experimental rhinovirus-16 colds: a randomized, double-blind, controlled trial. Am. J. Med. 2000, 108, 453–459. [Google Scholar] [CrossRef]
- Clarithromycin page in United Kingdom National Health Service. https://www.nhs.uk/medicines/clarithromycin/about-clarithromycin/ (accessed 04-Jul-2024).
- Hao, W.; Bernard, K.; Patel, N.; Ulbrandt, N.; Feng, H.; Svabek, C.; Wilson, S.; Stracener, C.; Wang, K.; Suzich, J.; Blair, W.; Zhu, Q. Infection and propagation of human rhinovirus C in human airway epithelial cells. J. Virol. 2012, 86, 13524–13532. [Google Scholar] [CrossRef]
- Basta, H.A.; Sgro, J.Y.; Palmenberg, A.C. Modeling of the human rhinovirus C capsid suggests a novel topography with insights on receptor preference and immunogenicity. Virology. 2014, 448, 176–184. [Google Scholar] [CrossRef]
- Wang, J.; Hu, Y.; Zheng, M. Enterovirus A71 Antivirals: Past, Present, and Future. Acta. Pharm. Sin. B. 2022, 12, 1542–1566. [Google Scholar] [CrossRef]
- Jia, J.; Zhu, F.; Chen, Y.Z.; et al. Mechanisms of drug combinations: Interaction and network perspectives. Nat. Rev. Drug. Discov. 2009, 8, 11–128. [Google Scholar] [CrossRef]
- Van Waardenburg, R.C.; de Jong, L.A.; Schellens, J.H.; et al. Platinated DNA adducts enhance poisoning of DNA topoisomerase I by camptothecin. J. Biol. Chem. 2004, 29, 54502–54509. [Google Scholar] [CrossRef] [PubMed]
- Lai, G.H.; Zhang, Z.; Sirica, A.E. Celecoxib acts in a cyloooxygenase-2-independent manner and in synergy with emodin to suppress rat cholangiocarcinoma growth in vitro through a mechanism involving enhanced akt inactivation and increased activation of caspase-9 and -3. Mol. Cancer. Ther. 2003, 2, 262–271. [Google Scholar]
- Cottagnoud, P.; Cottagnoud, M.; Täuber, M.G. Vancomycin acts systematically with gentamicin against penicillin-resistant pneumococci by increasing the intracellular penetration of gentamicin. Antimicrob. Agents Chemother. 2003, 47, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Abdelnabi, R.; Delang, L.; Froeyen, M.; Luyten, W.; Neyts, J.; Mirabelli, C. New class of early-stage enterovirus inhibitors with a novel mechanism of action. Antiviral. Res. 2017, 147, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Ianevski, A.; Zusinaite, E.; Tenson, T.; Oksenych, V.; Wang, W.; Afset, J.E.; Bjørås, M.; Kainov, D.E. Novel synergistic anti-enteroviral drug combinations. Viruses. 2022, 14, 1866. [Google Scholar] [CrossRef] [PubMed]
- Rhoden, E.; Liu, H.M.; Wang-Chern, S.W.; Oberste, M.S. Anti-poliovirus potency of protease inhibitor AG-7404, and assessment of in vitro potency in combination with antiviral capsid inhibitor compounds. Antiviral. Res. 2013, 98, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Stoyanova, A.; Galabov, A.S. Effects of double combinations of enterovirus replication inhibitors against coxsackie B viruses. Acta. Virol. 2021, 65, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Nikolaeva-Glomb, L.; Galabov, A.S. Synergistic combinations against the replication in vitro of coxsackievirus B1. Antivir. Res. 2004, 62, 9–19. [Google Scholar] [CrossRef]
- Tan, Y.W.; Yam, W.K.; Kooi, R.J.W.; Westman, J.; Arbrandt, G.; Chu, J.J.H. . Novel capsid binder and PI4KIIIbeta inhibitors for EV-A71 replication inhibition. Sci. Rep. 2021, 11, 9719. [Google Scholar] [CrossRef]
- Lonberg-Holm, K.; Gosser, L.B.; Kauer, J.C. Early alteration of poliovirus in infected cells and its specific inhibition. J. Gen. Virol. 1975, 27, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Wang Y, Li G, Yuan S, Gao Q, Lan K, Altmeyer R, Zou G. In Vitro assessment of combinations of enterovirus inhibitors against enterovirus 71. Antimicrob Agents Chemother. 2016, 60, 5357–5367. [Google Scholar] [CrossRef]
- Hung, H.C.; Wang, H.C.; Shih, S.R.; Teng, I.F.; Tseng, C.P.; Hsu, J.T. Synergistic inhibition of enterovirus 71 replication by interferon and rupintrivir. J. Infect. Dis. 2011, 203, 1784–1790. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, B.; Zhai, Y.; Sun, Y.; Rao, Z. Peptidyl aldehyde NK-1.8k suppresses enterovirus 71 and enterovirus 68 infection by targeting protease 3C. Antimicrob. Agents Chemother. 2015, 59, 2636–46. [Google Scholar] [CrossRef] [PubMed]
- Kang H, Kim C, Kim DE, Song JH, Choi M, Choi K, Kang M, Lee K, Kim HS, Shin JS, Kim J, Han SB, Lee MY, Lee SU, Lee CK, Kim M, Ko HJ, van Kuppeveld FJ, Cho S. Synergistic antiviral activity of gemcitabine and ribavirin against enteroviruses. Antiviral Res. 2015, 124, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yang Q, Jie Q, Shaw N, Li L, Rao Z, Yin Z, Lou Z. Studies on inhibition of proliferation of enterovirus-71 by compound YZ-LY-0. Int. J. Biol. Sci. 2015, 11, 1337–1347. [Google Scholar] [CrossRef]
- Vassileva-Pencheva, R.; Galabov, A.S. Avoiding drug-resistance development by novel approach of combining anti-enteroviral substances against coxsackievirus B1 infection in mice. Antiviral. Res. 2010, 85, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Vassileva-Pencheva, R.; Galabov; A. S. Effectiveness of the consecutive alternating administration course of a triple antiviral combination in coxsackievirus B3 infections in mice. Drug. Res. (Stuttg). 2016, 66, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Stoyanova, A.; Nikolova, I.; Pürstinger, G.; Dobrikov, G.; Dimitrov, V.; Philipov, S.; Galabov, A.S. . Anti-enteroviral triple combination of viral replication inhibitors: activity against coxsackievirus B1 neuroinfection in mice. Antivir. Chem. Chemother. 2015, 24, 136–147. [Google Scholar] [CrossRef]
- Galabov AS, Nikolova I, Vassileva-Pencheva R, Stoyanova A. Antiviral Combination Approach as a Perspective to Combat Enterovirus Infections. Pril. (Makedon. Akad. Nauk. Umet. Odd. Med. Nauki.). 2015, 36, 91–99. [Google Scholar] [CrossRef]
- Stoyanova, A.; Nikolova, I.; Galabov, A.S. Effect of consecutive altenarting administration (CAA) of triple anti-enteroviral combination on coxsackievirus B1 neuroinfection in mice. Antiviral. Res. 2015, 121, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Stoyanova A, Galabov AS. Effect of consecutive alternating administration of a triple combination of anti-enteroviral compounds in mice infected with coxsackievirus B3. Pathog. Dis. 2020, 78. [Google Scholar] [CrossRef]
- Stoyanova A, Galabov AS. Consecutive alternating administration as an effective anti-coxsackievirus B3 in vivo treatment scheme. Arch. Virol. 2021, 166, 1869–1875. [Google Scholar] [CrossRef] [PubMed]
- Nikolaeva, L.; Galabov, A.S. Antiviral effect of combination of enviroxime and disoxaril on coxsackievirus B1 Infection. Acta. Virol. 2000, 44, 73–78. [Google Scholar] [PubMed]
- Ianevski, A.; Frøysa, I.T.; Lysvand, H.; Calitz, C.; Smura, T.; Schjelderup Nilsen, H.J.; Høyer, E.; Afset, J.E.; Sridhar, A.; Wolthers, K.C.; Zusinaite, E.; Tenson, T.; Kurg, R.; Oksenych, V.; Galabov, A.S.; Stoyanova, A.; Bjørås, M.; Kainov, D.E. The combination of pleconaril, rupintrivir, and remdesivir efficiently inhibits enterovirus infections in vitro, delaying the development of drug-resistant virus variants. Antiviral Res. 2024, 224, 105842. [Google Scholar] [CrossRef] [PubMed]
- Hayden FG, Herrington DT, Coats TL, Kim K, Cooper EC, Villano SA, Liu S, Hudson S, Pevear DC, Collett M, McKinlay M; Pleconaril Respiratory Infection Study Group. Efficacy and safety of oral pleconaril for treatment of colds due to picornaviruses in adults: results of 2 double-blind, randomized, placebo-controlled trials. Clin. Infect. Dis. 2003, 36, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Hayden FG, Turner RB, Gwaltney JM, Chi-Burris K, Gersten M, Hsyu P, Patick AK, Smith GJ 3rd, Zalman LS. Phase II, randomized, double-blind, placebo-controlled studies of ruprintrivir nasal spray 2-percent suspension for prevention and treatment of experimentally induced rhinovirus colds in healthy volunteers. Antimicrob. Agents Chemother. 2003, 47, 3907–3916. [Google Scholar] [CrossRef] [PubMed]
- Abdelnabi, R.; Baggen, J.; Mirabelli, C.; Neyts, J. Antiviral strategies against (non-polio) picornaviruses. In New drug development for known and emerging viruses, 1st ed. Wiley-VCH GmbH. 2022.
- Chang, Y.K.; Chen, K.H.; Chen, K.T. Hand, foot and mouth disease and herpangina caused by enterovirus A71 infections: A Review of enterovirus A71 molecular epidemiology, pathogenesis, and current vaccine development. Rev. Inst. Med. Trop. Sao Paulo. 2018, 60. [Google Scholar] [CrossRef] [PubMed]
- Holm-Hansen, C.C.; Midgley, S.E.; Fischer, T.K. Global emergence of enterovirus D68: A Systematic review. Lancet. Infect. Dis. 2016, 16. [Google Scholar] [CrossRef]
- Royston, L.; Tapparel, C. Rhinoviruses and respiratory enteroviruses: Not as simple as ABC. Viruses. 2016, 8, 16. [Google Scholar] [CrossRef]
- Haston, J.C.; Dixon, T.C. Nonpolio enterovirus infections in neonates. Pediatr. Ann. 2015, 44. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Tijsma, A.; Mirabelli, C.; Baggen, J.; Wahedi, M.; Franco, D.; De Palma, A.; Leyssen, P.; Verbeken, E.; van Kuppeveld, F.J.M.; Neyts, J.; Thibaut, H.J. Intra-Host emergence of an enterovirus A71 variant with enhanced PSGL1 usage and neurovirulence. Emerg. Microbes. Infect. 2019, 8, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.; Liu, C.C.; Chow, Y.H.; Chou, A.H.; Klein, M. . Review of enterovirus 71 vaccines. Clin. Infect. Dis. 2015, 60, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Higgins, P.G. Enteroviral conjunctivitis and its neurological complications. Arch. Virol. 1982, 73, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Lugo, D.; Krogstad, P. Enteroviruses in the early 21st century: New manifestations and challenges. Curr. Opin. Pediatr. 2016, 28, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Disease Outbreak News (7th of June 2023) from World Health Organization site. https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON474 (accessed 04-Jul-2024).
- Messacar, K.; Abzug, M.J.; Dominguez, S.R. The Emergence of enterovirus-D68. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Benschop, K.S.; Albert, J.; Anton, A.; Andrés, C.; Aranzamendi, M.; Armannsdóttir, B.; Bailly, J.L.; Baldanti, F.; Baldvinsdóttir, G.E.; Beard, S.; Berginc, N.; Böttcher, S.; Blomqvist, S.; Bubba, L.; Calvo, C.; Cabrerizo, M.; Cavallero, A.; Celma, C.; Ceriotti, F.; Costa, I.; Cottrell, S.; Del Cuerpo, M.; Dean, J.; Dembinski, J.L.; Diedrich, S.; Diez-Domingo, J.; Dorenberg, D.; Duizer, E.; Dyrdak, R.; Fanti, D.; Farkas, A.; Feeney, S.; Flipse, J.; De Gascun, C.; Galli, C.; Georgieva, I.; Gifford, L.; Guiomar, R.; Hönemann, M.; Ikonen, N.; Jeannoël, M.; Josset, L.; Keeren, K.; López-Labrador, F.X.; Maier, M.; McKenna, J.; Meijer, A.; Mengual-Chuliá, B.; Midgley, S.E.; Mirand, A.; Montes, M.; Moore, C.; Morley, U.; Murk, J.L.; Nikolaeva-Glomb, L.; Numanovic, S.; Oggioni, M.; Palminha, P.; Pariani, E.; Pellegrinelli, L.; Piralla, A.; Pietsch, C.; Piñeiro, L.; Rabella, N.; Rainetova, P.; Uceda Renteria, S.C.; Romero, M.P.; Reynders, M.; Roorda, L.; Savolainen-Kopra, C.; Schuffenecker, I.; Soynova, A.; Swanink, C.M.; Ursic, T.; Verweij, J.J.; Vila, J.; Vuorinen, T.; Simmonds, P.; Fischer, T.K.; Harvala, H. Re-Emergence of enterovirus D68 in Europe after easing the COVID-19 lockdown, September 2021. Euro. Surveill. 2021, 26, 2100998. [Google Scholar] [CrossRef] [PubMed]
- To, K.K.W.; Yip, C.C.Y.; Yuen, K.Y. Rhinovirus - From Bench to bedside. J. Formos. Med. Assoc. 2017, 116, 496–504. [Google Scholar] [CrossRef]
- Ljubin-Sternak, S.; Meštrović, T.; Ivković-Jureković, I.; Kolarić, B.; Slović, A.; Forčić, D.; Tot, T.; Mijač, M.; Vraneš, J. The Emerging role of rhinoviruses in lower respiratory tract infections in children - Clinical and molecular epidemiological study from Croatia, 2017–2019. Front. Microbiol. 2019, 10, 2737. [Google Scholar] [CrossRef]
- Mirabelli, C.; Scheers, E.; Neyts, J. Novel therapeutic approaches to simultaneously target rhinovirus infection and asthma/COPD pathogenesis. F1000Res. 2017, 6, 1860. [Google Scholar] [CrossRef]
- Egorova, A.; Ekins, S.; Schmidtke, M.; Makarov, V. Back to the future: Advances in development of broad-spectrum capsid-binding inhibitors of enteroviruses. Eur. J. Med. Chem. 2019, 178, 606–622. [Google Scholar] [CrossRef] [PubMed]
- Limpens, R.W.; van der Schaar, H.M.; Kumar, D.; Koster, A.J.; Snijder, E.J.; van Kuppeveld, F.J.; Bárcena, M. The Transformation of enterovirus replication structures: A Three-dimensional study of single- and double-membrane compartments. mBio. 2011, 2. [Google Scholar] [CrossRef]
- Wolff, G.; Melia, C.E.; Snijder, E.J.; Bárcena, M. Double-membrane vesicles as platforms for viral replication. Trends. Microbiol. 2020, 28, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Lennemann, N.J.; Evans, A.S.; Coyne, C.B. Imaging-based reporter systems to define CV-B-induced membrane remodeling in living cells. Viruses. 2020, 12, 1074. [Google Scholar] [CrossRef] [PubMed]
- Belov, G.A.; Sztul, E. Rewiring of cellular membrane homeostasis by picornaviruses. J. Virol. 2014, 88, 9478–9489. [Google Scholar] [CrossRef] [PubMed]
- Ledford, R.M.; Patel, N.R.; Demenczuk, T.M.; Watanyar, A.; Herbetz, T.; Collet, M.S.; Pevear, D.C. VP1 Sequencing of all human rhinovirus serotypes: Insights into genus phylogeny and susceptibility to antiviral capsid-binding compounds. J. Virol. 2004, 78, 3663–3674. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Lin, T.M.; Wang, S.Y.; Wang, J.R. The Role of conserved arginine and proline residues in enterovirus VP1 protein. J. Microbiol. Immunol. Infect. 2022, 55, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Oberste, M.S.; Peñaranda, S.; Maher, K.; Pallansch, M.A. Complete genome sequences of all members of the species human enterovirus A. J. Gen. Virol. 2004, 85 Pt 6, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Arita, M.; Kojima, H.; Nagano, T.; Okabe, T.; Wakita, T.; Shimizu, H. Phosphatidylinositol 4-kinase III beta is a target of enviroxime-like compounds for antipoliovirus activity. J. Virol. 2011, 85, 2364–2372. [Google Scholar] [CrossRef]
- Feil, S.C.; Hamilton, S.; Krippner, G.Y.; Lin, B.; Luttick, A.; McConnell, D.B.; Nearn, R.; Parker, M.W.; Ryan, J.; Stanislawski, P.C.; Tucker, S.P.; Watson, K.G.; Morton, C.J. An Orally available 3-ethoxybenzisoxazole capsid binder with clinical activity against human rhinovirus. ACS. Med. Chem. Lett. 2012, 3, 303–307. [Google Scholar] [CrossRef]
- Deng, C.L.; Yeo, H.; Ye, H.Q.; Liu, S.Q.; Shang, B.D.; Gong, P.; Alonso, S.; Shi, P.Y.; Zhang, B. Inhibition of enterovirus 71 by adenosine analog NITD008. J. Virol. 2014, 88, 11915–11923. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, L.; Zhai, Y.; Ma, J.; Nie, Q.; Li, T.; Yin, Z.; Sun, Y.; Shang, L. Inhibition of enterovirus 71 replication by an α-hydroxy-nitrile derivative NK-1.9k. Antiviral. Res. 2017, 141, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Wang, K.; Zhao, K.; Hua, S.C.; Du, J. The Structure, function, and mechanisms of action of enterovirus non-structural protein 2C. Front. Microbiol. 2020, 11, 615965. [Google Scholar] [CrossRef] [PubMed]
- Visser, L.J.; Langereis, M.A.; Rabouw, H.H.; Wahedi, M.; Muntjewerff, E.M.; de Groot, R.J.; van Kuppeveld, F.J.M. Essential role of enterovirus 2A protease in counteracting stress granule formation and the induction of type I interferon. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wu, S.; Xiao, T.; Li, Y.; Su, Z.; Wei, W.; Hao, F.; Hu, G.; Lin, F.; Chen, X.; Gu, Z.; Lin, T.; He, H.; Li, J.; Chen, S. Design, synthesis, and evaluation of a novel macrocyclic anti-EV71 agent. Bioorg. Med. Chem. 2020, 28, 115551. [Google Scholar] [CrossRef] [PubMed]
- Deutschmann-Olek, K.M.; Yue, W.W.; Bezerra, G.A.; Skern, T. Defining substrate selection by rhinoviral 2A proteinase through its crystal structure with the inhibitor zVAM.fmk. Virology. 2021, 562, 128–141. [Google Scholar] [CrossRef]
- Lanko, K.; Sun, L. .; Froeyen, M.; Leyssen, P.; Delang, L.; Mirabelli, C.; Neyts, J. Comparative analysis of the molecular mechanism of resistance to vapendavir across a panel of picornavirus species. Antiviral. Res. 2021, 195, 105177. [Google Scholar] [CrossRef]
- El Kazzi, P.; Yaacoub, C.; Fajloun, Z.; Vanelle, P.; Decoly, E.; Coutard, B.; Barral, K. 2C Protein of enterovirus: Key protein of viral replication and antiviral target. Virologie (Montrouge). 2023, 27, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jiang, X.; He, S.; Jiang, H.; Feng, F.; Liu, W.; Qu, W.; Sun, H. Rational design of multitarget-directed ligands: Strategies and emerging paradigms. J. Med. Chem. 2019, 62, 8881–8914. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Liu, F.; Li, S.; Shi, D. Rational multitargeted drug design strategy from the perspective of a medecinal chemist. J. Med. Chem. 2021, 64, 10581–10605. [Google Scholar] [CrossRef]
- Chaves, S.; Resta, S.; Santos, M.A. Design, synthesis, and in vitro evaluation of hydroxybenzimidazole-donepezil analogues as multitarget-directed ligands for the treatment of Alzheimer’s disease. Molecules. 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Abdolmaleki, A; Ghasemi, J. B. Dual-acting of hybrid compounds - A New dawn in the discovery of multi-target drugs: lead generation approaches. Curr. Top. Med. Chem. 2017, 17, 1096–1114. [Google Scholar] [CrossRef] [PubMed]
- Csermely, P.; Agoston, V.; Pongor, S. The Efficiency of multi-target drugs: The Network approach might help drug design. Trends. Pharmacol. Sci. 2005, 26, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Puls, L.N.; Eadens, M.; Messersmith, W. Current status of SRC inhibitors in solid tumor malignancies. Oncologist. 2011, 16, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Boran, A.D.; Iyengar, R. Systems approaches to polypharmacology and drug discovery. Curr. Opin. Drug. Discov. Devel. 2010, 13, 297–309. [Google Scholar] [PubMed]
- Ikram, N.; Mirza; M. U.; Vanmeert, M.; Froeyen, M.; Salo-Ahen, O.M.H.; Tahir, M.; Qazi, A.; Ahmad, S. Inhibition of oncogenic kinases: An in vitro validated computational approach identified potential multi-target anticancer compounds. Biomolecules. 2019, 9, 124. [Google Scholar] [CrossRef]
- Kumar, N.; Gupta, S.; Chand Yadav, T.; Pruthi, V.; Kumar Varadwaj, P.; Goel, N. Extrapolation of phenolic compounds as multi-target agents against cancer and inflammation. J. Biomol. Struct. Dyn. 2019, 37, 2355–2369. [Google Scholar] [CrossRef] [PubMed]
- Sunil, D.; Kamath, P.R. Multi-target directed indole based hybrid molecules in cancer therapy: An Up-to-date evidence-based review. Curr. Top. Med. Chem. 2017, 17, 959–985. [Google Scholar] [CrossRef]
- Sbaraglini, M.L.; Talevi, A. Hybrid compounds as anti-infective agents. Curr. Top. Med. Chem. 2017, 17, 1080–1095. [Google Scholar] [CrossRef]
- Naik, B.; Gupta, N.; Ojha, R.; Singh, S.; Prajapati, V.K.; Prusty, D. High throughput virtual screening reveals SARS-CoV-2 multi-target binding natural compounds to lead instant therapy for COVID-19 treatment. Int. J. Biol. Macromol. 2020, 160, 1–17. [Google Scholar] [CrossRef]
- Tassini, S.; Sun, L.; Lanko, K.; Crespan, E.; Langron, E.; Falchi, F.; Kissova, M.; Armijos-Rivera, JI.; Delang, L.; Mirabelli, C.; Neyts, J.; Pieroni, M.; Cavalli, A.; Cstantino, G.; Maga, G.; Vergani, P.; Leyssen, P.; Radi, M. Discovery of multi-target agents active as broad-spectrum antivirals and correctors of cystic fibrosis transmembrane conductance regulator (CFTR) for associated pulmonary diseases. J. Med. Chem. 2017, 60, 1400–1416. [Google Scholar] [CrossRef] [PubMed]
- Ambure, P.; Bhat, J.; Puzyn, T.; Roy, K. Identifying natural compounds as multi-target-directed ligands against Alzheimer’s disease: An In Silico approach. J. Biomol. Struct. Dyn. 2019, 37, 1282–1306. [Google Scholar] [CrossRef] [PubMed]
- Scotti, L.; Mendonca Junior, F.J.; Ishiki, H.M.; Ribeiro, F.F.; Singla, R.K.; Barbosa Filho, J.M.; Da Silva, M.S.; Scotti, M.T. Docking studies for multi-target drugs. Curr. Drug. Targets. 2017, 18, 592–604. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).