1. Introduction
Mites of the family Syringophilidae Lavoipierre, 1953 belong to widely distributed parasites of birds, spending their entire lives inside feather quills. In this unique habitat, they feed, undergo their entire development, and copulate [
1,
2,
3,
4,
5]. The dispersing forms are adult fertilised females, which move to a new host during breeding season, from parents to offspring (vertical transfer). Horizontal transfer of parasites can also occur during host mating or through frequent contact between host individuals, especially among social birds or between predators and their prey [
6,
7,
8,
9]. The infestation rate of the host population by Syringophilidae mites is undoubtedly dependent on the host behaviour; it is relatively high in social species (reaching up even to 70%) and low in solitary species [
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20]. It is worth noting that in the field studies provided in Poland, the examined population of the Eurasian Starling was infested by quill mites on a quite high level (the prevalence = 54%) [
21].
Syringophilids, living and feeding on the tissue fluids of birds, are not considered to significantly reduce bird fitness [
20,
21]. However, our understanding of the harm caused by syringophilids is still incomplete. Veterinarians have observed clinical symptoms of feather picking in domestic birds attributed to quill mites, as reported in various studies [
22,
23,
24]. Gritschenko [
25] suggested that
S. bipectinatus feeding induces itching, leading chickens to peck at affected feathers. This behaviour was thought to cause feather loss due to muscle tone relaxation, allowing new feathers to displace old, infested ones. Despite these findings, recent research shows no signs of quill mites causing skin or feather morphological changes in wild birds, even under heavy infestation [
15,
20,
21]. On the other hand, the studies provided by Skoracki et al. [
26], suggest that quill mites could act as vectors for the bacterium
Anaplasma phagocytophilum, an obligate intracellular pathogen. These studies specifically found this pathogen in syringophiline mites, parasitising Eurasian Starling (Sturnidae) and Blackbirds (Turdidae). From an epidemiological perspective, the vertical transmission of syringophilids could potentially accelerate the spread of various avian diseases within bird populations.
Syringophilinae mites exhibit high specificity towards their host groups. Each mite species typically parasitises a narrow host range, usually confined to hosts within a single order. For example,
Aulobia and
Syringophilopsis are found exclusively in birds of the order Passeriformes, while
Syringophilus and
Colinophilus parasitise Galliformes, and
Creagonycha and
Niglarobia are associated with Charadriiformes [
27,
28,
29]. Additionally, Syringophilinae species are specific to the type of feather they inhabit. For instance,
Syringophiloidus prefers secondary feathers,
Syringophilopsis is found in primary and secondary feathers,
Neoaulonastus inhabits secondary feathers and coverts, and
Aulonastus occupies coverts and contour feathers [
4].
The family Syringophilidae is divided into two subfamilies, Syringophilinae and Picobiinae, significantly differing from each other in terms of morphology, biology, and ecology [
1,
2,
4,
5,
30]. Mites from the Syringophilinae subfamily, the subject of this article, mainly inhabit the feathers of wings (flight feathers, coverts) and tail. Relatively rarely, and usually smaller-sized species, can additionally be found in the under- and upper-tail coverts (e.g., some species of the genus
Peristerophila) and contour feathers (e.g., mites of the genus
Aulonastus) [
2,
4].
The subfamily Syringophilinae currently includes 328 species grouped in 50 genera [
31,
32]. They have been found on host representatives belonging to many orders of neognathous birds (Neognathae), whereas in paleognathous birds (Paleognathae), they are known only among representatives of the order Tinamiformes [
33,
34]. However, Skoracki et al. [
35], suggest that their presence on representatives of this order is the result of a switching from hosts belonging to neognathous birds. The order Passeriformes has its own syringophiline fauna grouped into 12 genera [
2,
4,
5,
8,
36,
37,
38]. Among this diversity, four genera of syringophilines have been recorded from starlings so far. In this paper, we provide detailed information on the host spectrum and distribution for all described species of syringophilines with new host and locality records. Additionally, we describe three new species within the genera
Aulonastus and
Syringophiloidus collected from starlings captured in Indonesia, Papua New Guinea, and Tanzania.
2. Materials and Methods
The mite material used in the present study was collected from dry bird skins housed in the ornithological collection of the Bavarian State Collection of Zoology, Munich, Germany (Staatliche Naturwissenschaftliche Sammlungen Bayerns SNSB-ZSM). Before mounting, specimens were softened and cleared in Nesbitt’s solution at room temperature for three to four days, according to the protocol introduced by Krantz and Walter [
39] and Skoracki [
4].
Identification of mite specimens and drawing preparations were carried out with a ZEISS Axioscope2™ light microscope with differential interference contrast (DIC) optics and a camera lucida. All measurements are given in micrometres. The nomenclature for the idiosomal setation follows Grandjean [
40], as adapted for Prostigmata by Kethley [
41], leg setation is that of Grandjean [
42], and general morphological terms follow Skoracki [
4].
Specimen depositories are cited using the following abbreviations: AMU—Adam Mickiewicz University, Department of Animal Morphology, Poznań, Poland; SNSB-ZSM—Bavarian State Collection of Zoology, Munich, Germany.
The common and scientific names of birds after Clements et al. [
43] and del Hoyo et al. [
44]. Zoogeographical regions after Holt et al. [
45] and Ficetola et al. [
46].
3. Results
3.1. Descriptions
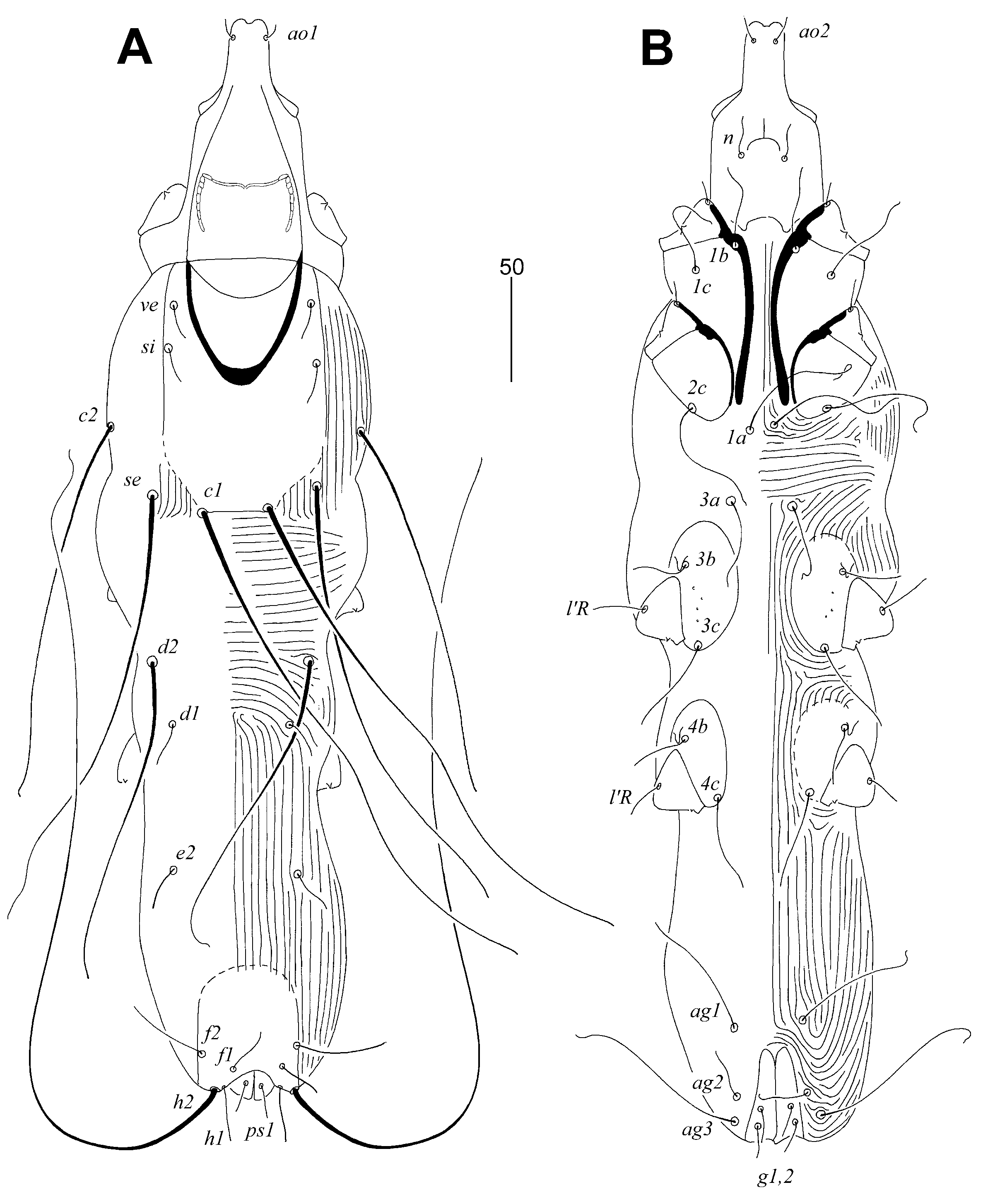
3.1.1. Aulonastus indonesianus Marcisova, Patan and Skoracki sp. n.
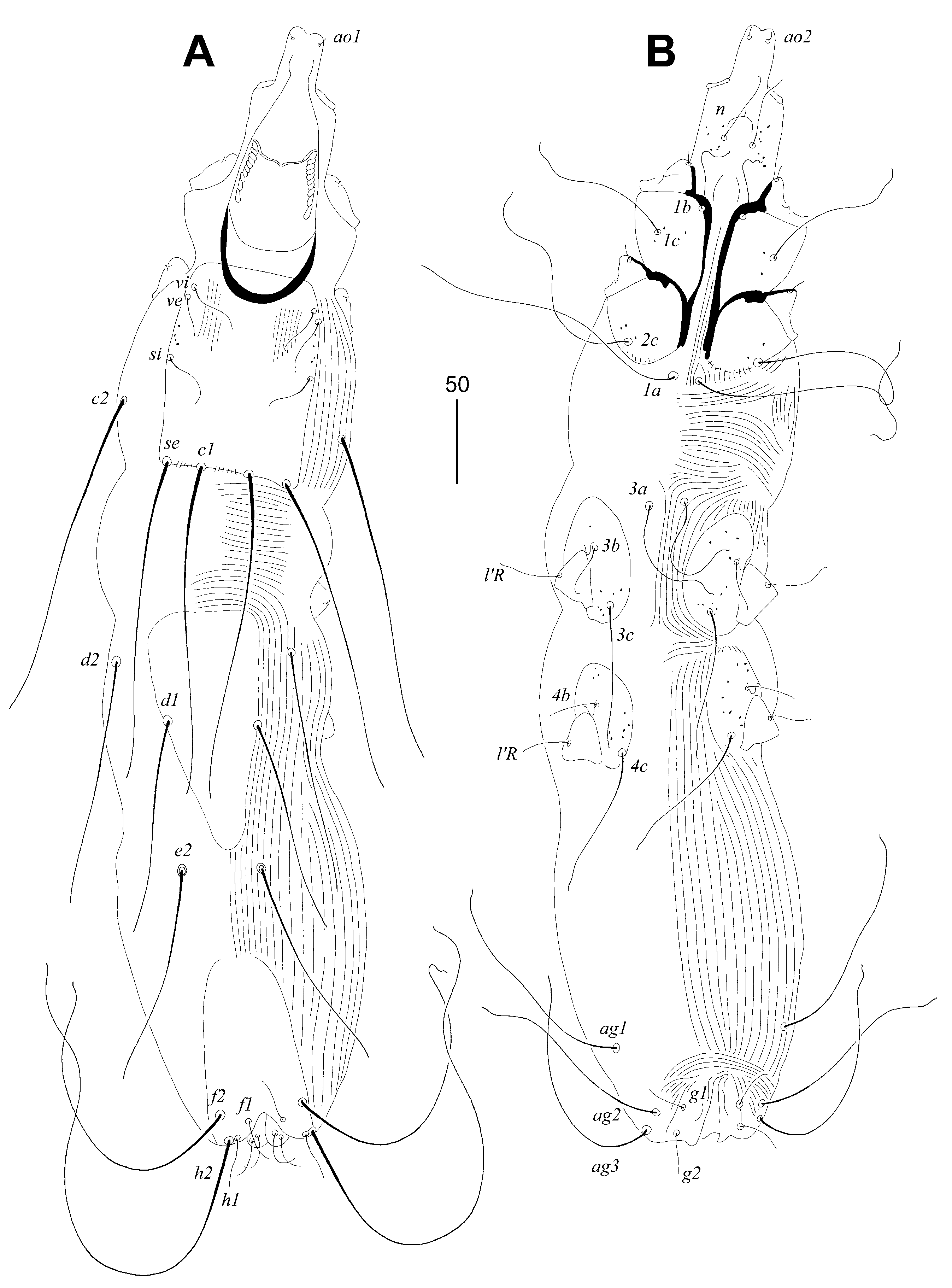
Female, holotype (
Figure 1 and
Figure 2A–C). Total body length 530 (555–595 in 45 paratypes). Gnathosoma. Infracapitulum apunctate. Movable cheliceral digit 130 (130–135) long. Stylophore 175 (170–185) long, exposed portion of stylophore apunctate, 130 (130–140) long. Each medial branch of peritremes with two chambers, each lateral branch with seven chambers (
Figure 2A). Idiosoma. Propodonotal shield well sclerotised, apunctate, bearing bases of setae
ve,
si and
c1, margin between bases of setae
se and
c1 indistinct. Bases of setae
c1 situated slightly posterior to level of setal bases
se. Propodonotal setae
ve and
si short and subequal in length. Hysteronotal shield absent. Bases of setae
d1 situated closer to
d2 than to
e2. Setae
c1 about 1.5 times longer than
c2. Setae
d2 distinctly longer than
d1 and
e2. Pygidial shield apunctate and with rounded anterior margin. Genital plate absent. Genital setae
g1 and
g2 and pseudanal setae
ps1 equal in length. Coxal fields I–II well sclerotised, III–IV weakly sclerotised, all apunctate. Body cuticular striations as in
Figure 1. Legs. Solenidia of legs I as in
Figure 2B. Fan-like setae of legs III and IV with six tines (
Figure 2C). Lengths of setae:
ve 20 (20–30),
si 20 (25–30),
se 210 (185–210),
c1 265 (270),
c2 180 (190–225),
d1 20 (15–25),
d2 150 (145–195),
e2 25 (20–30),
f1 (20–25),
f2 50 (40–50),
h1 20 (15–30),
h2 415 (430–470),
ag1 60 (50–70),
ag2 (30–45),
ag3 (90–100),
ps1 20 (15–20),
g1 and
g2 20 (15–20),
3b 25 (20),
3c 45 (40),
l’RI 5 (10),
l’RII (15),
l’RIII (20–30),
l’RIV 15 (20),
tc’III–IV 40 (35–40),
tc”III–IV 65 (60–70).
Male. (
Figure 2D-G). Total body length 380–385 in five paratypes. Gnathosoma. Infracapitulum apunctate. Movable cheliceral digits 100–105 long. Stylophore 145–150 long; exposed portion of stylophore with striae ornament, apunctate, 115–120 long. Each medial branch of peritremes with two chambers, each lateral branch with eight or nine chambers (
Figure 2D). Idiosoma. Propodonotal shield trapezoidal in shape, weakly sclerotised and apunctate, bearing bases of setae
ve,
si and
c1. Propodonotal setae
ve and
si subequal in length. Bases of setae
se situated distinctly anterior to level of setae
c1. Hysteronotal and pygidial shields absent. Setae
d2 1.4–2 times longer than
d1 and
e2. Setae
h2 about twice as long as
f2. Coxal fields weakly sclerotised and apunctate. Body cuticular striations as in
Figure 2G. Lengths of setae:
ve 15–20,
si 15–20,
se 25–30,
c1 30–35,
c2 30–35,
d1 15–20,
d2 20–30,
e2 15–20,
f2 15–20,
h2 30–35,
ag1 30–40,
ag2 20–30.
Type Material
Female holotype and paratypes: 45 females and five males (reg. no. AMU MS 21-0910-060) from the Common Hill Myna Gracula religiosa Linnaeus (host at SNSB-ZSM, uncatalogued); Indonesia, Malay Archipelago, Java, 1908, coll. W. Elbert.
Type Material Deposition
Holotype and most paratypes are deposited in the SNSB-ZSM, except ten females and 3 males in the AMU.
Additional Material
Eleven females (reg. no. AMU MS 21-0910-052) from the White-necked Myna Streptocitta albicollis (Vieillot) (host at SNSB-ZSM, uncatalogued); Indonesia, Malay Archipelago, Celebes Isl., 1875, coll. Riedel.
Differential Diagnosis
Aulonastus indonesianus sp. n. is morphologically similar to the recently described
Aulonastus darwini Skoracki, Sikora, Unsoeld and Hromada, 2022 collected from two host species of the genus
Geospiza (Thraupidae) [
47]. In females of both species, the infracapitulum is apunctate; setae
ve and
si are subequal in length; setae
c1 are longer than
se; the genital plate is absent; fan-like setae have six or seven tines, and all coxal fields are apunctate. This new species differs from
A. darwini by the following features: in females of
A. indonesianus, the stylophore is 170–185 long; each lateral branch of the peritremes has seven chambers; the propodonotal shield bearing bases of setae
ve,
si and
c1; bases of setae
c1 are situated slightly posterior to level of setal bases
se, and the hysteronotal shield is absent. In females of
A. darwini, the stylophore is 130–135 long; each lateral branch of the peritremes has four or five chambers; the propodonotal shield bearing bases of setae
ve,
si,
se and
c1; bases of setae
c1 and
se are situated at the same transverse level, and the hysteronotal shield is present and fused with the pygidial shield.
Etymology
The name “indonesianus” is taken from the region where the hosts were captured—Indonesia.
3.1.2. Aulonastus anais Skoracki and Patan sp. n.
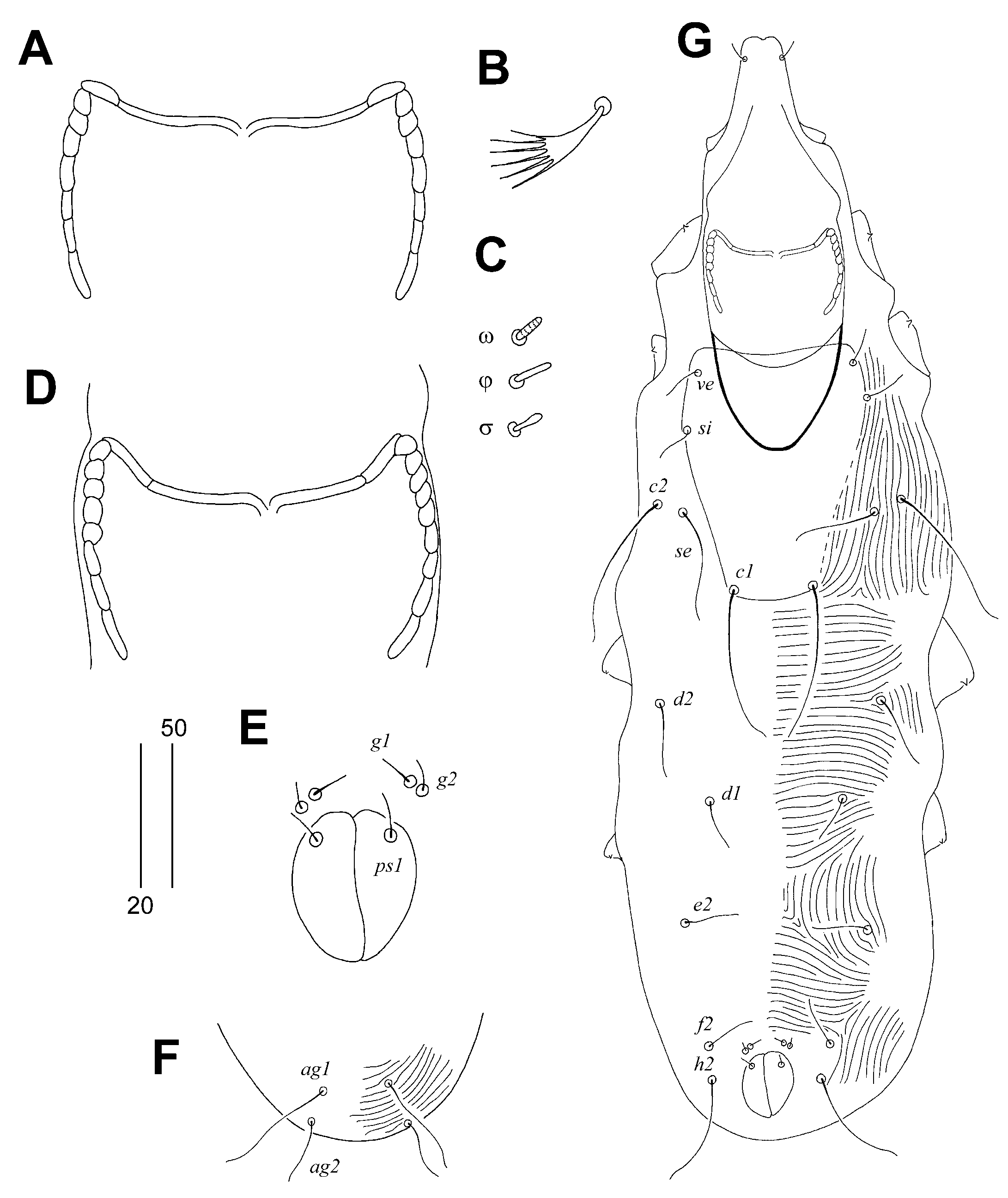
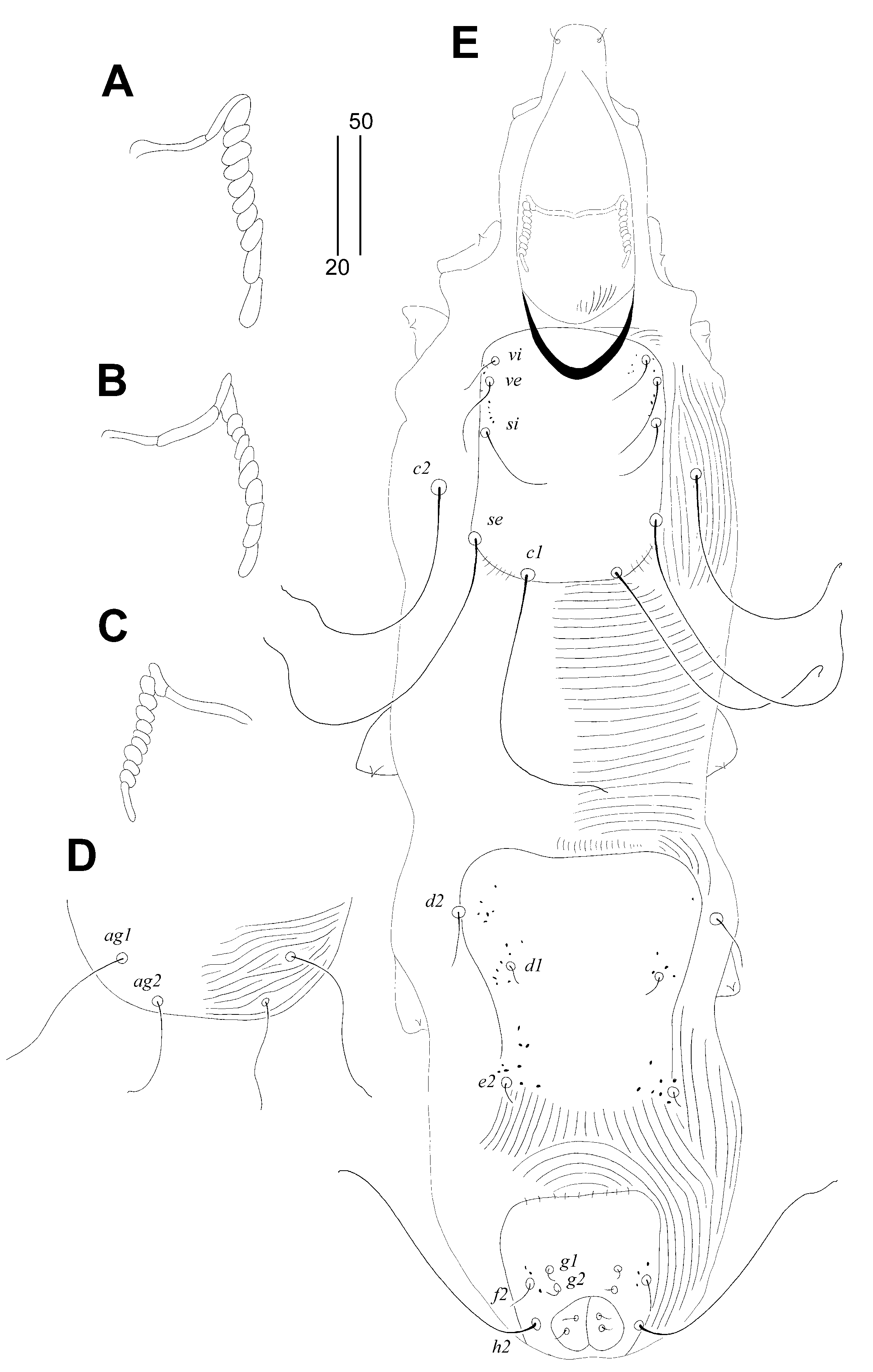
Female, holotype (
Figure 3 and A-C). Total body length 590 (550–600 in 11 paratypes). Gnathosoma. Infracapitulum densely punctate. Movable cheliceral digit 120 (120–125) long. Stylophore 170 (165–170) long, exposed portion of stylophore apunctate, 130 (125–130) long. Each medial branch of peritremes with one or two chambers, each lateral branch with four chambers (
Figure 4A). Idiosoma. Propodonotal shield well sclerotised, bearing bases of setae
ve,
si and
c1 sparsely punctate near bases of setae
ve and
si, margin between bases of setae
se and
c1 indistinct. Bases of setae
c1 and
se situated at same transverse level. Propodonotal setae
ve and
si short and subequal in length or setae
si slightly (1.3 times) longer than
ve. Bases of setae
d1 situated closer to
d2 than to
e2. Setae
c1 1.5 times longer than
c2. Setae
d2 distinctly longer than
d1 and
e2. Hysteronotal shield narrow and apunctate, not fused with pygidial shield, situated between bases of setae
d1 and
e2. Pygidial shield apunctate with indistinct anterior margin. Genital plate absent. Genital setae
g1 and
g2 equal in length. Coxal fields I–II well sclerotised, III–IV weakly sclerotised, all punctate. Body cuticular striations as in
Figure 3. Legs. Solenidia of legs I as in
Figure 4B. Fan-like setae of legs III and IV with eight tines (
Figure 4C). Lengths of setae:
ve 15 (15–20),
si 20 (20–25),
se 220 (205–235),
c1 245 (220–240),
c2 165 (150–175),
d2 110 (110–130),
d1 20 (20–25),
e2 35 (35–50),
f1 30 (30–35),
f2 65 (50–65),
h1 30 (30–35),
h2 (370–400),
ag1 50 (50–60),
ag2 35 (30–40),
ag3 95 (80–110),
ps1 25 (20–25),
g1 and
g2 25 (25),
tc’III–IV 35 (35),
tc”III–IV 55 (55–60),
3b 20 (20),
3c 35 (30–35),
4c 35 (30–35),
l’RIII 25 (20–25),
l’RIV 20 (20).
Male. (
Figure 4D, E). Total body length 380 in one paratype. Gnathosoma. Infracapitulum apunctate. Stylophore 135 long; exposed portion of stylophore with striae ornament, apunctate, 110 long. Each medial branch of peritremes with three chambers, each lateral branch with five or six chambers (
Figure 4D). Idiosoma. All dorsal shields weakly sclerotised and apunctate. Propodonotal shield trapezoidal in shape, bearing bases of setae
ve,
si and
c1. Propodonotal setae
ve and
si subequal in length. Bases of setae
c1 and
se situated at same transverse level. Hysteronotal shield fused to pygidial shield; anterior margin concave and reaching level of setal bases
d1. Setae
d1,
d2 and
e2 subequal in length. Setae
h2 about three times longer than
f2. Coxal fields weakly sclerotised and apunctate. Body cuticular striations as in
Figure 4E. Legs. Fan-like setae of legs III and IV with five or six tines. Lengths of setae:
ve 10,
si 10,
se 25,
c1 45,
c2 25,
d1 10,
d2 15,
e2 10,
f2 15,
h2 50.
Type Material
Female holotype, 11 female paratypes and one male paratype (reg. no. AMU MS 21-0910-045) from the Golden Myna Mino anais (Lesson) (host reg. no. SNSB-ZSM 11.602; female); Papua New Guinea, August 1910, coll. L. von Wiedenfeld.
Type Material Deposition
Holotype and most paratypes are deposited in the SNSB-ZSM, except five females and one male in the AMU.
Differential Diagnosis
This new species is morphologically similar to the above described species, A. indonesianus, and can be easily distinguished by the following features: in females of A. anais, the infracapitulum is densely punctate; each lateral branch of the peritremes has four chambers; bases of setae c1 and se are situated at same transverse level; the hysteronotal shield reduced to small and narrow shield, situated between bases of setae d1 and e2; and all coxal fields are punctate. In females of A. indonesianus, the infracapitulum is apunctate; each lateral branch of the peritremes has seven chambers; bases of setae c1 are situated slightly posterior to level of setal bases se; the hysteronotal shield is absent; and all coxal fields are apunctate.
Etymology
The name “anais” is taken from the species name of the host, Mino anais.
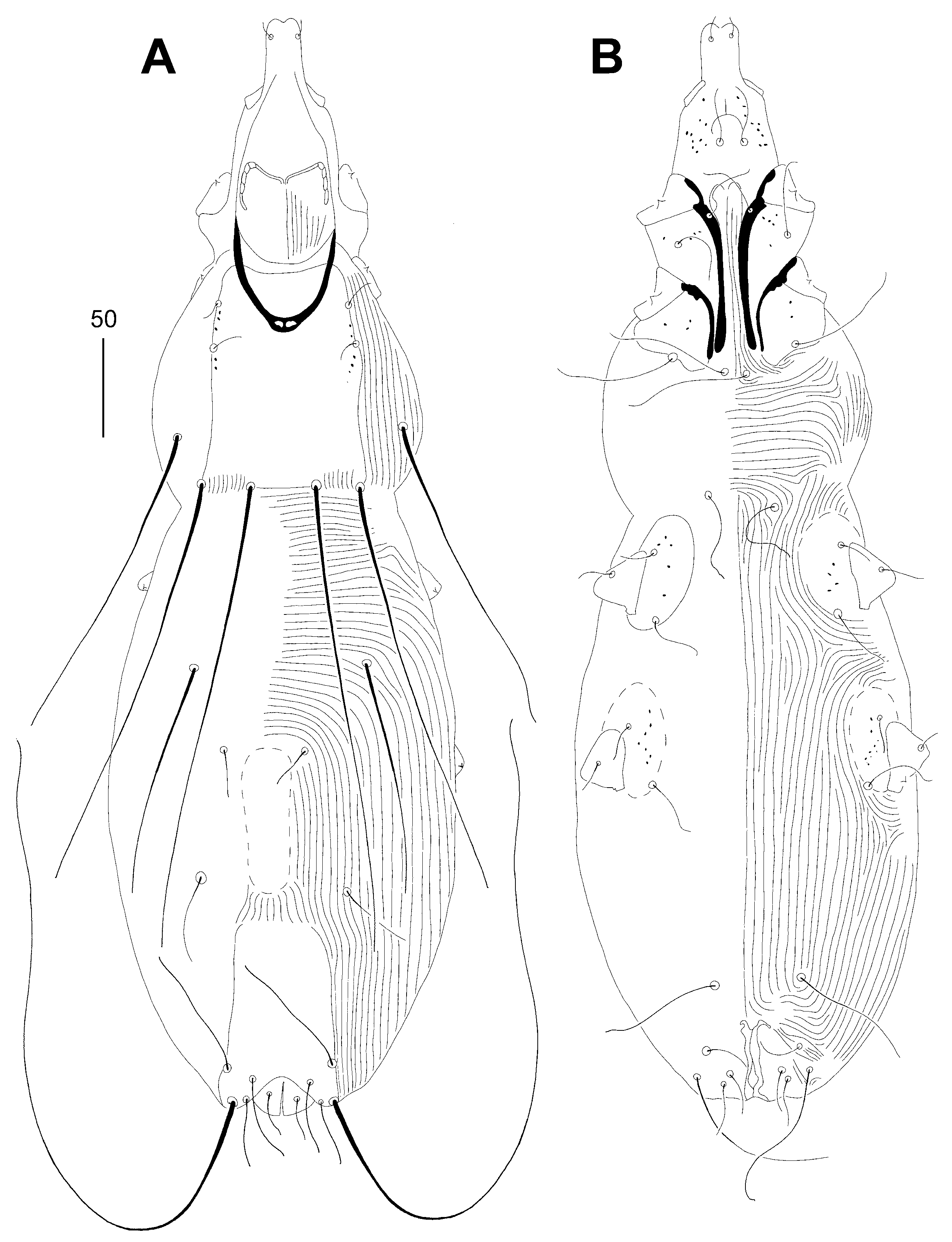
3.1.3. Syringophiloidus poeopterus Skoracki and Patan sp. n.
Female, holotype (
Figure 5 and A). Total body length 675 (620–670 in three paratypes). Gnathosoma. Infracapitulum apunctate. Stylophore 160 (155–160) long, exposed portion of stylophore apunctate, 130 (125–130) long. Each medial branch of peritremes with two chambers, each lateral branch with ten chambers (
Figure 6A). Idiosoma. Propodonotal shield well sclerotised, rectangular in shape, sparsely punctate between bases of setae
ve and
si. Propodonotal setae
vi,
ve and
si short, smooth, and subequal in length. Bases of setae
c1 and
se situated at same transverse level. Hysteronotal shield well sclerotised and apunctate; anterior margin reaching above level of setal bases
d2, posterior margin not fused to pygidial shield and not reaching bases of setae
e2. Bases of setae
d1 situated closer to
d2 than to
e2. Setae
d1,
d2 and
e2 subequal in length. Pygidial shield apunctate and with rounded anterior margin. Genital plate absent. Agenital setae
ag1–3 subequal in length. Genital setae
g1 and
g2 equal in length. Pseudanal setae
ps1 and
ps2 equal in length. Coxal fields I–IV well sclerotised, I–II sparsely punctate or apunctate, III–IV punctate. Body cuticular striations as in
Figure 5. Legs. Fan-like setae of legs III and IV with eight or nine tines. Lengths of setae:
vi 30 (25–30),
ve 30 (30-35),
si 30 (30–40),
se 195 (190–200),
c1 185 (180-195),
c2 220 (195–215),
d1 125 (115–130),
d2 (125–145),
e2 (130–135),
f1 20 (20–25),
h1 20 (20–25),
h2 280 (270-305),
ag1 120 (120–145),
ag2 130 (110–135),
ag3 150 (140–150),
ps1 and
ps2 30 (25–30),
g1 and
g2 30 (25–30),
4b 25 (25–30),
4c (80),
l’RIII 35 (35-40),
l’RIV 25 (25).
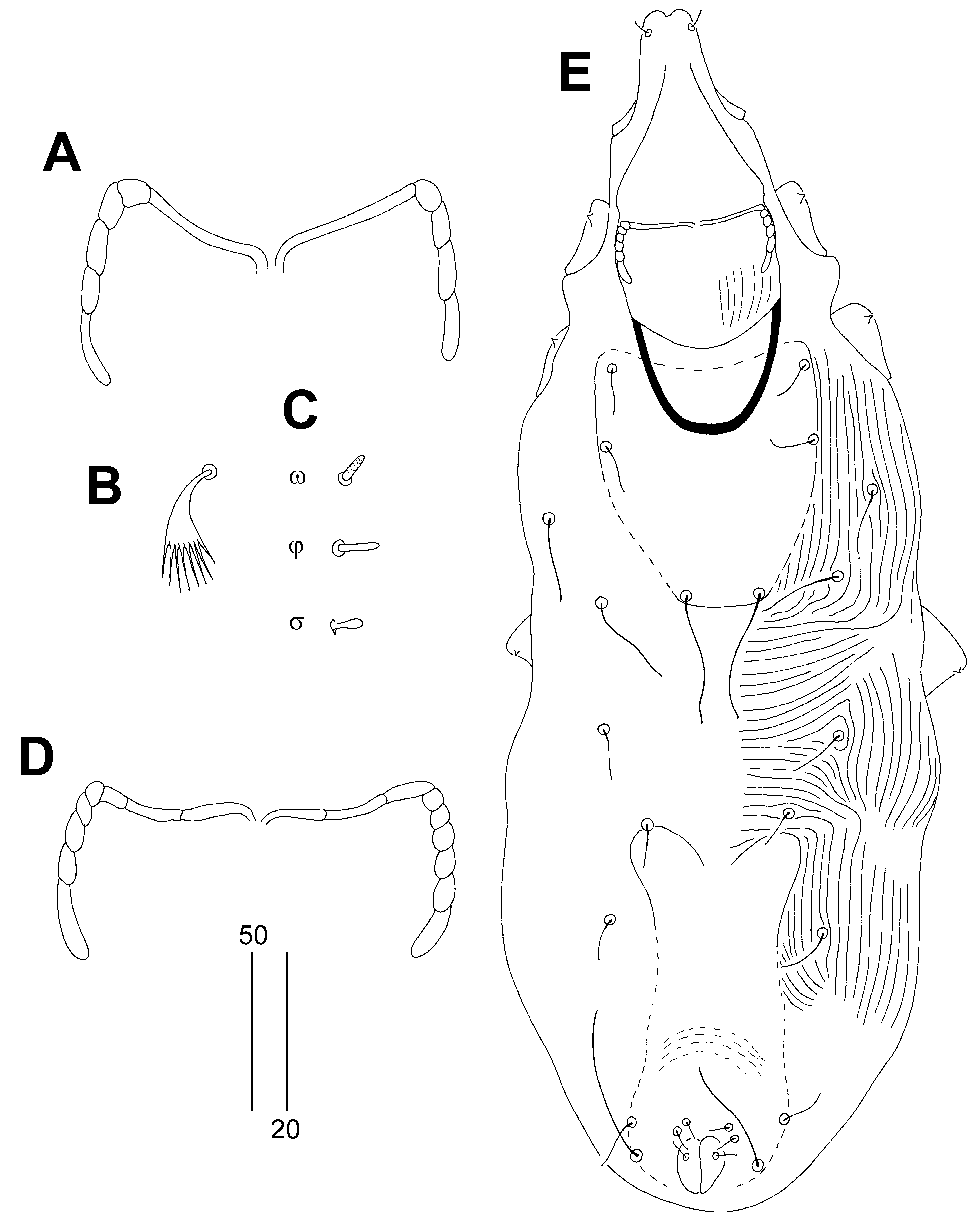
Male (
Figure 6B-E). Total body length 475–560 in two paratypes. Gnathosoma. Infracapitulum apunctate. Stylophore 160 (155–160) long, exposed portion of stylophore apunctate, 135–145 long. Each medial branch of peritremes has two or three chambers, each lateral branch has ten chambers (
Figure 6B, C). Idiosoma. Propodonotal shield well sclerotised, rectangular in shape, sparsely punctate between bases of setae
ve and
si. Propodonotal setae
vi,
ve and
si short, smooth, and subequal in length. Bases of setae
c1 situated posterior to level of setae
se. Hysteronotal shield well sclerotised, large and punctate, anterior margin reaching above level of setal bases
d2, posterior margin not fused to pygidial shield and reaching bases of setae
e2. Bases of setae
d1 situated closer to
d2 than to
e2. Setae
d2 2.5 times longer than
d1 and
e2. Pygidial shield with indistinct anterior margin. Agenital setae
ag1 1.4 times longer than
ag2. Body cuticular striations as in
Figure 6E. Lengths of setae:
vi 20–35,
ve 25–40,
si 30–35,
se 130–145,
c1 115–140,
c2 100–150,
d1 10,
d2 20–25,
e2 10,
f2 10–20,
h2 110–120,
ag1 50–75,
ag2 35–40.
Type Material
Female holotype, three female paratypes and two male paratypes (reg. no AMU MS 21-1012-042) from the quill of wing covert of the Abbott’s Starling Poeoptera femoralis; Tanzania, Arusha Region, Arusha National Park, Mt. Meru, 2,164 m a.s.l., 16 November 1958, coll. Nagy.
Types Deposition
Holotype deposited in the SNSB-ZSM, paratypes in the AMU.
Differential Diagnosis
This new species is morphologically similar to
Syringophiloidus saponai Skoracki, Patan and Unsoeld, 2024 recorded from four host species of the genus
Lamprotornis in Kenya, Tanzania and Ethiopia [
48], by the presence of short setae
vi,
ve and
si (all shorter than 70).
S. poeopterus can be easily distinguished from
S. saponai by the shorter hysteronotal setae
d1,
d2 and
e2 which are 115–130, 125–145, and 130–135, respectively (
vs the length of setae
d1,
d2 and
e2 are 190–205, 260–285, and 200–215, respectively in
S. saponai). Additionally, in females of
S. poeopterus genital plate is absent (
vs genital plate is present in
S. saponai).
Etymology
The name “poeopterus” is taken from the generic name of the host, Poeoptera.
3.1.4. Syringophiloidus presentalis Chirov and Kravtsova, 1995
Host and distribution. Sturnidae: the Eurasian Starling
Sturnus vulgaris Linnaeus from Kyrgyzstan [
49], Poland, Slovakia, and France [
4].
3.1.5. Syringophiloidus saponai Skoracki, Patan and Unsoeld, 2024
Hosts and distribution. Sturnidae: the Greater Blue-eared Glossy-Starling
Lamprotornis chalybaeus Hemprich and Ehrenberg from Kenya, Tanzania, and Ethiopia; the Superb Starling
Lamprotornis superbus Rüppell from Tanzania and Kenya; the Lesser Blue-eared Glossy-Starling
Lamprotornis chloropterus Swainson from Tanzania; the Ashy Starling
Lamprotornis unicolor (Shelley) from Tanzania [
48]; the Pale-winged Starling
Onychognathus nabouroup (Daudin) from Namibia, and the Red-winged Starling
Onychognathus morio (Linnaeus) from Tanzania (current study).
New Material Examined
Four females and one male (reg. no AMU MS 21-1012-031) from the Pale-winged Starling Onychognathus nabouroup (Daudin) (host reg. no. SNSB-ZSM 57.20); Namibia, 5 November 1956, no other data. Five females and one male (reg. no. AMU MS 21-1012-034) from the Red-winged Starling Onychognathus morio (Linnaeus) (host reg. no. SNSB-ZSM 64.717); Tanzania, Morogoro District, 6 May 1962, coll. Th. Andersen.
3.1.6. Syringophiloidus graculae Fain, Bochkov and Mironov, 2000
Hosts and distribution. Sturnidae: the Common Hill Myna
Gracula religiosa Linnaeus from SE Asia [
50]; the Golden-crested Myna
Ampeliceps coronatus Blyth from Indochina, and the Coleto
Sarcops calvus (Linnaeus) from the Philippines (current study).
New Material Examined
Seventeen females and two males (reg. no. ZISP AVB 05-0726-010) from the Golden-crested Myna Ampeliceps coronatus Blyth; Indochina, no other data. Twelve females and one male (reg. no. AMU MS 21-0910-054) from the Coleto Sarcops calvus (Linnaeus) (host reg. no. SNSB-ZSM 26.213); Philippines, Cebu Isl., 1879, coll. Burger. Four females (reg. no. AMU MS 21-0910-055) from same host species (host reg no. SNSB-ZSM 26.215) and locality.
3.1.7. Syringophilopsis sturni Chirov and Kravtsova, 1995
Host and distribution. Sturnidae: the Eurasian Starling
Sturnus vulgaris Linnaeus from Kyrgyzstan [
49], Kazakhstan [
52], Poland [
53], and Ukraine [
4].
3.1.8. Syringophilopsis parasturni Skoracki, Patan and Unsoeld, 2024
Hosts and distribution. Sturnidae: the Chestnut-bellied Starling
Lamprotornis pulcher (Müller) from Senegal; the Bronze-tailed Glossy-Starling
Lamprotornis chalcurus Nordmann from Senegal [
48]; the Black-bellied Glossy-Starling
Notopholia corusca (Nordmann) from Tanzania, and the Abbott’s Starling
Poeoptera femoralis (Richmond) from Tanzania (current study).
New Material Examined
Seven females and two males (reg. no. AMU MS 22-0821-012) from the Black-bellied Glossy-Starling Notopholia corusca (Nordmann) (org. Lamprotornis corruscus) (host in the SNSB-ZSM, uncatalogued); Tanzania, Tanga Region, Tanga, March 1893, coll. O. Neumann. Seven females and one male (reg. no. AMU MS 21-1012-041) from the Abbott’s Starling Poeoptera femoralis (Richmond) (host in the SNSB-ZSM, uncatalogued); Tanzania, Arusha Region, Arusha National Park, Mt. Meru, 2,560 m a.s.l., 2 November 1959, coll. Nagy. Nine females (reg. no AMU MS 21-1012-042) from same host species (host reg, no SNSB-ZSM 59.148) and locality, 2,164 m a.s.l., coll. Nagy.
3.1.9. Krantziaulonastus buczekae (Skoracki, 2002)
Host and distribution. Sturnidae: the Eurasian Starling
Sturnus vulgaris Linnaeus from Poland [
4,
51].
The world fauna of quill mites of the subfamily Syringophilinae associated with Starlings is summarised in
Table 1.
4. Discussion
The bird family Sturnidae (Starlings and Mynas) includes approximately 125 species divided into 36 genera [
54]. Their distribution is confined to the Old World, naturally occurring in Europe, Asia, Africa, northern Australia, and the Pacific islands, except for anthropogenic introductions and/or invasions in regions such as New Zealand and both Americas. The centres of biodiversity of this family are identified in Southeast Asia and Africa [
54,
55]. Our study has identified four genera of quill mites belonging to the subfamily Syringophilinae, which are prevalent across a wide array of passerine birds.
Mite species belonging to the genus
Syringophiloidus, have been recorded on hosts across 22 passerine families [
4,
27,
56]. Currently, we have recorded four species residing on starlings observed across the Oriental, Palearctic, and Ethiopian regions, as well as on both basal and crown starling lineages (see [
55]). This pattern suggest that the genus
Syringophiloidus established a symbiotic relationship with starlings before their worldwide diversification.
Similarly, species from the genus
Syringophilopsis predominantly inhabit passerine birds, having been documented in up to 27 families [
27]. Although two species from this genus have only been recorded on starlings occurring in the Ethiopian and Palaearctic regions, the absence of records from the Oriental region may be due to insufficient specimen examination. It is conceivable that future research will uncover the presence of this genus in the Oriental region as well. Similar to
Syringophiloidus, it is plausible that
Syringophilopsis established its association with starlings before their global dispersal.
The distribution of mites from the genera
Aulonastus (two species) and
Krantziaulonastus (one species) presents a notable scenario. Representatives of the genus
Aulonastus are found in the body feathers of the rather basal Asian jungle starlings lineage in the Oriental region [
55]. In contrast, in Europe, the Eurasian Starling (
Sturnus vulgaris), a member of the Eurasian savannah starlings clade, hosts a member of a different genus,
Krantziaulonastus, occupying the same ecological niche. Interestingly, neither of these syringophiline genera have been recorded in Africa, where different crown starling clades have diversified. However, syringophilids belonging to the subfamily Picobiinae occupy their body feather quills, utilising the same niche.
5. Conclusions
Our dataset elucidates the host specificity of these quill mite species, revealing a combination of mono- and oligoxenous tendencies. The latter are consistently restricted to hosts from specific zoogeographical regions and infect closely related genera. This specificity indicates a nuanced symbiotic relationship between quill mites and their avian hosts, likely shaped by intricate coevolutionary dynamics.
Author Contributions
Conceptualization, I.M., M.S., M.P., M.H and B.S.; methodology, M.S. and M.P.; investigation, I.M., M.S. and M.P.; resources and material collection, I.M., M.S. and M.P.; visualization, M.S. and M.P.; writing—original draft preparation, I.M., M.S., M.P., M.H. and B.S.; writing—review and editing, I.M., M.S., M.P., M.H. and B.S.; supervision, M.S. and B.S.; project administration and funding acquisition, I.M., M.S., M.P., M.H. and B.S. All authors have read and agreed to the published version of the manuscript.
Funding
Slovak Research and Development Agency under the contract APVV-22-0440 (to I.M., M.H., B.S., and M.S); the Agency of the Ministry of Education, Research and Sport of the Slovak Republic and Slovak Academy of Sciences 1/0876/21 (to I.M., M.H. and M.S), and by the AMU Excellence Initiative—Research University, grant no. 118/34/UAM/0056 (to M.P.).
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the use of only dead animals (specimens deposited in the ornithological collection).
Data Availability Statement
All necessary data (such as localities) are available in the text of this article.
Acknowledgments
We would like to thank Dr Markus Unsöld (Bavarian State Collection of Zoology, Munich, Germany) for making available samples of feathers for the present study. We also thank the anonymous reviewers for their critical review of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lavoipierre, M.M.J. The undescribed male and female of the Pigeon quill mite, Syringophilus columbae Hirst, 1920. Trans. Roy. Soc. Trop. Med. Hyg. 1953, 47, 7.
- Kethley, J.B. A revision of the family Syringophilidae (Prostigmata: Acarina). Contrib. Am. Entomol. Inst. 1970, 5, 1–76.
- Kethley, J.B. Population regulation in quill mites (Acari: Syringophilidae). Ecology 1971, 52, 1113–1118.
- Skoracki, M. Quill mites (Acari: Syringophilidae) of the Palaearctic region. Zootaxa 2011, 2840, 1–414. [CrossRef]
- Skoracki, M.; Sikora, B.; Spicer, G.S. A review of the subfamily Picobiinae Johnston and Kethley, 1973 (Acariformes: Prostigmata: Syringophilidae). Zootaxa 2016, 4113, 1–95. [CrossRef]
- Hromada, M.; Klimovicova, M.; Unsöld, M.; Skoracki, M. Host-parasite relationships in the system composed by cuckoos and quill mites. Syst. Appl. Acarol., 2016, 21, 528–536. [CrossRef]
- Sikora, B.; Unsoeld, M.; Melzer, R.R.; Friedrich, S; Skoracki, M. First Records of Picobiine Mites Associated with Birds-of-Paradise: Can Interspecific Sexual Behaviour of Hosts Play a Role in the Distribution of Quill Mite Parasites? Animals 2023, 13, 1509. [CrossRef]
- Skoracki, M.; Unsoeld, M.; Kosicki, J.Z.; Melzer, R.R.; Friedrich, S.; Sikora, B. Enigmatic host-mite relationships: Unraveling the distribution of quill mites on Birds-of-Paradise. Int. J. Parasitol. 2024, 54, 415–427. [CrossRef]
- Nattress, B. Horizontal transmission of Syringophilopsis kirgizorum (Acari: Cheyletoidea: Syringophilidae). Acarina 2011, 19, 270.
- Casto, S.D. Dispersal of the quill mite Syringophiloidus minor (Berlese) (Acarina: Syringophilidae). J. Med. Entomol. 1976, 13, 357–360.
- Skoracki, M.; Hebda, G. Quill mites (Acari: Syringophilidae) from Aegithalos caudatus (Passeriformes: Aegithalidae). Zootaxa 2004, 691, 1–6. [CrossRef]
- Skirnisson, K.; Nielsen, Ó.K. Quill mite infestation of rock ptarmigan Lagopus muta (Aves: Phasianidae) in relation to year and host age, sex, body condition, and density. Parasitol. Res. 2019, 118, 2643–2650. [CrossRef]
- Skoracki, M.; Hromada, M; Sikora, B. Castosyringophilus meropis sp. n. (Acariformes: Syringophilidae) – A new quill mite species parasitising the world population of Merops apiaster Linnaeus (Coraciiformes: Meropidae). Folia Parasitol. 2017, 64, 24. [CrossRef]
- Skoracki, M.; Kosicki, J.Z.; Sikora, B.; Töpfer, T.; Hušek, J.; Unsöld, M.; Hromada, M. The Occurrence of Quill Mites (Arachnida: Acariformes: Syringophilidae) on Bee-Eaters (Aves: Coraciiformes: Meropidae: Merops) of Two Sister Clades. Animals 2021, 11, 3500. [CrossRef]
- Grossi, A.; Proctor, H. The distribution of quill mites (Betasyringophiloidus seiuri) among flight feathers of the ovenbird (Seiurus aurocapilla). J. Parasitol. 2020, 106, 82–89. [CrossRef]
- Skoracki, M.; Hromada, M.; Tryjanowski, P. Description of a new species of quill mite Syringophiloidus weiszi sp. n. (Acari, Prostigmata, Syringophilidae) from great grey shrike Lanius excubitor. Acta Parasitol. 2001, 46, 30–34.
- Skoracki, M.; Møller, A.P.; Tryjanowski, P. A new species of parasitic mites of the genus Syringophiloidus Kethley, 1970 (Acari: Syringophilidae) from the Barn Swallow Hirundo rustica Linnaeus, 1758. Parasite 2003, 10, 17–20. [CrossRef]
- Kaszewska-Gilas, K.; Kosicki, J.Z.; Hromada, M.; Skoracki, M. Global Studies of the Host-Parasite Relationships between Ectoparasitic Mites of the Family Syringophilidae and Birds of the Order Columbiformes. Animals 2021, 11, 3392. [CrossRef]
- Marciniak-Musial, N.; Skoracki, M.; Kosicki, J.Z.; Unsöld, M.; Sikora, B. Host-Parasite Relationships of Quill Mites (Syringophilidae) and Parrots (Psittaciformes). Diversity 2023, 15: 1. [CrossRef]
- Szymański, P.; Niśkiewicz, M.; Budka, M.; Zampa, L.; Osiejuk, T.S.; Skoracki, M. Quill mites of the family Syringophilidae (Acariformes: Prostigmata) parasitising (Columbiformes: Columbidae) doves of the genus Turtur. Syst. Appl. Acarol. 2023, 28, 1466–1475. [CrossRef]
- Skoracki, M.; Michalik, J.; Sikora, B. Prevalence and habitat preference of quill mites (Acari, Syringophilidae) parasitising forest passerine birds in Poland. Acta Parasitol. 2010, 55, 188–193.
- Rebrassier, R.E.; Martin, E.D. Syringophilus bipectinatus, a quill mite of poultry. Science 1932, 76, 128.
- Schwabe, O. A quill mite of poultry – a case report. J. Am. Vet. Med. Assoc. 1956, 129, 481–482.
- Hwang, J.C. Case reports of the quill mite, Syringophilus bipectinatus in poultry. Proc. Helminthol. Soc. Washington 1959, 26, 47–50.
- Gritsenko, E.F. The biology and ecology of the quill mite Syringophilus bipectinatus Heller, 1880, In: Milan, D.; Rosicky, B. (eds.). Proceedings of the 3rd International Congress of Acarology, Prague, Czechoslovakia. Springer, Dordrecht, The Netherlands 1971, pp. 515–516.
- Skoracki, M.; Michalik, J.; Skotarczak, B.; Rymaszewska, A.; Sikora, B.; Hofman, T.; Wodecka, B.; Sawczuk, M. First detection of Anaplasma phagocytophilum in quill mites (Acari: Syringophilidae) parasitising passerine birds in Poland. Microbes Infect. 2006, 8, 303–307. [CrossRef]
- Skoracki, M.; Spicer, G.S.; OConnor, B.M. A systematic review of the subfamily Syringophilinae (Acari: Syringophilidae) of the Nearctic region. Part 1: Quill mites associated with passerines (Aves: Passeriformes). Zootaxa 2016, 4084, 451–494. [CrossRef]
- Skoracki, M.; Sikora, B. Quill mites (Acari: Syringophilidae) associated with galliform birds (Aves: Galliformes). Zootaxa 2011, 2966, 13–30. [CrossRef]
- Skoracki, M.; Dabert, J.; Schmaschke, R. Observations on the quill mites (Acari: Syringophilidae) from charadriiform birds. Zootaxa 2006, 1156, 51–64. [CrossRef]
- Johnston, D.E.; Kethley, J.B. A numerical phenetic study of the quill mites of the family Syringophilidae (Acari). J. Parasitol. 1973, 59, 520–530. [CrossRef]
- Glowska, E.; Chrzanowski, M.; Kaszewska, K. Checklist of the quill mites (Acariformes: Syringophilidae) of the World. Zootaxa 2015, 3968, 1–81. [CrossRef]
- Zmudzinski, M.; Skoracki, M.; Sikora, B. An Updated Checklist of Quill Mites of the Family Syringophilidae (Acariformes: Prostigmata). 2023. Available online: https://figshare.com/articles/dataset/An_updated_checklist_of_quill_mites_of_the_family_Syringophilidae_Acariformes_Prostigmata_/16529574 (accessed on 27 July 2024).
- Skoracki, M.; Sikora, B. Tinamiphilopsis elegans gen. nov. et sp. nov., a first record of the quill mites (Acari: Syringophilidae) from tinamou birds (Tinamiformes: Tinamidae). Acta Parasitol. 2004, 49, 348–352.
- Skoracki, M.; Sikora, B.; Ozminski, M. A new quill mite species (Acari: Syringophilidae) parasitising tinamous (Aves: Tinamiformes). Syst. Parasitol. 2012, 81, 109–113. [CrossRef]
- Skoracki, M.; Fajfer, M.; Hromada, M.; Hušek, J.; Sikora, B. Tinamiphilopsis temmincki sp. n., a New Quill Mite Species from Tataupa Tinamou, and the Early History of Syringophilid Mites. Animals 2023, 13, 2728. [CrossRef]
- Bochkov, A.V.; Fain, A.; Skoracki, M. New quill mites of the family Syringophilidae (Acari: Cheyletoidea). Syst. Parasitol. 2004, 57, 135–150. [CrossRef]
- Skoracki, M.; Sikora, B. Neosyringophilopsis, a new genus of the subfamily Syringophilinae (Acari: Syringophilidae). Zootaxa 2005, 1052, 21–28. [CrossRef]
- Skoracki, M.; Bochkov, A.V. Quill mites from Kazakhstan. Zootaxa 2010, 2546, 52–68. [CrossRef]
- Krantz, G.W.; Walter, D.E. A Manual of Acarology, 3rd ed.; Texas Tech University Press: Lubbock, TX, USA, 2009.
- Grandjean, F. Les segments post-larvaires de l’hystérosoma chez les Oribates (Acariens). Bull. Soc. Zool. Fr. 1939, 64, 273–284.
- Kethley, J.B. Acarina: Prostigmata (Actinedida). In Soil Biology Guide; Dindal, D.L., Ed.; John Wiley & Sons: New York, NY, USA, 1990; pp. 667–756.
- Grandjean, F. Observations Sur Les Acariens de La Famille Des Stigmaeidae. Arch. Sci. Phys. Nat. 1944, 26, 103–131.
- Clements, J.F.; Rasmussen, P.C.; Schulenberg, T.S.; Iliff, M.J.; Fredericks, T.A.; Gerbracht, J.A.; Lepage, D.; Spencer, A.; Billerman, S.M.; Sullivan, B.L.; et al. The eBird/Clements Checklist of Birds of the World. 2023. Available online: https://www.birds.cornell.edu/clementschecklist/download/ (accessed on 27 July 2024).
- del Hoyo, J.; Collar, N.J.; Christie, D.A.; Elliott, A.; Fishpool, L.D.C.; Boesman, P.; Kirwan, G.M. HBW and BirdLife International Illustrated Checklist of the Birds of the World. Volume 2: Passerines; Lynx Edicions and BirdLife International: Barcelona, Spain; Cambridge, UK, 2016.
- Holt, B.G.; Lessard, J.P.; Borregaard, M.K.; Fritz, S.A.; Araújo, M.B.; Dimitrov, D.; Fabre, P.H.; Graham, C.H.; Graves, G.R.; Jønsson, K.A.; Nogués-Bravo, D.; Wang, Z.; Whittaker, R.J.; Fjeldså, J.; Rahbek, C. An update of Wallace’s zoogeographic regions of the world. Science 2013, 339, 74–78. [CrossRef]
- Ficetola, G.; Mazel, F.; Thuiller, W. Global determinants of zoogeographical boundaries. Nat. Ecol. Evol. 2017, 1, 0089. [CrossRef]
- Skoracki, M.; Sikora, B.; Unsoeld, M.; Hromada, M. Mite Fauna of the Family Syringophilidae (Acariformes: Prostigmata) Parasitizing Darwin’s Finches in Galápagos Archipelago. Diversity 2022, 14, 585. [CrossRef]
- Skoracki, M.; Patan, M.; Unsoeld, M.; Hromada, M.; Kwieciński, Z.; Marcisova, I. Diversity of Quill Mites of the Family Syringophilidae (Acariformes: Prostigmata) Parasitizing Starlings of the Genus Lamprotornis (Passeriformes: Sturnidae). Diversity 2024, 16, 51. [CrossRef]
- Chirov, P.A.; Kravtsova, N.T. A new genus and new species of mites of the family Syringophilidae. Parazitologiya 1995, 29, 370–379.
- Fain, A.; Bochkov, A.V.; Mironov, S.V. New genera and species of quill mites of the family Syringophilidae (Acari: Prostigmata). Bull. Inst. Roy. Sci. Nat. Belg. 2000, 70, 33–70.
- Skoracki, M. Three new species of quill mites of the genus Aulonastus Kethley, 1970 (Acari, Prostigmata, Syringophilidae) from passerine birds. Acta Parasitol. 2002, 47, 300–305.
- Bochkov, A.V; Mironov, S.V. Quill mites of the family Syringophilidae Lavoipierre, 1953 (Acariformes: Prostigmata) parasitic on birds (Aves) of the fauna of the former USSR. Acarina 1998, 6, 3–16.
- Skoracki, M. Quill mites of the genus Syringophilopsis (Acari, Syringophilidae) from passeriform birds of Poland with descriptions of five new species. Acta Parasitol. 2004, 49, 45–62.
- Winkler, D.W.; Billerman, S.M.; Lovette, I.J. Starlings (Sturnidae), version 1.0. In Birds of the World (Billerman, S.M.; Keeney, B.K.; Rodewald, P.G.; Schulenberg, T.S. (eds.). Cornell Lab of Ornithology, Ithaca, NY, USA, 2020.
- Fjeldså, J.; Christidis, L.; Ericson, P.G.P. The Largest Avian Radiation: The Evolution of Perching Birds, or the Order Passeriformes. Lynx Edicions, Barcelona, Spain, 2020.
- Glowska, E.; Laniecka, I.; Ostrowska, K.; Gebhard, C.A.; Olechnowicz, J.; Dabert, M. Evaluation of Genetic Diversity in Quill Mites of the Genus Syringophiloidus Kethley, 1970 (Prostigmata: Syringophilidae) with Six New-to-Science Species. Animals 2023, 13, 3877. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).