Submitted:
15 July 2024
Posted:
16 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
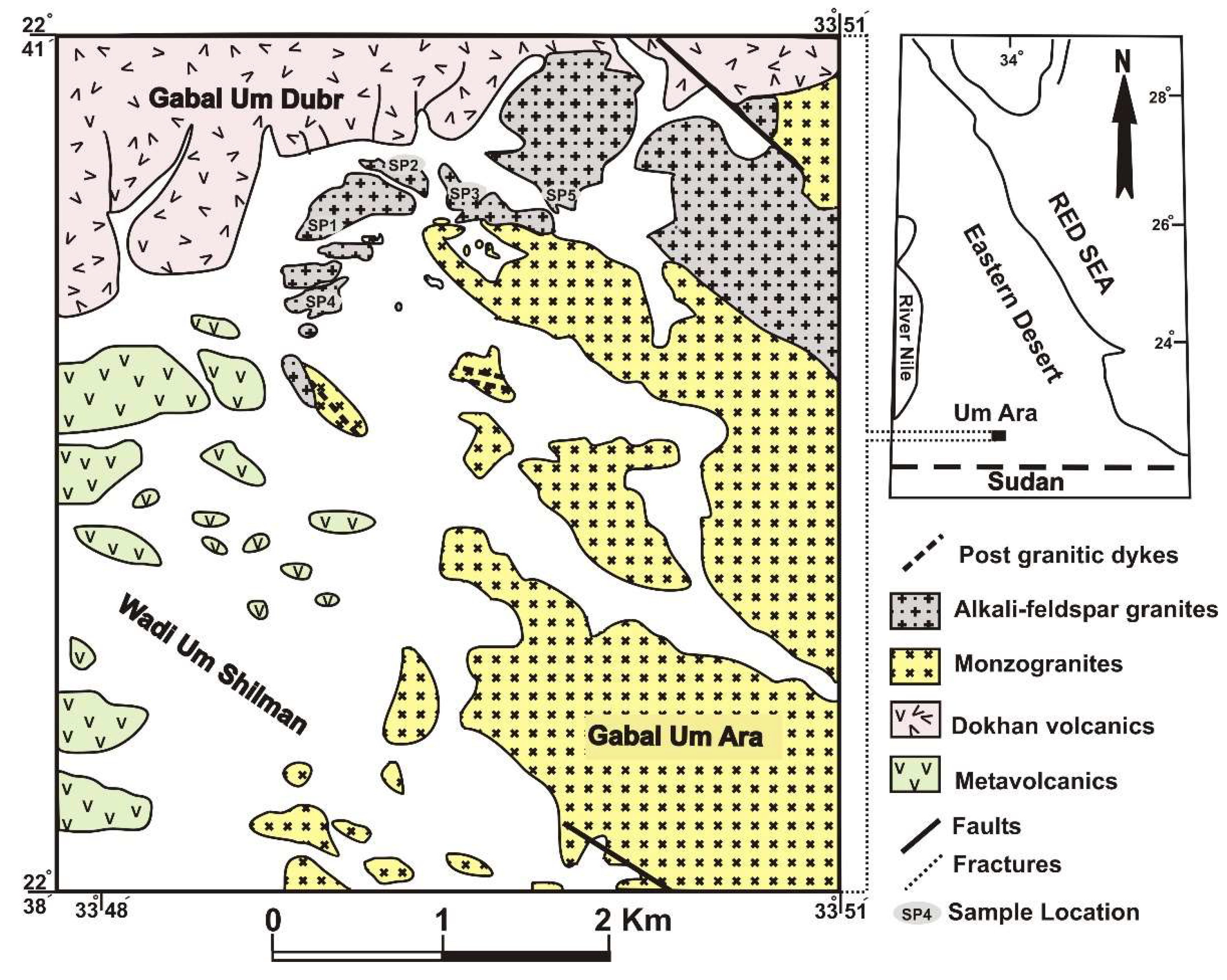
2. Geological and Mineralogical Background
3. Analytical Procedures
4. Results
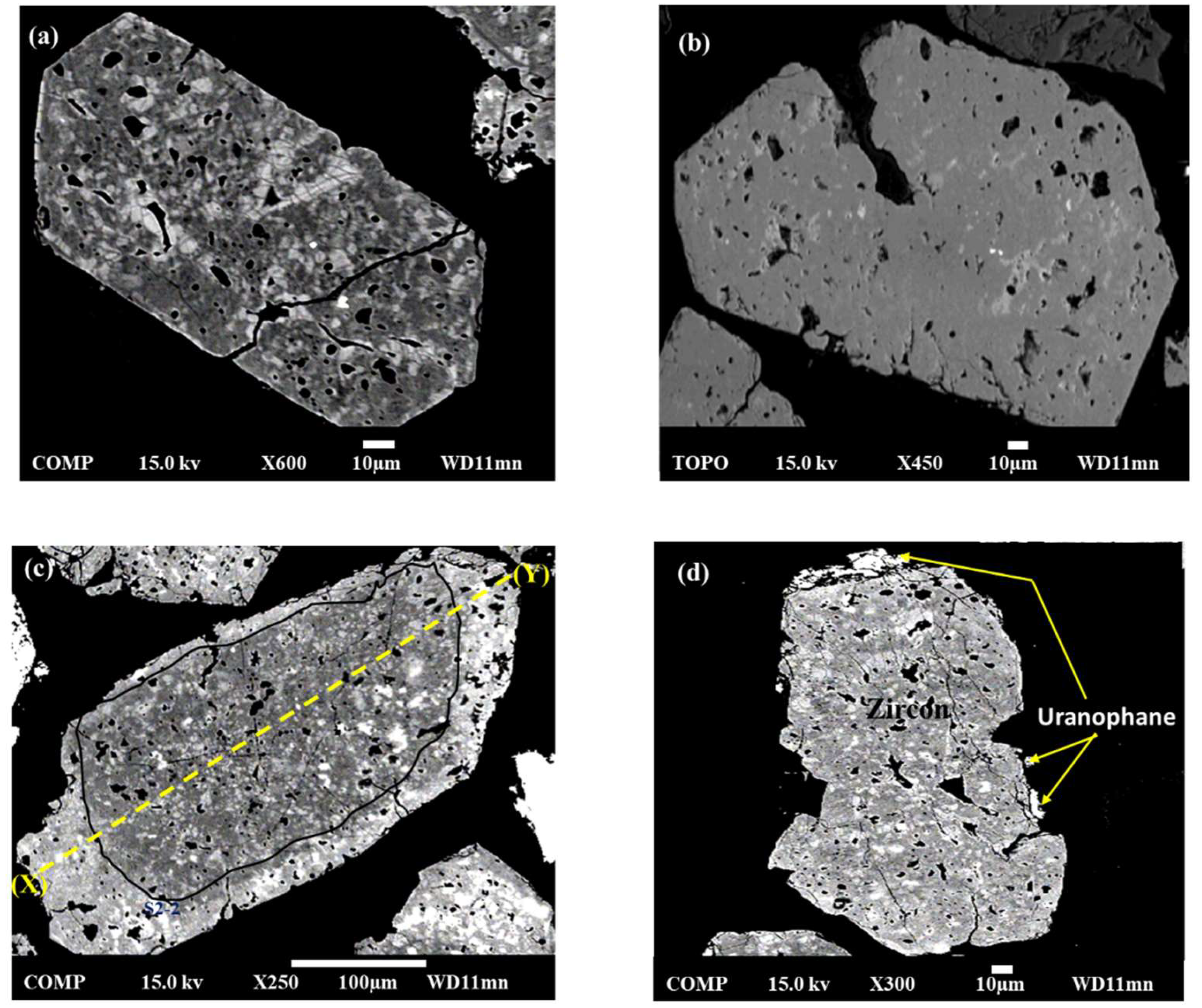
4.1. Textural Observations
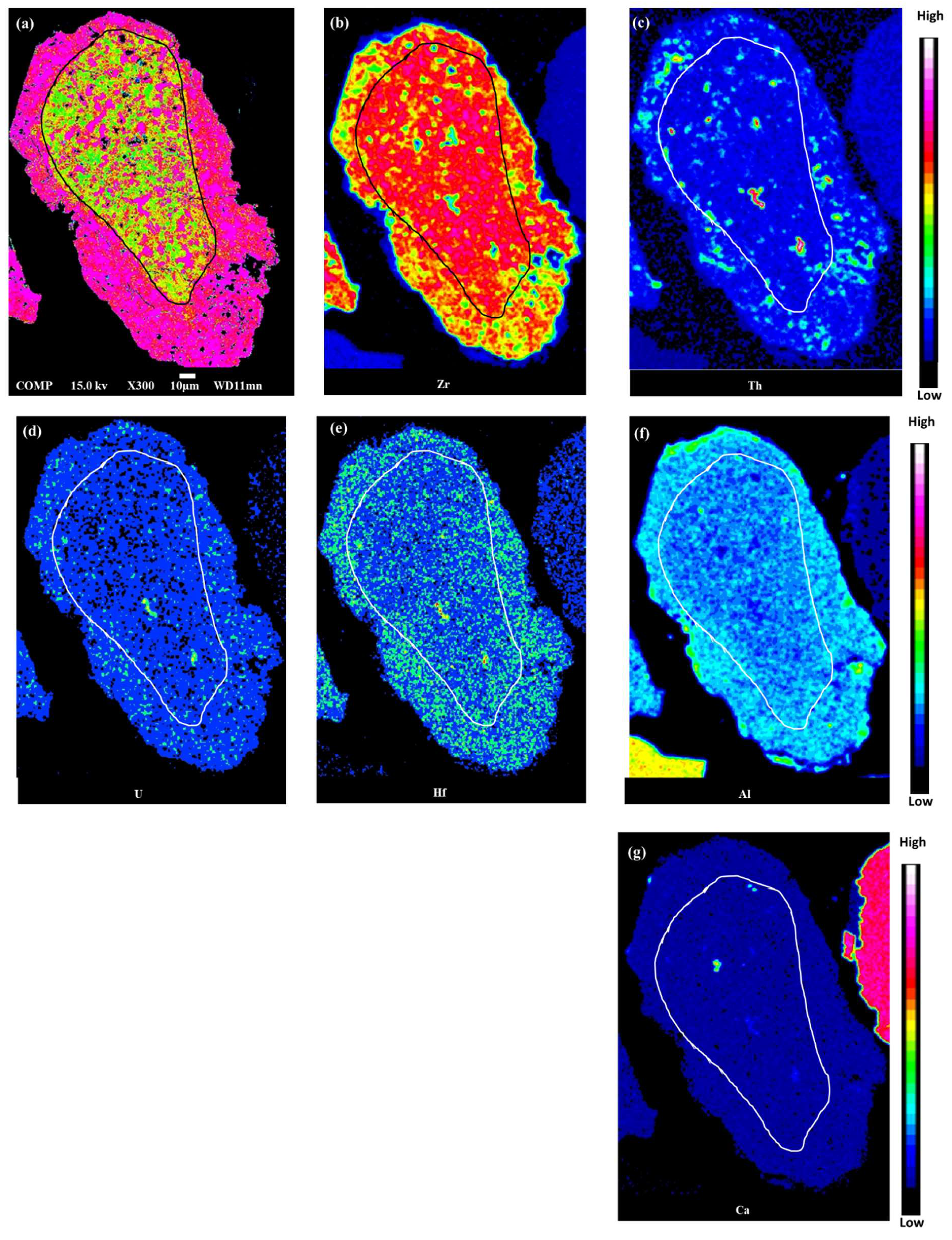
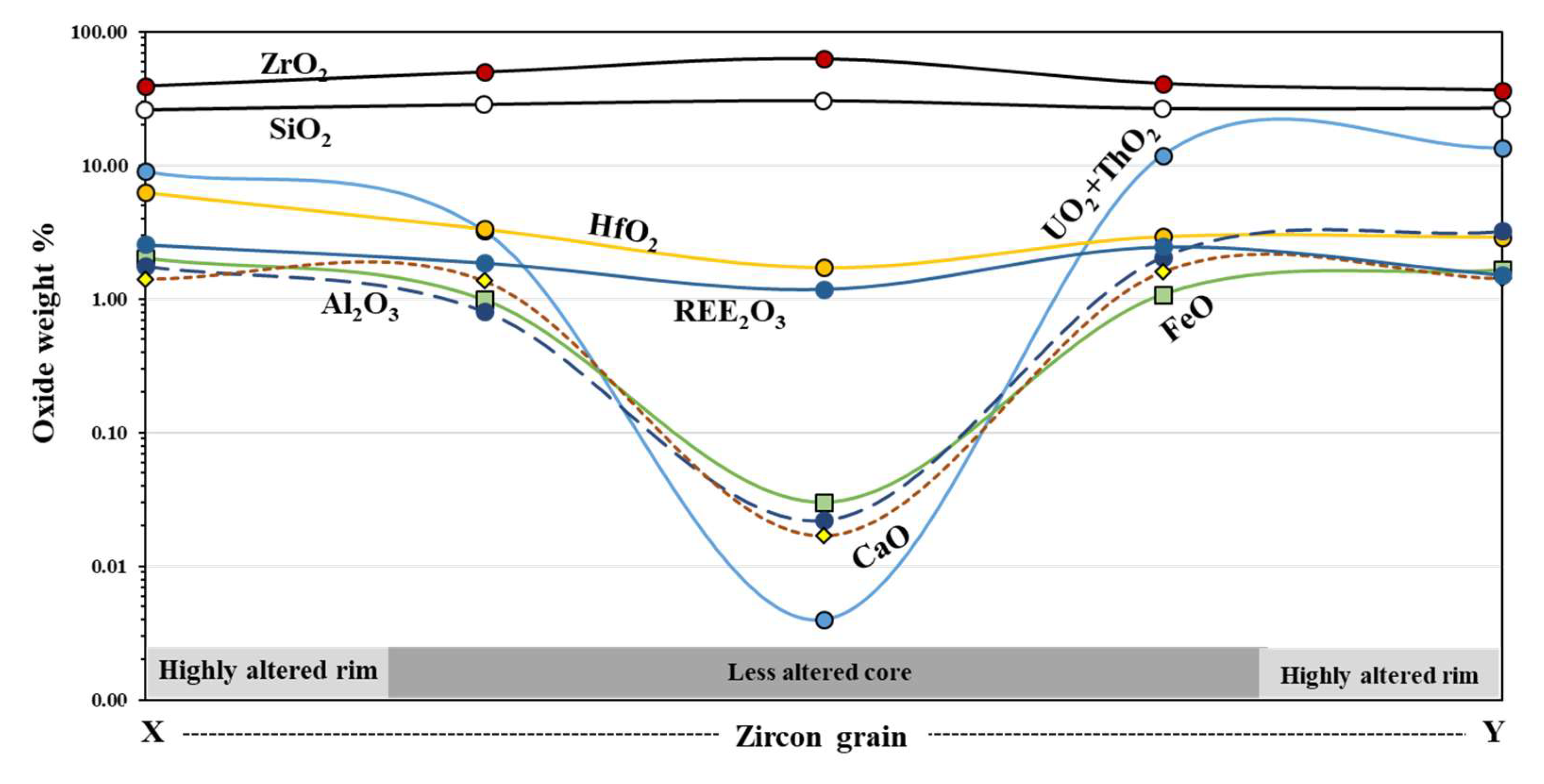
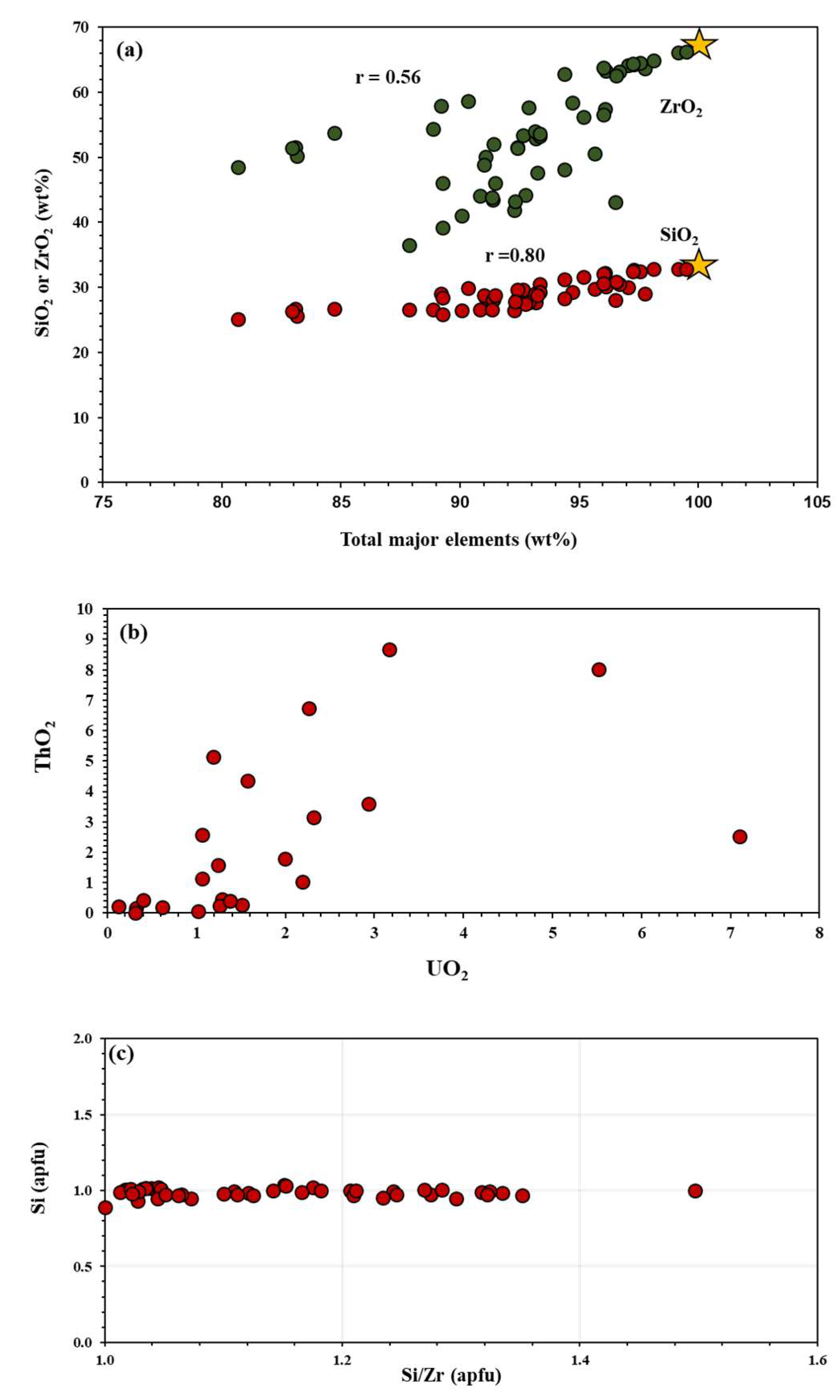
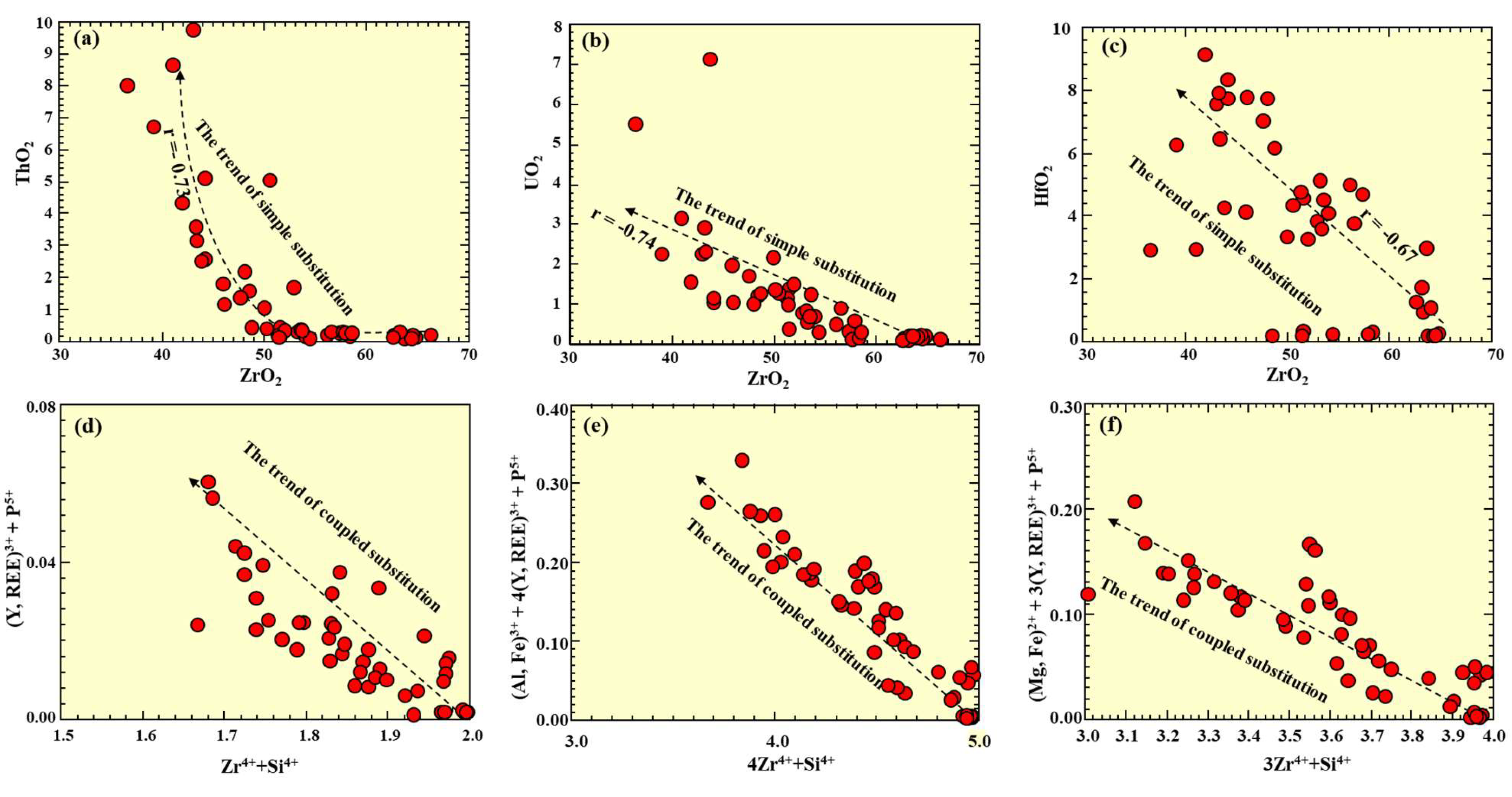
4.2. Major and Trace Elements Composition
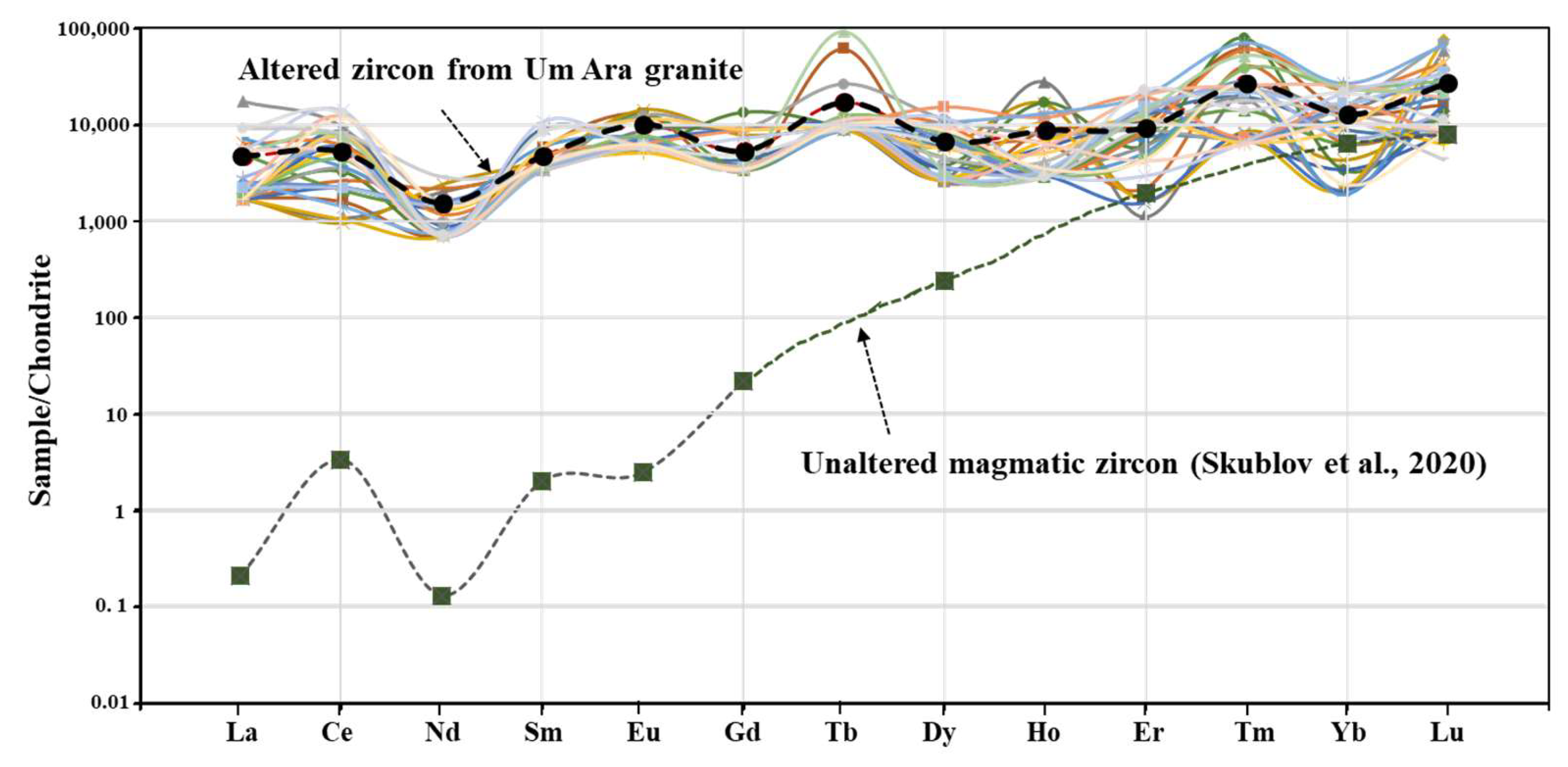
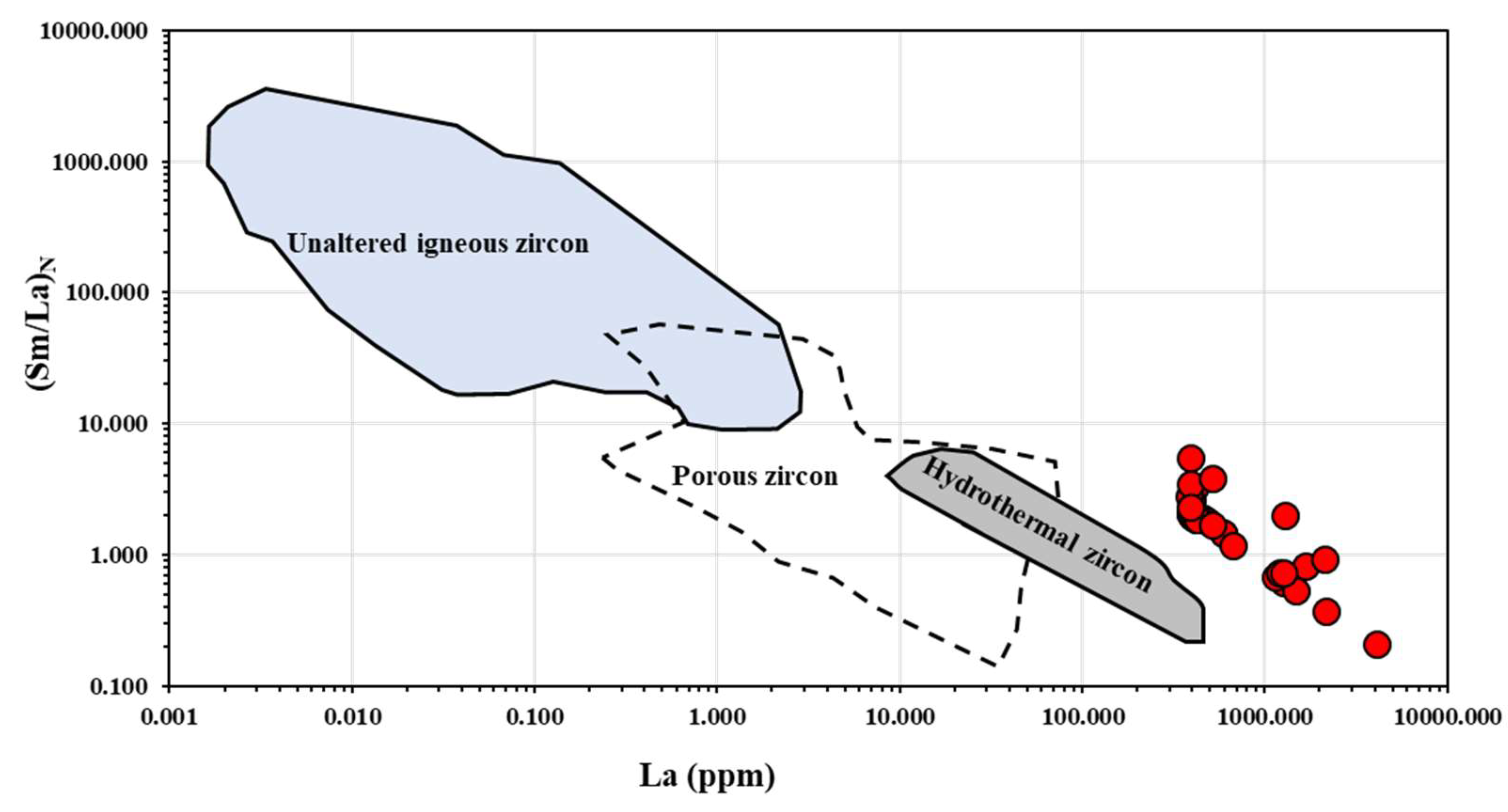
4.3. REE Pattern of Zircon
5. Discussion
5.1. Zircon Alteration
5.2. Timing of ‘Non-Formula’ Element Uptake in Um Ara Zircon
6. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abd El-Naby, H. High and low temperature alteration of uranium and thorium minerals, Um Ara granites, south Eastern Desert, Egypt. Ore Geol. Rev. 2009, 35, 436–446. [Google Scholar] [CrossRef]
- Abdalla, H.M.; Ishihara, S.; Matsueda, H.; Abdel-Monem, A.A. On the albite-enriched granitoids at Um Ara area, Southeastern Desert, Egypt. 1. Geochemical, ore potentiality and fluid inclusion studies. J. Geochem. Explor. 1996, 57, 127–138. [Google Scholar] [CrossRef]
- Bouvier, A.-S.; Ushikubo, T.; Kita, N.T.; Cavoise, A.J.; Kozdon, R.; Valley, J.W. Isotopes and trace elements as a petrogenetic tracer in zircon: insights from Archean TTGs and sanukitoids. Contrib Mineral Petrol 2012, 163, 745–768. [Google Scholar] [CrossRef]
- Caruba, R.; Iacconi, P. Les zircons de Narssârssuk (Groënland) - L’eau et les groupements OH dans les zircons métamictes. Chem Geol 1983, 38, 75–92. [Google Scholar] [CrossRef]
- Claiborne, Lily, L.; Miller, C.F.; Wooden, J.L. Trace element composition of igneous zircon: a thermal and compositional record of the accumulation and evolution of a large silicic batholith, Spirit Mountain, Nevada. Contrib Mineral Petrol 2010, 160, 511–531. [Google Scholar] [CrossRef]
- Dawood, Y.H. Uranium-series disequilibrium dating of secondary uranium ore from south Eastern Desert of Egypt. Appl Radiat Isot 2001, 881–887. [Google Scholar] [CrossRef]
- Delattre, S.; Utsunomiya, S.; Ewing, R.C.; Boeglin, J.-L.; Braun, J.; Balan, E.; Calas, G. Dissolution of radiation-damaged zircon in lateritic soils. Amer Miner 2007, 92, 1978–1989. [Google Scholar] [CrossRef]
- Ewing, R.C.; Meldrum, A.; Wang, L.; Weber, W.J.; Corrales, L.R. Radiation effects in zircon. Rev. Mineral. Geochem. 2003, 53, 387–425. [Google Scholar] [CrossRef]
- Frondel, C. Hydroxyl substitution in thorite and zircon. Amer Miner 1953, 38, 1007–1018. [Google Scholar]
- Geisler, T.; Schaltegger, U.; Tomaschek, F. Re-equilibration of zircon in aqueous fluids and melts. Elements 2007, 3, 43–50. [Google Scholar] [CrossRef]
- Geisler, T.; Burakov, B.E.; Zirlin, V.; Nikolaeva, L.; Pöml, P. A Raman spectroscopic study of high-uranium zircon from the Chernobyl “lava”. European Journal of Mineralogy 2005, 17, 883–894. [Google Scholar] [CrossRef]
- Geisler, T.; Rashwan, A.A.; Rahn, M.K.W.; Poller, U.; Zwingmann, H.; Pidgeon, R.T.; Schleicher, H.; Tomaschek, F. Low-temperature hydrothermal alteration of natural metamict zircons from the Eastern Desert, Egypt. Mineralogical Magazine 2003, 67, 485–508. [Google Scholar] [CrossRef]
- Geisler, T.; Pidgeon, R.T.; Kurtz, R.; van Bronswijk, W.; Schleicher, H. Experimental hydrothermal alteration of partially metamict zircon. Amer Miner 2003a, 86, 1496–1518. [Google Scholar] [CrossRef]
- Geisler, T. Isothermal annealing of partially metamict zircon: evidence or a three-stage recovery process. Physics and Chemistry of Minerals 2002, 29, 420–429. [Google Scholar] [CrossRef]
- Grimes, C.B.; John, B.E.; Cheadle, M.J.; Mazdab, F.K.; Wooden, J.L.; Swapp, S.; Schwartz, J.J. On the occurrence, trace element geochemistry, and crystallization history of zircon from in situ ocean lithosphere. Contrib Mineral Petrol 2009, 158, 757–783. [Google Scholar] [CrossRef]
- Finch, R.J.; Hanchar, J.M. Structure and chemistry of zircon and zircon-group minerals. Rev. Mineral. Geochem. 2003, 53, 1–25. [Google Scholar] [CrossRef]
- Harlov, D.E.; Anczkiewicz, R.; Dunkley, D.J. Metasomatic alteration of zircon at lower crustal P-T conditions utilizing alkali- and F-bearing fluids: Trace element incorporation, depletion, and resetting the zircon geochronometer. Geochimica et Cosmochimica Acta 2023, 352. [Google Scholar] [CrossRef]
- Hay, D.C.; Dempster, T.J. Zircon behaviour during low-temperature metamorphism. J. Petrol. 2009, 50, 571–589. [Google Scholar] [CrossRef]
- Hoskin, P.W.O. Trace-element composition of hydrothermal zircon and the alteration of Hadean zircon from the Jack Hills, Australia. Geochim. Cosmochim. Acta 2005, 69, 637–648. [Google Scholar] [CrossRef]
- Hoskin, P.W.O.; Kinny, P.D.; Wyborn, D.; Chappell, B.W. Identifying accessory mineral saturation during differentiation in granitoid magmas: an integrated approach. Journal of Petrology 2000, 41, 1365–1396. [Google Scholar] [CrossRef]
- Hoskin, P.W.O.; Schaltegger, U. The composition of zircon and igneous and metamorphic petrogenesis. Reviews in Mineralogy and Geochemistry 2003, 53, 27–62. [Google Scholar] [CrossRef]
- Kempe, D.; Gruner, T.; Nasdala, L.; Wolf, D. Relevance of cathodoluminescence for the interpretation of U-Pb zircon ages, with an example of an application to a study of zircons from the Saxonian Granulite Complex, Germany. In Cathodoluminescence in Geosciences; Pagel, M.; Barbin, V.; Blanc, P.; Ohnenstetter, D., Eds., Springer, Berlin, Heidelberg, New York, 2000, pp. 415–455.
- Kirkland, C.; Smithies, R.; Taylor, R.; Evans, N.; McDonald, B. Zircon Th/U ratios in magmatic environs. Lithos 2015, 212, 397–414. [Google Scholar] [CrossRef]
- Lenting, C.; Geisler, T.; Gerdes, A.; Kooijman, E.; Scherer, E.E.; Zeh, A. The behavior of the Hf isotope system in radiation damaged zircon during experimental hydrothermal alteration. Amer Miner 2010, 95, 1343–1348. [Google Scholar] [CrossRef]
- Lewerentz, A.; Harlov, D.E.; Scherstén, A.; Whitehouse, M.J. Baddeleyite formation in zircon by Ca-bearing fluids in silica-saturated systems in nature and experiment: resetting of the U–Pb geochronometer. Contrib Mineral Petrol 2019, 174, 64–10. [Google Scholar] [CrossRef]
- Marsellos, A.E.; Garver, J.I. Radiation damage and uranium concentration in zircon as assessed by Raman spectroscopy and neutron irradiation. Amer Miner 2010, 95, 1192–1201. [Google Scholar] [CrossRef]
- Moussa, E.M.; Stern, R.J.; Manton, W.I.; Ali, K.A. SHRIMP zircon dating and Sm/Nd isotopic investigations of Neoproterozoic granitoids, Eastern Desert, Egypt. Precambr Res 2007, 160, 341–356. [Google Scholar] [CrossRef]
- Murakami, T.; Chakoumakos, B.C.; Ewing, R.C.; Lumpkin, G.R.; Weber, W.J. Alpha-decay event damage in zircon. Amer Miner 1991, 76, 1510–1532. [Google Scholar]
- Nasdala, L.; Kronz, A.; Wirth, R.; Váczi, T.; Pérez-Soba, C.; Willner, A.; Kennedy, A.K. The phenomenon of deficient electron microprobe totals in radiation-damaged and altered zircon. Geochim Cosmochim Acta 2009, 73, 1637–1650. [Google Scholar] [CrossRef]
- Pérez -Soba, C.; Villaseca, C.; Del Tánago, J.G.; Nasdala, L. The composition of zircon in the peraluminous Hercynian granites of the Spanish Central System batholith. Canad Mineral 2007, 45, 509–527. [Google Scholar] [CrossRef]
- Pointer, C.M.; Ashworth, J.R.; Ixer, R.A. The zircon-thorite mineral group in metasomatized granite, Ririwai, Nigeria 1. Zoning, alteration and exsolution in zircon. Mineral Petrol 1988, 39, 21–37. [Google Scholar] [CrossRef]
- Reddy, S.M.; Timms, N.; Kinny, P.D.; Buchan, C.; Trimby, P.; Blake, K. Crystal-plastic deformation of zircon: a defect in the assumption of chemical robustness. Geology 2006, 34, 257–260. [Google Scholar] [CrossRef]
- Scott, V.D.; Love, G.; Reed, S.J.B. Quantitative Electron-Probe Microanalysis (2nd edition). Ellis Horwood. London and New York, 1995, 311p.
- Seydoux-Guillaume, A.M.; Bingen, B.; Paquette, J.L.; Bosse, V. Nanoscale evidence for uranium mobility in zircon and the discordance of U-Pb chronometers. Earth Planet Sc Lett 2015, 409, 43–48. [Google Scholar] [CrossRef]
- Skublov, S.G.; Berezin, A.V.; Li, X.-H.; Li, Q.-L.; Salimgaraeva, L.I.; Travin, V.V.; Rezvukhin, D.I. Zircons from a Pegmatite Cutting Eclogite (Gridino, Belomorian Mobile Belt): U-Pb-O and Trace Element Constraints on Eclogite Metamorphism and Fluid Activity. Geosciences 2020, 10, 197. [Google Scholar] [CrossRef]
- Smith, D.G.W.; de, St. Jorre, L.; Reed S, J.B.; Long, J.V.P. Zonally metamictized and other zircons from Thor Lake, Northwest Territories. Canad Mineral 1991, 29, 301–309. [Google Scholar]
- Soman, A.; Geisler, T.; Tomaschek, F.; Grange, M.; Berndt, J. Alteration of crystalline zircon solid solutions: A case study on zircon from an alkaline pegmatite from Zomba-Malosa, Malawi. Contrib Mineral Petr 2010, 160, 909–930. [Google Scholar] [CrossRef]
- Speer, J.A. Zircon. Mineralogical Society of America. Rev. Mineral. 1982, 5, 67–l12. [Google Scholar]
- Tomaschek, F.; Kennedy, A.K.; Villa, I.M.; Lagos, M.; Ballhaus, C. Zircons from Syros, Cyclades, Greece—recrystallization and mobilization of zircon during high-pressure metamorphism. J Petrol 2003, 44, 1977–2002. [Google Scholar] [CrossRef]
- Törnroos, R. Metamict zircon from Mozambique. Bull. Geol. Soc. Finland 1985, 57, 181–195. [Google Scholar] [CrossRef]
- Trail, D.; Watson, E.B.; Tailby, N.D. Ce and Eu anomalies in zircon as proxies for the oxidation state of magmas. Geochim Cosmochim Acta 2012, 97, 70–87. [Google Scholar] [CrossRef]
| Element | Line | Std. | Crystal | Counting time (Sec.) |
Limit Of Quantification (LOQ) (wt.%) |
|---|---|---|---|---|---|
| Si | Kα1 | Wollastonite | PETH | 10 | 0.066 |
| Zr | Lα1 | Zr oxide | PETJ | 10 | 0.042 |
| U | Mα1 | UO2 | PETH | 10 | 0.081 |
| Th | Mα1 | ThO2 | PETH | 10 | 0.102 |
| Hf | Lα1 | HfO2 | LIFH | 10 | 0.033 |
| Pb | Mα1 | PbVGe Oxides | PETH | 10 | 0.015 |
| Fe | Kα1 | Fe2O3 | LIFH | 10 | 0.033 |
| Al | Kα1 | Y-garnet | TAP | 10 | 0.027 |
| Ca | Kα1 | Wollastonite | PETJ | 10 | 0.051 |
| Mg | Kα1 | MgO | TAP | 10 | 0.051 |
| Ti | Kα1 | Ilmenite | PETJ | 10 | 0.024 |
| P | Kα1 | LaPO4 | TAP | 10 | 0.081 |
| Y | Lα1 | Y-garnet | TAP | 10 | 0.039 |
| AS | Lα1 | Cal-STD | LIF | 10 | 0.039 |
| La | Lα1 | LaPO4 | LIF | 10 | 0.045 |
| Ce | Lα1 | CePO4 | LIFH | 10 | 0.069 |
| Nd | Lβ1 | NdPO4 | LIFH | 10 | 0.036 |
| Sm | Lβ1 | SmPO4 | LIFH | 10 | 0.057 |
| Eu | Lβ1 | EuPO4 | LIFH | 10 | 0.036 |
| Gd | Lβ1 | GdPO4 | LIF | 10 | 0.075 |
| Tb | Lβ1 | TbPO4 | LIFH | 10 | 0.036 |
| Dy | Lβ1 | DyPO4 | LIFH | 10 | 0.075 |
| Ho | Lβ1 | HoPO4 | LIFH | 10 | 0.018 |
| Er | Lβ1 | ErPO4 | LIFH | 10 | 0.018 |
| Tm | Lα1 | TmPO4 | LIFH | 10 | 0.018 |
| Yb | Lα1 | YbPO4 | LIFH | 10 | 0.039 |
| Lu | Lα1 | LuPO4 | LIFH | 10 | 0.021 |
| Mineral type | Less altered Zircon | Altered Zircon | Uranophane | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | SP1 (N*=1) |
SP2-1 (N*=2) |
SP2-2 (N*=8) |
SP3-1 (N*=7) |
SP3-2 (N*=8) |
SP4-1 (N*=8) |
SP5 (N*=8) |
SP4-2B (N*=8) |
U1 (N=3) |
U3 (N=3) |
| Oxides (wt.%) | ||||||||||
| SiO2 | 32.853 | 32.846 | 30.518 | 30.913 | 28.44 | 26.48 | 25.84 | 26.66 | 14.327 | 13.696 |
| ZrO2 | 66.273 | 64.848 | 63.184 | 62.605 | 50.04 | 41.08 | 39.2 | 36.52 | 0.037 | 0.006 |
| UO2 | 0.083 | 0.118 | 0.085 | 0.013 | 2.195 | 3.166 | 2.262 | 5.518 | 66.685 | 60.288 |
| ThO2 | 0.103 | 0.05 | - | 0.038 | 1.035 | 8.685 | 6.73 | 8.01 | - | - |
| HfO2 | - | 0.075 | 1.716 | 1.275 | 3.342 | 2.931 | 6.257 | 2.905 | 0.157 | - |
| PbO | - | - | - | 0.092 | - | - | 0.104 | - | - | |
| FeO | 0.152 | 0.145 | 0.035 | 0.625 | 0.992 | 1.086 | 2.035 | 1.662 | 0.219 | 0.284 |
| Al2O3 | - | - | 0.029 | - | 0.813 | 2.062 | 1.768 | 3.224 | 0.15 | 0.289 |
| CaO | 0.057 | 0.043 | - | 0.051 | 1.368 | 1.597 | 1.416 | 1.426 | 6.764 | 6.143 |
| MgO | - | - | - | - | 0.059 | 0.055 | - | 0.074 | 0.029 | 0.069 |
| TiO2 | - | - | 0.053 | 0.064 | 0.026 | - | - | - | 0.014 | - |
| P2O5 | - | - | - | - | 0.248 | 0.417 | 0.39 | 0.128 | - | - |
| Y2O3 | - | - | - | - | 0.518 | - | 0.564 | - | - | - |
| As2O3 | - | - | - | - | 0.06 | 0.061 | 0.22 | 0.128 | 0.034 | 0.055 |
| La2O3 | - | - | 0.046 | 0.047 | 0.07 | 0.15 | 0.25 | 0.054 | - | 0.056 |
| Ce2O3 | - | - | 0.079 | 0.07 | 0.103 | 1.024 | 0.941 | 0.856 | 0.183 | - |
| Nd2O3 | - | - | 0.106 | 0.126 | 0.04 | 0.039 | 0.037 | 0.036 | 0.118 | 0.06 |
| Sm2O3 | - | - | 0.059 | 0.075 | 0.063 | 0.184 | 0.144 | 0.066 | - | - |
| EU2O3 | - | - | 0.056 | 0.091 | 0.04 | 0.037 | 0.038 | 0.045 | - | - |
| Gd2O3 | - | - | 0.198 | 0.175 | 0.081 | 0.164 | 0.11 | 0.078 | - | - |
| Tb2O3 | - | - | 0.037 | 0.037 | 0.037 | 0.039 | 0.037 | 0.039 | - | 0.089 |
| Dy2O3 | - | - | 0.077 | 0.162 | 0.288 | 0.328 | 0.177 | 0.241 | - | - |
| Ho2O3 | - | - | 0.175 | 0.104 | 0.082 | 0.02 | 0.018 | 0.018 | - | - |
| Er2O3 | - | - | 0.02 | 0.02 | 0.356 | 0.053 | 0.413 | 0.076 | - | - |
| Tm2O3 | - | - | 0.062 | 0.023 | 0.193 | 0.018 | 0.037 | 0.072 | - | 0.035 |
| Yb2O3 | - | - | 0.26 | 0.078 | 0.489 | 0.393 | 0.395 | 0.044 | - | - |
| Lu2O3 | - | - | 0.112 | 0.033 | 0.182 | 0.089 | 0.03 | 0.021 | - | 0.075 |
| Total | 99.52 | 98.13 | 96.91 | 96.56 | 91.25 | 90.16 | 89.31 | 88.01 | 88.72 | 81.15 |
| Mineral type | Less altered Zircon | Altered Zircon | Uranophane | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | SP1 (N*=1) |
SP2-1 (N*=2) |
SP2-2 (N*=8) |
SP3-1 (N*=7) |
SP3-2 (N*=8) |
SP4-1 (N*=8) |
SP5 (N*=8) |
SP4-2B (N*=8) |
U1 (N=3) |
U3 (N=3) |
| Structural formula |
Based on O = 4 | Based on O = 7 | ||||||||
| Si | 1.0065 | 1.0167 | 0.9816 | 0.9908 | 0.9912 | 0.9757 | 0.9688 | 1.0001 | 1.5076 | 1.5490 |
| Al | - | - | 0.0008 | - | 0.0334 | 0.0895 | 0.0781 | 0.1424 | 0.0186 | 0.0385 |
| P | - | - | - | - | 0.0073 | 0.0130 | 0.0124 | 0.0041 | - | - |
| As | - | - | - | - | 0.0013 | 0.0014 | 0.0050 | 0.0029 | 0.0022 | 0.0038 |
| T-site | 1.0065 | 1.0167 | 0.9824 | 0.9908 | 1.0332 | 1.0796 | 1.0643 | 1.1495 | ||
| Zr | 0.9900 | 0.9788 | 0.9910 | 0.9785 | 0.8504 | 0.7381 | 0.7167 | 0.6680 | 0.0019 | 0.0003 |
| U | 0.0006 | 0.0008 | 0.0006 | 0.0001 | 0.0170 | 0.0260 | 0.0189 | 0.0461 | 1.5616 | 1.5174 |
| Th | 0.0007 | 0.0004 | - | 0.0003 | 0.0082 | 0.0728 | 0.0574 | 0.0684 | - | - |
| Hf | - | 0.0007 | 0.0158 | 0.0117 | 0.0332 | 0.0308 | 0.0670 | 0.0311 | 0.0047 | - |
| Pb | - | - | - | - | 0.0009 | 0.0001 | - | 0.0011 | - | - |
| Fe | 0.0039 | 0.0038 | 0.0008 | 0.0168 | 0.0289 | 0.0335 | 0.0638 | 0.0521 | 0.0193 | 0.0269 |
| Ca | 0.0006 | 0.0014 | - | 0.0018 | 0.0511 | 0.0630 | 0.0569 | 0.0573 | 0.7626 | 0.7444 |
| Mg | - | - | - | - | 0.0031 | 0.0009 | - | 0.0041 | 0.0045 | 0.0116 |
| Ti | - | - | 0.0013 | 0.0015 | 0.0001 | - | - | - | 0.0011 | - |
| Y | - | - | - | 0.0000 | 0.0096 | - | 0.0112 | - | - | - |
| La | - | - | 0.0004 | 0.0004 | 0.0009 | 0.0020 | 0.0035 | 0.0002 | - | 0.0023 |
| Ce | - | - | 0.0007 | 0.0005 | 0.0013 | 0.0138 | 0.0129 | 0.0117 | 0.0070 | - |
| Nd | - | - | 0.0012 | 0.0014 | 0.0002 | 0.0002 | 0.0002 | 0.0005 | 0.0044 | 0.0024 |
| Sm | - | - | 0.0007 | 0.0008 | 0.0004 | 0.0023 | 0.0019 | 0.0009 | - | - |
| Eu | - | - | 0.0006 | 0.0003 | 0.0002 | 0.0002 | 0.0002 | 0.0002 | - | - |
| Gd | - | - | 0.0021 | 0.0010 | 0.0002 | 0.0020 | 0.0014 | 0.0010 | - | - |
| Tb | - | - | 0.0003 | 0.0003 | 0.0003 | 0.0004 | 0.0002 | 0.0002 | - | 0.0033 |
| Dy | - | - | 0.0001 | 0.0002 | 0.0032 | 0.0039 | 0.0021 | 0.0029 | - | - |
| Ho | - | - | 0.0018 | 0.0017 | 0.0009 | 0.0001 | 0.0002 | 0.0002 | - | - |
| Er | - | - | 0.0001 | 0.0001 | 0.0039 | 0.0006 | 0.0049 | 0.0009 | - | - |
| Tm | - | - | 0.0006 | 0.0001 | 0.0021 | 0.0001 | 0.0004 | 0.0008 | - | 0.0012 |
| Yb | - | - | 0.0025 | 0.0008 | 0.0052 | 0.0044 | 0.0045 | 0.0003 | - | - |
| Lu | - | - | 0.0011 | 0.0008 | 0.0019 | 0.0010 | 0.0003 | 0.0001 | - | 0.0026 |
| A-Site | 1.00 | 0.99 | 1.02 | 1.02 | 1.02 | 1.00 | 1.02 | 0.95 | ||
| Total | 2.002 | 2.003 | 2.004 | 2.010 | 2.056 | 2.076 | 2.089 | 2.098 | 3.896 | 3.904 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




