Submitted:
16 July 2024
Posted:
16 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Fundamental Concepts for the Study of Plant-Derived Bioactive Compounds
2.1. The Importance of the Phytocomplex
2.2. Vehicle, Matrix and Food Matrix
3. Polyphenols: General Properties and Lipophilic Phenols in Wine
3.1. Bioavailability
3.2. Polyphenols as Wine Matrix Constituents
3.3. Tyrosol,Hydroxytyrosol and Resveratrol
3.3.1. When Matrix Effect is Respected: the case of Tyrosol and Hydroxytyrosol
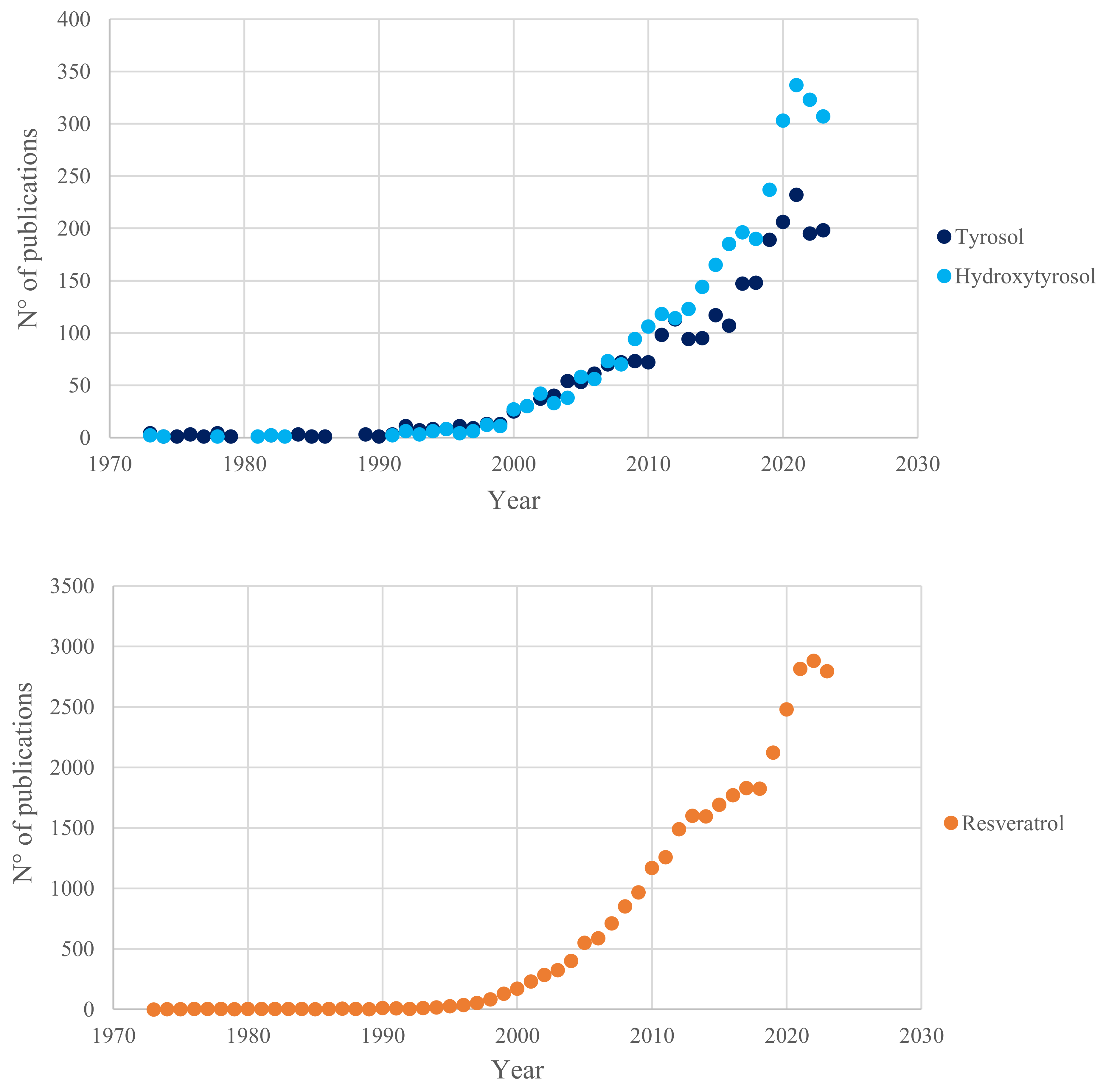
3.3.2. When Matrix Effect is Disregarded: Resveratrol
4. Alcohol and Alcoholic Beverages
4.1. Absorption and Metabolismof Ethanol
4.2. Physiological Effects and Toxicity of Alcohol
The Dark Side of the Matrix Effect: Alcohol and Smoke
4.3. When the Matrix Effect Is Ignored: Alcohol Beverages Specificity
5. In Vivo Studies on Polyphenols Biological Activities
6. When Natural Vehicle in Wine is Targeted: Wine Non-Alcoholic Alternatives
7. Conclusions and future perspectives
Abbreviations
| ADH | Alcohol dehydrogenase |
| ALD | Alcohol-related liver disease |
| ALDH | Acetaldehyde dehydrogenase |
| AMPK | 5’ adenosine monophosphate-activated protein kinase |
| BBB | Blood-Brain Barrier |
| BMD | Bone Mineral Density |
| CO2 | Carbon dioxide |
| CYP2E1 | Cytochrome P450 isoform 2E1 |
| EFSA | European Food Safety Agency |
| EMA | European Medicines Agency |
| EtG | Ethyl glucuronide |
| EtOH | Ethanol |
| EtS | Ethyl sulfate |
| FAEE | Fatty acid ethyl ester |
| FMD | Flow Mediated Dilatation |
| GABA | Gamma-aminobutyric acid |
| H2O | Water |
| HDL-C | High Density Lipoprotein – Cholesterol |
| HNC | Head and Neck Cancer |
| HTyr | Hydroxytyrosol |
| IL | Interleukin |
| LDL | Low Density Lipoprotein |
| MCP | Monocyte Chemoattractant Protein |
| MeOH | Methanol |
| MEOS | Microsomal ethanol-oxidizing system |
| MUFAs | Mono-Unsaturated Fatty Acids |
| PEth | Phosphatidylethanol |
| PUFAs | Poly-Unsaturated Fatty Acids |
| ROS | Reactive Oxygen Species |
| Rsv | Resveratrol |
| RWE | Red Wine Extract |
| TAG | Triacylglycerol |
| TNF | Tumor Necrosis Factor |
| Tyr | Tyrosol |
| USDA | United States Department of Agriculture |
| UV | Ultraviolet |
| WHO | World Health Organization |
References
- Addolorato, G., Capristo, E., Greco, A. V., Stefanini, G. F., &Gasbarrini, G. (1997). Energy expenditure, substrate oxidation, and body composition in subjects with chronic alcoholism: new findings from metabolic assessment. Alcoholism, clinical and experimental research, 21(6), 962–967.
- Ahlemeyer, B., &Krieglstein, J. (2003). Pharmacological studies supporting the therapeutic use of Ginkgo biloba extract for Alzheimer's disease. Pharmacopsychiatry, 36 Suppl 1, S8–S14. [CrossRef]
- Alemán-Jiménez, C., Domínguez-Perles, R., Medina, S., Prgomet, I., López-González, I., Simonelli-Muñoz, A., Campillo-Cano, M., Auñón, D., Ferreres, F., & Gil-Izquierdo, Á. (2021). Pharmacokinetics and bioavailability of hydroxytyrosol are dependent on the food matrix in humans. European journal of nutrition, 60(2), 905–915. [CrossRef]
- Anderson, L. A., Cantwell, M. M., Watson, R. G., Johnston, B. T., Murphy, S. J., Ferguson, H. R., McGuigan, J., Comber, H., Reynolds, J. V., & Murray, L. J. (2009). The association between alcohol and reflux esophagitis, Barrett's esophagus, and esophageal adenocarcinoma. Gastroenterology, 136(3), 799–805. [CrossRef]
- Arranz, S., Chiva-Blanch, G., Valderas-Martínez, P., Medina-Remón, A., Lamuela-Raventós, R. M., & Estruch, R. (2012). Wine, beer, alcohol and polyphenols on cardiovascular disease and cancer. Nutrients, 4(7), 759–781. [CrossRef]
- Baan, R., Straif, K., Grosse, Y., Secretan, B., Ghissassi, F. E., Bouvard, V., Altieri, A., &Cogliano, V. (2007). Carcinogenicity of alcoholic beverages. Lancet Oncology/Lancet. Oncology, 8(4), 292–293. [CrossRef]
- Baiano, A., Terracone, C., Gambacorta, G., & La Notte, E. (2009). Phenolic content and antioxidant activity of Primitivo wine: comparison among winemaking technologies. Journal of food science, 74(3), C258–C267. [CrossRef]
- Bandiwadekar, A., Jose, J., Gopan, G., Augustin, V., Ashtekar, H., & Khot, K. B. (2024). Transdermal delivery of resveratrol loaded solid lipid nanoparticle as a microneedle patch: a novel approach for the treatment of Parkinson's disease. Drug delivery and translational research. [CrossRef]
- Barrajón-Catalán, E., Herranz-López, M., Joven, J., Segura-Carretero, A., Alonso-Villaverde, C., Menéndez, J. A., & Micol, V. (2014). Molecular promiscuity of plant polyphenols in the management of age-related diseases: far beyond their antioxidant properties. Advances in experimental medicine and biology, 824, 141–159. [CrossRef]
- Beaumont, P., Courtois, A., Atgié, C., Richard, T., & Krisa, S. (2022). In the shadow of resveratrol: biological activities of epsilon-viniferin. Journal of physiology and biochemistry, 78(2), 465–484. [CrossRef]
- Bernardi, S., Del Bo’, C., Marino, M., Gargari, G., Cherubini, A., Andrés-Lacueva, C., Hidalgo-Liberona, N., Peron, G., González-Dominguez, R., Kroon, P., Kirkup, B., Porrini, M., Guglielmetti, S., & Riso, P. (2020). Polyphenols and Intestinal Permeability: Rationale and Future Perspectives. Journal of agricultural and food chemistry, 68(7), 1816–1829. [CrossRef]
- Bertelli, A.A.E. (2007). Wine, research and cardiovascular disease: instructions for use. Atherosclerosis, 195(2), 242–247. [CrossRef]
- Bertelli, A. A., Giovannini, L., Bernini, W., Migliori, M., Fregoni, M., Bavaresco, L., & Bertelli, A. (1996). Antiplatelet activity of cis-resveratrol. Drugs under experimental and clinical research, 22(2), 61–63.
- Bertelli, A. A., Giovannini, L., Giannessi, D., Migliori, M., Bernini, W., Fregoni, M., & Bertelli, A. (1995). Antiplatelet activity of synthetic and natural resveratrol in red wine. International journal of tissue reactions, 17(1), 1–3.
- Bertelli, A. A., Giovannini, L., Stradi, R., Urien, S., Tillement, J. P., & Bertelli, A. (1996). Kinetics of trans- and cis-resveratrol (3,4',5-trihydroxystilbene) after red wine oral administration in rats. International journal of clinical pharmacology research, 16(4-5), 77–81.
- Bertelli, A., Biagi, M., Corsini, M., Baini, G., Cappellucci, G., & Miraldi, E. (2021). Polyphenols: From Theory to Practice. Foods (Basel, Switzerland), 10(11), 2595. [CrossRef]
- Bertuccioli, A., Cardinali, M., Di Pierro, F., Zonzini, G. B., & Matera, M. R. (2022). Ketogenic and Low FODMAP Diet in Therapeutic Management of a Young Autistic Patient with Epilepsy and Dysmetabolism Poorly Responsive to Therapies: Clinical Response and Effects of Intestinal Microbiota. International journal of molecular sciences, 23(15), 8829. [CrossRef]
- Biagi, M., Pecorari, R., Appendino, G., Miraldi, E., Magnano, A. R., Governa, P., Cettolin, G., & Giachetti, D. (2016). Herbal Products in Italy: The Thin Line between Phytotherapy, Nutrition and Parapharmaceuticals; A Normative Overview of the Fastest Growing Market in Europe. Pharmaceuticals (Basel, Switzerland), 9(4), 65. [CrossRef]
- Bladé, C., Arola, L., &Salvadó, M. J. (2010). Hypolipidemic effects of proanthocyanidins and their underlying biochemical and molecular mechanisms. Molecular nutrition & food research, 54(1), 37–59. [CrossRef]
- Boban, M., Modun, D., Music, I., Vukovic, J., Brizic, I., Salamunic, I., Obad, A., Palada, I., &Dujic, Z. (2006). Red wine induced modulation of vascular function: separating the role of polyphenols, ethanol, and urates. Journal of cardiovascular pharmacology, 47(5), 695–701. [CrossRef]
- Boffetta, P., &Hashibe, M. (2006). Alcohol and cancer. The Lancet. Oncology, 7(2), 149–156. [CrossRef]
- Boronat, A., Mateus, J., Soldevila-Domenech, N., Guerra, M., Rodríguez-Morató, J., Varon, C., Muñoz, D., Barbosa, F., Morales, J. C., Gaedigk, A., Langohr, K., Covas, M. I., Pérez-Mañá, C., Fitó, M., Tyndale, R. F., & de la Torre, R. (2019). Cardiovascular benefits of tyrosol and its endogenous conversion into hydroxytyrosol in humans. A randomized, controlled trial. Free radical biology & medicine, 143, 471–481. [CrossRef]
- Brizuela, L., Dayon, A., Doumerc, N., Ader, I., Golzio, M., Izard, J. C., Hara, Y., Malavaud, B., & Cuvillier, O. (2010). The sphingosine kinase-1 survival pathway is a molecular target for the tumor-suppressive tea and wine polyphenols in prostate cancer. FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 24(10), 3882–3894. [CrossRef]
- Buljeta, I., Pichler, A., Šimunović, J., &Kopjar, M. (2023). Beneficial Effects of Red Wine Polyphenols on Human Health: Comprehensive Review. Current issues in molecular biology, 45(2), 782–798. [CrossRef]
- Butterweck, V. (2003). Mechanism of action of St John's wort in depression : what is known?. CNS drugs, 17(8), 539–562. [CrossRef]
- Cai, Y., Yang, L., Hu, G., Chen, X., Niu, F., Yuan, L., Liu, H., Xiong, H., Arikkath, J., & Buch, S. (2016). Regulation of morphine-induced synaptic alterations: Role of oxidative stress, ER stress, and autophagy. The Journal of cell biology, 215(2), 245–258. [CrossRef]
- Capuano, E., Oliviero, T., & van Boekel, M. A. J. S. (2018). Modeling food matrix effects on chemical reactivity: Challenges and perspectives. Critical reviews in food science and nutrition, 58(16), 2814–2828. [CrossRef]
- Cardiovascular disease risk factors: new areas for research. Report of a WHO Scientific Group. (1994). PubMed, 841, 1–53. Available online: https://pubmed.ncbi.nlm.nih.gov/8184600.
- Cardona, F., Andrés-Lacueva, C., Tulipani, S., Tinahones, F. J., & Queipo-Ortuño, M. I. (2013). Benefits of polyphenols on gut microbiota and implications in human health. The Journal of nutritional biochemistry, 24(8), 1415–1422. [CrossRef]
- Cederbaum A. I. (2012). Alcohol metabolism. Clinics in liver disease, 16(4), 667–685. [CrossRef]
- Cerrato-Alvarez, M., Bernalte, E., Bernalte-García, M. J., & Pinilla-Gil, E. (2019). Fast and direct amperometric analysis of polyphenols in beers using tyrosinase-modified screen-printed gold nanoparticles biosensors. Talanta, 193, 93–99. [CrossRef]
- Chan, M. M., Mattiacci, J. A., Hwang, H. S., Shah, A., &Fong, D. (2000). Synergy between ethanol and grape polyphenols, quercetin, and resveratrol, in the inhibition of the inducible nitric oxide synthase pathway. Biochemicalpharmacology, 60(10), 1539–1548. [CrossRef]
- Chen, A., Zhang, L., Xu, J., & Tang, J. (2002). The antioxidant (-)-epigallocatechin-3-gallate inhibits activated hepatic stellate cell growth and suppresses acetaldehyde-induced gene expression. The Biochemical journal, 368(Pt 3), 695–704. [CrossRef]
- Chen, C. H., Budas, G. R., Churchill, E. N., Disatnik, M. H., Hurley, T. D., &Mochly-Rosen, D. (2008). Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science (New York, N.Y.), 321(5895), 1493–1495. [CrossRef]
- Clinton, S. K., Giovannucci, E. L., &Hursting, S. D. (2020). The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and Future Directions. The Journal of nutrition, 150(4), 663–671. [CrossRef]
- Comporti, M., Signorini, C., Leoncini, S., Gardi, C., Ciccoli, L., Giardini, A., Vecchio, D., & Arezzini, B. (2010). Ethanol-induced oxidative stress: basic knowledge. Genes & nutrition, 5(2), 101–109. [CrossRef]
- Corder, R., Mullen, W., Khan, N. Q., Marks, S. C., Wood, E. G., Carrier, M. J., & Crozier, A. (2006). Oenology: red wine procyanidins and vascular health. Nature, 444(7119), 566. [CrossRef]
- Cucciniello, R., Tomasini, M., Russo, A., Falivene, L., Gambuti, A., & Forino, M. (2023). Experimental and theoretical studies on the acetaldehyde reaction with (+)-catechin. Food chemistry, 426, 136556. [CrossRef]
- D'Angelo, C., Franceschelli, S., Quiles, J. L., & Speranza, L. (2020). Wide Biological Role of Hydroxytyrosol: Possible Therapeutic and Preventive Properties in Cardiovascular Diseases. Cells, 9(9), 1932. [CrossRef]
- Dinu, M., Pagliai, G., Casini, A., & Sofi, F. (2018). Mediterranean diet and multiple health outcomes: an umbrella review of meta-analyses of observational studies and randomised trials. European journal of clinical nutrition, 72(1), 30–43. [CrossRef]
- Ditano-Vázquez, P., Torres-Peña, J. D., Galeano-Valle, F., Pérez-Caballero, A. I., Demelo-Rodríguez, P., Lopez-Miranda, J., Katsiki, N., Delgado-Lista, J., & Alvarez-Sala-Walther, L. A. (2019). The Fluid Aspect of the Mediterranean Diet in the Prevention and Management of Cardiovascular Disease and Diabetes: The Role of Polyphenol Content in Moderate Consumption of Wine and Olive Oil. Nutrients, 11(11), 2833. [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA), Turck, D., Bresson, J. L., Burlingame, B., Dean, T., Fairweather-Tait, S., Heinonen, M., Hirsch-Ernst, K. I., Mangelsdorf, I., McArdle, H. J., Naska, A., Neuhäuser-Berthold, M., Nowicka, G., Pentieva, K., Sanz, Y., Siani, A., Sjödin, A., Stern, M., Tomé, D., Vinceti, M., … van Loveren, H. (2017). Safety of hydroxytyrosol as a novel food pursuant to Regulation (EC) No 258/97. EFSA journal. European Food Safety Authority, 15(3), e04728. [CrossRef]
- European Commission. (2017). Commission Implementing Decision (EU) 2017/2373 of 14 December 2017 authorising the placing on the market of hydroxytyrosol as a novel food ingredient under Regulation (EC) No 258/97 of the European Parliament and of the Council. Off. J. Eur. Union, 50, 56-59.
- European Medicines Agency; Herbal Medicinal Products; (n.d.). Available online: https://www.ema.europa.eu/en/human-regulatory-overview/herbal-medicinal-products.
- European Parliament; Consolidated text: Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001 (Text with EEA relevance) Text with EEA relevance. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02015R2283-20210327.
- Faria, A., Meireles, M., Fernandes, I., Santos-Buelga, C., Gonzalez-Manzano, S., Dueñas, M., de Freitas, V., Mateus, N., &Calhau, C. (2014). Flavonoid metabolites transport across a human BBB model. Food chemistry, 149, 190–196. [CrossRef]
- Fernandes, I., Pérez-Gregorio, R., Soares, S., Mateus, N., & de Freitas, V. (2017). Wine Flavonoids in Health and Disease Prevention. Molecules (Basel, Switzerland), 22(2), 292. [CrossRef]
- Ferraz da Costa, D. C., Pereira Rangel, L., Quarti, J., Santos, R. A., Silva, J. L., & Fialho, E. (2020). Bioactive Compounds and Metabolites from Grapes and Red Wine in Breast Cancer Chemoprevention and Therapy. Molecules (Basel, Switzerland), 25(15), 3531. [CrossRef]
- Forman, H. J., Davies, K. J., &Ursini, F. (2014). How do nutritional antioxidants really work: nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free radical biology & medicine, 66, 24–35. [CrossRef]
- Freedman, N. D., Abnet, C. C., Leitzmann, M. F., Hollenbeck, A. R., &Schatzkin, A. (2007). Prospective investigation of the cigarette smoking-head and neck cancer association by sex. Cancer, 110(7), 1593–1601. [CrossRef]
- Freedman, N. D., Schatzkin, A., Leitzmann, M. F., Hollenbeck, A. R., & Abnet, C. C. (2007). Alcohol and head and neck cancer risk in a prospective study. British journal of cancer, 96(9), 1469–1474. [CrossRef]
- GBD 2020 Alcohol Collaborators (2022). Population-level risks of alcohol consumption by amount, geography, age, sex, and year: a systematic analysis for the Global Burden of Disease Study 2020. Lancet, 400(10347), 185–235. [CrossRef]
- Gál, R., Halmosi, R., Gallyas, F., Jr, Tschida, M., Mutirangura, P., Tóth, K., Alexy, T., & Czopf, L. (2023). Resveratrol and beyond: The Effect of Natural Polyphenols on the Cardiovascular System: A Narrative Review. Biomedicines, 11(11), 2888. [CrossRef]
- Gammon, M. D., Schoenberg, J. B., Ahsan, H., Risch, H. A., Vaughan, T. L., Chow, W. H., Rotterdam, H., West, A. B., Dubrow, R., Stanford, J. L., Mayne, S. T., Farrow, D. C., Niwa, S., Blot, W. J., & Fraumeni, J. F., Jr (1997). Tobacco, alcohol, and socioeconomic status and adenocarcinomas of the esophagus and gastric cardia. Journal of the National Cancer Institute, 89(17), 1277–1284. [CrossRef]
- Gandini, S., Botteri, E., Iodice, S., Boniol, M., Lowenfels, A. B., Maisonneuve, P., & Boyle, P. (2008). Tobacco smoking and cancer: a meta-analysis. International journal of cancer, 122(1), 155–164. [CrossRef]
- García-Conesa, M. T., Chambers, K., Combet, E., Pinto, P., Garcia-Aloy, M., Andrés-Lacueva, C., de Pascual-Teresa, S., Mena, P., Konic Ristic, A., Hollands, W. J., Kroon, P. A., Rodríguez-Mateos, A., Istas, G., Kontogiorgis, C. A., Rai, D. K., Gibney, E. R., Morand, C., Espín, J. C., & González-Sarrías, A. (2018). Meta-Analysis of the Effects of Foods and Derived Products Containing Ellagitannins and Anthocyanins on Cardiometabolic Biomarkers: Analysis of Factors Influencing Variability of the Individual Responses. International journal of molecular sciences, 19(3), 694. [CrossRef]
- Geng, T., Zhao, X., Ma, M., Zhu, G., & Yin, L. (2017). Resveratrol-Loaded Albumin Nanoparticles with Prolonged Blood Circulation and Improved Biocompatibility for Highly Effective Targeted Pancreatic Tumor Therapy. Nanoscale researchletters, 12(1), 437. [CrossRef]
- Giovannelli, L., Pitozzi, V., Luceri, C., Giannini, L., Toti, S., Salvini, S., Sera, F., Souquet, J. M., Cheynier, V., Sofi, F., Mannini, L., Gori, A. M., Abbate, R., Palli, D., &Dolara, P. (2011). Effects of de-alcoholised wines with different polyphenol content on DNA oxidative damage, gene expression of peripheral lymphocytes, and haemorheology: an intervention study in post-menopausal women. European journal of nutrition, 50(1), 19–29. [CrossRef]
- Goldstein, B. Y., Chang, S. C., Hashibe, M., La Vecchia, C., & Zhang, Z. F. (2010). Alcohol consumption and cancers of the oral cavity and pharynx from 1988 to 2009: an update. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation (ECP), 19(6), 431–465. [CrossRef]
- González-Domínguez, R., Jáuregui, O., Mena, P., Hanhineva, K., Tinahones, F. J., Angelino, D., & Andrés-Lacueva, C. (2020). Quantifying the human diet in the crosstalk between nutrition and health by multi-targeted metabolomics of food and microbiota-derived metabolites. International journal of obesity (2005), 44(12), 2372–2381. [CrossRef]
- Gorelik, S., Ligumsky, M., Kohen, R., & Kanner, J. (2008). The stomach as a "bioreactor": when red meat meets red wine. Journal of agricultural and food chemistry, 56(13), 5002–5007. [CrossRef]
- Governa, P., Manetti, F., Miraldi, E., & Biagi, M. (2021). Effects of in vitro simulated digestion on the antioxidant activity of different Camellia sinensis (L.) Kuntze leaves extracts. European Food Research & Technology, 248(1), 119–128. [CrossRef]
- Grønbaek M. (2004). Epidemiologic evidence for the cardioprotective effects associated with consumption of alcoholic beverages. Pathophysiology : the official journal of the International Society for Pathophysiology, 10(2), 83–92. [CrossRef]
- Guerrero, R. F., Valls-Fonayet, J., Richard, T., & Cantos-Villar, E. (2020). A rapid quantification of stilbene content in wine by ultra-high pressure liquid chromatography–Mass spectrometry. Food Control, 108, 106821. [CrossRef]
- Habold, C., Momken, I., Ouadi, A., Bekaert, V., & Brasse, D. (2011). Effect of prior treatment with resveratrol on density and structure of rat long bones under tail-suspension. Journal of bone and mineral metabolism, 29(1), 15–22. [CrossRef]
- Hashibe, M., Brennan, P., Benhamou, S., Castellsague, X., Chen, C., Curado, M. P., Dal Maso, L., Daudt, A. W., Fabianova, E., Fernandez, L., Wünsch-Filho, V., Franceschi, S., Hayes, R. B., Herrero, R., Koifman, S., La Vecchia, C., Lazarus, P., Levi, F., Mates, D., Matos, E., … Boffetta, P. (2007). Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Journal of the National Cancer Institute, 99(10), 777–789. [CrossRef]
- Hashibe, M., Brennan, P., Chuang, S. C., Boccia, S., Castellsague, X., Chen, C., Curado, M. P., Dal Maso, L., Daudt, A. W., Fabianova, E., Fernandez, L., Wünsch-Filho, V., Franceschi, S., Hayes, R. B., Herrero, R., Kelsey, K., Koifman, S., La Vecchia, C., Lazarus, P., Levi, F., … Boffetta, P. (2009). Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology, 18(2), 541–550. [CrossRef]
- He, X., Andersson, G., Lindgren, U., & Li, Y. (2010). Resveratrol prevents RANKL-induced osteoclast differentiation of murine osteoclast progenitor RAW 264.7 cells through inhibition of ROS production. Biochemical and biophysical research communications, 401(3), 356–362. [CrossRef]
- Heier, C., Xie, H., & Zimmermann, R. (2016). Nonoxidative ethanol metabolism in humans-from biomarkers to bioactive lipids. IUBMB life, 68(12), 916–923. [CrossRef]
- Hendriks, H.F.J. (2020). Alcohol and Human Health: What Is the Evidence? Annual review of food science and technology, 11, 1–21. [CrossRef]
- Holford N., H. (1987). Clinical pharmacokinetics of ethanol. Clinical pharmacokinetics, 13(5), 273–292. [CrossRef]
- Hollman, P. C., & Katan, M. B. (1999). Dietary flavonoids: intake, health effects and bioavailability. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 37(9-10), 937–942. [CrossRef]
- Holstege, A., Bedossa, P., Poynard, T., Kollinger, M., Chaput, J. C., Houglum, K., &Chojkier, M. (1994). Acetaldehyde-modified epitopes in liver biopsy specimens of alcoholic and nonalcoholic patients: localization and association with progression of liver fibrosis. Hepatology (Baltimore, Md.), 19(2), 367–374–374.
- Hong, S., Khil, H., Lee, D. H., Keum, N., & Giovannucci, E. L. (2020). Alcohol Consumption and the Risk of Prostate Cancer: A Dose-Response Meta-Analysis. Nutrients, 12(8), 2188. [CrossRef]
- Hrelia, S., Di Renzo, L., Bavaresco, L., Bernardi, E., Malaguti, M., & Giacosa, A. (2022). Moderate Wine Consumption and Health: A Narrative Review. Nutrients, 15(1), 175. [CrossRef]
- Hyun, J., Han, J., Lee, C., Yoon, M., & Jung, Y. (2021). Pathophysiological Aspects of Alcohol Metabolism in the Liver. International journal of molecular sciences, 22(11), 5717. [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans (2012). Personal habits and indoor combustions. IARC monographs on the evaluation of carcinogenic risks to humans, 100(Pt E), 1–538.
- Joven, J., Micol, V., Segura-Carretero, A., Alonso-Villaverde, C., Menéndez, J. A., & Bioactive Food Components Platform (2014). Polyphenols and the modulation of gene expression pathways: can we eat our way out of the danger of chronic disease?. Critical reviews in food science and nutrition, 54(8), 985–1001. [CrossRef]
- Karković Marković, A., Torić, J., Barbarić, M., &Jakobušić Brala, C. (2019). Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules (Basel, Switzerland), 24(10), 2001. [CrossRef]
- Kitada, M., Ogura, Y., Monno, I., &Koya, D. (2020). Supplementation with Red Wine Extract Increases Insulin Sensitivity and Peripheral Blood Mononuclear Sirt1 Expression in Nondiabetic Humans. Nutrients, 12(10), 3108. [CrossRef]
- Krittanawong, C., Isath, A., Rosenson, R. S., Khawaja, M., Wang, Z., Fogg, S. E., Virani, S. S., Qi, L., Cao, Y., Long, M. T., Tangney, C. C., & Lavie, C. J. (2022). Alcohol Consumption and Cardiovascular Health. The American journal of medicine, 135(10), 1213–1230.e3. [CrossRef]
- Kubo, A., Levin, T. R., Block, G., Rumore, G. J., Quesenberry, C. P., Jr, Buffler, P., & Corley, D. A. (2009). Alcohol types and sociodemographic characteristics as risk factors for Barrett's esophagus. Gastroenterology, 136(3), 806–815. [CrossRef]
- Kutleša, Z., & Budimir Mršić, D. (2016). Wine and bone health: a review. Journal of bone and mineral metabolism, 34(1), 11–22. [CrossRef]
- Kwo, P. Y., Ramchandani, V. A., O'Connor, S., Amann, D., Carr, L. G., Sandrasegaran, K., Kopecky, K. K., & Li, T. K. (1998). Gender differences in alcohol metabolism: relationship to liver volume and effect of adjusting for body mass. Gastroenterology, 115(6), 1552–1557. [CrossRef]
- Lands W. E. (1995). Alcohol and energy intake. The American journal of clinical nutrition, 62(5 Suppl), 1101S–1106S. [CrossRef]
- Laposata, E. A., & Lange, L. G. (1986). Presence of nonoxidative ethanol metabolism in human organs commonly damaged by ethanol abuse. Science (New York, N.Y.), 231(4737), 497–499. [CrossRef]
- Larsen, B. A., Klinedinst, B. S., Le, S. T., Pappas, C., Wolf, T., Meier, N. F., Lim, Y. L., & Willette, A. A. (2022). Beer, wine, and spirits differentially influence body composition in older white adults-a United Kingdom Biobank study. Obesity science & practice, 8(5), 641–656. [CrossRef]
- Lekakis, J., Rallidis, L. S., Andreadou, I., Vamvakou, G., Kazantzoglou, G., Magiatis, P., Skaltsounis, A. L., &Kremastinos, D. T. (2005). Polyphenolic compounds from red grapes acutely improve endothelial function in patients with coronary heart disease. European journal of cardiovascular prevention and rehabilitation : official journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology, 12(6), 596–600. [CrossRef]
- Lieber C., S. (1994). Hepatic and metabolic effects of ethanol: pathogenesis and prevention. Annals of medicine, 26(5), 325–330. [CrossRef]
- Lieber C., S. (1997). Ethanol metabolism, cirrhosis and alcoholism. Clinicachimica acta; international journal of clinical chemistry, 257(1), 59–84. [CrossRef]
- Lu, Y., &Cederbaum, A. I. (2008). CYP2E1 and oxidative liver injury by alcohol. Free radical biology & medicine, 44(5), 723–738. [CrossRef]
- Lu, Z., Zhang, Y., Liu, H., Yuan, J., Zheng, Z., & Zou, G. (2007). Transport of a Cancer Chemopreventive Polyphenol, Resveratrol: Interaction with Serum Albumin and Hemoglobin. Journal of Fluorescence, 17(5), 580–587. [CrossRef]
- Lubin, J. H., Purdue, M., Kelsey, K., Zhang, Z. F., Winn, D., Wei, Q., Talamini, R., Szeszenia-Dabrowska, N., Sturgis, E. M., Smith, E., Shangina, O., Schwartz, S. M., Rudnai, P., Neto, J. E., Muscat, J., Morgenstern, H., Menezes, A., Matos, E., Mates, I. N., Lissowska, J., … Hayes, R. B. (2009). Total exposure and exposure rate effects for alcohol and smoking and risk of head and neck cancer: a pooled analysis of case-control studies. American journal of epidemiology, 170(8), 937–947. [CrossRef]
- Maasland, D. H., van den Brandt, P. A., Kremer, B., Goldbohm, R. A., & Schouten, L. J. (2014). Alcohol consumption, cigarette smoking and the risk of subtypes of head-neck cancer: results from the Netherlands Cohort Study. BMC cancer, 14, 187. [CrossRef]
- Maciel, M. E., Castro, J. A., & Castro, G. D. (2011). Inhibition of rat mammary microsomal oxidation of ethanol to acetaldehyde by plant polyphenols. Human & experimental toxicology, 30(7), 656–664. [CrossRef]
- Manach, C., Williamson, G., Morand, C., Scalbert, A., &Rémésy, C. (2005). Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. The American journal of clinical nutrition, 81(1 Suppl), 230S–242S. [CrossRef]
- Mannari, C., Bertelli, A. A. E., Stiaccini, G., & Giovannini, L. (2010). Wine, sirtuins and nephroprotection: not only resveratrol. Medical hypotheses, 75(6), 636–638. [CrossRef]
- Marron, M., Boffetta, P., Zhang, Z. F., Zaridze, D., Wünsch-Filho, V., Winn, D. M., Wei, Q., Talamini, R., Szeszenia-Dabrowska, N., Sturgis, E. M., Smith, E., Schwartz, S. M., Rudnai, P., Purdue, M. P., Olshan, A. F., Eluf-Neto, J., Muscat, J., Morgenstern, H., Menezes, A., McClean, M., … Hashibe, M. (2010). Cessation of alcohol drinking, tobacco smoking and the reversal of head and neck cancer risk. International journal of epidemiology, 39(1), 182–196. [CrossRef]
- Martínez-González, M. A., Gea, A., & Ruiz-Canela, M. (2019). The Mediterranean Diet and Cardiovascular Health. Circulation research, 124(5), 779–798. [CrossRef]
- Matsuzaki, K., Kumatoriya, K., Tando, M., Kometani, T., &Shinohara, M. (2022). Polyphenols from persimmon fruit attenuate acetaldehyde-induced DNA double-strand breaks by scavenging acetaldehyde. Scientific reports, 12(1), 10300. [CrossRef]
- Medina-Remón, A., Casas, R., Tressserra-Rimbau, A., Ros, E., Martínez-González, M. A., Fitó, M., Corella, D., Salas-Salvadó, J., Lamuela-Raventos, R. M., Estruch, R., & PREDIMED Study Investigators (2017). Polyphenol intake from a Mediterranean diet decreases inflammatory biomarkers related to atherosclerosis: a substudy of the PREDIMED trial. British journal of clinical pharmacology, 83(1), 114–128. [CrossRef]
- Meng, X., Maliakal, P., Lu, H., Lee, M. J., & Yang, C. S. (2004). Urinary and plasma levels of resveratrol and quercetin in humans, mice, and rats after ingestion of pure compounds and grape juice. Journal of agricultural and food chemistry, 52(4), 935–942. [CrossRef]
- Miró-Casas, E., Covas, M. I., Fitó, M., Farré-Albadalejo, M., Marrugat, J., & de la Torre, R. (2003). Tyrosol and hydroxytyrosol are absorbed from moderate and sustained doses of virgin olive oil in humans. European journal of clinical nutrition, 57(1), 186–190. [CrossRef]
- Moreno-Indias, I., Sánchez-Alcoholado, L., Pérez-Martínez, P., Andrés-Lacueva, C., Cardona, F., Tinahones, F., & Queipo-Ortuño, M. I. (2016). Red wine polyphenols modulate fecal microbiota and reduce markers of the metabolic syndrome in obese patients. Food & function, 7(4), 1775–1787. [CrossRef]
- Nair M. S. (2015). Spectroscopic study on the interaction of resveratrol and pterostilbene with human serum albumin. Journal of photochemistry and photobiology. B, Biology, 149, 58–67. [CrossRef]
- Office International de la Vigne et du Vin (OIV) (2003). Specificity of wine and scientific research. Resolution OENO 8/2003.
- Pal, S., Naissides, M., & Mamo, J. (2004). Polyphenolics and fat absorption. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity, 28(2), 324–326. [CrossRef]
- Pandeya, N., Williams, G., Green, A. C., Webb, P. M., Whiteman, D. C., & Australian Cancer Study (2009). Alcohol consumption and the risks of adenocarcinoma and squamous cell carcinoma of the esophagus. Gastroenterology, 136(4), 1215–e2. [CrossRef]
- Pelucchi, C., Gallus, S., Garavello, W., Bosetti, C., & La Vecchia, C. (2008). Alcohol and tobacco use, and cancer risk for upper aerodigestive tract and liver. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation (ECP), 17(4), 340–344. [CrossRef]
- Pérez-Mañá, C., Farré, M., Rodríguez-Morató, J., Papaseit, E., Pujadas, M., Fitó, M., Robledo, P., Covas, M. I., Cheynier, V., Meudec, E., Escudier, J. L., & de la Torre, R. (2015). Moderate consumption of wine, through both its phenolic compounds and alcohol content, promotes hydroxytyrosol endogenous generation in humans. A randomized controlled trial. Molecular nutrition & food research, 59(6), 1213–1216. [CrossRef]
- Pezzuto J., M. (2019). Resveratrol: Twenty Years of Growth, Development and Controversy. Biomolecules & therapeutics, 27(1), 1–14. [CrossRef]
- Pikaar, N. A., Wedel, M., & Hermus, R. J. (1988). Influence of several factors on blood alcohol concentrations after drinking alcohol. Alcohol and alcoholism (Oxford, Oxfordshire), 23(4), 289–297.
- Purdue, M. P., Hashibe, M., Berthiller, J., La Vecchia, C., Dal Maso, L., Herrero, R., Franceschi, S., Castellsague, X., Wei, Q., Sturgis, E. M., Morgenstern, H., Zhang, Z. F., Levi, F., Talamini, R., Smith, E., Muscat, J., Lazarus, P., Schwartz, S. M., Chen, C., Neto, J. E., … Hayes, R. B. (2009). Type of alcoholic beverage and risk of head and neck cancer--a pooled analysis within the INHANCE Consortium. American journal of epidemiology, 169(2), 132–142. [CrossRef]
- Rana, A., Samtiya, M., Dhewa, T., Mishra, V., & Aluko, R. E. (2022). Health benefits of polyphenols: A concise review. Journal of food biochemistry, 46(10), e14264. [CrossRef]
- Reis, A., Perez-Gregorio, R., Mateus, N., & de Freitas, V. (2021). Interactions of dietary polyphenols with epithelial lipids: advances from membrane and cell models in the study of polyphenol absorption, transport and delivery to the epithelium. Critical reviews in food science and nutrition, 61(18), 3007–3030. [CrossRef]
- Renaud, S., & de Lorgeril, M. (1992). Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet (London, England), 339(8808), 1523–1526. [CrossRef]
- Rotches-Ribalta, M., Andres-Lacueva, C., Estruch, R., Escribano, E., & Urpi-Sarda, M. (2012). Pharmacokinetics of resveratrol metabolic profile in healthy humans after moderate consumption of red wine and grape extract tablets. Pharmacological research, 66(5), 375–382. [CrossRef]
- Sakavitsi, M. E., Breynaert, A., Nikou, T., Lauwers, S., Pieters, L., Hermans, N., &Halabalaki, M. (2022). Availability and Metabolic Fate of Olive Phenolic Alcohols Hydroxytyrosol and Tyrosol in the Human GI Tract Simulated by the In Vitro GIDM-Colon Model. Metabolites, 12(5), 391. [CrossRef]
- Sancho, M., & Mach, N. (2014). Efecto de lospolifenoles del vino sobre la prevención del cáncer [Effects of wine polyphenols on cancer prevention]. Nutricionhospitalaria, 31(2), 535–551. [CrossRef]
- Santangelo, C., Vari, R., Scazzocchio, B., De Sanctis, P., Giovannini, C., D'Archivio, M., & Masella, R. (2018). Anti-inflammatory Activity of Extra Virgin Olive Oil Polyphenols: Which Role in the Prevention and Treatment of Immune-Mediated Inflammatory Diseases?. Endocrine, metabolic & immune disorders drug targets, 18(1), 36–50. [CrossRef]
- Santos-Buelga, C., González-Manzano, S., & González-Paramás, A. M. (2021). Wine, Polyphenols, and Mediterranean Diets. What Else Is There to Say?. Molecules (Basel, Switzerland), 26(18), 5537. [CrossRef]
- Santos-Buelga, C., González-Paramás, A. M., Oludemi, T., Ayuda-Durán, B., & González-Manzano, S. (2019). Plant phenolics as functional food ingredients. Advances in food and nutrition research, 90, 183–257. [CrossRef]
- Scalbert, A., Johnson, I. T., & Saltmarsh, M. (2005). Polyphenols: antioxidants and beyond. The American journal of clinical nutrition, 81(1 Suppl), 215S–217S. [CrossRef]
- Schutte, R., Papageorgiou, M., Najlah, M., Huisman, H. W., Ricci, C., Zhang, J., Milner, N., & Schutte, A. E. (2020). Drink types unmask the health risks associated with alcohol intake - Prospective evidence from the general population. Clinical nutrition (Edinburgh, Scotland), 39(10), 3168–3174. [CrossRef]
- Schwingshackl, L., Morze, J., & Hoffmann, G. (2020). Mediterranean diet and health status: Active ingredients and pharmacological mechanisms. British journal of pharmacology, 177(6), 1241–1257. [CrossRef]
- Sessa, M., Balestrieri, M. L., Ferrari, G., Servillo, L., Castaldo, D., D'Onofrio, N., Donsì, F., & Tsao, R. (2014). Bioavailability of encapsulated resveratrol into nanoemulsion-based delivery systems. Food chemistry, 147, 42–50. [CrossRef]
- Seth, D., Haber, P. S., Syn, W. K., Diehl, A. M., & Day, C. P. (2011). Pathogenesis of alcohol-induced liver disease: classical concepts and recent advances. Journal of gastroenterology and hepatology, 26(7), 1089–1105. [CrossRef]
- Setshedi, M., Wands, J. R., & Monte, S. M. (2010). Acetaldehyde adducts in alcoholic liver disease. Oxidative medicine and cellular longevity, 3(3), 178–185. [CrossRef]
- Sheng, Y. Sheng, Y., Meng, G., Li, G., & Wang, J. (2024). Red wine alleviates atherosclerosis-related inflammatory markers in healthy subjects rather than in high cardiovascular risk subjects: A systematic review and meta-analysis. Medicine, 103(23), e38229. [CrossRef]
- Singla, R. K., Dubey, A. K., Garg, A., Sharma, R. K., Fiorino, M., Ameen, S. M., Haddad, M. A., & Al-Hiary, M. (2019). Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. Journal of AOAC International, 102(5), 1397–1400. [CrossRef]
- Soldevila-Domenech, N., Boronat, A., Mateus, J., Diaz-Pellicer, P., Matilla, I., Pérez-Otero, M., Aldea-Perona, A., & de la Torre, R. (2019). Generation of the Antioxidant Hydroxytyrosol from Tyrosol Present in Beer and Red Wine in a Randomized Clinical Trial. Nutrients, 11(9), 2241. [CrossRef]
- Spaak, J., Tomlinson, G., McGowan, C. L., Soleas, G. J., Morris, B. L., Picton, P., Notarius, C. F., & Floras, J. S. (2010). Dose-related effects of red wine and alcohol on heart rate variability. American journal of physiology. Heart and circulatory physiology, 298(6), H2226–H2231. [CrossRef]
- Stiller, A., Garrison, K., Gurdyumov, K., Kenner, J., Yasmin, F., Yates, P., & Song, B. H. (2021). From Fighting Critters to Saving Lives: Polyphenols in Plant Defense and Human Health. International journal of molecular sciences, 22(16), 8995. [CrossRef]
- Stranieri, C., Guzzo, F., Gambini, S., Cominacini, L., & Fratta Pasini, A. M. (2022). Intracellular Polyphenol Wine Metabolites Oppose Oxidative Stress and Upregulate Nrf2/ARE Pathway. Antioxidants (Basel, Switzerland), 11(10), 2055. [CrossRef]
- Talavéra, S., Felgines, C., Texier, O., Besson, C., Gil-Izquierdo, A., Lamaison, J. L., &Rémésy, C. (2005). Anthocyanin metabolism in rats and their distribution to digestive area, kidney, and brain. Journal of agricultural and food chemistry, 53(10), 3902–3908. [CrossRef]
- Timmers, P. R. H. J., Wilson, J. F., Joshi, P. K., &Deelen, J. (2020). Multivariate genomic scan implicates novel loci and haem metabolism in human ageing. Nature communications, 11(1), 3570. [CrossRef]
- Tuma, D. J., & Casey, C. A. (2003). Dangerous byproducts of alcohol breakdown--focus on adducts. Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism, 27(4), 285–290.
- United States Department of Agriculture (USDA), National Agricultural Library (NAL) Thesaurus (Created 2006-01-19, last modified 2015-12-22). Food matrix. Available online: https://agclass.nal.usda.gov/vocabularies/nalt/concept?uri=https://lod.nal.usda.gov/nalt/17238.
- Urpi-Sarda, M., Casas, R., Chiva-Blanch, G., Romero-Mamani, E. S., Valderas-Martínez, P., Arranz, S., Andres-Lacueva, C., Llorach, R., Medina-Remón, A., Lamuela-Raventos, R. M., & Estruch, R. (2012). Virgin olive oil and nuts as key foods of the Mediterranean diet effects on inflammatory biomakers related to atherosclerosis. Pharmacological research, 65(6), 577–583. [CrossRef]
- Urpi-Sarda, M., Zamora-Ros, R., Lamuela-Raventos, R., Cherubini, A., Jauregui, O., de la Torre, R., Covas, M. I., Estruch, R., Jaeger, W., & Andres-Lacueva, C. (2007). HPLC-tandem mass spectrometric method to characterize resveratrol metabolism in humans. Clinical chemistry, 53(2), 292–299. [CrossRef]
- van Bussel, B. C. T., Henry, R. M. A., Schalkwijk, C. G., Dekker, J. M., Nijpels, G., Feskens, E. J. M., & Stehouwer, C. D. A. (2018). Alcohol and red wine consumption, but not fruit, vegetables, fish or dairy products, are associated with less endothelial dysfunction and less low-grade inflammation: the Hoorn Study. European journal of nutrition, 57(4), 1409–1419. [CrossRef]
- van Mierlo, L. A., Zock, P. L., van der Knaap, H. C., & Draijer, R. (2010). Grape polyphenols do not affect vascular function in healthy men. The Journal of nutrition, 140(10), 1769–1773. [CrossRef]
- Varoni, E. M., Lodi, G., & Iriti, M. (2015). Ethanol versus Phytochemicals in Wine: Oral Cancer Risk in a Light Drinking Perspective. International journal of molecular sciences, 16(8), 17029–17047. [CrossRef]
- Vauzour, D., Vafeiadou, K., Rodriguez-Mateos, A., Rendeiro, C., & Spencer, J. P. (2008). The neuroprotective potential of flavonoids: a multiplicity of effects. Genes & nutrition, 3(3-4), 115–126. [CrossRef]
- Vioque, J., Barber, X., Bolumar, F., Porta, M., Santibáñez, M., de la Hera, M. G., Moreno-Osset, E., & PANESOES Study Group (2008). Esophageal cancer risk by type of alcohol drinking and smoking: a case-control study in Spain. BMC cancer, 8, 221. [CrossRef]
- Visioli, F., Panaite, S. A., & Tomé-Carneiro, J. (2020). Wine's Phenolic Compounds and Health: A Pythagorean View. Molecules (Basel, Switzerland), 25(18), 4105. [CrossRef]
- Wallerath, T., Li, H., Gödtel-Ambrust, U., Schwarz, P. M., &Förstermann, U. (2005). A blend of polyphenolic compounds explains the stimulatory effect of red wine on human endothelial NO synthase. Nitric oxide : biology and chemistry, 12(2), 97–104. [CrossRef]
- Wannamethee, S. G., Shaper, A. G., & Whincup, P. H. (2005). Alcohol and adiposity: effects of quantity and type of drink and time relation with meals. International journal of obesity (2005), 29(12), 1436–1444. [CrossRef]
- Warleta, F., Quesada, C. S., Campos, M., Allouche, Y., Beltrán, G., &Gaforio, J. J. (2011). Hydroxytyrosol protects against oxidative DNA damage in human breast cells. Nutrients, 3(10), 839–857. [CrossRef]
- Weaver, S. R., Rendeiro, C., McGettrick, H. M., Philp, A., & Lucas, S. J. E. (2021). Fine wine or sour grapes? A systematic review and meta-analysis of the impact of red wine polyphenols on vascular health. European journal of nutrition, 60(1), 1–28. [CrossRef]
- Welch, A., MacGregor, A., Jennings, A., Fairweather-Tait, S., Spector, T., & Cassidy, A. (2012). Habitual flavonoid intakes are positively associated with bone mineral density in women. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research, 27(9), 1872–1878. [CrossRef]
- Wilkinson, P. K., Sedman, A. J., Sakmar, E., Kay, D. R., & Wagner, J. G. (1977). Pharmacokinetics of ethanol after oral administration in the fasting state. Journal of pharmacokinetics and biopharmaceutics, 5(3), 207–224. [CrossRef]
- Willett, W. C., Sacks, F., Trichopoulou, A., Drescher, G., Ferro-Luzzi, A., Helsing, E., &Trichopoulos, D. (1995). Mediterranean diet pyramid: a cultural model for healthy eating. The American journal of clinical nutrition, 61(6 Suppl), 1402S–1406S. [CrossRef]
- Williamson, G. (2017). The role of polyphenols in modern nutrition. Nutrition bulletin, 42(3), 226–235. [CrossRef]
- Wojtowicz J., S. (2023). Long-Term Health Outcomes of Regular, Moderate Red Wine Consumption. Cureus, 15(10), e46786. [CrossRef]
- Wong, R. H., Thaung Zaw, J. J., Xian, C. J., & Howe, P. R. (2020). Regular Supplementation With Resveratrol Improves Bone Mineral Density in Postmenopausal Women: A Randomized, Placebo-Controlled Trial. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research, 35(11), 2121–2131. [CrossRef]
- World Health Organization, WHO Consultation on Selected Medicinal Plants, WHO Consultation on Selected Medicinal Plants (2nd: 1999; Ravello-Salerno, Italy), WHO Consultation on Selected Medicinal Plants (3rd: 2001; Ottawa, Ont.) & WHO Consultation on Selected Medicinal Plants (4th: 2005; Salerno-Paestum, Italy). (2006). WHO monographs on selected medicinal plants. World Health Organization. Available online: https://iris.who.int/handle/10665/42052.
- Wu, D., & Cederbaum, A. I. (2003). Alcohol, oxidative stress, and free radical damage. Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism, 27(4), 277–284.
- Xie, C., & Feng, Y. (2022). Alcohol consumption and risk of Alzheimer's disease: A dose-response meta-analysis. Geriatrics & gerontology international, 22(4), 278–285. [CrossRef]
- Yan, Y., Yang, J. Y., Mou, Y. H., Wang, L. H., Zhou, Y. N., & Wu, C. F. (2012). Differences in the activities of resveratrol and ascorbic acid in protection of ethanol-induced oxidative DNA damage in human peripheral lymphocytes. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 50(2), 168–174. [CrossRef]
- Ye, J., Chen, X., & Bao, L. (2019). Effects of wine on blood pressure, glucose parameters, and lipid profile in type 2 diabetes mellitus: A meta-analysis of randomized interventional trials (PRISMA Compliant). Medicine, 98(23), e15771. [CrossRef]
- Zakhari, S. (2006). Overview: how is alcohol metabolized by the body?. Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism, 29(4), 245–254.
- Zeka, A., Gore, R., & Kriebel, D. (2003). Effects of alcohol and tobacco on aerodigestive cancer risks: a meta-regression analysis. Cancer causes & control : CCC, 14(9), 897–906. [CrossRef]
- Zhao, J., Zhou, G., Yang, J., Pan, J., Sha, B., Luo, M., Yang, W., Liu, J., & Zeng, L. (2023). Effects of resveratrol in an animal model of osteoporosis: a meta-analysis of preclinical evidence. Frontiers in nutrition, 10, 1234756. [CrossRef]
- Zhou, M., Wang, S., Zhao, A., Wang, K., Fan, Z., Yang, H., Liao, W., Bao, S., Zhao, L., Zhang, Y., Yang, Y., Qiu, Y., Xie, G., Li, H., & Jia, W. (2012). Transcriptomic and metabonomic profiling reveal synergistic effects of quercetin and resveratrol supplementation in high fat diet fed mice. Journal of proteome research, 11(10), 4961–4971. [CrossRef]
- Zupančič, Š., Lavrič, Z., & Kristl, J. (2015). Stability and solubility of trans-resveratrol are strongly influenced by pH and temperature. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur PharmazeutischeVerfahrenstechnike.V, 93, 196–204. [CrossRef]
| Reference | N° of studies | Treatment | Results | Outcomes |
|---|---|---|---|---|
| Sheng et al., 2024 | 12 | Wine (242 – 94.308 mg/L/die) |
↓ CICAM-1 ↓ VCAM-1 ↓ TNF-α ↓ CCR2 ↑ αMβ2 (Mac-1) |
Improvement of the markers associated with atherosclerotic inflammation in healthy patients, but not in those with CVD |
| Xie and Feng, 2022 | 4 | Red wine vs white wine and/or other alcoholic beverages | / | Red wine decreased the risk of Alzheimer’s Disease |
| Weaver et al., 2021 | 4 | Grape extract (700 – 1 400 mg/die) |
↓ blood pressure ↑ FMD |
Improvement of vascular health, particularly in at risk human populations |
| Hong et al., 2020 | 11 | Wine (0 – 84.9 g/die) |
/ | There isn’t a statical significant correlation between wine assumption and prostate cancer |
| Ye et al., 2019 | 9 | Wine (118 – 300 mL/die) |
↓ total cholesterol ↓ diastolic blood pressure |
Improvement of some cardiovascular parameters in type 2 diabetes mellitus patients |
| García-Conesa et al., 2018 | 99 | Red wine / grape extract (100 – 2 000 mg/die) Red wine (250 – 400 mL/die) |
↓ total cholesterol ↓ blood pressure ↑ HDL-C ↑ FMD ↓ TAGs |
Improvement of cardiovascular health Support in the treatment of metabolic disorders |
| Sancho and Mach, 2014 | 6 | Red wine (moderate consumption, max one glass/die) |
/ | Decrease in the risk of developing Non-Hodgkin Lymphoma, epithelial ovarian cancer, prostate cancer, lung cancer, breast cancer, esophageal adenocarcinoma (Barret’s esophagus) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





