Submitted:
16 July 2024
Posted:
16 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
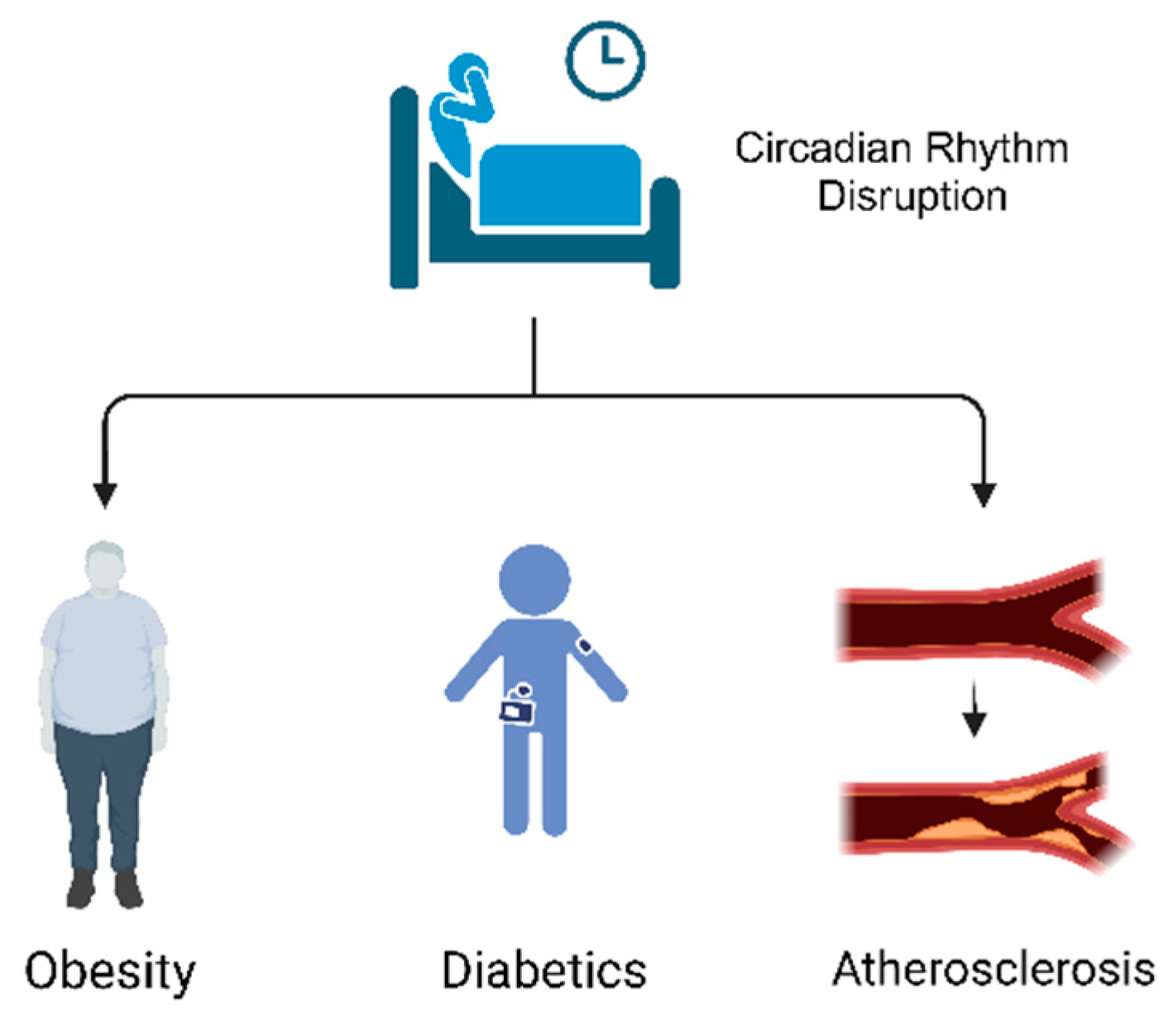
2. Relationship between Circadian Rhythm Disruption and CMDs
2.1. Obesity
2.2. Diabetics
2.3. Atherosclerosis
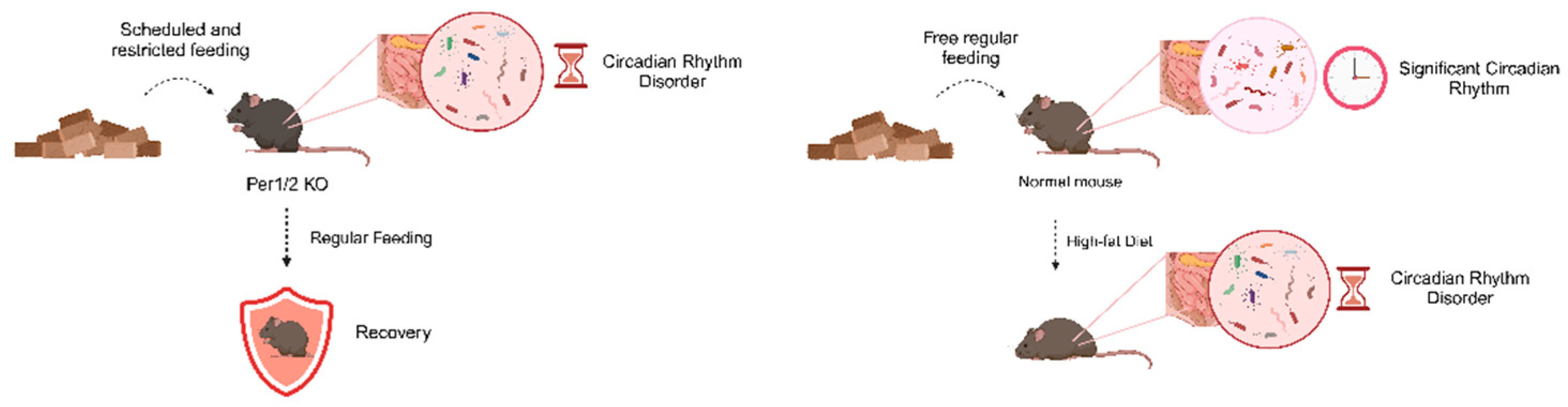
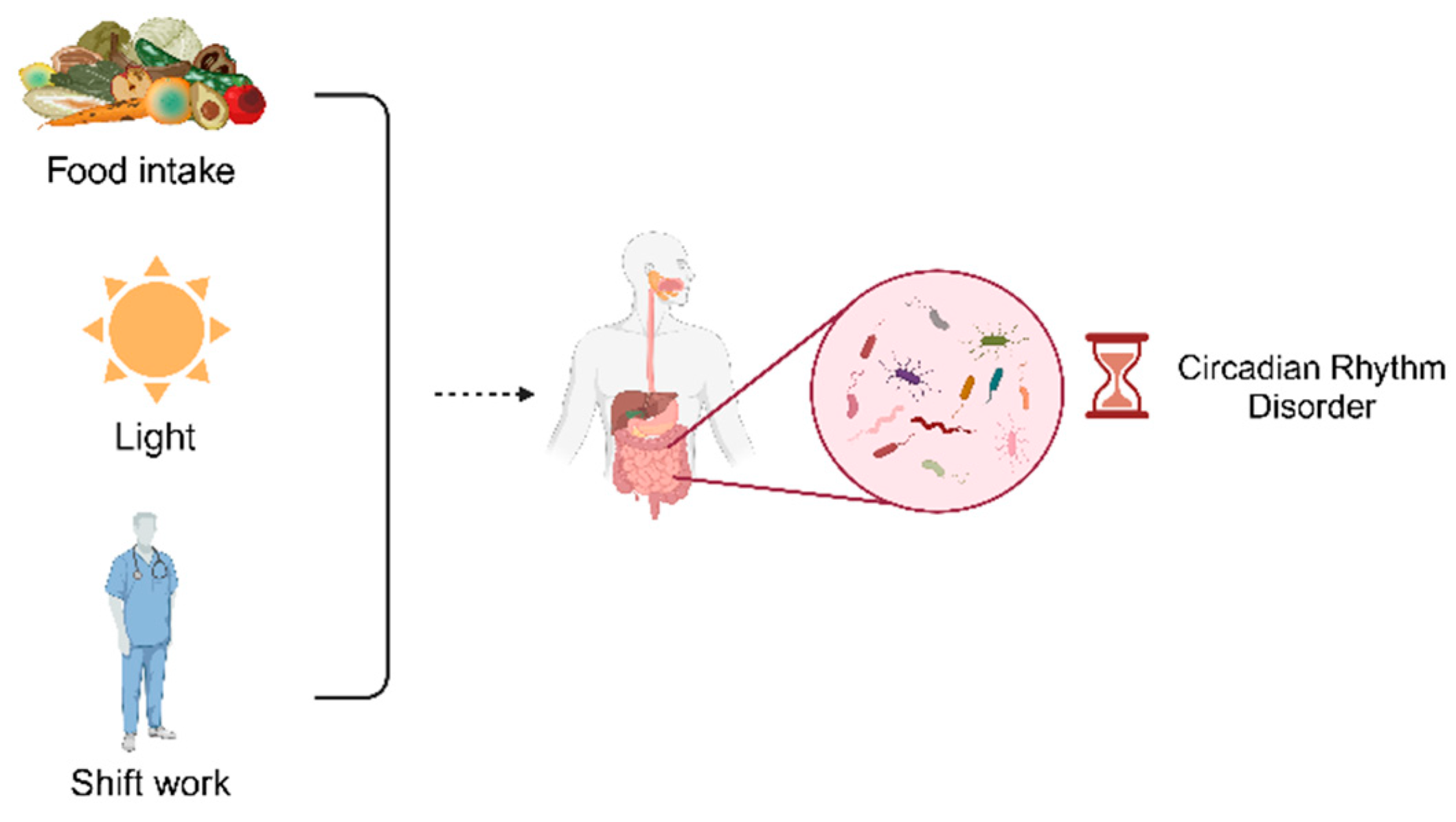
3. Circadian Rhythm Disruptions Lead to Gut Microbiota Dysbiosis
3.1. Food Intake and Circadian Rhythm
3.2. Light and Circadian Rhythm
3.3. Shift Work and Circadian Rhythm
4. Relationship between Gut Microbiota Dysbiosis and CMDs
4.1. Gut Microbiota Dysbiosis Leads to CMDs
4.2. Pathogenesis of CMDs Caused by Gut Microbiota Dysbiosis
4.2.1. Metabolism-Independent Pathway
4.2.2. Metabolism-Dependent Pathway
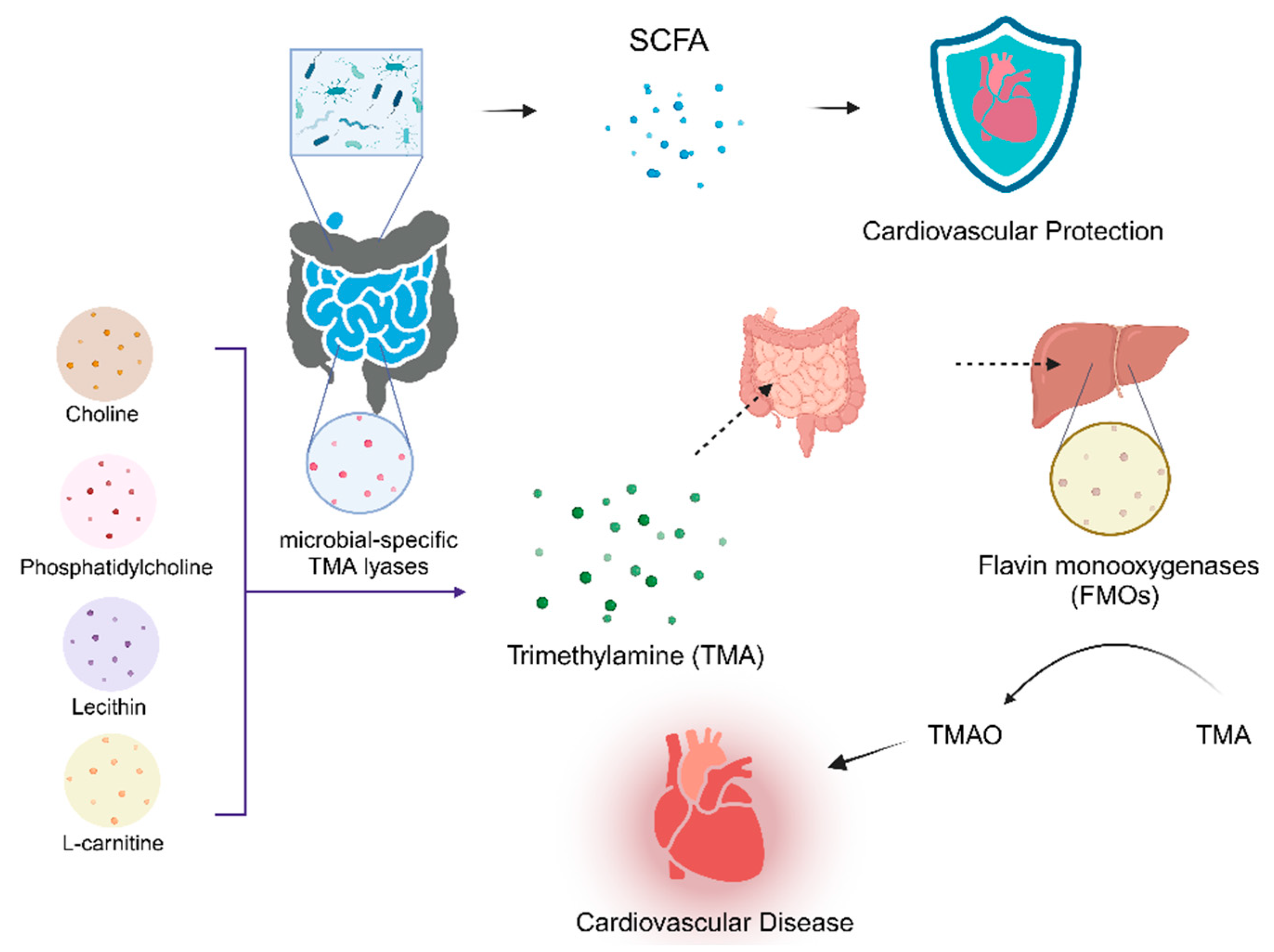
5. Crosstalk between Circadian Rhythms and Gut Microbiota:the Potential Role in CMDs
6. CMDs Treatment and Prevention
6.1. Treatment Associated with the Gut Microbiota
6.2. Treatment Associated with Dysfunction of Both Circadian Rhythms and the Gut Microbiota
7. Discussion
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, F.; Moellering, D.R.; Garvey, W.T. The Progression of Cardiometabolic Disease: Validation of a New Cardiometabolic Disease Staging System Applicable to Obesity. Obesity (Silver Spring) 2014, 22, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Després, J.-P.; et al.; Writing Group Members Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016, 133, e38–360. [Google Scholar] [CrossRef] [PubMed]
- Koronowski, K.B.; Kinouchi, K.; Welz, P.-S.; Smith, J.G.; Zinna, V.M.; Shi, J.; Samad, M.; Chen, S.; Magnan, C.N.; Kinchen, J.M.; et al. Defining the Independence of the Liver Circadian Clock. Cell 2019, 177, 1448–1462. [Google Scholar] [CrossRef] [PubMed]
- Patke, A.; Young, M.W.; Axelrod, S. Molecular Mechanisms and Physiological Importance of Circadian Rhythms. Nat Rev Mol Cell Biol 2020, 21, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Feng, M.; Zhang, S.; Dong, Z.; Wang, Y.; Zhang, W.; Liu, C. Angptl8 Mediates Food-Driven Resetting of Hepatic Circadian Clock in Mice. Nat Commun 2019, 10, 3518. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Lamaze, A.; De, J.; Mena, W.; Chélot, E.; Martin, B.; Hardin, P.; Kadener, S.; Emery, P.; Rouyer, F. Reconfiguration of a Multi-Oscillator Network by Light in the Drosophila Circadian Clock. Curr Biol 2018, 28, 2007–2017. [Google Scholar] [CrossRef] [PubMed]
- Inder, M.L.; Crowe, M.T.; Porter, R. Effect of Transmeridian Travel and Jetlag on Mood Disorders: Evidence and Implications. Aust N Z J Psychiatry 2016, 50, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Reid, K.J.; Abbott, S.M. Jet Lag and Shift Work Disorder. Sleep Medicine Clinics 2015, 10, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Schilperoort, M.; van den Berg, R.; Dollé, M.E.T.; van Oostrom, C.T.M.; Wagner, K.; Tambyrajah, L.L.; Wackers, P.; Deboer, T.; Hulsegge, G.; Proper, K.I.; et al. Time-Restricted Feeding Improves Adaptation to Chronically Alternating Light-Dark Cycles. Sci Rep 2019, 9, 7874. [Google Scholar] [CrossRef]
- Roenneberg, T.; Pilz, L.K.; Zerbini, G.; Winnebeck, E.C. Chronotype and Social Jetlag: A (Self-) Critical Review. Biology (Basel) 2019, 8, 54. [Google Scholar] [CrossRef]
- Asher, G.; Sassone-Corsi, P. Time for Food: The Intimate Interplay between Nutrition, Metabolism, and the Circadian Clock. Cell 2015, 161, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Burgess, H.J.; Molina, T.A. Home Lighting before Usual Bedtime Impacts Circadian Timing: A Field Study. Photochem Photobiol 2014, 90, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Tahara, Y.; Yamazaki, M.; Sukigara, H.; Motohashi, H.; Sasaki, H.; Miyakawa, H.; Haraguchi, A.; Ikeda, Y.; Fukuda, S.; Shibata, S. Gut Microbiota-Derived Short Chain Fatty Acids Induce Circadian Clock Entrainment in Mouse Peripheral Tissue. Sci Rep 2018, 8, 1395. [Google Scholar] [CrossRef] [PubMed]
- Leone, V.; Gibbons, S.M.; Martinez, K.; Hutchison, A.L.; Huang, E.Y.; Cham, C.M.; Pierre, J.F.; Heneghan, A.F.; Nadimpalli, A.; Hubert, N.; et al. Effects of Diurnal Variation of Gut Microbes and High-Fat Feeding on Host Circadian Clock Function and Metabolism. Cell Host Microbe 2015, 17, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Bushman, F.D.; FitzGerald, G.A. Rhythmicity of the Intestinal Microbiota Is Regulated by Gender and the Host Circadian Clock. Proc Natl Acad Sci U S A 2015, 112, 10479–10484. [Google Scholar] [CrossRef] [PubMed]
- Voigt, R.M.; Summa, K.C.; Forsyth, C.B.; Green, S.J.; Engen, P.; Naqib, A.; Vitaterna, M.H.; Turek, F.W.; Keshavarzian, A. The Circadian Clock Mutation Promotes Intestinal Dysbiosis. Alcohol Clin Exp Res 2016, 40, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Patke, A.; Murphy, P.J.; Onat, O.E.; Krieger, A.C.; Özçelik, T.; Campbell, S.S.; Young, M.W. Mutation of the Human Circadian Clock Gene CRY1 in Familial Delayed Sleep Phase Disorder. Cell 2017, 169, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Daut, R.A.; Hartsock, M.J.; Tomczik, A.C.; Watkins, L.R.; Spencer, R.L.; Maier, S.F.; Fonken, L.K. Circadian Misalignment Has Differential Effects on Affective Behavior Following Exposure to Controllable or Uncontrollable Stress. Behav Brain Res 2019, 359, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Hor, C.N.; Yeung, J.; Jan, M.; Emmenegger, Y.; Hubbard, J.; Xenarios, I.; Naef, F.; Franken, P. Sleep-Wake-Driven and Circadian Contributions to Daily Rhythms in Gene Expression and Chromatin Accessibility in the Murine Cortex. Proc Natl Acad Sci U S A 2019, 116, 25773–25783. [Google Scholar] [CrossRef]
- Kuehn, B.M. Resetting the Circadian Clock Might Boost Metabolic Health. JAMA 2017, 317, 1303–1305. [Google Scholar] [CrossRef]
- Allen, N.C.; Philip, N.H.; Hui, L.; Zhou, X.; Franklin, R.A.; Kong, Y.; Medzhitov, R. Desynchronization of the Molecular Clock Contributes to the Heterogeneity of the Inflammatory Response. Sci Signal 2019, 12, eaau1851. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Deschasaux, M.; Bouter, K.E.; Prodan, A.; Levin, E.; Groen, A.K.; Herrema, H.; Tremaroli, V.; Bakker, G.J.; Attaye, I.; Pinto-Sietsma, S.-J.; et al. Depicting the Composition of Gut Microbiota in a Population with Varied Ethnic Origins but Shared Geography. Nat Med 2018, 24, 1526–1531. [Google Scholar] [CrossRef] [PubMed]
- Maier, L.; Pruteanu, M.; Kuhn, M.; Zeller, G.; Telzerow, A.; Anderson, E.E.; Brochado, A.R.; Fernandez, K.C.; Dose, H.; Mori, H.; et al. Extensive Impact of Non-Antibiotic Drugs on Human Gut Bacteria. Nature 2018, 555, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Santos-Marcos, J.A.; Haro, C.; Vega-Rojas, A.; Alcala-Diaz, J.F.; Molina-Abril, H.; Leon-Acuña, A.; Lopez-Moreno, J.; Landa, B.B.; Tena-Sempere, M.; Perez-Martinez, P.; et al. Sex Differences in the Gut Microbiota as Potential Determinants of Gender Predisposition to Disease. Mol Nutr Food Res 2019, 63, e1800870. [Google Scholar] [CrossRef]
- Cotillard, A.; Kennedy, S.P.; Kong, L.C.; Prifti, E.; Pons, N.; Le Chatelier, E.; Almeida, M.; Quinquis, B.; Levenez, F.; Galleron, N.; et al. Dietary Intervention Impact on Gut Microbial Gene Richness. Nature 2013, 500, 585–588. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut Bacteria Selectively Promoted by Dietary Fibers Alleviate Type 2 Diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Quercia, S.; Turroni, S.; Fiori, J.; Soverini, M.; Rampelli, S.; Biagi, E.; Castagnetti, A.; Consolandi, C.; Severgnini, M.; Pianesi, M.; et al. Gut Microbiome Response to Short-Term Dietary Interventions in Reactive Hypoglycemia Subjects. Diabetes Metab Res Rev 2017, 33. [Google Scholar] [CrossRef]
- Erickson, M.L.; Malin, S.K.; Wang, Z.; Brown, J.M.; Hazen, S.L.; Kirwan, J.P. Effects of Lifestyle Intervention on Plasma Trimethylamine N-Oxide in Obese Adults. Nutrients 2019, 11, 179. [Google Scholar] [CrossRef]
- Moreira, G.C.; Cipullo, J.P.; Ciorlia, L.A.S.; Cesarino, C.B.; Vilela-Martin, J.F. Prevalence of Metabolic Syndrome: Association with Risk Factors and Cardiovascular Complications in an Urban Population. PLoS One 2014, 9, e105056. [Google Scholar] [CrossRef]
- Tuteja, S.; Ferguson, J.F. Gut Microbiome and Response to Cardiovascular Drugs. Circ Genom Precis Med 2019, 12, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Schuster, H. Improving Lipid Management--to Titrate, Combine or Switch. Int J Clin Pract 2004, 58, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Li, H.; Zhou, H.; Zhang, X.; Zhang, A.; Xie, Y.; Li, Y.; Lv, S.; Zhang, J. Role and Effective Therapeutic Target of Gut Microbiota in Heart Failure. Cardiovasc Ther 2019, 2019, 5164298. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Sun, B.; Yu, D.; Zhu, C. Gut Microbiota: An Important Player in Type 2 Diabetes Mellitus. Front Cell Infect Microbiol 2022, 12, 834485. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Jia, H.; Feng, Q.; Wang, D.; Liang, D.; Wu, X.; Li, J.; Tang, L.; Li, Y.; et al. The Oral and Gut Microbiomes Are Perturbed in Rheumatoid Arthritis and Partly Normalized after Treatment. Nat Med 2015, 21, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Z.; Wang, Y.; Li, Y.; Ye, C.; Ruhn, K.A.; Behrendt, C.L.; Olson, E.N.; Hooper, L.V. The Intestinal Microbiota Programs Diurnal Rhythms in Host Metabolism through Histone Deacetylase 3. Science 2019, 365, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Adamovich, Y.; Rousso-Noori, L.; Zwighaft, Z.; Neufeld-Cohen, A.; Golik, M.; Kraut-Cohen, J.; Wang, M.; Han, X.; Asher, G. Circadian Clocks and Feeding Time Regulate the Oscillations and Levels of Hepatic Triglycerides. Cell Metab 2014, 19, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Wang, J.; Klein, P.S.; Lazar, M.A. Nuclear Receptor Rev-Erbalpha Is a Critical Lithium-Sensitive Component of the Circadian Clock. Science 2006, 311, 1002–1005. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Chen, J.; Wang, J.; Yao, J.; Huang, Y.; Zhang, G.; Bao, Z. Circadian Clock Genes in the Metabolism of Non-Alcoholic Fatty Liver Disease. Front Physiol 2019, 10, 423. [Google Scholar] [CrossRef]
- Dang, F.; Sun, X.; Ma, X.; Wu, R.; Zhang, D.; Chen, Y.; Xu, Q.; Wu, Y.; Liu, Y. Insulin Post-Transcriptionally Modulates Bmal1 Protein to Affect the Hepatic Circadian Clock. Nat Commun 2016, 7, 12696. [Google Scholar] [CrossRef]
- Lau, P.; Fitzsimmons, R.L.; Raichur, S.; Wang, S.-C.M.; Lechtken, A.; Muscat, G.E.O. The Orphan Nuclear Receptor, RORalpha, Regulates Gene Expression That Controls Lipid Metabolism: Staggerer (SG/SG) Mice Are Resistant to Diet-Induced Obesity. J Biol Chem 2008, 283, 18411–18421. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Huang, X.; Song, B.-L.; Yang, H. The Biogenesis of Lipid Droplets: Lipids Take Center Stage. Prog Lipid Res 2019, 75, 100989. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, J.E.; Livelo, C.; Trujillo, A.S.; Chandran, S.; Woodworth, B.; Andrade, L.; Le, H.D.; Manor, U.; Panda, S.; Melkani, G.C. Time-Restricted Feeding Restores Muscle Function in Drosophila Models of Obesity and Circadian-Rhythm Disruption. Nat Commun 2019, 10, 2700. [Google Scholar] [CrossRef] [PubMed]
- Schäbler, S.; Amatobi, K.M.; Horn, M.; Rieger, D.; Helfrich-Förster, C.; Mueller, M.J.; Wegener, C.; Fekete, A. Loss of Function in the Drosophila Clock Gene Period Results in Altered Intermediary Lipid Metabolism and Increased Susceptibility to Starvation. Cell Mol Life Sci 2020, 77, 4939–4956. [Google Scholar] [CrossRef] [PubMed]
- Baron, K.G.; Reid, K.J.; Kim, T.; Van Horn, L.; Attarian, H.; Wolfe, L.; Siddique, J.; Santostasi, G.; Zee, P.C. Circadian Timing and Alignment in Healthy Adults: Associations with BMI, Body Fat, Caloric Intake and Physical Activity. Int J Obes (Lond) 2017, 41, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Kovac, J.; Kolbe, I.; Ehrhardt, L.; Leliavski, A.; Husse, J.; Salinas, G.; Lingner, T.; Tsang, A.H.; Barclay, J.L.; Oster, H. Hepatic Gene Therapy Rescues High-Fat Diet Responses in Circadian Clock Mutant Mice. Mol Metab 2017, 6, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Marcheva, B.; Ramsey, K.M.; Buhr, E.D.; Kobayashi, Y.; Su, H.; Ko, C.H.; Ivanova, G.; Omura, C.; Mo, S.; Vitaterna, M.H.; et al. Disruption of the Clock Components CLOCK and BMAL1 Leads to Hypoinsulinaemia and Diabetes. Nature 2010, 466, 627–631. [Google Scholar] [CrossRef]
- Perelis, M.; Marcheva, B.; Ramsey, K.M.; Schipma, M.J.; Hutchison, A.L.; Taguchi, A.; Peek, C.B.; Hong, H.; Huang, W.; Omura, C.; et al. Pancreatic β Cell Enhancers Regulate Rhythmic Transcription of Genes Controlling Insulin Secretion. Science 2015, 350, aac4250. [Google Scholar] [CrossRef]
- Qian, J.; Block, G.D.; Colwell, C.S.; Matveyenko, A.V. Consequences of Exposure to Light at Night on the Pancreatic Islet Circadian Clock and Function in Rats. Diabetes 2013, 62, 3469–3478. [Google Scholar] [CrossRef]
- Gale, J.E.; Cox, H.I.; Qian, J.; Block, G.D.; Colwell, C.S.; Matveyenko, A.V. Disruption of Circadian Rhythms Accelerates Development of Diabetes through Pancreatic Beta-Cell Loss and Dysfunction. J Biol Rhythms 2011, 26, 423–433. [Google Scholar] [CrossRef]
- Tahira, K.; Ueno, T.; Fukuda, N.; Aoyama, T.; Tsunemi, A.; Matsumoto, S.; Nagura, C.; Matsumoto, T.; Soma, M.; Shimba, S.; et al. Obesity Alters the Expression Profile of Clock Genes in Peripheral Blood Mononuclear Cells. Arch Med Sci 2011, 7, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Touitou, Y.; Reinberg, A.; Touitou, D. Association between Light at Night, Melatonin Secretion, Sleep Deprivation, and the Internal Clock: Health Impacts and Mechanisms of Circadian Disruption. Life Sci 2017, 173, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Panda, S. Circadian Physiology of Metabolism. Science 2016, 354, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Li, Y.; Han, H.; Chen, S.; Gao, J.; Liu, G.; Wu, X.; Deng, J.; Yu, Q.; Huang, X.; et al. Melatonin Reprogramming of Gut Microbiota Improves Lipid Dysmetabolism in High-Fat Diet-Fed Mice. J Pineal Res 2018, 65, e12524. [Google Scholar] [CrossRef] [PubMed]
- Cipolla-Neto, J.; Amaral, F.G. do Melatonin as a Hormone: New Physiological and Clinical Insights. Endocr Rev 2018, 39, 990–1028. [Google Scholar] [CrossRef] [PubMed]
- Bazwinsky-Wutschke, I.; Wolgast, S.; Mühlbauer, E.; Albrecht, E.; Peschke, E. Phosphorylation of Cyclic AMP-Response Element-Binding Protein (CREB) Is Influenced by Melatonin Treatment in Pancreatic Rat Insulinoma β-Cells (INS-1). J Pineal Res 2012, 53, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.A.; Kinote, A.; Ignacio-Souza, L.M.; de Araújo, T.M.; Razolli, D.S.; Doneda, D.L.; Paschoal, L.B.; Lellis-Santos, C.; Bertolini, G.L.; Velloso, L.A.; et al. Melatonin Acts through MT1/MT2 Receptors to Activate Hypothalamic Akt and Suppress Hepatic Gluconeogenesis in Rats. Am J Physiol Endocrinol Metab 2013, 305, E230–E242. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Li, Y.; Han, H.; Ma, J.; Liu, G.; Wu, X.; Huang, X.; Fang, R.; Baba, K.; Bin, P.; et al. Administration of Exogenous Melatonin Improves the Diurnal Rhythms of the Gut Microbiota in Mice Fed a High-Fat Diet. mSystems 2020, 5, e00002-20. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Lugo, M.J.; Cano, P.; Jiménez-Ortega, V.; Fernández-Mateos, M.P.; Scacchi, P.A.; Cardinali, D.P.; Esquifino, A.I. Melatonin Effect on Plasma Adiponectin, Leptin, Insulin, Glucose, Triglycerides and Cholesterol in Normal and High Fat-Fed Rats. Journal of pineal research 2010, 49. [Google Scholar] [CrossRef]
- Downs, J.L.; Urbanski, H.F. Aging-Related Sex-Dependent Loss of the Circulating Leptin 24-h Rhythm in the Rhesus Monkey. J Endocrinol 2006, 190, 117–127. [Google Scholar] [CrossRef]
- Kettner, N.M.; Mayo, S.A.; Hua, J.; Lee, C.; Moore, D.D.; Fu, L. Circadian Dysfunction Induces Leptin Resistance in Mice. Cell Metab 2015, 22, 448–459. [Google Scholar] [CrossRef]
- Hackl, M.T.; Fürnsinn, C.; Schuh, C.M.; Krssak, M.; Carli, F.; Guerra, S.; Freudenthaler, A.; Baumgartner-Parzer, S.; Helbich, T.H.; Luger, A.; et al. Brain Leptin Reduces Liver Lipids by Increasing Hepatic Triglyceride Secretion and Lowering Lipogenesis. Nat Commun 2019, 10, 2717. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhu, Y.; Schultz, R.D.; Li, N.; He, Z.; Zhang, Z.; Caron, A.; Zhu, Q.; Sun, K.; Xiong, W.; et al. Partial Leptin Reduction as an Insulin Sensitization and Weight Loss Strategy. Cell Metab 2019, 30, 706–719. [Google Scholar] [CrossRef] [PubMed]
- Ravussin, Y.; Edwin, E.; Gallop, M.; Xu, L.; Bartolomé, A.; Kraakman, M.J.; LeDuc, C.A.; Ferrante, A.W. Evidence for a Non-Leptin System That Defends against Weight Gain in Overfeeding. Cell Metab 2018, 28, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Caratti, G.; Iqbal, M.; Hunter, L.; Kim, D.; Wang, P.; Vonslow, R.M.; Begley, N.; Tetley, A.J.; Woodburn, J.L.; Pariollaud, M.; et al. REVERBa Couples the Circadian Clock to Hepatic Glucocorticoid Action. J Clin Invest 2018, 128, 4454–4471. [Google Scholar] [CrossRef]
- Shimba, A.; Cui, G.; Tani-Ichi, S.; Ogawa, M.; Abe, S.; Okazaki, F.; Kitano, S.; Miyachi, H.; Yamada, H.; Hara, T.; et al. Glucocorticoids Drive Diurnal Oscillations in T Cell Distribution and Responses by Inducing Interleukin-7 Receptor and CXCR4. Immunity 2018, 48, 286–298. [Google Scholar] [CrossRef]
- Quagliarini, F.; Mir, A.A.; Balazs, K.; Wierer, M.; Dyar, K.A.; Jouffe, C.; Makris, K.; Hawe, J.; Heinig, M.; Filipp, F.V.; et al. Cistromic Reprogramming of the Diurnal Glucocorticoid Hormone Response by High-Fat Diet. Mol Cell 2019, 76, 531–545. [Google Scholar] [CrossRef]
- Wu, T.; Yang, L.; Jiang, J.; Ni, Y.; Zhu, J.; Zheng, X.; Wang, Q.; Lu, X.; Fu, Z. Chronic Glucocorticoid Treatment Induced Circadian Clock Disorder Leads to Lipid Metabolism and Gut Microbiota Alterations in Rats. Life Sci 2018, 192, 173–182. [Google Scholar] [CrossRef]
- van den Berg, R.; Mook-Kanamori, D.O.; Donga, E.; van Dijk, M.; van Dijk, J.G.; Lammers, G.-J.; van Kralingen, K.W.; Prehn, C.; Adamski, J.; Romijn, J.A.; et al. A Single Night of Sleep Curtailment Increases Plasma Acylcarnitines: Novel Insights in the Relationship between Sleep and Insulin Resistance. Arch Biochem Biophys 2016, 589, 145–151. [Google Scholar] [CrossRef]
- Brum, M.C.B.; Dantas Filho, F.F.; Schnorr, C.C.; Bertoletti, O.A.; Bottega, G.B.; da Costa Rodrigues, T. Night Shift Work, Short Sleep and Obesity. Diabetol Metab Syndr 2020, 12, 13. [Google Scholar] [CrossRef]
- Pugliese, G.; Barrea, L.; Laudisio, D.; Salzano, C.; Aprano, S.; Colao, A.; Savastano, S.; Muscogiuri, G. Sleep Apnea, Obesity, and Disturbed Glucose Homeostasis: Epidemiologic Evidence, Biologic Insights, and Therapeutic Strategies. Curr Obes Rep 2020, 9, 30–38. [Google Scholar] [CrossRef]
- Ling, C.; Bacos, K.; Rönn, T. Epigenetics of Type 2 Diabetes Mellitus and Weight Change - a Tool for Precision Medicine? Nat Rev Endocrinol 2022, 18, 433–448. [Google Scholar] [CrossRef]
- Mullington, J.M.; Cunningham, T.J.; Haack, M.; Yang, H. Causes and Consequences of Chronic Sleep Deficiency and the Role of Orexin. Front Neurol Neurosci 2021, 45, 128–138. [Google Scholar] [CrossRef]
- Kalsbeek, A.; la Fleur, S.; Fliers, E. Circadian Control of Glucose Metabolism. Mol Metab 2014, 3, 372–383. [Google Scholar] [CrossRef]
- Swirski, F.K.; Nahrendorf, M. Leukocyte Behavior in Atherosclerosis, Myocardial Infarction, and Heart Failure. Science 2013, 339, 161–166. [Google Scholar] [CrossRef]
- Englund, A.; Kovanen, L.; Saarikoski, S.T.; Haukka, J.; Reunanen, A.; Aromaa, A.; Lönnqvist, J.; Partonen, T. NPAS2 and PER2 Are Linked to Risk Factors of the Metabolic Syndrome. J Circadian Rhythms 2009, 7, 5. [Google Scholar] [CrossRef]
- Yang, Y.-D.; Zeng, Y.; Li, J.; Zhou, J.-H.; He, Q.-Y.; Zheng, C.-J.; Reichetzeder, C.; Krämer, B.K.; Hocher, B. Association of BMAL1 Clock Gene Polymorphisms with Fasting Glucose in Children. Pediatr Res 2023, 94, 653–659. [Google Scholar] [CrossRef]
- McAlpine, C.S.; Swirski, F.K. Circadian Influence on Metabolism and Inflammation in Atherosclerosis. Circulation Research 2016, 119, 131–141. [Google Scholar] [CrossRef]
- Corella, D.; Asensio, E.M.; Coltell, O.; Sorlí, J.V.; Estruch, R.; Martínez-González, M.Á.; Salas-Salvadó, J.; Castañer, O.; Arós, F.; Lapetra, J.; et al. CLOCK Gene Variation Is Associated with Incidence of Type-2 Diabetes and Cardiovascular Diseases in Type-2 Diabetic Subjects: Dietary Modulation in the PREDIMED Randomized Trial. Cardiovasc Diabetol 2016, 15, 4. [Google Scholar] [CrossRef]
- Dupuis, J.; Langenberg, C.; Prokopenko, I.; Saxena, R.; Soranzo, N.; Jackson, A.U.; Wheeler, E.; Glazer, N.L.; Bouatia-Naji, N.; Gloyn, A.L.; et al. New Genetic Loci Implicated in Fasting Glucose Homeostasis and Their Impact on Type 2 Diabetes Risk. Nat Genet 2010, 42, 105–116. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom Control of Microbiota Diurnal Oscillations Promotes Metabolic Homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, K.; MacSharry, J.; Casey, P.G.; Shanahan, F.; Joyce, S.A.; Gahan, C.G.M. Unconjugated Bile Acids Influence Expression of Circadian Genes: A Potential Mechanism for Microbe-Host Crosstalk. PLoS One 2016, 11, e0167319. [Google Scholar] [CrossRef] [PubMed]
- Zarrinpar, A.; Chaix, A.; Yooseph, S.; Panda, S. Diet and Feeding Pattern Affect the Diurnal Dynamics of the Gut Microbiome. Cell Metab 2014, 20, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xie, C.; Lu, S.; Nichols, R.G.; Tian, Y.; Li, L.; Patel, D.; Ma, Y.; Brocker, C.N.; Yan, T.; et al. Intermittent Fasting Promotes White Adipose Browning and Decreases Obesity by Shaping the Gut Microbiota. Cell Metab 2017, 26, 672–685. [Google Scholar] [CrossRef]
- Deaver, J.A.; Eum, S.Y.; Toborek, M. Circadian Disruption Changes Gut Microbiome Taxa and Functional Gene Composition. Front Microbiol 2018, 9, 737. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, H.-Y.; Hu, X.-M.; Zhang, Y.; Zhang, S.-Y. Current Understanding of Gut Microbiota Alterations and Related Therapeutic Intervention Strategies in Heart Failure. Chin Med J (Engl) 2019, 132, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Levy, M.; Korem, T.; Dohnalová, L.; Shapiro, H.; Jaitin, D.A.; David, E.; Winter, D.R.; Gury-BenAri, M.; Tatirovsky, E.; et al. Microbiota Diurnal Rhythmicity Programs Host Transcriptome Oscillations. Cell 2016, 167, 1495–1510. [Google Scholar] [CrossRef] [PubMed]
- Montagner, A.; Korecka, A.; Polizzi, A.; Lippi, Y.; Blum, Y.; Canlet, C.; Tremblay-Franco, M.; Gautier-Stein, A.; Burcelin, R.; Yen, Y.-C.; et al. Hepatic Circadian Clock Oscillators and Nuclear Receptors Integrate Microbiome-Derived Signals. Sci Rep 2016, 6, 20127. [Google Scholar] [CrossRef] [PubMed]
- Parkar, S.G.; Kalsbeek, A.; Cheeseman, J.F. Potential Role for the Gut Microbiota in Modulating Host Circadian Rhythms and Metabolic Health. Microorganisms 2019, 7, 41. [Google Scholar] [CrossRef]
- Van Cauter, E.; Polonsky, K.S.; Scheen, A.J. Roles of Circadian Rhythmicity and Sleep in Human Glucose Regulation. Endocr Rev 1997, 18, 716–738. [Google Scholar] [CrossRef]
- Chu, H.; Khosravi, A.; Kusumawardhani, I.P.; Kwon, A.H.K.; Vasconcelos, A.C.; Cunha, L.D.; Mayer, A.E.; Shen, Y.; Wu, W.-L.; Kambal, A.; et al. Gene-Microbiota Interactions Contribute to the Pathogenesis of Inflammatory Bowel Disease. Science 2016, 352, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; Pollard, E.L. Microbiota Transfer Therapy Alters Gut Ecosystem and Improves Gastrointestinal and Autism Symptoms: An Open-Label Study. Microbiome 2017, 5. [Google Scholar] [CrossRef]
- Tremaroli, V.; Bäckhed, F. Functional Interactions between the Gut Microbiota and Host Metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Baker, S.S.; Gill, C.; Liu, W.; Alkhouri, R.; Baker, R.D.; Gill, S.R. Characterization of Gut Microbiomes in Nonalcoholic Steatohepatitis (NASH) Patients: A Connection between Endogenous Alcohol and NASH. Hepatology 2013, 57, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Caro, F.; Robins, W.; Mekalanos, J.J. Antagonism toward the Intestinal Microbiota and Its Effect on Vibrio Cholerae Virulence. Science 2018, 359, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Liao, S.X.; He, Y.; Wang, S.; Xia, G.H.; Liu, F.T.; Zhu, J.J.; You, C.; Chen, Q.; Zhou, L.; Pan, S.Y. Dysbiosis of Gut Microbiota With Reduced Trimethylamine-N-Oxide Level in Patients With Large-Artery Atherosclerotic Stroke or Transient Ischemic Attack. Journal of the American Heart Association 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Karbach, S.H.; Schönfelder, T.; Brandão, I.; Wilms, E.; Hörmann, N.; Jäckel, S.; Schüler, R.; Finger, S.; Knorr, M.; Lagrange, J.; et al. Gut Microbiota Promote Angiotensin II-Induced Arterial Hypertension and Vascular Dysfunction. J Am Heart Assoc 2016, 5, e003698. [Google Scholar] [CrossRef]
- Kim, S.; Goel, R.; Kumar, A.; Qi, Y.; Lobaton, G.; Hosaka, K.; Mohammed, M.; Handberg, E.M.; Richards, E.M.; Pepine, C.J.; et al. Imbalance of Gut Microbiome and Intestinal Epithelial Barrier Dysfunction in Patients with High Blood Pressure. Clin Sci (Lond) 2018, 132, 701–718. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Ye, L.; Li, J.; Jin, L.; Wang, W.; Li, S.; Bao, M.; Wu, S.; Li, L.; Geng, B.; et al. Metagenomic and Metabolomic Analyses Unveil Dysbiosis of Gut Microbiota in Chronic Heart Failure Patients. Sci Rep 2018, 8, 635. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Dai, Z.; Wang, Z.; Deng, Z.; Zhang, J.; Pu, J.; Cao, W.; Pan, T.; Zhou, Y.; Yang, Z.; et al. Gut Microbiota Dysbiosis Contributes to the Development of Chronic Obstructive Pulmonary Disease. Respir Res 2021, 22, 274. [Google Scholar] [CrossRef]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Roberts, A.B.; Buffa, J.A.; Levison, B.S.; Zhu, W.; Org, E.; Gu, X.; Huang, Y.; Zamanian-Daryoush, M.; Culley, M.K.; et al. Non-Lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell 2015, 163, 1585–1595. [Google Scholar] [CrossRef]
- Wang, Y.; Kuang, Z.; Yu, X.; Ruhn, K.A.; Kubo, M.; Hooper, L.V. The Intestinal Microbiota Regulates Body Composition through NFIL3 and the Circadian Clock. Science 2017, 357, 912–916. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The Gut Microbiota as an Environmental Factor That Regulates Fat Storage. Proc Natl Acad Sci U S A 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [PubMed]
- Sandek, A.; Bauditz, J.; Swidsinski, A.; Buhner, S.; Weber-Eibel, J.; von Haehling, S.; Schroedl, W.; Karhausen, T.; Doehner, W.; Rauchhaus, M.; et al. Altered Intestinal Function in Patients with Chronic Heart Failure. J Am Coll Cardiol 2007, 50, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, A.L.; Bäckhed, F. Role of Gut Microbiota in Atherosclerosis. Nat Rev Cardiol 2017, 14, 79–87. [Google Scholar] [CrossRef]
- Mitra, S.; Drautz-Moses, D.I.; Alhede, M.; Maw, M.T.; Liu, Y.; Purbojati, R.W.; Yap, Z.H.; Kushwaha, K.K.; Gheorghe, A.G.; Bjarnsholt, T.; et al. In Silico Analyses of Metagenomes from Human Atherosclerotic Plaque Samples. Microbiome 2015, 3, 38. [Google Scholar] [CrossRef]
- Xu, H.; Wang, X.; Feng, W.; Liu, Q.; Zhou, S.; Liu, Q.; Cai, L. The Gut Microbiota and Its Interactions with Cardiovascular Disease. Microb Biotechnol 2020, 13, 637–656. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Bäckhed, F.; Landmesser, U.; Hazen, S.L. Intestinal Microbiota in Cardiovascular Health and Disease: JACC State-of-the-Art Review. J Am Coll Cardiol 2019, 73, 2089–2105. [Google Scholar] [CrossRef]
- Lau, K.; Srivatsav, V.; Rizwan, A.; Nashed, A.; Liu, R.; Shen, R.; Akhtar, M. Bridging the Gap between Gut Microbial Dysbiosis and Cardiovascular Diseases. Nutrients 2017, 9, 859. [Google Scholar] [CrossRef]
- Geovanini, G.R.; Libby, P. Atherosclerosis and Inflammation: Overview and Updates. Clin Sci (Lond) 2018, 132, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Lawler, P.R.; Bhatt, D.L.; Godoy, L.C.; Lüscher, T.F.; Bonow, R.O.; Verma, S.; Ridker, P.M. Targeting Cardiovascular Inflammation: Next Steps in Clinical Translation. Eur Heart J 2021, 42, 113–131. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Ogawa, A.; Mizoguchi, E.; Shimomura, Y.; Andoh, A.; Bhan, A.K.; Blumberg, R.S.; Xavier, R.J.; Mizoguchi, A. IL-22 Ameliorates Intestinal Inflammation in a Mouse Model of Ulcerative Colitis. J Clin Invest 2008, 118, 534–544. [Google Scholar] [CrossRef]
- Wang, X.; Ota, N.; Manzanillo, P.; Kates, L.; Zavala-Solorio, J.; Eidenschenk, C.; Zhang, J.; Lesch, J.; Lee, W.P.; Ross, J.; et al. Interleukin-22 Alleviates Metabolic Disorders and Restores Mucosal Immunity in Diabetes. Nature 2014, 514, 237–241. [Google Scholar] [CrossRef]
- Zheng, Y.; Valdez, P.A.; Danilenko, D.M.; Hu, Y.; Sa, S.M.; Gong, Q.; Abbas, A.R.; Modrusan, Z.; Ghilardi, N.; de Sauvage, F.J.; et al. Interleukin-22 Mediates Early Host Defense against Attaching and Effacing Bacterial Pathogens. Nat Med 2008, 14, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Avery, E.G.; Bartolomaeus, H.; Maifeld, A.; Marko, L.; Wiig, H.; Wilck, N.; Rosshart, S.P.; Forslund, S.K.; Müller, D.N. The Gut Microbiome in Hypertension: Recent Advances and Future Perspectives. Circ Res 2021, 128, 934–950. [Google Scholar] [CrossRef]
- Brown, J.M.; Hazen, S.L. The Gut Microbial Endocrine Organ: Bacterially Derived Signals Driving Cardiometabolic Diseases. Annu Rev Med 2015, 66, 343–359. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal Microbiota Metabolism of L-Carnitine, a Nutrient in Red Meat, Promotes Atherosclerosis. Nat Med 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Seldin, M.M.; Meng, Y.; Qi, H.; Zhu, W.; Wang, Z.; Hazen, S.L.; Lusis, A.J.; Shih, D.M. Trimethylamine N-Oxide Promotes Vascular Inflammation Through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor-κB. J Am Heart Assoc 2016, 5, e002767. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Kitai, T.; Hazen, S.L. Gut Microbiota in Cardiovascular Health and Disease. Circ Res 2017, 120, 1183–1196. [Google Scholar] [CrossRef]
- Du, Y.; Li, X.; Su, C.; Wang, L.; Jiang, J.; Hong, B. The Human Gut Microbiome - a New and Exciting Avenue in Cardiovascular Drug Discovery. Expert Opin Drug Discov 2019, 14, 1037–1052. [Google Scholar] [CrossRef] [PubMed]
- Blacher, E.; Levy, M.; Tatirovsky, E.; Elinav, E. Microbiome-Modulated Metabolites at the Interface of Host Immunity. J Immunol 2017, 198, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Shi, X.; Yang, J.; Zhao, Y.; Xue, L.; Xu, L.; Cai, J. Gut Microbes in Cardiovascular Diseases and Their Potential Therapeutic Applications. Protein Cell 2021, 12, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, F.H.; Fåk, F.; Nookaew, I.; Tremaroli, V.; Fagerberg, B.; Petranovic, D.; Bäckhed, F.; Nielsen, J. Symptomatic Atherosclerosis Is Associated with an Altered Gut Metagenome. Nat Commun 2012, 3, 1245. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Santisteban, M.M.; Rodriguez, V.; Li, E.; Ahmari, N.; Carvajal, J.M.; Zadeh, M.; Gong, M.; Qi, Y.; Zubcevic, J.; et al. Gut Dysbiosis Is Linked to Hypertension. Hypertension 2015, 65, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Berni Canani, R.; Di Costanzo, M.; Leone, L. The Epigenetic Effects of Butyrate: Potential Therapeutic Implications for Clinical Practice. Clin Epigenetics 2012, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.V.; Frassetto, A.; Kowalik, E.J.; Nawrocki, A.R.; Lu, M.M.; Kosinski, J.R.; Hubert, J.A.; Szeto, D.; Yao, X.; Forrest, G.; et al. Butyrate and Propionate Protect against Diet-Induced Obesity and Regulate Gut Hormones via Free Fatty Acid Receptor 3-Independent Mechanisms. PLoS One 2012, 7, e35240. [Google Scholar] [CrossRef] [PubMed]
- Rath, S.; Heidrich, B.; Pieper, D.H.; Vital, M. Uncovering the Trimethylamine-Producing Bacteria of the Human Gut Microbiota. Microbiome 2017, 5, 54. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; DuGar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; Wu, Y. Gut Flora Metabolism of Phosphatidylcholine Promotes Cardiovascular Disease. Nature 2011, 472. [Google Scholar] [CrossRef]
- Bennett, B.J.; de Aguiar Vallim, T.Q.; Wang, Z.; Shih, D.M.; Meng, Y.; Gregory, J.; Allayee, H.; Lee, R.; Graham, M.; Crooke, R.; et al. Trimethylamine-N-Oxide, a Metabolite Associated with Atherosclerosis, Exhibits Complex Genetic and Dietary Regulation. Cell Metab 2013, 17, 49–60. [Google Scholar] [CrossRef]
- Warrier, M.; Shih, D.M.; Burrows, A.C.; Ferguson, D.; Gromovsky, A.D.; Brown, A.L.; Marshall, S.; McDaniel, A.; Schugar, R.C.; Wang, Z.; et al. The TMAO-Generating Enzyme Flavin Monooxygenase 3 Is a Central Regulator of Cholesterol Balance. Cell Rep 2015, 10, 326–338. [Google Scholar] [CrossRef]
- Randrianarisoa, E.; Lehn-Stefan, A.; Wang, X.; Hoene, M.; Peter, A.; Heinzmann, S.S.; Zhao, X.; Königsrainer, I.; Königsrainer, A.; Balletshofer, B.; et al. Relationship of Serum Trimethylamine N-Oxide (TMAO) Levels with Early Atherosclerosis in Humans. Sci Rep 2016, 6, 26745. [Google Scholar] [CrossRef]
- Martin, F.-P.J.; Wang, Y.; Sprenger, N.; Yap, I.K.S.; Lundstedt, T.; Lek, P.; Rezzi, S.; Ramadan, Z.; van Bladeren, P.; Fay, L.B.; et al. Probiotic Modulation of Symbiotic Gut Microbial-Host Metabolic Interactions in a Humanized Microbiome Mouse Model. Mol Syst Biol 2008, 4, 157. [Google Scholar] [CrossRef]
- Bergeron, N.; Williams, P.T.; Lamendella, R.; Faghihnia, N.; Grube, A.; Li, X.; Wang, Z.; Knight, R.; Jansson, J.K.; Hazen, S.L.; Krauss, R.M. Diets High in Resistant Starch Increase Plasma Levels of Trimethylamine-N-Oxide, a Gut Microbiome Metabolite Associated with CVD Risk. The British journal of nutrition 2016, 116. [Google Scholar] [CrossRef] [PubMed]
- Ea, G.; Ja, S. The Human Microbiome: Our Second Genome. Annual review of genomics and human genetics 2012, 13. [Google Scholar] [CrossRef]
- Ve, B.; Ra, G.-R.; Ag, C.; Ns, V.; Bp, Z.; Zj, S.; Jj, R.; Mc, Z.; Ap, N.; Kp, D.; et al. Trimethylamine-N-Oxide Promotes Age-Related Vascular Oxidative Stress and Endothelial Dysfunction in Mice and Healthy Humans. Hypertension (Dallas, Tex. : 1979) 2020, 76. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, W.H.W.; Buffa, J.A.; Fu, X.; Britt, E.B.; Koeth, R.A.; Levison, B.S.; Fan, Y.; Wu, Y.; Hazen, S.L. Prognostic Value of Choline and Betaine Depends on Intestinal Microbiota-Generated Metabolite Trimethylamine-N-Oxide. Eur Heart J 2014, 35, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.W.; Wang, Z.; Shrestha, K.; Borowski, A.G.; Wu, Y.; Troughton, R.W.; Klein, A.L.; Hazen, S.L. Intestinal Microbiota-Dependent Phosphatidylcholine Metabolites, Diastolic Dysfunction, and Adverse Clinical Outcomes in Chronic Systolic Heart Failure. Journal of cardiac failure 2015, 21. [Google Scholar] [CrossRef] [PubMed]
- Li, X.S.; Obeid, S.; Klingenberg, R.; Gencer, B.; Mach, F.; Räber, L.; Windecker, S.; Rodondi, N.; Nanchen, D.; Muller, O.; Miranda, M.X. Gut Microbiota-Dependent Trimethylamine N-Oxide in Acute Coronary Syndromes: A Prognostic Marker for Incident Cardiovascular Events beyond Traditional Risk Factors. European heart journal 2017, 38. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Wang, Z.; Fan, Y.; Levison, B.; Hazen, J.E.; Donahue, L.M.; Wu, Y.; Hazen, S.L. Prognostic Value of Elevated Levels of Intestinal Microbe-Generated Metabolite Trimethylamine-N-Oxide in Patients with Heart Failure: Refining the Gut Hypothesis. J Am Coll Cardiol 2014, 64, 1908–1914. [Google Scholar] [CrossRef]
- Senthong, V.; Wang, Z.; Fan, Y.; Wu, Y.; Hazen, S.L.; Tang, W.H.W. Trimethylamine N-Oxide and Mortality Risk in Patients With Peripheral Artery Disease. J Am Heart Assoc 2016, 5, e004237. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.L.; Martoni, C.J.; Ganopolsky, J.G.; Labbé, A.; Prakash, S. The Human Microbiome and Bile Acid Metabolism: Dysbiosis, Dysmetabolism, Disease and Intervention. Expert Opin Biol Ther 2014, 14, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.L.; Martoni, C.J.; Parent, M.; Prakash, S. Cholesterol-Lowering Efficacy of a Microencapsulated Bile Salt Hydrolase-Active Lactobacillus Reuteri NCIMB 30242 Yoghurt Formulation in Hypercholesterolaemic Adults. Br J Nutr 2012, 107, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Lekawanvijit, S.; Adrahtas, A.; Kelly, D.J.; Kompa, A.R.; Wang, B.H.; Krum, H. Does Indoxyl Sulfate, a Uraemic Toxin, Have Direct Effects on Cardiac Fibroblasts and Myocytes? Eur Heart J 2010, 31, 1771–1779. [Google Scholar] [CrossRef] [PubMed]
- Nagatomo, Y.; Tang, W.H.W. Intersections Between Microbiome and Heart Failure: Revisiting the Gut Hypothesis. J Card Fail 2015, 21, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Lam, V.; Su, J.; Hsu, A.; Gross, G.J.; Salzman, N.H.; Baker, J.E. Intestinal Microbial Metabolites Are Linked to Severity of Myocardial Infarction in Rats. PLoS One 2016, 11, e0160840. [Google Scholar] [CrossRef] [PubMed]
- Cason, C.A.; Dolan, K.T.; Sharma, G.; Tao, M.; Kulkarni, R.; Helenowski, I.B.; Doane, B.M.; Avram, M.J.; McDermott, M.M.; Chang, E.B.; et al. Plasma Microbiome-Modulated Indole- and Phenyl-Derived Metabolites Associate with Advanced Atherosclerosis and Postoperative Outcomes. J Vasc Surg 2018, 68, 1552–1562. [Google Scholar] [CrossRef]
- Wicherski, J.; Schlesinger, S.; Fischer, F. Association between Breakfast Skipping and Body Weight-A Systematic Review and Meta-Analysis of Observational Longitudinal Studies. Nutrients 2021, 13, 272. [Google Scholar] [CrossRef] [PubMed]
- Effects of Intermittent Fasting on Health, Aging, and Disease. N Engl J Med 2020, 382, 978. [CrossRef] [PubMed]
- Deleu, S.; Machiels, K.; Raes, J.; Verbeke, K.; Vermeire, S. Short Chain Fatty Acids and Its Producing Organisms: An Overlooked Therapy for IBD? EBioMedicine 2021, 66, 103293. [Google Scholar] [CrossRef]
- Sun, J.; Furio, L.; Mecheri, R.; van der Does, A.M.; Lundeberg, E.; Saveanu, L.; Chen, Y.; van Endert, P.; Agerberth, B.; Diana, J. Pancreatic β-Cells Limit Autoimmune Diabetes via an Immunoregulatory Antimicrobial Peptide Expressed under the Influence of the Gut Microbiota. Immunity 2015, 43, 304–317. [Google Scholar] [CrossRef]
- Witkowski, M.; Weeks, T.L.; Hazen, S.L. Gut Microbiota and Cardiovascular Disease. Circ Res 2020, 127, 553–570. [Google Scholar] [CrossRef]
- Barnes, D.; Park, K.T. Donor Considerations in Fecal Microbiota Transplantation. Curr Gastroenterol Rep 2017, 19, 10. [Google Scholar] [CrossRef]
- Fuentes, S.; de Vos, W.M. How to Manipulate the Microbiota: Fecal Microbiota Transplantation. Adv Exp Med Biol 2016, 902, 143–153. [Google Scholar] [CrossRef]
- Gregory, J.C.; Buffa, J.A.; Org, E.; Wang, Z.; Levison, B.S.; Zhu, W.; Wagner, M.A.; Bennett, B.J.; Li, L.; DiDonato, J.A.; et al. Transmission of Atherosclerosis Susceptibility with Gut Microbial Transplantation. J Biol Chem 2015, 290, 5647–5660. [Google Scholar] [CrossRef]
- Drew, L. Microbiota: Reseeding the Gut. Nature 2016, 540, S109–S112. [Google Scholar] [CrossRef]
- Chehoud, C.; Dryga, A.; Hwang, Y.; Nagy-Szakal, D.; Hollister, E.B.; Luna, R.A.; Versalovic, J.; Kellermayer, R.; Bushman, F.D. Transfer of Viral Communities between Human Individuals during Fecal Microbiota Transplantation. mBio 2016, 7, e00322. [Google Scholar] [CrossRef]
- Tamburini, S.; Shen, N.; Wu, H.C.; Clemente, J.C. The Microbiome in Early Life: Implications for Health Outcomes. Nat Med 2016, 22, 713–722. [Google Scholar] [CrossRef]
- Sun, B.; Ma, T.; Li, Y.; Yang, N.; Li, B.; Zhou, X.; Guo, S.; Zhang, S.; Kwok, L.-Y.; Sun, Z.; et al. Bifidobacterium Lactis Probio-M8 Adjuvant Treatment Confers Added Benefits to Patients with Coronary Artery Disease via Target Modulation of the Gut-Heart/-Brain Axes. mSystems 2022, 7, e0010022. [Google Scholar] [CrossRef]
- Hutchison, E.R.; Kasahara, K.; Zhang, Q.; Vivas, E.I.; Cross, T.-W.L.; Rey, F.E. Dissecting the Impact of Dietary Fiber Type on Atherosclerosis in Mice Colonized with Different Gut Microbial Communities. NPJ Biofilms Microbiomes 2023, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Marques, F.Z. How Dietary Fibre, Acting via the Gut Microbiome, Lowers Blood Pressure. Curr Hypertens Rep 2022, 24, 509–521. [Google Scholar] [CrossRef]
- Evans, M.; Dai, L.; Avesani, C.M.; Kublickiene, K.; Stenvinkel, P. The Dietary Source of Trimethylamine N-Oxide and Clinical Outcomes: An Unexpected Liaison. Clin Kidney J 2023, 16, 1804–1812. [Google Scholar] [CrossRef]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.W.M.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of Intestinal Microbiota from Lean Donors Increases Insulin Sensitivity in Individuals with Metabolic Syndrome. Gastroenterology 2012, 143, 913–916. [Google Scholar] [CrossRef]
- Hu, X.-F.; Zhang, W.-Y.; Wen, Q.; Chen, W.-J.; Wang, Z.-M.; Chen, J.; Zhu, F.; Liu, K.; Cheng, L.-X.; Yang, J.; et al. Fecal Microbiota Transplantation Alleviates Myocardial Damage in Myocarditis by Restoring the Microbiota Composition. Pharmacol Res 2019, 139, 412–421. [Google Scholar] [CrossRef]
- Bastos, R.M.C.; Simplício-Filho, A.; Sávio-Silva, C.; Oliveira, L.F.V.; Cruz, G.N.F.; Sousa, E.H.; Noronha, I.L.; Mangueira, C.L.P.; Quaglierini-Ribeiro, H.; Josefi-Rocha, G.R.; et al. Fecal Microbiota Transplant in a Pre-Clinical Model of Type 2 Diabetes Mellitus, Obesity and Diabetic Kidney Disease. Int J Mol Sci 2022, 23, 3842. [Google Scholar] [CrossRef]
- Chen, L.; Guo, L.; Feng, S.; Wang, C.; Cui, Z.; Wang, S.; Lu, Q.; Chang, H.; Hang, B.; Snijders, A.M.; et al. Fecal Microbiota Transplantation Ameliorates Type 2 Diabetes via Metabolic Remodeling of the Gut Microbiota in Db/Db Mice. BMJ Open Diabetes Res Care 2023, 11, e003282. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.T.; Kang, J.H.; Lee, M.S. Characterization of Lactobacillus Plantarum PH04, a Potential Probiotic Bacterium with Cholesterol-Lowering Effects. Int J Food Microbiol 2007, 113, 358–361. [Google Scholar] [CrossRef]
- Yadav, H.; Jain, S.; Sinha, P.R. Antidiabetic Effect of Probiotic Dahi Containing Lactobacillus Acidophilus and Lactobacillus Casei in High Fructose Fed Rats. Nutrition 2007, 23, 62–68. [Google Scholar] [CrossRef]
- Asemi, Z.; Zare, Z.; Shakeri, H.; Sabihi, S.-S.; Esmaillzadeh, A. Effect of Multispecies Probiotic Supplements on Metabolic Profiles, Hs-CRP, and Oxidative Stress in Patients with Type 2 Diabetes. Ann Nutr Metab 2013, 63, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Moludi, J.; Kafil, H.S.; Qaisar, S.A.; Gholizadeh, P.; Alizadeh, M.; Vayghyan, H.J. Effect of Probiotic Supplementation along with Calorie Restriction on Metabolic Endotoxemia, and Inflammation Markers in Coronary Artery Disease Patients: A Double Blind Placebo Controlled Randomized Clinical Trial. Nutr J 2021, 20, 47. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zhao, D.; Liu, X. Nutritional Supplements Improve Cardiovascular Risk Factors in Overweight and Obese Patients: A Bayesian Network Meta-Analysis. Front Nutr 2023, 10, 1140019. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, J.; Quan, G.; Wang, X.; Yang, L.; Zhong, L. Lactobacillus Acidophilus ATCC 4356 Prevents Atherosclerosis via Inhibition of Intestinal Cholesterol Absorption in Apolipoprotein E-Knockout Mice. Appl Environ Microbiol 2014, 80, 7496–7504. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, S.; Zhao, Z.; Song, X.; Qu, H.; Liu, H. Phenylacetylglutamine Is Associated with the Degree of Coronary Atherosclerotic Severity Assessed by Coronary Computed Tomographic Angiography in Patients with Suspected Coronary Artery Disease. Atherosclerosis 2021, 333, 75–82. [Google Scholar] [CrossRef]
- Di Francesco, A.; Di Germanio, C.; Bernier, M.; de Cabo, R. A Time to Fast. Science 2018, 362, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Regmi, P.; Chaudhary, R.; Page, A.J.; Hutchison, A.T.; Vincent, A.D.; Liu, B.; Heilbronn, L. Early or Delayed Time-Restricted Feeding Prevents Metabolic Impact of Obesity in Mice. J Endocrinol 2021, 248, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.J.; et al. Time-Restricted Feeding without Reducing Caloric Intake Prevents Metabolic Diseases in Mice Fed a High-Fat Diet. Cell Metab 2012, 15, 848–860. [Google Scholar] [CrossRef]
- Takahashi, M.; Ozaki, M.; Kang, M.-I.; Sasaki, H.; Fukazawa, M.; Iwakami, T.; Lim, P.J.; Kim, H.-K.; Aoyama, S.; Shibata, S. Effects of Meal Timing on Postprandial Glucose Metabolism and Blood Metabolites in Healthy Adults. Nutrients 2018, 10, 1763. [Google Scholar] [CrossRef]
| Treatment | Species/Strain | Effect | Reference |
|---|---|---|---|
| FMT | Human | Transplantation of feces from lean healthy individuals into patients with metabolic syndrome for 6 weeks resulted in a significant enhancement of peripheral and hepatic insulin sensitivity respectively, and this enhancement was independent of differences in body weight | [163] |
| With regard to FMT in men with metabolic syndrome, shifting the gut microbiota from a lean donor improved insulin sensitivity while increasing butyrate-producing gut bacteria | [163] | ||
| Treatment of Clostridium difficile infection | [153] | ||
| C57BL/6J mouse | FMT restored gut microbial homeostasis in mice and prevented cardiac cell damage in a mouse model of myocarditis | [164] | |
| Administration of FMT in BTBRob/ob mice, a model of diabetic nephropathy, attenuated weight gain, inflammation, and insulin resistance, a finding that was accompanied by an increase in the number of Odoribacteraceae bacteria | [165] | ||
| FMT in diabetic db/db mice increased the abundance of Ruminococcaceae and Porphyromonas, restored the integrity of the intestinal barrier, and ameliorated inflammation | [166] | ||
| Human | Patients consuming Bifidobacterium bifidum and probiotics containing Lactobacillus acidophilus have shown significant reductions in lipid and/or blood glucose levels | [167,168] | |
| Probiotics | improve metabolic status in diabetic patients | [169] | |
| After 12 weeks of supplementation with Lactobacillus rhamnosus and calorie intake restriction in patients with Coronary Artery Disease(CAD), the patients lost significant weight and showed anti-inflammatory effects that were superior to those of calorie intake restriction alone | [170] | ||
| CAD patients treated with the probiotic strain Bifidobacterium lactis Probio-M8 in combination with conventional therapy showed significant improvements in angina, anxiety and depressive symptoms, as well as reductions in interleukin 6 and LDL-C levels, when compared to the control group. | [159] | ||
| Improvement of glycemic status (fasting glucose, fasting insulin levels, insulin resistance) in overweight or obese patients | [171] | ||
| C57BL/6J mouse | Treatment with Lactobacillus acidophilus ATCC 4358 attenuated atherosclerosis in ApoE-/- mice | [172] | |
| Probiotics can effectively reduce the burden of atherosclerotic plaque and have an anti-atherosclerotic effect | [173] | ||
| Rats | Administration of a single cholesterol-lowering probiotic strain to hypercholesterolemic rats affects weight gain, lipid markers, and hepatic steatosis | [30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





