Submitted:
16 July 2024
Posted:
17 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
- -
- To compare the CGP of recurrent thymoma patients vs non-recurrent thymoma patients;
- -
- To explore the CGP of both primary and recurrent thymomas and identify associations with clinic-pathological variables;
- -
- To evaluate actionable mutations detected in thymomas as target for new therapeutic approaches.
2. Materials and Methods
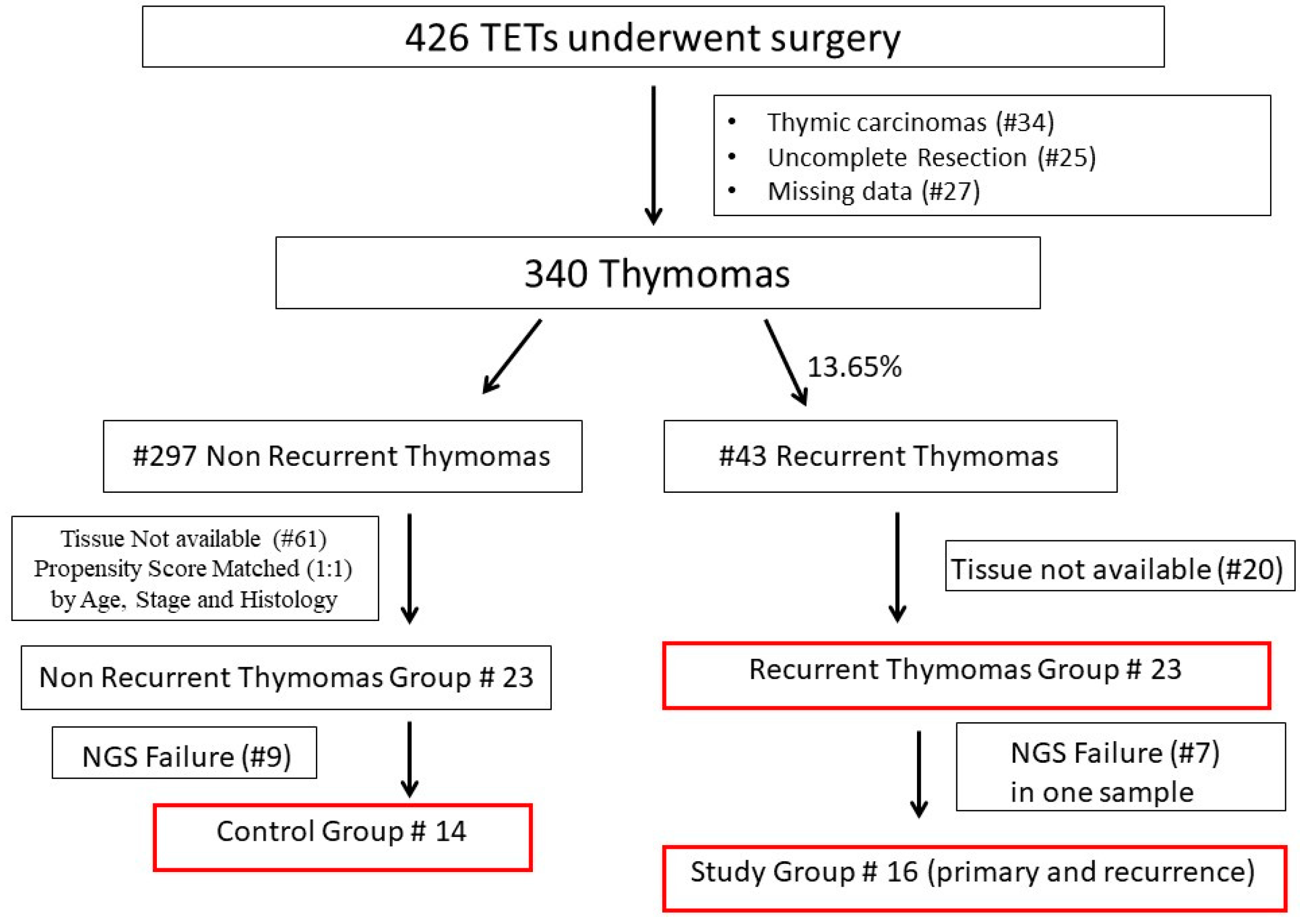
2.1. Study Design and Selection of Cases
2.2. Pathological Review
2.3. Comprehensive Genomic Profiling and Bioinformatics Analysis
2.4. Statistical Analysis
3. Results
3.1. Clinical and Pathological Characteristics
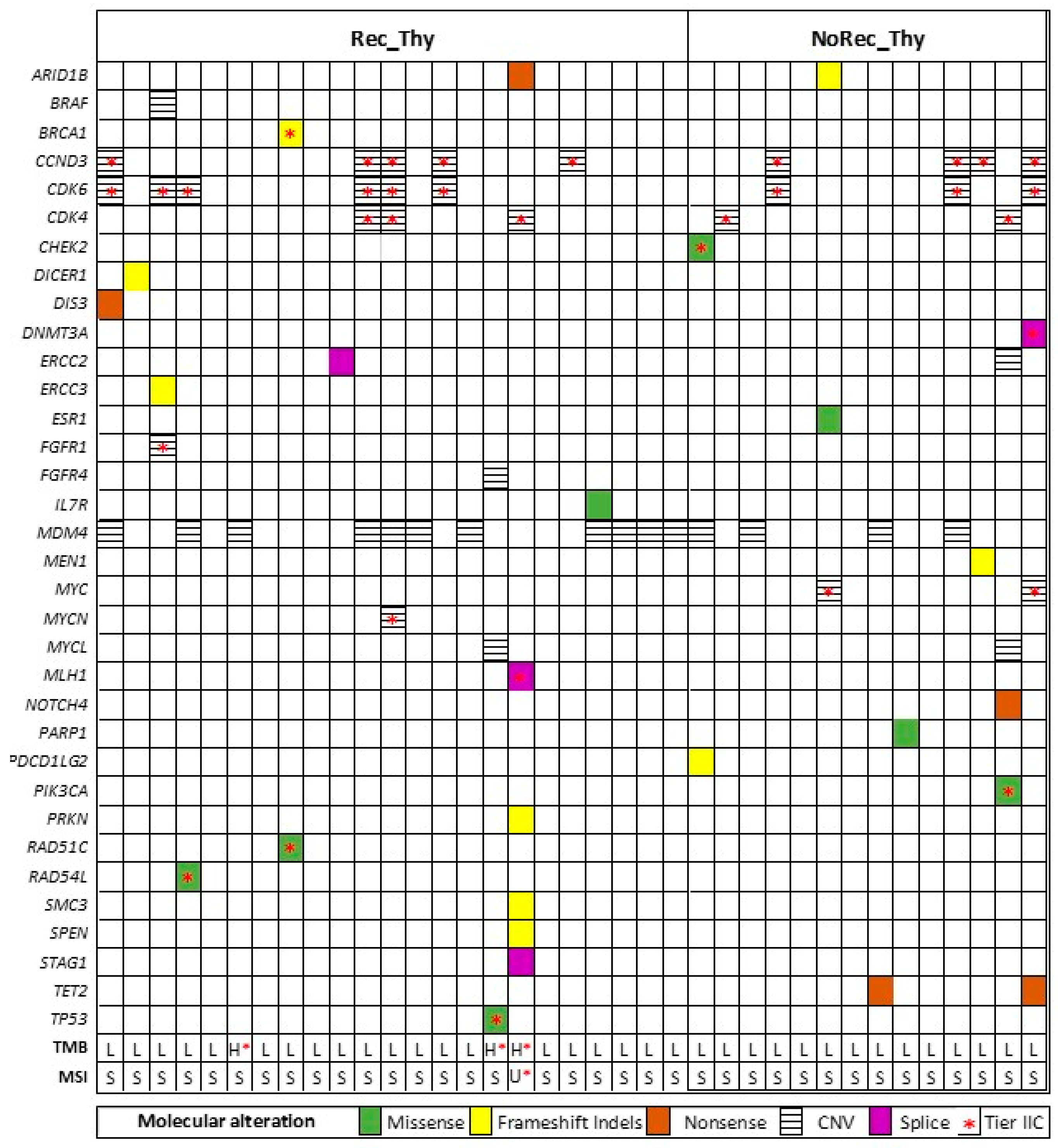
3.2. Overall Genomic Results (Entire Cohort)
3.3. CGP Differences in Recurrent Thymoma vs Non Recurrent Thymoma
3.4. CGP Differences in Primary vs Recurrent Thymoma and Inter-Relationship with Clinic-Pathological Variables
3.5. Actionable Mutations for New Therapeutic Approaches
4. Discussion
Limitations, Points of Strength and Future Clinical Applications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Jong WK, Blaauwgeers JL, Schaapveld M, Timens W, Klinkenberg TJ, Groen HJ. Thymic epithelial tumours: a population-based study of the incidence, diagnostic procedures and therapy. Eur J Canc 2008. [CrossRef]
- Marx A, Chan JK, Coindre JM, et al. The 2015 World Health Organization classification of tumors of the thymus: continuity and changes. J Thorac Oncol. 2015. [CrossRef]
- Regnard JF, Magdeleinat P, Dromer C, Dulmet E, de Montpreville V, Levi JF, Levasseur P. Prognostic factors and long-term results after thymoma resection: a series of 307 patients. J Thorac Cardiovasc Surg. 1996. [CrossRef] [PubMed]
- Sandri A, Cusumano G, Lococo F, Alifano M, Granone P, Margaritora S, Cesario A, Oliaro A, Filosso P, Regnard JF, Ruffini E. Long-term results after treatment for recurrent thymoma: a multicenter analysis. J Thorac Oncol. 2014. [CrossRef] [PubMed]
- Margaritora S., Cesario A., Cusumano G., Lococo F., Porziella V., Meacci E., Evoli A., Granone P. Single-centre 40-year results of redo operation for recurrent thymomas. Eur. J. Cardiothorac. Surg. 2011. [CrossRef]
- Chiappetta M, Lococo F, Zanfrini E, Moroni R, Aprile V, Guerrera F, Nachira D, Congedo MT, Ambrogi MC, Korasidis S, Lucchi M, Filosso PL, Ruffini E, Sperduti I, Meacci E, Margaritora S. The International Thymic Malignancy Interest Group Classification of Thymoma Recurrence: Survival Analysis and Perspectives. J Thorac Oncol. 2021. Epub 2021 Jul 10. [CrossRef] [PubMed]
- Mizuno T., Okumura M., Asamura H., Yoshida K., Niwa H., Kondo K., Horio H., Matsumura A., Yokoi K. Surgical management of recurrent thymic epithelial tumors a retrospective analysis based on the Japanese nationwide database. J. Thorac. Oncol. 2015. [CrossRef]
- Chiappetta M, Grossi U, Sperduti I, Margaritora S, Marulli G, Fiorelli A, Sandri A, Mizuno T, Cusumano G, Hamaji M, Cesario A, Lococo F. Which Is the Best Treatment in Recurrent Thymoma? A Systematic Review and Meta-Analysis. Cancers (Basel). 2021. [CrossRef] [PubMed] [PubMed Central]
- Giaccone G., Wilmink H., Paul M.A., van der Valk P. Systemic treatment of malignant thymoma: A decade experience at a single institution. Am. J. Clin. Oncol. 2006. [CrossRef]
- Bott M., Wang H., Travis W., Riely G.J., Bains M., Downey R., Rusch V., Huang J. Management and outcomes of relapse after treatment for thymoma and thymic carcinoma. Ann. Thorac. Surg. 2011. [CrossRef]
- Shimada M, Taniguchi H, Yamaguchi H, Gyotoku H, Sasaki D, Kaku N, Senju C, Senju H, Imamura E, Takemoto S, Yamamoto K, Sakamoto N, Obase Y, Tsuchiya T, Fukuda M, Soda H, Ashizawa K, Fukuoka J, Nagayasu T, Yanagihara K, Mukae H. Genetic profile of thymic epithelial tumors in the Japanese population: an exploratory study examining potential therapeutic targets. Transl Lung Cancer Res. 2023. Epub 2023 Mar 23. [CrossRef] [PubMed] [PubMed Central]
- Kurokawa K, Shukuya T, Greenstein RA, Kaplan BG, Wakelee H, Ross JS, Miura K, Furuta K, Kato S, Suh J, Sivakumar S, Sokol ES, Carbone DP, Takahashi K. Genomic characterization of thymic epithelial tumors in a real-world dataset. ESMO Open. 2023. [CrossRef]
- Girard N, Basse C, Schrock A, Ramkissoon S, Killian K, Ross JS. Comprehensive Genomic Profiling of 274 Thymic Epithelial Tumors Unveils Oncogenic Pathways and Predictive Biomarkers. Oncologist. 2022. [CrossRef]
- Agrafiotis AC, Brandão M, Berghmans T, Durieux V, Jungels C. Immunotherapy and Targeted Therapies Efficacy in Thymic Epithelial Tumors: A Systematic Review. Biomedicines. 2023. [CrossRef]
- Koga K., Matsuno Y., Noguchi M., et al. A review of 79 thymomas: modification of staging system and reappraisal of conventional division into invasive and non-invasive thymoma. Pathol Int, 44 (1994), pp. 359-367.
- Brierley J., Gospodarowicz M.K., Wittekind C. TNM Classification of Malignant Tumours (8th ed.), John Wiley & Sons, Inc, Hoboken, NJ (2017).
- Marx A., Ströbel P., Badve S.S., et al. ITMIG consensus statement on the use of the WHO histological classification of thymoma and thymic carcinoma: refined definitions, histological criteria, and reporting J Thorac Oncol, 9 (2014), pp. 596-611.=.
- Liu H, Gu Z, Qiu B, Detterbeck FC, Roden AC, Ruffini E, Okumura M, Girard N, Xiang Y, Liu Y, Du Z, Hao Y, Fu J, Zhang P, Pang L, Chen K, Wang Y, Yu Z, Mao T, Fang W; AME Thoracic Surgery Cooperative Group. A Recurrence Predictive Model for Thymic Tumors and Its Implication for Postoperative Management: a Chinese Alliance for Research in Thymomas Database Study. J Thorac Oncol. 2020. Epub 2019 Nov 11. [CrossRef] [PubMed]
- Li MM, Datto M, Duncavage EJ, Kulkarni S, Lindeman NI, Roy S, Tsimberidou AM, Vnencak-Jones CL, Wolff DJ, Younes A, Nikiforova MN. Standards and Guidelines for the Interpretation and Reporting of Sequence Variants in Cancer: A Joint Consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn. 2017. [CrossRef] [PubMed] [PubMed Central]
- https://cancer.sanger.ac.uk/signatures/, last accessed 02/2024 (last accessed, 06/2024).
- https://www.oncokb.org/ (last accessed, 06/2024).
- https://www.ncbi.nlm.nih.gov/clinvar/ (last accessed, 06/2024).
- Kuzbari Z, Bandlamudi C, Loveday C, Garrett A, Mehine M, George A, Hanson H, Snape K, Kulkarni A, Allen S, Jezdic S, Ferrandino R, Westphalen CB, Castro E, Rodon J, Mateo J, Burghel GJ, Berger MF, Mandelker D, Turnbull C. Germline-focused analysis of tumour-detected variants in 49,264 cancer patients: ESMO Precision Medicine Working Group recommendations. Ann Oncol. 2023. Epub 2022 Dec 16. [CrossRef] [PubMed]
- Chiappetta M, Sassorossi C, Nachira D, Lococo F, Meacci E, Ruffini E, Guerrera F, Lyberis P, Aprile V, Lucchi M, Ambrogi MC, Bacchin D, Dell'Amore A, Marino C, Comacchio G, Roca G, Rea F, Margaritora S. Survival outcome after surgery in patients with thymoma distant recurrence. J Thorac Oncol. 2024. [CrossRef]
- Ak N, Aydiner A. Nivolumab treatment for metastatic thymic epithelial tumors. J Oncol Pharm Pract. 2021. Epub 2020 Oct 25. [CrossRef] [PubMed]
- Song X, Fan J, Zhu L, Wang Z, He Y, Zhou C. The efficacy and safety of immunotherapy in thymic epithelial tumors: more effective, more risky: a systematic review. J Thorac Dis. 2021. [CrossRef]
- Montella L, Ottaviano M, Morra R, Pietroluongo E, De Placido P, Tortora M, Sorrentino C, Facchini G, De Placido S, Giuliano M, Palmieri G. The Never-Ending History of Octreotide in Thymic Tumors: A Vintage or A Contemporary Drug? Cancers (Basel). 2022. [CrossRef]
- Morfouace M, Stevovic A, Vinches M, Golfinopoulos V, Jin DX, Holmes O, Erlich R, Fayette J, Croce S, Ray-Coquard I, Girard N, Blay JY. First results of the EORTC-SPECTA/Arcagen study exploring the genomics of rare cancers in collaboration with the European reference network EURACAN. ESMO Open. 2020. [CrossRef]
- Morfouace M, Novello S, Stevovic A, Dooms C, Janžič U, Berghmans T, Dziadziuszko R, Gorlia T, Felip E, Paz-Ares L, Mazieres J, O'Brien M, Bironzo P, Vansteenkiste J, Lacroix L, Dingemans AC, Golfinopoulos V, Besse B. Results of screening in early and advanced thoracic malignancies in the EORTC pan-European SPECTAlung platform. Sci Rep. 2022. [CrossRef]
- Jovanovic D, Markovic J, Ceriman V, Peric J, Pavlovic S, Soldatovic I. Correlation of genomic alterations and PD-L1 expression in thymoma. J Thorac Dis. 2020. [CrossRef]
- Conforti F, Pala L, Giaccone G, De Pas T. Thymic epithelial tumors: From biology to treatment. Cancer Treat Rev. 2020. [CrossRef]
- Jardim DL, Millis SZ, Ross JS, Woo MS, Ali SM, Kurzrock R. Cyclin Pathway Genomic Alterations Across 190,247 Solid Tumors: Leveraging Large-Scale Data to Inform Therapeutic Directions. Oncologist. 2021. [CrossRef]
- Baldi A, Ambrogi V, Mineo D, Mellone P, Campioni M, Citro G, Mineo TC. Analysis of cell cycle regulator proteins in encapsulated thymomas. Clin Cancer Res. 2005. [CrossRef]
- https://ascopubs.org/doi/10.1200/JCO.2018.36.15_suppl.8519.
- Aesif SW, Aubry MC, Yi ES, Kloft-Nelson SM, Jenkins SM, Spears GM, Greipp PT, Sukov WR, Roden AC. Loss of p16INK4A Expression and Homozygous CDKN2A Deletion Are Associated with Worse Outcome and Younger Age in Thymic Carcinomas. J Thorac Oncol. 2017. [CrossRef]
- https://www.cbioportal.org/ (last accessed 06/2024).
- Markey MP. Regulation of MDM4. Front Biosci (Landmark Ed). 2011. [CrossRef] [PubMed]
- Yi EJ, Park JH, Lee HW, Cho SY, Na II, Kang MC. BRCA1 gene mutation in thymic malignant melanoma. Ann Thorac Surg. 2013. [CrossRef]
- Nicodème F, Geffroy S, Conti M, Delobel B, Soenen V, Grardel N, Porte H, Copin MC, Laï JL, Andrieux J. Familial occurrence of thymoma and autoimmune diseases with the constitutional translocation t(14;20)(q24.1;p12.3). Genes Chromosomes Cancer. 2005. [CrossRef]
- Enkner F, Pichlhöfer B, Zaharie AT, Krunic M, Holper TM, Janik S, Moser B, Schlangen K, Neudert B, Walter K, Migschitz B, Müllauer L. Molecular Profiling of Thymoma and Thymic Carcinoma: Genetic Differences and Potential Novel Therapeutic Targets. Pathol Oncol Res. 2017. [CrossRef]
- Principe DR, Kamath SD, Munshi HG, Mohindra NA. Metastatic Thymoma Harboring a Deleterious BRCA2 Mutation Derives Durable Clinical Benefit from Olaparib. Oncologist. 2020. [CrossRef]
- Cimpean AM, Raica M, Encica S, Cornea R, Bocan V. Immunohistochemical expression of vascular endothelial growth factor A (VEGF), and its receptors (VEGFR1, 2) in normal and pathologic conditions of the human thymus. Ann Anat. 2008. [CrossRef]
- Thomas A, Rajan A, Berman A, Tomita Y, Brzezniak C, Lee MJ, Lee S, Ling A, Spittler AJ, Carter CA, Guha U, Wang Y, Szabo E, Meltzer P, Steinberg SM, Trepel JB, Loehrer PJ, Giaccone G. Sunitinib in patients with chemotherapy-refractory thymoma and thymic carcinoma: an open-label phase 2 trial. Lancet Oncol. 2015. [CrossRef]
- Radovich M, Solzak JP, Hancock BA, Conces ML, Atale R, Porter RF, Zhu J, Glasscock J, Kesler KA, Badve SS, Schneider BP, Loehrer PJ. A large microRNA cluster on chromosome 19 is a transcriptional hallmark of WHO type A and AB thymomas. Br J Cancer. 2016. [CrossRef]
- Rajan A, Carter CA, Berman A, Cao L, Kelly RJ, Thomas A, Khozin S, Chavez AL, Bergagnini I, Scepura B, Szabo E, Lee MJ, Trepel JB, Browne SK, Rosen LB, Yu Y, Steinberg SM, Chen HX, Riely GJ, Giaccone G. Cixutumumab for patients with recurrent or refractory advanced thymic epithelial tumours: a multicentre, open-label, phase 2 trial. Lancet Oncol. 2014. [CrossRef]
- Zucali PA, De Pas T, Palmieri G, Favaretto A, Chella A, Tiseo M, Caruso M, Simonelli M, Perrino M, De Vincenzo F, Toffalorio F, Damiano V, Pasello G, Garbella E, Ali M, Conforti F, Ottaviano M, Cioffi A, De Placido S, Giordano L, Bertossi M, Destro A, Di Tommaso L, Santoro A. Phase II Study of Everolimus in Patients With Thymoma and Thymic Carcinoma Previously Treated With Cisplatin-Based Chemotherapy. J Clin Oncol. 2018. [CrossRef]
- Abu Zaid MI, Radovich M, Althouse S, Liu H, Spittler AJ, Solzak J, Badve S, Loehrer PJ Sr. A phase II study of buparlisib in relapsed or refractory thymomas. Front Oncol. 2022. [CrossRef]
| Rec_Thy (n = 23 pts) | No Rec_Thy (n=14 pts) | |
| GENDER M F |
13 (56.5%) 10 (43.5%) |
10 (71.4%) 4 (28.6%) |
| AGE (median, range) | 51y (27y-83y) | 59y (16y-82y) |
| MG | 8 (34.8%) | 7 (50.0%) |
| MASAOKA* II III IV |
5 (21.8%) 12 (52.2%) 3 (13.0%) |
5 (35.7%) 7 (52.2%) 2 (14.3%) |
| NEOADJUVANT TREATMENT | 10/20 (50.0%) | 6/14 (42.9%) |
| HISTOLOGY WHO AB B1 B2 B3 |
0 (0%) 9 (39.2%) 7 (30.4%) 7 (30.4%) |
1 (7.1%) 2 (14.3%) 9 (64.3%) 2 (14.3%) |
| ^RISK CLASS* Low-Risk High-Risk |
5 (25.0%) 15 (75.0%) |
5 (35.7%) 9 (64.3%) |
| STAGE* II III IV |
4 (20.0%) 11 (55.0%) 5 (25.0%) |
6 (42.8%) 5 (35.7%) 3 (21.5%) |
| DFI (median, range)** | 32m (6m-132m) | / |
| ADJUVANT TREATMENT | 7 (30.0%) | 7 (50.0%) |
| GROUP | All Patients (#37) | Rec_Thy(#23) | NoRec_Thy (#14) | p-value |
|---|---|---|---|---|
| Pathway cell cycle | 26 (70%) | 17 (73.9%) | 9 (64.3%) | p=0.53 |
| Pathway DNA repair | 8 (22%) | 5 (21.7%) | 3 (21.4%) | p=0.98 |
| At least 1 alteration | 30 (81%) | 19 (82.6%) | 11 (78.6%) | p=0.76 |
| Clinically relevant alteration | 18 (49%) | 10 (43%) | 8 (57%) | p=0.83 |
| GROUP | Primary_Thy | Recurrent_Thy | p-value |
|---|---|---|---|
| Pathway cell cycle | 6 (37.5 %) | 9 (56.2%) | p=0.30 |
| Pathway DNA repair | 2 (12.5%) | 3 (18.7%) | p=0.23 |
| At least 1 alteration | 9 (56.2%) | 11 (68.7%) | p=0.84 |
| Pathway cell cycle | Pathway DNA repair | At least 1 alteration | |
|---|---|---|---|
| Rec_Thy (n=23) | 11/23 (47.8%) | 3/23 (13.0%) | 14/23 (60.9%) |
| Masaoka Stage II (n=5) III-IV (n=18) |
p=0.121 5/5 (100.0%) 12/18 (66.6%) |
p=0.019 3/5 (60.0%) 2/18 (11.1%) |
p=0.351 5/5 (100.0%) 14/18 (77.8%) |
| Age <51 (n=11) >51 (n=12) |
p=0. 896 5/11 (45.6%) 6/12 (50.0%) |
p=0.635 1/11 (9.1%) 2/12 (16.7%) |
p=0. 582 8/11 (72.7%) 6/12 (50.0%) |
| Miastenia Gravis Yes (n=8) No (n=15) |
p=0.661 3/8 (37.5%) 8/15 (53.3%) |
p=0.960 1/8 (12.5%) 2/15 (13.3%) |
p=0.695 4/8 (50.0%) 10/15 (66.7%) |
| RISK Class* Low (n=5) High (n=18) |
p=0.121 4/5 (80.0%) 9/18 (50.0%) |
p=0.770 1/5 (20.0%) 4/18 (22.2%) |
p=0.201 5/5 (100.0%) 11/18 (61.1%) |
| DFI <32 months (n=9) >32 months (n=14) |
p=0.022 9/9 (100.0%) 8/14 (57.1%) |
p=0.960 2/9 (22.2%) 3/14 (21.4%) |
p=0.082 9/9 (100.0%) 10/14 (71.4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





