Introduction
Catheter ablation (CA) is a widely used treatment for atrial fibrillation (AF). The initial approach involves the use of radiofrequency (RF) energy to circumferentially isolate the pulmonary veins (PVs) from the left atrium (LA); this technique is known as pulmonary vein isolation (PVI). PVI provides satisfactory AF-free rates for paroxysmal AF.[
1] However, for persistent AF and long-standing persistent AF, PVI alone does not yield acceptable success rates or optimal freedom from AF.[
2] Thus, various adjunctive ablation techniques have been developed to enhance ablation outcomes. These techniques include linear isolation, regional ablation of electrically high-excitability zones, and ablation of non-PV foci.[
3,
4,
5,
6] In such cases, CA for AF tends to require a longer procedure time than other catheter treatments such as those for simpler arrhythmias or percutaneous coronary intervention. Thus, anticoagulation is essential during the ablation procedure, specifically for long procedural times.
Activated clotting time (ACT) is widely utilized in CA, including AF ablation. The ACT provides a comprehensive assessment of clotting using whole blood, thus encompassing platelet and phospholipid functions.[
7] The method for measuring ACT is not standardized. Generally, clotting occurs in a tube or cartridge, with detection achieved through electromagnetic or photo-optical methods, often using clotting accelerators such as celite, kaolin, or a combination thereof. Consequently, the ACT measured by different machines varies. Determining the optimal ACT during AF ablation requires adjustment of the specific ACT measurement machine used.
The guidelines recommend an ACT range of 300–350 s. However, these guidelines do not provide specific statements regarding ACT measurement methods. Significant differences in ACT measurements using different ACT machines have been reported.[
8] Optimizing ACT adjustments during AF ablation for different ACT measurement machines is essential. The aims of the present study were twofold: to 1) determine the desirable ACT range using electromagnetic machine combined with a tube and 2) to adjust the ACT measurement using an electromagnetic machine and a photo-optical machine.
Methods
Patients
Initially, to determine the ACT range measured by electromagnetic methods (described later in detail) during the AF ablation procedure without any complications, ACT data at pre-ablation and during procedures from 2015 to 2018 were extracted from the database. A total of 3364 ACT data points during AF ablation in 1161 patients were analyzed. The pre-ACT data were analyzed using 853 measurements.
Second, we analyzed the ACT in patients with clinically significant complications over a 5-year period.
Third, to disclose the differences in ACT between the two measurement methods (electromagnetic and photo-optical detection systems), ACT was measured using the two methods with the same blood collected during and just before AF ablation in 52 patients from 2023 to 2024.
This study was conducted in accordance with the principles of the Declaration of Helsinki 2000 and approved by the Institutional Ethics Committee of the Okayama Heart Clinic for Human Research (approval number, HS2). Written informed consent for the use of clinical data without personal information was obtained from all patients. The present study did not include Patient and Public Involvement.
ACT Measurements
This study employed two methods. The first method involved electromagnetic detection using a rotation tube (EM system; Hemochron Response, Soma Tech Intl., Bloomfield, CT, USA). Cerite was used to accelerate clotting. This method was used in our clinic for a long time before the introduction of another method, as described below. This method was referred to as the EM system. Another method involved photo-optical detection using a cartridge plunged into blood (PO system; ACT CA-300TM, APEL, Kawaguchi, Saitama, Japan). This system used cerite and kaolin as clotting accelerators. Blood was sampled directly from the venous root into a syringe and transferred into a tube or cartridge for each method. This method was referred to as the PO system.
ACT was then measured immediately by ACT measurement systems in accordance with the manufacturer’s instructions.
Anticoagulation Regimen
After confirmation of the absence of a risk of excessive bleeding, based on the pre-ACT value, a heparin bolus was administered just before septal puncture, taking into consideration age, sex, and body weight (120–130 U/kg for a pre-ACT ≥150 s and 140–150 U/kg for a pre-ACT <150 s).[
9,
10] After the heparin bolus, continuous heparinized saline infusion (400 U/h) was administered via a peripheral vein to maintain the ACT within 300–400 s in order to avoid thrombus formation. ACT values were obtained at baseline (pre-ACT), 15 min after the pre-ACT measurement, and at 30-min intervals thereafter. An additional heparin bolus was administered intravenously when the 15-min ACT or subsequent 30-min interval ACT measurements, under continuous heparinized saline infusion, were 250–274 s at 3000 U and 275–299 s at 2000 U, respectively. The final ACT measurement was performed before catheter removal.
After termination of the AF ablation procedure, protamine sulfate 20 and 30 mg was administered intravenously when the ACT was 300–350 s and >350 s, respectively. When bleeding at the puncture site did not stop after the initial protamine sulfate administration noted above, additional protamine sulfate (10 mg for patients who received anticoagulation therapy with warfarin and 20–30 mg, depending on bleeding status, for patients on novel oral anticoagulants was administered at 4-min intervals until bleeding ceased.
AF Ablation
The methods of PVI have been described in detail in another study.[
11] PVI was performed with the double LASSO technique using an electroanatomical integration mapping system (Ensite-NavX System, ABBOTT Japan, Tokyo, Japan). Ablation was performed using an open-irrigated ablation catheter (CoolFlex
TM/FlexAbility
TM/TactiCath
TM/TactiFlex
TM, ABBOTT) via a steerable sheath. The PVI endpoint was defined as follows: 1) elimination of the PV potentials recorded by the two-ring catheters within the ipsilateral PVs and lack of LA capture during intra-PV, isthmus, and PV atrium pacing for at least 30 min after isolation; and 2) no recurrence of PV spikes within all PVs after an intravenous bolus administration of 20–40 mg of adenosine triphosphate during sinus rhythm or coronary sinus pacing. Thus, PVI was established.
Additional PVI ablation was performed when required. These circumstances included prophylactic cavotricuspid isthmus ablation, superior vena cava isolation, LA linear ablation, LA low-voltage area ablation, and ablation of the complex fractionated atrial electrograms in the right and LA. The decision to select and perform these procedures was at the discretion of the operator.
Complications
Thromboembolic complications: Cerebrovascular accidents and transient ischemic attacks were considered thromboembolic complications after ruling out intracranial hemorrhage. Pulmonary and deep venous embolisms were considered thromboembolic complications.
Major bleeding complications: Cardiac tamponade, pericardial effusion, and bleeding requiring treatment were considered as major bleeding complications. Cardiac tamponade was defined by characteristic clinical features with considerable pericardial effusion requiring drainage with an echo-free space of >1 cm on cardiac echocardiography. Other major bleeding complications were defined as bleeding requiring blood transfusion and hematoma requiring surgical intervention.
Minor bleeding complications were defined as bleeding that did not require blood transfusion or hematoma that did not require surgical intervention. Small pericardial effusions that did not require drainage were classified as minor bleeding complications.
Minor bleeding complications that occurred after protamine neutralization of heparin associated with ACT normalization were excluded to analyze the relationship between ACT and complications.
Statistical Analysis
Statistical analysis was performed using R version 3.2.2. provided by the R Foundation for Statistics Computing (Vienna, Austria).[
12] The power analysis for paired comparison and correlation analysis was conducted using G*Power 3.1.9.7.[
13] ACT distribution patterns in patients without any complications were determined using histogram and Q-Q plots. The Kolmogorov-Smirnov test could not be applied because many tied values were included in the ACT data. Agreements in ACT data between the EM and PO systems were examined using simple regression analysis and Bland-Altman plots. Data are presented as means ± 1 standard deviation (SD) or as medians and interquartile ranges. Statistical significance was set at p < 0.05.
Results
ACT distribution obtained during the procedure without any complications
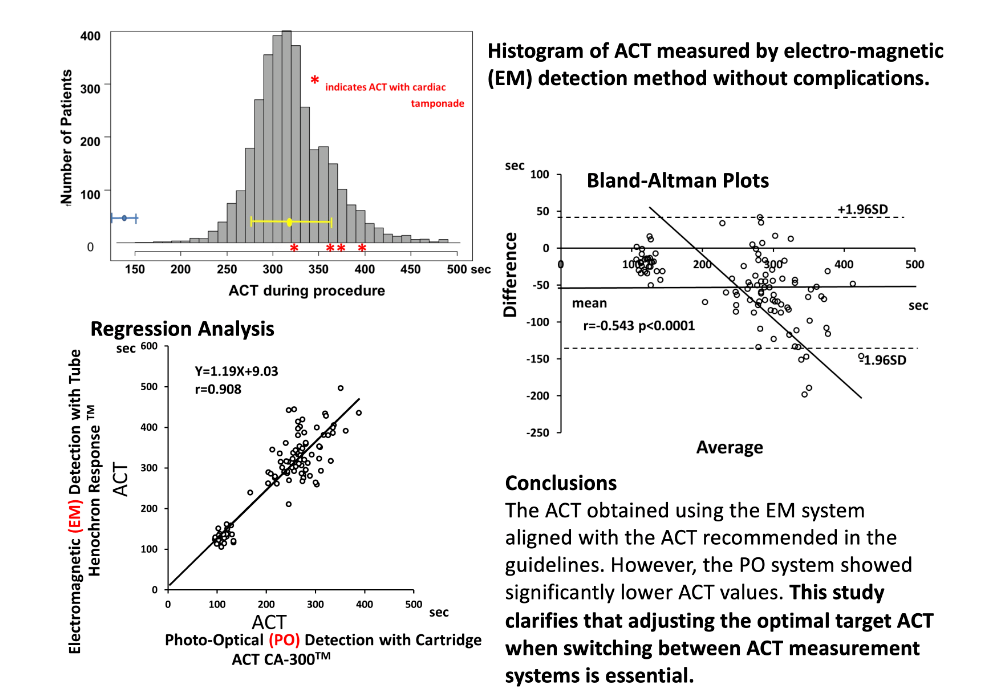
The distribution pattern of ACT obtained through the EM system during ablation procedures and at the end of ablation without any complications in 3363 measurements across 1161 patients is presented in
Figure 1. The histogram and Q-Q plots revealed that the ACT distribution pattern was approximately normal. The average ± 1 SD of ACT was 320 ± 44 s. The ACT ranged from 156 to 487 sec. ACT >300 s was observed in 2% (694/3364) and >400 s was observed in 3.7% (124/3364) of cases.
Pre-ablation ACT measured through the EM detection with tube method was 143 ± 28 s.
ACT Values during Procedure with Complications
Major bleeding complications: Cardiac tamponade was observed in 4 out of the 1982 ablations (0.20%) over 4 years. The maximal EM-ACT during the procedure associated with cardiac tamponade ranged from 330 to 391, The respective ACT values were 330, 373, 375 and 391 sec. These ACT values overlapped with the ACT histogram without complications in
Figure 1. No other major bleeding or thromboembolic complications were observed during ablation. Minor bleeding complications occurred but were observed after catheter removal with protamine neutralization associated with pre-ablation ACT levels. Therefore, these minor complications could not be analyzed.
Comparison between ACT Measured by Electromagnetic Methods and Photo-Optical Methods
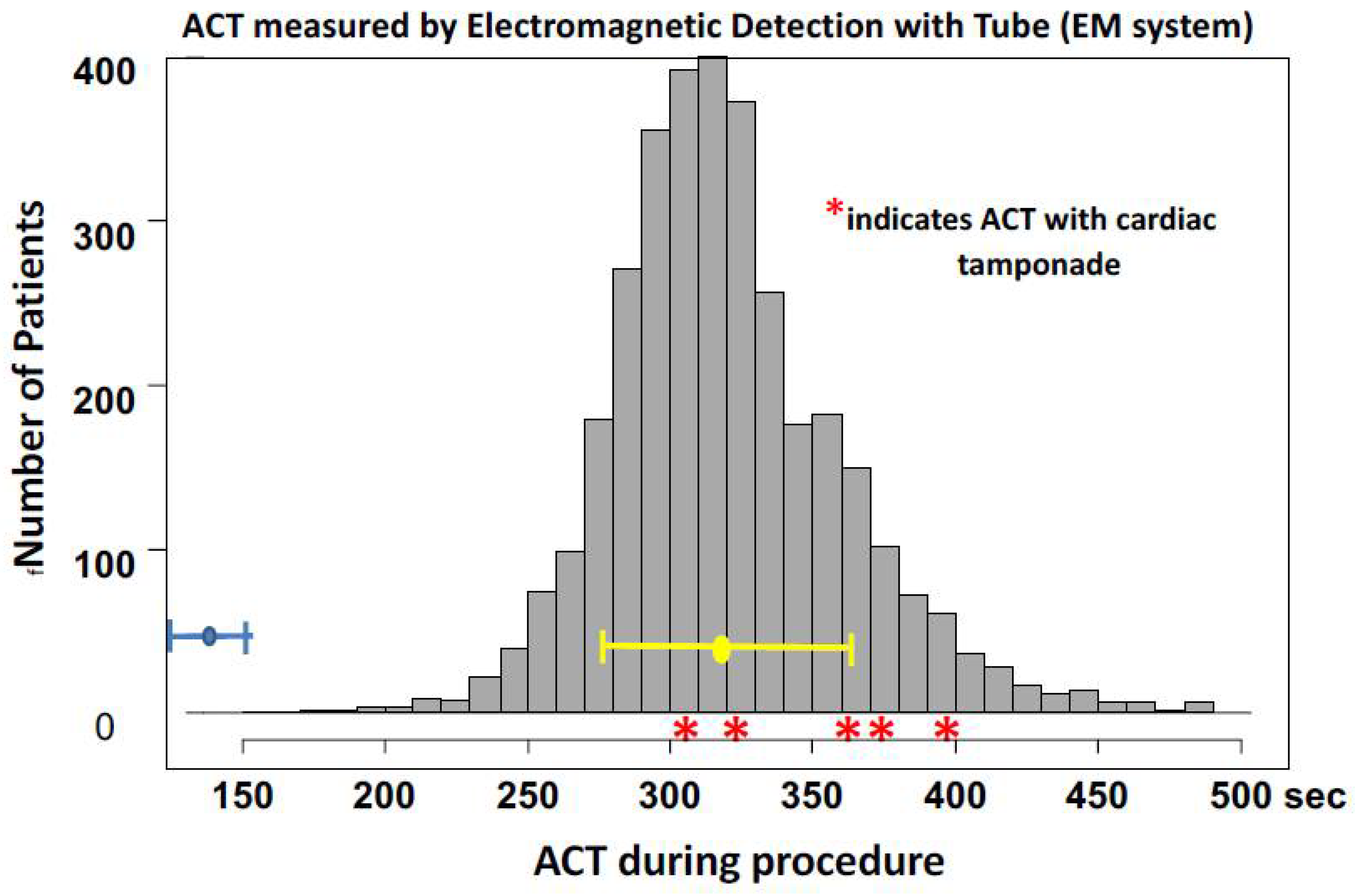
Linear Regression Analysis
Figure 2 shows the results of the regression analysis. Regression analysis revealed the following: EM-ACT=1.19 × PO-ACT + 90.3 (regression coefficient, p<0.001; intercept, p<0.001). Correlation analysis showed a strong correlation between the ACT measured using the EM system and the ACT measured using the PO system (r=0.908, p<0.001).
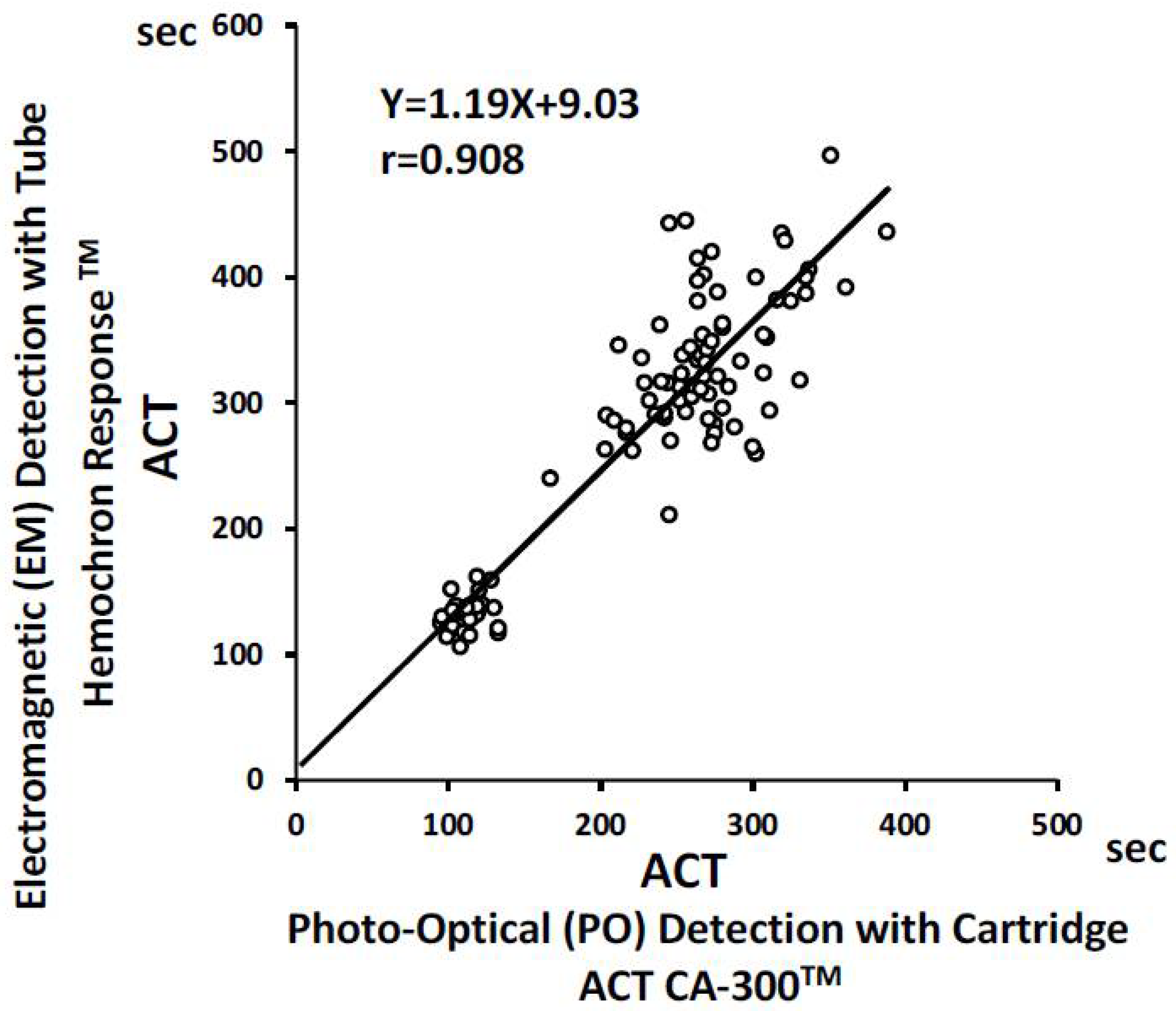
Bland-Altman Plots
The Bland-Altman plots are presented in
Figure 3. The average of the difference between the EM and PO ACTs was 50 s and almost all differences were located in ±1.96 SD of the differences range. Differences increased with an increase in the average ACT.
Discussion
The main findings of the present study are as follows: 1) ACT, as measured by the EM system in patients without any AF ablation complications, was ranged from 156 to 487 s (323 ± 48 s), which was consistent with the recommended optimal target ACT range. 2) Four patients (0.20%, 4/1982) had an ACT of 330–391 s, overlapping ACT range found in patients without complications. 3) Considerable differences in ACT were observed between the EM and PO systems, indicating that adjustments were required for the different methods.
To date, there are no established methods for measuring the ACT. Additionally, no reference blood sample is available to calibrate the measurement machines.[
7] Moreover, there are different systems, either photo-optical or electromagnetic, for clotting detection. Cerite, kaolin, and a combination of agents that accelerate clotting were used. Clotting was detected in the tubes or cartridges. These considerations lead to considerable differences in the ACT among the different methods.
In agreement with the consideration of ACT variations among different measurement methods, the present results clearly show that there are non-negligible variances between the two ACT measurement methods: an electromagnetic system with cerite and a tube, and photo-optical methods with cerite-kaolin and a cartridge. The average difference between the two methods was 51 ± 46 s and the difference increased with an increase in ACT. These results indicate that the linear regression equation is mathematically appropriate for adjusting the ACT between the two methods.
One guideline recommends >300 s as the optimal ACT during AF ablation. Several studies have used an ACT >300 to <350–400 as an adequate range to be maintained by heparin or fractionated heparin during AF ablation.[
14,
15,
16,
17,
18,
19,
20,
21] However, although there are considerable differences in ACT between ACT measurement systems, machines, and reagents, no reports have disclosed ACT measurement methods. One study analyzed six randomized studies with a total of 5216 patients to clarify the optimal ACT range to be maintained during percutaneous coronary intervention.[
17] ACT was measured by the Hemochron assay (International Technidyne Corporation) and HemoTech assay (HemoTec Comp) in 95 and 5%, respectively; ACT in the range of 350 to 375 s provided the lowest ischemic event rate or greater relative risk reduction in comparison with rates observed between 171 and 295 s. However, significant differences between the Hemochron ACT and HemoTec ACT were observed and associated with linear regression as follows; HemoTac ACT=0.63 × Hemochron ACT + 48.67.[
8] Different target ACT ranges in percutaneous coronary intervention have been demonstrated and recommended, as follows: Hemochron ACT, 250–275 sec and HemoTec ACT, 300–350 sec. The present study compared the two ACT measurement systems. One (Hemochron Response) involved electromagnetic detection using a rotation tube and cerite as an acceleration agent for clotting. Another method (Act CA-300) involved photo-optical detection using a cartridge plunged in blood cerite with kaolin as an accelerator for clotting. The former was almost identical to the Hemochron ACT, and the latter was the HemoTec ACT. The present results of the relationships between the Hemochron Response ACT and CA-300ACT were in good agreement with the relationship between the Hemochron ACT and HemoTech ACT. The present results clarify that the evaluation and determination of the optimal ACT range is essential for recognizing the type of ACT measurement system, and one system should not be extrapolated to the other. The ACT was adjusted using an appropriate equation.
The histogram of ACT values obtained using the EM system (Hemochron Response
TM) during AF ablation without any complications indicated that the recommended optimal target ACT range of 300 (–350) s was in good agreement with the expert consensus statement.[
22] When this range was adjusted using the PO method (ACT CA-300
TM), the adjusted range was 250–285 s. The adjusted Hemochron response to ACT CA-300 was identical to that of Hemochron ACT 300–350 and HemoTech ACT 250–275 in percutaneous coronary intervention.[
23,
24,
25] There are numerous ACT measurement machines, which can be divided into the Hemochron type (EM type) and HemoTech type (PO type). The present results clarify that the target optimal ACT should be adjusted when the ACT measurement machine is changed, using the same blood samples for each machine.
The ACT during procedure associated with cardiac tamponade ranged from 330 s to 391 s, which overlapped with the ACT during procedures without complications. In cardiopulmonary bypass for cardiac surgery, an ACT over 400–480 s is recommended.[
26] Therefore, cardiac tamponade was not related to excessive anticoagulation by heparin, and ACT did not indicate over-anticoagulation by heparin in these patients with cardiac tamponade. Cardiac tamponade occurs due to complicated mechanisms, including LA pathological conditions, the ablation catheter used, and technical factors. Thus, the present results indicate that an ACT range of 300-400 s, as measured by the EM system was optimal.
Limitations
First, we did not compare various ACT measurement machines. Therefore, we cannot extrapolate our findings to other measurement machines. However, the present study provides information on the differences between Hemochron and HemoTech machines. Second, we did not perform brain magnetic resonance imaging to detect subclinical brain thromboembolisms. However, there is insufficient information concerning clinically unrecognized cerebral embolisms complicating CA for AF and their long-term effects. Therefore, this limitation does not represent a clinically significant deficit.
Conclusions
The ACT obtained using the EM system aligned with the ACT recommended in the guidelines. It is important to adjust the optimal target ACT when switching between ACT measurement systems; therefore, a standard ACT measurement system is required to ensure consistency.
What is already known on this topic:
Activated clotting time (ACT) is widely utilized to monitor anticoagulation status with heparin during atrial fibrillation ablation. However, ACT measurements have been demonstrated to be affected by the measurement methods used.
What this study adds:
The ACT obtained using the electromagnetic system aligned with the ACT recommended in the guidelines. However, the photo-optical detection system showed significantly lower ACT values compared to those obtained with the electromagnetic system.
How this study might affect research, practice, or policy:
This study clarifies that adjusting the optimal target ACT when switching between ACT measurement systems is essential.
Author Contributions
All authors meet the criteria for authorship. Contribution of each author to the four criteria of contribution noted below was as follows: H.S. contributed to 1), 2), 3), and 4). H.Y. contributed to 1), 2), 3), and 4). S.O contributed to 1), 3), and 4). K.O. contributed to 1), 3), and 4). Y.F. contributed to 2) and 3). S.H. contributed to 3) and 4). T.M. contributed to 3) and 4). S.H. contributed to 3) and 4). S.K. contributed to 1), 2), 3), and 4). Criteria of author contribution: 1. Made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data. 2. Participated in drafting the article or revising it critically for important intellectual content. 3. Gave final approval of the version to be published. 4. Agreed to be accountable for all aspects of the work to ensure that questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
The examination and analytical procedures adhered to the principles of the Declaration of Helsinki and were approved by the Institutional Ethics Committee for Human Research of the Okayama Heart Clinic (ID, TK1). Written informed consent for the use of data without personally identifiable information was obtained from all patients.
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author, S. K., upon reasonable request.
Conflicts of Interest
There are no conflicts of interest in connection with the present study.
References
- Medi C, Sparks PB, Morton JB, et al. Pulmonary vein antral isolation for paroxysmal atrial fibrillation: results from long-term follow-up. J Cardiovasc Electrophysiol. 2011 Feb;22:137-41. [CrossRef]
- Schreiber D, Rostock T, Frohlich M, et al. Five-year follow-up after catheter ablation of persistent atrial fibrillation using the stepwise approach and prognostic factors for success. Circ Arrhythm Electrophysiol. 2015 Apr;8:308-17.
- Takahashi Y, O'Neill MD, Hocini M, et al. Characterization of electrograms associated with termination of chronic atrial fibrillation by catheter ablation. J Am Coll Cardiol. 2008 Mar 11;51:1003-10.
- Tsai CF, Tai CT, Hsieh MH, et al. Initiation of atrial fibrillation by ectopic beats originating from the superior vena cava: electrophysiological characteristics and results of radiofrequency ablation. Circulation. 2000 Jul 4;102:67-74.
- Rolf S, Kircher S, Arya A, et al. Tailored atrial substrate modification based on low-voltage areas in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2014 Oct;7:825-33. [CrossRef]
- Jadidi AS, Lehrmann H, Keyl C, et al. Ablation of Persistent Atrial Fibrillation Targeting Low-Voltage Areas With Selective Activation Characteristics. Circ Arrhythm Electrophysiol. 2016 Mar;9.
- Horton S, Augustin S. Activated clotting time (ACT). Methods Mol Biol. 2013;992:155-67.
- Avendano A, Ferguson JJ. Comparison of Hemochron and HemoTec activated coagulation time target values during percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1994 Mar 15;23:907-10.
- Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: Executive summary. Europace. 2018 Jan 1;20:157-208.
- Yamaji H, Murakami T, Hina K, et al. Adequate Initial Heparin Dosage for Atrial Fibrillation Ablation in Patients Receiving Non-Vitamin K Antagonist Oral Anticoagulants. Clin Drug Investig. 2016 Oct;36:837-48.
- Yamaji H, Murakami T, Hina K, et al. Usefulness of dabigatran etexilate as periprocedural anticoagulation therapy for atrial fibrillation ablation. Clin Drug Investig. 2013 Jun;33:409-18.
- Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013 Mar;48:452-8.
- Faul F, Erdfelder E, Buchner A, et al. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009 Nov;41:1149-60. [CrossRef]
- Di Biase L, Burkhardt JD, Santangeli P, et al. Periprocedural stroke and bleeding complications in patients undergoing catheter ablation of atrial fibrillation with different anticoagulation management: results from the Role of Coumadin in Preventing Thromboembolism in Atrial Fibrillation (AF) Patients Undergoing Catheter Ablation (COMPARE) randomized trial. Circulation. 2014 Jun 24;129:2638-44.
- Di Biase L, Gaita F, Toso E, et al. Does periprocedural anticoagulation management of atrial fibrillation affect the prevalence of silent thromboembolic lesion detected by diffusion cerebral magnetic resonance imaging in patients undergoing radiofrequency atrial fibrillation ablation with open irrigated catheters? Results from a prospective multicenter study. Heart Rhythm. 2014 May;11:791-8.
- Cappato R, Marchlinski FE, Hohnloser SH, et al. Uninterrupted rivaroxaban vs. uninterrupted vitamin K antagonists for catheter ablation in non-valvular atrial fibrillation. Eur Heart J. 2015 Jul 21;36:1805-11.
- Chew DP, Bhatt DL, Lincoff AM, et al. Defining the optimal activated clotting time during percutaneous coronary intervention: aggregate results from 6 randomized, controlled trials. Circulation. 2001 Feb 20;103:961-6.
- Konduru SV, Cheema AA, Jones P, et al. Differences in intraprocedural ACTs with standardized heparin dosing during catheter ablation for atrial fibrillation in patients treated with dabigatran vs. patients on uninterrupted warfarin. J Interv Card Electrophysiol. 2012 Dec;35:277-84; discussion 84.
- Nagao T, Inden Y, Yanagisawa S, et al. Differences in activated clotting time among uninterrupted anticoagulants during the periprocedural period of atrial fibrillation ablation. Heart Rhythm. 2015 Sep;12:1972-8.
- Hohnloser SH, Camm J, Cappato R, et al. Uninterrupted edoxaban vs. vitamin K antagonists for ablation of atrial fibrillation: the ELIMINATE-AF trial. Eur Heart J. 2019 Sep 21;40:3013-21.
- Kirchhof P, Haeusler KG, Blank B, et al. Apixaban in patients at risk of stroke undergoing atrial fibrillation ablation. Eur Heart J. 2018 Aug 21;39:2942-55.
- Tzeis S, Gerstenfeld EP, Kalman J, et al. 2024 European Heart Rhythm Association/Heart Rhythm Society/Asia Pacific Heart Rhythm Society/Latin American Heart Rhythm Society expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. 2024 Mar 30;26.
- Zucker M, Johari V, Bush V, et al. Cooagulation. In: Nichils J, editor. Evidence-based practice for point-of care testing. Washington DC, USA: AACCPress; 2006. p. 21-9.
- Ferguson JJ, 3rd. Conventional antithrombotic approaches. Am Heart J. 1995 Sep;130:651-7. [CrossRef]
- Klein LW, Agarwal JB. When we "act" on ACT levels: activated clotting time measurements to guide heparin administration during and after interventional procedures. Cathet Cardiovasc Diagn. 1996 Feb;37:154-7.
- Shore-Lesserson L, Baker RA, Ferraris V, et al. STS/SCA/AmSECT Clinical Practice Guidelines: Anticoagulation during Cardiopulmonary Bypass. J Extra Corpor Technol. 2018 Mar;50:5-18.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).