1. Introduction
The skin, the body's largest organ, plays a crucial role in numerous functions. Beyond its sensory capacity, it acts as a dynamic interface between the internal and external environments, enabling the continuous adaptation and acclimatization of the organism throughout its lifespan [
1,
2].
Collagen, a protein primarily synthesized by fibroblasts in the connective tissues, constitutes the most abundant component of the extracellular matrix of the skin, representing over 75% of the dry weight of a young and healthy human dermis [
3,
4]. Fibroblasts, as connective tissue cells within the dermis, play a pivotal role in the synthesis and organization of the collagen matrix. These cells exhibit sensitivity to both physical and chemical stimuli, which can trigger fibroblast activation and proliferation. Chemical stimuli operate via a key-lock mechanism, wherein small ligands bind to receptors located on the fibroblast extracellular membrane, leading to their activation. On the other hand, physical stimuli directly influence the interactions between collagen and fibroblasts [
3,
4].
Thus, collagen is a cornerstone in the skin`s extracellular matrix, essential for maintaining structural integrity and physiological functions. It retains water and plays a pivotal role in maintaining the skin's smoothness, firmness, and resilience [
4]. This enables the skin to effectively respond to the ever-changing onslaught of environmental stressors [
5].
The aging process of the skin is an ongoing phenomenon, marked by a gradual decline in structural and physiological functions, often exacerbated by environmental factors and dermatological disorders [
6,
7].
Aging precipitates a decline in collagen synthesis within mature skin, attributed to diminished activity of enzymes involved in collagen post-translational processing, a reduction in the population of collagen-synthesizing fibroblasts, and a decrease in skin vasculature [
8,
9,
10]. Consequently, the skin's biomatrix begins to deteriorate as the collagen scaffold loses its strength and stability [
4,
11,
12].
Extrinsic factors such as sunlight exposure, smoking, pollution, alcohol consumption, an unbalanced diet, and stress-related micronutrient deficiencies expedite this process, contributing to the process of collagen loss associated with aging skin [
4,
12,
13,
14]. The consequences of aging on the skin are manifold. It undergoes regressive changes, losing its integrity and becoming progressively thin and dry, leading to a compromised ability to retain enough moisture. Additionally, the reduction in dermal thickness and elasticity over time manifests as lines and wrinkles, further contributing to the visible signs of aging [
15].
To counteract age-related changes and rejuvenate the biomatrix, hydrolyzed collagen has emerged as a popular and promising nutraceutical for skin anti-aging. Collagen supplements are available in various forms, including gels, liquids, capsules and powder [
2,
16,
17,
18,
19].
While some studies suggest potential benefits of collagen supplementation, it is crucial to elucidate its potential implications for skin health and aging as well as to consider individual factors and preferences [
19,
20,
21,
22].
A tailored enzymatic digestion of native collagen preserves the collagen-specific secuence Gly-Pro-Hyp (GPH) and yields a low molecular weight (LMW) hydrolysate, enriched with this active tripeptide that seems to exert beneficial effects in various tissues, including skin, muscles, joints and bones [
23,
24,
25]. Therefore, COLLinstant LMW, a novel cosmeceutical with low molecular weight (≤ 1000 Da) collagen peptides distinguishes itself, in contrast to other regular collagen hydrolysates, by containing a high proportion of small GPH active peptides that could improve the bioavailability, mode of action and thereby, the efficacy of the product.
The objective of the present study is to assess the effectiveness of daily supplementation with COLLinstant® LMW over a period of six weeks in ameliorating visible signs of aging. This includes assessing its impact on skin wrinkle reduction, as well as its potential to enhance skin elasticity and moisturization. COLLinstant® LMW was administered orally in a single-center, randomized, double-blind, placebo-controlled clinical trial. A secondary objective involves comparing skin improvement, assessing product satisfaction, and monitoring adverse events among middle-aged female volunteers.
2. Materials and Methods
2.1. Study Design and Ethical Aspects
This was a 6-week, prospective, randomized, placebo-controlled, double-blind, monocentric study performed at GALA Laboratories in Don Benito-Villanueva (Badajoz, Spain). This study is listed on the Clinical Trials.gov registry (NCT06321770).
Participants were individually randomized (1:1 ratio) to a strategy of receiving either COLLinstant LMW (collagen group) or a placebo regimen and were followed up for 6 weeks. Subjects were instructed to follow the administration guidelines provided in the manufacturer's package for both product regimens and, if necessary, investigator guidance.
The investigation was performed according to the ethical guidelines detailed in the Declaration of Helsinki (amendment of the 64th General Assembly, Fortaleza, Brazil, October 2013) and with national regulations of Spain, and in full compliance with the applicable principles of good clinical practice (GCP) and International Council for Harmonisation (ICH) of Technical Requirements for Pharmaceuticals for Human Use [
26]. The trial protocol was approved (code 075-2022) by the Clinical Research Ethics Committee at the University Hospital of Cáceres (Caceres, Spain) and written informed consent was obtained from all subjects prior to any study procedures being initiated.
2.2. Investigational Product
The preparation under study was COLLinstant® LMW (Viscofan DE GmbH, Weinheim, Germany), an oral food supplement based on bovine bioactive hydrolyzed type I and III-collagen peptides.
Following ICH-GCP requirements and applicable local regulations[
26], the investigational product and placebo were formulated as powder-containing sachets for oral suspension that were identical in appearance and odor.
Each sachet of the investigational product (COLLinstant® LMW) contained low molecular weight hydrolyzed 2.5 g collagen peptides. Other ingredients, which were also contained in the placebo, were 467 mg lemon flavour, 150 mg anhydrous citric acid, 8.5 mg sucralose and 7.1 mg stevia (97%). The placebo did not contain any nutrients.
2.3. Study Subjects
We recruited a total of 80 women (aged 30-65 years) with phototypes I-IV (Fitzpatrick scale) [
27], who were mentally and physically healthy, had a BMI 20.0-29.9 kg/m2 and displayed visible signs of natural and photoaging on their face (crow´s feet) rated from moderate to severe [
28].
The Fitzpatrick scale is a numerical classification system for human skin color, ranging from type I to type VI, based on the amount of melanin in the skin. This classification informs the skin's susceptibility to burns and its ability to tan [
27,
29].
During the screening phase, participants met all inclusion and exclusion criteria and agreed to refrain from prolonged exposure to ultraviolet (UV) radiation throughout the study duration. Subjects were excluded if they were pregnant or lactating, had acute or chronic skin diseases or dermatological disorders, used natural health supplements for skin improvement within one month prior to the study commencement, followed a low protein diet, had planned or unavoidable UV radiation exposure, had tattoos on or near the test area, used systemic corticosteroids or applied topical alpha hydroxy acids near the test site within four weeks of enrollment, used topical medications near the test area within six weeks of enrollment, underwent Botulinum toxin A (Botox) treatment or filler injection (collagen, hyaluronic acid, etc.) near the test sites within two years of enrolment, were cognitively impaired and/or unable to provide informed consent, or had any other condition that, in the medical investigator's opinion, might adversely affect the individual's ability to complete the study or its measures or pose significant risk to the individual.
2.4. Study Schedule and Biometric Evaluation
All participants (test and placebo groups) were instructed to consume the content of one sachet daily, in the morning, on an empty stomach for 6 weeks. It was required that the product was dissolved in at least 100 ml of water, juice or other liquid.
For all women participating in the study, skin parameters were assessed at baseline (T0), and biometric changes were also evaluated after 6 weeks of treatment with the products (T6).
Measurement of skin wrinkling parameters (volume, area and depth) was evaluated at the crow`s feet region and changes were analyzed and digitally photographed in all patients by VisioFace® 1000D (equipped with a high-resolution reflex camera) [
30].
According to Mödinger et al [
31] subjects underwent acclimatization for a minimum of 30 minutes in the air-conditioned measurement room set at a temperature of 21 ± 1°C and a relative humidity of 50 ± 5%.
Skin elasticity at the crow's feet region was quantified using a Cutometer® dual MPA 580 (Courage & Khazaka), a non-invasive instrument designed to assess skin biomechanical properties [
32]. This device evaluates skin elasticity by applying negative force that mechanically deforms the skin. The operational principle involves using a probe with negative pressure (450 mbar) to suction the skin, drawing the test area into the probe's aperture. A non-contact optical measurement system then determines the depth of skin penetration. The evaluated parameters (R0, R2, R5, R7 y R9) are key indicators used to characterize skin biomechanical properties following suction force application [
33,
34,
35,
36,
37,
38,
39].
R0 represents the final distension of the initial curve, reflecting the passive response of the skin to suction force and correlating with skin firmness. It is calculated as the difference between the highest point of amplitude at the end of the suction phase and the baseline reading (R0 = Uf). The R2 parameter is related to the gross elasticity/viscoelasticity, representing the skin's resistance to mechanical suction force relative to its ability to recover (R2 = Ua/Uf). R5, referred to as net elasticity, signifies the ratio of elastic deformation during suction to rapid recovery during relaxation (R5 = Ur/Ue), indicating higher skin elasticity with increasing values. R7 is related to biological elasticity, quantifying the immediate elastic recovery within the first 0.1 seconds compared to the total deformation after suction (R7 = Ur/Uf) and can be interpreted as another marker of elasticity, with aging causing its reduction. Lastly, R9 denotes residual deformation at the conclusion of the measurement cycle, reflecting skin fatigue following repeated suction (R9 = R3 - R0) [
32,
37,
38,
40,
41]. The measurements were carried out in triplicate.
Furthermore, stratum corneum hydration was assessed at each study visit using the electrical capacitance method with a Corneometer® CM 825 (Courage & Khazaka, Cologne, Germany). A minimum of five measurements were taken per measurement area at four distinct locations (middle forehead, both cheekbones, and the chin area), and the average value was used for subsequent analysis [
31,
39,
41].
2.5. Safety and Self-Reported Measures
Safety was evaluated based on adverse reactions reported by the patients during the treatment period. Adverse events were documented at the final visit following six weeks of treatment.
After 6 weeks of treatment, the volunteers filled out questionnaires, to subjectively assess their perception of different parameters such as efficacy, organoleptic properties, tolerability and satisfaction since the last time they took the product. The Spanish version of the Treatment Satisfaction Questionnaire with Medication (TSQM) [
42], and a 3-point Likert scale with the following items: Dissatisfied, slightly satisfied and very satisfied, were used.
2.6. Statistical Methods
All statistical analyses were performed using IBM® SPSS® Statistics for Windows (version 27.0) and JASP [
43]. The analysis of the distribution and normality tests of the variables were carried out using the Shapiro-Wilk tests.
All measured data are presented as mean ± SD (standard deviation). Categorical variables were summarized using the number and percentages of patients in each category. Skin parameters (wrinkling, elasticity, and hydration) were evaluated descriptively at baseline (T0) and after 6 weeks of collagen supplementation (T6). The efficacy was assessed based on relative changes in these parameters, calculated as the differences between means at T0 and T6.
Graphical representations of the results were generated using box-and-whisker plots. The box spans from the 25th to the 75th percentile of the data. Whiskers extend from the edges of the box to the minimum and maximum values that are not considered outliers. Outliers, defined as values more than 1.5 times the interquartile range from the box, are depicted as symbols beyond the whiskers.
The between-group comparison (placebo group vs. verum group) was carried out to determine population homogeneity at baseline (T0); and, to analyse treatment-related differences after 6 weeks (at T6) by means of the Mann-Whitney U test, a non-parametric test applied to two independent samples. Comparisons between categorical variables are performed using the Chi-square test.
The within-group mean changes of skin parameters between the initial (T0) and final (T6) visits were evaluated by the non-parametric Wilcoxon signed-rank test, for paired data. The threshold of statistical significance was set, in all cases, for a value of p < 0.05.
3. Results
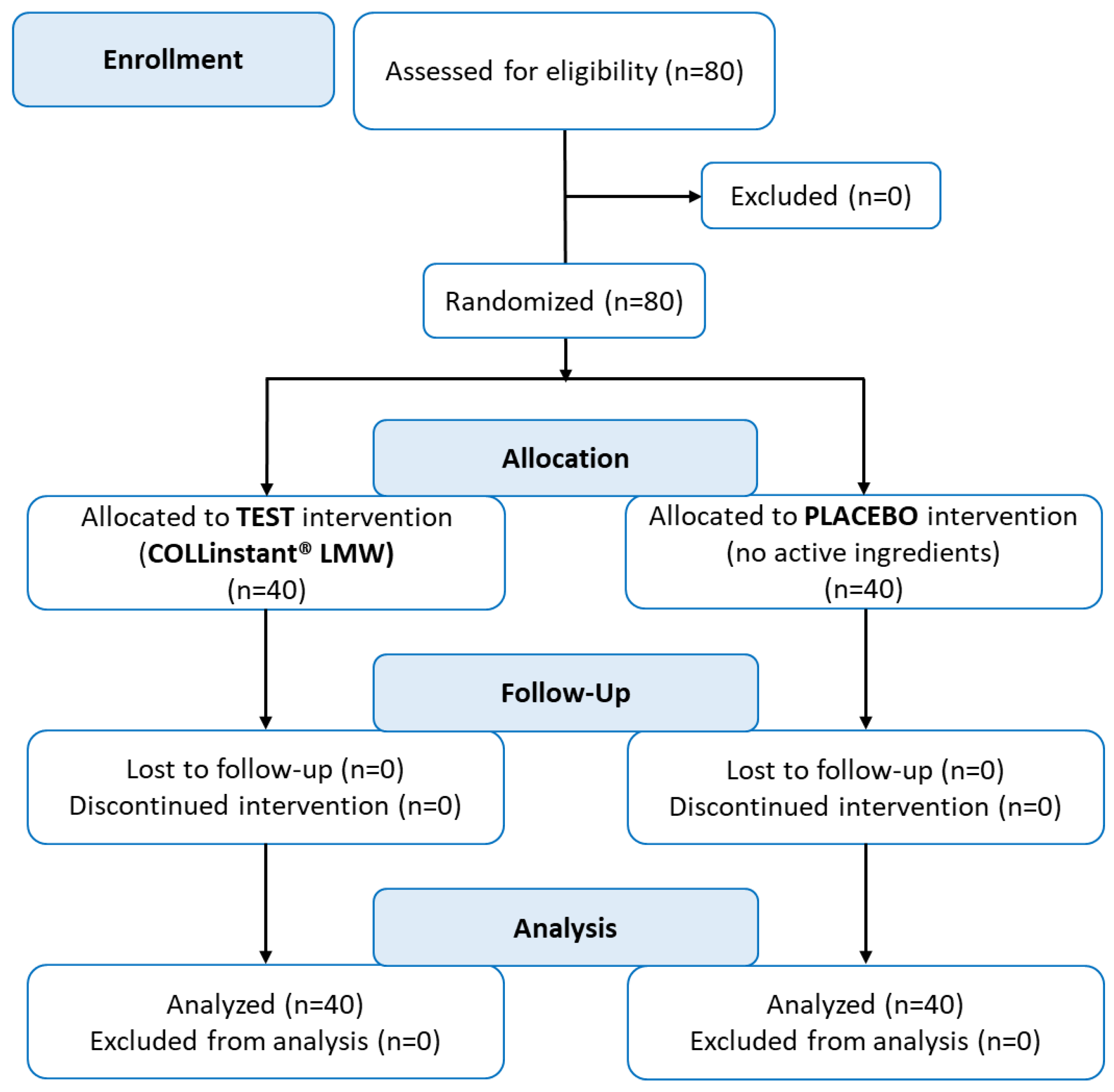
3.1. Consort (Consolidated Standards of Reporting Trials) Flowchart of the Controlled Interventional Trial
Eighty women aged 30 to 60 years were included in the statistical analysis. No subjects were excluded during screening or throughout the study, and no protocol violations occurred. Compliance during the trial was excellent; therefore, all volunteers (n= 80) who were screened for eligibility and requested to participate, completed the study protocol, and could therefore be analysed. The flow of subjects through the controlled intervention trial is depicted in a diagram according to CONSORT guidelines (
Figure 1) [
44].
3.2. Characteristics of the Population
Participants (n= 80) were randomized at baseline (T0) and allocated to the verum group (n= 40) or the placebo group (n= 40). Demographic and general features of the volunteers did not show any significant difference between the test product and the placebo group at baseline (
Table 1).
The investigational treatment group included 40 women who received the bioactive collagen peptide-based food supplement orally.
The mean age in the placebo group (n= 40) was 47 ± 7.7 years (age range between 32 and 60 years); and in the experimental group (n= 40), 45 ± 7.1 years (age range between 30 and 58 years), with no significant differences between the two groups (p = 0.22).
The predominant skin type in both groups (placebo and experimental) was "normal skin" (67.5% in the experimental group vs. 75% in the placebo group), followed by "sensitive skin" (30% in the experimental group vs. 20% in the placebo group), with no significant differences between the two groups (p = 0.27).
Among all participants, the most represented Fitzpatrick skin classification was phototype III (slightly brown skin and brown hair) that was predominant in both groups (90% in the placebo group; 85% in the verum group). No significant differences were detected between the two groups for this dermatological parameter (p = 0.20).
Homogeneity tests between groups revealed no significant differences for the mean initial skin parameters at baseline (T0) between the placebo group and the collagen group (
Table 2).
3.3. Skin Wrinkling
The descriptive analysis of skin wrinkling biometric parameters (volume, area and depth) of the facial crow`s feet region, before intake of the product (at T0), and after 6 weeks of intake (at T6), is summarized in
Table 3. The mean value of three determinations was used for analysis. At baseline the mean skin wrinkling biometric parameters were similar between both groups of treatment (
Table 2). Regarding the intraindividual comparison from baseline (T0-T6), all biometric parameters (volume, area and depth) considerably improved at T6, after 6 weeks of treatment in the collagen group, whereas they remained unchanged in the placebo group during the same period (
Table 3).
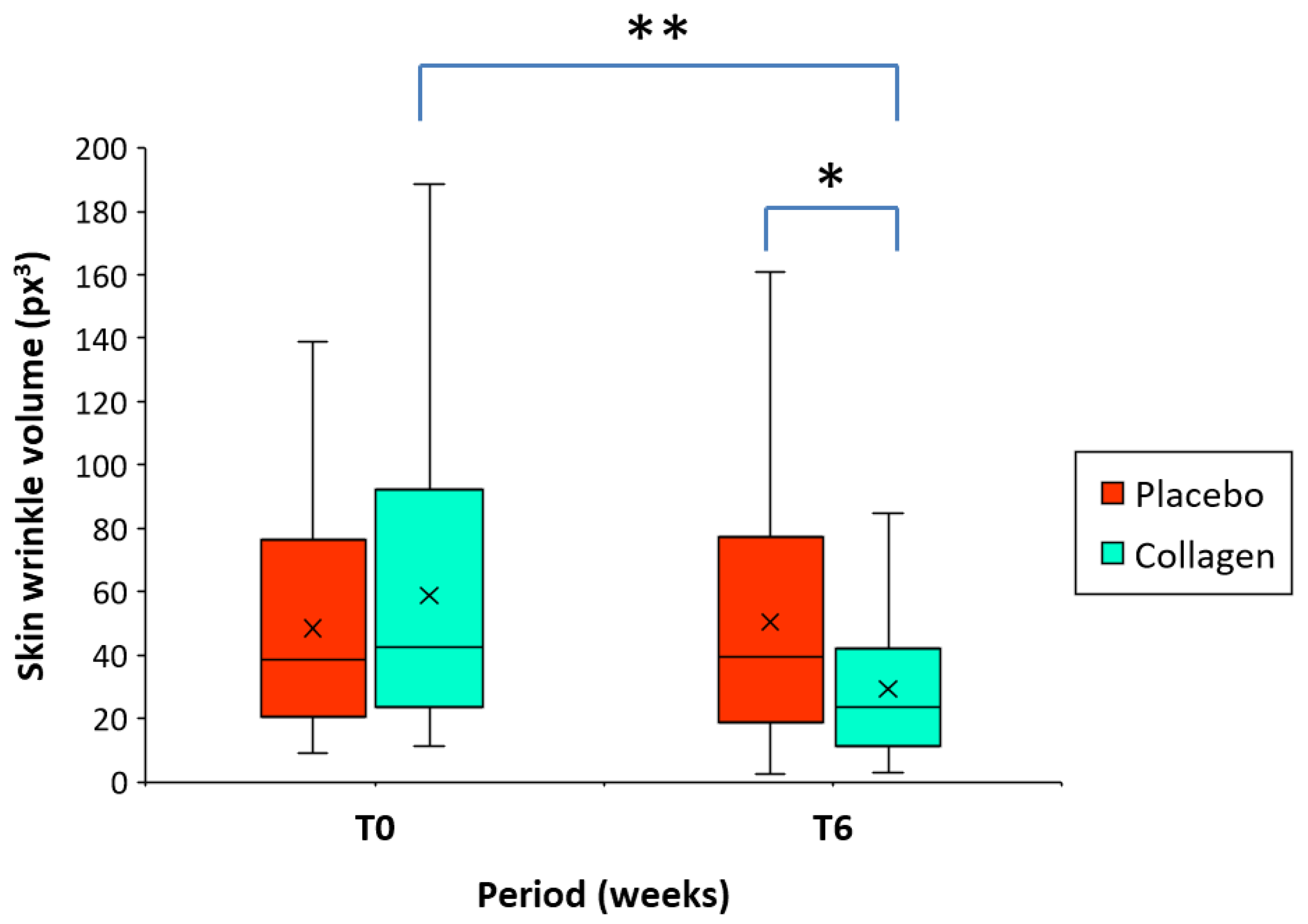
At baseline (T0), no significant differences were found for the mean skin wrinkle volume at the crow`s feet region between the placebo group (48.50 px³) and the collagen group (58.77 px³) (
Table 2). After 6 weeks of intake of the study products, at T6, the mean volume of wrinkles significantly decreased from baseline (T0-T6) by -45.9% [-90.8% – (-2.9%)] in the collagen group (58.77 vs. 30.24 px³; p < 0.001) but remained relatively unchanged by 0.32% (-90.4% - 20.9%) in the placebo group (48.50 vs. 50.30 px³) (
Table 3 and
Figure 2). The difference between groups (placebo vs. collagen) at T6 proves to be significant (p < 0.01) in favor of the test product (
Table 3 and
Figure 2).
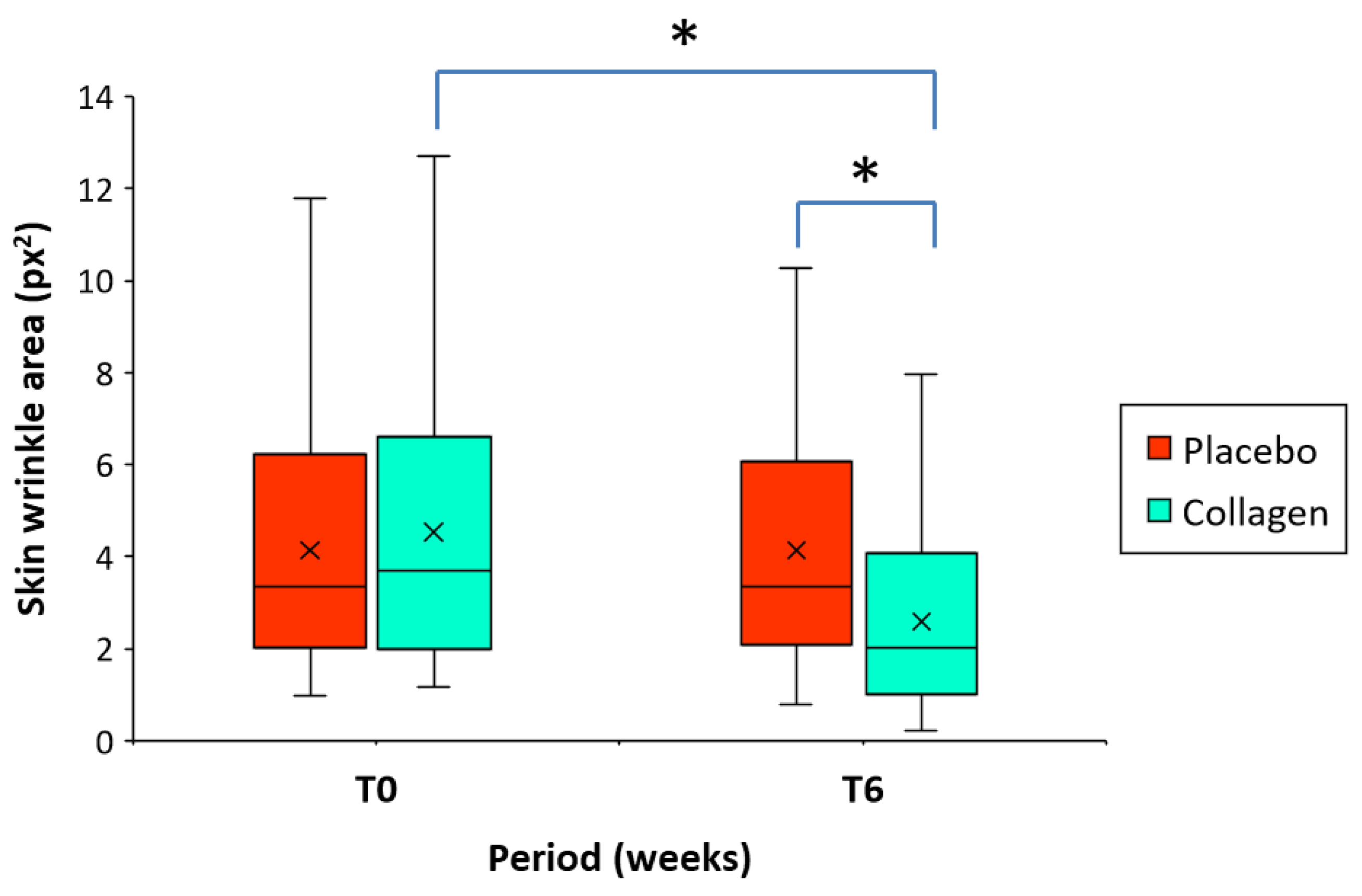
Starting at baseline from similar initial mean wrinkle area of 4.13 px
2 (placebo group) and 4.53 px
2 (collagen group) (
Table 2), after an intake of 6 weeks of either placebo or collagen, the area of skin wrinkle at crow`s feet region significantly decreased from baseline (T0-T6) by -43.8% [-94.4% – (-2.6%)] (4.53 vs. 2.60 px
2; p < 0.001) in the collagen group, but the intraindividual difference was almost negligible by 1.66% (-24.6% – 41.7%), in the placebo group (4.13 vs. 4.14 px
2) (
Table 3 and
Figure 3). At T6, the intergroup comparison (placebo group vs. collagen group) of the mean wrinkle area proved to be significant (p < 0.001) in favor of the test product (
Table 3 and
Figure 3).
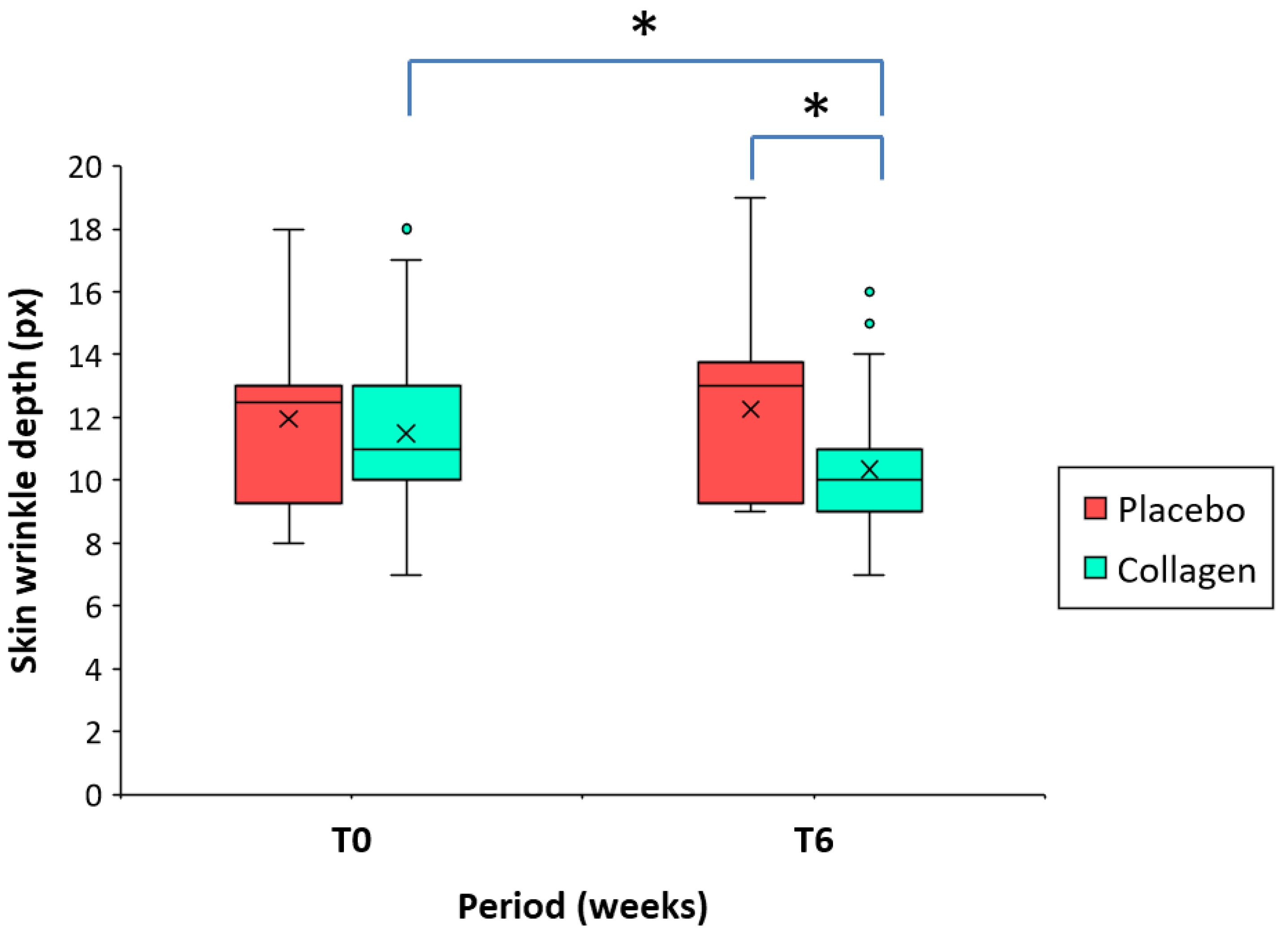
Table 2 shows that mean wrinkle depth values were similar at baseline of 11.95 px (placebo) and 11.50 px (collagen group). However, in line with improvements in volume and area, skin wrinkle depth decreased during intake of the investigational product. As shown in
Table 3 and
Figure 4, at T6, the depth of wrinkles significantly decreased from baseline (T0-T6) by -9.0% (-40.0% – 0.0%) (11.50 vs. 10.35 px; p < 0.001) in the collagen group. On the other hand, the intraindividual variation was non-significant and limited to 3.3% (-33.3% – 40.0%) (11.95 vs. 12.25 px) in the placebo group. The differences between the groups at T6 (placebo group vs. collagen group) of the mean wrinkle depth proved also to be highly significant (p < 0.001) in favor of the test product (
Table 3 and
Figure 4).
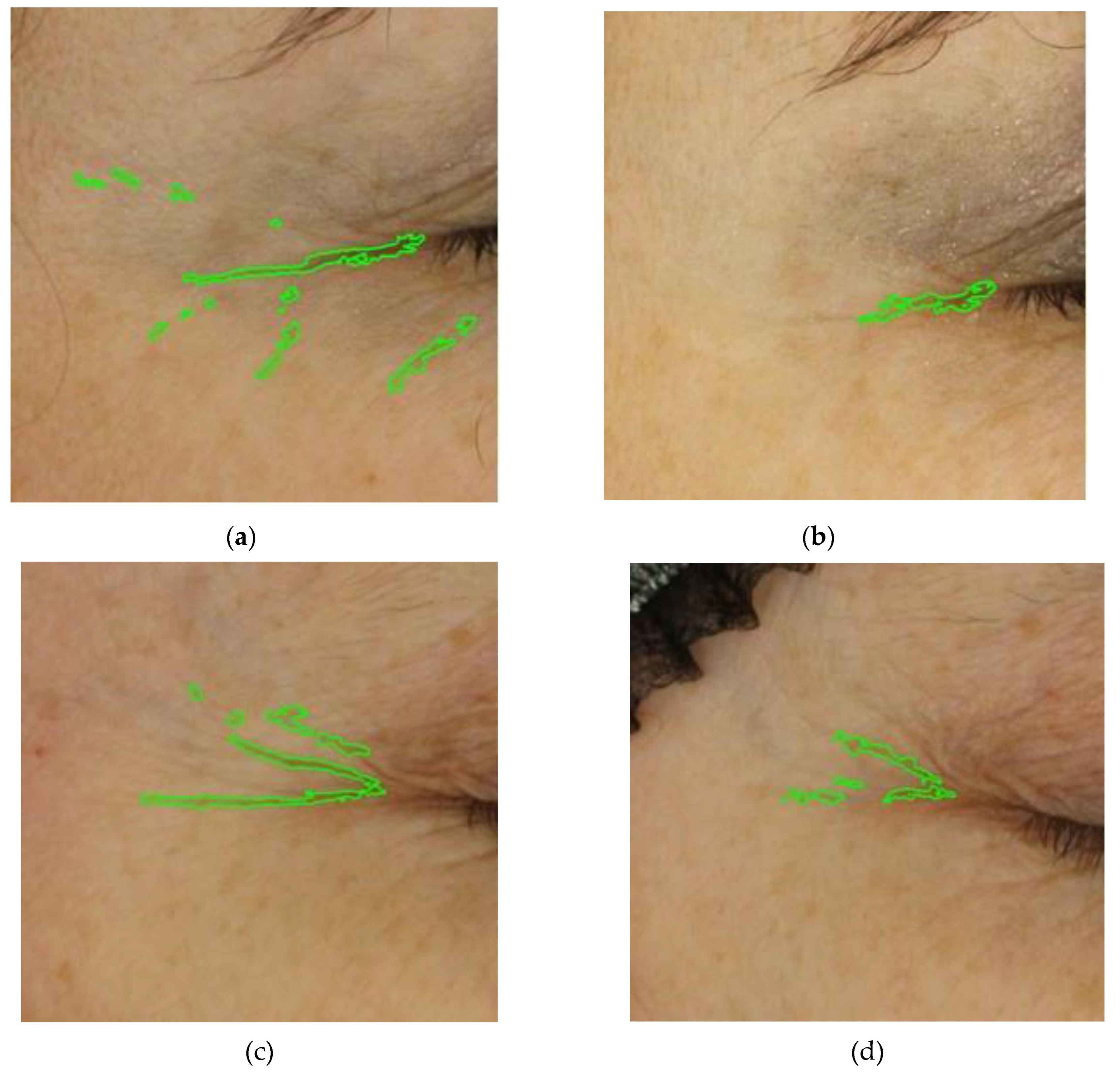
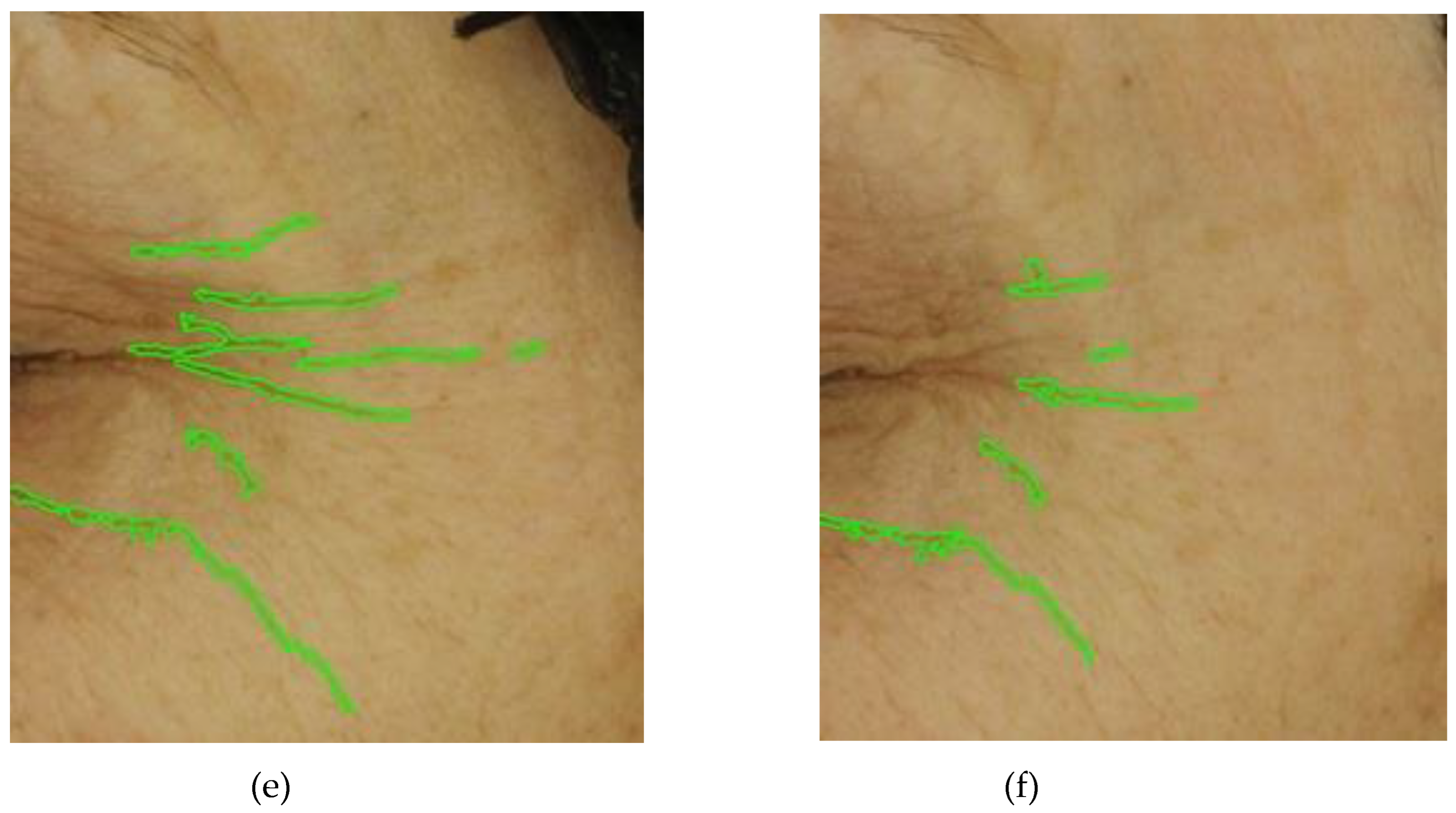
Figure 5 illustrates photographs of three volunteers showcasing the improvement from the initial visit at T0, in facial wrinkling following the consumption of the investigational product COLLinstant
® LMW for six weeks. The skin assessment was conducted using objective and validated methods (Visioface 1000D). In volunteer no. 13, there was a percentage change in volume, area, and depth of -59.18%, -51.91%, and -15.4%, respectively. For volunteer no. 60, the corresponding changes were -64.8%, -46.2%, and -40.0%, and for volunteer no. 70, the changes were -37.1%, -31.4%, and -7.2%, respectively.
3.4. Skin Elasticity
The descriptive analysis of skin elasticity obtained from the Cutometer
® device before intake of the product (at T0) and after 6 weeks of intake (at T6) is summarized in
Table 4. The mean value of three determinations at the crow's feet region was used for analysis. At baseline (T0) the skin elasticity parameters were similar between both groups of treatment (
Table 2).
Results showed that skin firmness (R0) significantly increased in both groups of treatment. Compared to baseline (T0), the assessment of total elongation and skin firmness (R0) showed statistically significant lower values (p < 0.001) after 6 weeks in both the group of treatment receiving placebo and the collagen group (
Table 4). At the end of the study, at T6, we could not demonstrate significant differences in the R0 parameter between both group means (
Table 4).
The percentage of variation in mean skin firmness (R0) from baseline was -8.6% (-50.8% - 24.1%) in the placebo and -10.7% (-61.3 % - 29.0 %) in the group that received the food supplement.
The assessment of the remaining skin elasticity parameters (R2, R5, R7 and R9) showed a moderate improvement, but the differences were not statistically significant (
Table 4).
At T6, the assessment of the rest of skin elasticity parameters (R2, R5, R7 and R9) obtained with the Cutometer
® device did not show any statistical significance between groups (
Table 4) indicating that the food supplement did not show an improvement in most skin elasticity parameters in our study.
3.5. Skin Hydration
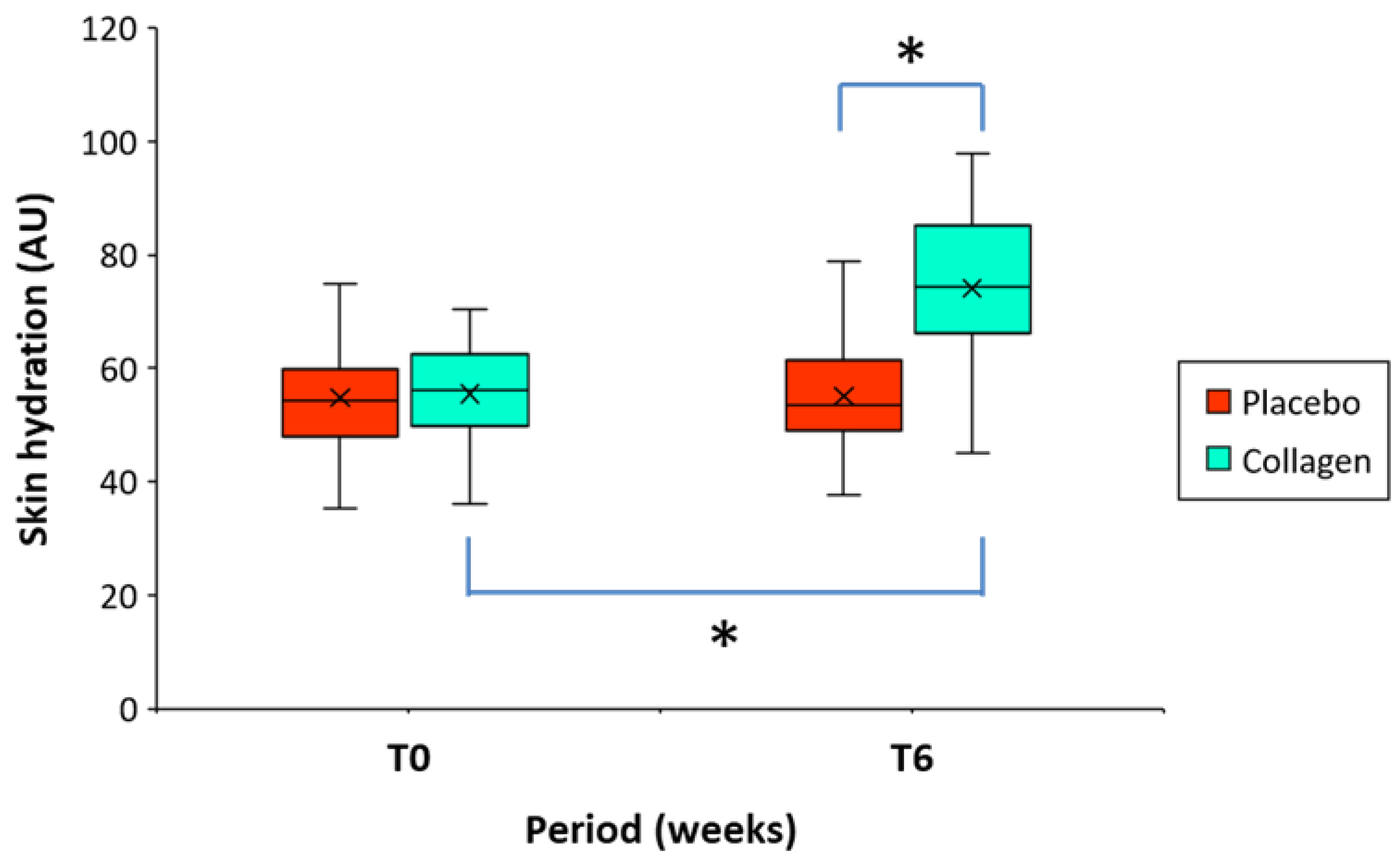
The descriptive analysis of skin hydration before intake of the product (at T0) and after 6 weeks of intake (at T6) is summarized in
Table 5. The mean value of five determinations at four different locations (middle forehead, both right and left cheek and the chin) was used for analysis. At baseline (T0) the skin hydration values were similar between both groups of treatment, 54.77 AU (placebo group) vs. 55.50 AU (collagen group) (
Table 2).
Corneometric methodology corroborated the improvement in skin hydration in the volunteers who received the test product. Compared to baseline (T0), it has been detected a statistically significant improvement in skin hydration by +34.4% (9.2% - 98.0%) (55.50 AU vs. 74.13 AU, p < 0.001) in the volunteers who received the investigational product for 6 weeks (T0-T6) (
Table 5). On the other hand, the percentage change in skin hydration was non-significant and limited to +1.0% (-30.3% - 17.6%) (54.77 vs. 55.03 AU) in the placebo group (
Table 5 and
Figure 6). The differences between the groups at T6 (placebo group vs. collagen group) of the mean hydration values proved to be highly significant (p < 0.001) in favor of the test product (
Table 5 and
Figure 6)
.
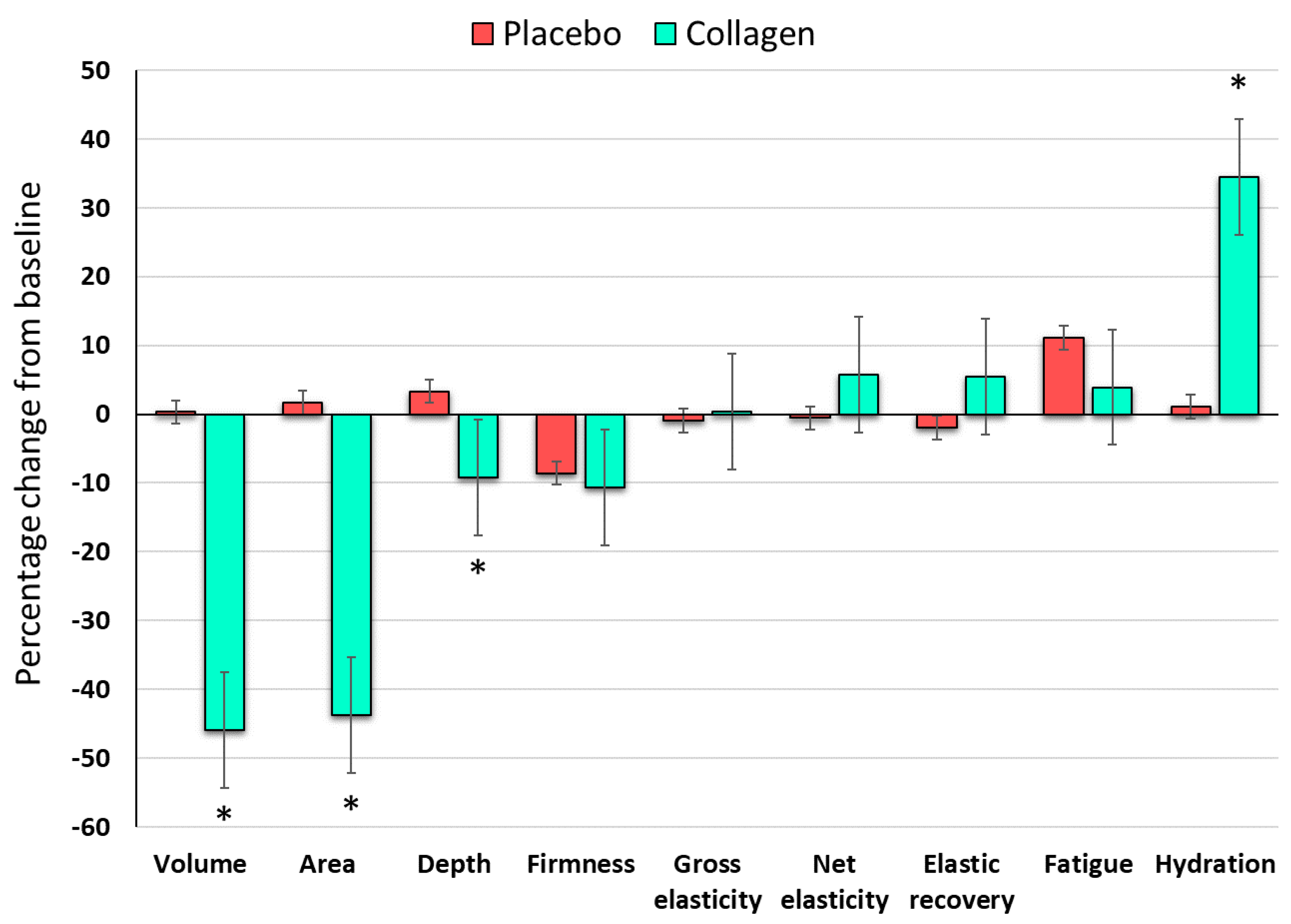
3.6. Overall Assessment of the Efficacy
Compared with placebo, the efficacy of the test product is summarized in
Figure 7. The differences between the relative changes (T0-T6) in skin wrinkle parameters (volume, area and dept), skin elasticity and skin hydration are illustrated. There was a statistically significant improvement (p < 0.001) in the skin wrinkle parameters and skin hydration in the collagen group. Regarding skin elasticity, the food supplement had only slight beneficial effects on skin elasticity parameters but we could not demonstrate statistical significance.
3.7. Safety and Subjective Rating
During the study intervention, the collagen supplement did not cause any side effects and proved to be safe and well tolerated during the entire period of application.
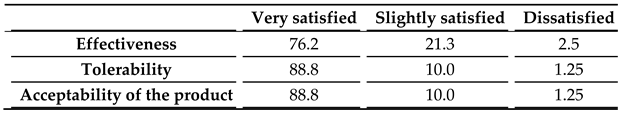
Over two thirds of the volunteers rated the overall effectiveness of COLLinstant® LMW as good, a survey result that was matched by the ratings of the treating physicians. Over 90% of the volunteers in the experimental group indicated a high degree of satisfaction with the ability of the supplement to improve skin hydration or wrinkles, confirming the robust effect of the treatment. In addition, 87.5% of the volunteers in this group was highly satisfied with the brief time period it takes for the supplement to start showing its effect, underlining the significance of obtaining quick results with a collagen food supplement.
Table 6.
Results of the survey conducted by the principal investigators with the volunteers who received the food supplement.
Table 6.
Results of the survey conducted by the principal investigators with the volunteers who received the food supplement.
4. Discussion
Skin aging involves an intricate number of biological processes characterized by changes that affect several components of the skin over time, influencing its appearance as an individual ages [
45]. Intrinsic skin aging, driven by factors like genomic instability, cellular senescence, and telomere shortening, contributes to declining skin function and appearance. Additionally, external factors such as UV radiation, pollution, tobacco smoke, and alcohol consumption accelerate this process, leading to premature, hastening this process and contributing to early skin aging [
45,
46].
The psychosocial impact of skin aging has spurred the demand for effective interventions, including topical creams, injectable fillers, and collagen supplements [
2,
47].
While topically applied collagen from skincare products such as creams, lotions, and serums often fail to penetrate the deeper layers of the skin [
2,
48], collagen supplements, particularly those containing hydrolyzed collagen, have emerged as a safe and cost-effective option, gaining popularity in recent years as a strategy to enhance skin health and maintain a youthful appearance [
4,
19,
47,
49,
50,
51].
In this context, COLLinstant® LMW, a novel cosmeceutical containing low molecular weight collagen peptides, was selected for the clinical trial. The study specifically aimed to assess the efficacy, safety, and tolerability of 2.5 g COLLinstant® LMW over a 6-week period, specifically assessing various skin parameters. We have demonstrated a significant reduction in some skin wrinkle biomarker indicators such as volume, area, and depth at crow's feet region. Additionally, compared with placebo, increased skin hydration and moderate improvement in skin elasticity were also found. However, we were unable to demonstrate differences in the parameters that assessed skin elasticity between the control group and the investigational group. The effects were exclusive to COLLinstant® LMW, as the subjects had no other form of cosmetic treatment.
Ingesting hydrolyzed collagen and in particular, low molecular weight collagen hydrolysate, has been shown as a promising and useful strategy to improve skin hydration and elasticity and thus, to counteract the changes associated with skin aging [
2,
4,
52]. COLLinstant® LMW is orally administered, making them easy to incorporate into daily routines. Nevertheless, not all sources of hydrolyzed collagen are equally effective, and further studies are warranted to determine the optimal source and therapeutic duration against skin aging. Different types of collagen used in these supplements, such as fish, porcine, chicken, and bovine collagen, may exhibit varying effects based on their source [
2,
4,
53,
54].
In the context of skincare and nutraceuticals, hydrolyzed bovine collagen has become a popular ingredient. COLLinstant® LMW has been the first bovine collagen hydrolysate in the market, providing a beneficial amino acid profile that serves as building material and stimulator for the synthesis of new collagen, elastin and hyaluronic acid in the skin as corroborated in different studies [
54]. The remarkable improvements in skin parameters observed in this investigation may be attributed to the substantial similarity between the collagen peptides derived from the bovine collagen complex and those naturally present in human collagen. Indeed, the hydrolysis of bovine collagen yields specific bioactive short-chain peptides that closely match the amino acid profile of human collagen I, as well as elastin and hyaluronic acid within the skin [
4,
55,
56,
57,
58,
59,
60]. This chemical resemblance likely contributes to the efficacy of the supplementation in enhancing skin health and appearance.
During the aging process, the relationship between enzymes and collagen turnover is complex. A pivotal molecular event is the dysregulation of extracellular matrix turnover, particularly the degradation of collagen fibers by matrix metalloproteinases (MMPs) and other proteases [
45]. Consequently, the skin undergoes structural changes, including a thinning and a reduction in epidermal thickness, along with damage to the dermis [
61,
62]. These structural changes result in regressive changes such as dehydration and loss of elasticity, leading to dry and loose skin with the appearance of furrows or wrinkles [
45,
63,
64]. According to the findings by Oesser et al. [
65], the observed improvements in our study are likely attributable to changes in protein turnover and the restoration of collagen synthesis within the dermal stratum of the skin. This was notably manifested in our study through increases in the volume, area, and depth of skin wrinkles following oral supplementation with collagen.
The importance of dosage and duration of the treatment emerged as crucial factors to be considerated. In contrast with the higher dosages and longer durations recommended for standard molecular weight collagen hydrolysates to achieve clear beauty or health benefits [
17,
66] notably, a daily intake of 2.5 g COLLinstant® LMW for 6 weeks was sufficient to yield beneficial effects on some biometric parameters of skin health in this study.
Regarding improvement on skin elasticity, our findings indicated a positive trend (as measured by the Cutometer) in the group treated with the investigational product. However, the observed differences did not attain statistical significance when compared with the placebo group. In order to obtain efficacy in enhancing skin elasticity through hydrolysed collagen supplementation a more prolonged treatment, exceeding 8 weeks, could be necessary [
54].
Collagen is characterized by a triple helix structure formed by the repetition of glycine every third residue, and particularly by proline and hydroxyproline in the other residues [
67,
68]. In this context, COLLinstant® LMW has a unique composition enriched with bioactive glycine- and proline-rich peptides that may help to increase efficacy of the product.
It has been demonstrated that the administration of collagen peptides can positively impact on various skin conditions and aging [
4,
54,
56]. These amino acids are crucial in the structure of collagen, and their presence in the form of bioactive peptides enhances gastrointestinal absorption [
3], making it highly bioavailable and detectable in human blood shortly after ingestion [
69,
70]. While normal weight collagen is degraded in the gastrointestinal tract but not readily cleaved into bioactive peptides, GPH and PH enriched in LMW collagen easily cross the intestinal barrier into the blood stream via the peptide transporter PEPT1 and remain intact over the entire gastrointestinal pathway [
23,
71,
72].
The efficacy of bioactive collagen di- and tripeptides in enhancing skin health appears to be attributed to several key mechanisms. First, the ability of these compounds to induce collagen expression through the mitogen-activated protein kinase 38 (p38 MAPK) pathway.[
73] Secondly, an increased availability of essential free amino acids to promote the synthesis of collagen and elastin fibers. Third, they have the potential to stimulate fibroblasts to produce collagen and hyaluronic acid [
3,
4,
74,
75,
76,
77].
The tested product of collagen supplementation was demonstrated to be safe, and no adverse effects were reported. Furthermore, the beneficial effects of collagen intake were not only fully validated through objective testing methods but also in the subjective assessments provided by the volunteers.
5. Conclusions
Exploring its potential as a natural intervention to uphold skin health and combating signs of aging, low molecular weight collagen peptides (COLLinstant® LMW) were investigated in a randomized, placebo-controlled clinical trial, following a daily oral supplementation of 2.5g of these peptides.
LMW collagen hydrolysate with a high concentration of these characteristic peptides has major advantages over standard molecular weight products, including quicker and more efficient peptide uptake, higher bioavailability, as well as enhanced stability and efficacy, especially in the skin.
The study substantiated the efficacy of these nutrients in restoring altered skin biometric parameters, as objectively assessed. We observed a significant reduction in wrinkles, a considerable increase in skin hydration, and modest improvements in skin elasticity. Regular supplementation may contribute to achieving smoother and more radiant skin.
A continuous supplementation has the potential to enhance skin texture by providing essential building materials in this tissue, stimulating the synthesis of new collagen, elastin, and hyaluronic acid. Importantly, the collagen supplementation regimen has been found to be devoid of any adverse effects, proving to be safe and well-tolerated throughout the entire administration period.
Author Contributions
Conceptualization, J.A.C.-N., L.Q., B.B. and R.G.-B.; methodology, J.A.C.-N., B.G.-M., L.Q., B.B. and R.G.-B.; validation, J.A.C.-N., B.G.-M. and R.G.-B.; formal analysis J.A.C.-N.; investigation, J.A.C.-N. and B.G.-M.; resources, B.G.-M., L.Q. and B.B.; data curation, J.A.C.-N.; writing—original draft preparation, J.A.C.-N. and R.G.-B.; supervision, J.A.C.-N., B.G.-M. and B.B.; project administration, J.A.C.-N.; funding acquisition, L.Q. and B.B. All authors have read and agreed to the published version of the manuscript.”.
Funding
This research was funded by Nutraresearch S.L. (Barcelona, Spain), the company that manufactures the active collagen peptides; and Viscofan BioEngineering (Viscofan DE GmbH, Weinheim, Germany), the company owning IP rights of COLLinstant® LMW, the nutraceutical product used in this study. The APC was funded by Nutraresearch S.L. (Barcelona, Spain).
Conflicts of Interest
L.Q. and B.B. are full-time employees of Viscofan BioEngineering (Viscofan DE GmbH, Weinheim, Germany). The remaining authors (J.A.C.-N., B.G.-M. and R.G.-B) declare no conflicts of interest. The sponsors had no influence in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Skin, Mucosa and Menopause; 2015.
- Evans, M.; Lewis, E.D.; Zakaria, N.; Pelipyagina, T.; Guthrie, N. A Randomized, Triple-Blind, Placebo-Controlled, Parallel Study to Evaluate the Efficacy of a Freshwater Marine Collagen on Skin Wrinkles and Elasticity. J Cosmet Dermatol 2021, 20, 825–834. [Google Scholar] [CrossRef]
- Sibilla, S.; Godfrey, M.; Brewer, S.; Budh-Raja, A.; Genovese, L. An Overview of the Beneficial Effects of Hydrolysed Collagen as a Nutraceutical on Skin Properties: Scientific Background and Clinical Studies. Open Nutraceuticals Journal 2015, 8. [Google Scholar] [CrossRef]
- Bolke, L.; Schlippe, G.; Gerß, J.; Voss, W. A Collagen Supplement Improves Skin Hydration, Elasticity, Roughness, and Density: Results of a Randomized, Placebo-Controlled, Blind Study. Nutrients 2019, 11. [Google Scholar] [CrossRef]
- Quan, T.; Fisher, G.J. Role of Age-Associated Alterations of the Dermal Extracellular Matrix Microenvironment in Human Skin Aging: A Mini-Review. Gerontology 2015, 61. [Google Scholar] [CrossRef]
- Tobin, D.J. Introduction to Skin Aging. J Tissue Viability 2017, 26. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Gao, X.; Guo, Y.; Xie, W. Research Progress on Bioactive Factors against Skin Aging. Int J Mol Sci 2024, 25, 3797. [Google Scholar] [CrossRef]
- Calleja-Agius, J.; Muscat-Baron, Y.; Brincat, M.P. Skin Ageing. Menopause Int 2007, 13. [Google Scholar] [CrossRef] [PubMed]
- Castelo-Branco, C.; Duran, M.; González-Merlo, J. Skin Collagen Changes Related to Age and Hormone Replacement Therapy. Maturitas 1992, 15. [Google Scholar] [CrossRef]
- Rocha, M.S.; Aquino, L.L. de; Barbosa, L.L.; Souza, I.L. de; Carvalho, E.M. de; Brítez, L.E.O.; Gonçalves, G. de O.; Lopes, V.B.; Silva, J.N.F. Clinical Studies and Meta-Analysis on the Effects of Collagen, Vitamin, and Nutrient Supplementation for the Rejuvenation of Collagenic Fibers: A Systematic Review. International Journal of Nutrology 2024, 17. [Google Scholar] [CrossRef]
- Sato, K. The Presence of Food-Derived Collagen Peptides in Human Body-Structure and Biological Activity. Food Funct 2017, 8. [Google Scholar] [CrossRef]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The Skin Aging Exposome. J Dermatol Sci 2017, 85. [Google Scholar] [CrossRef]
- Lee, D.E.; Huh, C.S.; Ra, J.; Choi, I.D.; Jeong, J.W.; Kim, S.H.; Ryu, J.H.; Seo, Y.K.; Koh, J.S.; Lee, J.H.; et al. Clinical Evidence of Effects of Lactobacillus Plantarum HY7714 on Skin Aging: A Randomized, Double Blind, Placebo-Controlled Study. J Microbiol Biotechnol 2015, 25. [Google Scholar] [CrossRef]
- Nistico, S.P.; Silvestri, M.; Zingoni, T.; Tamburi, F.; Bennardo, L.; Cannarozzo, G. Combination of Fractional CO2Laser and Rhodamine-Intense Pulsed Light in Facial Rejuvenation: A Randomized Controlled Trial. Photobiomodul Photomed Laser Surg 2021, 39. [Google Scholar] [CrossRef]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.G.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased Collagen Production in Chronologically Aged Skin: Roles of Age-Dependent Alteration in Fibroblast Function and Defective Mechanical Stimulation. American Journal of Pathology 2006, 168. [Google Scholar] [CrossRef]
- Schwartz, S.R.; Park, J. Ingestion of BioCell Collagen®, a Novel Hydrolyzed Chicken Sternal Cartilage Extract; Enhanced Blood Microcirculation and Reduced Facial Aging Signs. Clin Interv Aging 2012, 7. [Google Scholar] [CrossRef]
- de Miranda, R.B.; Weimer, P.; Rossi, R.C. Effects of Hydrolyzed Collagen Supplementation on Skin Aging: A Systematic Review and Meta-Analysis. Int J Dermatol 2021, 60. [Google Scholar] [CrossRef]
- Branquinho França, A.; Batista dos Santos, A.; Mônica dos Santos Morais, K.; Cardoso Morais, G. Benefits of Hydrolyzed Collagen Type 1 Supplementation on Skin Health. Health and Society 2023, 3. [Google Scholar] [CrossRef]
- Pogačnik, T.; Žmitek, J.; Hristov, H.; Keršmanc, P.; Butina, M.R.; Žmitek, K. The Effect of a 12-Week Dietary Intake of Food Supplements Containing Collagen and MSM on Dermis Density and Other Skin Parameters: A Double-Blind, Placebo-Controlled, Randomised Four-Way Study Comparing the Efficacy of Three Test Products. J Funct Foods 2023, 110, 105838. [Google Scholar] [CrossRef]
- Wang, H. A Review of the Effects of Collagen Treatment in Clinical Studies. Polymers 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, M.; Abdul, N.S.; Qamar, Z.; Bahri, B.M. Al; Al Ghalayini, K.Z.K.; Kakti, A. Collagen Structure, Synthesis, and Its Applications: A Systematic Review. Cureus 2022. [Google Scholar] [CrossRef]
- Lupu, M.; Gradisteanu Pircalabioru, G.; Chifiriuc, M.; Albulescu, R.; Tanase, C. Beneficial Effects of Food Supplements Based on Hydrolyzed Collagen for Skin Care (Review). Exp Ther Med 2019. [Google Scholar] [CrossRef]
- Sontakke, S.B.; Jung, J.H.; Piao, Z.; Chung, H.J. Orally Available Collagen Tripeptide: Enzymatic Stability, Intestinal Permeability, and Absorption of Gly-Pro-Hyp and Pro-Hyp. J Agric Food Chem 2016, 64. [Google Scholar] [CrossRef] [PubMed]
- Yazaki, M.; Ito, Y.; Yamada, M.; Goulas, S.; Teramoto, S.; Nakaya, M. aki; Ohno, S.; Yamaguchi, K. Oral Ingestion of Collagen Hydrolysate Leads to the Transportation of Highly Concentrated Gly-Pro-Hyp and Its Hydrolyzed Form of Pro-Hyp into the Bloodstream and Skin. J Agric Food Chem 2017, 65. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Ko, E.J.; Lee, Y.H.; Kim, B.G.; Shin, H.J.; Seo, D.B.; Lee, S.J.; Kim, B.J.; Kim, M.N. Effects of Collagen Tripeptide Supplement on Skin Properties: A Prospective, Randomized, Controlled Study. Journal of Cosmetic and Laser Therapy 2014, 16. [Google Scholar] [CrossRef] [PubMed]
- ICH International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Official Web Site: ICH. Ich.Org 2018, 41.
- Gupta, V.; Sharma, V.K. Skin Typing: Fitzpatrick Grading and Others. Clin Dermatol 2019, 37. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, A.; Carruthers, J.; Hardas, B.; Kaur, M.; Goertelmeyer, R.; Jones, D.; Rzany, B.; Cohen, J.; Kerscher, M.; Flynn, T.C.; et al. A Validated Grading Scale for Crow’s Feet. Dermatologic Surgery 2008, 34. [Google Scholar] [CrossRef] [PubMed]
- Czajka, A.; Kania, E.M.; Genovese, L.; Corbo, A.; Merone, G.; Luci, C.; Sibilla, S. Daily Oral Supplementation with Collagen Peptides Combined with Vitamins and Other Bioactive Compounds Improves Skin Elasticity and Has a Beneficial Effect on Joint and General Wellbeing. Nutrition Research 2018, 57. [Google Scholar] [CrossRef]
- Bazargan, A.S.; Shemshadi, M.; Ziaeifar, E.; Taheri, A.; Roohaninasab, M.; Goodarzi, A.; Mirhashemi, M. Evaluation of Effectiveness of Tranexamic Acid as Mesotherapy in Improvement of Periorbital Wrinkling in a Trial Study. J Cosmet Dermatol 2023, 22. [Google Scholar] [CrossRef]
- Yvonne Mödinger; Christiane Schön; Katrin Vogel; Marianne Brandt; Stepah Bielfeldt; Klaus-Peter Wilhelm Evaluation of a Food Supplement with Collagen Hydrolysate and Micronutrients on Skin Appearance and Beauty Effects: A Randomized, Double-Blind, PlaceboControlled Clinical Study with Healthy Subjects. Journal of Clinical and Cosmetic Dermatology 2021, 5. [CrossRef]
- Ryu, H.S.; Joo, Y.H.; Kim, S.O.; Park, K.C.; Youn, S.W. Influence of Age and Regional Differences on Skin Elasticity as Measured by the Cutometer®. Skin Research and Technology 2008, 14. [Google Scholar] [CrossRef]
- Stroumza, N.; Bosc, R.; Hersant, B.; Hermeziu, O.; Meningaud, J.P. Benefits of Using the Cutometer to Evaluate the Effectiveness of Skin Treatments in Plastic and Maxillofacial Surgery. Rev Stomatol Chir Maxillofac Chir Orale 2015, 116. [Google Scholar]
- Ohshima, H.; Kinoshita, S.; Oyobikawa, M.; Futagawa, M.; Takiwaki, H.; Ishiko, A.; Kanto, H. Use of Cutometer Area Parameters in Evaluating Age-Related Changes in the Skin Elasticity of the Cheek. Skin Research and Technology 2013, 19. [Google Scholar] [CrossRef]
- Granger, C.; Aladren, S.; Delgado, J.; Garre, A.; Trullas, C.; Gilaberte, Y. Prospective Evaluation of the Efficacy of a Food Supplement in Increasing Photoprotection and Improving Selective Markers Related to Skin Photo-Ageing. Dermatol Ther (Heidelb) 2020, 10. [Google Scholar] [CrossRef]
- Saito, M.; Tanaka, M.; Misawa, E.; Yao, R.; Nabeshima, K.; Yamauchi, K.; Abe, F.; Yamamoto, Y.; Furukawa, F. Oral Administration of Aloe Vera Gel Powder Prevents Uvb-Induced Decrease in Skin Elasticity via Suppression of Overexpression of Mmps in Hairless Mice. Biosci Biotechnol Biochem 2016, 80. [Google Scholar] [CrossRef]
- Salomão Calixto, L.; Picard, C.; Savary, G.; Campos, P.M.B.G.M. Skin Characterization and Immediate Effects of Different Dermocosmetic Treatments in French and Brazilian Skin. J Cosmet Dermatol 2020, 19. [Google Scholar] [CrossRef]
- Abbas, D.B.; Lavin, C.V.; Fahy, E.J.; Griffin, M.; Guardino, N.; King, M.; Chen, K.; Lorenz, P.H.; Gurtner, G.C.; Longaker, M.T.; et al. Standardizing Dimensionless Cutometer Parameters to Determine In Vivo Elasticity of Human Skin. Adv Wound Care (New Rochelle) 2022, 11. [Google Scholar] [CrossRef]
- Bianchi, F.M.; Angelinetta, C.; Rizzi, G.; Praticò, A.; Villa, R. Evaluation of the Efficacy of a Hydrolyzed Collagen Supplement for Improving Skin Moisturization, Smoothness, and Wrinkles. Journal of Clinical and Aesthetic Dermatology 2022, 15. [Google Scholar]
- Woo, M.S.; Moon, K.J.; Jung, H.Y.; Park, S.R.; Moon, T.K.; Kim, N.S.; Lee, B.C. Comparison of Skin Elasticity Test Results from the Ballistometer® and Cutometer®. Skin Research and Technology 2014, 20. [Google Scholar] [CrossRef]
- Agache’s Measuring the Skin; 2017.
- Atkinson, M.J.; Sinha, A.; Hass, S.L.; Colman, S.S.; Kumar, R.N.; Brod, M.; Rowland, C.R. Validation of a General Measure of Treatment Satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), Using a National Panel Study of Chronic Disease. Health Qual Life Outcomes 2004, 2. [Google Scholar] [CrossRef]
- JASP Team JASP (Version 0.17.1). JASP - Free and User-Friendly Statistical Software 2023.
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 Statement. Obstetrics & Gynecology 2010, 115, 1063–1070. [Google Scholar] [CrossRef]
- Quan, T. Molecular Insights of Human Skin Epidermal and Dermal Aging. J Dermatol Sci 2023, 112. [Google Scholar] [CrossRef]
- Liang, Y.; Su, W.; Wang, F. Skin Ageing: A Progressive, Multi-Factorial Condition Demanding an Integrated, Multilayer-Targeted Remedy. Clin Cosmet Investig Dermatol 2023, 16. [Google Scholar] [CrossRef]
- Honigman, R.; Castle, D.J. Aging and Cosmetic Enhancement. Clin Interv Aging 2006, 1. [Google Scholar] [CrossRef]
- Lee, Y.I.; Lee, S.G.; Jung, I.; Suk, J.; Lee, M.H.; Kim, D.U.; Lee, J.H. Effect of a Topical Collagen Tripeptide on Antiaging and Inhibition of Glycation of the Skin: A Pilot Study. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Lordan, R. Dietary Supplements and Nutraceuticals Market Growth during the Coronavirus Pandemic – Implications for Consumers and Regulatory Oversight. PharmaNutrition 2021, 18. [Google Scholar] [CrossRef]
- Martinez, M.J.; Dixit, D.; White, M.W.; Rieder, E.A. Motivations for Seeking Cosmetic Enhancing Procedures of the Face: A Systematic Review. Dermatologic Surgery 2023, 49. [Google Scholar] [CrossRef]
- Schagen, S.K.; Zampeli, V.A.; Makrantonaki, E.; Zouboulis, C.C. Discovering the Link between Nutrition and Skin Aging. Dermatoendocrinol 2012, 4. [Google Scholar] [CrossRef]
- de Miranda, R.B.; Weimer, P.; Rossi, R.C. Effects of Hydrolyzed Collagen Supplementation on Skin Aging: A Systematic Review and Meta-Analysis. Int J Dermatol 2021, 60. [Google Scholar] [CrossRef]
- Wang, H. A Review of the Effects of Collagen Treatment in Clinical Studies. Polymers 2021, 13. [Google Scholar] [CrossRef]
- Pu, S.Y.; Huang, Y.L.; Pu, C.M.; Kang, Y.N.; Hoang, K.D.; Chen, K.H.; Chen, C. Effects of Oral Collagen for Skin Anti-Aging: A Systematic Review and Meta-Analysis. Nutrients 2023, 15. [Google Scholar] [CrossRef]
- Avila Rodríguez, M.I.; Rodríguez Barroso, L.G.; Sánchez, M.L. Collagen: A Review on Its Sources and Potential Cosmetic Applications. J Cosmet Dermatol 2018, 17. [Google Scholar] [CrossRef]
- Choi, F.D.; Sung, C.T.; Juhasz, M.L.W.; Mesinkovsk, N.A. Oral Collagen Supplementation: A Systematic Review of Dermatological Applications. J Drugs Dermatol 2019, 18. [Google Scholar]
- Garcez Duarte, M. Collagen Supplementation for Health of the Skin, Cartilage and Muscles-Current Myths and Truths. Ann Nutr Metab 2017, 71. [Google Scholar]
- León-López, A.; Morales-Peñaloza, A.; Martínez-Juárez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Álvarez, G. Hydrolyzed Collagen-Sources and Applications. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Czech, I.; As, S. Collagen Peptides – Source, Properties and Benefits. Seagarden AS 2016. [Google Scholar]
- Farage, M.A.; Miller, K.W.; Maibach, H.I. Textbook of Aging Skin; 2010.
- Robins, S.P. Biochemistry and Functional Significance of Collagen Cross-Linking. In Proceedings of the Biochemical Society Transactions; 2007; Vol. 35. [Google Scholar]
- Calleja-Agius, J.; Brincat, M.; Borg, M. Skin Connective Tissue and Ageing. Best Pract Res Clin Obstet Gynaecol 2013, 27. [Google Scholar] [CrossRef]
- Rostkowska, E.; Poleszak, E.; Wojciechowska, K.; Dos Santos Szewczyk, K. Dermatological Management of Aged Skin. Cosmetics 2023, 10. [Google Scholar] [CrossRef]
- Krutmann, J.; Grether-Beck, S.; Makrantonaki, E.; Schikowski, T. Skin Aging Exposome. Dermatologie 2023, 74. [Google Scholar] [CrossRef]
- Oesser, S.; Adam, M.; Babel, W.; Seifert, J. Oral Administration of 14C Labeled Gelatin Hydrolysate Leads to an Accumulation of Radioactivity in Cartilage of Mice (C57/BL). Journal of Nutrition 1999, 129. [Google Scholar] [CrossRef]
- Asserin, J.; Lati, E.; Shioya, T.; Prawitt, J. The Effect of Oral Collagen Peptide Supplementation on Skin Moisture and the Dermal Collagen Network: Eevidence from an Ex Vivo Model and Randomized, Placebo-Controlled Clinical Trials. J Cosmet Dermatol 2015, 14. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu Rev Biochem 2009, 78. [Google Scholar] [CrossRef]
- Li, P.; Wu, G. Roles of Dietary Glycine, Proline, and Hydroxyproline in Collagen Synthesis and Animal Growth. Amino Acids 2018, 50. [Google Scholar] [CrossRef]
- Ohara, H.; Matsumoto, H.; Ito, K.; Iwai, K.; Sato, K. Comparison of Quantity and Structures of Hydroxyproline-Containing Peptides in Human Blood after Oral Ingestion of Gelatin Hydrolysates from Different Sources. J Agric Food Chem 2007, 55. [Google Scholar] [CrossRef]
- Proksch, E.; Segger, D.; Degwert, J.; Schunck, M.; Zague, V.; Oesser, S. Oral Supplementation of Specific Collagen Peptides Has Beneficial Effects on Human Skin Physiology: A Double-Blind, Placebo-Controlled Study. Skin Pharmacol Physiol 2013, 27. [Google Scholar] [CrossRef]
- Yamamoto, S.; Hayasaka, F.; Deguchi, K.; Okudera, T.; Furusawa, T.; Sakai, Y. Absorption and Plasma Kinetics of Collagen Tripeptide after Peroral or Intraperitoneal Administration in Rats. Biosci Biotechnol Biochem 2015, 79. [Google Scholar] [CrossRef]
- Mari, W.K.; Muneshige, S.; Shin, K.; Yasuki, T.; Hideyuki, S.; Fumiki, M.; Hitoshi, S.; Yuji, F.; Michio, K. Absorption and Effectiveness of Orally Administered Low Molecular Weight Collagen Hydrolysate in Rats. J Agric Food Chem 2010, 58. [Google Scholar] [CrossRef]
- Morikiri, Y.; Matsuta, E.; Inoue, H. The Collagen-Derived Compound Collagen Tripeptide Induces Collagen Expression and Extends Lifespan via a Conserved P38 Mitogen-Activated Protein Kinase Cascade. Biochem Biophys Res Commun 2018, 505. [Google Scholar] [CrossRef]
- Sato, K.; Asai, T.T.; Jimi, S. Collagen-Derived Di-Peptide, Prolylhydroxyproline (Pro-Hyp): A New Low Molecular Weight Growth-Initiating Factor for Specific Fibroblasts Associated With Wound Healing. Front Cell Dev Biol 2020, 8. [Google Scholar] [CrossRef]
- Asai, T.T.; Oikawa, F.; Yoshikawa, K.; Inoue, N.; Sato, K. Food-Derived Collagen Peptides, Prolyl-Hydroxyproline (Pro-Hyp), and Hydroxyprolyl-Glycine (Hyp-Hly) Enhance Growth of Primary Cultured Mouse Skin Fibroblast Using Fetal Bovine Serum Free from Hydroxyprolyl Peptide. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef]
- Campos, L.D.; Santos Junior, V. de A.; Pimentel, J.D.; Carregã, G.L.F.; Cazarin, C.B.B. Collagen Supplementation in Skin and Orthopedic Diseases: A Review of the Literature. Heliyon 2023, 9. [Google Scholar] [CrossRef]
- Hee Shin, J.; Hyang Kim, A.; Woo Lee, H.; Il Kim, J.; Kwang Lee, H. The Beneficial Effects of Collagen Tripeptide on Deep Wrinkling and Skin Moisturization: A Randomized Controlled Trial. Journal of Food and Nutrition Research 2021, 9. [Google Scholar] [CrossRef]
Figure 1.
Flow chart of subject’s recruitment, randomization and follow-up.
Figure 1.
Flow chart of subject’s recruitment, randomization and follow-up.
Figure 2.
Boxplot representing skin wrinkle volume at crow`s feet region before (T0) and after intake of the product for 6 weeks (T6) in the group of women receiving a low molecular weight (LMW) collagen preparation or placebo (n= 40/group). The mean value (x) is also represented. The asterisks indicate statistically significant differences in the intergroup comparison (*p < 0.01; **p < 0.001).
Figure 2.
Boxplot representing skin wrinkle volume at crow`s feet region before (T0) and after intake of the product for 6 weeks (T6) in the group of women receiving a low molecular weight (LMW) collagen preparation or placebo (n= 40/group). The mean value (x) is also represented. The asterisks indicate statistically significant differences in the intergroup comparison (*p < 0.01; **p < 0.001).
Figure 3.
Boxplot representing skin wrinkle area at crow`s feet region before (T0) and after intake of the product for 6 weeks (T6) in the group of women receiving a low molecular weight (LMW) collagen preparation or placebo (n= 40/group). The mean value (x) is also represented. The asterisk (*) indicates statistically significant differences in the intergroup comparison (*p < 0.001).
Figure 3.
Boxplot representing skin wrinkle area at crow`s feet region before (T0) and after intake of the product for 6 weeks (T6) in the group of women receiving a low molecular weight (LMW) collagen preparation or placebo (n= 40/group). The mean value (x) is also represented. The asterisk (*) indicates statistically significant differences in the intergroup comparison (*p < 0.001).
Figure 4.
Boxplot representing skin wrinkle depth at crow`s feet region before (T0) and after intake of the product for a 6-week period (T6) in the group of women receiving a low molecular weight (LMW) collagen preparation or placebo (n= 40/group). The mean value (x) is also represented. The asterisk (*) indicates statistically significant differences in the intergroup comparison (p < 0.001).
Figure 4.
Boxplot representing skin wrinkle depth at crow`s feet region before (T0) and after intake of the product for a 6-week period (T6) in the group of women receiving a low molecular weight (LMW) collagen preparation or placebo (n= 40/group). The mean value (x) is also represented. The asterisk (*) indicates statistically significant differences in the intergroup comparison (p < 0.001).
Figure 5.
Appearance of wrinkles at the crow's feet region during the treatment period with the investigational product. The photographs were taken of three volunteers, no. 13 (top), no. 60 (middle) and no. 70 (bottom), at baseline (images a, c and e) and at T6, after 6 weeks of oral supplementation with the test product (images b, d and f). Data obtained from Visioface® 1000 D.
Figure 5.
Appearance of wrinkles at the crow's feet region during the treatment period with the investigational product. The photographs were taken of three volunteers, no. 13 (top), no. 60 (middle) and no. 70 (bottom), at baseline (images a, c and e) and at T6, after 6 weeks of oral supplementation with the test product (images b, d and f). Data obtained from Visioface® 1000 D.
Figure 6.
Boxplot representing skin hydration before (T0) and after intake of the product for a 6-week period (T6) in the group of women receiving a low molecular weight (LMW) collagen preparation or placebo (n= 40/group). The mean value (x) is also represented. The asterisk (*) indicates statistically significant differences in the intergroup comparison (p < 0.001).
Figure 6.
Boxplot representing skin hydration before (T0) and after intake of the product for a 6-week period (T6) in the group of women receiving a low molecular weight (LMW) collagen preparation or placebo (n= 40/group). The mean value (x) is also represented. The asterisk (*) indicates statistically significant differences in the intergroup comparison (p < 0.001).
Figure 7.
Percentage change of biometric skin parameters between the baseline visit and the end of the 6 weeks of the interventional period (T0-T6) in the placebo group (red bars) and in the group taking the investigational product (green bars). Error bars indicate the standard error of the mean. The asterisk (*) indicates p < 0.001 for intergroup comparison (placebo group vs. collagen group).
Figure 7.
Percentage change of biometric skin parameters between the baseline visit and the end of the 6 weeks of the interventional period (T0-T6) in the placebo group (red bars) and in the group taking the investigational product (green bars). Error bars indicate the standard error of the mean. The asterisk (*) indicates p < 0.001 for intergroup comparison (placebo group vs. collagen group).
Table 1.
Demographics and general characteristics of women randomly allocated to the placebo and the test group.
Table 1.
Demographics and general characteristics of women randomly allocated to the placebo and the test group.
| |
|
Placebo (n=40) |
Collagen (n=40) |
p |
| Age, years |
|
47 ± 7.7 |
45 ± 7.1 |
0.22 |
| Height (m) |
|
1.68 ± 0.06 |
1.69 ± 0.07 |
0.68 |
| Weight (kg) |
|
69.1 ± 9.13 |
68.5± 9.27 |
0.59 |
| BMI (kg/m2) |
|
24.5 ± 2.7 |
24.1 ± 2.6 |
0.71 |
| |
Normal |
30 (75) |
27 (67.5) |
|
| Skin type, n (%) |
Sensitive |
8 (20) |
12 (30) |
|
| |
Dry |
0 (0) |
1 (2.5) |
0.27 |
| |
Oiled |
2 (5) |
0 (0) |
|
| Skin phototype, |
II |
0 (0) |
3 (7.5) |
|
| (Fitzpatricka), n (%) |
III |
36 (90) |
34 (85) |
0.20 |
| |
IV |
4 (10) |
3 (7.5) |
|
Table 2.
Skin biometric parameters at baseline (T0).
Table 2.
Skin biometric parameters at baseline (T0).
| |
|
Placebo (n= 40) |
Collagen (n= 40) |
|
| |
Parameter |
Mean (SD) |
Mean (SD) |
p value* |
| Skin wrinkling |
Volume (px³) |
48.50 (35.2) |
58.77 (47.7) |
0.52 |
| |
Area (px2) |
4.13 (2.6) |
4.53 (3.1) |
0.72 |
| |
Depth (px) |
11.95 (2.7) |
11.50 (2.4) |
0.45 |
| Elasticity |
R0 (mm) |
0.40 (0.09) |
0.38 (0.10) |
0.25 |
| |
R2 (%) |
53.28 (15.7) |
53.14 (11.6) |
0.89 |
| |
R5 (%) |
48.59 (17.4) |
48.16 (13.9) |
0.96 |
| |
R7 (%) |
34.03 (14.5) |
32.1 (10.2) |
0.95 |
| |
R9 (mm) |
0.07 (0.03) |
0.07 (0.02) |
0.27 |
| Hydration (AU) |
|
54.77 (9.4) |
55.5 (8.6) |
0.52 |
Table 3.
Skin crow`s feet parameters (volume, area, and depth) assessed by VisioFace® 1000D.
Table 3.
Skin crow`s feet parameters (volume, area, and depth) assessed by VisioFace® 1000D.
| |
|
Placebo (n= 40) |
|
Collagen (n= 40) |
|
|
| Parameter |
Time-point |
Mean (SD) |
p value* |
Mean (SD) |
p value* |
Test/Placebo p valueƗ
|
|
Volume (px³)
|
Baseline |
48.50 (35.2)) |
|
58.77 (47.7) |
|
|
| |
Week 6 |
50.30 (40.4) |
0.13 |
30.24 (24.4) |
0.001 |
0.01 |
|
Area (px2)
|
Baseline |
4.13 (2.6) |
|
4.53 (3.1) |
|
|
| |
Week 6 |
4.14 (2.5) |
0.92 |
2.60 (2.1) |
0.001 |
0.001 |
|
Depth (px)
|
Baseline |
11.95 (2.7) |
|
11.50 (2.4) |
|
|
| |
Week 6 |
12.25 (2.6) |
0.23 |
10.35 (2.1) |
0.001 |
0.001 |
Table 4.
Mechanical characteristics of the skin through the analysis of elasticity parameters (mean ± SD) at the crow's feet region, before intake of the study products at baseline (T0) and after 6 weeks of treatment (T6) as assessed by Cutometer®.
Table 4.
Mechanical characteristics of the skin through the analysis of elasticity parameters (mean ± SD) at the crow's feet region, before intake of the study products at baseline (T0) and after 6 weeks of treatment (T6) as assessed by Cutometer®.
| |
|
Placebo (n= 40) |
|
Test group (n= 40) |
|
|
| Elasticity Parameter |
Time-point |
Mean (SD) |
p value* |
Mean (SD) |
p value* |
Test/Placebo p valueƗ
|
| R0 (mm) |
Baseline |
0.402 (0.09) |
|
0.380 (0.09) |
|
|
| |
Week 6 |
0.362 (0.09) |
0.001 |
0.329 (0.07) |
0.001 |
0.07 |
| R2 (%) |
Baseline |
53.28 (15.7) |
|
53.14 (11.66) |
|
|
| |
Week 6 |
51.31 (18.1) |
0.31 |
51.86 (13.7) |
0.68 |
0.53 |
| R5 (%) |
Baseline |
48.59 (17.5) |
|
48.16 (13.9) |
|
|
| |
Week 6 |
45.84 (17.6) |
0.15 |
48.54 (15.5) |
0.95 |
0.39 |
| R7 (%) |
Baseline |
34.03 (14.5) |
|
32.09 (10.2) |
|
|
| |
Week 6 |
31.65 (13.7) |
0.09 |
32.27 (13.2) |
0.65 |
0.63 |
| R9 (mm) |
Baseline |
0.073 (0.03) |
|
0.070 (0.02) |
|
|
| |
Week 6 |
0.066 (0.02) |
0.14 |
0.065 (0.02) |
0.13 |
0.41 |
Table 5.
Skin hydration values (mean ± SD) before intake of the study products at baseline (T0) and after 6 weeks of treatment (T6).
Table 5.
Skin hydration values (mean ± SD) before intake of the study products at baseline (T0) and after 6 weeks of treatment (T6).
| |
|
Placebo (n= 40) |
|
Collagen (n= 40= |
|
|
| |
Time-point |
Mean (SD) |
p value* |
Mean (SD) |
p value* |
Test/Placebo p valueƗ
|
| Skin hydration(AU) |
Baseline |
54.77 (9.4) |
|
55.50 (8.6) |
|
|
| |
Week 6 |
55.03 (9.5) |
0.79 |
74.13 (11.9) |
0.001 |
0.001 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).