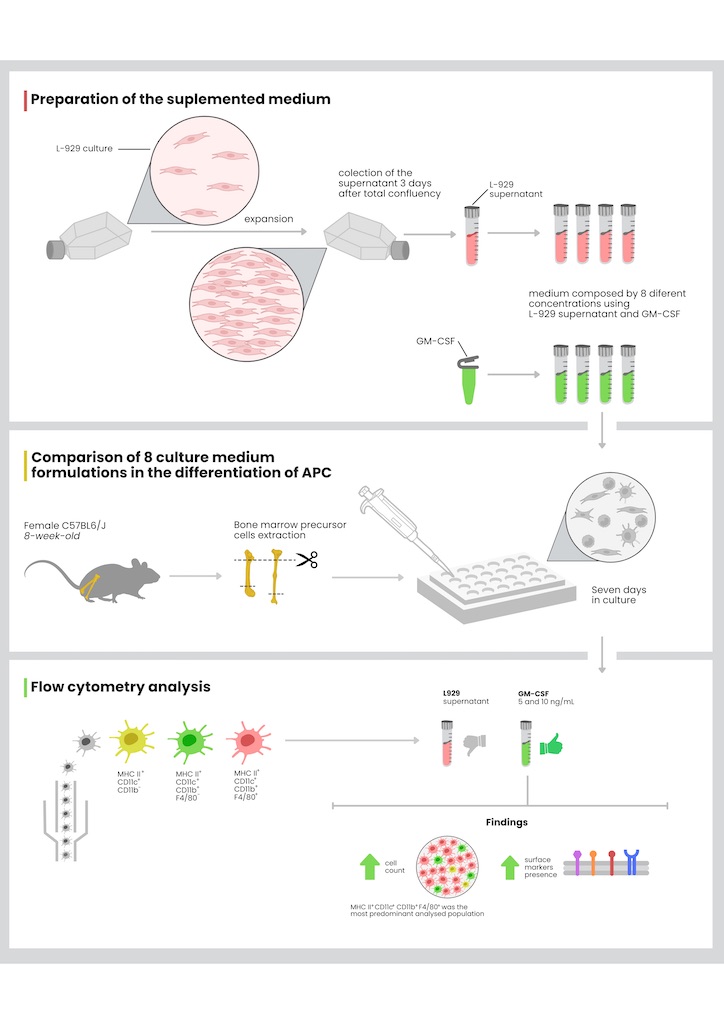
1. Introduction
When the body is threatened by pathogens or even by cellular and non-cellular components of the host itself, antigen-presenting cells (APCs) are responsible for initiating the adaptive response. Monocytes, macrophages, and dendritic cells (DCs), members of this group, are known to express various surface proteins used as molecular markers for identification. Surface proteins such as MHC II, CD11c, CD11b, and F4/80 play a significant role in the functionality of these populations, and the expression of some of them is used as a parameter to validate the quality of therapies such as cell-based [
1,
2]
APC-dependent cellular therapy emerges as a promising therapeutic avenue for diverse pathologies, including cancer, autoimmune disorders, and infectious diseases, highlighting the crucial dependence on protocols for obtaining or differentiating specific cell populations. This promise is reflected in ongoing clinical trials targeting the treatment of various diseases, paving the way for new therapeutic options for patients (
Table 1). This therapeutic approach leverages the inherent antigen processing and presentation capabilities of APCs, predominantly DCs, to orchestrate a targeted immune response against specific pathogens or tumor cells. Unlike tolerogenic therapies that suppress immune responses, this strategy aims to elicit a robust and antigen-specific immune response, for example against cancer. High cost and time consumption in the development of APC-therapies are some of the issues limiting progress in this field. Therefore, it is necessary that new protocols be developed to optimize and make better use of available resources.
In the bone marrow, hematopoietic stem cells (HSCs) give rise to several cell types, including DCs and macrophages [
3]. It is already known that cellular differentiation can be directly influenced by various chemicals and physical factors, such as Colony Stimulating Factors (CSFs), temperature, and gravity [
9,
10,
11]. There are several methods for differentiating APCs in vitro from HSCs. The use of Macrophage Colony Stimulating Factor (M-CSF) and Granulocyte-Monocyte Colony Stimulating Factor (GM-CSF) are the most common approaches [
12,
13,
14]. It is also known that the concentrations of M-CSF and GM-CSF influence the type and percentage of differentiated cells [
9]. GM-CSF is the most commonly used cytokine to differentiate DCs and macrophages and the concentration of this substance in medium can directly influence cell type and function [
15]. In addition, some protocols indicate conditioned medium with L-929 supernatant (SL-929) for macrophage differentiation [
16,
17]. L-929, a fibroblast cell line, is known for secreting both GM-CSF and M-CSF in different concentrations. However, there is currently no specific protocol using supernatant of L-929 (SL-929) that demonstrates the same effectiveness for APC profiles other than macrophages.
Understanding the phenotypic profile of these cells is critical to assessing their functional capabilities. The refinement of the protocol using both, SL-929 or GM-CSF, may be important to assist in the development of research that requires more specific cell profiles. With the aim of assisting in the development of new APC-dependent therapies and optimizing the use of resources, this study investigates the impact of different concentrations of GM-CSF and SL-929 on the differentiation of progenitor cells into distinct APC populations. Therefore, we hypothesized that minor changes in GM-CSF and SL-929 concentrations can generate different cellular profiles that can be useful to developing or improving new therapeutic strategies.
2. Materials and Methods
2.1. Reagent and Animals
The reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA), eBioscience (Waltham, MA, USA) and Cultilab (Campinas, SP, BR). All experiments were conducted in accordance with the Ethical Principles on Animal Research, adopted by the Brazilian College of Animal Experimentation (Colégio Brasileiro de Experimentação Animal – COBEA), with the prior approval by the Ethics Committee on the Use of Animals (CEUA/UNICAMP – Universidade Estadual de Campinas; protocol number: 5074-1/2018). Females C57BL/6J mice, 6 – 8 weeks old, from the Multidisciplinary Centre for Biological Research in Universidade Estadual de Campinas, were used in this study. Mice were kept in specific-pathogen free condition, in a controlled temperature and photoperiod environment, with autoclaved food and water ad libitum throughout the experiment. The animals were kept in the Animal Facility of the Biology Institute, Department of Genetic, Evolution, Microbiology and Immunology, UNICAMP, Campinas, São Paulo, Brazil.
2.2. L-929 Culture and Supernatant
2x106 L-929 cells (murine fibroblasts) were placed in 25 cm cell culture flasks. The culture was incubated with 20 mL of IMDM (Iscove’s Modified Dulbecco’s Medium) containing 10% of serum fetal bovine (SFB), 50 mg / mL of L-glutamine, penicillin and streptomycin solution (Gibco Inc., Billings, MT, USA) at 37 ° C, in an oven with 5% CO2. The medium has not been replaced during the experiment. The supernatant was collected two days after total confluence. The pH was measured and the supernatant of 10 flasks of L-929 cells were used for the differentiation of the cells. The culture medium was supplemented with L-929 supernatant at concentrations of 1%, 5%, 10%, and 25% of the total culture medium volume.
2.3. Generation of APCs
Bone marrow-derived precursors were used in the generation of cells, according to a previous report [
18]. Briefly, femurs were collected and the BM cells were flushed out with IMDM medium (Iscove’s Modified Dulbecco’s Medium - I7633/Sigma) supplemented with fetal bovine serum (Cultilab - 10%, v/v) and Antibiotic Antimycotic Solution (MFCD00130520/Sigma - 50 mg/ml) - referred to as complete medium.
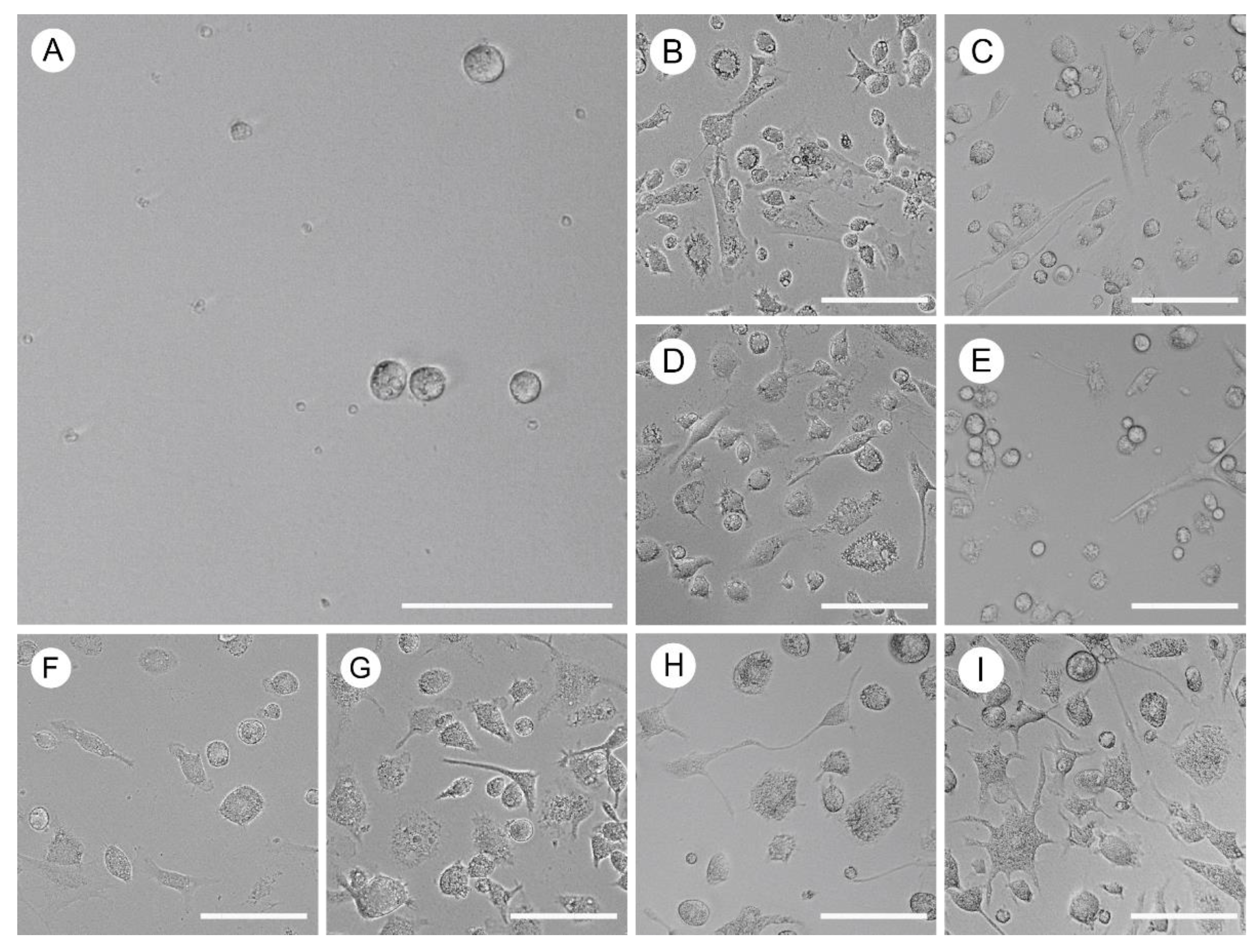
2 x 105 cells were seeded in 96-well culture plate containing complete medium supplemented with GM-CSF (1 ng/ml, 5 ng/ml, 10 ng/ml or 25 ng/ml) or L-929 supernatant (v/v: 1%, 5%, 10% or 25%). Fresh medium was added every 2 days of culture. In the control group, cells were plated only with a complete medium without stimulus. For light microscopy analysis, cells were cultured in 35 mm Petri dishes with the same medium and conditions as the 96-well plates. Live cell images were captured on day 7 of culture using a light microscope at 400x magnification. On the same day, cells were removed from the 96-well plates, centrifuged, and marked for flow cytometry.
2.4. Flow Cytometry
Each treatment group was individually homogenized in 1 mL of sterile phosphate-buffered saline (PBS, 0.02 M) to wash and remove the culture medium. Cell viability was assessed using Trypan Blue (Sigma). The following antibodies were used for immunostaining (all from eBioscience, USA): anti-MHC II (clone M5/114.15.2, PerCP Cy5.5, #107626), anti-CD11c (clone N418, APC, #117310), anti-CD11b (clone M1/70, PE, #557397), and anti-F4/80 (clone BM8, PE-Cy7, #25-4801-82). The cells were incubated with these antibodies for 30 minutes at 4°C in the dark. After incubation, cells were fixed with 1% paraformaldehyde, washed to remove all fixative, and resuspended in PBS for analysis. Data were acquired from 20,000 events per sample using a FACSVerse flow cytometer (BD Biosciences, located at the Department of Genetics, Evolution, Microbiology and Immunology, Campinas, São Paulo, Brazil) and analyzed with FlowJo VX software (Becton, Dickinson & Company, New Jersey, USA).
2.5. Statistical Analysis
Values were analyzed using the GraphPad Prism software package (San Diego, CA, USA; v. 6.0). Data are presented as mean ± standard error (SEM). An unpaired Student’s t-test was used to compare each treatment with the control. A p-value < 0.05 indicates statistical significance.
3. Results
3.1. GM-CSF Induces an Increase in the Number of Cells Better than the SL-929
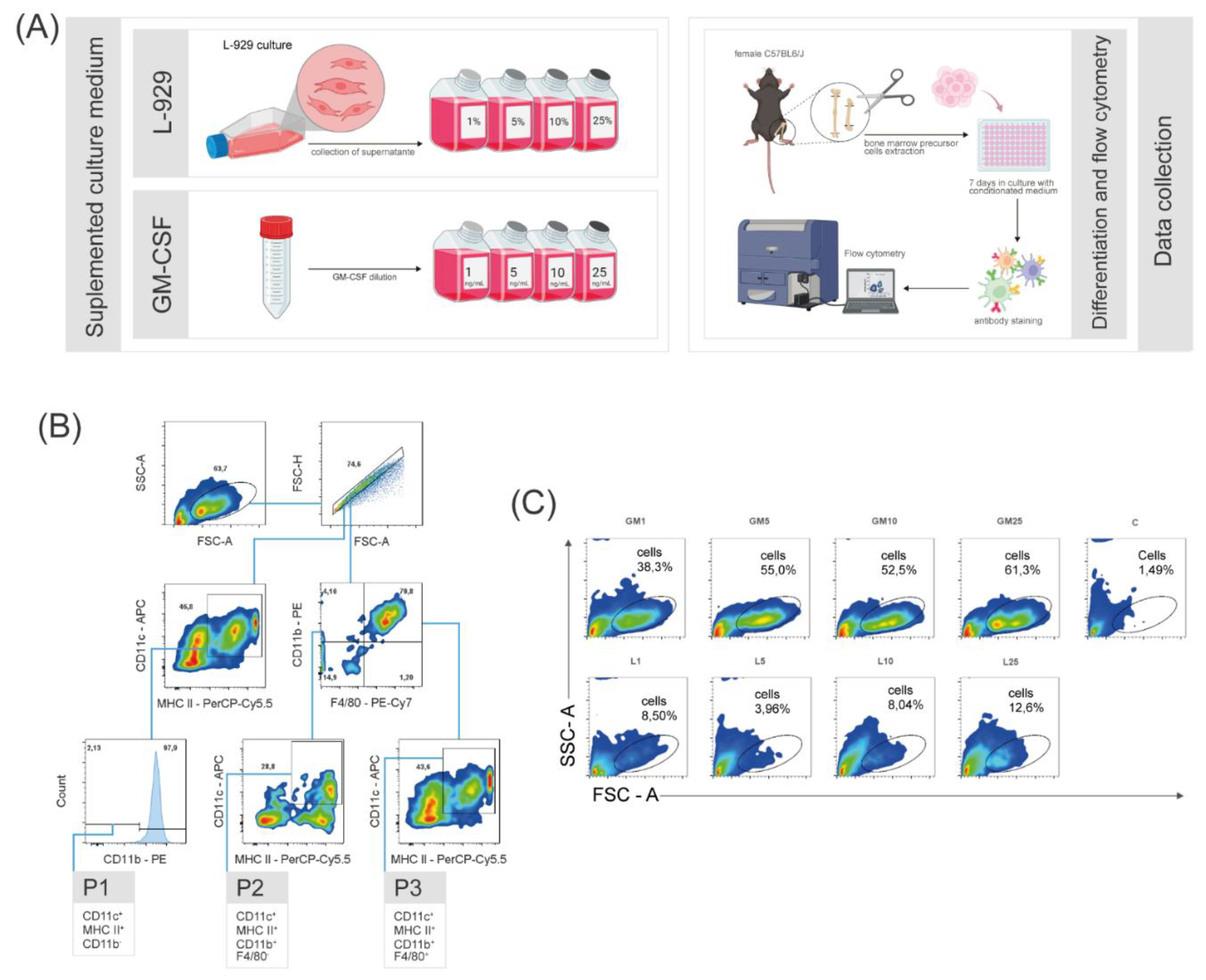
To delineate the primary distinctions among differentiated cell populations, a comparative study was conducted utilizing eight distinct culture medium formulations. The formulations consisted of four concentrations of GM-CSF and supernatant of L-929 cell line, allowing for a comprehensive analysis of the impact of these factors on cellular profiles. For this investigation, all bone marrow cells were harvested from the femur and tibia of two eight-week-old female C57BL/6J mice. The total cells were cultured for seven days under the influence of conditioned medium, subsequently light microscopy images were registered (
Figure 1) and finally the cells were labeled with specific antibodies for evaluation using flow cytometry. Due to limitations in the experimental performance, definite classification of the populations as dendritic cells or macrophages could not be achieved. Therefore, the data suggests the presence of either one or the other population. Hence, we chose to categorize them as APCs, highlighting their generalist surface markers. These targeted populations were categorized into three groups designated as: P1 (expressing CD11c
+ MHC II
+ CD11b
-), P2 (CD11c
+ MHC II
+ CD11b
+ F4/80
-), and P3 (CD11c
+ MHC II
+ CD11b
+ F4/80
+) (
Figure 2B). While the precise phenotypic characteristics of these profiles remain undetermined, it is suggested that P1 corresponds to a conventional type 1 DC (cDC1 - lacking CD11b expression), P2 aligns with a conventional type 2 DC (cDC2 - expressing CD11b), and P3 represents a monocyte-derived DC (moDC - expressing both CD11b and F4/80).
Our observations revealed a significant and noteworthy increase in the cell count within the target population of interest following GM-CSF stimulation. (
Figure 2C). Furthermore, the data revealed that SL-929 did not exhibit superior differentiation-promoting qualities within the observed populations compared to GM-CSF.
3.2. CD11c+ MHCII+ CD11b+ F4/80+P3 Identified as Predominant Population
Among the three observed populations, the population proportion of P3 was higher at all observed concentrations for both stimuli. Under GM-CSF stimulation, the observed increase compared to control was even more substantial. Notably, GM-CSF proved to be more efficient in enhancing cell count compared to L-929 and control (Figure 4). Upon observing this population under GM-CSF stimulation at 10 ng/mL, it was observed that the density of CD11b and F4/80 did not exhibit a significant increase, similarly to the control group (Figure 4C). Furthermore, our results demonstrate that SL-929 stimulates the expression of MHC II in these cells, but its values were lower than those presented by GM-CSF and were not able to differentiate these cells with success. The distinct cell profile induced by GM-CSF, compared to that of L-929, particularly at the pronounced 5 ng/mL concentration, highlights its unique capability.
Population P1 exhibited an advantage under the influence of GM-CSF 10 ng/mL, manifesting a considerably more pronounced differentiation within this cohort in comparison to other treatments, concomitantly leading to a substantial elevation in the expression of the surface protein CD11c (Figure 4, A). While SL-929 also displayed the capability to enhance the expression of these proteins compared to control, their magnitudes were found to be up to six times lesser when juxtaposed with those induced by GM-CSF and the total number of differentiated cells was not increased.
SL-929 also failed to promote differentiation of cells belonging to the P2 population, unlike GM-CSF (Figure 4B). Notably, the lowest tested concentration of GM-CSF (1 ng/mL) yielded a quantitatively diminished differentiation in comparison to higher concentrations. However, this does not unequivocally infer a dose-dependent relationship, as the concentration of 5 ng/mL exhibited the most prominent percentages when contrasted with higher doses. A discernible elevation in CD11c expression was noted across all populations subjected to treatments, with no significant disparities observed in expressions among them.
Figure 3.
Number of cells and mean fluorescence intensity (MFI) of surface proteins from the three populations of interest acquired by flow cytometry. (A) The P1 population (CD11c+ MHC II+ CD11b-) was favored by GM-CSF (10 ng/mL), showing up to six times higher levels of CD11c and MHC II surface proteins compared to cells conditioned with L-929 supernatant. (B) The P2 population (CD11c+ MHC II+ CD11b+) exhibited the lowest percentage with GM-CSF at 1 ng/mL, but this effect was not dose-dependent, as 5 ng/mL resulted in the highest percentage. (C) The P3 population (CD11c+ MHC II+ CD11b+ F4/80+) was the most predominant. GM-CSF at 5 ng/mL was the most effective for this profile. L-929 supernatant stimulated MHC II expression, but less effectively than GM-CSF. In all populations, L-929 supernatant did not significantly increase the number of differentiated cells, similar to the unconditioned control group. These findings suggest that GM-CSF is more effective in promoting the differentiation of APCs, highlighting the importance of optimizing culture conditions for specific therapeutic applications. Statistical significance was determined using t-tests comparing each treatment group to the control. GM - granulocyte-macrophage colony-stimulating factor groups; L - L-929 supernatant conditioned medium; MFI - mean fluorescence intensity.
Figure 3.
Number of cells and mean fluorescence intensity (MFI) of surface proteins from the three populations of interest acquired by flow cytometry. (A) The P1 population (CD11c+ MHC II+ CD11b-) was favored by GM-CSF (10 ng/mL), showing up to six times higher levels of CD11c and MHC II surface proteins compared to cells conditioned with L-929 supernatant. (B) The P2 population (CD11c+ MHC II+ CD11b+) exhibited the lowest percentage with GM-CSF at 1 ng/mL, but this effect was not dose-dependent, as 5 ng/mL resulted in the highest percentage. (C) The P3 population (CD11c+ MHC II+ CD11b+ F4/80+) was the most predominant. GM-CSF at 5 ng/mL was the most effective for this profile. L-929 supernatant stimulated MHC II expression, but less effectively than GM-CSF. In all populations, L-929 supernatant did not significantly increase the number of differentiated cells, similar to the unconditioned control group. These findings suggest that GM-CSF is more effective in promoting the differentiation of APCs, highlighting the importance of optimizing culture conditions for specific therapeutic applications. Statistical significance was determined using t-tests comparing each treatment group to the control. GM - granulocyte-macrophage colony-stimulating factor groups; L - L-929 supernatant conditioned medium; MFI - mean fluorescence intensity.
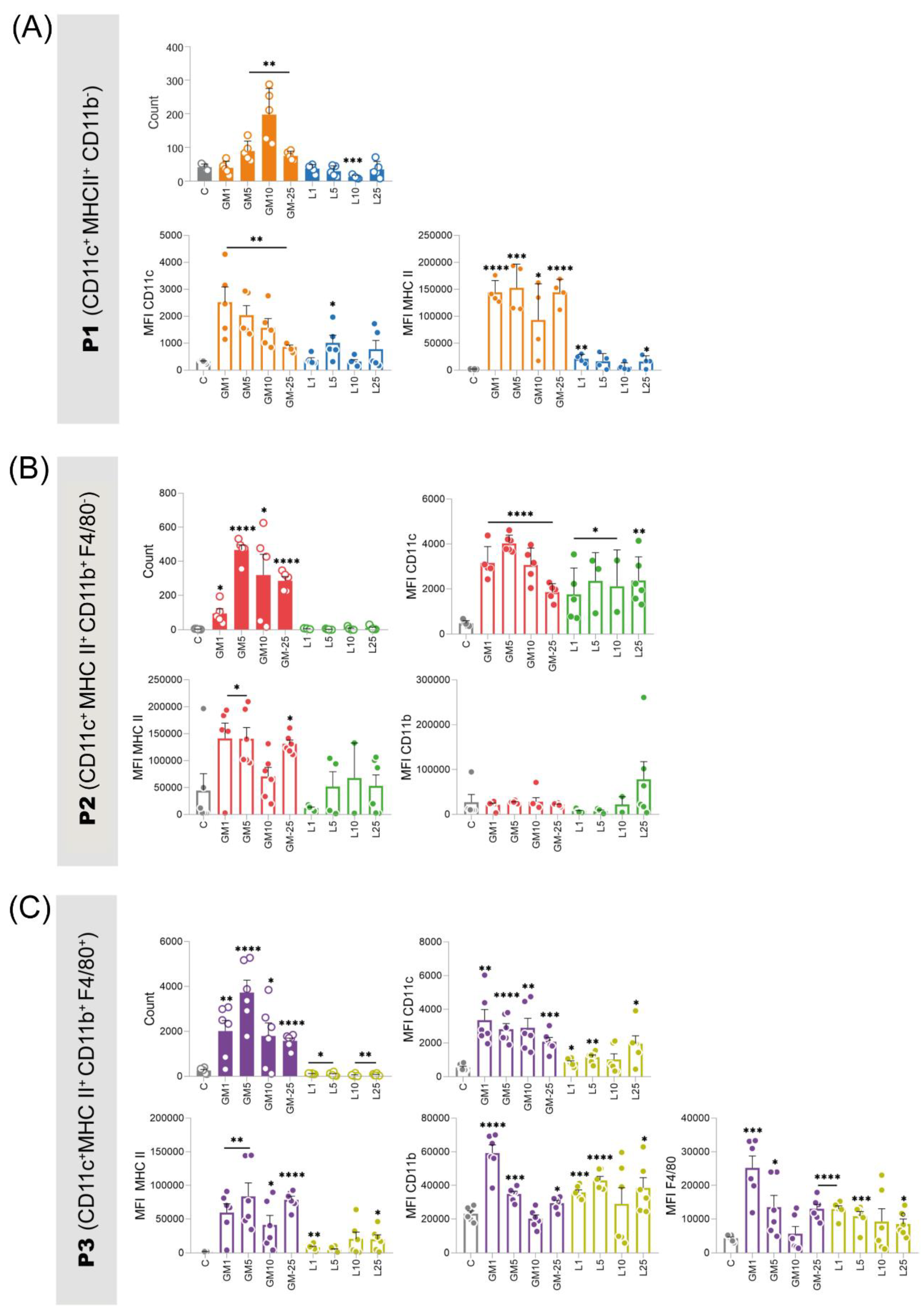
Conversely, the presence of MHC II was more pronounced in cells exposed to GM-CSF stimulation, except at the concentration of 10 ng/mL. No discernible effects on CD11b presence were attributed to the employed treatments. The concurrent elevation of MHC II and CD11c, as observed in this study, implies potential enhancements in cellular phagocytic and adhesion capabilities within the populations under scrutiny. Our findings underscore that GM-CSF, particularly at a concentration of 5 and 10 ng/mL, stands as the most promising candidate for inducing differentiation in the three observed populations from this research.
4. Discussion
In this study, we identified three distinct populations following in vitro differentiation of murine bone marrow precursor cells using GM-CSF and L-929 supernatant: P1 cells (CD11c
+, MHC II
+, CD11b
-), P2 cells (CD11c
+, MHC II
+, CD11b
+, F4/80
-) and P3 cells (CD11c
+, MHC II
+, CD11b
+, F4/80
+). Due to limitations of the employed methodology, precise identification of the cell types represented by each profile was not possible. However, we propose that these profiles correspond to three dendritic cell subsets based on their surface markers: P1 as conventional DC type 1 (cDC1), P2 as conventional DC type 2 (cDC2), and P3 as monocyte-derived DC (moDC). The most abundant population in our cultures corresponds to moDCs. Notably, the co-expression of CD11b and F4/80 in P3, while characteristic of macrophages, has also been reported in moDCs [
19].
The observed increase in the number of populations differentiated using GM-CSF compared to SL-929 could be attributed to the CSF levels in the medium. It is pertinent to highlight that GM-CSF levels in SL-929 were found to be at low concentrations, as reported by Englen et al. [
20], suggesting a potential impact of this component insufficiency on the differentiation process. A total of 2,549 secreted proteins by L-929 were documented [
21], thereby compelling to consider the possibility that they might exert an influence on the differentiation process.
Previous studies have shown that the concentration of 10 ng/mL of GM-CSF yields the best population ratio of DCs after BMPCs differentiation [
9]. In the present study, we demonstrate that, in addition to 10 ng/mL, 5 ng/mL may also be suitable depending on the desired cellular profile, potentially reducing development costs. It is important to highlight that the reduction of MHC II and CD11c proteins on the cell surface can negatively impact the functional performance of DCs [
22,
23,
24]. Our study demonstrated that the use of SL-929 yielded the lowest levels of these proteins, thus indicating that its implementation is not recommended for obtaining the DC subtypes.
5. Conclusions
In this study, we conducted a comprehensive exploration of the effects of colony growth factors present in GM-CSF and L-929 supernatant on the differentiation process of bone marrow precursor cells. Our findings underscore a remarkable and significant increase in the count of differentiated cells across all three target populations upon GM-CSF stimulation. Remarkably, the concentrations of 5 ng/mL and 10 ng/mL of GM-CSF proved notably effective in enhancing both cell count and surface protein levels. Our results substantiate that GM-CSF demonstrates superiority over L-929 supernatant in promoting differentiation within observed populations. Furthermore, our study unveiled a noteworthy prominence of the P3 population, which exhibited a substantial response to GM-CSF stimulation. This effect was most pronounced at a concentration of 10 ng/mL, suggesting the potential for a distinct differentiation profile. Our observations contribute to advancing our understanding of the impacts of GM-CSF and L-929 supernatant on cellular differentiation. Moreover, they offer insights into the potential applications of these findings in the context of cell-based immunotherapy and the exploration of roles of cell populations in various pathologies. As a next step, further investigations may delve into the underlying mechanisms driving these differentiation responses and their potential therapeutic implications.
Author Contributions
C.R.D.C.: supervision, methodology, project administration, writing (original drafting, reviewing and editing) funding acquisition. L.V.: supervision, writing (reviewing and editing), funding acquisition. F.C.P.: visualization, investigation, formal analysis, data curation, writing (reviewing and editing). A.P.B.: visualization, investigation, formal analysis, data curation, writing (reviewing and editing). G.S.C.: investigation, visualization, formal analysis, data curation, writing (original draft and editing). All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the following Brazilian foundations: Conselho Nacional de Desenvolvimento Científico e Tecnológico (National Council for Scientific and Technological Development – CNPq #158888/2018-9), Fundação de Amparo à Pesquisa do Estado de São Paulo (The São Paulo Research Foundation – FAPESP #2015/04194-0; #2022/03543-4) and Fundação de Desenvolvimento da Unicamp (Foundation for the Development of Unicamp – FAEPEX #2230/24).
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee CEUA of the Universidade Estadual de Campinas, Instituto de Biologia (5074-1/2018).
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
The graphical abstract and Figure 2(A) were created with BioRender.com.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as potential conflict of interest.
References
- ROCHE, Paul A.; FURUTA, Kazuyuki. The ins and outs of MHC class II-mediated antigen processing and presentation. Nature Reviews Immunology, v. 15, n. 4, p. 203-216, 2015. [CrossRef]
- WU, Jiaxi et al. Critical role of integrin CD11c in splenic dendritic cell capture of missing-self CD47 cells to induce adaptive immunity. Proceedings of the National Academy of Sciences, v. 115, n. 26, p. 6786-6791, 2018. [CrossRef]
- SEITA, Jun; WEISSMAN, Irving L. Hematopoietic stem cell: self-renewal versus differentiation. Wiley Interdisciplinary Reviews: Systems Biology and Medicine, v. 2, n. 6, p. 640-653, 2010.
- HONG, Wan Xing et al. Neoadjuvant intratumoral immunotherapy with TLR9 activation and anti-OX40 antibody eradicates metastatic cancer. Cancer research, v. 82, n. 7, p. 1396-1408, 2022.
- SLINGLUFF JR, Craig L. et al. Clinical and immunologic results of a randomized phase II trial of vaccination using four melanoma peptides either administered in granulocyte-macrophage colony-stimulating factor in adjuvant or pulsed on dendritic cells. Journal of Clinical Oncology, v. 21, n. 21, p. 4016-4026, 2003. [CrossRef]
- BELL, G. M. et al. Autologous tolerogenic dendritic cells for rheumatoid and inflammatory arthritis. Annals of the rheumatic diseases, v. 76, n. 1, p. 227-234, 2017.
- GIANNOUKAKIS, Nick et al. Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes care, v. 34, n. 9, p. 2026-2032, 2011. [CrossRef]
- LEAL, Lorna et al. Effect of intranodally administered dendritic cell-based HIV vaccine in combination with pegylated interferon α-2a on viral control following ART discontinuation: a phase 2A randomized clinical trial. Frontiers in Immunology, v. 12, p. 767370, 2021. [CrossRef]
- NA, Yi Rang et al. GM-CSF grown bone marrow derived cells are composed of phenotypically different dendritic cells and macrophages. Molecules and cells, v. 39, n. 10, p. 734-741, 2016.
- MASIELLO, Maria Grazia et al. Physical constraints in cell fate specification. A case in point: Microgravity and phenotypes differentiation. Progress in biophysics and molecular biology, v. 134, p. 55-67, 2018. [CrossRef]
- MACE, Thomas A. et al. Differentiation of CD8+ T cells into effector cells is enhanced by physiological range hyperthermia. Journal of leukocyte biology, v. 90, n. 5, p. 951-962, 2011. [CrossRef]
- INABA, Kayo et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. Journal of Experimental Medicine, v. 176, n. 6, p. 1693-1702, 1992. [CrossRef]
- XU, Yuekang et al. Differential development of murine dendritic cells by GM-CSF versus Flt3 ligand has implications for inflammation and trafficking. The Journal of Immunology, v. 179, n. 11, p. 7577-7584, 2007. [CrossRef]
- LACEY, Derek C. et al. Defining GM-CSF–and macrophage-CSF–dependent macrophage responses by in vitro models. The Journal of Immunology, v. 188, n. 11, p. 5752-5765, 2012.
- SUN, Li et al. GM-CSF quantity has a selective effect on granulocytic vs. monocytic myeloid development and function. Frontiers in immunology, v. 9, p. 1922, 2018. [CrossRef]
- BOLTZ-NITULESCU, George et al. Differentiation of rat bone marrow cells into macrophages under the influence of mouse L-929 cell supernatant. Journal of leukocyte biology, v. 41, n. 1, p. 83-91, 1987. [CrossRef]
- TROUPLIN, Virginie et al. Bone marrow-derived macrophage production. JoVE (Journal of Visualized Experiments), n. 81, p. e50966, 2013.
- LUTZ, Manfred B. et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. Journal of Immunological Methods, v. 223, n. 1, p. 77-92, 1999. [CrossRef]
- BOŠNJAK, B.; DO, K. T. H.; FÖRSTER, R.; HAMMERSCHMIDT, S. I. Imaging dendritic cell functions*. Immunological Reviews, [s. l.], v. 306, n. 1, p. 137–163, 2021. [CrossRef]
- ENGLEN, M.D.; VALDEZ, Y.E.; LEHNERT, N.M.; et al. Granulocyte/macrophage colony-stimulating factor is expressed and secreted in cultures of murine L-929 cells. Journal of Immunological Methods, v. 184, n. 2, p. 281–283, 1995. [CrossRef]
- HEAP, Rachel E; MARÍN-RUBIO, José Luis; PELTIER, Julien; et al. Proteomics characterisation of the L-929 cell supernatant and its role in BMDM differentiation. Life Science Alliance, v. 4, n. 6, p. e202000957, 2021. [CrossRef]
- DUNNE, J. L.; BALLANTYNE, C. M.; BEAUDET, A. L.; LEY, K. Control of leukocyte rolling velocity in TNF-α–induced inflammation by LFA-1 and Mac-1. Blood, [s. l.], v. 99, n. 1, p. 336–341, 2002.
- MORELLI, A. E.; LARREGINA, A. T.; SHUFESKY, W. J.; ZAHORCHAK, A. F.; LOGAR, A. J.; PAPWORTH, G. D.; WANG, Z.; WATKINS, S. C.; FALO, L. D., Jr; THOMSON, A. W. Internalization of circulating apoptotic cells by splenic marginal zone dendritic cells: Dependence on complement receptors and effect on cytokine production. Blood, [s. l.], v. 101, n. 2, p. 611–620, 2003. [CrossRef]
- WOHN, C.; LE GUEN, V.; VOLUZAN, O.; FIORE, F.; HENRI, S.; MALISSEN, B. Absence of MHC class II on cDC1 dendritic cells triggers fatal autoimmunity to a cross-presented self-antigen. Science Immunology, [s. l.], v. 5, n. 45, 2020.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).