Introduction
Antimicrobial resistance (AMR) arises when conventional drugs become ineffective against germs such as bacteria, fungi, viruses, and parasites, and second or third-line treatments are required (1). There are several major causes that contribute to its development and spread. Among them, we can find inappropriate use of antibiotics (2), that is the leading cause, inadequate infection prevention and control, massive use in agriculture (3) and the lack of new antibiotic development in last years (4).
It is expected that in 2050 bacterial infections will be the leading cause of death, surpassing cancer and diabetes. and it will be an economic burden worldwide (5). This is why many healthcare organizations have taken several actions in the last years. In 2018, the World Health Organization (WHO) has raised awareness among the scientific community regarding the urgency of developing new drugs; WHO has classified antibiotic-resistant bacteria into three categories, according to the urgency of needs of new treatments: critical, high, and medium priority (6,7). A fair number of bacteria resistant to carbapenems and fluoroquinolones are included in this list, such as carbapenem-resistant Acinetobacter baumanii, carbapenem-resistant Enterobacteriaceae (CRE), carbapenem-resistant Pseudomonas aeruginosa (CRPsA), fluoroquinolone-resistant Campylobacter spp. and fluoroquinolone-resistant Salmonellae, and their impact on public health is significant.
Furthermore, antibiotics are not even safe drugs. The attention on antibiotics is also focused on their adverse events. For example, Committee for Medicinal Product for Human Use (CHMP) of the European Medicines Agency (EMA) imposed severe restrictions on the use of fluoroquinolones due to their potential and severe side effects (8).
In this contest, several studies have been shown that Antimicrobial Stewardship Programs (ASPs) can be an effective strategy and have a positive impact on antibiotic consumption, appropriateness of use, decrease in AMR incidence, and side effects (9–12). Consequentially, WHO has recommended the implementation of ASPs in hospitals to contain infections and antibiotic use (13). Also, the European Centre for Disease Prevention and Control (ECDC) has highlighted the importance of ASPs in reducing the inappropriate use of antibiotics (14). Accordingly with European guidelines, in 2017, the Italian National Plan of Antimicrobial-Resistance Contrast (PNCAR) stated that ASPs were essential to reduce the inappropriate use of antibiotics and that a “One Health” approach was necessary (15). PNCAR 2022-2025 (16) has called on Italian hospitals to specifically reduce the consumption of fluoroquinolones and carbapenems, as well as the total consumption of antibiotics. Therefore, it is important to evaluate the impact of ASPs on antibiotic consumption and AMR spread in hospitals.
In this study, we aim to measure the impact of ASPs on antibiotics consumption and AMR spread from May 2023 to December 2023 in a Southern Italy hospital.
Materials and Methods
Study Design
This work compares AMR spread and antibiotic consumption of two different periods: the first, May-December 2022 (IP), and the second, May-December 2023 (IIP).
Implementing ASPs and Guidelines
In March 2023, an Antimicrobial Stewardship Committee was established in our hospital. The group consisted of clinicians, surgeons, pharmacists, nurses, an infectious disease physician, microbiologists and the hospital director.
Starting from May 2023, during each scheduled meetings, antibiotic consumption and AMR diffusion data were shown, and interventions aimed at improving the situation were discussed and applied. For example, the Antimicrobial Stewardship group provided educational interventions on hand hygiene, environmental cleaning, and prophylaxis treatment in accordance with ESCMID guidelines (17).
Detecting AMR Spread
Active Screening Culture (ASC) and Passive Screening Culture (PSC) were firstly enhanced between IP and IIP, according to ESCMID guidelines (17).
ASC consisted in programming rectal swabs for CRE and nasal swabs for MRSA on all surgical patients; PSC was used instead to detect CRPsA, MRSA, pandrug-resistant (PDR) bacteria, and vancomycin-resistant Enterococcus (VanA).
A New Way of Requesting Antibiotics
From April 2023 onwards, a change was made in the way meropenem and fluoroquinolones were prescribed. Previously, physicians were allowed to mention just the therapeutic indication in the prescription. Instead, since May 2023, physicians can only prescribe meropenem if the infection falls under the WHO recommendations mentioned in “The AWaRe Book” (18). These recommendations include sepsis and septic shock, bacterial meningitis, non-responsive to piperacillin/tazobactam (PT), severe cases of abdominal infection complicated by sepsis, extended-spectrum beta-lactamase (ESBL) infection, febrile neutropenia, and non-responsive to PT pneumonia.
Ciprofloxacin and levofloxacin can only be prescribed if the germ sensitivity is certified by an antibiogram or if the patient is allergic to beta-lactams.
Data Collection
Clinical microbiology laboratory data for ASC and PSC were used to calculate the incidence rates of antimicrobial resistance (AMR) during inpatient (IP) and outpatient (IIP) care. The study focused on bacteria such as CRE, CRPsA, MRSA, PDR, and VanA. Both hospitalizations and data consumption were obtained from hospital database. Hospitalizations were evaluated across all departments. Consumption of antibiotics was standardized using the Anatomical Therapeutic Chemical (ATC) classification system and the Defined Daily Dose (DDD) of antibiotics was calculated according to the WHO Collaborating Centre for Drug Statistics Methodology (19). Finally, the number of hospitalizations was matched with DDD to express antibiotic consumption in DDD/100 bed-days, as requested by the PNCAR 2022-2025 guidelines (16).
T-test and chi-square test were conducted, as appropriate.
Results
Active and Passive Screening Cultures
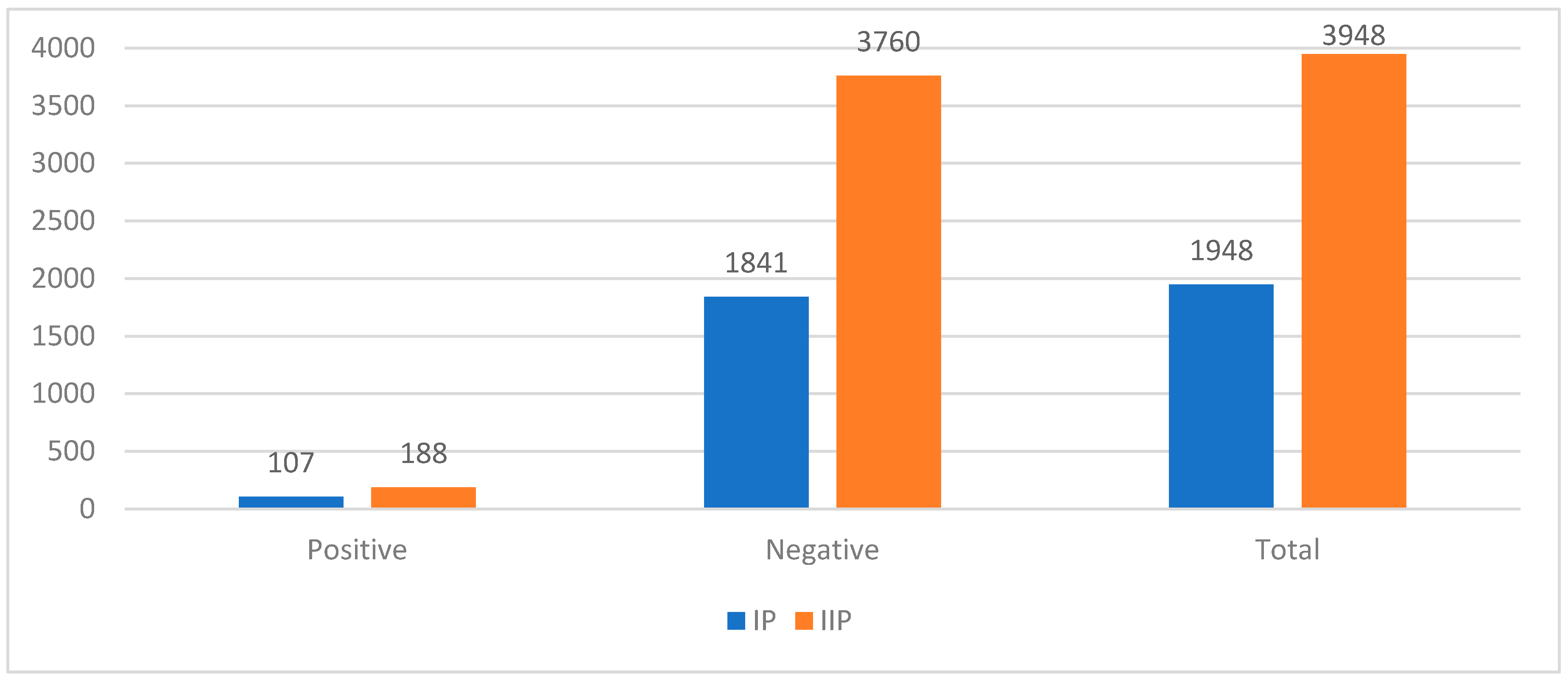
The number of rectal swabs for detecting CRE and nasal swabs for MRSA increased by 102.7% and 117.3%, respectively, between IP and IIP. However, the positivity rate decreased from 5.5% to 4.8% for CRE (p-value > 0.05) (
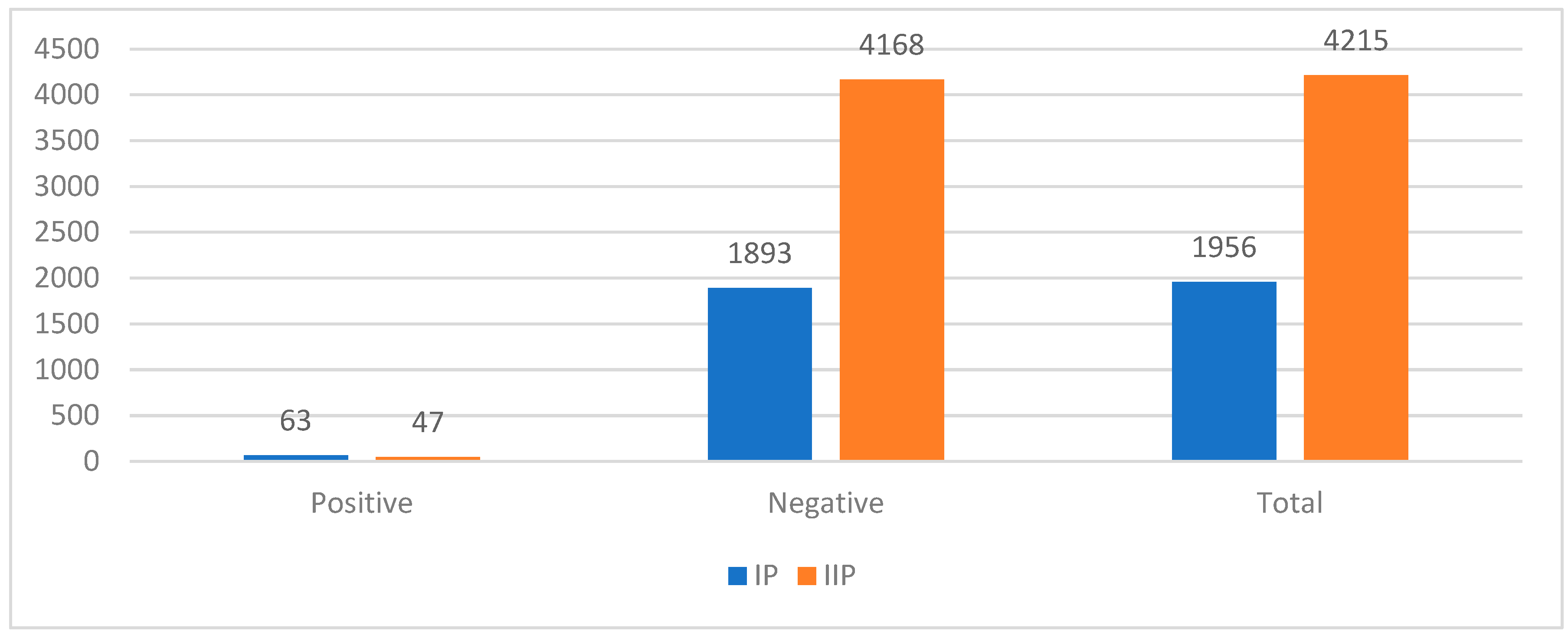
Figure 1) and from 3.2% to 1.1% for MRSA (p-value < 0.05) (
Figure 2). On the other hand, PSC activity showed a significant decrease in the number of infections. There was a reduction of 60.0% in CRPsA infections, 40.4% in MRSA infections, 21% in PDR infections, and 37.5% in VanA infections between IP and IIP.
Hospitalization and Antibiotic Consumption
The number of patients admitted to hospitals increased from 109,584 to 135,874, which is a 24% increase. However, during the same period, the total usage of antibiotics decreased from 52.1 to 38.6 (p-value < 0.05) DDD (defined daily doses) per 100 bed-days. Specifically, the use of meropenem decreased from 1.6 to 1.0 (p-value < 0.05) DDD/100 bed-days (-35.9%), ciprofloxacin decreased from 2.5 to 0.5 (p-value < 0.05) DDD/100 bed-days, and levofloxacin decreased from 4.8 to 0.5 (p-value < 0.05) DDD/100 bed-days. The most significant change was seen in the usage of cefazolin, which decreased from 4.5 DDD/100 bed-days (p-value < 0.05) to 1.6 DDD/100 bed-days (-64.4%).
Discussion
Antibiotics are drugs included in WHO Essential Medicine List (20). Nevertheless, their administration must be particularly careful. In fact, overuse and inappropriate prescriptions of antibiotics can cause various side effects and the rise of Antimicrobial Resistance (3,21). Antibiotic resistance occurs when bacteria develop mechanisms to withstand the effects of antibiotics, making them ineffective against infections. This can happen through various mechanisms, such as altering the target site of the antibiotic (22), producing enzymes that break down the antibiotic (23), or actively pumping out the antibiotic from the cell (24).
Actually, AMR is a global public health crisis, and it’s causing more deaths each year. It is among the top 10 global public health issues, and it has a significant impact on the worldwide economy, according to the World Bank (25,26). Also, it is estimated that in 2050 bacterial infections will be the leading cause of death, surpassing cancer and diabetes (5). This scenario forces healthcare organizations at every level and all stakeholders to make their contribution to stem the AMR spread. In the last years, WHO has worked on various fronts to raise awareness among the scientific community of this inexorable problem. Firstly, WHO published its “Priority List”, classifying bacteria into three categories (critical, high and medium) based on the urgency of the need for new treatments (6,7). That’s because another factor exacerbating antibiotic resistance is the lack of new antibiotic discoveries (4). In the past few decades, there has been a significant decline in the development of new antibiotics. According to a report by the Centers for Disease Control and Prevention (CDC), only three new classes of antibiotics have been approved since 1980 (27). Furthermore, WHO has also divided antibiotics into three categories, according to the AWaRe classification (28), to direct clinicians towards a more cautious use of these drugs and recommended the implementation of Antimicrobial Stewardship Programs (ASPs) to promote optimal antimicrobial use at both national and local levels (13). Studies have shown that ASPs are effective in containing AMR, reducing antibiotic consumption and side effects, and lowering healthcare costs (29–32). Also European Centre for Disease Prevention and Control (ECDC) has highlighted the importance of ASPs in reducing the inappropriate use of antibiotics (14). Consequently, Italian Ministry of Health applied these recommendations and is calling for a decrease in the use of fluoroquinolones, meropenem, and the total amount of antibiotics in 2025 (16). So, this study aims to demonstrate how the implementation of ASPs represents an effective strategy in reducing antibiotic consumption and limiting the spread of AMR.
Antimicrobial Stewardship Committee of the Hospital was formed in March 2023 and it is formed by different healthcare specialists. The Committee, discussed, approved, applied and checked different ASPs, such as: implementing infection control measures, environmental cleaning, diffusion of surgical prophylaxis protocols and individual interventions, by taking simple actions such as washing their hands regularly or avoiding close contact with people who have infections. But mainly, we decreased antibiotic consumption according to PNCAR 2022-2025. To respect its indications, we restricted meropenem and fluoroquinolones indications: meropenem can only be prescribed according to “The WHO AWaRe Book” indications (18) and fluoroquinolones only if the germ sensitivity is certified by an antibiogram or if the patient is allergic to beta-lactams. Furthermore, AMR spread was measured with Active Screening Culture (ASC) and Passive Screening Culture (PSC). Antibiotic consumptions are expressed according to ATC/DDD classification (19) and divided for 100 bed-days, as PNCAR specifies (16); incidence rates of CRE, CRPsA, MRSA, PDR and VanA are calculated to indicate AMR spread. The results showed that - despite a higher number of hospitalizations during the intervention period, the DDD/100 bed-days decreased from 52.1 to 38.6 (p-value < 0.05). Additionally, rectal swabs for CRE and nasal swabs for MRSA increased by 102.7% and 117.3%, respectively, and the positivity rate decreased from 5.8% to 5.0% for CRE and from 3.3% to 1.1% for MRSA.
Conclusion
In conclusion, antibiotic resistance is a complex issue that requires a comprehensive approach involving governments, healthcare providers, industries, and individuals. ASPs are a crucial measure in the fight against AMR. They have proven to reduce antibiotic consumption, lower healthcare costs, and limit the spread of AMR. Specifically, ASPs applied in our hospital demonstrated that rapid effects on reducing antibiotic consumption and limiting AMR spread could be obtained in few months. Furthermore, it is possible to notice how the decrease in antibiotics has led to a decrease in positivity rates in screening activities. However, statistical evaluations are needed to prove the correlation and more efforts are necessary in professional training to improve treatments, healthcare and reduce related costs. Moreover, we need to change our mindset about antibiotics and recognize that they should not be chosen for mild infections, and we should prioritize prevention strategies such as vaccination and good hygiene practices. By working together, we can reduce the spread of resistant bacteria and ensure that future generations have access to effective treatments for infectious diseases.
References
- CDC. Centers for Disease Control and Prevention. 2022 [cited 2024 Feb 20]. What Exactly is Antibiotic Resistance? Available from: https://www.cdc.gov/drugresistance/about.html.
- Hand K. Antibiotic stewardship. Clin Med. 2013 Oct;13(5):499–503. [CrossRef]
- McEwen SA, Collignon PJ. Antimicrobial Resistance: a One Health Perspective. Microbiol Spectr. 2018 Mar 29;6(2). [CrossRef]
- Lack of innovation set to undermine antibiotic performance and health gains [Internet]. [cited 2024 Jul 7]. Available from: https://www.who.int/news/item/22-06-2022-22-06-2022-lack-of-innovation-set-to-undermine-antibiotic-performance-and-health-gains.
- O’neill JIM. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Rev Antimicrob Resist. 2014;
- Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018 Mar 1;18(3):318–27. [CrossRef]
- Asokan GV, Ramadhan T, Ahmed E, Sanad H. WHO Global Priority Pathogens List: A Bibliometric Analysis of Medline-PubMed for Knowledge Mobilization to Infection Prevention and Control Practices in Bahrain. Oman Med J. 2019 May;34(3):184–93. [CrossRef]
- Quinolone- and fluoroquinolone-containing medicinal products - referral | European Medicines Agency [Internet]. [cited 2024 Feb 23]. Available from: https://www.ema.europa.eu/en/medicines/human/referrals/quinolone-and-fluoroquinolone-containing-medicinal-products.
- Nathwani D, Varghese D, Stephens J, Ansari W, Martin S, Charbonneau C. Value of hospital antimicrobial stewardship programs [ASPs]: a systematic review. Antimicrob Resist Infect Control. 2019;8:35. [CrossRef]
- Rehman IU, Asad MM, Bukhsh A, Ali Z, Ata H, Dujaili JA, et al. Knowledge and Practice of Pharmacists toward Antimicrobial Stewardship in Pakistan. Pharm Basel Switz. 2018 Oct 23;6(4):116. [CrossRef]
- Ture Z, Güner R, Alp E. Antimicrobial stewardship in the intensive care unit. J Intensive Med. 2022 Nov 15;3(3):244–53. [CrossRef]
- Wang H, Wang H, Yu X, Zhou H, Li B, Chen G, et al. Impact of antimicrobial stewardship managed by clinical pharmacists on antibiotic use and drug resistance in a Chinese hospital, 2010–2016: a retrospective observational study. BMJ Open. 2019 Aug 2;9(8):e026072. [CrossRef]
- WHO Global Strategy for Containment of Antimicrobial Resistance [Internet]. [cited 2024 Feb 20]. Available from: https://www.who.int/publications-detail-redirect/who-global-strategy-for-containment-of-antimicrobial-resistance.
- Proposals for EU guidelines on the prudent use of antimicrobials in humans [Internet]. 2017 [cited 2024 Feb 20]. Available from: https://www.ecdc.europa.eu/en/publications-data/proposals-eu-guidelines-prudent-use-antimicrobials-humans.
- Salute M della. Piano Nazionale di Contrasto dell’Antimicrobico-Resistenza (PNCAR) 2017-2020 [Internet]. [cited 2024 Feb 20]. Available from: https://www.salute.gov.it/portale/documentazione/p6_2_2_1.jsp?id=2660&lingua=italiano.
- EpiCentro. Piano Nazionale di Contrasto all’Antibiotico-Resistenza (PNCAR) 2022-2025 [Internet]. [cited 2024 Feb 22]. Available from: https://www.epicentro.iss.it/antibiotico-resistenza/pncar-2022.
- Tacconelli E, Cataldo MA, Dancer SJ, De Angelis G, Falcone M, Frank U, et al. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin Microbiol Infect. 2014 Jan;20:1–55. [CrossRef]
- The WHO AWaRe (Access, Watch, Reserve) antibiotic book [Internet]. [cited 2024 Feb 22]. Available from: https://www.who.int/publications-detail-redirect/9789240062382.
- ATCDDD - ATC/DDD Index [Internet]. [cited 2024 Jul 7]. Available from: https://atcddd.fhi.no/atc_ddd_index/.
- WHO Model Lists of Essential Medicines [Internet]. [cited 2024 Jul 7]. Available from: https://www.who.int/groups/expert-committee-on-selection-and-use-of-essential-medicines/essential-medicines-lists.
- Ventola CL. The Antibiotic Resistance Crisis. Pharm Ther. 2015 Apr;40(4):277–83.
- Lambert PA. Bacterial resistance to antibiotics: modified target sites. Adv Drug Deliv Rev. 2005 Jul 29;57(10):1471–85. [CrossRef]
- Bush K, Bradford PA. β-Lactams and β-Lactamase Inhibitors: An Overview. Cold Spring Harb Perspect Med. 2016 Aug 1;6(8):a025247. [CrossRef]
- Soto SM. Role of efflux pumps in the antibiotic resistance of bacteria embedded in a biofilm. Virulence. 2013 Apr 1;4(3):223–9. [CrossRef]
- Ten health issues WHO will tackle this year [Internet]. [cited 2024 Feb 26]. Available from: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019.
- World Bank [Internet]. [cited 2024 Feb 26]. Drug-Resistant Infections: A Threat to Our Economic Future. Available from: https://www.worldbank.org/en/topic/health/publication/drug-resistant-infections-a-threat-to-our-economic-future.
- CDC. Antimicrobial Resistance. 2024 [cited 2024 Jul 7]. 2019 Antibiotic Resistance Threats Report. Available from: https://www.cdc.gov/antimicrobial-resistance/data-research/threats/index.html.
- 2021 AWaRe classification [Internet]. [cited 2024 Jul 7]. Available from: https://www.who.int/publications/i/item/2021-aware-classification.
- Pollack LA, Srinivasan A. Core Elements of Hospital Antibiotic Stewardship Programs From the Centers for Disease Control and Prevention. Clin Infect Dis Off Publ Infect Dis Soc Am. 2014 Oct 15;59(Suppl 3):S97-100. [CrossRef]
- D’Agata EMC, Tran D, Bautista J, Shemin D, Grima D. Clinical and Economic Benefits of Antimicrobial Stewardship Programs in Hemodialysis Facilities. Clin J Am Soc Nephrol CJASN. 2018 Sep 7;13(9):1389–97. [CrossRef]
- Ibrahim NH, Maruan K, Khairy HAM, Hong YH, Dali AF, Neoh CF. Economic Evaluations on Antimicrobial Stewardship Programme: A Systematic Review. J Pharm Pharm Sci. 2017;20:397–406. [CrossRef]
- Global action plan on antimicrobial resistance [Internet]. [cited 2024 Feb 26]. Available from: https://www.who.int/publications-detail-redirect/9789241509763.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).