Submitted:
17 July 2024
Posted:
17 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
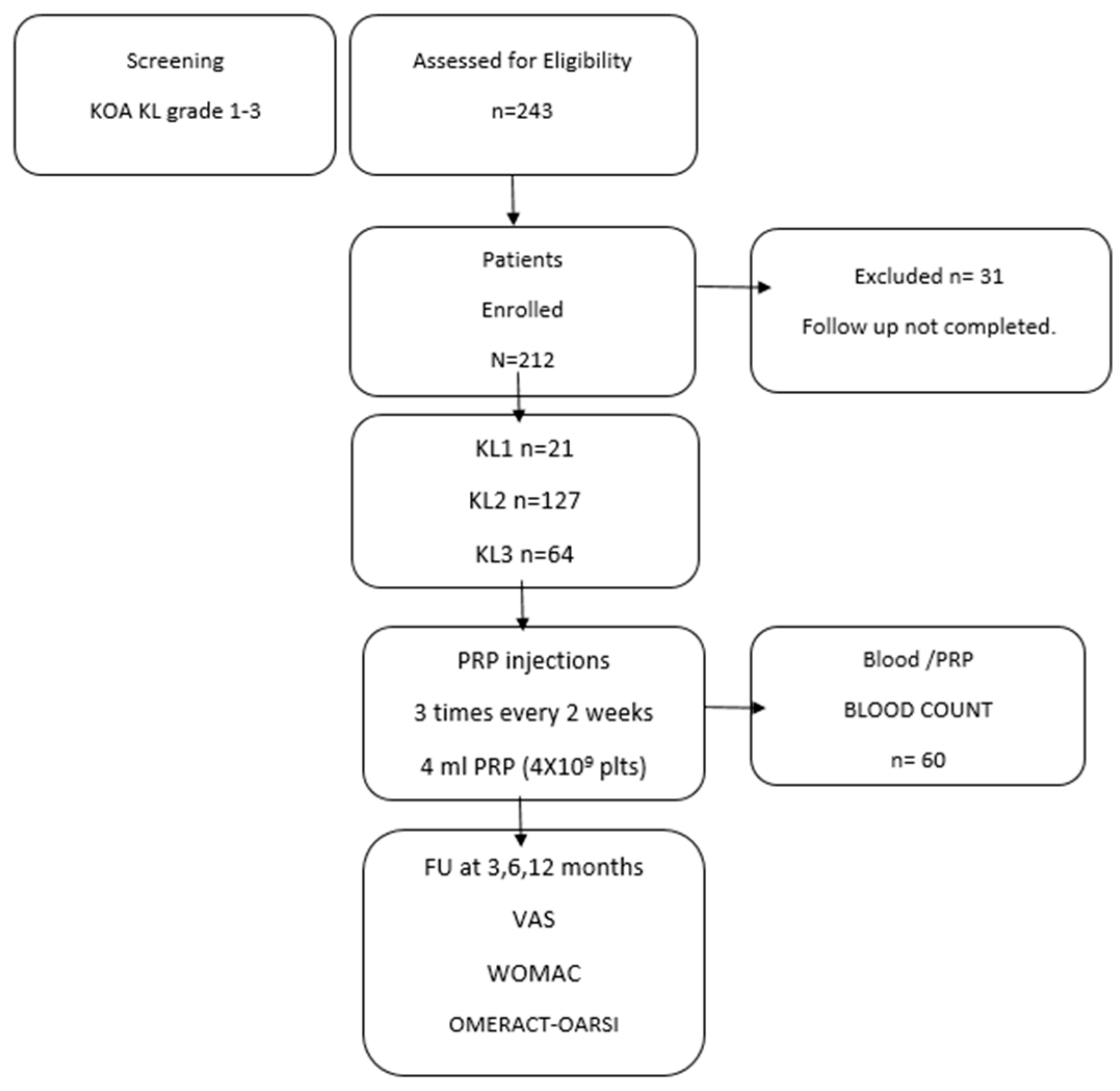
2. Materials and Methods
2.1. Patients
- Age: > 18 years
- Hgb: > 12 g/dl
- PLT (minimum value): ≥120,000/µl
- WBC < 10,000/mm3
- No corticosteroid therapies for more than one month
- No NSAIDs for at least one week
2.2. PRP Preparation and Protocol
2.3. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Biological Characteristics of Injected PRP
3.3. Clinical Outcome after PRP According to the OMERCAT-OARSI, VAS and WOMAC Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990-2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [CrossRef]
- Primorac, D.; Molnar, V.; Rod, E.; Jeleč, Ž.; Čukelj, F.; Matišić, V.; Vrdoljak, T.; Hudetz, D.; Hajsok, H.; Borić, I. Knee Osteoarthritis: A Review of Pathogenesis and State-Of-The-Art Non-Operative Therapeutic Considerations. Genes (Basel) 2020, 11, 1–35. [CrossRef]
- Gangadharan, S.B.; Satapathy, S.; Dixit, T.; C, Dr.S.; Ravindran, S.; Parida, P.K. Platelet-Rich Plasma Treatment for Knee Osteoarthritis: A Systematic Investigation. Multidisciplinary Reviews 2024, 6, 2023ss015. [CrossRef]
- Everts, P.A.; van Erp, A.; DeSimone, A.; Cohen, D.S.; Gardner, R.D. Platelet Rich Plasma in Orthopedic Surgical Medicine. Platelets 2021, 32, 163–174. [CrossRef]
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.F.; Mautner, K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int J Mol Sci 2020, 21, 1–36. [CrossRef]
- Laver, L.; Filardo, G.; Sanchez, M.; Magalon, J.; Tischer, T.; Abat, F.; Bastos, R.; Cugat, R.; Iosifidis, M.; Kocaoglu, B.; et al. The Use of Injectable Orthobiologics for Knee Osteoarthritis: A European ESSKA-ORBIT Consensus. Part 1—Blood-derived Products (Platelet-rich Plasma). Knee Surgery, Sports Traumatology, Arthroscopy 2024. [CrossRef]
- Zahir, H.; Dehghani, B.; Yuan, X.; Chinenov, Y.; Kim, C.; Burge, A.; Bandhari, R.; Nemirov, D.; Fava, P.; Moley, P.; et al. In Vitro Responses to Platelet-Rich-Plasma Are Associated with Variable Clinical Outcomes in Patients with Knee Osteoarthritis. Scientific Reports 2021 11:1 2021, 11, 1–13. [CrossRef]
- Gato-Calvo, L.; Magalhaes, J.; Ruiz-Romero, C.; Blanco, F.J.; Burguera, E.F. Platelet-Rich Plasma in Osteoarthritis Treatment: Review of Current Evidence. Ther Adv Chronic Dis 2019, 10, 1–18. [CrossRef]
- Andia, I.; Maffulli, N. Platelet-Rich Plasma for Managing Pain and Inflammation in Osteoarthritis. Nat Rev Rheumatol 2013, 9, 721–730. [CrossRef]
- Everts, P.A.; Mazzola, T.; Mautner, K.; Randelli, P.S.; Podesta, L. Modifying Orthobiological PRP Therapies Are Imperative for the Advancement of Treatment Outcomes in Musculoskeletal Pathologies. Biomedicines 2022, 10. [CrossRef]
- Everts, P.A.; Lana, J.F.; Onishi, K.; Buford, D.; Peng, J.; Mahmood, A.; Fonseca, L.F.; van Zundert, A.; Podesta, L. Angiogenesis and Tissue Repair Depend on Platelet Dosing and Bioformulation Strategies Following Orthobiological Platelet-Rich Plasma Procedures: A Narrative Review. Biomedicines 2023, 11. [CrossRef]
- Sprugel, K.H.; Mcpherson, J.M.; Clowes, A.W.; Ross, R. Effects of Growth Factors in Vivo I. Cell Ingrowth Into Porous Subcutaneous Chambers; 1987; Vol. 129;
- Liang, Y.; Li, J.; Wang, Y.; He, J.; Chen, L.; Chu, J.; Wu, H. Platelet Rich Plasma in the Repair of Articular Cartilage Injury: A Narrative Review. Cartilage 2022, 13. [CrossRef]
- Gupta, A.; Jeyaraman, M.; Potty, A.G. Leukocyte-Rich vs. Leukocyte-Poor Platelet-Rich Plasma for the Treatment of Knee Osteoarthritis. Biomedicines 2023, 11.
- Di Martino, A.; Boffa, A.; Andriolo, L.; Romandini, I.; Altamura, S.A.; Cenacchi, A.; Roverini, V.; Zaffagnini, S.; Filardo, G. Leukocyte-Rich versus Leukocyte-Poor Platelet-Rich Plasma for the Treatment of Knee Osteoarthritis: A Double-Blind Randomized Trial. Am J Sports Med 2022, 50, 609–617. [CrossRef]
- Zhou, Y.; Li, H.; Cao, S.; Han, Y.; Shao, J.; Fu, Q.; Wang, B.; Wu, J.; Xiang, D.; Liu, Z.; et al. Clinical Efficacy of Intra-Articular Injection with P-PRP Versus That of L-PRP in Treating Knee Cartilage Lesion: A Randomized Controlled Trial. Orthop Surg 2023, 15, 740–749. [CrossRef]
- Everts, P.A.; Malanga, G.A.; Paul, R. V.; Rothenberg, J.B.; Stephens, N.; Mautner, K.R. Assessing Clinical Implications and Perspectives of the Pathophysiological Effects of Erythrocytes and Plasma Free Hemoglobin in Autologous Biologics for Use in Musculoskeletal Regenerative Medicine Therapies. A Review. Regen Ther 2019, 11, 56. [CrossRef]
- Xiong, Y.; Gong, C.; Peng, X.; Liu, X.; Su, X.; Tao, X.; Li, Y.; Wen, Y.; Li, W. Efficacy and Safety of Platelet-Rich Plasma Injections for the Treatment of Osteoarthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front Med (Lausanne) 2023, 10. [CrossRef]
- Carlsson, A.M. Assessment of Chronic Pain. I. Aspects of the Reliability and Validity of the Visual Analogue Scale. Pain 1983, 16, 87–101. [CrossRef]
- Pham, T.; van der Heijde, D.; Altman, R.D.; Anderson, J.J.; Bellamy, N.; Hochberg, M.; Simon, L.; Strand, V.; Woodworth, T.; Dougados, M. OMERACT-OARSI Initiative: Osteoarthritis Research Society International Set of Responder Criteria for Osteoarthritis Clinical Trials Revisited. Osteoarthritis Cartilage 2004, 12, 389–399. [CrossRef]
- Saita, Y.; Kobayashi, Y.; Nishio, H.; Wakayama, T.; Fukusato, S.; Uchino, S.; Momoi, Y.; Ikeda, H.; Kaneko, K. Predictors of Effectiveness of Platelet-Rich Plasma Therapy for Knee Osteoarthritis: A Retrospective Cohort Study. J Clin Med 2021, 10. [CrossRef]
- Magalon, J.; Chateau, A.L.; Bertrand, B.; Louis, M.L.; Silvestre, A.; Giraudo, L.; Veran, J.; Sabatier, F. DEPA Classification: A Proposal for Standardising PRP Use and a Retrospective Application of Available Devices. BMJ Open Sport Exerc Med 2016, 2. [CrossRef]
- Delong, J.M.; Russell, R.P.; Mazzocca, A.D. Platelet-Rich Plasma: The PAW Classification System. Arthroscopy - Journal of Arthroscopic and Related Surgery 2012, 28, 998–1009. [CrossRef]
- Bansal, H.; Leon, J.; Pont, J.L.; Wilson, D.A.; Bansal, A.; Agarwal, D.; Preoteasa, I. Platelet-Rich Plasma (PRP) in Osteoarthritis (OA) Knee: Correct Dose Critical for Long Term Clinical Efficacy. Sci Rep 2021, 11. [CrossRef]
- Patel, S.; Gahlaut, S.; Thami, T.; Chouhan, D.K.; Jain, A.; Dhillon, M.S. Comparison of Conventional Dose Versus Superdose Platelet-Rich Plasma for Knee Osteoarthritis: A Prospective, Triple-Blind, Randomized Clinical Trial. Orthop J Sports Med 2024, 12. [CrossRef]
- Qiao, X.; Yan, L.; Feng, Y.; Li, X.; Zhang, K.; Lv, Z.; Xu, C.; Zhao, S.; Liu, F.; Yang, X.; et al. Efficacy and Safety of Corticosteroids, Hyaluronic Acid, and PRP and Combination Therapy for Knee Osteoarthritis: A Systematic Review and Network Meta-Analysis. BMC Musculoskelet Disord 2023, 24. [CrossRef]
- Ivander, G.; Anggono, Y. A Comparison of Intra-Articular Hyaluronic Acid and Platelet-Rich Plasma for Knee Osteoarthritis: A Systematic Review. Orthop Rev (Pavia) 2024, 16. [CrossRef]
- Migliorini, F.; Driessen, A.; Quack, V.; Sippel, N.; Cooper, B.; Mansy, Y. El; Tingart, M.; Eschweiler, J. Comparison between Intra-Articular Infiltrations of Placebo, Steroids, Hyaluronic and PRP for Knee Osteoarthritis: A Bayesian Network Meta-Analysis. Arch Orthop Trauma Surg 2021, 141, 1473–1490. [CrossRef]
- Berrigan, W.A.; Bailowitz, Z.; Park, A.; Reddy, A.; Liu, R.; Lansdown, D. A Higher Platelet Dose May Yield Better Clinical Outcomes for PRP in the Treatment of Knee Osteoarthritis: A Systematic Review. Arthroscopy 2024. [CrossRef]
- Prost, D.; Bardot, T.; Baud, A.; Calvo, A.; Aumont, S.; Collado, H.; Borne, J.; Rajon, O.; Ponsot, A.; Malaterre, A.; et al. Long Term Improvement of Knee Osteoarthritis after Injection of Single High/Very High Volume of Very Pure PRP: A Retrospective Analysis of Patients Optimally Managed in Dedicated Centers. Regen Ther 2024, 25, 203–212. [CrossRef]
- Everts, P.A.; Lana, J.F.; Onishi, K.; Buford, D.; Peng, J.; Mahmood, A.; Fonseca, L.F.; van Zundert, A.; Podesta, L. Angiogenesis and Tissue Repair Depend on Platelet Dosing and Bioformulation Strategies Following Orthobiological Platelet-Rich Plasma Procedures: A Narrative Review. Biomedicines 2023, 11. [CrossRef]
- Bennell, K.L.; Paterson, K.L.; Metcalf, B.R.; Duong, V.; Eyles, J.; Kasza, J.; Wang, Y.; Cicuttini, F.; Buchbinder, R.; Forbes, A.; et al. Effect of Intra-Articular Platelet-Rich Plasma vs Placebo Injection on Pain and Medial Tibial Cartilage Volume in Patients With Knee Osteoarthritis: The RESTORE Randomized Clinical Trial. JAMA 2021, 326, 2021–2030. [CrossRef]
- Johal, H.; Khan, M.; Yung, S. hang P.; Dhillon, M.S.; Fu, F.H.; Bedi, A.; Bhandari, M. Impact of Platelet-Rich Plasma Use on Pain in Orthopaedic Surgery: A Systematic Review and Meta-Analysis. Sports Health 2019, 11, 355–366. [CrossRef]
- Gupta, A.; Maffulli, N.; Jain, V.K. Red Blood Cells in Platelet-Rich Plasma: Avoid If at All Possible. Biomedicines 2023, 11. [CrossRef]
- Gupta, A.; Jeyaraman, M.; Potty, A.G. Leukocyte-Rich vs. Leukocyte-Poor Platelet-Rich Plasma for the Treatment of Knee Osteoarthritis. Biomedicines 2023, 11. [CrossRef]
- Xu, Z.; Yin, W.; Zhang, Y.; Qi, X.; Chen, Y.; Xie, X.; Zhang, C. Comparative Evaluation of Leukocyte-and Platelet-Rich Plasma and Pure Platelet-Rich Plasma for Cartilage Regeneration. Sci Rep 2017, 7. [CrossRef]
- Peng, Y.; Guanglan, W.; Jia, S.; Zheng, C. Leukocyte-Rich and Leukocyte-Poor Platelet-Rich Plasma in Rotator Cuff Repair: A Meta-Analysis. Int J Sports Med 2022, 0. [CrossRef]
- Lana, J.F.; Huber, S.C.; Purita, J.; Tambeli, C.H.; Santos, G.S.; Paulus, C.; Annichino-Bizzacchi, J.M. Leukocyte-Rich PRP versus Leukocyte-Poor PRP - The Role of Monocyte/Macrophage Function in the Healing Cascade. J Clin Orthop Trauma 2019, 10, S7–S12. [CrossRef]
- Fedorova, N. V.; Ksenofontov, A.L.; Serebryakova, M. V.; Stadnichuk, V.I.; Gaponova, T. V.; Baratova, L.A.; Sud’ina, G.F.; Galkina, S.I. Neutrophils Release Metalloproteinases during Adhesion in the Presence of Insulin, but Cathepsin G in the Presence of Glucagon. Mediators Inflamm 2018, 2018. [CrossRef]
- Kim, J.H.; Park, Y.B.; Ha, C.W.; Roh, Y.J.; Park, J.G. Adverse Reactions and Clinical Outcomes for Leukocyte-Poor Versus Leukocyte-Rich Platelet-Rich Plasma in Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Orthop J Sports Med 2021, 9. [CrossRef]
- Belk, J.W.; Kraeutler, M.J.; Houck, D.A.; Goodrich, J.A.; Dragoo, J.L.; McCarty, E.C. Platelet-Rich Plasma Versus Hyaluronic Acid for Knee Osteoarthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. American Journal of Sports Medicine 2021, 49, 249–260. [CrossRef]
- Xiong, Y.; Gong, C.; Peng, X.; Liu, X.; Su, X.; Tao, X.; Li, Y.; Wen, Y.; Li, W. Efficacy and Safety of Platelet-Rich Plasma Injections for the Treatment of Osteoarthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front Med (Lausanne) 2023, 10, 1204144. [CrossRef]
- Ogle, M.E.; Segar, C.E.; Sridhar, S.; Botchwey, E.A.; Coulter, W.H. Monocytes and Macrophages in Tissue Repair: Implications for Immunoregenerative Biomaterial Design. Exp Biol Med 2016, 241, 1084–1097. [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [CrossRef]
- Caballero-Sánchez, N.; Alonso-Alonso, S.; Nagy, L. Regenerative Inflammation: When Immune Cells Help to Re-Build Tissues. FEBS J 2024, 291, 1597–1614. [CrossRef]
- Groppa, E.; Colliva, A.; Vuerich, R.; Kocijan, T.; Zacchigna, S. Immune Cell Therapies to Improve Regeneration and Revascularization of Non-Healing Wounds. Int J Mol Sci 2020, 21, 1–22. [CrossRef]
- Forbes, S.J.; Rosenthal, N. Preparing the Ground for Tissue Regeneration: From Mechanism to Therapy. Nat Med 2014, 20, 857–869. [CrossRef]
- Julier, Z.; Park, A.J.; Briquez, P.S.; Martino, M.M.; Julier, Z.; Park, A.J.; Briquez, P.S.; Martino, M.M. Promoting Tissue Regeneration by Modulating the Immune System. Acta Biomater 2017, 53, 13–28. [CrossRef]
- Chisari, Em.; Rehak, L.; Khan, W.S.; Maffulli, N. The Role of the Immune System in Tendon Healing: A Systematic Review. Br Med Bull 2020, 133, 49–54. [CrossRef]
- Chisari, E.; Rehak, L.; Khan, W.S.; Maffulli, N. Tendon Healing in Presence of Chronic Low-Level Inflammation: A Systematic Review. Br Med Bull 2019, 132, 97–116. [CrossRef]
- Rehak, L.; Giurato, L.; Meloni, M.; Panunzi, A.; Manti, G.M.; Uccioli, L. The Immune-Centric Revolution in the Diabetic Foot: Monocytes and Lymphocytes Role in Wound Healing and Tissue Regeneration—A Narrative Review. J Clin Med 2022, 11.
- Hopper, N.M.; Wardale, J.; Rushton, N. Mononuclear Cells Enhance Cell Migration out of Human Articular Cartilage. J Tissue Eng Regen Med 2012, 6.
- Hopper, N.; Wardale, J.; Brooks, R.; Power, J.; Rushton, N. Peripheral Blood Mononuclear Cells Enhance Cartilage Repair in in Vivo Osteochondral Defect Model. 2015, 1–16. [CrossRef]
- Hopper, N.; Wardale, J.; Howard, D.; Brooks, R.; Rushton, N.; Henson, F. Peripheral Blood Derived Mononuclear Cells Enhance the Migration and Chondrogenic Differentiation of Multipotent Mesenchymal Stromal Cells. Stem Cells Int 2015, 2015. [CrossRef]
- Yuan, Z.; Jiang, D.; Yang, M.; Tao, J.; Hu, X.; Yang, X.; Zeng, Y. Emerging Roles of Macrophage Polarization in Osteoarthritis: Mechanisms and Therapeutic Strategies. Orthop Surg 2024, 16, 532–550. [CrossRef]
- Zhu, Y.; Fu, W. Peripheral Blood-Derived Stem Cells for the Treatment of Cartilage Injuries: A Systematic Review. Front Bioeng Biotechnol 2022, 10, 956614. [CrossRef]
- Abdine, N.M.; Moustafa, K.A.; Bakery, R.H.E.; Sarhan, N.E.; Salah, E.F. Effect of Intra-Articular Injection of Peripheral Blood Mononuclear Cells Versus Platelet-Rich Plasma on Restoration of Collagen Fibers of the Articular Cartilage in a Rat Model of Knee Osteoarthritis. Egyptian Journal of Histology 2023, 46, 1861–1869. [CrossRef]
- Bohaud, C.; Contreras-Lopez, R.; De La Cruz, J.; Terraza-Aguirre, C.; Wei, M.; Djouad, F.; Jorgensen, C. Pro-Regenerative Dialogue Between Macrophages and Mesenchymal Stem/Stromal Cells in Osteoarthritis. Front Cell Dev Biol 2021, 9, 2367. [CrossRef]
- Kanda, K.; Asawa, Y.; Inaki, R.; Fujihara, Y.; Hoshi, K.; Hikita, A. Requirement of Direct Contact between Chondrocytes and Macrophages for the Maturation of Regenerative Cartilage. Scientific Reports 2022 11:1 2021, 11, 1–17. [CrossRef]
- Li, M.; Yin, H.; Yan, Z.; Li, H.; Wu, J.; Wang, Y.; Wei, F.; Tian, G.; Ning, C.; Li, H.; et al. The Immune Microenvironment in Cartilage Injury and Repair. Acta Biomater 2022, 140, 23–42. [CrossRef]
- Roh, Y.H.; Kim, W.; Park, K.U.; Oh, J.H. Cytokine-Release Kinetics of Platelet-Rich Plasma According to Various Activation Protocols. Bone Joint Res 2016, 5, 37. [CrossRef]
- Mishra, A.; Woodall, J.; Vieira, A. Treatment of Tendon and Muscle Using Platelet-Rich Plasma. Clin Sports Med 2009, 28, 113–125. [CrossRef]
- Mishra, A.; Tummala, P.; King, A.; Lee, B.; Kraus, M.; Tse, V.; Jacobs, C.R. Buffered Platelet-Rich Plasma Enhances Mesenchymal Stem Cell Proliferation and Chondrogenic Differentiation. Tissue Eng Part C Methods 2009, 15, 431–435. [CrossRef]
- Han, B.; Woodell-May, J.; Ponticiello, M.; Yang, Z.; Nimni, M. The Effect of Thrombin Activation of Platelet-Rich Plasma on Demineralized Bone Matrix Osteoinductivity. J Bone Joint Surg Am 2009, 91, 1459–1470. [CrossRef]

| n=212 | |
|---|---|
|
Age (mean +/- SD) Range (years) |
56.09 +/- 9.23 19-89 |
|
Gender Male n (%) Female n (%) |
42% 58% |
|
BMI (kg/m2) Range BMI |
25.3 +/-4.1 23-28.3 |
| Side | |
| Left /Right Knee (%) | 44%/56% |
| KL Grade | |
| Grade 1 n (%) | 21 out 212 (9,9 %) |
| Grade 2 n (%) | 127 out 212 (59,9 %) |
| Grade 3 n (%) | 64 out 212 (30,2 %) |
| Basal Platelets (X103/µl) | 225+/- 37 |
| VAS score | 6.49 +/- 1.15 |
| WOMAC score | 41.98 +/- 6.8 |
| Blood | PRP | |
|---|---|---|
| Blood Volume /PRP volume* | 20 ml | 4.1 +/- 0.3 |
| Platelet Conc. (103/µl) | 235+/- 37 | 960+/- 108 |
| Platelet dose (Billions 109) | 4.7 +/-0.79 | 3.9+/- 0.2 |
| WBC (103/µl) | 10.3 +/- 2.3 | 3 +/- 0.22 |
| Granulocyte (103/µl) | 7.5 +/- 0.89 | 0.5 +/- 0.05 |
| Limphocytes (103/µl) | 2.7+/- 1.6 | 2.1+/- 0.8 |
| Monocytes (103/µl) | 0.78+/- 1.8 | 0.42+/- 0.49 |
| PB-MNC (103/µl) | 3.56 +/- 1.2 | 2.38 +/- 0.98 |
| RBC (106/µl) | 5.40+/- 2.9 | 0.053 +/- 0.01 |
| Platelets conc fold | 4 X | |
| Platelet Recovery Rate% | 82,1 +/- 0,4 | |
| % Mononuclear cells/WBC | 80 % |
| Non-Responder | Responder | Patients | Responder Rate | |
|---|---|---|---|---|
| Overall | ||||
| 3 months | 61 | 151 | n=212 | 71,2% |
| 6 months | 56 | 156 | n=212 | 73,6 % |
| 12 months | 66 | 147 | n=212 | 69,3, % |
| KL Grade 1 | ||||
| 3 months | 6 | 15 | n= 21 | 71,4% |
| 6 months | 5 | 16 | n= 21 | 76,2% |
| 12 months | 6 | 15 | n= 21 | 71,4% |
| KL Grade 2 | ||||
| 3 months | 36 | 91 | n= 127 | 71,7 % |
| 6 months | 33 | 94 | n= 127 | 74,0% |
| 12 months | 39 | 88 | n= 127 | 69,3% |
| KL Grade 3 | ||||
| 3 months | 19 | 45 | n=64 | 70,3% |
| 6 months | 18 | 46 | n=64 | 71,9% |
| 12 months | 20 | 44 | n=64 | 68,8% |
| Basal- Pretreatment | 3m | 6m | 12m | |
|---|---|---|---|---|
| VAS pain | ||||
| Overall | 6.49 +/- 1.15 | 4.3 +/- 1.8* | 2.69 +/- 0.98* | 3.79 +/- 0.78* |
| KL1 | 6.02 +/- 1.09 | 4.66 +/- 1.23* | 2.81 +/- 0.6* | 3.81+/- 0.3* |
| KL2 | 6.41 +/- 0.65 | 4.15+/- 0.98* | 2.6+/- 0.53* | 3.65+/- 0.89* |
| KL3 | 6.58+/- 1.09 | 4.99+/- 1.2* | 2.4+/- 0.99* | 3.48+/- 1.1* |
| WOMAC | ||||
| Overall | 41.98 +/- 6.8 | 36.55+/- 13.5* | 34.9 +/- 11.8* | 39.3 +/- 8,05 |
| KL1 | 40.65 +/- 11.9 | 36.99 +/- 9.5* | 35.8 +/- 12.3* | 38.2 +/- 9,99 |
| KL2 | 41.65 +/- 9.5 | 35.05 +/- 9.99* | 34.1 +/- 14.0* | 38.1 +/- 8,67 |
| KL3 | 40.65 +/- 3.9 | 36.18 +/- 4.89* | 35.8 +/- 9.06* | 40.13 +/- 12.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





