Submitted:
17 July 2024
Posted:
18 July 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and Methods
The Study Patients
Inclusion and Exclusion Criteria
Race/Ethnicity, Patients’ Demographics and Body Mass Index (BMI)
Data Availability
Statistical Analysis
DNA Mismatch Repair Protein Expression (MMRP)
Results
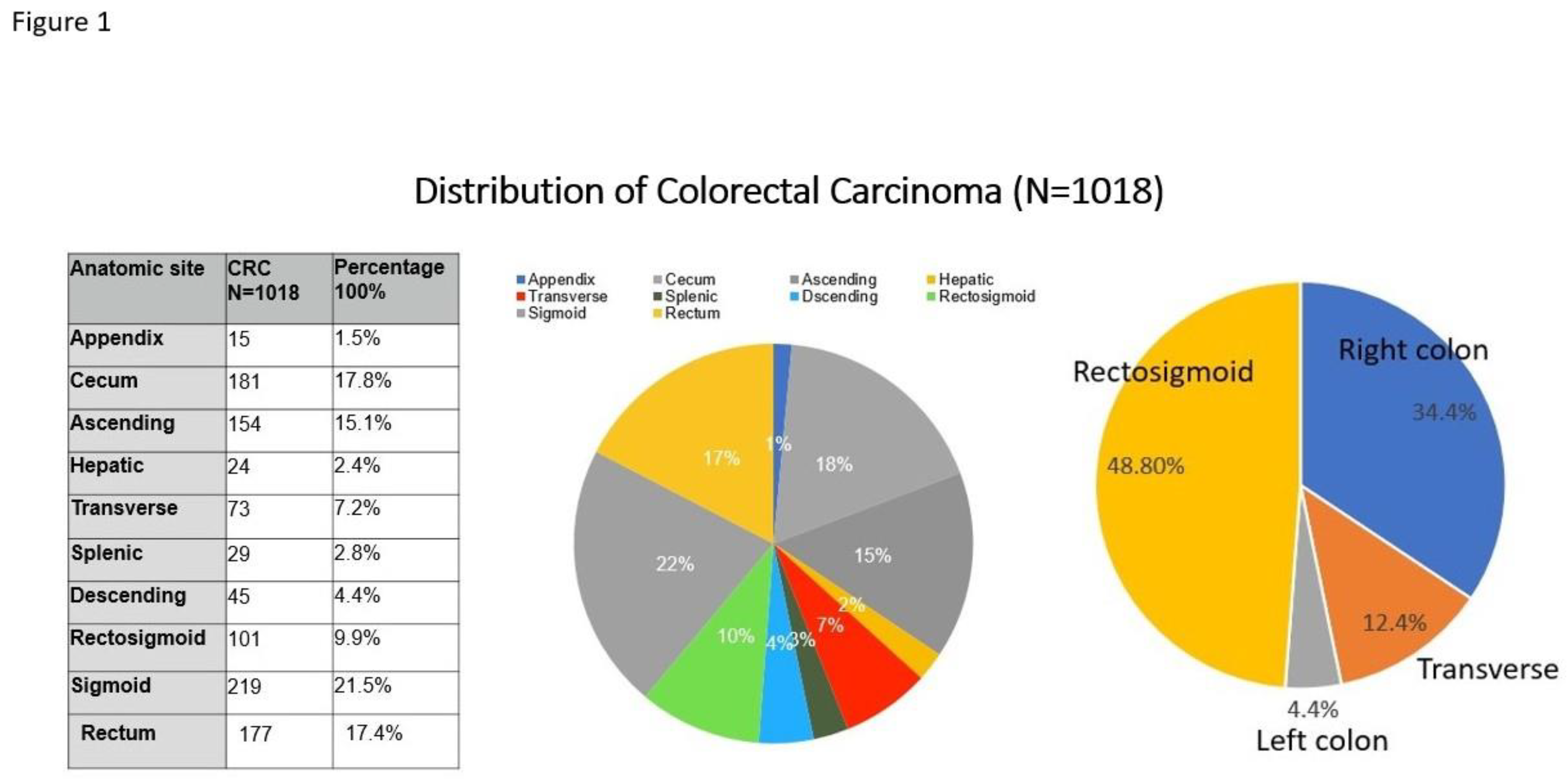
Distribution of CRC Based on Anatomic Sites and Tumor Stages
Sex and Marital Status Differences in CRC
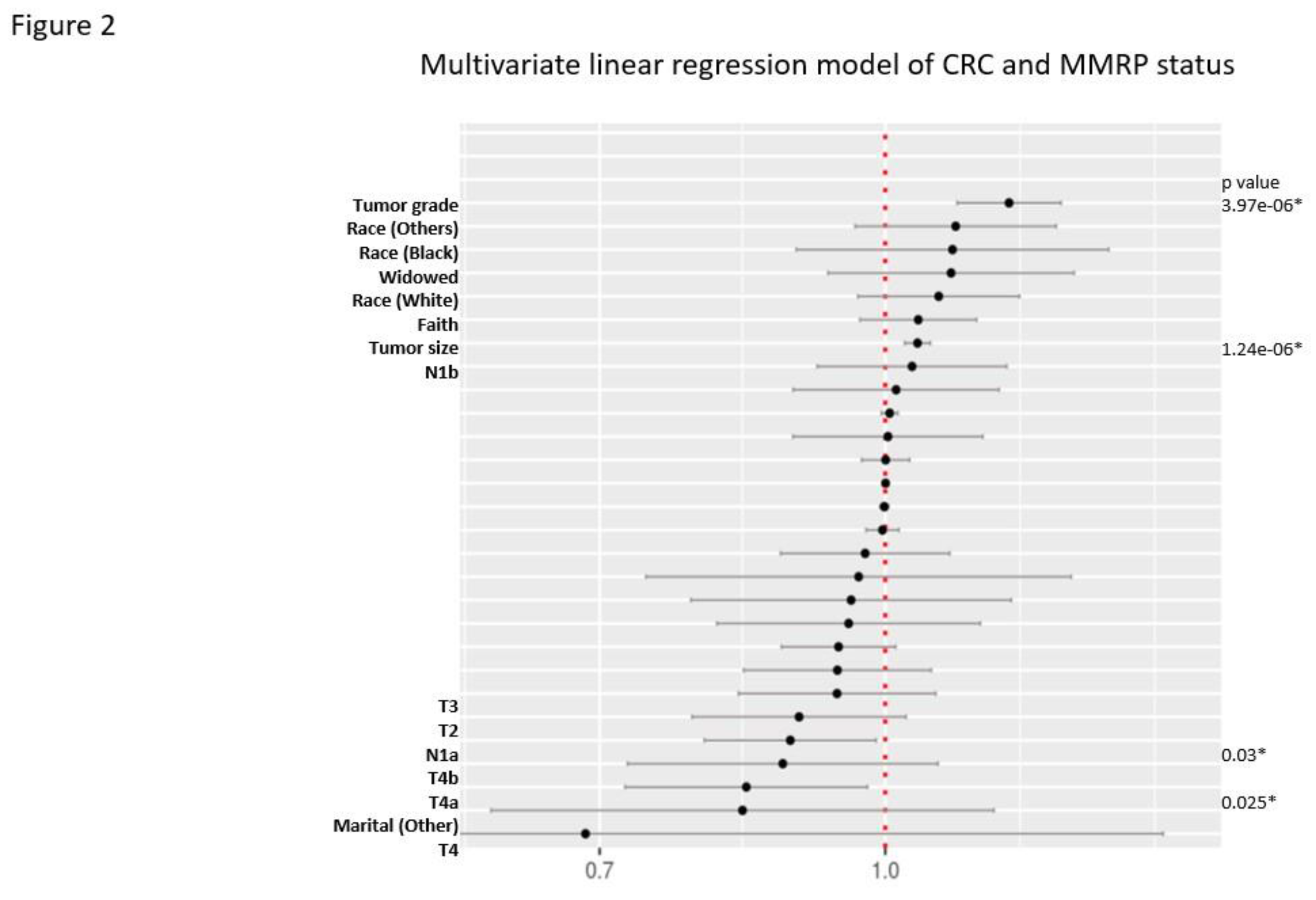
MMRP Status in CRC
Discussion
Conclusions
Funding
Authorship contribution statement
Financial disclosure
References
- Society, A.C., Colorectal Cancer Facts & Figures 2023-2025 . . 2022.
- Baidoun, F., et al., Colorectal Cancer Epidemiology: Recent Trends and Impact on Outcomes. Curr Drug Targets, 2021. 22(9): p. 998-1009.
- Arnold, M., et al., Global patterns and trends in colorectal cancer incidence and mortality. Gut, 2017. 66(4): p. 683-691. [CrossRef]
- Lu, L., et al., A global assessment of recent trends in gastrointestinal cancer and lifestyle-associated risk factors. Cancer Commun (Lond), 2021. 41(11): p. 1137-1151. [CrossRef]
- Siegel, R.L., et al., Colorectal cancer statistics, 2023. CA Cancer J Clin, 2023. 73(3): p. 233-254. [CrossRef]
- Baraibar, I., et al., Sex and gender perspectives in colorectal cancer. ESMO Open, 2023. 8(2): p. 101204. [CrossRef]
- Wong, M.C.S., et al., Differences in Incidence and Mortality Trends of Colorectal Cancer Worldwide Based on Sex, Age, and Anatomic Location. Clin Gastroenterol Hepatol, 2021. 19(5): p. 955-966.e61. [CrossRef]
- Ramai, D., et al., Gender and racial disparities in colorectal cancer incidence and mortality: a national cancer registry study. Int J Colorectal Dis, 2021. 36(8): p. 1801-1804. [CrossRef]
- van Erning, F.N., et al., Gender differences in tumor characteristics, treatment and survival of colorectal cancer: A population-based study. Cancer Epidemiol, 2023. 86: p. 102441.
- Montiel Ishino, F.A., et al., A National Study of Colorectal Cancer Survivorship Disparities: A Latent Class Analysis Using SEER (Surveillance, Epidemiology, and End Results) Registries. Front Public Health, 2021. 9: p. 628022. [CrossRef]
- Li, Q., et al., The influence of marital status on stage at diagnosis and survival of patients with colorectal cancer. Oncotarget, 2015. 6(9): p. 7339-47. [CrossRef]
- Martínez, M.E., et al., Differences in marital status and mortality by race/ethnicity and nativity among California cancer patients. Cancer, 2016. 122(10): p. 1570-8. [CrossRef]
- Wang, Y., et al., Sex differences in the association between marital status and the risk of cardiovascular, cancer, and all-cause mortality: a systematic review and meta-analysis of 7,881,040 individuals. Glob Health Res Policy, 2020. 5: p. 4. [CrossRef]
- Zito Marino, F., et al., Microsatellite Status Detection in Gastrointestinal Cancers: PCR/NGS Is Mandatory in Negative/Patchy MMR Immunohistochemistry. Cancers (Basel), 2022. 14(9). [CrossRef]
- Sargent, D.J., et al., Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol, 2010. 28(20): p. 3219-26. [CrossRef]
- Kumarasinghe, A.P., et al., DNA mismatch repair enzyme immunohistochemistry in colorectal cancer: a comparison of biopsy and resection material. Pathology, 2010. 42(5): p. 414-20. [CrossRef]
- André, T., et al., Nivolumab plus low-dose ipilimumab in previously treated patients with microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: 4-year follow-up from CheckMate 142. Ann Oncol, 2022. 33(10): p. 1052-1060. [CrossRef]
- André, T., et al., Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med, 2020. 383(23): p. 2207-2218. [CrossRef]
- Thibodeau, S.N., G. Bren, and D. Schaid, Microsatellite instability in cancer of the proximal colon. Science, 1993. 260(5109): p. 816-9. [CrossRef]
- Amin, M.B., et al., AJCC Cancer Staging Manual. 8th ed. 2017: Springer Cham.
- Zhang, P., Mendoza, Art., Bakhtar, O., Wixom, C., Muller, S., Sadeghi, S., Clement, A., Kabakibi, L., Schwab, M., Race/ethnicity and social determinants of health in endometrial carcinomaand DNA mismatch repair protein expression status. Obstetrics & Gynecology, 2024. In review.
- Sun, B.L., Current Microsatellite Instability Testing in Management of Colorectal Cancer. Clin Colorectal Cancer, 2021. 20(1): p. e12-e20. [CrossRef]
- Sahin, I.H., et al., Immune checkpoint inhibitors for the treatment of MSI-H/MMR-D colorectal cancer and a perspective on resistance mechanisms. Br J Cancer, 2019. 121(10): p. 809-818. [CrossRef]
- Uribe, Y., et al., Intersectionality Between Epigenetics and Cancer Health Disparities Stemming from Social Determinants of Health (SDoH) Through a Gynecologic Oncology Lens: A Narrative Review. Clin Obstet Gynecol, 2023. 66(1): p. 53-62. [CrossRef]
| T-stage | T1 | T2 | T3 | T4 | p |
|---|---|---|---|---|---|
| (N=156)(15.3%) | (N=143)(14%) | (N=496)(48.7%) | (N=223)(21.9%) | ||
| Marital status | <0.01 | ||||
| - Divorced | 10 ( 6.4%) | 17 (11.9%) | 61 (12.3%) | 33 (14.8%) | |
| - Married | 96 (61.5%) | 81 (56.6%) | 244 (49.2%) | 103 (46.2%) | |
| - Other | 1 ( 0.6%) | 1 ( 0.7%) | 5 ( 1.0%) | 2 ( 0.9%) | |
| - Single | 41 (26.3%) | 32 (22.4%) | 103 (20.8%) | 60 (26.9%) | |
| - Widowed | 8 ( 5.1%) | 12 ( 8.4%) | 83 (16.7%) | 25 (11.2%) | |
| BMI | 26.0 [23.0;31.0] | 27.0 [23.0;30.0] | 25.0 [22.0;30.0] | 24.0 [21.0;29.0] | <0.01 |
| MMRP status | <0.01 | ||||
| - Proficient | 83 (91.2%) | 97 (89.8%) | 309 (78.0%) | 147 (81.7%) | |
| - Deficient | 8 ( 8.8%) | 11 (10.2%) | 87 (22.0%) | 33 (18.3%) | |
| Faith (religious belief) | 88 (59.9%) | 85 (60.7%) | 324 (66.8%) | 141 (64.7%) | 0.34 |
| Sex (gender) | 0.65 | ||||
| - Female | 82 (52.6%) | 69 (48.3%) | 269 (54.2%) | 116 (52.0%) | |
| - Male | 74 (47.4%) | 74 (51.7%) | 227 (45.8%) | 107 (48.0%) | |
| Patient's age (year) | 63.0 [54.5;72.0] | 65.0 [55.0;78.0] | 70.0 [60.0;81.0] | 67.0 [59.0;76.5] | <0.01 |
| Tumor size (cm) | 1.0 [ 0.5; 2.0] | 3.0 [ 1.5; 4.0] | 4.6 [ 3.3; 5.8] | 5.1 [ 3.8; 7.3] | <0.01 |
| Histologic grade | <0.01 | ||||
| - Grade 1 | 82 (52.6%) | 50 (35.0%) | 118 (23.8%) | 37 (16.6%) | |
| - Grade 2 | 70 (44.9%) | 87 (60.8%) | 332 (66.9%) | 141 (63.2%) | |
| - Grade 3 | 4 ( 2.6%) | 6 ( 4.2%) | 46 ( 9.3%) | 45 (20.2%) | |
| Lymph node number | 17.0 [12.0;21.0] | 17.0 [14.0;23.0] | 19.0 [14.0;26.0] | 18.0 [14.0;25.0] | <0.01 |
| N stage (TNM-N) | <0.01 | ||||
| - No data | 12 ( 7.7%) | 9 ( 6.3%) | 2 ( 0.4%) | 3 ( 1.3%) | |
| - No nodal metastasis | 124 (79.5%) | 100 (69.9%) | 264 (53.2%) | 55 (24.7%) | |
| - 1a | 9 ( 5.8%) | 20 (14.0%) | 70 (14.1%) | 37 (16.6%) | |
| - 1b | 6 ( 3.8%) | 6 ( 4.2%) | 69 (13.9%) | 42 (18.8%) | |
| - 1c | 1 ( 0.6%) | 0 ( 0.0%) | 14 ( 2.8%) | 7 ( 3.1%) | |
| - 2a | 1 ( 0.6%) | 5 ( 3.5%) | 43 ( 8.7%) | 36 (16.1%) | |
| - 2b | 3 ( 1.9%) | 3 ( 2.1%) | 34 ( 6.9%) | 43 (19.3%) | |
| BRAF | 1 (14.3%) | 2 (40.0%) | 26 (49.1%) | 10 (30.3%) | 0.17 |
| MLH | 2 (66.7%) | 6 (100.0%) | 32 (91.4%) | 8 (80.0%) | 0.35 |
| Lynch syndrome | 4 (50.0%) | 3 (27.3%) | 16 (18.4%) | 5 (15.2%) | 0.14 |
| Gender | Female | Male | Total | p |
|---|---|---|---|---|
| (N=536)(52.7%) | (N=482)(47.3%) | (N=1018) | ||
| Marital status | <0.01 | |||
| - Divorced | 72 (13.4%) | 49 (10.2%) | 121 (11.9%) | |
| - Married | 238 (44.4%) | 286 (59.3%) | 524 (51.5%) | |
| - Other | 6 ( 1.1%) | 3 ( 0.6%) | 9 ( 0.9%) | |
| - Single | 120 (22.4%) | 116 (24.1%) | 236 (23.2%) | |
| - Widowed | 100 (18.7%) | 28 ( 5.8%) | 128 (12.6%) | |
| BMI | 25.0 [22.0;30.0] | 26.0 [23.0;30.0] | 26.0 [22.0;30.0] | 0.10 |
| MMRP | <0.01 | |||
| - Proficient | 329 (78.0%) | 307 (87.0%) | 636 (82.1%) | |
| - Deficient | 93 (22.0%) | 46 (13.0%) | 139 (17.9%) | |
| Faith (religious belief) | 369 (70.3%) | 269 (57.8%) | 638 (64.4%) | <0.01 |
| Patient's age (year) | 69.0 [56.0;79.0] | 67.0 [59.0;77.0] | 68.0 [58.0;78.0] | 0.55 |
| Tumor size (cm) | 4.0 [ 2.5; 5.7] | 4.1 [ 2.2; 5.5] | 4.0 [ 2.3; 5.6] | 0.75 |
| Histologic grade | 0.06 | |||
| - Grade 1 | 138 (25.7%) | 149 (30.9%) | 287 (28.2%) | |
| - Grade 2 | 336 (62.7%) | 294 (61.0%) | 630 (61.9%) | |
| - Grade 3 | 62 (11.6%) | 39 ( 8.1%) | 101 ( 9.9%) | |
| Tumor stage (TNM-T) | 0.65 | |||
| - 1 | 82 (15.3%) | 74 (15.4%) | 156 (15.3%) | |
| - 2 | 69 (12.9%) | 74 (15.4%) | 143 (14.0%) | |
| - 3 | 269 (50.2%) | 227 (47.1%) | 496 (48.7%) | |
| - 4 | 116 (21.6%) | 107 (22.2%) | 223 (21.9%) | |
| Nodal stage (TNM-N) | 0.59 | |||
| - No data | 10 ( 1.9%) | 16 ( 3.3%) | 26 ( 2.6%) | |
| - No nodal metastasis | 282 (52.6%) | 261 (54.1%) | 543 (53.3%) | |
| - 1a | 74 (13.8%) | 62 (12.9%) | 136 (13.4%) | |
| - 1b | 70 (13.1%) | 53 (11.0%) | 123 (12.1%) | |
| - 1c | 9 ( 1.7%) | 13 ( 2.7%) | 22 ( 2.2%) | |
| - 2a | 46 ( 8.6%) | 39 ( 8.1%) | 85 ( 8.3%) | |
| - 2b | 45 ( 8.4%) | 38 ( 7.9%) | 83 ( 8.2%) | |
| Marital Status | Divorced | Married | Other | Single | Widowed | p |
|---|---|---|---|---|---|---|
| (N=121) | (N=524) | (N=9) | (N=236) | (N=128) | ||
| BMI | 25.0 [22.0;31.0] | 26.0 [22.0;30.0] | 25.0 [21.0;27.0] | 25.0 [22.0;29.5] | 25.5 [22.0;30.0] | 0.77 |
| MMRP | <0.01 | |||||
| - Proficient | 67 (76.1%) | 347 (85.9%) | 6 (75.0%) | 155 (87.1%) | 61 (62.9%) | |
| - Deficient | 21 (23.9%) | 57 (14.1%) | 2 (25.0%) | 23 (12.9%) | 36 (37.1%) | |
| Faith (religious belief) | 68 (57.6%) | 346 (67.7%) | 2 (28.6%) | 126 (54.5%) | 96 (78.0%) | <0.01 |
| Sex (gender) | <0.01 | |||||
| - Female | 72 (59.5%) | 238 (45.4%) | 6 (66.7%) | 120 (50.8%) | 100 (78.1%) | |
| - Male | 49 (40.5%) | 286 (54.6%) | 3 (33.3%) | 116 (49.2%) | 28 (21.9%) | |
| Patient's age (year) | 66.0 [59.0;75.0] | 67.0 [56.5;76.0] | 77.0 [77.0;81.0] | 64.0 [54.0;73.0] | 84.0 [76.0;90.0] | <0.01 |
| Tumor size (cm) | 4.8 [ 3.3; 6.5] | 3.9 [ 2.1; 5.4] | 5.2 [ 3.5; 5.5] | 3.5 [ 2.0; 5.1] | 4.5 [ 3.3; 6.4] | <0.01 |
| Histologic grade | 0.56 | |||||
| - Grade 1 | 31 (25.6%) | 150 (28.6%) | 1 (11.1%) | 72 (30.5%) | 33 (25.8%) | |
| - Grade 2 | 77 (63.6%) | 325 (62.0%) | 6 (66.7%) | 145 (61.4%) | 77 (60.2%) | |
| - Grade 3 | 13 (10.7%) | 49 ( 9.4%) | 2 (22.2%) | 19 ( 8.1%) | 18 (14.1%) | |
| T stage (TNM-T) | <0.01 | |||||
| - 1 | 10 ( 8.3%) | 96 (18.3%) | 1 (11.1%) | 41 (17.4%) | 8 ( 6.2%) | |
| - 2 | 17 (14.0%) | 81 (15.5%) | 1 (11.1%) | 32 (13.6%) | 12 ( 9.4%) | |
| - 3 | 61 (50.4%) | 244 (46.6%) | 5 (55.6%) | 103 (43.6%) | 83 (64.8%) | |
| - 4 | 33 (27.3%) | 103 (19.7%) | 2 (22.2%) | 60 (25.4%) | 25 (19.5%) | |
| N stage (TNM-N) | 0.64 | |||||
| - No data | 4 ( 3.3%) | 15 ( 2.9%) | 0 ( 0.0%) | 5 ( 2.1%) | 2 ( 1.6%) | |
| - No nodal metastasis | 59 (48.8%) | 290 (55.3%) | 5 (55.6%) | 117 (49.6%) | 72 (56.2%) | |
| - 1a | 19 (15.7%) | 67 (12.8%) | 1 (11.1%) | 32 (13.6%) | 17 (13.3%) | |
| - 1b | 16 (13.2%) | 58 (11.1%) | 0 ( 0.0%) | 29 (12.3%) | 20 (15.6%) | |
| - 1c | 0 ( 0.0%) | 13 ( 2.5%) | 0 ( 0.0%) | 8 ( 3.4%) | 1 ( 0.8%) | |
| - 2a | 8 ( 6.6%) | 41 ( 7.8%) | 2 (22.2%) | 25 (10.6%) | 9 ( 7.0%) | |
| - 2b | 15 (12.4%) | 40 ( 7.6%) | 1 (11.1%) | 20 ( 8.5%) | 7 ( 5.5%) | |
| BRAF mutation | 5 (41.7%) | 15 (34.9%) | 0 ( 0.0%) | 6 (28.6%) | 13 (61.9%) | 0.17 |
| MLH gene methylation | 7 (77.8%) | 15 (83.3%) | 1 (100.0%) | 9 (90.0%) | 16 (100.0%) | 0.43 |
| Lynch syndrome | 4 (19.0%) | 17 (29.8%) | 0 ( 0.0%) | 5 (21.7%) | 2 ( 5.6%) | 0.07 |
| MMRP status | Proficient | Deficient | Total | p |
|---|---|---|---|---|
| (N=636) | (N=111) | (N=747) | ||
| Race /ethnicity | 0.04 | |||
| - Asian | 91 (14.3%) | 6 ( 5.4%) | 97 (13.0%) | |
| - Black | 25 ( 3.9%) | 3 ( 2.7%) | 28 ( 3.7%) | |
| - Hispanic | 121 (19.0%) | 17 (15.3%) | 138 (18.5%) | |
| - Other | 90 (14.2%) | 16 (14.4%) | 106 (14.2%) | |
| - White | 309 (48.6%) | 69 (62.2%) | 378 (50.6%) | |
| Marital status | <0.01 | |||
| - Divorced | 67 (10.5%) | 17 (15.3%) | 84 (11.2%) | |
| - Married | 347 (54.6%) | 40 (36.0%) | 387 (51.8%) | |
| - Other | 6 ( 0.9%) | 2 ( 1.8%) | 8 ( 1.1%) | |
| - Single | 155 (24.4%) | 18 (16.2%) | 173 (23.2%) | |
| - Widowed | 61 ( 9.6%) | 34 (30.6%) | 95 (12.7%) | |
| BMI | 26.0 [22.0;30.0] | 25.0 [22.0;28.5] | 26.0 [22.0;30.0] | 0.43 |
| Faith (religious belief) | 388 (62.7%) | 68 (63.6%) | 456 (62.8%) | 0.95 |
| Gender (sex) | <0.01 | |||
| - Female | 329 (51.7%) | 77 (69.4%) | 406 (54.4%) | |
| - Male | 307 (48.3%) | 34 (30.6%) | 341 (45.6%) | |
| Patient age (year) | 65.5 ± 14.0 | 74.3 ± 13.2 | 67.9 ± 14.2 | <0.01 |
| Anatomic site | <0.01 | |||
| - Right colon | 182 (28.7%) | 80 (72.0%) | 262 (35.1%) | |
| - Left colon | 54 (8.4%) | 6 (5.4%) | 60 (8.0%) | |
| - Sigmoid & Rectum | 364 (57.2%) | 9 (8.1%) | 373 (49.9%) | |
| - Transverse | 36 ( 5.7%) | 16 (14.4%) | 52 ( 7.0%) | |
| Tumor size (cm) | 4.0 [ 2.5; 5.5] | 6.0 [ 4.5; 7.5] | 4.0 [ 2.3; 5.5] | <0.01 |
| Histologic grade | <0.01 | |||
| - Grade 1 | 180 (28.3%) | 17 (15.3%) | 197 (26.4%) | |
| - Grade 2 | 411 (64.6%) | 60 (54.1%) | 471 (63.1%) | |
| - Grade 3 | 45 ( 7.1%) | 34 (30.6%) | 79 (10.6%) | |
| Tumor stage (TNM-T) | <0.01 | |||
| - 1 | 83 (13.1%) | 4 ( 3.6%) | 87 (11.6%) | |
| - 2 | 97 (15.3%) | 8 ( 7.2%) | 105 (14.1%) | |
| - 3 | 309 (48.6%) | 71 (64.0%) | 380 (50.9%) | |
| - 4 | 147 (23.1%) | 28 (25.2%) | 175 (23.4%) | |
| Nodal stage (TNM-N) | 0.01 | |||
| No data | 12 ( 1.9%) | 0 ( 0.0%) | 12 ( 1.6%) | |
| N0 | 304 (47.8%) | 68 (61.3%) | 372 (49.8%) | |
| - 1a | 107 (16.8%) | 6 ( 5.4%) | 113 (15.1%) | |
| - 1b | 76 (11.9%) | 18 (16.2%) | 94 (12.6%) | |
| - 1c | 14 ( 2.2%) | 2 ( 1.8%) | 16 ( 2.1%) | |
| - 2a | 60 ( 9.4%) | 7 ( 6.3%) | 67 ( 9.0%) | |
| - 2b | 63 ( 9.9%) | 10 ( 9.0%) | 73 ( 9.8%) | |
| BRAF | 7 (14.9%) | 32 (65.3%) | 39 (40.6%) | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





