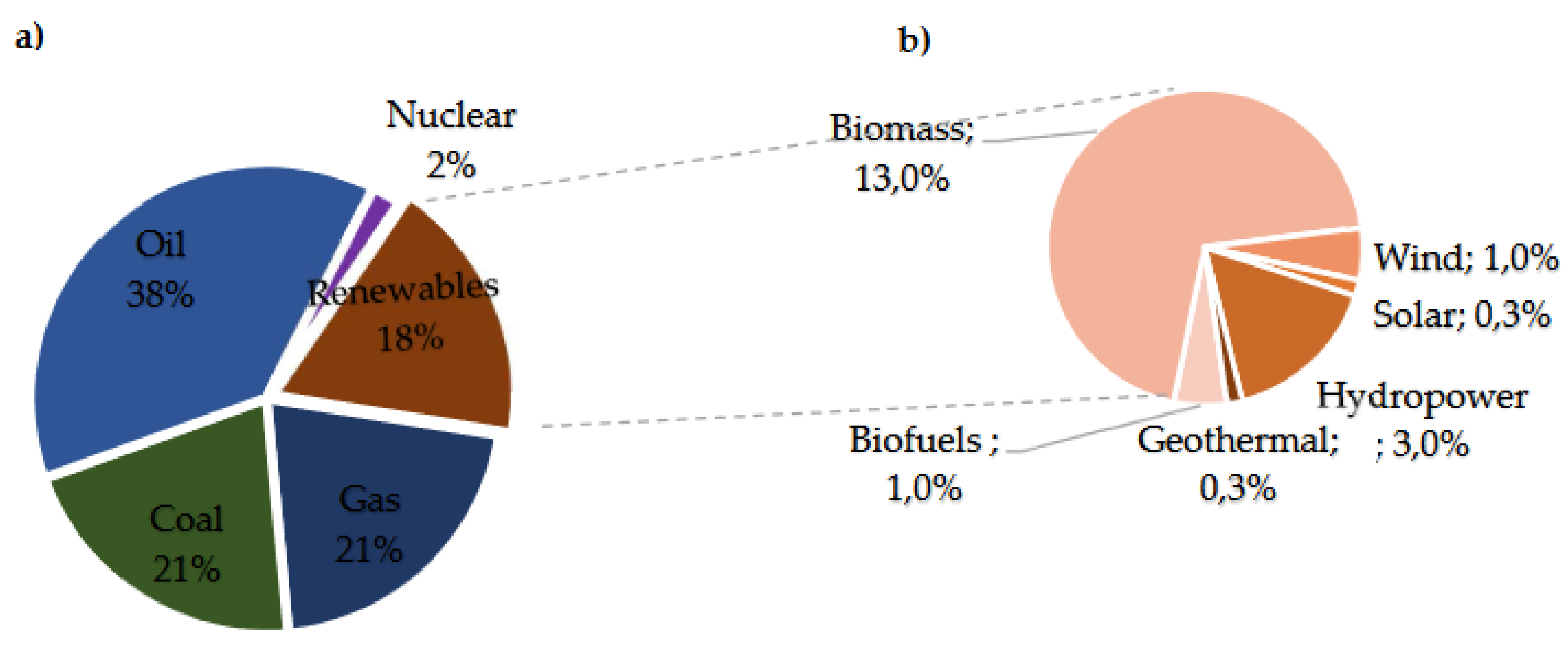1. Introduction
The COVID-19 pandemic has disproportionately affected various communities and economies globally, and the energy sector is no exception [2]. This crisis has highlighted the critical role of energy in maintaining homes, health systems, and digital infrastructure. It has challenged the resilience of energy networks and has catalyzed investments aimed at achieving deeper decarbonization in heating and transportation. Rising energy demand, compounded by the environmental impact of extensive fossil fuel use, significant demographic expansion (with a growth of over 1.5 million in the last two decades) [3], and the need for economic and social advancement, has hastened the advancement of economical and sustainable energy solutions. Since 1973, energy consumption has doubled in developed nations, yet the demand continues to outpace supply even as reserves continue to decline (
Figure 1) [3,4,5].
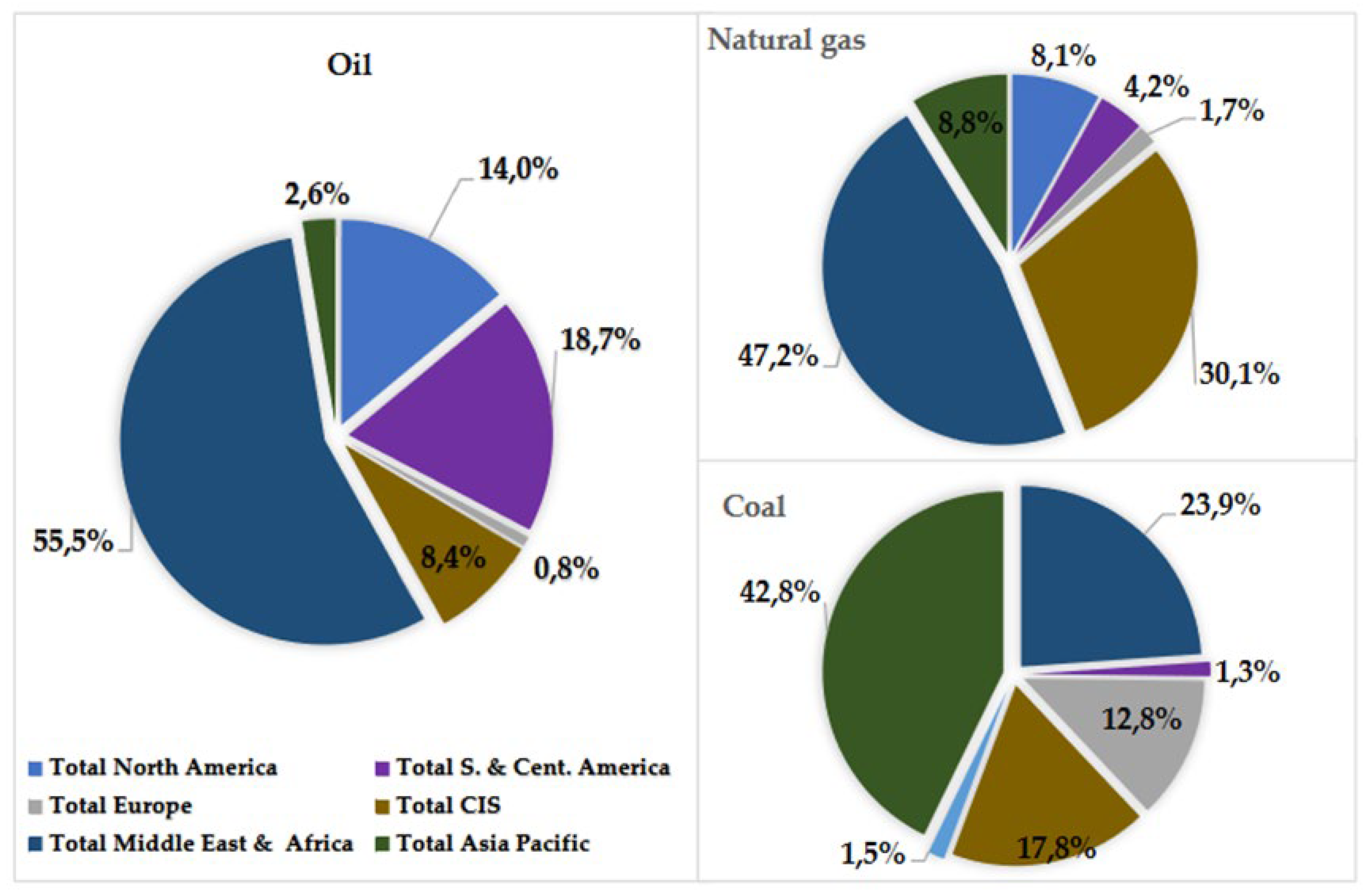
The global reliance on fossil fuels carries a range of drawbacks, including socio-political conflicts arising from their localized extraction and the inevitable depletion of these resources in the foreseeable future. Energy needs are predominantly supplied through the utilization of non-renewable sources such as coal, oil, and natural gas. However, the finite nature of these resources means they could be depleted sooner than anticipated. According to data from BP’s "Statistical Review of World Energy" in July 2021 [5], the world’s current fossil fuel reserves are substantial: 244 billion tons of oil, 6642 billion cubic feet of natural gas, and 1074 billion tons of coal. These amounts may appear significant initially, projections suggest that natural gas reserves could be exhausted by 2068, with oil reserves depleted by 2065. As shown in
Figure 2, coal is allocated in more regions compared to natural gas and particularly oil, of which 75% of reserves are held by OPEC nations, predominantly located in the Middle East [5].
In 2021, renewable energies contributed 18% to primary energy production, 8% larger from 2006, as indicated in figure 2.a. Among renewables, approximately 5% stemmed from hydropower, wind, solar, and biofuels. Biomass, mainly used for consumption, represented about 10% of total renewable energy, with a smaller fraction allocated to energy, fuels, and chemical feedstocks. Hydropower accounted for 3% of global final energy consumption, while other renewables, including wind, biofuels, and geothermal sources, constituted 2.0% in 2021. This growth was particularly evident in developed countries, as depicted in
Figure 2.b of the Supply – Key World Energy Statistics 2021 – Analysis [6]. This article first focuses on the general aspects of biomass, subsequent bio-oil production, its properties, and the various methods of upgrading pyrolytic bio-oil to improve its calorific value, pH, viscosity, degree of deoxygenation (DOD), and other attributes. Secondly, particular emphasis is placed on the process of converting model molecules and bio-oil via HDO using catalysts based on nickel and nickel combined with other active elements. Through these phases, readers can gain a deeper understanding of the HDO process and the reaction mechanisms involved. Finally, the different equipment used to obtain an improved HDO product from bio-oil is discussed, providing valuable insights for the practical application of this reaction in pyrolysis bio-oil production.
2. Biomass as Renewable Energy Source
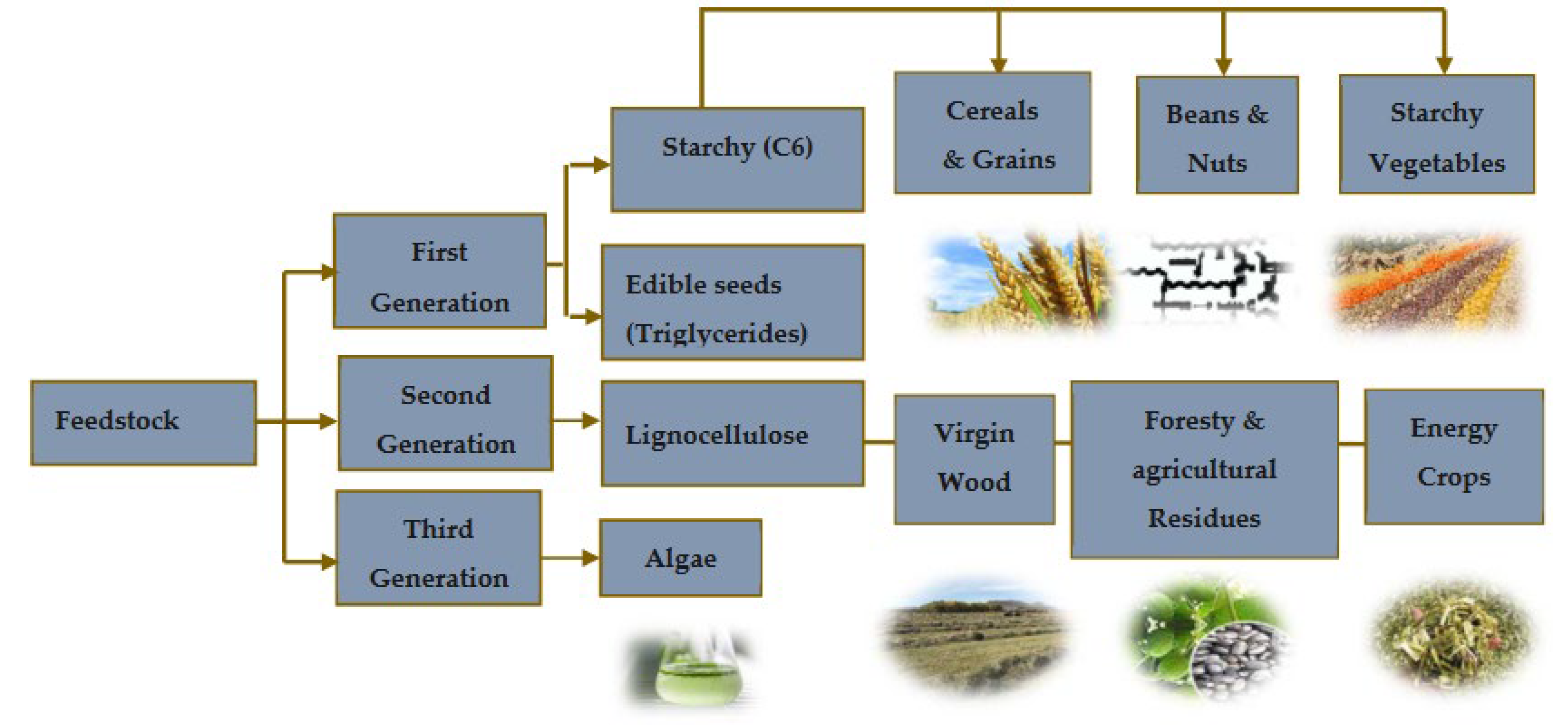
Biomass can be defined as organic matter derived from plants or animals, which is produced through biological process, whether induced or spontaneous. This material can be broadly categorized into non-lignocellulosic or lignocellulosic forms, including woody, herbaceous, aquatic residues, manure, agricultural sub-products and more [7]. Its classification as a renewable energy source stems from its ability to regenerate at a rate that exceeds its consumption[8]. The energy contained in biomass originates from the photosynthesis process of plants. Through valorization techniques like anaerobic digestion, combustion, pyrolysis, and gasification, this energy can be released artificially.
From the energy aspect, one key advantage of using biomass is its capacity not to upsurge CO2 emissions. Unlike fossil fuels, the emissions from biomass combustion derive from carbon that was recently extracted from the atmosphere during the plant’s growth cycle. This means that the release of CO2 does not disrupt the balance of atmospheric carbon concentration, thus mitigating the greenhouse effect. However, there is ongoing debate regarding the comprehensive assessment of energy consumption throughout the production process and its impact on greenhouse gas emissions. To address this, employing analytical methodologies like life cycle assessment and carbon footprint analysis becomes imperative in evaluating the environmental sustainability of various biofuel production techniques. Biomass is classified into different generations according to the various types of feedstock used Figure 7. Biomass classifications are alterable and depend on their variety, composition and quantity [9].
Figure 3.
Classification of biomass feedstock in three generations from [11]. .
Figure 3.
Classification of biomass feedstock in three generations from [11]. .
The chemical composition significantly influences the reactivity of biomass, so it must be well-known in order to optimise the transformation process, both for obtaining biofuels and chemical products, with high selectivity and efficiency [10].
2.1. Fast Pyrolysis: Pyrolytic Bio-Oil, Composition and Properties
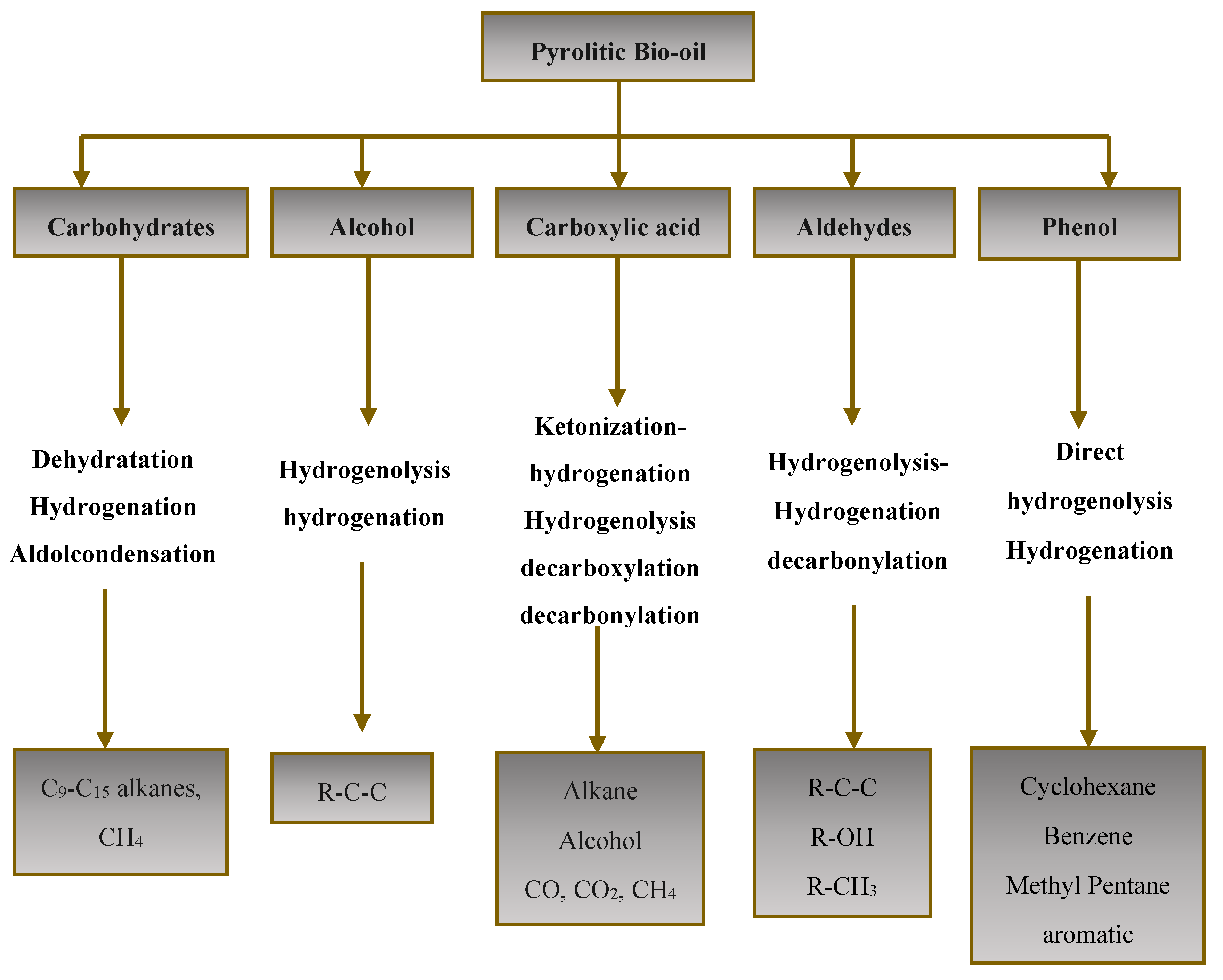
Fast pyrolysis is a thermal decomposition process where biomass is rapidly heated to high temperatures without oxygen. This process targets the breakdown of hemicellulose, cellulose and lignin. It occurs in a temperature range of 300–700°C at a faster heating rate of 10–200°C/s, a short residence time between 0.5 and 10 s, and a feedstock with a fine particle size (< 1 mm) [11], this method yields char, a solid residue with carbon, along with volatiles and gases [12]. After cooling to room temperature, some of these volatiles condense into a tar or pyrolytic bio-oil, around 30-40 wt% of the original biomass weight and containing diverse organic compounds with approximately 25 wt% water content sourced from both biomass moisture and decomposition [13].
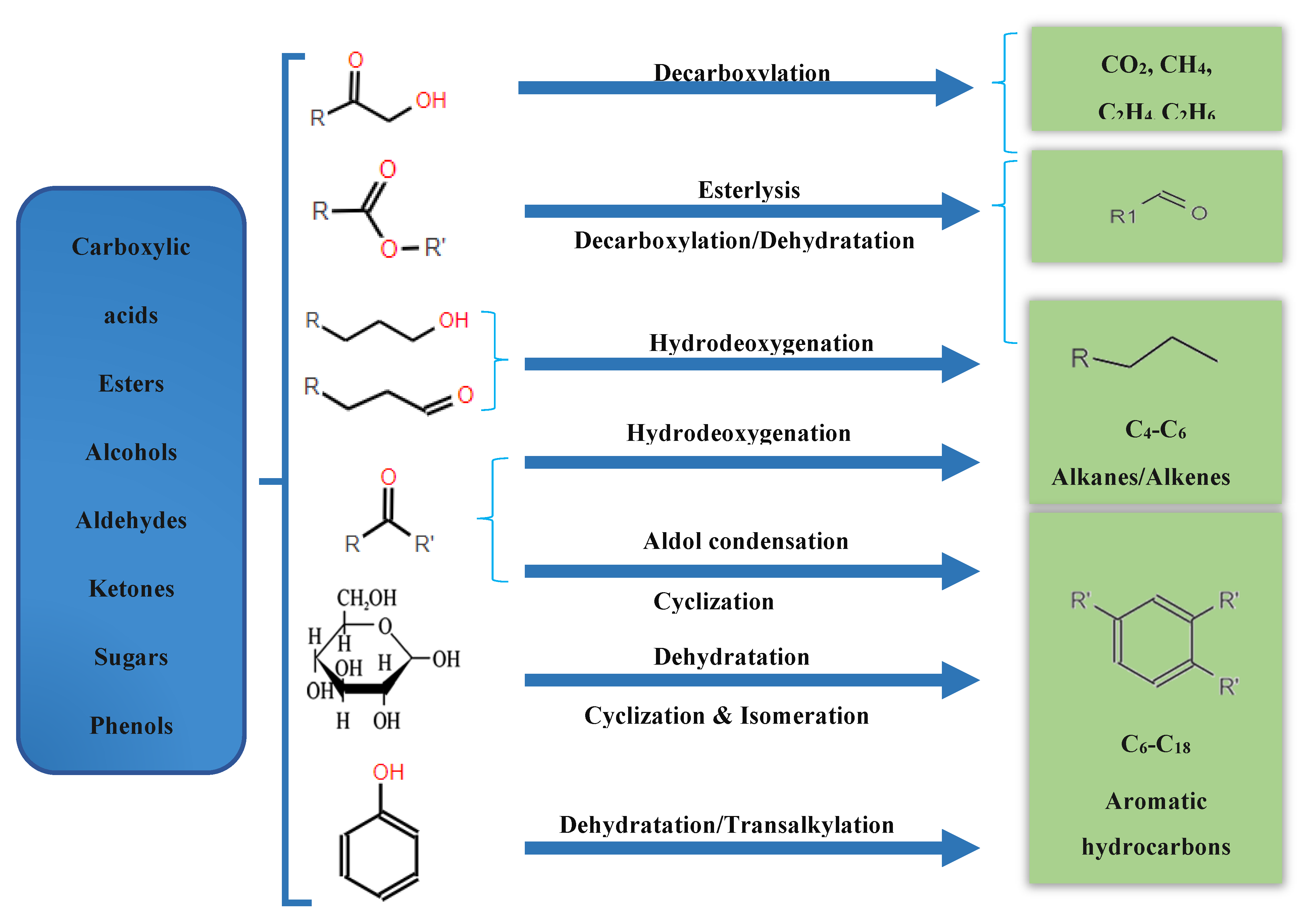
In general, organics compounds are classified into aldehydes and ketones (~15.0wt%), acids (~35.0wt%), phenols (~20.0wt%), Alcohols (~8.0wt%), furans (~2.75wt%) and others (~20.0wt%)[14,15]. All these oxygenated compounds are challenging for direct applications of bio-oil as a result of its physical and chemical properties, such as high water content, high acidity, high proportion of oxygen and low calorific value (LHV: 15-20 MJ/kg), almost a half compared to petroleum as shown in Table 2 [16,17].
Table 1.
Typical properties of pine wood, bio-oil from fast pyrolysis and fossil-based oil from [18,20,21].
Table 1.
Typical properties of pine wood, bio-oil from fast pyrolysis and fossil-based oil from [18,20,21].
| Physical Properties |
Pine Wood Bio-Oil |
Fast Pyrolysis Bio-Oil |
Fossil Petroleum |
| Moisture content (wt%) |
15 – 30 |
15 – 30 |
0.1 |
| pH |
- |
2-3.7 |
- |
| Specific gravity |
- |
1.2 |
0.94 |
Elemental analysis (wt%) |
C |
49 |
54 – 58 |
83-86 |
| H |
6 |
5.5 – 7.0 |
11 |
| O |
44 |
35 – 40 |
1 |
| N |
0.06 |
0 – 0.2 |
0.3 |
| Ash |
0.3 |
0 – 0.2 |
0.1 |
| High heating value (HHV) (MJ/kg) |
20 |
16 – 19 |
40 |
| Viscosity (@50 ºC, cP) |
-
|
40-100 |
180 |
| Solid content (wt%) |
- |
0.2 – 1 |
1 |
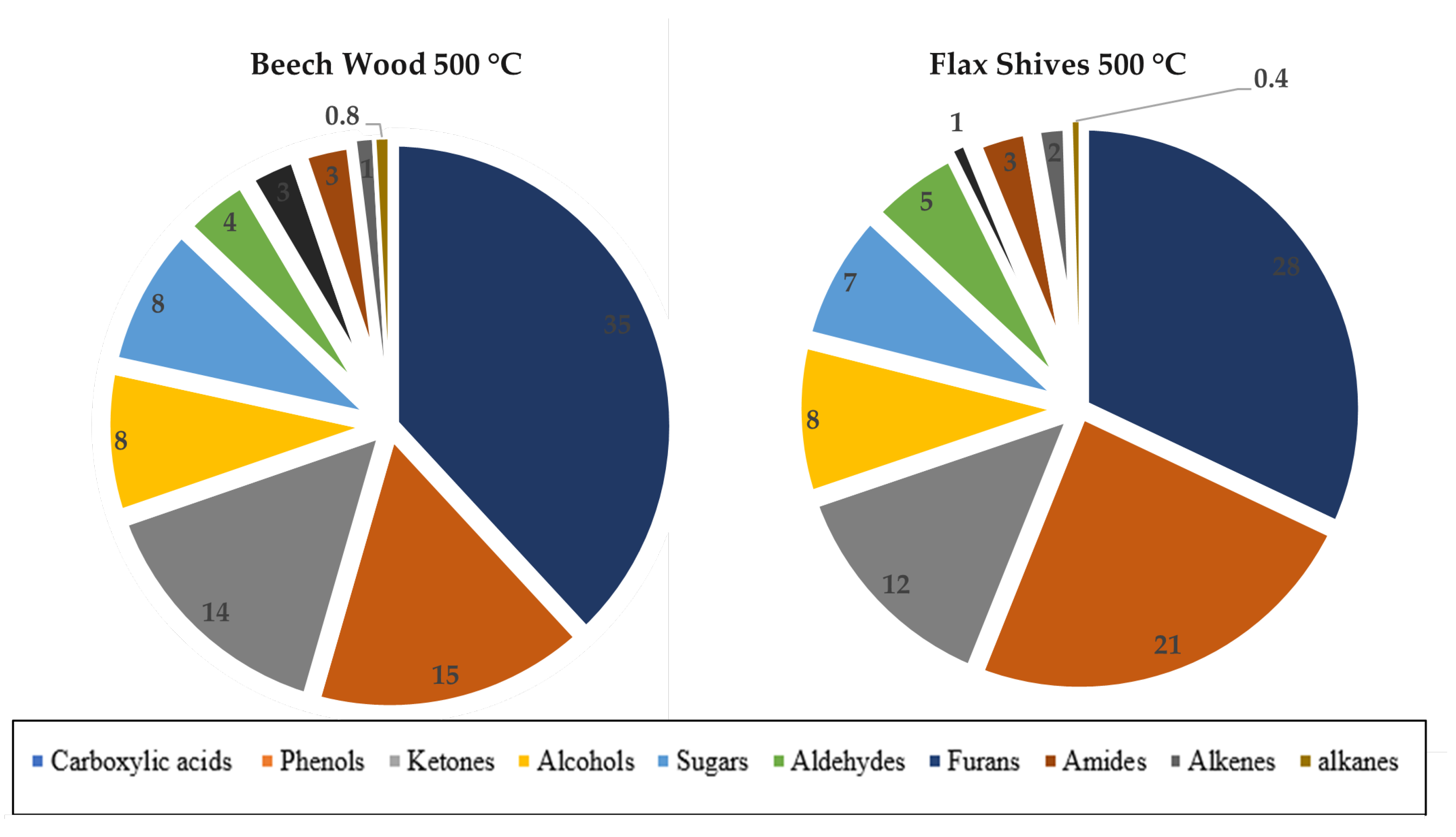
Therefore, the bio-oil must be upgraded by physical or chemical methods. The specific distribution of chemical families in pyrolytic beechwood oil at 500°C has been found in the literature and can be seen in
Figure 4. It is clear that acids are the primary chemical family in the pyrolytic bio-oil followed by phenols, ketones, alcohols and anhydrosugars [15].
3. Bio-Oil Upgrading Methods
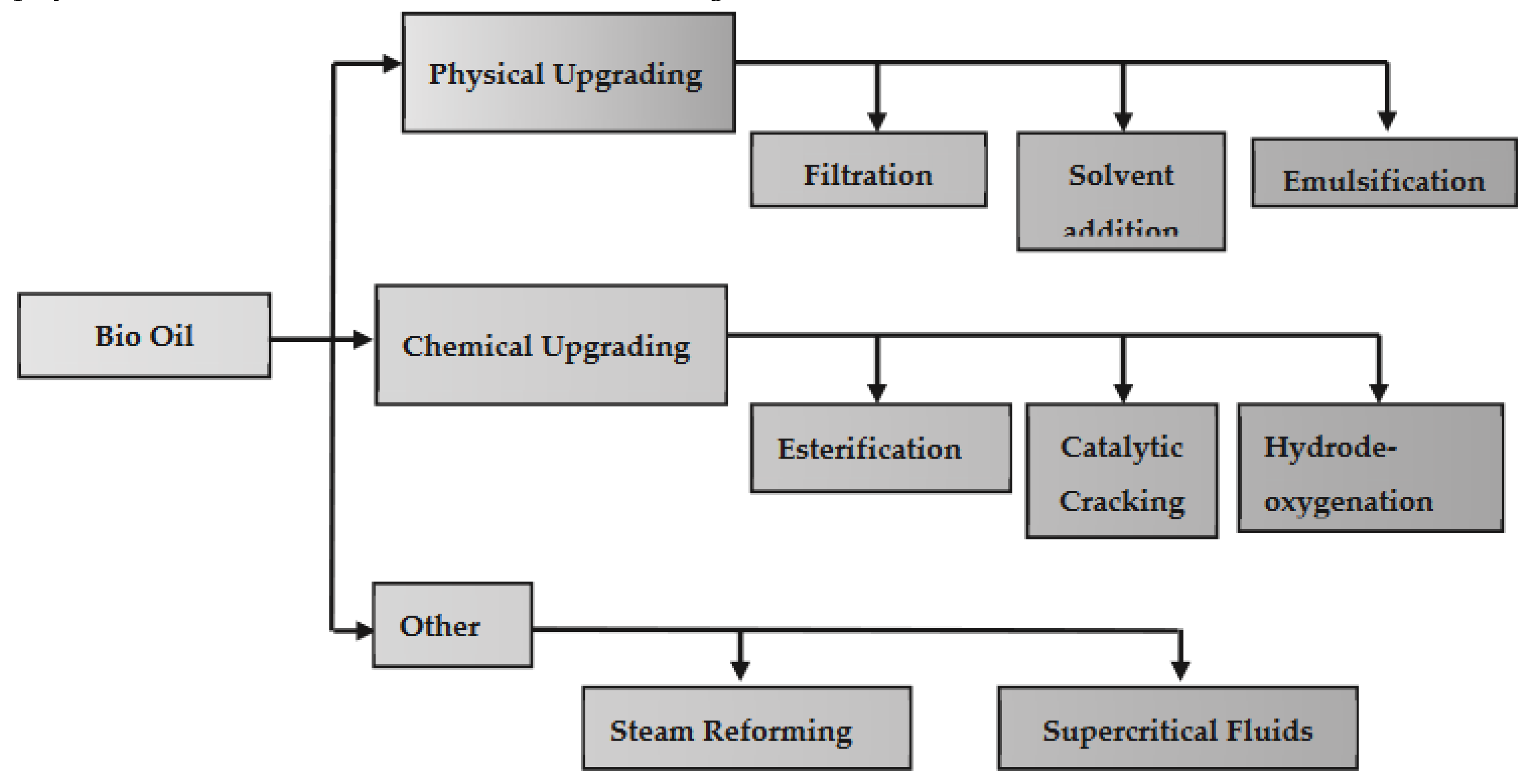
As discussed above the direct application of Pyrolitic bio-oil, as petroleum-based fuel is inconceivable [21,22]. The limitations of bio-oil can be removed by different upgrading methods, however intensive research has been done so far on these methods. Here, recent development in bio-oil upgrading methods is presented. Bio-oil upgradation can be classified as the following three types, physical, chemical and others, shown in the
Figure 5.
3.1. Emulsification
Emulsification improves the low solubility of bio-oil in hydrocarbon fuels by mixing it with diesel and surfactants, leading a more stable mixture and improving fuel properties [25,21], Emulsification method depends on the hydrophilic-lipophilic balance (HLB) of surfactants, which can either lipophilic (4-8) or hydrophilic (9-15) [21,22]. Successful emulsification needs immiscible bio-oil and diesel, surfactants to aid mixing and stirring required for dispersion [21,22]. Bio-oil emulsification with diesel may be difficult because of phase separation, and requires precise proportions of bio-oil (10-50%), diesel (50-90%) and surfactants (1-10%) [26,27]. This process increases the calorific value, pH and stability of the bio-oil [28]. Ikura et al. [29] reported that increased bio-oil content increases the viscosity of the emulsion, yielding optimal stability at 10-20 wt%of bio-oil and 4-6 wt% of surfactant. The addition of alcohols such as methanol, ethanol and n-butanol as co-surfactants improves stability [30]. Farooq et al. [31] demonstrated that the use of Span 80 and Tween 60 increased the heating value of ether extracted emulsified bio-oil (EEO) up to 44 MJ/kg, maintaining stability for 40 days. In addition, emulsifiers such as Span 60, Span 80, Tween 80 and lecithin are common in bio-oil emulsification, and sometimes need to be mixed to obtain optimal HLB values, although differences in thermal stability must be considered [26,29,30,32,33]. Also, emulsified bio-oil may act as a lubricant, with increased bio-oil content increasing lubrication [34].
3.2. Esterification
Esterification transforms the carboxylic acids in bio-oils into more stable esters by reacting them with alcohols and often using zeolite-supported catalysts, leading to a fuel suitable for combustion engines [35]. This process reduces the acid number, density and water content of bio-oil, while enhancing its calorific value [36]. Usually, bio-oil is esterified using alcohols such as ethanol or methanol at 50-60°C for 1-5 hours, which reduces the viscosity and increases the calorific value. Methanol is preferable due to its efficiency as a solvent [36,37]. Prasertpong et al. reported catalytic esterification with ethanol using 12-tungsto-silicic acid reduced acidity by 38-85% and increased calorific values by about 32% [38,39]. Despite these improvements, the calorific value of the improved bio-oil of 17.6 and 23.2 MJ/kg suggests further improvement is needed [39].
3.2. Solvent Addition
Bio-oil, which contains active oxygen compounds and a high viscosity, presents challenges as a transport fuel due to increasing viscosity over time caused by condensation and polymerisation [21]. Solvent additions such as methanol, ethanol, ethyl acetate and acetone help to homogenise the blend, reduce the viscosity and density and increase the calorific value [40]. In particular, alcohols avoid phase separation by increasing the solubility of hydrophobic compounds, resulting in a top layer of enriched bio-oil [41]. Feng et al. researched phenol hydrogenation over palladium catalysts on activated carbon, discovering that the polarity of the solvent affects the conversion of phenol. Phenol was converted fully to hexane and water at 250°C, but only partially to methanol or ethanol, highlighting the synergistic effects of various factors on phenol hydrogenation. Both water and hexane were identified as effective solvents for upgrading the pyrolysis bio-oil, enhancing phenol conversion [42].
3.2. Steam Reforming (SR)
Steam reforming turns hydrocarbons into CO and H2 by reacting them with steam at high temperatures, involving also water-gas shift (WGS) and methanation reactions, influenced by operational conditions [43]. H2 production via steam reforming from bio-oil requires a high-temperature reactor [44]. Some studies have explored the conversion of bio-oil to syngas using fluidised bed reactors and Ni catalysts, simulating methane steam reforming at 600-900°C [45]. Bio-oil, represented as CaHbOc, xH2O, undergoes several reactions: steam reforming, WGS catalytic cracking, Boudouard reaction and methanation [46]. These reactions together produce H2, CO2, CO, CH4 and solid carbon. Transition metals such as Ni, Co and Cu are preferred as catalysts due to their lower cost compared to noble metals such as Rh, Pt and Ru [47]. Yan Ding et al. proved the effectiveness of NiO/NaF catalysts for hydrogen production on various oxygen-containing volatile organic compounds (OVOCs), showing high H2 selectivity for formic acid, formaldehyde and methanol at specific temperatures [45].
3.2. Catalytic Cracking (Zeolite)
Catalytic cracking involves treating the steam with a suitable catalyst to breakdown the organic compounds and produce an oxygen-free product [49]. A variety of catalysts, such as solid acids, zeolites, alumina and metal oxides, are used for this purpose, either integrated in the biomass or supplied separately. Catalysts based on zeolite, in particular HZSM-5 [48,49], are notable for their porous structure and efficiency in biomass conversion, in particular for aromatics production, due to their acidity, thermal stability and suitable pore size. Studies by Kurnia et al. proved that CuNi/zeolite, Cu/zeolite and Ni/zeolite catalysts significantly increased the bio-oil yield compared to pyrolysis without catalysts. Catalyst structure and acidity influenced bio-oil composition, affecting product distribution, coke formation and deoxygenation rate. In addition, zeolites with high alumina content can turn oxygenated compounds into aromatic hydrocarbons, with catalyst regeneration possible by calcination. Catalysts based on alkali metals and Ni have been effective in reducing tar formation during pyrolysis, although carbon deposition in the catalyst structure can hinder activity [49,50,51,50,51,52,53].
3.2. Super Critical Fluids
The SCF upgrading method improves the calorific value and reduces the viscosity of bio-oil by using the unique properties of supercritical states to dissolve typically insoluble materials in liquid or gaseous solvents [56]. SCFs are used in bio-oil production, especially in hydrothermal liquefaction of biomass, together with catalysts and solvents such as ethanol, methanol, CO2 and water [54,55]. For example, Duan et al. [56] reported enhanced calorific value of algal bio-oil during gasification in supercritical water with a Ru/C-Rh/C catalyst. Similarly, Kazmi et al. documented the complete transformation of carboxylic acids to esters in supercritical ethanol [57]. Lee et al. demonstrated improved bio-oil quality in supercritical ethanol using Ni-based catalysts [58], while Prajitno et al. achieved remarkable improvements in bio-oil properties in supercritical ethanol without external catalysts [59]. Furthermore, Zhang et al. found that combining hydrodeoxygenation with supercritical fluid systems significantly increased the calorific value of bio-oil and improved deoxygenation levels [60]. These studies highlight the potential of supercritical fluid systems in bio-oil upgrading, offering avenues to improve biofuel properties and energy efficiency.
Table 2 summarizes the essentials of the comparison of the different methods of improving bio-oils.
4. Hydrodeoxygenation
Considerable research efforts have been dedicated to enhancing the quality and utilization efficiency of pyrolysis oil through methods such as hydrodeoxygenation (HDO), emulsification, hydrocracking, reforming for hydrogen production, and others. Among these techniques, HDO stands out due to its comparative advantages over the latter three methods. While emulsification can be relatively corrosive, hydrocracking often yields low results, and hydrogen reforming is both expensive and still in its developmental stages. In contrast, HDO offers a promising solution by effectively saturating aromatic components and alkenes, thereby increasing the calorific value of the bio-oil through elevated H/C ratio and reduces O/C ratio in organic compounds, oxygen is removed in the form of water (which is environmentally friendly unlike CO2 in the case of deoxygenation) [25].
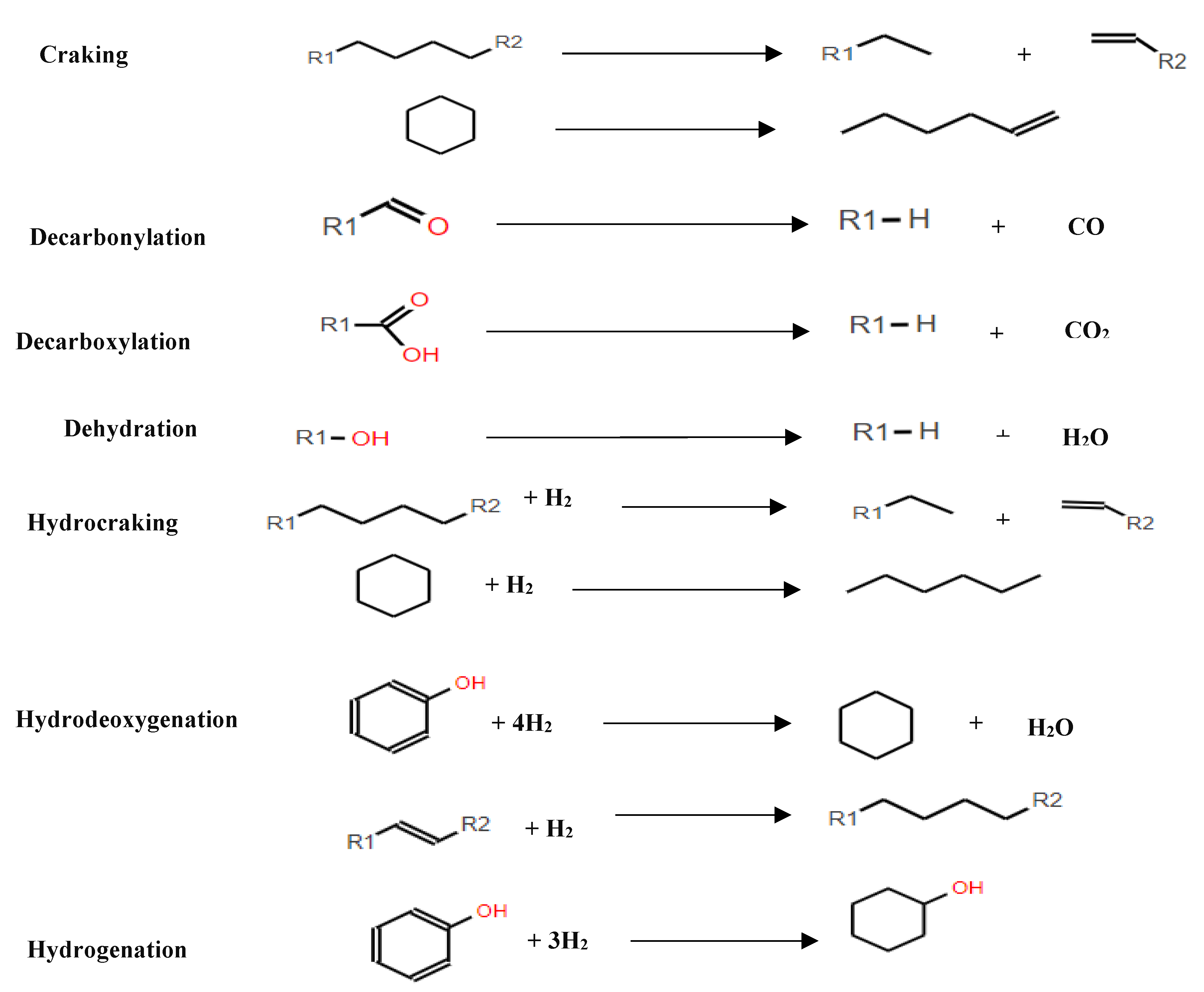
This underscores the significance of HDO as a valuable approach for improving the quality and energy density of pyrolysis oil, presenting opportunities for more efficient utilization of biofuels. Selecting the right catalyst is crucial for an efficient hydrodeoxygenation process. Lignin-based model compounds are commonly used for testing catalytic performance due to the complex structure of lignin-derived bio-oil, which contains many different molecules[39,64]. During HDO, various reaction pathways such as decarbonylation, hydrogenation, cracking, and hydrocracking influence product selectivity (
Figure 6), determining the desirability of the outcomes. Hydrodeoxygenation and hydrogenation are particularly important because they are directly linked to producing transportation fuels.
5. Supported Catalyst for the HDO
Catalyst supports, also known as carriers, play crucial roles in the hydrodeoxygenation (HDO) reaction. Apart from dispersing and stabilizing the promoter and active phases, they also offer secondary functional sites, such as Brønsted and Lewis acid sites, which are essential for the deoxygenation reaction [25]. A wide array of noble, non-noble, and transition metal catalysts, in various forms like sulphide, oxide, carbide, and phosphide, have been assessed for their HDO activity.
In HDO, Hydrogenation occurs only at metallic sites, while acidic sites are required for dehydration, deoxygenation, and hydrogenolysis. Hence, bifunctional catalysts are highly sought-after in HDO applications [66,67]. The stability of metal under operating condition is a critical parameter in catalyst design, and the the choice of support is based on its acid–base and structural–morphological properties. Notably, the support material significantly impacts the overall reactivity of the catalyst. Aspects such as surface area, mesoporous–microporous volume, acid–base properties, and electronic interactions with metals strongly influence HDO effciency. Addictionally, tuning of the catalyst composition plays a vital role in minimizing its deactivation. Therefore, meticulous catalyst design is vital in bio-oil upgrading by HDO [68].
5.1. Sulphide Catalysts
CoMoS2 and NiMoS2 are widely used in both conventional hydrotreatments and also HDO reactions. In these processes, either Cobalt (Co) or Nickel (Ni) serves as a promoter, facilitating electron transfer to the Molybdenum (Mo) atom. This electron transfer weakens the bond between Mo and S, resulting in the creation of an S vacancy site, which is known to be an active site during the HDO process[13].
In the treatment of petroleum crude oil, CoMoS2[69] and NiMoS2 [60] can persist in sulfide form, owing to the presence of H2 and hydrogen sulfide (H2S) in the reactor. These components are typically derived directly from the thiols present in petroleum oil. However, in the case of biomass, which generally has limited or zero sulfur content, maintaining sulfide form is challenging. Without a sulfur source added to the system, CoMoS2 and NiMoS2 are often converted into their respective oxide forms [39].
Table 3 presents the HDO of various feedstocks over sulphide catalysts.
While the addition of extra sulfur could prevent this conversion, it poses risks such as catalyst poisoning during post-processing and sulfur oxide (SOx) emissions upon combustion. Thus, finding a balance between maintaining catalyst activity and avoiding environmental harm is crucial in these processes.
Liu et al [78] reported that 99.3% oxygen removal efficiency from the p-cresol at 300°C was achieved using a dispersed unsupported MoS
2 catalyst. All the hydrothermally synthesized MoS
2 catalysts show much higher activity in the HDO of p-cresol than the commercial MoS
2 sample. It seems that the catalytic activity of MoS
2 is strongly related to the surface area and morphology [77] and the high catalytic activity of molybdenum sulphide in the catalytic HDO of phenol at 450 °C and 5 MPa H
2. The reaction mechanism for the HDO of phenol over MoS
2 was proposed by Chaihad et al. [77], as shown in
Figure 7.
5.1. Oxide Catalysts
Previous research has highlighted the effectiveness of oxide catalysts like Mo, Ni, W, and V in catalyzing the HDO reaction. These catalysts operate on a basic mechanism where a balance between low hydrogen (H2) pressure prevents the conversion of active species into inactive ones, while high H2 pressure is crucial for preventing coke formation. The catalytic activity of oxides in HDO primarily relies on acidic sites, with Lewis acidity playing a significant role in the initial chemisorption stage. The presence of oxygenated compounds allows chemisorption, and the availability of acidic sites is influenced by Brønsted acidity[79]. In a study by Mathew et al.[80], bimetallic Pt-WOx/Al2O3 catalysts with varying WOx loadings were examined for their effectiveness in converting benzyl alcohol to toluene through HDO. It was found that moderate WOx loadings resulted in the most significant improvements in HDO activity and selectivity.
It had been reported previously in the literature that oxide catalysts, such as Mo, Ni, W, and V, exhibit significant catalytic activity in the HDO reaction. Based on the basic mechanism of oxide catalysts, a low H2 pressure is needed to limit the transformation of active species into inactive species; however, a high pressure of H2 is a necessity to avoid coke formation during the HDO process. Generally, the catalytic activity of oxides in the HDO mainly depends on the acidic sites. At the initial chemisorption stage, the Lewis acidity is the key factor and the oxygen lone pair of the oxygenated compounds can be chemisorbed, and the availability of acidic sites on the oxide catalyst is affected by the Brønsted acidity [79]. Mathew Jon et al.[80] studied Bimetallic Pt-WOx/Al2O3 catalysts at varying WOx loadings were tested for the HDO of benzyl alcohol to toluene. Moderate WOx loadings led to the greatest enhancements in HDO activity and selectivity.
5.1. Transition Metal Catalysts
Transition metal catalysts, including noble metals and nickel (Pt, Pd, Ru Rh and Ni), favour HDO and hydrogenation reactions, and their reaction rate is proportional to H2 pressure. Compared with sulphide-based catalysts, it is not necessary to have an additional source of sulphur to maintain the active form [81]. The main disadvantage of transition metal catalysts is related to their high sensitivity to sulphur; therefore, the removal of sulphur-containing compounds from the bio-oil is required prior to HDO treatment [79].
In a previous study by Gutierrez et al.[69], it was observed that the catalytic activity in HDO of guaiacol in hexadecane at 100°C and 80 bars H2 was influenced by the catalytic metals as follows : Rh/ZrO2 > CoMoS2/Al2O3 > Pd/ZrO2 > Pt/ZrO2. Up to now, the basic mechanism of the transition metal in the HDO reaction is still unclear. It is accepted that the metal plays a role in hydrogen donation, but no conclusion has been established as to the activation mechanism for oxygen compounds.
5.1. Phosphide, Carbide, and Nitride Catalysts
Phosphide catalysts have garnered significant attention for their potential in the hydrodeoxygenation (HDO) of bio-oil, mainly due to their efficiency in oil hydrotreating, characterised by low activation energies and high catalytic activity [23,39,75,82,83,84]. A study by Gonçalves et al.[85] compared the effects of silica (SiO2) and tetragonal zirconia (ZrO2) as supports for nickel metal and nickel phosphide (Ni(0) and Ni2P) catalysts on the HDO of m-cresol at 340 °C and 4 MPa. The results showed that Ni2P phase was considerably more active than metallic Ni, and that zirconia supports improved the deoxygenation properties more effectively than silica supports, probably due to the oxophilic sites of zirconia (Zr3+/Zr4+) that enhance the adsorption of m-cresol.
Additional insights were provided by Berenguer et al. [86] that studied the catalytic HDO of m-cresol using Ni2P supported on hierarchical zeolite (h-ZSM-5) at 200 °C and 25 bar H2. This setup induced the formation of strong Lewis acid sites, proportional to the Ni2P charge, complemented by new Brönsted acid sites due to P-OH units[82,83,87]. The Ni2P/h-ZSM-5 catalyst showed high selectivity (>97%) for converting m-cresol to methylcyclohexane, significantly improving compared to the Ni2P/SiO2 reference catalyst. This demonstrates the synergistic effects between the metal phosphide and the solid acid support, with catalyst activity displaying a dependence on Ni2P dispersion and optimal activity observed for particle sizes of 4 nm.
In parallel, alternative catalysts, such as carbides and nitrides, are being explored for their cost-efficiency and properties similar to traditional HDO catalysts. The research by Boullosa-Eiras et al.[88] investigated the performance of several catalysts, including carbide, nitride, phosphide and molybdenum oxide supported on titanium, in the HDO of phenol at 350 °C and 25 bar H2. Molybdenum carbide on titanium dioxide (Mo2C/TiO2) showed the highest activity with marked selectivity towards benzene. Moreover, molybdenum phosphide on titanium dioxide (MoP/TiO2) proved propensity to hydrogenate the aromatic ring and exhibited considerable selectivity towards methylcyclopentane, suggesting the influence of the acidic surface chemistry on the reaction behaviour.
Although phosphide, nitride and carbide catalysts demonstrate efficient performance in bio-oil HDO [88,89,90], their industrial scale adoption is still in the early stages, and they do not yet match the performance of commercial sulphide catalysts in industrial applications. Further research and development are needed to optimise these new catalysts for wider industrial deployment.
5.1. Ni2P Promoted Catalysts
A new class of hydrotreating catalysts known as transition metal phosphides has been developed and widely applied in processes like hydrodesulphurisation (HDS) and hydrodenitrogenation (HDN), which are closely related to hydrodeoxygenation (HDO) [64,91]. Among them, nickel phosphide (Ni2P) has emerged as a particularly effective material due to its unique structural and chemical properties. Ni2P is characterised by a high density of Ni-Ni and Ni-P bonds, which contribute to its superior electronic conductivity, thermal stability and chemical durability [82]. In addition, Ni2P displays a reduced propensity for hydrogenolysis of C-C bonds, a typically undesirable reaction that leads to the formation of short-chain hydrocarbons with low octane values [83].
The effectiveness of Ni2P reaches out to its role in facilitating the easy dissociation of hydrogen due to the electronic (ligand) and geometrical (assembly) effects imparted by the presence of phosphorus. This feature significantly enhances the hydrogenation activity of Ni species, making Ni2P a useful catalyst in the HDO process [87]. Recent studies focusing on the HDO of lignin-derived bio-oil model compounds have shown that Ni2P is a bifunctional catalyst system. In this system, Ni metal sites actively promote the hydrogenation of aromatic rings and the hydrogenolysis of C-O bonds, depending on the reaction conditions. At the same time, the phosphorus species of the catalyst generate Brønsted acid sites through the formation of P-OH groups, thus facilitating the direct HDO of methoxy or hydroxyl groups [85,92].
Additional comparative analyses have underscored the higher activity of Ni
2P-based catalysts compared to other transition metal phosphide catalysts in guaiacol HDO. Also, compared to Ni-based monometallic catalysts, Ni
2P-based catalysts have consistently demonstrated superior catalytic performance [87]. Zhu et al.[83] specifically examined the impact of Ni and Ni
2P active phases on the HDO of m-cresol at 275 °C for 120 min in a packed bed reactor under a hydrogen pressure of 3.0 MPa. The results of this study revealed that the Ni
2P-based catalysts exhibit high and stable activity, reaching m-cresol conversion rates of over 94.7% at temperatures between 250 and 275 °C (
Figure 8).
These results confirm the pivotal role of Ni
2P in enhancing the field of catalytic HDO, emphasising its potential to outperform traditional monometallic catalysts and other transition metal phosphide catalysts in complex biochemical conversions. Moreover, the catalytic performance of Ni2P-based catalysts for HDO of model compounds such as guaiacol [87], phenol [96], anisole [86], cresol [88] and real pyrolysis oil [25] has been extensively explored (
Table 4).
Studies investigating the catalytic activity of Ni2P-based catalysts have demonstrated their remarkable selectivity towards saturated hydrocarbons suitable for use as transport molecules. For instance, Wang et al.[93] explored the HDO of furfural at 240 °C and 2.0 MPa for 4 hours using MoP or Ni2P catalysts. They found that using Ni2P_0.5% as catalyst resulted in a high selectivity of 83.1% towards 2-methylfuran, together with a complete conversion of furfural. Similarly, Berenguer et al.[86] carried out the HDO of phenol in a batch reactor at 200 °C, 4 MPa and 2 h reaction time using Ni2P supported on Al-SBA-15. Their results showed a complete conversion of phenol (100%) and a high selectivity of 91% towards cyclohexane.
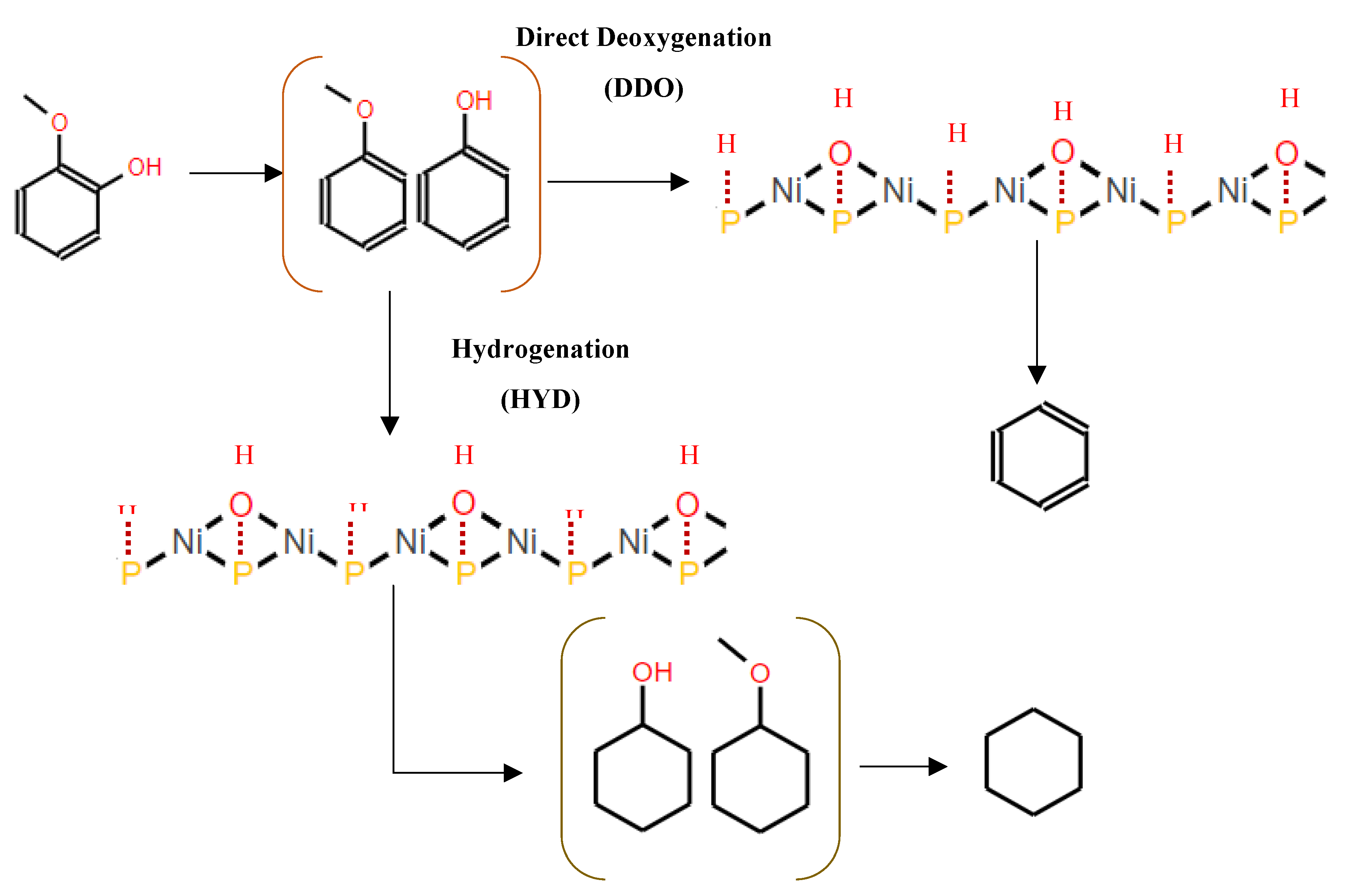
Moreover, Moon et al.[87] proposed a mechanism for the conversion of guaiacol to cyclohexane and benzene (
Figure 9). According to their model, the conversion of guaiacol over Ni
2P-based catalysts initiates with direct deoxygenation, which produces anisole and phenol as primary intermediate species. At low pressure, direct deoxygenation (DDO) of these intermediate species prevails, leading to the formation of benzene. However, at high hydrogen pressure, hydrogenation of the aromatic ring becomes dominant, leading to the formation of cyclohexanol and methoxycyclohexane. Subsequent deoxygenation processes result in cyclohexane as the final hydrocarbon product observed over the Ni
2P catalyst.
Interestingly, both reaction pathways highlight the dual catalytic roles of Ni2P-based catalysts. Deoxygenation reactions (C–O cleavage) are promoted by the Brønsted acid sites generated from surface hydroxyl groups (OH), while hydrogenation reactions are favored by the Ni redox sites of the Ni2P-based catalysts [87,93]. This mechanistic understanding provides valuable insights into the catalytic behavior of Ni2P-based catalysts and their potential applications in the conversion of lignin-derived bio-oils into valuable transportation fuels.
The activity of Ni2P catalyst was also investigated in a series of experiments for guaiacol HDO compared to a wide variety of other metal phosphides including Co2P, Fe2P, WP, and MoP. An order of turnover frequency of active sites, Ni2P > Co2P > Fe2P, WP, and MoP, was observed by CO titrating chemisorption[94].
Table 4 presents the HDO of various feedstocks over Ni and Ni-modified supported catalyst.
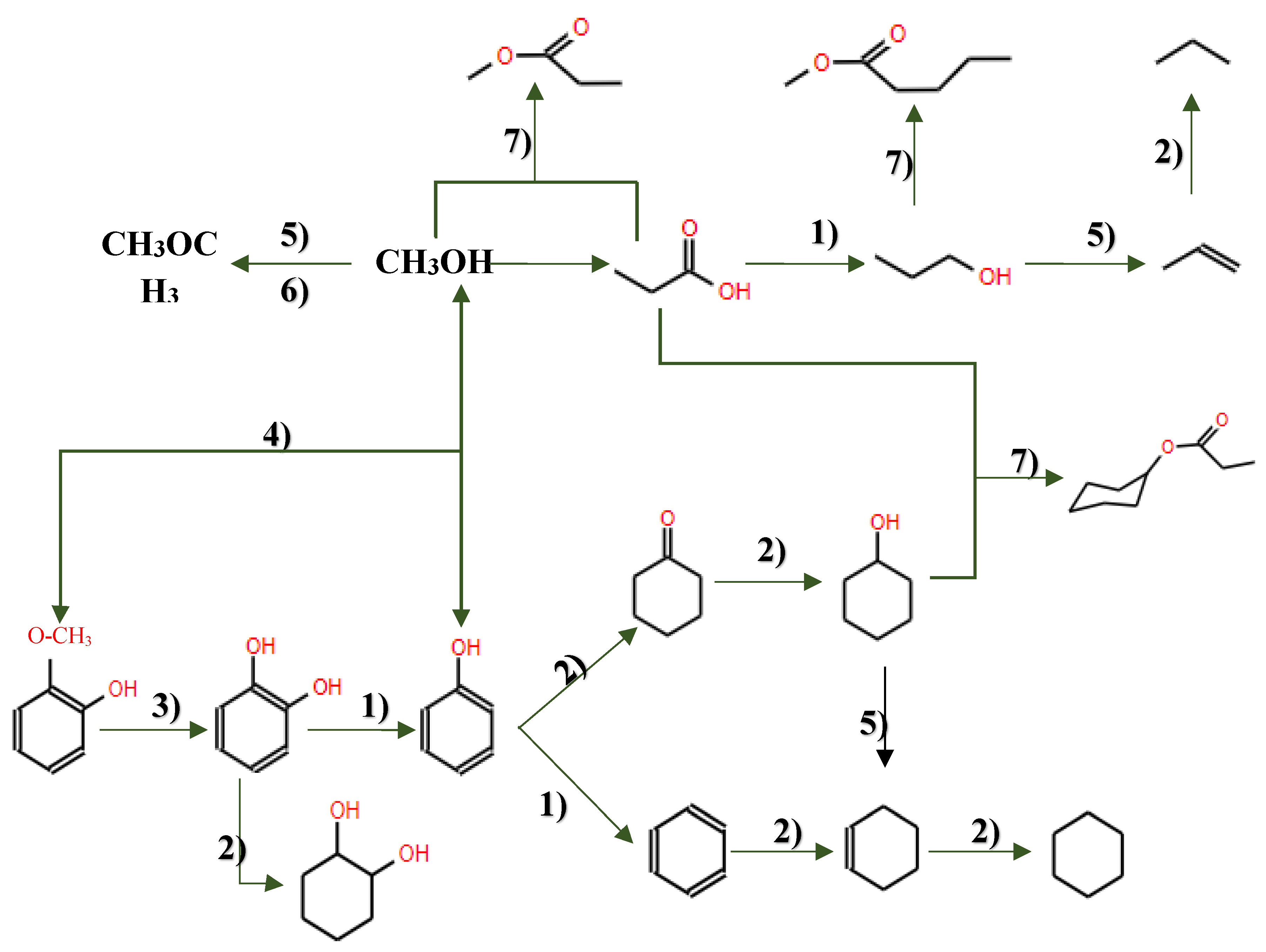
6. Hydrodeoxygenation of Model Compounds
The complexity of bio-oil composition poses significant challenges in understanding its HDO pathways, due to the simultaneous occurrence of numerous reactions during the upgrading process. Consequently, many researchers have chosen to use model compounds instead of full-scale pyrolysis oil in their studies. These model compounds cover a wide range of classifications, including phenols such as guaiacol[87], phenol[114] and cresol[86]), as well as aldehydes such as furfural[115], ethers such as anisole[116,117], furans such as 2-methylfuran[118], and carboxylic acids such as acetic acid[109], ketones such as acetone[119], among others. For example, Zhu et al.[83] investigated the HDO of m-cresol using Ni-based catalysts in a fixed-bed reactor, achieving a complete conversion to methylcyclohexane (MCH) at 250°C. These experiments with model compounds are essential for gaining comprehensive insights into HDO reaction mechanisms and networks, as well as for the selection and design of efficient catalysts [83].
At present, model compounds are meticulously chosen to represent the most active components of bio-oil, a factor contributing to its inherent instability. These compounds, characterised by a wide range of functional groups, facilitate the exploration of relative activities and selectivity in various reactions, encompassing dehydration, decarboxylation, hydrogenation, hydrogenolysis and hydrocracking (see
Figure 6). Additionally, dimeric compounds are selected as model compounds to provide valuable information about the cleavage of major linkage types prevalent in bio-oil. Through the utilisation of these model compounds, researchers can further investigate the fundamental reactions underlying HDO, as depicted in
Figure 10, and design strategies to optimize the performance of catalysts for the efficient conversion of bio-oil into valuable fuels and chemicals.
6.1. Hydrodeoxygenation of Phenols and Alkylated Phenols (Guaiacols)
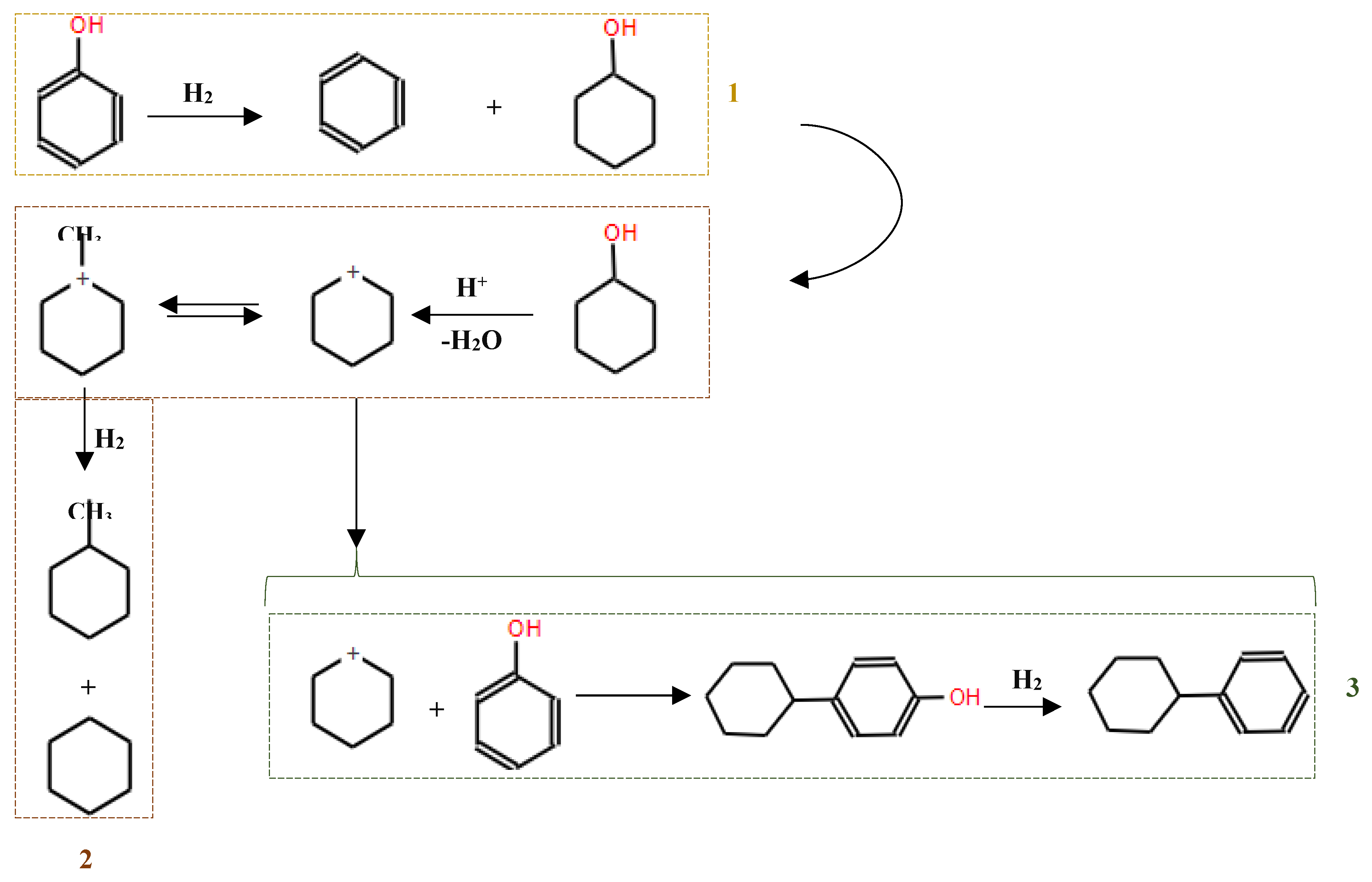
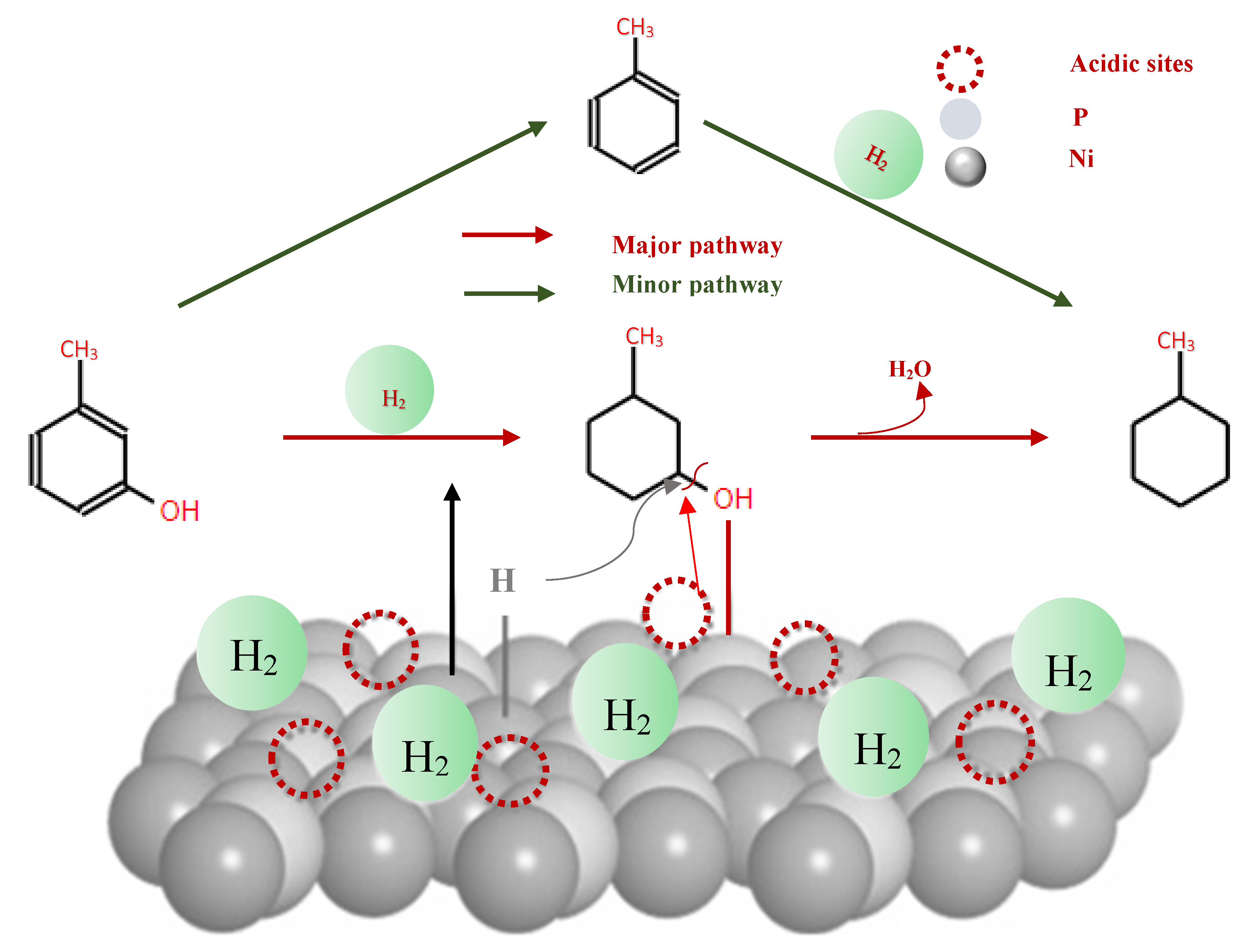
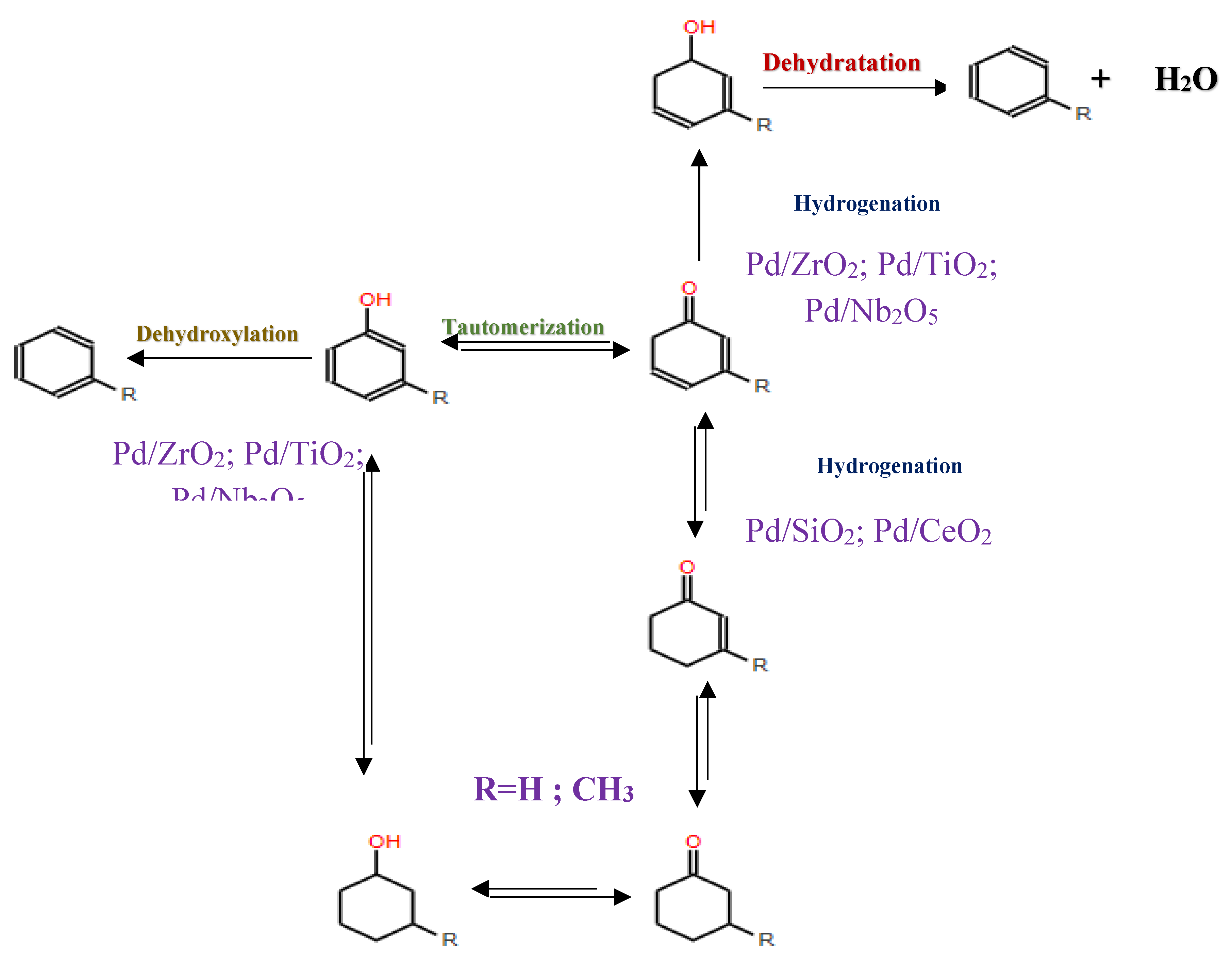
Phenolic monomers, such as phenols, guaiacols and syringols, represent fundamental by-products resulting from lignin degradation. Among these, phenol and its alkylated derivatives (cresol and 2-ethylphenol) stand out as the most prevalent lignin-derived phenolic monomers. The HDO process, essencial for converting lignin-derived phenols, primarily involves the cleavage of the C-OH bond. This process can follow two different chemical routes, leading to the production of cycloalkanes and arenes: (i) hydrogenation of the aromatic ring followed by deoxygenation of the alcohols to produce cycloalkanes, and (ii) direct deoxygenation to generate arenes by cleaving the C-OH bond[114]. Hydrogenation, dehydration and hydrogenolysis reactions represent the three main categories of reactions involved in the HDO of phenolic compounds [102].
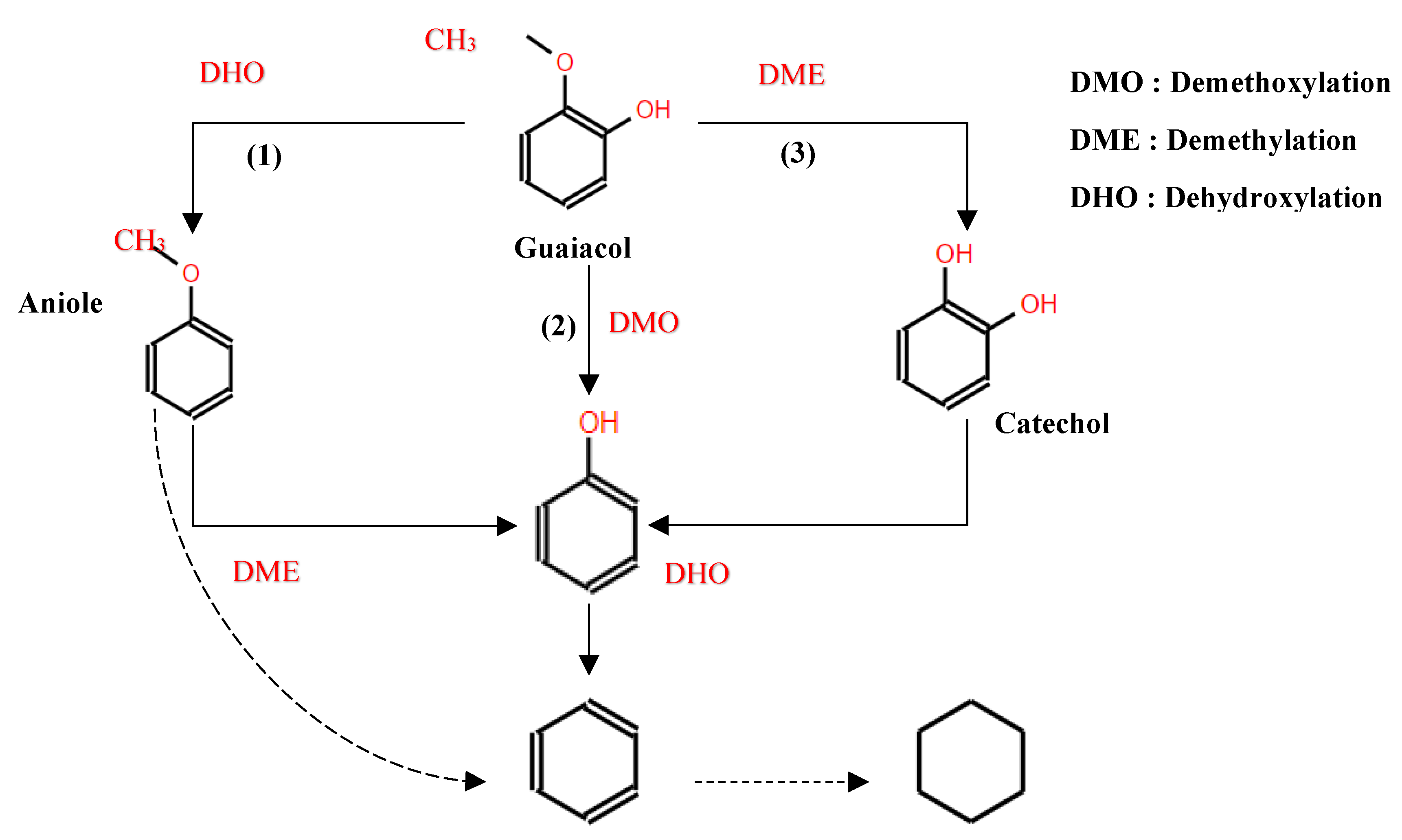
In studies focusing on the HDO of phenols, Lan et al [121] investigated the HDO of guaiacol, revelating that Ni2P/SiO2 exhibed a higher selectivity towards benzene. This selectivity was achieved through demethoxylation and dehydroxylation via phenol and anisole intermediates (over 97%), compared to MoP/SiO2 catalyst. They also observed a significant influence Ni2P particle size on the turnover frequencies of guaiacol HDO, as illustrated in their proposed reaction pathways depicted in
Figure 11. Additionally, Gutiérrez-Rubio et al [98] explored the HDO of bio-derived anisole using different catalysts, including nickel supported on 2D zeolites: L-ZSM-5 and PI-ZSM-5), achieving high guaiacol conversion rates of 75% and 78%, respectively.
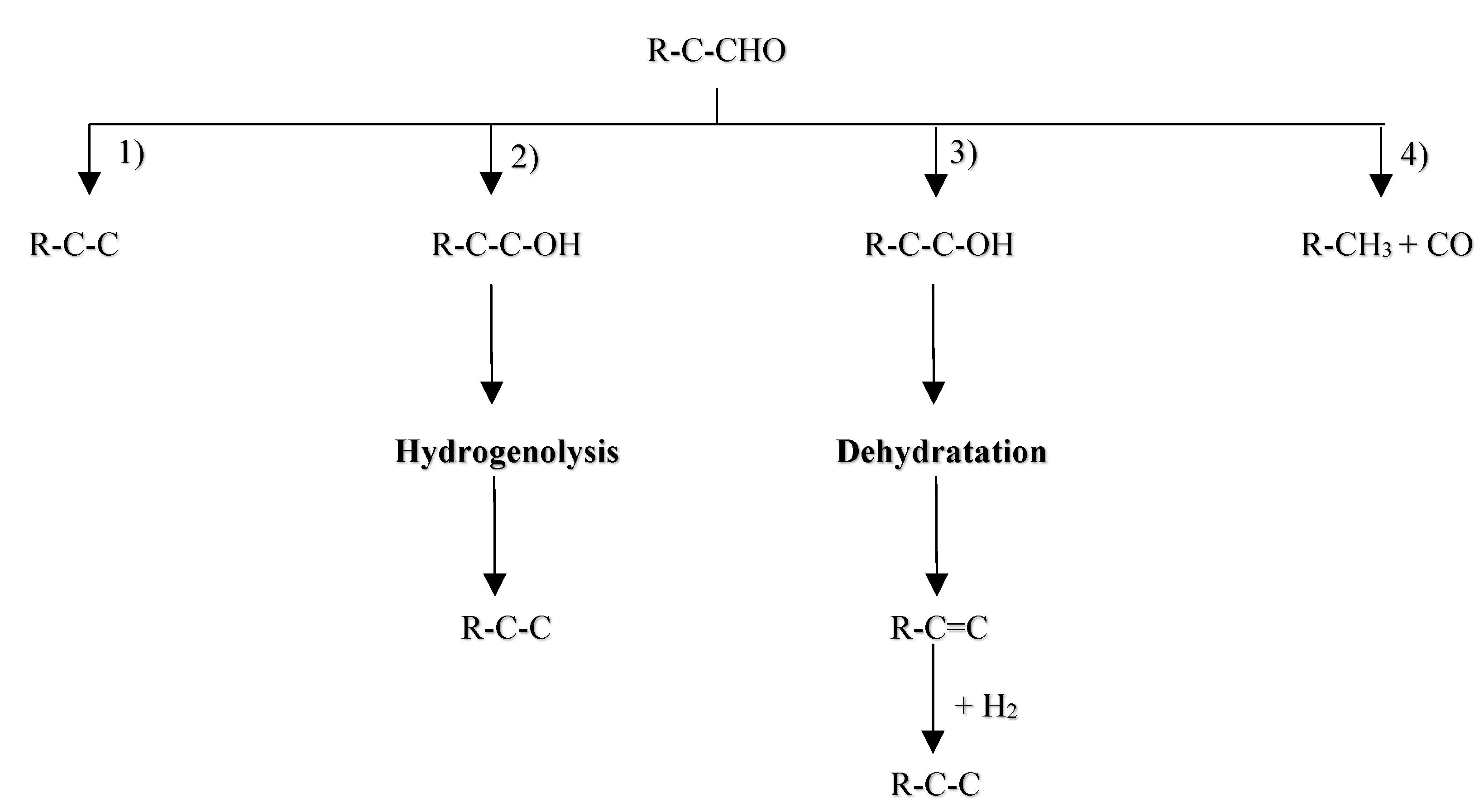
6.1. Hydrodeoxygenation of Ketones, Aldehydes, and Alcohols
Pyrolysis oil is composed of a diverse array of components, including ketones10-15%, carboxylic acids, phenols, guaiacols, and aldehydes 0-5% [15]. Among the oxygenated compounds found in pyrolysis oil, furan, tetrahydrofuran (THF), furanone, furfural, furfuryl alcohol, and 5-hydroxymethyl furfural (HMF) present notable challenges in terms of deoxygenation. The HDO of aldehydes, such as furfural, can theoretically progress through four major routes[122] as depicted in figure 12 and enumerated as following:
Direct hydrogenolysis of the C=O bond[97];
Hydrogenation of C=O bond to form alcohols, followed by hydrogenolysis of the C-O bond to produce alkanes (hydrogenation-hydrogenolytic mechanism);
Hydrogenation of C=O bond to form alcohols, subsequent dehydration to generate olefins, and then rehydrogenation of the C=C bond to yield corresponding alkanes (hydrogenation-hydrogenolytic mechanism);
Decarbonylation of C=O to generate CO and alkane with one fewer carbon;
These routes involve the conversion of the carbonyl group into a methyl group or CO. The conversion of alcohols encompasses steps 2 and 3, ketone conversion entails all steps but is not depicted in figure 12 due to differing molecular formulas. These mechanistic insights serve as a basis for understanding the intricate transformations involved in the HDO of aldehydes, highlighting both the challenges and opportunities in developing efficient catalysts for the valorization of pyrolysis oil constituents.
Figure 12.
Main reaction pathways of HDO of aldehydes and alcohols, R stands for alkyl groups adapted from [123].
Figure 12.
Main reaction pathways of HDO of aldehydes and alcohols, R stands for alkyl groups adapted from [123].
In the realm of hydrodeoxygenation (HDO), various studies have investigated the conversion of oxygenated organic compounds into valuable chemicals and fuels. For instance, Lan et al. [97] conducted a study on the HDO of furfural using bifunctional Ni2P catalysts in a flow fixed-bed reactor at 1 bar pressure and 200°C, and a weight hourly space velocity (WHSV) of 3 h−1, they achieved a 90% conversion rate of furfural and identified a highly selective pathway leading to 2-methylfuran (MF), as illustrated in
Figure 13.
Complementary research by Wang et al.[124] explored the HDO of furfural over a modified Cu/ZSM-5 catalyst incorporated with nickel (Ni). Their findings revealed that nickel’s moderate addition enhanced both the adsorption of furfural and hydrogen, thereby accelerating the conversion process. This modification resulted in a significant increase in selectivity for 2-Methylfuran (2-MeF), reaching 78.8 wt% at 220°C after 30 minutes of reaction. The reaction pathway involved a sequence of hydrogenation followed by deoxygenation steps.
Simultaneously, Lino et al.[125] investigated the HDO of 2-methyltetrahydrofuran using Ni2P supported on SiO2 in a continuous-flow fixed bed quartz reactor at 0.5 MPa of N2 and high nickel loading, which were conducive to furfural conversion at elevated temperatures. A notable finding was that a higher partial pressure of H2 markedly favored the hydrogenation process, leading to a total furan yield surpassing 100% and a selectivity of 85% to n-pentane at 350°C.
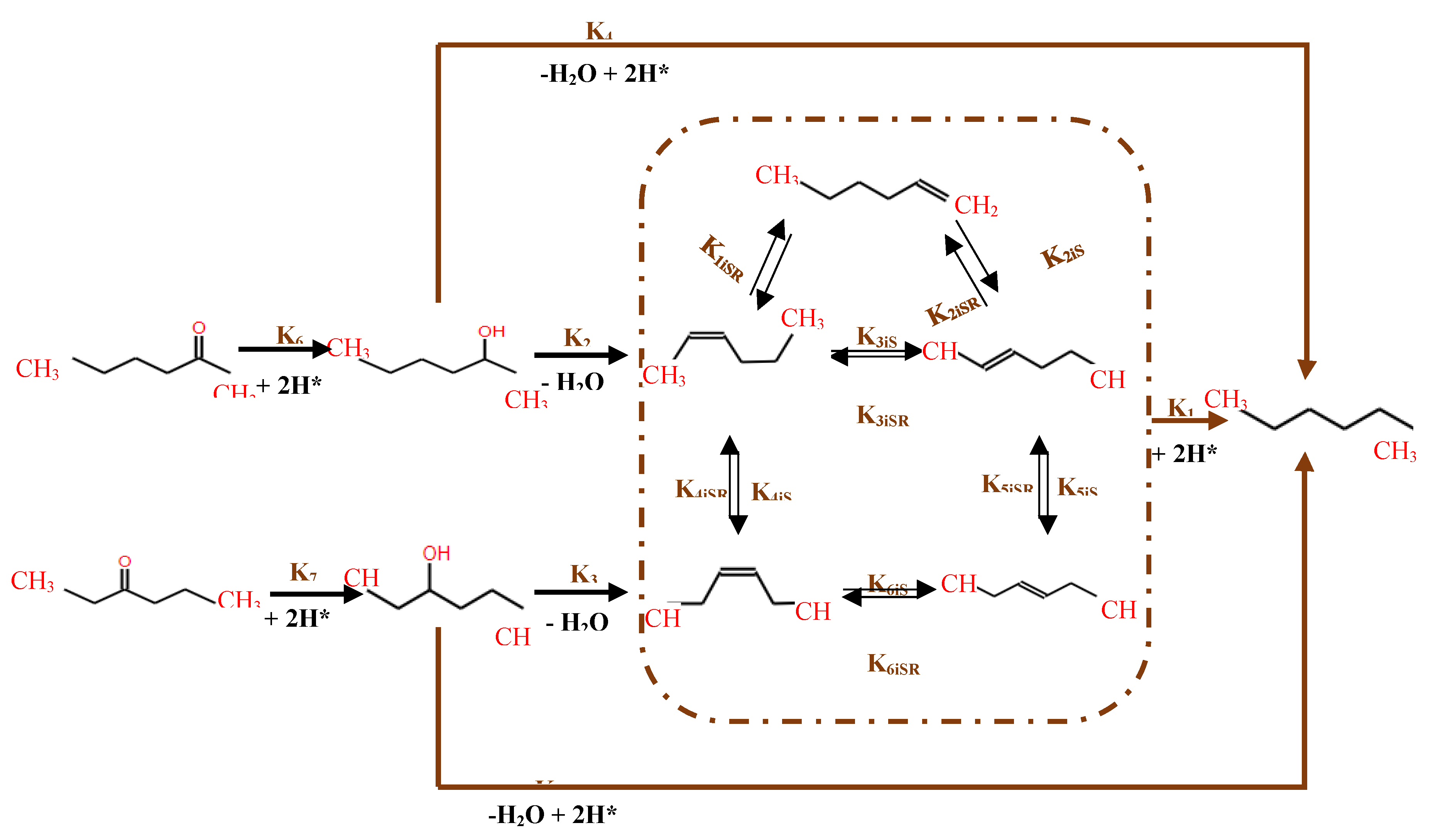
Further explorations in HDO were conducted by researchers using NiMo/γ-Al2O3 catalysts supported on sulphides in a a batch reactor[126] at 5 MPa H2 and 250°C, primary transformations of 2-hexanone and 3-hexanone involved hydrogenation into their respective alcohols, followed by dehydration to form olefins. These were subsequently hydrogenated into saturated alkanes, predominantly hexane. An alternative pathway featured the direct hydrogenolysis of the C-O bond, crucial for the conversion of secondary hexanol into hexane at 275°C (figure 14). Notably, while the presence of various hexene isomers was consistent with literature finding [127], only trace amounts of 1-hexene were detected, suggesting its formation through isomerization of 2-hexene. Ultimately, this sequence culminated in the hydrogenation of all hexene isomers into hexane, delineating the primary product pathway from alcohols and ketones [126,127].
Figure 14.
Proposed reaction scheme of the HDO of model compounds: 2-hexanone, 2-hexanol, 3-hexanone and 3-hexanol over NiMo/-Al2O3 catalyst, adapted from [126].
Figure 14.
Proposed reaction scheme of the HDO of model compounds: 2-hexanone, 2-hexanol, 3-hexanone and 3-hexanol over NiMo/-Al2O3 catalyst, adapted from [126].
These studies collectively underscore the influence of catalyst composition, reactor conditions, and hydrogen pressure in optimizing HDO processes, each contributing uniquely to the enhanced conversion rates, selectivity, and understanding of reaction mechanisms in converting ketones, aldehydes, and alcohols compounds into hydrocarbons.
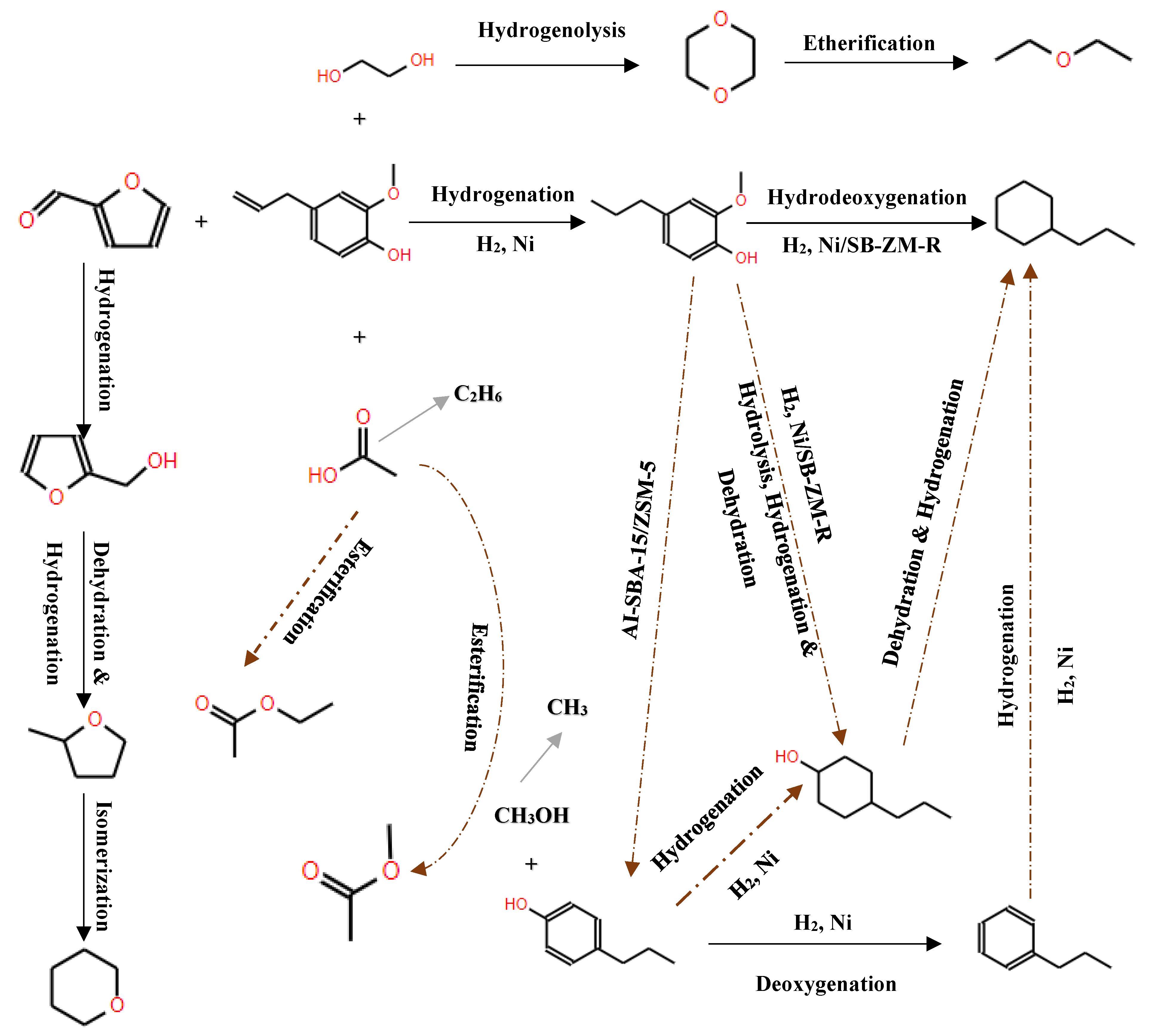
6.1. Hydrodeoxygenation of Carboxylic Acids
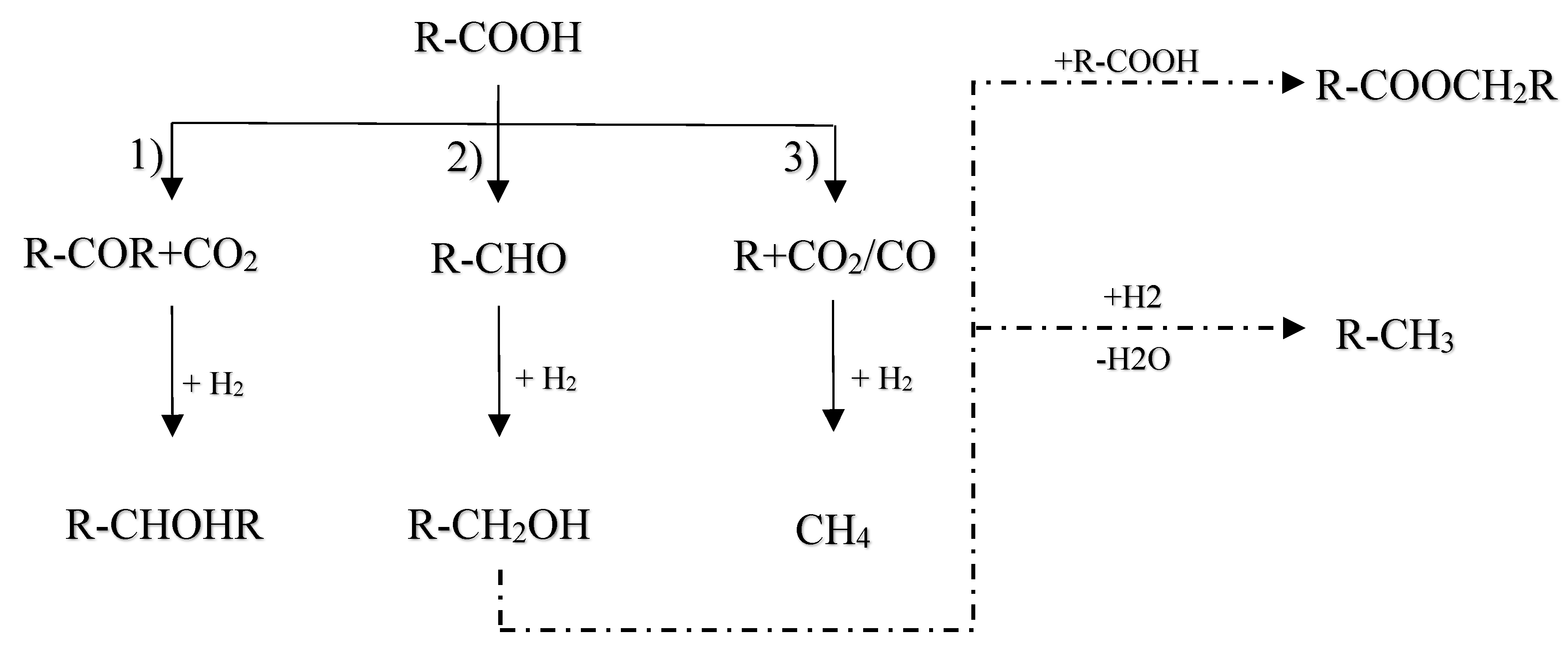
The inherent acidity of bio-oils, often manifested by a pH between 2 and 3 [128], primarily arises from the presence of carboxylic acids such as acetic and formic acids. This acidic nature significantly contributes to the corrosive properties of bio-oils, particularly under elevated temperatures, as noted in [109]. In the HDO of these carboxylic acids, literature describes three principal reaction pathways (as delineated in
Figure 15):
Ketonization by C-O bond cleavage to generate ketones, and further by hydrogenation to produce alcohols;
Hydrogenolysis by the C-O bond cleavage to yield aldehyde, followed by further hydrogenation to produce alcohols, and then dehydration and hydrogenation to obtain alkane, or the alcohols react with carboxylic acids to form esters;
Decomposition (decarboxylation and decarbonylation) of carboxylic acids by breaking C-C bond to yield alkanes with one less carbon, CO and CO2. Also, CO can be further hydrogenated to methane.
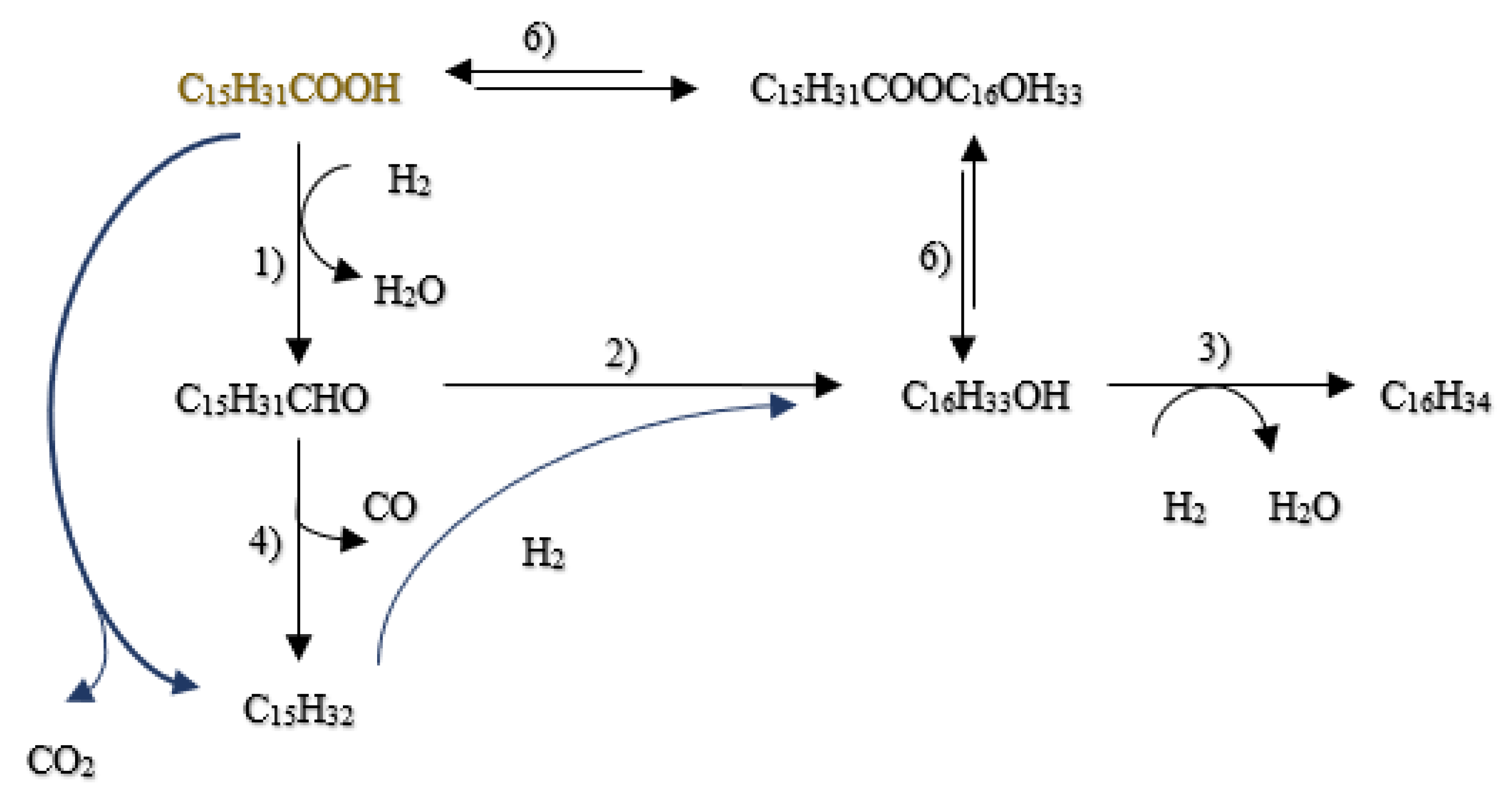
A detailed investigation into the HDO of acetic acid using reduced sulfided Ni-Mo(R)/ZSM-5 catalysts [129] showed that HDO of palmitic acid can occur effectively below 300°C with an initial hydrogen pressure of 35 bar. Ater 4 hours, the conversion of palmitic acid neared 99%, producing a range of isomerized paraffins with carbon numbers from 6 to 16, as identified by GC-MS. This observation suggests that the catalyst facilitates both hydrodeoxygenation and hydroisomerization reactions concurrently on palmitic acid.
Furthermore, Peroni et al.[130] examined the HDO of formic acid using a temperature-programmed reaction (TPR) approach with a Ni2P/Al2O3 catalyst in a bed flow reactor under various temperatures and residence times. Their study demonstrated a complete conversion of palmitic acid, where the use of Ni2P supported on Al2O3 increased the selectivity towards decarbonylation and decarboxylation processes. Remarkably, this resulted in pentadecane being the predominant product, constituting about 78% of yield. According to the hydrodeoxygenation mechanism presented in
Figure 16, initial products included pentadecane and hexadecane, which subsequently underwent decarbonylation to yield n-pentadecane and carbon monoxide according to [131].
These studies collectively illuminate the nuanced dynamics of HDO mechanisms under varying conditions and catalyst formulations, elucidating how such parameters dictate the efficiency, selectivity, and range of products formed during the deoxygenation of carboxylic acids in bio-oils.
6.1. Hydrodeoxygenation of Carbohydrates
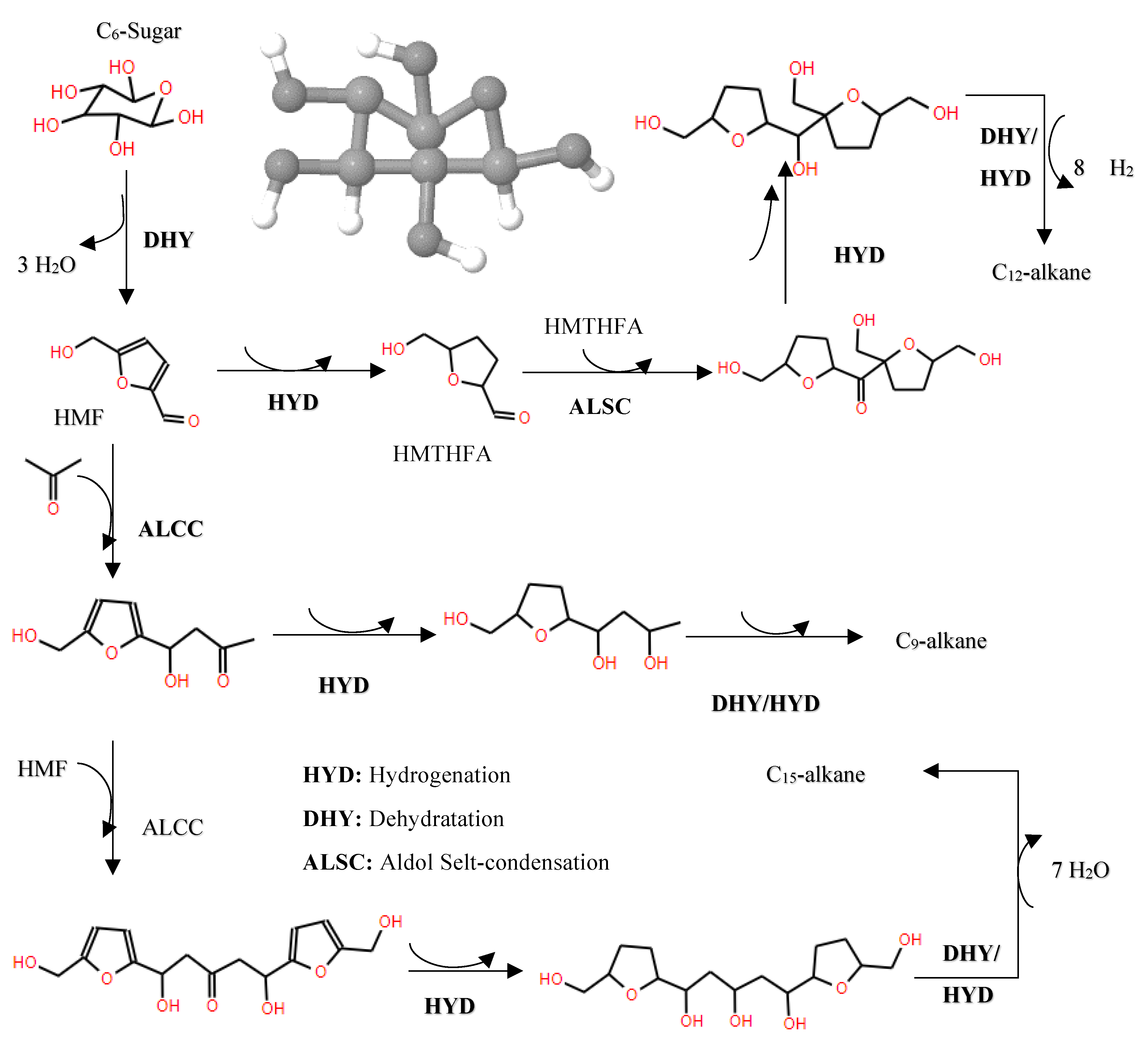
In the domain of hydrodeoxygenation of carbohydrates, a detailed understanding of the reaction pathways and catalyst functionalities is critical for optimizing the production of biofuels and chemicals. Initially, the HDO of carbohydrates such as C6 sugars (e.g., fructose) typically begins with dehydration to form 5-hydroxymethylfurfural (HMF), a pivotal intermediate in the conversion process. Subsequently, HMF undergoes hydrogenation to yield 5-(hydroxymethyl)tetrahydrofuran-2-carbaldehyde (HMTHFA), as delineated in [132]. These intermediates can participate in further reactions such as aldol condensation, leading to the formation of larger molecules, and are subject to multi-step hydrogenation and dehydration processes to synthesize C9-C15 alkanes, as illustrated in
Figure 17.
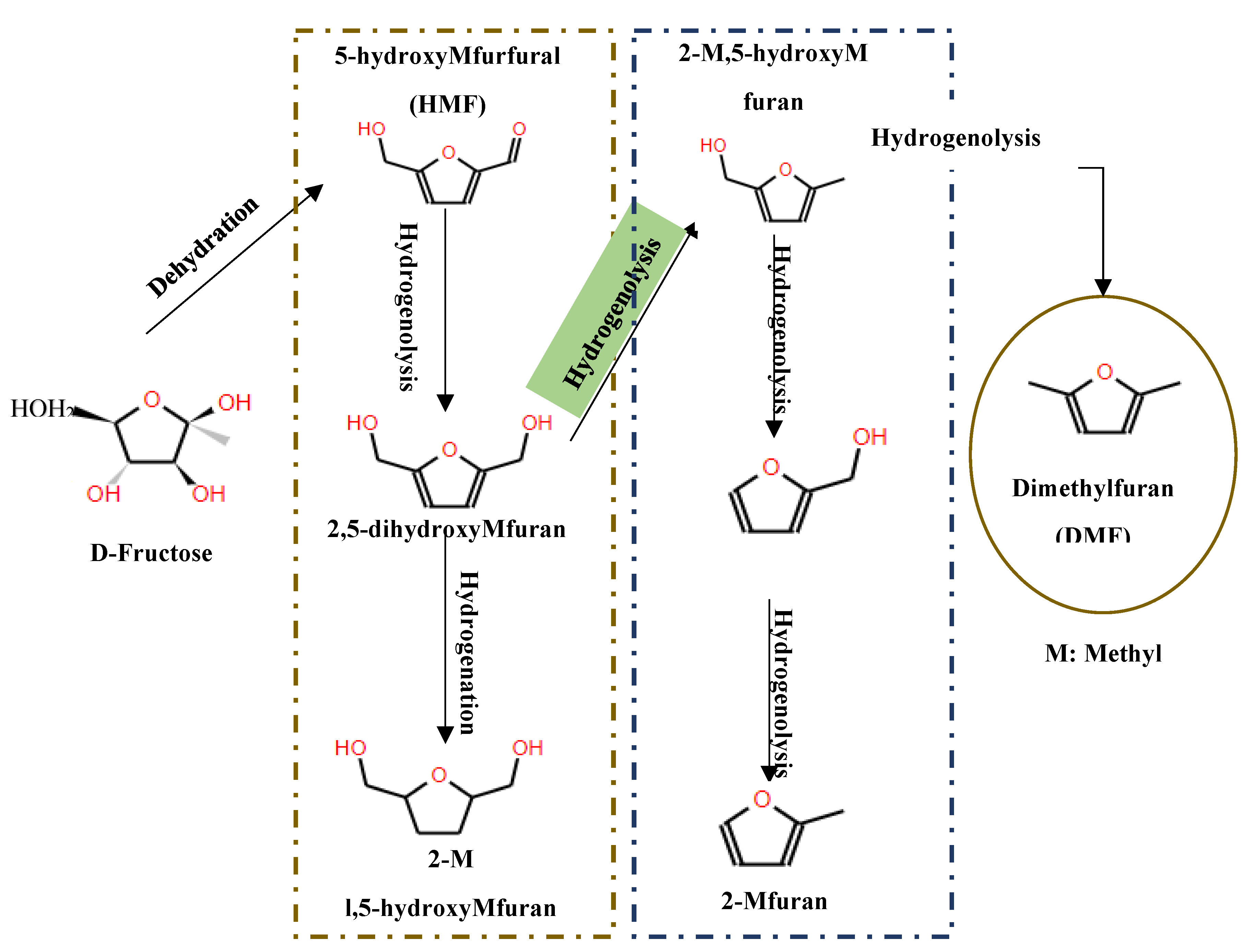
A variety of catalysts with metal and acid sites, such as CuRu/C, have been used for the initial dehydration of fructose (C6-sugar) to HMF, followed by hydrogenolysis of the C-O bonds inside HMF to produce 2,5-dimethylfuran (DMF)[133] as illustrated in
Figure 18. This step is crucial as DMF serves as a gateway for other hydrogenated products. In addition, research involving different metal sites supported on silica, including Ni, Cu, Fe, Co, Pt, Pd and Re, has demonstrated that all these metals are capable of producing DMF from HMF using 1-propanol as solvent in a tubular flow reactor at 180 °C and 33 bar, according to the results of [133] and [134]. Moreover, the transformation of DMF can yield a variety of products depending on the metal employed; for example, ring-opening and 2,5-dimethyltetrahydrofuran (DMTHF) products have been specifically observed with Pd/C catalysts.
Parallel to these developments, other catalyst such as acid catalysts (Zr-P, SiO2-Al2O3, WOX/ZrO2, γ-Al2O3, and HY zeolite) have also been used for aqueous-phase HDO of carbohydrate (xylose) at 160°C in a batch reactor [135]. The dehydration of xylose predominantly leads to the production of furfural. The efficiency of this conversion, particularly the selectivity towards furfural after 30 minutes, is significantly; about 20% influenced by the ratio of Brønsted to Lewis acid sites on the catalyst. Notably, catalysts like Zr-P exhibit a furfural selectivity substantially higher than those with predominately Lewis acid sites, even at modest xylose conversion levels. This high selectivity is comparable to that achieved with ion-exchange polymer resins featuring high concentrations of Brønsted acid sites, similar to Zr-P and HCl. The presence of Lewis acid sites, however, tends to catalyze side reactions between xylose and furfural, leading to the formation of undesired by-products such as humins.
These studies collectively emphasize the importance of catalyst composition—balancing metal and acid sites—and reaction conditions in steering the HDO pathways from simple sugars to complex hydrocarbons and chemicals. Understanding these dynamics facilitates the development of tailored catalyst systems that enhance selectivity and yield, crucial for the efficient conversion of biomass into valuable biofuels and chemical feedstocks.
7. Hydrodeoxygenation of Mixtures
Research into the hydrodeoxygenation of binary mixtures containing aromatic compounds has shown intriguing results concerning the interaction and competitive adsorption behaviors on catalyst surfaces. Teles et al.[136] conduced studies on binary mixtures (phenol/anisole and m-cresol/anisole) using Pd catalysts supported on varios oxides like SiO2, CeO2, ZrO2, TiO2. Their findings indicated that the hydroxyl (OH) groups in phenol and m-cresol exhibited stronger adsorption to the catalyst surfaces compared to the methoxy groups of anisole. This preferential adsorption facilitated the reactions of the hydroxyl-containing molecules. Despite the apparent competition for active sites on the catalyst, the interaction between the model compounds was characterized as weak, as depicted in
Figure 19. Similarly, Funkenbusch et al. [137] observed comparable behavior in the HDO of other blends (anisole/m-cresol and anisole/phenol) over Pt/Al2O3 and Pd/C catalysts, reinforcing the concept that the nature of functional groups significantly influences adsorption and subsequent reactivity in these systems.
Extending the complexity of the reagent mixtures, Roldugina et al.[138] explored the HDO of a synthetic bio-oil mixture containing guaiacol, water, n-dodecane and methanol, using a ruthenium catalyst supported on aluminium modified hexagonal mesoporous silica (Ru/Al-HMS), performed under a hydrogen pressure of 6.0 MPa and temperature of 250°C. The upgraded bio-oil exhibited a conversion rate of 38% at 210°C, yielding major products such as cyclohexanol (47%), phenol (18%), benzene (15%) and cyclohexane (7%). However, when the temperature was increased to 290°C and the hydrogen pressure was reduced to 2.5 MPa, the conversion of guaiacol dropped to 19%, with phenol being the predominant product with a selectivity of 70%.
These studies collectively demonstrate the critical roles of catalyst composition, reactant structure, and reaction conditions in dictating the dynamics of HDO processes. The selective adsorption of functional groups and the subsequent reactivity highlight the nuanced interactions within the catalytic environment, essential for optimizing conversion and selectivity in the transformation of complex bio-oil mixtures to valuable chemical products.
7.1. Hydrodeoxygenation of Mixtures over Zeolites and Non-Noble Metal Catalysts
Investigations on the hydrodeoxygenation (HDO) of bio-oil mixtures have revealed the effectiveness of non-noble metal catalysts supported on solids with varying acidity and textural properties due to their high HDO activity and reduced-cost. Sankaranarayanan et al.[139] addressed on the catalytic transformation of mixtures containing guaiacol and propionic acid over Ni-based catalysts supported on solids such as hierarchical ZSM-5 (h-ZSM-5), SBA-15, and Al-SBA-15. These catalysts assisted the in-situ formation of methanol from guaiacol demethoxylation and the generation of other alcohols such as cyclohexanol, enabling the esterification of carboxylic acids without external alcohol addition. This approach allowed for significant upgrading of carboxylic acids directly within the HDO process, demonstrating the high efficacy of Ni/h-ZSM-5 in both HDO and esterification, with methyl propionate emerging as a major product. Methyl propionate has been identified as the major product of propionic acid transformation over all catalysts (
Figure 20).
Further exploring on the role of Ni-based catalysts, Chen et al. [140] examined the impact of catalyst composition on the HDO of a mixture of eugenol with light bio-oil fractions using Ni/SB-ZM-R, Ni/SB-R, Ni/ZM-R. They observed that the addition of different molecular fractions significantly affected the selectivity of products like propyl-cyclohexane. Among the catalysts tested, Ni/SB-ZM-R showed superior performance due to its optimal pore structure and acidity. The presence of other compounds, like ethylene glycol, increased the selectivity for propylcyclohexane from 67.9% up to 90%, whereas additives like furfural and acetic acid (1%) significantly decreased its selectivity to 36%, indicating their adverse effects on HDO efficiency. The study delineated two different reaction pathways that could be seen for the eugenol: i) eugenol was converted into 2-methoxy-4-propyl-phenol and then converted into the final product propyl-cyclohexane via HDO and ii) 2-methoxy-4-propyl-phenol was firstly converted into 4-propenebenzene, and then converted into propyl-cyclohexane by hydrogenation. For the other pathway, 4-propylcyclohexanol was obtained by hydrolysis, dehydration and hydrodehydration, leading to final products such as propyl-cyclohexane (
Figure 21).
In another investigation focusing on a mixture of guaiacol and acetic acid, the researchers used a Ni2P/ZSM-5 catalyst[109]. This study revealed a partial HDO inhibition of guaiacol due to competitive adsorption of acetic acid on the active sites of the catalyst. Ni2P incorporation altered the acidic properties of ZSM-5 by introducing new acid sites of moderate strength, consisting of Niδ+ species (Lewis acid sites) and residual P-OH groups (weak Brønsted acid sites). This modification affected differently the adsorption capacity and performance of the catalyst in microporous and mesoporous structures [98]. However, this system also facilitated positive interactions such as esterification, leading to the production of guaiacol acetate, and acylation reactions producing acetophenones, especially apocynin [98].
Collectively, these studies illustrate the nuanced interplay between catalyst properties, reagent composition and reaction pathways in the HDO of complex bio-oil blends. They emphasise the importance of catalyst design and selection based on specific feedstock compositions and desired chemical performance, highlighting the potential of tailored catalyst systems to optimise both performance and selectivity in renewable energy applications.
8. Vegetal Bio-Oil Hydrodeoxygenation over Zeolites and Non-Noble Metal Catalysts
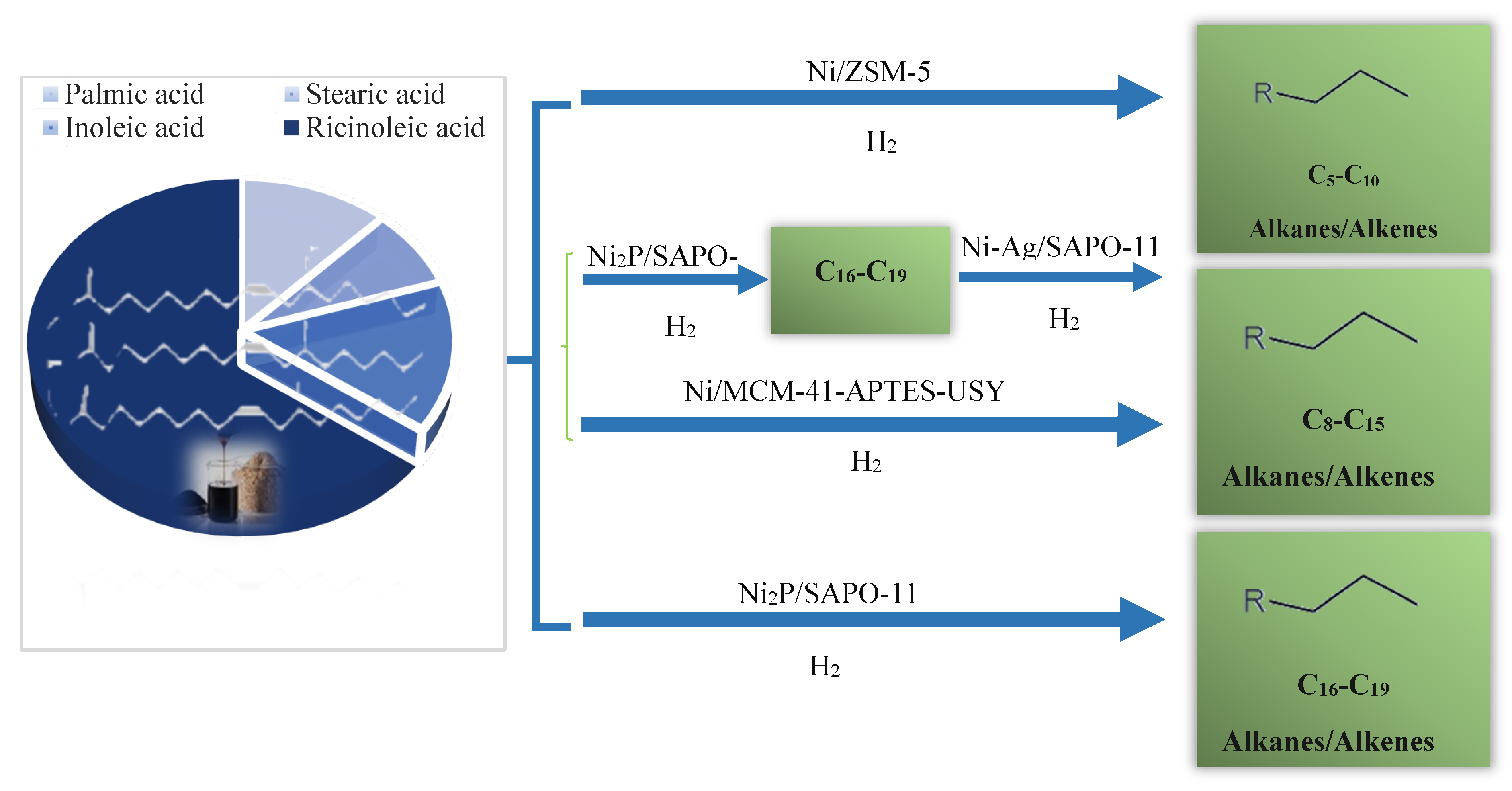
Hydrodeoxygenation from vegetable bio-oils, in particular using zeolites and non-noble metal catalysts, has demonstrated significant progress in improving the yield and quality of bio-derived fuels. For instance, Tang et al.[141] documented HDO of jatropha oil using a 5 wt.% Ni-Fe/SAPO-11 magnetic catalyst in a stainless-steel reactor, where they reported high HDO activity. The catalyst effectively produced various hydrocarbon types in the product oil, including linear alkanes (59.83%), isoparaffins (4.16%), aromatics (8.41%) and naphthenes (12.03%). In particular, the oil contained a high proportion of biofuel (C8-C16; 55.04%), and the overall deoxygenation rate achieved was 95.48% after 6 hours of reaction figure 25.
Likewise, Wu et al.[142] examined the HDO of fatty acid methyl ester (FAME) over a Ni/SAPO-11-X catalyst. They obtained a high selectivity for C15-C18 products, reaching 94%. They achieved high selectivity for C15-C18 products, reaching 94%, with the oxygen content in the feed removed mainly in the form of CO2 and CO. In comparison to a lower performance using a Pt/Al2O3-SAPO-11 catalyst[143], indicating the performance of the Ni-based catalyst in promoting deoxygenation.
Figure 22.
Hydroprocessing castor oil by Ni-based bifunctional catalysts with variable acidity, adapted from [144].
Figure 22.
Hydroprocessing castor oil by Ni-based bifunctional catalysts with variable acidity, adapted from [144].
Additional investigations by Luo et al.[145] on the HDO of Jatropha curcas oil with Ni2P/MCM-41 and Ni2P/Zr-MCM-41 catalysts at 20 wt% in a trickling bed reactor resulted in high HDO activity. Significant conversion rate reached 93.90%, and the product oil was predominantly composed of linear paraffins (85.36%), among which the diesel fraction (C15-C20) exceeded 61.90%, especially when using the Ni2P/Zr-MCM-41 catalyst with a Ni2P loading of 20%. (
https://www.sciencedirect.com/science/article/pii/S096195342300226X)
Following these results, another study focused on the synthesis of aviation biofuel by HDO of Jatropha oil on nickel-based bimetallic catalysts in a fixed-bed reactor[146]. This process resulted in a high yield of aviation range alkanes (C6-C18) at 63.5 wt.%, with smaller fractions of i-paraffins, aromatics and naphthenes. The predominant products, C15-C18 alkanes, were formed through hydrodeoxygenation, decarbonylation or decarboxylation reactions, while the i-paraffins and aromatics were products of isomerisation and aromatisation reactions.
These studies collectively demonstrate the promising potential of using specific zeolite supported non-noble and bimetallic catalysts for the efficient production of high-value deoxygenated biofuels. The selection of catalyst composition and operating conditions plays a crucial role in determining the yield, product distribution and overall efficiency of the HDO process, underlining the importance of developing tailor-made catalysts for the production of renewable fuels.
9. True Bio-Oil Hydrodeoxygenation over Zeolites and Non-Noble Metal Catalysts
HDO of biomass-derived pyrolysis oils has attracted increasing interest from researchers aiming to improve the quality and utility of bio-oils. A remarkable investigation on the HDO of fast pyrolysis beech wood oil (FPBO) using a nickel-based catalyst revealed the propensity for higher gas production at elevated temperatures, showing remarkable yields of carbon dioxide (by decarboxylation) and methane (by cleavage of the C-C bond) [63]. Gas chromatographic analyses indicated complete conversion of ketones, furfural and aldehydes, while aromatic compounds remained stable. In particular, the study showed that bimetallic nickel-chromium catalysts altered the properties of the upgraded bio-oil more efficiently than monometallic nickel catalysts, achieving a yield improvement of up to 42%.
Following the topic of effective catalysts in HDO processes, Ismail et al. [147] reported the selective formation of fuel components such as BTX (benzene, xylene, toluene) using a Ni/Ni-H-Beta zeolite catalyst in a multi-stage catalytic pyrolysis reactor. The operational optimal conditions identified were 400°C, an initial hydrogen pressure of 4 MPa and a reaction time of 20 minutes, yielding a dominant product set of alkylated benzenes and BTX compounds in the range of C7 - C9 hydrocarbons, constituting approximately 75% of the products. The resulting bio-oil showed a significant increase in the higher heating value (HHV), from about 20 MJ/kg in its original state to 41.5 MJ/kg after upgrading.
Further understanding of bio-oil upgrading, supplementary investigations with a Ni2P/HZSM-5 catalyst demonstrated effective deoxygenation pathways for crude bio-oil from beechwood pyrolysis [25]. The catalyst, with a Ni:P ratio of 1:2 and a Ni2P content of 5%, showed superior performance, resulting in the production of a bio-oil rich in aromatic hydrocarbons and phenolic compounds (
Figure 23), suitable for use as a gasoline additive. This improved oil also showed a higher generation of gases such as CO, CH4 and CO2, similar results were obtained by other authors such as Wang et al.[23,102].
In another study, Shafaghat et al.[148] examined the HDO of crude bio-oil using supercritical fluids (ethanol, methanol, 2-propanol) in a high-pressure batch reactor equipped with a nickel catalyst supported on HBeta zeolite (10 wt.%). The use of supercritical methanol was especially effective, increasing the calorific value of the crude bio-oil from 12.61 MJ/kg to 24.17 MJ/kg. The maximum deoxygenation attained under these conditions was 46.37%, with a HHV reaching 26.04 MJ/kg at a hydrogen pressure of 20 bar and a reaction duration of 4 hours.
These studies provide a collective illustration of advances in catalyst design and operating parameters that significantly influence the efficiency and outcomes of the HDO process. By optimising these factors, researchers can improve the conversion of crude bio-oils into fuels of higher quality, utility and economic value.
10. Catalyst Deactivation
In the hydrodeoxygenation (HDO) process, catalyst deactivation poses a considerable challenge, mainly due to mechanisms such as carbonaceous deposition, sintering and poisoning of catalytic sites. These phenomena affect the performance of HDO catalysts by reducing their effective surface area and altering their chemical functionality. In particular, the presence of nitrogen-, sulphur- and phosphorus-containing compounds from the biomass, together with the role of inherent and reaction produced water in the bio-oil, contribute to catalyst instability at high temperatures [123]. These types of deactivation will be developed further in the following sections.
10.1. Deactivation due to Coking
Coke formation occurs as a result of the polymerisation of unsaturated hydrocarbons and the polycondensation of oxygenated organic compounds, causing coke deposits to form on the active sites of the catalyst and blocking the pores. This phenomenon is particularly problematic for materials with small pores, such as zeolites, which limit access to the reactants. With their strong interactions with active metal sites and oxide supports, phenolic compounds play an important role as precursors to coke formation [88,102,114].
The catalyst nature, types of bio-oil compounds or model compounds [149], and obtained intermediates, as well as reaction conditions such as temperature, pressure and time, considerably influence coke formation. Additionally, compounds containing more than one oxygen atom greatly contribute to coke formation due to their propensity for self-polymerization[149]. For example, He et al.[116] reported catalyst deactivation due to coke formation in the HDO of anisole over Ni-Mo/SiO2 at 410°C and 0.1 MPa, where the high temperatures led to the production of undesirable by-products such as biphenyl and anthracene, the main coke precursors.
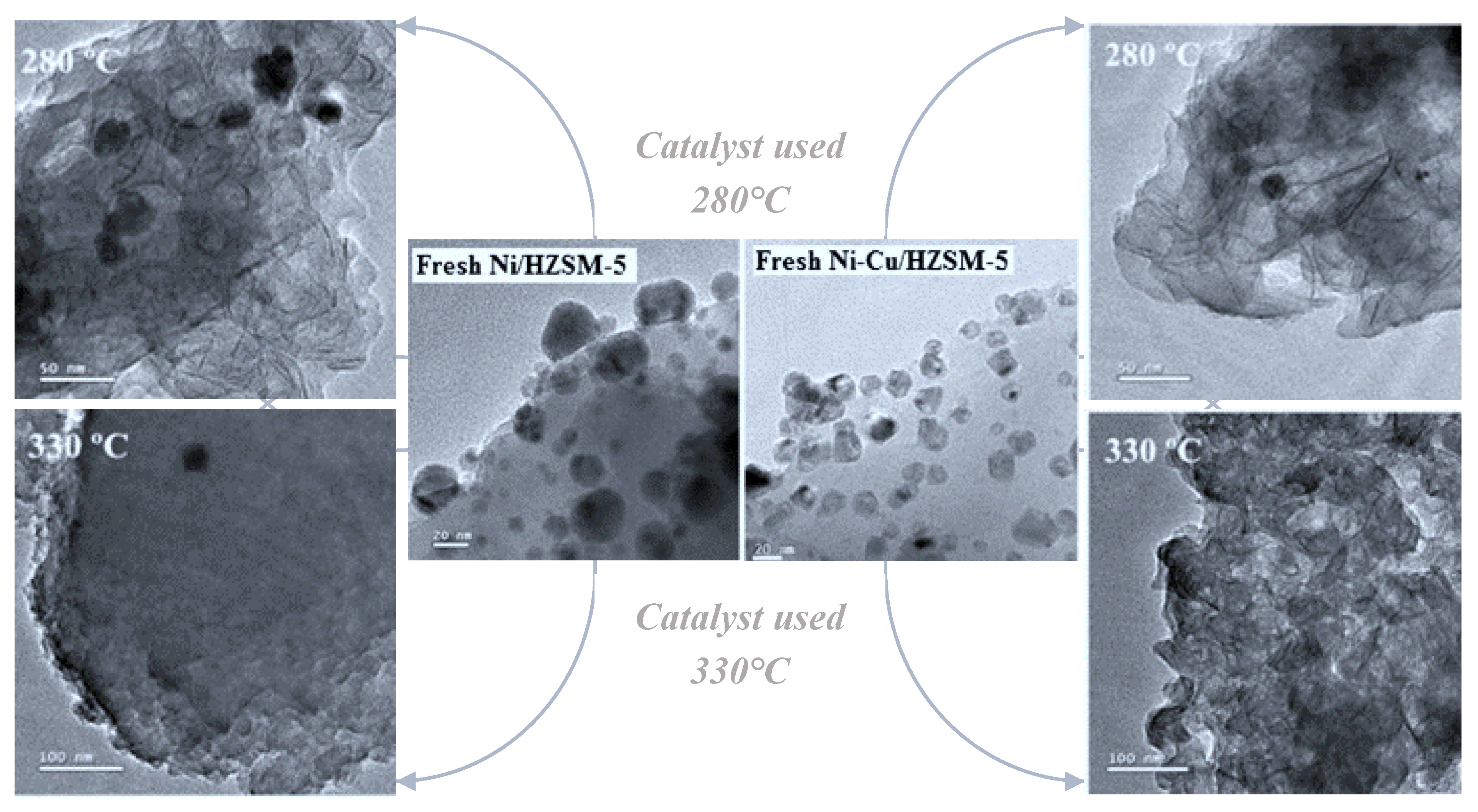
Additionally, both the Lewis and Brønsted acid sites of zeolite-supported catalysts are susceptible to deactivation by coke formation. The strong acid sites and the confined porous structure of these catalysts promote conditions propitious for coke deposition. Li et al. [150] examined the mechanisms of coke formation on two specific catalysts, Ni/HZSM-5 and Ni-Cu/HZSM-5 (
Figure 24), during HDO of lignin-derived bio-oil at 250 to 330°C under 2 MPa hydrogen pressure. The authors reported a drastic reduction in catalytic performance due to coke formation on the catalyst surfaces, attributed to the high acidity of the HZSM-5 zeolite. Under this environment, Lewis acid sites rapidly become capped with oxygenated hydrocarbons, while Brønsted acid sites donate protons to these oxygenates, facilitating the formation of carbocations, precursors of soluble coke according to [151].
Additionally, operating conditions such as temperature, H2 pressure and contact time, along with post-reaction treatments like calcination and reduction, significantly influence coke formation during hydroprocessing (
Figure 5). For instance, low H2 pressure and reaction temperature are known to predispose the process towards coke formation rather than fostering the desired hydrogenation reactions. Li et al. [152] investigated the effect of reaction temperature on coke formation, finding that elevated temperatures resulted in severe coke formation despite improvements in HDO efficiency.
Furthermore, previous treatments such as calcination and reduction can regenerate the spent catalyst, restoring its activity almost to the levels of a fresh catalyst. In their study, Zhu et al. Investigated coke formation using a Pt/H-Beta catalyst during the HDO of anisole at 400°C and atmospheric pressure. They revealed that the acidic nature of the catalyst promoted coke formation; however, the addition of metal to the acidic zeolite improved the catalyst stability and moderately reduce coke formation due to its strong hydrogenation activity[67]. Addictionally, maintaining a high hydrogen partial pressure during HDO has been showed to reduce coke formation[153].
Infantes-Molina et al.[154] investigated a comparative study on coke formation between a cobalt phosphide catalyst and a nickel phosphide catalyst during HDO of dibenzofuran DBF. Their findings showed that although both catalysts possessed high acid sites densities, Ni2P catalyst showed both a greater HDO activity and fewer deactivation attributed to a higher quantity of weak acidic sites. In contrast, in the cobalt phosphide catalyst, the substantial presence of strongly acidic sites caused severe coke formation, reducing its HDO activity. This comparative analysis emphasises the significant impact of the acid strength of the catalysts on the degree of coke formation, underscoring the importance of carefully selecting and designing catalysts to minimise deactivation and maximise process efficiency in hydrodeoxygenation operations.
10.1. Deactivation due to Sintering
Sintering or thermal aging of the catalyst, is a critical phenomenon that occurs as the reaction temperature increases due to the exothermic nature of HDO process. This process is characterized by loss of catalytic surface area resulting from the growth of crystallites, pores collapses inside these crystallites, or degradation and structural collapse of the support material [64,149] . Although not the main focus of much research, notable observations have been made on the catalytic performance evolution under conditions that favour sintering.
Ni catalysts supported on acidic supports, such as transition metal oxides (Al2O3, TiO2), exhibit higher thermal stability compared to those supported on non-acidic or weak acidic supports such as SiO2 [119]. This enhanced stability is attributed to the interactions between Ni and the support material. Lan et al. [121, p. 2] explored the sintering of active Ni on Ni2P/SiO2 during HDO of guaiacol at 300°C, they observed a significant reduction in catalytic activity, where conversion rates decreased from 100% to 10% in as little as 8 hours. This drastic loss underscores the susceptibility of certain catalysts to thermal degradation under HDO conditions.
Additionally, Ni-doped mesoporous supports, such as Al-SBA-15, Ti-SBA-15, Al-MCM-41 and Ti-MCM-41, provide a dual advantage that mitigates some sintering effects: the enhancement of Ni-support interactions and the confinement offered by the pore size, which limits the crystallite and atom migration and reduces the rate of crystallite growth as they approach the pore diameters [64]. These characteristics are essential for maintaining the structural integrity and activity of Ni-based catalysts subjected to thermal stress during HDO, illustrating the importance of selecting suitable support materials to improve catalyst longevity and efficiency.
10.1. Deactivation due to Poisoning
The poisoning of active sites on catalyst surface is a key factor in catalyst performance and is influenced by the adsorption strength of chemical species competing with the reactant for these sites[64].Poisoning occurs through the strong chemisorption of various chemical species - including reagents, intermediates, products and impurities - on the catalytic sites [155]. In lignin-derived bio-oil HDO, common poisons include oxygen-containing compounds such as water and CO. In particular, water, which is produced during the HDO process, can significantly reduce the efficiency of the catalyst by competing with the reagents for adsorption on the active sites of the catalyst [156].
Recent studies have revealed the detrimental effects of water poisoning on catalytic performance. Water not only adsorbs on active sites, but can also alter the chemical structures of these sites, leading to a decreased catalytic activity. For instance, Li et al.[117] investigated the HDO of anisole at 300°C and 1.5 MPa using NiP/SiO2 and NiMoP/SiO2 catalysts and revealed that the water generated as a by-product led to the formation of nickel metal oxides and phosphate oxides (resulting from the oxidation of NiP), which are significantly less active than the original phosphide forms. In addition, Mortensen et al.[157] also reported that in their phenol and octanol HDO deactivation studies, water caused the deactivation of Ni-MoS2/ZrO2 catalysts by undergoing competitive adsorption on the active sites, which facilitated the conversion of sulphide to sulphate at the edges of the MoS2 catalyst.
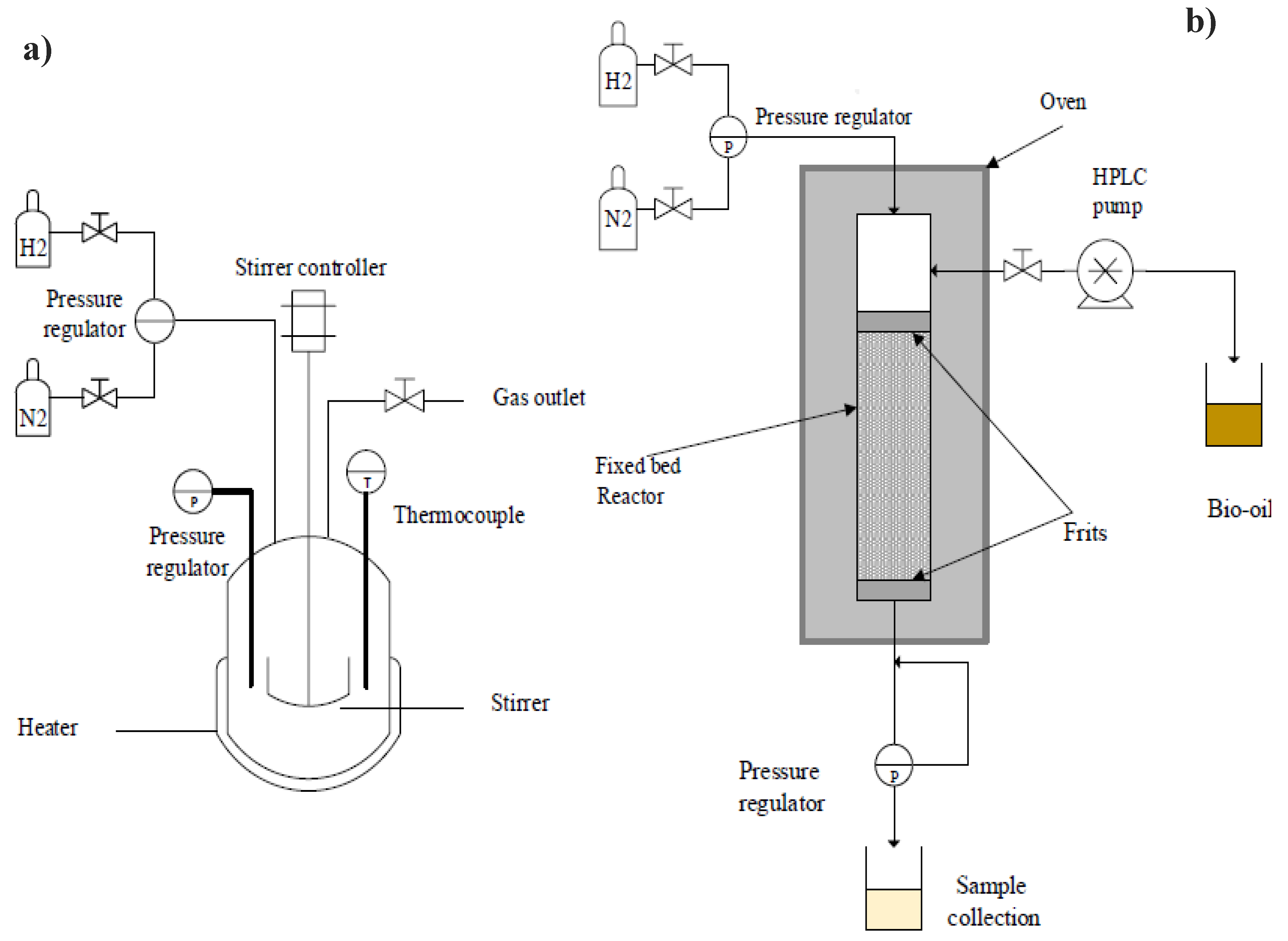
11. Hydrodeoxygenation Set Up
HDO process is typically carried out at reaction temperatures between 250 and 500 °C [88,158,159] and high hydrogen pressures in various reactor configurations, such as batch reactors in the liquid phase (
Figure 16a) and continuous flow fixed-bed reactors in gaz phase operating between 5 and 50 MPa [125,158]. And in the case of a continuous reactor, liquid hourly space velocity values (LHSV) between 0.05 and 2 h-1. For the reaction parameters in both the batch and continuous set-up, the temperature and presure were generally proved to be a key factor in governing the final oxygen content of the upgraded bio-oil.
Bukhtiyarova et al. [158] highlighted the fundamental disparities between batch and continuous flow reactors, outlining their distinctive operating characteristics and their implications for catalytic processes. A batch reactor, depicted in
Figure 16a, functions as a transitory reactor in which the reaction mixture and catalyst are loaded and subsequently submitted to high-temperature and high-pressure conditions. Contrarily, a continuous flow reactor (
Figure 16b) functions as a steady-state reactor, in which reactants are continuously fed into the reactor entrance and traverse the catalyst bed. Particularly, after the reaction, the unreacted reactant/product mixture exits the reactor outlet, distinguishing it from the batch reactor. The comparison between batch and continuous flow reactors underscores crucial differences in their operational dynamics and implications for catalytic processes
Table 5.
Figure 25.
Schematic diagram of the most common reactors used in the HDO ; batch reactor (a) and continuous flow reactor (b), adapted from[158,159].
Figure 25.
Schematic diagram of the most common reactors used in the HDO ; batch reactor (a) and continuous flow reactor (b), adapted from[158,159].
Usually, researchers like [89,158,160] advocate for conducting reactions in continuous flow rea Usually, researchers like [89,158,160] advocate for conducting reactions in continuous flow reactors due to numerous advantages over batch reactors, such as better mixing of reagents, superior interfacial mass and energy transfer characteristics, reduced operational costs, mitigation of byproduct generation through enhanced control over reaction parameters, simplified scalability, and heightened process safety. When applied to the HDO of bio-oil, continuous reactors have demonstrated distinct characteristics, including a tendency toward relatively higher temperatures and H2 consumption. Despite these variances, continuous reactors exhibit a propensity for achieving elevated DOD rates, primarily attributed to the generation H2O as a byproduct during the reaction.ctors due to numerous advantages over batch reactors, such as better mixing of reagents, superior interfacial mass and energy transfer characteristics, reduced operational costs, mitigation of byproduct generation through enhanced control over reaction parameters, simplified scalability, and heightened process safety. When applied to the HDO of bio-oil, continuous reactors have demonstrated distinct characteristics, including a tendency toward relatively higher temperatures and H2 consumption. Despite these variances, continuous reactors exhibit a propensity for achieving elevated DOD rates, primarily attributed to the generation H2O as a byproduct during the reaction.
Additionally, continuous reactors typically yield lower levels of coke formation, indicating their potential to deliver more sustainable and efficient bio-oil upgrading processes. Consequently, it is plausible that the utilization of continuous reactors may lead to the production of superior quality upgraded bio-oil and sustain prolonged catalyst activity. Leveraging the benefits conferred by continuous reactors holds promise for optimizing the efficacy and sustainability of bio-oil refining technologies, thereby contributing to the advancement of renewable energy solutions.
12. Conclusion
Biomass, derived from plants or animals, undergoes fast pyrolysis to yield pyrolytic bio-oil, containing 30-40 wt% organic chamicals and 25 wt% water. Challenges like high water content, acidity, oxygen proportion, and low calorific value hinder direct use. Hydrodeoxygenation (HDO) is crucial as it saturates aromatic components and alkenes, raising the bio-oil’s calorific value by enhancing the H/C ratio and reducing the O/C ratio, removing oxygen as water. Despite advancements, biomass pyrolysis oils require further research, catalyst optimization, and understanding of complex reactions routes (decarbonylation, hydrogenation, cracking, hydrocracking) that play a crucial role for determining the quality and selectivity of the final upgraded bio-oil products before practical applications. Successful HDO hinges on suitable catalysts and model compounds, pivotal for enhancing biofuel properties and advancing sustainable energy solutions.
Catalytic carriers are essential for HDO, facilitating active phase dispersion and providing essential functional sites for effective deoxygenation reactions. Various catalyst types including sulfides (e.g., CoMoS2, NiMoS2), oxides (e.g., MoOx, NiOx), carbides (e.g., Mo2C), phosphides (e.g., MoP), and noble metals (e.g., Pt, Pd) have been assessed for HDO efficacy. Sulphide catalysts operate via electron transfer, necessitating sulfur sources for optimal performance, while oxide and bimetallic catalysts like Pt-WOx/Al2O3 use acid sites to enhance activity. Transition metals such as Pt, Pd, and Ni offer sulfur-free pathways but are susceptible to sulfur contamination. Ni2P distinguishes itself with high electronic conductivity and chemical durability, eliminating the need for additional sulfur sources. Phosphorus in Ni2P enhances hydrogenation and fosters Brønsted acid sites, crucial for efficient deoxygenation, showing superior performance in converting bio-oil model compounds with high selectivity and stability. Nickel-modified catalysts, especially Ni2P on supports like ZSM-5 or SBA-15, demonstrate enhanced efficiency and stability, highlighting Ni2P’s role in advancing HDO technology.
The HDO of bio-oil involves complex reaction pathways, which researchers are attempting to clarify using model compounds representing key constituents of bio-oil. Studies on phenolic compounds such as guaiacol reveal various pathways, such as hydrogenation and deoxygenation, that influence the selectivity of products such as cycloalkanes or arenes. Research on aldehydes such as furfural illustrates multiple pathways, such as hydrogenolysis and decarbonylation, which influence products such as 2-methylfuran. Carboxylic acids, such as acetic acid, present pathways such as ketonisation and hydrogenolysis, where catalyst conditions affect the distribution of products. Mixtures present additional challenges; for example, hydroxyl-containing molecules influence pathways and selectivity, while combinations such as guaiacol and propionic acid show potential for simultaneous hydrodeoxygenation and esterification. Catalyst design and understanding of reaction mechanisms are crucial for optimising HDO, where reactor conditions such as temperature and pressure improve conversion rates and selectivity, while acidity facilitates reactions such as esterification and hydrolysis. Higher temperatures favor certain reactions like hydrogenation, while higher hydrogen pressures enhance hydrogenation processes, leading to improved conversion efficiencies.
In HDO, catalyst deactivation involves coking, sintering and poisoning of the catalytic sites. Coking is a consequence of hydrocarbon polymerisation, which is influenced by the type of catalyst, bio-oil composition and conditions. Catalysts supported by zeolite are susceptible due to the strong acid sites and their confined structure. Sintering reduces the surface area at higher temperatures, which is achieved with Ni doped mesoporous supports. Poisoning by species such as water and CO decreases catalytic efficiency and alters active site structures. Coking dominates, requiring adaptation of catalyst design and operations to mitigate its impact. HDO challenges include gas-liquid phase equilibrium at elevated temperatures and pressures, accelerated coke formation by highly acidic catalysts, and the influence of pore size on reaction rate. Bio-oil viscosity poses equipment problems, catalyst recycling remains problematic and L- glucose appears as a coke precursor. These complexities require further research to optimise HDO processes.
A comparison between batch and continuous flow reactors highlights the operational differences affecting the catalytic processes. Continuous flow reactors offer advantages such as improved mixing, parameter control and increased safety, achieving higher HDO rates and reduced coke formation compared to batch systems. Continuous reactors promise sustainable upgrading of bio-oil, leveraging prolonged catalyst activity and efficiency to advance renewable energy solutions.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
ALCC: Aldol crossed-condensation
ALSC: Aldol Selt-condensation
BXT: Benzene, Xylene, Toluene
CGE: Carbon gasification efficiency
CSO: Camelina oil
DBF: Dibenzofuran
DDO: Direct deoxygenation
DHO : Dehydroxylation
DHY: Dehydratation
DOD: Degree deoxygenation
DME : Demethylation
DMO: Demethoxylation
EEO: Ether extracted bio-oil
FAME: Fatty acid methyl ester
FPBO: Beech wood fast pyrolysis-oil
HBA: Hydrogen bond acceptor
HBD: Hydrogen bond donor
HDO: Hydrodeoxygenation
HDN: Hydrodenitrogenation
HGD : Hydrogenolysis
HGE: Hydrogen gasification efficiency
HHV: High heating value
HLB: Hydrophilic-lipophilic balance
HMF: 5-hydroxymethyl furfural
HMTHFA: 5-(hydroxymethyl) tetrahydrofuran-2-carbaldehyde
HYD: Hydrogenation
LHV: Low Heating value
MCH: Methylcyclohexane
MSW: Municipal solid waste
Ni2P: Nickel phosphide
OVOCs: oxygen-containing volatile organic compounds
SCF: Supercritical fluid
SR: Steam reforming
TAN: Total acid number
THF: Tetrahydrofuran
TPR: Temperature-programmed reaction
WHSV: Weight hourly space velocity
WGS: Water gas shift
TEM: Transmission electron microscopy
References
- A. Tshikovhi et M. Tshwafo, « Technologies and Innovations for Biomass Energy Production », Sustainability, vol. 15, p. 12121, août 2023. [CrossRef]
- « World Energy Transitions Outlook 2023 ». Consulté le: 19 avril 2024. [En ligne]. Disponible sur: https://www.irena.org/Digital-Report/World-Energy-Transitions-Outlook-2023.
- « The Times ‘Future of Energy’: We can humanise energy, and we must do so urgently », World Energy Council. Consulté le: 17 février 2022. [En ligne]. Disponible sur: https://www.worldenergy.org/news-views/entry/the-times-future-of-energy-we-can-humanise-energy-and-we-must-do-so-urgently.
- « The Oil and Gas Industry in Energy Transitions – Analysis », IEA. Consulté le: 19 avril 2024. [En ligne]. Disponible sur: https://www.iea.org/reports/the-oil-and-gas-industry-in-energy-transitions.
- BP, « Statistical Review of World Energy 2021 », no 70, p. 72, juill. 2021.
- Renewables 2021, « Renewables Global Status Report », REN21. Consulté le: 23 février 2022. [En ligne]. Disponible sur: https://www.ren21.net/reports/global-status-report/.
- D. Kaloudas, N. Pavlova, et R. Penchovsky, « Lignocellulose, algal biomass, biofuels and biohydrogen: a review », Environ Chem Lett, vol. 19, no 4, p. 2809-2824, août 2021. [CrossRef]
- « What is biomass? Advantages and Disadvantages - Aquae Foundation ». Consulté le: 24 février 2022. [En ligne]. Disponible sur: https://www.fundacionaquae.
- S. Bardhan, S. Gupta, M. E. Gorman, et A. Haider, « Biorenewable chemicals: Feedstocks, technologies and the conflict with food production », Renewable and Sustainable Energy Reviews, vol. 51, nov. 2015. [CrossRef]
- C. González Rebollar, « Hidrodesoxigenación de compuestos aromáticos oxigenados sobre catalizadores de metal precioso soportado ». 2015. [En ligne]. Disponible sur: https://www.google.com/search?q=hidrodeoxigenacion+de+compuestos+aromaticos&rlz=1C1GCEA_en&oq=HIDRODEOXIGENA&aqs=chrome.0.69i59j69i57.8759j0j7&sourceid=chrome&ie=UTF-8.
- D. Barik, « Chapter 3 - Energy Extraction From Toxic Waste Originating From Food Processing Industries », in Energy from Toxic Organic Waste for Heat and Power Generation, D. Barik, Éd., in Woodhead Publishing Series in Energy., Woodhead Publishing, 2019, p. 17-42. [CrossRef]
- S. Nanda et F. Berruti, « A technical review of bioenergy and resource recovery from municipal solid waste », Journal of Hazardous Materials, vol. 403, p. 123970, févr. 2021. [CrossRef]
- V. N. Bui, D. Laurenti, P. Delichère, et C. Geantet, « Hydrodeoxygenation of guaiacol: Part II: Support effect for CoMoS catalysts on HDO activity and selectivity », Applied Catalysis B: Environmental, vol. 101, no 3, p. 246-255, janv. 2011. [CrossRef]
- A. Bridgwater, « Fast pyrolysis of biomass for the production of liquids », Biomass Combustion Science, Technology and Engineering, p. 130-171, avr. 2013. [CrossRef]
- C. Mohabeer, L. Abdelouahed, S. Marcotte, et B. Taouk, « Comparative analysis of pyrolytic liquid products of beech wood, flax shives and woody biomass components », Journal of Analytical and Applied Pyrolysis, vol. 127, juill. 2017. [CrossRef]
- J. A. Oyebanji, P. Okekunle, O. Lasode, et S. Oyedepo, « Chemical composition of bio-oils produced by fast pyrolysis of two energy biomass », Biofuels, vol. 9, p. 1-9, févr. 2017. [CrossRef]
- A. M. Berenguer Ruiz, « “Hidrodesoxigenación catalítica de bio-oils de pirólisis sobre fosfuros metálicos soportados” ». 2017.
- A. Dimitriadis et S. Bezergianni, « Hydrothermal liquefaction of various biomass and waste feedstocks for biocrude production: A state of the art review », Renewable and Sustainable Energy Reviews, vol. 68, p. 113-125, févr. 2017. [CrossRef]
- P. Lahijani, M. Mohammadi, A. R. Mohamed, F. Ismail, K. T. Lee, et G. Amini, « Upgrading biomass-derived pyrolysis bio-oil to bio-jet fuel through catalytic cracking and hydrodeoxygenation: A review of recent progress », Energy Conversion and Management, vol. 268, p. 115956, sept. 2022. [CrossRef]
- J. Ellens, « Design, optimization and evaluation of a free-fall biomass fast pyrolysis reactor and its products », Master of Science, Iowa State University, Digital Repository, Ames, 2009. [CrossRef]
- N. L. Panwar et A. S. Paul, « An overview of recent development in bio-oil upgrading and separation techniques », Environmental Engineering Research, vol. 26, no 5, oct. 2021. [CrossRef]
- T. Shan Ahamed, S. Anto, T. Mathimani, K. Brindhadevi, et A. Pugazhendhi, « Upgrading of bio-oil from thermochemical conversion of various biomass – Mechanism, challenges and opportunities », Fuel, vol. 287, p. 119329, mars 2021. [CrossRef]
- J. Wang, « Catalytic hydro-deoxygenation of bio-oil from biomass pyrolysis: comprehension of reaction pathways ».
- M. Zhang et al., « A review of bio-oil upgrading by catalytic hydrotreatment: Advances, challenges, and prospects », Molecular Catalysis, vol. 504, p. 111438, mars 2021. [CrossRef]
- J. WANG, « Catalytic hydro-deoxigenation of model molecules and bio-oil from biomass pyrolysis: comprehension of reaction pathways », Institut National des sciences appliquées, INSA Rouen, INSA Rouen, 2020.
- J. A. Martin, C. A. Mullen, et A. A. Boateng, « Maximizing the Stability of Pyrolysis Oil/Diesel Fuel Emulsions », Energy Fuels, vol. 28, no 9, p. 5918-5929, sept. 2014. [CrossRef]
- Q. Lu, X. Zhu, W. Li, Y. Zhang, et D. Chen, « On-line catalytic upgrading of biomass fast pyrolysis products », Sci. Bull., vol. 54, no 11, p. 1941-1948, juin 2009. [CrossRef]
- Y. Y. Chong et al., Emulsification of Bio-Oil and Diesel, vol. 56. 2017. [CrossRef]
- M. Ikura, « Emulsification of pyrolysis derived bio-oil in diesel fuel », Biomass and Bioenergy, vol. 24, no 3, p. 221-232, mars 2003. [CrossRef]
- H. Sanli, E. Alptekin, et M. Canakci, « Using low viscosity micro-emulsification fuels composed of waste frying oil-diesel fuel-higher bio-alcohols in a turbocharged-CRDI diesel engine », Fuel, vol. 308, p. 121966, janv. 2022. [CrossRef]
- A. Farooq, H. Shafaghat, J. Jae, S.-C. Jung, et Y.-K. Park, « Enhanced stability of bio-oil and diesel fuel emulsion using Span 80 and Tween 60 emulsifiers », Journal of Environmental Management, vol. 231, p. 694-700, févr. 2019. [CrossRef]
- F. Mustan, N. Politova-Brinkova, D. Rossetti, P. Rayment, et S. Tcholakova, « Oil soluble surfactants as efficient foam stabilizers », Colloids and Surfaces A: Physicochemical and Engineering Aspects, vol. 633, p. 127874, janv. 2022. [CrossRef]
- H. Wang et J. Liu, « Emulsification and corrosivity study of bio-oil and vacuum gas oil mixtures with a novel surfactant system », Fuel, vol. 333, p. 126460, févr. 2023. [CrossRef]
- M. Y. Lim et J. R. Stokes, « Lubrication of non-ionic surfactant stabilised emulsions in soft contacts », Biotribology, vol. 28, p. 100199, déc. 2021. [CrossRef]
- Y. Yang et al., « Synthesis of hierarchical ZSM-5 zeolites templated by sodium alginate toward enhanced catalytic activity for esterification », Journal of Solid State Chemistry, vol. 292, p. 121686, déc. 2020. [CrossRef]
- J. Wang, J. Chang, et J. Fan, « Catalytic esterification of bio-oil by ion exchange resins », Journal of Fuel Chemistry and Technology, vol. 38, no 5, p. 560-564, oct. 2010. [CrossRef]
- Y. Y. Chong, S. Thangalazhy-Gopakumar, S. Gan, L. Y. Lee, et H. K. Ng, « Esterification and neutralization of bio-oil from palm empty fruit bunch fibre with calcium oxide », Bioresource Technology Reports, vol. 12, p. 100560, déc. 2020. [CrossRef]
- P. Prasertpong, C. Jaroenkhasemmeesuk, J. R. Regalbuto, J. Lipp, et N. Tippayawong, « Optimization of process variables for esterification of bio-oil model compounds by a heteropolyacid catalyst », Energy Reports, vol. 6, p. 1-9, nov. 2020. [CrossRef]
- S. F. J. C. Mai Attia, « Upgrading of Oils from Biomass and Waste: Catalytic Hydrodeoxygenation », MDPI, p. 28, août 2020. [CrossRef]
- A. Oasmaa, E. Kuoppala, J.-F. Selin, S. Gust, et Y. Solantausta, « Fast Pyrolysis of Forestry Residue and Pine. 4. Improvement of the Product Quality by Solvent Addition », Energy Fuels, vol. 18, no 5, p. 1578-1583, sept. 2004. [CrossRef]
- L. K.-E. Park, S. Ren, S. Yiacoumi, X. P. Ye, A. P. Borole, et C. Tsouris, « Separation of Switchgrass Bio-Oil by Water/Organic Solvent Addition and pH Adjustment », Energy Fuels, vol. 30, no 3, p. 2164-2173, mars 2016. [CrossRef]
- G. Feng, Z. Liu, P. Chen, et H. Lou, « Influence of solvent on upgrading of phenolic compounds in pyrolysis bio-oil », RSC Adv., vol. 4, no 91, p. 49924-49929, oct. 2014. [CrossRef]
- M. Abou Rjeily, C. Gennequin, H. Pron, E. Abi-Aad, et J. H. Randrianalisoa, « Pyrolysis-catalytic upgrading of bio-oil and pyrolysis-catalytic steam reforming of biogas: a review », Environ Chem Lett, vol. 19, no 4, p. 2825-2872, août 2021. [CrossRef]
- R. K. Gollakota et al., « Catalytic hydrodeoxygenation of bio-oil and model compounds - Choice of catalysts, and mechanisms », Renewable and Sustainable Energy Reviews, vol. 187, p. 113700, nov. 2023. [CrossRef]
- Y. Ding et al., « Recyclable regeneration of NiO/NaF catalyst: Hydrogen evolution via steam reforming of oxygen-containing volatile organic compounds », Energy Conversion and Management, vol. 258, p. 115456, avr. 2022. [CrossRef]
- S. Douvartzides, N. D. Charisiou, W. Wang, V. G. Papadakis, K. Polychronopoulou, et M. A. Goula, « Catalytic fast pyrolysis of agricultural residues and dedicated energy crops for the production of high energy density transportation biofuels. Part I: Chemical pathways and bio-oil upgrading », Renewable Energy, vol. 185, p. 483-505, févr. 2022. [CrossRef]
- R. Xing et al., « Steam reforming of fast pyrolysis-derived aqueous phase oxygenates over Co, Ni, and Rh metals supported on MgAl2O4 », Catalysis Today, vol. 269, p. 166-174, juill. 2016. [CrossRef]
- N. Chaihad, S. Karnjanakom, A. Abudula, et G. Guan, « Zeolite-based cracking catalysts for bio-oil upgrading: A critical review », Resources Chemicals and Materials, avr. 2022. [CrossRef]
- N. Chaihad et al., « Catalytic upgrading of bio-oils over high alumina zeolites », Renewable Energy, vol. 136, p. 1304-1310, juin 2019. [CrossRef]
- W. N. R. W. Isahak, M. W. M. Hisham, M. A. Yarmo, et T. Yun Hin, « A review on bio-oil production from biomass by using pyrolysis method », Renewable and Sustainable Energy Reviews, vol. 16, no 8, p. 5910-5923, oct. 2012. [CrossRef]
- R. Kumar, V. Strezov, T. Kan, H. Weldekidan, J. He, et S. Jahan, « Investigating the Effect of Mono- and Bimetallic/Zeolite Catalysts on Hydrocarbon Production during Bio-oil Upgrading from Ex Situ Pyrolysis of Biomass », Energy Fuels, vol. 34, no 1, p. 389-400, janv. 2020. [CrossRef]
- Kurnia et al., « In-situ catalytic upgrading of bio-oil derived from fast pyrolysis of lignin over high aluminum zeolites », Fuel Processing Technology, vol. 167, p. 730-737, déc. 2017. [CrossRef]
- « The reduction and control technology of tar during biomass gasification/pyrolysis: An overview ». Consulté le: 15 juillet 2022. [En ligne]. Disponible sur: https://ideas.repec.org/a/eee/rensus/v12y2008i2p397-416.html.
- Y. H. Chan, S. Yusup, A. T. Quitain, Y. Uemura, et S. K. Loh, « Fractionation of pyrolysis oil via supercritical carbon dioxide extraction: Optimization study using response surface methodology (RSM) », Biomass and Bioenergy, vol. 107, p. 155-163, déc. 2017. [CrossRef]
- W. Li et al., « Upgrading of low-boiling fraction of bio-oil in supercritical methanol and reaction network », Bioresource Technology, vol. 102, no 7, p. 4884-4889, avr. 2011. [CrossRef]
- P.-G. Duan, S.-C. Li, J.-L. Jiao, F. Wang, et Y.-P. Xu, « Supercritical water gasification of microalgae over a two-component catalyst mixture », Science of The Total Environment, vol. 630, p. 243-253, juill. 2018. [CrossRef]
- W. W. Kazmi, J.-Y. Park, G. Amini, et I.-G. Lee, « Upgrading of esterified bio-oil from waste coffee grounds over MgNiMo/activated charcoal in supercritical ethanol », Fuel Processing Technology, vol. 250, p. 107915, nov. 2023. [CrossRef]
- J.-H. Lee, G. Amini, J.-Y. Park, et I.-G. Lee, « Supercritical ethanol-assisted catalytic upgrading of bio-tar using mesoporous SBA-15 supported Ni-based catalysts », Journal of the Energy Institute, vol. 114, p. 101591, juin 2024. [CrossRef]
- H. Prajitno, R. Insyani, J. Park, C. Ryu, et J. Kim, « Non-catalytic upgrading of fast pyrolysis bio-oil in supercritical ethanol and combustion behavior of the upgraded oil », Applied Energy, vol. 172, p. 12-22, juin 2016. [CrossRef]
- M. Zhang, H. Wang, X. Han, Y. Zeng, et C. C. Xu, « Catalytic HDO of pyrolysis oil in supercritical ethanol with CoMoP and CoMoW catalysts supported on different carbon materials using formic acid as in-situ hydrogen sources », Biomass and Bioenergy, vol. 174, p. 106814, juill. 2023. [CrossRef]
- R. Shomal et Y. Zheng, « Development of Processes and Catalysts for Biomass to Hydrocarbons at Moderate Conditions: A Comprehensive Review », Nanomaterials, vol. 13, p. 2845, oct. 2023. [CrossRef]
- M. Zhou et al., « MOF-derived NiM@C catalysts (M = Co, Mo, La) for in-situ hydrogenation/hydrodeoxygenation of lignin-derived phenols to cycloalkanes/cyclohexanol », Fuel, vol. 329, p. 125446, déc. 2022. [CrossRef]
- C. C. Schmitt et al., « Hydrotreatment of Fast Pyrolysis Bio-oil Fractions Over Nickel-Based Catalyst », Top Catal, vol. 61, no 15, p. 1769-1782, oct. 2018. [CrossRef]
- M. M. Ambursa, J. C. Juan, Y. Yahaya, Y. H. Taufiq-Yap, Y.-C. Lin, et H. V. Lee, « A review on catalytic hydrodeoxygenation of lignin to transportation fuels by using nickel-based catalysts », Renewable and Sustainable Energy Reviews, vol. 138, p. 110667, mars 2021. [CrossRef]
- C. Ranga, V. I. Alexiadis, J. Lauwaert, R. Lødeng, et J. W. Thybaut, « Effect of Co incorporation and support selection on deoxygenation selectivity and stability of (Co)Mo catalysts in anisole HDO », Applied Catalysis A: General, vol. 571, p. 61-70, févr. 2019. [CrossRef]
- A. Kumar, M. Jindal, S. Maharana, et B. Thallada, « Lignin Biorefinery: New Horizons in Catalytic Hydrodeoxygenation for the Production of Chemicals », Energy Fuels, vol. 35, no 21, p. 16965-16994, nov. 2021. [CrossRef]
- X. Zhu, L. L. Lobban, R. G. Mallinson, et D. E. Resasco, « Bifunctional transalkylation and hydrodeoxygenation of anisole over a Pt/HBeta catalyst », Journal of Catalysis, vol. 281, no 1, p. 21-29, juill. 2011. [CrossRef]
- P. Yan, J. Mensah, M. Drewery, E. Kennedy, T. Maschmeyer, et M. Stockenhuber, « Role of metal support during ru-catalysed hydrodeoxygenation of biocrude oil », Applied Catalysis B: Environmental, vol. 281, p. 119470, févr. 2021. [CrossRef]
- A. Gutierrez, R. K. Kaila, M. L. Honkela, R. Slioor, et A. O. I. Krause, « Hydrodeoxygenation of guaiacol on noble metal catalysts », Catalysis Today, vol. 147, no 3, p. 239-246, oct. 2009. [CrossRef]
- A. V. Vutolkina et al., « Hydrodeoxygenation of guaiacol via in situ H2 generated through a water gas shift reaction over dispersed NiMoS catalysts from oil-soluble precursors: Tuning the selectivity towards cyclohexene », Applied Catalysis B: Environmental, vol. 312, p. 121403, sept. 2022. [CrossRef]
- H. Zhu et al., « A theoretical study on hydrodeoxygenation of phenol over MoS2 supported single-atom Fe catalyst », Molecular Catalysis, vol. 530, p. 112650, sept. 2022. [CrossRef]
- V. N. Bui, D. Laurenti, P. Afanasiev, et C. Geantet, « Hydrodeoxygenation of guaiacol with CoMo catalysts. Part I: Promoting effect of cobalt on HDO selectivity and activity », Applied Catalysis B: Environmental, vol. 101, no 3, p. 239-245, janv. 2011. [CrossRef]
- J. Cao, Y. Zhang, X. Liu, C. Zhang, et Z. Li, « Comparison of Co-Mo-S and remote control model for designing efficient Co-doped MoS2 hydrodeoxygenation catalysts », Fuel, vol. 334, p. 126640, févr. 2023. [CrossRef]
- C. Wang, D. Wang, Z. Wu, Z. Wang, C. Tang, et P. Zhou, « Effect of W addition on the hydrodeoxygenation of 4-methylphenol over unsupported NiMo sulfide catalysts », Applied Catalysis A: General, vol. 476, p. 61-67, avr. 2014. [CrossRef]
- R. W. S. Lima, T. L. R. Hewer, R. M. B. Alves, et M. Schmal, « Surface Analyses of adsorbed and deposited species on the Ni-Mo catalysts surfaces after Guaiacol HDO. Influence of the alumina and SBA-15 supports. », Molecular Catalysis, vol. 511, p. 111724, juill. 2021. [CrossRef]
- T. A. Zepeda et al., « Positive phosphorous effect during co-processing of pyrolysis bio-oils and S-content model compounds over sulfide NiMo/P/HMS-Al catalysts », Fuel Processing Technology, vol. 211, p. 106599, janv. 2021. [CrossRef]
- Y. Yang, A. Gilbert, et C. (Charles) Xu, « Hydrodeoxygenation of bio-crude in supercritical hexane with sulfided CoMo and CoMoP catalysts supported on MgO: A model compound study using phenol », Applied Catalysis A: General, vol. 360, no 2, p. 242-249, juin 2009. [CrossRef]
- Y. Liu, K. Wu, X. Guo, W. Wang, et Y. Yang, « A comparison of MoS2 catalysts hydrothermally synthesized from different sulfur precursors in their morphology and hydrodeoxygenation activity », Journal of Fuel Chemistry and Technology, vol. 46, no 5, p. 535-542, mai 2018. [CrossRef]
- T. M. H. Dabros et al., « Transportation fuels from biomass fast pyrolysis, catalytic hydrodeoxygenation, and catalytic fast hydropyrolysis », Progress in Energy and Combustion Science, vol. 68, p. 268-309, sept. 2018. [CrossRef]
- Mathew Jon Rasmussen, « Metal Oxide Catalysts for Hydrodeoxygenation and Aldol Condensation ». Consulté le: 13 octobre 2023. [En ligne]. Disponible sur: https://www.proquest.com/openview/80085c10d494a1b4bfeab3409e8e9297/1?cbl=18750&diss=y&pq-origsite=gscholar&parentSessionId=S9M6ZhGgSl9pn7BNW4FWiwfDknRfFzhQrAunnWbryNA%3D.
- M. Attia, S. Farag, et J. Chaouki, « Upgrading of Oils from Biomass and Waste: Catalytic Hydrodeoxygenation », Catalysts, vol. 10, no 12, Art. no 12, déc. 2020. [CrossRef]
- Z. Ran et al., « Phosphorus vacancies enriched Ni2P nanosheets as efficient electrocatalyst for high-performance Li–O2 batteries », Electrochimica Acta, vol. 337, p. 135795, mars 2020. [CrossRef]
- T. Zhu et al., « Comparative study of hydrodeoxygenation performance over Ni and Ni2P catalysts for upgrading of lignin-derived phenolic compound », Fuel, vol. 331, p. 125663, janv. 2023. [CrossRef]
- Pawnprapa Pitakjakpipop, « Effect of Support for Ni2P Catalysts on Hydrodeoxygenation of Bio-Oil Using Anisole and Guaiacol as Model Compounds ». 2017. [En ligne]. Disponible sur: https://etda.libraries.psu.edu/files/final_submissions/14633.
- V. O. O. Gonçalves, P. M. de Souza, T. Cabioc’h, V. T. da Silva, F. B. Noronha, et F. Richard, « Hydrodeoxygenation of m-cresol over nickel and nickel phosphide based catalysts. Influence of the nature of the active phase and the support », Applied Catalysis B: Environmental, vol. 219, p. 619-628, déc. 2017. [CrossRef]
- A. Berenguer et al., « Catalytic hydrodeoxygenation of m-cresol over Ni 2 P/hierarchical ZSM-5 », Catalysis Today, vol. 304, p. 72-79, avr. 2018. [CrossRef]
- J.-S. Moon, E.-G. Kim, et Y.-K. Lee, « Active sites of Ni2P/SiO2 catalyst for hydrodeoxygenation of guaiacol: A joint XAFS and DFT study », Journal of Catalysis, vol. 311, p. 144-152, mars 2014. [CrossRef]
- S. Boullosa-Eiras, R. Lødeng, H. Bergem, M. Stöcker, L. Hannevold, et E. A. Blekkan, « Catalytic hydrodeoxygenation (HDO) of phenol over supported molybdenum carbide, nitride, phosphide and oxide catalysts », Catalysis Today, vol. 223, p. 44-53, mars 2014. [CrossRef]
- Y. Saito, H. Ishitani, M. Ueno, et S. Kobayashi, « Selective Hydrogenation of Nitriles to Primary Amines Catalyzed by a Polysilane/SiO 2 -Supported Palladium Catalyst under Continuous-Flow Conditions », ChemistryOpen, vol. 6, janv. 2017. [CrossRef]
- S. Meng et al., « Synthesis and characterization of molybdenum carbide catalysts on different carbon supports », Catalysis Today, vol. 402, p. 266-275, sept. 2022. [CrossRef]
- C. Costa, A. L. Soldati, G. Pecchi, J. F. Bengoa, S. G. Marchetti, et V. Vetere, « Preparation and characterization of a supported system of Ni 2 P/Ni 12 P 5 nanoparticles and their use as the active phase in chemoselective hydrogenation of acetophenone », Nanotechnology, vol. 29, no 21, p. 215702, mai 2018. [CrossRef]
- I. V. Deliy et al., « Support Effect on the Performance of Ni2P Catalysts in the Hydrodeoxygenation of Methyl Palmitate », Catalysts, vol. 8, no 11, Art. no 11, nov. 2018. [CrossRef]
- Y. Wang, F. Liu, H. Han, L. Xiao, et W. Wu, « Metal Phosphide:A Highly Efficient Catalyst for the Selective Hydrodeoxygenation of Furfural to 2-Methylfuran », ChemistrySelect, vol. 3, no 27, p. 7926-7933, 2018. [CrossRef]
- S. T. Oyama, X. Wang, Y.-K. Lee, K. Bando, et F. G. Requejo, « Effect of Phosphorus Content in Nickel Phosphide Catalysts Studied by XAFS and Other Techniques », Journal of Catalysis, vol. 210, no 1, p. 207-217, août 2002. [CrossRef]
- Q. Tan, Y. Cao, et J. Li, « Prepared multifunctional catalyst Ni2P/Zr-SBA-15 and catalyzed Jatropha Oil to produce bio-aviation fuel », Renewable Energy, vol. 150, p. 370-381, mai 2020. [CrossRef]
- B. Jiang, T. Zhu, H. Song, et F. Li, « Hydrodeoxygenation and hydrodesulfurization over Fe promoted Ni2P/SBA-15 catalyst », Journal of Alloys and Compounds, vol. 806, p. 254-262, oct. 2019. [CrossRef]
- X. Lan, R. Pestman, E. J. M. Hensen, et T. Weber, « Furfural hydrodeoxygenation (HDO) over silica-supported metal phosphides – The influence of metal–phosphorus stoichiometry on catalytic properties », Journal of Catalysis, p. S0021951721000336, févr. 2021. [CrossRef]
- S. Gutiérrez-Rubio et al., « Guaiacol hydrodeoxygenation over Ni2P supported on 2D-zeolites », Catalysis Today, vol. 345, p. 48-58, avr. 2020. [CrossRef]
- I. V. Shamanaev et al., « Hydroconversion of methyl palmitate over Ni-phosphide catalysts on SAPO-11 and ZSM-5 composite supports », Microporous and Mesoporous Materials, vol. 359, p. 112667, sept. 2023. [CrossRef]
- L. K. H. Pham et al., « Formation and activity of activated carbon supported Ni2P catalysts for atmospheric deoxygenation of waste cooking oil », Fuel Processing Technology, vol. 185, p. 117-125, mars 2019. [CrossRef]
- Gwang-Nam Yunb, So-Jin Ahn, et Atsushi Takagaki, « Infrared Spectroscopic Studies of the Hydrodeoxygenation of Y-Valerolactone on Ni2P/MCM-41 », 28/7/2018, p. 31. [CrossRef]
- S. Wang et al., « Synthesis of highly active carbon-encapsulated Ni2P catalysts by one-step pyrolysis–phosphidation for hydrodeoxygenation of phenolic compounds », Catal. Sci. Technol., vol. 12, no 5, p. 1586-1597, mars 2022. [CrossRef]
- Y. Li, X. Zhang, H. Zhang, B. Chen, et K. J. Smith, « Enhanced stability of Pd-Ni2P/SiO2 catalysts for phenol hydrodeoxygenation in the presence of H2O », Journal of the Taiwan Institute of Chemical Engineers, vol. 80, p. 215-221, nov. 2017. [CrossRef]
- A. Berenguer et al., « Catalytic hydrodeoxygenation of m-cresol over Ni2P/hierarchical ZSM-5 », Catalysis Today, août 2017. [CrossRef]
- X. Fan et al., « Benzene, toluene and xylene (BTX) from in-situ gas phase hydrodeoxygenation of guaiacol with liquid hydrogen donor over bifunctional non-noble-metal zeolite catalysts », Renewable Energy, vol. 152, p. 1391-1402, juin 2020. [CrossRef]
- M. de Oliveira Camargo, J. L. Castagnari Willimann Pimenta, M. de Oliveira Camargo, et P. A. Arroyo, « Green diesel production by solvent-free deoxygenation of oleic acid over nickel phosphide bifunctional catalysts: Effect of the support », Fuel, vol. 281, p. 118719, déc. 2020. [CrossRef]
- I. Aziz, P. Sugita, N. Darmawan, A. A. Dwiatmoko, et W. Rustyawan, « Hydrodeoxygenation of palm fatty acid distillate (PFAD) over natural zeolite-supported nickel phosphide catalyst: Insight into Ni/P effect », Case Studies in Chemical and Environmental Engineering, p. 100571, déc. 2023. [CrossRef]
- N. Kochaputi et al., « Catalytic Behaviors of Supported Cu, Ni, and Co Phosphide Catalysts for Deoxygenation of Oleic Acid », Catalysts, vol. 9, no 9, Art. no 9, sept. 2019. [CrossRef]
- S. Gutiérrez-Rubio, I. Moreno, D. P. Serrano, et J. M. Coronado, « Hydrotreating of Guaiacol and Acetic Acid Blends over Ni2P/ZSM-5 Catalysts: Elucidating Molecular Interactions during Bio-Oil Upgrading », ACS Omega, vol. 4, no 25, p. 21516-21528, déc. 2019. [CrossRef]
- Y. Liu, L. Yao, H. Xin, G. Wang, D. Li, et C. Hu, « The production of diesel-like hydrocarbons from palmitic acid over HZSM-22 supported nickel phosphide catalysts », Applied Catalysis B: Environmental, vol. 174-175, p. 504-514, sept. 2015. [CrossRef]
- M. Mukhtarova, M. A. Golubeva, et A. L. Maximov, « In situ Ni2P catalyst for the selective processing of terephthalic acid into BTX fraction », Applied Catalysis A: General, vol. 678, p. 119734, mai 2024. [CrossRef]
- R. Sun, L. Xiao, et W. Wu, « In-situ carbon-encapsulated Ni2P@C catalysts for reductive amination of furfural », Molecular Catalysis, vol. 553, p. 113710, janv. 2024. [CrossRef]
- Q. Zhang et al., « Design of a highly active TiO2-supported Ni2P@C catalyst with special flower-like radial channels for quick p-cresol hydrodeoxygenation », Journal of Catalysis, vol. 432, p. 115338, avr. 2024. [CrossRef]
- P. M. de Souza et al., « Hydrodeoxygenation of phenol using nickel phosphide catalysts. Study of the effect of the support », Catalysis Today, vol. 356, p. 366-375, oct. 2020. [CrossRef]
- J. Wang, L. Abdelouahed, M. Jabbour, et B. Taouk, « Catalytic hydro-deoxygenation of acetic acid, 4-ethylguaiacol, and furfural from bio-oil over Ni<span class="mathjax-formula">$_{2}$</span>P/HZSM-5 catalysts », Comptes Rendus. Chimie, vol. 24, no S1, p. 1-17, 2021. [CrossRef]
- T. He et al., « Gas phase hydrodeoxygenation of anisole and guaiacol to aromatics with a high selectivity over Ni-Mo/SiO2 », Catalysis Communications, vol. 102, p. 127-130, déc. 2017. [CrossRef]
- K. Li, R. Wang, et J. Chen, « Hydrodeoxygenation of Anisole over Silica-Supported Ni2P, MoP, and NiMoP Catalysts », Energy Fuels, vol. 25, no 3, p. 854-863, mars 2011. [CrossRef]
- Z. Moravvej, F. Farshchi Tabrizi, M. R. Rahimpour, et A. Behrad Vakylabad, « Exploiting the potential of cobalt molybdenum catalyst in elevated hydrodeoxygenation of furfural to 2-methyl furan », Fuel, vol. 332, p. 126193, janv. 2023. [CrossRef]
- J. Wang et al., « Catalytic upgrading of bio-oil: Hydrodeoxygenation study of acetone as molecule model of ketones », The Canadian Journal of Chemical Engineering, vol. 99, no 5, p. 1082-1093, 2021. [CrossRef]
- W. Jin, L. Pastor-Pérez, D. Shen, A. Sepúlveda-Escribano, S. Gu, et T. Ramirez Reina, « Catalytic Upgrading of Biomass Model Compounds: Novel Approaches and Lessons Learnt from Traditional Hydrodeoxygenation – a Review », ChemCatChem, vol. 11, no 3, p. 924-960, 2019. [CrossRef]
- X. Lan, E. J. M. Hensen, et T. Weber, « Hydrodeoxygenation of guaiacol over Ni2P/SiO2–reaction mechanism and catalyst deactivation », Applied Catalysis A: General, vol. 550, p. 57-66, janv. 2018. [CrossRef]
- A. Modak, A. Deb, T. Patra, S. Rana, S. Maity, et D. Maiti, « ChemInform Abstract: A General and Efficient Aldehyde Decarbonylation Reaction by Using a Palladium Catalyst. », Chemical communications (Cambridge, England), vol. 48, p. 4253-5, mars 2012. [CrossRef]
- Z. He et X. Wang, « Hydrodeoxygenation of model compounds and catalytic systems for pyrolysis bio-oils upgrading », Catalysis for Sustainable Energy, vol. 1, no 2013, p. 28-52, oct. 2012. [CrossRef]
- C. Wang et al., « Ni − Promoted Cu/ZSM-5 for selective hydrodeoxygenation of furfural to produce 2 − Methylfuran », Fuel, vol. 353, p. 129233, déc. 2023. [CrossRef]
- A. Iino, A. Cho, A. Takagaki, R. Kikuchi, et S. Ted Oyama, « Kinetic studies of hydrodeoxygenation of 2-methyltetrahydrofuran on a Ni2P/SiO2 catalyst at medium pressure », Journal of Catalysis, vol. 311, p. 17-27, mars 2014. [CrossRef]
- B. Hočevar, M. Grilc, M. Huš, et B. Likozar, « Mechanism, ab initio calculations and microkinetics of hydrogenation, hydrodeoxygenation, double bond migration and cis–trans isomerisation during hydrotreatment of C6 secondary alcohol species and ketones », Applied Catalysis B: Environmental, vol. 218, p. 147-162, déc. 2017. [CrossRef]
- J. F. Harrod et A. J. Chalk, « Homogeneous Catalysis. I. Double Bond Migration in n-Olefins, Catalyzed by Group VIII Metal Complexes », J. Am. Chem. Soc., vol. 86, no 9, p. 1776-1779, mai 1964. [CrossRef]
- M. Balat, « An Overview of the Properties and Applications of Biomass Pyrolysis Oils », Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, vol. 33, no 7, p. 674-689, janv. 2011. [CrossRef]
- Chao-Wei Lee, Po-Yi Lin, et Bing-Hung Chen, « Hydrodeoxygenation of palmitic acid over zeolite-supported nickel catalysts », p. 8, 2020. doi: https://www.sciencedirect.com/science/article/abs/pii/S0920586120302923.
- M. Peroni, G. Mancino, E. Baráth, O. Y. Gutiérrez, et J. A. Lercher, « Bulk and γ-Al2O3-supported Ni2P and MoP for hydrodeoxygenation of palmitic acid », Applied Catalysis B: Environmental, vol. 180, p. 301-311, janv. 2016. [CrossRef]
- J. Chen et al., « Selective production of alkanes and fatty alcohol via hydrodeoxygenation of palmitic acid over red mud-supported nickel catalysts », Fuel, vol. 314, p. 122780, avr. 2022. [CrossRef]
- G. Huber, J. Chheda, C. Barrett, et J. Dumesic, « Production of Liquid Alkanes by Aqueous-Phase Processing of Biomass-Derived Carbohydrates », Science (New York, N.Y.), vol. 308, p. 1446-50, juill. 2005. [CrossRef]
- Y. Román-Leshkov, C. J. Barrett, Z. Y. Liu, et J. A. Dumesic, « Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates », Nature, vol. 447, no 7147, Art. no 7147, juin 2007. [CrossRef]
- J. Luo, L. Arroyo-Ramírez, J. Wei, H. Yun, C. B. Murray, et R. J. Gorte, « Comparison of HMF hydrodeoxygenation over different metal catalysts in a continuous flow reactor », Applied Catalysis A: General, vol. 508, p. 86-93, nov. 2015. [CrossRef]
- R. Weingarten, G. A. Tompsett, W. C. Conner, et G. W. Huber, « Design of solid acid catalysts for aqueous-phase dehydration of carbohydrates: The role of Lewis and Brønsted acid sites », Journal of Catalysis, vol. 279, no 1, p. 174-182, avr. 2011. [CrossRef]
- C. A. Teles et al., « Hydrodeoxygenation of Lignin-Derived Compound Mixtures on Pd-Supported on Various Oxides », ACS Sustainable Chem. Eng., vol. 9, no 38, p. 12870-12884, sept. 2021. [CrossRef]
- L. T. Funkenbusch, M. E. Mullins, M. A. Salam, D. Creaser, et L. Olsson, « Catalytic hydrotreatment of pyrolysis oil phenolic compounds over Pt/Al2O3 and Pd/C », Fuel, vol. 243, p. 441-448, mai 2019. [CrossRef]
- E. A. Roldugina, S. V. Kardashev, A. L. Maksimov, et E. A. Karakhanov, « Hydrodeoxygenation of Bio-oil Components Containing a Guaiacol Fragment in the Presence of a Ruthenium-Suppoting Mesoporous Aluminosilicate Catalyst », Russ J Appl Chem, vol. 95, no 12, p. 1756-1766, déc. 2022. [CrossRef]
- T. M. Sankaranarayanan et al., « Cross-reactivity of guaiacol and propionic acid blends during hydrodeoxygenation over Ni-supported catalysts », Fuel, vol. 214, p. 187-195, févr. 2018. [CrossRef]
- G.Chen, J. Liu, X. Li, J. Zhang, H. Yin, et Z. Su, « Investigation on catalytic hydrodeoxygenation of eugenol blend with light fraction in bio-oil over Ni-based catalysts », Renewable Energy, vol. 157, p. 456-465, sept. 2020. [CrossRef]
- H. Tang et al., « Production of jet fuel range hydrocarbons using a magnetic Ni–Fe/SAPO-11 catalyst for solvent-free hydrodeoxygenation of jatropha oil », Biomass and Bioenergy, vol. 177, p. 106927, oct. 2023. [CrossRef]
- Y. Wu et al., « Synthesis of Ni/SAPO-11-X zeolites with graded secondary pore structure and its catalytic performance for hydrodeoxygenation-isomerization of FAME for green diesel production », Renewable Energy, vol. 218, p. 119372, déc. 2023. [CrossRef]
- R. Zarchin, M. Rabaev, R. Vidruk-Nehemya, M. V. Landau, et M. Herskowitz, « Hydroprocessing of soybean oil on nickel-phosphide supported catalysts », Fuel, vol. 139, p. 684-691, janv. 2015. [CrossRef]
- S. Liu, Q. Zhu, Q. Guan, L. He, et W. Li, « Bio-aviation fuel production from hydroprocessing castor oil promoted by the nickel-based bifunctional catalysts », Bioresource Technology, vol. 183, p. 93-100, mai 2015. [CrossRef]
- N. Luo, Y. Cao, J. Li, W. Guo, et Z. Zhao, « Preparation of Ni2P/Zr-MCM-41 catalyst and its performance in the hydrodeoxygenation of Jatropha curcas oil », Journal of Fuel Chemistry and Technology, vol. 44, no 1, p. 76-83, janv. 2016. [CrossRef]
- R. Yang et al., « Transformation of Jatropha Oil into High-Quality Biofuel over Ni–W Bimetallic Catalysts », ACS Omega, vol. 4, no 6, p. 10580-10592, juin 2019. [CrossRef]
- O. Ismail et al., « Selective formation of fuel BXT compounds from catalytic hydrodeoxygenation of waste biomass over Ni-decorated beta-zeolite », Bioresource Technology Reports, vol. 24, p. 101616, déc. 2023. [CrossRef]
- H. Shafaghat, J. M. Kim, I.-G. Lee, J. Jae, S.-C. Jung, et Y.-K. Park, « Catalytic hydrodeoxygenation of crude bio-oil in supercritical methanol using supported nickel catalysts », Renewable Energy, vol. 144, p. 159-166, déc. 2019. [CrossRef]
- V. S. Prabhudesai, L. Gurrala, et R. Vinu, « Catalytic Hydrodeoxygenation of Lignin-Derived Oxygenates: Catalysis, Mechanism, and Effect of Process Conditions », Energy Fuels, vol. 36, no 3, p. 1155-1188, févr. 2022. [CrossRef]
- Y. Li et al., « Coke formation on the surface of Ni/HZSM-5 and Ni-Cu/HZSM-5 catalysts during bio-oil hydrodeoxygenation », Fuel, vol. 189, p. 23-31, févr. 2017. [CrossRef]
- E. Laurent, A. Centeno, et B. Delmon, « Coke Formation during the Hydrotreating of Biomass Pyrolysis Oils: Influence of Guaiacol Type Compounds », in Studies in Surface Science and Catalysis, vol. 88, B. Delmon et G. F. Froment, Éd., in Catalyst Deactivation 1994, vol. 88. , Elsevier, 1994, p. 573-578. [CrossRef]
- Y. Li, C. Zhang, Y. Liu, X. Hou, R. Zhang, et X. Tang, « Coke Deposition on Ni/HZSM-5 in Bio-oil Hydrodeoxygenation Processing », Energy Fuels, vol. 29, no 3, p. 1722-1728, mars 2015. [CrossRef]
- R. J. French et al., « Optimizing Process Conditions during Catalytic Fast Pyrolysis of Pine with Pt/TiO 2 —Improving the Viability of a Multiple-Fixed-Bed Configuration », ACS Sustainable Chem. Eng., vol. 9, no 3, p. 1235-1245, janv. 2021. [CrossRef]
- A. Infantes-Molina, E. Moretti, E. Segovia, A. Lenarda, et E. Rodriguez-Castellon, « Pd-Nb binfunctional catalysts supported on silica and zirconium phosphate heterostructures for O-removal of dibenzofurane », Catalysis Today, vol. 277, janv. 2016. [CrossRef]
- NPTEL – Chemical Engineering – Catalyst Science and Technology, « Solid catalysts ». [En ligne]. Disponible sur: https://www.bitmesra.ac.in/UploadedDocuments/admince/files/ARE%20Module%202%20Notes.pdf.
- A. Popov et al., Deactivation of Mo-based hydrodeoxygenation catalysts: the effect of water., vol. 238. 2009, p. 133.
- P.M. Mortensen, J.-D. Grunwaldt, P. A. Jensen, et A. D. Jensen, « Influence on nickel particle size on the hydrodeoxygenation of phenol over Ni/SiO2 », Catalysis Today, vol. 259, p. 277-284, janv. 2016. [CrossRef]
- M. V. Bukhtiyarova, A. L. Nuzhdin, et G. A. Bukhtiyarova, « Comparative Study of Batch and Continuous Flow Reactors in Selective Hydrogenation of Functional Groups in Organic Compounds: What Is More Effective? », International Journal of Molecular Sciences, vol. 24, no 18, Art. no 18, janv. 2023. [CrossRef]
- A. Aho, N. Kumar, K. Eränen, T. Salmi, M. Hupa, et D. Yu. Murzin, « Catalytic pyrolysis of woody biomass in a fluidized bed reactor: Influence of the zeolite structure », Fuel, vol. 87, no 12, p. 2493-2501, sept. 2008. [CrossRef]
- R.J. Madon et M. Boudart, « Experimental criterion for the absence of artifacts in the measurement of rates of heterogeneous catalytic reactions », Ind. Eng. Chem. Fund., vol. 21, no 4, p. 438-447, nov. 1982. [CrossRef]
- F. Ibnu P, W. Lestari, R. Putra, A. Aqna, I. Cahyani, et G. T. M. Kadja, « Heterogeneous Catalyst based on Nickel Modified into Indonesian Natural Zeolite in Green Diesel Production from Crude Palm Oil », International Journal of Technology, vol. 13, p. 931, oct. 2022. [CrossRef]
Figure 1.
Fossil fuels reserves from [2].
Figure 1.
Fossil fuels reserves from [2].
Figure 2.
Estimated Renewable Share of Total Final Energy Consumption 2021 from [3,4,5].
Figure 2.
Estimated Renewable Share of Total Final Energy Consumption 2021 from [3,4,5].
Figure 4.
Chemical composition of bio-oils from different feedstock pyrolysis and influence of temperature on the lumped classes of pine pyrolysis bio-oils adapted from [17,158].
Figure 4.
Chemical composition of bio-oils from different feedstock pyrolysis and influence of temperature on the lumped classes of pine pyrolysis bio-oils adapted from [17,158].
Figure 5.
Upgrading methods of bio-oil adapted from [21,23,24].
Figure 5.
Upgrading methods of bio-oil adapted from [21,23,24].
Figure 6.
Typical reactions involved in HDO, adapted from [23,39,60,64,65].
Figure 6.
Typical reactions involved in HDO, adapted from [23,39,60,64,65].
Figure 7.
A possible reaction mechanism for the HDO of phenol over MoS2. Adapted from reference [
77].
Figure 7.
A possible reaction mechanism for the HDO of phenol over MoS2. Adapted from reference [
77].
Figure 8.
Catalytic hydrodeoxygenation performance of m-cresol over Ni2P/SiO2 catalyst. Adapted from [85].
Figure 8.
Catalytic hydrodeoxygenation performance of m-cresol over Ni2P/SiO2 catalyst. Adapted from [85].
Figure 9.
Proposed reaction pathway for HDO of guaiacol to hydrocarbons over Ni2P-based catalyst. Adapted from [64,87].
Figure 9.
Proposed reaction pathway for HDO of guaiacol to hydrocarbons over Ni2P-based catalyst. Adapted from [64,87].
Figure 10.
Possible reactions in the HDO of model molecules adapted from [120].
Figure 10.
Possible reactions in the HDO of model molecules adapted from [120].
Figure 11.
Reaction pathways and selectivity of aromatic products in the HDO of Guaiacol over Ni2P/SiO2 at 300 °C adapted from [90].
Figure 11.
Reaction pathways and selectivity of aromatic products in the HDO of Guaiacol over Ni2P/SiO2 at 300 °C adapted from [90].
Figure 13.
Reaction pathways in the HDO of furfural according to [115]. FOL: furfuryl alcohol, MF: 2-methylfuran, THF: tetrahydrofuran, THMF: tetrahydro-2-methylfuran, THFOL: tetrahydrofurfuryl alcohol, adapted from [97].
Figure 15.
Main reaction pathways of HDO of carboxylic acids, R stands for alkyl groups, adapted from [123].
Figure 15.
Main reaction pathways of HDO of carboxylic acids, R stands for alkyl groups, adapted from [123].
Figure 16.
Proposed reaction network over Ni2P/Al2O3 catalyst; the reaction steps are hydrogenolysis (1), hydrogenation (2), dehydration-hydrogenation (3), decarbonylation (4), decarboxylation (5) and esterification (6), adapted from [123].
Figure 16.
Proposed reaction network over Ni2P/Al2O3 catalyst; the reaction steps are hydrogenolysis (1), hydrogenation (2), dehydration-hydrogenation (3), decarbonylation (4), decarboxylation (5) and esterification (6), adapted from [123].
Figure 17.
Reaction pathways for the conversion of carbohydrates biomass-derived glucose into liquid alkanes, adapted from [124].
Figure 17.
Reaction pathways for the conversion of carbohydrates biomass-derived glucose into liquid alkanes, adapted from [124].
Figure 18.
Reaction pathways for the conversion of carbohydrates) formed by removal of oxygen atoms from hexoses (Fructose), adapted from [132].
Figure 18.
Reaction pathways for the conversion of carbohydrates) formed by removal of oxygen atoms from hexoses (Fructose), adapted from [132].
Figure 19.
Reaction Scheme for Hydrodeoxygenation of Phenolic Compounds (Phenol and m-Cresol) for the Pd Catalysts Supported on Various Oxides, adapted from [136].
Figure 19.
Reaction Scheme for Hydrodeoxygenation of Phenolic Compounds (Phenol and m-Cresol) for the Pd Catalysts Supported on Various Oxides, adapted from [136].
Figure 4.
Product distribution and major Pathways of guaiacol and propionic acid transformations in HDO conditions : 1. Hydrogenolysis, 2. Hydrogenation, 3. Demethylation, 4. Demethoxylation, 5. Dehydration, 6. Etherification, 7. Esterification [131]
Figure 4.
Product distribution and major Pathways of guaiacol and propionic acid transformations in HDO conditions : 1. Hydrogenolysis, 2. Hydrogenation, 3. Demethylation, 4. Demethoxylation, 5. Dehydration, 6. Etherification, 7. Esterification [131]
Figure 21.
Major reaction pathways of eugenol and acetic acid, ethylene glycol and furfural transformations during HDO process, adapted from [140].
Figure 21.
Major reaction pathways of eugenol and acetic acid, ethylene glycol and furfural transformations during HDO process, adapted from [140].
Figure 23.
Main reaction pathways of bio-oil hydrotreatment over Ni2P/HZSM-5 catalysts, Adapted from [23].
Figure 23.
Main reaction pathways of bio-oil hydrotreatment over Ni2P/HZSM-5 catalysts, Adapted from [23].
Figure 24.
TEM images of fresh and spent catalysts at different reaction temperatures adapted from[150].
Figure 24.
TEM images of fresh and spent catalysts at different reaction temperatures adapted from[150].
Table 2.
Advantages and disadvantages of various upgrading technologies currently developed for upgrading of pyrolysis bio-oil.
Table 2.
Advantages and disadvantages of various upgrading technologies currently developed for upgrading of pyrolysis bio-oil.
| Upgrading Methods |
Objetives |
Advantages |
Disadvantages |
Ref |
| Emulsification |
Enhancing the miscibility of bio-oils with diesel fuel.
Use bio-oils in combustion engines. |
Simple operation steps |
High energy input
High-cost of surfactant
Corrosion problems
|
[31] |
| Solvent Addition |
Reduce the ageing effect: Alcohol: methanol, ethanol and isopropanol are used. |
Easy Operation and increases in bio- oil’s lower heating value, reduces density and viscosity |
Decrease in the bio-flashoil’s point. Unfavourable materials cannotbe removed (Oxygen) |
[42,61] |
| Steam reforming |
Production d’hydrogène à partir de reformage de biohuile |
High yield
Better regeneration of the catalyst |
Costly
Fully developed reactors
High operating temperature |
[47] |
Hydrotreatment
(HDO)
|
Removal of sulphur, nitrogen, and oxygen heteroatom. |
Utilizing compressed hydrogen to remove oxygen, increasing heating value and lowering bio-crude oil viscosity, moderate reaction condition |
Harsh conditions, complicated equipment, easy reactor blockage and catalyst deactivation |
[62,63,25], [63] |
| Esterification |
Organic acids (from acid, acetic acid, propionic acid, etc.) in bio-oil can be converted to their corresponding esters |
The most practical approach (simplicity, the low cost of some solvents, and their beneficial effects on the oil properties) |
Low oil production and poor performance |
[57] |
| Catalytic crackings |
Break down larger hydrocarbon molecules into smaller hydrocarbon molecules, and often involve subsequent hydrogenation |
Makes large quantities of light products.
High yield of light products |
High cost, harsh, hydrogen consumption
High pressure-resistant reactor required
Catalyst deactivation, reactor clogging.
|
[48,61] |
| Supercritical fluid |
Obtain high yields and qualities of the bio-oil.
some organic solvents, such as ethanol, methanol, water and CO2 are used |
Higher oil yield, better fuel quality (Lower oxygen content, lower viscosity) |
High-cost of solvent
High-pressure resistant reactor required
|
[58] |
Table 3.
HDO of different compounds over sulphide catalyst.
Table 3.
HDO of different compounds over sulphide catalyst.
| Catalyst |
Oxygenate Compound |
Deoxygenated Compound |
Reference |
| NiMoS |
Guaiacol |
Phenol, catechol, cyclohexne, |
[70,39] |
| MoS2 |
Phenol |
Benzene |
[71] |
| NiM@C |
Guaiacol |
Cyclohexanol, Phenol, cyclohexane |
[62] |
| CoMoZ |
Anisole |
Benzene, Toluene, xylenes |
[65] |
| CoMoS/ Al2O3 |
Guaiacol |
Cyclohexene, Cyclohexane Benzene |
[72] |
| CoMoS |
P-cresol |
Toluene, Methylcyclohexane,
3-methylcyclohexene |
[73] |
| Ni-Mo |
4-methylphenol |
Toluene methylcyclohexane, and 3-4 methylcyclohexene |
[74] |
| NiMo/SBA-15 |
Guaiacol |
Benzene, cyclohexene, cyclohexane, Phenol |
[75] |
| NiMoP/HMS |
Guaiacol |
Biphenyl, Clohexylbenzene, Dicyclohexyl, Tetrahydrodibenzothiophene.
|
[76] |
| Co–Mo–P/MgO |
Phenol |
Benzene, cyclohexyl-benzene, cyclohexyl-phenol. |
[77] |
Table 4.
Summary of HDO results of bio-oil and model compound using Ni and Ni-modified supported catalyst.
Table 4.
Summary of HDO results of bio-oil and model compound using Ni and Ni-modified supported catalyst.
| Catalysts |
Feedstock |
T
(oC) |
P
(MPa) |
T
(h) |
Set Up |
Conversionsmol. % |
Major products |
Selectivitymol. % |
Refs |
| Ni2P/SiO2
|
M-cresol |
250 |
3 |
1 |
Batch |
94.7 |
Hydrocarbons |
~96.3 |
[83] |
| Ni2P/Zr-SBA-15 |
Bio Oil |
330 |
4,5 |
4 |
Fixed-bed |
98 |
Hydrocarbons |
67.80 |
[95] |
| Ni2P/Fe-SBA-15 |
Benzofuran |
300 |
3.0 |
7 |
Fixed-bed |
91.7 |
Hydrocarbons |
83.3 |
[96] |
| Ni2P/SiO2
|
Furfural |
200 |
0.1 |
2 |
Fixed-bed |
90 |
Hydrocarbons |
~60 |
[97] |
| Ni2P/2D ZSM-5 |
Guaiacol |
260 |
4 |
2 |
Batch |
78 |
Cyclohexane |
95.0 |
[98] |
| Ni2P/Al2O3-ZSM-5 |
Methyl palmitate |
340 |
2 |
- |
Continuous flow reactor |
80.3 |
Iso-alkanes (i-C15-i-C16) |
62.1 |
[99] |
| Ni2P/AC |
Waste cooking oil |
300 |
0.1 |
~1 |
Continuous flow reactor |
85 |
Hydrocarbons(n-alkanes) |
~60 |
[100] |
| Ni2P/MCM-41 |
γ-Valerolactone |
350 |
0.5 |
3 |
Continuous flow reactor |
~100.0 |
Hydrocarbons (Butane) |
88.0 |
[101] |
| Ni2P@C(x) |
Phenol |
250 |
2 |
2 |
Batch |
100 |
Cyclohexane |
100 |
[102] |
| PdNi2P/SiO2
|
Phenol |
220 |
2 |
3 |
Fixed-bed |
100 |
Cyclohexane |
98 |
[103] |
| Ni2P/HZSM-5 |
M-cresol |
200 |
2.5 |
6 |
Batch reactor |
97 |
Methylcyclohexane |
88 |
[104] |
| Ni2P/HZS M-5 |
4-ethylguaiacol, |
400 |
0.5 |
8 |
Continuousflow reactor |
84 |
Hydrocarbons |
65.10 |
[25] |
| Ni/HZSM-5&La |
Guaiacol |
350 |
2 |
0.83 |
Fixed-bed |
97.79 |
Hydrocarbons |
61.75 |
[105] |
| Ni2P/H-ZSM-5 |
Oleic acid |
300 |
5 |
6 |
Batch reactor |
65 |
Hydrocarbons |
29 |
[106] |
| NiP(2:1)/NZ0.5 |
PFAD |
350 |
4 |
2 |
Fixed-bed |
100 |
Hydrocarbons |
93.32 |
[107] |
| Ni2P/USYZ |
Oleic Acid |
340 |
1 |
1 |
Batch |
|
Hydrocarbons |
48 |
[108] |
| Ni2P/ZSM-5 |
Blends |
260 |
0.4 |
- |
Batch |
|
Cyclohexane ðane |
|
[109] |
| Ni2P/HZSM-22 |
Palmitic acid |
350 |
0.1 |
2.5 |
Fixed-bed |
99.6 |
Hydrocarbons |
42.9 |
[110]
|
| Ni2P/HZSM |
Bio oil |
450 |
0.5 |
1.30 |
Fixed-bed |
80 |
Hydrocarbons |
28.87 |
[25] |
| In-situ Ni2P |
Terephthalic acid |
400 |
7 |
6 |
Autoclave reactor |
98 |
Benzene-toluene-xylene |
100 |
[111] |
| Ni2P@C-T |
Furfural |
150 |
1 |
4 |
Batch |
100 |
N-butyl furfurylamine |
85 |
[112] |
| Ni2P@C/FLRC-TiO2
|
p-cresol |
275 |
2 |
1.5 |
Batch |
100 |
4-methylcyclohexanol |
90.8 |
[113] |
Table 5.
The main characteristics of batch and continuous flow reactors, adapted from [161,134].
Table 5.
The main characteristics of batch and continuous flow reactors, adapted from [161,134].
| Batch Reactor |
Continuous Flow Reactor |
| The reaction occurs in the liquid phase. |
The reaction occurs in the gas phase. |
| Precise Temperature control and vigorous stirring are essential for uniform temperature and composition |
The composition of the gas at the outlet remain constant over time. |
| Concentrations of reactants and products evolve over time, leading to higher product yields with extended reaction times. |
Precise control of reactant molar ratio is achievable by controlling the flow rates of reactants. |
| Long synthesis can result in catalyst deactivation without knowing it has happened |
The change of residence time without changing the catalyst in the reactor. |
| Catalyst deactivation is typically addressed by catalyst reactivation through repeated the catalytic runs with a washed catalyst. |
Catalyst deactivation is determined by the long-term stability test with online gas mixture measurements. |
| Catalyst particles disturb the sampling procedure by possibly blocking the sampling port. |
There is no need to start and stop the continuous process for the production of the target product in a high yield. |
| Batch synthesis should be repeated several times to produce a high amount of the desired product. |
| Typical HDO temperatures range from 250°C to 400°C. |
Typical HDO temperatures range from 250°C to 550°C. |
| Operating pressures are generally in the range of 1 to 150 bar. |
Operating pressures are generally in the range of 1 to 150 bar. |
| Stirring typically, 300-600 RPM to ensure uniform temperature and mixing of hydrogen with the feedstock. |
Liquid Hourly Space Velocity (LHSV) typically ranges from 0.1 to 2.0 h⁻¹. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).