Submitted:
18 July 2024
Posted:
19 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
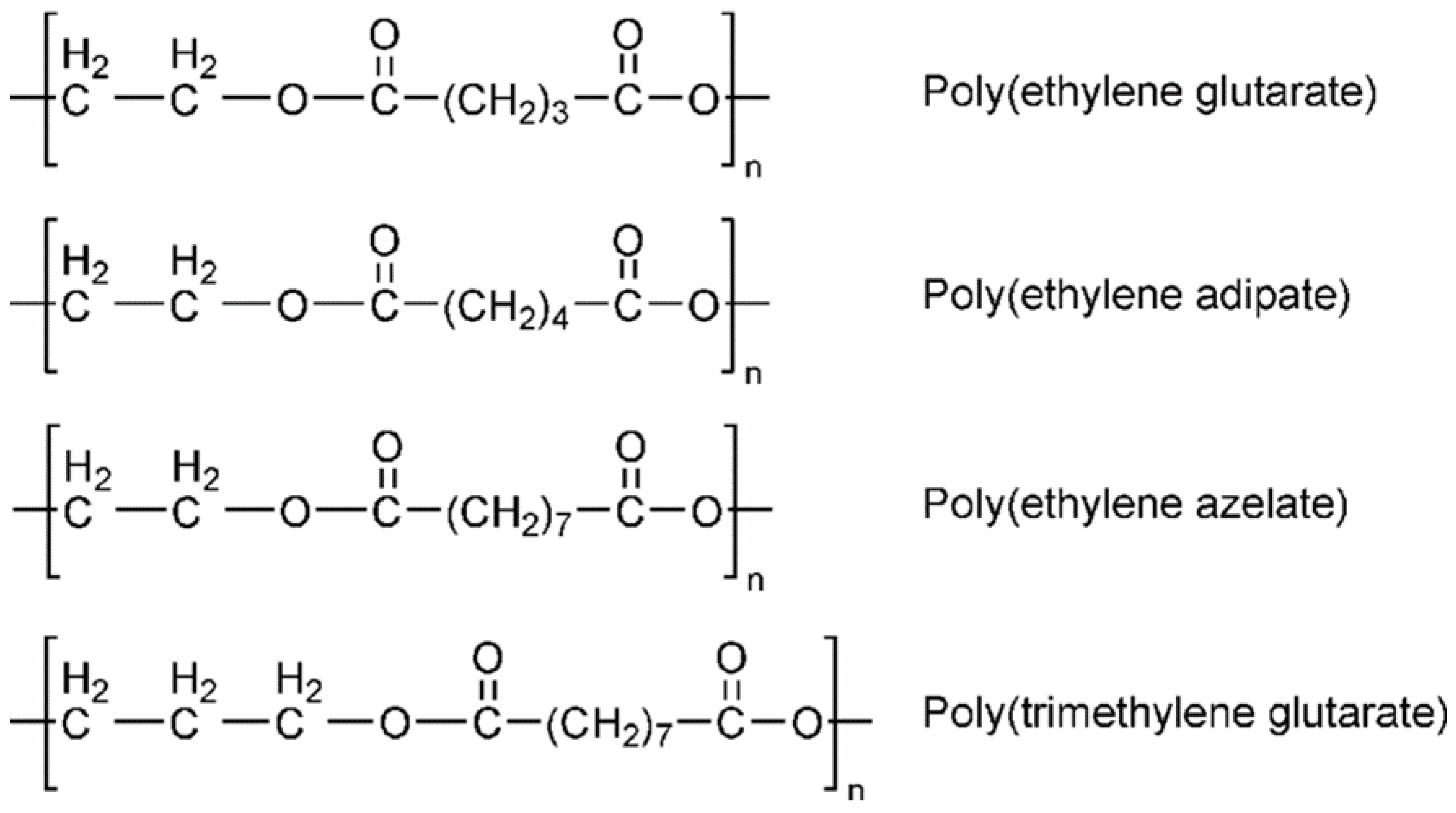
2.1. Aliphatic Polyesters and Copolyesters Containing CHDM and Their Applications
- 1-
- Sutures
- 2-
- Adhesion prevention barriers
- 3-
- UV resistant
2.2. Thermally Stable Aromatic Polyesters and Copolyesters Containing CHDM
3. Preparation of CHDM Based Advanced Polymers
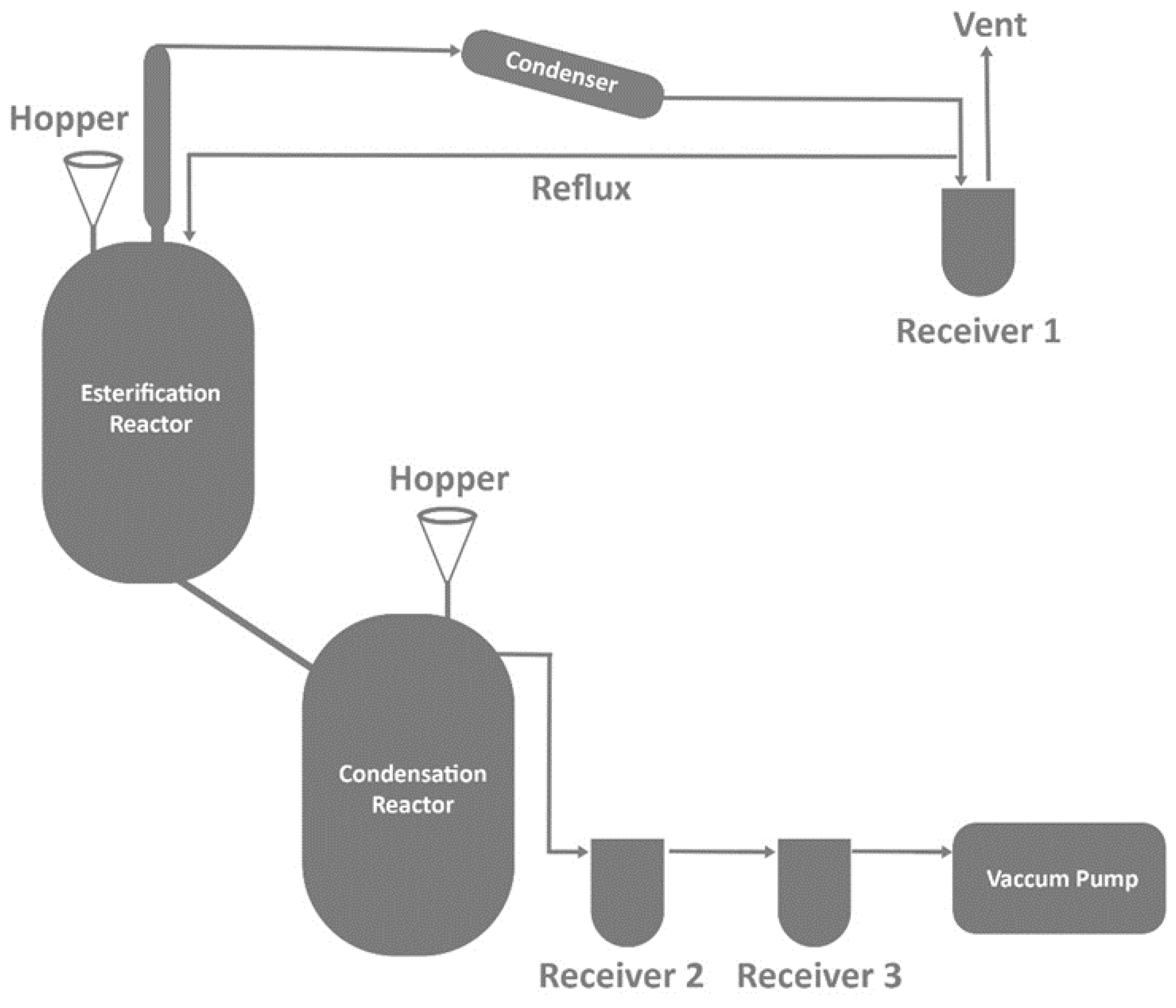
3.1. Solution Polymerization
3.2. Melt Polymerization
3.3. Ring Opening Polymerization
3.4. Solid State Polycondensation (SSP) of Polyesters and Copolyesters
4. Synthesis of Cyclic Compound Based Advanced Hompolyesters and Copolyesters
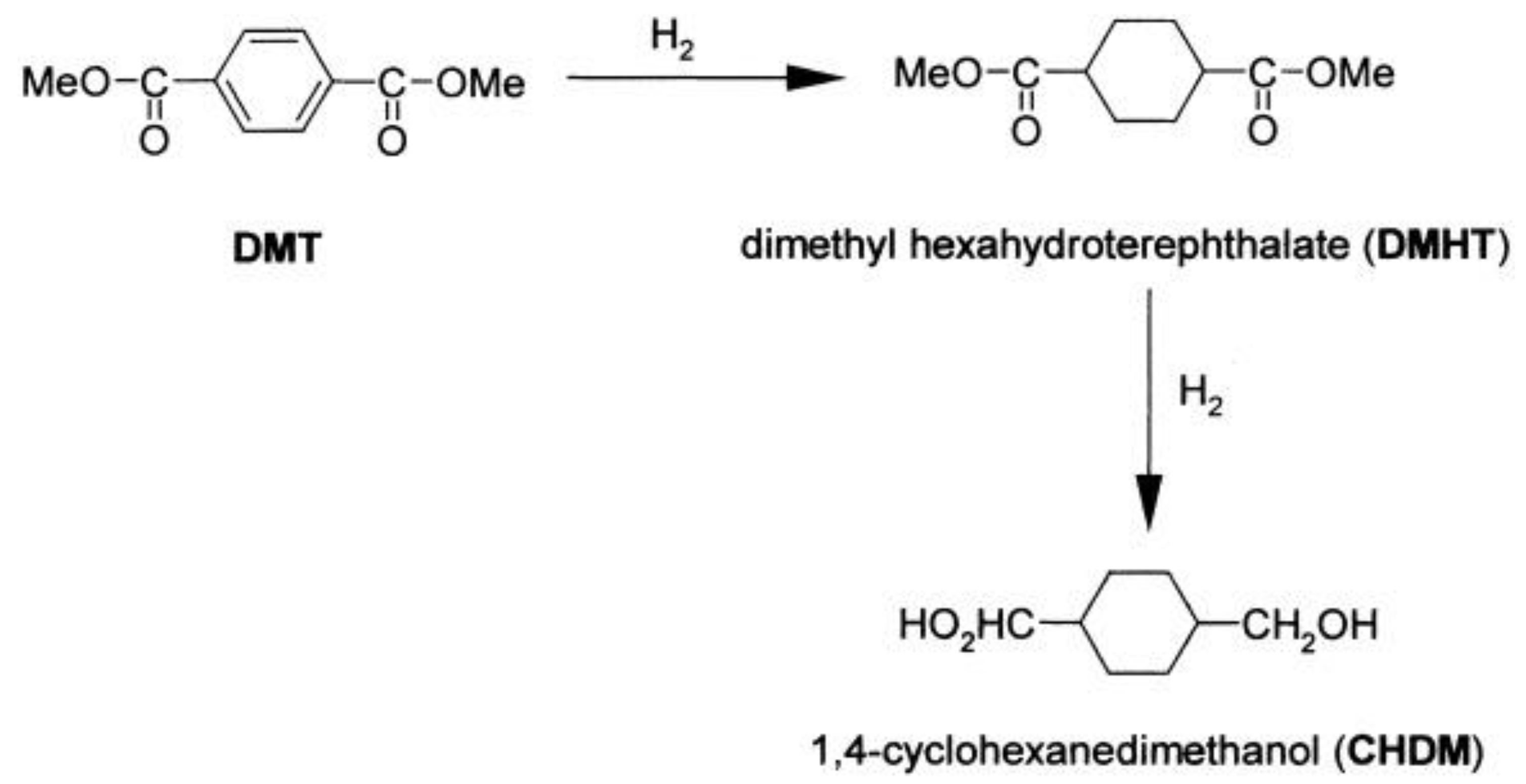
4.1. Synthesis and Properties of 1,4-Cyclohexanedimethanol (CHDM) Based Conventional Homopolyesters (PCT&PCN)
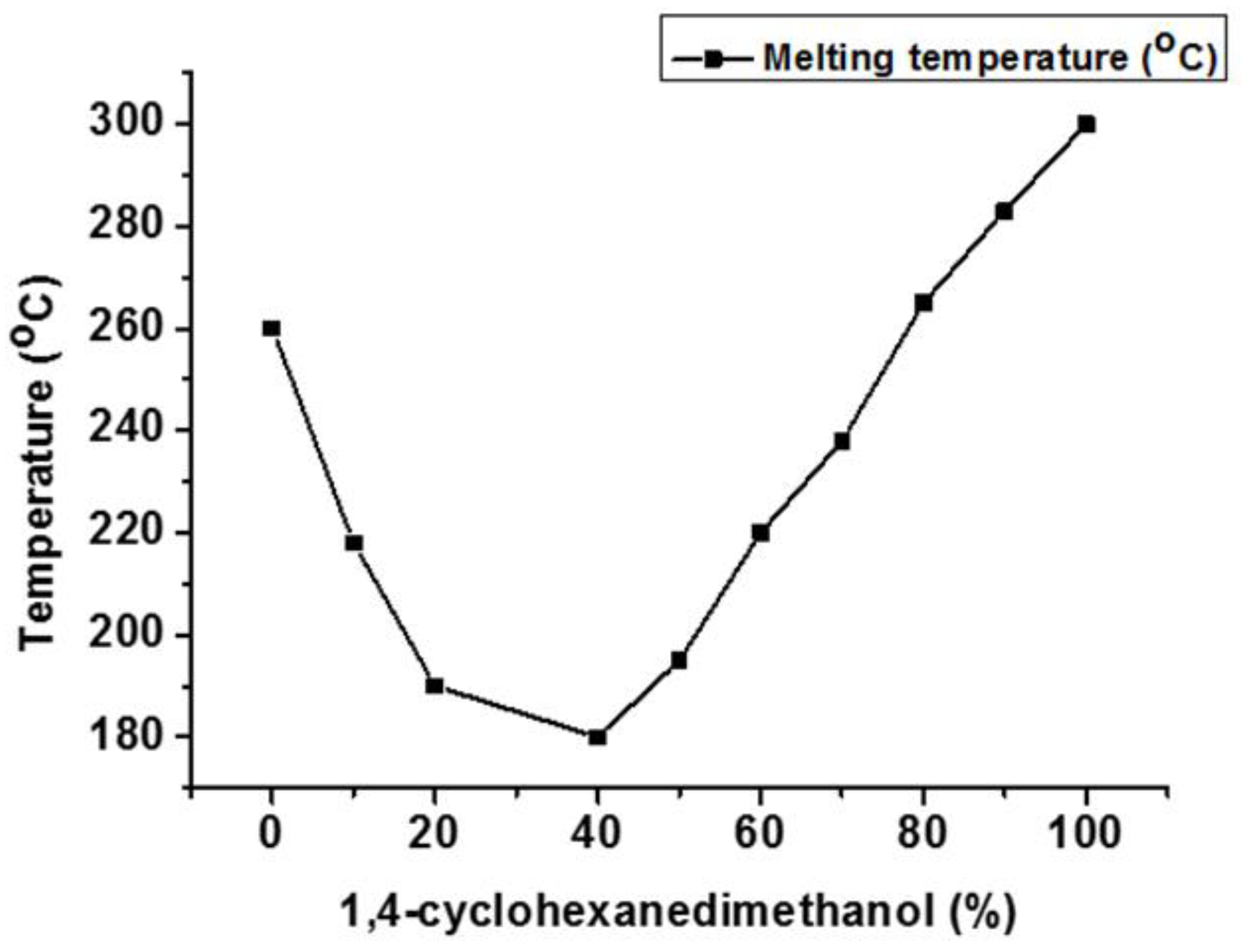
4.2. Second Diol Modified PCT Copolyesters
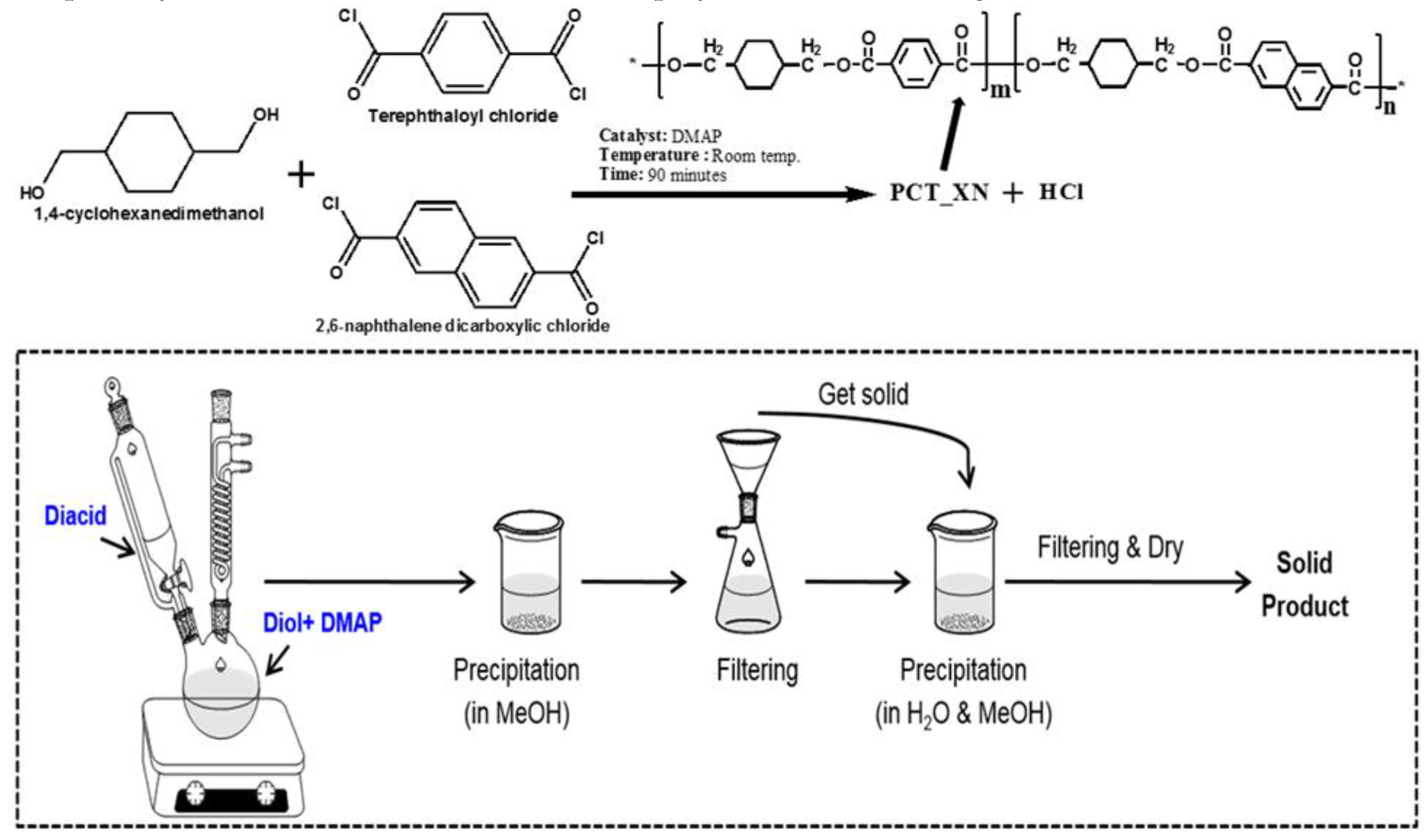
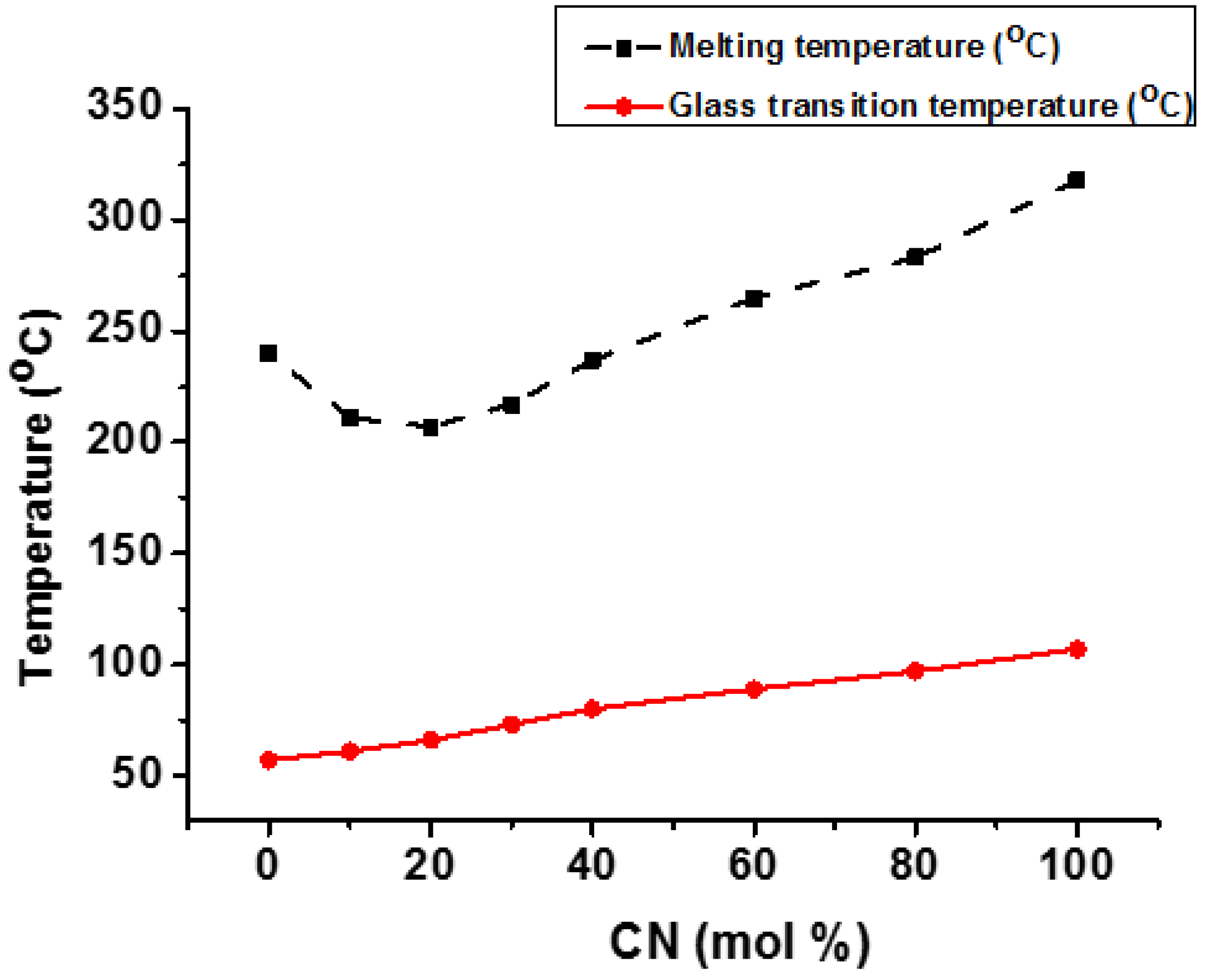
4.3. Second Diacid Modified PCT Copolyesters and Their Applications
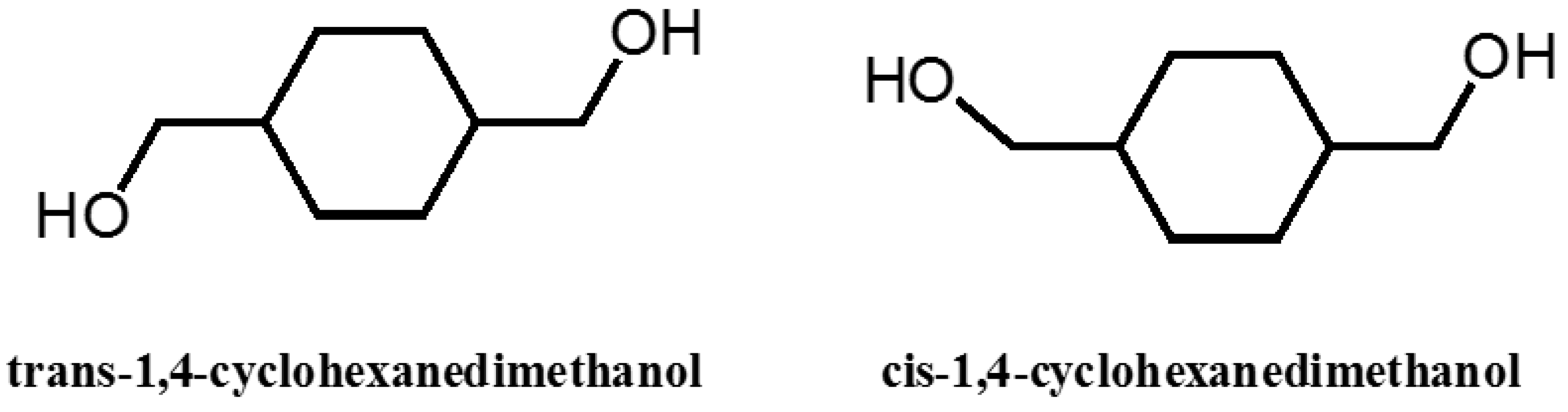
4.4. Effect of Stereochemistry of Monomers on Synthesized Polyesters
5. Polymeric Substrates for Flexible Electronics
6. Future Recommendations for 1,4-Cycloheanedimethanol (CHDM) and Cyclic Monomer-Based Advanced Polyesters
7. Conclusions
Funding
Acknowledgments
References
- Carothers, B.W.H.; Hill, J.W. Studies of polymerization and ring formation. The use of molecular evaporation as a means for propagating chemical reactions. J. Am. Chem. Soc. 1932, 54, 1557–1559. [Google Scholar] [CrossRef]
- Hussain, F.; Shaban, S.M.; Kim, J.; Kim, D.-H. One-pot synthesis of highly stable and concentrated silver nanoparticles with enhanced catalytic activity. Korean J. Chem. Eng. 2019, 36, 988–995. [Google Scholar] [CrossRef]
- Cichosz, S.; Masek, A.; Zaborski, M. Polymer-based sensors: A review. Polym. Test. 2018, 67, 342–348. [Google Scholar] [CrossRef]
- Shen, W.; Zhang, X.; Huang, Q.; Xu, Q.; Song, W. Preparation of solid silver nanoparticles for inkjet printed flexible electronics with high conductivity. Nanoscale 2014, 6, 1622–1628. [Google Scholar] [CrossRef]
- Hussain, F.; Khurshid, M.F.; Masood, R.; Ibrahim, W. Developing antimicrobial calcium alginate fibres from neem and papaya leaves extract. J. Wound Care 2017, 26, 778–783. [Google Scholar] [CrossRef]
- McIntyre, J.E. The historical development of polyesters. In Modern polyesters: chemistry and technology of polyesters and copolyesters; J. Scheirs and T. E. Long, Ed.; Wiley Sussex: England, 2004; pp. 1–28. ISBN 0471498564. [Google Scholar]
- Al., H. et US Patent. 3,897,392 1975. [CrossRef]
- Turner, S.R.; Walter, F.; Voit, B.I.; Mourey, T.H. Hyperbranched aromatic polyesters with carboxylic acid terminal groups. Macromolecules 1994, 27, 1611–1616. [Google Scholar] [CrossRef]
- G. Bier Polyarylates (polyesters from aromatic dicarboxylic acids and bisphenols). 1974, 15, 527–535.
- Boland, C.S.; Khan, U.; Ryan, G.; Barwich, S.; Charifou, R.; Harvey, A.; Backes, C.; Li, Z.; Ferreira, M.S.; Mobius, M.E.; et al. Sensitive electromechanical sensors using viscoelastic graphene-polymer nanocomposites. Science (80-. ). 2016, 354, 1257–1260. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.K.; Arora, H. Surgical gown: A critical review. J. Ind. Text. 2009, 38, 205–231. [Google Scholar] [CrossRef]
- Turner, S.R. Development of amorphous copolyesters based on 1,4-cyclohexanedimethanol. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 5847–5852. [Google Scholar] [CrossRef]
- Rex, J.; Dickson, J.T.; Lothian, E.; I, E. “US Patent” 2,465,319. 1949.
- Bang, H.J.; Kim, H.Y.; Jin, F.L.; Park, S.J. Fibers spun from 1,4-cyclohexanedimethanol-modified polyethylene terephthalate resin. J. Ind. Eng. Chem. 2011, 17, 805–810. [Google Scholar] [CrossRef]
- Pech-May, N.W.; Vales-Pinzón, C.; Vega-Flick, A.; Cifuentes, Á.; Oleaga, A.; Salazar, A.; Alvarado-Gil, J.J. Study of the thermal properties of polyester composites loaded with oriented carbon nanofibers using the front-face flash method. Polym. Test. 2016, 50, 255–261. [Google Scholar] [CrossRef]
- Shih, W.K. Shrinkage modeling of polyester shrink film. Polym. Eng. Sci. 1994, 34, 1121–1128. [Google Scholar] [CrossRef]
- Huang, J.; Wang, J.; Qiu, Y.; Wu, D. Mechanical properties of thermoplastic polyester elastomer controlled by blending with poly(butylene terephthalate). Polym. Test. 2016, 55, 152–159. [Google Scholar] [CrossRef]
- Khankrua, R.; Pivsa-Art, S.; Hiroyuki, H.; Suttiruengwong, S. Thermal and mechanical properties of biodegradable polyester/silica nanocomposites. Energy Procedia 2013, 34, 705–713. [Google Scholar] [CrossRef]
- Bae, J.; Lee, S.; Kim, B.C.; Cho, H.H.; Chae, D.W. Polyester-based thermoplastic elastomer/MWNT composites: Rheological, thermal, and electrical properties. Fibers Polym. 2013, 14, 729–735. [Google Scholar] [CrossRef]
- Park, S.; Hussain, F.; Kang, S.; Jeong, J.; Kim, J. Synthesis and properties of copolyesters derived from 1,4-cyclohexanedimethanol, terephthalic acid, and 2,6-naphthalenedicarboxylic acid with enhanced thermal and barrier properties. Polym. 2018, 42, 662–669. [Google Scholar] [CrossRef]
- Zhang, J.B.; Wang, X.L.; He, Q.X.; Zhao, H.B.; Wang, Y.Z. A novel phosphorus-containing poly(1,4-cyclohexylenedimethylene terephthalate) copolyester: Synthesis, thermal stability, flammability and pyrolysis behavior. Polym. Degrad. Stab. 2014, 108, 12–22. [Google Scholar] [CrossRef]
- Koo, J.M.; Hwang, S.Y.; Yoon, W.J.; Lee, Y.G.; Kim, S.H.; Im, S.S. Structural and thermal properties of poly(1,4-cyclohexane dimethylene terephthalate) containing isosorbide. Polym. Chem. 2015, 6, 6973–6986. [Google Scholar] [CrossRef]
- W. Chester, I. Duling Popolyesters. US Pat. 3,436,376 1969.
- Kasmi, N.; Terzopoulou, Z.; Papageorgiou, G.Z.; Bikiaris, D.N. Poly(1,4-cyclohexanedimethylene 2,6-naphthalate) polyester with high melting point: Effect of different synthesis methods on molecular weight and properties. Express Polym. Lett. 2018, 12, 227–237. [Google Scholar] [CrossRef]
- Meehan, S.J.; Sankey, S.W.; Jones, S.M.; MacDonald, W.A.; Colquhoun, H.M. Cocrystalline copolyimides of poly(ethylene 2,6-naphthalate). ACS Macro Lett. 2014, 3, 968–971. [Google Scholar] [CrossRef]
- Cavallo, D.; Mileva, D.; Portale, G.; Zhang, L.; Balzano, L.; Alfonso, G.C.; Androsch, R. Mesophase-mediated crystallization of poly(butylene-2,6-naphthalate): An example of ostwald’s rule of stages. ACS Macro Lett. 2012, 1, 1051–1055. [Google Scholar] [CrossRef]
- B. Hu, and Ottenbrite, R.M. Biaxially oriented poly(ethylene 2,6-naphthalene) film: manufacture, properties and commercial applications. In Modern polyesters: Chemistry and technology of polyesters and copolyesters; J. Scheirs and T. E. Long, Ed.; Wiley Sussex: England, 2004; pp. 335–359. ISBN 0471498564. [Google Scholar]
- Callander, D.D. Properties and Applications of Poly(Ethylene 2,6-Naphthalene), its Copolyesters and Blends. In Modern polyesters: Chemistry and technology of polyesters and copolyesters; Long, S. and T.E., Ed.; Wiley Sussex: England, 2004; pp. 323–334. ISBN 0471498564. [Google Scholar]
- Wu, W.; Wagner, M.H.; Qian, Q.; Pu, W.; Kheirandish, S. Morphology and barrier mechanism of biaxially oriented poly(ethylene terephthalate)/poly(ethylene 2,6-naphthalate) blends. J. Appl. Polym. Sci. 2006, 101, 1309–1316. [Google Scholar] [CrossRef]
- Kibler, C.J.; Bell, A.; Smith, J.G. Polyesters of 1,4-cyclohexanedimethanol. J. Polym. Sci. Part A 1964, 2, 2115–2125. [Google Scholar] [CrossRef]
- Koo, J.M.; Hwang, S.Y.; Yoon, W.J.; Lee, Y.G.; Kim, S.H.; Im, S.S. Structural and thermal properties of poly(1,4-cyclohexane dimethylene terephthalate) containing isosorbide. Polym. Chem. 2015, 6, 6973–6986. [Google Scholar] [CrossRef]
- Kasmi, N.; Majdoub, M.; Papageorgiou, G.Z.; Bikiaris, D.N. Synthesis and crystallization of new fully renewable resources-based copolyesters: Poly(1,4-cyclohexanedimethanol-co-isosorbide 2,5-furandicarboxylate). Polym. Degrad. Stab. 2018, 152, 177–190. [Google Scholar] [CrossRef]
- Hussain, F.; Park, S.; Jeong, J.; Kang, S.; Kim, J. Structure–property relationship of poly(cyclohexane 1,4-dimethylene terephthalate) modified with high trans-1,4-cyclohexanedimethanol and 2,6-naphthalene dicarboxylicacid. J. Appl. Polym. Sci. 2020, 137, 48950. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, J.; Wang, J.; Liu, X.; Zhang, R.; Hu, G.; Na, H.; Zhu, J. Soft segment free thermoplastic polyester elastomers with high performance. J. Mater. Chem. A 2015, 3, 13637–13641. [Google Scholar] [CrossRef]
- Celli, A.; Marchese, P.; Sisti, L.; Dumand, D.; Sullalti, S.; Totaro, G. Effect of 1,4-cyclohexylene units on thermal properties of poly(1,4-cyclohexylenedimethylene adipate) and similar aliphatic polyesters. Polym. Int. 2013, 62, 1210–1217. [Google Scholar] [CrossRef]
- Alan Bei, and J.G.S.C.J.K. US Patent 2,901, 466 1959.
- Celli, A.; Marchese, P.; Sullalti, S.; Berti, C.; Barbiroli, G. Eco-friendly poly(butylene 1,4-cyclohexanedicarboxylate): Relationships between stereochemistry and crystallization behavior. Macromol. Chem. Phys. 2011, 212, 1524–1534. [Google Scholar] [CrossRef]
- Rosado, M.T.S.; Maria, T.M.R.; Castro, R.A.E.; Canotilho, J.; Silva, M.R.; Eusébio, M.E.S. Molecular structure and polymorphism of a cyclohexanediol: trans-1,4-cyclohexanedimethanol. CrystEngComm 2014, 16, 10977–10986. [Google Scholar] [CrossRef]
- S. R. Turner, R. W. Seymour, J.R.D. Amorphous and crystalline polyesters based on 1,4-cyclohexanedimethanol. In Modern polyesters: Chemistry and technology of polyesters and copolyesters; Long, J.S. and T.E., Ed.; Wiley Sussex: England, 2004; pp. 267–292. ISBN 0471498564. [Google Scholar]
- Martinez de Ilarduya and, S. Munoz Guerra Polyesters based on cyclohexanedimethanol. In Handbook of engineering and specialty thermoplastics; S. thomas, V.P.M., Ed.; Wiley, 2011; pp. 181–182.
- Robert, H. Hasek and M. B. Knowles, Kingsport, T. Preparation of trans-1,4-cyclohexanedimethanol. US Pat. 2,917,549, 1949. [Google Scholar]
- Raja, R.; Khimyak, T.; Thomas, J.M.; Hermans, S.; Johnson, B.F.G. Single-Step, Highly Active, and Highly Selective Nanoparticle Catalysts for the Hydrogenation of Key Organic Compounds. Angew. Chemie Int. Ed. 2001, 40, 4638–4642. [Google Scholar] [CrossRef]
- Raja, R.; Khimyak, T.; Thomas, J.M.; Hermans, S.; Johnson, B.F.G. Single-step, highly active, and highly selective nanoparticle catalysts for the hydrogenation of key organic compounds. Angew. Chem. Int. Ed. Engl. 2001, 40, 4638–4642. [Google Scholar] [CrossRef]
- Hungria, A.B.; Raja, R.; Adams, R.D.; Captain, B.; Thomas, J.M.; Midgley, P.A.; Golovko, V.; Johnson, B.F.G. Single-step conversion of dimethyl terephthalate into cyclohexanedimethanol with Ru5PtSn, a trimetallic nanoparticle catalyst. Angew. Chemie - Int. Ed. 2006, 45, 4782–4785. [Google Scholar] [CrossRef]
- Li, M. US Patent. 9,550,721,B2, 2017. [Google Scholar]
- Guo, X.; Xin, J.; Lu, X.; Ren, B.; Zhang, S. Preparation of 1,4-cyclohexanedimethanol by selective hydrogenation of a waste PET monomer bis(2-hydroxyethylene terephthalate). RSC Adv. 2015, 5, 485–492. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, Z.; Liu, Y.; Li, G.; Wang, A.; Cong, Y.; Zhang, T.; Wang, F.; Li, N. Synthesis of 1,4-cyclohexanedimethanol, 1,4-cyclohexanedicarboxylic acid and 1,2-cyclohexanedicarboxylates from formaldehyde, crotonaldehyde and acrylate/fumarate. Angew. Chemie - Int. Ed. 2018, 57, 6901–6905. [Google Scholar] [CrossRef]
- Al., S. et. US Patent 5,387, 752 1995.
- Lecomte, H.A.; Liggat, J.J.; Curtis, A.S.G. Synthesis and characterization of novel biodegradable aliphatic poly(ester amide)s containing cyclohexane units. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 1785–1795. [Google Scholar] [CrossRef]
- Tsai, Y.; Jheng, L.C.; Hung, C.Y. Synthesis, properties and enzymatic hydrolysis of biodegradable alicyclic/aliphatic copolyesters based on 1,3/1,4-cyclohexanedimethanol. Polym. Degrad. Stab. 2010, 95, 72–78. [Google Scholar] [CrossRef]
- Hunsen, M.; Azim, A.; Mang, H.; Wallner, S.R.; Ronkvist, A.; Wenchun, X.; Gross, R.A. A cutinase with polyester synthesis activity. Macromolecules 2007, 40, 148–150. [Google Scholar] [CrossRef]
- Barrett, D.G.; Merkel, T.J.; Luft, J.C.; Yousaf, M.N. One-step syntheses of photocurable polyesters based on a renewable resource. Macromolecules 2010, 43, 9660–9667. [Google Scholar] [CrossRef]
- Cai, X.; Yang, X.; Zhang, H.; Wang, G. Modification of biodegradable poly(butylene carbonate) with 1,4-cyclohexanedimethylene to enhance the thermal and mechanical properties. Polym. Degrad. Stab. 2017, 143, 35–41. [Google Scholar] [CrossRef]
- Berti, C.; Celli, A.; Marchese, P.; Barbiroli, G.; Di Credico, F.; Verney, V.; Commereuc, S. Novel copolyesters based on poly(alkylene dicarboxylate)s: 2. Thermal behavior and biodegradation of fully aliphatic random copolymers containing 1,4-cyclohexylene rings. Eur. Polym. J. 2009, 45, 2402–2412. [Google Scholar] [CrossRef]
- Park, S.A.; Choi, J.; Ju, S.; Jegal, J.; Lee, K.M.; Hwang, S.Y.; Oh, D.X.; Park, J. Copolycarbonates of bio-based rigid isosorbide and flexible 1,4-cyclohexanedimethanol: Merits over bisphenol-A based polycarbonates. Polymer (Guildf). 2017, 116, 153–159. [Google Scholar] [CrossRef]
- Arévalo-Alquichire, S.; Valero, M. Castor Oil Polyurethanes as Biomaterials. In Elastomers; InTech, 2017; pp. 137–157.
- Brunelle, et al. US Patent 6,084, 055 2000.
- Brunelle, D.J.; Jang, T. Optimization of poly(1,4-cyclohexylidene cyclohexane-1,4-dicarboxylate) (PCCD) preparation for increased crystallinity. Polymer (Guildf). 2006, 47, 4094–4104. [Google Scholar] [CrossRef]
- Pal, R.S.; Pal, Y.; Singh, V. Isolation and characterization of n-octa decanoic acid from whole aerial parts of Centella asiatica Linn. Int. J. Pharm. Technol. 2016, 8, 18989–18994. [Google Scholar]
- Catia Bastioli, Marco Foa, Giovanni Floridi, Giandomenico Cella, Fernanda Farachi, T.M. US Patent 6,727,342 B1 2004, 2.
- John Rex Dickson, J.T.D. US Patent. 2,465,319, 1949. [Google Scholar]
- Kibler, C.J.; Bell, A.; Smith, J.G. Polyesters of 1, 4-Cyclohexanedimethano. J. Polym. Sci. PART A 1964, 2, 2115–2125. [Google Scholar]
- , S. R. Turner, R. W. Seymour, J.R.D. Amorphous and crystalline polyesters based on 1,4-cyclohexanedimethanol. In Modern polyesters: Chemistry and technology of polyesters and copolyesters; Long, J.S. and T.E., Ed.; Wiley Sussex: England, 2004; pp. 267–288. ISBN 0471498564. [Google Scholar]
- Nathalie Gonzalez-Vidal, Antoxon Martinez De Ilarduya, S. M. Poly(ethylene-co-1,4-cyclohexylenedimethylene terephthalate) copolyesters obtained by ring opening polymerization. J. Polym. Sci. Part A Polym. Chem. 2009, 19, 5954–5966. [Google Scholar] [CrossRef]
- Velmathi, S.; Nagahata, R.; Takeuchi, K. Extremely Rapid Synthesis of Aliphatic Polyesters by Direct polycondensation of 1:1 mixtures of dicarboxylic acids and diols using microwaves. Polym. J. 2007, 39, 841–844. [Google Scholar] [CrossRef]
- Jeong, Y.G.; Jo, W.H.; Lee, S.C. Synthesis and isodimorphic cocrystallization behavior of poly(1,4-cyclohexylenedimethylene terephthalate-co-1,4-cyclohexylenedimethylene 2,6-naphthalate) copolymers. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 177–187. [Google Scholar] [CrossRef]
- Hu, L.; Wu, L.; Song, F.; Li, B.G. Kinetics and modeling of melt polycondensation for synthesis of poly[(butylene succinate)-co-(butylene terephthalate)], 1 - esterification. Macromol. React. Eng. 2010, 4, 621–632. [Google Scholar] [CrossRef]
- Lodha, A.; Ghadage, R.S.; Ponrathnam, S. Polycondensation reaction kinetics of wholly aromatic polyesters. Polymer (Guildf). 1997, 38, 6167–6174. [Google Scholar] [CrossRef]
- Shah, T.H.; Bhatty, J.I.; Gamlen, G.A.; Dollimore, D. Aspects of the chemistry of poly(ethylene terephthalate): 5. Polymerization of bis(hydroxyethyl)terephthalate by various metallic catalysts. Polymer (Guildf). 1984, 25, 1333–1336. [Google Scholar] [CrossRef]
- Karayannidis, G.P.; Roupakias, C.P.; Bikiaris, D.N.; Achilias, D.S. Study of various catalysts in the synthesis of poly(propylene terephthalate) and mathematical modeling of the esterification reaction. Polymer (Guildf). 2003, 44, 931–942. [Google Scholar] [CrossRef]
- Shotyk, W.; Krachler, M. Contamination of bottled waters with antimony leaching from PET increases with storage. Environ. Sci. Technol. 2007, 41, 1560–1563. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.; Kotek, R.; Tonelli, A. Review of conventional and novel polymerization processes for polyesters. Prog. Polym. Sci. 2006, 31, 1009–1037. [Google Scholar] [CrossRef]
- Muller, R. Keys to the trematoda. Trans. R. Soc. Trop. Med. Hyg. 2003, 97, 124. [Google Scholar] [CrossRef]
- Kwolek, S.L.; Morgan, P.W. Preparation of polyamides, polyurethanes, polysulfonamides, and polyesters by low temperature solution polycondensation. J. Polym. Sci. Part A Gen. Pap. 1964, 2, 2693–2703. [Google Scholar] [CrossRef]
- Giol, E.D.; Van den Brande, N.; Van Mele, B.; Van Vlierberghe, S.; Dubruel, P. Single-step solution polymerization of poly(alkylene terephthalate)s: synthesis parameters and polymer characterization. Polym. Int. 2018, 67, 292–300. [Google Scholar] [CrossRef]
- Hussain, F.; Jeong, J.; Park, S.; Kang, S.-J.; Kim, J. Single-step solution polymerization and thermal properties of copolyesters based on high trans-1,4-cyclohexanedimethanol, terephthaloyl dichloride, and 2,6-naphthalene dicarboxylic chloride. Polym. 2019, 43, 475–484. [Google Scholar] [CrossRef]
- Liu, Y.; Turner, S.R. Synthesis and properties of cyclic diester based aliphatic copolyesters. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 2162–2169. [Google Scholar] [CrossRef]
- Jérôme, C.; Lecomte, P. Recent advances in the synthesis of aliphatic polyesters by ring-opening polymerization. Adv. Drug Deliv. Rev. 2008, 60, 1056–1076. [Google Scholar] [CrossRef] [PubMed]
- Bednarek, M. Branched aliphatic polyesters by ring-opening (co)polymerization. Prog. Polym. Sci. 2016, 58, 27–58. [Google Scholar] [CrossRef]
- Macdonald, J.P.; Shaver, M.P. An aromatic/aliphatic polyester prepared via ring-opening polymerisation and its remarkably selective and cyclable depolymerisation to monomer. Polym. Chem. 2016, 7, 553–559. [Google Scholar] [CrossRef]
- Wan, X.H.; Yang, Y.; Tu, H.L.; Lan, H.; Tan, S.; Zhou, Q.F.; Turner, S.R. Synthesis, characterization, and ring opening polymerization of poly(1,4-cyclohexylenedimethylene terephthalate) cyclic oligomers. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 1828–1833. [Google Scholar] [CrossRef]
- Achilias, D.S.; Chondroyiannis, A.; Nerantzaki, M.; Adam, K.V.; Terzopoulou, Z.; Papageorgiou, G.Z.; Bikiaris, D.N. Solid State Polymerization of Poly(Ethylene Furanoate) and Its Nanocomposites with SiO2 and TiO2. Macromol. Mater. Eng. 2017, 302, 1–15. [Google Scholar] [CrossRef]
- Culbert, B.; Christel, A. Continuous solid-state polycondensation of polyesters. In Modern Polyesters: Chemistry and Technology of Polyesters and Copolyesters; Long, J.S. and T.E., Ed.; Wiley Sussex: England, 2004; ISBN 9780470090688. [Google Scholar]
- Tate, S.; Watanabe, Y.; Chiba, A. Synthesis of ultra-high molecular weight poly(ethylene terephthalate) by swollen-state polymerization. Polymer (Guildf). 1993, 34, 4974–4977. [Google Scholar] [CrossRef]
- Willem F., H. Borman, Pittsfield, M. US Patent 3,953,404 1976. [CrossRef]
- Kasmi, N.; Papageorgiou, G.Z.; Achilias, D.S.; Bikiaris, D.N. Solid-State polymerization of poly(Ethylene Furanoate) biobased Polyester, II: An efficient and facile method to synthesize high molecular weight polyester appropriate for food packaging applications. Polymers (Basel). 2018, 10, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Dröscher, M.; Wegner, G. Poly(ethylene terephthalate): a solid state condensation process. Polymer (Guildf). 1978, 19, 43–47. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, X.J.; Zhang, J.; Feng, L.F.; Wang, J.J. Experimental and modeling study of the solid state polymerization of poly(ethylene terephthalate) over a wide range of temperatures and particle sizes. J. Appl. Polym. Sci. 2013, 127, 3814–3822. [Google Scholar] [CrossRef]
- Göltner, W. Solid-State Polycondensation of Polyester Resins: Fundamentals and Industrial Production. In Modern Polyesters: Chemistry and Technology of Polyesters and Copolyesters; Long, J.S. and T.E., Ed.; Wiley Sussex, 2004; pp. 195–242 ISBN 9780470090688.
- Papaspyrides, C.D.; Vouyiouka, S.N. Solid-state polymerization. Prog. Polym. Sci. 2005, 30, 10–37. [Google Scholar] [CrossRef]
- Steinborn-Rogulska, I.; Rokicki, G. Solid-state polycondensation (SSP) as a method to obtain high molecular weight polymers: Part II. Synthesis of polylactide and polyglycolide via SSP. Polimery 2013, 58, 85–92. [Google Scholar] [CrossRef]
- Jeong, Y.G.; Jo, W.H.; Lee, S.C. Synthesis and crystallization behavior of poly(m-methylene 2,6-naphthalate-co-1,4-cyclohexylenedimethylene 2,6-naphthalate) copolymers. Macromolecules 2003, 36, 4051–4059. [Google Scholar] [CrossRef]
- Jian Yang, Wengang Li, Aifang Yu, Peng Xi, Xiang’an Huang, S. L. Sequence distribution, thermal properties, and crystallization studies of poly(trimethylene terephthalate-co-1,4-cyclohexylene dimethylene terephthalate) copolyesters. J. Appl. Polym. Sci. 2008, 111, 2751–2760. [Google Scholar] [CrossRef]
- Hill, A.J.; Weinhold, S.; Stack, G.M.; Tant, M.R. Effect of copolymer composition on free volume and gas permeability in poly(ethylene terephthalate)-poly(1,4 cyclohexylenedimethylene terephthalate) copolyesters. Eur. Polym. J. 1996, 32, 843–849. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, W.; Zhang, J. Alkali resistance of poly(ethylene terephthalate) (PET) and poly(ethylene glycol-co-1,4-cyclohexanedimethanol terephthalate) (PETG) copolyesters: The role of composition. Polym. Degrad. Stab. 2015, 120, 232–243. [Google Scholar] [CrossRef]
- Jeong, Y.G.; Jo, W.H. Cocrystallization of Poly (1,4-cyclohexylenedimethylene terephthalate-co-hexamethylene terephthalate) Copolymers. Macromol. Res. 2004, 12, 459–465. [Google Scholar] [CrossRef]
- Kliesh, H.; Oliver Klein, M.K.; Kuhmann, B. White, biaxially oriented polyester film with a high portion of cyclohexanedimethanol and a primary and secondary dicarboxylic acid portion and a method for its production and its use. US Pat. 2012/0196111 A1, 2012. [Google Scholar]
- Sublett USPatent. 5,616,404, 1997. [CrossRef]
- Dickerson et al. US Patent. 5,656,715, 1997.
- Sublett. US Patent 5,552, 512 1996.
- Gandini, A.; Lacerda, T.M.; Carvalho, A.J.F.; Trovatti, E. Progress of Polymers from Renewable Resources: Furans, Vegetable Oils, and Polysaccharides. Chem. Rev. 2016, 116, 1637–1669. [Google Scholar] [CrossRef]
- Delidovich, I.; Hausoul, P.J.C.; Deng, L.; Pfützenreuter, R.; Rose, M.; Palkovits, R. Alternative Monomers Based on Lignocellulose and Their Use for Polymer Production. Chem. Rev. 2016, 116, 1540–1599. [Google Scholar] [CrossRef]
- Pellis, A.; Herrero Acero, E.; Gardossi, L.; Ferrario, V.; Guebitz, G.M. Renewable building blocks for sustainable polyesters: new biotechnological routes for greener plastics. Polym. Int. 2016, 65, 861–871. [Google Scholar] [CrossRef]
- Jeong, J.; Hussain, F.; Park, S.; Kang, S.-J.; Kim, J. High Thermal Stability, High Tensile Strength, and Good Water Barrier Property of Terpolyester Containing Biobased Monomer for Next-Generation Smart Film Application: Synthesis and Characterization. Polymers (Basel). 2020, 12, 2458. [Google Scholar] [CrossRef] [PubMed]
- Hussain, F.; Jeong, J.; Park, S.; Kang, S.J.; Khan, W.Q.; Kim, J. Synthesis and unique characteristics of biobased high Tg copolyesters with improved performance properties for flexible electronics and packaging applications. J. Ind. Eng. Chem. 2021, 100, 119–125. [Google Scholar] [CrossRef]
- Vanhaecht, B.; Teerenstra, M.N.; Suwier, D.R.; Willem, R.; Biesemans, M.; Koning, C.E. Controlled stereochemistry of polyamides derived from cis/trans-1,4-cyclohexanedicarboxylic acid. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 833–840. [Google Scholar] [CrossRef]
- Tsai, Y.; Fan, C.H.; Hung, C.Y.; Tsai, F.J. Amorphous copolyesters based on 1,3/1,4-cyclohexanedimethanol: Synthesis, characterization and properties. J. Appl. Polym. Sci. 2008, 109, 2598–2604. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Jia, Z.; Sun, L.; Zhang, Y.; Zhu, J. Modification of poly(ethylene 2,5-furandicarboxylate) (PEF) with 1, 4-cyclohexanedimethanol: Influence of stereochemistry of 1,4-cyclohexylene units. Polymer (Guildf). 2018, 137, 173–185. [Google Scholar] [CrossRef]
- Berti, C.; Celli, A.; Marchese, P.; Marianucci, E.; Barbiroli, G.; Di Credico, F. Influence of molecular structure and stereochemistry of the 1,4-cyclohexylene ring on thermal and mechanical behavior of poly(butylene 1,4-cyclohexanedicarboxylate). Macromol. Chem. Phys. 2008, 209, 1333–1344. [Google Scholar] [CrossRef]
- Jeong, Y.G.; Jo, W.H.; Lee, S.C. Crystal structure determination of poly(1,4-trans-cylcohexylenedimethylene 2,6-naphthalate) by X-ray diffraction and molecular modeling. Macromolecules 2003, 36, 5201–5207. [Google Scholar] [CrossRef]
- Lyman, D.J. Polyurethanes: Effect of cis-trans Isomerism on proeprties of polyurethane. J. Polym. Sci. 1961, 55, 507–514. [Google Scholar] [CrossRef]
- Sakellarides, S.L. Poly(ethylene naphthalate) PEN. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Inc, 2002; Vol. 11, pp. 88–114.
- Light, R.R.; Seymour, R.W. Effect of sub-Tg relaxations on the gas transport properties of polyesters. Polym. Eng. Sci. 1982, 22, 857–864. [Google Scholar] [CrossRef]
- Boye, C.A. X-ray diffraction studies of poly (1,4-cyclohexylenedimethylene terephthalate). Joural Polym. Sci. 1961, 55, 275–284. [Google Scholar] [CrossRef]
- Jeong, Y.G.; Jo, W.H.; Lee, S.C. Synthesis and crystallization behavior of Poly(m -methylene 2,6-naphthalate-co-1,4-cyclohexylenedimethylene 2,6-naphthalate) copolymers. Macromolecules 2003, 36, 4051–4059. [Google Scholar] [CrossRef]
- Turner, S.R.; Seymour, R.W.; Smith, T.W. Cyclohexanedimethanol polyesters. In Encyclopedia of Polymer Science and Technology; 2001; Vol. 2, pp. 127–134.
- Sublett, I.B.J. US Patent. 1996, 8–11.
- Sandhya, T.E.; Ramesh, C.; Sivaram, S. Copolyesters Based on Poly(butylene terephthalate)s Containing Cyclohexyl Groups: Synthesis, Structure and Crystallization. Macromol. Symp. 2003, 199, 467–482. [Google Scholar] [CrossRef]
- MacDonald, W.A. Engineered films for display technologies. J. Mater. Chem. 2004, 14, 4–10. [Google Scholar] [CrossRef]
- I-Chun Cheng and Sigurd Wagner Overview of flexible electronics. In Flexible Electronics : Materials and Applications Electronic Materials : Science and Technology; Salleo, W.S.W. and A., Ed.; Springer: New York, 2009; pp. 1–28 ISBN 0387743626.
- Lewis, J.S.; Weaver, M.S. Thin film permeation-barrier technology for flexible organic light-emitting devices. IEEE J. Sel. Top. Quantum Electron. 2004, 10, 45–57. [Google Scholar] [CrossRef]
- Hussain, F.; Park, S.; Jeong, J.; Jeong, E.; Kang, S.; Yoon, K.; Kim, J. Fabrication and characterization of poly(1,4-cyclohexanedimthylene terephthalate-co-1,4-cyclohxylenedimethylene 2,6-naphthalenedicarboxylate) (PCTN) copolyester film: A novel copolyester film with exceptional performances for next generation. J. Appl. Polym. Sci. 2021, 138, 49840. [Google Scholar] [CrossRef]
- Hussain, F.; Jeong, J.; Park, S.; Jeong, E.; Kang, S.-J.; Yoon, K.; Kim, J. Fabrication and characterization of a novel terpolyester film: An alternative substrate polymer for flexible electronic devices. Polymer (Guildf). 2020, 210, 123019–123026. [Google Scholar] [CrossRef]
- Hussain, F.; Fayzan Shakir, H.M.; Ali, A.; Rehan, Z.A.; Zubair, Z. Development and characterization of cyclic compound-based biaxially stretched smart polymeric film for futuristic flexible electronic devices. Eur. Polym. J. 2022, 172, 111243. [Google Scholar] [CrossRef]
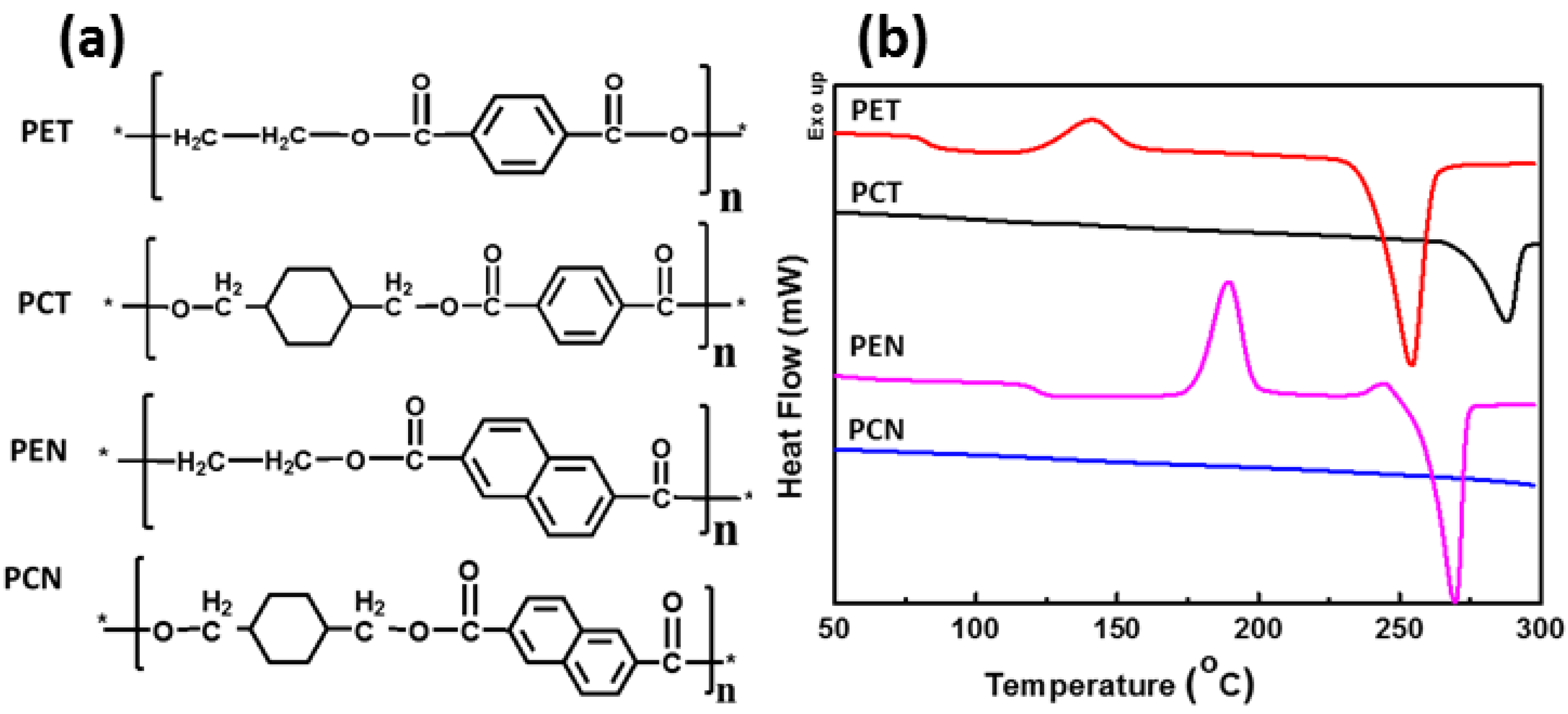
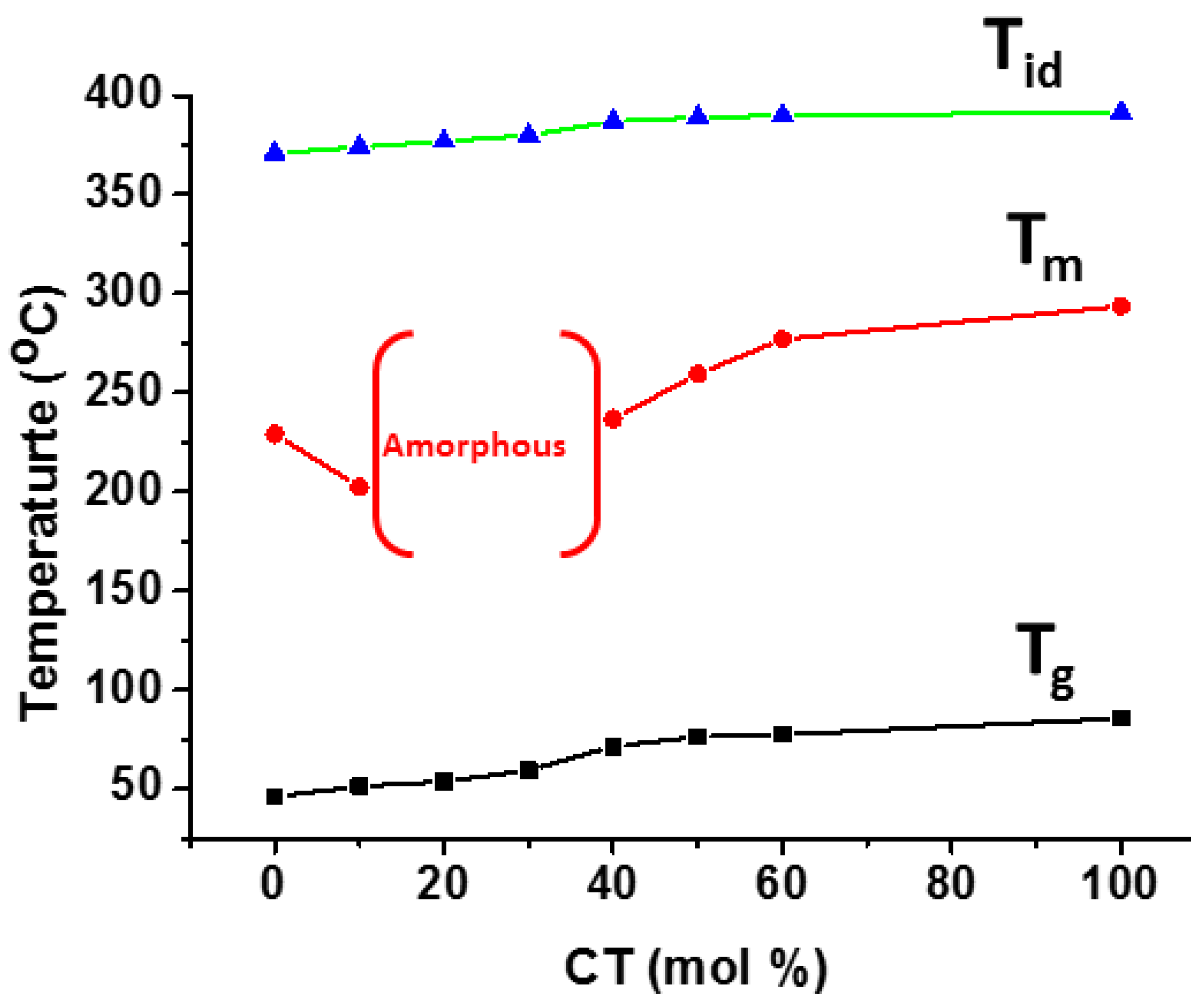
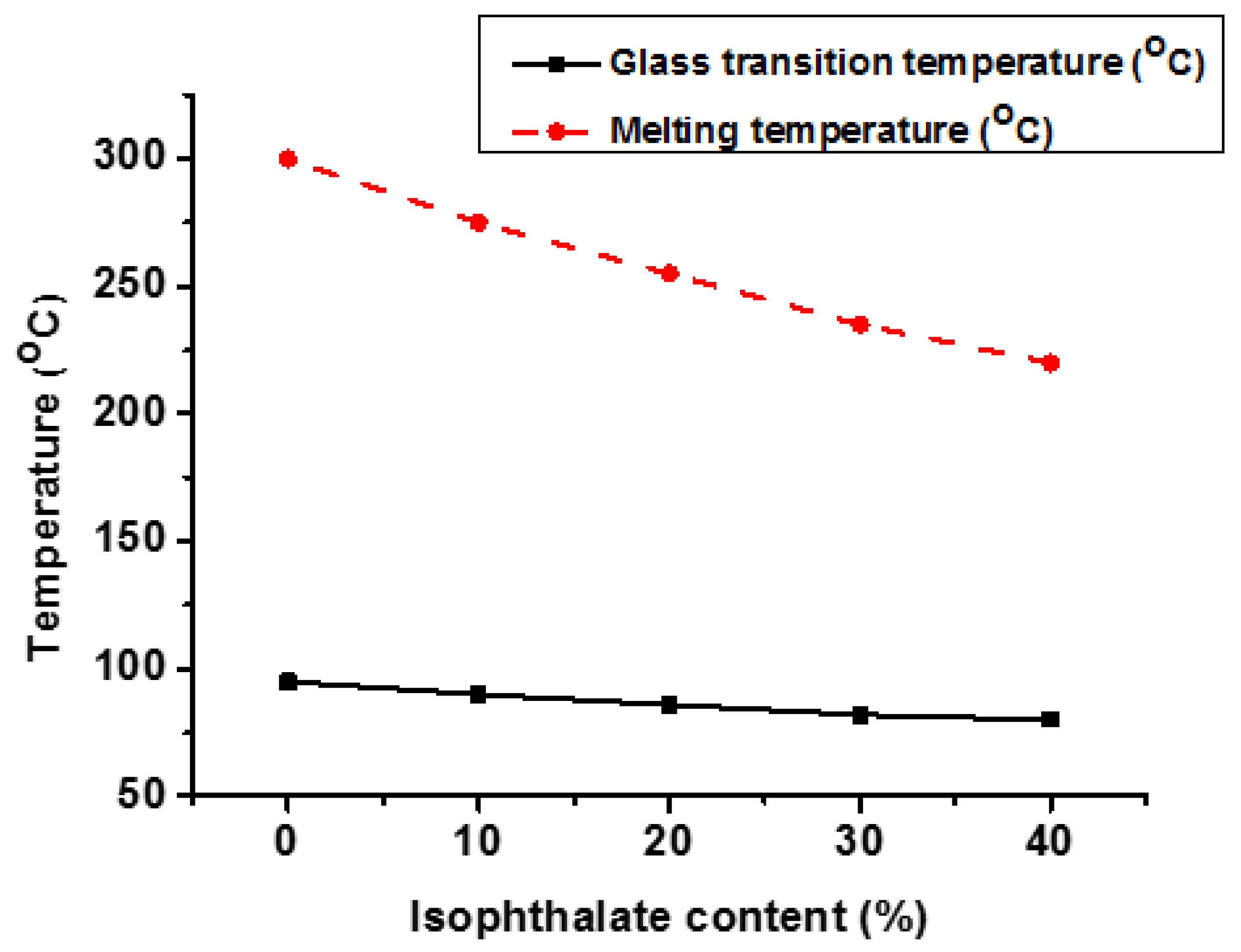
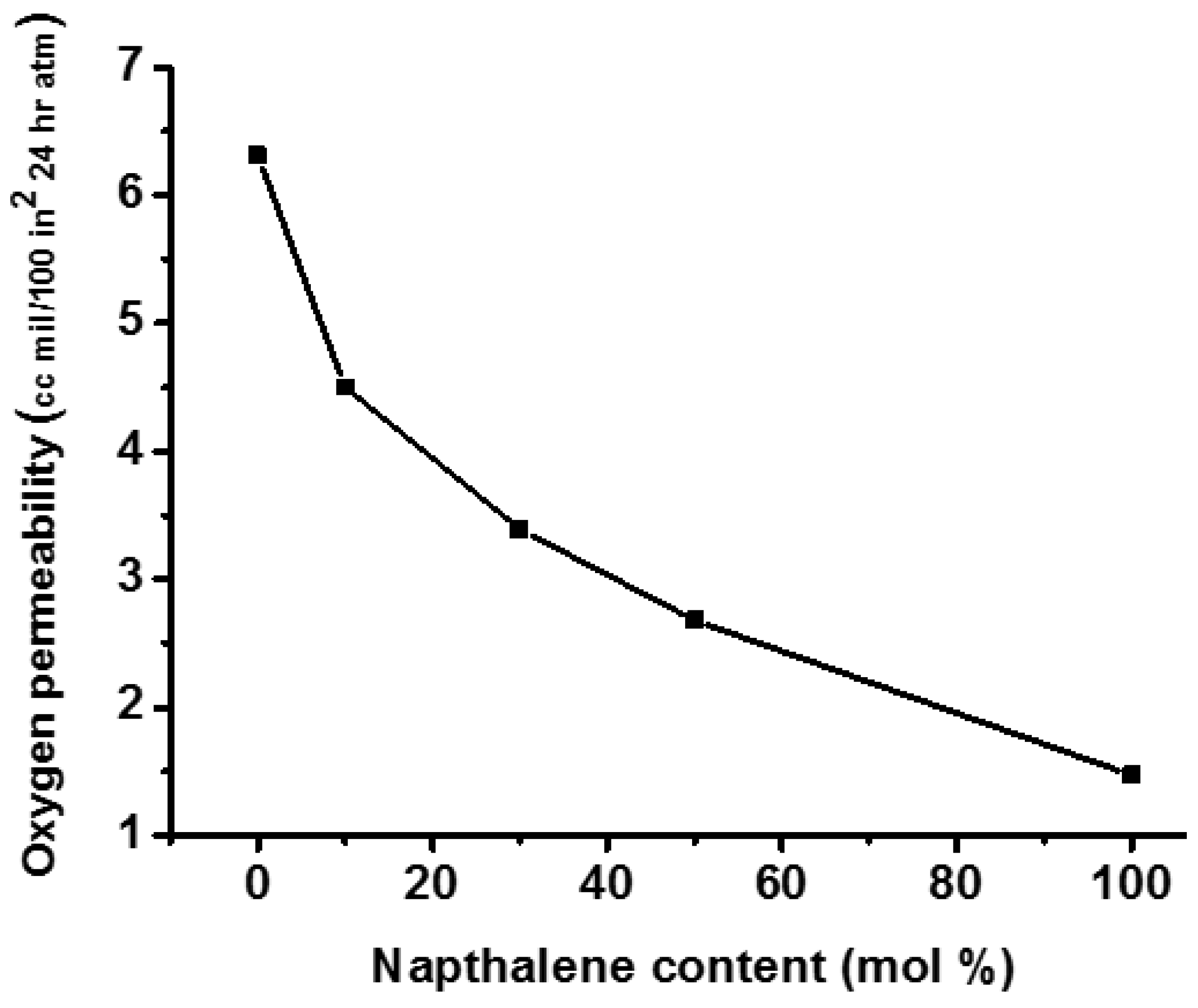
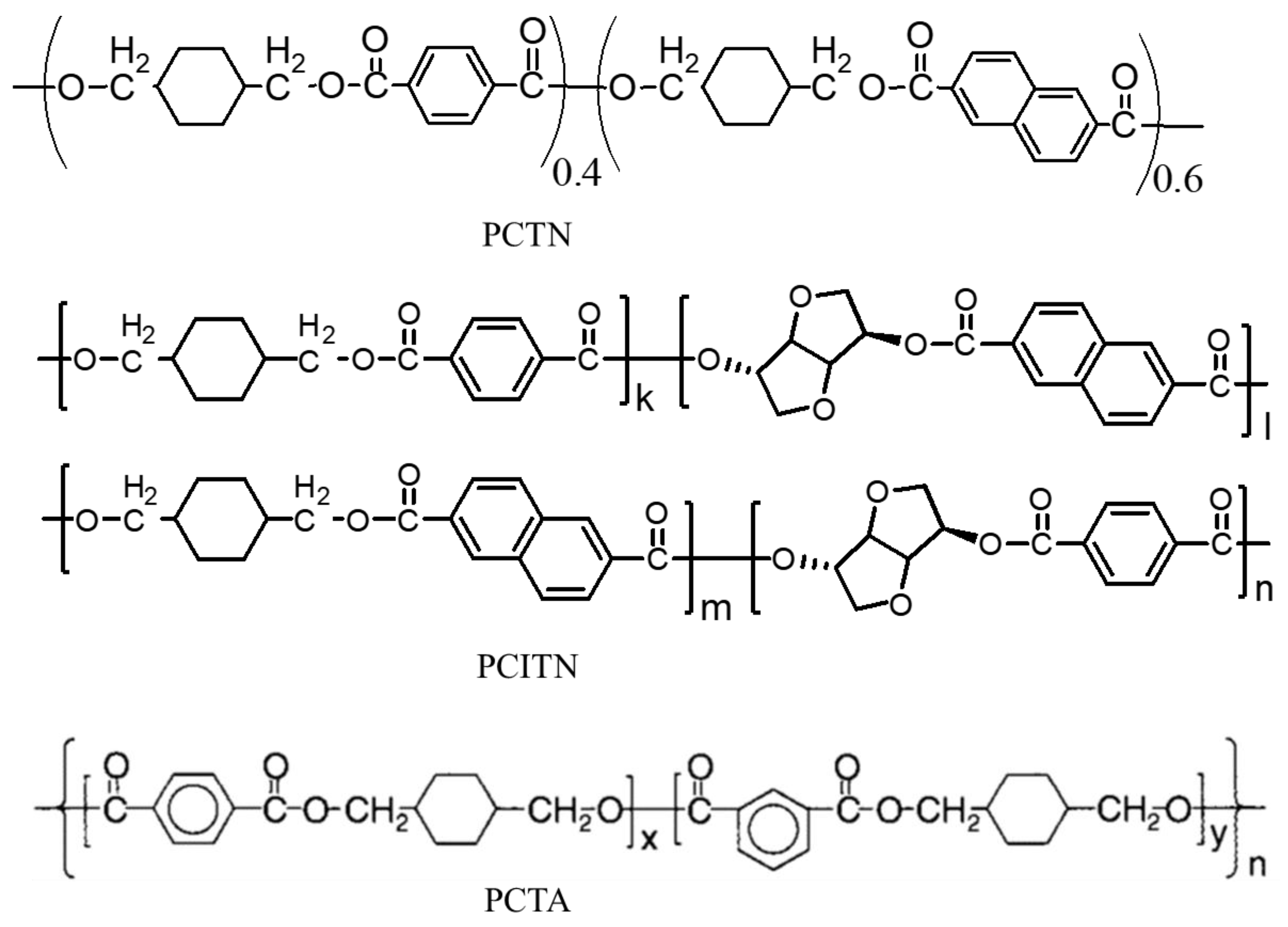
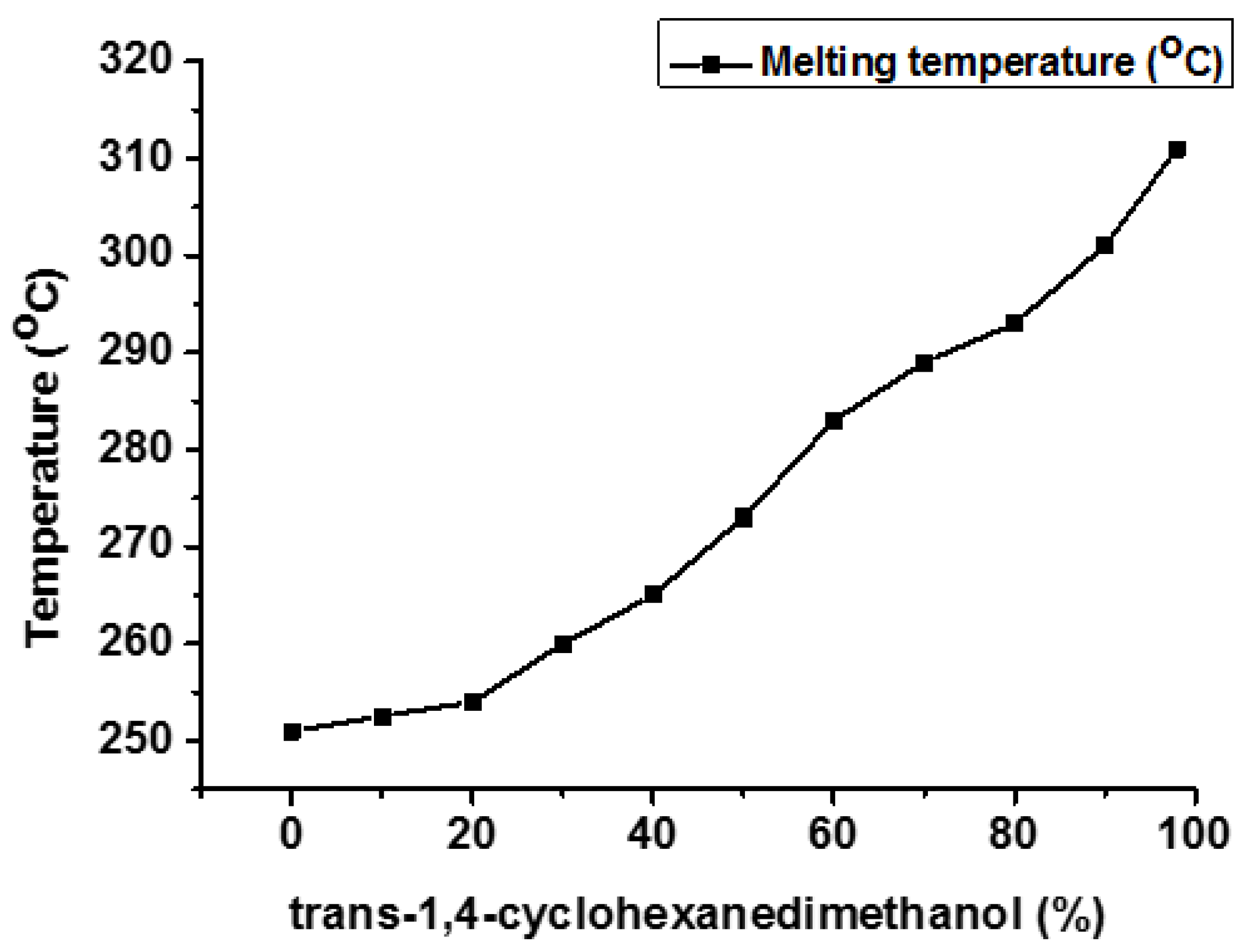
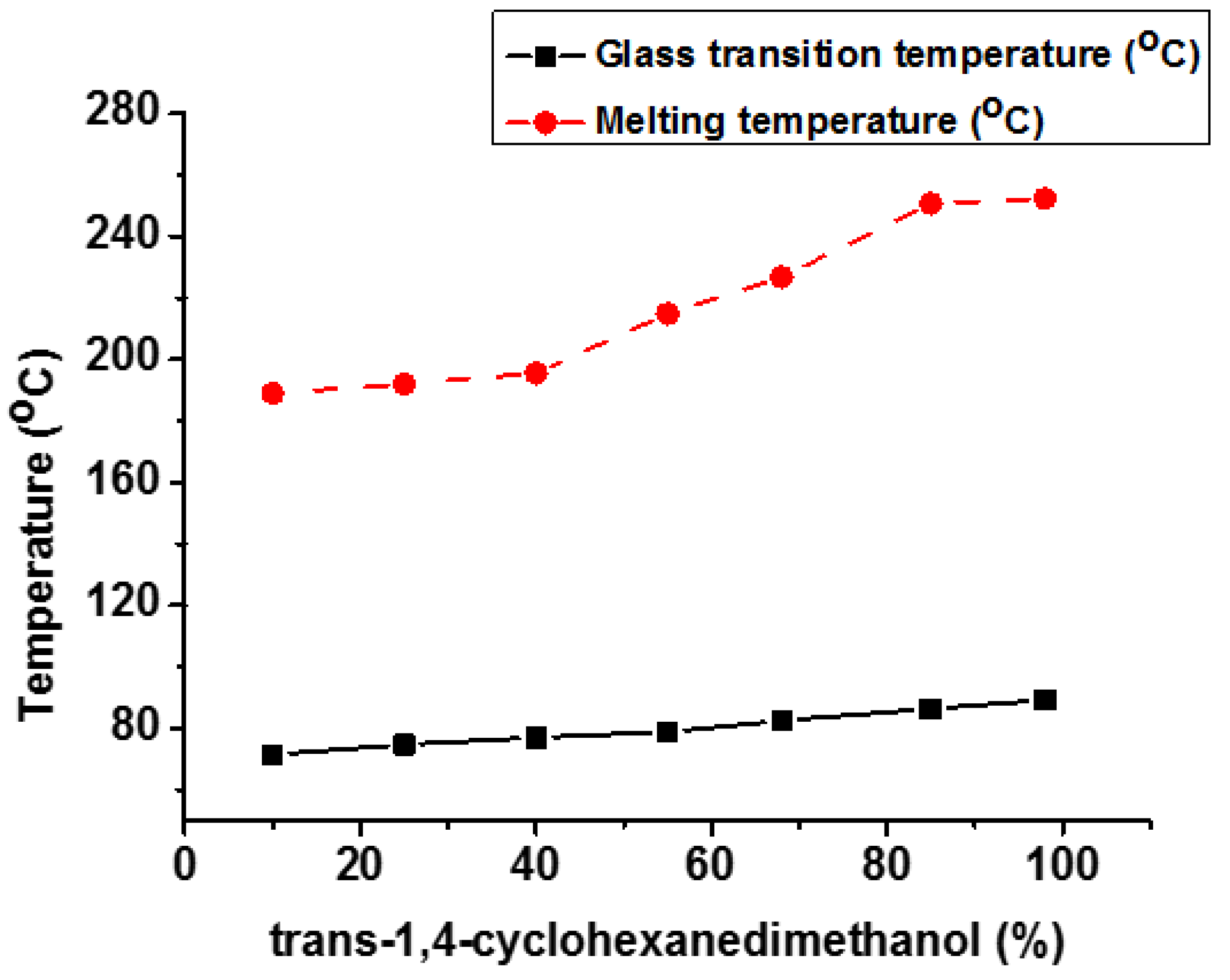
| Properties | PET | PCT | PEN | PCN |
|---|---|---|---|---|
| Monomers | EG, TPA | CHDM, TPA | EG, NDA | CHDM, NDA |
| Tg (oC) | 80 | 88 | 122 | 139 |
| Tm (oC) | 260 | 297 | 269 | 320 |
| Tc (oC) | 140 | - | 189 | - |
| Mw | 44,800 | 53,200 | 50,600 | 49,195 |
| Mn | 20,600 | 23,600 | 23,600 | 28,821 |
| Polymer disparity index | 2.17 | 2.25 | 2.14 | 1.71 |
| Lattice structure | Triclinic | Triclinic | Triclinic | triclinic |
| Density (g/cm3) | 1.337 | 1.197 | 1.198 | 1.313 |
| Intrinsic Viscosity(dl/g) | 0.70 | 0.79 | 0.84 | 0.70 |
| Young’s modulus, MPa | 3900 | 3660 | 5200 | - |
| Tensile strength, MPa | 45 | 52 | 60 | - |
| Break elongation, % | 150 | 250 | 65 | - |
| UV absorbance (360 nm, %) | 1 | 0.90 | 17 | - |
| Oxygen permeability (cm3-mil/100 in.2-24 h-atm) | 9.0 | 40 | 3.1 | 1.47 |
| Hydrolysis resistance, h | 50 | - | 200 | - |
| Samples | Tg (oC)a | Tm (oC)b | ∆Hm (J g-1)c | Crystallinity (%) |
|---|---|---|---|---|
| PCTN_0 | 83.79 | 285.76 | 48.53 | 47.6 |
| PCTN_18 | 84.75 | 261.69 | 31.94 | 31.3 |
| PCTN_26 | 85.12 | 246.81 | 31.43 | 30.81 |
| PCTN_36 | 85.35 | 235.85 | 39.49 | 38.7 |
| PCTN_47 | 88.62 | 238.21 | 35.54 | 34.8 |
| PCTN_56 | 92.62 | 255.17 | 27.16 | 26.6 |
| PCTN_65 | 107.54 | 278.61 | 28.33 | 27.8 |
| PCTN_83 | 114.30 | 310.21 | 39.37 | 38.6 |
| Copolyester composition |
Tg (oC) | Tm (oC) | IV (dl g-1) |
|---|---|---|---|
| PCT | 88.0 | 295.3 | 0.85 |
| PCTA-48 | 66 | 225 | 0.75 |
| PCTN_30 | 97.91 | 244.88 | 0.79 |
| PCTN_70 | 110.28 | 279.76 | 0.76 |
| PCTS_17 | - | 286.0 | 1.04 |
| PCTS_25 | - | 280.0 | 0.93 |
| PCTSA_17 | - | 268 | 0.91 |
| PCTSA_25 | - | 270 | 0.94 |
| PETg30N-30 | 85 | - | 0.73 |
| PETg30S-30 | 44 | - | 0.65 |
| property | PET (Malinex) |
PEN (Teonex) |
Glass | PI (Kapton) |
Steel |
|---|---|---|---|---|---|
| Optical property (%transmission 400-700 nm) | >85 | 0.85 | >92 | yellow | 0.0 |
| Tg (OC) | 80 | 121 | - | 410 | - |
| Water absorption (%) | 0.4 | 0.4 | 0.0 | 1.8 | 0.0 |
| Permeable to oxygen | yes | yes | no | yes | no |
| Young’s modulus (GPa) | 5.3 | 6.1 | 80 | 2.5 | 200 |
| Tensile strength, (MPa) | 225 | 275 | 27-62 | 231 | 370 |
| CLTE -55 TO 85 OC (ppm/OC) | 15 | 13 | 4 | 30-60 | 10 |
| Maximum processing temperature (OC) | 80 | 180 | 600 | 300 | 1000 |
| Deform after device fabrication | yes | yes | no | yes | No |
| Roll to roll processing? | likely | likely | unlikely | likely | Yes |
| Prebake required? | yes | yes | may be | yes | no |
| Electrical conductivity | none | none | none | none | high |
| Upper working temperature | 115-170 | 155 | 600 | 250-320 | 1400 |
| Thermal conductivity (W/m. OC) | 0.1 | 0.1 | 1 | 0 | 16 |
| Safe bending radius (cm) | - | 4 | 40 | 4 | 4 |
| Refractive index | 1.66 | 1.75 | 1.52 | 1.50 | 2.76 |
| Coefficient of hydrolytic expansion (ppm/%RH) | - | 11 | 0 | 11 | 0 |
| Thermal conductivity (W/m OC) | 0.1 | 0.1 | 1 | 0.1-0.2 | 16 |
| Density g/cm3 | 1.4 | 1.36 | 2.70 | 1.43 | 7.8 |
| Property | PI (Kapton) |
PCTN (Uniaxially stretched) |
PCTN (Biaxially Stretched) |
PCITN (Randomly Oriented) |
PCITN (Uniaxially stretched) |
| Glass transition temperature (oC) | 360 | 127 | 124.3 | 120.4 | 140 |
| Melting temperature (oC) | - | 279 | 276.8 | 279 | 275 |
| Commercial availability | Yes | No | No | No | No |
| Transmission (300-800 nm), % | Yellow | 87 | 94 | 86.7 | 86 |
| CTE (-55 to 85 oC) (ppm oC-1) | 30-60 | 6.0 | 13.6 | - | 5.8 |
| Young’s modulus (GPa) | 2.5 | 2.1 | 2.8 | 2.2 | 2.6 |
| Birefringence (△n) | - | 0.09 | 0.003 | 0.08 | 0.09 |
| Water absorption (%) (Randomly Oriented) |
1.8 | 0.37 | 0.16 | 0.21 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





