1. Introduction
In a world where health and well-being are constant priorities, the quest for functional foods that offer additional health benefits has become a significant area of scientific interest. Dairy products, such as yogurt, have demonstrated effectiveness as vehicles for delivering bioactive compounds that can enhance health and prevent diseases [
1]. The incorporation of nanoliposomes as efficient carriers of bioactive compounds has emerged as a promising strategy to improve the stability and bioavailability of these compounds in dairy products [
2,
3,
4]. One such bioactive compound is fucoxanthin, a marine carotenoid found in brown algae with antioxidant properties and potential neuroprotective effects [
5]. Fucoxanthin has received considerable attention due to its ability to inhibit oxidative stress and reduce the risk of chronic and neurodegenerative diseases [
4,
5,
6]. However, its application in dairy products has been limited by its low water solubility and susceptibility to degradation during processing and storage. To overcome these challenges, encapsulating fucoxanthin in nanoliposomes offers a promising strategy. Nanoliposomes are controlled release systems that can protect and stabilize bioactive compounds, enhancing their solubility and bioavailability [
6].
Nanoliposomes are spherical nanostructures primarily composed of phospholipids. They have emerged as promising delivery vehicles for various bioactive compounds, including carotenoids [
4,
7]. The absorption of carotenoids in the gastrointestinal system is influenced by several factors, including their water solubility and the presence of lipids. Recent studies have demonstrated the potential of nanoliposomes as carotenoid delivery vehicles, showing significant improvements in the bioavailability and absorption of these compounds compared to conventional formulations [
8,
9]. Additionally, encapsulation in nanoliposomes can protect carotenoids from oxidative degradation and improve their stability during food processing and storage [
7,
10,
11]. The impact of storage on the antioxidant, physicochemical, and rheological properties of yogurt enriched with antioxidant compounds is a crucial aspect to consider in the development of functional dairy products [
12,
13]. As enriched yogurt is stored for extended periods, various factors can influence its characteristics and quality. During storage, the antioxidant compounds in yogurt may be affected by oxidation, which could decrease their antioxidant capacity [
14].
Prolonged storage of enriched yogurt can lead to changes in its physicochemical properties, such as acidity, pH, viscosity, and texture. The acidification of yogurt during storage can affect its taste and acceptability [
15,
16]. Additionally, changes in the structure of the dairy matrix and the distribution of added compounds can affect the homogeneity and stability of the product. The rheology of yogurt, i.e., its behavior under deformation and flow, can change during storage due to the reorganization of the dairy structure. This can manifest in changes in the yogurt’s viscosity, elasticity, and fluidity [
17,
18]. The formation of whey and phase separation can also occur during prolonged storage, affecting the product’s texture and consistency [
17,
18]. Therefore, it is important to conduct stability studies during storage to evaluate how these changes impact the quality and acceptability of enriched yogurt ([
19,
20]. These studies will provide a better understanding of the mechanisms of deterioration during storage and help develop strategies to improve the stability and quality of yogurt enriched with antioxidant compounds, analyzing the impact of nanoliposome addition on the antioxidant, physicochemical, rheological, and sensory properties.
Thus, the present study aims to develop and evaluate yogurt enriched with fucoxanthin-loaded nanoliposomes, investigating the impact of storage on its antioxidant, physicochemical, and rheological properties under storage condition. This multidisciplinary approach combines the encapsulation technology of bioactive compounds with food science and nutrition, aiming to provide a functional dairy product that can enhance the health and well-being of consumers. The results obtained will provide valuable insights into the feasibility of this fortification strategy and its implications for the formulation of functional foods in the future.
2. Results and Discussion
2.1. Morphological Study and Particle Size Measurement
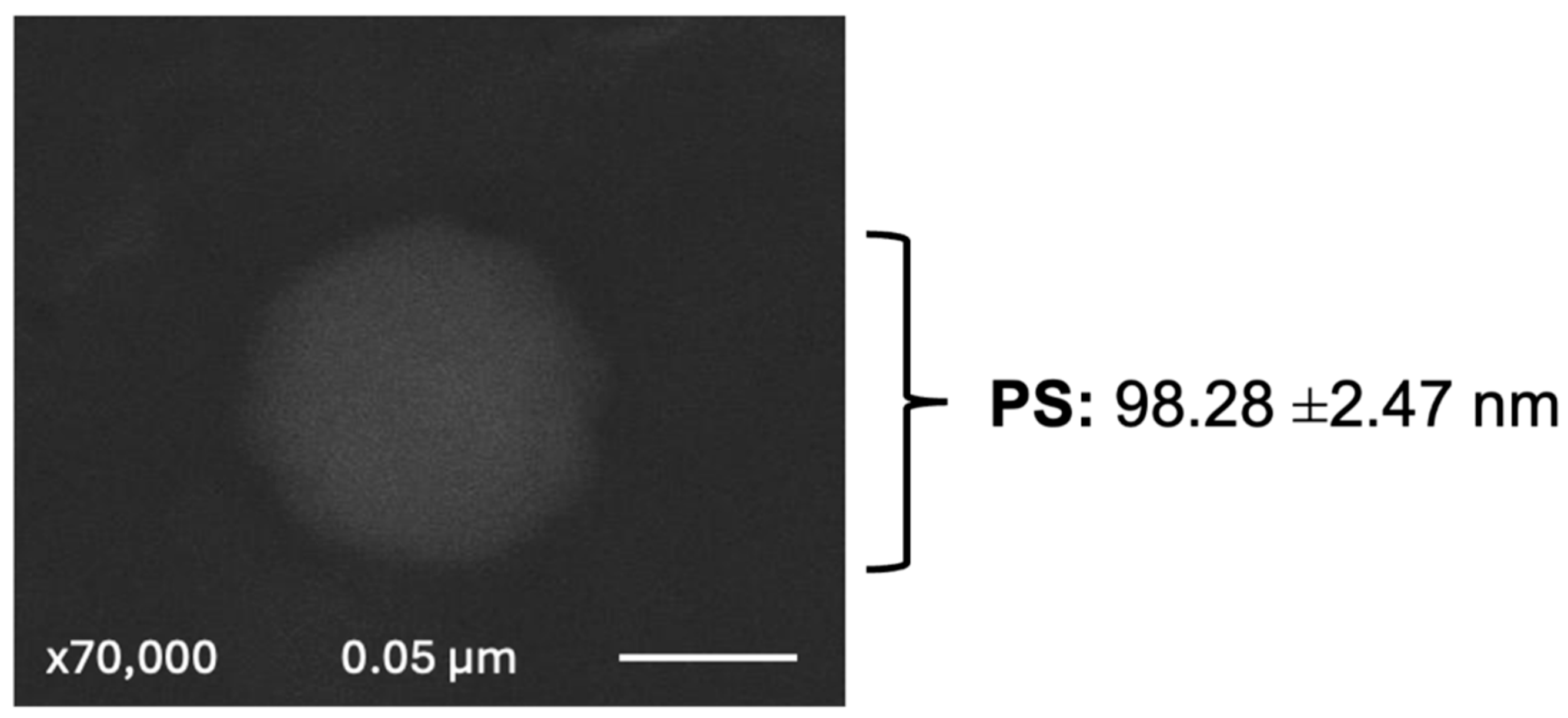
Scanning electron microscopy (SEM) was employed to visually validate the uniformity, size, shape, and integrity of the nanoliposoma vehicles, as shown in
Figure 1. This technique offers a superior resolution to traditional methods, providing detailed visualization of liposomal vesicles and verifying their structural integrity, uniformity, and homogeneity, utilizing SEM ensures the quality of liposomal vehicles. Fucoxanthin-loaded nanoliposomes (FXN-LN) exhibited a particle size of 98.28 ± 2.47 nm, which directly influences the absorption, distribution, release, and internalization of fucoxanthin. This extends the half-life of nanoliposomes in the bloodstream and reduces their absorption in the liver and spleen [
21]. The reduction in particle size of the nanoliposomes is achieved through the ultrasound-assisted encapsulation method, which uses the mechanical energy generated during ultrasound treatment to decrease the size of the liposomal vesicles, thereby improving their uniformity and distribution. These results are consistent with those obtained by Pan et al. [
22] and Rodríguez-Ruiz et al. [
23] for the astaxanthin encapsulation.
The morphological analysis reveals nanoliposomes with homogeneous distribution and size. The shape of the lipid vesicles indicates that all particles exhibit similar size and structure, which are typical characteristics of ideal nanoliposomes [
24]. Moreover, no deformations, aggregations, or structural aberrations are observed in the nanoliposomes. In research, the development of new delivery systems for bioactive compounds in food matrices is a highly attractive strategy for enhancing the biological functions of these molecules. The nanoscale size of liposomes facilitates their internalization into cells and biological tissues, improving aspects such as bioavailability, especially in complex membranes like the blood-brain barrier. Their well-defined spherical shape provides greater structural support and stability, allowing uniform distribution of encapsulated compounds within various matrices. The absence of deformations in the vesicles ensures the stability of the encapsulated compounds during storage [
25,
26].
2.2. Encapsulation Efficiency
The encapsulation efficiency (EE) is a measure that indicates the percentage of fucoxanthin successfully encapsulated within the liposomal vehicles relative to the total amount of fucoxanthin used in the encapsulation process (free fucoxanthin). EE is a key indicator for estimating the stability of the liposomal system, this suggests that fucoxanthin is well protected within the liposomes, which is crucial for maintaining its stability during storage and administration. The encapsulation efficiency of fucoxanthin in nanoliposomes (95.33 ± 1.34%) is considered high and efficient, indicating that the encapsulation process used in this study is highly effective. The results suggest that the process parameters, such as lipid ratio, encapsulation technique, and operating conditions, have been adequately controlled to maximize the incorporation of fucoxanthin into the nanoliposomes. This high encapsulation percentage not only ensures the protection and stability of fucoxanthin but also opens doors for future research and practical applications in various fields. The ability to consistently maintain a high EE reinforces the viability of these nanoliposomes as efficient delivery vehicles for bioactive compounds, offering a robust platform for use in commercial and therapeutic products.
Due to the nonpolar nature of fucoxanthin, it is considered that its encapsulation occurred in the hydrophobic part of the vehicles, specifically in the lipid membrane, between the hydrophobic tails of the fatty acids that constitute the phospholipids. This characteristic increases the incorporation of fucoxanthin into the liposomal vehicles. This characteristic and encapsulation efficiency percentage were observed in studies by Pan et al. [
22] and Taksima et al. [
27] for astaxanthin, with an encapsulation efficiency ranging from 95-97%. The hydrophobic nature of carotenoids increases the affinity for the hydrophobic part of phosphatidylcholine molecules, encapsulating it within the lipid bilayer, thereby facilitating its interaction and encapsulation [
24,
25,
26,
27]. Finally, the high EE of fucoxanthin suggests that nanoliposomes have great potential for applications in the pharmaceutical and food industries, fortifying foods with fucoxanthin and enhancing their functional value. In the pharmaceutical industry, these nanoliposomes could be used to develop dietary supplements and medications with antioxidant and anti-inflammatory benefits.
2.3. Stability of Nanoliposomes by Centrifugation
The stability of fucoxanthin-loaded nanoliposomal carriers, indicates a high degree of stability (92.67 ± 2.34%), suggesting that the nanoliposomes are effective in maintaining their structural integrity and functional efficacy under the given conditions. When comparing the stability of these nanoliposomes to other systems reported in scientific literature, several key points emerge. According to a study by Sun et al. [
8], nanoliposomes encapsulating natural antioxidants, such as resveratrol and curcumin, demonstrated stability percentages ranging from 85% to 90% over a period of one month at room temperature. The observed stability of 92.67% for fucoxanthin-loaded nanoliposomes is slightly higher, indicating better preservation of encapsulated compounds under similar conditions. Research by Mozafari et al. [
28] on nanoliposomes encapsulating bioactive compounds reported stability values of approximately 88% over a similar timeframe. The higher stability percentage in the current study suggests that the formulation and preparation method for fucoxanthin-loaded nanoliposomes may be more effective in maintaining structural integrity compared to the methods used in the referenced studies. The lipid composition of nanoliposomes plays a crucial role in their stability. Studies by Mozafari [
29] highlight that the inclusion of cholesterol in the lipid bilayer can significantly enhance the stability of nanoliposomes. The 92.67% stability observed in the current study might be attributed to an optimized lipid composition, possibly including cholesterol or other stabilizing agents. The stability of nanoliposomes is also influenced by storage conditions. A study by Xu et al. [
30] on nanoliposomes containing polyphenols reported stability percentages around 87% when stored at 4°C. The stability of fucoxanthin-loaded nanoliposomes at 92.67% could imply that the storage conditions were optimized, possibly involving controlled temperature and light exposure to minimize degradation. The observed stability of 92.67% for fucoxanthin-loaded nanoliposomes is noteworthy and compares favorably with other nano-liposomal systems reported in the literature.
The stability of nanoliposomes is crucial for their incorporation into functional foods, such as enriched yogurt. High stability ensures that nanoliposomes maintain their structure during the processing and storage of yogurt, preserving their encapsulating properties and preventing the premature release of bioactive compounds. Stability contributes to the homogeneous distribution of bioactive compounds within the yogurt, guaranteeing a uniform concentration of the active ingredient in each serving, thus enhancing the consistency and efficacy of the functional product. Stable nanoliposomes minimize undesirable changes in the sensory properties of yogurt, such as flavor, texture, and color, as the degradation of unstable nanoliposomes could release compounds that negatively affect the product’s acceptability to consumers. Furthermore, the stability of nanoliposomes influences the preservation of biological activity by providing a protective barrier for bioactive compounds, shielding them from adverse factors such as light, oxygen, and temperature, thereby preserving their biological activity throughout the product’s shelf life. This stability also allows for controlled and sustained release of bioactive compounds, improving their bioavailability and efficacy, ensuring that health benefits are maintained over time.
The stability of nanoliposomes ensures that bioactive compounds reach their biological target in their most effective form, optimizing their antioxidant and anti-inflammatory effects in the organism. When compared to the delivery of other antioxidant compounds, resveratrol-loaded nanoliposomes exhibited a stability of 90% and significantly improved the bioavailability of resveratrol in functional foods, suggesting that fucoxanthin-loaded nanoliposomes with a stability of 92.67% could have a similar or superior impact [
28,
29]. Curcumin-loaded nanoliposomes, with a stability of 88%, were successfully utilized in enriched dairy products, preserving their bioactive properties, indicating that the higher stability of fucoxanthin-loaded nanoliposomes could better conserve their health benefits when incorporated into yogurt [
8]. Polyphenol-loaded nanoliposomes, with a stability of 87%, maintained their antioxidant activity in functional foods, suggesting that the high stability of fucoxanthin-loaded nanoliposomes will not only preserve their biological activity but also potentially enhance the antioxidant benefits of enriched yogurt [
30]. These studies indicate that the high stability of nanoliposomes is a determinant factor for their successful incorporation into enriched yogurt, ensuring the protection and controlled release of bioactive compounds, preserving their biological activity, and enhancing the functional properties of yogurt, indicating a promising application in the functional food industry.
2.4. In Vitro Release
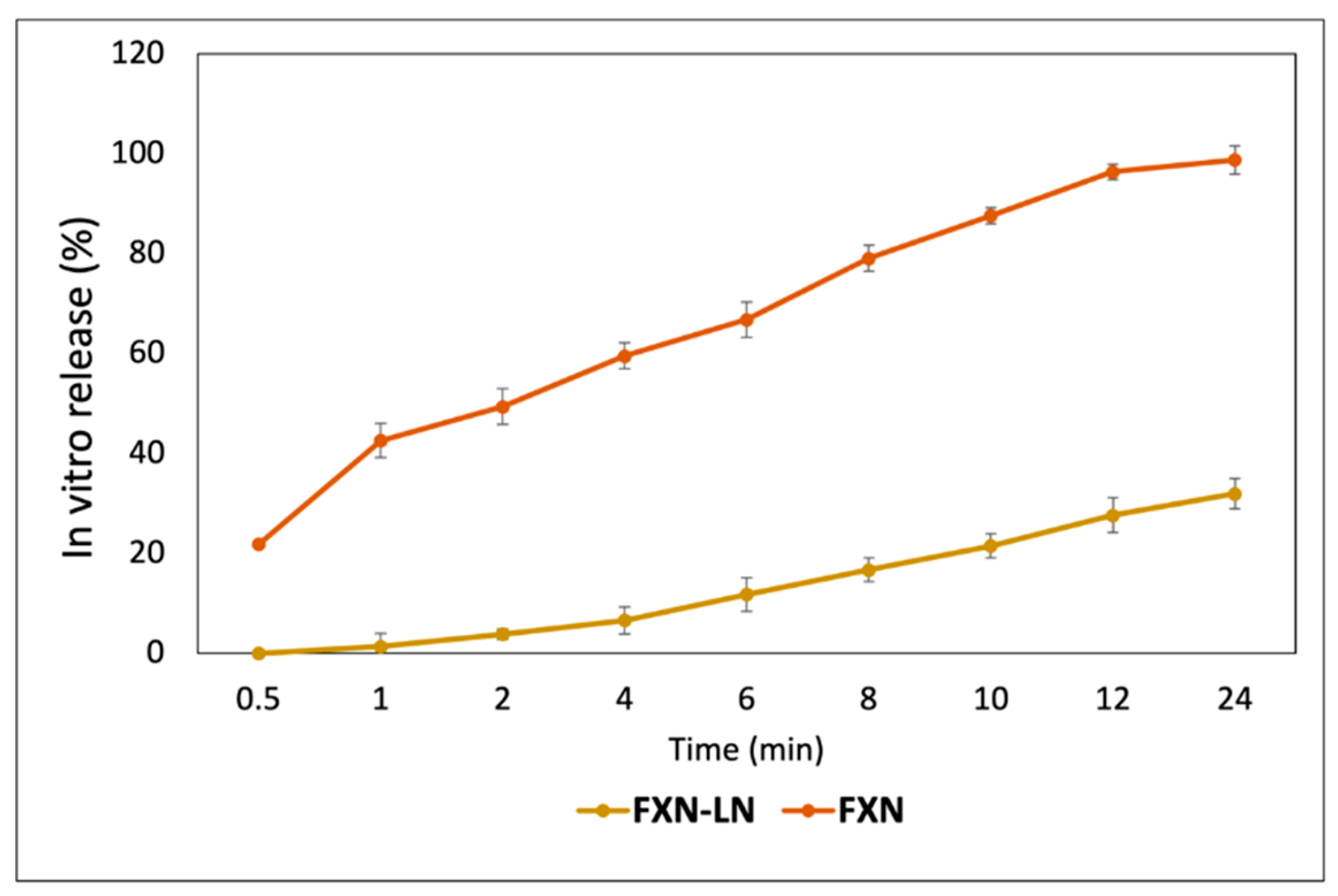
As a shown in
Figure 2, the in vitro release profile results demonstrate that free fucoxanthin exhibits a rapid and significant release, reaching 98.78% within 24 hours, with an initial increase of 21.87% at 0.5 hours. This percentage quickly rises, reaching 42.60% at 1 hour and 66.85% at 6 hours. By 10 hours, the release reaches 87.64%, and finally, at 24 hours, almost complete release is observed. In contrast, fucoxanthin encapsulated in nanoliposomes shows a slower and more controlled release, achieving only 31.98% at the end of the same period, with no release in the first 0.5 hours. The release begins to gradually increase, reaching 1.38% at 1 hour and rising to 6.62% at 4 hours. At 6 hours, the release is 11.81%, and it continues to increase at a constant rate, reaching 21.54% at 10 hours. This difference indicates that nanoliposomes are effective in delaying and maintaining a sustained release of fucoxanthin, which could be beneficial in applications requiring prolonged release of the compound, such as in drug delivery or nutritional supplements. Nanoliposome encapsulation provides a controlled and gradual release, whereas the free form offers immediate and rapid availability of fucoxanthin. In other words, the release of free fucoxanthin is much faster, with a high percentage of release achieved in the initial hours, indicating immediate and rapid availability in its free form. Meanwhile, the release of fucoxanthin encapsulated in nanoliposomes is significantly slower and controlled. Both forms of fucoxanthin show a continuous increase in the percentage of release over time, but the magnitude and rate of increase differ significantly.
The effectiveness of nanoliposomes in delaying and maintaining a sustained release of fucoxanthin has been well-documented in scientific literature. This characteristic is beneficial for applications requiring prolonged release of the compound, such as in drug delivery or nutritional supplements. Nanoliposomes enable a controlled and gradual release, in contrast to free fucoxanthin, which offers immediate and rapid availability. Studies have shown that nanoliposome-based formulations can increase stability, improve epithelial permeability and bioavailability, and prolong the drug’s half-life in the bloodstream, while minimizing adverse side effects [
31,
32,
33]. Additionally, the ability of nanoliposomes to encapsulate both hydrophobic and hydrophilic drugs and release them in a controlled manner has been highlighted as a significant advantage in various biomedical and food applications [
34,
35]. These systems have shown efficacy in enhancing drug stability and improving targeted drug delivery, which is crucial in treating complex diseases such as cancer [
36].
2.5. Identification of Nanoliposomal Vehicles in Freeze-Dried Yogurt-Enriched by Scanning Electron Microscopy
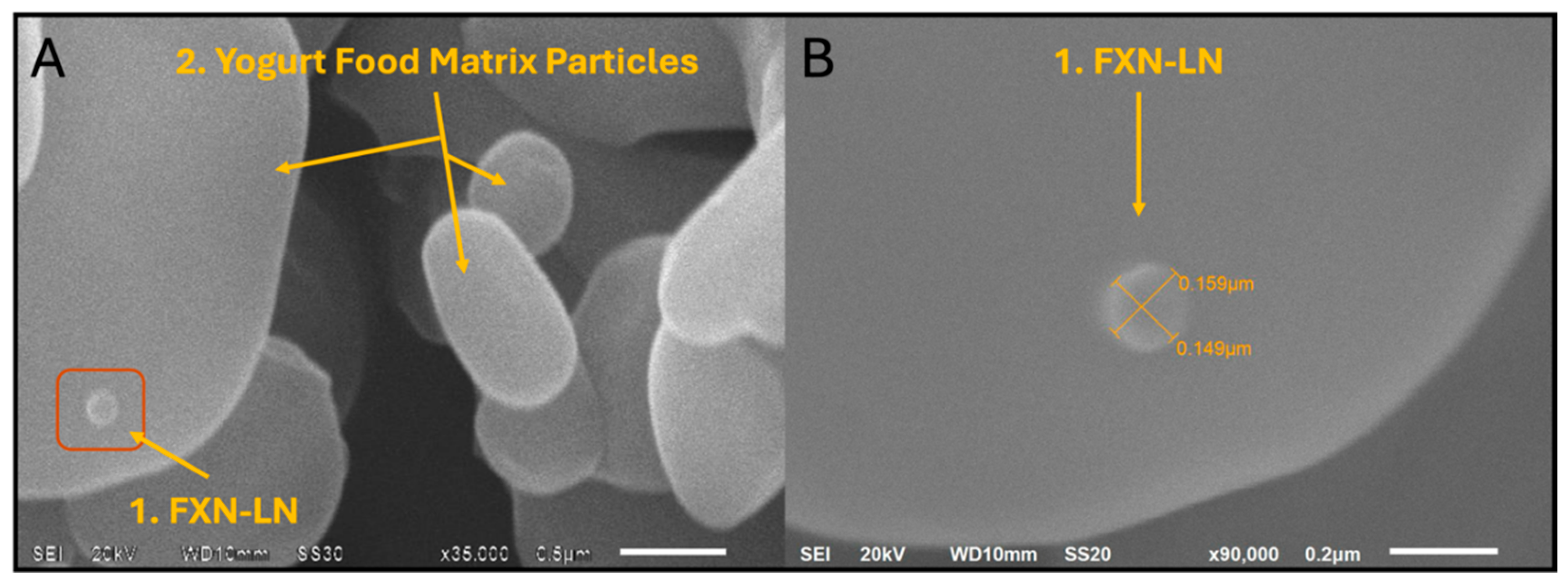
The results obtained through scanning electron microscopy (SEM) revealed the presence of nano-liposomes in the enriched yogurt, indicating successful integration of these delivery vehicles into the food matrix (
Figure 3). Direct observation of nanoliposomes in yogurt is an important indicator of their stability in this complex environment made up of micro- and macromolecules. The particle size of the nanoliposomes ranges between 0.149 and 0.159 μm, suggesting that these nanoliposomes maintain a relatively uniform size [
28,
30]. This uniformity in size is crucial to ensure homogeneous distribution of nanoliposomes in yogurt, which could significantly impact their effectiveness as delivery vehicles for fucoxanthin. Preserving the morphology and size of the nanoliposomes in enriched yogurt is essential as it ensures proper protection of encapsulated fucoxanthin and their controlled release. The stability of nanoliposomes in the yogurt matrix suggests that they can withstand food processing and storage conditions, which is essential to ensure the efficacy of the final product in terms of its therapeutic potential. Overall, the successful detection of nanoliposomes in enriched yogurt, along with their uniform particle size, points towards the viability of this delivery system in functional food formulation. These findings support the notion that yogurt enriched with fucoxanthin-loaded nanoliposomes could offer a promising nutritional and therapeutic option for improving brain health and providing additional benefits for the consumer [
37,
38,
39,
40].
2.6. Impact of Cold Storage Conditions on Antioxidant, Physicochemical, and Rheological Properties of Different Formulation of Yogurt
2.6.1. Antioxidant Properties
Cold storage is a common practice to prolong the shelf life of food and preserve its functional and sensory properties. In this study, the antioxidant properties of yogurt formulations (Y-C, Y-FXN-5, Y-FXN-10) were evaluated under a 21-day cold storage period (
Table 1). The antioxidant properties were assessed using the DPPH, ABTS, and FRAP methods. The DPPH and ABTS methods measure the antioxidant capacity of a substance to donate electrons or hydrogen atoms and neutralize free radicals.
The results show a decrease in antioxidant activity for the different formulations as the storage period progresses. The evaluation of free radical scavenging activity using the DPPH assay was as follows: For Y-C, the antioxidant activity decreased from 24.77% to 19.23% by day 21. Initially, Y-FXN-5 exhibited an antioxidant activity of 34.99%, which decreased to 31.06% at the end of the storage period. Finally, Y-FXN-10 showed a much higher initial antioxidant activity (52.96%), which reduced to 50.34% by day 21. Thus, the results indicate that the Y-FXN-10 formulation maintained the highest DPPH free radical inhibitory activity. The ABTS assay also measures antioxidant capacity but, unlike DPPH, can react with a broader range of antioxidant molecules. The results show that the antioxidant activity of the control (Y-C) decreased from 33.72% to 25.98% after 21 days. In Y- FXN-5, the initial antioxidant activity was 43.38%, decreasing to 39.27% at the end of the storage period. Y-FXN-10 started with a high value of 97.97% and ended at 88.42%. Regarding the reducing power of the formulations measured by FRAP, the control (Y-C) showed a decrease from 2.85 mmol ET/g to 2.25 mmol ET/g, while Y-FXN-5 showed an initial capacity of 3.20 mmol ET/g, slightly reducing to 3.16 mmol ET/g. The Y-FXN-10 formulation presented the highest initial capacity (3.31 mmol ET/g), slightly decreasing to 3.04 mmol ET/g by day 21. These results indicate that the addition of fucoxanthin-loaded nanoliposomes (Y-FXN-5 and Y-FXN-10) significantly enhances the antioxidant properties of yogurt compared to the control (Y-C), with Y-FXN-10 showing the greatest antioxidant stability during cold storage. It is observed that the nanoliposomes might be providing additional protection, possibly due to the controlled release of fucoxanthin and protection against oxidation.
The results are expressed as mean ± standard deviation (n = 3). Means with different lowercase letters (a-h: time effects) within the same treatment are significantly different (p < 0.05). Y-C= Control Yogurt without nanocapsules; Y-FXN-5= Yogurt enriched with 5% nanocapsules; Y-FXN-10= Yogurt enriched with 10% nanocapsules.
Yogurt formulations without antioxidant additives or functional ingredients such as nanoliposomes typically show a decrease in their antioxidant capacity during storage. This is due to the natural degradation of antioxidant compounds like vitamins, polyphenols, and bioactive peptides. Studies by Corrêa et al. [
41] report that the antioxidant capacity in unenriched yogurts significantly decreases during storage, similar to what was observed in the Y-C formulation of our study. Bourne [
42] and Lee & Lucey [
43] reported a decrease in antioxidant capacity in natural yogurts due to the oxidation of lipids and proteins during cold storage. In our study, the Y-C formulation showed a significant decrease in antioxidant capacity (DPPH, ABTS, FRAP) over the 21-day storage period, which is consistent with Lee & Lucey [
43] findings.
On the other hand, natural antioxidants have been incorporated into yogurts to enhance their functional properties. According to studies by Jeong et al. [
44], yogurt enriched with green tea extract showed a significant improvement in initial antioxidant capacity but a decrease during storage. Ranadheera et al. [
45] reported that the addition of polyphenol-rich fruit extracts derived from pomegranate and açaí increased the initial antioxidant capacity of yogurt. However, antioxidant capacity decreased in both studies over time due to the degradation of bioactive compounds that were not protected or encapsulated. The use of nanoliposomal carriers to effectively transport bioactive compounds and achieve controlled release of antioxidants is considered a relatively new and promising strategy. Studies by Gómez-Estaca et al. [
46] report that curcumin-loaded nanoliposomes significantly improved the stability and antioxidant capacity of yogurt during storage. Encapsulation showed a lesser decrease in antioxidant capacity compared to non-encapsulated yogurts. Similarly, Wu et al. [
46] found that resveratrol-loaded nanoliposomes not only improved the initial antioxidant capacity of yogurt but also maintained these properties during a prolonged cold storage period. The results of both studies are consistent with our findings aimed at improving the stability of antioxidants in yogurt. Based on previous studies and the results obtained in our research, the incorporation of nanoliposomes in yogurts can be an effective strategy to develop functional dairy products such as yogurt with enhanced antioxidant benefits, potentially contributing to consumer health and extending the product’s shelf life. These findings suggest that the incorporation of fucoxanthin-loaded nanoliposomes in yogurt not only improves its initial antioxidant properties but also helps maintain these properties during cold storage, making the product more stable and potentially healthier for consumers. However, these results are not sufficient to establish the therapeutic potential of yogurt formulations to delay cellular oxidation and premature aging. Additionally, the erythroprotective activities (Hemolysis inhibition, photohemolysis inhibition, and membrane stabilization test) will provide complementary information on cellular antioxidant activity.
The
Table 2 show the results of erythroprotective activities. Hemolysis inhibition (HI %) was significantly higher in the yogurt formulations enriched with nanoliposomes (Y-FXN-5 and Y-FXN-10) compared to the control yogurt (Y-C). Y-FXN-10 exhibited the highest hemolysis inhibition at 82.41%, followed by Y-FXN-5 at 63.83%, whereas the control only reached 17.29%. These data are consistent with the results for the percentage of photohemolysis inhibition, another method of inducing free radical production. Here, Y-FXN-10 also showed a notably higher erythroprotective potential at 82.4%, which is significantly higher than that of the control (61.07%) and Y-FXN-5 (54.93%). Regarding the membrane stabilization test, for Heat-IH, Y-FXN-10 presented significantly higher values (46.8%) compared to Y-C (25.5%) and Y-FXN-5 (25.17%). Although the difference is not as marked as in the other parameters, these results indicate that the incorporation of nanoliposomal vehicles at a 10% concentration contributes to the stability of erythrocytes under thermal stress conditions. While stabilizing the membrane by Hypo-IH, the Y-FXN-10 formulation again exhibited the highest inhibition (93.62%), followed by Y-C (81.46%) and Y-FXN-5 (80. 87%).
The results obtained clearly demonstrate that the addition of FXN-LN significantly (p<0.05) enhances the erythroprotective potential of the yogurt formulations. Hemolysis inhibition, photohemolysis inhibition and membrane stabilization test (heat-induced hemolysis inhibition, hypotonicity-induced hemolysis inhibition) were the parameters that showed the most notable differences between the enriched formulations and the control, indicating that the antioxidant compounds present in the enriched yogurt have a protective effect against oxidative damage. This can be attributed to fucoxanthin being a potent antioxidant, and its encapsulation in nanoliposomes can improve its stability and bioavailability. Therefore, the incorporation of nanoliposomes loaded with fucoxanthin not only enhances the antioxidant capacity, stability, and bioavailability of fucoxanthin in yogurt formulations but also potentiates its protective effect under various stress conditions, such as light and heat exposure, which are common in the food industry. The tested formulations could be considered to have therapeutic potential against oxidative damage [
39]. Additionally, these nanoscale vesicles could confer an antioxidant power comparable to a food preservative, preventing lipid peroxidation and extending the product’s shelf life.
The results are expressed as mean ± standard deviation (n = 3). Means with different lowercase letters (a-c: time effects) within the same treatment are significantly different (p < 0.05). Y-C= Control Yogurt without nanocapsules; Y-FXN-5= Yogurt enriched with 5% nanocapsules; Y-FXN-10= Yogurt enriched with 10% nanocapsules. HI: Hemolysis inhibition; PHI: Photohemolysis inhibition. Heat-IH: Inhibition of heat-induced hemolysis; Hypo-IH: Inhibition of hypotonicity-induced hemolysis.
2.6.2. Physicochemical Properties
2.6.2.1. Color
The stability of nanoliposomal carriers loaded with fucoxanthin significantly influences the color characteristics of enriched yogurt (
Table 3). The L* values for control yogurt (Y-C) decrease from 93.12 to 87.43 over 21 days, indicating gradual darkening likely due to microbial activity and oxidation. Yogurt with 5% fucoxanthin (Y-FXN-5) shows relatively stable initial L* values (93.29) that drop to 82.65 by day 21, suggesting that while nanoliposomes do not significantly affect initial lightness, they result in faster darkening over time. Yogurt with 10% fucoxanthin (Y-FXN-10) starts at 92.12 and decreases to 81.52, indicating higher concentrations of fucoxanthin lead to more pronounced darkening. The a* values for Y-C remain negative, showing a slight greenish tint that increases over time, while Y-FXN-5 and Y-FXN-10 show initial redness that diminishes, with the higher fucoxanthin concentration showing more significant decreases. The b* values for Y-C increase from 6.52 to 8.72, indicating a steady rise in yellow hue, whereas Y-FXN-5 and Y-FXN-10 show less pronounced increases, suggesting fucoxanthin stabilizes the yellow component.
The cold storage of enriched yogurts significantly impacts the color and quality of the product over time. Analyzing the color data (L*, a*, b*) for different yogurts (Y-C, Y-FXN-5, Y-FXN-10) on days 0, 7, 14, and 21, the following trends are observed: in terms of lightness (L*), the control yogurt (Y-C) shows a gradual decrease from 93.12 to 87.43, indicating darkening during storage, likely due to oxidation and microbial activity; yogurt with 5% fucoxanthin (Y-FXN-5) shows a faster decrease from 93.29 to 82.65, suggesting that fucoxanthin may be accelerating color change; and yogurt with 10% fucoxanthin (Y-FXN-10) shows the most significant decrease from 92.12 to 81.52, indicating that higher concentrations of fucoxanthin lead to greater darkening. In the red/green coordinate (a*), the control yogurt maintains negative values, indicating a slight greenish tint that intensifies over time, while yogurts with fucoxanthin show a decrease in positive values, indicating a reduction in redness towards a more neutral tone, more pronounced in Y-FXN-10. In the yellow/blue coordinate (b*), the control yogurt increases from 6.52 to 8.72, suggesting an increase in yellow hue, possibly due to fermentation and other biochemical processes; yogurt with 5% fucoxanthin shows a more moderate increase, suggesting that fucoxanthin stabilizes the color; and yogurt with 10% fucoxanthin shows the least increase in yellow hue, indicating that higher concentrations of fucoxanthin inhibit this development. Regarding quality, the stability of nanoliposomes is crucial in maintaining the structural integrity of yogurt during storage, improving consistency and texture compared to control yogurt, which may be affected by oxidation and microbial activity. Storage time also affects the sensory properties of yogurt, such as flavor, texture, and color, and the use of fucoxanthin appears to stabilize color and potentially improve long-term sensory acceptance. Nanoliposomes help preserve the biological activity of bioactive compounds by providing controlled and sustained release, ensuring health benefits are maintained during the storage period. Previous studies have demonstrated that the use of nanoliposomes and other encapsulation systems can improve the stability and quality of enriched dairy products during storage. Mozafari et al. [
28,
29] and Xu et al. [
30] highlighted that the encapsulation of bioactive compounds in nanoliposomes helps maintain color stability and biological activity, similar to the findings of this study with fucoxanthin. In conclusion, cold storage significantly impacts the color and quality of enriched yogurts, and the incorporation of fucoxanthin-loaded nanoliposomes improves color stability and preserves product quality during storage, making these yogurts more stable and potentially more attractive to consumers.
Studies from 2010 to 2024 consistently show that nanoliposomal encapsulation can enhance color stability in dairy products. Mozafari et al. [
28,
29] demonstrated nanoliposomes maintain color integrity, reducing color changes during storage. Xu et al. [
30] highlighted that natural antioxidants like polyphenols in nanoliposomes protect against oxidative changes, supporting the stability observed in L* values in Y-FXN-5 and Y-FXN-10. Sun et al. [
8] found higher concentrations of bioactive compounds lead to more significant color changes due to increased interactions with the yogurt matrix, similar to the more noticeable changes in L*, a*, and b* values in Y-FXN-10. These findings underscore the potential of nanoliposomal encapsulation in improving the quality and stability of functional dairy products by maintaining lightness and reducing undesirable color changes, with higher concentrations of fucoxanthin leading to more pronounced effects.
The transmittance-analyzed colorimetry data reveals notable changes in the yogurt samples enriched with fucoxanthin-loaded nanoliposomes over 21 days of cold storage (
Table 4). In the control sample (Y-C), the percentage transmittance of all measured wavelengths (Red, Green, Blue, Orange) increased gradually, indicating a slight but steady clarification of the yogurt. For the yogurt enriched with 5% fucoxanthin (Y-FXN-5), there was a significant reduction in transmittance across all wavelengths during the initial 7 days, followed by a stabilization and slight decrease, suggesting the dispersion and possible aggregation of nanoliposomes affecting light passage. The yogurt with 10% fucoxanthin (Y-FXN-10) exhibited an initial increase in transmittance for all wavelengths, particularly in the red and orange spectra, possibly due to the higher concentration of fucoxanthin enhancing color intensity. Over time, the transmittance remained high, especially notable at 660 nm (red) and 610 nm (orange), indicating sustained color retention and stability of nanoliposomes in the matrix. These trends highlight that higher concentrations of fucoxanthin-loaded nanoliposomes not only influence the initial color intensity of the yogurt but also contribute to maintaining its color stability during storage, with a more pronounced effect at 10% enrichment compared to 5%.
The results are expressed as mean ± standard deviation (n = 3). Means with different lowercase letters (a-d: time effects) within the same treatment are significantly different (p < 0.05). Y-C= Control Yogurt without nanocapsules; Y-FXN-5= Yogurt enriched with 5% nanocapsules; Y-FXN-10= Yogurt enriched with 10% nanocapsules.
In comparison with other studies, similar trends are observed where nanoliposome encapsulation enhances the stability and retention of antioxidant properties in food matrices. For instance, quercetin-loaded nanoliposomes showed sustained antioxidant effects and improved physicochemical stability, similar to the color stability observed in fucoxanthin-loaded yogurt samples. Quercetin encapsulation in nanoliposomes not only improved the retention of antioxidant activity but also influenced the release profiles, providing a prolonged protective effect against oxidative damage [
48]. Other study on rosemary oleoresin-loaded nanoliposomes demonstrated enhanced antioxidant stability and significant improvements in oxidative stability when applied to dried oysters. The rosemary oleoresin nanoliposomes maintained high antioxidant activity over an extended storage period, paralleling the sustained color stability seen in the fucoxanthin-enriched yogurt samples. This reinforces the efficacy of nanoliposome encapsulation in preserving the functional properties of antioxidants during storage [
49]. These comparisons indicate that nanoliposome technology consistently enhances the stability and effectiveness of encapsulated antioxidants across different food systems. The sustained color stability in yogurt with fucoxanthin-loaded nanoliposomes aligns well with findings from studies involving other antioxidants, underlining the versatility and potential of nanoliposome applications in food preservation.
2.6.2.2. Electric Conductivity
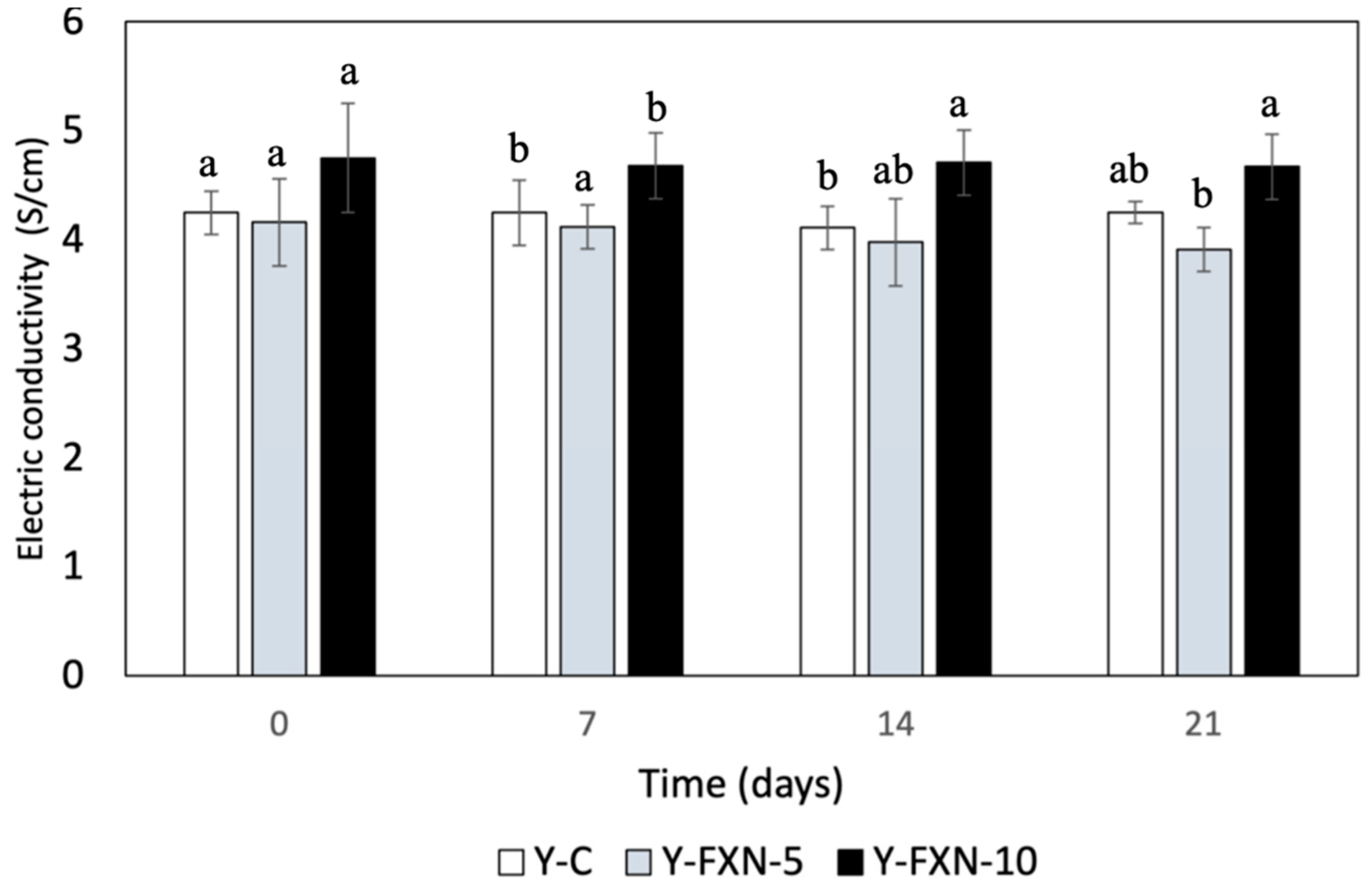
The data in the
Figure 4 indicates the effect of fucoxanthin-loaded nanoliposomes and cold storage time on the electrical conductivity of different yogurt formulations over a period of 21 days. The control yogurt (Y-C) maintained a relatively stable electrical conductivity around 4.25 throughout the storage period, indicating minimal changes in its physicochemical properties. In contrast, the yogurt with a lower concentration of fucoxanthin-loaded nanoliposomes (Y-FXN-5) showed a slight decrease in electrical conductivity from 4.16 to 3.91, suggesting that the addition of fucoxanthin at this concentration may have impacted the ionic balance or stability of the yogurt matrix over time. The yogurt with a higher concentration of fucoxanthin-loaded nanoliposomes (Y-FXN-10) demonstrated a small reduction in electrical conductivity from 4.75 to 4.67, which was less pronounced compared to Y-FXN-5. This indicates that higher levels of fucoxanthin might have a stabilizing effect, reducing the extent of change in electrical conductivity. Overall, while the incorporation of fucoxanthin-loaded nanoliposomes appears to influence the electrical conductivity of yogurt formulations during refrigerated storage, the impact is more significant at lower concentrations of fucoxanthin.
Electrical conductivity data (S/cm) provide crucial information about a solution’s ability to conduct electrical current and can reflect changes in the composition and structure of a sample over storage time. The addition of nanoliposomes to yogurt affects electrical conductivity by modifying the yogurt matrix and altering the ionic balance within the product. A stable conductivity indicates that the yogurt matrix and its physicochemical properties remain constant, which is crucial for product quality over time. Furthermore, electrical conductivity can serve as an indicator of the homogeneity of the dispersion of nanoliposomes within yogurt. If the conductivity changes significantly, it could signal a redistribution of components or an unwanted interaction between ingredients, which could affect both the texture and flavor of the yogurt. Therefore, maintaining stable electrical conductivity is essential to ensure that nanoliposome-enriched yogurt retains its sensory and nutritional properties throughout its shelf life [
50].
This finding is similar with other studies that have explored the effects of bioactive compounds and nanocarriers on the physicochemical properties of yogurt. In research by Wu et al. [
52]; Hasan et al., [
52] and Linder and Arab-Tehrany, [
53] demonstrated that the inclusion of curcumin-loaded nanoliposomes led to decreased electrical conductivity in yogurt, suggesting interactions between the nanocarriers and the yogurt matrix that affect ionic strength and stability [
52]. Similarly, studies by Linder and Arab-Tehrany, [
53], Verma et al. [
54] and Liu et al. [
55] found that encapsulating antioxidants in nanoliposomes altered the electrical properties of dairy products, highlighting the importance of concentration and formulation methods. These results could be suggested that the observed changes in electrical conductivity with fucoxanthin-loaded nanoliposomes are consistent with broader trends in the use of nanotechnology in dairy product enhancement.
2.6.2.3. pH
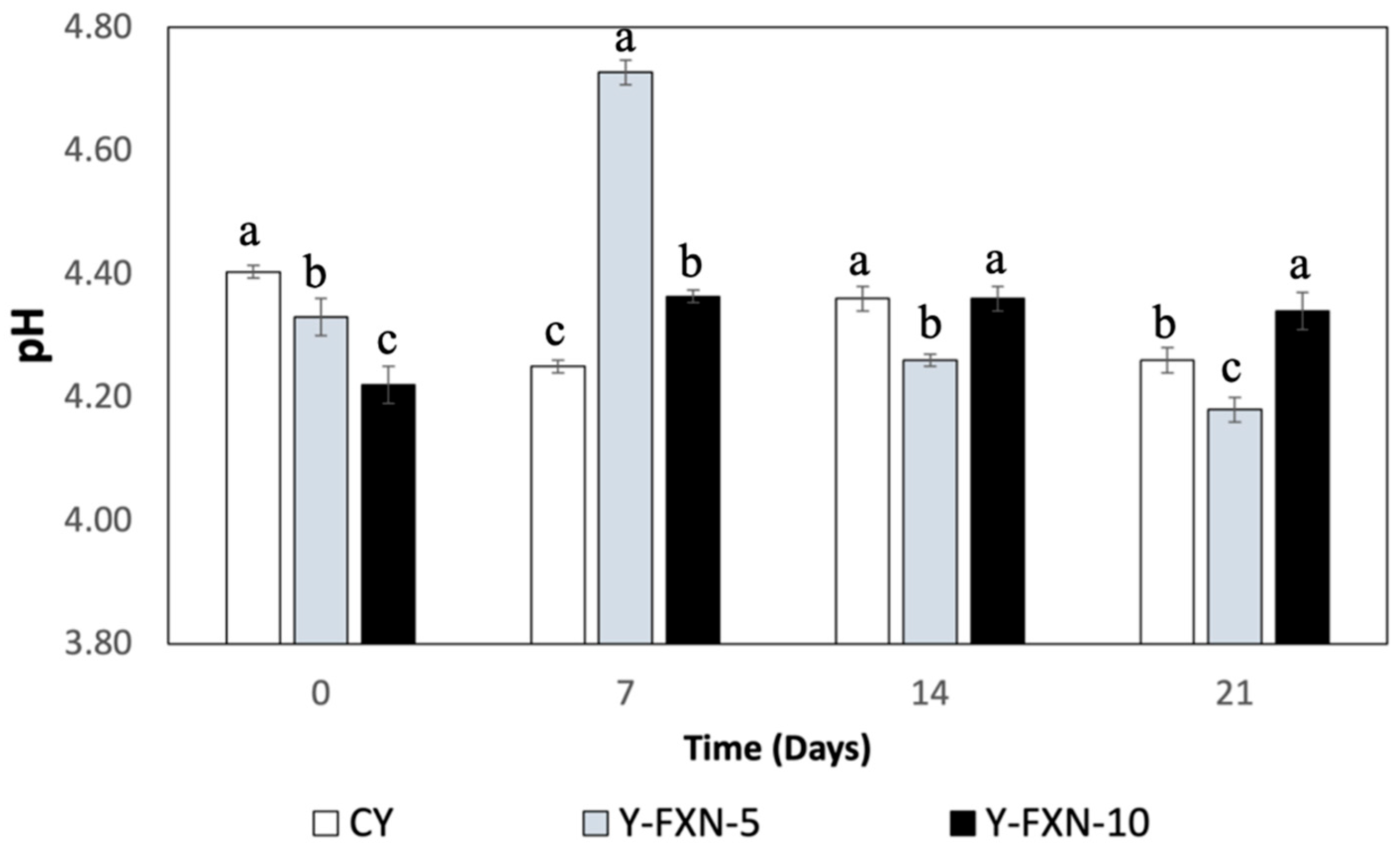
The analysis of the data regarding the effect of the addition of fucoxanthin-loaded nanoliposomes and cold storage for 21 days on the pH of various yogurt formulations reveals several notable trends (
Figure 5). Initially, the pH of the Y-C slightly decreases from 4.40 to 4.26, which is expected due to the metabolic activity of lactic cultures during storage. In the formulations with 5% fucoxanthin nanoliposomes (Y-FXN-LN-5%), the pH shows a more pronounced decreasing trend, starting at 4.33 and reducing to 4.18 by the end of the storage period, indicating higher acidogenic activity or possible acid release from the nanoliposomes. Conversely, the yogurt formulation with 10% of FXN-LN exhibits a less pronounced pH decrease, from 4.22 to 4.34, suggesting possible pH stabilization due to the higher concentration of nanoliposomes, which might be interfering with the activity of lactic cultures or acting as a buffer. Overall, these results indicate that the addition of fucoxanthin nanoliposomes and the increase in their concentration influence the acidification dynamics of yogurt during cold storage, with differentiated effects according to the concentration of nanoliposomes used.
Previous studies indicates that the addition of fucoxanthin-loaded nanoliposomes in yogurt formulations significantly impacts pH stability during cold storage. In our study, formulations with 5% fucoxanthin nanoliposomes exhibited a greater decrease in pH compared to those with 10%, suggesting higher acidogenic activity in the former. These findings are consistent with previous research demonstrating that encapsulating carotenoids, such as fucoxanthin, in nanoliposomes and other delivery systems enhances their stability and bioaccessibility. For instance, studies have shown that fucoxanthin nanoliposomes improve carotenoid stability during storage, maintaining their bioactivity and delaying degradation [
56,
57,
58]. Additionally, the use of chitosan-based delivery systems has proven effective in preserving carotenoid stability in various food matrices, providing sustained release and enhancing bioaccessibility [
59]. Together, these studies support the notion that nanoencapsulation not only improves carotenoid stability but also influences the physicochemical properties of the final product, such as pH, during storage in food product.
2.6.2.4. Titratable Acidity
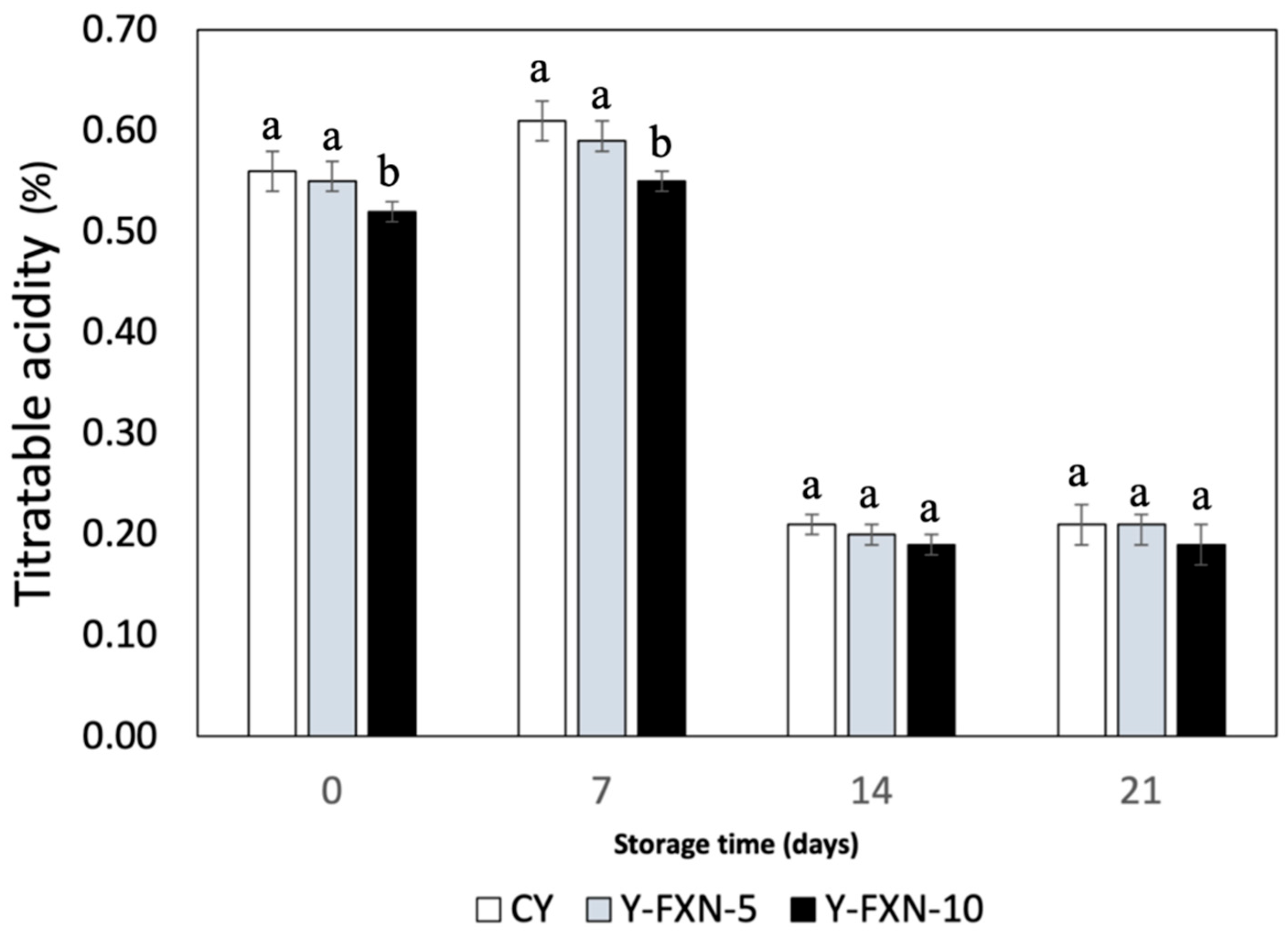
The data presented in the
Figure 6 indicates the titratable acidity (Lactic acid percentage) of different yogurt formulations over a 21-day cold storage period. Initially, on day 0, the control yogurt (Y-C) has a titratable acidity of 0.56%, while the yogurts with 5% and 10% fucoxanthin-loaded nanoliposomes (Y-FXN-LN-5% and Y-FXN-LN-10%) show slightly lower acidity levels of 0.55% and 0.52%, respectively. By day 7, all samples exhibit an increase in acidity, with Y-C reaching 0.61%, Y-FXN-LN-5% at 0.59%, and Y-FXN-LN-10% at 0.55%, indicating active lactic acid bacterial fermentation. Interestingly, by day 14, the acidity of all formulations decreases significantly, suggesting a possible stabilization or reduction in fermentation activity, with values of 0.21% for Y-C, 0.20% for Y-FXN-LN-5%, and 0.19% for Y-FXN -LN-10%. This trend continues through day 21, with the acidity remaining stable at these lower levels. Overall, the addition of fucoxanthin-loaded nanoliposomes appears to slightly reduce initial acidity but does not significantly alter the long-term stabilization of titratable acidity during cold storage, suggesting that nanoliposome inclusion maintains yogurt quality over time.
The addition of fucoxanthin-loaded nanoliposomes to yogurt formulations results in a slight reduction in titratable acidity at the initial stage and during the 21-day storage period. These results were observed in findings from other studies, which have shown that fucoxanthin can influence the physicochemical properties of yogurt, including its acidity. e.g., a study on goat milk yogurt fortified with fucoxanthin demonstrated that while fucoxanthin did not significantly affect the chemical composition or physicochemical properties, it did result in changes in the acidity and color of the yogurt during storage [
60,
61,
62]. Similarly, another study highlighted that the addition of encapsulated fucoxanthin to yogurt improved its structural and functional properties without negatively impacting its acidity. These comparisons indicate that fucoxanthin’s incorporation into yogurt, whether in nanoliposome form or otherwise, can help maintain the quality and stability of the product over extended storage periods, while slightly altering its initial acidity. The incorporation of fucoxanthin-loaded nanoliposomes into yogurt affects the percentage of lactic acid by initially reducing the titratable acidity, as observed from the data in the provided table. This reduction suggests that the nanoliposomes might have a stabilizing effect on the under-storage condition, leading to a slower acid production by the lactic acid bacteria. Over time, however, the titratable acidity in all yogurt formulations stabilizes [
60]. This effect on lactic acid percentage is crucial for the quality of enriched yogurt as it impacts its taste, texture, and shelf life. Lower initial acidity can lead to a milder flavor, which might be preferred by some consumers. Additionally, the stabilizing effect of nanoliposomes can help maintain the structural integrity of the yogurt, enhancing its texture and consistency over time. This can be particularly beneficial in maintaining the quality of the yogurt during extended storage periods, ensuring that the product remains appealing to consumers while delivering the added health benefits of fucoxanthin [
60].
3.6.2.5. Syneresis Susceptibility (STS)
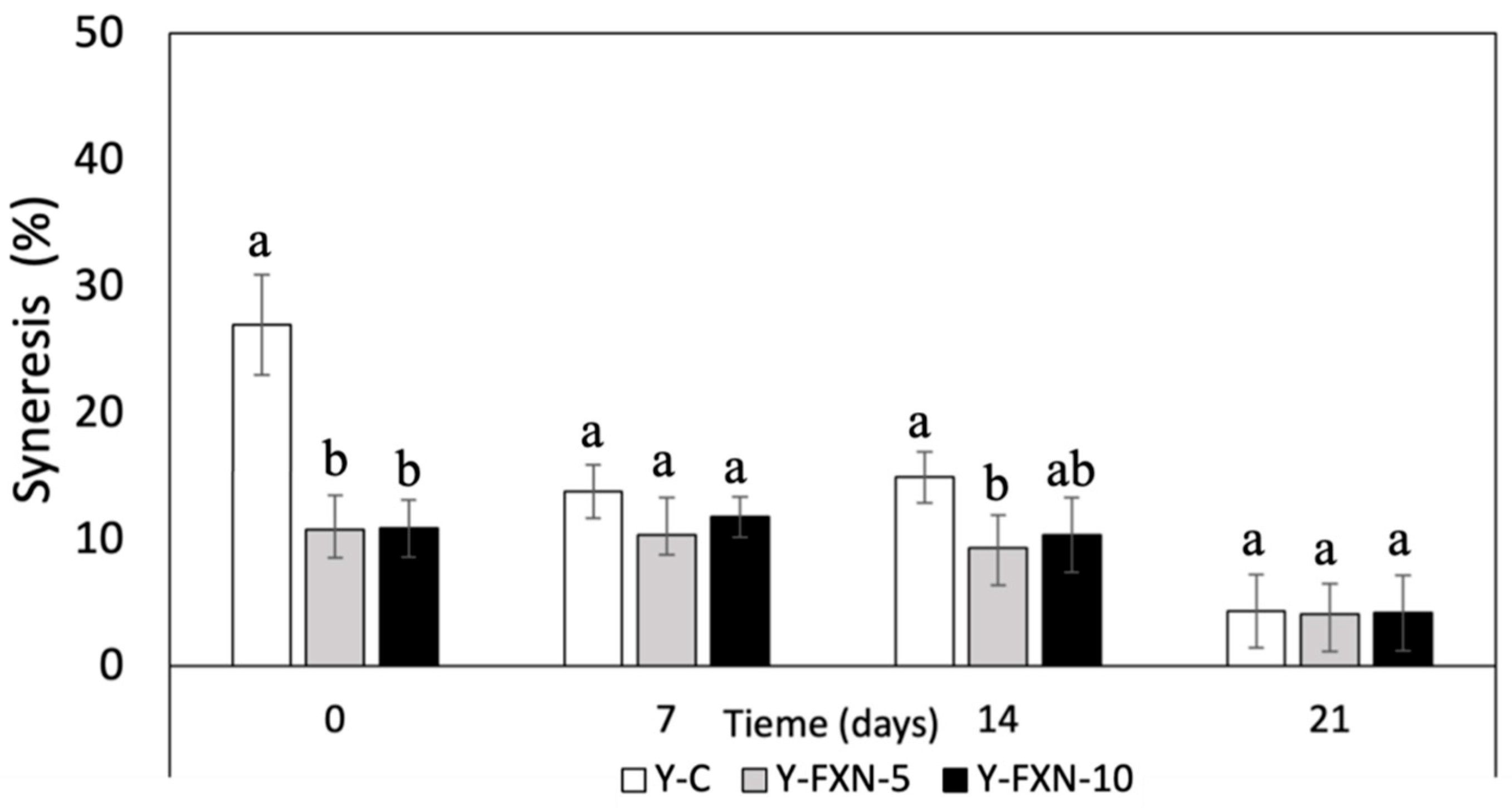
The data indicates that the addition of fucoxanthin-loaded nanoliposomes significantly influences the syneresis of yogurt formulations over the 21-day cold storage period (
Figure 7). The control yogurt (Y-C) starts with a syneresis value of 26.47 %, which dramatically decreases to 4.31 times by day 21, indicating substantial whey separation. In contrast, both Y-FXN-5 and Y-FXN-10 formulations exhibit much lower initial syneresis values (10.76 and 10.87, respectively) and maintain relatively stable and low syneresis levels throughout the storage period. This suggests that the incorporation of fucoxanthin-loaded nanoliposomes stabilizes the yogurt matrix, reducing whey separation compared to the control. Consequently, these fortified formulations likely retain better texture and consistency, enhancing the overall quality of the yogurt during refrigerated storage. This improved stability can be attributed to the structural benefits provided by the nanoliposomes, which may enhance the interaction between yogurt proteins and fucoxanthin, leading to a more cohesive gel network.
The incorporation of nanoliposomes into the yogurt matrix significantly affects the syneresis results by enhancing the structural stability of the yogurt. Nanoliposomes, being nanoscale lipid-based vesicles, can interact with yogurt components at a microscopic level, creating a more cohesive and stable gel network. This improved network reduces the separation of whey from the yogurt matrix, which is observed as decreased syneresis. Several components (protein, carbohydrates, and lipids) in the yogurt interact with nanoliposomes to reduce syneresis. e.g., Proteins, particularly casein micelles, play a crucial role in the gel structure of yogurt. Nanoliposomes can interact with these proteins, enhancing their ability to form a stable network that traps water more effectively. This interaction likely strengthens the protein matrix, preventing the expulsion of whey. In other hands, polysaccharides, such as those from the yogurt cultures or added stabilizers like pectin, can form a complex with nanoliposomes, further enhancing the viscosity and water-holding capacity of the yogurt. This results in reduced syneresis as the water is more effectively retained within the gel structure. In addition, polysaccharides such as exopolysaccharides contribute to improving the water retention capacity and viscosity of yogurt, which decreases syneresis. This effect has been demonstrated in recent studies that highlight how nanoliposomes improve the physicochemical properties of yogurt by acting as emulsifiers and protein network stabilizers. Meanwhile, the lipid content from nanoliposomes can integrate into the yogurt matrix, contributing to the overall texture and stability. Lipids from nanoliposomes might interact with both proteins and carbohydrates, forming a more homogenous mixture that resists phase separation creating a stronger more cohesive gel network, thereby reducing syneresis and improving the textural quality of the yogurt during storage [
63,
64,
65].
2.6.2.6. Water-Holding Capacity (WHC)
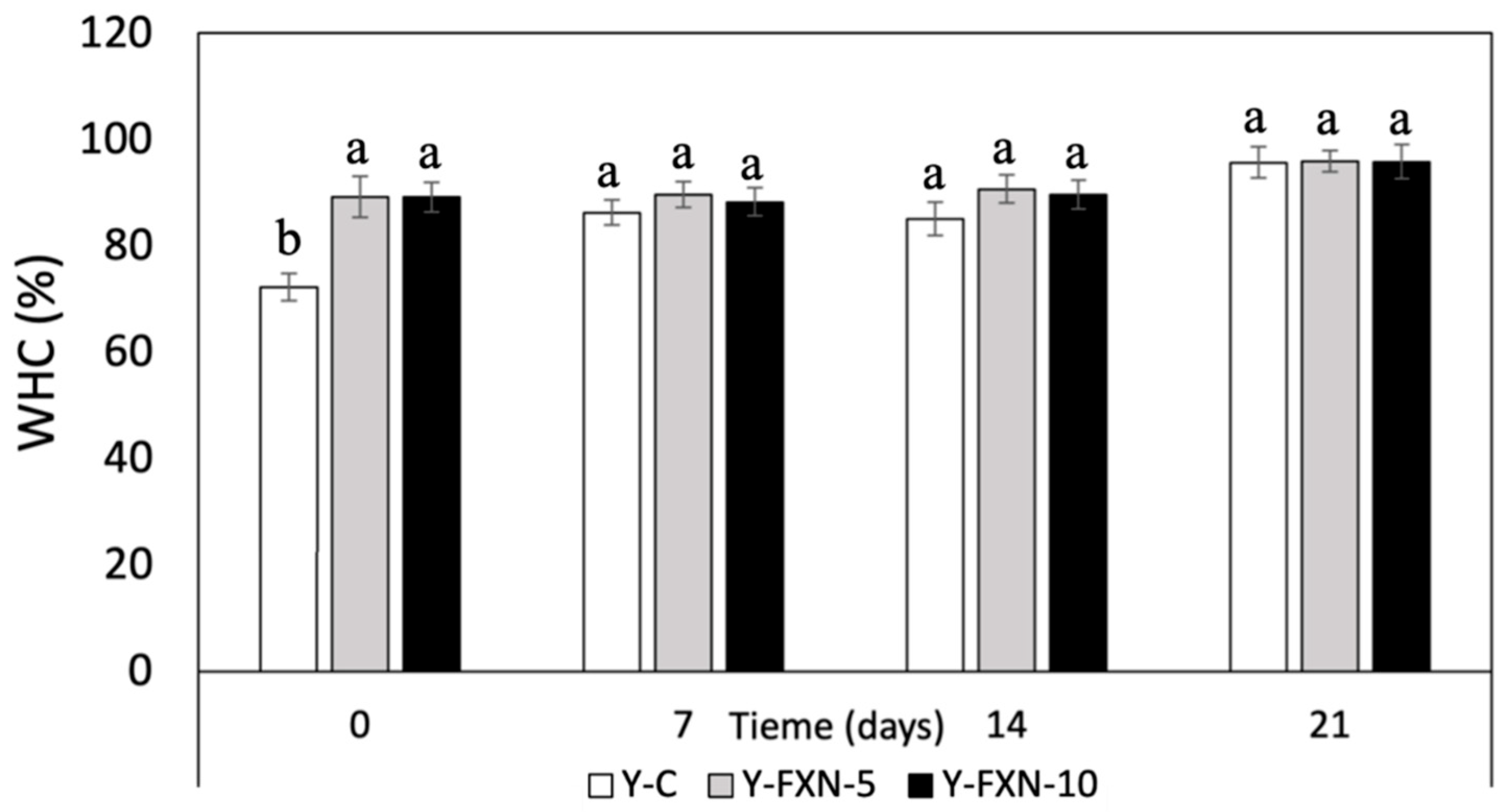
The addition of fucoxanthin-loaded nanoliposomes to yogurt formulations (Y-FXN-5 and Y-FXN-10) had a positive impact on water holding capacity over 21 days of cold storage compared to control yogurt (
Figure 8). From day 0 to day 21, the water holding capacity of Y-C increased from 73.53% to 95.69%, while nanoliposome-enriched formulations consistently showed higher values from the experimental initial. Y-FXN-5 exhibited water retention ranging from 89.24% to 95.92%, and Y-FXN-10 showed a similar trend, with values between 89.13% and 95.82%. This indicates that the inclusion of fucoxanthin not only enhances water holding capacity from the outset but also maintains this advantage during storage. These results suggest that fucoxanthin-enriched formulations offer better quality in terms of yogurt stability and texture, likely due to the structuring and functional properties of nanoliposomes, providing a better sensory experience and potentially greater consumer acceptance compared to unfortified yogurt.
The addition of nanoliposomal carriers to yogurt can enhance product quality by favorably interacting with proteins, amino acids, casein, carbohydrates, and lipids, resulting in improved water holding capacity, reduced syneresis, and a more stable and pleasant texture. These combined effects can lead to higher quality yogurt and consumer acceptability [
66]. Yogurt quality is often assessed by parameters such as syneresis and water holding capacity. Syneresis, the separation of whey from yogurt, is a negative indicator of yogurt stability, while high water holding capacity is desirable as it indicates better texture and consistency. Nanoparticles, due to their size and functional properties, can beneficially interact with yogurt’s food matrix components, such as proteins (casein and whey proteins), amino acids, carbohydrates, and lipids. These interactions offer several advantages. Nanoparticles can form a more stable network with yogurt proteins, particularly casein, the primary protein in yogurt. This enhanced protein network can reduce syneresis and increase water holding capacity, providing a creamier and more homogeneous texture [
68,
69,
70]. Lipid-based self-assembling vesicles can help preserve essential amino acids during storage, thereby improving yogurt’s nutritional value. Moreover, interactions with amino acids can contribute to the stability of yogurt’s protein structure [
67,
68]. Similarly, these carriers can interact with carbohydrates in yogurt, such as lactose, improving product viscosity and stability. These interactions can help maintain a more uniform consistency and reduce syneresis. Finally, the lipids in nanoliposomes can integrate with the natural lipids in yogurt, enhancing texture and mouthfeel [
68,
70]. The presence of nanoliposomes can also act as emulsifiers, helping to maintain a stable emulsion and reducing phase separation.
The incorporation of fucoxanthin-loaded nanoliposomes to yogurt has been shown to significantly improve water holding capacity and reduce syneresis compared to unfortified control yogurt. The use of polymerized whey proteins (PWP) has similarly demonstrated improved stability and reduced syneresis during 21 days of refrigerated storage, maintaining greater yogurt coherence compared to control [
68]. Likewise, adding dill extract to yogurt increased water holding capacity and reduced syneresis, enhancing physicochemical and antioxidant properties during storage [
68,
70]. These studies corroborate that incorporating functional agents like nanoliposomes, polymerized proteins, or plant extracts can improve yogurt quality by interacting with food matrix components, stabilizing proteins, casein, and other macronutrients, resulting in a product with better texture and reduced syneresis [
68,
69,
70].
2.6.2.7. Viscosity
The results reveal that the addition of fucoxanthin-loaded nanoliposomes and the duration of cold storage significantly influence the viscosity of yogurt formulations (
Figure 9). The control yogurt (Y-C) shows an initial viscosity of 10300, peaking at 12715 after 7 days, then gradually decreasing to 10599 at 14 days, and finally dropping to 9009 at 21 days. In contrast, the formulations with nanoliposomes (Y-FXN-5 and Y-FXN-10) display a different trend. Y-FXN-5 starts with a viscosity of 4690, increases to 8473 after 7 days, then drops sharply to 2657 at 14 days, and further reduces to 1771 at 21 days. Similarly, Y-FXN-10 begins at 6372, peaks at 7323 on 7th day , then decreases to 2242 by day 14, and slightly increases to 2812 by day 21. These results indicate that the inclusion of nanoliposomes loaded with fucoxanthin generally leads to a lower viscosity compared to the control yogurt. The sharp decline in viscosity after 7 days for the fortified yogurts suggests a potential destabilization or breakdown of the yogurt matrix, which could impact the overall quality, making them less viscous and potentially altering the texture and mouthfeel compared to the unfortified yogurt. This implies that while fortification with fucoxanthin could offer nutritional benefits, it also necessitates careful consideration of its impact on the physical properties and storage stability of yogurt [
4,
11].
The interaction of nanoliposomes with yogurt components such as casein, proteins, carbohydrates, and lipids at a chemical and biochemical level significantly affects the yogurt’s structure and behavior as a non-Newtonian fluid. Nanoliposomes can disrupt casein micelles through electrostatic and hydrophobic interactions, reducing the micellar stability and leading to decreased viscosity [
14]. They may also act as surfactants, affecting protein aggregation and the hydration properties of polysaccharides, thus altering the yogurt matrix’s thickening and stabilizing functions [
14,
19]. Additionally, nanoliposomes can integrate into fat globules or interact with free fatty acids, impacting the size distribution and stability of these globules. These interactions collectively contribute to changes in the yogurt’s shear-thinning behavior, resulting in a less viscous and potentially less stable gel network. Consequently, the fortified yogurt displays altered rheological properties and texture compared to the unfortified control, influencing its overall quality and sensory attributes [
67,
71,
72].
Casein micelles are the primary protein structures in yogurt, responsible for its gel-like texture. Nanoliposomes can interact with these micelles through electrostatic and hydrophobic interactions, potentially altering their size and stability. The encapsulation of bioactive compounds like fucoxanthin in nanoliposomes can lead to competitive binding sites on casein, which can disrupt the micellar structure and reduce viscosity [
42]. Nanoliposomes might also influence the aggregation behavior of proteins. They can act as a surfactant, reducing protein-protein interactions and leading to a more dispersed protein network, which contributes to a decrease in viscosity. Yogurt often contains polysaccharides (e.g., pectin, guar gum) that contribute to its viscosity and texture. Nanoliposomes can interact with these polysaccharides, potentially affecting their ability to thicken and stabilize the yogurt matrix. The presence of nanoliposomes might lead to a competition for water molecules, affecting the hydration and gelation properties of these carbohydrates [
67,
68]. Yogurt contains fat globules that contribute to its creaminess and mouthfeel. Nanoliposomes, being lipid-based carriers, can integrate into the fat globule membrane or interact with free fatty acids, potentially altering the size distribution and stability of fat globules. This can impact the overall rheology of the yogurt. These biochemical interactions can result in a yogurt that is less viscous and has a different texture compared to unfortified yogurt. This can lead to a smoother, less thick product, which might be perceived differently in terms of mouthfeel and overall sensory experience [
73,
74].
Comparing the results from the provided table with other research on yogurt fortified with nanoliposomes highlights several key insights. The significant decrease in viscosity observed in the fortified yogurts (Y-FXN-5 and Y-FXN-10) over time aligns with findings from various studies. Research indicates that nanoliposomes can disrupt the protein network, particularly casein micelles, leading to a reduction in viscosity and altering the rheological properties of yogurt. For instance, studies have shown that the incorporation of nanoliposomes can lead to a more fluid consistency due to interactions with proteins and fats, affecting the overall stability and texture of the yogurt [
63,
73]. These changes underscore the importance of balancing nutritional enhancements with the maintenance of desirable physical properties in yogurt formulations.
The findings of the viscosity data of yogurt formulations enriched with nanoliposomes throw important observations regarding quality and syneresis. The lower viscosity of nanoliposome-enriched yogurts (Y-FXN-5 and Y-FXN-10) indicates a higher susceptibility to syneresis, as viscosity is crucial for serum retention in yogurt. Aziminezhad et al. [
75] note that viscosity isn’t the only factor affecting syneresis; water-holding capacity and gel structure stability also play significant roles. Additional studies by Najgebauer-Lejko et al. [
76], Shokery et al. [
73], and de Campo et al. [
77] emphasize viscosity as a key parameter for yogurt quality. Research by Anuyahong et al. [
13], Liu et al. [
18], and Athar et al. [
78] suggests that increased total solids content and gel firmness can enhance viscosity and reduce syneresis, reinforcing the importance of viscosity in maintaining yogurt quality and optimizing nanoemulsion production processes. The control yogurt (Y-C) demonstrates higher viscosity, suggesting better water retention and lower susceptibility to syneresis compared to nanoliposome-enriched formulations (Y-FXN-5 and Y-FXN-10). Studies by Aziminezhad et al. [
75] highlight that viscosity, although critical, is not the sole determinant of syneresis; other factors such as water-holding capacity and gel structure stability are also influential. Additional research, including works by Najgebauer-Lejko et al. [
76], Shokery et al. [
73], and de Campo et al. [
77], emphasizes the importance of viscosity in yogurt quality. Furthermore, studies by Anuyahong et al. [
13], Liu et al. [
18], and Athar et al. [
78] suggest that higher total solids content and gel firmness can increase viscosity and reduce syneresis, underscoring the role of viscosity in evaluating dairy product quality and optimizing nanoemulsion production processes.
2.6.2.8. Textural Properties (Firmness and Consistency)
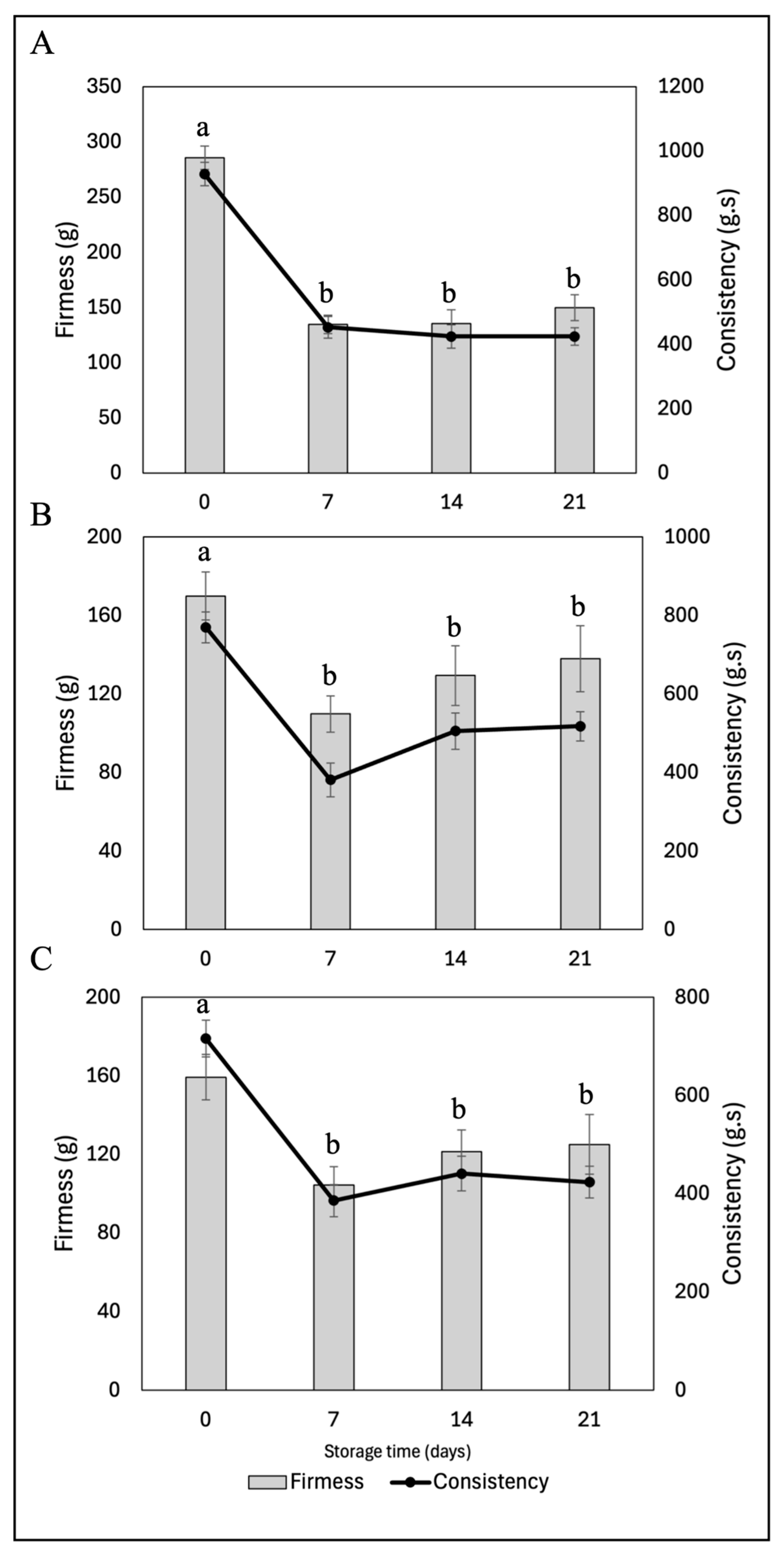
The incorporation of fucoxanthin-loaded nanoliposomes into yogurts could have varying effects on the firmness of the final product during cold storage, depending on the specific concentration of encapsulated fucoxanthin and interactions with the food matrix (
Figure 10). The results indicate notable differences between the different formulations. According to the data from the control yogurt (Y-C), the initial firmness is 285.59 g, which significantly decreases to 149.58 g after 21 days. This considerable decline indicates that the texture of the yogurt loses firmness over time, potentially negatively impacting the perceived quality of the product. Similarly, the yogurt with FXN-5 (Y-FXN-5) shows an initial firmness of 169.89 g, which decreases to 137.85 g after the same storage period. However, this reduction is not as drastic as in Y-C. This suggests that the addition of FXN-5 may contribute to greater firmness stability during storage compared to Y-C. Conversely, the incorporation of 10% nanocapsules results in an initial firmness of 134.44 g, dramatically decreasing to 54.82 g after 21 days, unlike the addition of 5% FXN-LN. This yogurt experiences the greatest reduction in firmness among the studied formulations, indicating a significant loss of consistency during cold storage. This result suggests that the FXN-10 formulation might not be ideal for maintaining yogurt firmness during prolonged storage.
Firmness in dairy products such as yogurt is a crucial indicator of texture and, therefore, yogurt quality. A significant decrease in firmness can result in a more liquid or less creamy texture, which may be perceived negatively by consumers. Yogurt Y-C maintains relatively high firmness after long storage periods. According to consumer standards for yogurt, this property and effect might be perceived as higher quality compared to the other formulations. However, the diverse preferences of consumers and the various presentations of yogurt today, such as spoonable yogurt and drinkable yogurt, must be considered [
13,
79]. These results are desirable for the formulation of a drinkable yogurt. Therefore, in terms of overall product acceptance, initial firmness and its stability during storage are determining factors for consumer acceptance and sensory analysis. Yogurt Y-C, with its higher initial firmness and lower reduction in firmness, will likely offer a more consistent and satisfactory texture, potentially resulting in higher sensory acceptance. Yogurt Y-FXN-5, although having lower initial firmness than Y-C, shows better stability during storage, which could be favorable in terms of sensory acceptance.
On the other hand, yogurt Y-FXN-10, due to its significant reduction in firmness, might be perceived as less fresh or of lower quality, negatively affecting its acceptance by consumers [
66]. The nanoliposomes incorporated into the different yogurt formulations might interact with various components of the food matrix, such as caseins, proteins, lipids, and carbohydrates [
79]. This interaction could affect the structure and stability of the yogurt’s protein network, contributing to firmness stability in some cases [
67,
80]. Additionally, the interactions between nanoliposomes and the carbohydrates present in yogurt may alter the viscosity and texture of the dairy products, influencing their firmness and stability during storage [
9,
81]. The incorporation of nanoliposomes not only impacts the firmness of yogurt but also affects its consistency, a parameter related to the final quality of dairy products [
66]. The consistency of yogurt after 21 days of cold storage shows significant variations among the formulations. For Y-C, the initial consistency is 928.35 g·s, reducing to 424.14 g·s by the end of the storage period. This reduction suggests that the yogurt becomes less viscous and loses body over time, which could negatively impact its perceived quality. As previously mentioned, this is subject to consumer perception. Meanwhile, Y-FXN-5 starts with a consistency of 769.54 g·s, decreasing to 517.21 g·s after 21 days of storage.
Although a reduction in consistency is observed, the decrease is less pronounced than in the control yogurt, indicating that the FXN-5 formulation helps maintain better consistency during the storage period. Y-FXN-10 has an initial consistency of 611.50 g·s, which significantly decreases to 199.48 g·s by the end of the storage period. This formulation shows the greatest loss of consistency, indicating a notable reduction in the viscosity and body of the product during cold storage without freezing. This result suggests that the Y-FXN-10 formulation may not be suitable for maintaining the consistency of yogurt during prolonged storage. Among the three tested formulations, while Y-FXN-5 has a slightly higher consistency value (517.21 g·s), it is important to consider the relative reduction from the initial to the final value. The Y-C formulation has a higher initial value (928.35 g·s) and maintains a notably high consistency throughout the storage period, suggesting that its structure is better preserved compared to the nanoliposome formulations [
82,
83].
2.6.2.9. Rheological Properties
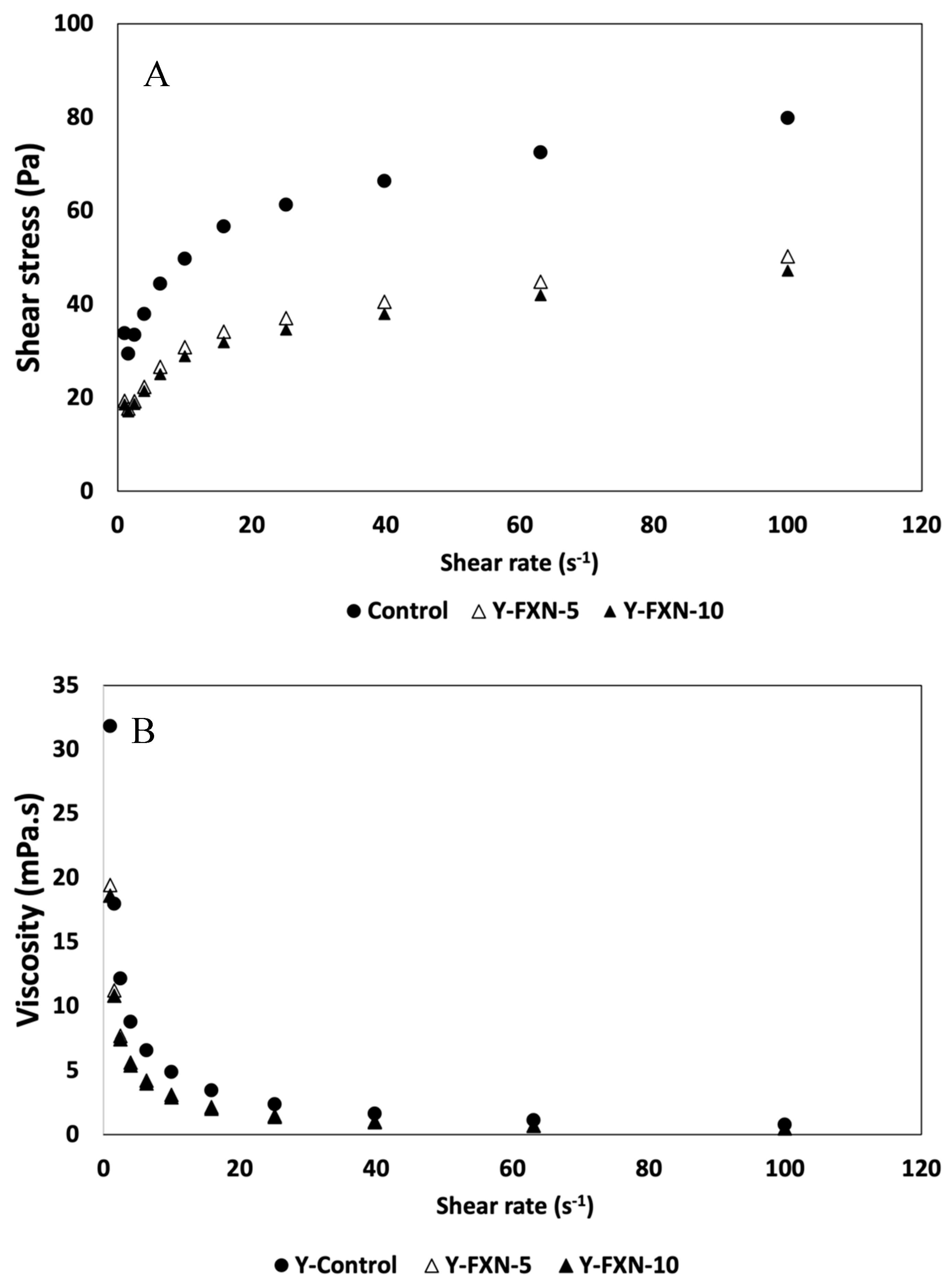
This study evaluates how these properties evolve over 21 days of cold storage, providing valuable insights into the structural behavior of yogurts with added nanoliposomes (
Figure 11). Initially, the Y-C shows increasing shear stress values with the increase in shear rate, indicating good initial stability and firmness (day 0), suggesting a more rigid structure. In contrast, these values decrease significantly in yogurts supplemented with nanoliposomes. The addition of just 5% nanoliposomes significantly reduces the shear stress compared to the control. Here, the nanoliposomes could interact with the yogurt matrix components, possibly reducing structural rigidity and increasing product fluidity. Similar results were observed for the effect of adding 10% nanoliposomal carriers, resulting in lower shear stress in Y-FXN-10 than in the control.
Regarding the second rheological parameter, the results reveal that the addition of nanoliposomes substantially decreases viscosity compared to the Y-C. For instance, at the lowest shear rate (1.00 s⁻¹), the viscosity of the control yogurt is 31.8 mPa·s. In contrast, the yogurts enriched with 5% and 10% nanoliposomes (Y-FXN-5 and Y-FXN-10) exhibit reduced viscosities of 19.42 mPa·s and 18.60 mPa·s, respectively. This significant reduction can be attributed to the interaction between the nanoliposomes and the components of the yogurt matrix, which likely alters the protein network and leads to a less rigid structure. The lower viscosity suggests that the nanoliposomes act as lubricants within the yogurt matrix, facilitating easier flow [
72]. All yogurt samples exhibit shear-thinning behavior, where viscosity decreases with increasing shear rate. This behavior is typical of yogurt and indicates that the structure breaks down under shear, allowing the yogurt to flow more easily. The yogurts with added nanoliposomes display a more pronounced shear-thinning behavior compared to the control. At a shear rate of 10.00 s⁻¹, the control yogurt has a viscosity of 4.9 mPa·s. Comparing the results with the viscosity data of Y-FXN-5 and Y-FXN-10, which have viscosities of 3.09 mPa·s and 2.89 mPa·s, respectively, it is evident that the nanoliposomes make the yogurt structure more susceptible to deformation under shear stress. Concerning the impact of 21-day storage, it is suggested that nanoliposomes help maintain a stable and less rigid structure over time. The stable viscosity values of the yogurts with added nanoliposomes also imply a reduction in syneresis, as a more fluid structure can better retain whey, improving water retention capacity. This property is beneficial for the texture and consistency of the yogurt, making it more appealing to consumers [
72,
74].
Yogurt firmness and viscosity are a critical parameter for the perceived quality of yogurt. A very fluid yogurt can be perceived as low quality, while an excessively firm one may not be palatable. Therefore, these parameters and their interpretation must be considered carefully [
12,
71]. Nanoliposomes appear to decrease initial firmness, which could enhance the perception of creaminess and smoothness, desirable characteristics in high-quality yogurt. There is a strong indication that a reduction in shear stress in yogurts with nanoliposomes could reduce syneresis, as a more fluid structure can better retain serum and increase its water retention capacity. The incorporation of nanoliposomes could benefit yogurt texture, making the consistency more pleasant to consume [
51,
55].
According to the color data of enriched yogurts, it is plausible that nanoliposomes, depending on their composition, do not significantly alter these characteristics, maintaining consumer acceptance [
74,
84]. Ghorbanzade et al. [
85] demonstrated the effectiveness of nanoencapsulating fish oil using nanoliposomes. The results showed a reduction in acidity, syneresis, and peroxide values, and greater stability of DHA and EPA. Yogurts with nanoencapsulated fish oil exhibited superior sensory characteristics compared to those containing non-encapsulated fish oil. This suggests that nanoencapsulation improves the stability and sensory acceptance of the product. Nanoliposomes can interact with casein micelles and other proteins, modifying the gel structure and reducing rigidity, reflected in lower shear stress. This indicates that nanoliposomes could act as emulsifiers, better distributing lipids and carbohydrates in the yogurt matrix, contributing to a more homogeneous and smoother texture. The integration of nanoliposomes in yogurts not only improves the rheological and sensory properties of the product but also ensures the stability and bioaccessibility of bioactive compounds like DHA. Scientific evidence supports the use of nanoencapsulation techniques to enhance the quality of enriched dairy products, although more research is needed to fully understand their behavior in the food system [
86].
2.6.2.10. Sensorial Analysis
Table 5 provides a comprehensive sensory analysis detailing the evaluation conducted by a group of panelists on the sensory attributes of three different yogurt formulations (Y-C, Y-FXN-LN-5, and Y-FXN-LN-10). The attributes assessed include color, flavor, aftertaste, aroma, consistency, texture, appearance, and overall acceptance. The sensory scale used ranges from 0 (very unpleasant) to 9 (very pleasant). The incorporation of fucoxanthin-loaded nanoliposomes in the yogurt, depending on the concentration, has a positive effect on various sensory attributes. In particular, an increase in the fucoxanthin concentration (10%) improves the color, flavor, aftertaste, aroma, texture, appearance, and overall acceptance of the yogurt. Similarly, a 5% fucoxanthin concentration also enhances some attributes compared to the control. However, this 5% concentration is not as effective in terms of acceptance by the panelists as the 10% fucoxanthin concentration.
Means with different lowercase letters (a-c: yogurt formulations) within the same sensory quality attributes are significantly different (p < 0.05). Sensory scale: 0 being very unpleasant and 9 being very pleasant. Values in the same column with different letters are significantly different. Y-C: control sample (unfortified yogurt); Y-FXN-5: 5% fucoxanthin-enriched yogurt; Y-FXNt-10: 10% fucoxanthin-enriched yogurt.
In terms of the color attribute, the yogurt enriched with 10% fucoxanthin (Y-FXN-LN-10) was the highest-rated attribute, surpassing that of Y-C. However, the yogurt with a 5% fucoxanthin concentration (Y-FXN-LN-5) received the lowest score. This indicates that a higher concentration of fucoxanthin can significantly improve the color of the yogurt, making it more visually appealing. Similarly, Y-FXN-LN-10 received the highest score for flavor, followed by the control. Y-FXN-LN-5 had the lowest score, although it remains high on the sensory scale. This suggests that higher concentrations of fucoxanthin can enhance the flavor of the yogurt. The aftertaste, which is the lingering flavor perception in the mouth after consuming the yogurt, was best rated in the yogurt enriched with 10% fucoxanthin. This indicates that fucoxanthin not only improves the initial flavor but also contributes to a more pleasant aftertaste. It is important to note that a good aftertaste is generally pleasant, complementing the initial flavor of the yogurt. Conversely, an unpleasant aftertaste can deter consumers from repurchasing the product. Regarding aroma, consistency, texture, appearance, and overall appearance of the formulations, Y-FXN-LN-10 and Y-C were equally appreciated, while the 5% yogurt received a lower score. These results suggest that fucoxanthin is a beneficial additive for improving the sensory quality of yogurt at 10% concentrations.
According to the data provided by the sensory attribute analysis, the Y-FXN-LN-10 formulation has the highest acceptability with a score of 8.36 on the sensory scale. This formulation also obtained the highest scores in several sensory attributes (color, flavor, aftertaste, texture, appearance, and overall acceptance), indicating a greater preference among the panelists. The attribute “Overall Acceptance” is the most important, as this information is crucial for concluding which formulation was most accepted by the panelists. Although other attributes such as flavor, texture, and appearance are critical, overall acceptance integrates a holistic evaluation of all these characteristics, reflecting the global perception of the product. The incorporation of nanoliposomes into yogurt significantly improves the overall acceptance of the yogurt compared to the control yogurt, which is an artisanal and natural yogurt without additives [
87,
88]. Based on the overall acceptance of the product, additional analyses are recommended to address various challenges in the development and distribution of yogurt in the food industry. It would be necessary to obtain regulatory approvals to ensure that nanoliposomes are safe for human consumption. Additionally, consumer perception needs to be addressed to overcome potential consumer reluctance towards products with nanotechnology by emphasizing safety and naturalness [
89,
90].
3. Materials and Methods
3.1. Chemical Reagents
All chemical reagents utilized in this study, including food-grade fucoxanthin (FXN), soy phosphatidylcholine (SPC), cholesterol, DPPH (1,1-diphenyl-2-picrylhydrazyl), ABTS [2,2’ -azinobis (3-ethylbenzothiazoline)-6-sulfonic acid], dimethyl sulfoxide (DMSO), sodium acetate buffer, FeCl3 (ferric chloride), TPTZ (2,4,6-tripyridyl-s-triazine), Triton X-100, AAPH [2,2′-azobis (2-methylpropionamidine) dihydrochloride], and PBS (phosphate-buffered saline), were sourced from Sigma-Aldrich Co., St. Louis, MO, USA. All other solvents used were of analytical grade and the highest commercial quality.
3.2. Biological Material and Ethical Considerations
All techniques involving human red blood cells (RBCs) are subject to international regulations (FDA: CFR - Code of Federal Regulations Title 21, Part 640 for human blood and blood products, Subpart B Red Blood Cells, Sec. 640.14 Blood Tests [21CFR640.14]) and Mexican Additional Standards (NOM-253-SSA1-2012). The RBCs membrane was donated by the clinical analysis laboratory of the University of Guadalajara, accredited by ISO-IEC 17.025 (NMX-EC-17025) and ISO 15.189 developed by the ISO/TC 212 technical committee (Clinical Laboratory Testing and In vitro Diagnostic Systems), with ISO/IEC 17.025 and ISO 9001 as reference standards [
91,
92,
93,
94]. Human red blood cells were collected from healthy adult volunteers (aged 20 to 40 years) containing approximately 4.7 to 6.1 x106 cells/μL. Prior to the procedure, informed consent was obtained from each individual. The venous puncture technique was applied to collect human red blood cells in a sterile vial with EDTA used as anticoagulant. The use of erythrocyte membranes as a cellular model aims to evaluate the erythroprotective potential of yogurt formulation. Therefore, this study does not involve volunteers [
95,
96]. According to university procedure, study approval was obtained by the authors (CI 2023-47).
3.3. Synthesis of Fucoxanthin-Loaded Nanoliposomes
Fucoxanthin-Loaded Nanoliposomes (FXN-LN) were prepared using the ultrasonic film dispersion technique [
21]. A quantity of 1 mg of fucoxanthin was dissolved in 5 mL of ethanol/ sodium phosphate buffer (0.05 mol/L, pH 7.4). Lipid materials (SPC and Chol, 5:1 w/w) for the formation of liposomal vehicles were added to the fucoxanthin solution and dissolved into ethanol. This solution underwent rotary evaporation (Heidolph Laborata 4000, Germany) to remove completely the ethanol. Once the solvent was removed, the sample was hydrated with 25 mL of sodium phosphate buffer (0.05 mol/L, pH 7.4), agitated for 30 minutes at 50°C. Subsequently, the suspension underwent homogenization assisted by high-power ultrasonic pulses (Branson Digital Sonifier Qsonica, LLC. U.S.A.), 15 seconds per pulse, three pulses with a one-minute rest interval at an amplitude of 30% at 400w and 500mHz to reduce the particular size. Finally, the nanoliposomes are freeze-dried to later be integrated into an amber glass bottle under a nitrogen atmosphere and stored at 4°C prior to the study.
3.4. Morphological Study
The morphological study of FXN-LN was conducted using scanning electron microscopy (HT7700, Hitachi, Tokyo, Japan). The lyophilized fucoxanthin-loaded nanoliposomal carriers were placed onto a copper tape to form a film. The samples were then coated with gold and analyzed by SEM [
97,
98].
3.5. Encapsulation Efficiency
The encapsulation efficiency measurement was conducted using the extraction methodology outlined by Pan et al. [
22]. A volume of 400 μL of FXN-LN solution was mixed with 1 mL of petroleum ether, followed by constant agitation (45 rpm) for 5 minutes at 30°C. The resulting mixture was centrifuged at 3000 rpm for 5 minutes to recover the supernatant. The upper portion was collected and subjected to rotary evaporation to remove the petroleum ether. After evaporation, the residual sample was resuspended in chloroform. The content of free fucoxanthin was quantified at 460 nm using a 96-well microplate reader (UV-Vis spectrophotometer, Thermo Fisher Scientific Inc. Multiskan GO, NY, USA). The encapsulation efficiency (%) was calculated using the following equation: The encapsulation efficiency (%) will be calculated using the following equations:
3.6. Particle Size Measurement
The particle size measurement was estimated using Dynamic Light Scattering (DLS) (Nano-ZS90, Malvern Instruments, Worcestershire, United Kingdom) at 25°C with a 90º detector angle. The nanoliposomes were diluted 100 times in PBS. Each determination was performed in triplicate [
22].
3.7. Centrifugal Stability Measurement
Stability of nanoliposome samples was examined as per the method of Ghorbanzade et al. [
85] with slight modification. A volume of 5 mL of nanoliposomes were subjected to centrifugation at 3500 g for 15 min. Nanoliposome stability (NS) was calculated as follows:
where,
Fev is the final volume of the bottom phase and
Iev is the initial volume of liposomal dispersion.
3.8. In Vitro Release
In vitro release studies were conducted using 10 mL of pure fucoxanthin solution and FXN-LN at the same concentration (1 mg). This volume was placed into a dialysis bag (8000-14000 Da). The dialysis membrane was immersed in 100 mL of PBS (0.05 mol/L, pH 7.4) at 37°C with constant agitation at 100 rpm. Aliquots of 1 mL were withdrawn from the release medium at intervals of 0.5, 1, 2, 4, 6, 8, 10, 12, and 24 hours. The concentration of released fucoxanthin was measured in a microplate reader at a wavelength of 460 nm. The results will be reported as the percentage of fucoxanthin released. All release tests were conducted in triplicate [
22]. The release rate of Ant was obtained using the following equation:
3.9. Preparation of Functional Yogurt
Artisanal yogurt was obtained from a local store (Ameca, Jalisco, Mexico). Two formulations of yogurt enriched with 5 and 10% nano-liposomes loaded with fucoxanthin were prepared in separate 100 g yogurt samples [
85]. The formulations were stored in glass containers in a refrigerator at 4°C for subsequent analysis. The yogurt enriched with nanoliposomes was placed in airtight containers to prevent exposure to air and light. The containers were stored at 4°C, simulating refrigeration conditions for a period of 21 days. Samples of the enriched yogurt were taken as part of periodic sampling during the storage period every 7 days. Under this condition, the effect of cold storage conditions on the stability, physicochemical and rheological characteristics of the formulations can systematically be evaluated, providing important information for their application in refrigerated food products.
3.10. Effect of Cold Storage Conditions on the Antioxidant, Physicochemical and Rheological Properties of Yogurt Enriched with FXN-LN
3.10.1. Antioxidant Properties
The measurement of the antioxidant properties of fucoxanthin-loaded nanoliposome was carried out for 21 days under cold storage. The antioxidant activity was evaluated using three methods. The FRAP assay, based on Benzie and Strain [
99], involved preparing a working solution from sodium acetate buffer, triazine-TPTZ in HCl, and ferric chloride, and measuring the reduction of TPTZ at 638 nm. The DPPH• assay, following 96. Brand-Williams et al. [
100], required dissolving DPPH• in ethanol, adjusting its absorbance, and mixing it with the sample to measure absorbance at 515 nm. The ABTS assay, modified from Re et al. [
101], involved preparing an ABTS+• solution with potassium persulfate, adjusting its absorbance, and mixing it with the sample to measure absorbance at 734 nm. All assays were performed in triplicate and measurements were taken using a microplate reader (Thermo Fisher Scientific Inc. Multiskan GO, NY, USA).
3.10.2. Erythroprotective Potential
The evaluation of erythroprotective potential was conducted through a series of assays assessing oxidative inhibition by AAPH-induced hemolysis, ultraviolet radiation-induced hemolysis, and membrane stabilization test (heat-induced hemolysis inhibition and hypotonicity-induced hemolysis inhibition). The antihemolytic activity was evaluated by inducing oxidative hemolysis using the free radical generator AAPH [2,2′-azobis-(2-methylpropionamidine)], following the methods of Ruiz-Cruz et al. [
96]. Human erythrocytes were obtained from healthy adults aged 20-45 years, who were informed about the procedure and provided informed consent. A 2% erythrocyte suspension was prepared, and an AAPH solution was made at 40 mM (pH 7.4). The reaction was initiated by mixing 100 μL of erythrocytes (2%), 100 μL of the sample, and 100 μL of AAPH. Controls were prepared as follows: 100 μL of erythrocytes + 100 μL of PBS + 100 μL of AAPH (positive control, hemolysis) and 100 μL of erythrocytes + 200 μL of PBS (negative control, no hemolysis). Samples and controls were incubated at 37 °C for 3 hours with constant shaking at 45 rpm. After incubation, 1 mL of PBS was added to each sample, including the controls, and they were centrifuged at 1500 rpm for 10 minutes. Finally, 300 µL of supernatant was collected, and the hemoglobin released by oxidation was quantified at 540 nm using a 96-well microplate reader (Multiskan Go, Thermo Scientific, Waltham, MA, USA).
Photohemolysis of human erythrocytes will be induced using UV-A (315-395 nm) and UV-B (280-315 nm) light at an intensity of 0.85 mW/cm², with the UV light chamber maintained at 18 ±1 °C. The photoprotective effect of fucoxanthin against UV-induced peroxidation was evaluated. Fucoxanthin was dissolved in DMSO and diluted in PBS:DMSO (90:10). A 1% erythrocyte suspension was used, with 3 mL aliquots placed in sterile vials and 150 µL of the sample added. Positive controls were erythrocytes exposed to UV radiation, and negative controls were non-irradiated erythrocytes. Samples were pre-incubated at 37 °C for 30 minutes, then exposed to UV-A and UV-B for intervals of 0, 15, 30, 60, and 120 minutes at 10 cm from the lamps. Post-irradiation, samples were centrifuged at 2000 x g for 10 minutes, and 300 µL of each sample was placed in a 96-well microplate for measurement at 540 nm [
4].
Heat-induced hemolysis and hypotonicity-induced hemolysis assays were conducted to evaluate the membrane stabilization (%) of various tested yogurt formulation. Encapsulated fucoxanthin carried in yogurt stabilizes erythrocyte membranes by inhibiting the release of hemoglobin constituents, making it a useful control in the Membrane-Stabilizing Capacity Assay to assess the potential of antioxidants as erythroprotective agents. The heat-induced hemolysis assay followed Agarwal et al. [
98] methodology with minor modifications, involving the incubation of erythrocyte suspensions and samples at 55°C for 30 minutes, followed by PBS addition and centrifugation, with absorbance measured at 540 nm. The hypotonicity-induced hemolysis assay also followed Agarwal et al. [
102] modified methodology, using distilled water as the hypotonic solution, and involved a similar incubation and centrifugation process, with different controls used for comparison.
3.10.3. Physicochemical Properties
3.10.3.1. Color
The colorimetric assay of the yogurt samples was evaluated using a colorimeter Color flex, Hunter Associates Laboratory, Inc., USA. The instrument was calibrated with a white tile calibration plate (L* = 93.45; a* = -1.00; b* = 2.15). The results were expressed as L*, a*, and b* color values, where L* represents luminosity; a* represents redness (+) to greenness (-); b* represents yellowness (+) to blueness (-) of the yogurt [
85].
3.10.3.2. Electrical Conductivity
Measurements of the electrical conductivity of the yogurt samples will be carried out using the method described in Alimentarius, Codex [
50] with slight modifications.
3.10.3.2. pH
The pH was assessed using a digital pH meter (HI 2211 PH/MV, HANNA) calibrated before measurement with reference buffer solutions (pH 4.0 and 7.0), according to AOCS [
103], by immersing an electrode in the solution. The sample (1 g) was mixed in 10 mL of distilled water for analysis, and the reading was recorded.
3.10.3.3. Titratable Acidity
Titratable acidity and pH were measured according to the official AOAC 942.15 method [
104]. Titratable acidity was expressed as grams of lactic acid per 100 grams of product after mixing 10 g of yogurt sample with 10 mL of hot distilled water and titrating with 0.1 N NaOH using a 0.5% phenolphthalein indicator.
3.10.3.4. Syneresis Susceptibility (STS)
To determine syneresis of different yogurt formulation, a quantity of 20 g of yogurt was centrifuged at 500 rpm for 5 minutes. The separated liquid was collected in a graduated cylinder [
105]. The percentage of syneresis was calculated using the following equation:
3.10.3.5. Water Holding Capacity (WHC)
To determine water holding capacity (WHC), 2.5 g of sample were taken (Anderson et al., 1969) and homogenized with 30 mL of distilled water at 30°C in a water bath for 30 minutes. The mixture was then centrifuged at 3000 x g for 30 minutes. The supernatant was decanted and dried in an oven (3489M-1, Barnstead International, Dubuque, Iowa, USA) at 90°C for 24 hours. Additionally, the weight of the precipitate in the cylinders was recorded. The WHC results were expressed as a percentage of water holding capacity [
105].
3.10.3.6. Texture
The texture of the yogurt was evaluated using a TA-XT2 texture analyzer (Stable Micro Systems, Godalming, United Kingdom) with accompanying software. The probe utilized was cylindrical with a diameter of 25 mm (P25/L), and the test conditions included a pre-test speed of 5 mm/s, a test speed of 3.0 mm/s, target mode set to strain, a duration of 3 seconds, and a trigger force of 0.5 g using a 2000 g calibration weight. The test was conducted directly on a 40 g sample cup and performed in triplicate. All experiments were carried out at a temperature of 5°C. The primary textural properties of the yogurt were measured, including firmness or gel strength (peak compression force during penetration) and adhesiveness (negative force area) [
42].
3.10.4. Rheological Analysis
Measurements were conducted using a rotational viscometer (Thermo Haake DC 10, model VT 550, Karlsruhe, Germany), with concentric cylinders (NV ST 807-0713 CE and NV 807-0702), and the data were collected by the Pro Rheowin software program © (version 2.93, Haake). These analyses were performed in triplicate. The temperature used was 25 °C, and the shear rate ranged from 0 to 2000 s
-1 (upward curve) and from 2000 to 0 s
-1 (downward curve); each curve was obtained over 3 minutes. The flow behavior was described using the Power Law model, and the thixotropic behavior of the yogurt was evaluated by calculating the area of the hysteresis loop between the upward and downward flow curves [
66].
3.10.5. Sensory Analysis
Thirty panelists aged between 20 and 40 years were selected to participate in the sensory panel. The sensory evaluation (color, flavor, aftertaste, scent, consistency, texture, appearance, and overall acceptance were based on 10-point hedonic scales (0: dislike extremely to 9: like extremely). Each sample was individually rated, and the samples were presented to the panelists in individual plastic containers. The yogurts (coded with 3 digits) were randomly presented to the panel group in each session [
106].
3.11. Statistical Analysis
The results will be reported as means ± standard deviation (SD) of at least three repetitions (n > 3). All data were analyzed using JMP software v16 for Mac and expressed as mean ± SD (standard deviation). One-way and two-way ANOVA were performed to explore the interaction between the various factors evaluated. Tukey’s test was applied with a confidence level of p < 0.05.
4. Conclusions
The nanoliposomal vehicles that encapsulated fucoxanthin developed in this study were produced by applying a simple, low-cost ultrasonic film dispersion technique that could be scaled up industrially. The incorporation of fucoxanthin-loaded nanoliposomes into yogurt has revealed significant findings about the bioactive potential to enhance and increase the therapeutic properties of yogurt. The nanoliposomes showed high encapsulation efficiency and stability, allowing for controlled and sustained release of fucoxanthin. This encapsulation method not only protects fucoxanthin from degradation during storage, but also increases its solubility and bioavailability. Fucoxanthin nanoliposome-enriched yogurt formulations demonstrated significant improvements in antioxidant capacity. It was confirmed that yogurt with nanoliposomes retained higher antioxidant activity without being affected by cold storage compared to yogurt without enrichment. On the other hand, although the incorporation of nanoliposomes decreases the viscosity and firmness of yogurt, which could affect the texture of the product, an improvement in pH stability and titratable acidity was observed. Furthermore, there was a reduction in syneresis while increasing its water retention capacity, thereby improving product stability during storage. The formulation with 10% fucoxanthin nanoliposomes not only improved the antioxidant properties of yogurt but also had a positive impact on sensory properties such as color, taste, and overall acceptance, suggesting a higher likelihood of consumer acceptance. The incorporation of fucoxanthin-loaded nanoliposomes into yogurt could be a viable strategy to enhance the functionality and stability of the product. However, challenges related to texture need to be addressed to fully optimize the formulation. These findings open the door to future research on optimizing the interaction between nanoliposomes and the yogurt matrix, as well as exploring other bioactive compounds encapsulated in nanoliposomes for applications in functional foods. Finally, these results suggest that nanoliposomal vehicles are suitable for carries fucoxanthin, their incorporation into food matrices is key to developing functional foods. Regulatory approvals and consumer perceptions regarding nanotechnology-based products must be addressed, emphasizing their safety and health benefits.
Author Contributions
M.A.R.-G.: Conceptualization and investigation; R.I.G.-V and C.L.D.-T.-S.: Supervision and project administration; G.L.-V. and R.G.L.-B.: Writing-review-preparation of the original draft; A.T.B-M and R.D.I-G: Editing and Proofreading; M.G-L and M.G.A-N: data curation; F.V.V-V and B.V-R: Collaborations and Networking. All authors have read and agreed to the send version of the manuscript.
Funding
This research received no external funding or not-for-profit sectors. The study was funded with the authors’ own resources.
Institutional Review Board Statement
This research was conducted in accordance with the Declaration of Helsinki of 1975. The work was supported by the clinical laboratory holds accreditation from ISO-IEC 17025 (NMX-EC-17025) and ISO 15189, as established by the technical committee ISO/TC 212 (Clinical Laboratory Testing and In Vitro Diagnostic Systems), with reference to ISO/IEC 17025 and ISO 9001 standards. Ethical approval: As per university protocol, the study was approved by the authors (CI 2023-47).
Informed Consent Statement
Written informed consent has been obtained from the participants to publish this paper. Therefore, all participants provided their informed consent before participating in the study.
Data Availability Statement
The data supporting the findings of this research are provided within the article. Additional details can be obtained from the corresponding authors upon request.
Acknowledgments
The authors extend their sincere gratitude to the Department of Research and Postgraduate in Food at Universidad de Sonora and the Department of Medical and Life Sciences at Cienega University Center (CUCIÉNEGA), Universidad de Guadalajara, for supplying the essential resources and infrastructure. Additionally, the authors are thankful to the artisans from Ameca, Jalisco, for providing the raw materials.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Rashwan, A. K. , Osman, A. I., & Chen, W. (2023). Natural nutraceuticals for enhancing yogurt properties: A review. Environmental Chemistry Letters, 21, 1907–1931. [CrossRef]
- Alkobeisi, F. , Varidi, M. J., Varidi, M., & Nooshkam, M. (2022). Quinoa flour as a skim milk powder replacer in concentrated yogurts: Effect on their physicochemical, technological, and sensory properties. Food Science and Nutrition, 10, 1113–1125. [CrossRef]
- Almutairi, B. , Turner, M. S., Fletcher, M. T., & Sultanbawa, Y. (2021). The impact of commercial prebiotics on the growth, survival and nisin production by Lactococcuslactis 537 in milk. LWT - Food Science and Technology, 137, Article 110356. [CrossRef]
- González-Vega, R.I.; Robles-García, M.Á.; Mendoza- Urizabel, L.Y.; Cárdenas-Enríquez, K.N.; Ruiz-Cruz, S.; Gutiérrez- Lomelí, M.; Iturralde-García, R.D.; Avila-Novoa, M.G.; Villalpando- Vargas, F.V.; Del-Toro-Sánchez, C.L. Impact of the ABO and RhD Blood Groups on the Evaluation of the Erythroprotective Potential of Fucoxanthin, β-Carotene, Gallic Acid, Quercetin and Ascorbic Acid as Therapeutic Agents against Oxidative Stress. Antioxidants 2023, 12, 2092. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Jian-Ping, Y.; Chou-Fei, W.; Jiang-Hai, W. Fucoxanthin, a Marine Carotenoid Present in Brown Seaweeds and Diatoms: Metabolism and Bioactivities Relevant to Human Health. Mar. Drugs. 2011, 9:1806-1828. [CrossRef]
- Lourenço-Lopes, C.; Fraga-Corral, M.; Jimenez-Lopez, C.; Carpena, M.; Pereira, A.G.; Garcia-Oliveira, P.; Prieto, M.A.; Simal-Gandara, J. Biological action mechanisms of fucoxanthin extracted from algae for application in food and cosmetic industries. Trends Food Sci. Technol. 2021, 117, 163–181. [Google Scholar] [CrossRef]
- Rostamabadi, H. , Reza Falsafi, S., Mahdi Jafari, S. 2019. Nanoencapsulation of carotenoids within lipid-based nanocarriers. Journal of controlled release. 298: 38-67. [CrossRef]
- Sun, Y. , Chi, J., Ye, X., Wang, S., Liang, J., Yue, P., Xiao, H., & Gao, X. (2021). Nanoliposomes as delivery system for anthocyanins: Physicochemical characterization, cellular uptake, and antioxidant properties. LWT, 139, 110554. [CrossRef]
- Bhosale, S. , Fulpagare, Y. G., & Desale, R. J. (2019). Nanoliposomes: Applications in food and dairy industry. 6(11), 79-84. [CrossRef]
- Cheng, X.; Zang, M.; Wang, S.; Zhao, X.; Zhai, G.; Wang, L.; Li, X.; Zhao, Y.; Yue, Y. Physicochemical and Antioxidant Properties of Nanoliposomes Loaded with Rosemary Oleoresin and Their Oxidative Stability Application in Dried Oysters. Bioengineering 2022, 9, 818. [Google Scholar] [CrossRef] [PubMed]
- Barkallah, M. , Dammak, M., Louati, I., Hentati, F., Hadrich, B., Mechichi, T., et al. (2017). Effect of Spirulina platensis fortification on physicochemical, textural, antioxidant and sensory properties of yogurt during fermentation and storage. LWT-Food Science & Technology, 84, 323–330. [CrossRef]
- da Silva, D. F. , Junior, N. N. T., Gomes, R. G., dos Santos Pozza, M. S., Britten, M., & Matumoto-Pintro, P. T. (2017). Physical, microbiological and rheological properties of probiotic yogurt supplemented with grape extract. Journal of Food Science & Technology, 54(6), 1608–1615. [CrossRef]
- Anuyahong, T. , Chusak, C., Adisakwattana, S. (2020). Incorporation of anthocyanin-rich riceberry rice in yogurts: Effect on physicochemical properties, antioxidant activity and in vitro gastrointestinal digestion. LWT. 129:109571. [CrossRef]
- Megrous, S. , Al-Dalali, S., Yang, Z. 2024. Physicochemical and functional properties of yoghurt supplemented with bioactive low-molecular-weight casein hydrolysates. International Dairy Journal. 155, 105956. [CrossRef]
- Lee, W. J. , & Lucey, J. A. (2010). Formation and physical properties of yogurt. Asian-Australasian Journal of Animal Sciences, 23, 1127–1136. [CrossRef]
- Oh, N. S. , Lee, J. Y., Joung, J. Y., Kim, K. S., Shin, Y. K., Lee, K. W., et al. (2016). Microbiological characterization and functionality of set-type yogurt fermented with potential prebiotic substrates Cudrania tricuspidata and Morus alba L. leaf extracts. Journal of Dairy Science, 99(8), 6014–6025. [CrossRef]
- Mani-López, E., Palou, E., & López-Malo, A. (2014). Probiotic viability and storage stability of yogurts and fermented milks prepared with several mixtures of lactic acid bacteria. Journal of Dairy Science, 97(5), 2578–2590. [CrossRef]
- Liu, D. , & Lv, X. X. (2019). Effect of blueberry flower pulp on sensory, physicochemical properties, lactic acid bacteria, and antioxidant activity of set-type yogurt during refrigeration. Journal of Food Processing and Preservation, 43(1), e13856. [CrossRef]
- Liu, X. T., Zhang, H., Wang, F., Luo, J., Guo, H. Y., & Ren, F. Z. (2014). Rheological and structural properties of differently acidified and renneted milk gels. Journal of Dairy Science, 97, 3292–3299. [CrossRef]
- Brodziak, A., Krol, J., Barłowska, J., Teter, A., & Florek, M. (2020). Changes in the physicochemical parameters of yoghurts with added whey protein in relation to the starter bacteria strains and storage time. Animals, 10(8), 1350. [CrossRef]
- Tamjidi, F.; Shahedi, M.; Varshosaz, J.; Nasirpour, A. Design and characterization of astaxanthin-loaded nanostructured lipid carriers. Innov. Food Sci. Emerg. 2014, 26, 366–374. [Google Scholar] [CrossRef]
- Pan, L. H. , Liu, F., Luo, S. Z., & Luo, J. P. (2019). Pomegranate juice powder as sugar replacer enhanced quality and function of set yogurts: Structure, rheological property, antioxidant activity and in vitro bioaccessibility. LWT-Food Science and Technology, 115, 108479. [CrossRef]
- Rodriguez-Ruiz, V. , Salatti-Dorado J.Á., Barzegari A., Nicolas-Boluda A., Houaoui A., Caballo C., Caballero-Casero N., Sicilia D., Venegas J.B., Pauthe E., et al. Astaxanthin-loaded nanostructured lipid carriers for preservation of antioxidant activity. Molecules. 2018;23:2601. [CrossRef]
- Hu, F. , Liu W., Yan L., Kong F., Wei K. Optimization and characterization of poly(lactic-co-glycolic acid) nanoparticles loaded with astaxanthin and evaluation of anti-photodamage effect in vitro. R. Soc. Open Sci. 2019;6:191184. [CrossRef]
- Wang, Q. , Zhao Y., Guan L., Zhang Y., Dang Q., Dong P., Li J., Liang X. Preparation of astaxanthin-loaded DNA/chitosan nanoparticles for improved cellular uptake and antioxidation capability. Food Chem. 2017;227:9–15. [CrossRef]
- Liu, C. , Zhang S., McClements D.J., Wang D., Xu Y. Design of Astaxanthin-Loaded Core-Shell Nanoparticles Consisting of Chitosan Oligosaccharides and Poly(lactic- co-glycolic acid): Enhancement of Water Solubility, Stability, and Bioavailability. J. Agric. Food Chem. 2019;67:5113–5121. [CrossRef]
- Taksima, T.; Limpawattana, M.; Klaypradit, W. Astaxanthin encapsulated in beads using ultrasonic atomizer andapplication in yogurt as evaluated by consumer sensory profile. LWT-Food Sci. Technol. 2015, 62, 431–437. [Google Scholar] [CrossRef]
- Mozafari, M.R. (2010). Nanoliposomes: Preparation and Analysis. In: Weissig, V. (eds) Liposomes. Methods in Molecular Biology, vol 605. Humana Press. [CrossRef]
- Mozafari MR (ed) (2006) Nanocarrier technologies: frontiers of nanotherapy. Springer, The Netherlands. [CrossRef]
- Thien Trung Le, Thi Thanh Que Phan, John Van Camp, Koen Dewettinck. Milk and Dairy Polar Lipids: Occurrence, Purification, and Nutritional and Technological Properties. 2015, 91-143. [CrossRef]
- Aguilar-Pérez, K.M.; Medina, D.I.; Narayanan, J.; Parra-Saldívar, R.; Iqbal, H.M.N. Synthesis and Nano-Sized Characterization of Bioactive Oregano Essential Oil Molecule-Loaded Small Unilamellar Nanoliposomes with Antifungal Potentialities. Molecules 2021, 26, 2880. [Google Scholar] [CrossRef] [PubMed]
- Matos,J.;Afonso,C.; Cardoso, C.; Serralheiro, M.L.; Bandarra, N.M. Yogurt Enriched with Isochrysis galbana: An Innovative Functional Food. Foods 2021, 10, 1458. [CrossRef]
- Peng, C.H.; Chang, C.H.; Peng, R.Y.; Chyau, C.C. Improved membrane transport of astaxanthin by liposomal encapsulation. Eur. J. Pharm. Biopharm. 2010, 75, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Risaliti, L.; Kehagia, A.; Daoultzi, E.; Lazari, D.; Bergonzi, M.C.; Vergkizi-Nikolakaki, S.; Bilia, A.R. Liposomes loaded with Salvia triloba and Rosmarinus officinalis essential oils: In vitro assessment of antioxidant, antiinflammatory and antibacterial activities. J. Drug Deliv. Sci. Technol. 2019, 51, 493–498. [Google Scholar] [CrossRef]
- Hammoud, Z.; Gharib, R.; Fourmentin, S.; Elaissari, A.; Greige-Gerges, H. New findings on the incorporation of essential oil components into liposomes composed of lipoid S100 and cholesterol. Int. J. Pharm. 2019, 561, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Hamad I, Harb AA, Bustanji Y. Liposome-Based Drug Delivery Systems in Cancer Research: An Analysis of Global Landscape Efforts and Achievements. Pharmaceutics. 2024; 16(3):400. [CrossRef]
- Zarrabi, A.; Alipoor Amro Abadi, M.; Khorasani, S.; Mohammadabadi, M.-R.; Jamshidi, A.; Torkaman, S.; Taghavi, E.; Mozafari, M.R.; Rasti, B. Nanoliposomes and Tocosomes as Multifunctional Nanocarriers for the Encapsulation of Nutraceutical and Dietary Molecules. Molecules 2020, 25, 638. [Google Scholar] [CrossRef]
- Wang, C. , Wang, E., Bai, Y., Lu, Y., Qi, H., 2023. Encapsulated fucoxanthin improves the structure and functional properties of fermented yogurt during cold storage. Food Chemistry. 419: 136076. [CrossRef]
- Shehata, M.K. , Ismail, A.A., Kamel, M.A. 2023. Nose to Brain Delivery of Astaxanthin–Loaded Nanostructured Lipid Carriers in Rat Model of Alzheimer’s Disease: Preparation, in vitro and in vivo Evaluation. Int J Nanomedicine. 2023; 18: 1631–1658.
- Jafari, Z. , Bigham, A., Sadeghi, S., Dehdashti, S. M., Rabiee, N., Abedivash, A., Bagherzadeh, M., Nasseri, B., Karimi-Maleh, H., Sharifi, E., Varma, R. S., & Makvandi, P. (2022). Nanotechnology-abetted astaxanthin formulations in multimodel therapeutic and biomedical applications. Journal of Medicinal Chemistry, 65(2). [CrossRef]
- Corrêa, R. C. G. , Barros, L., Fernandes, Â., Sokovic, M., Bracht, A., Peralta, R. M., & Ferreira, I. C. F. R. (2018). A natural food ingredient based on ergosterol: Optimization of the extraction from Agaricus blazei, evaluation of bioactive properties and incorporation in yogurts. Food & Function, 9(3), 1465-1474. [CrossRef]
- Bourne, M. C. 2002. Food Texture and Viscosity: Concept and Measurement. 2nd ed. Academic Press, San Diego, CA.
- Lee, J-W and Lucey, J.A. 2006. Impact of Gelation Conditions and Structural Breakdown on the Physical and Sensory Properties of Stirred Yogurts. J. Dairy Sci. 89:2374–2385. [CrossRef]
- Jeong, C. H. , Ryu, H., Zhang, T., Lee, C. H., Seo, H. G., & Han, S. G. (2018). Green tea powder supplementation enhances fermentation and antioxidant activity of set-type yogurt. Food Science and Biotechnology, 27(5), 1419-1427. [CrossRef]
- Zahoor, I. , Allai, F.M. (2020). Food Antioxidants: Functional Aspects and Preservation During Food Processing. In: Ahmad, S., Al-Shabib, N. (eds) Functional Food Products and Sustainable Health. Springer, Singapore. [CrossRef]
- Gómez-Estaca, J.; Balaguer, M.P.; López-Carballo, G.; Gavara, R.; Hernández-Muñoz, P. Improving Antioxidant and Antimicrobial Properties of Curcumin by Means of Encapsulation in Gelatin through Electrohydrodynamic Atomization. Food Hydrocoll. 2017, 70, 313–320. [Google Scholar]
- Kristl, J., K. Teskac, C. Caddeo, Z. Abramovic, and M. Sentjurc. 2009. Improvements of cellular stress response on resveratrol in liposomes. European Journal of Pharmaceutics and Biopharmaceutics 73 (2):253–9. [CrossRef]
- Abd El-Emam, M.M.; Mostafa, M.; Farag, A.A.; Youssef, H.S.; El-Demerdash, A.S.; Bayoumi, H.; Gebba, M.A.; El-Halawani, S.M.; Saleh, A.M.; Badr, A.M.; et al. The Potential Effects of Quercetin-Loaded Nanoliposomes on Amoxicillin/Clavulanate-Induced Hepatic Damage: Targeting the SIRT1/Nrf2/NF-κB Signaling Pathway and Microbiota Modulation. Antioxidants 2023, 12, 1487. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zang, M.; Wang, S.; Zhao, X.; Zhai, G.; Wang, L.; Li, X.; Zhao, Y.; Yue, Y. Physicochemical and Antioxidant Properties of Nanoliposomes Loaded with Rosemary Oleoresin and Their Oxidative Stability Application in Dried Oysters. Bioengineering 2022, 9, 818. [Google Scholar] [CrossRef] [PubMed]
- Alimentarius, Codex (2010). Codex standard for fermented milks (CODEX STAN 243-2003), Adopted in 2003, Revision 2008. Rome: FAO.
- Wu, Y. , Wang, K., Liu, Q., Liu, X., Mou, B., Lai, O.-M., Tan, C.-P., & Cheong, L.-Z. (2022). Selective antibacterial activities and storage stability of curcumin-loaded nanoliposomes prepared from bovine milk phospholipid and cholesterol. Food Chemistry, 367, 130700. [CrossRef]
- Hasan, M. , Elkhoury, K., Kahn, C. J. F., Arab-Tehrany, E., & Linder, M. (2019). Preparation, Characterization, and Release Kinetics of Chitosan-Coated Nanoliposomes Encapsulating Curcumin in Simulated Environments. Molecules, 24(10), 2023. [CrossRef]
- Linder, M. , & Arab-Tehrany, E. (2018). The Positive Role of Curcumin-Loaded Salmon Nanoliposomes on the Culture of Primary Cortical Neurons. Marine Drugs, 16(7), 218. [CrossRef]
- Verma, S. , Singh, S.K., Syan, N.P., Mathur, N., Valecha, V. 2010. Nanoparticle vesicular systems: a versatile tool for drug delivery, J. Chem. Pharm. Res. 2 (2): 496–509.
- Liu, W. , Ye, A., Liu, C., Liu, W., Singh, H. 2012. Structure and integrity of liposomes pre- pared from milk-or soybean-derived phospholipids during in vitro digestion, Food. Res. Int. 48 (2): 499–506.
- Yakoubi, S.; Kobayashi, I.; Uemura, K.; Nakajima, M.; Isoda, H.; Ksouri, R.; Saidani-Tounsi, M.; Neves, M.A. Essential-Oil-Loaded Nanoemulsion Lipidic-Phase Optimization and Modeling by Response Surface Methodology (RSM): Enhancement of Their Antimicrobial Potential and Bioavailability in Nanoscale Food Delivery System. Foods 2021, 10, 3149. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; He, N.; Yang, T.; Cai, S.; Zhang, Y.; Lin, J.; Huang, M.; Chen, W.; Zhang, Y.; Hong, Z. Fucoxanthin Loaded in Palm Stearin- and Cholesterol-Based Solid Lipid Nanoparticle-Microcapsules, with Improved Stability and Bioavailability In Vivo. Mar. Drugs 2022, 20, 237. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, K.; Inbaraj, B.S.; Chen, B.-H. Recent Advances on Nanoparticle Based Strategies for Improving Carotenoid Stability and Biological Activity. Antioxidants 2021, 10, 713. [Google Scholar] [CrossRef] [PubMed]
- Verardi, A.; Sangiorgio, P.; Lopresto, C.G.; Casella, P.; Errico, S. Enhancing Carotenoids’ Efficacy by Using Chitosan-Based Delivery Systems. Nutraceuticals 2023, 3, 451–480. [Google Scholar] [CrossRef]
- Núñez de González, M.T.; Attaie, R.; Woldesenbet, S.; Mora-Gutierrez, A.; Jung, Y. Fucoxanthin as a Biofunctional Compound in Goat Milk Yogurt: Stability and Physicochemical Effects. Fermentation 2023, 9, 273. [Google Scholar] [CrossRef]
- Mok, I.K.; Lee, J.K.; Kim, J.H.; Pan, C.H.; Kim, S.M. 2018. Fucoxanthin bioavailability from fucoxanthin-fortified milk: In vivo and in vitro study. Food Chem. 258, 79–86. [CrossRef]
- Mok, I.K.; Yoon, J.R.; Pan, C.H.; Kim, S.M. Development, quantification, method validation, and stability study of a novel fucoxanthin-fortified milk. J. Agric. Food Chem. 2016, 64, 6196–6202. [Google Scholar] [CrossRef] [PubMed]
- Gürbüz Z, Erkaya-Kotan T, Şengül M. 2021. Evaluation of physicochemical, microbiological, texture and microstructure characteristics of set-style yoghurt supplemented with quince seed mucilage powder as a novel natural stabiliser. International Dairy Journal 114:104938 10.1016/j.idairyj.2020.104938.
- Gyawali R, Ibrahim SA. 2016. Effects of hydrocolloids and processing conditions on acid whey production with reference to Greek yogurt. Trends in Food Science & Technology 56(S1):61–76 DOI 10.1016/j.tifs.2016.07.013.
- Mehra R, Kumar H, Rafiq S, Kumar N, Buttar HS, Leicht K, Okpala COR, Korzeniowska M. 2022. Enhancing yogurt products’ ingredients: preservation strategies, processing conditions, analytical detection methods, and therapeutic delivery—an overview. PeerJ 10:e14177 DOI 10.7717/peerj.14177.
- Aportela-Palacios, A., Sosa-Morales, M., & Vélez-Ruiz, J. (2005). Rheological and physicochemical behavior of fortified yogurt, with fiber and calcium. Journal of Texture Studies, 36(3), 333–349. [CrossRef]
- Mahomud et al., (2016)Role of whey protein-casein complexes on yoghurt texture. Reviews in Agricultural Science, 5:1- 12, 2017. [CrossRef]
- Bierzuńska, P.; Cais-Sokolińska, D.; Yiğit, A. Storage Stability of Texture and Sensory Properties of Yogurt with the Addition of Polymerized Whey Proteins. Foods 2019, 8, 548. [Google Scholar] [CrossRef] [PubMed]
- Gharibzahedi, S.M.T.; Chronakis, I.S. 2018. Crosslinking of milk proteins by microbial transglutaminase: Utilization in functional yogurt products. Food Chem. 245, 620–632. [CrossRef]
- Vital, A.C.P.; Goto, P.A.; Hanai, L.N.; Gomes-da-Costa, S.M.; de Abreu Filho, B.A.; Nakamura, C.V.; Matumoto-Pintro, P.T. Microbiological, functional and rheological properties of low-fat yogurt supplemented with Pleurotus ostreatus aqueous extract. LWT-Food Sci. Technol. 2015, 64, 1028–1035. [Google Scholar] [CrossRef]
- Guggisberg, D. , Cuthbert-Steven, J., Piccinali, P., Bütikofer, U., & Eberhard, P. 2009. Rheological, microstructural and sensory characterization of low-fat and whole milk set yoghurt as influenced by inulin addition. International Dairy. Volume 19 (2):107-115. [CrossRef]
- Shaker, R. , Jumah, R., & Abu-Jdayil, B. (2000). Rheological properties of plain yogurt during coagulation process: Impact of fat content and preheat treatment of milk. Journal of Food Engineering, 44(3), 175–180. [CrossRef]
- Shokery ES, El-Ziney MG, Yossef AH, Mashaly RI (2017). Effect of Green Tea and Moringa Leave Extracts Fortification on the Physicochemical, Rheological, Sensory and Antioxidant Properties of Set-Type Yoghurt. J Adv Dairy Res 5: 179. [CrossRef]
- Staffolo, M. D., Bertola, N., & Martino, M. (2004). Influence of dietary fiber addition on sensory and rheological properties of yogurt. International Dairy Journal, 14 (3), 263–268. [CrossRef]
- Aziminezhad, H. , Esmaeilzadeh Kenari, R., & Raftani Amiri, Z. (2024). Nanoemulsions of red quinoa and ginseng extracts with chitosan wall: Investigating the antioxidant properties and its effect on the shelf life of dairy cream. Food Science & Nutrition, 00, 1–16. [CrossRef]
- Najgebauer-Lejko, D. , Żmudziński, D., Ptaszek, A., Socha, R. (2013). Textural properties of yogurts with green tea and Pu-erh tea additive. International Journal of Food Science & Technology. 49 (4). [CrossRef]
- de Campo, C. , Assis, R. Q., da Silva, M. M., Costa, T. M. H., Paese, K., Guterres, S. S., et al. (2019). Incorporation of zeaxanthin nanoparticles in yogurt: Influence on physico- chemical properties, carotenoid stability and sensory analysis. Food Chemistry, 301, 125230. [CrossRef]
- Athar, I.H. , Shah M.A., Khan U.N. 2000. Effect of various stabilizers on whey separation (syneresis)and quality of yogurt. Pakisatn Journal of Biological Science, 3:1336-1338. [CrossRef]
- Raftani Amiri, Z. , Rezaei Erami, S., Jafari, S. M., & Ahmadian, S. (2024). Physicochemical properties of yogurt enriched with nanoliposomes containing bitter melon extract. LWT - Food Science and Technology, 198, 116091. [CrossRef]
- Asaduzzaman, M. , Mahomud, M. S., & Haque, M. E. (2021). Heat-induced interaction of milk proteins: Impact on yoghurt structure. International Journal of Food Science, 2021, Article 5569917. [CrossRef]
- Tavakoli, H. , Hosseini, O., Jafari, S. M., & Katouzian, I. (2018). Evaluation of physicochemical and antioxidant properties of yogurt enriched by olive leaf phenolics within nanoliposomes. Journal of Agricultural and Food Chemistry, 66(35). [CrossRef]
- Borjizadeh, Z. , Ahari, H., Özdal, T., Khosravi-Darani, K., & Mohammadi Nafchi, A. (2024). Saffron nanoencapsulation (Crocus sativus) and its role in food science: Types and techniques. ACS Food Science & Technology, 4(6), 1310–1333. [CrossRef]
- Radi, U. M. A. , & Doosh, K. S. (2023). Study of the physiochemical and sensory properties of therapeutic low-cholesterol yoghurt fortified with zinc nanoparticles. IOP Conference Series: Earth and Environmental Science, 1262(6), 062029. [CrossRef]
- Schram, L.B.; Nielsen, C.J.; Porsgaard, T.; Nielsen, N.S.; Holm, R.; Mu, H. Food matrices affect the bioavailability of (n-3) polyunsaturated fatty acids in a single meal study in humans. Food Res. Int. 2007, 40, 1062–1068. [Google Scholar] [CrossRef]
- Ghorbanzade, T. , Mahdi Jafari, M.S., Akhavan, S., Hadavi, R. 2017. Nano-encapsulation of fish oil in nano-liposomes and its application in fortification of yogurt. Food Chemistry 216 (2017) 146–152. [CrossRef]
- Domoto, N.; Koenen, M.E.; Havenaar, R.; Mikajiri, A.; Chu, B.S. 2013. The bioaccessibility of eicosapentaenoic acid was higher from phospholipid food products than from mono and triacylglycerol food products in a dynamic gastrointestinal model. Food Sci. Nutr. 1, 409–415.
- Sahin, O. I., Dundar, A. N., Ozdemir, S., Uzuner, K., Parlak, M. E., Dagdelen, A. F., & Saricaoglu, F. T. (2022). Nanophytosomes as a protection system to improve the gastrointestinal stability and bioavailability of phycocyanin. Food Bioscience, 50(Part A), 102052. [CrossRef]
- Mahfoudhi, N. , Ksouri, R., & Hamdi, S. (2016). Nanoemulsions as potential delivery systems for bioactive compounds in food systems: Preparation, characterization, and applications in food industry. En Nanotechnology in the Agri-Food Industry. 365-403. [CrossRef]
- McClements, D. J. (2018). Encapsulation, protection, and delivery of bioactive proteins and peptides using nanoparticle and microparticle systems: A review. Advances in Colloid and Interface Science, 253, 1-22. [CrossRef]
- Li, S. , Lo, C.-Y., Pan, M.-H., Lai, C.-S., & Ho, C.-T. (2013). Black tea: Chemical analysis and stability. Food & Function, 4(1), 10-18. [CrossRef]
-
ISO-IEC 17,025; Mexican Standard PROY-NMX-EC17,025 prepared by the Technical Committee for National Standardization of the Quality System INMC/CTNN 9. ISO: Geneva, Switzerland, 2022; Available online: https://www.iso.org/obp/ui/#iso:std: iso-iec:17025:ed-3:v2:es (accessed on 28 October 2022).
-
ISO 15,189; Medical laboratories. ISO: Geneva, Switzerland, 2022; Available online: https://www.iso.org/obp/ui/#iso:std:iso: 15189:ed-4:v1:en (accessed on 28 October 2022).
-
ISO/TC 212; Clinical laboratory testing and in vitro diagnostic test systems. ISO: Geneva, Switzerland, 2022. Available online: https://www.iso.org/committee/54916.html (accessed on 28 October 2022).
-
ISO 9001; Quality Management. ISO: Geneva, Switzerland, 2015; Available online: https://www.iso.org/obp/ui/#iso:std:iso: 9001:ed-5:v1:en (accessed on 28 October 2022).
- Peña-Medina, R.L.; Fimbres-Olivarría, D.; Enríquez-Ocaña, L.F.; Martínez-Córdova, L.R.; Del-Toro- Sánchez, C.L.; López-Elías, J.A.; González-Vega, R.I. Erythroprotective potential of phycobiliproteins extracted from Porphyridium cruentum. Metabolites. 2023, 13, 366. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cruz, S.; González-Vega, R.I.; Robles-Zepeda, R.E.; Reyes-Díaz, A.; López-Elías, J.A.; Alvarez-Ainza, M.L.; Cinco-Moroyoqui, F.J.; Moreno-Corral, R.A.; Wong-Corral, F.J.; Borboa-Flores, J.; et al. Association of Different ABO and Rh Blood Groups with the Erythroprotective Effect of Extracts from Navicula Incerta and Their Anti-Inflammatory and Antiproliferative Properties. Metabolites 2022, 12, 1203. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, B.; Maji, R.; Roychowdhury, S.; Ghosh, S. Toxicological concerns of engineered nanosize drug delivery systems. Am. J. Ther. 2016, 23, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Damari, S.P.; Shamrakov, D.; Varenik, M.; Koren, E.; Nativ-Roth, E.; Barenholz, Y.; Regev, O. Practical aspects in size and morphology characterization of drug-loaded nano-liposomes. Int. J. Pharm. 2018, 547, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, H.; Shanmugam, V.K. Anti-inflammatory activity screening of Kalanchoe pinnata methanol extract T and its validation using a computational simulation approach. Inform. Med. Unlocked 2019, 14, 6–14. [Google Scholar] [CrossRef]
- AOCS (2007). Official methods and recommended practices of the American oil chemist’s society (6th ed.). Champaign, IL: AOCS Press.
- AOAC (2000). The official methods of analysis (17th ed.). Maryland, USA: Association of Official Analytical Chemists.
- Achanta, K. , Aryana, K. J., & Boeneke, C. A. (2007). Fat free plain set yogurts fortified with various minerals. LWT – Food Science and Technology, 40(3), 424–429. [CrossRef]
- Lawless, H. T. , & Heymann, H. (2010). Sensory evaluation of food: Principles and practices. Springer Science & Business Media.
Figure 1.
Morphology and particle size of nanoliposomes loaded with fucoxanthin by scanning electron microscopy (SEM). Expansion to x70,000. Scale bar = 0.05 µm. PS = Particle Size.
Figure 1.
Morphology and particle size of nanoliposomes loaded with fucoxanthin by scanning electron microscopy (SEM). Expansion to x70,000. Scale bar = 0.05 µm. PS = Particle Size.
Figure 2.
In vitro release of free fucoxanthin solution (FXN) and fucoxantina-loaded nanoliposomes (FXN-LN) in PBS (pH 7.4) at 37°C. Data are presented as mean ± standard deviation (n = 3).
Figure 2.
In vitro release of free fucoxanthin solution (FXN) and fucoxantina-loaded nanoliposomes (FXN-LN) in PBS (pH 7.4) at 37°C. Data are presented as mean ± standard deviation (n = 3).
Figure 3.
Scanning Electron Microscopy (SEM) micrograph of freeze-dried yogurt enriched with fucoxanthin-loaded nanoliposomes (FXN-LN). (A) Magnification of x35,000. Scale bar = 0.5 µm. (B) Magnification of x90,000. Scale bar = 0.2 µm. Nanoliposome found with a particle size ranging from 0.149-0.159 µm. (1) fucoxanthin-loaded nanoliposomes; (2) Yogurt food matrix particles.
Figure 3.
Scanning Electron Microscopy (SEM) micrograph of freeze-dried yogurt enriched with fucoxanthin-loaded nanoliposomes (FXN-LN). (A) Magnification of x35,000. Scale bar = 0.5 µm. (B) Magnification of x90,000. Scale bar = 0.2 µm. Nanoliposome found with a particle size ranging from 0.149-0.159 µm. (1) fucoxanthin-loaded nanoliposomes; (2) Yogurt food matrix particles.
Figure 4.
Effect of fucoxanthin-loaded nanoliposome addition and cold storage time on the electrical conductivity of yogurt formulations. All data were analyzed using two-way ANOVA with interactions. Means with different lowercase letters (a-c: pH effects) within the same treatment are significantly different (p < 0.05). Y-C= Control Yogurt without nanocapsules; Y-Ant-5= Yogurt enriched with 5% nanocapsules; Y-Ant-10= Yogurt enriched with 10% nanocapsules. Bars represent the standard deviation of at least three replicates (n > 3) per concentration.
Figure 4.
Effect of fucoxanthin-loaded nanoliposome addition and cold storage time on the electrical conductivity of yogurt formulations. All data were analyzed using two-way ANOVA with interactions. Means with different lowercase letters (a-c: pH effects) within the same treatment are significantly different (p < 0.05). Y-C= Control Yogurt without nanocapsules; Y-Ant-5= Yogurt enriched with 5% nanocapsules; Y-Ant-10= Yogurt enriched with 10% nanocapsules. Bars represent the standard deviation of at least three replicates (n > 3) per concentration.
Figure 5.
Effect of Fucoxanthin-loaded nanoliposome addition and cold storage time on the pH of yogurt formulations. All data were analyzed using two-way ANOVA with interactions. Means with different lowercase letters (a-c: pH effects) within the same treatment are significantly different (p < 0.05). Y-C= Control Yogurt without nanocapsules; Y-FXN-5= Yogurt enriched with 5% nanocapsules; Y-FXN-10= Yogurt enriched with 10% nanocapsules. Bars represent the standard deviation of at least three replicates (n > 3) per concentration.
Figure 5.
Effect of Fucoxanthin-loaded nanoliposome addition and cold storage time on the pH of yogurt formulations. All data were analyzed using two-way ANOVA with interactions. Means with different lowercase letters (a-c: pH effects) within the same treatment are significantly different (p < 0.05). Y-C= Control Yogurt without nanocapsules; Y-FXN-5= Yogurt enriched with 5% nanocapsules; Y-FXN-10= Yogurt enriched with 10% nanocapsules. Bars represent the standard deviation of at least three replicates (n > 3) per concentration.
Figure 6.
Effect of nanoliposome addition and cold storage time on the titratable acidity of yogurt formulations. All data were analyzed using two-way ANOVA with interactions. Means with different lowercase letters (a-c: pH effects) within the same treatment are significantly different (p < 0.05). Y-C= Control Yogurt without nanocapsules; Y-FXN-5= Yogurt enriched with 5% nanocapsules; Y-FXN-10= Yogurt enriched with 10% nanocapsules. Bars represent the standard deviation of at least three replicates (n > 3) per concentration.
Figure 6.
Effect of nanoliposome addition and cold storage time on the titratable acidity of yogurt formulations. All data were analyzed using two-way ANOVA with interactions. Means with different lowercase letters (a-c: pH effects) within the same treatment are significantly different (p < 0.05). Y-C= Control Yogurt without nanocapsules; Y-FXN-5= Yogurt enriched with 5% nanocapsules; Y-FXN-10= Yogurt enriched with 10% nanocapsules. Bars represent the standard deviation of at least three replicates (n > 3) per concentration.
Figure 7.
Effect of Fucoxanthin-loaded nanoliposome addition and cold storage time on the syneresis susceptibility of yogurt formulations. All data were analyzed using two-way ANOVA with interactions. Means with different lowercase letters (a-c: storage time effects) within the same treatment are significantly different (p < 0.05). Y-C= Control Yogurt without nanocapsules; Y-FXN-5= Yogurt enriched with 5% nanocapsules; Y-FXN-10= Yogurt enriched with 10% nanocapsules. Bars represent the standard deviation of at least three replicates (n > 3) per concentration.
Figure 7.
Effect of Fucoxanthin-loaded nanoliposome addition and cold storage time on the syneresis susceptibility of yogurt formulations. All data were analyzed using two-way ANOVA with interactions. Means with different lowercase letters (a-c: storage time effects) within the same treatment are significantly different (p < 0.05). Y-C= Control Yogurt without nanocapsules; Y-FXN-5= Yogurt enriched with 5% nanocapsules; Y-FXN-10= Yogurt enriched with 10% nanocapsules. Bars represent the standard deviation of at least three replicates (n > 3) per concentration.
Figure 8.
Effect of the addition of nanoliposomes and cold storage time on the water-holding capacity of yogurt formulations. All data were analyzed using two-way ANOVA with interactions. Means with different lowercase letters (a-c: storage time effects) within the same treatment are significantly different (p < 0.05). Y-C= Control Yogurt without nanocapsules; Y-FXN-5= Yogurt enriched with 5% nanocapsules; Y-FXN-10= Yogurt enriched with 10% nanocapsules. Bars represent the standard deviation of at least three replicates (n > 3) per concentration.
Figure 8.
Effect of the addition of nanoliposomes and cold storage time on the water-holding capacity of yogurt formulations. All data were analyzed using two-way ANOVA with interactions. Means with different lowercase letters (a-c: storage time effects) within the same treatment are significantly different (p < 0.05). Y-C= Control Yogurt without nanocapsules; Y-FXN-5= Yogurt enriched with 5% nanocapsules; Y-FXN-10= Yogurt enriched with 10% nanocapsules. Bars represent the standard deviation of at least three replicates (n > 3) per concentration.
Figure 9.
Effect of fucoxanthin-loaded nanoliposome addition and cold storage time on the viscosity (cP) of yogurt formulations. Means with different lowercase letters (a-c: storage time effects) within the same treatment are significantly different (p < 0.05). Y-C= Control Yogurt without nanocapsules; Y-Ant-5= Yogurt enriched with 5% nanocapsules; Y-Ant-10= Yogurt enriched with 10% nanocapsules. Bars represent the standard deviation of at least three replicates (n > 3) per concentration.
Figure 9.
Effect of fucoxanthin-loaded nanoliposome addition and cold storage time on the viscosity (cP) of yogurt formulations. Means with different lowercase letters (a-c: storage time effects) within the same treatment are significantly different (p < 0.05). Y-C= Control Yogurt without nanocapsules; Y-Ant-5= Yogurt enriched with 5% nanocapsules; Y-Ant-10= Yogurt enriched with 10% nanocapsules. Bars represent the standard deviation of at least three replicates (n > 3) per concentration.
Figure 10.
Effect of adding nanoliposome vehicles to yogurts under different cold storage times on their textural properties (firmness and consistency). (A) Y-C: Control Yogurt; (B) Y-FXN-5: Yogurt-enriched with 5% of fucoxanthin-loaded nanoliposome; (C) Y-FXN-10: Yogurt-enriched with 10% of fucoxanthin-loaded nanoliposome.
Figure 10.
Effect of adding nanoliposome vehicles to yogurts under different cold storage times on their textural properties (firmness and consistency). (A) Y-C: Control Yogurt; (B) Y-FXN-5: Yogurt-enriched with 5% of fucoxanthin-loaded nanoliposome; (C) Y-FXN-10: Yogurt-enriched with 10% of fucoxanthin-loaded nanoliposome.
Figure 11.
Effect of the incorporation of nanoliposomes under cold storage time on the rheological parameters: (A) Shear stress and (B) viscosity of different yogurt formulations. All results were analyzed in triplicate (n > 3).
Figure 11.
Effect of the incorporation of nanoliposomes under cold storage time on the rheological parameters: (A) Shear stress and (B) viscosity of different yogurt formulations. All results were analyzed in triplicate (n > 3).
Table 1.
Effect of cold storage on the antioxidant properties (DPPH, ABTS, FRAP) of yogurt formulations added with nanoliposomes loaded with fucoxanthin.
Table 1.
Effect of cold storage on the antioxidant properties (DPPH, ABTS, FRAP) of yogurt formulations added with nanoliposomes loaded with fucoxanthin.
| Samples |
Storage time (days) |
Free radical-scavenging (%) |
mmol TE/g |
| DPPH• |
ABTS+• |
FRAP |
| Y-C |
0 |
24.77 e±1.25 |
33.72 f±1.75 |
2.85 c±0.13 |
| 7 |
24.32 e±2.45 |
30.36 fg±2.35 |
2.42 d±0.11 |
| 14 |
21.68 f±1.74 |
28.41 g±2.95 |
2.23 d±0.10 |
| 21 |
19.23 f±2.84 |
25.98 g±1.24 |
2.25 d±0.09 |
| Y-FXN-5 |
0 |
34.99 c±0.95 |
43.38 d±1.64 |
3.11 ab±0.07 |
| 7 |
34.12 c±1.81 |
41.64 de±1.74 |
3.21 a±0.11 |
| 14 |
32.58 cd±2.76 |
39.32 e±1.63 |
3.03 b±0.03 |
| 21 |
30.23 d±1.63 |
39.64 e±2.87 |
2.98 b±0.08 |
| Y-FXN-10 |
0 |
52.96 a±1.24 |
97.97 a±2.93 |
3.16 a±0.05 |
| 7 |
50.23 b±0.35 |
92.54 b±2.26 |
3.14 a±0.03 |
| 14 |
51.25 ab±1.55 |
90.23 bc±1.15 |
3.15 a±0.06 |
| 21 |
50.34 b±1.23 |
88.42 c±0.74 |
3.04 b±0.03 |
Table 2.
Effect of the cold storage condition on erythroprotective potential of yogurt formulations added with nanoliposomes loaded with fucoxanthin.
Table 2.
Effect of the cold storage condition on erythroprotective potential of yogurt formulations added with nanoliposomes loaded with fucoxanthin.
| Samples |
HI (%) |
PHI (%) |
Heat-IH |
Hypo-IH |
| Y-C |
17.29 c ±2.67 |
61.07 b ±2.53 |
25.50 b ±2.35 |
81.46 b ±1.23 |
| Y-FXN-5 |
63.83 b ±2.34 |
54.93 c ±1.98 |
25.17 b ±2.87 |
80.87 b ±2.85 |
| Y-FXN-10 |
82.41 a ±1.54 |
82.40 a ±2.63 |
46.80 a ±3.63 |
93.62 a ±2.16 |
Table 3.
Colorimetric analysis of yogurt enriched with 5 and 10% of Fucoxanthin-loaded nanoliposomes during 21 days of cold storage.
Table 3.
Colorimetric analysis of yogurt enriched with 5 and 10% of Fucoxanthin-loaded nanoliposomes during 21 days of cold storage.
| Characteristics |
Experiments |
Day 0 |
Day 7 |
Day 14 |
Day 21 |
| L* |
Y-C |
93.12ab±2.25 |
94.23a ±1.17 |
91.36b ±1.23 |
87.43c ±1.25 |
| Y-FXN-5 |
93.29a ±1.36 |
93.67a ±1.37 |
89.25b ±1.73 |
82.65c ±1.75 |
| Y-FXN-10 |
92.12a ±0.73 |
91.13a ±1.26 |
87.26b ±1.26 |
81.52c ±1.24 |
| a* |
Y-C |
-2.25a ±0.13 |
-2.33ab ±0.04 |
-2.42b ±0.04 |
-2.63c ±0.03 |
| Y-FXN-5 |
1.22a ±0.02 |
0.86b ±0.04 |
0.52c ±0.04 |
0.67b ±0.02 |
| Y-FXN-10 |
2.20a ±0.02 |
0.83b ±0.03 |
0.47d ±0.02 |
0.52c ±0.03 |
| b* |
Y-C |
6.52a ±1.36 |
8.12b ±0.13 |
8.56c ±0.12 |
8.72d ±0.03 |
| Y-FXN-5 |
5.63a ±1.14 |
6.44ab ±0.43 |
6.89bc ±0.04 |
7.11c ±0.12 |
| Y-FXN-10 |
4.75a ±0.64 |
4.37ab ±0.98 |
4.92bc ±0.48 |
5.35c ±0.16 |
Table 4.
Transmittance-analyzed Colorimetry (%) of yogurt enriched with 5 and 10% of Fucoxanthin-loaded nanoliposomes during 21 days of cold storage.
Table 4.
Transmittance-analyzed Colorimetry (%) of yogurt enriched with 5 and 10% of Fucoxanthin-loaded nanoliposomes during 21 days of cold storage.
| Samples |
Abs (nm) |
Day 0 |
Day 7 |
Day 14 |
Day 21 |
| Y-C |
Red (660) |
40.70a±2.10 |
45.05b±1.75 |
49.53c ±1.84 |
53.67c ±2.53 |
| Green (565) |
17.20a ±1.47 |
19.65b ±0.94 |
22.24bc ±1.73 |
24.56c ±1.75 |
| Blue (468) |
27.57a ±1.83 |
30.74ab ±1.23 |
34.14bc ±1.26 |
37.32c ±1.24 |
| |
Orange (610) |
25.67a ±1.24 |
29.35b ±1.52 |
33.23bc ±1.17 |
36.34c ±1.24 |
| Y-FXN-5 |
Red (660) |
75.23a ±1.23 |
71.57b ±2.24 |
49.46c ±1.36 |
42.98d ±1.52 |
| Green (565) |
61.63a ±2.52 |
45.67b ±1.62 |
21.15c ±1.73 |
19.86d ±2.63 |
| Blue (468) |
57.27a ±2.02 |
52.27b ±2.42 |
30.74c ±1.83 |
28.22c ±2.36 |
| |
Orange (610) |
62.23a ±1.02 |
56.87b ±1.63 |
32.37c ±2.84 |
28.22c ±2.05 |
| Y-FXN-10 |
Red (660) |
61.45c ±1.46 |
68.72b ±1.27 |
92.90a ±2.84 |
93.71a ±3.71 |
| Green (565) |
40.13c ±1.47 |
42.64c ±2.76 |
69.19b ±1.47 |
72.45a ±1.34 |
| Blue (468) |
42.75c ±2.84 |
48.87b ±2.68 |
84.83a ±2.74 |
84.91a ±1.74 |
| |
Orange (610) |
75.16b ±2.65 |
52.40c ±2.25 |
81.91a ±1.34 |
84.26a ±2.42 |
| The results are expressed as mean ± standard deviation (n = 3). Means with different lowercase letters (a-c: time effects) within the same treatment are significantly different (p < 0.05). Y-C= Control Yogurt without nanocapsules; Y-FXN-5= Yogurt enriched with 5% nanocapsules; Y-FXN-10= Yogurt enriched with 10% nanocapsules. |
Table 5.
Effect of nanoencapsulation on the sensory characteristics of fucoxanthin-enriched yogurt.
Table 5.
Effect of nanoencapsulation on the sensory characteristics of fucoxanthin-enriched yogurt.
| Sensory quality attributes |
Treatments |
| Y-C |
Y-FXN-5 |
Y-FXN-10 |
| Color |
8.56a ±0.16 |
7.491a ±0.26 |
8.73a ±0.47 |
| Flavor |
7.83a ±0.23 |
7.64.73b ±0.42 |
8.09c ±0.83 |
| Aftertaste |
7.24b ±0.26 |
8.00a ±0.25 |
8.18a ±1.08 |
| Scent |
8.61a ±0.45 |
7.73ab ±0.45 |
8.64b ±0.67 |
| Consistency |
8.29a ±0.35 |
7.55ab ±0.34 |
8.19b ±0.94 |
| Texture |
8.22a ±0.23 |
7.91a ±0.65 |
8.36a ±0.92 |
| Appearance |
8.29a ±0.32 |
7.64a ±0.32 |
8.82b ±0.40 |
| General acceptance |
7.88a ±0.15 |
8.00a ±0.21 |
8.36a ±0.81 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).