Submitted:
17 July 2024
Posted:
19 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
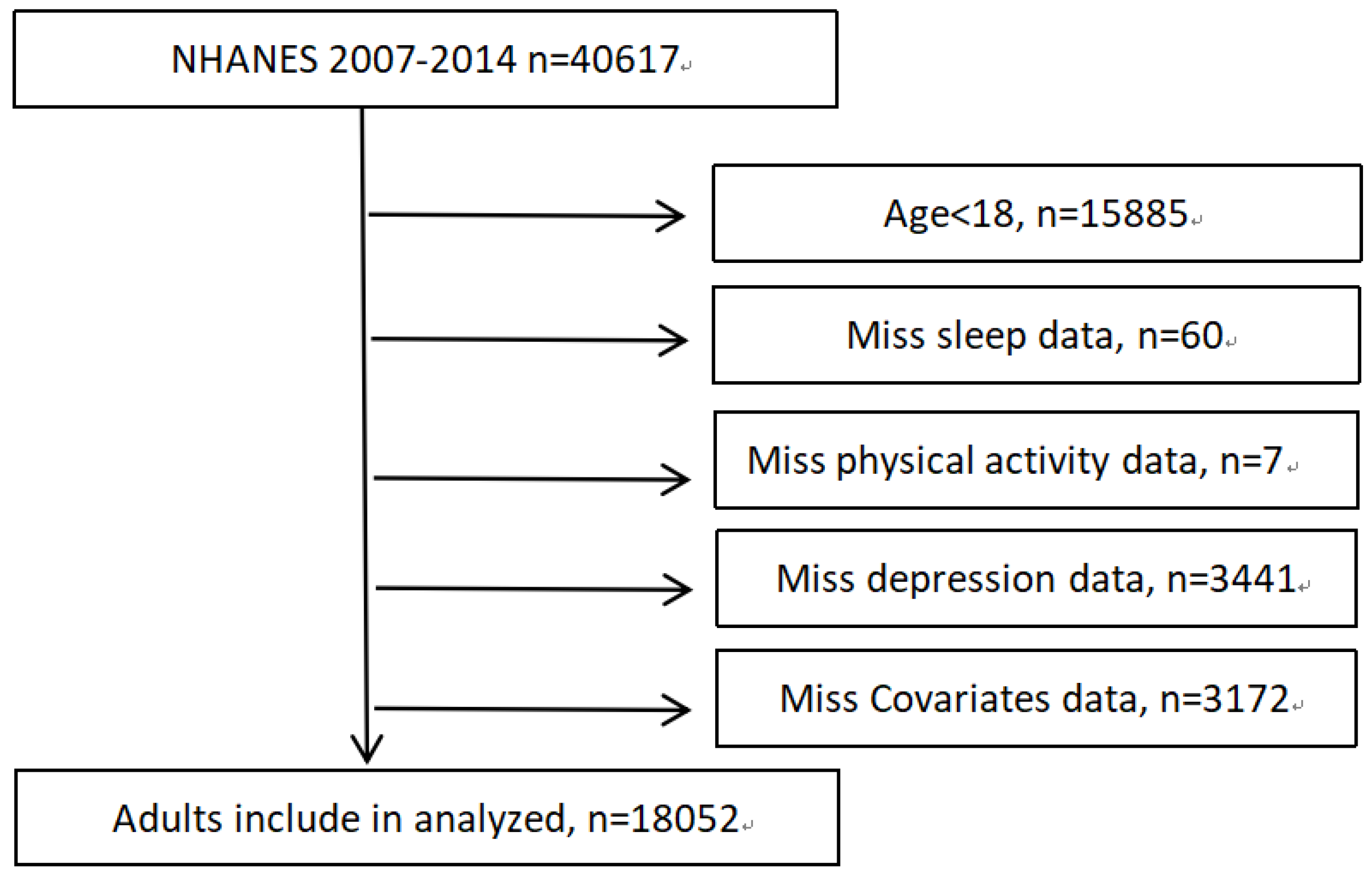
2.1. Study Population
2.2. Physical Activity and Sleep Duration
2.3. Depressive Symptoms
2.4. Assessment of covariates
2.5. Patient and public involvement
2.6. Statistical analysis
3. Results
3.1. Independent Associations of Physical Activity (PA) and Sleep Duration with Symptoms of Depression
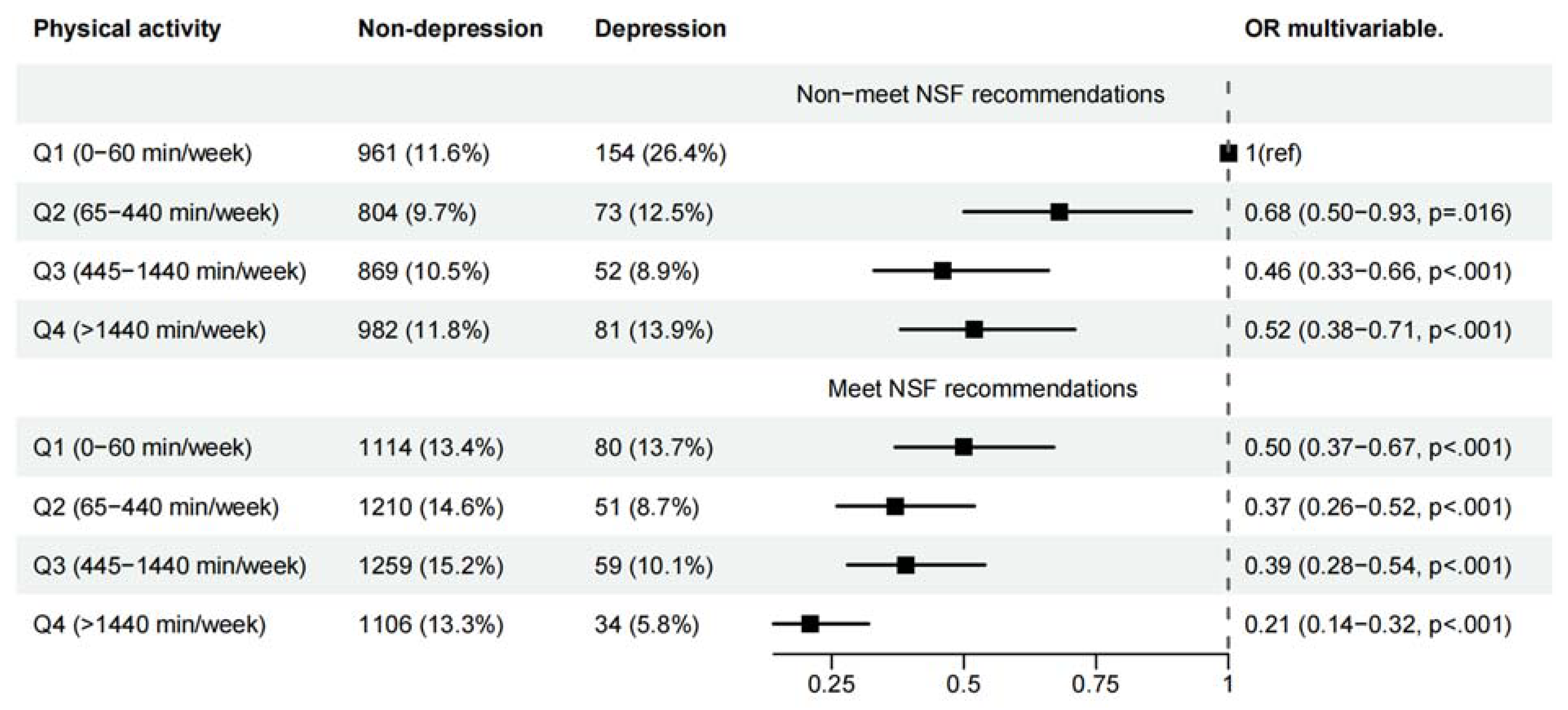
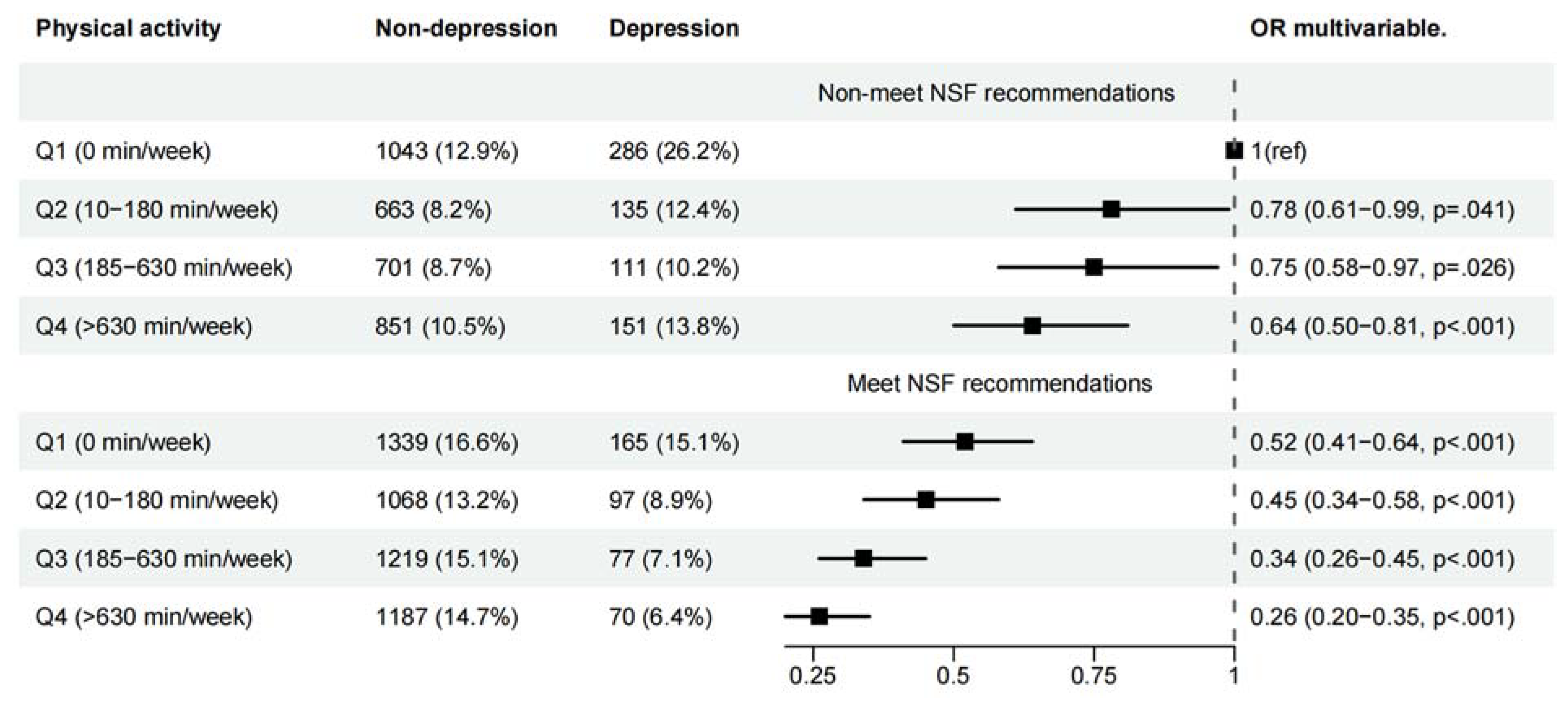
3.2. Joint Associations of PA and Sleep Duration with Symptoms of Depression
4. Discussion
5. Conclusion
References
- Patel V, Chisholm D, Parikh R, Charlson FJ, Degenhardt L, Dua T, et al. Addressing the burden of mental, neurological, and substance use disorders: key messages from Disease Control Priorities, 3rd edition. LANCET. 2016; 387: 1672-85. [CrossRef]
- Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022; 9: 137-50.
- Malhi GS, Mann JJ. Depression. LANCET. 2018; 392: 2299-312.
- Lund C, De Silva M, Plagerson S, Cooper S, Chisholm D, Das J, et al. Poverty and mental disorders: breaking the cycle in low-income and middle-income countries. LANCET. 2011; 378: 1502-14. [CrossRef]
- Baglioni C, Nanovska S, Regen W, Spiegelhalder K, Feige B, Nissen C, et al. Sleep and mental disorders: A meta-analysis of polysomnographic research. PSYCHOLOGICAL BULLETIN. 2016; 142: 969-90. [CrossRef]
- Choi KW, Chen CY, Stein MB, Klimentidis YC, Wang MJ, Koenen KC, et al. Assessment of Bidirectional Relationships Between Physical Activity and Depression Among Adults: A 2-Sample Mendelian Randomization Study. JAMA Psychiatry. 2019; 76: 399-408.
- Kandola A, Lewis G, Osborn D, Stubbs B, Hayes JF. Depressive symptoms and objectively measured physical activity and sedentary behaviour throughout adolescence: a prospective cohort study. Lancet Psychiatry. 2020; 7: 262-71. [CrossRef]
- Firth J, Solmi M, Wootton RE, Vancampfort D, Schuch FB, Hoare E, et al. A meta-review of "lifestyle psychiatry": the role of exercise, smoking, diet and sleep in the prevention and treatment of mental disorders. World Psychiatry. 2020; 19: 360-80. [CrossRef]
- Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1.9 million participants. Lancet Global Health. 2018; 6: e1077-86. [CrossRef]
- Rock CL, Thomson CA, Sullivan KR, Howe CL, Kushi LH, Caan BJ, et al. American Cancer Society nutrition and physical activity guideline for cancer survivors. CA-A CANCER JOURNAL FOR CLINICIANS. 2022; 72: 230-62. [CrossRef]
- Schmitz KH, Campbell AM, Stuiver MM, Pinto BM, Schwartz AL, Morris GS, et al. Exercise is medicine in oncology: Engaging clinicians to help patients move through cancer. CA-A CANCER JOURNAL FOR CLINICIANS. 2019; 69: 468-84. [CrossRef]
- He F, Li Y, Hu Z, Zhang H. Association of domain-specific physical activity with depressive symptoms: A population-based study. EUROPEAN PSYCHIATRY. 2022; 66: e5. [CrossRef]
- Espana-Romero V, Artero EG, Lee DC, Sui X, Baruth M, Ruiz JR, et al. A prospective study of ideal cardiovascular health and depressive symptoms. PSYCHOSOMATICS. 2013; 54: 525-35. [CrossRef]
- Hughes KC, Gao X, Molsberry S, Valeri L, Schwarzschild MA, Ascherio A. Physical activity and prodromal features of Parkinson disease. NEUROLOGY. 2019; 93: e2157-69. [CrossRef]
- Chang SC, Pan A, Kawachi I, Okereke OI. Risk factors for late-life depression: A prospective cohort study among older women. PREVENTIVE MEDICINE. 2016; 91: 144-51. [CrossRef]
- Schuch FB, Vancampfort D, Firth J, Rosenbaum S, Ward PB, Silva ES, et al. Physical Activity and Incident Depression: A Meta-Analysis of Prospective Cohort Studies. AMERICAN JOURNAL OF PSYCHIATRY. 2018; 175: 631-48. [CrossRef]
- Bloomberg M, Brocklebank L, Hamer M, Steptoe A. Joint associations of physical activity and sleep duration with cognitive ageing: longitudinal analysis of an English cohort study. The Lancet Healthy Longevity. 2023; 4: e345-53. [CrossRef]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA-JOURNAL OF THE AMERICAN MEDICAL ASSOCIATION. 2003; 289: 3095-105.
- Divney AA, Murillo R, Rodriguez F, Mirzayi CA, Tsui EK, Echeverria SE. Diabetes Prevalence by Leisure-, Transportation-, and Occupation-Based Physical Activity Among Racially/Ethnically Diverse U.S. Adults. DIABETES CARE. 2019; 42: 1241-7. [CrossRef]
- Celis-Morales CA, Lyall DM, Anderson J, Iliodromiti S, Fan Y, Ntuk UE, et al. The association between physical activity and risk of mortality is modulated by grip strength and cardiorespiratory fitness: evidence from 498 135 UK-Biobank participants. EUROPEAN HEART JOURNAL. 2017; 38: 116-22. [CrossRef]
- Kocevska D, Lysen TS, Dotinga A, Koopman-Verhoeff ME, Luijk M, Antypa N, et al. Sleep characteristics across the lifespan in 1.1 million people from the Netherlands, United Kingdom and United States: a systematic review and meta-analysis. Nature Human Behaviour. 2021; 5: 113-22.
- Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, et al. National Sleep Foundation's sleep time duration recommendations: methodology and results summary. Sleep Health. 2015; 1: 40-3.
- Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, et al. National Sleep Foundation's updated sleep duration recommendations: final report. Sleep Health. 2015; 1: 233-43. [CrossRef]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. JOURNAL OF GENERAL INTERNAL MEDICINE. 2001; 16: 606-13.
- Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA-JOURNAL OF THE AMERICAN MEDICAL ASSOCIATION. 1999; 282: 1737-44.
- Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. INTERNATIONAL JOURNAL OF METHODS IN PSYCHIATRIC RESEARCH. 2012; 21: 169-84. [CrossRef]
- Park LT, Zarate CJ. Depression in the Primary Care Setting. NEW ENGLAND JOURNAL OF MEDICINE. 2019; 380: 559-68.
- Harvey SB, Overland S, Hatch SL, Wessely S, Mykletun A, Hotopf M. Exercise and the Prevention of Depression: Results of the HUNT Cohort Study. AMERICAN JOURNAL OF PSYCHIATRY. 2018; 175: 28-36. [CrossRef]
- Vancampfort D, Firth J, Schuch FB, Rosenbaum S, Mugisha J, Hallgren M, et al. Sedentary behavior and physical activity levels in people with schizophrenia, bipolar disorder and major depressive disorder: a global systematic review and meta-analysis. World Psychiatry. 2017; 16: 308-15.
- Goldstein AN, Walker MP. The role of sleep in emotional brain function. Annual Review of Clinical Psychology. 2014; 10: 679-708. [CrossRef]
- Plante DT. The Evolving Nexus of Sleep and Depression. AMERICAN JOURNAL OF PSYCHIATRY. 2021; 178: 896-902. [CrossRef]
- Ben SE, Rossi A, Harvey AG, Walker MP. Overanxious and underslept. Nature Human Behaviour. 2020; 4: 100-10.
- Zhao Y, Yang L, Sahakian BJ, Langley C, Zhang W, Kuo K, et al. The brain structure, immunometabolic and genetic mechanisms underlying the association between lifestyle and depression. Nature Mental Health. 2023; 1: 736-50.
- O'Neil A, Scovelle AJ, Milner AJ, Kavanagh A. Gender/Sex as a Social Determinant of Cardiovascular Risk. CIRCULATION. 2018; 137: 854-64. [CrossRef]
- Xia S, Du X, Guo L, Du J, Arnott C, Lam C, et al. Sex Differences in Primary and Secondary Prevention of Cardiovascular Disease in China. CIRCULATION. 2020; 141: 530-9. [CrossRef]
- Whipple MO, Pinto AJ, Abushamat LA, Bergouignan A, Chapman K, Huebschmann AG, et al. Sex Differences in Physical Activity Among Individuals With Type 2 Diabetes Across the Life Span: A Systematic Review and Meta-analysis. DIABETES CARE. 2022; 45: 2163-77. [CrossRef]
- Kuehner C. Why is depression more common among women than among men? Lancet Psychiatry. 2017; 4: 146-58.
- Salk RH, Hyde JS, Abramson LY. Gender differences in depression in representative national samples: Meta-analyses of diagnoses and symptoms. PSYCHOLOGICAL BULLETIN. 2017; 143: 783-822. [CrossRef]
| All (%) | Male (N=8889(49.24%)) | Female(N=9163(50.76)) | |||||
|---|---|---|---|---|---|---|---|
| Non-depression | Depression | p | Non-depression | Depression | p | ||
| (N=8305) | (N=584(6.57)) | (N=8071) | (N=1092(11.92)) | ||||
| Age, year | |||||||
| 20-44 | 7721 (42.77) | 3587 (43.2) | 233 (39.9) | <.001 | 3442 (42.6) | 459 (42.0) | <.001 |
| 45-65 | 6184 (34.26) | 2768 (33.3) | 254 (43.5) | 2696 (33.4) | 466 (42.7) | ||
| >=65 | 4147 (22.97) | 1950 (23.5) | 97 (16.6) | 1933 (23.9) | 167 (15.3) | ||
| Race/ethnicity | |||||||
| Mexican American | 2532 (14.03) | 1191 (14.3) | 76 (13) | 0.042 | 1102 (13.7) | 163 (14.9) | <.001 |
| Other Hispanic | 1726 (9.56) | 720 (8.7) | 65 (11.1) | 788 (9.8) | 153 (14.0) | ||
| Non-Hispanic White | 8357 (46.29) | 3875 (46.7) | 283 (48.5) | 3728 (46.2) | 471 (43.1) | ||
| Non-Hispanic Black | 3769 (20.88) | 1717 (20.7) | 121 (20.7) | 1687 (20.9) | 244 (22.3) | ||
| Other race | 1668 (9.24) | 802 (9.7) | 39 (6.7) | 766 (9.5) | 61 (5.6) | ||
| BMI | |||||||
| <25 | 5289 (29.30) | 2337 (28.1) | 162 (27.7) | <.001 | 2550 (31.6) | 240 (22.0) | <.001 |
| 25-30 | 5971 (33.08) | 3179 (38.3) | 182 (31.2) | 2345 (29.1) | 265 (24.3) | ||
| >=30 | 6792 (37.62) | 2789 (33.6) | 240 (41.1) | 3176 (39.4) | 587 (53.8) | ||
| Education | |||||||
| Less than college | 8466 (46.90) | 3978 (47.9) | 378 (64.7) | <.001 | 3475 (43.1) | 635 (58.2) | <.001 |
| College or more | 9586 (53.10) | 4327 (52.1) | 206 (35.3) | 4596 (56.9) | 457 (41.8) | ||
| PIR | |||||||
| <1 | 3913 (21.68) | 1557 (18.7) | 215 (36.8) | <.001 | 1690 (20.9) | 451 (41.3) | <.001 |
| 1-1.99 | 4675 (25.90) | 2387 (28.7) | 55 (9.4) | 2121 (26.3) | 112 (10.3) | ||
| 2-3.99 | 4789 (26.53) | 2135 (25.7) | 200 (34.2) | 2105 (26.1) | 349 (32.0) | ||
| >=4 | 4675 (25.90) | 2226 (26.8) | 114 (19.5) | 2155 (26.7) | 180 (16.5) | ||
| Marital status | |||||||
| Married | 10674 (59.13) | 5468 (65.8) | 279 (47.8) | <.001 | 4464 (55.3) | 463 (42.4) | <.001 |
| Single | 7378 (40.87) | 2837 (34.2) | 305 (52.2) | 3607 (44.7) | 629 (57.6) | ||
| Smoking status | |||||||
| No | 9826 (54.43) | 3901 (47) | 185 (31.7) | <.001 | 5253 (65.1) | 487 (44.6) | <.001 |
| Yes | 8226 (45.57) | 4404 (53) | 399 (68.3) | 2818 (34.9) | 605 (55.4) | ||
| Years for NHANES | |||||||
| 2007-2008 | 4576 (25.35) | 2101 (25.3) | 154 (26.4) | 0.903 | 2042 (25.3) | 279 (25.5) | 0.707 |
| 2009-2010 | 4704 (26.06) | 2169 (26.1) | 152 (26) | 2091 (25.9) | 292 (26.7) | ||
| 2011-2012 | 4179 (23.15) | 1959 (23.6) | 139 (23.8) | 1848 (22.9) | 233 (21.3) | ||
| 2013-2014 | 4593 (25.44) | 2076 (25) | 139 (23.8) | 2090 (25.9) | 288 (26.4) | ||
| Hypertension | |||||||
| No | 11557 (64.02) | 5449 (65.6) | 323 (55.3) | <.001 | 5223 (64.7) | 562 (51.5) | <.001 |
| Yes | 6495 (35.98) | 2856 (34.4) | 261 (44.7) | 2848 (35.3) | 530 (48.5) | ||
| Diabetes | |||||||
| No | 15673 (86.82) | 7251 (87.3) | 471 (80.7) | <.001 | 7099 (88) | 852 (78) | <.001 |
| Yes | 2379 (13.18) | 1054 (12.7) | 113 (19.3) | 972 (12) | 240 (22) | ||
| Arthritis | |||||||
| No | 13157 (72.88) | 6525 (78.6) | 372 (63.7) | <.001 | 5704 (70.7) | 556 (50.9) | <.001 |
| Yes | 4895 (27.12) | 1780 (21.4) | 212 (36.3) | 2367 (29.3) | 536 (49.1) | ||
| Heart disease | |||||||
| No | 16639 (92.17) | 7564 (91.1) | 477 (81.7) | <.001 | 7640 (94.7) | 958 (87.7) | <.001 |
| Yes | 1413 (7.83) | 741 (8.9) | 107 (18.3) | 431 (5.3) | 134 (12.3) | ||
| Stroke | |||||||
| No | 17429 (96.55) | 8046 (96.9) | 544 (93.2) | <.001 | 7823 (96.9) | 1016 (93) | <.001 |
| Yes | 623 (3.45) | 259 (3.1) | 40 (6.8) | 248 (3.1) | 76 (7) | ||
| Pulmonary disease | |||||||
| No | 14736 (81.63) | 7078 (85.2) | 402 (68.8) | <.001 | 6530 (80.9) | 726 (66.5) | <.001 |
| Yes | 3316 (18.37) | 1227 (14.8) | 182 (31.2) | 1541 (19.1) | 366 (33.5) | ||
| n | event (%) | OR For Depression (95%CI) | |||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||
| Male | |||||
| Physical activity a | |||||
| Q1 (0-60 min/week) | 2,309 | 234(10.13) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 (65-440 min/week) | 2,138 | 124(5.80) | 0.61 (0.49-0.78) ** | 0.63 (0.50-0.80) ** | 0.70 (0.55-0.90) * |
| Q3 (445-1440 min/week) | 2,239 | 111(4.96) | 0.50 (0.39-0.64) ** | 0.52 (0.40-0.66) ** | 0.59 (0.46-0.77) ** |
| Q4 (>1440 min/week) | 2,203 | 115(5.22) | 0.40 (0.31-0.51) ** | 0.42 (0.32-0.53) ** | 0.49 (0.38-0.63) ** |
| Sleep time duration recommendations b | |||||
| Non-meet NSF | 3,976 | 360(9.05) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Meet NSF | 4,913 | 224(4.56) | 0.50 (0.42-0.59) ** | 0.51 (0.42-0.61) ** | 0.54 (0.45-0.65) ** |
| Female | |||||
| Physical activity a | |||||
| Q1 (0 min/week) | 2,833 | 451(15.92) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 (10-180 min/week) | 1,963 | 232(11.82) | 0.76 (0.64-0.91) * | 0.77 (0.64-0.92) * | 0.82 (0.68-0.98) * |
| Q3 (185-630 min/week) | 2,108 | 188(8.96) | 0.60 (0.50-0.73) ** | 0.63 (0.52-0.77) ** | 0.71 (0.58-0.86) ** |
| Q4 (>630 min/week) | 2,259 | 221(9.78) | 0.53 (0.44-0.64) ** | 0.54 (0.45-0.65) ** | 0.59 (0.48-0.71) ** |
| Sleep time duration recommendations b | |||||
| Non-meet NSF | 3,914 | 683(17.33) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Meet NSF | 5,222 | 409(7.83) | 0.44 (0.39-0.51) ** | 0.46 (0.41-0.53) ** | 0.49 (0.43-0.57) ** |
|
Note: *p<0.05, **p<0.001 Model 1 was adjusted for age, race, education level, marital status and poverty ratio. Model 2 was adjusted for smoking status, BMI and years of NHANES in addition to model 1. Model 3 was adjusted for Model 2 plus stroke, diabetes, arthritis pulmonary disease, hypertension and heart disease. aAnalyses were adjusted for sleep time duration in addition to all models. bAnalyses were adjusted for physical activity in addition to all models Abbreviations: CI, confidence interval; OR, odds ratio. | |||||
| n | Event (%) | Quintiles of physical activity | Hazard ratio per one quartile a change in physical activity | P-trend | P-int | ||||
|---|---|---|---|---|---|---|---|---|---|
| Q1((lowest) | Q2 | Q3 | Q4((highest) | ||||||
| Male | |||||||||
| Sleep time duration recommendations | |||||||||
| Non-meet NSF | 3,976 | 360(9.05) | 1.00 (reference) | 0.67 (0.49-0.92) * | 0.44 (0.31-0.63) ** | 0.49 (0.36-0.68) ** | 0.77 (0.69-0.86) ** | <.001 | 0.046 |
| Meet NSF | 4,913 | 224(4.56) | 1.00 (reference) | 0.75 (0.51-1.10) | 0.82 (0.56-1.19) | 0.45 (0.29-0.70) ** | 0.81 (0.71-0.92) * | 0.001 | |
| Female | |||||||||
| Sleep time duration recommendations | |||||||||
| Non-meet NSF | 3,914 | 683(17.33) | 1.00 (reference) | 0.77 (0.60-0.99) * | 0.74 (0.57-0.96) * | 0.63 (0.50-0.81) ** | 0.86 (0.80-0.93) ** | <.001 | 0.380 |
| Meet NSF | 5,222 | 409(7.83) | 1.00 (reference) | 0.86 (0.65-1.14) | 0.68 (0.50-0.91) * | 0.52 (0.38-0.70) ** | 0.80 (0.73-0.89) ** | <.001 | |
|
Note: *p<0.05, **p<0.001 Analyses were adjusted for age, race, education level, marital status, poverty ratio, smoking status, BMI, years of NHANES, stroke, diabetes, arthritis pulmonary disease, hypertension and heart disease. P-trend has calculated separately in different sleep duration groups. P-int (p for interaction) has calculated by the likelihood ratio test. | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





