1. Introduction
Breast cancer is a heterogeneous disease characterized by different molecular subtypes, each associated with distinct clinical outcomes and therapeutic responses. The tumor microenvironment (TME) plays a pivotal role in shaping the progression and response to therapy in breast cancer [
1]. It is a dynamic and complex system composed of cancer cells, stromal cells, extracellular matrix, and infiltrating immune cells. Among these components, tumor-infiltrating lymphocytes (TILs) and cytokines, particularly interleukins (ILs), are critical mediators of immune responses and have significant prognostic and therapeutic implications in particular in triple-negative breast cancer [
2,
3].
Interleukins are a group of cytokines that facilitate communication between immune cells and can exhibit both pro-inflammatory and anti-inflammatory properties [
4]. Their dual role in cancer biology involves modulating tumor growth, metastasis, and the immune landscape within the TME. IFN-γ, for instance, is secreted by activated lymphocytes such as cytotoxic CD8+ T cells and type I CD4+ T helper cells (Th1) [
5,
6]. It can strengthen the Th1-mediated antitumor immune response through a positive feedback loop, activating macrophages and enhancing antigen presentation by dendritic cells [
7]. High levels of IFN-γ are associated with improved survival in breast cancer patients due to its role in orchestrating effective antitumor immunity. However, IFN-γ also has protumorigenic roles by sending anti-apoptotic and proliferative signals, leading to immune escape mechanisms of tumor cells [
8].
Previous research has demonstrated that higher IFN-γ mRNA expression significantly impacts metastasis-free survival (MFS) in the basal-like subtype of breast cancer, with higher IFN-γ mRNA expression being associated with longer MFS. Furthermore, our studies have shown that elevated expression of an IFN-γ-related gene signature correlates with favorable outcomes across the whole cohort of 461 patients with early breast cancer [
5]. This suggests a broader protective role for IFN-γ and its associated pathways in enhancing antitumor immunity and improving clinical prognosis.
One of the key cytokines that contribute to this protective role is IL-12, which promotes Th1 responses and enhances the cytotoxic activity of Natural Killer (NK)- cells and CD8+ T cells, stimulating the secretion of cytokines including IFN-γ [
4,
9].
It has been shown to inhibit tumor growth in a breast cancer mouse model by stimulating antitumor immunity and apoptosis in tumor cells [
10]. Similarly, IL-21 enhances the proliferation and function of NK cells and CD8+ T cells, boosting antitumor immune responses and reducing tumor burden in preclinical breast cancer studies [
11].
Conversely, certain interleukins predominantly exhibit pro-inflammatory properties. IL-4, IL-5, and IL-13 are associated with the Th2 immune response, which can create an immunosuppressive environment that promotes tumor progression. IL-4 and IL-13, in particular, support breast cancer cell survival, proliferation, and metastasis. In a preclinical breast cancer mouse model, IL-4 has been shown to activate monocytes and tumor-associated macrophages (TAM), thereby facilitating breast cancer metastasis to the lung [
12].
IL-10 is known for its ability to suppress effective anti-tumor immune responses, thereby enhancing tumor immune evasion and progression [
13]. In addition, IL-17 plays a critical role in chronic inflammation and has been reported to promote angiogenesis and tumor growth in breast cancer [
14,
15]. CXCL1 (C-X-C motif ligand 1), a chemokine that recruits neutrophils to the tumor site, is associated with tumor progression and metastasis [
1,
16]. High CXCL1 expression correlates with advanced disease and poor prognosis in breast cancer.
The present study aims to elucidate the prognostic impact of pro- and anti-inflammatory interleukin mRNA-expression signatures in early breast cancer. Additionally, it investigates correlations between these interleukin signatures and immune cell markers such as CD8, IgKC and CD20 and the immune checkpoint Programmed Cell Death Protein 1 (PD-1) to provide insights into their role in modulating the TME and influencing clinical outcomes in breast cancer.
2. Results
The prognostic significance of both anti-inflammatory and pro-inflammatory interleukin signatures for metastasis-free survival was investigated in a cohort of 461 patients with early breast cancer and long term follow up. The analysis included Kaplan-Meier curves, univariate, and multivariate Cox regression analyses to assess these signatures in the whole cohort and across different molecular subtypes of breast cancer.
2.1. Anti-Inflammatory Interleukin Signature
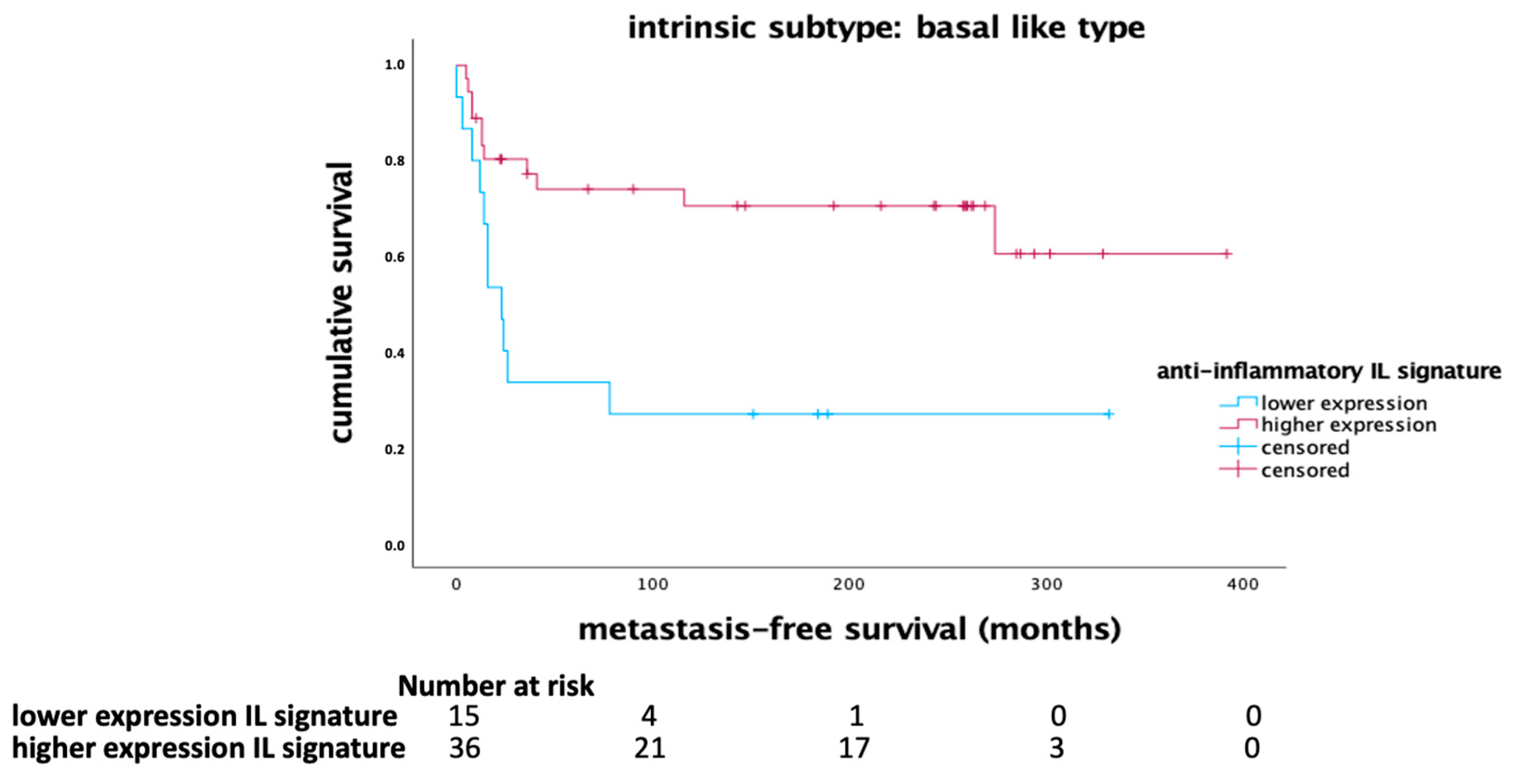
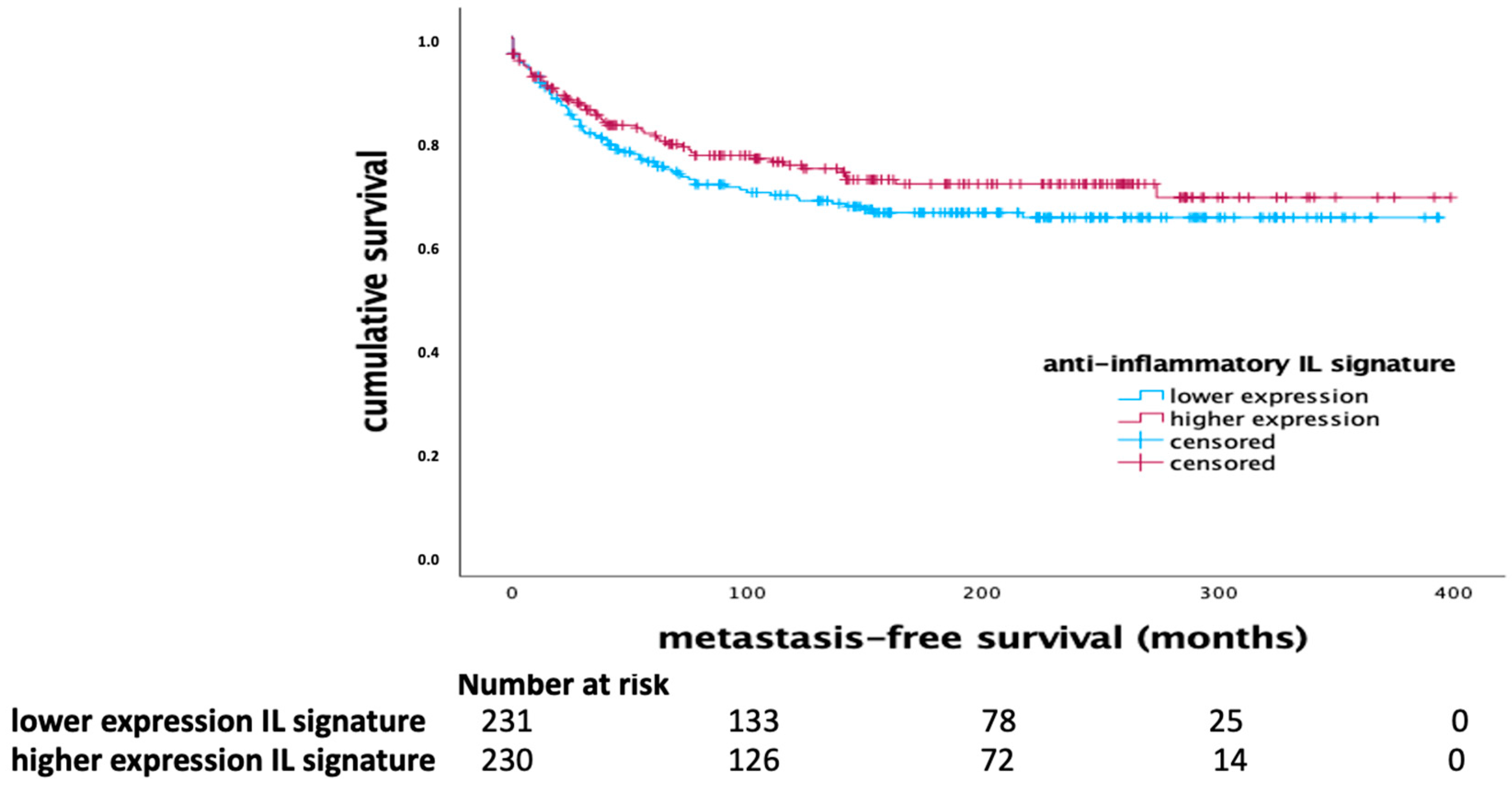
The anti-inflammatory interleukin signature, comprising IL-12, IL-21, and IFN-γ, demonstrated a significant impact on MFS in the basal-like breast cancer subtype. Kaplan-Meier survival analysis indicated that higher expression of this anti-inflammatory signature correlated with longer MFS in patients with basal-like tumors (p = 0.004, Log Rank) as shown in
Figure 1. In the whole cohort, there was a tendency towards a favorable outcome being associated with higher mRNA expression of the anti-inflammatory signature (
Figure 2).
The prognostic significance of the anti-inflammatory signature was further confirmed in a multivariate Cox regression analysis, which was adjusted for clinical-pathological variables such as age, tumor size, lymph node status, tumor grade, and the proliferation marker Ki-67. The results showed that the anti-inflammatory interleukin signature retained its independent prognostic significance (HR 0.463, 95% CI 0.290-0.741; p = 0.001), as summarized in
Table 2. Using multivariate Cox regression analysis, grading and the proliferation marker Ki67 were identified as further independent prognostic factors. These findings suggest that higher expression levels of IL-12, IL-21, and IFN-γ are associated with better outcomes especially in patients with basal-like breast cancer.
2.2. Pro-Inflammatory Interleukin Signature
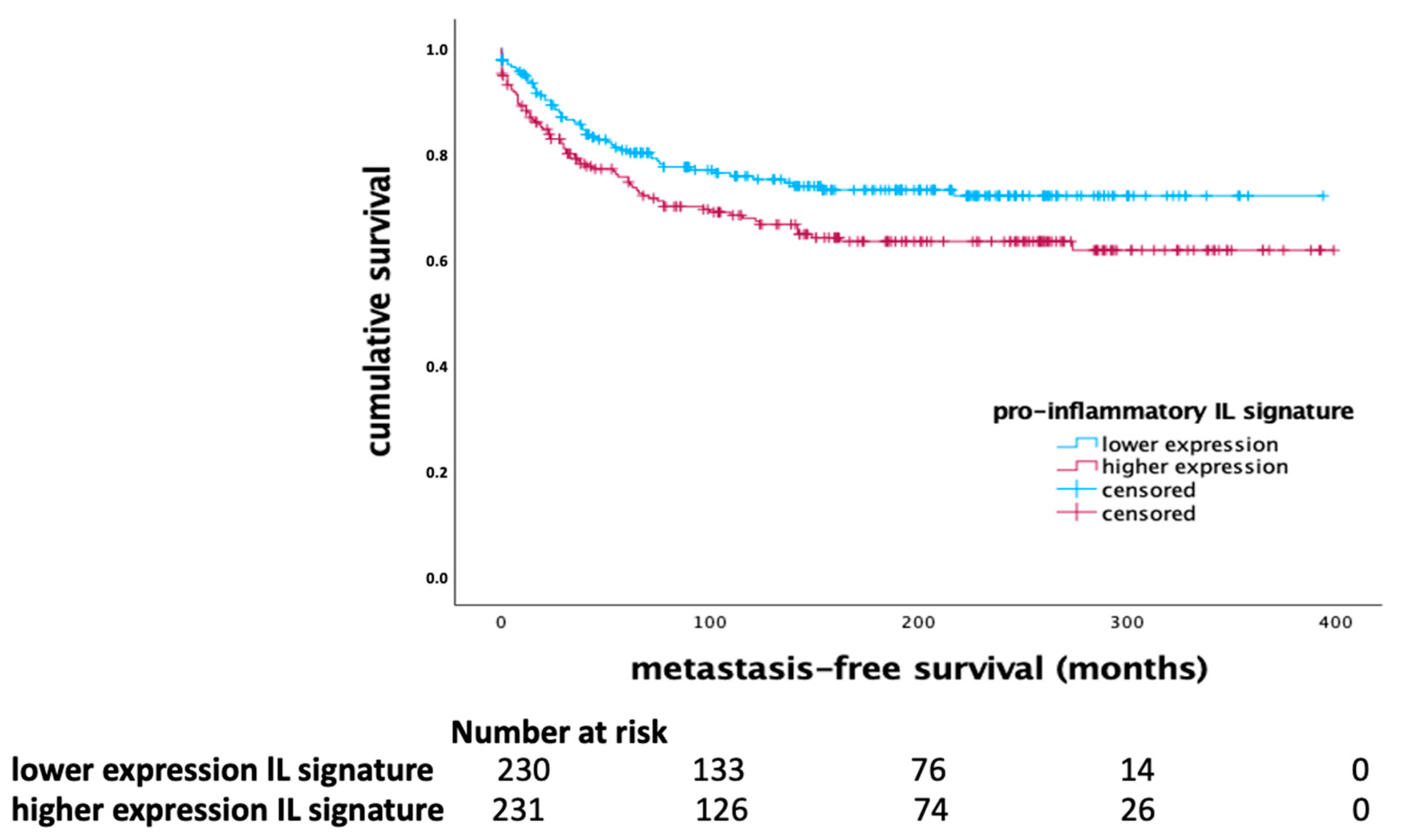
In contrast, the pro-inflammatory interleukin signature, including IL-4, IL-5, IL-10, IL-13, IL-17, and CXCL1, showed a significant prognostic effect across the whole cohort of breast cancer patients. Higher expression of pro-inflammatory interleukins was associated with shorter MFS (p = 0.034, Log Rank), as indicated in
Figure 3.
This suggests that elevated levels of these cytokines correlate with poorer clinical outcomes, highlighting their role in promoting tumor progression and metastasis. The multivariate Cox regression analysis for the pro-inflammatory interleukin signature is detailed in
Table 3, indicating that this signature did not retain independent significance when adjusted for other clinical variables.
Table 1.
Univariate Cox Regression analysis.
Table 1.
Univariate Cox Regression analysis.
| |
|
HR |
95% CI |
p- value |
| lower |
upper |
| Pro-inflammatory IL-Signature |
High vs. low |
1.446 |
1.025 |
2.041 |
0.036 |
| Anti-inflammatory IL-Signature |
High vs. low |
0.802 |
0.569 |
1.129 |
0.206 |
| Age |
<50 vs. ≥50 |
0.851 |
0.578 |
1.253 |
0.415 |
| Tumor size |
T2-4 vs. T1 |
2.219 |
1.513 |
3.254 |
<0.001 |
| Lymph node status |
N1,2,3 vs. N0 |
2.288 |
1.598 |
3.276 |
<0.001 |
| Grade |
GIII vs. GI/II |
4.946 |
2.020 |
12.109 |
<0.001 |
| Ki-67 |
>20% vs. <20% |
1.926 |
1.254 |
2.957 |
0.03 |
Table 2.
Multivariate Cox Regression analysis (anti-inflammatory IL-signature).
Table 2.
Multivariate Cox Regression analysis (anti-inflammatory IL-signature).
| |
|
HR |
95% CI |
p- value |
| lower |
upper |
| Anti-inflammatory IL-Signature |
High vs. low |
0.463 |
0.290 |
0.741 |
0.001 |
| Age |
<50 vs. ≥50 |
1.265 |
0.729 |
2.195 |
0.403 |
| Tumor size |
T2-4 vs. T1 |
1.604 |
0.995 |
2.585 |
0.052 |
| Lymph node status |
N1,2,3 vs. N0 |
1.479 |
0.926 |
2.365 |
0.102 |
| Grade |
GIII vs. GI/II |
2.489 |
1.527 |
4.057 |
<0.001 |
| Ki-67 |
>20% vs. <20% |
1.660 |
1.025 |
2.688 |
0.039 |
Table 3.
Multivariate Cox Regression analysis (pro-inflammatory IL-signature).
Table 3.
Multivariate Cox Regression analysis (pro-inflammatory IL-signature).
| |
|
HR |
95% CI |
p- value |
| lower |
upper |
| Pro-inflammatory IL-Signature |
High vs. low |
1.098 |
0.714 |
1.689 |
0.671 |
| Age |
<50 vs. ≥50 |
1.242 |
0.716 |
2.156 |
0.440 |
| Tumor size |
T2-4 vs. T1 |
1.510 |
0.938 |
2.430 |
0.090 |
| Lymph node status |
N1,2,3 vs. N0 |
1.207 |
0.762 |
1.912 |
0.422 |
| Grade |
GIII vs. GI/II |
2.218 |
1.369 |
3.595 |
0.001 |
| Ki-67 |
>20% vs. <20% |
1.516 |
0.944 |
2.436 |
0.085 |
2.3. Validation of IL-Signatures in Independent Cohorts
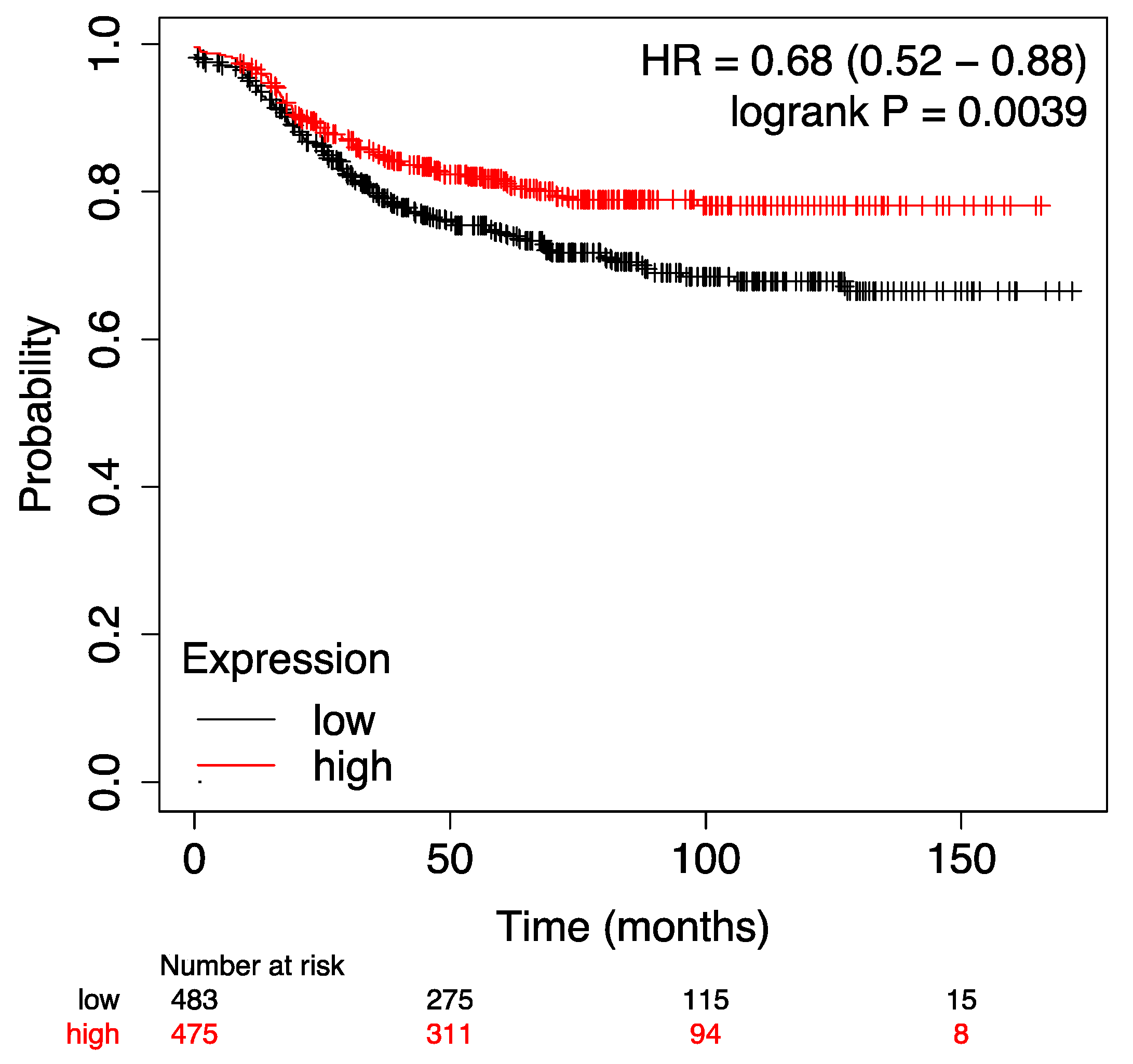
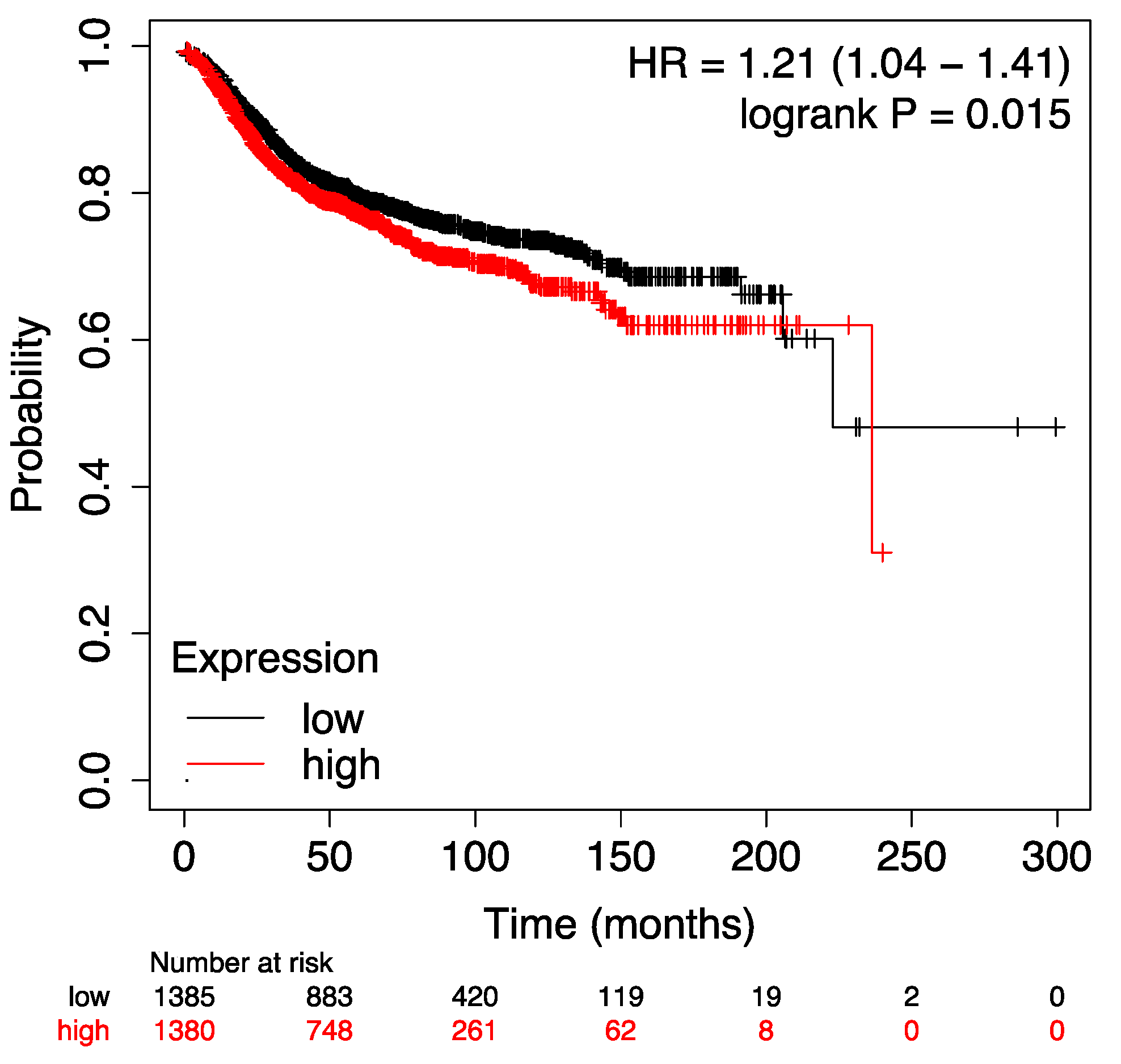
To validate our results in a larger, independent cohort, we used publicly available gene-expression data with associated tumor characteristics and follow-up data [
17,
18]. The prognostic significance of both the anti-inflammatory and the pro-inflammatory signatures was analyzed in relation to MFS. The prognostic significance of both the anti-inflammatory signature (p = 0.0039, Log Rank) and the pro-inflammatory signature (p = 0.015, Log Rank) was successfully validated in previously published datasets (suppl.
Figure A1 and
Figure A2).
2.4. Correlation with Immune Markers and Immune Checkpoints
To further elucidate the role of these interleukin signatures within the TME, correlations between the interleukin signatures and immune cell markers (CD8, IgKC, and CD20) as well as immune checkpoints (PD-1) were analyzed using the Spearman-Rho correlation coefficient.
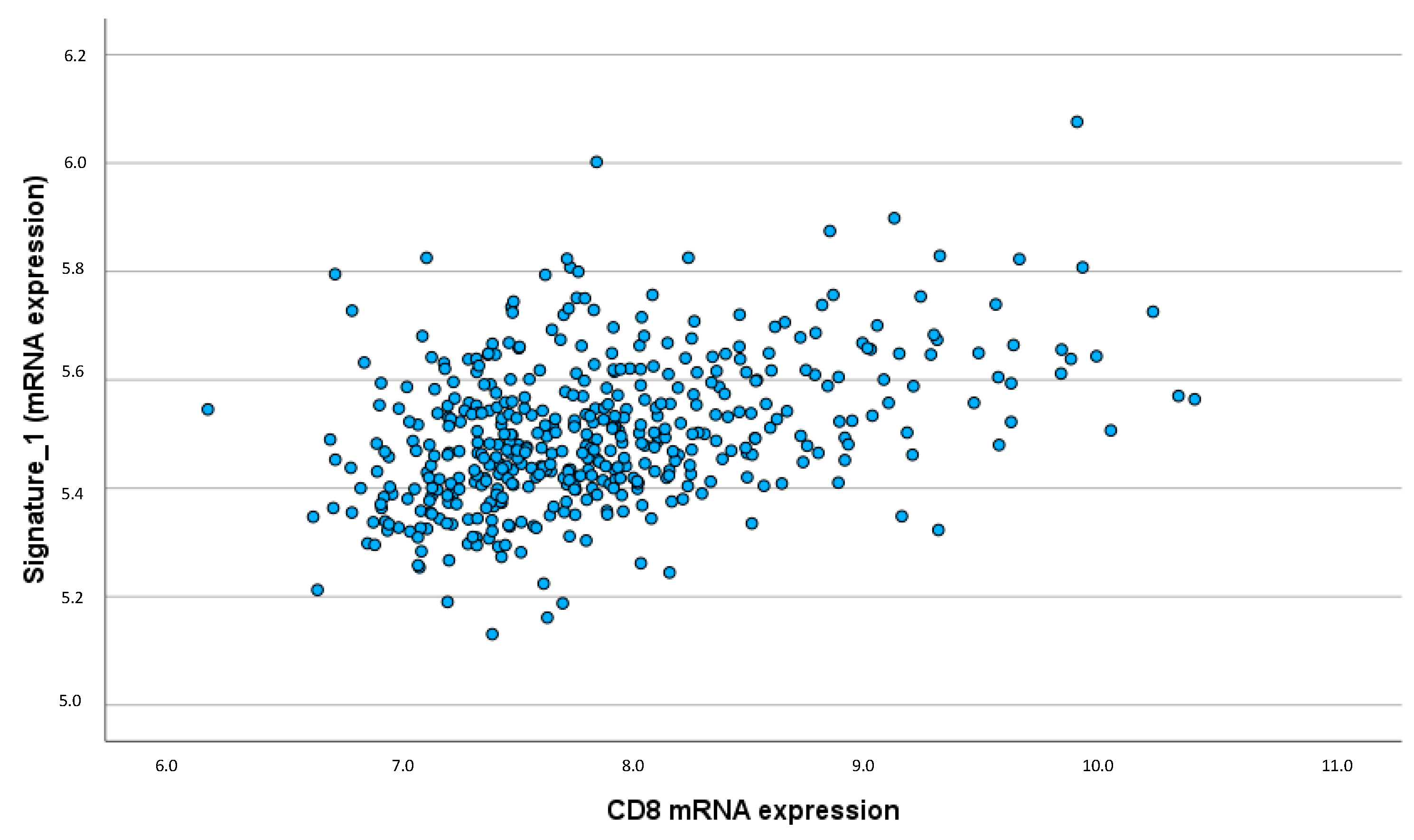
The anti-inflammatory interleukin signature showed a strong and significant correlation with CD8 expression (ρ = 0.391; p < 0.001), indicating that higher CD8 expression is associated with increased levels of IL-12, IL-21, and IFN-γ (
Figure 4). This emphasizes that the anti-inflammatory cytokines may enhance the presence and activity of cytotoxic T-cells within the TME. Similarly, positive correlations were observed between the anti-inflammatory interleukin signature and B cell-associated markers such as IgKC (ρ = 0.157; p < 0.001) and CD20 (ρ = 0.160; p < 0.001), implying a potential role in modulating humoral immune responses in the TME.
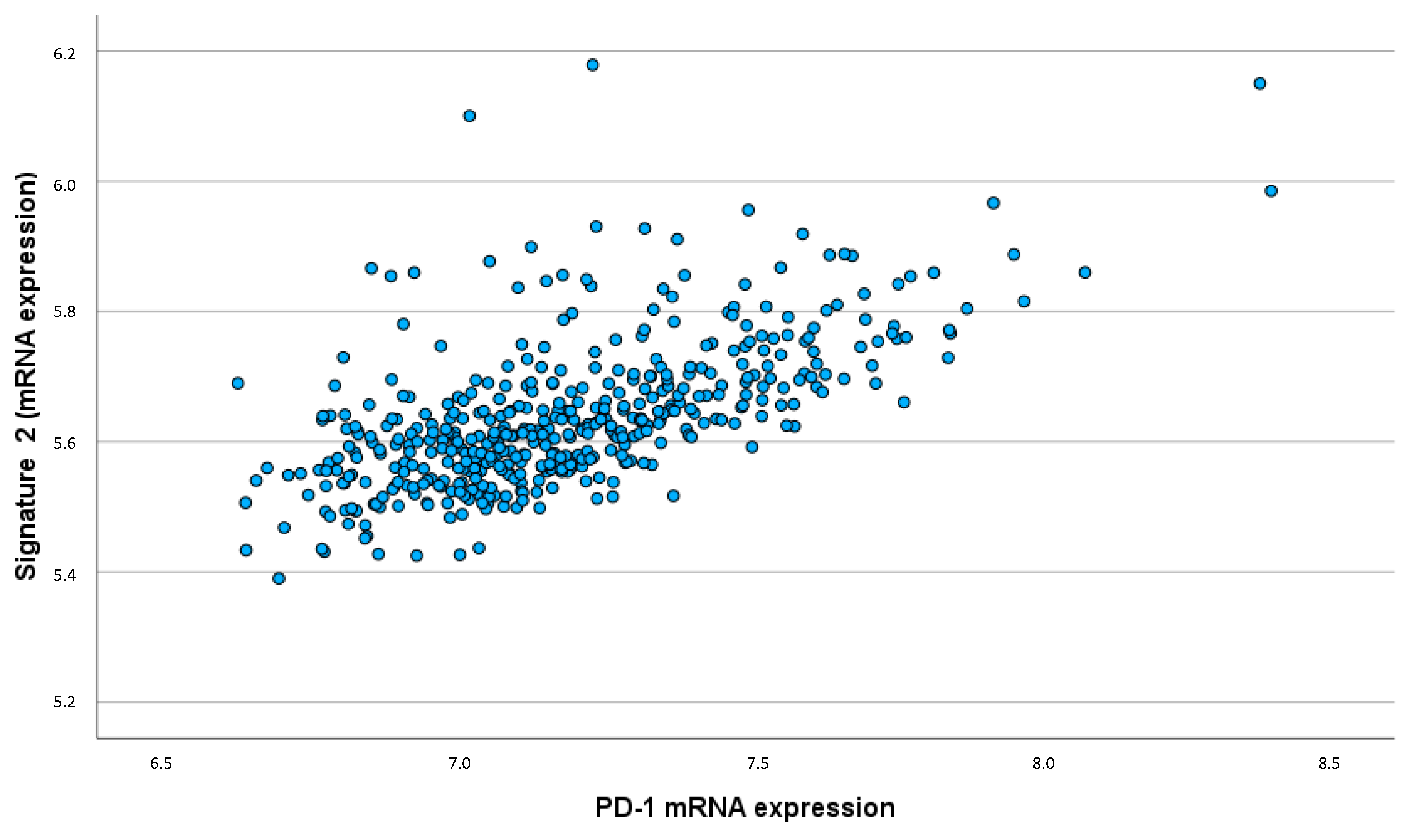
Additionally, the pro-inflammatory interleukin signature showed a significant correlation with PD-1 expression (ρ = 0.627; p < 0.001). This strong correlation indicates that higher levels of pro-inflammatory cytokines are associated with increased expression of immune checkpoint molecules, which may contribute to immune evasion mechanisms within the tumor (
Figure 5).
Overall, these results highlight the contrasting roles of anti-inflammatory and pro-inflammatory interleukins in breast cancer prognosis and their potential interactions with the immune microenvironment.
3. Discussion
The present study highlights the complex role of a pro- and anti-inflammatory interleukin signature in early breast cancer, emphasizing their prognostic significance and interaction within the tumor microenvironment. The findings underscore the dual nature of interleukins in modulating immune responses, tumor progression, and metastasis, offering insights into potential therapeutic targets for improving clinical outcomes.
Anti-Inflammatory Interleukin Signature: Protective Role in Basal-like Subtype
The anti-inflammatory interleukin signature, comprising IL-12, IL-21, and IFN-γ, demonstrated a significant prognostic impact, particularly within the basal-like breast cancer subtype. This subtype is known for its aggressive nature and poor prognosis, yet our findings suggest that a robust anti-inflammatory immune response may improve metastasis-free survival (MFS) in these patients. The independent prognostic significance of the anti-inflammatory signature, as revealed by multivariate Cox regression analysis, indicates that these cytokines play a critical role in enhancing antitumor immunity.
These results support the results of a previous gene expression analysis which showed that higher IFN-γ expression was associated with a better outcome (longer MFS), particularly in the basal-like subgroup. In addition, a protective effect of an IFN-γ -associated gene signature was shown in the whole cohort of early breast cancer: higher expression of the IFN-γsignature, which covers the IFN-γsignaling pathway, was associated with a favorable prognosis [
5].
IL-12 is known to promote Th1 responses and enhance the cytotoxic activity of NK cells and CD8+ T cells, leading to increased secretion of IFN-γ. This cytokine cascade stimulates antitumor immunity, as evidenced by its ability to inhibit tumor growth and metastasis in breast cancer models. In a preclinical breast cancer mouse model, it was shown that inhibition of the transcription factor YAP led to an activation of CD8 + cytotoxic T cells, as well as to an increase in IL12 levels, which overall led to reduced tumor growth [
19].
Using a preclinical mouse model, Mansurov et al. showed that intravenous administration of a collagen-binding domain fused to IL-12 (CBD-IL-12) had a synergistic effect with Immune Checkpoint Inhibitors (ICPi) in mice with immunologically cold tumors, leading to significant tumor regression [
20].
IL-21 is another important cytokine that plays a significant role in modulating the immune response within the TME of breast cancer. It is produced by activated CD4+ T cells and influences a variety of immune cells, including NK cells, CD8+ T cells, and B cells [
21]. IL-21 increases the proliferation and function of NK cells and CD8+ T cells, thereby enhancing their cytotoxic effects against tumor cells [
22]. Additionally, IL-21 promotes the differentiation of B cells into plasma cells, which can produce antibodies that target tumor cells. By supporting both cellular and humoral immune responses, IL-21 contributes to a comprehensive antitumor effect within the TME.
The strong correlation between the anti-inflammatory interleukin signature and CD8+ T cell markers suggests that these cytokines enhance cytotoxic immune responses within the TME. These findings align with previous studies showing that high levels of TILs, particularly CD8+ T cells, are associated with better prognosis in breast cancer [
23,
24,
25]. In interaction with the cellular immune response, the humoral immune response also plays a crucial prognostic role within the TME. A previous retrospective immunohistochemical study demonstrated that tumor-infiltrating IgKC and CD38-positive B-lymphocytes were associated with better prognosis in patients with early triple-negative breast cancer [
26]. In this context, another important finding of our retrospective study is the correlation of the anti-inflammatory signature with B cell-associated markers such as IgKC and CD20, indicating that anti-inflammatory interleukins may also modulate humoral immune responses.
Pro-Inflammatory Interleukin Signature Adversely Affected Prognosis
In contrast, the pro-inflammatory interleukin signature, including IL-4, IL-5, IL-10, IL-13, IL-17, and CXCL1, was associated with shorter MFS across the whole cohort. This finding suggests that elevated levels of these cytokines correlate with poorer clinical outcomes, reflecting their role in promoting tumor progression and metastasis.
IL-4, IL-5 and IL-13, which are related to the Th2 immune response, can create an immunosuppressive environment that favours tumor growth [
27,
28]. These cytokines support breast cancer cell survival and proliferation, contributing to a more aggressive disease phenotype. In particular, higher levels of IL-4 were associated with tumor cell resistance to proapoptotic signaling in an
in vitro cancer model [
27]. Interestingly, in a clinical study by König et al., which evaluated cytokine levels in the serum of breast cancer patients in relation to the presence of circulating tumor cells, it was shown that elevated serum levels of both IL-4 and IL-5 were associated with a poorer prognosis [
29]. Specifically, IL-4 was found to be related to negative estrogen receptor (ER) status and poor differentiation, while IL-5 was associated with positive nodal status [
29].
IL-10, while primarily anti-inflammatory, can suppress effective antitumor immune responses, facilitating tumor immune evasion and progression. A retrospective study by LLanes-Fernandez et al. revealed that IL-10 can suppress T-cell activity, and its association with apoptosis markers suggests an increase in tumor aggressiveness [
30]. IL-17, involved in chronic inflammation, promotes angiogenesis and tumor growth, further exacerbating disease severity [
31]. In a retrospective study by Popovic et al, a negative association between IL-17 and ER expression was shown, which in turn suggests a link between increased IL-17 levels and a more aggressive tumor biology [
32]. The chemokine CXCL1 which is expressed in tumor-associated fibroblasts is associated with poor prognosis. Zou et al. showed that increased CXCL1 expression in breast cancer stroma was associated with higher tumor grade, breast cancer recurrence and decreased patient survival [
33].
The strong correlation between the pro-inflammatory interleukin signature and PD-1 expression highlights the potential for immune suppression and tumor immune escape. PD-1 is an immune checkpoint molecule that inhibits T-cell activity, allowing tumors to evade immune surveillance. The association between pro-inflammatory cytokines and increased PD-1 expression suggests a mechanism by which these cytokines contribute to an immunosuppressive TME, promoting tumor progression and metastasis.
Therapeutic Implications and Future Directions
The distinct prognostic roles of pro- and anti-inflammatory interleukins in breast cancer highlight the importance of a nuanced approach to immunotherapy. Targeting the immune-inflammatory axis represents a promising strategy for modulating the TME and enhancing antitumor immunity.
Future research should focus on elucidating the mechanisms by which these interleukins interact with immune cells within the TME, as well as their potential as biomarkers for predicting response to immunotherapy. Understanding the complex interplay between cytokines and immune checkpoints, such as PD-1, will be crucial for developing effective combination therapies that harness the full potential of the immune system to combat breast cancer.
The present study provides valuable insights into the prognostic implications of pro- and anti-inflammatory interleukin mRNA expression signatures in early breast cancer. However, it is essential to acknowledge both the strengths and potential limitations of this work.
One of the main strengths of this study is the large cohort size of 461 patients and the long-term follow-up period, with a median follow-up time of 12.75 years, allowing for a comprehensive assessment of survival outcomes. By integrating established prognostic factors such as tumor grade, size, nodal status, and molecular subtypes, the study enhances the clinical relevance and applicability of the findings. The dual focus on both pro- and anti-inflammatory cytokines offers a balanced perspective on the complex role of the immune response in breast cancer, elucidating the contrasting effects of different cytokines on tumor progression and patient outcomes.
Despite these strengths, the study has several limitations. The retrospective design of the study may introduce biases related to patient selection and data collection. As a single-center study, the findings may have limited generalizability to other populations and clinical settings, necessitating multi-center studies for validation. While the study provides strong associations between interleukin expression and clinical outcomes, functional validation experiments to elucidate the mechanistic roles of specific interleukins were not performed. Additionally, the study does not extensively analyze the interaction between interleukin expression and specific treatment modalities, which could provide more targeted clinical insights.
In summary, while this study significantly contributes to understanding the prognostic role of interleukin signatures in early breast cancer, it also highlights areas for further research and validation. Addressing these limitations in future studies will be crucial for translating these findings into clinical practice and improving patient outcomes.
4. Materials and Methods
Patient Cohort
This study cohort included 461 patients with early breast cancer who underwent surgery at the Department of Gynecology and Obstetrics at the University Medical Center Mainz between 1986 and 2000. Adequate tumor tissue (fresh frozen) was available for successful Affymetrix microarray analysis. The cohort comprised three subgroups with different systemic treatments:
N0 cohort: 200 node-negative patients who received no further adjuvant therapy after surgery and radiation.
Tamoxifen cohort: 165 patients treated with tamoxifen as a single adjuvant therapy.
Chemotherapy cohort: 96 patients treated with either cyclophosphamide, methotrexate, fluorouracil (CMF; n = 34) or epirubicin, cyclophosphamide (EC; n = 62) in the adjuvant setting.
Table 4.
Patient’s characteristics.
Table 4.
Patient’s characteristics.
| |
number of patients
(n = 461) |
Percentage (%) |
| Age at diagnosis |
|
|
</=50
>50
|
104
357
|
22.6
77.4 |
| Tumor size |
|
|
T1
T2
T3
T4
missing value
|
188
214
19
39
1 |
40.8
46.4
4.1
8.5
0.2 |
| Tumor grade |
|
|
GI
GII
GIII
|
63
287
111 |
13.7
62.3
24 |
| Lymph node status |
|
|
N0
N1
N2
Nx
|
254
140
49
18 |
55.1
30.4
10.6
3.9 |
| Tumor type |
|
|
Invasive ductal (NST)
Invasive lobular
others
|
291
79
91 |
63.1
17.1
19.7
|
| ER |
|
|
positive
negative
missing value
|
381
79
1 |
82.6
17.1
0.2 |
| PR |
|
|
positive
negative
missing value
|
346
114
1 |
75.1
24.7
0.2 |
| HER2 |
|
|
positive
negative
missing value
|
46
358
57 |
10
77.7
12.3 |
| Ki-67 |
|
|
>20 %
≤ 20 %
missing value
|
138
250
73 |
29.9
54.2
15.8 |
| Molecular subtypes |
|
|
Luminal A-like
Luminal B-like
Basal-like
HER2-positive
|
189
182
51
39 |
41
39.5
11.1
8.5 |
| Distant metastasis |
|
|
Yes
No
|
133
328 |
28.9
71.1 |
| Treatment collective |
|
|
N0, untreated
endocrine treatment (tamoxifen)
chemotherapy:
|
200
165
96:
|
43.4
35.8
20.8:
|
mRNA Isolation and Gene Expression Analysis
The mRNA isolation and gene expression analysis were performed as previously described [
5,
34]. Tumor samples were frozen and stored at -80°C. Approximately 50 mg of frozen breast tumor tissue was fragmented in liquid nitrogen. RLT buffer was added, and the homogenate was centrifuged through a QIAshredder column (Qiagen). Total RNA was isolated from the eluate using the RNeasy kit (Qiagen) according to the manufacturer’s instructions. RNA yield was determined by UV absorbance, and RNA quality was assessed using an Agilent 2100 Bioanalyzer RNA 6000 LabChip Kit (Agilent Technologies).
For mRNA expression analysis, fresh frozen tumor tissue from 461 breast cancer samples was used to generate HG-U133A arrays (Affymetrix, Santa Clara, CA, USA) and measure the relative transcript frequencies of the selected genes. Labeled cRNA was synthesized from 5 µg of total RNA using Roche Microarray cDNA Synthesis, Microarray RNA Target Synthesis (T7), and Microarray Target Purification Kits (Roche Applied Science) following the manufacturer’s instructions. Raw expression data (CEL files) were standardized by multiarray analysis (fRMA). The complete record of 461 samples used in the current study with updated follow-up are filed at the NCBI in the GEO database under accession number GSE158309.
The gene expression data included the following single genes and corresponding probe sets related to pro- and anti-inflammatory interleukin signatures:
Pro-inflammatory Interleukin signature:
Anti-inflammatory Interleukin signature:
An overall inflammatory signature was calculated as the average of the expression values of the pro-inflammatory interleukins (IL-4, IL-5, IL-10, IL-13, IL-17, CXCL1) and anti-inflammatory interleukins (IL-12, IL-21, IFN-γ). Scores above the median of the inflammatory signature were defined as high expression, and scores below the median were defined as low expression.
To validate our results on a larger, independent cohort, we used publicly available gene-expression data of both the anti- and pro-inflammatory signatures with associated tumor characteristics, as well as follow-up data [
17,
18].
Molecular Subtypes
Intrinsic subtypes were determined according to the model proposed by Haibe-Kains et al., which includes estrogen receptor gene (ESR1), HER2, and Aurora kinase A (AURKA). ER and HER2 status were determined from the bimodally distributed mRNA levels of ESR1 (205225_at) and ERBB2 (216836_s_at), respectively. The cut-off values for ESR1 and ERBB2 were determined using model-based clustering and the upper quartile plus interquartile range of mRNA levels. The median mRNA expression of AURKA (208079_s_at) was used as the cut-off value.
This resulted in the following molecular subtypes:
ESR1-positive, HER2-negative, low proliferation (AURKA low) luminal A-like.
ESR1-positive, HER2-negative, high proliferation (AURKA high) luminal B-like.
HER2-positive.
ESR1-negative, HER2-negative basal-like.
Table 4 shows the absolute and relative frequencies of these molecular subtypes within the patient cohort.
Statistical Analysis
Statistical analyses were performed using SPSS version 28.0 (SPSS Inc, Chicago, IL, USA) and Stata version 17. The prognostic significance of pro- and anti-inflammatory interleukin expression for metastasis-free survival (MFS) was examined by Kaplan-Meier survival analysis (≤ median vs. > median) and univariate and multivariate Cox regression analysis. Multivariate Cox regression analysis was adjusted for tumor stage (T1 vs. T2, T3, T4), histological grade (GI + GII vs. GIII), Ki-67 (≤ 20% vs. > 20%), and lymph node status (negative vs. positive). Significance of Kaplan-Meier survival analysis was assessed using the log-rank test. Correlations were analyzed using the Spearman-Rho correlation coefficient.
5. Conclusions
This study highlights the prognostic relevance of pro- and anti-inflammatory interleukin mRNA expression signatures in early breast cancer. The anti-inflammatory interleukin signature, particularly in the basal-like subtype, is associated with improved metastasis-free survival, suggesting a protective role in enhancing antitumor immunity. Conversely, the pro-inflammatory interleukin signature correlates with poorer outcomes, indicating its role in promoting tumor progression and immune evasion. These findings underscore the potential of targeting the immune-inflammatory axis in breast cancer therapy. Future research should focus on validating these results in larger cohorts and exploring the underlying mechanisms to develop targeted therapeutic strategies that improve patient outcomes.
Author Contributions
Conceptualization, Anne-Sophie Heimes and Marcus Schmidt; Data curation, Anne-Sophie Heimes, Marcus Schmidt; Formal analysis, Anne-Sophie Heimes and Ina Shehaj; Investigation, Marcus Schmidt; Methodology, Anne-Sophie Heimes, Ina Shehaj and Marcus Schmidt; Project administration, Anne-Sophie Heimes; Resources, Walburgis Brenner, Annette Hasenburg and Marcus Schmidt; Supervision, Marcus Schmidt; Validation, Anne-Sophie Heimes, Ina Shehaj and Marcus Schmidt; Visualization, Anne-Sophie Heimes, Ina Shehaj; Writing – original draft, Anne-Sophie Heimes and Marcus Schmidt; Writing – review & editing, Ina Shehaj, Katrin Almstedt, Slavomir Krajnak, Roxana Schwab, Kathrin Stewen, Antje Lebrecht,, Walburgis Brenner, Annette Hasenburg and Marcus Schmidt.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was approved by the Ethics Committee of Rhineland-Palatinate, Germany [no. 837.139.05 (4797)]. Written informed consent was obtained from all patients, and all clinical investigations were conducted ethically in accordance with ethical and legal standards and in consideration of the Declarations of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge Martina Seehase and Susanne Gebhard for excellent administrative and technical support.
Conflicts of Interest
A.-S. Heimes received honoraria from Pfizer Pharma GmbH, Roche Pharma AG, Medupdate GmbH, Streamedup! GmbH and travel reimbursement from Daiichy Sankyo GmbH and Lilly. I. Shehaj received funding and travel reimbursement from Olympus and AURIKAMED. K. Almstedt received speaker honoraria from Roche Pharma AG, Pfizer Pharma GmbH, Seagen and AstraZeneca. K. Stewen received honoraria from Astra Zeneca, Roche Pharma AG, StreamedUp! GmbH, and Pierre Fabre. R. Schwab received honoraria from Roche Pharma AG, AstraZeneca, and Streamedup! GmbH. S. Krajnak received speaker honoraria from Roche Pharma AG and Novartis Pharma GmbH Germany, research funding from Novartis Pharma GmbH Germany and travel reimbursement from PharmaMar and Novartis Pharma GmbH Germany. A. Hasenburg received honoraria from AstraZeneca, Celgene, GSK, LEO Pharma, MedConcept GmbH, Med update GmbH, Medicultus, Pfizer, Promedicis GmbH, Softconsult, Roche Pharma AG, Streamedup!GmbH, and Tesaro Bio Germany GmbH. She is a member of the advisory board of AstraZeneca, GSK, LEO Pharma, PharmaMar, Promedicis GmbH, Roche Pharma AG, Tesaro Bio Germany GmbH, MSD Sharp and Dohme GmbH. M. Schmidt reports personal fees from AstraZeneca, BioNTech, Daiichi Sankyo, Eisai, Gilead, Lilly, MSD, Novartis, Pantarhei Bioscience, Pfizer, Roche, and SeaGen outside the submitted work. Institutional research funding from AstraZeneca, BioNTech, Eisai, Genentech, German Breast Group, Novartis, Palleos, Pantarhei Bioscience, Pierre Fabre, and SeaGen. In addition, Marcus Schmidt has a patent for EP 2390370 B1 issued and a patent for EP 2951317 B1 issued. All other authors declare that they have no conflicts of interest.
Appendix A
Figure A1.
Kaplan Meier Plot of the anti-inflammatory IL signature in terms of MFS of an unselected breast cancer cohort using publicy available gene expression data showing that a higher expression of the anti-inflammatory signature was significantly associated with an improved outcome (longer MFS).
Figure A1.
Kaplan Meier Plot of the anti-inflammatory IL signature in terms of MFS of an unselected breast cancer cohort using publicy available gene expression data showing that a higher expression of the anti-inflammatory signature was significantly associated with an improved outcome (longer MFS).
The above mentioned validation signature contained the following probe sets: 207160_at (IL-12A); 1560725_at (IL12A-AS1); 207901_at (IL-12B); 221271_at (IL-21); 210354_at (IFNG) [
17,
18].
Figure A2.
Kaplan Meier Plot of the pro-inflammatory IL signature in terms of MFS of an unselected breast cancer cohort using publicy available gene expression data showing that a higher expression of the pro-inflammatory signature was significantly associated with worse prognosis (shorter MFS).
Figure A2.
Kaplan Meier Plot of the pro-inflammatory IL signature in terms of MFS of an unselected breast cancer cohort using publicy available gene expression data showing that a higher expression of the pro-inflammatory signature was significantly associated with worse prognosis (shorter MFS).
The above mentioned validation signature contained the following probe sets: 207539_s_at (IL-4); 207538_at (IL-4); 207952_at (IL-5); 207433_at (IL-10); 206569_at (IL-10B); 207844_at (IL-13); 208402_at (IL-17A); 204470_at (CXCL1) [
17,
18].
References
- So, J.Y. et al. Triple negative breast cancer (TNBC): Non-genetic tumor heterogeneity and immune microenvironment: Emerging treatment options. Pharmacol Ther 2022, 237, 108253. [Google Scholar]
- Denkert, C. et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol 2018, 19, 40–50. [Google Scholar]
- Denkert, C. et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 2010, 28, 105–13. [Google Scholar]
- Habanjar, O. et al., Crosstalk of Inflammatory Cytokines within the Breast Tumor Microenvironment. Int J Mol Sci, 2023, 24.
- Heimes, A.S. et al., Prognostic Significance of Interferon-gamma and Its Signaling Pathway in Early Breast Cancer Depends on the Molecular Subtypes. Int J Mol Sci, 2020, 21.
- Castro, F. et al. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front Immunol 2018, 9, 847. [Google Scholar]
- Jorgovanovic, D. et al. Roles of IFN-gamma in tumor progression and regression: a review. Biomark Res 2020, 8, 49. [Google Scholar]
- Sharma, P. et al. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar]
- Tait Wojno, E.D., C. A. Hunter, and J.S. Stumhofer, The Immunobiology of the Interleukin-12 Family: Room for Discovery. Immunity 2019, 50, 851–870. [Google Scholar]
- Yang, S.X. et al. Interleukin-12 activated CD8(+) T cells induces apoptosis in breast cancer cells and reduces tumor growth. Biomed Pharmacother 2016, 84, 1466–1471. [Google Scholar]
- Park, Y.K. et al. Interleukin-21 increases direct cytotoxicity and IFN-gamma production of ex vivo expanded NK cells towards breast cancer cells. Anticancer Res 2012, 32, 839–46. [Google Scholar]
- Rodriguez-Tirado, C. et al., Interleukin 4 Controls the Pro-Tumoral Role of Macrophages in Mammary Cancer Pulmonary Metastasis in Mice. Cancers (Basel), 2022, 14.
- Llanes-Fernandez, L. et al. Association between the expression of IL-10 and T cell activation proteins loss in early breast cancer patients. J Cancer Res Clin Oncol 2009, 135, 255–64. [Google Scholar]
- Zhu, X. et al. IL-17 expression by breast-cancer-associated macrophages: IL-17 promotes invasiveness of breast cancer cell lines. Breast Cancer Res 2008, 10, R95. [Google Scholar]
- Wang, L. et al. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med 2009, 206, 1457–64. [Google Scholar]
- Wang, N. et al. CXCL1 derived from tumor-associated macrophages promotes breast cancer metastasis via activating NF-kappaB/SOX4 signaling. Cell Death Dis 2018, 9, 880. [Google Scholar]
- Gyorffy, B. Survival analysis across the entire transcriptome identifies biomarkers with the highest prognostic power in breast cancer. Comput Struct Biotechnol J 2021, 19, 4101–4109. [Google Scholar] [CrossRef]
- Gyorffy, B. Integrated analysis of public datasets for the discovery and validation of survival-associated genes in solid tumors. Innovation (Camb) 2024, 5, 100625. [Google Scholar] [CrossRef]
- Wang, C. et al. YAP/STAT3 inhibited CD8(+) T cells activity in the breast cancer immune microenvironment by inducing M2 polarization of tumor-associated macrophages. Cancer Med 2023, 12, 16295–16309. [Google Scholar]
- Mansurov, A. et al. Collagen-binding IL-12 enhances tumour inflammation and drives the complete remission of established immunologically cold mouse tumours. Nat Biomed Eng 2020, 4, 531–543. [Google Scholar]
- Petersen, C.C. et al. Interleukin-21 restrains tumor growth and induces a substantial increase in the number of circulating tumor-specific T cells in a murine model of malignant melanoma. Cytokine 2010, 49, 80–8. [Google Scholar]
- Frederiksen, K.S. et al. IL-21 induces in vivo immune activation of NK cells and CD8(+) T cells in patients with metastatic melanoma and renal cell carcinoma. Cancer Immunol Immunother 2008, 57, 1439–49. [Google Scholar] [PubMed]
- Mahmoud, S.M.A. et al. Tumor-Infiltrating CD8(+) Lymphocytes Predict Clinical Outcome in Breast Cancer. Journal of Clinical Oncology 2011, 29, 1949–1955. [Google Scholar]
- Peng, G.L. et al. CD8(+) cytotoxic and FoxP3(+) regulatory T lymphocytes serve as prognostic factors in breast cancer. Am J Transl Res 2019, 11, 5039–5053. [Google Scholar]
- Rody A, H.U. Pusztai L, Liedtke C, Gaetje R, Ruckhaeberle E, Solbach C, Hanker L, Ahr A, Metzler D, Engels K, Karn T, Kaufmann M. T-cell metagene predicts a favorable prognosis in estrogen receptor-negative and HER2-positive breast cancers. Breast Cancer Res 2009, 11, R15. [Google Scholar]
- Heimes, A.S. et al., Prognostic Impact of CD38- and IgkappaC-Positive Tumor-Infiltrating Plasma Cells in Triple-Negative Breast Cancer. Int J Mol Sci, 2023, 24.
- Li, Z. et al. Endogenous interleukin-4 promotes tumor development by increasing tumor cell resistance to apoptosis. Cancer Res 2008, 68, 8687–94. [Google Scholar] [PubMed]
- Prokopchuk, O. et al. Interleukin-4 enhances proliferation of human pancreatic cancer cells: evidence for autocrine and paracrine actions. Br J Cancer 2005, 92, 921–8. [Google Scholar] [PubMed]
- Konig, A. et al. Determination of Interleukin-4, -5, -6, -8 and -13 in Serum of Patients with Breast Cancer Before Treatment and its Correlation to Circulating Tumor Cells. Anticancer Res 2016, 36, 3123–30. [Google Scholar]
- Llanes-Fernandez, L. et al. Relationship between IL-10 and tumor markers in breast cancer patients. Breast 2006, 15, 482–9. [Google Scholar]
- Fabre, J.A.S. et al., The Interleukin-17 Family of Cytokines in Breast Cancer. Int J Mol Sci, 2018, 19.
- Popovic, M. et al. Interleukin 17 in early invasive breast cancer. Front Oncol 2023, 13, 1171254. [Google Scholar]
- Zou, A. et al. Elevated CXCL1 expression in breast cancer stroma predicts poor prognosis and is inversely associated with expression of TGF-beta signaling proteins. BMC Cancer 2014, 14, 781. [Google Scholar]
- Heimes, A.S. et al., Prognostic Impact of LAG-3 mRNA Expression in Early Breast Cancer. Biomedicines, 2022, 10.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).