Submitted:
18 July 2024
Posted:
19 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
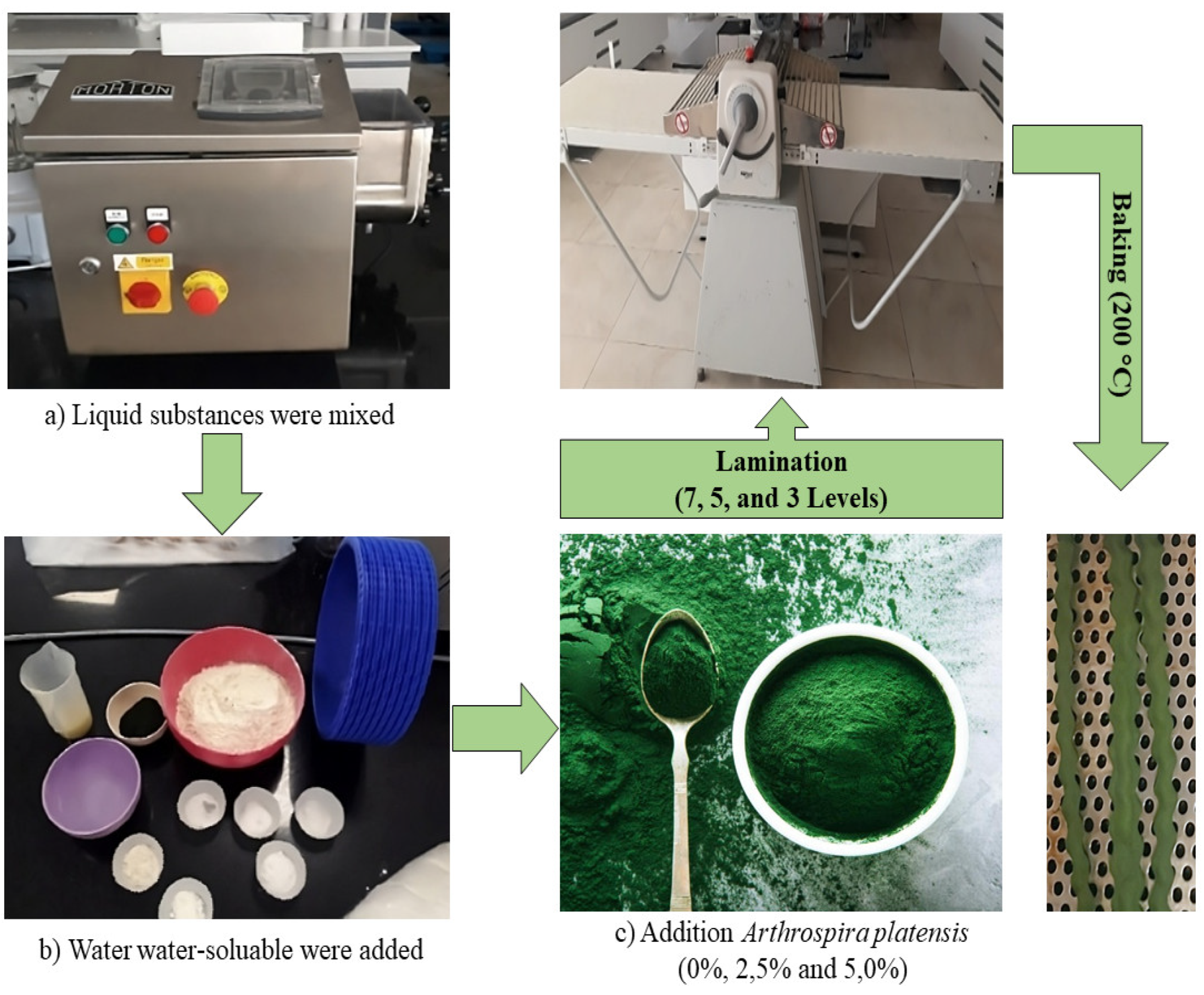
2.2. Manufacturing of Functional Crackers
2.3. Sensory Analyses
2.4. Physicochemical Analysis
2.5. Statistical Analysis
2.6. Shelf Life
3. Results and Discussion
3.1. Composition of Arthrospira Platensis
3.2. Sensory, Physical, and Chemical Structure of the Crackers
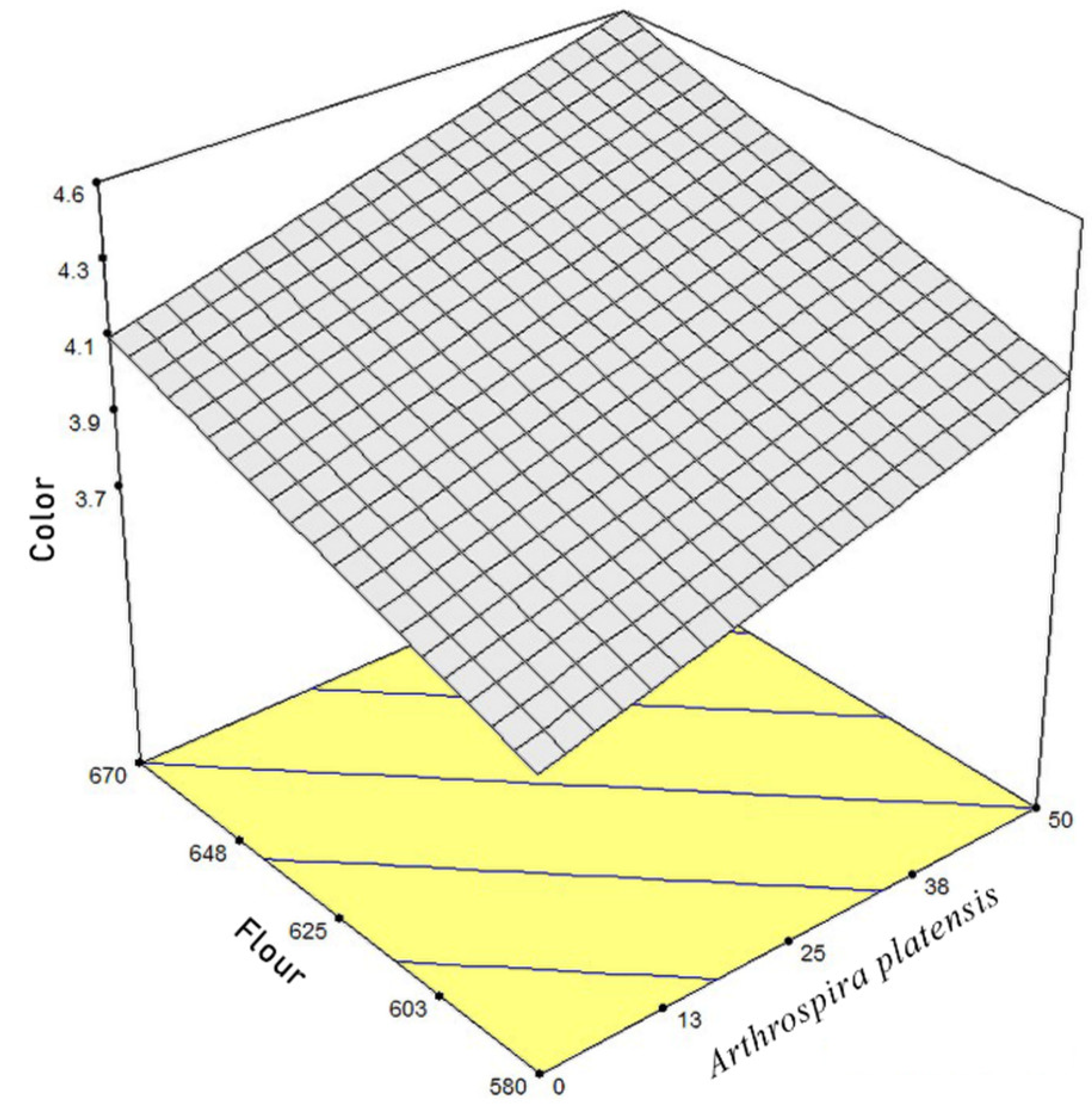
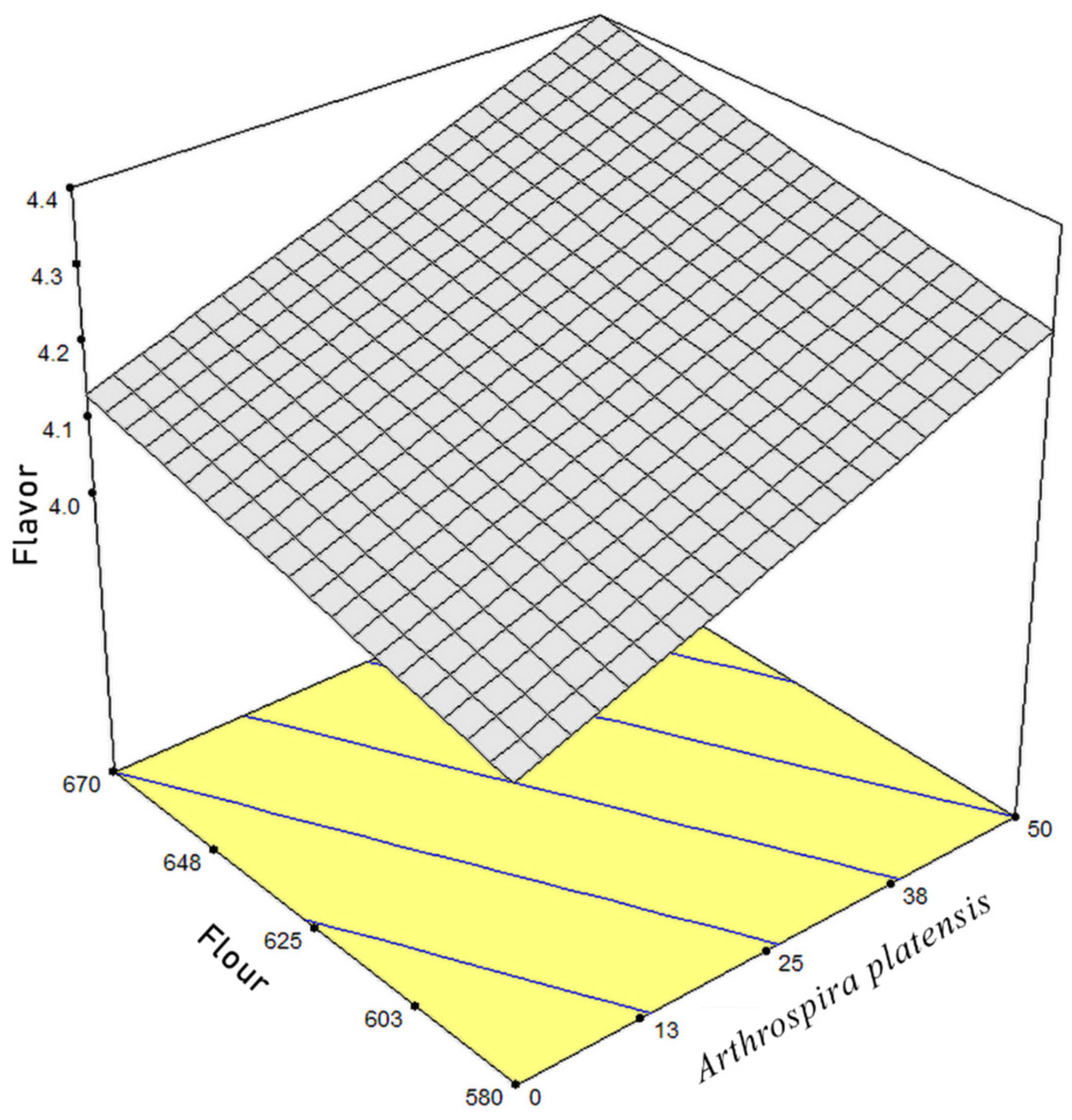
3.3. Hunter Color, Moisture, and Hardness of Cracker Samples
3.4. Optimization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, D.; Van Der Horst, K.; Jacquier, E.F.; Afeiche, M.C.; Eldridge, A.L. Snacking Patterns in Children: A Comparison between Australia, China, Mexico, and the US. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Lafarga, T.; Hayes, M. Effect of Pre-Treatment on the Generation of Dipeptidyl Peptidase-IV-and Prolyl Endopeptidase-Inhibitory Hydrolysates from Bovine Lung. Irish Journal of Agricultural and Food Research 2017, 56, 12–24. [Google Scholar] [CrossRef]
- Bharat Helkar, P.; Sahoo, A. Review: Food Industry By-Products Used as a Functional Food Ingredients. International Journal of Waste Resources 2016, 6. [Google Scholar] [CrossRef]
- Bimbo, F.; Bonanno, A.; Nocella, G.; Viscecchia, R.; Nardone, G.; De Devitiis, B.; Carlucci, D. Consumers’ Acceptance and Preferences for Nutrition-Modified and Functional Dairy Products: A Systematic Review. Appetite 2017, 113, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Urala, N.; Lähteenmäki, L. Attitudes behind Consumers’ Willingness to Use Functional Foods. Food Quality and Preference 2004, 15, 793–803. [Google Scholar] [CrossRef]
- Bernaerts, T.M.M.; Gheysen, L.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. The Potential of Microalgae and Their Biopolymers as Structuring Ingredients in Food: A Review. Biotechnology Advances 2019, 37, 107419. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.; Johnston, C.; Wharton, C. Plant-Based Diets: Considerations for Environmental Impact, Protein Quality, and Exercise Performance. Nutrients 2018, 10. [Google Scholar] [CrossRef]
- Geada, P.; Moreira, C.; Silva, M.; Nunes, R.; Madureira, L.; Rocha, C.M.R.; Pereira, R.N.; Vicente, A.A.; Teixeira, J.A. Algal Proteins: Production Strategies and Nutritional and Functional Properties. Bioresource technology 2021, 332. [Google Scholar] [CrossRef] [PubMed]
- Matos, J.; Cardoso, C.; Bandarra, N.M.; Afonso, C. Microalgae as Healthy Ingredients for Functional Food: A Review. Food and Function 2017, 8, 2672–2685. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive Compounds in Seaweed: Functional Food Applications and Legislation. Journal of Applied Phycology 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Nethravathy, M.U.; Mehar, J.G.; Mudliar, S.N.; Shekh, A.Y. Recent Advances in Microalgal Bioactives for Food, Feed, and Healthcare Products: Commercial Potential, Market Space, and Sustainability. Comprehensive Reviews in Food Science and Food Safety 2019, 18, 1882–1897. [Google Scholar] [CrossRef]
- Schlösser, U.G. Sammlung von Algenkulturen. Berichte der Deutschen Botanischen Gesellschaft 1982, 95, 181–276. [Google Scholar] [CrossRef]
- Teuling, E.; Schrama, J.W.; Gruppen, H.; Wierenga, P.A. Characterizing Emulsion Properties of Microalgal and Cyanobacterial Protein Isolates. Algal Research 2019, 39, 101471. [Google Scholar] [CrossRef]
- Niccolai, A.; Venturi, M.; Galli, V.; Pini, N.; Rodolfi, L.; Biondi, N.; D’Ottavio, M.; Batista, A.P.; Raymundo, A.; Granchi, L.; et al. Development of New Microalgae-Based Sourdough “Crostini”: Functional Effects of Arthrospira platensis (Spirulina) Addition. Scientific Reports 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gantar, M.; Svirčev, Z. Microalgae and Cyanobacteria: Food for Thought. Journal of Phycology 2008, 44, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Champagne, C.; Allen, R.; Cole, R.; Armstrong, N.; McGraw, S.; Moylan, E.; Miketinas, D. Baseline Diet Adequacy of Army Soldiers Supports the Need for a Planned Dining Facility Intervention. Journal of Nutrition Education and Behavior 2018, 50, S109–S110. [Google Scholar] [CrossRef]
- Thivani, M.; Mahendran, T.; Kanimoly, M. Study on the Physico-Chemical Properties, Sensory Attributes and Shelf Life of Pineapple Powder Incorporated Biscuits. Ruhuna Journal of Science 2016, 7, 32. [Google Scholar] [CrossRef]
- Gün, D.; Çelekli, A.; Bozkurt, H.; Kaya, S. Optimization of Biscuit Enrichment with the Incorporation of Arthrospira platensis: Nutritional and Sensory Approach. Journal of Applied Phycology 2022, 34, 1555–1563. [Google Scholar] [CrossRef]
- Çelekli, A.; Alslibi, Z.A.; Bozkurt, H. Influence of Incorporated Spirulina platensis on the Growth of Microflora and Physicochemical Properties of Ayran as a Functional Food. Algal Research 2019, 44, 101710. [Google Scholar] [CrossRef]
- Çelekli, A.; Özbal, B.; Bozkurt, H. Challenges in Functional Food Products with the Incorporation of Some Microalgae. Foods 2024, 13. [Google Scholar] [CrossRef]
- Devi, S.; Varkey, A.; Sheshshayee, M.S.; Preston, T.; Kurpad, A. V. Measurement of Protein Digestibility in Humans by a Dual-Tracer Method. American Journal of Clinical Nutrition 2018, 107, 984–991. [Google Scholar] [CrossRef] [PubMed]
- AACC Approved Methods of the American Association of Cereal Chemists, Volumes 1-2. Approved Methods of the American Association of Cereal Chemists, American Association of Cereal Chemists. Approved Methods Committee. Food Sci. Technol. Leb. 2000, 2, 1200. [Google Scholar]
- Anonymous EN ISO 8589, Sensory Analysis; General Guidance for the Design of Test Rooms. International Organization for Standardization 2010.
- Stefanowicz, P. Sensory Evaluation of Food Principles and Practices; Springer Science & Business Media, 2013; Vol. 24; ISBN 1441964886.
- ASTM Standard Test Methods for Water Vapor Transmission of Materials- E96/E96-05. Philadelphia. 2005, i, 1–14.
- Hussain, M.A.; Basahy, A.Y. Nutrient Composition and Amino Acid Pattern of Cowpea (Vigna unguiculata (L.) Walp, Fabaceae) Grown in the Gizan Area of Saudi Arabia. International Journal of Food Sciences and Nutrition 1998, 49, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Anonymous (TS EN ISO 2171), Cereals and Milled Cereals Products – Determination of Total Ash., Switzerland. International Standardization Organization, 1993.
- Bahloul, N.; Boudhrioua, N.; Kouhila, M.; Kechaou, N. Effect of Convective Solar Drying on Colour, Total Phenols and Radical Scavenging Activity of Olive Leaves (Olea europaea L.). International Journal of Food Science and Technology 2009, 44, 2561–2567. [Google Scholar] [CrossRef]
- Yadav, D.N.; Sharma, G.K.; Bawa, A.S. Optimization of Soy-Fortified Instant Sooji Halwa Mix Using Response Surface Methodology. Journal of Food Science and Technology 2007, 44, 297–300. [Google Scholar]
- Kathiravan, T. *; Kumar, S. Optimization of Pulsed Electric Field Processing Conditions for Passion Fruit Juice (Passiflora edulis) Using Response Surface Methodology. International Journal of Advanced Research 2013, 1, 399–411. [Google Scholar]
- Yamaguchi, K. Recent Advances in Microalgal Bioscience in Japan, with Special Reference to Utilization of Biomass and Metabolites: A Review. Journal of Applied Phycology 1997, 8, 487–502. [Google Scholar] [CrossRef]
- Gouveia, L.; Batista, A.P.; Sousa, I.; Raymundo, A.; Bandarra, N.M. In: Food Chemistry Research Developments. 2008.
- Prabhasankar, P.; Ganesan, P.; Bhaskar, N.; Hirose, A.; Stephen, N.; Gowda, L.R.; Hosokawa, M.; Miyashita, K. Edible Japanese Seaweed, Wakame (Undaria pinnatifida) as an Ingredient in Pasta: Chemical, Functional and Structural Evaluation. Food Chemistry 2009, 115, 501–508. [Google Scholar] [CrossRef]
- Hall, A.C.; Fairclough, A.C.; Mahadevan, K.; Paxman, J.R. Ascophyllum nodosum Enriched Bread Reduces Subsequent Energy Intake with No Effect on Post-Prandial Glucose and Cholesterol in Healthy, Overweight Males. A Pilot Study. Appetite 2012, 58, 379–386. [Google Scholar] [CrossRef]
- Batista, A.P.; Niccolai, A.; Fradinho, P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Microalgae Biomass as an Alternative Ingredient in Cookies: Sensory, Physical and Chemical Properties, Antioxidant Activity and in Vitro Digestibility. Algal Research 2017, 26, 161–171. [Google Scholar] [CrossRef]
- Minh, N.P. Effect of Saccharomyces cerevisiae, Spirulina and Preservative Supplementation to Sweet Bread Quality in Bakery. International journal of multidisciplinary research and development 2014, 1, 36–44. [Google Scholar]
- Gouveia, L.; Coutinho, C.; Mendonça, E.; Batista, A.P.; Sousa, I.; Bandarra, N.M.; Raymundo, A. Functional Biscuits with PUFA-Ω3 from Isochrysis Galbana. Journal of the Science of Food and Agriculture 2008, 88, 891–896. [Google Scholar] [CrossRef]
- Gouveia, L.; Batista, A.P.; Miranda, A.; Empis, J.; Raymundo, A. Chlorella vulgaris Biomass Used as Colouring Source in Traditional Butter Cookies. Innovative Food Science and Emerging Technologies 2007, 8, 433–436. [Google Scholar] [CrossRef]
- Zen, C.K.; Tiepo, C.B.V.; da Silva, R.V.; Reinehr, C.O.; Gutkoski, L.C.; Oro, T.; Colla, L.M. Development of Functional Pasta with Microencapsulated Spirulina: Technological and Sensorial Effects. Journal of the Science of Food and Agriculture 2020, 100, 2018–2026. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.S.; Hussein, A.M.S. Exploring the Suitability of Incorporating Tiger Nut Flour as Novel Ingredient in Gluten-Free Biscuit. Polish Journal of Food and Nutrition Sciences 2014, 64, 27–33. [Google Scholar] [CrossRef]
- Ho, D. Development of High-Protein Algae Dark Chocolate Using Arthrospira platensis. 2019, Doctoral dissertation, Tunku Abdul Rahman University College.
- Dapčević Hadnadev, T.R.; Torbica, A.M.; Hadnadev, M.S. Influence of Buckwheat Flour and Carboxymethyl Cellulose on Rheological Behaviour and Baking Performance of Gluten-Free Cookie Dough. Food and Bioprocess Technology 2013, 6, 1770–1781. [Google Scholar] [CrossRef]
- Özbal, B.; Çelekli, A.; Gün, D.; Bozkurt, H. Effect of Arthrospira platensis Incorporation on Nutritional and Sensory Attributes of White Chocolate. International Journal of Gastronomy and Food Science 2022, 28, 1878–450. [Google Scholar] [CrossRef]
- Kim, E.H.J.; Corrigan, V.K.; Wilson, A.J.; Waters, I.R.; Hedderley, D.I.; Morgenstern, M.P. Fundamental Fracture Properties Associated with Sensory Hardness of Brittle Solid Foods. Journal of Texture Studies 2012, 43, 49–62. [Google Scholar] [CrossRef]
- Ha, E.; Zemel, M.B. Functional Properties of Whey, Whey Components, and Essential Amino Acids: Mechanisms Underlying Health Benefits for Active People (Review). Journal of Nutritional Biochemistry 2003, 14, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Lupatini Menegotto, A.L.; Souza, L.E.S. de; Colla, L.M.; Costa, J.A.V.; Sehn, E.; Bittencourt, P.R.S.; Moraes Flores, É.L. de; Canan, C.; Colla, E. Investigation of Techno-Functional and Physicochemical Properties of Spirulina platensis Protein Concentrate for Food Enrichment. Lwt 2019, 114, 108267. [Google Scholar] [CrossRef]
- Grinstead, G.S.; Tokach, M.D.; Dritz, S.S.; Goodband, R.D.; Nelssen, J.L. Effects of Spirulina Platensis on Growth Performance of Weanling Pigs. Animal Feed Science and Technology 2000, 83, 237–247. [Google Scholar] [CrossRef]
- Kumar, A.; Elavarasan, K.; Hanjabam, M.D.; Binsi, P.K.; Mohan, C.O.; Zynudheen, A.A.; Kumar K, A. Marine Collagen Peptide as a Fortificant for Biscuit: Effects on Biscuit Attributes. Lwt 2019, 109, 450–456. [Google Scholar] [CrossRef]
- Morsy, O.M.; Sharoba, A.M.; El-Desouky, A.I.; Bahlol, H.E.M.; Abd El Mawla, E.M. Production and Evaluation of Some Extruded Food Products Using Spirulina Algae. Annals of Agricultural Science, Moshtohor 2014, 52, 495–510. [Google Scholar] [CrossRef]
- Niccolai, A.; Chini Zittelli, G.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae of Interest as Food Source: Biochemical Composition and Digestibility. Algal Research 2019, 42, 101617. [Google Scholar] [CrossRef]
- Naulbunrang, J.; Kawngdang, A.; Manon, S.; Thepjaikat, T.; Technol, T. Utilization of Pumpkin Powder in Bakery Products. 2006, 71–79.
| Trials |
Arthrospira platensis (g/kg) |
Flour (g/kg) |
Water (g/kg) |
Raw Materials (g/kg) |
| 1 | 0 | 670 | 180 | 125 |
| 2 | 0 | 625 | 260 | 115 |
| 3 | 25 | 625 | 220 | 130 |
| 4 | 25 | 670 | 260 | 45 |
| 5 | 0 | 580 | 220 | 200 |
| 6 | 25 | 625 | 220 | 130 |
| 7 | 50 | 670 | 220 | 60 |
| 8 | 25 | 625 | 220 | 130 |
| 9 | 0 | 670 | 220 | 110 |
| 10 | 0 | 625 | 180 | 155 |
| 11 | 50 | 580 | 220 | 150 |
| 12 | 25 | 625 | 220 | 130 |
| 13 | 25 | 580 | 180 | 215 |
| 14 | 50 | 625 | 260 | 165 |
| 15 | 50 | 625 | 180 | 145 |
| 16 | 25 | 580 | 260 | 135 |
| 17 | 25 | 625 | 220 | 130 |
| Trials | Hunter Color | Moisture % |
aw | Hardness (N) | ||
| L* | a* | b* | ||||
| 1 | 48.58 | -1.40 | 26.37 | 1.71 | 0.03 | 463 |
| 2 | 80.84 | 3.09 | 25.84 | 3.57 | 0.08 | 395 |
| 3 | 47.95 | -3.56 | 26.03 | 2.32 | 1.80 | 425 |
| 4 | 47.59 | -1.98 | 25.38 | 2.30 | 1.70 | 421 |
| 5 | 79.72 | 1.97 | 24.13 | 3.50 | 1.96 | 306 |
| 6 | 47.28 | -0.18 | 23.20 | 4.56 | 2.60 | 421 |
| 7 | 39.37 | -0.88 | 20.79 | 1.80 | 2.30 | 516 |
| 8 | 46.47 | -2.39 | 25.67 | 2.20 | 1.60 | 357 |
| 9 | 83.35 | 1.04 | 20.92 | 3.67 | 1.96 | 320 |
| 10 | 83.76 | 0.93 | 20.84 | 4.60 | 2.80 | 356 |
| 11 | 35.62 | 0.69 | 20.16 | 2.80 | 1.96 | 404 |
| 12 | 47.18 | -2.23 | 26.20 | 2.60 | 1.25 | 335 |
| 13 | 48.25 | -2.68 | 26.18 | 0.23 | 0.12 | 291 |
| 14 | 34.60 | -3.78 | 20.53 | 1.60 | 0.20 | 577 |
| 15 | 33.60 | -0.80 | 20.91 | 2.35 | 0.38 | 244 |
| 16 | 43.19 | 0.48 | 27.05 | 1.30 | 0.57 | 148 |
| 17 | 43.65 | -2.10 | 25.60 | 1.87 | 0.63 | 287 |
| Constituents | Control | Optimum recipe | Difference % |
| Protein (g/100 g) | 13.34 | 16.60 | 24.7 |
| Carbohydrate | 73.5 | 71.3 | -3.0 |
| Fatty Acid | 6.0 | 7.0 | 16.7 |
| Total amino acid (mg/100g) | 11052 | 15949 | 53.83 |
| L-Isoleucine | 418 | 758 | 81 |
| L-Leucine | 888 | 1272 | 43.2 |
| L-Methionine | 178 | 298 | 67 |
| L-Threonine | 223 | 467 | 109 |
| L-Phenylalanine | 670 | 891 | 33 |
| Tryptophan | 90 | 187 | 107 |
| L-Valine | 473 | 792 | 67 |
| L-Alanine | 318 | 694 | 118 |
| L-Arginine | 250 | 633 | 153 |
| L-Aspartic Acid | 353 | 903 | 156 |
| L-Phenylalanine | 670 | 891 | 33 |
| L-Glutamic Acid | 3618 | 4268 | 18 |
| L-Histidine | 183 | 319 | 74 |
| L-Methionine | 178 | 298 | 67 |
| L-Proline | 1.755 | 1973 | 12 |
| L-Serine | 415 | 623 | 50 |
| L-Tyrosine | 355 | 529 | 49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





