Submitted:
18 July 2024
Posted:
19 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Yield Performance
2.2. Cutting time
2.3. Morphological characteristics
3. Discussion
3.1. Performance yield
3.4. Cutting time
3.3. Morphological differentiation
4. Materials and Methods
4.1. Place and duration of study
4.2. Experimental units
4.3. Experimental material
4.4. Sampling
4.5. Evaluation characteristics
- − Cycle 1: In the 2017 - 2018 season.
- − Cycle 2: During the 2018 - 2019 campaign.
4.5. Statistical analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loka, D.; Harper, J.; Humphreys, M.; Gasior, D.; Wootton-Beard, P.; Gwynn-Jones, D.; Scullion, J.; Doonan, J.; Kingston-Smith, A.; Dodd, R.; et al. Impacts of Abiotic Stresses on the Physiology and Metabolism of Cool-Season Grasses: A Review. Food and Energy Security 2019, 8, e00152. [Google Scholar] [CrossRef]
- Finn, J.A.; Suter, M.; Haughey, E.; Hofer, D.; Lüscher, A. Greater Gains in Annual Yields from Increased Plant Diversity than Losses from Experimental Drought in Two Temperate Grasslands. Agriculture, Ecosystems & Environment 2018, 258, 149–153. [Google Scholar] [CrossRef]
- Mroncz, A.; Isselstein, J.; Komainda, M.; Leuschner, C. Microtopography Causes Small-Scale Variation in Harvest and Forage Quality of High-Yielding Silage Grassland in Northern Germany. Grassland Research 2024, 3, 33–42. [Google Scholar] [CrossRef]
- Stewart, A.V.; Goldson, S.L.; Marris, J.W.; Rubenstein, J.M.; Rolston, M.P. Analytical Purity of Old New Zealand Forage Seed Samples and Detection of Fungal and Insect Contaminants Including Ryegrass Endophyte and Argentine Stem Weevil. New Zealand Journal of Agricultural Research 2023, 66, 270–283. [Google Scholar] [CrossRef]
- Victoria Arellano, A.D.; Silva, G.M. da; Guatimosim, E.; Dorneles, K. da R.; Moreira, L.G.; Dallagnol, L.J. Las Semillas Recubiertas Con Trichoderma Atroviride y El Suelo Modificado Con Silicio Mejoran La Resistencia de Lolium Multiflorum Frente a Pyricularia Oryzae. Biological Control 2021, 154, 104499. [Google Scholar] [CrossRef]
- Ambus, J.V.; Awe, G.O.; Faccio Carvalho, P.C. de; Reichert, J.M. Integrated Crop-Livestock Systems in Lowlands with Rice Cultivation Improve Root Environment and Maintain Soil Structure and Functioning. Soil and Tillage Research 2023, 227, 105592. [Google Scholar] [CrossRef]
- Augustyniak, A.; Perlikowski, D.; Rapacz, M.; Kościelniak, J.; Kosmala, A. Conocimiento Del Proteoma Celular de Las Formas de Introgresión de Lolium Multiflorum/Festuca Arundinacea Para Descifrar Los Mecanismos Cruciales de La Aclimatación al Frío En Gramíneas Forrajeras. Plant Science 2018, 272, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Vallejos-Cacho, R.; Vallejos-Fernandez, L.A.; Alvarez-García, W.Y.; Tapia-Acosta, E.A.; Saldanha-Odriozola, S.; Quilcate-Pairazaman, C.E. Sustainability of Lolium Multiflorum L. “Ecotype Cajamarquino”, Associated with Trifolium Repens L., at Three Harvest Times in the Northern Highlands of Peru 2024.
- Cepeda Cepeda, E.B. Efecto del cultivo mixto de bacterias y levaduras sobre las características físicoquímicas y biomasa de Rye grass (Lolium multiflorum) en Andisoles, parroquia Columbe cantón Colta. 2022.
- Vanek, S.J.; Meza, K.; Ccanto, R.; Olivera, E.; Scurrah, M.; Fonte, S.J. Participatory Design of Improved Forage/Fallow Options across Soil Gradients with Farmers of the Central Peruvian Andes. Agriculture, Ecosystems & Environment 2020, 300, 106933. [Google Scholar] [CrossRef]
- Prerostova, S.; Zupkova, B.; Petrik, I.; Simura, J.; Nasinec, I.; Kopecky, D.; Knirsch, V.; Gaudinova, A.; Novak, O.; Vankova, R. Respuestas Hormonales Asociadas Con La Aclimatación al Estrés Por Congelación En Lolium Perenne. Environmental and Experimental Botany 2021, 182, 104295. [Google Scholar] [CrossRef]
- Perlikowski, D.; Augustyniak, A.; Masajada, K.; Skirycz, A.; Soja, A.M.; Michaelis, Ä.; Wolter, G.; Kosmala, A. Alteraciones Estructurales y Metabólicas En Sistemas Radiculares Bajo Condiciones Hídricas Limitadas En Gramíneas Forrajeras Del Complejo Lolium-Festuca. Plant Science 2019, 283, 211–223. [Google Scholar] [CrossRef]
- Wu, C.; Song, M.; Zhang, T.; Zhou, C.; Liu, W.; Jin, T.; Zhao, N. La Mutación En El Sitio Diana y El Citocromo P450 Confieren Resistencia a Múltiples Herbicidas En Raigrás Italiano (Lolium Multiflorum Lam.) de China. Crop Protection 2022, 161, 106068. [Google Scholar] [CrossRef]
- Kim, M.; Sung, K. Comparison of Causality of Temperature and Precipitation on Italian Ryegrass (Lolium Multiflorum Lam.) Yield between Cultivation Fields via Multi-Group Structural Equation Model Analysis in the Republic of Korea. Agriculture 2019, 9, 254. [Google Scholar] [CrossRef]
- Navarro-Zamora, L.A.; Villalobos-Villalobos, L.A. Composición morfológica del forraje ryegrass anual (Lolium multiflorum) cv. jumbo en respuesta a tres fórmulas nitrogenadas. Nutrición Animal Tropical 2021, 15, 99–122. [Google Scholar] [CrossRef]
- Maleki, K.; Soltani, E.; Seal, C.E.; Colville, L.; Pritchard, H.W.; Lamichhane, J.R. The Seed Germination Spectrum of 486 Plant Species: A Global Meta-Regression and Phylogenetic Pattern in Relation to Temperature and Water Potential. Agricultural and Forest Meteorology 2024, 346, 109865. [Google Scholar] [CrossRef]
- Schaeffer, A.H.; Silveira, D.C.; Schaeffer, O.A.; Lângaro, N.C.; Vargas, L. Seed Germination Behavior of Glyphosate-Resistant and Susceptible Italian Ryegrass (Lolium Multiflorum Lam.). Weed Biology and Management 2021, 21, 3–10. [Google Scholar] [CrossRef]
- Faisca, L.D.; Peres, M.T.P.; Fernandes, S.R.; Bonnet, O.J.F.; Batista, R.; Deiss, L.; Monteiro, A.L.G. A New Insight about the Selection and Intake of Forage by Ewes and Lambs in Different Production Systems on Pasture. Small Ruminant Research 2023, 221, 106949. [Google Scholar] [CrossRef]
- Yüce, İ.; Tatar, M.; Kökten, K.; Sarikaya, M.F.; Çi̇lesi̇z, Y.; Karaköy, T. Determination of Herbage Yield and Quality of Some Italian Ryegrass Varieties in Sivas Ecological Conditions. ISPEC Journal of Agricultural Sciences 2024, 8, 36–44. [Google Scholar] [CrossRef]
- Nunes, P.A. de A.; Bredemeier, C.; Bremm, C.; Caetano, L.A.M.; de Almeida, G.M.; de Souza Filho, W.; Anghinoni, I.; Carvalho, P.C. de F. Grazing Intensity Determines Pasture Spatial Heterogeneity and Productivity in an Integrated Crop-Livestock System. Grassland Science 2019, 65, 49–59. [Google Scholar] [CrossRef]
- Brooker, R.; Brown, L.K.; George, T.S.; Pakeman, R.J.; Palmer, S.; Ramsay, L.; Schöb, C.; Schurch, N.; Wilkinson, M.J. Active and Adaptive Plasticity in a Changing Climate. Trends in Plant Science 2022, 27, 717–728. [Google Scholar] [CrossRef]
- Abraha, A.B.; Truter, W.F.; Annandale, J.G.; Fessehazion, M.K. Forage Yield and Quality Response of Annual Ryegrass (Lolium Multiflorum) to Different Water and Nitrogen Levels. African Journal of Range & Forage Science 2015, 32, 125–131. [Google Scholar] [CrossRef]
- Bridges, K.M.; Fultz, L.M.; Alison, M.W.; Han, K.-J.; Macoon, B.; Pitman, W.D. Quantifying Soil Health in a Topographically Diverse Warm-Season Perennial Pasture over-Seeded with a Mix of Cool-Season Annuals. Agriculture, Ecosystems & Environment 2019, 282, 58–68. [Google Scholar] [CrossRef]
- Cui, E.; Cui, B.; Fan, X.; Li, S.; Gao, F. Los Cultivos Intercalados de Raigrás (Lolium Multiflorum L.) y Mostaza de La India (Brassica Juncea L.) Pueden Mejorar La Fitorremediación de Los Antibióticos y Los Genes de Resistencia a Los Antibióticos, Pero No de Los Metales Pesados. Science of The Total Environment 2021, 784, 147093. [Google Scholar] [CrossRef] [PubMed]
- Joniec, J.; Oleszczuk, P.; Jezierska-Tys, S.; Kwiatkowska, E. Effect of Reclamation Treatments on Microbial Activity and Phytotoxicity of Soil Degraded by the Sulphur Mining Industry. Environmental Pollution 2019, 252, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Y.; Wang, J.; Condron, L.M.; Guo, B.; Liu, J.; Qiu, G.; Li, H. Impact of Ryegrass Cover Crop Inclusion on Soil Phosphorus and pqqC- and phoD-Harboring Bacterial Communities. Soil and Tillage Research 2023, 234, 105823. [Google Scholar] [CrossRef]
- Han, K. j.; Smith, D.J.; Pitman, W. d. Potential of Cool-Season Species as Cover Crops and Forage in the Southeastern United States. Crop, Forage & Turfgrass Management 2018, 4, 170038. [Google Scholar] [CrossRef]
- Pandey, V.C.; Gajić, G.; Sharma, P.; Roy, M. 4 - Soil and Phytomanagement for Adaptive Phytoremediation Practices. In Adaptive Phytoremediation Practices; Pandey, V.C., Gajić, G., Sharma, P., Roy, M., Eds.; Elsevier, 2022; pp. 135–179 ISBN 978-0-12-823831-8.
- Shi, G.; Hu, J.; Zhang, S.; Ni, G.; Shi, W.; Hu, C.; Zhao, X. La Aplicación de Se Exógeno Mejoró La Eficiencia de Remediación de Lolium Multiflorum Lam Cultivado En Suelos Cocontaminados Con Nonilfenol-Cd. Journal of Environmental Chemical Engineering 2022, 10, 108962. [Google Scholar] [CrossRef]
- Gao, R.; Duan, Y.; Zhang, J.; Ren, Y.; Li, H.; Liu, X.; Zhao, P.; Jing, Y. Effects of Long-Term Application of Organic Manure and Chemical Fertilizer on Soil Properties and Microbial Communities in the Agro-Pastoral Ecotone of North China. Front. Environ. Sci. 2022, 10. [Google Scholar] [CrossRef]
- Liu, M.; Che, Y.; Wang, L.; Zhao, Z.; Zhang, Y.; Wei, L.; Xiao, Y. Rice Straw Biochar and Phosphorus Inputs Have More Positive Effects on the Yield and Nutrient Uptake of Lolium Multiflorum than Arbuscular Mycorrhizal Fungi in Acidic Cd-Contaminated Soils. Chemosphere 2019, 235, 32–39. [Google Scholar] [CrossRef]
- Oliva, M.; Valqui Valqui, L.; Meléndez Mori, J.; Milla, M.; Leiva, S.; Collazos, R.; Maicelo, J. Influence of Arboreal Native Species on Silvopastoral Systems on the Yield and Nutritional Value of Lolium Multiflorum and Trifolium Repens. Scientia Agropecuaria 2018, 9, 579–583. [Google Scholar] [CrossRef]
- Paye, W.S.; Szogi, A.A.; Shumaker, P.D.; Billman, E.D. Annual Ryegrass (Lolium Multiflorum Lam.) Growth Response to Nitrogen in a Sandy Soil Amended with Acidified Manure and Municipal Sludge after “Quick Wash” Treatment. Agronomy 2023, 13, 2655. [Google Scholar] [CrossRef]
- Hu, J.; Hassi, U.; Gebremikael, M.T.; Dumack, K.; De Swaef, T.; Wesemael, W.; Sleutel, S.; De Neve, S. Root Traits Explain Multitrophic Interactions of Belowground Microfauna on Soil Nitrogen Mineralization and Plant Productivity. Soil Biology and Biochemistry 2023, 184, 109093. [Google Scholar] [CrossRef]
- Montenegro, L.F.; Descalzo, Adriana. M.; Rizzo, S.; Rossetti, L.; García, P.T.; Pérez, C.D. Mejora Del Estado Antioxidante, Las Vitaminas Liposolubles, La Composición de Ácidos Grasos y La Estabilidad Lipídica En La Carne de Carpa Herbívora (Ctenopharyngodon Idella Val) Alimentada Con Raigrás Fresco (Lolium Multiflorum Lam). Aquaculture 2022, 553, 738067. [Google Scholar] [CrossRef]
- Fernández-Habas, J.; Real, D.; Vanwalleghem, T.; Fernández-Rebollo, P. LANZA® Tedera Is Strongly Suppressed by Competition from Lolium Multiflorum and Is Best Adapted to Light-Textured Soils. Agronomy 2023, 13, 965. [Google Scholar] [CrossRef]
- Rupngam, T.; Messiga, A.J.; Karam, A. Phosphorus Mobility in Heavily Manured and Waterlogged Soil Cultivated with Ryegrass (Lolium Multiflorum). Agronomy 2023, 13, 2168. [Google Scholar] [CrossRef]
- Brede, A.D.; Brede, J.L. Establishment Clipping of Tall Fescue and Companion Annual Ryegrass. Agronomy Journal 1988, 80, 27–30. [Google Scholar] [CrossRef]
- Ollerenshaw, J.H.; Incoll, L.D. Leaf Photosynthesis in Pure Swards of Two Grasses (Lolium Perenne and Lolium Multiflorum) Subjected to Contrasting Intensities of Defoliation. Annals of Applied Biology 1979, 92, 133–142. [Google Scholar] [CrossRef]
- Mavlyutov, Y.; Kostenko, S.; Shamustakimova, A.; Klimenko, I. Análisis de Variabilidad Genética de Cultivares Rusos de Raigrás (Lolium) a Partir de Marcadores SCoT. Journal of Genetic Engineering and Biotechnology 2022, 20, 163. [Google Scholar] [CrossRef]
- Beckie, H.J.; Jasieniuk, M. Chapter 12 - Lolium Rigidum and Lolium Multiflorum. In Biology and Management of Problematic Crop Weed Species; Chauhan, B.S., Ed.; Academic Press, 2021; pp. 261–283 ISBN 978-0-12-822917-0.
- SENAMHI - Pronostico Meteorologico. Available online: https://www.senamhi.gob.pe/?p=pronostico-meteorologico (accessed on 6 June 2024).
- UPOV Unión Internacional Para La Protección de Las Obtenciones Vegetales (UPOV). Available online: https://www.upov.int/portal/index.html.es (accessed on 27 June 2024).
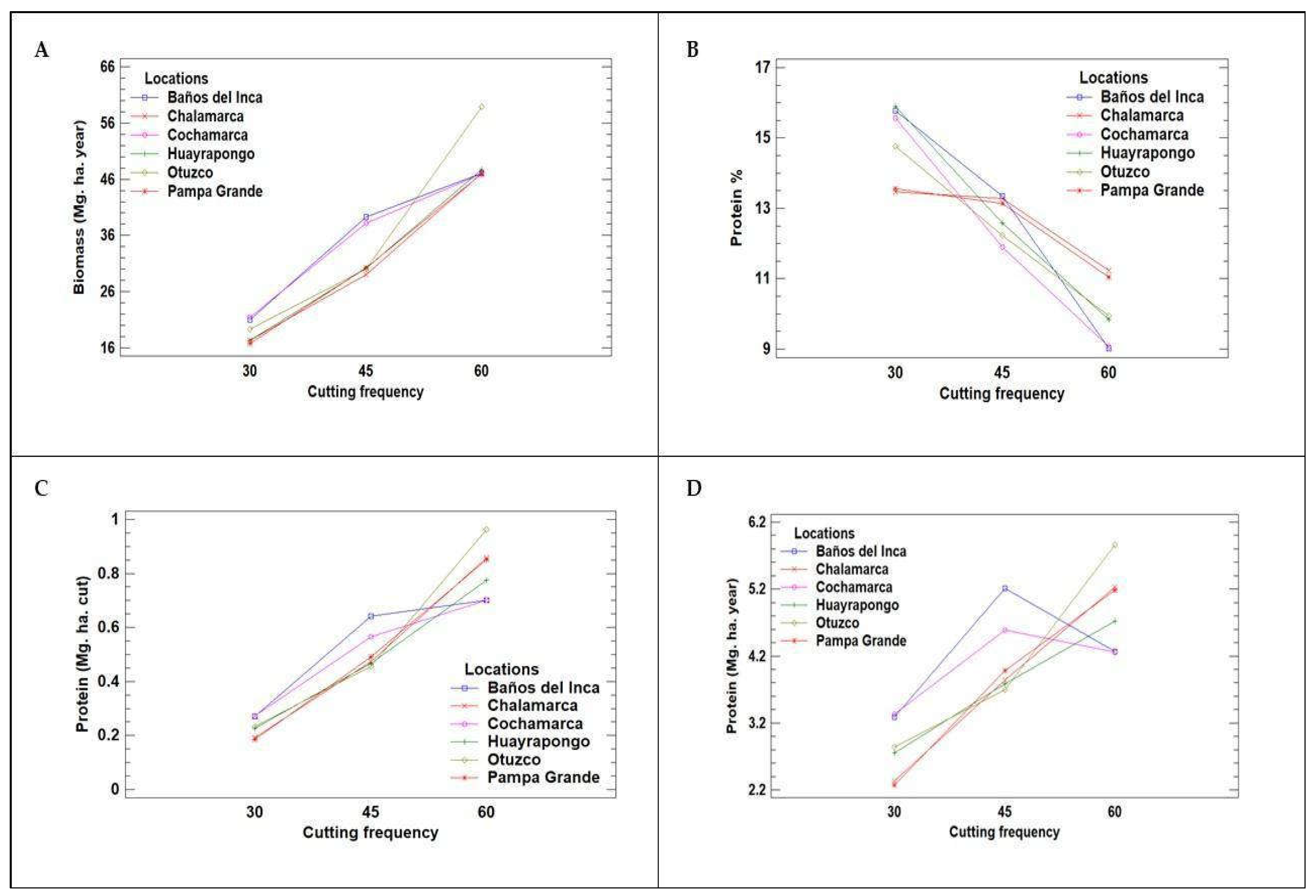
| Factors | Green forage (Mg. ha) | Biomass (Mg. ha) | Seeds (kg. ha) | Protein % |
|---|---|---|---|---|
| Genotype | ||||
| LM-43 | 20.15 b | 3.64 b | 189.95 b | 11.95 b |
| LM-58 | 20.97 a | 4.49 a | 259.23 a | 13.48 a |
| Standar error | 0.21 | 0.06 | 13.56 | 0.29 |
| p-value | 0.0104 | <0.001 | 0.0047 | 0.0037 |
| Locations | ||||
| Baños del Inca | 22.19 a | 4.3 a | 251.98 | 13.31 |
| Chalamarca | 18.83 c | 3.95 b | 218.24 | 12.50 |
| Cochamarca | 21.26 ab | 3.77 b | 207.95 | 13.04 |
| Huayrapongo | 22.10 a | 4.35 a | 217.40 | 12.18 |
| Otuzco | 20.71 b | 4.06 ab | 254.75 | 13.34 |
| Pampa Grande | 18.26 c | 3.95 b | 197.20 | 11.94 |
| Standar error | 0.36 | 0.11 | 23.49 | 0.48 |
| p-value | <0.001 | 0.0061 | 0.8743 | 0.7580 |
| Factors | Biomass | Protein | |||
|---|---|---|---|---|---|
| Mg. ha. cut | Mg. ha. year | % | Mg. ha. cut | Mg. ha. year | |
| Cutting time | |||||
| 30 days | 1.55 c | 18.87 c | 14.84 a | 0.23 c | 2.80 b |
| 45 days | 4.05 b | 32.83 b | 12.74 b | 0.52 b | 4.19 a |
| 60 days | 8.07 a | 49.10 a | 10.03 c | 0.81 a | 4.91 a |
| SE | 0.24 | 1.60 | 0.44 | 0.036 | 0.26 |
| p-value | |||||
| Cuts (C) | 0.0000 | 0.0000 | 0.0001 | 0.000 | 0.0006 |
| Locations (L) | 0.4008 | 0.5376 | 0.9755 | 0.9599 | 0.8896 |
| C x L | 0.6303 | 0.6192 | 0.0036 | 0.2076 | 0.1817 |
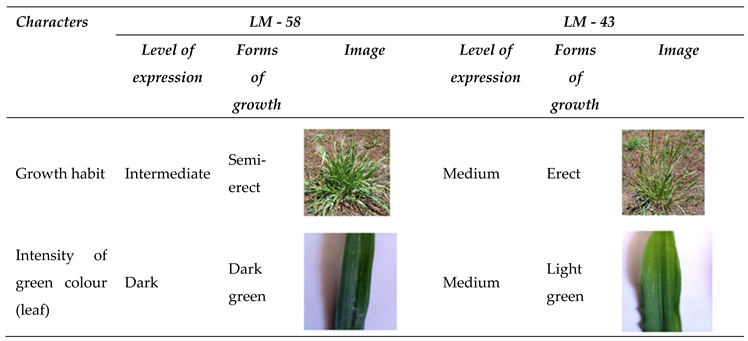
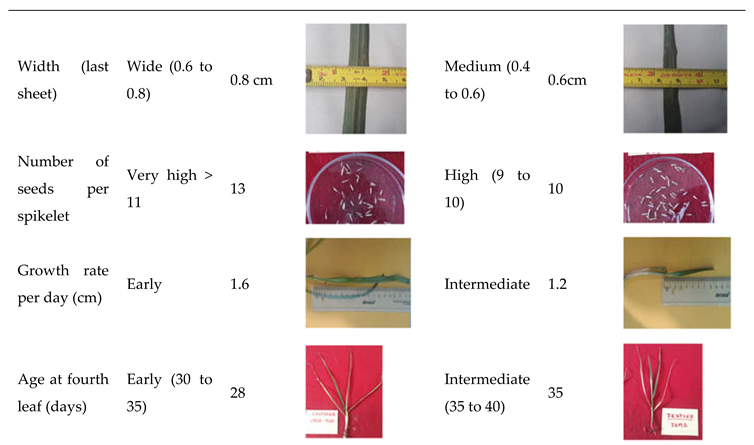
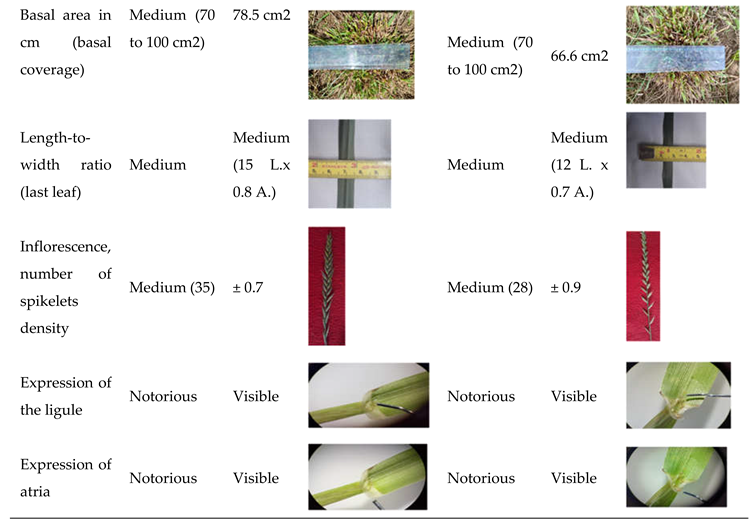
| Character | Observation period | States | Score |
|---|---|---|---|
| Growth habit | Plant | Semi-erect | 3 |
| Leaf length in vegetative state (33 to 38) | Leaflet | Long | 7 |
| Leaf width in vegetative state (0.8-1.0) | Limbo | Media | 5 |
| The intensity of the green colour | Leaflet | Dark | 7 |
| Width after vernalization (basal coverage) | Plant | Medium | 5 |
| Height (after vernalization) | Plant | High (60 - 70) | 7 |
| Tendency to form inflorescences (without vernalization) | Plant | Medium | 5 |
| Flag leaf length | Leaflet | Medium | 5 |
| Width | Leaflet | Wide (0.6 to 0.8) | 7 |
| Length/width ratio | Leaflet | Media | 5 |
| Length of most extended stem, including inflorescences (when fully developed) | Stem | Long (45 to 55) | 7 |
| Length including inflorescences (when fully developed) | Panicle | Long (25 to 30) | 7 |
| Number of spikelets | Panicle | Very high (>11) | 9 |
| Number of spikelets density | Panicle | Medium | 5 |
| External length of basal spikelet | Glumes | Long (0.8 to 1.0) | 7 |
| Length of basal spikelet, excluding the ridge | Glumes | Long (1.5 to 2.0) | 7 |
| Growth rate (cm/day) | Plant | High (1.4 to 1.8) | 3 |
| Form | Leaflet | Lanceolate medium | 3 |
| Length at 30 days | Plant | Long (33 to 39) | 7 |
| Presence of atria | Leaflet | Media | 5 |
| Expression of the ligule | Limbo | Regularly noted | 7 |
| Age at fourth leaf (cm) | Leaflet | High (35 to 40) | 5 |
| Number of seeds per spikelet | Grain | High (12 to 14) | 7 |
| Short slogan | Grain | Dark cream | 5 |
| Length of the locker | Grain | Cut | |
| Time of beginning of flowering (green fodder) | Flowering | High (60 to 70 days) | 5 |
| Growing season (grain production) | Harvest | High (100 to 110 days) | 7 |
| Presence of edges | Grain | Present | 3 |
| Campaign | Characters | |||||
|---|---|---|---|---|---|---|
| Colour of the slogan | Grain presence of edge | |||||
| Level of expression | Off-type plants | Maximum number | Level of expression | Off-type plants | Maximum number | |
| First | Dark cream | 0 | 3 | Present | 0 | 3 |
| Second | Dark cream | 0 | 3 | Present | 0 | 3 |
| Campaign | Characters | |||||||
|---|---|---|---|---|---|---|---|---|
| Vegetative cycle | Start of flowering | |||||||
| Level of expression |
Average (days) |
DS. | CV. | Level of expression |
Average (days) |
DS. | CV. | |
| First | Semi-early | 105.0 | 2.94 | 2.80% | Precoz | 40.2 | 1.17 | 2.91% |
| Second | Semi-early | 105.8 | 2.87 | 2.73% | Precoz | 39.7 | 1.03 | 2.59% |
| Locations |
Agricultural campaign |
Altitude | District | Geographical coordinates | Chemical composition of the soil | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
P (ppm) |
K (ppm) |
pH |
OM (%) |
Al (mEq/100g) |
||||||
| Otuzco | 2018-2019 | 2680 | Baños del Inca | -7.131402 | -78.469430 | 16.22 | 225 | 4.5 | 1.54 | 0.10 |
| Cochamarca | 2016-2017 | 2820 | Gregorio Pita | -7.181967 | -78.467969 | 8.11 | 195 | 4.3 | 2.13 | 0.90 |
| Huayrapongo | 2015-2016 | 2650 | Baños del Inca | -7.181967 | -78.467970 | 33.70 | 271 | 6.1 | 3.82 | 0.00 |
| Pampa Grande | 2014-2015 | 2680 | Cajabamba | -7.611059 | -78.070150 | 6.20 | 170 | 3.8 | 3.55 | 0.50 |
| Chalamarca | 2014-2015 | 2760 | Chota | -7.279996 | -78.218141 | 18.22 | 186 | 4.9 | 4.12 | 0.70 |
| Baños del Inca | 2014-2015 | 2650 | Cajamarca | -7.159426 | -78.461260 | 10.90 | 330 | 7.0 | 2.25 | 0.00 |
| Period (years) | Activity | Description |
|---|---|---|
| 1995 | Collection and installation of experimental material. | Sowing (germination beds). |
| 1996 – 1998 | Characterisationof genotypes | Selection and sowing of 680 seedlings (genotypes). |
| 1999 – 2000 | Selection multiplication | Characterisation of genotypes (growth habit, anthesis and pests and diseases). |
| 2001 – 2003 | Multiplication selection | Characterisation and selection of the best genotypes. |
| 2004 – 2006 | Research | Selection and evaluation of elite genotypes in Forage green, Dry matter, and seed. |
| 2006 – 2008 | Research | Polycrosses between the 10 elite genotypes, in experimental field. To fix superior characters. |
| 2009 - 2011 | Field testing by growers | Selection and evaluation of 10 superior genotypes in forage production, nutritive value and tolerance to pests and diseases. |
| 2011 – 2016 | Adaptation and efficiency trials in growers' fields. | First campaign - Producers' field adaptation and efficiency trials. |
| 2016 – 2019 | Adaptation and efficiency trials in growers' fields. | Second campaign - Adaptation and efficiency trials in growers' fields. |
| Parts | Features |
|---|---|
| Plant | Vegetative growth habit (without vernalization); Tendency to form inflorescences (without vernalization); Width after vernalization (basal area in cm) Height after vernalization Length of most extended stem, including inflorescences (when fully developed) |
| Leaflet | Leaf length (in a vegetative state); Leaf width (in a vegetative state); The intensity of the green colour Flag leaf length (last leaf); Width (previous sheet); Length/width ratio (previous leaf); Leaf shape; Presence of atria; Expression of the ligule; Motto colour. |
| Inflorescence | Length, including inflorescences (when fully developed); Number of spikelets; Number of spikelets density; Length of outer glume of basal spikelet; Length of basal spikelet, excluding the ridge; Number of seeds per spikelet; Grain presence of edge; Time of beginning of flowering. |
| Other | Growth rate per day; Plant length at 30 days; Growing season for grain-seed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





