Introduction
The study of biodiversity and ecological dynamics holds paramount importance in understanding and preserving our natural world. In this light, documenting species richness and species abundance becomes a critical tool for ecological research and conservation efforts. With the advent of technology, camera traps have emerged as an invaluable asset in this realm, offering insights into the lives of elusive and nocturnal wildlife that would otherwise remain hidden from our eyes [
1]. Species diversity, distribution, and population abundance are strongly influenced by changes in habitat and its use over time [
2,
3]. Effective monitoring of species planning and implementation of conservation strategies, as well as understanding the demographics of wildlife are critical [
4]. The Jhalana reserve forest represents a unique ecosystem that has yet to be thoroughly explored and understood. The significance of this study lies not only in its novelty but also in its potential to uncover unprecedented data regarding species distribution, human intrusion patterns, and the prey base for apex predators like leopards within this relatively new reserve. Through a comprehensive two-year survey employing camera traps, this research aims to illuminate the intricate tapestry of life that thrives in the shadows of Jhalana, offering critical insights that could guide future conservation strategies and enhance our understanding of species dynamics in newly established protected areas.
The deployment of camera traps across various ecosystems has revolutionized the field of wildlife research and conservation, offering unparalleled insights into the behaviors, distributions, and populations of myriad species, often with a focus on elusive and nocturnal animals. This methodological advancement is well-documented in the literature, with studies highlighting its efficacy in recording species occurrences— a cornerstone of biodiversity surveys essential for determining species distribution and informing the International Union for Conservation of Nature (IUCN) status [
5]. Furthermore, photographic capture-recapture techniques facilitated by camera traps have become a gold standard in estimating the abundance and density of several secretive large carnivores, including leopards [
6,
7,
8], striped hyenas (
Hyaena hyaena)[
9,
10,
11,
12], tigers (
Panthera tigris)[
8,
13,
14], snow leopards (
P. uncia)[
15], and jaguars (
P. onca)[
16]. Beyond their application in the study of mammalian fauna [
17,
18,
19], camera traps have also proven effective in documenting avifauna within various habitats, including forests and grasslands [
20,
21].
Despite the widespread application and success of camera traps in diverse environments and for various species, a noticeable gap persists in the baseline data available for certain areas, notably the Jhalana reserve forest (JRF). Established in 2017, Jhalana has remained relatively unexplored from a scientific standpoint, lacking comprehensive data that could inform conservation efforts and enhance our understanding of its ecological dynamics [
22]. This absence of baseline data represents a critical knowledge gap, underscoring the necessity of this study to employ camera traps within the reserve. By doing so, we aim to not only contribute to filling this gap but also to provide a foundation upon which future research and conservation strategies can be built, tailored to the unique environmental and biological context of the Jhalana reserve forest.
JRF is located in the heart of of Jaipur city in north-western India, and is a northern tropical dry deciduous forest type. Apart from a limited number of species-specific studies on spiders [
23], gray langurs (
Presbitys entellus)[
24], vegetation [
25], forest ecosystem services [
26], and our studies on the leopard population (
Panthera pardus fusca), [
22,
27,
28,
29,
30,
31,
32], no further studies have been conducted to document the species richness of JRF. Leopards have existed for centuries in this small island habitat, now surrounded by 3.9 million people in Jaipur’s urban areas. However, there is no systematic documentation of the diversity of birds and mammals and the associated abundance in the JRF. The paucity of data on the ecology and wildlife of the JRF prompted us to conduct a baseline study on local wildlife diversity.
After studying the geography and climatic conditions, we discovered that there are no perennial streams, wells, or other natural water sources in the JRF. The only water sources available are artificial waterholes created by the Rajasthan Forest Department and the local nature lovers, villagers, and tourists. Wildlife has been observed to be water dependent and concentrate at these artificial waterholes. We set up camera traps at all of these waterholes and in the outlying areas. It is known that the frequency of revisiting specific camera stations is a consequence of site fidelity [
33].
At the heart of our investigation into the ecological dynamics of the Jhalana Reserve Forest (JRF) lies a series of focused research questions aimed at elucidating the biodiversity and anthropogenic impacts within this relatively unexplored sanctuary. Our primary research objectives are twofold: first, to assess the diversity of animal species and their relative abundance within the reserve; and second, to investigate the extent and nature of human activities impacting this ecosystem. Complementing these objectives is an evaluation of the efficacy of camera trap methodology in capturing comprehensive photographic records of the species residing in JRF, thus providing a robust framework for generating essential baseline data.
Our hypothesis posits that the camera trap method serves as an effective tool not only for cataloguing animal diversity and their relative abundance but also for recording human intrusion in reserve forests situated within human-dominated landscapes. We anticipate that the insights gleaned from our study will furnish the forest department with valuable data to inform and refine strategies aimed at rewilding, curtailing human intrusion, and bolstering the prey base for the leopards of Jhalana.
To address our research aims, we employed a rigorous methodological framework leveraging commercially manufactured Cuddeback camera traps (X-Change Color Model 1279), strategically deployed across the reserve from November 2017 to November 2019. This approach was tailored to the specific ecological characteristics and challenges of the semi-arid region of Northwest India where JRF is located. Notably, the reserve’s lack of perennial water sources, which concentrates species activity around artificial waterholes, guided our stratified deployment of cameras at these critical sites to maximize record yield.
Our approach is underscored by its novelty and comprehensiveness - representing the first exhaustive study of its kind within JRF. By covering all locations within the reserve and compiling data without disturbing the wildlife, our study stands as a pioneering effort in the conservation and understanding of this unique ecosystem. Furthermore, our methodological choices — informed by the proven efficacy of camera traps in various studies and tailored to the specific context of JRF — directly address the previously identified gap in baseline data.
In aligning our study with these objectives, we anticipate providing foundational insights that will significantly contribute to the conservation and management strategies for Jhalana Reserve Forest, drawing on our extensive, novel dataset to elucidate not only the patterns of wildlife diversity and distribution but also the intricate interplay between these natural inhabitants and human activities within the reserve.
Methods
Study Area
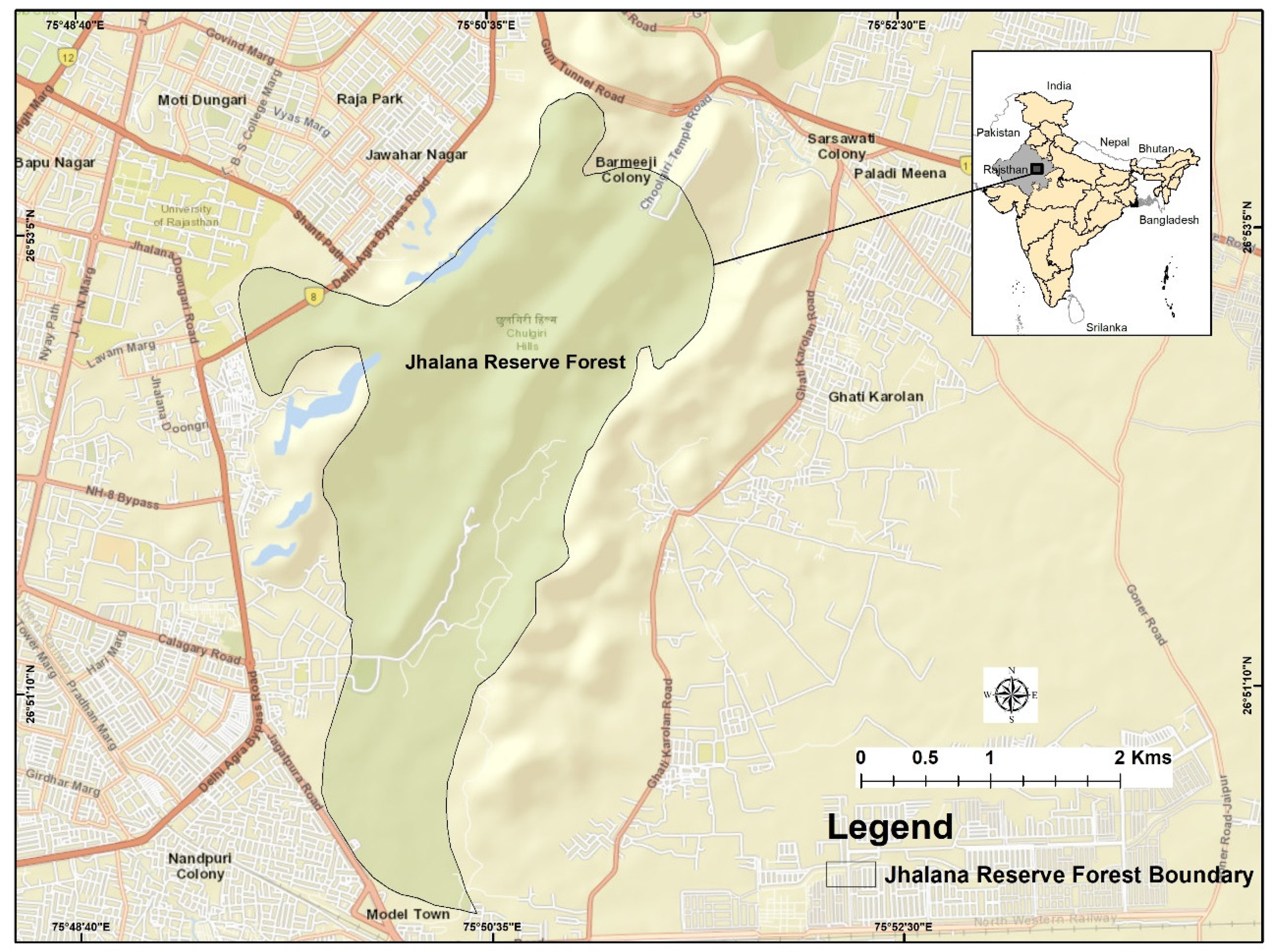
The study was conducted from November 2017 to November 2019 at Jhalana Reserve Forest (JRF), located at 26°51′N 75°49′E, 516 meters above sea level (ASL), in the southeast corner of Jaipur city, India (
Figure 1).
JRF was declared a Reserve Forest in 1961 under the provisions of the Rajasthan Forest Act, 1953, encompassing a total area of 29 km². In 2017, it was designated as a leopard reserve. During the 1980s, the main valley was planted with Acacia tortilis and A. senegal. Most ephemeral streams flow in a south-westerly direction, while higher elevations in the north form low, flat hills. Elevations in the plains range from 280 meters in the south to 530 meters in the northeast. JRF lacks defined buffer or core areas, and a 2-meter-high wall with a 3-meter-high fence separates the forest area from surrounding neighborhoods and villages.
JRF is characterized by a semi-arid tropical dry deciduous forest. Tourist access is permitted through jeep safaris on three designated routes. Due to the continuous interface between the forest and human habitats, and its recent designation as a forest reserve, human encroachments into the reserve are common, as are wildlife incursions into adjacent villages and urban areas.
Field Methods
We utilized commercially manufactured Cuddeback cameras (X-Change Color Model 1279) equipped with motion sensors. No bait or lure was used to attract wildlife at any site [
34]. The cameras were set with a minimum time delay of 15 seconds between captures. Each recording period lasted two weeks, and each camera was assigned a unique identification number. A total of 21 cameras were deployed, all of which recorded the date and time of each photo (
Figure 2). Overexposed images with distorted perspectives and lack of clarity were discarded (N=89, 0.55%) and not included in our analyses.
The cameras were deployed in an optimally stratified manner. Cameras within the reserve were left untouched during the capture cycles, while cameras on the periphery were removed during the day and reinstalled at night to prevent theft or damage. Cameras were installed at a height of 45 to 50 cm above the ground to cover access routes to the waterholes. In the outlying areas, camera traps were enclosed in boxes securely attached to iron bars to ensure safety. The trap locations were mapped using a GPS device.
We recorded the number of species (mean ± SD), their abundance, distribution, and human activities. Individual leopards were identified based on their facial markings to estimate their abundance [
22]. Of the 39 species recorded in the study area, 10 wildlife species were classified as gregarious (two or more individuals in one photo,
Table 1), and the remaining 29 were classified as solitary. Since photographs of gregarious species indicate higher species abundance estimates and wider distribution [
35], we recorded one data point per photograph when more than one individual of the same species was observed.
To understand the impact of designating the area as a reserve forest, the study was conducted over two consecutive years.
Statistical Analysis
Field data points collected from camera traps were used to compute the Abundance and Shannon Diversity Index at the geolocations of the camera traps. These values were then mapped using inverse distance weighted (IDW) interpolation. The geolocations varied annually based on changes in camera trap locations and waterholes.
Statistical comparisons were performed using R version 4.3.3. For this analysis, random points (N>100) were collected each year across the visible study area. Additional mapping processes were conducted using the QGIS 3.34 spatial environment.
Results
Over the study period from 2017 to 2019, a total of 16,328 photos were captured across 23,208 trap hours, averaging 0.7 (± 0.31 SD) images per hour. We recorded 39 species, including 18 bird species, 14 mammal species, 6 domestic animal species, and humans (
Table 1). The relative abundance (± SD) for each species was derived from the number of photographic records. There were notable differences in taxonomic groups between the two study years: birds accounted for 25.15% and 68.33% of observations, respectively; mammals for 42.53% and 27.75%; domestic animals were only documented in 2017-2018, comprising 4.28% of observations; and humans were documented 28.04% in 2017-2018 and 3.92% in 2018-2019.
The Indian peafowl (Pavo cristatus) was the most frequently observed species, accounting for 24.6% of sightings in 2017-2018 and 39.9% in 2018-2019, followed by nilgai (Boselaphus tragocamelus) at 27.3% and 15.7%, humans (Homo sapiens) at 28.0% and 3.9%, and Indian leopards (Panthera pardus fusca) at 3.8% and 4.8%.
Among the 14 mammal species, we observed seven carnivores, four artiodactyls, one rodent, one hedgehog, and one primate. Some species likely present in the reserve were not recorded due to low numbers or their absence in the areas where cameras were deployed. Eighteen bird species were photographed at waterholes, with jungle babblers (Turdoides striata), rufous treepies (Dendrocitta vagabunda), red-vented bulbuls (Pycnonotus cafer), red-wattled lapwings (Vanellus indicus), and blue rock pigeons (Columba livia) being some of the notable observations. Raptors such as the shikra (Accipiter badius), Indian eagle owl (Bubo bengalensis), and long-legged buzzard (Buteo rufinus) were also recorded.
Among wild carnivores, the Indian leopard, striped hyena, Bengal fox (Vulpes bengalensis), golden jackal (Canis aureus), small Indian civet (Viverricula indica), and Indian mongoose (Herpestes edwardsii) were observed. Herbivores frequently recorded included nilgai, chital (Axis axis), sambar deer (Rusa unicolor), and black-naped hare (Lepus nigricollis). Other vertebrates such as the crested porcupine (Hystrix indica) and fruit bat (Pteropus giganteus) were also present, along with the gray langur (Semnopithecus entellus), the only primate recorded.
Domestic animals documented included cattle (Bos taurus), goats (Capra aegagrus hircus), feral dogs (Canis lupus familiaris), cats (Felis catus), and domestic pigs (Sus scrofa domesticus), with grazing activity observed primarily in the peripheral areas. Human activity, divided into villagers, forestry officers, and ecotourism, was recorded within the reserve. Human presence decreased from 28.4% in the first year to 3.92% in the second year of the study.
We observed an increase in the relative abundance of several wildlife species in 2018-2019 compared to 2017-2018, including the Indian peafowl, rufous treepie, jungle babbler, red-vented bulbul, Indian leopard, and small Indian civet. Over the two-year period, 661 images of leopards were captured, identifying 25 individual leopards (8 males and 17 females).
Of the 39 species recorded, two were classified as “Vulnerable” (Indian leopard, sambar deer), one as “Near Threatened” (striped hyena), and the remaining 36 as “Least Concern” according to the IUCN Red List [
36].
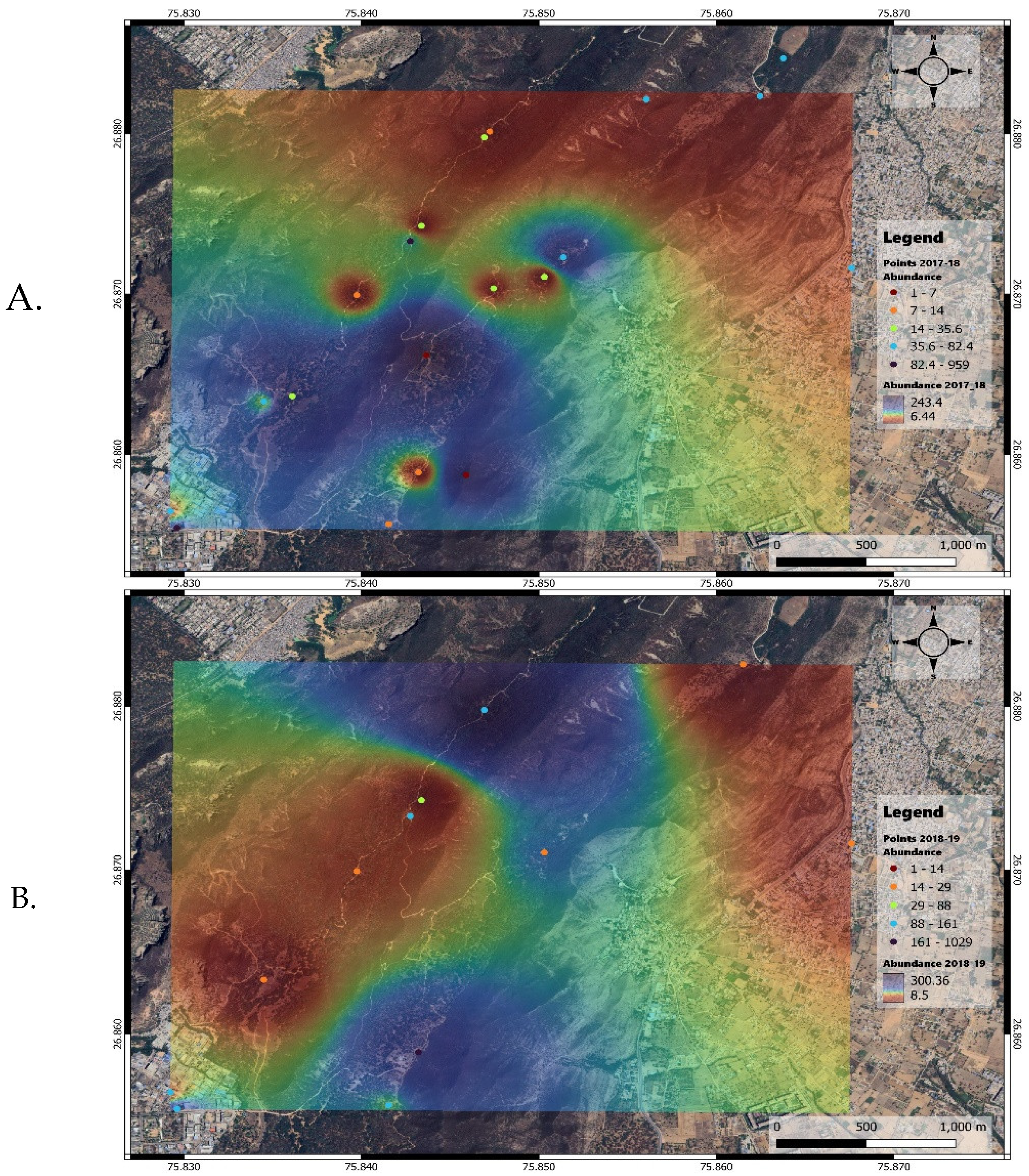
The abundance maps for 2017-2018 showed higher abundance density in the southern and southwestern parts of the study area, whereas in 2018-2019, the abundance density was more evenly distributed across the north and south parts (
Figure 3a, b).
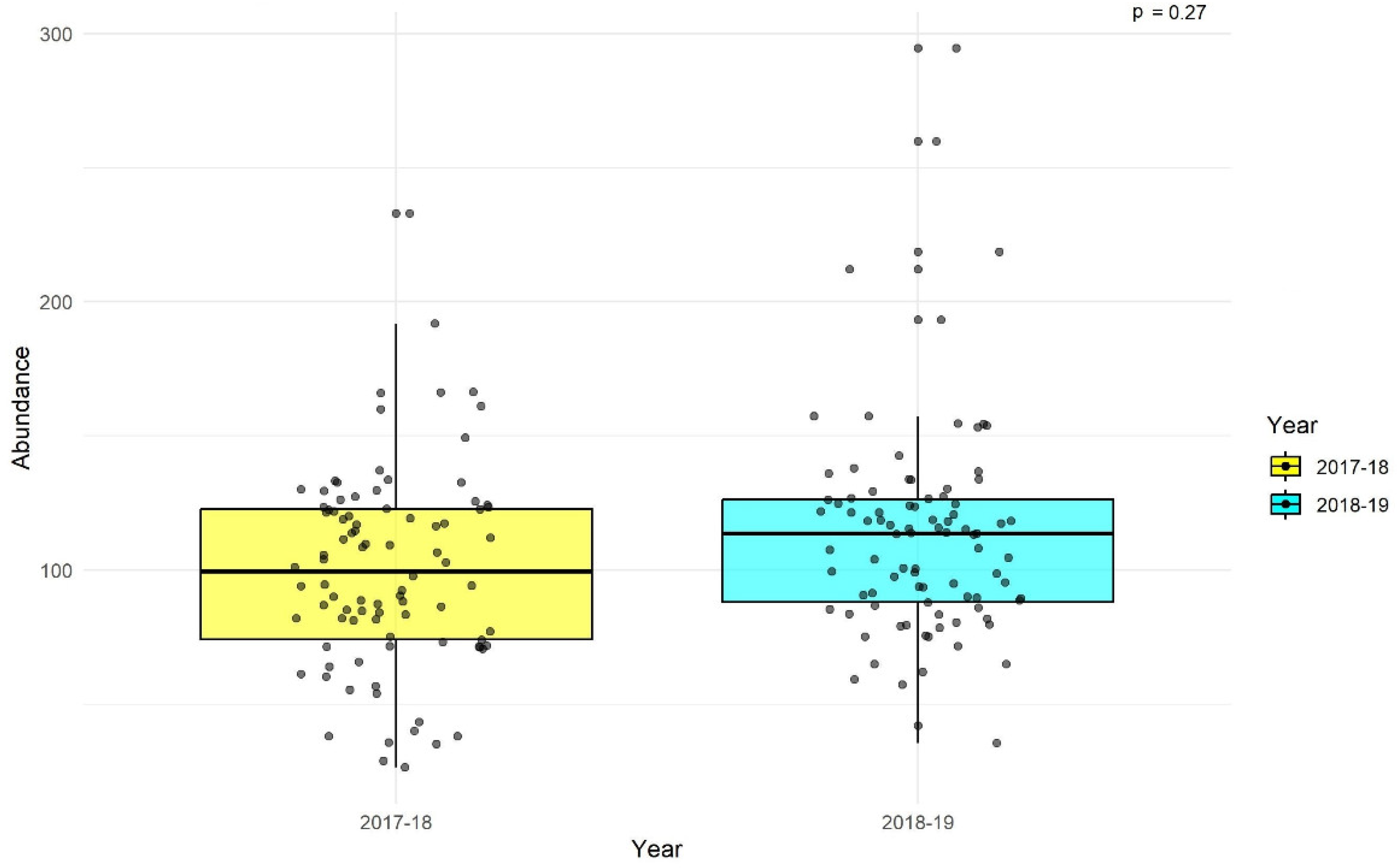
There was no significant difference in abundance between the two years (p = 0.27;
Figure 4).
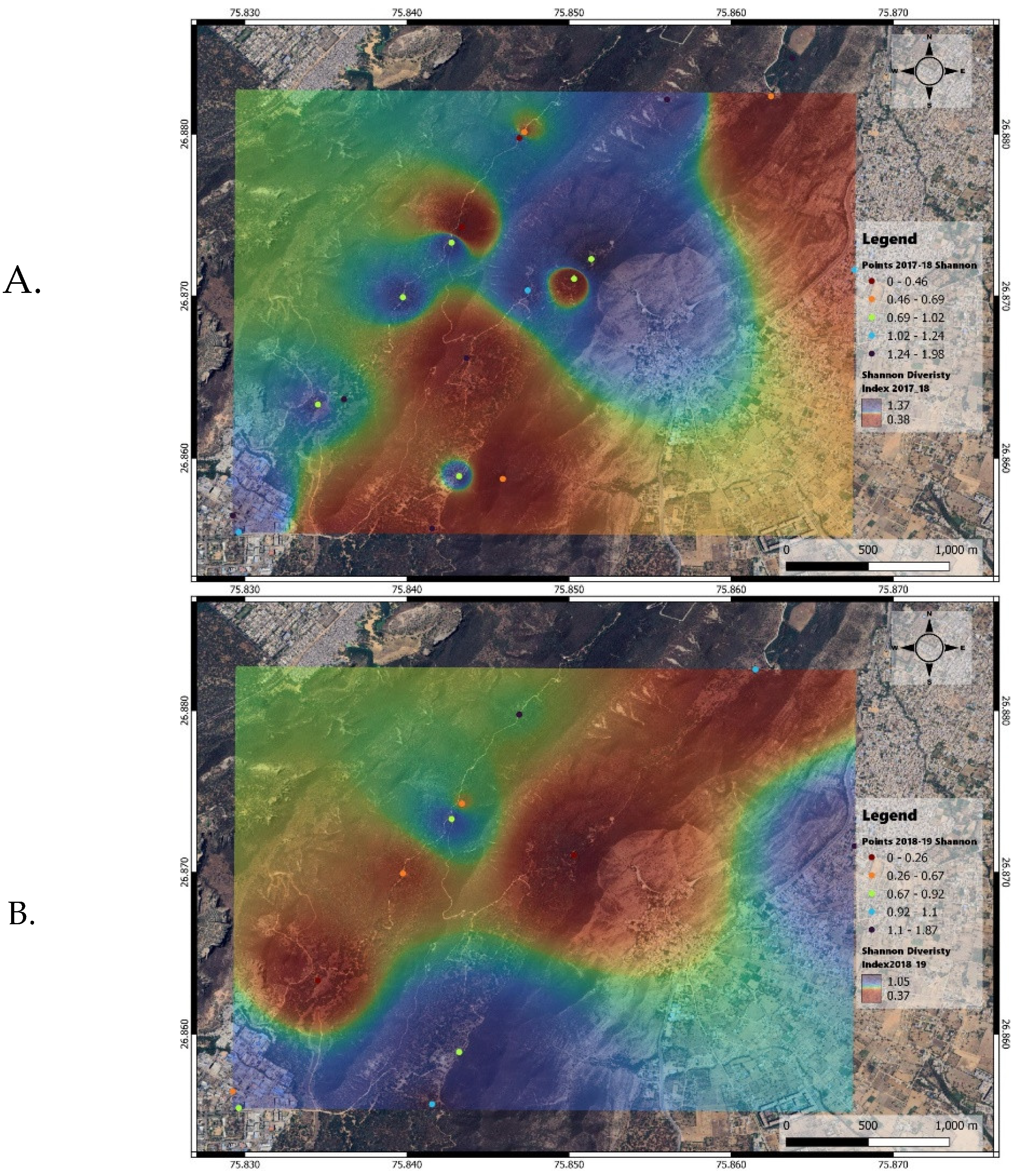
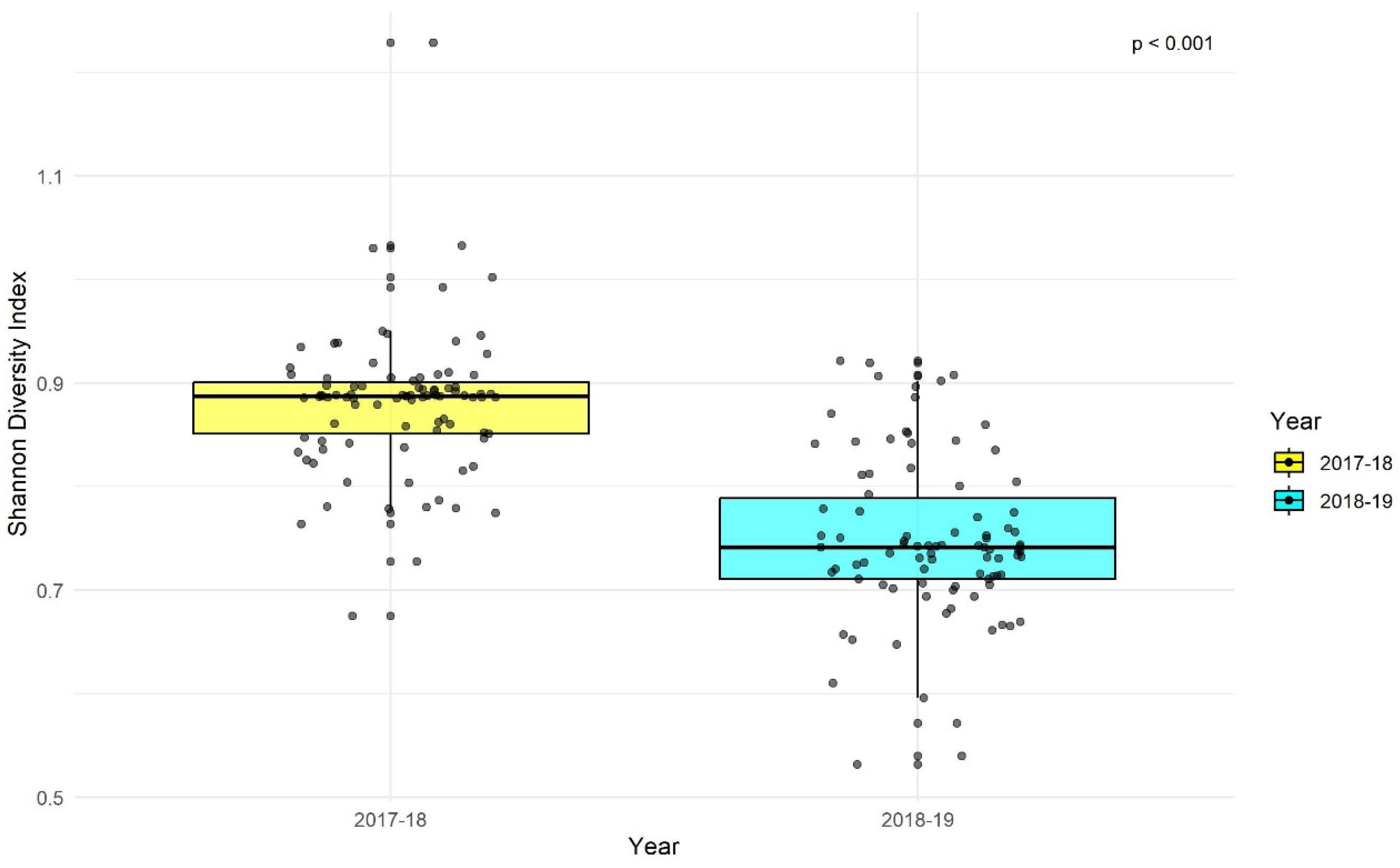
The Shannon diversity index maps indicated higher diversity in the northern and central parts of the study area in 2017-2018, while in 2018-2019, diversity was higher in the southern, central, and southeastern parts (
Figure 5a, b).
There was a significant difference in Shannon diversity index values between the two years (p < 0.01;
Figure 6). This change in diversity has implications for the prey community of the apex predator, the Indian leopard, and the resource dependence of other species, likely influenced by changes in waterhole locations.
Discussion
This study is the first to scientifically document the species richness within the JRF, capturing both wildlife and anthropogenic activities to understand the implications of declaring JRF as India’s first leopard reserve. Despite the semi-arid, human-exploited landscape, JRF hosts seven carnivore species, although wild herbivores are not naturally present. These findings are consistent with camera trap studies in human-dominated landscapes, such as those in Maharashtra [
37].
In the absence of tigers and wolves (
Canis lupus pallipes), the leopard serves as the apex predator in JRF. Our results align with those of Karanth and Sunquist [
38] and Reddy et al. [
39], who noted that leopards thrive where large or medium-sized prey and competitors like tigers or dholes (
Cuon alpinus) are absent. Leopards were photographed at all camera traps and were well distributed throughout the reserve and surrounding areas. Specific observations included a male leopard with an injury in December 2017, who later recovered. Unlike other studies [
37,
40], we captured both flanks and facial markings of leopards, enabling individual identification of 17 females and 8 males [
22]. We recorded three females with cubs, indicating a resident and reproductive population, akin to findings by Athreya et al. [
37].
The diet composition of JRF leopards shows a dependence on human-associated and domestic animals such as feral dogs, cats, goats, and cattle [
28]. Domestic animals constituted 89% of the leopards’ prey. A survey in Jaipur city reported 36,850 domestic dogs [
41], a significant prey source for leopards. Camera trap data corroborate these findings with numerous images of domestic animals within JRF.
Eighteen villages surround JRF, and the local community relies on the forest for livelihood, engaging in activities such as grazing and firewood collection, leading to frequent human-wildlife interactions. The Rajasthan Forest Department’s vigilance has reduced human activity within the reserve from 28.04% in 2017-18 to 3.9% in 2018-19 (
Table 1), resulting in increased relative abundance of dominant wildlife species in 2018-19 (
Table 1). This supports O’Brien and Kinnaird’s [
5] assertion that camera traps are effective for monitoring ground-dwelling birds and other wildlife, though they may underrepresent arboreal species.
Striped hyenas, Bengal foxes, and small Indian civets were commonly observed and well-distributed in JRF. Golden jackals and Indian mongooses were occasionally spotted at waterholes. Hyenas were frequently seen near the southeastern border, close to human habitations. The construction of a 3-meter high wall on the reserve’s border in 2018 by the Rajasthan Forest Department has reduced domestic animal intrusions to zero in 2018-19, though leopards can still traverse the fence.
A 2018 survey indicated 90% support for conservation efforts [
27]. However, as domestic livestock is a significant food source for carnivores in human-modified habitats [
28,
42,
43], conservation strategies must balance human and wildlife needs. Our results show that integrating wildlife into human-modified landscapes while considering human tolerance is crucial [
44]. Research should extend beyond reserve boundaries to include adjacent areas, particularly in JRF where urban neighborhoods are contiguous with the forest.
Forest workers are primarily active in central JRF, involved in activities such as road construction, tree planting, water tanker transport to waterholes, and ecotourism route maintenance. Ecotourism, regulated since June 2017 under “Project Leopard,” has contributed to human presence but is managed to minimize wildlife disturbance. Electric vehicles have been introduced to reduce noise impact [
31].
Several studies highlight the effects of ecotourism on wildlife behavior, including changes in feeding, hormonal responses, habituation, predation, and reproductive success [
45,
46,
47,
48]. Further research on the impact of tourism in JRF will aid in developing responsible tourism policies. Continuous monitoring of species richness is vital for tracking protected and endangered species [
49,
50].
Our study demonstrates that camera trapping is an effective method for estimating and recording the animal diversity of the Jhalana Reserve Forest (JRF). The findings reveal that human-dominated landscapes can sustain and support a consortium of predators, and wildlife in JRF shares resources with humans outside the reserve forest. Various conservation measures undertaken by the Rajasthan Forest Department have significantly reduced human intrusion, leading to an increase in the relative abundance of wildlife species in the JRF. Camera traps have proven to be an essential tool for ongoing monitoring of vulnerable and near-threatened species in JRF, and this approach can be valuable in other human-shared protected areas to assess the status of wildlife amidst anthropogenic activities. Our results underscore the importance of integrating wildlife into human-modified landscapes while considering human tolerance and the necessity of extending research beyond reserve boundaries to include adjacent areas.
Author Contributions
SK, RY – inception, planning, writing; SK – implementation; AM, RY –supervision, quality control, writing; Statistics -SR, RY.
Funding
No funding was received to for this research.
Ethics Approval
Relevant permissions to conduct the research were obtained from the Office of the Chief Wildlife Warden, Rajasthan Forest Department (Research permit no 2017/ 171).
Consent to participate
Agreed by all authors
Consent for publication
Agreed by all authors
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We thank the Rajasthan Forest Department for granting permissions to conduct research on the leopards of Jhalana and for installing camera traps. Sudarshan Sharma, Deputy Conservator of Forests, accompanied our field team in this study. Ranger Janeshwar Chaudhary, forester Rajkishore Yogi and volunteers Anand Kulkarni, Mahi Kulkarni, Sandra Lichinski, Siya Bhagat, Bablu Gurjar, Shubham Saini, Dayal Gurjar, Abhishek Gurjar, Abhinav Mudgal, and Ajay Arora helped us on site and with the analysis of the images. We thank Tejaswinee Shendye for analysis and Rajaram and Rohit Gangwal for species identification.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- MacKenzie:, D.I., Bailey, L.L., Hines, J.E. and Nichols, J.D. 2011. An integrated model of habitat and species occurrence dynamics. Methods in Ecology and Evolution 2:612-622. [CrossRef]
- Findlay, CS. and Houlahan, J. 1997. Anthropogenic Correlates of Species Richness in Southeastern Ontario Wetlands: Correlativos Antropogénicos de la Riqueza de Especies en Humedales del Sureste de Ontario. Conservation Biology, 11:1000-1009.
- Gurd, D.B., Nudds, T.D. and Rivard, D.H., 2001. Conservation of mammals in eastern North American wildlife reserves: how small is too small? Conservation Biology, 15:1355-1363.
- Caughley, G., 1977. Analysis of vertebrate populations. John Wiley, Chichester, England.
- O’Brien, T.G. and Kinnaird, M.F. 2008. A picture is worth a thousand words: the application of camera trapping to the study of birds. Bird Conservation International 18:S144-S162. [CrossRef]
- Balme, G.A., Hunter, L.T. and Slotow, R.O.B., 2009. Evaluating methods for counting cryptic carnivores. Journal of Wildlife Management 73:433-441. [CrossRef]
- Harihar, A., Pandav, B. and Goyal, S.P. 2009. Density of leopards (Panthera pardus) in the Chilla Range of Rajaji National Park, Uttarakhand, India. Mammalia 73:68-71. [CrossRef]
- Harihar, A., Pandav, B. and Goyal, S.P. 2011. Responses of leopard Panthera pardus to the recovery of a tiger Panthera tigris population. Journal of Applied Ecology 48:806-814. [CrossRef]
- Harihar, A., Ghosh, M., Fernandes, M., Pandav, B. and Goyal, S.P. 2010. Use of photographic capture-recapture sampling to estimate density of Striped Hyena (Hyaena hyaena): implications for conservation. Mammalia 74:83-87. [CrossRef]
- Gupta, S., Mondal, K., Sankar, K. and Qureshi, Q. 2009. Estimation of striped hyena Hyaena hyaena population using camera traps in Sariska Tiger Reserve, Rajasthan, India. Journal of the Bombay Natural History Society, 106:284.
- Singh, P., Gopalaswamy, A.M. and Karanth, K.U. 2010. Factors influencing densities of striped hyenas (Hyaena hyaena) in arid regions of India. Journal of Mammalogy 91:1152-1159. [CrossRef]
- Hadad, E., Balaban, A. and Yosef, R. 2023. Alloparenting by helpers in striped hyena (Hyaena hyaena). Animals 13:1914. [CrossRef]
- Karanth, K.U. and Nichols, J.D. 1998. Estimation of tiger densities in India using photographic captures and recaptures. Ecology 79:2852-2862.
- Karanth, K.U., Nichols, J.D., Kumar, N.S. and Hines, J.E. 2006. Assessing tiger population dynamics using photographic capture–recapture sampling. Ecology 87:2925-2937. [CrossRef]
- Janečka, J.E., Munkhtsog, B., Jackson, R.M., Naranbaatar, G., Mallon, D.P. and Murphy, W.J. 2011. Comparison of noninvasive genetic and camera-trapping techniques for surveying snow leopards. Journal of Mammalogy 92:771-783. [CrossRef]
- Soisalo, M.K. and Cavalcanti, S.M. 2006. Estimating the density of a jaguar population in the Brazilian Pantanal using camera-traps and capture–recapture sampling in combination with GPS radio-telemetry. Biological Conservation 129:487-496. [CrossRef]
- Martyr, D. 1997. Important findings by FFI team in Kerinci Seblat, Sumatra, Indonesia. Oryx 31:80–82.
- Zetra, B., Rafiastanto, A., Rombang, W.M. and Trainor, C.R. 2002. Rediscovery of the critically endangered Sumatran Ground Cuckoo Carpococcyx viridis. Forktail 2002:63-66.
- Dinata, Y., Nugroho, A., Haidir, IA. and Linkie, M. 2008. Camera trapping rare and threatened avifauna in west-central Sumatra. Bird Conservation International 18:30-37. [CrossRef]
- Ontiveros, Y., Kappa, F. M., Andino, N., Campos, C. M., Bordghi, C. E., and Giannoni, S. M. 2022. Mammal and bird diversity in a system of protected areas in Argentina. Biodiversity 23:61-71. [CrossRef]
- Trofino-Falasco, C., Simoy, M. V., Aranguren, M. F., Pizzarello, M. G., Cortelezzi, A., Vera, D. G., Simoy, M. I., Marinelli, C. B., Cepeda, R. E., Di Giacomo, A. S., and Berkunsky, I. 2023. How effective is camera trapping in monitoring grassland species in the southern Pampas ecoregion? Revista Mexicana de Biodiversidad 94:e945243. [CrossRef]
- Kumbhojkar, S., Yosef, R., Mehta, A. and Rakholia, S. 2020. A camera-trap home-range analysis of the Indian leopard (Panthera pardus fusca) in Jaipur, India. Animals 10:1600. [CrossRef]
- Chauhan, R., Sihag, V. and Singh, N.P., 2009. Distribution and biocontrol potential of chosen spiders. Journal of biopesticides 2:151-155. [CrossRef]
- Reena, M. and Ram, M.B. 1992. Rate of takeovers in groups of Hanuman langurs (Presbytis entellus) at Jaipur. Folia Primatologica 58:61-71. [CrossRef]
- Sultana, A., Hussain, M.S. and Rathore, D.K. 2014. Diversity of tree vegetation of Rajasthan, India. Tropical Ecology 55:403-410.
- Yosef, R., Rakholia, S., Mehta, A., Bhatt, A. and Kumbhojkar, S. 2022. Land Surface Temperature Regulation Ecosystem Service: A Case Study of Jaipur, India, and the Urban Island of Jhalana Reserve Forest. Forests 13:1101.
- Kumbhojkar, S., Yosef, R., Benedetti, Y. and Morelli, F. 2019. Human-leopard (Panthera pardus fusca) co-existence in Jhalana Forest Reserve, India. Sustainability 11:3912. [CrossRef]
- Kumbhojkar, S., Yosef, R., Kosicki, J.Z., Kwiatkowska, P.K. and Tryjanowski, P. 2021. Dependence of the leopard Panthera pardus fusca in Jaipur, India, on domestic animals. Oryx 55:692-698.
- Kumbhojkar, S., R. Yosef, A. Mehta & S.Rakholia. 2024, In Press. Ecosystem services of Leopards (Panthera pardus fusca) to the conurbation of Jaipur, India. Chapter 33 In “Ecology of Tropical cities: Nature & Social Sciences applied to the conservation of urban biodiversity” (F. Angeoletto, P. Tryjanowski, K. Fellowes, eds). Springer Nature.
- Yosef, R., Dabi, H. and Kumbhojkar, S. 2021. Thanatological behavior of a female Leopard (Panthera pardus fusca). Acta Ethologica 24:137-140. [CrossRef]
- Yosef, R., Kumbhojkar, S., Sharma, S. and Morelli, F. 2021. Electric vehicles minimize disturbance to mammals. European Journal of Wildlife Research 67:74. [CrossRef]
- Yosef, R., Kumbhojkar, S., Gurjar, B. and Kosicki, J.Z. 2022. Magnetic alignment in free-ranging Indian Leopard (Panthera pardus fusca). Plos one 17:e0266129. [CrossRef]
- Larrucea E.S., Brussard, P.F., Jaeger, M.M. and Barrett, R.H. 2007. Cameras, coyotes, and the assumption of equal detectability. Journal of Wildlife Management 71:1682-1689. [CrossRef]
- Tarugara, A., Clegg, B.W., Gandiwa, E. and Muposhi, V.K. 2019. Cost-benefit analysis of increasing sampling effort in a baited-camera trap survey of an African leopard (Panthera pardus) population. Global Ecology and Conservation 18:e00627. [CrossRef]
- Treves, A., Mwima, P., Plumptre, A.J. and Isoke, S. 2010. Camera-trapping forest–woodland wildlife of western Uganda reveals how gregariousness biases estimates of relative abundance and distribution. Biological Conservation 143:521-528. [CrossRef]
- Stein, A.B., Gerngross, P., Al Hikmani, H., Balme, G., Bertola, L., Drouilly, M., Farhadinia, M.S., Feng, L., Ghoddousi, A., Henschel, P., Jhala, Y., Khorozyan, I., Kittle, A., Laguardia, A., Luo, S.-J., Mann, G., Miquelle, D., Moheb, Z., Raza, H., Rostro-García, S., Shivakumar, S., Song, D. & Wibisono, H. 2024. Panthera pardus. The IUCN Red List of Threatened Species 2024: e.T15954A254576956. Accessed on 17 July 2024.
- Athreya, V., Odden, M., Linnell, J.D., Krishnaswamy, J. and Karanth, U., 2013. Big cats in our backyards: persistence of large carnivores in a human dominated landscape in India. PloS one 8:e57872. [CrossRef]
- Karanth, K.U. and Sunquist, M.E. 1995. Prey selection by tiger, leopard and dhole in tropical forests. Journal of Animal Ecology 1:439-450. [CrossRef]
- Reddy, C.S., Yosef, R., Calvi, G. and Fornasari, L. 2019. Inter-specific competition influences apex predator–prey populations. Wildlife Research 46:628-638. [CrossRef]
- Kalle, R., Ramesh, T., Qureshi, Q. and Sankar, K. 2011. Density of tiger and leopard in a tropical deciduous forest of Mudumalai Tiger Reserve, southern India, as estimated using photographic capture–recapture sampling. Acta Theriologica 56:335-342. [CrossRef]
- Hiby, L.R., Reece, J.F., Wright, R., Jaisinghani, R., Singh, B. and Hiby, E.F. 2011. A mark-resight survey method to estimate the roaming dog population in three cities in Rajasthan, India. BMC Veterinary Research 7:1-10. [CrossRef]
- Chauhan DS and Goyal SP. 2000. A study on distribution, relative abundance and food habits of Leopard (Panthera pardus) in Garhwal Himalayas. Report of the Wildlife Institute of India, Dehradun.
- Vijayan, S. and Pati, B.P. 2002. Impact of changing cropping patterns on man-animal conflicts around Gir Protected Area with specific reference to Talala Sub-District, Gujarat, India. Population and Environment 23:541-559. [CrossRef]
- Breitenmoser U, Angst C, Landry JM, Breitenmoser-Wursten C, Linnell JDC. 2005. Non-lethal techniques for reducing depredation. Pp 49–71 In People and Wildlife. Conflict or Co-existence? (Woodroffe R, Thirgood S, Rabinowitz A, eds). New York: Cambridge University Press,.
- Lott, D.F. and McCoy, M. 1995. Asian rhinos Rhinoceros unicornis on the run? Impact of tourist visits on one population. Biological Conservation 73:23-26.
- Fowler, GS. 1999. Behavioral and hormonal responses of Magellanic penguins (Spheniscus magellanicus) to tourism and nest site visitation. Biological Conservation 90:143-149. [CrossRef]
- Romero, L.M. and Wikelski, M. 2002. Exposure to tourism reduces stress-induced corticosterone levels in Galapagos marine iguanas. Biological Conservation 108:371-374. [CrossRef]
- Lacy, K.E. and Martins, E.P. 2003. The effect of anthropogenic habitat usage on the social behaviour of a vulnerable species, Cyclura nubila. Animal Conservation Forum 6:3-9. [CrossRef]
- Jones, J.P., 2011. Monitoring species abundance and distribution at the landscape scale. Journal of Applied Ecology 48:9-13. [CrossRef]
- Gray, T.N., Phan, C., Pin, C. and Prum, S. 2012. Establishing a monitoring baseline for threatened large ungulates in eastern Cambodia. Wildlife Biology 18:406-413. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).