Case Presentation
The patient, a 70-year-old female with a medical history significant for hypertension, hypothyroidism, chronic back pain, and a left hip prosthetic joint infection, underwent a total left hip replacement in 2023 at an outside hospital. This procedure was complicated by multiple revisions in January and February of 2024. After the final revision in February, which the patient tolerated well, she was discharged to a rehabilitation facility. The following day, she developed bleeding at the surgical site. A computed tomography (CT) scan of the pelvis without contrast revealed a dislocation of the femoral head component, which extended superiorly into the pelvis beyond the iliac crest. Consequently, the patient was transferred to our hospital.
Upon arrival, a CT scan of the hip without contrast demonstrated a failed left total hip arthroplasty with prosthetic hip dislocation and intrapelvic migration of the femoral component. Additionally, periprosthetic fractures of the left superior pubic ramus/pubic root and inferior pubic ramus were noted, along with a large left hip effusion that decompressed posterolaterally into the subcutaneous tissue. The patient then underwent a complex revision of the left total hip arthroplasty, which included resection of the proximal femur for osteomyelitis, resection of a draining sinus, and extensive irrigation and debridement, followed by placement of an antibiotic spacer. Cultures obtained during the surgery were positive for methicillin-resistant *Streptococcus epidermidis* (MRSE). Postoperatively, the patient was commenced on a six-week regimen of intravenous vancomycin, administered twice daily, with an anticipated treatment completion in April 2024, in preparation for a two-stage hip revision post-infection clearance.
The patient initially tolerated the treatment well; however, in the fifth week, she developed a diffuse erythematous pruritic rash on her abdomen. The rash rapidly spread to the rest of her body, prompting her admission to our emergency department two days later. Upon evaluation, the patient reported chills but denied shortness of breath, facial swelling, chest pain, or other symptoms. She was afebrile and hemodynamically stable, with a diffuse maculopapular rash that spared the palms and soles. There was no evidence of skin desquamation or mucous membrane involvement. Initial laboratory tests revealed an elevated white blood cell count of 15.31 k/uL, with an absolute neutrophil count of 11.65 k/uL and an absolute eosinophil count of 1.49 k/uL. Vancomycin was immediately discontinued, and the infectious disease team was consulted to manage the ongoing need for antibiotic therapy given the incomplete course for the prosthetic joint infection. The patient expressed discomfort with the peripherally inserted central catheter (PICC) line, prompting a switch to oral linezolid 600 mg twice daily for the remainder of her antibiotic course.
A diagnosis of vancomycin-induced hypersensitivity reaction, consistent with drug reaction with eosinophilia and systemic symptoms (DRESS), was considered. The severity of the reaction was classified as mild due to the absence of end organ damage, which was monitored by kidney and liver function tests showing blood urea nitrogen (BUN) levels of 15-21 mg/dL, creatinine (Cr) levels of 0.85-0.93 mg/dL, alanine aminotransferase (ALT) levels of 15-20 U/L, and aspartate aminotransferase (AST) levels of 14-18 U/L. Due to the mild nature of the disease, systemic steroids were initially deemed unnecessary. However, the rash became more erythematous and "beefy" in appearance, leading to the initiation of systemic corticosteroids with a one-time dose of intravenous methylprednisolone 80 mg, which improved the patient’s symptoms.
Prior to discharge, dermatology was consulted to consider a skin biopsy to confirm the diagnosis of DRESS; however, it was not performed at that time. The patient was advised to follow up outpatient to monitor thyroid function, given the risk of hypothyroidism occurring four to twelve weeks following a DRESS reaction. She received only a short course of steroids; hence, no taper was required. With her symptoms significantly improved and transitioned to oral antibiotics, the patient was discharged home.
Investigations
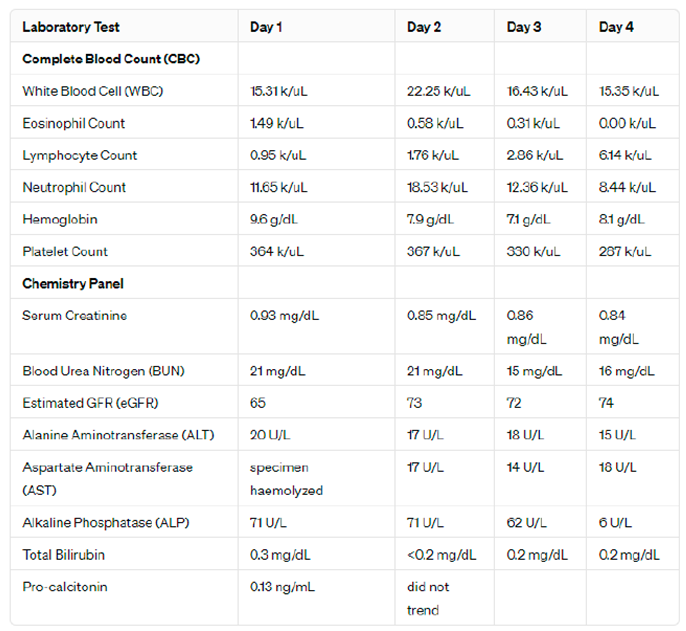
Throughout the patient's hospitalization, comprehensive laboratory and imaging investigations were essential in assessing her condition, suspected to be DRESS syndrome induced by vancomycin. Daily complete blood counts (CBC) revealed significant fluctuations; initial white blood cell (WBC) counts were elevated at 15.31 k/uL, peaking at 22.25 k/uL before stabilizing. This elevation in WBC was accompanied by a distinct pattern in eosinophil counts, which were initially high at 1.49 k/uL, supporting the diagnosis of DRESS but decreased rapidly to 0.00 k/uL, potentially indicating a response to treatment or the transient nature of eosinophilia in this syndrome. Detailed lab values are presented in
Table 1, which illustrates the dynamic changes in CBC and chemistry panel results throughout the patient's stay.
Lymphocyte counts progressively increased from 0.95 k/uL to 6.14 k/uL, reflecting an evolving immune response characteristic of an active hypersensitivity reaction. Neutrophil counts mirrored this trend, suggesting an acute phase reaction. Hemoglobin levels showed a downward trend, indicative of potential anemia of chronic disease or acute blood loss, and platelet counts demonstrated a slight but consistent decrease, remaining within normal limits.
Renal function was monitored through serum creatinine and blood urea nitrogen (BUN) levels, which remained stable, indicating no renal impairment despite systemic involvement. Liver function tests, including alanine aminotransferase (ALT) and aspartate aminotransferase (AST), were minimally elevated, suggesting mild liver involvement without significant hepatotoxicity. Alkaline phosphatase and total bilirubin levels remained within normal limits, reinforcing the lack of severe liver dysfunction.
The sole imaging study conducted, a chest X-ray, showed no signs of pleural effusion, consolidation, or pneumothorax, ruling out acute cardiopulmonary conditions and indicating mild linear lingular scarring from past inflammation. The X-ray also noted a borderline-enlarged cardiomediastinal silhouette and severe degenerative changes in the glenohumeral joints, more pronounced on the right, aligning with the patient’s history of chronic pain. The results and images from the chest X-ray are included below for detailed review.
The limitations of these investigations were notable, particularly the transient nature of eosinophilia and its rapid resolution, which might lead to under-recognition of its diagnostic significance in DRESS. Furthermore, the absence of a skin biopsy limited histopathological confirmation of the diagnosis, which is considered the gold standard for identifying DRESS and differentiating it from other drug reactions. The imaging provided was limited in scope, focusing on ruling out acute thoracic complications but not offering information on other potentially involved systems or detailed cardiac evaluation, which could be pertinent given the borderline cardiomediastinal enlargement noted.
Overall, the investigations during the hospital stay provided critical data for managing the suspected DRESS syndrome. They facilitated monitoring of organ functions and the progression of hematological abnormalities associated with the syndrome. However, the investigative approach had its constraints, primarily the lack of specific tests such as viral serologies and a comprehensive skin or organ biopsy, which could have offered more definitive diagnostic and prognostic information. This case underscores the necessity for timely and repeated assessments in suspected DRESS to capture the dynamic changes typical of this syndrome and to adapt clinical management accordingly.
Differential Diagnosis
Vancomycin Allergy / Vancomycin induced reaction
Vancomycin causes several different types of hypersensitivity reactions, ranging from localized skin reactions to generalized cardiovascular collapse. The most common adverse reaction is vancomycin infusion reaction (VIR). VIR is a rate-dependent infusion reaction, not a true allergic reaction.
VIR is an idiopathic reaction, which is not thought to involve drug-specific antibodies. VIR is a form of pseudoallergic drug reaction, which is an adverse drug reaction with signs and symptoms that mimic immunologic drug allergies but in which IgE-mediated immunologic mechanisms have not been demonstrated.
The most important symptoms of SJS/TEN are those that affect the skin. In the first few days, before the skinsymptoms start, SJS/TEN causes
- -
Fever (often higher than 102 F or 39C)
- -
Flu like illness
- -
Itching or burning eyes
- -
Joint Pain
- -
Cough
Kawasaki disease is an arteritis that predominantly affects the medium and small arteries, but the aorta and large arteries may also be involved. The disease usually occurs in children and is often associated with a mucocutaneous lymph node syndrome. The coronary arteries may also be involved. A small number of cases have also been reported in adults [
11].
Approximately one-fourth of adult KD cases have occurred in patients with human immunodeficiency virus (HIV) infection [
12].One review found that cervical lymphadenopathy, hepatitis, and arthralgia were all more common in adults with KD than in children, and meningitis, thrombocytosis, and CA aneurysms were less common [
13]
Churg-Strauss syndrome, now known as eosinophilic granulomatosis with polyangiitis (EGPA), is a rare autoimmune condition characterized by asthma, sinusitis, and elevated eosinophil levels. Skin manifestations include palpable purpura, nodules, and livedo reticularis, reflecting vasculitis and eosinophilic infiltration.
Hyper eosinophilic syndrome (HES) is characterized by persistent eosinophilia and end-organ damage, including the skin. Common dermatologic manifestations include eczema, urticaria, angioedema, and erythematous papules or plaques. These skin changes result from eosinophil infiltration and cytokine release, necessitating prompt recognition and targeted therapy to prevent complications.
Treatment
Initial treatment of DRESS is to discontinue the offending drug, Vancomycin was discontinued upon admission for this patient. The consideration once Vancomycin was discontinued was antibiotic choice to continue to cover the patient prior to her two-stage hip replacement since she only made it to the fifth week of a six-week course of IV antibiotics. Linezolid 600 mg BID was started, with end of treatment scheduled for the same time as the original antibiotic course. This antibiotic was selected due to its comparable coverage, and the patient's preference of an oral antibiotic due to discomfort from her PICC line. Although corticosteroids are not indicated with Mild DRESS due to no organ involvement, throughout admission the patients rash became increasingly erythematous and continued to spread eventually affecting her face at which point an initial 125 mg IM dose of Methylprednisolone was given, and the following day another dose but of 80 mg IM was administered with significant improvement to the patients overall comfort. Due to systemic steroids having been given in a very short course, a taper was unnecessary upon discharge.
Discussion
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome is a dermatological emergency with a fatality rate of roughly 10% [
1]. The estimated incidence of drug exposure fluctuates between 1 in 1000 to 1 in 10,000 [
2,
3]. The onset tends to be delayed, often within 2-8 weeks of initiating treatment with the presumptive offending medication [
6]. This case fits the typical timeline with eruptive skin findings beginning week 5 of therapy. Most studies show a predominance in Females, with the Male-to-Female ratio of affected individuals being 0.80 in one study [
6], and 0.71 in another [
10]. Median age at diagnosis was estimated to be 51.4 in males, and 55.7 for females [
10].
DRESS syndrome pathophysiology is not well understood, however, it has been speculated to be an immune reaction with viral involvement [
4]. During the early stages of the disease, circulating B cells and serum immunoglobulin levels decrease, while regulatory T-cell populations are expanded [
4]. While T-cells have been hypothesized to contribute to the pathogenesis of DRESS and SJS/TEN via a hypersensitivity-like reaction, one differentiating factor between the two, is the upregulation of T-regulatory cells in the acute phase of DRESS, but not in SJS/TEN [
10]. Immunosuppression can cause viral reactivation, such as HHV-6, resulting in a more severe systemic immunological response. DRESS is linked to elevated levels of inflammatory cytokines, including Interleukin 5, which peak before eosinophilia [
5]. This increase in inflammatory cytokines may be linked to the common manifestation of end-organ damage in DRESS.
While antiepileptic drugs represent the largest group of associated drugs implicated in this syndrome, followed by the gout medication Allopurinol, and sulfonamides, antibiotics have also been implicated, with Vancomycin being the most frequently observed in the RegiSCAR study [
6].
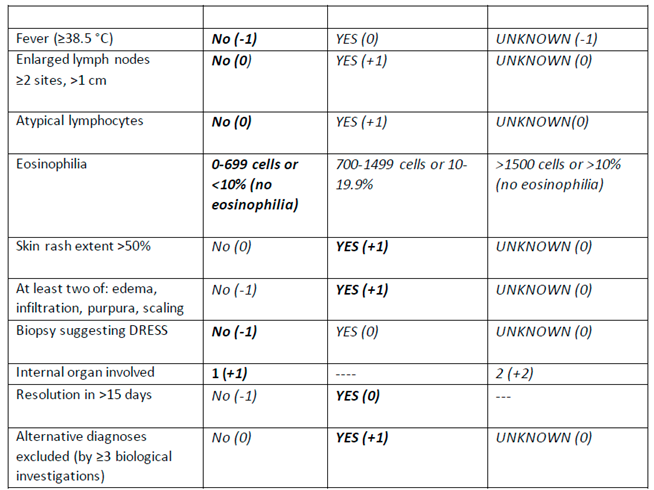
The European Registry of Severe Cutaneous Adverse Reaction Criteria (RegiSCAR) was developed to help diagnose DRESS due to its variable clinical presentation [
6]. The patient received two RegiSCAR points
Negative tests for ANA, blood culture, and hepatitis and normal renal function tests. This score classified the risk of vancomycin-induced DRESS syndrome as "Possible case."
Vancomycin is a glycopeptide antibiotic having a half-life of 6–10 days [
7]. It is effective against both gram-positive and gram-negative bacteria, including those from the Flavobacterium genus (7). Parenteral vancomycin is now used to treat infections such as sepsis, pneumonia, cellulitis, endocarditis, and meningitis due to the rise of Methicillin-resistant Staphylococcus aureus (MRSA) [
8,
9]. Vancomycin has been linked to severe hypersensitivity responses, including Linear IgA Bullous Dermatosis, DRESS, and SJS/TEN [
8].
References
- Chen Y-C, Chiu H-C, Chu C-Y. Drug reaction with eosinophilia and systemic symptoms: a retrospective study of 60 cases. Arch Dermatol. 2010;146(12):1373–9.
- Bocquet H, Bagot M, Roujeau JC. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (drug rash with Eosinophilia and systemic symptoms: DRESS). In: Seminars in cutaneous medicine and surgery: 1996: WB Saunders; 1996. p. 250–7.
- Chiou CC, Yang LC, Hung SI, Chang YC, Kuo TT, Ho HC, Hu S, Hong HS, Chung WH. Clinicopathlogical features and prognosis of drug rash with eosinophilia and systemic symptoms: a study of 30 cases in Taiwan. J Eur Acad Dermatol Venereol. 2008;22(9):1044–9.
- Hirahara K, Kano Y, Mitsuyama Y, Takahashi R, Kimishima M, Shiohara T. Differences in immunological alterations and underlying viral infections in two well-defined severe drug eruptions. Clin Exp Dermatol. 2010;35(8):863–8.
- Choquet-Kastylevsky G, Intrator L, Chenal C, Bocquet H, Revuz J, Roujeau J. Increased levels of interleukin 5 are associated with the generation of eosinophilia in drug-induced hypersensitivity syndrome. Br J Dermatol. 1998;139(6):1026–32.
- Kardaun S, Sekula P, Valeyrie-Allanore L, Liss Y, Chu C, Creamer D, Sidoroff A, Naldi L, Mockenhaupt M, Roujeau J. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169(5):1071–80.
- Deck D, Winston L. Chapter 43. Beta-Lactam & Other Cell Wall-& membraneactive antibiotics. In: Basic & clinical pharmacology. 12th ed. New York: McGraw-Hill; 2012.
- Minhas JS, Wickner PG, Long AA, Banerji A, Blumenthal KG. Immunemediated reactions to vancomycin: a systematic case review and analysis. Ann Allergy Asthma Immunol. 2016;116(6):544–53.
- Korman T, Turnidge J, Grayson M. Vancomycin vintage: my favourite DRESS. Intern Med J. 2015;45(2):233–4.
- Shiohara T, Mizukawa Y. Drug-induced hypersensitivity syndrome (DiHS)/drug reaction with eosinophilia and systemic symptoms (DRESS): An update in 2019. Allergol Int. 2019;68(3):301-308. [CrossRef]
- Gomard-Mennesson E, Landron C, Dauphin C, et al. Kawasaki disease in adults: report of 10 cases. Medicine (Baltimore) 2010; 89:149.
- Sève P, Lega JC. [Kawasaki disease in adult patients]. Rev Med Interne 2011; 32:17.
- Wolff AE, Hansen KE, Zakowski L. Acute Kawasaki disease: not just for kids. J Gen Intern Med 2007; 22:681.
Table 1.
Daily Lab Values during hospitalization.
Table 1.
Daily Lab Values during hospitalization.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).