Submitted:
18 July 2024
Posted:
19 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
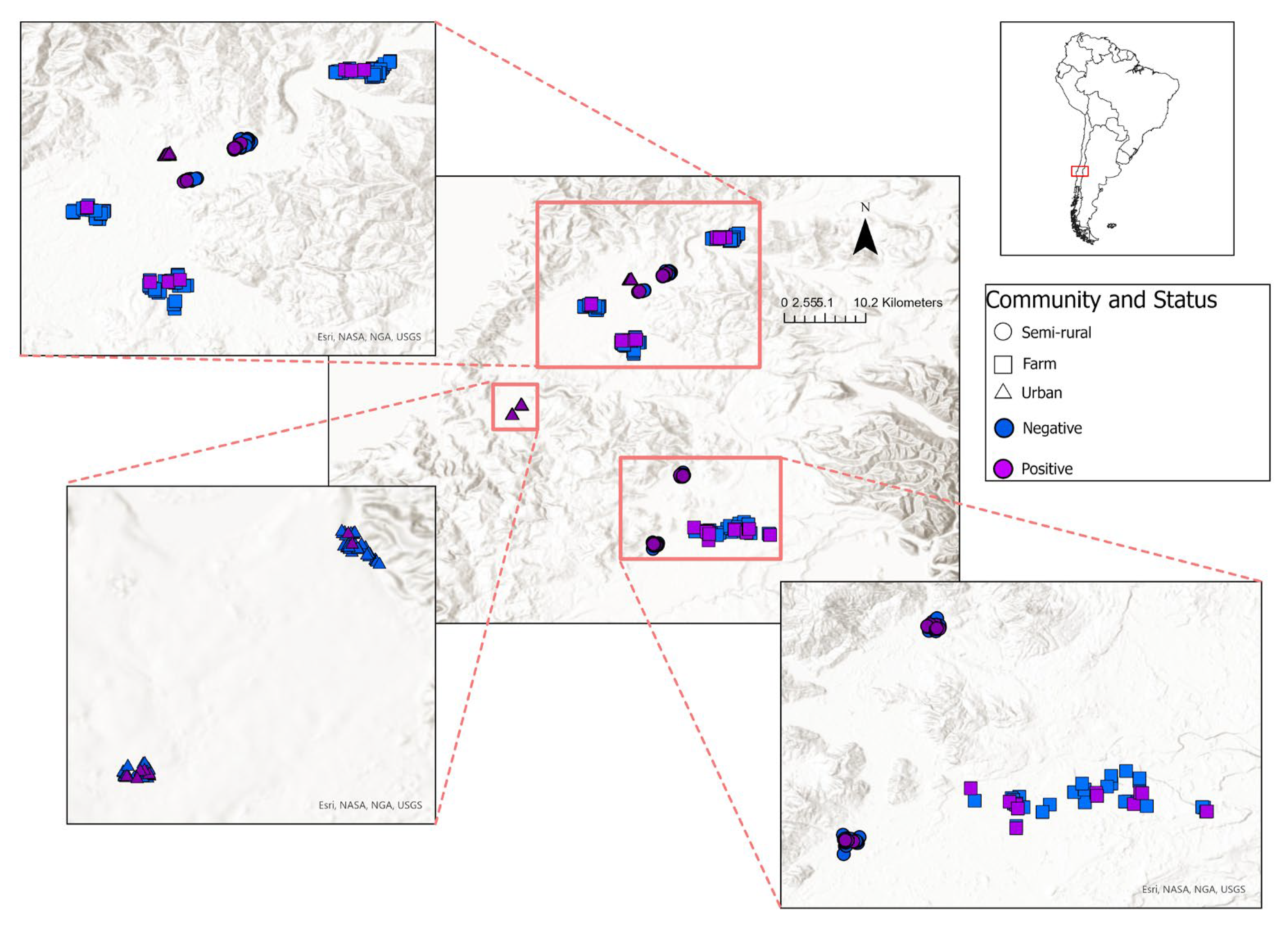
2.1. Study Area
2.2. Data Collection
2.3. Extreme Gradient Boosting Model
3. Results
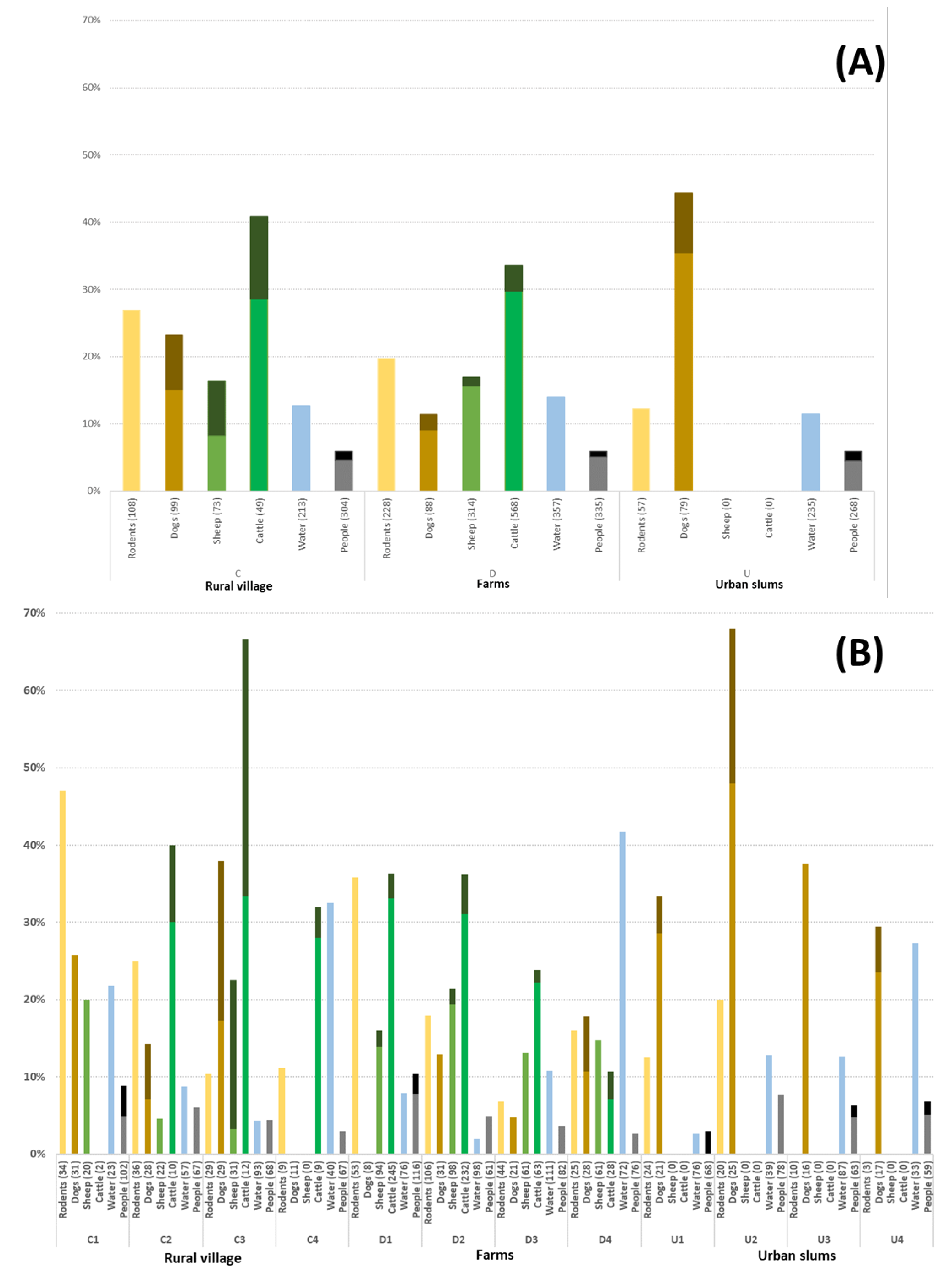
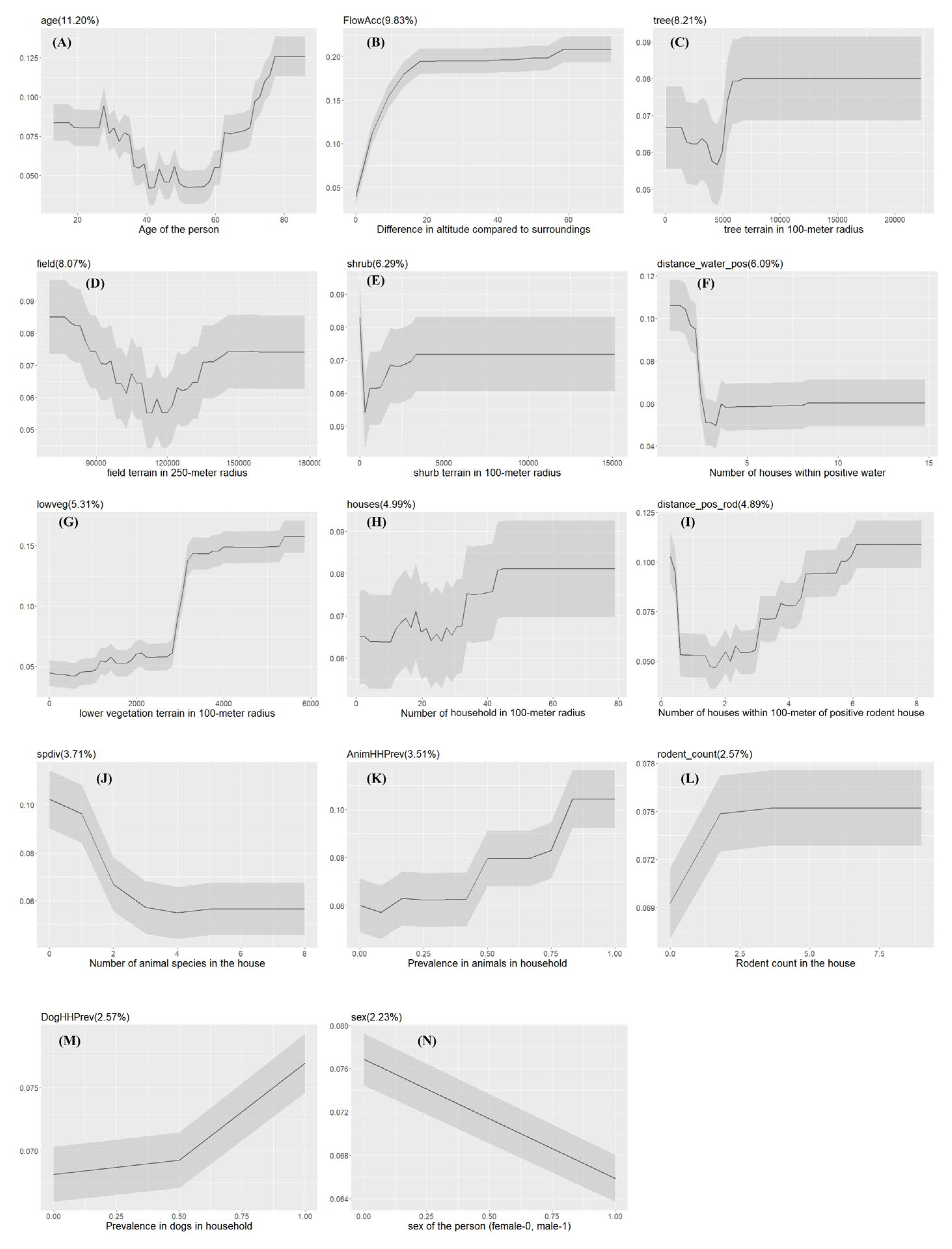
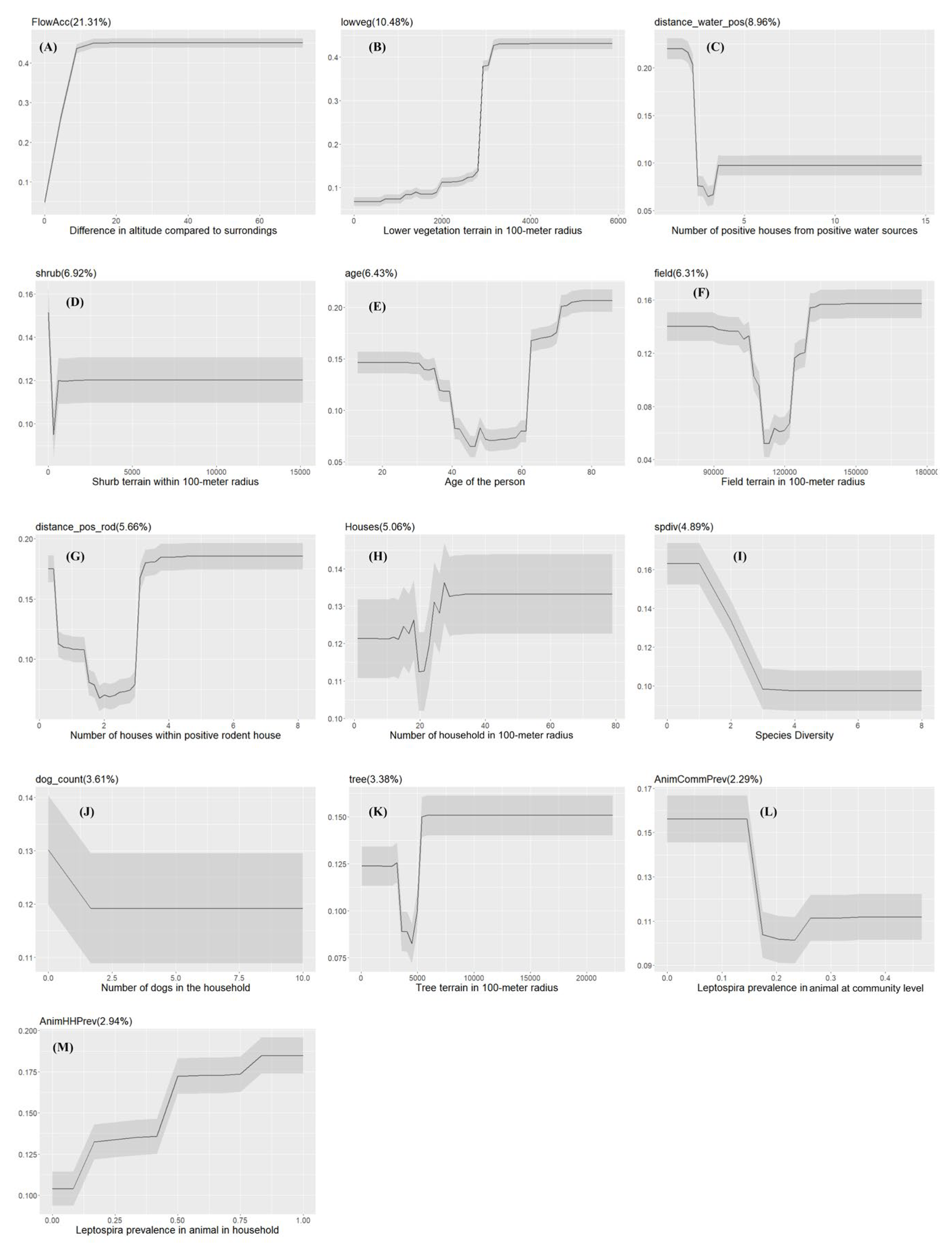
3.1. Seroprevalence of Leptospirosis
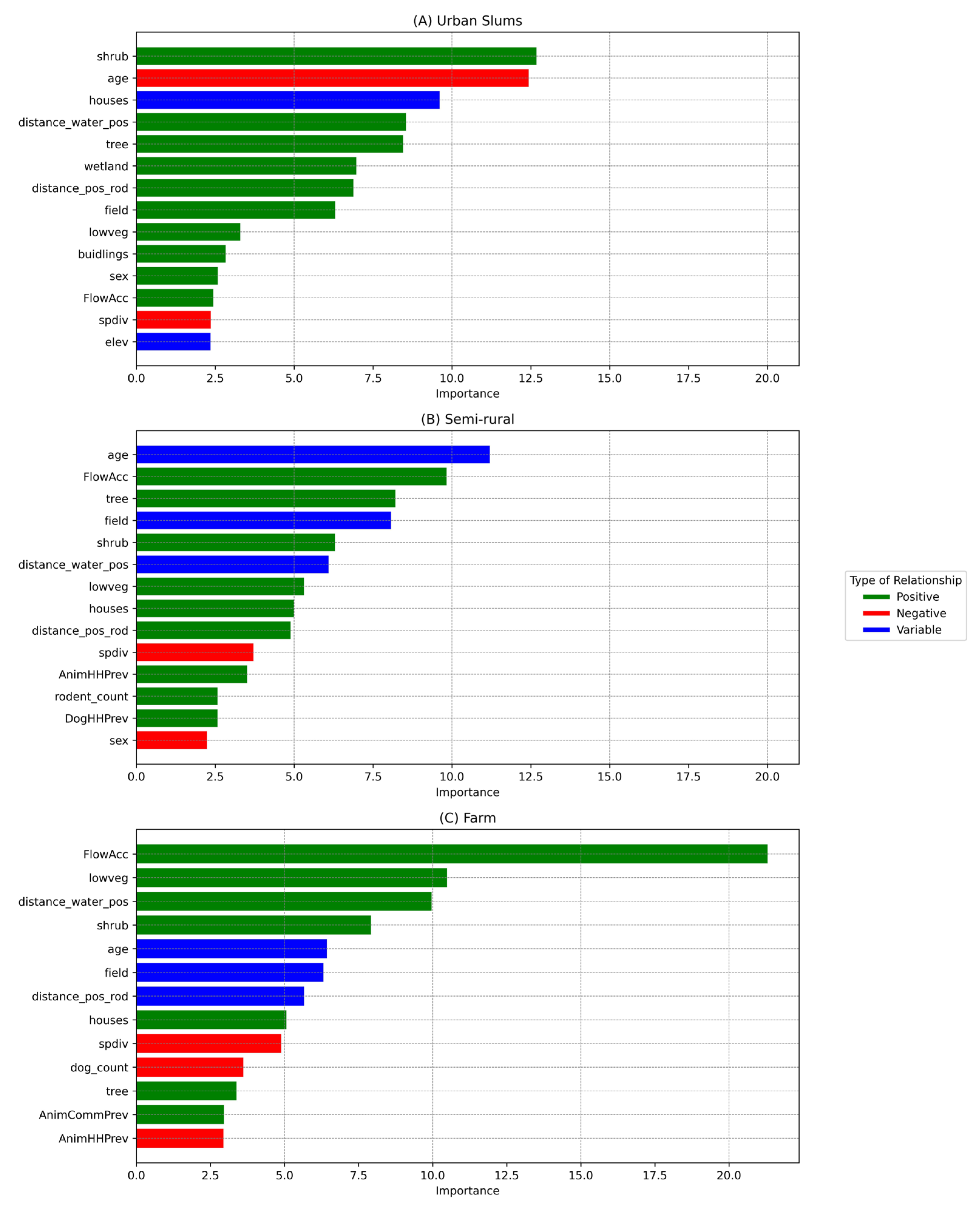
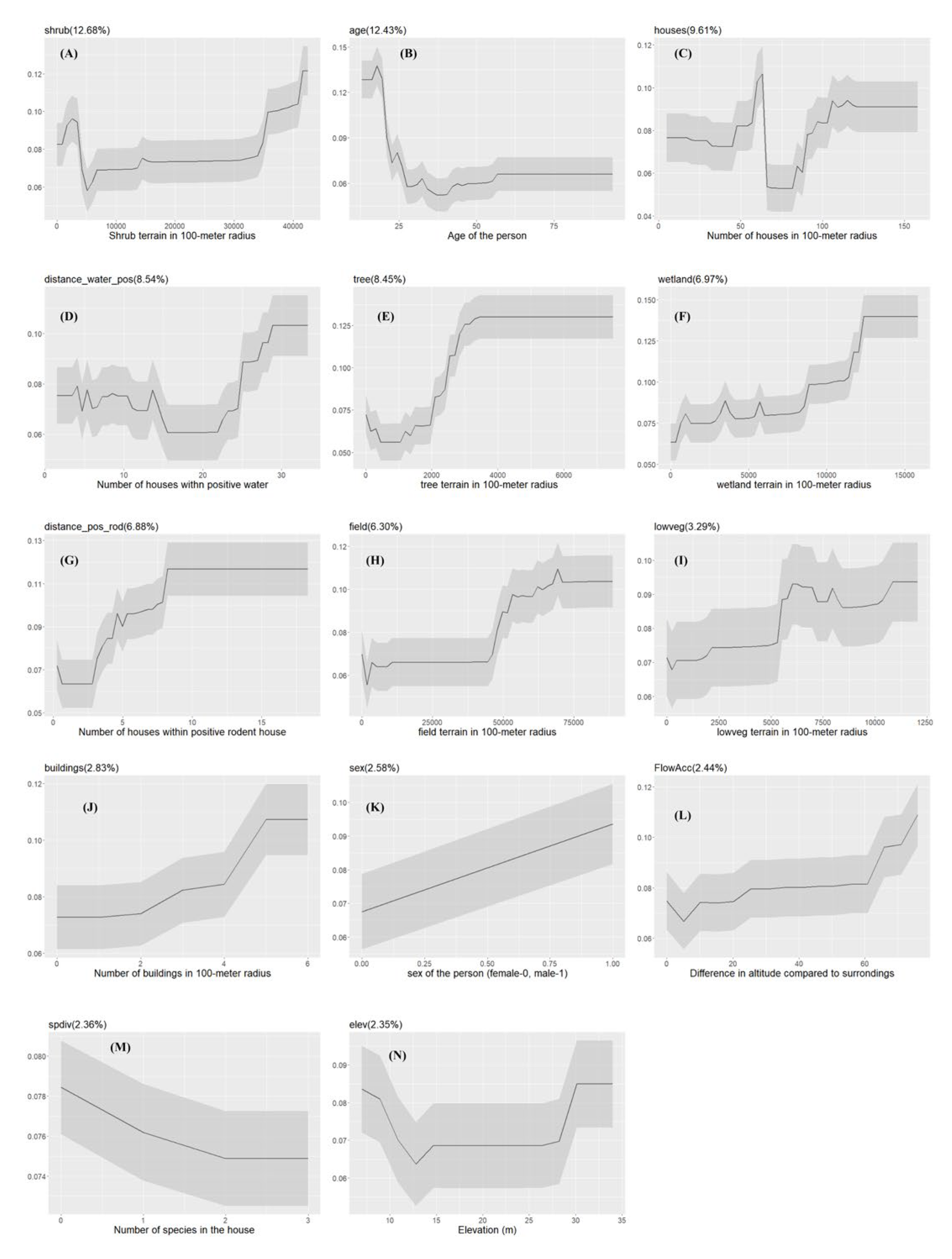
3.2. Urban Slum Community
3.3. Semi-Rural Community
3.4. Farm Community
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Variables | Number of Participants | MAT positive | seroprevalence (%) | 95% CI |
|---|---|---|---|---|
| Sex of the person | ||||
| Male | 387 | 25 | 6.46 | 4.22-9.39 |
| Female | 520 | 29 | 5.57 | 3.77-7.91 |
| Person swim | ||||
| Yes | 629 | 43 | 6.84 | 4.99-9.09 |
| No | 278 | 11 | 3.96 | 1.99-6.97 |
| Positive rodents in the household | ||||
| Yes | 407 | 29 | 7.13 | 4.82-10.07 |
| No | 500 | 25 | 5.00 | 3.26-7.29 |
| Positive dog in the household | ||||
| Yes | 128 | 10 | 7.81 | 4.13-7.51 |
| No | 779 | 44 | 5.64 | 3.81-13.90 |
| Positive Cattle in the household | ||||
| Yes | 299 | 16 | 5.35 | 3.09-8.54 |
| No | 608 | 38 | 6.25 | 4.46-8.48 |
| Positive sheep in the household | ||||
| Yes | 282 | 13 | 4.61 | 2.48-7.75 |
| No | 625 | 41 | 6.56 | 4.75-8.79 |
| work in garden | ||||
| Yes | 269 | 11 | 4.09 | 2.06-7.20 |
| No | 638 | 43 | 6.73 | 4.92-8.97 |
| Clean barn | ||||
| Yes | 337 | 23 | 6.82 | 4.37-10.06 |
| No | 570 | 31 | 5.44 | 3.73-7.63 |
| Clean sewage drains | ||||
| Yes | 46 | 1 | 2.27 | 0.05-11.53 |
| No | 861 | 53 | 6.15 | 4.64-7.97 |
| Person slaughter | ||||
| Yes | 123 | 9 | 7.31 | 3.40-13.43 |
| No | 784 | 45 | 5.74 | 4.22-7.60 |
| Person milk animals | ||||
| Yes | 48 | 4 | 8.33 | 2.32-19.98 |
| No | 859 | 50 | 5.82 | 4.35-7.60 |
| Clean animal at birth | ||||
| Yes | 96 | 5 | 5.21 | 1.71-11.73 |
| No | 811 | 49 | 6.04 | 4.50-7.91 |
References
- Crecelius, E.M. and M.W. Burnett, Leptospirosis. J. Spec. Oper. Med., 2020. 20(4): p. 121.
- Haake, D.A., Levett, P.N. (2015). Leptospirosis in Humans. In: Adler, B. (eds) Leptospira and Leptospirosis. Current Topics in Microbiology and Immunology, vol 387. Springer, Berlin, Heidelberg.
- Costa, F., et al., Global Morbidity and Mortality of Leptospirosis: A Systematic Review. PLoS Negl Trop Dis, 2015. 9(9): p. e0003898. [CrossRef]
- Luna, J., et al., Assessment of Risk Factors in Synanthropic and Wild Rodents Infected by Pathogenic Leptospira spp. Cap-tured in Southern Chile. Animals, 2020. 10(11): p. 2133.
- Guerra, M.A., Leptospirosis: Public health perspectives. Biologicals, 2013. 41(5): p. 295-297. [CrossRef]
- Ellis, W.A., Animal leptospirosis. Curr Top Microbiol Immunol, 2015. 387: p. 99-137.
- Bradley, E.A. and G. Lockaby, Leptospirosis and the Environment: A Review and Future Directions. Pathogens, 2023. 12(9): p. 1167.
- Montes, V. and G. Monti, Pathogenic Leptospira spp. Seroprevalence and Herd-Level Risk Factors Associated with Chilean Dairy Cattle. Animals, 2021. 11(11): p. 3148.
- Harrison, N.A. and W.R. Fitzgerald, Leptospirosis--can it be a sexually transmitted disease? Postgrad Med J, 1988. 64(748): p. 163-4.
- Bolin, C.A. and P. Koellner, Human-to-Human Transmission of Leptospira interrogans by Milk. J. Infect. Dis., 1988. 158(1): p. 246-247.
- Muñoz-Zanzi, C., et al., Leptospira Contamination in Household and Environmental Water in Rural Communities in South-ern Chile. Int. J. Environ. Res. Public Health, 2014. 11(7): p. 6666-6680.
- Pappas, G., et al., The globalization of leptospirosis: worldwide incidence trends. Int J Infect Dis, 2008. 12(4): p. 351-7. [CrossRef]
- Miller, D.A., M.A. Wilson, and G.W. Beran, Relationships between prevalence of Leptospira interrogans in cattle, and regional, climatic, and seasonal factors. Am J Vet Res, 1991. 52(11): p. 1766-8. [CrossRef]
- Romero, E.C., C.C. Bernardo, and P.H. Yasuda, Human leptospirosis: a twenty-nine-year serological study in São Paulo, Bra-zil. Rev Inst Med Trop Sao Paulo, 2003. 45(5): p. 245-8.
- Mwachui, M.A., et al., Environmental and Behavioural Determinants of Leptospirosis Transmission: A Systematic Review. PLoS Negl Trop Dis, 2015. 9(9): p. e0003843.
- Muñoz-Zanzi, C., et al., Household characteristics associated with rodent presence and Leptospira infection in rural and ur-ban communities from Southern Chile. Am J Trop Med Hyg, 2014. 90(3): p. 497-506.
- Alexander, A.D., The distribution of leptospirosis in Latin America. Bull World Health Organ, 1960. 23(1): p. 113-25.
- Zamora, J., et al., [Serological survey of human leptospirosis in a high risk population in Chile]. Rev Med Chil, 1990. 118(3): p. 247-52.
- Lelu, M., et al., Seroepidemiology of leptospirosis in dogs from rural and slum communities of Los Rios Region, Chile. BMC Vet Res, 2015. 11(1): p. 31. [CrossRef]
- Mason, M.R., et al., Distribution and Diversity of Pathogenic Leptospira Species in Peri-domestic Surface Waters from South Central Chile. PLoS Negl Trop Dis, 2016. 10(8): p. e0004895. [CrossRef]
- Munoz-Zanzi, C., C. Campbell, and S. Berg, Seroepidemiology of toxoplasmosis in rural and urban communities from Los Rios Region, Chile. Infect Ecol Epidemiol, 2016. 6: p. 30597.
- Fick, S.E. and R.J. Hijmans, WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol., 2017. 37(12): p. 4302-4315.
- Chen, T. and C. Guestrin, XGBoost: A Scalable Tree Boosting System, in Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining. 2016, Association for Computing Machinery: San Francisco, California, USA. p. 785–794.
- Ogunleye, A. and Q.G. Wang, XGBoost Model for Chronic Kidney Disease Diagnosis. IEEE/ACM Trans Comput Biol Bioinform, 2020. 17(6): p. 2131-2140. [CrossRef]
- Shaheed, K., et al., Optimized Xception Learning Model and XgBoost Classifier for Detection of Multiclass Chest Disease from X-ray Images. Diagnostics (Basel), 2023. 13(15). [CrossRef]
- Aydin ZE, Ozturk ZK. Performance analysis of xgboost classifier with missing data. In: Proceedings of the 1st international conference on computing and machine intelligence, Vol. 2. 2021.
- Putatunda, S. and K. Rama, A Comparative Analysis of Hyperopt as Against Other Approaches for Hyper-Parameter Opti-mization of XGBoost, in Proceedings of the 2018 International Conference on Signal Processing and Machine Learning. 2018, Asso-ciation for Computing Machinery: Shanghai, China. p. 6–10.
- Davagdorj, K., et al., XGBoost-Based Framework for Smoking-Induced Noncommunicable Disease Prediction. Int J Environ Res Public Health, 2020. 17(18).
- Srinivas, P. and R. Katarya, hyOPTXg: OPTUNA hyper-parameter optimization framework for predicting cardiovascular dis-ease using XGBoost. Biomed. Signal Process. Control, 2022. 73: p. 103456.
- Farooq, Z., et al., Artificial intelligence to predict West Nile virus outbreaks with eco-climatic drivers. Lancet Reg Health Eur, 2022. 17: p. 100370.
- Jin, H. and C.X. Ling, Using AUC and accuracy in evaluating learning algorithms. IEEE Transactions on Knowledge and Data Engineering, 2005. 17(3): p. 299-310.
- R Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Aus-tria. 2020.
- Chen T, H.T., Benesty M, Khotilovich V, Tang Y, Cho H, Chen K, Mitchell R, Cano I, Zhou T, Li M, Xie J, Lin M, Geng Y, Li Y, Yuan J, xgboost: Extreme Gradient Boosting. . 2023.
- Karpagam, K.B. and B. Ganesh, Leptospirosis: a neglected tropical zoonotic infection of public health importance-an updated review. Eur J Clin Microbiol Infect Dis, 2020. 39(5): p. 835-846. [CrossRef]
- Notobroto, H.B., Y.A. Mirasa, and F.S. Rahman, Sociodemographic, behavioral, and environmental factors associated with the incidence of leptospirosis in highlands of Ponorogo Regency, Province of East Java, Indonesia. Clin. Epidemiol. Glob. Health, 2021. 12. [CrossRef]
- Romero, M., J. Sanchez, and L. Hayek, Prevalencia de anticuerpos contra Leptospira en población urbana humana y canina del Departamento del Tolima. Revista de Salud Pública, 2010. 12: p. 268-275. [CrossRef]
- Alvarado-Esquivel, C., et al., Leptospira Exposure and Gardeners: A Case-Control Seroprevalence Study. J Clin Med Res, 2016. 8(1): p. 25-8.
- Benschop, J., et al., Sero-prevalence of leptospirosis in workers at a New Zealand slaughterhouse. N Z Med J, 2009. 122(1307): p. 39-47.
- Carrero, S., et al., Seroprevalencia de infección por Leptospira y factores de riesgo en estudiantes de una universidad de Co-lombia. Nova, 2017. 15: p. 131.
- Dias, J.P., et al., Factors associated with Leptospira sp infection in a large urban center in northeastern Brazil. Rev Soc Bras Med Trop, 2007. 40(5): p. 499-504. [CrossRef]
- Wynwood, S.J., et al., Leptospirosis from water sources. Pathog Glob Health, 2014. 108(7): p. 334-8. [CrossRef]
- Goarant, C., Leptospirosis: risk factors and management challenges in developing countries. Res Rep Trop Med, 2016. 7: p. 49-62. [CrossRef]
- Davignon, G., et al., Leptospirosis: toward a better understanding of the environmental lifestyle of Leptospira. Front. Water, 2023. 5.
- Moseley, M., et al., Mixed Leptospira Infections in a Diverse Reservoir Host Community, Madagascar, 2013-2015. Emerg Infect Dis, 2018. 24(6): p. 1138-1140.
- Cucchi, K., et al., Hydroclimatic drivers of highly seasonal leptospirosis incidence suggest prominent soil reservoir of patho-genic Leptospira spp. in rural western China. PLoS Negl Trop Dis, 2019. 13(12): p. e0007968.
- Cunha, M., et al., Rainfall and other meteorological factors as drivers of urban transmission of leptospirosis. PLoS Negl Trop Dis, 2022. 16(4): p. e0007507. [CrossRef]
- Kocher, A., et al., Biodiversity and vector-borne diseases: Host dilution and vector amplification occur simultaneously for Amazonian leishmaniases. Mol. Ecol., 2023. 32(8): p. 1817-1831. [CrossRef]
- Chiani, Y.T., et al., Presence of Leptospira spp. in a Mosaic of Wetlands Used for Livestock Raising under Differing Hydrocli-matic Conditions. Appl Environ Microbiol, 2023. 89(6): p. e0197122.
- Caley, P. and D. Ramsey, Estimating disease transmission in wildlife, with emphasis on leptospirosis and bovine tuberculosis in possums, and effects of fertility control. J. Appl. Ecol, 2001. 38(6): p. 1362-1370. [CrossRef]
- Bacallao, J., et al., Socioeconomic factors and vulnerability to outbreaks of leptospirosis in Nicaragua. Int J Environ Res Public Health, 2014. 11(8): p. 8301-18. [CrossRef]
- Zakharova, O.I., et al., Ecological and Socio-Economic Determinants of Livestock Animal Leptospirosis in the Russian Arctic. Front Vet Sci, 2021. 8: p. 658675. [CrossRef]
- Baquero, O.S. and G. Machado, Spatiotemporal dynamics and risk factors for human Leptospirosis in Brazil. Sci Rep, 2018. 8(1): p. 15170.
- Hagan, J.E., et al., Spatiotemporal Determinants of Urban Leptospirosis Transmission: Four-Year Prospective Cohort Study of Slum Residents in Brazil. PLoS Negl Trop Dis, 2016. 10(1): p. e0004275.
- Reis, R.B., et al., Impact of environment and social gradient on Leptospira infection in urban slums. PLoS Negl Trop Dis, 2008. 2(4): p. e228.
- Kembhavi, R.S., G.D. Velhal, and A.K. Shah, Epidemiological determinants of leptospirosis in rural and urban districts of Maharashtra, India. J Family Med Prim Care, 2021. 10(9): p. 3361-3367.
- Sluydts, V., et al., Ecology and distribution of Leptospira spp., reservoir hosts and environmental interaction in Sri Lanka, with identification of a new strain. PLoS Negl Trop Dis, 2022. 16(9): p. e0010757.
- Daniels, M.E., et al., Waterborne exposure during non-consumptive domestic use of surface water: a population study across WASH service levels in rural India. J Water Health, 2023. 21(6): p. 751-762.
- Galan, D.I., et al., Epidemiology of human leptospirosis in urban and rural areas of Brazil, 2000-2015. PLoS One, 2021. 16(3): p. e0247763.
- Awoniyi, A.M., et al., Population dynamics of synanthropic rodents after a chemical and infrastructural intervention in an urban low-income community. Sci Rep, 2022. 12(1): p. 10109.
- Céspedes Z, M., et al., Prevalencia de Leptospirosis y factores de riesgo en personas con antecedentes de fiebre en la Provincia de Manu, Madre de Dios, Perú. Rev. Peru Med. Exp. Salud Publica, 2003. 20: p. 80-185.
- Maze, M.J., et al., Risk factors for human acute leptospirosis in northern Tanzania. PLoS Negl Trop Dis, 2018. 12(6): p. e0006372. [CrossRef]
- Brockmann, S.O., et al., Risk factors for human Leptospira seropositivity in South Germany. Springerplus, 2016. 5(1): p. 1796. [CrossRef]
- Anderson, T., et al., Animals Exposed to Leptospira Serogroups Not Included in Bacterins in the United States and Puerto Ri-co. Trop Med Infect Dis, 2023. 8(3).
- Harran, E., et al., Identification of Pathogenic Leptospira kirschneri Serogroup Grippotyphosa in Water Voles (Arvicola ter-restris) from Ruminant Pastures in Puy-de-Dome, Central France. Pathogens, 2023. 12(2).
| Type | Variable Name | Description | Source |
|---|---|---|---|
| Socio-demographic and household characteristics | sex | Sex of the person | Questionnaire |
| age | Age of the person (in years) | ||
| clean_barn | Person cleans barns | ||
| clean_drain | Person cleans drains in the field | ||
| slaughter | Person butchers meat | ||
| milking | Person milks cows | ||
| clean_birth | Person cleans cow birth products | ||
| clean_water_drain | Person cleans water drains | ||
| clean_field | Person cleans fields | ||
| swim | Person swims | ||
| season | Sampling season | ||
| house | Number of houses within 100-meter radius | Derived from worldview-2 satellite imagery | |
| buildings | Number buildings within 100-meter radius | ||
| Environmental | elev | Altitude of sampled household | Derived from worldview-2 satellite imagery |
| FlowAcc | Difference in altitude compared to surroundings (higher numbers means greater slope downward) | ||
| tree | Square meters of tree-dominated terrain within 100-meter radius | ||
| lowveg | Square meters of lower-vegetation terrain within 100-meter radius (e.g., bushes and other short plants) | ||
| shrub | Square meters of shrub-dominated terrain within 100-meter radius | ||
| wetland | Square meters of wetland terrain within 100-meter radius | ||
| field | Square meters of field terrain within 250-meter radius | ||
| bio1 | Annual mean temperature | worldclim.org | |
| bio2 | Mean Diurnal Range (Mean of monthly (max temp – min temp)) | ||
| bio12 | Annual Precipitation | ||
| bio15 | Precipitation Seasonality (Coefficient of Variation) | ||
| puddle_pos_com | Proportion of Leptospira positive puddles in the community | Laboratory testing | |
| water_prev_com | Proportion of Leptospira positive water samples in the community (all water source types) | ||
| distance_pos_water | Number of households within 100 meters with Leptospira-positive water samples weighted inversely by distance from house | Derived from worldview-2 satellite imagery | |
| Animal | rodent_count | Number of rodents trapped in the household | Questionnaire |
| rod_pos | Presence of Leptospira positive rodents in the household | Derived | |
| rodent_count_com | Number of rodents trapped in the community | Questionnaire | |
| RodHHPrev | Leptospira prevalence in rodents at household level | Derived | |
| rodent_prev_com | Leptospira prevalence in rodents in the community | Derived | |
| distance_pos_rod | Number of households within 100-meter with Leptospira-positive rodents weighted inversely by distance from house | Derived from worldview-2 satellite imagery | |
| spdiv | Number of different domestic animal species in the household | Derived | |
| bov_count | Number of bovines in the household | Questionnaire | |
| bov_pos | Presence of seropositive bovines in the household | Derived | |
| BovHHPrev | Leptospira seroprevalence in bovines at household level | ||
| bov_com_pos | Number of seropositive bovines in the community | ||
| bov_com_prev | Leptospira seroprevalence in bovines at community level | ||
| ovi_count | Number of ovines in the household | Questionnaire | |
| ovi_pos | Presence of seropositive ovines in the household | Derived | |
| OviHHPrev | Leptospira seroprevalence in ovines at household level | ||
| ovi_pos_com | Number of seropositive ovines in the community | ||
| OviComPrev | Leptospira seroprevalence in ovines at community level | ||
| dog_count | Number of dogs in the household | Questionnaire | |
| dog_pos | Presence of seropositive dogs in the household | Derived | |
| DogHHPrev | Leptospira seroprevalence in dogs at household level | ||
| dog_com_pos | Number of seropositive dogs in the community | ||
| DogComPrev | Leptospira seroprevalence in dogs at community level | ||
| Anim_pos | Presence of seropositive animals in the household | ||
| AnimalHHPrev | Leptospira seroprevalence in farm animals at household level | ||
| animal_pos_com | Number of overall seropositive farm animals in the community | ||
| AnimCommPrev | Leptospira seroprevalence in farm animals at community level |
| Parameter | Description | Range | Interval |
|---|---|---|---|
| scale_pos_weight | Weight of positive class to address class imbalance | Neg/pos | Fixed |
| nrounds | Number of boosting rounds or iterations during the training process. | 100-600 | 50 |
| learning_rate | Learning rate for gradient boosting | 0-1 | 0.01 |
| max_depth | Maximum depth of the decision tree | 0-10 | 1 |
| min_child_weight | Minimum sum of instance weight (Hessian) needed in a child | 0-10 | 1 |
| gamma | Minimum loss reduction required to make a further partition on a leaf node | 0-5 | 0.1 |
| subsample | Fraction of training data to randomly sample during training | 0-1 | 0.1 |
| colsample_bytree | Fraction of features to be randomly sampled for each tree | 0-1 | 0.1 |
| objective | Learning task and objective function (binary classification in this case) | Binary:logistic | |
| Max_delta_step | Introduce an ‘absolute’ regularization capping the weight before apply eta correction. | 1-10 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





