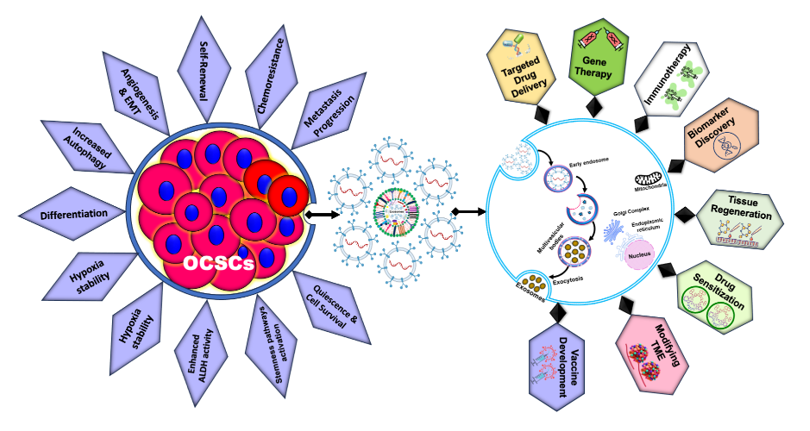
Background
Oral cancer (OC) presents a significant global health burden, with rising incidence rates. It predominantly affects the mucosal surfaces of the oral cavity, lips, gums, tongue, and throat [
1,
2]. It is a significant public health problem in developing countries including India, where it is the most prevalent cancer among men. It is the 6
th most common cancer globally, with India is having the highest number of oral cancer cases [
3,
4]. Despite advances in therapeutic modalities such as surgery, radiation therapy, chemotherapy and immunotherapy [
5], the survival rates for oral cancer, particularly those with advanced or recurrent disease, remains poor and have not substantially improved over the last few decades [
6]. However, five-year survival remains low (40-50%), mainly due to metastatic invasion, local recurrence and chemoresistance [
7,
8,
9]. Oral cancer spreads in two primary ways: it invades nearby tissues directly and establishes distant metastases by seeding pre-metastatic niches with secreted elements like exosomes [
10]. One of the major hurdles in effectively treating oral cancer lies in its intrinsic heterogeneity and chemoresistance, is often attributed due to the presence of subpopulations of cells called cancer stem cells (CSCs). CSCs are a small subset of cancer cells with the ability to self-renew and differentiate, contributing to cancer initiation, metastasis, and resistance to conventional therapies [
11,
12].
Recent studies have increasingly highlighted the significance of molecular pathways and signals mediated by exosomes-small extracellular vesicles (EVs) (50-150 nm) released from host cells that facilitate intercellular communication through the transfer of proteins, lipids, and nucleic acids (DNA, mRNA, non-coding RNAs) [
13]. These exosomes show great potential in regulating the disease progression and metastasis. They are present in various body fluids, including blood, urine, saliva, and cerebrospinal fluid, in cancer stem cells (CSCs) and can be altered in response to various physiological and pathological conditions, highlighting their potential as biomarkers for cancer diagnostic and therapeutic. Moreover, exosomes are being explored as therapeutic agents and drug delivery vehicles due to their natural ability to cross biological barriers and deliver their cargo to specific target cells.
Recent research has highlighted the intricate relationship between CSCs and exosomes, revealing that CSC-derived exosomes play crucial roles in modulating the tumor microenvironment, promoting angiogenesis, immune evasion, and metastasis [
14,
15,
16,
17]. Emerging evidence further suggests that exosomes interact with the tumor microenvironment (TME) and may be valuable in diagnosing and treating oral cancer patients. Exosomes derived from oral cancer stem cells (OCSCs) are believed to play crucial roles in creating a tumor-promoting microenvironment, regulating angiogenesis and stemness, mediating drug resistance, and enhancing the metastatic potential of tumor cells [
10,
18,
19]. Understanding the complex interplay between CSCs and exosomes is essential for developing novel therapeutic strategies targeting these elements to inhibit cancer progression and overcome therapeutic resistance. This review provides an overview of the current understanding of CSCs and exosomes, emphasizing their roles in oral cancer and their potential as targets for cancer therapies.
Cancer Stem Cells: Biological Functions and Characteristics
Cancer stem cells (CSCs), also known as cancer stem-like cells, constitute a small, heterogeneous, and highly tumorigenic subset of tumor cells, accounting for approximately 0.05-3% of the tumor population. These cells are endowed with the capacity for unregulated growth, proliferation, and self-renewal, enabling them to differentiate into multiple cell types. Such capabilities are critical for tumor invasion, aggressive metastasis, recurrence, and the development of drug resistance, issues central to cancer pathology and treatment challenges [
20,
21,
22,
23]. CSCs are particularly noteworthy for their ability to self-renew-a process where CSCs generate new cells that retain stem-like properties, crucial for maintaining the CSC population within tumors. These cells can undergo either symmetric division, producing two identical stem-like daughter cells, or asymmetric division, yielding one stem cell and one differentiated non-stem cancer cell [
24]. This divisional versatility not only helps preserve their numbers but also contributes to the expansion of the tumor mass, thereby enhancing tumor growth and the potential for recurrence [
25,
26].
The concept of CSCs can be traced back to the foundational theories of Virchow and Cohnheim in the 1870s. Later, the definitive identification of CSCs occurred in 1997 when Bonnet and colleagues isolated a subpopulation in acute myeloid leukemia characterized by the expression of specific surface markers (CD34+ but CD38-) [
27].
Techniques such as the dye efflux method have been essential for identifying CSCs by isolating "side population" cells capable of excluding certain dyes. Moreover, the ability of CSCs to form spherical colonies in non-adherent, differential growth factor conditions is another critical hallmark for their identification. Several studies have highlighted the role of putative CSC markers in various cancers including those of the skin, brain, lung, liver, breast, cervix, prostate, ovary, colorectal region, and head and neck [
11,
12,
28,
29,
30,
31,
32]. CSCs express distinctive cell surface markers that vary across cancer types, for instance, ALDH+, CD44+, and CD133+ in oral cancer, [
33]; CD34+ in leukemia [
27], and CD200+ and CD166+ in colorectal cancer [
34,
35]. Based on these stemness markers, CSCs can be specifically isolated and characterized. These markers are crucial not only for the isolation of CSCs but also for distinguishing them from non-CSC tumor cells.
Moreover, CSCs are characterized by dysregulated signaling pathways, such as Hedgehog, Wnt/β-catenin, PI3K/AKT/mTOR, NOTCH, and JAK-STAT, which play pivotal roles in maintaining their pathological state [
36,
37,
38,
39,
40,
41,
42]. These aberrations contribute significantly to the deregulation of CSCs, promoting the epithelial-mesenchymal transition (EMT). EMT is a critical process that facilitates increased stemness, enhanced self-renewal capabilities, and greater invasive and metastatic potential. It also plays a significant role in chemoresistance and the potential for tumor relapse. Given the distinctive surface markers and altered signalling pathways exhibited by CSCs, they represent critical targets in the development of cancer therapeutics. Targeting these pathways could potentially curb the stemness and the associated malignant traits of tumors, thereby offering a promising avenue for enhancing cancer treatment efficacy and patient outcomes.
Oral Cancer-Derived Stem Cells
Oral cancer remains a major global health challenge, characterized by its aggressive behavior, high recurrence rate, metastasis and drug resistance [
43]. Central to these challenges are cancer stem cells (CSCs), which play critical roles in tumor initiation, progression, and resistance to conventional therapies [
44]. Oral cancer stem cells (OCSCs) are identified by specific surface markers such as CD44, CD133, and ALDH1, which not only facilitate their isolation but also serve as potential therapeutic targets [
12,
45].
OCSCs play a crucial role in the metastasis and tumor recurrence, owing to their ability to self-renew and differentiate into diverse cell types that make up the tumor mass [
46]. These cells efficiently drive tumor growth and metastasis through epithelial-to-mesenchymal transition (EMT), a process that enhances the migratory and invasive properties of cancer cells [
47]. EMT is also linked to increased resistance to apoptosis and altered cellular metabolism, making OCSCs particularly resistant to standard cancer therapies [
48,
49]. The resistance of OCSCs to therapies such as chemotherapy and radiation is partly due to their enhanced DNA repair capabilities and ability to remain in a quiescent state, making them less susceptible to treatments targeting rapidly dividing cells. Additionally, OCSCs express high levels of drug efflux pumps, which actively transport chemotherapeutic agents out of the cells, reducing drug efficacy [
50,
51,
52].
To address these challenges, targeted therapies that specifically eliminate OCSCs without harming normal tissues are imperative. Emerging data focuses on pathways regulating OCSC properties, including Notch, Wnt, and Hedgehog signalling pathways, which are crucial for maintaining stemness and survival of these cells. Additional pathways, such as the NF-κB, AP-1, PI3K/Akt and JAK/STAT pathways, also play significant roles in regulating OCSC stemness and resistance to therapy [
37,
41,
53,
54]. Inhibitors targeting these pathways are currently being tested in preclinical trials and offer promising avenues for potentially curative oral cancer therapies [
55]. Recently, it has been shown that Spalt-like transcription factor 4 (SALL4), a downstream target of methyltransferase-like 3 (METTL3), activates the Wnt/β-catenin pathway post-radiation therapy, enhancing the CSC phenotype and leading to radio-resistance in oral cancer. This METTL3/SALL4 axis offers a potential therapeutic target to eliminate radioresistant oral cancer cells [
56]. Thus, the identification and understanding of OCSCs have profound implications for managing oral cancer. Targeting the root of tumor propagation and resistance, new therapeutic strategies aimed at OCSCs hold the promise of more effective and lasting treatments. This approach aligns with the broader goal of precision medicine, which aims to tailor treatments based on individual patient characteristics and the genetic profile of their tumors. Further research and clinical trials will be crucial in translating these findings into effective clinical therapies that can significantly improve outcome of oral cancer patient.
Exosomes and Their Cargo
Exosomes have garnered significant attention in recent years due to their critical roles in intercellular communication and their potential therapeutic applications [
57]. These small, membrane-bound extracellular vesicles (EVs) carry a diverse array of biomolecules, including proteins, lipids, nucleic acids, and metabolites, making them significant components of cellular interactions during numerous physiological and pathological processes [
58,
59,
60]. Initially discovered in the late 1980s as a mechanism for sheep reticulocytes to dispose of transferrin receptors during maturation [
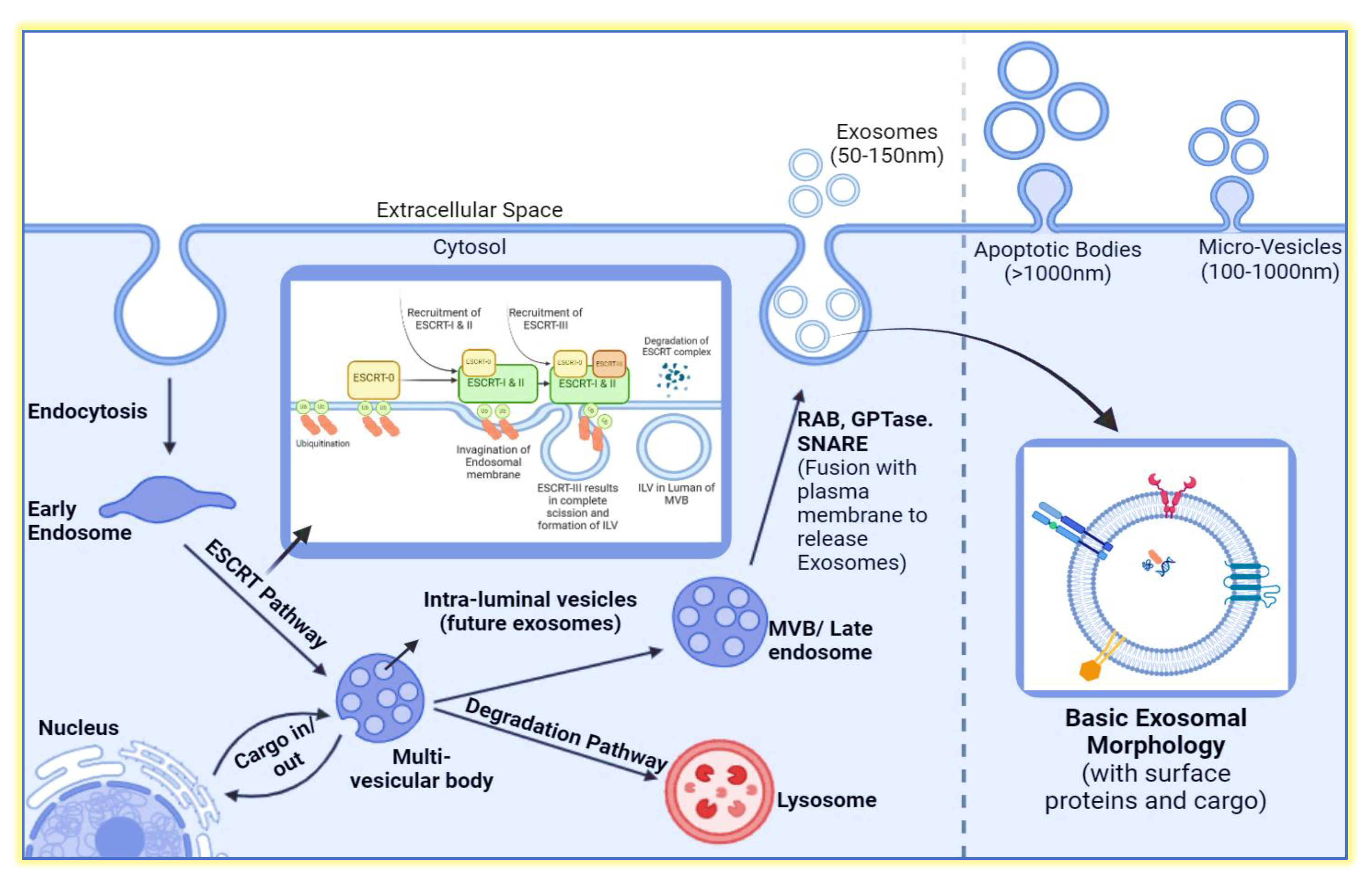
61], exosomes have since been recognized for their broader roles in cell-to-cell communication. EVs are categorized into three main types based on their origin and size: apoptotic bodies (1,000-5,000 nm), formed during programmed cell death; microvesicles (200-1,000 nm), which bud directly from the plasma membrane; and exosomes (50-150 nm), originating from the endosomal pathway (
Figure 1). This classification underscores the diverse nature and roles of EVs in cellular communication.
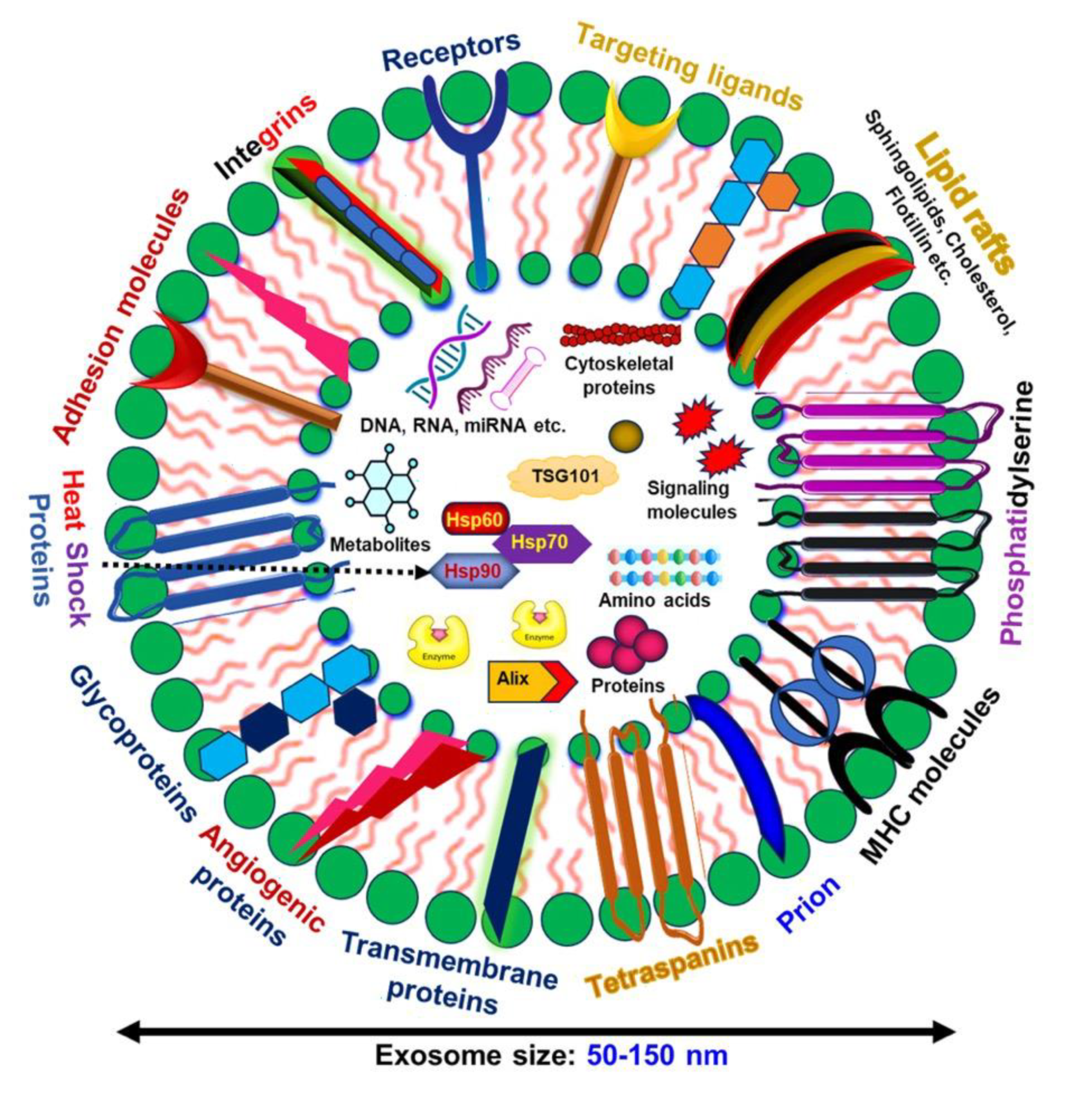
Exosomes are bi-layered vesicles that resemble the plasma membrane of their origin cell, formed through endocytosis [
62]. Key components of exosomes include cholesterol, sphingomyelin, ceramides, receptors, and targeting ligands, which provide structural stability. Proteins such as CD9, CD63, and CD81 (tetraspanins) serve as markers for exosome identification, facilitating their interaction with recipient cells. Heat shock proteins (HSP70, HSP90) in exosomes assist in maintaining protein stability under stress conditions. Proteins involved in exosome biogenesis, such as Alix and Tsg101, and adhesion molecules like integrins (Integrin-α and Integrin-β), play roles in exosome docking and fusion with target cells (Figure1).
Lipids are crucial for the structural integrity of exosomes, with an enriched composition of cholesterol, sphingomyelin, and ceramides conferring rigidity and stability. These lipids influence the interaction and uptake of exosomes by recipient cells. Nucleic acids, including DNA, mRNA, and non-coding RNAs such as microRNAs (miRNAs), are key components of exosome cargo. These nucleic acids can modulate gene expression and influence cellular behaviour in recipient cells. The transfer of miRNAs via exosomes regulates numerous biological processes, including cell proliferation, apoptosis, and immune responses, highlighting their potential as therapeutic delivery vehicles.
Recent studies have elucidated the diverse functions of exosomes in intercellular communication, particularly their roles in cancer progression, metastasis, immune response modulation, and therapeutic delivery [
63,
64,
65,
66,
67,
68]. Exosomes can carry oncogenic proteins and RNAs that promote tumor progression and create a favorable microenvironment for cancer cells. Conversely, exosomes from healthy cells are being explored for their potential to deliver therapeutic agents and modulate immune responses against tumors. The evolving understanding of exosome biology underscores their importance in both physiological and pathological contexts, making them a focal point of current biomedical research.
Exosome Biogenesis
Exosome biogenesis is a complex, tightly regulated process crucial for cell-to-cell communication in various physiological and pathological contexts. The formation of an exosome begins with the invagination (endocytosis) of the plasma membrane to produce an early endosome, which subsequently undergoes further invaginations and sorting of different cargos, leading to the formation of multivesicular bodies (MVBs), also known as late endosomes (
Figure 2). Within these MVBs, intraluminal vesicles (ILVs) are formed, containing cargos such as proteins, DNA, RNA, and enzymes. Upon fusion of MVBs with the plasma membrane, ILVs are released as exosomes into the extracellular environment. Alternatively, MVBs can fuse with lysosomes, leading to cargo degradation [
68,
69]. From here, the MVBs can go through one of the two fates; Either releasing ILVs into the extracellular space in the form of exosomes, micro vesicles and apoptotic bodies or underdo degradation after fusing themselves with Lysosome [
70].
Recent studies highlight that cargo selection during exosome formation is a selective process driven by proteins and lipids. The ESCRT (Endosomal Sorting Complex Required for Transport) machinery, along with proteins like ALIX and TSG101, plays a key role in sorting ubiquitinated proteins into ILVs [
71,
72]. Tetraspanins, such as CD63 and CD81, are also crucial for sorting specific bioactive molecules into exosomes, influencing their composition and function. The ESCRT machinery, comprising four protein complexes (ESCRT-0 to ESCRT-III), orchestrates ILV formation and cargo sorting. Proteins destined for ILVs are labeled with ubiquitin and recognized by the ESCRT-0 complex, which recruits ESCRT-I and ESCRT-II for cargo sorting and endosomal membrane budding. ESCRT-III polymerization then facilitates bud scission, releasing ILVs into the MVB lumen (see
Figure 2).
The lipid composition of exosomes, enriched with cholesterol, ceramide, and sphingolipids, dictates membrane curvature and vesicle formation, playing a role in the selective packaging of signaling molecules. Ceramide, produced by neutral sphingomyelinase, is particularly emphasized in exosome biogenesis independent of the ESCRT machinery, suggesting alternative pathways. The functional implications of exosome biogenesis are vast, affecting normal physiological processes such as immune responses and tissue repair, as well as the pathogenesis of diseases, including cancer. Understanding exosome biogenesis has expanded our knowledge of cellular communication and opened new avenues for biomarker discovery and the development of novel therapeutic strategies.
Isolation and Characterization of CSC-Derived Exosomes
CSC-derived exosomes significantly influence the tumor microenvironment, promote metastasis, and confer drug resistance, making their isolation and characterization vital for developing targeted cancer therapies. Understanding and improving methods for isolating high-purity CSC-derived exosomes are essential for diagnostics and therapeutic development.
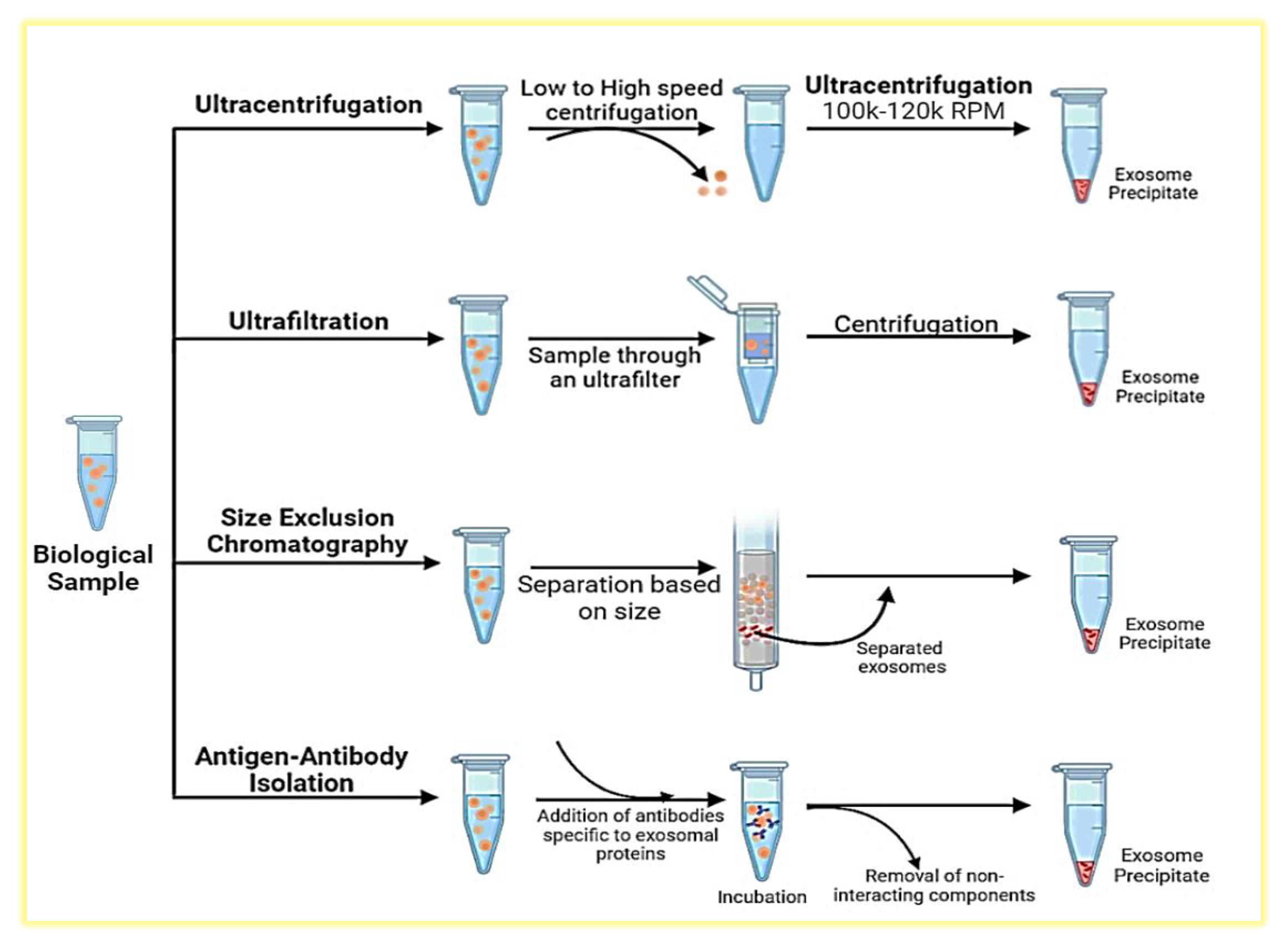
CSCs are isolated from tumor tissues or cell lines using flow cytometry or functional sphere formation assays, ensuring a population rich in CSC-specific stemness markers. These cells are cultured in exosome-depleted medium to promote the exclusive production of CSC-derived exosomes. Once the CSCs reach 70-80% confluency, the medium is collected and subjected to a series of centrifugation steps: a low-speed centrifugation at 300-500g to remove cellular debris, followed by a higher speed centrifugation at 2,000g to eliminate larger vesicles. The supernatant is then ultracentrifuged at 100,000g, resulting in a pellet of crude exosomes. This pellet is resuspended in phosphate-buffered saline (PBS) and further purified through a second ultracentrifugation or through size-exclusion chromatography (SEC) column (
Figure 3). SEC, utilizing materials such as Sephadex or BiogelP, separates exosomes based on size and is particularly effective for achieving high purity, essential for downstream analyses.
Characterization of exosomes involves morphological and molecular analyses. Electron microscopy, both Transmission and Scanning, is used to examine the size and structure of exosomes, typically ranging from 50-150nm [
73,
74]. For protein analysis, immunoblotting is employed to detect specific exosomal markers such as CD63, CD9, and TSG101, confirming the exosomal identity and purity. Additionally, ELISA is used to quantify these markers, enhancing the understanding of the surface protein composition of the exosomes. Further nanoparticle tracking analysis (NTA) is used to determine the concentration and size distribution of exosomes, offering quantitative insights that are vital for further biological and clinical evaluations. [
75,
76]. For additional specificity, immunoaffinity capture methods are employed, targeting exosomal surface proteins (e.g., CD63, CD9) with specific antibodies linked to magnetic beads, allowing for the selective isolation of CSC-derived exosomes. Together, these methodologies not only facilitate the detailed study of CSC-derived exosomes but also enhance our understanding of their biological roles and potential as targets for cancer therapy.
Role of CSC-Exos in Cancer Development and Drug Resistance
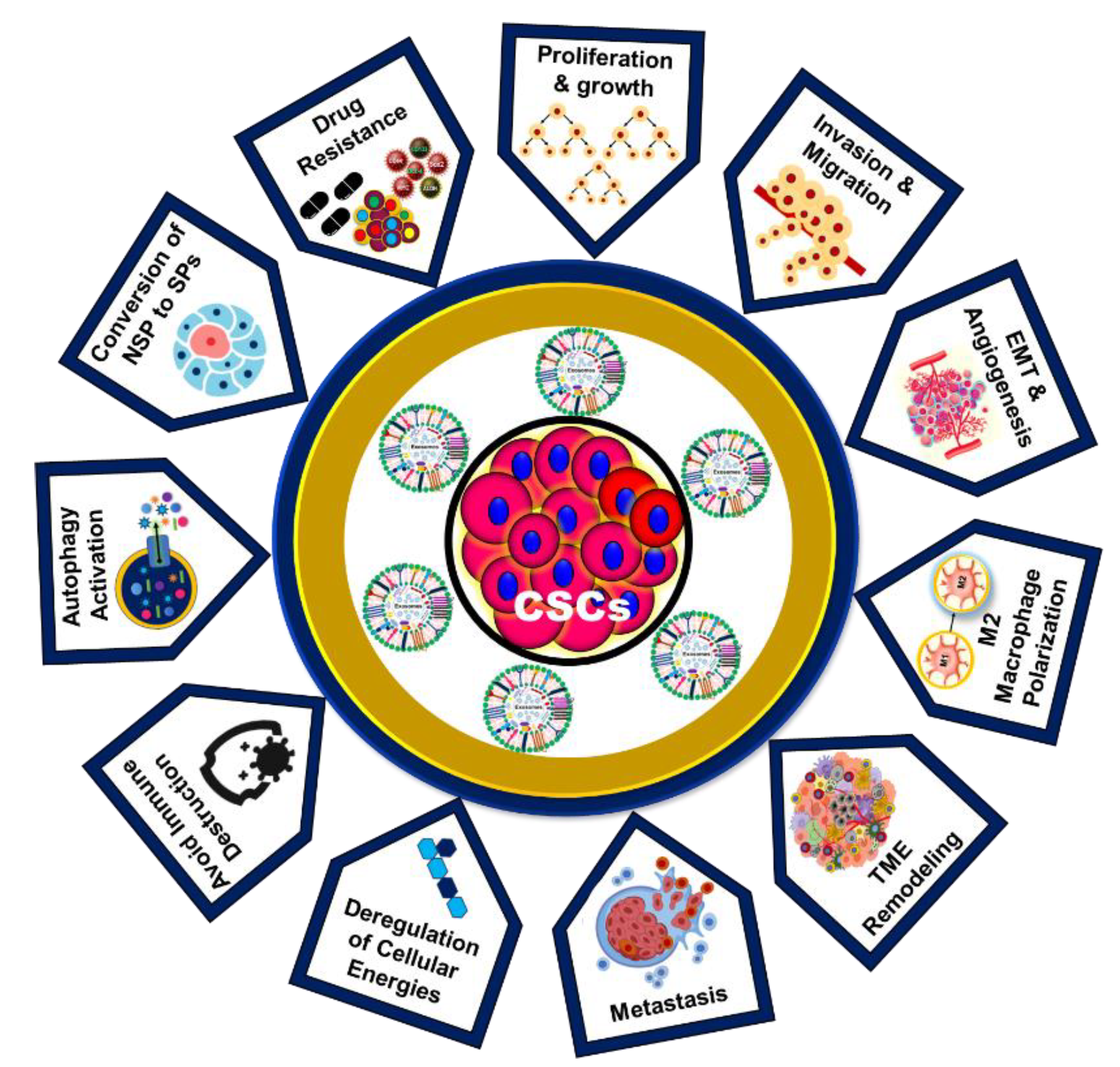
The role of CSC-Exos in cancer development represents a cutting-edge frontier in oncology research, offering novel insights and therapeutic opportunities. The unique ability of CSC-Exos to modulate the tumor microenvironment, influence immune responses, and confer drug resistance distinguishes them from other extracellular vesicles (
Figure 4). Unlike bulk cancer cells, CSCs possess the remarkable capacity for self-renewal and differentiation, and their exosomes reflect this unique biology, carrying distinct molecular cargos that drive tumorigenesis and metastasis by modulating the tumor microenvironment [
35].
Studies indicate that exosomes from diffuse large B-cell lymphoma (DLBCL) cells can induce a CSC phenotype in recipient cells, promoting self-renewal and maintaining stemness through Hedgehog, Wnt, and β-catenin pathways provides a new understanding of how CSCs maintain their population and contribute to tumor heterogeneity (
Table 1) [
77,
78].
A notable study demonstrated that mesenchymal stem cell (MSC)-derived exosomes containing miRNA-222 promote quiescence in breast cancer cells, leading to chemo-radio therapy resistance [
79]. Additionally, CSC-Exos are instrumental in the metastatic cascade by preparing the pre-metastatic niches, promoting epithelial-mesenchymal transition (EMT), and increasing matrix metalloproteinases (MMPs) expression, which leads to extracellular matrix (ECM) degradation and tumor invasion. The altered expression of miRNA-200 in CSC-Exos further promotes EMT, enhances stemness, and drives tumor invasion and metastasis, contributing to a more aggressive and drug-resistant phenotype. Furthermore, CSC-Exos expressing IL-6, p-STAT3, TGF-β1, and β-catenin promote the generation of cancer-associated fibroblasts and M2 macrophages in colon cancer (
Table 1) [
80].
CSC-Exos are rich in oncogenic factors such as miRNAs, mRNAs, and proteins that enhance proliferation, survival, and resistance to apoptosis in recipient cells. For instance, CSC-Exos enriched with miR-21 and miR-126 have been shown to activate the PI3K/AKT and MAPK/ERK pathways, promoting tumor growth and survival [
81]. Additionally, CSC-Exos significantly enhance angiogenesis by transferring pro-angiogenic factors like VEGF and angiopoietin to endothelial cells, promoting blood vessel formation [
82]. These exosomes influence the tumor microenvironment by interacting with various stromal cells, including fibroblasts, immune cells, and endothelial cells, promoting the secretion of growth factors, cytokines, and ECM components that create a supportive niche for tumor growth and metastasis. By ensuring a conducive environment for cancer progression, CSC-Exos play a pivotal role in cancer development [
83,
84].
Moreover, CSC-Exos aid in immune evasion by inducing the polarization of macrophages towards an immunosuppressive M2 phenotype and inhibiting the activation of cytotoxic T cells and NK cells, thereby creating an immunosuppressive microenvironment conducive to tumor growth. For example, CSC-Exos carrying PD-L1 suppress T cell activity and promote immune evasion [
85]. Interactions between ovarian cancer stem cells and macrophages through the WNT pathway further promote pro-tumoral and malignant phenotypes in engineered microenvironments [
86]. CSC-Exos also transfer drug-resistance factors, including drug efflux transporters, anti-apoptotic proteins, and miRNAs, to sensitive cancer cells, thereby conferring resistance to chemo-radio therapy and targeted therapies. For instance, CSC-Exos carrying P-glycoprotein (P-gp), a well-known drug efflux transporter, reduce intracellular drug accumulation and efficacy [
15,
16,
17,
87,
88]. CSC-Exos express PD-L1, leading to the upregulation of PD-1 on CD8+ T cells and promoting their exhaustion. These exosomes contain TGF-β and tenascin-C, which activate mTOR signaling and glycolysis via HIF-1α. Additionally, CSC-Exos carry Notch1, inducing stemness in non-tumor cells and further exacerbating CD8+ T cell exhaustion (
Table 1). These findings indicate new therapeutic opportunities for cancer immunotherapy [
89].
Understanding these mechanisms of CSC-Exos provides insight into the complex biology of cancer and highlights novel therapeutic targets. Sustained research in this area is crucial for developing effective interventions to counteract the pro-tumorigenic effects of CSC-Exos. Such advancements hold the potential to significantly improve patient outcomes.
Emerging Roles of CSC-Exos in Oral Cancer Progression
The role of CSC-derived exosomes (CSC-Exos) in oral cancer is pivotal in understanding high loco-regional recurrence rates and resistance to chemotherapy. These challenges are primarily due to oral cancer stem cells (OCSCs), which possess unique properties that allow them to evade immune detection and eradication and escape antineoplastic treatment [
90]. Recent studies have demonstrated that CSC-derived EVs significantly contribute to tumor progression, drug resistance, metastasis, angiogenesis, promoting stem-like characteristics in non-CSCs and remodelling the tumor microenvironment (TME) [
78,
79]. Given their origin, these exosomes carry genetic material from parental cells, suggesting a specific role in oral cancer progression. Heather Hardin et al (2018) revealed that in the CSC model, exosomes from a CSC clonal line transferred lncRNA MALAT1, SLUG, and SOX2 to normal thyroid cells, but EMT was not induced. However, when these exosomes also transferred linc-ROR, EMT was induced in the thyroid cells, and siRNA targeting linc-ROR reduced invasion. This suggests that CSC-exos transfer lncRNAs, particularly linc-ROR, to induce EMT and influence the TME and metastatic niche, highlighting potential therapeutic targets [
91].
Earlier, Mori et al. (2011) showed a positive correlation between M2 TAMs and oral cancer pathological grade in patient specimens [
92]. It has also been demonstrated that oral cancer-derived exosomes containing miR-29a-3p promote M2 macrophage polarization. Exosomes from oral cancer cells co-cultured with macrophages increased the expression of M2 markers CD163, CD206, Arg-1, and IL-10. This conditioned medium enhanced oral cancer cell invasion, proliferation and promoting tumorigenesis [
93]. OC-derived exosomes can reprogram monocytes via the NF-κB pathway and macrophages via miR-29a-3p, mediating immunosuppression in the tumor microenvironment. They also carry EMT-promoting cargoes such as miR-21 and miR-155, conferring chemoresistance to recipient cells. Additionally, exosomal miR-146a enhances oral cancer stemness, contributing to their resistance to DNA-damaging drugs [
94,
95,
96]. These findings emphasize the importance of immunomodulation by exosomes in developing therapeutic strategies against oral cancer chemoresistance.
A recent study by Patricia Gonzalez-Callejo et al. (2023) identified specific immune cell subsets within EVs derived from HNCs. These EVs selectively targeted MHC-II–macrophages and PD1+ T cells in the HNC-TME [
18], serving as therapeutic markers for oral cancer progression and treatment responses [
97]. It has been further shown that mesenchymal stem cell-derived exosomes (MSC-Exos) overexpressing miR-126 enhance cell growth, migration, survival, and angiogenesis by targeting the PI3K/Akt and MAPK/ERK signaling pathways [
98]. Conversely, miRNA-101 overexpression in MSC-Exos suppressed oral cancer cell proliferation, migration, and invasion. CSC-derived EVs also promote macrophages to exhibit an M2 phenotype. For instance, glioblastoma CSC-generated exosomes (GDEs) preferentially target monocytes, promoting their conversion into immunosuppressive M2 macrophages through upregulated PD-L1 expression due to components of the STAT3 pathway [
99].
OCSC-EVs induce a cancer-associated fibroblast phenotype in normal gingival fibroblasts, enhancing the oncogenicity of oral cancer cells. Treatment with Ovatodiolide, alone or combined with cisplatin, significantly reduces tumor sphere formation and decreases EV cargos through mTOR, PI3K, STAT3, β-catenin, and miR-21-5p [
100]. OCSC-derived small EVs transport the lncRNA UCA1, which sequesters miR-134, modulating the PI3K/AKT pathway via LAMC2 to drive macrophages toward an immunosuppressive M2 phenotype, leading to tumor growth and inhibition of T-cell function [
19]. OCSC-derived exosomes polarize tumor-associated macrophages (TAMs) into M2 macrophages by secreting urothelial carcinoma-associated 1 (UCA1), targeting the LAMC2-PI3K/AKT signaling pathway. These EVs also suppress anti-tumor immunity, including CD4+ T cell activation and interferon-γ production [
19]. Further, OC-CSC-derived EVs specifically interact with M2 macrophages and PD1+ T cells, essential immune constituents in the CSC niche, contributing to an immunosuppression that hinders effective oral cancer therapy [
18].
Additionally, exosomal TGF-β from oral cancer cells promotes angiogenesis by interacting with epithelial cells and regulating TAM chemotaxis (Ludwig N et al., 2022). Oral cancer-derived exosomal thrombospondin 1 (THBS-1) activates M1-like macrophages through p38/Akt/SAPK/JNK signaling, enhancing cancer progression. These M1-like TAMs promote EMT and cancer stem cell formation through the IL-6/Jak/Stat3/THBS-1 axis [
101]. These studies underscore the multifaceted roles of CSC-derived exosomes in oral cancer, highlighting their potential as therapeutic targets and biomarkers for monitoring disease progression and treatment responses.
Therapeutic Potential of CSC-Derived Exosomes
CSCs are known for their self-renewal and differentiation abilities and are considered the root cause of tumor initiation, progression, relapse, and resistance to conventional therapies. Exosomes, nanosized vesicles carrying biomolecules, have emerged as key players in cell-to-cell communication within the tumor microenvironment. CSC-derived exosomes, in particular, have gained attention due to their significant therapeutic potential.
These exosomes contribute to tumor aggressiveness by reprogramming non-CSCs into stem-like cells, promoting tumor growth and metastasis. They carry molecules like Wnt proteins and non-coding RNAs that enhance chemoresistance and immune evasion, hindering current therapies. However, this characteristic also presents a therapeutic opportunity. By analyzing the unique cargo of CSC-Exos, researchers can identify novel biomarkers for early cancer detection and target vulnerabilities within the CSC population [
59,
61,
68].
CSC-derived exosomes can be engineered to deliver therapeutic payloads, including drugs, small RNAs, or immune-modulating agents, directly to cancer cells or the tumor microenvironment. Their ability to bypass biological barriers, target specific cell types, and modulate immune responses makes them promising candidates for novel cancer therapies. For instance, exosomes from breast cancer cell lines have been modified to carry doxorubicin, a chemotherapy drug, reducing cancer proliferation without causing side effects [
102].
Targeting CSC-derived exosomes offers a promising therapeutic strategy. Inhibiting the release or uptake of these exosomes could disrupt the supportive communication network within the tumor microenvironment, potentially sensitizing tumors to conventional therapies. Various approaches, including the use of inhibitors of exosome biogenesis and release, have been explored. For example, GW4869, an inhibitor of neutral sphingomyelinase, has shown efficacy in reducing exosome release and sensitizing tumors to chemotherapy [
103]. In a study focused on pancreatic cancer, exosomes derived from CSCs, were found to be enriched with Glypican-1 (GPC1). These exosomes served as biomarkers, effectively distinguishing between healthy individuals and those with benign or malignant pancreatic tumor [
104]. Furthermore, the potential of engineered exosomes loaded with siRNA targeting KRAS in reducing tumor growth in pancreatic cancer models [
105].
Furthermore, exosomes can serve as Trojan horses by being loaded with anti-cancer drugs or molecules that disrupt CSC signalling pathways, selectively targeting and eliminating CSCs. Recent research in pancreatic cancer demonstrated the potential of exosomes loaded with siRNA targeting KRAS, resulting in reduced tumor growth in models [
4]. CSC-Exos can be engineered in such a way that they carry certain chemicals which are able to manipulate the body’s immune response like cytokines. When administered, these chemical agents can increase the efficiency of pre-existing immunotherapies in order to fight various types of cancers [
106,
107].
Despite the potential of CSC-derived exosomes in cancer therapy, significant challenges remain. The heterogeneity of exosomes and their cargo, which can vary depending on cell type and environmental conditions, presents a major hurdle. Standardizing the isolation and characterization of CSC-Exos is crucial for developing consistent and effective therapeutic strategies. Additionally, the safety and stability of exosome-based therapies require thorough evaluation, including understanding the immune response to exogenous exosomes and their potential off-target effects. Advances in nanotechnology and bioengineering are expected to address these challenges, paving the way for clinical applications.
Table 1.
Functions of CSC-Derived Exosomes in Various Cancers.
Table 1.
Functions of CSC-Derived Exosomes in Various Cancers.
| CSC Source |
Cargo |
Study observations |
References |
Oral cancer stem cells
(OC-CSCs)
Head and neck cancer stem cells (HNC-CSCs)
Esophageal cancer stem cells (EC-CSCs) |
miRNAs
lncRNA UCA1
O-GlcNAc transferase (OGT) |
- ◦
Reduce in tumor growth, increases migration, invasion and cancer progression - ◦
Promote M2 macrophage polarization and suppress CD4+ T-cell activity by transferring urothelial carcinoma-associated 1 (UCA1) and targeting LAMC2-PI3K/AKT pathway - ◦
Macrophage-derived exosomal miR- 31-5p promotes tumorigenesis by targeting LATS2/Hippo signalling pathway - ◦
Modulate tumor microenvironment and anti-cancer immune response - ◦
Overexpression of PD-1 in CD8+ T cells and promotes immunosuppression |
[14,18,19,83,87,108,109] |
| Breast cancer stem cells (BC-CSCs) |
miRNAs
|
- ◦
Targeting TF-(ONECUT2) resulting in expression of CSC traits. - ◦
Enhance EMT, metastasis, drug resistance and survivability to tumor cells |
[110,111] |
| Glioblastoma stem cells (GB-CSCs) |
Tenascin C, miRNAs,
Linc01060, NOTCH1,
STAT3 |
- ◦
Inhibit T cell-induced immune response - ◦
Promotes proliferation, tumor angiogenesis, invasion and metastasis - ◦
Induce M2 macrophage polarization |
[99,112,113,114] |
Lung cancer stem cells
(LC-CSCs) |
miRNAs |
- ◦
Enhances migration capability of cancer cells |
[17] |
Gastric cancer stem cells
(GC-CSCs) |
miRNAs |
- ◦
Promotes metastasis |
[16] |
Colorectal cancer stem cells
(CC-CSCs) |
miRNAs
miRNA-146a-5p
Tri-phosphate RNAs |
- ◦
Enhances angiogenesis and vascular permeability - ◦
Decrease CD8+ T cell infiltration - ◦
Sustain MPO+neutrophil survival associated with cancer progression and poor survival |
[115,116,117] |
Pancreatic Cancer
(PC-CSCs) |
miRNAs, CD44v6 |
- ◦
Enhances chemoresistance and metastasis |
[118,119] |
| Renal carcinoma (RC-CSCs) |
miRNAs, MMPs |
- ◦
Promotes EMT, Angiogenesis, formation premetastatic niche |
[120,121] |
| Prostate Cancer (PC-CSCs) |
miRNAs |
- ◦
Contribution to the premetastatic niche, enhances survival of cancer cells |
[122] |
Author Contributions
PK conceptualized this article’s topic and theme and wrote the mini-review’s first draft. RL participated in collection of relevant data and wrote some parts of manuscript. SG conceptualized, designed, supervised, participated in data interpretation and critically reviewed and finalized the manuscript. SA supervised, critically reviewed and communicated the manuscript. All authors have read and approved the final manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors gratefully acknowledge the financial support of DHR-Young Scientist Fellowships from Department of Health and Research (ICMR), Government of India to Prabhat Kumar and Shilpi Gupta.
Conflicts of Interest
All authors have declared that there are no conflicts of interest.
Abbreviations
OC- Oral Cancer; CSCs - Cancer Stem Cells; OCSCs - Oral Cancer Stem Cells; EVs - Extracellular Vesicles; CSC-Exos- Cancer Stem Cell-derived Exosomes; TME- Tumor Microenvironment; OCSC-EVs- Oral Cancer Stem Cell-derived Extracellular Vesicles; MVBs - Multivesicular Bodies; ILVs - Intraluminal Vesicles; ESCRT - Endosomal Sorting Complex Required for Transport; ALIX- ALG-2-interacting protein X; TSG101- Tumor Susceptibility Gene 101; EMT- Epithelial-Mesenchymal Transition; lncRNAs- Long Non-Coding RNAs; siRNA- Small Interfering RNA; TAMs- Tumor-Associated Macrophages; MHC-II- Major Histocompatibility Complex Class II; PD1- Programmed Death-1; HNCs - Head and Neck Cancers; MSC-Exos- Mesenchymal Stem Cell-derived Exosomes; PI3K- Phosphoinositide 3-Kinase; Akt- Protein Kinase B; MAP - Mitogen-Activated Protein Kinase; ERK- Extracellular Signal-Regulated Kinase; GDEs- Glioblastoma CSC-generated Exosomes; STAT3- Signal Transducer and Activator of Transcription 3; NF-κB - Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells; UCA1- Urothelial Carcinoma-Associated 1; LAMC2- Laminin Subunit Gamma 2; THBS-1 - Thrombospondin 1; SAPK/JNK - Stress-Activated Protein Kinase/c-Jun N-terminal Kinase; IL-6 - Interleukin 6; Jak - Janus Kinase; GPC1 - Glypican-1; KRAS - Kirsten Rat Sarcoma Viral Oncogene Homolog.
References
- D’Cruz, A.K.; Vaish, R.; Dhar, H. Oral cancers: current status. Oral oncology 2018, 87, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Wang, Z. Oral Cancer. In Pharynx-Diagnosis and Treatment; IntechOpen: 2021.
- Borse, V.; Konwar, A.N.; Buragohain, P. Oral cancer diagnosis and perspectives in India. Sensors International 2020, 1, 100046. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Gharat, S.A.; Momin, M.M.; Bhavsar, C. Oral squamous cell carcinoma: current treatment strategies and nanotechnology-based approaches for prevention and therapy. Critical Reviews™ in Therapeutic Drug Carrier Systems 2016, 33. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: mechanisms of action and clinical strategies. Nature reviews cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Chi, A.C.; Day, T.A.; Neville, B.W. Oral cavity and oropharyngeal squamous cell carcinoma—an update. CA: a cancer journal for clinicians 2015, 65, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.B.; Thariat, J.; Bensadoun, R.J.; Barasch, A.; Murphy, B.A.; Kolnick, L.; Popplewell, L.; Maghami, E. Oral complications of cancer and cancer therapy: from cancer treatment to survivorship. CA: a cancer journal for clinicians 2012, 62, 400–422. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA: a cancer journal for clinicians 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Qie, Y.; Sun, X.; Yang, Y.; Yan, T. Emerging functions and applications of exosomes in oral squamous cell carcinoma. Journal of Oral Pathology & Medicine 2023, 52, 886–894. [Google Scholar]
- Gupta, S.; Kumar, P.; Das, B.C. HPV+ ve/− ve oral-tongue cancer stem cells: a potential target for relapse-free therapy. Translational Oncology 2021, 14, 100919. [Google Scholar] [CrossRef]
- Prince, M.; Sivanandan, R.; Kaczorowski, A.; Wolf, G.; Kaplan, M.; Dalerba, P.; Weissman, I.; Clarke, M.; Ailles, L. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proceedings of the National Academy of Sciences 2007, 104, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Wang, J.; Pan, S.; Zheng, L.; Wang, Z.-W.; Zhu, X. Nucleic acids and proteins carried by exosomes of different origins as potential biomarkers for gynecologic cancers. Molecular Therapy-Oncolytics 2022, 24, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, N.; Gao, Z.; Liu, W.; Pang, B.; Dong, X.; Li, Y.; Fan, T. The emerging role of exosomes in cancer chemoresistance. Frontiers in Cell and Developmental Biology 2021, 9, 737962. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M. Extracellular vesicle-mediated transport of non-coding RNAs between stem cells and cancer cells: implications in tumor progression and therapeutic resistance. Stem Cell Investigation 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.-P.; Li, A.-Q.; Jia, W.-H.; Ye, S.; Van Eps, G.; Yu, J.-M.; Yang, W.-J. MicroRNA expression profiling in exosomes derived from gastric cancer stem-like cells. Oncotarget 2017, 8, 93839. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, J.; Hu, H.; Tu, L.; Sun, Z.; Liu, Y.; Luo, F. Lung CSC-derived exosomal miR-210-3p contributes to a pro-metastatic phenotype in lung cancer by targeting FGFRL1. Journal of Cellular and Molecular Medicine 2020, 24, 6324–6339. [Google Scholar] [CrossRef]
- Gonzalez-Callejo, P.; Guo, Z.; Ziglari, T.; Claudio, N.M.; Nguyen, K.H.; Oshimori, N.; Seras-Franzoso, J.; Pucci, F. Cancer stem cell-derived extracellular vesicles preferentially target MHC-II–macrophages and PD1+ T cells in the tumor microenvironment. Plos one 2023, 18, e0279400. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ye, S.; Yao, Y.; Zhang, C.; Liu, W. Oral Cancer Stem Cell-Derived Small Extracellular Vesicles Promote M2 Macrophage Polarization and Suppress CD4+ T-Cell Activity by Transferring UCA1 and Targeting LAMC2. Stem cells international 2022, 2022, 5817684. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.-s.; Zhao, M.-j.; Chen, Y.; Zhu, J.-y.; Xie, C.-f.; Li, X.-t.; Geng, S.-s.; Zhong, C.-y.; Fu, J.-y.; Wu, J.-s. Low-dose phthalates promote breast cancer stem cell properties via the oncogene ΔNp63α and the Sonic hedgehog pathway. Ecotoxicology and Environmental Safety 2023, 252, 114605. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Mitra, P.; Saha, U.; Ghosh, A.; Biswas, N.K.; Roy, S.S.; Acharya, M.; Singh, S. NOTCH-pathway inactivation reprograms oral-stem-like cancer cells to JAK-STAT dependent state and provides the opportunity of synthetic lethality. 2022.
- Müller, L.; Tunger, A.; Plesca, I.; Wehner, R.; Temme, A.; Westphal, D.; Meier, F.; Bachmann, M.; Schmitz, M. Bidirectional crosstalk between cancer stem cells and immune cell subsets. Frontiers in immunology 2020, 11, 140. [Google Scholar] [CrossRef] [PubMed]
- Walcher, L.; Kistenmacher, A.-K.; Suo, H.; Kitte, R.; Dluczek, S.; Strauß, A.; Blaudszun, A.-R.; Yevsa, T.; Fricke, S.; Kossatz-Boehlert, U. Cancer stem cells—origins and biomarkers: perspectives for targeted personalized therapies. Frontiers in immunology 2020, 11, 1280. [Google Scholar] [CrossRef] [PubMed]
- Pattabiraman, D.R.; Weinberg, R.A. Tackling the cancer stem cells—what challenges do they pose? Nature reviews Drug discovery 2014, 13, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Batlle, E.; Clevers, H. Cancer stem cells revisited. Nature medicine 2017, 23, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Kakarala, M.; Wicha, M.S. Cancer stem cells: implications for cancer treatment and prevention. The Cancer Journal 2007, 13, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, D. Normal and leukemic CD34-negative human hematopoietic stem cells. Reviews in clinical and experimental hematology 2001, 5, 42–61. [Google Scholar] [CrossRef]
- Fang, Y.; Xiao, X.; Wang, J.; Dasari, S.; Pepin, D.; Nephew, K.P.; Zamarin, D.; Mitra, A.K. Cancer associated fibroblasts serve as an ovarian cancer stem cell niche through noncanonical Wnt5a signaling. NPJ Precision Oncology 2024, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Hwang, J.W.; Cho, Y.; Kim, S.; Han, S.H.; Yu, J.; Ha, S.; Kim, W.-Y.; Kim, S.-N.; Kim, I.S. A novel synthetic microtubule inhibitor exerts antiproliferative effects in multidrug resistant cancer cells and cancer stem cells. Scientific Reports 2021, 11, 10822. [Google Scholar] [CrossRef] [PubMed]
- Spisak, S.; Chen, D.; Likasitwatanakul, P.; Doan, P.; Li, Z.; Bala, P.; Vizkeleti, L.; Tisza, V.; De Silva, P.; Giannakis, M. Identifying regulators of aberrant stem cell and differentiation activity in colorectal cancer using a dual endogenous reporter system. Nature Communications 2024, 15, 2230. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Vishnoi, K.; Mahata, S.; Verma, G.; Srivastava, Y.; Masaldan, S.; Roy, B.G.; Bharti, A.C.; Das, B.C. Cervical cancer stem cells selectively overexpress HPV oncoprotein E6 that controls stemness and self-renewal through upregulation of HES1. Clinical Cancer Research 2016, 22, 4170–4184. [Google Scholar] [CrossRef] [PubMed]
- Visvader, J.E.; Lindeman, G.J. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nature reviews cancer 2008, 8, 755–768. [Google Scholar] [CrossRef]
- Gomez, K.E.; Wu, F.; Keysar, S.B.; Morton, J.J.; Miller, B.; Chimed, T.-S.; Le, P.N.; Nieto, C.; Chowdhury, F.N.; Tyagi, A. Cancer cell CD44 mediates macrophage/monocyte-driven regulation of head and neck cancer stem cells. Cancer research 2020, 80, 4185–4198. [Google Scholar] [CrossRef] [PubMed]
- Eini, L.; Naseri, M.; Karimi-Busheri, F.; Bozorgmehr, M.; Ghods, R.; Madjd, Z. Preventive cancer stem cell-based vaccination modulates tumor development in syngeneic colon adenocarcinoma murine model. Journal of cancer research and clinical oncology 2023, 149, 4101–4116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Shen, L.; Shi, H.; Pan, Z.; Wu, L.; Yan, Y.; Zhang, X.; Mao, F.; Qian, H.; Xu, W. Exosomes from human umbilical cord mesenchymal stem cells: identification, purification, and biological characteristics. Stem cells international 2016, 2016, 1929536. [Google Scholar] [CrossRef] [PubMed]
- Borlongan, M.C.; Saha, D.; Wang, H. Tumor microenvironment: A niche for cancer stem cell immunotherapy. Stem Cell Reviews and Reports 2024, 20, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Cierpikowski, P.; Lis-Nawara, A.; Bar, J. Prognostic value of WNT1, NOTCH1, PDGFRβ, and CXCR4 in oral squamous cell carcinoma. Anticancer research 2023, 43, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Komura, N.; Mabuchi, S.; Shimura, K.; Yokoi, E.; Kozasa, K.; Kuroda, H.; Takahashi, R.; Sasano, T.; Kawano, M.; Matsumoto, Y. The role of myeloid-derived suppressor cells in increasing cancer stem-like cells and promoting PD-L1 expression in epithelial ovarian cancer. Cancer Immunology, Immunotherapy 2020, 69, 2477–2499. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, J.; Srivastava, T.P.; Sahoo, O.S.; Karmakar, A.; Rai, A.K.; Sarma, A.; Gogoi, G.; Alqahtani, M.S.; Abbas, M.; Dhar, R. Cancer stem cells: Signaling pathways and therapeutic targeting. MedComm–Oncology 2023, 2, e62. [Google Scholar] [CrossRef]
- Xie, J.; Huang, L.; Lu, Y.-G.; Zheng, D.-L. Roles of the Wnt signaling pathway in head and neck squamous cell carcinoma. Frontiers in Molecular Biosciences 2021, 7, 590912. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F. Targeting cancer stem cell pathways for cancer therapy. Signal transduction and targeted therapy 2020, 5, 8. [Google Scholar] [CrossRef]
- Yuan, S.; Stewart, K.S.; Yang, Y.; Abdusselamoglu, M.D.; Parigi, S.M.; Feinberg, T.Y.; Tumaneng, K.; Yang, H.; Levorse, J.M.; Polak, L. Ras drives malignancy through stem cell crosstalk with the microenvironment. Nature 2022, 612, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.K.; Park, H.Y.; Park, S.-G.; Hwang, J.J.; Park, H.R.; Yi, J.M. Promoter methylation of cancer stem cell surface markers as an epigenetic biomarker for prognosis of oral squamous cell carcinoma. International Journal of Molecular Sciences 2022, 23, 14624. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Urbano, M.A.; Griñán-Lisón, C.; Marchal, J.A.; Núñez, M.I. CSC radioresistance: a therapeutic challenge to improve radiotherapy effectiveness in cancer. Cells 2020, 9, 1651. [Google Scholar] [CrossRef]
- Aquino, I.G.; Cuadra-Zelaya, F.J.M.; Bizeli, A.L.V.; Palma, P.V.B.; Mariano, F.V.; Salo, T.; Coletta, R.D.; Bastos, D.C.; Graner, E. Isolation and phenotypic characterization of cancer stem cells from metastatic oral cancer cells. Oral Diseases 2024. [Google Scholar] [CrossRef] [PubMed]
- Mannelli, G.; Gallo, O. Cancer stem cells hypothesis and stem cells in head and neck cancers. Cancer treatment reviews 2012, 38, 515–539. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.A.; Guo, W.; Liao, M.-J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, C.; Van Tubergen, E.; Inglehart, R.; D’silva, N. Biomarkers of epithelial-mesenchymal transition in squamous cell carcinoma. Journal of dental research 2013, 92, 114–121. [Google Scholar] [CrossRef]
- Smith, B.N.; Bhowmick, N.A. Role of EMT in metastasis and therapy resistance. Journal of clinical medicine 2016, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Jan, S.; Fatima, K.; Malik, F. Immune Cells: Critical Players in Drug Resistance. In Drug Resistance in Cancer: Mechanisms and Strategies; Springer: 2024; pp. 121-151.
- Olmedo, I.; Martínez, D.; Carrasco-Rojas, J.; Jara, J.A. Mitochondria in oral cancer stem cells: Unraveling the potential drug targets for new and old drugs. Life Sciences 2023, 122065. [Google Scholar] [CrossRef] [PubMed]
- Sha, J.; Bai, Y.; Ngo, H.X.; Okui, T.; Kanno, T. Overview of evidence-based chemotherapy for oral cancer: focus on drug resistance related to the epithelial-mesenchymal transition. Biomolecules 2021, 11, 893. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Tie, Y.; Alu, A.; Ma, X.; Shi, H. Targeted therapy for head and neck cancer: signaling pathways and clinical studies. Signal transduction and targeted therapy 2023, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Patni, A.P.; Harishankar, M.; Joseph, J.P.; Sreeshma, B.; Jayaraj, R.; Devi, A. Comprehending the crosstalk between Notch, Wnt and Hedgehog signaling pathways in oral squamous cell carcinoma-clinical implications. Cellular Oncology 2021, 44, 473–494. [Google Scholar] [CrossRef] [PubMed]
- Takebe, N.; Miele, L.; Harris, P.J.; Jeong, W.; Bando, H.; Kahn, M.; Yang, S.X.; Ivy, S.P. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nature reviews Clinical oncology 2015, 12, 445–464. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, H.; Yang, Z.; Liu, R.; Li, Y.; Hu, Y.; Zhao, S.; Gao, X.; Yang, X.; Wei, J. SALL4 promotes cancer stem-like cell phenotype and radioresistance in oral squamous cell carcinomas via methyltransferase-like 3-mediated m6A modification. Cell Death & Disease 2024, 15, 139. [Google Scholar]
- Zhang, Y.; Liu, J.; Liu, S.; Yu, L.; Liu, S.; Li, M.; Jin, F. Extracellular vesicles in oral squamous cell carcinoma: current progress and future prospect. Frontiers in Bioengineering and Biotechnology 2023, 11, 1149662. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annual review of biochemistry 2019, 88, 487–514. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: composition, biogenesis and function. Nature reviews immunology 2002, 2, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.M.; Adam, M.; Hammond, J.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). Journal of Biological Chemistry 1987, 262, 9412–9420. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: exosomes, microvesicles, and friends. Journal of Cell Biology 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Bebelman, M.P.; Smit, M.J.; Pegtel, D.M.; Baglio, S.R. Biogenesis and function of extracellular vesicles in cancer. Pharmacology & therapeutics 2018, 188, 1–11. [Google Scholar]
- Brinton, L.T.; Sloane, H.S.; Kester, M.; Kelly, K.A. Formation and role of exosomes in cancer. Cellular and molecular life sciences 2015, 72, 659–671. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nature nanotechnology 2021, 16, 748–759. [Google Scholar] [CrossRef]
- Urabe, F.; Kosaka, N.; Ito, K.; Kimura, T.; Egawa, S.; Ochiya, T. Extracellular vesicles as biomarkers and therapeutic targets for cancer. American Journal of Physiology-Cell Physiology 2020, 318, C29–C39. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Communication and Signaling 2021, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: biogenesis, biologic function and clinical potential. Cell & bioscience 2019, 9, 1–18. [Google Scholar]
- Kowal, J.; Tkach, M.; Théry, C. Biogenesis and secretion of exosomes. Current opinion in cell biology 2014, 29, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Teng, Y. Harnessing cancer stem cell-derived exosomes to improve cancer therapy. Journal of Experimental & Clinical Cancer Research 2023, 42, 131. [Google Scholar]
- Henne, W.M.; Buchkovich, N.J.; Emr, S.D. The ESCRT pathway. Developmental cell 2011, 21, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Piper, R.C.; Katzmann, D.J. Biogenesis and function of multivesicular bodies. Annu. Rev. Cell Dev. Biol. 2007, 23, 519–547. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Wang, B.; Kodali, M.C.; Chen, C.; Kim, E.; Patters, B.J.; Lan, L.; Kumar, S.; Wang, X.; Yue, J. In vivo evidence for the contribution of peripheral circulating inflammatory exosomes to neuroinflammation. Journal of neuroinflammation 2018, 15, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yan, P.; Xu, X.; Jiang, W. A unique dual recognition hairpin probe mediated fluorescence amplification method for sensitive detection of uracil-DNA glycosylase and endonuclease IV activities. Analyst 2016, 141, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Chiba, M.; Uehara, H.; Kuwata, H.; Niiyama, I. Extracellular miRNAs in the serum and feces of mice exposed to high-dose radiation. Biomedical Reports 2024, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Ohi, T.; Murakami, T.; Komiyama, T.; Miyoshi, Y.; Endo, K.; Satoh, M.; Asayama, K.; Inoue, R.; Kikuya, M. Association between tooth loss and cognitive impairment in community-dwelling older Japanese adults: a 4-year prospective cohort study from the Ohasama study. BMC oral health 2018, 18, 1–8. [Google Scholar] [CrossRef]
- Fatima, F.; Nawaz, M. Stem cell-derived exosomes: roles in stromal remodeling, tumor progression, and cancer immunotherapy. Chinese journal of cancer 2015, 34, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Koch, R.; Demant, M.; Aung, T.; Diering, N.; Cicholas, A.; Chapuy, B.; Wenzel, D.; Lahmann, M.; Güntsch, A.; Kiecke, C. Populational equilibrium through exosome-mediated Wnt signaling in tumor progression of diffuse large B-cell lymphoma. Blood, The Journal of the American Society of Hematology 2014, 123, 2189–2198. [Google Scholar] [CrossRef] [PubMed]
- Bliss, S.A.; Sinha, G.; Sandiford, O.A.; Williams, L.M.; Engelberth, D.J.; Guiro, K.; Isenalumhe, L.L.; Greco, S.J.; Ayer, S.; Bryan, M. Mesenchymal stem cell–derived exosomes stimulate cycling quiescence and early breast cancer dormancy in bone marrow. Cancer research 2016, 76, 5832–5844. [Google Scholar] [CrossRef]
- Huang, Y.-J.; Huang, T.-H.; Yadav, V.K.; Sumitra, M.R.; Tzeng, D.T.; Wei, P.-L.; Shih, J.-W.; Wu, A.T. Preclinical investigation of ovatodiolide as a potential inhibitor of colon cancer stem cells via downregulating sphere-derived exosomal β-catenin/STAT3/miR-1246 cargoes. American journal of cancer research 2020, 10, 2337. [Google Scholar] [PubMed]
- Zhang, Y.; Xu, W.; Guo, H.; Zhang, Y.; He, Y.; Lee, S.H.; Song, X.; Li, X.; Guo, Y.; Zhao, Y. NOTCH1 signaling regulates self-renewal and platinum chemoresistance of cancer stem–like cells in human non–small cell lung cancer. Cancer research 2017, 77, 3082–3091. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhou, L.; Yu, F.; Zhang, Y.; Li, P.; Wang, K. The functional roles of exosomal long non-coding RNAs in cancer. Cellular and Molecular Life Sciences 2019, 76, 2059–2076. [Google Scholar] [CrossRef] [PubMed]
- González-Callejo, P.; Gener, P.; Díaz-Riascos, Z.V.; Conti, S.; Cámara-Sánchez, P.; Riera, R.; Mancilla, S.; García-Gabilondo, M.; Peg, V.; Arango, D. Extracellular vesicles secreted by triple-negative breast cancer stem cells trigger premetastatic niche remodeling and metastatic growth in the lungs. International Journal of Cancer 2023, 152, 2153–2165. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Alečković, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; García-Santos, G.; Ghajar, C.M. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nature medicine 2012, 18, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, S.; Mehta, P.; Xie, Y.; Lei, Y.L.; Mehta, G. Ovarian cancer stem cells and macrophages reciprocally interact through the WNT pathway to promote pro-tumoral and malignant phenotypes in 3D engineered microenvironments. Journal for immunotherapy of cancer 2019, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, J.; Xie, D.; Pei, Q.; Wan, X.; Xing, H.R.; Ye, T. Characteristics of the PI3K/AKT and MAPK/ERK pathways involved in the maintenance of self-renewal in lung cancer stem-like cells. International Journal of Biological Sciences 2021, 17, 1191. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, Y. Efflux mechanism and pathway of verapamil pumping by human P-glycoprotein. Archives of biochemistry and biophysics 2020, 696, 108675. [Google Scholar] [CrossRef]
- Gholami, A. Cancer stem cell-derived exosomes in CD8+ T cell exhaustion. International Immunopharmacology 2024, 137, 112509. [Google Scholar] [CrossRef] [PubMed]
- Kleffel, S.; Schatton, T. Tumor dormancy and cancer stem cells: two sides of the same coin? Systems biology of tumor dormancy 2013, 145–179. [Google Scholar]
- Hardin, H.; Helein, H.; Meyer, K.; Robertson, S.; Zhang, R.; Zhong, W.; Lloyd, R.V. Thyroid cancer stem-like cell exosomes: regulation of EMT via transfer of lncRNAs. Laboratory Investigation 2018, 98, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Hiroi, M.; Shimada, J.; Ohmori, Y. Infiltration of m2 tumor-associated macrophages in oral squamous cell carcinoma correlates with tumor malignancy. Cancers 2011, 3, 3726–3739. [Google Scholar] [CrossRef]
- Cai, X.; Zhang, L.; Wei, W. Regulatory B cells in inflammatory diseases and tumor. International immunopharmacology 2019, 67, 281–286. [Google Scholar] [CrossRef]
- Kirave, P.; Gondaliya, P.; Kulkarni, B.; Rawal, R.; Garg, R.; Jain, A.; Kalia, K. Exosome mediated miR-155 delivery confers cisplatin chemoresistance in oral cancer cells via epithelial-mesenchymal transition. Oncotarget 2020, 11, 1157. [Google Scholar] [CrossRef] [PubMed]
- Law, Z.-J.; Khoo, X.H.; Lim, P.T.; Goh, B.H.; Ming, L.C.; Lee, W.-L.; Goh, H.P. Extracellular vesicle-mediated chemoresistance in oral squamous cell carcinoma. Frontiers in molecular biosciences 2021, 8, 629888. [Google Scholar] [CrossRef] [PubMed]
- Momen-Heravi, F.; Bala, S. Emerging role of non-coding RNA in oral cancer. Cellular signalling 2018, 42, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Singha, A.; Magesh, K.; Mahalingam, R.; Aravindhan, R.; Sivachandran, A. Therapeutic signature of stem cell derivative exosomes in oral cancer: a scoping review. Cureus 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ouyang, P.; He, G.; Wang, X.; Song, D.; Yang, Y.; He, X. Exosomes from microRNA-126 overexpressing mesenchymal stem cells promote angiogenesis by targeting the PIK3R2-mediated PI3K/Akt signalling pathway. Journal of Cellular and Molecular Medicine 2021, 25, 2148–2162. [Google Scholar] [CrossRef] [PubMed]
- Gabrusiewicz, K.; Li, X.; Wei, J.; Hashimoto, Y.; Marisetty, A.L.; Ott, M.; Wang, F.; Hawke, D.; Yu, J.; Healy, L.M. Glioblastoma stem cell-derived exosomes induce M2 macrophages and PD-L1 expression on human monocytes. Oncoimmunology 2018, 7, e1412909. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-H.; Wu, A.T.; Bamodu, O.A.; Yadav, V.K.; Chao, T.-Y.; Tzeng, Y.-M.; Mukhopadhyay, D.; Hsiao, M.; Lee, J.-C. Ovatodiolide suppresses oral cancer malignancy by down-regulating exosomal Mir-21/STAT3/β-catenin cargo and preventing oncogenic transformation of normal gingival fibroblasts. Cancers 2019, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Tian, Z.; Du, Z.; Wu, K.; Xu, G.; Dai, M.; Wang, Y.a.; Xiao, M. M1-like tumor-associated macrophages cascade a mesenchymal/stem-like phenotype of oral squamous cell carcinoma via the IL6/Stat3/THBS1 feedback loop. Journal of Experimental & Clinical Cancer Research 2022, 41, 10. [Google Scholar]
- Tian, Y.; Li, S.; Song, J.; Ji, T.; Zhu, M.; Anderson, G.J.; Wei, J.; Nie, G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014, 35, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015, 523, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, F.; Zhu, D.; Hu, S.; Cheng, K.; Li, Z. Engineering Exosomes and Exosome-like Nanovesicles for Improving Tissue Targeting and Retention. Fundamental Research 2024. [Google Scholar] [CrossRef]
- Morishita, M.; Takahashi, Y.; Matsumoto, A.; Nishikawa, M.; Takakura, Y. Exosome-based tumor antigens–adjuvant co-delivery utilizing genetically engineered tumor cell-derived exosomes with immunostimulatory CpG DNA. Biomaterials 2016, 111, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Dickman, C.T.; Lawson, J.; Jabalee, J.; MacLellan, S.A.; LePard, N.E.; Bennewith, K.L.; Garnis, C. Selective extracellular vesicle exclusion of miR-142-3p by oral cancer cells promotes both internal and extracellular malignant phenotypes. Oncotarget 2017, 8, 15252. [Google Scholar] [CrossRef] [PubMed]
- Shoff, M.; Booker, T.; Leavitt, B.; Harmon, D.; Kingsley, K.; Howard, K. Differential exosome miRNA expression in oral cancer stem cells. ExRNA 2020, 2, 1–9. [Google Scholar] [CrossRef]
- Santos, P.; Almeida, F. Role of exosomal miRNAs and the tumor microenvironment in drug resistance. Cells 2020, 9, 1450. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Dong, C.; Ruan, X.; Yan, W.; Cao, M.; Pizzo, D.; Wu, X.; Yang, L.; Liu, L.; Ren, X. Chemotherapy-induced extracellular vesicle miRNAs promote breast cancer stemness by targeting ONECUT2. Cancer research 2019, 79, 3608–3621. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Jiang, Y.; Hu, H.; Zhang, S.; Chen, Y. Extracellular vesicles as modulators of glioblastoma progression and tumor microenvironment. Pathology and Oncology Research 2024, 30. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Xiao, J.; Li, X.; Tao, Y.; Zhou, P.; Lyu, L.; Shi, Z.; Liang, X.; Jia, Z.; Jiang, S. Autophagy-related CMTM6 promotes glioblastoma progression by activating Wnt/β-catenin pathway and acts as an onco-immunological biomarker. The Journal of Gene Medicine 2024, 26, e3685. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Sarkar, S.; Dzikowski, L.; Rawji, K.S.; Khan, L.; Faissner, A.; Bose, P.; Yong, V.W. Brain tumor-initiating cells export tenascin-C associated with exosomes to suppress T cell activity. 2018, 7, e1478647.
- Cheng, W.C.; Liao, T.T.; Lin, C.C.; Yuan, L.T.E.; Lan, H.Y.; Lin, H.H.; Teng, H.W.; Chang, H.C.; Lin, C.H.; Yang, C.Y. RAB27B-activated secretion of stem-like tumor exosomes delivers the biomarker microRNA-146a-5p, which promotes tumorigenesis and associates with an immunosuppressive tumor microenvironment in colorectal cancer. International Journal of Cancer 2019, 145, 2209–2224. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Ye, A.; Ye, W.; Liao, X.; Qin, G.; Xu, Y.; Yin, Y.; Luo, H.; Yi, M.; Xian, L. Cancer-secreted exosomal miR-21-5p induces angiogenesis and vascular permeability by targeting KRIT1. Cell death & disease 2021, 12, 576. [Google Scholar]
- Hwang, W.-L.; Jiang, J.-K.; Yang, S.-H.; Huang, T.-S.; Lan, H.-Y.; Teng, H.-W.; Yang, C.-Y.; Tsai, Y.-P.; Lin, C.-H.; Wang, H.-W. MicroRNA-146a directs the symmetric division of Snail-dominant colorectal cancer stem cells. Nature cell biology 2014, 16, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, H.; Provaznik, J.; Hackert, T.; Zöller, M. Pancreatic cancer-initiating cell exosome message transfer into noncancer-initiating cells: the importance of CD44v6 in reprogramming. Journal of Experimental & Clinical Cancer Research 2019, 38, 1–20. [Google Scholar]
- Yang, Z.; Zhao, N.; Cui, J.; Wu, H.; Xiong, J.; Peng, T. Exosomes derived from cancer stem cells of gemcitabine-resistant pancreatic cancer cells enhance drug resistance by delivering miR-210. Cellular Oncology 2020, 43, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Grange, C.; Tapparo, M.; Collino, F.; Vitillo, L.; Damasco, C.; Deregibus, M.C.; Tetta, C.; Bussolati, B.; Camussi, G. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer research 2011, 71, 5346–5356. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, G.; Zhao, D.; Wang, J.; Bai, Y.; Peng, Q.; Wang, H.; Fang, R.; Chen, G.; Wang, Z. CD103-positive CSC exosome promotes EMT of clear cell renal cell carcinoma: role of remote MiR-19b-3p. Molecular cancer 2019, 18, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.A.; Andahur, E.I.; Valenzuela, R.; Castellón, E.A.; Fullá, J.A.; Ramos, C.G.; Triviño, J.C. Exosomes from bulk and stem cells from human prostate cancer have a differential microRNA content that contributes cooperatively over local and pre-metastatic niche. Oncotarget 2016, 7, 3993. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).