1. Introduction
Exploring natural compounds for their health-promoting properties remains a pivotal area of research within nutraceutical and pharmaceutical sciences. Among these compounds, anthocyanins, predominantly found in berries, have garnered significant attention due to their diverse biological activities [
1,
2]. This study focuses on anthocyanins isolated from two Andean berries: the Andean blueberry (
Vaccinium floribundum Kunth) and the Andean blackberry (
Rubus glaucus Benth) henceforth, these will be referred as berries.
Berries are rich in anthocyanins and traditionally consumed in the Andes for their reputed therapeutic benefits, including antioxidant, antimicrobial, and anti-inflammatory effects [
2,
3,
4].
Recent studies underscore the role of anthocyanins in disease prevention and health enhancement, particularly their capacity to combat oxidative stress, inflammation, and microbial infections [
5,
6]. The biochemical diversity of anthocyanins facilitates a wide range of cellular interactions, modulating various metabolic and physiological pathways [
7,
8]. However, the effectiveness of these interactions is often contingent upon the bioavailability and chemical stability of anthocyanins, which are influenced by their botanical sources and extraction methods [
9,
10] .
In this study, we employed analytical methods to elucidate the anthocyanin profiles of these berries. Additionally, we evaluated their antimicrobial efficacy against clinically relevant bacterial strains and investigated their antioxidant capacities, aiming to correlate the presence of specific anthocyanins with observed biological effects. Such comprehensive assessments are essential for substantiating berry-derived anthocyanins’ health claims and advancing their potential therapeutic applications. Moreover, our investigation assessed the anthocyanins’ antitumoral, anti-inflammatory, and hemolytic properties. This study describes the anthocyanin profiles and biological activities of Vaccinium floribundum Kunth and Rubus glaucus Benth berries, highlighting their potential as significant contributors to the health food and pharmaceutical sectors. The anthocyanin content and distinct biological activities emphasize the importance of these berries in bioactive research and their prospective applications in combating microbial resistance.
2. Materials and Methods
2.1. Plant Material
Berries were purchased at a local market in Ambato, Ecuador. These fruits were selected, washed with tap water, disinfected using 100 ppm of chlorine, and freeze-dried. The samples were stored at −20 °C and used as feedstock for the experiments in this study.
2.2. Anthocyanin Extraction
Berries were washed, grinded, and lyophilized to obtain powdered particles. Subsequently, 10 g of the powdered material was combined with a solution of 200 ml, comprising 96% ethanol and 1.5 mol/l HCl in an 85:15 v/v ratio. This mixture was then placed in an oil bath, where it was agitated and maintained at 70 °C for 60 minutes to facilitate the extraction of anthocyanins from the sample. The resulting mixture underwent separation using a solid-liquid filtration method connected to a vacuum pump. Following this, an additional 200 ml of the ethanol-acidic solution underwent successive introductions to the solid residue, with this process repeated three times. The accumulated liquid extracts were consolidated and transferred to a flask with a continuous agitation mechanism. This flask was subsequently connected to a rotary evaporator operating at 70 °C to eliminate the solvent over two hours under vacuum conditions. The derived extract was ultimately subjected to lyophilization, ensuring comprehensive desiccation and yielding the ultimate dried product.
2.3. Anthocyanins Characterization
The dry extract described previously was suspended in methanol-water (80:20) (15 mg/1 mL), ultrasonicated for 30 min, continuously shaken for two hours, and protected from light at room temperature. Later, samples were centrifuged for 10 min at 5000 rpm (10 °C), and the supernatant was filtered through a 0.45 μm Minisart filter (RephiLe Bioscience Ltd., Acton, MA, USA). The solid residue was re-extracted with the same volume, and supernatants were mixed. The extract was concentrated in a rotary evaporator at 30 °C, and the dry extract was stored at −20 °C until further analysis.
2.4. Spectrophotometric Determination of Anthocyanin
The dry extract described previously was suspended in methanol water (80:20) (15 mg/1 mL) and used to determine the total anthocyanin content. The pH differential method was used to determine the total anthocyanin (ACY), and the results were expressed as milligrams of Pelargonidin—chloride equivalents (PgEq) per gram of fruit FW (PgEq/g FW) [
11].
2.5. HPLC-DAD Analysis of Anthocyanins
The dry extract described previously was suspended in methanol-water (80:20) (15 mg/1 mL), filtered through a 0.45 μm Minisart filter (RephiLe Bioscience Ltd., Acton, MA, USA), and analyzed using HPLC-DAD-MS/MS. The HPLC-DAD methodology was performed according to previous studies [
12]. The HPLC system consisted of a Vanquish (Thermo Fisher Scientific, Massachusetts, EE. UU) fitted with a binary pump and DAD coupled with an LTQ-XL (Thermo Fisher Scientific, Massachusetts, EE. UU) controlled by Xcalibur Software. An Accucore Vanquish C18 column (1.5 μm, 100× 2.1 mm) (Merck KGaA, Darmstadt, Germany) thermostated at 40°C was used as stationary phase. In contrast, a solution of 0.1% formic acid (A) and acetonitrile (B) was used for elution. The flow rate was 0.2 mL/min. Double line detection was carried out in DAD at 280, 220, 330, and 370 nm as preferred wavelengths, and MS was operated in negative ion mode. The dependent data analysis (DDA) was performed on the five most intense ions with a normalized collision energy of 35. Spectra between m/z 50 and m/z 1500 were recorded in positive and negative ionization mode.
2.6. Antimicrobial Activity Assay
The antimicrobial efficacy of extracts against a panel of bacterial and yeast strains was assessed. Three Gram-positive microorganisms Staphylococcus aureus ATCC 25923, Enterococcus faecalis ATCC 29212, and Listeria monocytogenes ATCC 13932, as well as four Gram-negative bacterial strains, Pseudomonas aeruginosa ATCC 27853, Salmonella typhimurium ATCC 14028, Burkholderia cepacea ATCC 25416 and Escherichia coli ATCC 25922; and four yeast including Candida albicans ATCC 10231, Candida krusei ATCC 14243, Candida glabarata ATCC 66032 and Candida tropicalis ATCC 1369. All strains were obtained from the American Type Culture Collection (ATCC, Virginia, U.S.A.) and were maintained at -80°C with a glycerol supplementation of 25% (v/v).
The antimicrobial activity was evaluated by determining the Minimum Inhibitory Concentration (MIC) utilizing the agar dilution technique, described by the Clinical and Laboratory Standards Institute (CLSI) guidelines [
13]. Serial dilutions of the berries extracts were prepared within the concentration research range. These diluted extracts were then introduced into molten Mueller–Hinton agar, maintained in a water bath at 45°C. The resulting agar and extract solution mixture was rigorously mixed and subsequently poured into Petri dishes to solidify at room temperature.
Ciprofloxacin served as the positive control in this assay, while the negative control was the microorganism suspension alone (no berry extract). Colonies isolated from a 24-48 hour agar plate were used for the initial inoculum to create a direct broth suspension. This suspension was adjusted to a 0.5 McFarland standard, corresponding to approximately 1 × 108 colony-forming units (CFU)/mL. Subsequently, a 1:10 dilution of the 0.5 McFarland suspension in sterile broth yielded a 107 CFU/mL concentration. Two microliters (µL) of the bacterial suspension were dispensed onto the agar, resulting in a final inoculum of approximately 104 CFU per spot. The inoculated plates were allowed to stand at room temperature for 30 minutes to facilitate absorption of the spots into the agar. The plates were inverted and incubated at 37°C for 24 hours afterward. The MIC was documented as the lowest extract concentration capable of inhibiting bacterial growth.
2.7. Antioxidant Activity
Antioxidant activity was assessed by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay as described elsewhere [
14]. DPPH was prepared in 100% methanol HPLC grade to a final concentration of 0.2 mM. Briefly, seven serial dilutions of the extract were prepared in a 96-well microplate in duplicates with a final volume of 100 µL using 100% methanol. One replicate was used as blank after adding 100 µL of 100% methanol, whereas 100 µL of DPPH was added to the other replicate. Ascorbic acid was used as a positive control, and it was serially diluted from a stock of 50 µg/mL and prepared in the same way as described for the extracts. The samples were incubated in the dark for 40 min at room temperature, and the absorbance was measured at 515 nm using a Cytation5 multi-mode detection system (BioTek). The following formula was used to calculate the % DPPH scavenging activity:
IC50 values were calculated using the GraphPad Prism software. The assay was performed at least in triplicates.
2.8. Antibiofilm Activity
The antibiofilm activity of extracts was tested in various biofilm-forming microorganisms.
S. aureus ATCC 25923,
E. faecalis ATCC 29212,
L. monocytogenes ATCC 13932,
P. aeruginosa ATCC 9027,
B. cepacia ATCC 25416 and the fungal strain
C. tropicalis ATCC 13803 were grown in TSB+G (Tryptic Soy Broth medium supplemented with 1% Glucose) overnight at 37 °C. Then, the microbial suspension was prepared at 1:100 of the overnight cultures in the absence or presence of the berry extracts (50 to 0.1 mg/mL), and 150 ul aliquots were added to 96-polystyrene plates. The plates were incubated at 37 °C in static conditions for 24h. The medium containing free-floating microbes was removed by aspiration using a micropipette, and the wells were washed three times with PBS buffer 1x (pH 7.2). The plate was dried for 1h at 60 °C inside a laboratory oven. Subsequently, the biofilms were stained with 150ul of 0.1% crystal violet for 20 min at room temperature and washed three times with PBS buffer 1x (pH 7.2). Finally, 150 ul of 96% ethanol was added to each well for 30 min, and the absorbance was measured at 570 nm. The biofilm inhibition percentage was assessed with the following formula:
2.9. Anti-Tumoral Activity
Four tumoral cell lines, MDA-MB-231 and MCF-7 (human breast carcinoma), HeLa (human cervical carcinoma) and THJ29T (thyroid carcinoma), and one non-tumoral cell line, NIH3T3 (mouse NIH/Swiss embryo fibroblasts) were obtained from ATCC and cultured in Dulbecco’s modified eagle medium (DMEM/F12) (Corning, Corning in Manassas, VA, USA) supplemented with 10% fetal bovine serum (FBS) (Eurobio, Les Ulis, France) and 1% penicillin/streptomycin (Thermo Fisher Scientific, Gibco, Miami, FL, USA). All cell lines were maintained at 37 °C in a humidified atmosphere at 5% CO2. To analyze the effect of the extracts on cell proliferation, cells were seeded at a density of 1 × 104 cells/well in 96-well plates. Cells were incubated for 72 hours with 100 μL of the berries extract at 0.04 - 5 mg/mL. Following the specified incubation period, the 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) dye assay was performed using the standard procedure provided by Sigma, St. Louis, MO, USA. In summary, 10 μL of MTT solution (5 mg/mL) was added to each well. After allowing 1-2 hours for incubation in a humidified environment, the media was aspirated, and 50 μL of DMSO was added to each well to dissolve the formazan crystals. The mixture was agitated for 5 minutes before measuring the absorbance at 570 nm using a Cytation5 multi-mode detection system by BioTek, Winooski, VT, USA. Each data point was derived from quadruplicate samples, and the experiment was replicated at least four times. To determine the concentration of the compound required to inhibit 50% of cell proliferation (IC50), dose-response curves were generated in GraphPad Prism software developed by GraphPad Software, San Diego, CA, USA. The control group of untreated cells served as the basis for 100% cell proliferation.
2.10. Anti-Inflammatory Activity
RAW264.7 murine macrophage cells were obtained from ATCC and cultured in RPMI (Corning, Corning in Manassas, VA, USA) supplemented with 10% fetal bovine serum (FBS) (Eurobio, Les Ulis, France) and 1% penicillin/streptomycin (Thermo Fisher Scientific, Gibco, Miami, FL, USA). Cells were maintained at 37 °C in a humidified atmosphere with 5% CO2. To analyze the anti-inflammatory effects of the berries extracts RAW264.7 cells were cultured in 24 well plates at 4 × 105 cells per well. The macrophage cells were first exposed to the extracts before being stimulated with 1 μg/mL of lipopolysaccharide (LPS). Also, aspirin (ASA) at 0.8 mg/mL and dexamethasone (DEX) at 0.5 μg/mL were included in the assay. The nitric oxide (NO) production is indicated by the intensity of color produced from the reaction between the culture medium and the Griess reagent. The concentrations of the compounds used for the assay were chosen based on their IC50 values and did not significantly affect cell growth. The assay was performed at least in triplicate.
2.11. Hemolytic Activity
The hemolytic activity of
V. floribundum (
Vf) and
R. glaucus (
Rg) was measured according to a previously described protocol [
15]. Ten milliliters of defibrinated sheep blood were subjected to three consecutive washes with PBS 1X. A 1% erythrocyte suspension in PBS 1X was prepared after the washing steps. The erythrocyte suspension was then combined 1:1 with fruit extracts, positive (10% Triton X-100) or negative (PBS 1X) hemolysis controls, in a 96-well polypropylene plate and incubated at 37°C for 1h. After the incubation, the resulting mixture underwent centrifugation (5 min, 1700 x g), and the resulting supernatant was carefully transferred to a transparent flat bottom 96-well plate, allowing for the quantification of absorbance at 405 nm using a Cytation 5 plate reader. Each experiment was conducted with three technical replicates, and the entire protocol was replicated three times.
3. Results
3.1. Chemical Characterization
The total anthocyanin content in R. glaucus and V. floribundum was quantified, revealing 26.48 mg PgEq/gram and 51.76 mg PgEq/gram, respectively. This substantial difference in anthocyanin concentration between the two fruits aligns with the chromatographic analysis, indicating a higher number of identified anthocyanin compounds in V. floribundum than in R. glaucus.
The data presented in
Table 1 summarizes the identification of the most abundant peaks in
R. glaucus and
V. floribundum using both positive and negative ionization modes. Peak 1 at RT=1.18 min, identified as quinic acid, is present in both fruits. Peaks 2 at RT=3.91 min and 3 at RT=7.80 min, identified as quinic acid derivatives, are also present, with Peak 3 found in both fruits. Peaks 4 and 5 at RT=10.45 min remain unidentified but are observed in
R. glaucus.
Table 1 provides retention times (RT), mass-to-charge ratios ([M-H]- and [M+H]+), and detailed MS/MS fragmentation patterns for each peak. This data set offers a profile of the chemical composition of anthocyanins in these fruits, highlighting the differences and similarities in their anthocyanin content. As summarized in
Table 1, the compounds were identified through spectral comparisons against the GNPS and MzCloud databases, supplemented by literature references. The detailed chromatographic profiles, including peak identification and retention times (RT), are presented in Supplementary Material.
Figure S1 in the Supplementary Material presents the chromatographic images, visually confirming the findings. Both fruits exhibit a similar overall chemical profile, though
V. floribundum shows a greater diversity and abundance of anthocyanins. This observation is consistent with the quantified total anthocyanin content, reinforcing the reliability of the spectrometric and chromatographic data. Peak 9 (RT=14.67 min) is a significant finding, identified based on its fragmentation pattern similarity (score of 81.5%) with cyanidin in the MzCloud database.
The high anthocyanin content in
V. floribundum, as opposed to
R. glaucus, could be attributed to several factors, including genetic differences [
1], environmental conditions [
16,
17], and developmental stages of the fruits [
17]. In comparing the anthocyanin profiles of the two fruits, it is evident that
V. floribundum possesses a richer and more diverse array of anthocyanins. This diversity is reflected in the higher number of peaks identified in
V. floribundum’s chromatogram. Such diversity may enhance the fruit’s bioactivity [
18,
19], offering a more complex blend of health-promoting compounds.
3.2. Minimum Inhibitory Concentration
Antimicrobial susceptibility testing is essential for effectively controlling pathogens. Moreover, MICs help monitor the development of resistance and are relevant for determining optimal pharmacodynamic dosage. The antimicrobial activity of V. floribundum Kunth and Rubus glaucus was tested against twelve susceptible microbial strains of clinical importance.
The in vitro susceptibility tests for the bacteria strains showed that the R. glaucus extracts had strong inhibition effects against Gram-positive bacteria E. faecalis, S. aureus, and L. monocytogenes, with MIC values that ranged between 1 and 1.2 mg/mL. In contrast, the range of action for V. floribundum was considerably higher, between 2.1 and 2.5 mg/ml. For Gram-negative bacteria, MIC values for both berries ranged between 8 and 18 mg/ml.
Table 2 shows the MICs for each strain obtained using the agar dilution method which revealed noticeable differences between the two berries. Overall,
R. glaucus was more effective than
V. floribundum against all the tested bacteria, with the lowest MICs generated. All Gram-positive strains and Gram-negative strains were highly susceptible to both berries. The MICs varied depending on the type of berries and the bacterial species. MICs for Gram-positive strains were lower than Gram-negative for both berries. MIC of
R. glaucus against
E. faecalis was the lower value at 1 mg/ml, whereas the highest MIC obtained was 18mg/ml of the
V. floribundum against
S. typhimurium.
The low inhibitory activity observed in the Gram-negative bacteria may be due to an outer lipopolysaccharide membrane surrounding the bacteria’s cell wall. The absence of this membrane in Gram-positive bacteria may contribute to a greater permeability of the bioactive phytochemicals in the cells, leading to more significant bacterial inhibition [
23,
24]. MIC values of the positive control ciprofloxacin were as previously reported, within plus or minus one or two-fold dilution of the expected MIC [
25]. Proanthocyanidins found in berries, comprising units of (-)-epicatechin and/or (+)-catechin connected through type A and B interflavanic bonds, are known for their antibacterial effectiveness against Gram-negative and Gram-positive bacteria. These compounds interact with bacterial membranes, enhancing their permeability, which leads to eventual puncturing, disintegration, and, ultimately, the death of the bacterial cells [26].
Table 1. Anthocyanin identification in positive ionization mode in both fruits by HPLC-MS/MS. The table presents the retention times (RT), mass-to-charge ratios ([M-H]- and [M+H]+), and MS/MS fragmentation patterns for the most abundant peaks identified in R. glaucus and V. floribundum under both positive and negative ionization modes. Peaks 1, 2, and 3 were identified as quinic and quinic acid derivatives. Peaks 4 and 5 remain unidentified but are observed in R. glaucus. Identification was based on spectral comparisons against the GNPS and MzCloud databases, supplemented by literature references. Detailed chromatographic images are provided in Supplementary Material.The results obtained in our research on the antifungal activity of anthocyanins are particularly relevant, given the limited body of evidence available in the scientific literature. To date, only a couple of studies have evaluated the antimicrobial activity of anthocyanins, and these are based on the measurement of inhibition zones in millimeters, which is not directly comparable to the MIC determination we performed and expressed in mg/ml [27]. The lack of standardization in methodologies makes direct comparisons of results difficult. However, a recent study published in reported MIC values for anthocyanins in the range of 100 to 200 mg/ml, consistent with our findings and validating the relevance of our methodologies and results [28]. This study provides a valuable reference point and suggests that our contributions are significant in the context of antifungal evaluation of anthocyanins.
The mechanism of action for the antifungal activity of anthocyanins in Candida strains cannot be concluded with certainty; however, the microbicidal capacity of anthocyanins on cell membrane integrity has been previously described. The plausible mechanisms underlying the activity of anthocyanins include both membrane and intracellular interactions of their functional groups. Multiple modes of action likely cause the microbicidal potential of fruits and other berries containing polyphenols, as anthocyanins comprise diverse molecules, including weak organic acids, phenolic acids, and glycosides in various chemical forms [27].
Overall, our data suggest that R. glaucus has excellent potential to become a source of helpful antimicrobial agents in the pharmaceutical and food industries. However, further analyses are needed to clarify whether the antimicrobial activity of the extracts is attributed solely to a single bioactive compound or the complementary, synergistic, and additive effects of multiple phytochemicals. This phenomenon will depend on many factors, including geographical and environmental conditions [29,102]
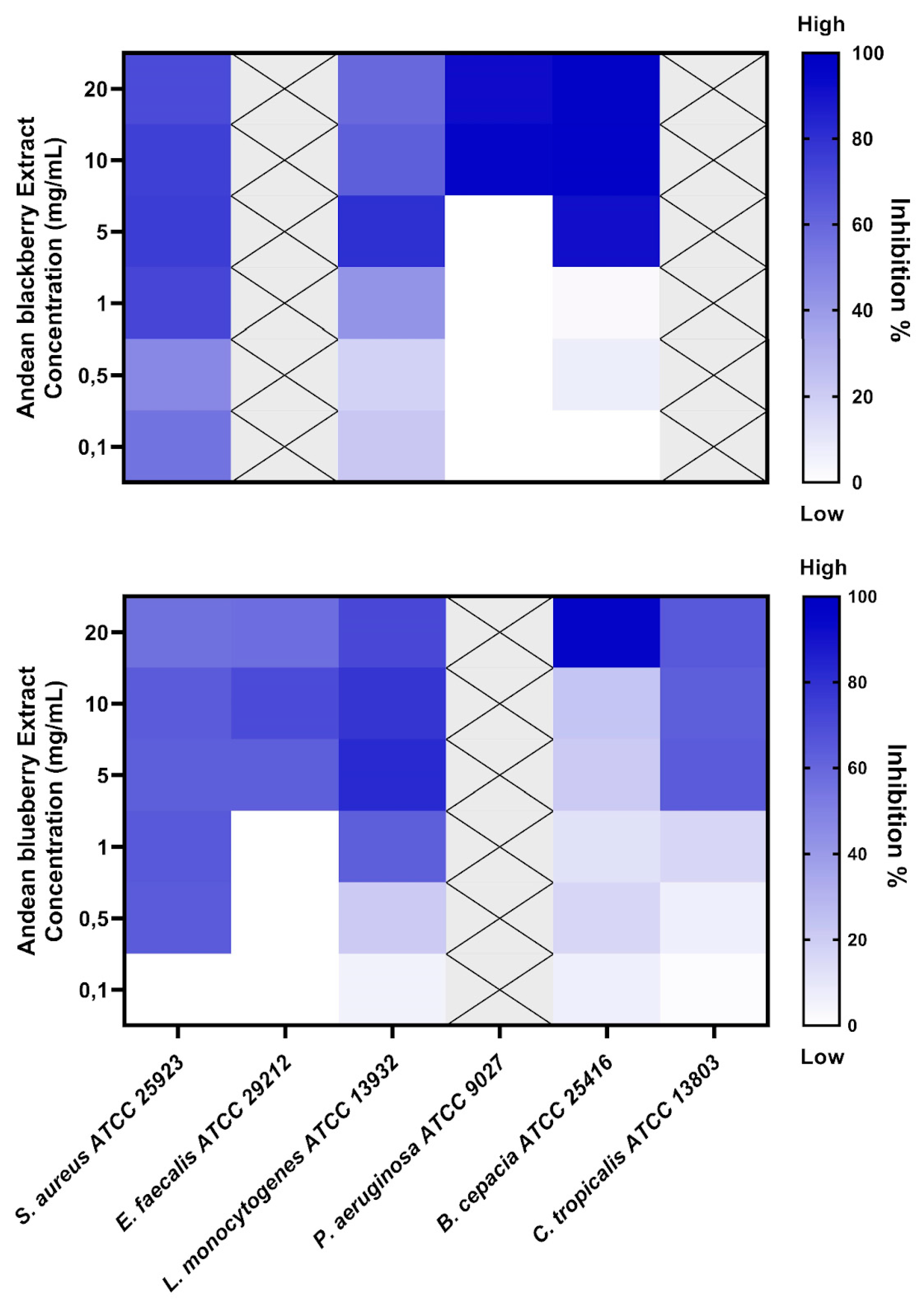
3.3. Biofilm Inhibition Assay
The extracts were tested to assess their biofilm inhibition activity against biofilm-forming microorganisms such as
S. aureus, E. faecalis, L.monocytogenes, P. aeruginosa, B. cepacia, and
C. tropicalis. The
R. glaucus extract displayed potential for biofilm inhibition in 4 strains, while the
V. floribundum extracts had biofilm inhibition activity against 5 of the tested microorganisms, as shown in
Figure 1
The minimum biofilm-inhibiting concentrations with 50% or more inhibition effectiveness (MBIC
50) are shown in
Table 3. MBIC values above 20 mg/ml were labeled “non-active” (NA). The
R. glaucus displayed the lowest MBIC value against
S. aureus (72% inhibition at 1 mg/ml concentration). The lowest MBIC
50 value for the
V. floribundum extract was 0,5 mg/ml for
S. aureus (64% inhibition percentage).
This study revealed that the
V. floribundum extract exhibited the lowest MBIC, at 0,5 mg/mL, against
S. aureus, leading to a 64% inhibition percentage of
S. aureus biofilm (
Table 3). The literature has supported similar MBIC and inhibitory percentages that align with our findings. In 2016, a study also found that blueberry extracts can exhibit an MBIC of 0,5 mg/mL and showcase a 68% inhibition percentage in a methicillin-sensitive (MSSA)
S. aureus strain [30]. Moreover, this study also showed that the same extract concentration can inhibit the growth of methicillin-resistant (MRSA)
S. aureus biofilms by 49% [30], which suggests that future anti-biofilm studies should include the assessment of
V. floribundum extract against antibiotic-resistant
S. aureus strains.
Liu et al. (2021) also assessed the effects of Brightwell blueberry extracts in L. monocytogenes biofilms and reported an MBIC of 2mg/mL (~ 84% inhibition rate), a slightly higher MBIC than the one obtained in this study [31]. However, it is worth noting that Liu et al. (2021) study utilized an optimized anthocyanin-concentration protocol where the crude extract was purified using AB-8 resin and freeze-dried, as described by Gao and coworkers [32]. Liu et al. (2021) suggest that the concentrated blueberry anthocyanin-rich extract can display enhanced antimicrobial effects compared to its crude extract counterpart [31]. In this regard, the difference in MBIC between Liu et al. (2021) results and our study could be attributed to the use of the described anthocyanin-concentration protocol and the slight variation in the phytochemical compositions in the fruits of both regions.
Our research is the first to assess the biofilm inhibition of blueberry and blackberry extracts against
C. tropicalis. In this study, our results showed an MBIC of 5 mg/mL and an inhibition percentage of 64% after treatment with the
V. floribundum extract. On the other hand, the
R. glaucus extract displayed no biofilm-inhibitory effects on
C. tropicalis at the tested concentrations. The literature addressing berry extracts and biofilm inhibition is very limited; one study evaluated the impact of a different berry extract (
Clery strawberries) against
C. albicans and reported a significant anti-biofilm potential [33]. Further research is needed regarding berry extracts and their
Candida spp—biofilm-inhibition potential. Similarly, literature addressing the biological activities of blackberry extracts is scarce. Wu et al. (2022) investigated the antimicrobial effects of blackberry extracts for eliminating planktonic cultures of periodontopathogens, also known for their biofilm-forming capabilities [34]. Similar to the Liu et al. (2021) study, Wu et al. (2022) employed an AB-8 resin macroporous-based purification process to recover anthocyanin-rich extracts from blackberry [
18]. They recorded an MBIC of 2mg/mL and an inhibition rate of ~93% after its application on
L. monocytogenes biofilm. In this case, the anthocyanin-concentration process could also have enhanced the activity of the reported blackberry extract. It could explain why the blackberry extract used by Wu et al. (2022) displayed biofilm inhibition activity, unlike the extract used in our study.
Implementing anthocyanin-concentration protocols for our berry extracts can benefit future research improvements. Additionally, anti-biofilm studies have addressed the fractionation and purification of specific active compounds in berry extracts to assess individual extract derivatives and their biological activities [35]. Moreover, studies that perform co-administration of blackberry extract and antibiotics like methicillin have also demonstrated favorable results [36].
3.4. Antioxidant activity
Both berries showed very similar antioxidant potential; however, it was 4 to 4.4-fold lower than the ascorbic acid control (
Table 4).
Our findings align with those presented in a study by Alarcón-Barrera et al. (2018), which demonstrated that V. floribundum possesses higher DPPH scavenging activity than R. glaucus [37].
While our study revealed that
V. floribundum Kunth exhibited an IC
50 of 21.77 µg/mL through the DPPH assay, a separate study by Prencipe et al. (2014) found that the acidified methanolic extract of these blueberries displayed an IC
50 of 0.694 µg/mL [38]. It should be noted, however, that the extraction method employed by Prencipe et al. (2014) may have contributed to this inconsistency, as they utilized maceration with EtOAc followed by dynamic maceration with HCl in MeOH [38]. Furthermore, Guevara-Terán (2022) emphasized the significance of considering the maturity stage of the plant when studying its antioxidant activity. Specifically, samples obtained at high altitudes (3641 m.a.s.l) exhibited notable variations in antioxidant activity depending on their maturity stage [
17]. Similar to Guevara-Terán’s (2022) findings regarding Andean blackberries, Samaniego (2020) observed that as these berries matured, their phytochemical components declined, reducing their antioxidant activity [
17,39]. Various methods, such as ABTS, modified TEAC assay, oxygen radical absorbance capacity (ORAC), and FRAC, have been used to demonstrate the antioxidant potential of
V. floribundum and
R. glaucus [
4,
24,39,40]. These studies underscore the importance of these berries as a natural source of anthocyanins and their potential health benefits as antioxidants.
3.5. Antitumoral Activity
The berries’ antitumoral activity was evaluated using the MTT assay to assess cell proliferation. Treatment with both extracts resulted in significant dose-dependent inhibition of cell proliferation for blackberry, with IC
50 values between 1.22 and 3.69 mg/mL and the highest effect against HeLa cells. We observed lower IC
50 values for R
. glaucus than
V. floribundum, except for MDA-MB-231 cells (
Table 5). Similar IC
50 values were obtained elsewhere for blackberry extracts against colon tumoral cells [41]. The authors stated that their results support the potential use of blackberry extracts as a source of bioactive compounds that may be beneficial in treating diseases related to oxidative stress. Also, the blueberry extract showed a dose-dependent inhibitory effect on cell proliferation for different tumor cell lines, decreasing cell adhesion and inhibiting migration of MDA-MB-231 and PC-3 tumor cells [42]. Both berries contain anthocyanins, which exhibit potent antioxidant activity and may inhibit carcinogen activation and DNA damage [43]; additionally, these anthocyanins are implicated in suppressing cancer cell growth by modulating cell signaling pathways and cell cycle regulators [44,45].
Similarly, research suggests that blueberry anthocyanins hold therapeutic potential in protecting liver cells against oxidative stress [46]. On the other hand, both extracts showed antiproliferative effects on non-tumoral NIH3T3 cells, with IC50 values of 2.22 mg/mL for R. glaucus and 2.60 mg/mL for V. floribundum. These results emphasize the need for further studies to evaluate the safety and efficacy of these extracts for cancer therapy.
3.6. Antiinflamatory Activity
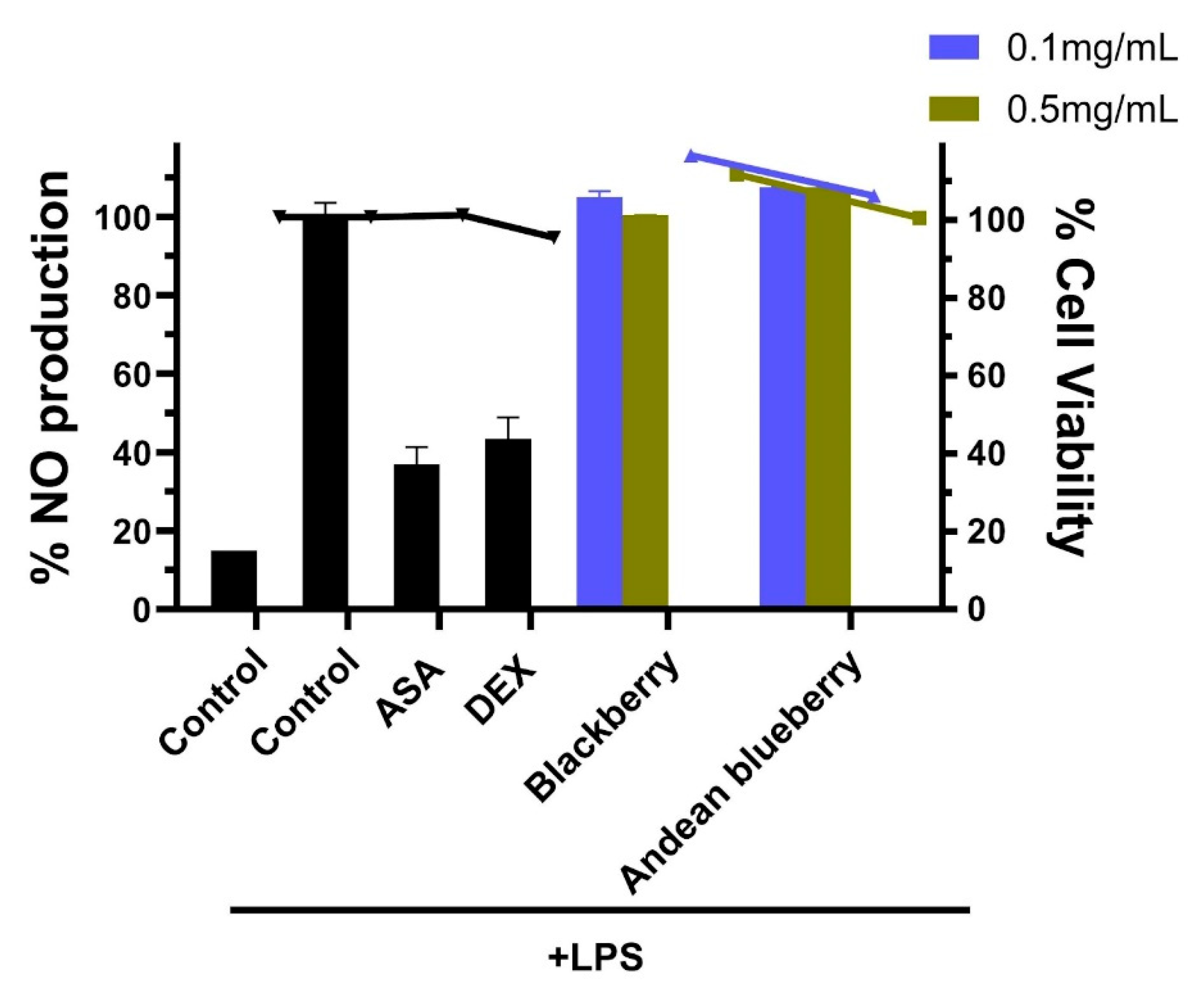
In the context of our study, the modulation of inflammatory responses, mainly through the inhibition of nitric oxide (NO) production in RAW264.7 cells, serves as an essential piece in evaluating the anti-inflammatory activity of the berries. Studies have shown that blueberry extracts exhibit suppression of pro-inflammatory cytokines and chemokines. In contrast, blackberry extracts inhibit the production of inflammatory mediators such as TNF-α, IL-6, IL-1β, COX-2, and iNOS, suggesting potential anti-inflammatory [47,48].
RAW264.7 cells were pretreated with the compounds before being stimulated with LPS to induce an inflammatory response. The NO levels in the culture media were then measured using the Griess method. LPS exposure activated RAW264.7 cells inflammation response, evidenced by a significant increase in NO production following 18 hours of LPS stimulation (
Figure 2). Pre-treatment with aspirin and dexamethasone before the LPS challenge effectively reduced NO production to 36.9% and 43.3%, respectively. Moreover, NO levels were notably higher in the LPS-stimulated group compared to the control group (media only). Our results show that pretreatment with 0.1-0.5 mg/mL of extracts did not significantly suppress NO production in LPS-induced RAW264.7 cells. The lack of significant suppression suggests that the anti-inflammatory activity of these extracts may vary depending on the anti-inflammatory activity of the berries, which can vary due to factors such as the fruit’s variety, ripeness, and processing [49].
3.7. Hemolytic Activity
Hemolysis, the rupture of red blood cells leading to the release of hemoglobin, is a crucial indicator of cytotoxicity. An assessment was conducted following a previously described protocol [
15]. The analysis of hemolytic activity (HA) in aqueous extracts of
R. glaucus (labeled Rg) and
V. floribundum (labeled Vf) reveals significant differences in their cytotoxic potential, influenced by the distinct profiles of bioactive compounds in each extract. The hemolytic assay results indicate that
R. glaucus exhibits a dose-dependent hemolytic activity, while
V. floribundum shows negligible hemolytic activity
Table 6.
In this study,
R. glaucus at 10 mg/ml demonstrated a hemolytic activity of 6.3%. At 50 mg/ml, it showed an increased HA to 10.2%, indicating a significant dose-dependent increase in hemolytic activity
Table 6. The higher hemolytic activity in
R. glaucus can be attributed to its bioactive compound profile, particularly delphinidin-3-pyranoside and cyanidin-3-pyranoside, identified in the extract (
Table 1). Previous research has shown that anthocyanins and other phenolic compounds in berries can exhibit cytotoxic effects, including hemolysis [50,51]. These studies suggest that the specific anthocyanins present in
R. glaucus contribute to its hemolytic activity. In contrast,
V. floribundum at 10 mg/ml exhibited no hemolytic activity. This negligible activity suggests that the aqueous extracts of
Vf are non-hemolytic at the tested concentration.
V. floribundum contains anthocyanins, such as cyanidin-3-arabinoside and delphinidin-3-pyranoside, but at lower intensities. This composition might contribute to its lack of hemolytic activity.
The contrasting hemolytic activities observed in R. glaucus and V. floribundum highlight the species-specific differences in bioactive compound profiles. With its higher hemolytic activity, R. glaucus contains anthocyanins and phenolics that can disrupt cell membranes. On the other hand, V. floribundum’s lack of hemolytic activity suggests a composition that either lacks cytotoxic compounds or contains protective compounds that prevent membrane disruption. These findings have significant implications for the potential therapeutic use of these extracts.
4. Conclusions
This study has evaluated the antimicrobial and antioxidant properties of V. floribundum Kunth and R. glaucus extracts, demonstrating their potential as natural antimicrobial agents with significant health benefits. The chemical characterization revealed a rich content of anthocyanins, particularly evident in their complex mass spectrometric profiles, suggesting a robust framework for their bioactivity. Notably, the antimicrobial activity assays highlighted that R. glaucus extracts were particularly effective, showing low MIC values against Gram-positive bacteria E. faecalis, S. aureus, and L. monocytogenes. These findings underscore the potential of R. glaucus as a natural antimicrobial source suitable for applications in both the pharmaceutical and food industries.
However, the extracts showed less activity against Gram-negative bacteria, likely due to the bacteria’s protective outer membrane, which may impede the penetration of bioactive compounds. This suggests the need for further studies to enhance the efficacy of these extracts against Gram-negative pathogens, potentially through formulation improvements or combination therapies.
Moreover, both berry extracts displayed significant antioxidant activity, though slightly less than standard ascorbic acids, indicating their potential as natural antioxidants to enhance food shelf life and safety. The antioxidant capacity and antimicrobial effects provide a compelling case for using these extracts in food preservation and as therapeutic agents. Regarding the antitumoral assay, the extracts showed a dose-dependent inhibition of cell proliferation across various cancer cell lines, with the most pronounced effects observed in HeLa cells. R. glaucus exhibited lower IC50 values compared to V. floribundum for most cell lines, indicating higher potency. The extracts also displayed antiproliferative effects on non-tumoral NIH3T3 cells, highlighting the need for further research to assess the safety and therapeutic potential of these compounds.
Additionally, our findings revealed no significant anti-inflammatory activity, suggesting variations in the fruit’s properties may influence its biological effects.
The hemolytic activity assay provided valuable insights into the cytotoxic potential of berries extracts. R. glaucus exhibits a dose-dependent hemolytic activity, likely due to its specific anthocyanin and phenolic content, while V. floribundum shows no significant hemolytic activity, corroborating its reputation as a safe, antioxidant-rich berry. The total anthocyanin content and the specific profiles of bioactive compounds play a crucial role in these effects. Future research should focus on isolating and characterizing the specific compounds responsible for these effects, further elucidating their mechanisms of action and potential therapeutic applications. Additionally, in vivo studies are necessary to confirm the safety and efficacy of these extracts in clinical settings.
In conclusion, our findings suggest that the studied berry extracts, particularly those from R. glaucus, offer promising potential for development into products that could contribute to health promotion and disease prevention. Future studies focusing on mechanistic pathways, dosage optimization, and clinical trials are necessary to fully integrate these natural products into the pharmaceutical and food sectors.
Supplementary Materials
The following supporting information can be downloaded at website of this paper posted on Preprints.org, Figure S1: title; Table S1:
Author Contributions
Conceptualization, Linda Guamán; Data curation, Johana Zuñiga; Formal analysis, Carlos Barba-Ostria; Investigation, Arianna Mayorga-Ramos; Methodology, Carlos Barba-Ostria, Saskya E. Carrera-Pacheco, Rebeca Gonzalez, Johana Zuñiga, Arianna Mayorga-Ramos and Eduardo Tejera; Resources, Linda Guamán; Validation, Carlos Barba-Ostria; Writing – original draft, Carlos Barba-Ostria, Saskya E. Carrera-Pacheco, Rebeca Gonzalez, Johana Zuñiga, Arianna Mayorga-Ramos and Eduardo Tejera; Writing – review & editing, Rebeca Gonzalez and Linda Guamán.
Funding
This research received no external funding.s
Data Availability Statement
The data presented in this study are openly available in FigShare at doi 10.6084/m9.figshare.26321551.
Conflicts of Interest
authors declare no conflicts of interest.
References
- Giné Bordonaba, J.; Chope, G.A.; Terry, L.A. Maximising blackcurrant anthocyanins: Temporal changes during ripening and storage in different genotypes. J. Berry Res. 2010, 1, 73–80. [Google Scholar] [CrossRef]
- Schreckinger, M.E.; Wang, J.; Yousef, G.; Lila, M.A.; Gonzalez de Mejia, E. Antioxidant capacity and in vitro inhibition of adipogenesis and inflammation by phenolic extracts of Vaccinium floribundum and Aristotelia chilensis. J. Agric. Food Chem. 2010, 58, 8966–8976. [Google Scholar] [CrossRef] [PubMed]
- Merecz-Sadowska, A.; Sitarek, P.; Kowalczyk, T.; Zajdel, K.; Jęcek, M.; Nowak, P.; Zajdel, R. Food Anthocyanins: Malvidin and Its Glycosides as Promising Antioxidant and Anti-Inflammatory Agents with Potential Health Benefits. Nutrients 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, E.; Guldas, M.; Ellergezen, P.; Acar, A.G.; Gurbuz, O. Obesity-associated Pathways of Anthocyanins. fst 2021, 41, 1–13. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, H.; Shao, S.; Sun, S.; Yang, D.; Lv, S. Anthocyanin: a review of plant sources, extraction, stability, content determination and modifications. Int. J. Food Sci. Technol. 2022, 57, 7573–7591. [Google Scholar] [CrossRef]
- Beltrán-Noboa, A.; Proaño-Ojeda, J.; Guevara, M.; Gallo, B.; Berrueta, L.A.; Giampieri, F.; Perez-Castillo, Y.; Battino, M.; Álvarez-Suarez, J.M.; Tejera, E. Metabolomic profile and computational analysis for the identification of the potential anti-inflammatory mechanisms of action of the traditional medicinal plants Ocimum basilicum and Ocimum tenuiflorum. Food Chem. Toxicol. 2022, 164, 113039. [Google Scholar] [CrossRef] [PubMed]
- Cadena-Cruz, J.E.; Guamán-Ortiz, L.M.; Romero-Benavides, J.C.; Bailon-Moscoso, N.; Murillo-Sotomayor, K.E.; Ortiz-Guamán, N.V.; Heredia-Moya, J. Synthesis of 4,4’-(arylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ols) and evaluation of their antioxidant and anticancer activities. BMC Chemistry 2021, 15, 38. [Google Scholar] [CrossRef] [PubMed]
- Spinardi, A.; Cola, G.; Gardana, C.S.; Mignani, I. Variation of anthocyanin content and profile throughout fruit development and ripening of highbush blueberry cultivars grown at two different altitudes. Front. Plant Sci. 2019, 10, 1045. [Google Scholar] [CrossRef]
- Wu, H.; Di, Q.-R.; Zhong, L.; Zhou, J.-Z.; Shan, C.-J.; Liu, X.-L.; Ma, A.-M. Enhancement on antioxidant, anti-hyperglycemic and antibacterial activities of blackberry anthocyanins by processes optimization involving extraction and purification. Front. Nutr. 2022, 9, 1007691. [Google Scholar] [CrossRef] [PubMed]
- Aita, S.; Capriotti, A.; Cavaliere, C.; Cerrato, A.; Giannelli Moneta, B.; Montone, C.; Piovesana, S.; Laganà, A. Andean Blueberry of the Genus Disterigma: A High-Resolution Mass Spectrometric Approach for the Comprehensive Characterization of Phenolic Compounds. Separations 2021, 8, 58. [Google Scholar] [CrossRef]
- Alcalde-Eon, C.; García-Estévez, I.; Rivas-Gonzalo, J.C.; Rodríguez de la Cruz, D.; Escribano-Bailón, M.T. Anthocyanins of the anthers as chemotaxonomic markers in the genus Populus L.. Differentiation between Populus nigra, Populus alba and Populus tremula. Phytochemistry 2016, 128, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Garzón, G.A.; Soto, C.Y.; López-R, M.; Riedl, K.M.; Browmiller, C.R.; Howard, L. Phenolic profile, in vitro antimicrobial activity and antioxidant capacity of Vaccinium meridionale swartz pomace. Heliyon 2020, 6, e03845. [Google Scholar] [CrossRef] [PubMed]
- Urbonaviciene, D.; Bobinaite, R.; Viskelis, P.; Bobinas, C.; Petruskevicius, A.; Klavins, L.; Viskelis, J. Geographic Variability of Biologically Active Compounds, Antioxidant Activity and Physico-Chemical Properties in Wild Bilberries (Vaccinium myrtillus L.). Antioxidants (Basel) 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Tiwari, V.; Vats, S.; Kumari, A.; Chunduri, V.; Kaur, S.; Kapoor, P.; Garg, M. Evaluation of Anthocyanin Content, Antioxidant Potential and Antimicrobial Activity of Black, Purple and Blue Colored Wheat Flour and Wheat-Grass Juice against Common Human Pathogens. Molecules 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Costa, E.M.; Mendes, M.; Morais, R.M.; Calhau, C.; Pintado, M.M. Antimicrobial, antiadhesive and antibiofilm activity of an ethanolic, anthocyanin-rich blueberry extract purified by solid phase extraction. J. Appl. Microbiol. 2016, 121, 693–703. [Google Scholar] [CrossRef] [PubMed]
- College of Food Science and Technology, Hebei Agricultural University, Baoding, Hebei, 071 000, P.R. China. Gao, Z. Extraction, separation, and purification of blueberry anthocyanin using ethyl alcohol. Kem. Ind. 2017, 66, 655–659. [Google Scholar] [CrossRef]
- González, O.A.; Escamilla, C.; Danaher, R.J.; Dai, J.; Ebersole, J.L.; Mumper, R.J.; Miller, C.S. Antibacterial effects of blackberry extract target periodontopathogens. J. Periodont. Res. 2013, 48, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Salaheen, S.; Peng, M.; Joo, J.; Teramoto, H.; Biswas, D. Eradication and Sensitization of Methicillin Resistant Staphylococcus aureus to Methicillin with Bioactive Extracts of Berry Pomace. Front. Microbiol. 2017, 8, 253. [Google Scholar] [CrossRef]
- Prencipe, F.P.; Bruni, R.; Guerrini, A.; Rossi, D.; Benvenuti, S.; Pellati, F. Metabolite profiling of polyphenols in Vaccinium berries and determination of their chemopreventive properties. J. Pharm. Biomed. Anal. 2014, 89, 257–267. [Google Scholar] [CrossRef]
- Vasco, C.; Riihinen, K.; Ruales, J.; Kamal-Eldin, A. Chemical composition and phenolic compound profile of mortiño (Vaccinium floribundum Kunth). J. Agric. Food Chem. 2009, 57, 8274–8281. [Google Scholar] [CrossRef]
- Lamdan, H.; Garcia-Lazaro, R.S.; Lorenzo, N.; Caligiuri, L.G.; Alonso, D.F.; Farina, H.G. Anti-proliferative effects of a blueberry extract on a panel of tumor cell lines of different origin. Exp. Oncol. 2020, 42, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Jazić, M.; Kukrić, Z.; Vulić, J.; Četojević-Simin, D. Polyphenolic composition, antioxidant and antiproliferative effects of wild and cultivated blackberries (Rubus fruticosus L.) pomace. Int. J. Food Sci. Technol. 2019, 54, 194–201. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, Y.; Tao, X.; Zhang, M.; Sun, A. Protective effect of blueberry anthocyanins in a CCL4-induced liver cell model. LWT - Food Science and Technology 2015, 60, 1105–1112. [Google Scholar] [CrossRef]
- Huang, W.; Yan, Z.; Li, D.; Ma, Y.; Zhou, J.; Sui, Z. Antioxidant and Anti-Inflammatory Effects of Blueberry Anthocyanins on High Glucose-Induced Human Retinal Capillary Endothelial Cells. Oxid. Med. Cell. Longev. 2018, 2018, 1862462. [Google Scholar] [CrossRef]
- Seeram, N.P. Berry fruits for cancer prevention: current status and future prospects. J. Agric. Food Chem. 2008, 56, 630–635. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).