1. Introduction
Organisms adapt to stress by stimulating the hypothalamic–pituitary–adrenal (HPA) axis to cope with environmental changes. The adrenal glands secrete corticosterone, the primary glucocorticoid in humans, which increases pulse and blood pressure, raises blood sugar levels, and suppresses excessive immune responses. However, excessive corticosterone levels weaken the prefrontal cortex and cause neuronal death in the hippocampus, leading to anxiety, depression, and other neurological and psychiatric disorders. Therefore, attempts have been made to reduce surplus corticosterone levels [
1].
Several nutrients, probiotics, and prebiotics are potential therapeutic agents for mitigating excessive stress responses [
2]. L-carnosine is an imidazole dipeptide composed of beta-alanine and L-histidine, and its concentration is very high in the skeletal muscle and brain of mammals. It can cross the blood–brain barrier or can be synthesized from beta-alanine and histidine in the brain. In practice, L-carnosine has been marketed as a nutritional supplement for its anti-aging and fatigue-relieving properties and ameliorating effects on lifestyle-related diseases (e.g., diabetes, hypertension, and atherosclerosis) through its antioxidant and pH-buffering properties [
3,
4]. In the brain, it has beneficial effects on various neuropsychiatric disorders such as ischemic stroke, cognitive impairment [
5,
6], autism spectrum syndrome [
7], schizophrenia [
8,
9], Alzheimer’s disease and dementia [
10,
11], attention deficit hyperactivity disorder [
12], and Gulf War syndrome [
13]. L-carnosine is involved in stress-induced corticosterone responses and anxiety behaviors in rodents. L-carnosine-administered mice subjected to restraint stress showed suppressed elevation of plasma corticosterone levels compared to that in the control Kunming mice [
14,
15]. Administration of L-carnosine to rats induces anxiolytic-like behavior in a dose-dependent manner.
Bone marrow stromal cell antigen (BST-1), also known as CD157, was first cloned as a glycosyl phosphatidylinositol-anchored protein involved in the growth of pre-B cells [
16]. CD157/BST-1 is a glycosyl phosphatidylinositol-anchored membrane protein that functions as an ADP ribosyl cyclase, and the loss of CD157 expression in mice results in anxiety-like behaviors and social behavioral deficits. It is a paralog of CD38 that catalyzes cyclic ADP-ribose to regulate intracellular Ca2+ [
17]. CD157/BST-1 is constitutively expressed in the myeloid cells of peripheral blood mononuclear cells and regulates humoral immune response [
18]. Moreover, CD157/BST-1 is associated with neuropsychiatric disorders, such as Parkinson’s disease, autism spectrum disorder (ASD), rapid eye movement sleep behavior disorder, major depressive disorder, restless leg syndrome/Willis–Ekbom disease, and Alzheimer’s disease [
19]. Although the physiological role of CD157 in the brain remains largely unexplored, an association between CD157/BST1 and ASD has been reported [
20,
21]. Homozygous CD157 knockout (CD157 KO) mice display social behavioral impairments and anxiety-related and depression-like behaviors, which can be restored by treatment with antidepressants or oxytocin [
22,
23,
24,
25]. These findings suggest that CD157 KO mice may be useful as a model of ASD with regard to modeling the behaviors associated with ASD symptoms. We have previously observed that chronic administration of L-carnosine ameliorates social behavioral deficits, which is a core symptom of ASD, in CD157 KO mice [
26]. However, the effects of L-carnosine on other comorbid symptoms of ASD, such as anxiety-related behaviors and altered stress responses, have not yet been investigated in CD157 KO mice. In this study, we examined the effect of chronically administered L-carnosine on corticosterone response induced by acute stress and anxiety-like behavior in CD157 KO mice.
2. Materials and Methods
2.1. Animals
C57BL6/N wild-type (WT) mice were obtained from Japan SLC Inc. (Hamamatsu, Japan) via the Sankyo Laboratory Service Corporation (Toyama, Japan). CD157 KO mice were developed as previously described [
18]. Homozygous CD157 KO mice were used in this study. WT and CD157 KO mice were housed at the Institute for Experimental Animals, Advanced Science Research Center, Kanazawa University, under standard conditions (22 °C; 12-h light/dark cycle, lights on at 8:45 a.m.) in standard mouse cages (300 × 160 × 110 mm) with sawdust bedding and access to food and water ad libitum. Mice weaned at 21–28 days of age were housed in same-sex groups of three to five animals until 11 weeks of age. Male mice were single-housed for 14 days before acute stress, and behavioral tests were conducted. Carnosine-treated mice were maintained on a steady dose of L-carnosine (Phytopharma Co., Ltd., Kanagawa, Japan) diluted in drinking water (0.09 g/100 mL) from weaning up till the behavioral test. Water intake and body weight were measured during the experiment. This study was conducted in accordance with the Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology of Japan. The protocol was approved by the Committee on Animal Experimentation of Kanazawa University (AP-143261). We divided into three groups, wild-type (WT) as control, CD157 KO mice with or without L- carnosine administration (KO-Car or KO-Water).
2.2. Acute Stress
Forced swimming was performed as previously described [
22]. Briefly, Mice were placed individually in a cylinder (height 25 cm, diameter 15 cm) filled up to a 10-cm depth with water (25 ± 1 °C) for 6 min. A restraint stress study was performed as mice were placed in a 50-mL polypropylene conical tube (Eppendorf, Hamburg, Germany) with air holes for 10 min.
2.3. Plasma Sampling and Enzymatic Detection of Corticosterone
Male mice were anesthetized immediately after acute stress by an intraperitoneal injection of pentobarbital (35 mg/kg). Blood samples (0.8–1 mL) were collected by cardiac puncture, and 8–10 μL of 0.1 g/mL ethylenediaminetetraacetic acid was added. The samples were centrifuged at 1,600 ×g for 15 min at 4 °C. Plasma samples (200–400 μL/mouse) were collected and stored at -80 °C until use.
2.4. Enzyme Immunoassay of Corticosterone
Immunoreactivity of plasma corticosterone was analyzed using a corticosterone EIA kit (Enzo Life Sciences, NY, USA), following the manufacturer’s instructions. The plasma samples (5 μL) were thawed and diluted to 1:80 in assay buffer. Fifty microliters of the sample were used for the assay. Blood samples were assayed without protein extraction as previously described [
25]. The assay had two linear ranges, covering a concentration range of 30–1000 pg/mL. The inter- and intra-assay coefficients of variation were < 5%.
2.5. Elevated Plus Maze
The mice in the homecage was placed in the experiment room for at least one hour for the habituation. Duration of elevated plus maze is five minutes. Behavior was measured using digital video system and ANY-maze software (Sloelting Co, Wood Dale, IL, USA).
2.6. Statistical Analysis
Statistical analysis was performed using Prism v.8 (GraphPad Software Inc., San Diego, CA, USA). The data are presented as mean ± standard error. The induction of corticosterone in the blood after the stress tests were compared with the baseline blood corticosterone levels in each group using an unpaired t-test. One-way analysis of variance (ANOVA) was used to assess the differences in blood corticosterone levels among groups. Subsequently, Tukey’s post hoc multiple comparison test was performed. We did not observe any effect of L-carnosine on blood corticosterone levels in WT mice; therefore, no further behavioral examination of WT mice was conducted. For the behavioral examination, an unpaired t-test was conducted to compare each index between the KO-Water, KO-Car, and WT-Water groups.
3. Results
3.1. L-Carnosine Mitigated Forced Swimming or Restraint Stress-Induced Elevation in Plasma Corticosterone Levels
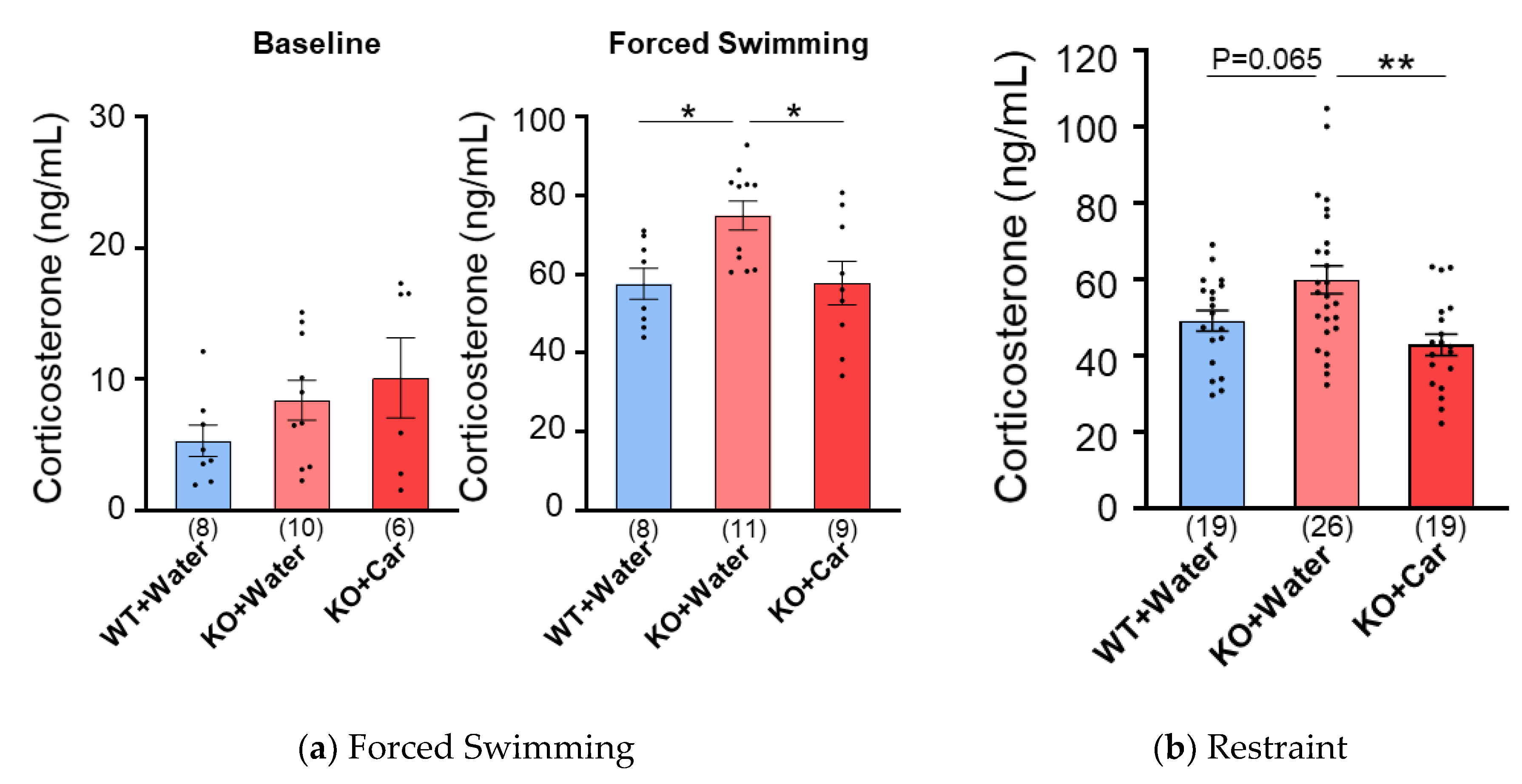
Acute stress increases plasma corticosterone levels. The effects of L-carnosine intake on plasma corticosterone levels after forced swimming were examined in WT and CD157 KO mice with (KO-C) or without (KO-W) chronic carnosine intake. While the basal corticosterone levels did not change between the groups (
Figure 1A, left panel), forced swimming stress increased plasma corticosterone levels in all groups (control vs. forced swimming, P < 0.0001 in all four groups, unpaired t-test). Corticosterone levels in KO-W mice were higher than those in WT mice, whereas corticosterone levels in KO-C mice were similar to those in WT mice. One-way ANOVA revealed a significant difference in corticosterone levels between the groups (F [2, 25] = 5.420, P = 0.011), and post hoc analysis with Bonferroni multiple comparisons test revealed significant differences in corticosterone levels between the WT and KO-W groups and between the KO-W and KO-C groups (WT vs. KO-W, P = 0.032; KO-W vs. KO-C, P = 0.028).
Next, we examined the effect of L-carnosine on restraint stress. A single bout of restraint stress caused an increase in plasma corticosterone levels from the baseline values (
Figure 1A, left) in all groups (
Figure 1B, P < 0.0001 in each group, unpaired t-test). Corticosterone levels in KO-W mice were higher than those in WT mice, whereas corticosterone levels in KO-C mice were similar to those in WT mice. One-way ANOVA revealed a significant difference in corticosterone levels between groups (F [2, 61] = 7.375, P = 0.001), and post hoc analysis with Bonferroni’s multiple comparison test revealed a tendency to significant difference between WT and KO-W mice (P = 0.065) and a highly significant difference between KO-W and KO-C mice (P = 0.001). These results showed that CD157 KO mice were more responsive than WT mice to acute physical stress and that oral supplementation of L-carnosine to CD157 KO mice mitigated acute stress-induced increases in blood corticosterone levels.
3.2. Elevated Plus Maze Test
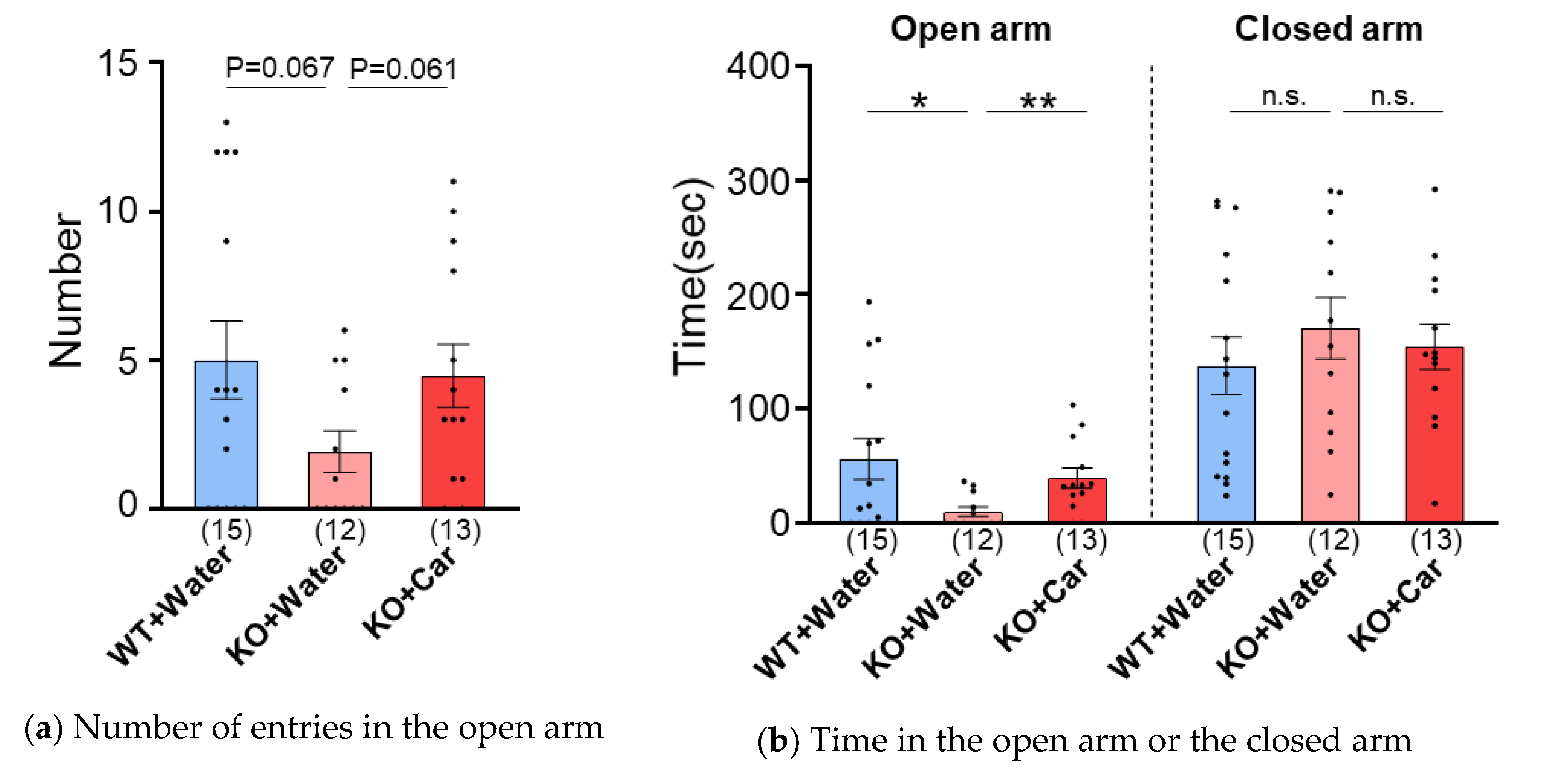
We examined whether L-carnosine uptake ameliorated anxiety-like behavior in CD157 KO mice. Anxiety was assessed using the elevated plus maze assay. The number of entries in the open arm decreased in KO-W mice compared to that in WT mice (P = 0.067, unpaired t-test), but increased in KO-C mice compared to that in KO-W mice (P = 0.061, unpaired t-test). The time spent in the open arms of KO-W mice was lower than that of WT and KO-C mice (P = 0.033 and 0.0073, unpaired t-test, respectively) and these were at a similar level between KO-C and WT mice (P = 0.85, unpaired t-test). Although one-way ANOVA revealed a significant difference between groups (F [2, 37] = 3.242, P =0.050), post hoc analysis with Bonferroni’s multiple comparisons test showed a significant difference in the corresponding test values only between WT and KO-W mice (P = 0.0468). On the other hand, the time spent in closed arm was not different between any groups (P = 0.39, WT vs KO-W, and P =0.63, WT vs KO-C, unpaired t-test). These results demonstrated that L-carnosine ameliorated anxiety-like behavior in ASD mice.
Figure 2.
Elevated plus maze test. (a) Numbers of entries in the open arms. (b) Time in the open arm or the closed arm. Numbers of animals are shown in bars. Data are the mean ± SEM. *p ≤.05. **p ≤.01.
Figure 2.
Elevated plus maze test. (a) Numbers of entries in the open arms. (b) Time in the open arm or the closed arm. Numbers of animals are shown in bars. Data are the mean ± SEM. *p ≤.05. **p ≤.01.
4. Discussion
Acute stress significantly increased corticosterone release in CD157 KO mice, whereas CD157 KO mice that were chronically administered L-carnosine showed corticosterone levels similar to those of WT mice. Furthermore, L-carnosine treatment reduced anxiety-like behaviors in CD157 KO mice.
We have previously reported that L-carnosine uptake improves social recognition behavior deficits in CD157 KO mice, probably through the activation of oxytocin neurons in the hypothalamus and increased secretion of oxytocin [
26]. Therefore, L-carnosine may reduce corticosterone secretion through activation of the oxytocinergic pathway. Exposure to various physiological and psychological stressors (immobilization, shaking, social defeat, forced swimming, or intracerebroventricular infusion of corticotropin-releasing factor [CRF]) can activate oxytocin neurons and facilitate the release of oxytocin in rodents [
27,
28]. Oxytocin innervates CRF neurons in the paraventricular nucleus to inhibit their activation, thereby inhibiting CRF secretion [
29]. Exogenous oxytocin reduces CRF secretion and mitigates physical and mental responses to acute stress [
30,
31]. Furthermore, oxytocin neurons modulate CRF neurons and project to the vagus nerve and solitary bundle nuclei, thereby stimulating parasympathetic neurons and directly relieving stress [
32]. Therefore, the administration of L-carnosine may relieve acute physical stress by decreasing corticosterone secretion through the oxytocinergic pathway.
Anxiety disorders occur frequently among individuals with ASD, with a meta-analysis estimating that approximately 40% of youths are affected by ASD [
33]. Children with ASD have higher anxiety levels than typically developing children, and anxiety levels increase with intelligence quotient and age [
34]. According to another meta-analysis, in autistic adults, although the prevalence rate is inconsistent depending on the study design, the estimated current prevalence rate of anxiety is high up to 27% [
35]. Numerous studies have investigated corticosterone responsiveness under physiological and/or psychosocial stress. Studies examining cortisol diurnal rhythms and cycles (cortisol arousal response, diurnal decline, and variability) indicate that relatively low-functioning individuals with autism show values different from those of typically developing individuals, but do not consistently show similar changes in high-functioning individuals with autism [
36]. In physically stressful environments, such as indoor cycling session, blood drawing, and simulated magnetic resonance imaging, patients with ASD respond more excessively to stressors than typically developed children. Furthermore, regarding psychosocial stressors, high reactivity has been observed in the playground during interactions with unfamiliar peers or short separation from guardian [
36]. Therefore, hypersecretion of cortisol can be predicted for some psychological or non-psychological stress in people with autism. Our study suggests that chronic intake of L-carnosine may reduce dysregulated responsiveness of the HPA axis and help dampen the stress response in individuals with ASD.
One limitation of our study is that we only explored the effect of oral supplementation of L-carnosine from weaning to adulthood. Adolescence may be a sensitive time window for improving neuronal circuitry deficit. Environmental enrichment experience during adolescence in rodents could restore behavioral and emotional deficits induced by aversive experiences during the early postnatal age. Therefore, further investigations are necessary regarding the duration and time window of L-carnosine supplementation for understanding the underlying role of L-carnosine in anxiolytic effects and stress responses.
5. Conclusions
This is the first study to demonstrate the anxiolytic effect of L-carnosine and the suppression of stress-induced corticosterone secretion by L-carnosine in an ASD mouse model. We found that L-carnosine supplementation may relieve anxiety by suppressing stress-induced hyperresponsivity, which appears in a subgroup of individuals with ASD.
Author Contributions
Conceptualization, T.T. and C.T.; methodology, T.T. and C.T.; validation, T.T., C.T. H.H. and Y.Y.; formal analysis, C.T. and K.F.; investigation, C.T., K.F., E.G. and Y.W.; resources, H.H.; writing—original draft preparation, T.T. and C.T.; writing—review and editing, T.T., C.T. H.H. and Y.Y.; funding acquisition, T.T. and C.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Japan Society for the Promotion of Science KAKENHI, grant number JP 22K07346 (T.T.), 21J40098JP and 24K10677 (C.T.), grants from the Life Science Innovation Center, University of Fukui, grant number LSI 23103 (T.T.) and Initiative for Realizing Diversity in the Research Environment, MEXT (C.T.).
Acknowledgments
This research is partially supported by the Moonshot Research and Development Program, grant number JPMJMS2297 from Japan Science and Technology Agency, JST.
Conflicts of Interest
This study was funded by Tokai Bussan Co., Ltd. (C.T.). The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Patel, V.; Vaishnaw, A.; Shirbhate, E.; Kore, R.; Singh, V.; Veerasamy, R.; Rajak, H. Cortisol as a Target for Treating Mental Disorders: A Promising Avenue for Therapy. Mini Rev Med Chem 2023. [CrossRef]
- Toyoda, A. Nutritional interventions for promoting stress resilience: Recent progress using psychosocial stress models of rodents. Anim Sci J 2020, 91, e13478. [Google Scholar] [CrossRef] [PubMed]
- Kohen, R.; Yamamoto, Y.; Cundy, K.C.; Ames, B.N. Antioxidant activity of carnosine, homocarnosine, and anserine present in muscle and brain. Proc Natl Acad Sci U S A 1988, 85, 3175–3179. [Google Scholar] [CrossRef] [PubMed]
- Davey, C.L. The significance of carnosine and anserine in striated skeletal muscle. Arch Biochem Biophys 1960, 89, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.H.; Noh, A.R.; Kim, K.A.; Akram, M.; Shin, Y.J.; Kim, E.S.; Yu, S.W.; Majid, A.; Bae, O.N. Modulation of mitochondrial function and autophagy mediates carnosine neuroprotection against ischemic brain damage. Stroke 2014, 45, 2438–2443. [Google Scholar] [CrossRef] [PubMed]
- Hisatsune, T.; Kaneko, J.; Kurashige, H.; Cao, Y.; Satsu, H.; Totsuka, M.; Katakura, Y.; Imabayashi, E.; Matsuda, H. Effect of Anserine/Carnosine Supplementation on Verbal Episodic Memory in Elderly People. J Alzheimers Dis 2016, 50, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Abraham, D.A.; Undela, K.; Narasimhan, U.; Rajanandh, M.G. Effect of L-Carnosine in children with autism spectrum disorders: a systematic review and meta-analysis of randomised controlled trials. Amino Acids 2021, 53, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Ghajar, A.; Khoaie-Ardakani, M.R.; Shahmoradi, Z.; Alavi, A.R.; Afarideh, M.; Shalbafan, M.R.; Ghazizadeh-Hashemi, M.; Akhondzadeh, S. L-carnosine as an add-on to risperidone for treatment of negative symptoms in patients with stable schizophrenia: A double-blind, randomized placebo-controlled trial. Psychiatry Res 2018, 262, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Tharoor, H.; Maran, S.; Chandan, A.K.; Pari, M.; Rao, S.; Durairaj, J. Cognitive and negative symptoms in schizophrenia with L-Carnosine adjuvant therapy - A randomized double-blind placebo-controlled study. Pharmacology research & perspectives 2023, 11, e01074. [Google Scholar] [CrossRef]
- Ding, Q.; Tanigawa, K.; Kaneko, J.; Totsuka, M.; Katakura, Y.; Imabayashi, E.; Matsuda, H.; Hisatsune, T. Anserine/Carnosine Supplementation Preserves Blood Flow in the Prefrontal Brain of Elderly People Carrying APOE e4. Aging Dis 2018, 9, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Masuoka, N.; Yoshimine, C.; Hori, M.; Tanaka, M.; Asada, T.; Abe, K.; Hisatsune, T. Effects of Anserine/Carnosine Supplementation on Mild Cognitive Impairment with APOE4. Nutrients 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Ghajar, A.; Aghajan-Nashtaei, F.; Afarideh, M.; Mohammadi, M.R.; Akhondzadeh, S. l-Carnosine as Adjunctive Therapy in Children and Adolescents with Attention-Deficit/Hyperactivity Disorder: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J Child Adolesc Psychopharmacol 2018, 28, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Baraniuk, J.N.; El-Amin, S.; Corey, R.; Rayhan, R.; Timbol, C. Carnosine treatment for gulf war illness: a randomized controlled trial. Global journal of health science 2013, 5, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; He, R.R.; Tsoi, B.; Li, X.D.; Li, W.X.; Abe, K.; Kurihara, H. Anti-stress effects of carnosine on restraint-evoked immunocompromise in mice through spleen lymphocyte number maintenance. PLoS One 2012, 7, e33190. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, B.; He, R.R.; Yang, D.H.; Li, Y.F.; Li, X.D.; Li, W.X.; Abe, K.; Kurihara, H. Carnosine ameliorates stress-induced glucose metabolism disorder in restrained mice. J Pharmacol Sci 2011, 117, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Kaisho, T.; Ishikawa, J.; Oritani, K.; Inazawa, J.; Tomizawa, H.; Muraoka, O.; Ochi, T.; Hirano, T. BST-1, a surface molecule of bone marrow stromal cell lines that facilitates pre-B-cell growth. Proc Natl Acad Sci U S A 1994, 91, 5325–5329. [Google Scholar] [CrossRef] [PubMed]
- Malavasi, F.; Deaglio, S.; Funaro, A.; Ferrero, E.; Horenstein, A.L.; Ortolan, E.; Vaisitti, T.; Aydin, S. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol Rev 2008, 88, 841–886. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Hirano, T. BST-1/CD157 regulates the humoral immune responses in vivo. Chem Immunol 2000, 75, 235–255. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S. Genetic polymorphisms of bone marrow stromal cell antigen-1 (BST-1/CD157): implications for immune/inflammatory dysfunction in neuropsychiatric disorders. Front Immunol 2023, 14, 1197265. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.; Al Mahmuda, N.; Munesue, T.; Hayashi, K.; Yagi, K.; Yamagishi, M.; Higashida, H. Association Study between the CD157/BST1 Gene and Autism Spectrum Disorders in a Japanese Population. Brain Sci 2015, 5, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Mo, W.; Liu, J.; Zhang, Z.; Yu, H.; Yang, A.; Qu, F.; Hu, P.; Liu, Z.; Hu, F. A study of single nucleotide polymorphisms in CD157, AIM2 and JARID2 genes in Han Chinese children with autism spectrum disorder. Nord J Psychiatry 2018, 72, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Lopatina, O.; Yoshihara, T.; Nishimura, T.; Zhong, J.; Akther, S.; Fakhrul, A.A.; Liang, M.; Higashida, C.; Sumi, K.; Furuhara, K.; et al. Anxiety- and depression-like behavior in mice lacking the CD157/BST1 gene, a risk factor for Parkinson’s disease. Front Behav Neurosci 2014, 8, 133. [Google Scholar] [CrossRef]
- Mizuno, A.; Cherepanov, S.M.; Kikuchi, Y.; Fakhrul, A.A.; Akther, S.; Deguchi, K.; Yoshihara, T.; Ishihara, K.; Shuto, S.; Higashida, H. Lipo-oxytocin-1, a Novel Oxytocin Analog Conjugated with Two Palmitoyl Groups, Has Long-Lasting Effects on Anxiety-Related Behavior and Social Avoidance in CD157 Knockout Mice. Brain Sci 2015, 5, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Higashida, H.; Liang, M.; Yoshihara, T.; Akther, S.; Fakhrul, A.; Stanislav, C.; Nam, T.S.; Kim, U.H.; Kasai, S.; Nishimura, T.; et al. An immunohistochemical, enzymatic, and behavioral study of CD157/BST-1 as a neuroregulator. BMC Neurosci 2017, 18, 35. [Google Scholar] [CrossRef]
- Kasai, S.; Yoshihara, T.; Lopatina, O.; Ishihara, K.; Higashida, H. Selegiline Ameliorates Depression-Like Behavior in Mice Lacking the CD157/BST1 Gene, a Risk Factor for Parkinson’s Disease. Front Behav Neurosci 2017, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Furuhara, K.; Gerasimenko, M.; Shabalova, A.; Cherepanov, S.M.; Minami, K.; Higashida, H.; Tsuji, C. Oral Supplementation with L-Carnosine Attenuates Social Recognition Deficits in CD157KO Mice via Oxytocin Release. Nutrients 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Olff, M.; Frijling, J.L.; Kubzansky, L.D.; Bradley, B.; Ellenbogen, M.A.; Cardoso, C.; Bartz, J.A.; Yee, J.R.; van Zuiden, M. The role of oxytocin in social bonding, stress regulation and mental health: an update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology 2013, 38, 1883–1894. [Google Scholar] [CrossRef]
- Windle, R.J.; Shanks, N.; Lightman, S.L.; Ingram, C.D. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology 1997, 138, 2829–2834. [Google Scholar] [CrossRef]
- Winter, J.; Jurek, B. The interplay between oxytocin and the CRF system: regulation of the stress response. Cell Tissue Res 2019, 375, 85-91, doi:10.1007/s00441-018-2866-2.Parker, K.J.; Buckmaster, C.L.; Schatzberg, A.F.; Lyons, D.M. Intranasal oxytocin administration attenuates the ACTH stress response in monkeys. Psychoneuroendocrinology 2005, 30, 924-929, doi:10.1016/j.psyneuen.2005.04.002.
- Windle, R.J.; Kershaw, Y.M.; Shanks, N.; Wood, S.A.; Lightman, S.L.; Ingram, C.D. Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. J Neurosci 2004, 24, 2974–2982. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, M.; Ebner, K.; Landgraf, R.; Holsboer, F.; Wotjak, C.T. Emotional stress triggers intrahypothalamic but not peripheral release of oxytocin in male rats. J Neuroendocrinol 1999, 11, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Karelina, K.; Norman, G.J. Oxytocin Influence on NTS: Beyond Homeostatic Regulation. J Neurosci 2009, 29, 4687–4689. [Google Scholar] [CrossRef] [PubMed]
- van Steensel, F.J.; Bogels, S.M.; Perrin, S. Anxiety disorders in children and adolescents with autistic spectrum disorders: a meta-analysis. Clin Child Fam Psychol Rev 2011, 14, 302–317. [Google Scholar] [CrossRef] [PubMed]
- van Steensel, F.J.A.; Heeman, E.J. Anxiety Levels in Children with Autism Spectrum Disorder: A Meta-Analysis. J Child Fam Stud 2017, 26, 1753–1767. [Google Scholar] [CrossRef] [PubMed]
- Hollocks, M.J.; Lerh, J.W.; Magiati, I.; Meiser-Stedman, R.; Brugha, T.S. Anxiety and depression in adults with autism spectrum disorder: a systematic review and meta-analysis. Psychol Med 2019, 49, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.L.; Corbett, B.A. A review of rhythm and responsiveness of cortisol in individuals with autism spectrum disorders. Psychoneuroendocrinology 2014, 49, 207–228. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).