Submitted:
19 July 2024
Posted:
19 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Information Source
2.3. Search
2.4. Eligibility Criteria and Study Selection
2.5. Data Collection
2.6. Quality Assessment
2.7. Statistical Analysis
3. Results
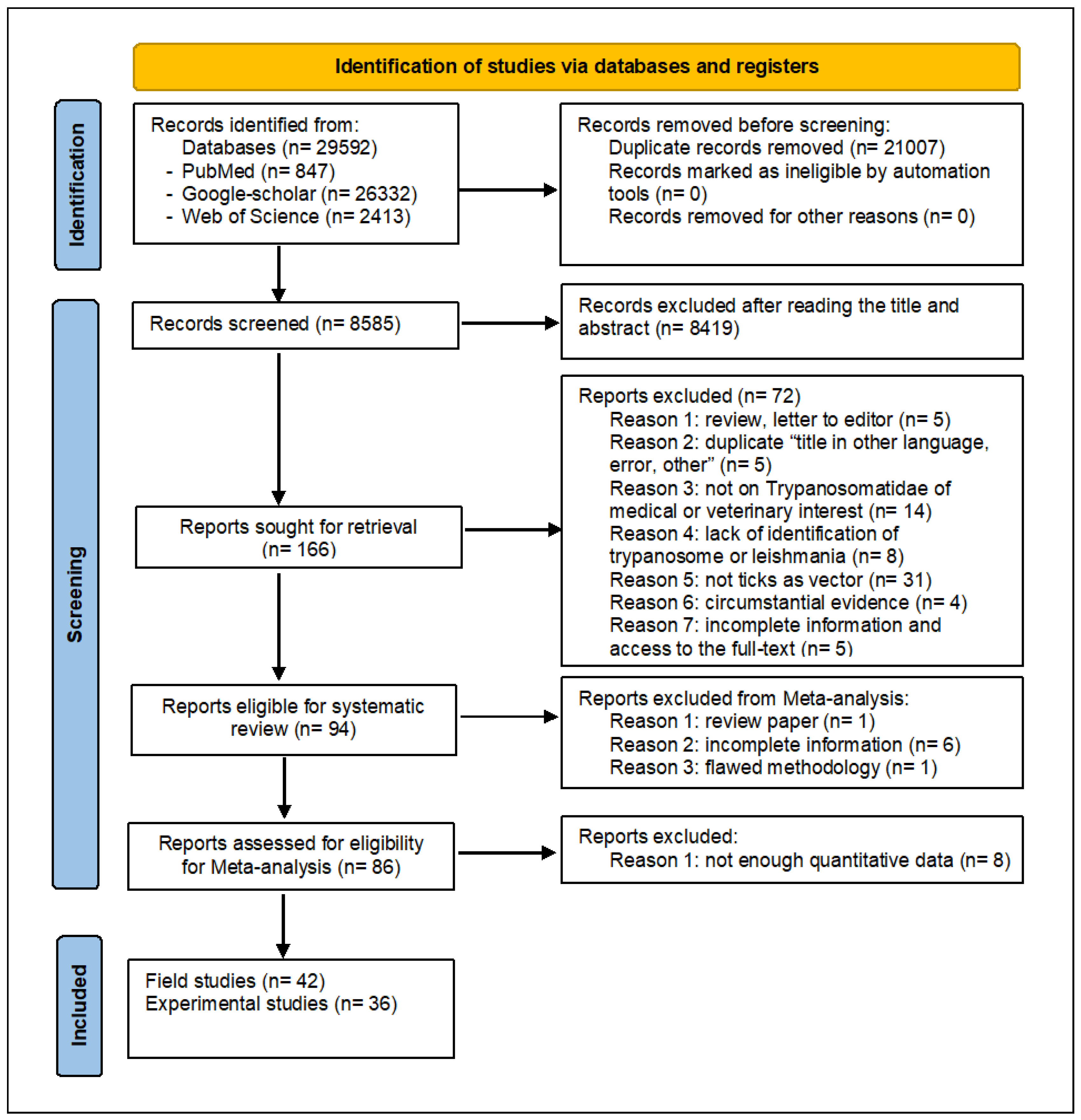
3.1. Selection Process Overview: Curating Datasets for Systematic Review
3.2. Exploring the Historical Presence in Tick of Trypanosomatidae Infecting Human and Animals of Veterinary Interests
3.2.1. Ticks as Vectors of Trypanosoma: Historical Aspects
3.2.2. Leishmania: a Parasite Transmitted by Ticks?
3.3. Observations of Trypanosomatidae of Medical and Veterinary Interest in Field-Collected Tick Specimens
3.3.1. Trypanosoma Carriage and Prevalence
- Trypanosoma cruzi
- Trypanosoma vivax
- Trypanosoma evansi
- Trypanosoma theileri and T. theileri-like
- Trypanosoma congolense
- Trypanosoma caninum
3.3.2. Leishmania Prevalence in Field Collected Ticks
- Leishmania infantum and L. chagasi (Syn L. infantum)
- Leishmania major
- Leishmania braziliensis
- Leishmania guyanensis
- Leishmania martiniquensis
- Other/unidentified Leishmania parasites
3.4. Experimental infection and Transmission of Trypanosomatidae by Ticks
3.4.1. Transmission of Trypanosoma following tick bites and feeding
- Trypanosoma cruzi
- Trypanosoma evansi
- Trypanosoma theileri and Trypanosoma theileri-like
3.4.2. Transmission of Leishmania Following Tick Bites and Feeding
- Leishmania infantum
3.4.3. Host Infection Following Ingestion of Infected Ticks or Injection with Trypanosoma-Infected Ticks
- Trypanosoma cruzi
- Trypanosoma theileri and T. theileri-like
- Other Trypanosoma
3.4.4. Infection after Ingestion or Injection with Leishmania- Infected Ticks Matertial
- Leishmania infantum and Leishmania chagasi (Syn: L. infantum)
- Leishmania
3.5. Transstadial Transmission in Ticks
3.5.1. Transstadial transmission of Trypanosoma
3.5.2. Transstadial Transmission of Leishmania
3.6. Transovarial Passage
3.6.1. Transovarial Passage of Trypanosoma
3.6.2. Transovarial passage of Leishmania
3.7. Other Circumstantial Evidence
4. Meta-analyses Results
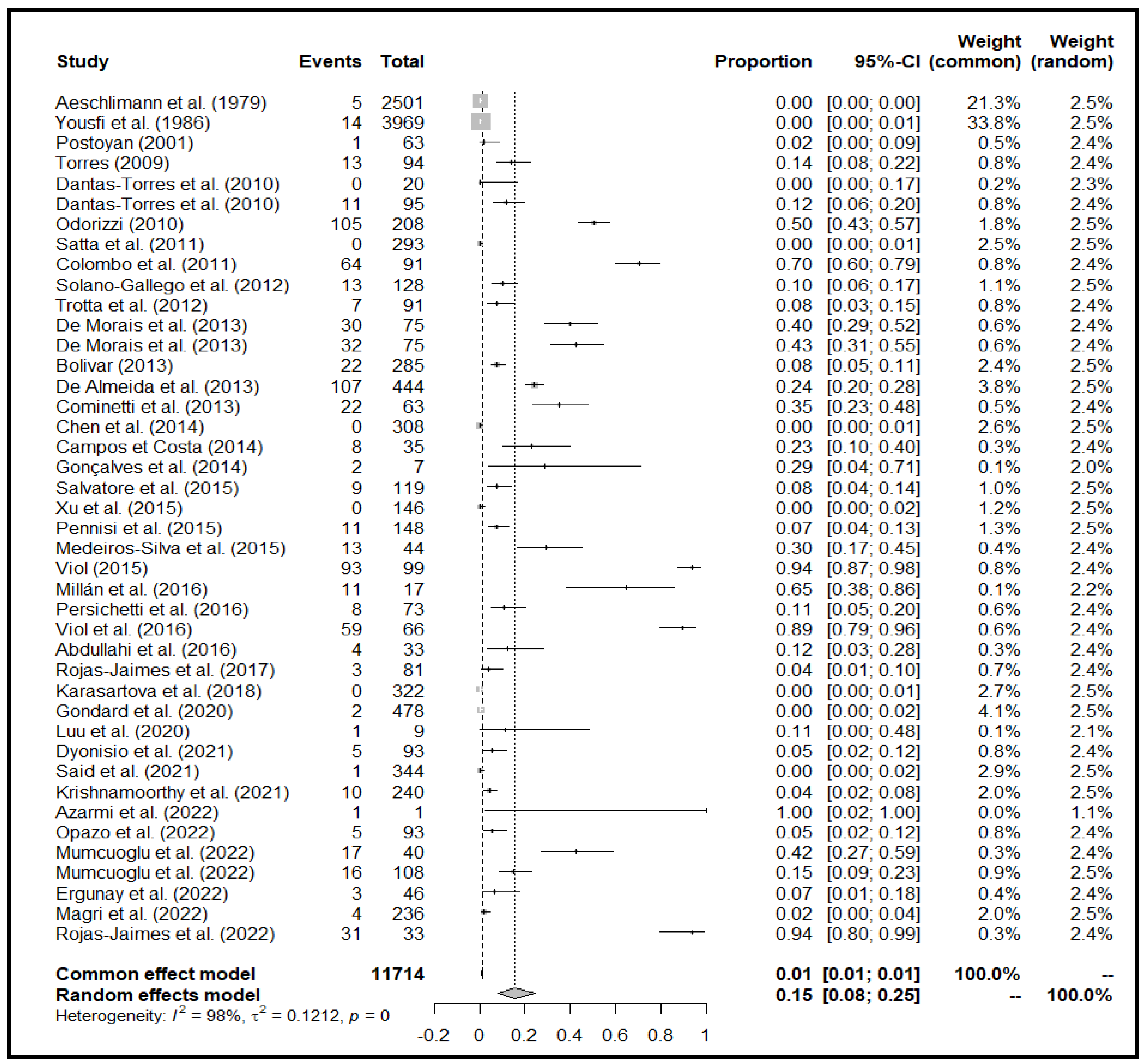
4.1. Field Detection
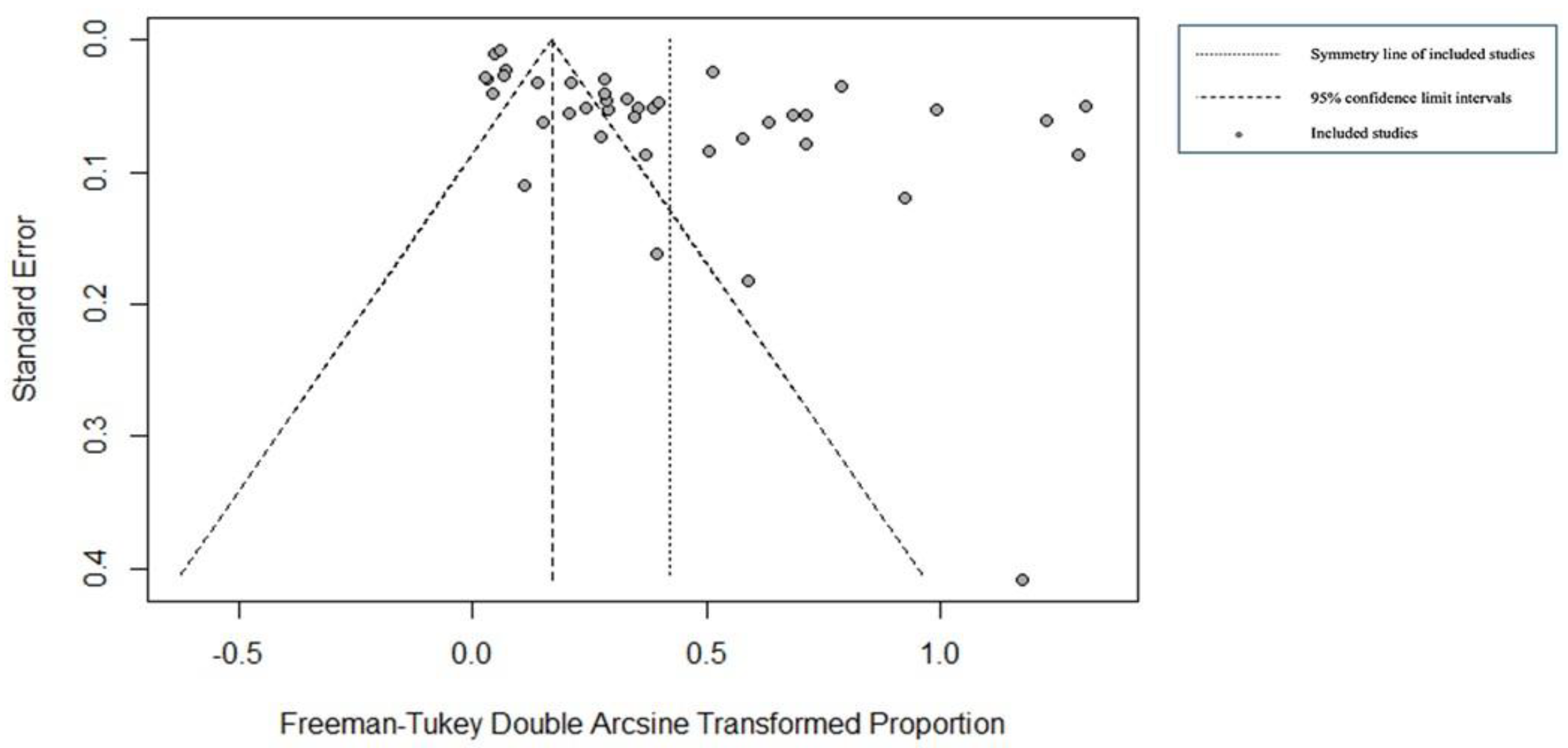
4.1.1. Overview of the Meta-Analysis
4.1.2. Detection Method
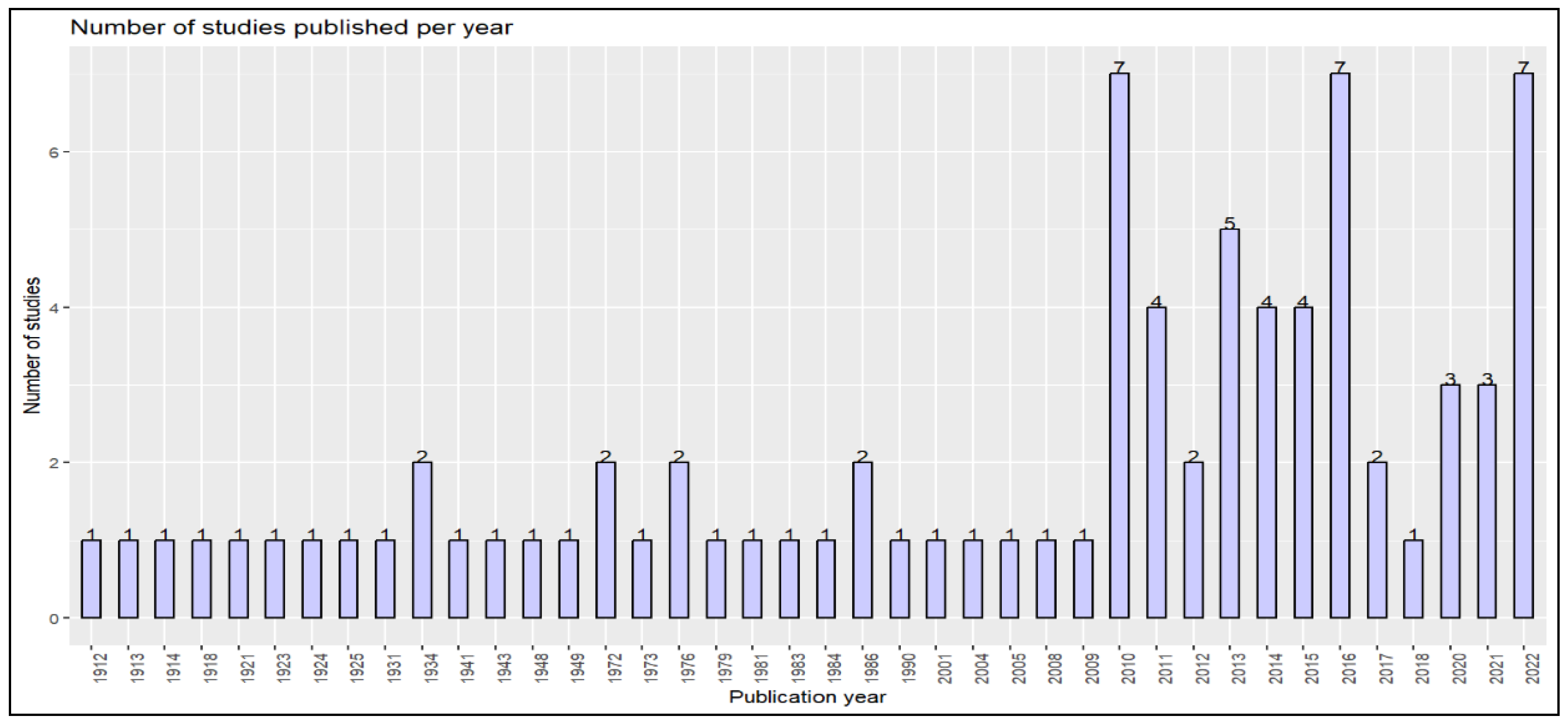
4.1.3. Temporal Analysis
4.1.4. Ticks Geographical Origin
4.1.5. Tick’s Genus
4.1.6. Detection Method, Genus and Organ Location
4.2. Experimental Studies
4.2.1. Ingestion after Blood Feeding on an Infected Host
- Ticks Family and Genus
- Parasite genus
- Host family
4.2.2. Transmission by injection of tick-infected material
- Tick's family, genus and species
- Parasite's genus and species
- Host family
4.2.3. Transmission Through Tick’s Blood Feeding on a Non-Infected Host
- Ticks family
- Parasite genus
- Donor and receiver host family
4.2.4. Vertical Transmission
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brites-Neto, J.; Duarte, K.M.R.; Martins, T.F. Tick-Borne Infections in Human and Animal Population Worldwide. Vet. world 2015, 8, 301. [Google Scholar] [CrossRef]
- Rajakaruna, R.S.; Eremeeva, M.E. Eco-Epidemiology of Tick-Borne Pathogens. One Heal. Human, Anim. Environ. Triad 2023, 325. [Google Scholar] [CrossRef]
- Guglielmone, A.A.; Robbins, R.G.; Apanaskevich, D.A.; Petney, T.N.; Estrada-Pena, A.; Horak, I.G.; Shao, R.F.; Barker, S.C. The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida) of the World: A List of Valid Species Names. Zootaxa 2010, 1–28. [Google Scholar] [CrossRef]
- Mans, B.J.; Featherston, J.; Kvas, M.; Pillay, K.-A.; Klerk, D.G. de; Pienaar, R.; Castro, M.H. de; Schwan, T.G.; Lopez, J.E.; Teel, P. Argasid and Ixodid Systematics: Implications for Soft Tick Evolution and Systematics, with a New Argasid Species List. Ticks Tick. Borne. Dis. 2019, 10, 219–240. [Google Scholar] [CrossRef] [PubMed]
- Mans, B.J. Paradigms in Tick Evolution. Trends Parasitol. 2023, 39, 475–486. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, P. Haemoprotozoa: Making Biological Sense of Molecular Phylogenies. Int. J. Parasitol. Wildl. 2017, 6, 241–256. [Google Scholar] [CrossRef]
- Akhoundi, M.; Kuhls, K.; Cannet, A.; Votýpka, J.; Marty, P.; Delaunay, P.; Sereno, D. A Historical Overview of the Classification, Evolution, and Dispersion of Leishmania Parasites and Sandflies. PLoS Negl. Trop. Dis. 2016, 10, e0004349. [Google Scholar] [CrossRef] [PubMed]
- Brun, R.; Blum, J.; Chappuis, F.; Burri, C. Human African Trypanosomiasis. Lancet 2010, 375, 148–159. [Google Scholar] [CrossRef]
- Kwakye-Nuako, G.; Mosore, M.-T.; Boakye, D.; Bates, P.A. Description, Biology, and Medical Significance of Leishmania (Mundinia) chancei n. sp.(Kinetoplastea: Trypanosomatidae) from Ghana and Leishmania (Mundinia) procaviensis n. sp.(Kinetoplastea: Trypanosomatidae) from Namibia. J. Parasitol. 2023, 109, 43–50. [Google Scholar] [CrossRef]
- Moncayo, Á.; Silveira, A.C. Current Epidemiological Trends of Chagas Disease in Latin America and Future Challenges: Epidemiology, Surveillance, and Health Policies. Am. Trypanos. Chagas Dis. 2017, 59–88. [Google Scholar] [CrossRef]
- Sereno, D. Leishmania (Mundinia) spp.: From Description to Emergence as New Human and Animal Leishmania Pathogens. New Microbes New Infect. 2019, 30, 100540. [Google Scholar] [CrossRef]
- Bayão T de S, Cupertino M do C, Mayers NAJ, Siqueira-Batista R. 2020. A systematic review of the diagnostic aspects and use of Trypanosoma rangeli as an immunogen for Trypanosoma cruzi infection. Rev Soc Bras Med Trop 53:e20190608. [CrossRef]
- Truc, P.; Büscher, P.; Cuny, G.; Gonzatti, M.I.; Jannin, J.; Joshi, P.; Juyal, P.; Lun, Z.-R.; Mattioli, R.; Pays, E. Atypical Human Infections by Animal Trypanosomes. PLoS Negl. Trop. Dis. 2013, 7, e2256. [Google Scholar] [CrossRef]
- Maurıcio, I.L.; Stothard, J.R.; Miles, M.A. The Strange Case of Leishmania chagasi. Parasitol. today 2000, 16, 188–189. [Google Scholar] [CrossRef] [PubMed]
- Desquesnes, M.; Gonzatti, M.; Sazmand, A.; Thévenon, S.; Bossard, G.; Boulangé, A.; Gimonneau, G.; Truc, P.; Herder, S.; Ravel, S. A Review on the Diagnosis of Animal Trypanosomoses. Parasit. Vectors 2022, 15, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Desquesnes, M.; Sazmand, A.; Gonzatti, M.; Boulangé, A.; Bossard, G.; Thévenon, S.; Gimonneau, G.; Truc, P.; Herder, S.; Ravel, S. Diagnosis of Animal Trypanosomoses: Proper Use of Current Tools and Future Prospects. Parasit. Vectors 2022, 15, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Itard, J.; Morel, P.C. Manual of Tropical Veterinary Parasitology; CAB International, 1989; ISBN 085198584X. [Google Scholar]
- Doherty ML, Windle H, Voorheis HP, Larkin H, Casey M, Clery D, Murray M. 1993. Clinical disease associated with Trypanosoma theileri infection in a calf in Ireland. Vet Rec 132:653–656. [CrossRef]
- Monzon, C.M.; Mancebo, O.A.; Jara, G.; Hoyos, C.B. Trypanosoma Theileri (Laveran, 1902) En Bovino de La Provincia de Formosa: Aislamiento, Cultivo y Alteraciones Hemáticas. 1993.
- Seifi HA. 1995. Clinical trypanosomiasis due to Trypanosoma theileri in a cow in Iran. [CrossRef]
- Radostits OM, Blood DC, Gay CC. Veterinary medicine. A textbook of the diseases of cattle, sheep, pigs, goats and horses. 8th Edition, Bailliere, Tindall, London, 1994, 1015-1026.
- Madeira, M.F.; Almeida, A.B.P.F.; Barros, J.H.S.; Oliveira, T.S.F.; Sousa, V.R.F.; Alves, A.S.; Miranda, L.F.C.; Schubach, A.O.; Marzochi, M.C.A. Trypanosoma Caninum, a New Parasite Described in Dogs in Brazil: Aspects of Natural Infection. J. Parasitol. 2014, 100, 231–234. [Google Scholar] [CrossRef]
- de Sousa, M.A. On Opportunist Infections by Trypanosoma Lewisi in Humans and Its Differential Diagnosis from T. Cruzi and T. Rangeli. Parasitol. Res. 2014, 113, 4471–4475. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.; Jenner, C.; Pender, J.; Rogers, D.; Slingenbergh, J.; Wint, W. The Programme against African Trypanosomiasis Information System (PAATIS). African Trypanos. 2002, 11–24. [Google Scholar] [CrossRef]
- Dantas-Torres, F. Ticks as Vectors of Leishmania Parasites. Trends Parasitol. 2011. [Google Scholar] [CrossRef]
- Koual, R.; Buysse, M.; Grillet, J.; et al. Phylogenetic Evidence for a Clade of Tick-Associated Trypanosomes. Parasites and vector. 2023. [Google Scholar] [CrossRef]
- McGhee, R.B.; Cosgrove, W.B. Biology and Physiology of the Lower Trypanosomatidae. Microbiol. Rev. 1980, 44, 140–173. [Google Scholar] [CrossRef] [PubMed]
- Becvar, T.; Vojtkova, B.; Siriyasatien, P.; Votypka, J.; Modry, D.; Jahn, P.; Bates, P.; Carpenter, S.; Volf, P.; Sadlova, J. Experimental Transmission of (Mundinia) Parasites by Biting Midges (Diptera: Ceratopogonidae). PLoS Pathog. 2021, 17, e1009654. [Google Scholar] [CrossRef] [PubMed]
- Hoare, C.A. The Trypanosomes of Mammals. A Zoological Monograph. Trypanos. mammals. A Zool. Monogr. 1972. [Google Scholar]
- Wenyon, C.M. The Transmission of Leishmania Infections: A Review. Trans. R. Soc. Trop. Med. Hyg. 1932, 25, 319–348. [Google Scholar] [CrossRef]
- O’Farrell, W.R. Hereditary Infection, with Special Reference to Its Occurrence in Hyalomma aegyptium Infected with Crithidia Hyalommae. Ann. Trop. Med. Parasitol. 1913, 7, 545–562. [Google Scholar] [CrossRef]
- Blanc, G.; Caminopetros, J. Transmission of Mediterranean EA by a Tick. Compte Rendu l’Academie des Sci. 1930, 191. [Google Scholar]
- Swellengrebel, N.H.; Strickland, C. The Development of Trypanosoma lewisi Outside the Vertebrate Host1. Parasitology 1910, 3, 360–389. [Google Scholar] [CrossRef]
- Brumpt, E. Le Trypanosoma Cruzi Évolue Chez Conorhinus Megistus, Cimex Lectularius, Cimex Boueti et Ornithodorus Moubata. Cycle Évolutif de Ce Parasite. Bull. la Société Pathol. Exot. 1912, 5, 360–367. [Google Scholar]
- Wheeler, C.M. Experimental Infection of Ornithodoros turicata (Duges) with a Brazilian Strain of Trypanosoma cruzi Chagas. Proc. Soc. Exp. Biol. Med. 1938, 38, 191–193. [Google Scholar] [CrossRef]
- Pifano, F. Amblyomma longyrostrum Infected in Nature by T. cruzi. Gac. Med. Caracas 1941, 48. [Google Scholar]
- Mazzotti, L.; Osorio, M.T. Experiments in the Transmission of T. cruzi to Four Species of Ornithodoros. Rev. Inst. Salubr. Enferm. Trop. 1943, 4, 163–165. [Google Scholar]
- Packchanian, A.A. The Fate of Some Pathogenic Trypanosomes in Triatoma and Ornithodorus. Am. J. Trop. Med. 1948, 28. [Google Scholar] [CrossRef] [PubMed]
- Lapierre, J. Evolution et Conservation de Trypanosoma cruzi Chez Ornithodorus moubata. Ann. Parasitol. Hum. comparée 1972, 47, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Neiva, A. Transmission of T. Cruzi by R. Sanguineus. Bras. Med. 1913, 27. [Google Scholar]
- Mayer, M.; Roca-lima, H.D. On the Behaviour of Schizotrypanum Cruzi in Warm-Blooded Animals and Arthropods. Beihefte zum Arch. fur Schiffs-und … 1914.
- Mayer, M. On the Length of Infection of Ornithodorus Moubata by S. Cruzi. Arch. fur Schiffs-und Tropenhygiene 1918, 22. [Google Scholar]
- Cross, H.E.; Patel, P.G. A Note on the Transmission of Surra by Ticks. Vet. J. 1922, 78, 469–471. [Google Scholar] [CrossRef]
- Cross, H.E. A Further Note on Surra Transmission Experiments with Tabanus Albimedius and Ticks. Trans. R. Soc. Trop. Med. Hyg. 1923, 16. [Google Scholar] [CrossRef]
- Yorke, W.; Macfie, J.W.S. Trypanosoma evansi and Ornithodorus crossi. Ann. Trop. Med. Parasitol. 1924, 18, 125–126. [Google Scholar] [CrossRef]
- Kahan, S. A Further Note on Surra Transmission Experiments with Ticks. A Furth. Note Surra Transm. Exp. with Ticks. 1925. [Google Scholar]
- Dunn, L.H. Attempts to Transmit Trypanosoma cruzi Chagas with Ticks of the Genus Ornithodoros. Am. J. Trop. Med. Hyg. 1934, s1-14, 283–289. [Google Scholar] [CrossRef]
- Burgdorfer, W.; Schmidt, M.L.; Hoogstraal, H. Detection of Trypanosoma Theileri in Ethiopian Cattle Ticks. Acta Trop. 1973, 30, 340–346. [Google Scholar] [PubMed]
- Shastri, U. V.; Deshpande, P.D. Hyalomma anatolicum anatolicum (Koch, 1844) as a Possible Vector for Transmission of Trypanosoma theileri, Laveran, 1902 in Cattle. Vet. Parasitol. 1981, 9, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Malamos, B. Experiments with Leishmania. IV. Experiments in the Transmission of Kala-Azar by Ticks, R. Sanguineus. Arch. fur Schiffs-und Tropenhygiene 1938, 42. [Google Scholar]
- Rioux, J.-A.; Lanotte, G.; Croset, H.; Houin, R.; Guy, Y.; Dedet, J.-P. Ecologie Des Leishmanioses Dans Le Sud de La France-3.-Réceptivité Comparée de Phlebotomus ariasi Tonnoir, 1921 et Rhipicephalus turanicus Pomerancev et Matikasvili, 1940 Vis-à-Vis de Leishmania donovani (Laveran et Mesnil, 1903). Ann. Parasitol.L. Hum. Comparée 1972, 47, 147–157. [Google Scholar] [CrossRef]
- McKenzie, K.K. A Study of the Transmission of Canine Leishmaniasis by the Tick, Rhipicephalus Sanguineus, and an Ultrastructural Comparison of the Promastigote. Oklahoma State Univ. Stillwater 1984. [Google Scholar]
- Azarmi, S.; Zahraei-ramazani, A.; Mohebali, M.; Rassi, Y.; Ahmad, A. Original Article PCR Positivity of Gerbils and Their Ectoparasites for Leishmania spp . in a Hy- Perendemic Focus of Zoonotic Cutaneous Leishmaniasis in Central Iran. 2022, 16, 124–135. [Google Scholar]
- Campos, J.H.F.; Costa, F.A.L. Participação Do Rhipicephalus Sanguineus No Ciclo Infeccioso Da Leishmaniose Visceral Canina Em Teresina, Piauí, Brasil. Rev. Inst. Med. Trop. Sao Paulo 2014, 56, 297–300. [Google Scholar] [CrossRef]
- Gonçalves, L.R.; Filgueira, K.D.; Ahid, S.M.M.; Pereira, J.S.; Vale, A.M. do; Machado, R.Z.; André, M.R. Study on Coinfecting Vector-Borne Pathogens in Dogs and Ticks in Rio Grande Do Norte, Brazil. Rev. Bras. Parasitol. Veterinária 2014, 23, 407–412. [Google Scholar] [CrossRef]
- Gondard, M.; Delannoy, S.; Pinarello, V.; Aprelon, R.; Devillers, E.; Galon, C.; Pradel, J.; Vayssier-Taussat, M.; Albina, E.; Moutailler, S. Upscaling the Surveillance of Tick-Borne Pathogens in the French Caribbean Islands. Pathogens 2020, 9, 1–37. [Google Scholar] [CrossRef]
- Medeiros-Silva, V.; Gurgel-Gonçalves, R.; Nitz, N.; D’Anduraim Morales, L.E.; Cruz, L.M.; Sobral, I.G.; Boité, M.C.; Ferreira, G.E.M.; Cupolillo, E.; Romero, G.A.S. Successful Isolation of Leishmania infantum from Rhipicephalus sanguineus Sensu Lato (Acari: Ixodidae) Collected from Naturally Infected Dogs. BMC Vet. Res. 2015, 11. [Google Scholar] [CrossRef]
- Millán, J.; Travaini, A.; Zanet, S.; López-Bao, J.V.; Trisciuoglio, A.; Ferroglio, E.; Rodríguez, A. Detection of Leishmania DNA in Wild Foxes and Associated Ticks in Patagonia, Argentina, 2000 Km South of Its Known Distribution Area. Parasites and Vectors 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Mumcuoglu, K.Y.; Arslan-Akveran, G.; Aydogdu, S.; Karasartova, D.; Koşar, A.; Savci, U.; Keskin, A.; Taylan-Ozkan, A. Pathogens in Ticks Collected in Israel: II. Bacteria and Protozoa Found in Rhipicephalus sanguineus Sensu Lato and Rhipicephalus turanicus. Ticks Tick. Borne. Dis. 2022, 13, 101986. [Google Scholar] [CrossRef] [PubMed]
- Mumcuoglu, K.Y.; Arslan-akveran, G.; Aydogdu, S.; Karasartova, D.; Kosar, N.; Semra, A.; Shacham, B.; Taylan-ozkan, A. Ticks and Tick-Borne Diseases Pathogens in Ticks Collected in Israel : I. Bacteria and Protozoa in Hyalomma aegyptium and Hyalomma dromedarii Collected from Tortoises and Camels. Ticks Tick. Borne. Dis. 2022, 13, 101866. [Google Scholar] [CrossRef] [PubMed]
- Paz, G.F.; Ribeiro, M.F.B.; Michalsky, É.M.; Da Rocha Lima, A.C.V.M.; França-Silva, J.C.; Barata, R.A.; Fortes-Dias, C.L.; Dias, E.S. Evaluation of the Vectorial Capacity of Rhipicephalus sanguineus (Acari: Ixodidae) in the Transmission of Canine Visceral Leishmaniasis. Parasitol. Res. 2010, 106, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, M.G.; Persichetti, M.F.; Serrano, L.; Altet, L.; Reale, S.; Gulotta, L.; Solano-Gallego, L. Ticks and Associated Pathogens Collected from Cats in Sicily and Calabria (Italy). Parasites and Vectors 2015, 8. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Jaimes, J.E.; Correa-Núñez, G.H.; Rojas-Palomino, N.; Cáceres-Rey, O. Detección de Leishmania (V) guyanensis En Ejemplares de Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) Recolectados En Pecaríes de Collar (Pecari tajacu). Biomedica 2017, 37, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, D.; Aureli, S.; Baldelli, R.; Di Francesco, A.; Tampieri, M.P.; Galuppi, R. Analisi Molecolare Di Leishmania infantum in Zecche Ixodes Ricinus Da Cani e Gatti in Italia. Vet. Ital. 2014, 50, 307–312. [Google Scholar] [CrossRef]
- Colombo, F.A.; Odorizzi, R.M.F.N.; Laurenti, M.D.; Galati, E.A.B.; Canavez, F.; Pereira-Chioccola, V.L. Detection of Leishmania (Leishmania) infantum RNA in Fleas and Ticks Collected from Naturally Infected Dogs. Parasitol. Res. 2011, 109, 267–274. [Google Scholar] [CrossRef]
- Trotta, M.; Nicetto, M.; Fogliazza, A.; Montarsi, F.; Caldin, M.; Furlanello, T.; Solano-Gallego, L. Detection of Leishmania infantum, Babesia canis, and Rickettsiae in Ticks Removed from Dogs Living in Italy. Ticks Tick. Borne. Dis. 2012, 3, 294–297. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Rossi, L.; Scroccaro, A.M.; Montarsi, F.; Caldin, M.; Furlanello, T.; Trotta, M. Detection of Leishmania infantum DNA Mainly in Rhipicephalus sanguineus Male Ticks Removed from Dogs Living in Endemic Areas of Canine Leishmaniosis. Parasit. Vectors 2012, 5, 1–6. [Google Scholar] [CrossRef]
- Viol, M.A.; Guerrero, F.D.; Oliveira, B.C.M. de; Aquino, M.C.C. de; Loiola, S.H.; Melo, G.D. de; Gomes, A.H. de S.; Kanamura, C.T.; Garcia, M.V.; Andreotti, R. Identification of Leishmania spp. Promastigotes in the Intestines, Ovaries, and Salivary Glands of Rhipicephalus sanguineus Actively Infesting Dogs. Parasitol. Res. 2016, 115, 3479–3484. [Google Scholar] [CrossRef] [PubMed]
- Viol, M.A. Considerações sobre a presença de formas promastigotas de Leishmania spp. em intestinos, ovários e glândulas salivares de carrapatos Rhipicephalus sanguineus em cães de áreas endêmicas. repositorio.unesp.br, 2015.
- Almeida, V. dos A.; Hora, T.N. da; Junior, N.F.L.; Carvalho, F.S.; Silva, A.L. da; Wenceslau, A.A.; Albuquerque, G.R.; Silva, F.L. Detection of Leishmania infantum DNA in Hamsters Infested with Ticks Collected from Naturally Infected Dogs. Rev Bras Med Vet 2016, 38, 329–333. [Google Scholar]
- Coutinho, M.T.Z.; Bueno, L.L.; Sterzik, A.; Fujiwara, R.T.; Botelho, J.R.; De Maria, M.; Genaro, O.; Linardi, P.M. Participation of Rhipicephalus sanguineus (Acari: Ixodidae) in the Epidemiology of Canine Visceral Leishmaniasis. Vet. Parasitol. 2005, 128, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Dabaghmanesh, T.; Asgari, Q.; Moemenbellah-Fard, M.D.; Soltani, A.; Azizi, K. Natural Transovarial and Transstadial Transmission of Leishmania infantum by Naïve Rhipicephalus sanguineus Ticks Blood Feeding on an Endemically Infected Dog in Shiraz, South of Iran. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 408–413. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; de Paiva-Cavalcanti, M.; Figueredo, L.A.; Melo, M.F.; da Silva, F.J.; da Silva, A.L.; Almeida, E.L.; Brandão-Filho, S.P. Cutaneous and Visceral Leishmaniosis in Dogs from a Rural Community in Northeastern Brazil. Vet. Parasitol. 2010, 170, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F.; Lorusso, V.; Testini, G.; De Paiva-Cavalcanti, M.; Figueredo, L.A.; Stanneck, D.; Mencke, N.; Brandão-Filho, S.P.; Alves, L.C.; Otranto, D. Detection of Leishmania infantum in Rhipicephalus sanguineus Ticks from Brazil and Italy. Parasitol. Res. 2010, 106, 857–860. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Martins, T.F.; de Paiva-Cavalcanti, M.; Figueredo, L.A.; Lima, B.S.; Brandão-Filho, S.P. Transovarial Passage of Leishmania infantum KDNA in Artificially Infected Rhipicephalus sanguineus. Exp. Parasitol. 2010, 125, 184–185. [Google Scholar] [CrossRef]
- de Morais, R.C.S.; Gonçalves-de-Albuquerque, S. da C.; Silva, Rô.P. e.; Costa, P.L.; Silva, K.G. da; Silva, F.J. da; Brandão-Filho, S.P.; Dantas-Torres, F.; Paiva-Cavalcanti, M. de Detection and Quantification of Leishmania braziliensis in Ectoparasites from Dogs. Vet. Parasitol. 2013, 196, 506–508. [Google Scholar] [CrossRef] [PubMed]
- de Morais, R.C.S.; Gonçalves, S. da C.; Costa, P.L.; da Silva, K.G.; da Silva, F.J.; Silva, R.P.E.; de Brito, M.E.F.; Brandão-Filho, S.P.; Dantas-Torres, F.; de Paiva-Cavalcanti, M. Detection of Leishmania infantum in Animals and Their Ectoparasites by Conventional PCR and Real Time PCR. Exp. Appl. Acarol. 2013, 59, 473–481. [Google Scholar] [CrossRef]
- Abdullahi, M.A.; Abubakar, Z.T.; Umar, L.M.; Sabo, H.M.; Onaolapo, A.Y.; Jibril, H.; Abubakar, A.; Haruna, I.L.; Muhammed, A.S.; Tasie, P.C. Amblyomma variegatum as Bioindicators for the Presence of Trypanosoma congolense in Semi-Extensively Managed White Fulani Cattle in Kaduna Metropolis, Kaduna State, Nigeria. IOSR J. Agric. Vet. Sci. 2016, 9, 42–45. [Google Scholar]
- Aeschlimann, A.; Burgdorfer, W.; Matile, H. Aspects Nouveaux Du Role De Vecteur Joue Par Ixodes Ricinus L. En Suisse. Note Preliminaire. Acta Trop. 1979, 36, 181–191. [Google Scholar] [PubMed]
- Bacellar, F.; Núncio, M.S.; Filipe, A.R. Forms of Trypanosoma in Tick Haemolymph. Rev. Port. Ciências Veterinárias 1990, 85, 158–159. [Google Scholar]
- Bolivar, A.M. Detection of Anaplasma Marginale and Trypanosoma Vivax in Ticks Collected from Cattle Using the Polimerase Chain Reaction. REDVET 2013, 14. [Google Scholar]
- Cominetti, M.C.; Almeida, R.F.C. de; Csordas, B.G.; Andreotti, R. Ticks as Bioindicators the Presence of Trypanosoma spp. in Campo Grande City, MS, Brazil; 2013.
- Dyonisio, G.H.S.; Batista, H.R.; Silva, R.E. da; Azevedo, R.C. de F. e; Costa, J. de O.J.; Manhães, I.B. de O.; Tonhosolo, R.; Gennari, S.M.; Minervino, A.H.H.; Marcili, A. Molecular Diagnosis and Prevalence of Trypanosoma Vivax (Trypanosomatida: Trypanosomatidae) in Buffaloes and Ectoparasites in the Brazilian Amazon Region. J. Med. Entomol. 2021, 58, 403–407. [Google Scholar] [PubMed]
- Ergunay, K.; Mutinda, M.; Bourke, B.; Justi, S.A.; Caicedo-Quiroga, L.; Kamau, J.; Mutura, S.; Akunda, I.K.; Cook, E.; Gakuya, F.; et al. Metagenomic Investigation of Ticks From Kenyan Wildlife Reveals Diverse Microbial Pathogens and New Country Pathogen Records. Front. Microbiol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, P.; Sudhagar, S.; Goudar, A.L.; Molecular survey and phylogenetic analysis of tick-borne pathogens in ticks infesting cattle from two South Indian states, Vet. Parsit. Reg. Stud. 2021. 25. [CrossRef]
- Luu, L.; Bown, K.J.; Palomar, A.M.; Kazimirova, M.; Bell-Sakyi, L. Isolation and Partial Characterisation of a Novel Trypanosoma from the Tick Ixodes ricinus. Ticks Tick. Borne. Dis. 2020, 11. [Google Scholar] [CrossRef]
- Martins, J.R.; Leite, R.C.; Doyle, R.L. Tripanosomatides like Trypanosoma theileri in the Cattle Tick Boophilus microplus. Rev. Bras. Parasitol. Vet. 2008, 17, 113–114. [Google Scholar] [CrossRef]
- Monod, R.Y.; Aeschlimann, A.; Derscheid, J.M. 3 Infections by Trypanosomes Observed in Hyalomma detritum, Ixodes ricinus and Rhipicephalus sanguineus (Acarina: Ixodidae). Schweiz. Arch. Tierheilkd. 1986, 128, 243–254. [Google Scholar]
- Opazo, A.; Bacigalupo, A.; Urrutia, S.; Chavez, G. Detection of Trypanosoma cruzi Infection by PCR in Canis lupus familiaris and Their Ectoparasites in Chile. Med. Vet. Entomol. 2022, 36, 88–96. [Google Scholar] [CrossRef]
- Postoyan, S.R. Сoхранение Жизнеспoсoбнoсти Trypanosoma Vivax Ziemann, 1905 в Клещах. Հայաստանի կենսաբանական հանդես 2001, 53, 128–130. [Google Scholar]
- Said, Y.; Lahmar, S.; Dhibi, M.; Rjeibi, M.R.; Jdidi, M.; Gharbi, M. First Survey of Ticks, Tick-Borne Pathogens (Theileria, Babesia, Anaplasma and Ehrlichia) and Trypanosoma evansi in Protected Areas for Threatened Wild Ruminants in Tunisia. Parasitol. Int. 2021, 81. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, R.F.; Garcia, M.V.; Cunha, R.C.; Matias, J.; e Silva, E.; Cepa Matos, M. de F.; Andreotti, R. Ixodid Fauna and Zoonotic Agents in Ticks from Dogs: First Report of Rickettsia Rickettsii in Rhipicephalus Sanguineus in the State of Mato Grosso Do Sul, Mid-Western Brazil. Exp. Appl. Acarol. 2013, 60, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, W.L.; Burgdorfer, W. Trypanosomes in Amblyomma Americanum from Oklahoma. J. Parasitol. 1976. [Google Scholar] [CrossRef]
- Brotánková, A.; Fialová, M.; Čepička, I.; Brzoňová, J.; Svobodová, M. Trypanosomes of the Trypanosoma theileri Group: Phylogeny and New Potential Vectors. Microorganisms 2022, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Oluwafemi, R.A.; Ilemobade, A.A.; Laseinde, E.A.O. The Impact of African Animal Trypanosomosis and Tsetse on the Livelihood and Wellbeing of Cattle and Their Owners in the BICOT Study Area of Nigeria. Sc Res Essays 2007, 2, 380–383. [Google Scholar]
- Awuoche, E.O.; Weiss, B.L.; Mireji, P.O.; Vigneron, A.; Nyambega, B.; Murilla, G.; Aksoy, S. Expression Profiling of Trypanosoma congolense Genes during Development in the Tsetse Fly Vector Glossina morsitans morsitans. Parasit. Vectors 2018, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Barros, J.H.S.; Almeida, A.; Figueiredo, F.B.; Sousa, V.R.F.; Fagundes, A.; Pinto, A.G.S.; Baptista, C.; Madeira, M.F. Occurrence of Trypanosoma caninum in Areas Overlapping with Leishmaniasis in Brazil: What Is the Real Impact of Canine Leishmaniasis Control? Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 419–423. [Google Scholar] [CrossRef]
- Azarmi, S.; Zahraei-Ramazani, A.; Mohebali, M.; Rassi, Y.; Akhavan, A.A.; Azarm, A.; Dehghan, O.; Elikaee, S.; Abdoli, R.; Mahmoudi, M. PCR Positivity of Gerbils and Their Ectoparasites for Leishmania spp. in a Hyperendemic Focus of Zoonotic Cutaneous Leishmaniasis in Central Iran. J. Arthropod. Borne. Dis. 2022, 16, 124. [Google Scholar] [CrossRef]
- Campos, J.H.F.; Costa, F.A.L. Participation of Ticks in the Infectious Cycle of Canine Visceral Leishmaniasis, in Teresina, Piauí, Brazil. Rev. Inst. Med. Trop. Sao Paulo 2014, 56, 297–300. [Google Scholar] [CrossRef]
- Dantas-Torres, F. Rhipicephalus Sanguineus E a Epidemiologia Da Leishmaniose Visceral Canina No Estado De Pernambuco 2009, 96.
- Giraud, P. Transmission of KA in the Mediterranean Region, with Special Reference to Ticks. Bull. la Société Pathol. Exot. 1934, 27. [Google Scholar]
- Magri, A.; Caffara, M.; Fioravanti, M.; Galuppi, R. Detection of Leishmania Sp . KDNA in Questing Ixodes Ricinus ( Acari , Ixodidae ) from the Emilia - Romagna Region in Northeastern Italy. 2022, 3331–3336. [CrossRef]
- Odorizzi, R.M.F.N. Investigação Do Papel de Vetores Secundários Da Leishmania (Leishmania) Infantum No Município de Mirandópolis-SP, Brasil; Universidade de São Paulo, 2010. [CrossRef]
- Persichetti, M.F.; Solano-Gallego, L.; Serrano, L.; Altet, L.; Reale, S.; Masucci, M.; Pennisi, M.G. Detection of Vector-Borne Pathogens in Cats and Their Ectoparasites in Southern Italy. Parasites and Vectors 2016, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Jaimes, J.; Correa-Núñez, G.H.; Donayre, L.; Lescano, A.G. Quantitative Detection of LeishmaniaLeishmania in Rhipicephalus microplus and Amblyomma sabanerae in the Peruvian Amazon Basin. Trop. Med. Infect. Dis. 2022. [Google Scholar] [CrossRef] [PubMed]
- Silva, O.A. da; Braga, G.M. da S. The Role of Rhipicephalus Sanguineus in the Transmission of Visceral Leishmaniasis Dogs: Epidemiological Aspects. PUBVET 2010, 4. [Google Scholar]
- Rojas-jaimes, J.; Zarate-rendon, D.; Montoya-arauco, G. Aislamiento e Identificación de Leishmania sp de Amblyomma naponense y Amblyomma humerale Colectadas de Pecari tajacu , Tayassu pecari y Chelonoide Denticulata Isolation and Identification of Leishmania sp of Amblyomma naponense and Amblyomma humerale Col. Amblyomma humerale 2022, 19, 189–192. [Google Scholar] [CrossRef]
- Satta, G.; Chisu, V.; Cabras, P.; Fois, F.; Masala, G. Pathogens and Symbionts in Ticks: A Survey on Tick Species Distribution and Presence of Tick-Transmitted Micro-Organisms in Sardinia, Italy. J. Med. Microbiol. 2011, 60, 63–68. [Google Scholar] [CrossRef]
- Karasartova, D.; Gureser, A.S.; Gokce, T.; Celebi, B.; Yapar, D.; Keskin, A.; Celik, S.; Ece, Y.; Erenler, A.K.; Usluca, S.; et al. Bacterial and Protozoal Pathogens Found in Ticks Collected from Humans in Corum Province of Turkey. PLoS Negl. Trop. Dis. 2018, 12, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, Q.; Liu, J.Q.; Xu, B.L.; ... Tick-Borne Pathogens and Associated Co-Infections in Ticks Collected from Domestic Animals in Central China. Parasites \& … 2014. [CrossRef]
- Paz, G.F.; Reis, I.A.; Avelar, D.M.; Ferreira, E.C. d. M.; Werneck, G.L. Ectoparasites and Anti-Leishmania Antibodies: Association in an Observational Case-Control Study of Dogs from a Brazilian Endemic Area. Prev. Vet. Med. 2013, 112, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhang, J.; Shi, Z.; Song, C.; Zheng, X.; Zhang, Y.; Hao, Y.; Dong, H.; Wei, L.; El-Mahallawy, H.S.; et al. Molecular Detection of Vector-Borne Agents in Dogs from Ten Provinces of China. Parasites and Vectors 2015, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F.; Latrofa, M.S.; Otranto, D. Quantification of Leishmania infantum DNA in Females, Eggs and Larvae of Rhipicephalus Sanguineus. Parasit. Vectors 2011, 4, 1–4. [Google Scholar] [CrossRef]
- Dantas-Torres, F. The Role of Dogs as Reservoirs of Leishmania Parasites, with Emphasis on Leishmania (Leishmania) infantum and Leishmania (Viannia) braziliensis. Vet. Parasitol. 2007, 149, 139–146. [Google Scholar] [CrossRef]
- Odorizzi, Rosa Maria Ferreira Noguerol. Investigação do papel de vetores secundários da Leishmania (Leishmania) infantum no Município de Mirandópolis-SP, Brasil. 2010. Thèse de doctorat. Universidade de São Paulo. [CrossRef]
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A Review. F1000Research 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Sereno, D.; Akhoundi, M.; Dorkeld, F.; Oury, B.; Momen, H.; Perrin, P. What Pre-Columbian Mummies Could Teach Us about South American Leishmaniases? Pathog. Dis. 2017, 75, ftx019. [Google Scholar] [CrossRef] [PubMed]
- Desbois, N.; Pratlong, F.; Quist, D.; Dedet, J.-P. Leishmania (Leishmania) martiniquensis n. sp.(Kinetoplastida:Trypanosomatidae), Description of the Parasite Responsible for Cutaneous Leishmaniasis in Martinique Island (French West Indies). Parasite 2014, 21. [Google Scholar] [CrossRef] [PubMed]
- Noyes, H.; Pratlong, F.; Chance, M.; Ellis, J.; Lanotte, G.; Dedet, J.-P. A Previously Unclassified Trypanosomatid Responsible for Human Cutaneous Lesions in Martinique (French West Indies) Is the Most Divergent Member of the Genus Leishmania Ss. Parasitology 2002, 124, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Dedet, J.P.; Roche, B.; Pratlong, F.; Cales-Quist, D.; Jouannelle, J.; Benichou, J.C.; Huerre, M. Diffuse Cutaneous Infection Caused by a Presumed Monoxenous Trypanosomatid in a Patient Infected with HIV. Trans. R. Soc. Trop. Med. Hyg. 1995, 89, 644–646. [Google Scholar] [CrossRef] [PubMed]
- Seblova, V.; Sadlova, J.; Vojtkova, B.; Votypka, J.; Carpenter, S.; Bates, P.A.; Volf, P. The Biting Midge Culicoides sonorensis (Diptera: Ceratopogonidae) Is Capable of Developing Late Stage Infections of Leishmania enriettii. PLoS Negl. Trop. Dis. 2015, 9, e0004060. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Jaimes, J.; Correa-Núñez, G.H.; Donayre, L.; Lescano, A.G. Quantitative Detection of Leishmania in Rhipicephalus microplus and Amblyomma sabanerae in the Peruvian Amazon Basin. Trop. Med. Infect. Dis. 2022, 7, 358. [Google Scholar] [CrossRef] [PubMed]
- Morzaria, S.P.; Latif, A.A.; Jongejan, F.; Walker, A.R. Transmission of a Trypanosoma sp to cattle by the tick Hyalomma anatolicum anatolicum. Vet. Parasitol. 1986, 19, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Latif, A.A.; Bakheit, M.A.; Mohamed, A.E.; Zweygarth, E. High Infection Rates of the Tick Hyalomma Anatolicum Anatolicum with Trypanosoma Theileri. ONDERSTEPOORT J. Vet. Res. 2004, 71, 251–256. [Google Scholar] [CrossRef]
- Krige, A.-S.; Thompson, R.C.A.; Clode, P.L. ‘Hang on a Tick’–Are Ticks Really the Vectors for Australian Trypanosomes? Trends Parasitol. 2019, 35, 596–606. [Google Scholar] [CrossRef]
- Taylor-lewis, E.G. Investigations on the Transmission of Trypanosoma Lewisi and T. Evansi through 5 Different Species of Hard Ticks. Investig. Transm. Trypanos. lewisi T. evansi through 5 Differ. species hard ticks. 1976. [Google Scholar]
- Mahmoud, N.E.; Habeeb, S.M.; Abdelrahman, K.A.; Ashry, H.M.; Hassan, M.R. Role of Tampan Tick Ornithodoros savignyi (Acari: Argasidae) in Transmitting Trypanosoma evansi in Laboratory Animals. Int. J. Vet. Sci. 2020, 9, 273–278. [Google Scholar] [CrossRef]
- Dujardin, J.-C.; Campino, L.; Cañavate, C.; Dedet, J.-P.; Gradoni, L.; Soteriadou, K.; Mazeris, A.; Ozbel, Y.; Boelaert, M. Spread of Vector-Borne Diseases and Neglect of Leishmaniasis, Europe. Emerg. Infect. Dis. 2008, 14, 1013. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, M.T.Z.; Linardi, P.M. Can Fleas from Dogs Infected with Canine Visceral Leishmaniasis Transfer the Infection to Other Mammals? Vet. Parasitol. 2007, 147, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Rakhshanpour, A.; Malmasi, A.; Mohebali, M.; Nabian, S.; Mirhendi, H.; Zarei, Z.; Dalimi, A.; Mohammadiha, A.; Akhoundi, B.; Azarm, A. Transmission of Leishmania Infantum by Rhipicephalus Sanguineus (Acari: Ixodidae) in Dogs. Iran. J. Parasitol. 2017, 12, 482. [Google Scholar] [PubMed]
- Joyeux, C.; Sautet, J. Observations Sur La Leishmaniose Canine Méditerranéenne. Bull. la Société Pathol. Exot. 1938, 31. [Google Scholar]
- Arganaraz, E.R.; Hubbard, G.B.; Ramos, L.A.; Ford, A.L.; Nitz, N.; Leland, M.M.; Vandeberg, J.L.; Teixeira, A.R.L. Blood-Sucking Lice May Disseminate Trypanosoma cruzi Infection in Baboons. Rev. Inst. Med. Trop. Sao Paulo 2001, 43, 271–276. [Google Scholar] [CrossRef]
- Votýpka, J.; Szabova, J.; Radrova, J.; Zídková, L.; Svobodova, M. Trypanosoma culicavium Sp. Nov., an Avian Trypanosome Transmitted by Culex Mosquitoes. Int. J. Syst. Evol. Microbiol. 2012, 62, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Baneth, G.; Samish, M.; Shkap, V. Life Cycle of Hepatozoon canis (Apicomplexa: Adeleorina: Hepatozoidae) in the Tick Rhipicephalus sanguineus and Domestic Dog (Canis familiaris). J. Parasitol. 2007, 93, 283–299. [Google Scholar] [CrossRef]
- Weilgama, D.J. Cyclical Development of Trypanosomes in the Tick and Their Transmission. Haemoprotozoan Dis. Domest. Anim. proceedings, Semin. Asian/Australian Reg. Haryana Agric. Univ., Hissar, India, Oct. 27-Nov. 1, 1980/edited by OP Gautam, RD Sharma, S. Dhar 1980.
- Arifdzhanov, K.A.; Nikitina, R.E. Detection of Crithidia Hyalomma (O’Farrel 1913) in Hyalomma a. Anatolicum (Koch, 1844). Zool. Cheskii 1961, 40, 20–24. [Google Scholar]
- Vergne, T.; Kamyinkird, K.; Desquesnes, M.; Jittapalapong, S. Attempted Transmission of Trypanosoma evansi to Rats and Mice by Direct Ingestion of Contaminated Blood and via Engorged Ticks. ACTA Protozool. 2011, 50, 133–136. [Google Scholar] [CrossRef]
- Bilgiç, H.B.; Bakirci, S.; Köse, O.; Aksulu, A.; Hacilarlioglu, S.; Karagenç, T. Determination the Role of Rhipicephalus sanguineus for Transmission of Leishmania major to Reservoir Animals. Türkiye Parazitolojii Derg. 2016, 40, 179. [Google Scholar] [CrossRef] [PubMed]
- Paz, G.F.; Ribeiro, M.F.B.; Magalhães, D.F. de; Sathler, K.P.B.; Morais, M.H.F.; Fiúza, V.O.P.; Brandão, S.T.; Werneck, G.L.; Fortes-Dias, C.L.; Dias, E.S. Association between the Prevalence of Infestation by Rhipicephalus sanguineus and Ctenocephalides felis felis and the Presence of Anti-Leishmania Antibodies: A Case–Control Study in Dogs from a Brazilian Endemic Area. Prev. Vet. Med. 2010, 97, 131–133. [Google Scholar] [CrossRef]
- Nakkoud, J.R.; Santos, C.M. dos; Aquino, D.R.R.R.A.; Favacho, A.R. de M. Um Olhar Para as Populações de Ectoparasitas Em Cães Com Leishmaniose Visceral Canina (LVC) Em Mato Grosso Do Sul–Potenciais Vetores de Transmissão Para Essa Doença. Ensaios e Ciência C Biológicas Agrárias e da Saúde 2022, 26, 43–47. [Google Scholar] [CrossRef]
- Bernardino, M. das G. da S.; Angelo, D.F. do S.; Silva, R.B.S.; Silva, E.G. da; Silva, L.F.F.; Vaz, A.F. de M.; Melo, M.A. de; Santos, C. de S.A.B.; Alves, C.J.; Azevedo, S.S. de High Seroprevalence and Associated Factors for Visceral Leishmaniasis in Dogs in a Transmission Area of Paraíba State, Northeastern Brazil. Rev. Bras. Parasitol. Veterinária 2020, 29. [Google Scholar] [CrossRef]
- Santos-Doni, T.R.; Viol, M.A.; Lima, V.M.F.; Oliveira, B.C.M.; Matos, L.V.S.; Costa, A.J. da; Gomes, J.F.; Bresciani, K.D.S. Canine Visceral Leishmaniasis and Rhipicephalus sanguineus: Evaluation and Comparison of Classical Techniques. Vet. Res. Commun. 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Piyasiri, S.B.; Dewasurendra, R.; Samaranayake, N.; Karunaweera, N. Diagnostic Tools for Cutaneous Leishmaniasis Caused by Leishmania donovani: A Narrative Review. Diagnostics 2023, 13, 2989. [Google Scholar] [CrossRef]
- Kariyawasam, K.; Edirisuriya, C.S.; Senerath, U.; Hensmen, D.; Siriwardana, H.; Karunaweera, N.D. Characteisation of Cutaneous Leishmaniasis in Matara District, Southern Sri Lanka: Evidence for Case Clustering. Pathog. Glob. Health 2015, 109, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Reimão, J.Q.; Coser, E.M.; Lee, M.R.; Coelho, A.C. Laboratory Diagnosis of Cutaneous and Visceral Leishmaniasis: Current and Future Methods. Microorganisms 2020, 8, 1632. [Google Scholar] [CrossRef]
- Caminade, C.; McIntyre, K.M.; Jones, A.E. Impact of Recent and Future Climate Change on Vector-borne Diseases. Ann. N. Y. Acad. Sci. 2019, 1436, 157–173. [Google Scholar] [CrossRef]
- de Angeli Dutra, D.; Salloum, P.M.; Poulin, R. Vector Microbiome: Will Global Climate Change Affect Vector Competence and Pathogen Transmission? Parasitol. Res. 2023, 122, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Macfie, J.W.S.; Thomson, J.G. A Trypanosome of the Canary (Serinus canarius Koch). Trans. R. Soc. Trop. Med. Hyg. 1929, 23. [Google Scholar] [CrossRef]
- Novy, F.G.; MacNeal, W.J.; Torrey, H.N. The Trypanosomes of Mosquitoes and Other Insects. J. Infect. Dis. 1907, 223–276. [Google Scholar] [CrossRef]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; Boer, M. den; Team, W.H.O.L.C. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS One 2012, 7, e35671. [Google Scholar] [CrossRef]
- Browne, A.J.; Guerra, C.A.; Alves, R.V.; Da Costa, V.M.; Wilson, A.L.; Pigott, D.M.; Hay, S.I.; Lindsay, S.W.; Golding, N.; Moyes, C.L. The Contemporary Distribution of Trypanosoma cruzi Infection in Humans, Alternative Hosts and Vectors. Sci. Data 2017, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Guglielmone, A.A.; Nava, S.; Robbins, R.G. Neotropical Hard Ticks (Acari: Ixodida: Ixodidae); Springer, 2021; ISBN 3030723526. [Google Scholar]
- Barros-Battesti, D.M.; Ramirez, D.G.; Landulfo, G.A.; Faccini, J.L.H.; Dantas-Torres, F.; Labruna, M.B.; Venzal, J.M.; Onofrio, V.C. Immature Argasid Ticks: Diagnosis and Keys for Neotropical Region. Rev. Bras. Parasitol. Veterinária 2013, 22, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Gürtler, R.E.; Cardinal, M.V. Reservoir Host Competence and the Role of Domestic and Commensal Hosts in the Transmission of Trypanosoma cruzi. Acta Trop. 2015, 151, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Alemayehu, B.; Alemayehu, M. Leishmaniasis: A Review on Parasite, Vector and Reservoir Host. Heal. Sci. J. 2017, 11, 1. [Google Scholar] [CrossRef]
- Sánchez-Montes, S.; Salceda-Sánchez, B.; Bermúdez, S.E.; Aguilar-Tipacamú, G.; Ballados-González, G.G.; Huerta, H.; Aguilar-Domínguez, M.; Mora, J.D. la; Licona-Enríquez, J.D.; Mora, D.D. la Rhipicephalus Sanguineus Complex in the Americas: Systematic, Genetic Diversity, and Geographic Insights. Pathogens 2021, 10, 1118. [Google Scholar] [CrossRef]
- Walker, J.B.; Keirans, J.E.; Horak, I.G. The Genus Rhipicephalus (Acari, Ixodidae): A Guide to the Brown Ticks of the World; Cambridge University Press, 2000; ISBN 0521480086. [Google Scholar]
- Kahl, O.; Gern, L.; Eisen, L.; Lane, R.S. Ecological Research on Borrelia burgdorferi sensu lato: Terminology and Some Methodological Pitfalls. 2002.
- Nuttall, P.A. Molecular Characterization of Tick-Virus Interactions. Front Biosci 2009, 14, 2466–2483. [Google Scholar] [CrossRef]
- Gargili, A.; Estrada-Peña, A.; Spengler, J.R.; Lukashev, A.; Nuttall, P.A.; Bente, D.A. The Role of Ticks in the Maintenance and Transmission of Crimean-Congo Hemorrhagic Fever Virus: A Review of Published Field and Laboratory Studies. Antiviral Res. 2017, 144, 93–119. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, C.R.; Fauquet, A.; Lahondère, C.; Araújo, R.N.; Pereira, M.H. Soft Ticks Perform Evaporative Cooling during Blood-Feeding. J. Insect Physiol. 2021, 130, 104197. [Google Scholar] [CrossRef] [PubMed]
- Mans, B.J.; Andersen, J.F.; Francischetti, I.M.B.; Valenzuela, J.G.; Schwan, T.G.; Pham, V.M.; Garfield, M.K.; Hammer, C.H.; Ribeiro, J.M.C. Comparative Sialomics between Hard and Soft Ticks: Implications for the Evolution of Blood-Feeding Behavior. Insect Biochem. Mol. Biol. 2008, 38, 42–58. [Google Scholar] [CrossRef] [PubMed]
- Antunes, S.; Rosa, C.; Couto, J.; Ferrolho, J.; Domingos, A. Deciphering Babesia-Vector Interactions. Front. Cell. Infect. Microbiol. 2017, 7, 429. [Google Scholar] [CrossRef] [PubMed]
- Tahir D, Meyer L, Fourie J, Jongejan F, Mather T, Choumet V, Blagburn B, Straubinger RK, Varloud M. 2020. Interrupted blood feeding in ticks: causes and consequences. Microorganisms 8:910. [CrossRef]
- Velásquez-Ortiz, N.; Ramírez, J.D. Understanding the Oral Transmission of Trypanosoma Cruzi as a Veterinary and Medical Foodborne Zoonosis. Res. Vet. Sci. 2020, 132, 448–461. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; ... & Moher, D.(2021). The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Bmj 2021. [CrossRef]
- Nakagawa, S.; Noble, D.W.A.; Senior, A.M.; Lagisz, M. Meta-Evaluation of Meta-Analysis: Ten Appraisal Questions for Biologists. BMC Biol. 2017, 15. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.; Hill, S. Supporting Implementation of Cochrane Methods in Complex Communication Reviews: Resources Developed and Lessons Learned for Editorial Practice and Policy. Heal. Res. Policy Syst. 2019, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.J. The Inverse of the Freeman–Tukey Double Arcsine Transformation. Am. Stat. 1978, 32, 138. [Google Scholar] [CrossRef]
- Freeman, M.F.; Tukey, J.W. Transformations Related to the Angular and the Square Root. Ann. Math. Stat. 1950, 607–611. [Google Scholar] [CrossRef]
| Species | Sampling year | Trypansoma species | Tick statut (P/N) |
Localization in the tick | Tick Detection method |
Host (Species) | Country | Continent | Author(s) |
|---|---|---|---|---|---|---|---|---|---|
| Amblyomma cajennense | 2013 | T. cruzi | N | H ; Gr | Microscopy, PCR | Dog (Canis lupus) | Brazil | South America | [92] |
| NS | T. vivax | N | H | Microscopy | Bovine (Bos taurus) | Cuba | South America | [90] | |
| NS | T. vivax | P | G | Microscopy | Bovine (Bos taurus) | Cuba | South America | [90] | |
| NS | T. vivax | P | Gr | PCR, Sequencing | buffalos | Brazil | South America | [83] | |
| Amblyomma longirostrum | NS | T. cruzi | P | NS | NS | Cercolabidae (Arboreal porcupine) | Venezuela | South America | [36] |
| Amblyomma spp. | 2013-2019 | T. cruzi | N | Gr | Metagenomics | * Wild ungulates Carnivores | Kenya | Africa | [84] |
| Amblyomma tigrinum | 2013 | T. cruzi | N | Gr | PCR, Sequencing | Canidae (Canis lupus familiaris) | Chile | South America | [89] |
| Amblyomma variegatum | NS | T. congolense | P | Gr | Parasitological analysis, PCR | Cattle | Nigeria | Africa | [78] |
| Haemaphysalis spp. | 2017-2018 | T. evansi | N | Gr | PCR, Sequencing | Cattle | India | Asia | [85] |
| Hyalomma detritum | 1982 | T. theileri | P | H | Microscopy | Bovine (Bos taurus) Dog (Canis lupus) | Algeria | Africa | [88] |
| Hyalomma dromedarii | 2015-2016 | T. evansi | N | Gr | PCR | **Wild ruminants species | Tunisia | Africa | [91] |
| Hyalomma excavatum | 2015-2016 | T. evansi | N | Gr | PCR | **Wild ruminants species | Tunisia | Africa | [91] |
| 1982 | T. theileri, T. evansi | N | H | Microscopy | Bovine (Bos taurus) Dog (Canis lupus ) | Algeria | Africa | [88] | |
| Hyalomma lusitanicum | 1982 | T. theileri, T. evansi | N | H | Microscopy | Bovine (Bos taurus); Dog (Canis lupus) | Algeria | Africa | [88] |
| Hyalomma marginatum | 2015-2016 | T. evansi | N | Gr | PCR | **Wild ruminants species | Tunisia | Africa | [91] |
| NS | T. theileri | P | H | Microscopy | Bovine (Bos taurus) | Portugal | Europe | [80] | |
| 1982 | T. theileri, T. evansi | N | H | Microscopy | Bovine (Bos taurus) Dog (Canis lupus) | Algeria | Africa | [88] | |
| Hyalomma spp. | 2017-2018 | T. evansi | N | Gr | PCR, Sequencing | Cattle | India | Asia | [85] |
| Ixodes ricinus | 2013 | T. caninum | P | Gr | Culture, PCR, Sequencing | Vegetation | Slovakia | Europe | [86] |
| 1979-1982 | T. theileri | P | H | Microscopy | Bovine (Bos taurus) Dog (Canis lupus) | Switzerland | Europe | [88] | |
| 2015-2016 | T. evansi | N | Gr | PCR | **Wild ruminants species | Tunisia | Africa | [91] | |
| Rhipicephalus (Boophilus) microplus | NS | T. theileri-like | P | H | ND | Bovine (Bos taurus) | Brazil | South America | [87] |
| NS | T. vivax | N | H | Microscopy | Bovine (Bos taurus) | Cuba | South America | [90] | |
| NS | T. vivax | P | G | Microscopy | Bovine (Bos taurus) | Cuba | South America | [90] | |
| NS | T. vivax | P | Gr | PCR | Bovine (Bos taurus) | Venezuela | South America | [81] | |
| NS | T. vivax | P | Gr | PCR, Sequencing | buffalos | Brazil | South America | [83] | |
| Rhipicephalus (Boophilus) spp. | 2017-2018 | T. evansi | P | Gr | PCR, Sequencing | Cattle | India | Asia | [85] |
| Rhipicephalus bursa | 2015-2016 | T. evansi | N | Gr | PCR | **Wild ruminants species | Tunisia | Africa | [91] |
| Rhipicephalus sanguineus | 2013 | T. cruzi | N | H ; Gr | Microscopy, PCR | Dog (Canis lupus) | Brazil | South America | [92] |
| 1982 | T. evansi | P | H | Microscopy | Bovine (Bos taurus) Dog (Canis lupus) | Algeria | Africa | [88] | |
| NS | T. evansi | P | Gr | PCR | Dog (Canis lupus) | Brazil | South America | [82] | |
| NS | T. vivax | P | Gr | PCR | Dog (Canis lupus) | Brazil | South America | [82] | |
| Rhipicephalus sanguineus sensu lato | 2013 | T. cruzi | P | Gr | PCR, Sequencing | Canidae (Canis lupus familiaris) | Chile | South America | [89] |
| 2015-2016 | T. evansi | P | Gr | PCR | Wild ruminants species | Tunisia | Africa | [91] | |
| Rhipicephalus spp. | 2013-2019 | T. cruzi | P | Gr | Metagenomics | * * * Wild ungulates Carnivores Regular domestic | Kenya | Africa | [84] |
| Rhipicephalus turanicus | 1982 | T. theileri, T. evansi | N | H | Microscopy | Bovine (Bos taurus) Dog (Canis lupus) | Algeria | Africa | [88] |
| Species | Sampling year | Leishmania species | Tick statut (P/N) |
Localisation in the tick | Tick Detection method |
Host (Species) | Host Detection method | Country | Continent | Author(s) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amblyomma cajennense | 2013 | L. chagasi (Syn L. infantum) | N | H ; Gr | Microscopy, PCR | Dog (Canis lupus) | ND | Brazil | South America | [92] | |
| Amblyomma ovale | 2008 | L. infantum | N | Gr | qPCR | Dog (Canis lupus) | IFAT | Brazil | South America | [73] | |
| Amblyomma sabanerae | NS | Leishmania sp. | P | Gr | qPCR | Collared peccary (Pecari tajacu) Tortoise (Chelonoidis denticulata) | ND | Peru | South America | [107] | |
| Amblyomma spp. | 2012 | L. guyanensis | P | Gr | PCR ; HRM-PCR | Tapiridae (Tapirus terrestris) Tayassuidae (Pecari tajacu) | ND | Peru | South America | [63] | |
| Amblyomma tigrinum | 2010-2013 | Leishmania sp. | P | Gr | PCR | Wild foxes (Pseudalopex griseus) | PCR, qPCR | Argentina | South America | [58] | |
| Amblyomma variegatum | 2014-2015 | L. martiniquensis | P | Gr | HT-qPCR | Cattle | ND | Guadeloupe | Island | [56] | |
| Dermacentor marginatus | 2007-2008 | L. infantum | N | Gr | PCR | Wild boars | ND | Italy | Europe | [108] | |
| 2007 | L. infantum | N | Gr | qPCR | Dog (Canis lupus) | ELISA, PCR | Italy | Europe | [66] | ||
| 2014 | Leishmania sp. | N | Gr | PCR | Human | ND | Turkey | Asia | [109] | ||
| Haemaphysalis longicornis | 2012 | L. infantum | N | Gr | PCR | sheep, cattle and dogs | ND | China | Asia | [110] | |
| Haemaphysalis parva | 2014 | Leishmania sp. | N | Gr | PCR | Human | ND | Turkey | Asia | [109] | |
| Haemaphysalis punctata | 2014 | Leishmania sp. | N | Gr | PCR | Human | ND | Turkey | Asia | [109] | |
| Haemaphysalis sulcata | 2007-2008 | L. infantum | N | Gr | PCR | Sheep; Goats | ND | Italy | Europe | [108] | |
| 2014 | Leishmania sp. | N | Gr | PCR | Human | ND | Turkey | Asia | [109] | ||
| Hyalomma aegyptium | 2015-2018 | L. infantum | P | Gr | PCR ; Sequencing | Tortoise (Testudo graeca) Arabian camels (Camelus dromedarius) | ND | Israel | Asia | [59] | |
| 2014 | Leishmania sp. | N | Gr | PCR | Human | ND | Turkey | Asia | [109] | ||
| Hyalomma dromedarii | 2015-2018 | L. infantum | P | Gr | PCR, Sequencing | Tortoise (Testudo graeca) Arabian camels (Camelus dromedarius) | ND | Israel | Asia | [59] | |
| Hyalomma excavatum | 2014 | Leishmania sp. | N | Gr | PCR | Human | ND | Turkey | Asia | [109] | |
| Hyalomma marginatum | 2007-2008 | L. infantum | N | Gr | PCR | Cattle | ND | Italy | Europe | [108] | |
| 2014 | Leishmania sp. | N | Gr | PCR | Human | ND | Turkey | Asia | [109] | ||
| Hyalomma spp. | 2014 | Leishmania sp. | N | Gr | PCR | Human | ND | Turkey | Asia | [109] | |
| Ixode ricinus | 2007 | L. infantum | P | Gr | qPCR | Dog (Canis lupus) | ELISA, PCR | Italy | Europe | [66] | |
| 2007-2008; 2010 | L. infantum | P | Gr | PCR | Dogs, horses, cat, bovine, humans | ND | Italy | Europe | [64] | ||
| 2011 and 2013 | L. infantum | P | Gr | PCR | Cat (Felis catus) | ND | Italy | Europe | [62] | ||
| Ixode ricinus(Cont.) | 2012-2013 | L. infantum | P | Gr | qPCR | Cat (Felis catus) | PCR, qPCR | Italy | Europe | [104] | |
| 2010 | L. infantum | P | Gr | PCR ; Sequencing | Flagging | ND | Italy | Europe | [102] | ||
| 2014 | Leishmania sp. | P | Gr | PCR | Human | ND | Turkey | Asia | [109] | ||
| Ixodes spp. | 2011 and 2013 | L. infantum | N | Gr | PCR | Cat (Felis catus) | ND | Italy | Europe | [62] | |
| 2012-2013 | L. infantum | P | Gr | qPCR | Cat (Felis catus) | PCR, qPCR | Italy | Europe | [104] | ||
| Ixodes ventalloi | 2011 and 2013 | L. infantum | P | Gr | PCR | Cat (Felis catus) | ND | Italy | Europe | [62] | |
| 2012-2013 | L. infantum | P | Gr | qPCR | Cat (Felis catus) | PCR, qPCR | Italy | Europe | [104] | ||
| Rhipicephalus (Boophilus) microplus | 2012 | L. guyanensis | P | Gr | PCR ; HRM-PCR | Tapiridae (Tapirus terrestris) Tayassuidae (Pecari tajacu) | ND | Peru | South America | [63] | |
| 2012 | L. infantum | N | Gr | PCR | Sheep, cattle and dogs | ND | China | Asia | [110] | ||
| Leishmania sp. | P | Gr | qPCR | Collared peccary (Pecari tajacu); Tortoise (Chelonoidis denticulata) | ND | Peru | South America | [105] | |||
| 2014-2015 | L. martiniquensis | P | Gr | HT-qPCR | Cattle | ND | Guadeloupe | Island | [56] | ||
| 2014-2015 | L. martiniquensis | P | Gr | HT-qPCR | Cattle | ND | Martinique | Island | [56] | ||
| Rhipicephalus bursa | 2014 | Leishmania sp. | N | Gr | PCR | Human | ND | Turkey | Asia | [109] | |
| 2007-2008 | L. infantum | N | Gr | PCR | Sheep, goats,cattle, horses, deer | ND | Italy | Europe | [108] | ||
| Rhipicephalus pusillus | 2011 and 2013 | L. infantum | P | Gr | PCR | Cat (Felis catus) | ND | Italy | Europe | [62] | |
| 2007-2008 | L. infantum | N | Gr | PCR | Hedgehogs | ND | Italy | Europe | [108] | ||
| 2012-2013 | L. infantum | P | Gr | qPCR | Cat (Felis catus) | PCR, qPCR | Italy | Europe | [104] | ||
| Rhipicephalus sanguineus | 2012-2013 | L. infantum | P | Gr | qPCR | Cat (Felis catus) | PCR, qPCR | Italy | Europe | [104] | |
| 2016-2017 | Leishmania major | P | Gr | PCR, Sequencing | Rodents (Rhombomys opimus; Nesokia indica) | ND | Iran | Asia | [98] | ||
| 2013 | L. chagasi (syn L. infantum) | P | H ; Gr | Microscopy, PCR | Dog (Canis lupus) | ND | Brazil | South America | [92] | ||
| NS | L. braziliensis | P | Gr | PCR, qPCR | Dog (Canis lupus) | ND | Brazil | South America | [76] | ||
| 2007 | L. infantum | P | Gr | PCR ; qPCR | Dog (Canis lupus) | IFAT, PCR, qPCR | Brazil | South America | [100] | ||
| 2007 | L. infantum | P | Gr, SG | PCR ; qPCR ; sequencing | Dog (Canis lupus) | ND | Italy | Europe | [74] | ||
| 2008 | L. infantum | P | Gr, SG | PCR; qPCR; sequencing | Dog (Canis lupus) | ND | Brazil | South America | [74] | ||
| 2007-2008 | L. infantum | P | Gr | PCR | Dog (Canis lupus) | ELISA | Brazil | South America | [103] | ||
| 2007-2008 | L. infantum | N | Gr | PCR | Dog (Canis lupus) | ND | Italy | Europe | [108] | ||
| 2008-2009 | L. infantum | P | Gr | PCR, RT-PCR, Sequencing | Dog (Canis lupus) | ELISA, PCR | Brazil | South America | [65] | ||
| Rhipicephalus sanguineus | 2007 | L. infantum | P | Gr | qPCR | Dog (Canis lupus) | ELISA, PCR | Italy | Europe | [66] | |
| 2006-2007 | L. infantum | P | Gr | qPCR | Dog (Canis lupus) | ELISA, PCR | Italy | Europe | [67] | ||
| 2011-2012 | L. infantum | N | NS | Infestation | Dog (Canis lupus) | IFAT, ELISA | Brazil | South America | [111] | ||
| NS | L. infantum | P | Gr | PCR, qPCR, seuqencing | Dog (Canis lupus) | PCR, qPCR | Brazil | South America | [77] | ||
| 2012 | L. infantum | P | Gr | PCR | Dog (Canis lupus) | IFAT, ELISA | Brazil | South America | [54] | ||
| 2013 | L. infantum | P | Gr | PCR, Sequencing | Dog (Canis lupus) | PCR, Sequencing | Brazil | South America | [55] | ||
| 2011 | L. infantum | P | G | PCR, RFLP, seuqencing, Parasit culture | Dog (Canis lupus) | DPP, IFAT, ELISA, PCR | Brazil | South America | [57] | ||
| 2011 | L. infantum | P | SG | PCR, RFLP, Sequencing, Parasit culture | Dog (Canis lupus) | DPP, IFAT, ELISA, PCR | Brazil | South America | [57] | ||
| 2011 and 2013 | L. infantum | P | Gr | PCR | Cat (Felis catus) | ND | Italy | Europe | [62] | ||
| 2002 | Leishmania sp. | P | G | Microscopy | Dog (Canis lupus) | IFAT | Brazil | South America | [106] | ||
| 2009 | Leishmania sp. | N | NS | present of tick or no | Dog (Canis lupus) | IFAT | Brazil | South America | [61] | ||
| 2012-2014 | Leishmania sp. | N | Gr | qPCR | Dog (Canis lupus) | qPCR | China | Asia | [112] | ||
| NS | Leishmania sp. | P | G ; O ;SG | IHC, qPCR, IHC | Dog (Canis lupus) | ND | Brazil | South America | [69] | ||
| NS | Leishmania sp. | P | G ; O ; SG | IHC, qPCR, IHC | Dog (Canis lupus) | ND | Brazil | South America | [68] | ||
| NS | L. kala azar£ | P | NS | NS | Human | NS | France | Europe | [101] | ||
| 2008 | L. infantum | N | Gr | qPCR | Dog (Canis lupus) | IFAT | Brazil | South America | [73] | ||
| Rhipicephalus sanguineus s.L. | NS | L. infantum | P | Gr | PCR, Sequencing | $Flagging & *Animals | ND | Israel | Asia | [59] | |
| Rhipicephalus turanicus | 2007-2008 | L. infantum | N | Gr | PCR | Sheep; Goats | ND | Italy | Europe | [108] | |
| 2014 | Leishmania sp. | N | Gr | PCR | Human | ND | Turkey | Asia | [109] | ||
| NS | L. infantum | P | Gr | PCR, Sequencing | $Flagging & *Animals | ND | Israel | Asia | [59] | ||
| Category | Variable | No. of studies | No. of tested | No. of positive | % [95% CI] | Heterogeneity | Univariate meta regression | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ꭕ2 | P-value | I2 (%) | P-value | R2 (%) | I2-res (%) | ||||||
| Sampling year |
2000 or before 2001-2010 2011-2019 2020 or after |
2 5 23 12 |
6470 480 3043 1721 |
19 130 518 96 |
0.22 [0.16-0.45] 12.19 [0.87-32.08] 21.13 [7.7-23.77] 11.65 [0.3-30.84] |
1.14 126.37 818.31 289.5 |
<0.0001 <0.0001 <0.0001 <0.0001 |
12.0 96.8 97.3 96.2 |
0.000*** |
0.00 | 98.74 |
| Continent |
Africa Asia Europe Island South America |
4 7 10 1 20 |
4392 1165 3693 478 1986 |
22 44 69 2 626 |
2.44 [0.00-9.54] 3.11 [0.00-20.83] 4.36 [1.49-9.06] 0.42 [0.01-1.26] 33.35 [14.27-37.83] |
23.24 131.61 158.52 0.000 828.68 |
< 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 |
85.8 95.4 94.3 -- 96.1 |
0.0056** |
23.77 | 97.78 |
| Host (Family) |
Several# Bovidae Canidae Felidae Other |
6 6 20 2 7 |
4894 1192 2184 221 722 |
43 44 595 19 57 |
2.83 [0.0-8.60] 3.92 [1.20-7.85] 27.92 [14.51-43.57] 8.47 [5.07-12.60] 21.23 [0.0-62.24]0 |
80.46 24.26 1071.56 0.81 252.480 |
< 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 |
93.8 88.2 98.2 0.0 97.6 |
0.0007** |
0.00 | 98.34 |
| Tick Genus |
Amblyomma Dermacentor Haemaphysalis Hyalomma Ixodes Rhipicephalus |
10 3 4 6 9 33 |
328 23 360 639 6906 3271 |
27 0 00 17 42 668 |
8.78 [0-29.41] 0.00 [0-5.25] 0.00 1.20 [0-14.55] 1.91 [0-10.06] 19.06 [9.64-30.39] |
100.12 0.41 1.58 70.55 71.41 1412.59 |
< 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 |
91.0 0.0 0.0 92.9 88.8 97.7 |
0.0001*** |
2.20 | 97.09 |
| Tick species |
A. cajennense A. ovale A. sabanerae A. tigrinum A. variegatum Amblyomma spp. D. marginatus H. aegyptium H. detritum H. dromedarii H. excavatum H. lusitanicum H. marginatum Hyalomma sp. Ha. longicornis Ha. parva Ha. punctata Ha. sulcata Haemaphysalis. sp. I. ricinus I. ventalloi Ixodes sp. R. (Boophilus) R. bursa R. pusillus R. sanguineus R. turanicus Rhipicephalus sp. |
1 1 1 2 2 2 3 2 1 2 3 1 4 2 1 1 1 2 1 8 1 1 6 3 2 21 4 1 |
131 10 10 28 165 43 21 32 1 45 291 3 171 81 308 41 6 4 13 6580 62 5 1025 23 18 2222 158 43 |
0 0 9 0 11 4 0 12 1 5 0 0 0 0 0 0 0 0 0 32 3 0 62 0 3 583 10 3 |
0.00 [0-7.04] 0.00 [0-16.52] 90.00 [61.91-100] 24.86 [0-95.91] 3.09 [0-24.26] 0.00 [0-0.64] 0.00 [0-5.25] 32.69 [11.66-56.75] 100 [0-100] 16.56 [0-88.80] 0.00 0 [0-50] 0.00 0.00 [0-2.36] 0.00 [0-0.58] 0.00 [0-4.15] 0.00 [0-26.80] 0.00 [0-78.74] 0.00 [0-12.82] 1.36 [0-10.39] 6.45 [1.42-14.19] 0.00 [0-31.73] 0.00 [0-3.92] 13.54 [0-47.12] 17.65 [2.57-39.97] 25.15 [11.67-41.19] 2.48 [0-1467] 6.98 [0.9-16.93] |
3.86 0.00 0.00 16.89 0.43 11.54 0.59 0.46 0.00 17.10 1.45 0.00 2.04 0.01 0.00 0.00 0.00 0.07 0.00 64.66 0.00 0.00 0.51 185.13 0.02 1092.57 23.70 0.00 |
< 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 |
-- 0.0 -- 94.1 91.30.0 0.0 0.0 -- 94.2 0.0 -- 0.0 0.0 -- -- -- 0.0 -- 87.6 -- -- 0.0 22.2 0.0 97.9 87.3 -- |
0.0001*** |
3.29 | 97.50 |
| Detection method |
Microscopy Molecular |
3 39 |
6533 5181 |
20 743 |
0.1 [0.0-0.23] 17.55 [9.31-27.44] |
3.47 1937.17 |
< 0.0001 < 0.0001 |
42.4 98.1 |
0.000*** | 5.37 | 98.84 |
| Parasite genus |
Leishmania Trypanosoma |
30 12 |
4569 7739 |
719 93 |
18.87 [9.12-30.75] 4.62 [1.14-9.79] |
1642.76 203.03 |
< 0.0001 < 0.0001 |
98.294.6 |
0.0046** | 7.10 | 98.88 |
| Parasite species |
L. braziliensis L. chagas (syn L. infantum) L. guyanensis L. infantum L. major L. martiniquensis Leishmania sp. T. caninum T. congolense T. cruzi T. evansi T. theileri T. vivax |
1 1 1 18 1 1 6 1 1 3 4 2 3 |
75 444 81 2124 1 578 683 9 33 583 892 6220 441 |
32 107 3 323 1 2 194 1 4 8 38 14 42 |
42.67 [31.64-64.66] 24.10 [20.23-28.19] 12.12 [2.79-25.84] 15.44 [7.18-25.89] 100 [0-100] 0.35 [0-1.04] 51.82 [8.42-93.57] 11.11 [0-41.77] 3.70 [0.47-9.18] 2.39 [0-10.25] 6.44 [0-23.33] 0.20 [0.12-0.36] 10.88 [2.91-22.73] |
0.00 0.00 0.00 679.35 0.08 0.00 0.00 0.00 0.00 895.52 13.00 73.71 22.82 |
< 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 |
-- -- -- 97.5 -- -- 94.4 -- -- 91.2 95.9 0.0 84.6 |
0.000*** | 9.25 |
98.97 |
| Localization in ticks |
H G GR GR-SG H-GR SG O |
2 3 35 1 1 2 2 |
6470 209 4532 95 444 110 165 |
19 164 478 11 107 35 74 |
0.29 [0.16-0.45] 74.32 [28.48-99.99] 13.45 [6.62-21.87] 11.58 [5.82-18.88] 24.10 [20.23-28.19] 31.79 [23.31-40.91] 44.48 [37.27-52.53] |
1.14 312.09 1234.08 0.00 0.00 0.00 0.67 |
< 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 |
12.0 97.3 97.2 -- -- 0.0 0.0 |
0.000*** | 0.00 | 98.89 |
| Acquisition of pathogens via tick blood feeding | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Category | Variable | No. of studies | No. of positive | % [95% CI] | Heterogeneity | Univariate meta regression | |||||||||||||
| ꭕ2 | P-value | I2 (%) | P-value | R2 (%) | I2-res (%) | ||||||||||||||
| Ticks Family | 0.0976 | 0.00 | 0.00 | ||||||||||||||||
| Argasidae | 16 | 16 | 100 [81.65-100] | 0.00 | 0.9966 | 0.0 | |||||||||||||
| Ixodidae | 21 | 15 | 78.01 [48.91-98.34] | 15.86 | 0.9966 | 0.0 | |||||||||||||
| Ticks Genus | 0.3905 | 0.00 | 0.00 | ||||||||||||||||
| Amblyoma | 1 | 1 | 100 [0-100] | 0.00 | 0.9938 | -- | |||||||||||||
| Hyalomma | 4 | 3 | 82.18 [16.67-100] | 2.78 | 0.9938 | 0.0 | |||||||||||||
| Ornithodoros | 15 | 15 | 100 [80.68-100] | 0.00 | 0.9938 | 0.0 | |||||||||||||
| Rhipicephalus | 16 | 11 | 74.57 [40.89-98.60] | 12.72 | 0.9938 | 0.0 | |||||||||||||
| Ticks species | 0.6118 | 0.00 | 0.00 | ||||||||||||||||
| A. americanum | 1 | 1 | 100 [0-100] | 0.00 | 0.9989 | -- | |||||||||||||
| B. decoloratus | 1 | 1 | 100 [0-100] | 0.00 | 0.9989 | -- | |||||||||||||
| D. andersoni | 2 | 2 | 100 [21.62-100] | 0.00 | 0.9989 | 0.0 | |||||||||||||
| H. A. anatolicum | 3 | 2 | 72.14 [2.45-100] | 2.47 | 0.9989 | 18.9 | |||||||||||||
| H. A. excavatum | 1 | 1 | 100 [0-100] | 0.00 | 0.9989 | -- | |||||||||||||
| H. dromaderii | 1 | 1 | 100 [0-100] | 0.00 | 0.9989 | -- | |||||||||||||
| H. impressum | 1 | 1 | 100 [0-100] | 0.00 | 0.9989 | -- | |||||||||||||
| O. amblus | 1 | 1 | 100 [0-100] | 0.00 | 0.9989 | -- | |||||||||||||
| O. crossi | 4 | 4 | 100 [48.72-100] | 0.00 | 0.9989 | 0.0 | |||||||||||||
| O. furcosis | 1 | 1 | 100 [0-100] | 0.00 | 0.9989 | -- | |||||||||||||
| O. hermsi | 2 | 0 | 0 [0-78.74] | 0.00 | 0.9989 | 0.0 | |||||||||||||
| O. lahorensis | 2 | 2 | 100 [21.26-100] | 0.00 | 0.9989 | 0.0 | |||||||||||||
| O. moubata | 7 | 7 | 100 [65.46-100] | 0.00 | 0.9989 | 0.0 | |||||||||||||
| O. parkeri | 1 | 1 | 100 [0-100] | 0.00 | 0.9989 | -- | |||||||||||||
| O. savignyi | 1 | 1 | 100 [0-100] | 0.00 | 0.9989 | -- | |||||||||||||
| O. talaje | 1 | 0 | 0 [0-100] | 0.00 | 0.9989 | -- | |||||||||||||
| O. turanicus | 2 | 2 | 100 [21.62-100] | 0.00 | 0.9989 | 0.0 | |||||||||||||
| O. venzualensis | 1 | 1 | 100 [0-100] | 0.00 | 0.9989 | -- | |||||||||||||
| R. sanguneus | 13 | 11 | 91.99 [58.62-100] | 6.26 | 0.9989 | 0.0 | |||||||||||||
| R. sanguneus s.I. | 2 | 0 | 0 [0-78.74] | 0.00 | 0.9989 | 0.0 | |||||||||||||
| R. pulchellus | 1 | 1 | 100 [0-100] | 0.00 | 0.9989 | -- | |||||||||||||
| Parasite genus | 0.0794 | 0.00 | 0.00 | ||||||||||||||||
| Leishmania | 14 | 9 | 69.10 [62.64-97.17] | 11.90 | 0.9974 | 0.0 | |||||||||||||
| Trypanosoma | 22 | 21 | 99.11 [80.73-100] | 3.53 | 0.9974 | 0.0 | |||||||||||||
| Parasite Species | 0.7192 | 0.00 | 0.00 | ||||||||||||||||
| L. donovani | 1 | 1 | 100 [0-100] | 0.00 | 0.9928 | -- | |||||||||||||
| L. chagasi (syn L.infantum) | 2 | 2 | 100 [21.26-100] | 0.00 | 0.9928 | 0.0 | |||||||||||||
| L. infantum | 5 | 4 | 87.58 [29.26-100] | 2.96 | 0.9928 | 0.0 | |||||||||||||
| L. kala azar£ | 4 | 1 | 17.82 [0-83.24] | 2.78 | 0.9928 | 0.0 | |||||||||||||
| L. major | 1 | 1 | 100 [0-100] | 0.00 | 0.9928 | -- | |||||||||||||
| Leishmania sp. | 1 | 1 | 100 [0-100] | 0.00 | 0.9928 | -- | |||||||||||||
| T. bruci | 1 | 1 | 100 [0-100] | 0.00 | 0.9928 | -- | |||||||||||||
| T. cruzi | 8 | 8 | 100 [68.71-100] | 0.00 | 0.9928 | 0.0 | |||||||||||||
| T. evansi | 7 | 6 | 92.94 [46.20-100] | 3.17 | 0.9928 | 0.0 | |||||||||||||
| T. gambiense | 1 | 1 | 100 [0-100] | 0.00 | 0.9928 | -- | |||||||||||||
| T. lewisi | 2 | 2 | 100 [21.26-100] | 0.00 | 0.9928 | 0.0 | |||||||||||||
| T. rhodesciense | 2 | 2 | 100 [21.26-100] | 0.00 | 0.9928 | 0.0 | |||||||||||||
| T. theileri | 4 | 3 | 82.18 [16.67-100] | 2.78 | 0.9928 | 0.0 | |||||||||||||
| T. theileri like | 1 | 1 | 100 [0-100] | 0.00 | 0.9928 | -- | |||||||||||||
| Donor host family | 0.9455 | 0.00 | 0.00 | ||||||||||||||||
| Bovidae | 4 | 3 | 82.18 [16.67-100] | 2.78 | 0.9471 | 0.0 | |||||||||||||
| Canidae | 13 | 9 | 75.34 [37.81-99.71] | 10.25 | 0.9471 | 0.0 | |||||||||||||
| Camelidae | 2 | 2 | 100 [21.26-100] | 0.00 | 0.9471 | 0.0 | |||||||||||||
| Rodentia | 15 | 14 | 93.73 [63.97-100] | 42.76 | 0.9471 | 0.0 | |||||||||||||
| ND | 2 | 2 | 100 [21.26-100] | 0.00 | 0.9471 | 0.0 | |||||||||||||
| Other | 1 | 1 | 100 [0-100] | 0.00 | 0.9471 | -- | |||||||||||||
| Ticks | 1 | 1 | 100 [0-100] | 0.00 | 0.9471 | -- | |||||||||||||
| Infection via the injection of ticks infected material | |||||||||||||||||||
| Ticks Family | 0.2884 | 0.00 | 0.00 | ||||||||||||||||
| Argasidae | 9 | 7 | 85.25 [41.62-100] | 5.76 | 0.6062 | 0.0 | |||||||||||||
| Ixodidae | 13 | 7 | 55.23 [18.49-89.66] | 11.96 | 0.6062 | 0.0 | |||||||||||||
| Ticks Genus | 0.5954 | 0.00 | 0.00 | ||||||||||||||||
| Hyalomma | 4 | 2 | 50.0 [0-100] | 3.70 | 0.5378 | 18.9 | |||||||||||||
| Ornithodoros | 9 | 8 | 85.25 [41.62-100] | 5.76 | 0.5378 | 0.0 | |||||||||||||
| Rhipicephalus | 7 | 4 | 59.68 [11.06-99.18] | 6.34 | 0.5378 | 5.4 | |||||||||||||
| Ticks species | 0.2229 | 0.00 | 0.00 | ||||||||||||||||
| B. decoloratus | 1 | 0 | 0 [0-100] | 0.00 | 0.8803 | -- | |||||||||||||
| D. andersoni | 2 | 0 | 0 [0-78.74] | 0.00 | 0.8803 | 0.0 | |||||||||||||
| H. a. anatolicum | 3 | 2 | 72.14 [2.45-100 | 2.47 | 0.8803 | 18.9 | |||||||||||||
| H. a. excavatum | 1 | 0 | 0 [0-100] | 0.00 | 0.8803 | -- | |||||||||||||
| H. dromaderii | 1 | 0 | 0 [0-100] | 0.00 | 0.8803 | -- | |||||||||||||
| H. impressum | 1 | 0 | 0 [0-100] | 0.00 | 0.8803 | -- | |||||||||||||
| O. amblus | 1 | 1 | 100 [0-100] | 0.00 | 0.8803 | -- | |||||||||||||
| O. crossi | 1 | 0 | 0 [0-100] | 0.00 | 0.8803 | -- | |||||||||||||
| O. furcosus | 1 | 1 | 100 [0-100] | 0.00 | 0.8803 | -- | |||||||||||||
| O. hermsi | 2 | 0 | 0 [0-78.74] | 0.00 | 0.8803 | 0.0 | |||||||||||||
| O. moubata | 4 | 4 | 100 [48.72-100] | 0.00 | 0.8803 | 0.0 | |||||||||||||
| O. perkeri | 1 | 1 | 100 [0-100] | 0.00 | 0.8803 | -- | |||||||||||||
| O. talaje | 1 | 0 | 0 [0-100] | 0.00 | 0.8803 | -- | |||||||||||||
| O. turanicus | 1 | 0 | 0 [0-100] | 0.00 | 0.8803 | -- | |||||||||||||
| O. turicata | 2 | 1 | 50.0 [0-100] | 1.85 | 0.8803 | 46.0 | |||||||||||||
| O. venzualensis | 1 | 1 | 100 [0-100] | 0.00 | 0.8803 | -- | |||||||||||||
| R. pulchellus | 1 | 0 | 0 [0-100] | 0.00 | 0.8803 | -- | |||||||||||||
| R. sanguineus | 6 | 5 | 90.82 [38.83-100] | 3.08 | 0.8803 | 0.0 | |||||||||||||
| R. sanguineus s.I. | 1 | 0 | 0 [0-100] | 0.00 | 0.8803 | -- | |||||||||||||
| Parasite genus | 0.7666 | 0.00 | 0.00 | ||||||||||||||||
| Leishmania | 7 | 5 | 78.10 [27.34-100] | 5.29 | 0.5774 | 0.0 | |||||||||||||
| Trypanosoma | 14 | 9 | 69.10 [32.64-97.17] | 11.9 | 0.5774 | 0.0 | |||||||||||||
| Donor host Family | 0.7557 | 0.00 | 0.00 | ||||||||||||||||
| Bovidae | 4 | 2 | 50.0 [0-100] | 3.70 | 0.5180 | 18.9 | |||||||||||||
| Camelidae | 1 | 1 | 100 [0-100] | 0.00 | 0.5180 | -- | |||||||||||||
| Canidae | 5 | 4 | 87.58 [29.26-100] | 2.96 | 0.5180 | 0.0 | |||||||||||||
| Rodentia | 11 | 8 | 68.26 [27.33-98.66] | 9.42 | 0.5180 | 0.0 | |||||||||||||
| Receiver host Family | 0.5494 | 0.00 | 0.00 | ||||||||||||||||
| Bovidae | 3 | 2 | 72.14 [2.45-100] | 2.47 | 0.5387 | 18.9 | |||||||||||||
| Canidae | 1 | 1 | 100 [0-100] | 0.00 | 0.5387 | -- | |||||||||||||
| Other | 1 | 0 | 0 [0-100] | 0.00 | 0.5387 | -- | |||||||||||||
| Rodentia | 15 | 11 | 63.49 [28.31-93.43] | 13.32 | 0.5387 | 0.0 | |||||||||||||
| Ticks | 2 | 2 | 100 [21.26-100] | 0.00 | 0.5387 | 0.0 | |||||||||||||
| Parasite species | 0.2614 | 0.00 | 0.00 | ||||||||||||||||
| L. chagasi (Syn L. infantum) | 1 | 1 | 100 [0-100] | 0.00 | 0.8397 | -- | |||||||||||||
| L. infantum | 1 | 1 | 100 [0-100] | 0.00 | 0.8397 | -- | |||||||||||||
| L. kala azar£ | 4 | 2 | 50.0 [0-100] | 3.70 | 0.8397 | 18.9 | |||||||||||||
| Leishmania sp. | 1 | 1 | 100 [0-100] | 0.00 | 0.8397 | -- | |||||||||||||
| T. brucei | 1 | 0 | 0 [0-100] | 0.00 | 0.8397 | -- | |||||||||||||
| T. cruzi | 6 | 6 | 100 [61.37-100] | 0.00 | 0.8397 | 0.0 | |||||||||||||
| T. evansi | 1 | 0 | 0 [0-100] | 0.00 | 0.8397 | -- | |||||||||||||
| T. lewisi | 1 | 0 | 0 [0-100] | 0.00 | 0.8397 | -- | |||||||||||||
| T. rhodeseinse | 1 | 0 | 0 [0-100] | 0.00 | 0.8397 | -- | |||||||||||||
| T. theileri | 4 | 3 | 82.18 [16.76-100] | 2.78 | 0.8397 | 0.0 | |||||||||||||
| T. theileri like | 1 | 0 | 0 [0-100] | 0.00 | 0.8397 | -- | |||||||||||||
| Transmission through blood feeding | |||||||||||||||||||
| Ticks Family | 0.4962 | 0.00 | 0.00 | ||||||||||||||||
| Argasidae | 10 | 3 | 23.71 [0-66.41] | 7.77 | 0.5314 | 0.9 | |||||||||||||
| Ixodidae | 11 | 5 | 34.33 [0.0-94] | 10.09 | 0.5314 | 0.0 | |||||||||||||
| Ticks Genus | 0.7745 | 0.00 | 0.00 | ||||||||||||||||
| Hyalomma | 4 | 2 | 50.0 [0-100] | 3.70 | 0.4677 | 18.9 | |||||||||||||
| Ornithodoros | 10 | 3 | 23.71 [0-66.41] | 7.77 | 0.4677 | 0.0 | |||||||||||||
| Rhipicephalus | 7 | 3 | 40.32 [0.48-88.94] | 6.32 | 0.4677 | 5.4 | |||||||||||||
| Parasite genus | 0.5011 | 0.00 | 0.00 | ||||||||||||||||
| Leishmania | 5 | 2 | 36.51 [0-92.81] | 4.44 | 0.9952 | 9.9 | |||||||||||||
| Trypanosoma | 16 | 8 | 33.22 [5.25-67.40] | 13.88 | 0.9952 | 0.0 | |||||||||||||
| Donnor host family | 0.0405* | 0.00 | 0.00 | ||||||||||||||||
| Bovidae | 4 | 2 | 50.0 [0-100] | 3.70 | 0.9017 | 18.9 | |||||||||||||
| Camelidae | 2 | 2 | 100 [21.26-100] | 0.00 | 0.9017 | 0.0 | |||||||||||||
| Canidae | 7 | 4 | 59.68 [11.06-99.18] | 6.34 | 0.9017 | 5.4 | |||||||||||||
| Rodentia | 8 | 0 | 0 [0-31.29] | 0.00 | 0.9017 | 0.0 | |||||||||||||
| Receiver host family | 0.0678 | 0.00 | 0.00 | ||||||||||||||||
| Bovidae | 3 | 2 | 72.14 [2.45-100] | 2.47 | 0.8461 | 18.9 | |||||||||||||
| Canidae | 3 | 2 | 72.14 [2.45-100] | 2.47 | 0.8461 | 18.9 | |||||||||||||
| Other | 2 | 2 | 100 [21.26-100] | 0.00 | 0.8461 | 0.0 | |||||||||||||
| Rodentia | 13 | 4 | 8.01 [0-41.38] | 6.26 | 0.8461 | 0.0 | |||||||||||||
| Ticks species | 0.6077 | 0.00 | 0.00 | ||||||||||||||||
| B. decoloratus | 1 | 0 | 0 [0-100] | 0.00 | 0.6280 | -- | |||||||||||||
| D. andersoni | 1 | 0 | 0 [0-100] | 0.00 | 0.6280 | -- | |||||||||||||
| H. a. anatolicum | 3 | 2 | 72.14 [2.45-100] | 2.47 | 0.6280 | 18.9 | |||||||||||||
| H. a. excavatum | 1 | 0 | 0 [0-100] | 0.00 | 0.6280 | -- | |||||||||||||
| H. dromaderii | 1 | 0 | 0 [0-100] | 0.00 | 0.6280 | -- | |||||||||||||
| H. impressum | 1 | 0 | 0 [0-100] | 0.00 | 0.6280 | -- | |||||||||||||
| O. crossi | 4 | 3 | 82.18 [16.67-100] | 2.78 | 0.6280 | 0.0 | |||||||||||||
| O. hermsi | 1 | 0 | 0 [0-100] | 0.00 | 0.6280 | -- | |||||||||||||
| O. lahorensis | 2 | 2 | 100 [21.26-100] | 0.00 | 0.6280 | 0.0 | |||||||||||||
| O. moubata | 3 | 0 | 0 [0-61.92] | 0.00 | 0.6280 | 0.0 | |||||||||||||
| O. savigny | 1 | 0 | 0 [0-100] | 0.00 | 0.6280 | -- | |||||||||||||
| O. talaje | 1 | 0 | 0 [0-100] | 0.00 | 0.6280 | -- | |||||||||||||
| O. turanicus | 1 | 0 | 0 [0-100] | 0.00 | 0.6280 | -- | |||||||||||||
| O. turicata | 1 | 0 | 0 [0-100] | 0.00 | 0.6280 | -- | |||||||||||||
| O. venzualensis | 1 | 0 | 0 [0-100] | 0.00 | 0.6280 | -- | |||||||||||||
| R. pulchellus | 1 | 0 | 0 [0-100] | 0.00 | 0.6280 | -- | |||||||||||||
| R. sanguineus | 6 | 3 | 50.0 [2.78-97.22] | 5.55 | 0.6280 | 9.9 | |||||||||||||
| Parasite species | 0.5529 | 0.00 | 0.00 | ||||||||||||||||
| L. infantum | 2 | 1 | 50.0 [0-100] | 1.85 | 0.5302 | 46.0 | |||||||||||||
| L. kala azar£ | 2 | 0 | 0 [0-78.74] | 0.00 | 0.5302 | 0.0 | |||||||||||||
| Leishmania sp. | 1 | 1 | 100 [0-100] | 0.00 | 0.5302 | -- | |||||||||||||
| T. cruzi | 6 | 1 | 9.18 [0-61.17] | 3.08 | 0.5302 | 0.0 | |||||||||||||
| T. evansi | 6 | 3 | 50.0 [2.78-97.22] | 5.55 | 0.5302 | 9.9 | |||||||||||||
| T. lewisi | 1 | 0 | 0 [0-100] | 0.00 | 0.5302 | -- | |||||||||||||
| T. theileri | 3 | 1 | 27.86 [0-97.55] | 2.47 | 0.5302 | 18.9 | |||||||||||||
| T. theileri like | 1 | 1 | 100 [0-100] | 0.00 | 0.5302 | -- | |||||||||||||
| Vertical transmission | |||||||||||||||||||
| Ticks Family | 0.2809 | 0.00 | 0.00 | ||||||||||||||||
| Argasidae | 1 | 0 | 0 [0-100] | 0.00 | 0.4606 | -- | |||||||||||||
| Ixodidae | 12 | 7 | 61.27 [22.41-94.58] | 10;79 | 0.4606 | 0.0 | |||||||||||||
| Ticks Genus | 0.5422 | 0.00 | 0.00 | ||||||||||||||||
| Hyalomma | 2 | 1 | 50.0 [0-100] | 1.85 | 0.3787 | 46.0 | |||||||||||||
| Ornithodoros | 1 | 0 | 0 [0-100] | 0.00 | 0.3787 | -- | |||||||||||||
| Rhipicephalus | 10 | 6 | 63.49 [21.05-97.58] | 8.88 | 0.3787 | 0.0 | |||||||||||||
| Ticks species | 0.4394 | 0.00 | 0.00 | ||||||||||||||||
| H. a. anatolicum | 2 | 1 | 50.0 [0-100] | 1.85 | 0.4143 | 46.0 | |||||||||||||
| O. moubata | 1 | 0 | 0 [0-100] | 0.00 | 0.4143 | -- | |||||||||||||
| R. pulchellus | 1 | 0 | 0 [0-100] | 0.00 | 0.4143 | -- | |||||||||||||
| R. sanguineus | 9 | 7 | 72.14 [27.07-100] | 7.40 | 0.4143 | 0.0 | |||||||||||||
| Parasite genus | 0.0634 | 0.00 | 0.00 | ||||||||||||||||
| Leishmania | 8 | 7 | 82.18 [35.16-100] | 5.55 | 0.6668 | 0.0 | |||||||||||||
| Trypanosoma | 5 | 1 | 12.42 [0-70.74] | 2.96 | 0.6668 | 0.0 | |||||||||||||
| Donor host family | 0.0425* | 0.00 | 0.00 | ||||||||||||||||
| Bovidae | 3 | 1 | 27.86 [0-97.55] | 2.47 | 0.8446 | 18.9 | |||||||||||||
| Canidae | 7 | 6 | 92.94 [46.20-100] | 3.17 | 0.8446 | 0.0 | |||||||||||||
| Rodentia | 3 | 0 | 0 [0-61.92] | 0.00 | 0.8446 | 0.0 | |||||||||||||
| Receiver host family | 0.3582 | 0.00 | 0.00 | ||||||||||||||||
| Bovidae | 2 | 1 | 50.0 [0-100] | 1.85 | 0.4748 | 46.0 | |||||||||||||
| Canidae | 1 | 1 | 100 [0-100] | 0.00 | 0.4748 | -- | |||||||||||||
| ND | 4 | 3 | 82.18 [16.67-100] | 2.78 | 0.4748 | 0.0 | |||||||||||||
| Rodentia | 5 | 1 | 12.42 [0-70.74] | 2.69 | 0.4748 | 0.0 | |||||||||||||
| Ticks | 1 | 1 | 100 [0-100] | 0.00 | 0.4748 | -- | |||||||||||||
| Parasite species | 0.3655 | 0.00 | 0.00 | ||||||||||||||||
| L. chagasi (Syn L. infantum) | 2 | 1 | 50.0 [0-100] | 1.85 | 0.5046 | 46.0 | |||||||||||||
| L. infantum | 3 | 3 | 100 [38.08-100] | 0.00 | 0.5046 | 0.0 | |||||||||||||
| L. major | 1 | 0 | 0 [0-100] | 0.00 | 0.5046 | -- | |||||||||||||
| L. kala azar£ | 1 | 1 | 100 [0-100] | 0.00 | 0.5046 | -- | |||||||||||||
| Leishmania sp. | 1 | 1 | 100 [0-100] | 0.00 | 0.5046 | -- | |||||||||||||
| T. cruzi | 1 | 0 | 0 [0-100] | 0.00 | 0.5046 | -- | |||||||||||||
| T. evansi | 1 | 0 | 0 [0-100] | 0.00 | 0.5046 | -- | |||||||||||||
| T.theileri | 3 | 1 | 27.86 [0-97.55] | 2.47 | 0.5046 | 18.9 | |||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





