Submitted:
19 July 2024
Posted:
22 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Key Pathogenetic Mechanisms and Cell Populations in the Development of Pulmonary Fibrosis
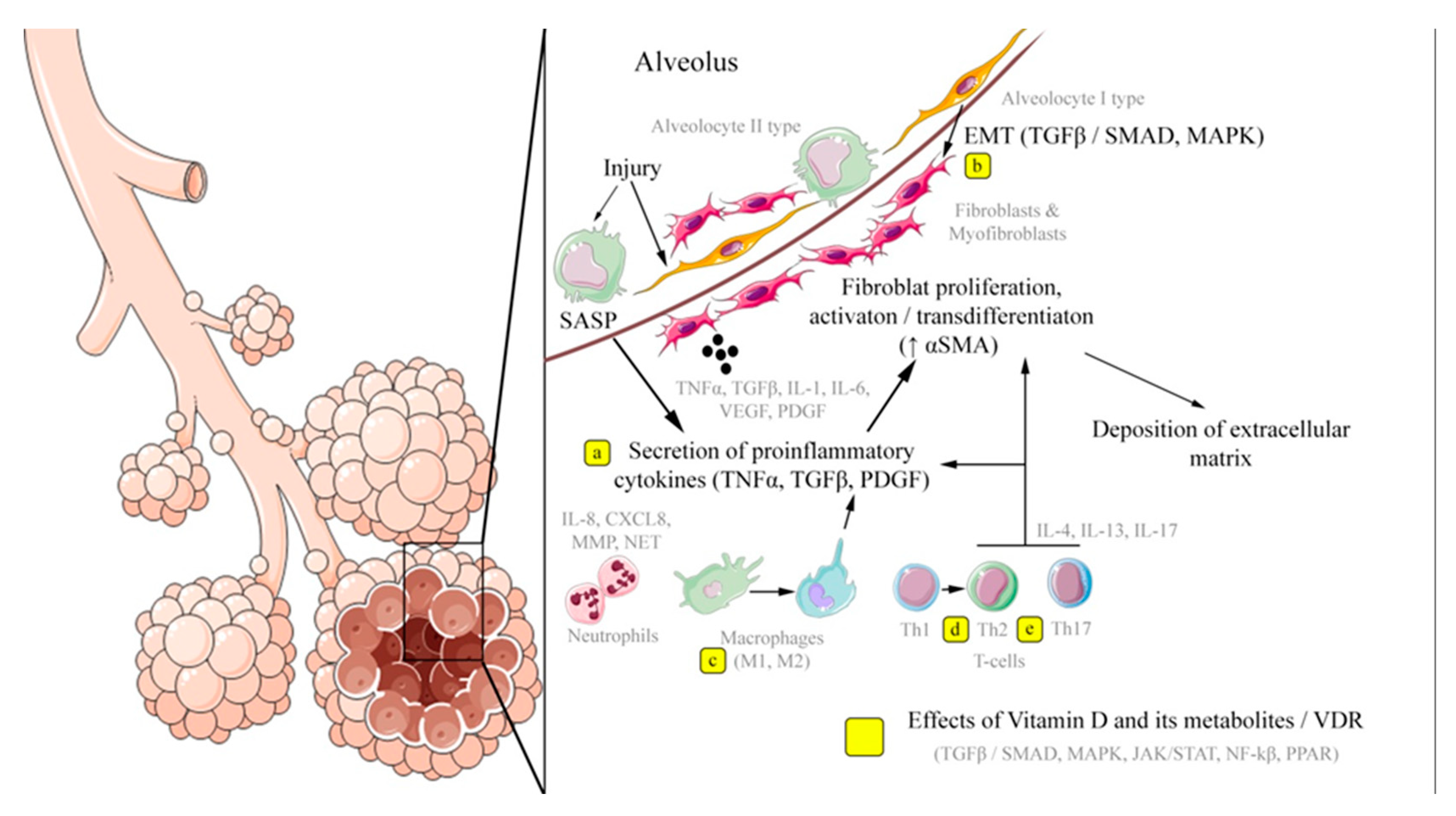
2.1. Alveolar Epithelium
2.2. Cells of the Stromal Microenvironment – Fibroblasts, Myofibroblasts and Vascular Endothelium
2.3. Immunocompetent Cells
2.4. Cell Aging and Apoptosis
3. Effects of Vitamin D and Its Analogues Implemented Through Ligand-Associated Activation of Vitamin D Receptors
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mei, Q.; Liu, Z.; Zuo, H.; Yang, Z.; Qu, J. Idiopathic Pulmonary Fibrosis: An Update on Pathogenesis. Front. Pharmacol. 2022, 12, 797292. [Google Scholar] [CrossRef] [PubMed]
- Glass, D.S.; Grossfeld, D.; Renna, H.A.; Agarwala, P.; Spiegler, P.; DeLeon, J.; Reiss, A.B. Idiopathic Pulmonary Fibrosis: Current and Future Treatment. Clinical Respiratory J. 2022, 16, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Cox, I.A.; Campbell, J.A.; Xia, Q.; Otahal, P.; De Graaff, B.; Corte, T.J.; Teoh, A.K.Y.; Walters, E.H.; Palmer, A.J. Mortality and Survival in Idiopathic Pulmonary Fibrosis: A Systematic Review and Meta-Analysis. ERJ Open Res. 2022, 8, 00591–02021. [Google Scholar] [CrossRef] [PubMed]
- Van Den Bosch, L.; Luppi, F.; Ferrara, G.; Mura, M. Immunomodulatory Treatment of Interstitial Lung Disease. Ther. Adv. Respir. Dis. 2022, 16, 175346662211170. [Google Scholar] [CrossRef] [PubMed]
- Kubo, H.; Nakayama, K.; Yanai, M.; Suzuki, T.; Yamaya, M.; Watanabe, M.; Sasaki, H. Anticoagulant Therapy for Idiopathic Pulmonary Fibrosis. Chest 2005, 128, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Pan, Y.; Xin, W.; Yan, C. The Potential Benefit of Endothelin Receptor Antagonists’ Therapy in Idiopathic Pulmonary Fibrosis: A Meta-Analysis of Results from Randomized Controlled Trials. Medicine 2022, 101, e29981. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Brown, K.K.; Costabel, U.; Cottin, V.; Du Bois, R.M.; Lasky, J.A.; Thomeer, M.; Utz, J.P.; Khandker, R.K.; McDermott, L.; et al. Treatment of Idiopathic Pulmonary Fibrosis with Etanercept: An Exploratory, Placebo-Controlled Trial. Am. J. Respir. Crit. Care Med. 2008, 178, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G. Idiopathic Pulmonary Fibrosis: Lessons from Clinical Trials over the Past 25 Years. Eur. Respir. J. 2017, 50, 1701209. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Wang, Z.; Zhu, H.; Liu, W.; Zhao, M.; Jiang, X.; Zhao, J.; Ren, C.; Zhang, Y.; Luo, L. Research Progress on Drugs Targeting the TGF-β Signaling Pathway in Fibrotic Diseases. Immunol. Res. 2022, 70, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Alkhatib, A.; Kolls, J.K.; Kondoh, Y.; Lasky, J.A. Pharmacotherapy and Adjunctive Treatment for Idiopathic Pulmonary Fibrosis (IPF). J. Thorac. Dis. 2019, 11, S1740–S1754. [Google Scholar] [CrossRef] [PubMed]
- Behr, J.; Prasse, A.; Kreuter, M.; Johow, J.; Rabe, K.F.; Bonella, F.; Bonnet, R.; Grohe, C.; Held, M.; Wilkens, H.; et al. Pirfenidone in Patients with Progressive Fibrotic Interstitial Lung Diseases Other than Idiopathic Pulmonary Fibrosis (RELIEF): A Double-Blind, Randomised, Placebo-Controlled, Phase 2b Trial. The Lancet Respiratory Medicine 2021, 9, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Cameli, P.; Alonzi, V.; d’Alessandro, M.; Bergantini, L.; Pordon, E.; Guerrieri, M.; Refini, R.M.; Sestini, P.; Bargagli, E. The Effectiveness of Nintedanib in Patients with Idiopathic Pulmonary Fibrosis, Familial Pulmonary Fibrosis and Progressive Fibrosing Interstitial Lung Diseases: A Real-World Study. Biomedicines 2022, 10, 1973. [Google Scholar] [CrossRef] [PubMed]
- Santos, G.; Fabiano, A.; Mota, P.C.; Rodrigues, I.; Carvalho, D.; Melo, N.; Novais-Bastos, H.; Alexandre, A.T.; Moura, C.S.; Guimarães, S.; et al. The Impact of Nintedanib and Pirfenidone on Lung Function and Survival in Patients with Idiopathic Pulmonary Fibrosis in Real-Life Setting. Pulmonary Pharmacology & Therapeutics 2023, 83, 102261. [Google Scholar] [CrossRef]
- Wolters, P.J.; Collard, H.R.; Jones, K.D. Pathogenesis of Idiopathic Pulmonary Fibrosis. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 157–179. [Google Scholar] [CrossRef] [PubMed]
- Jarzebska, N.; Karetnikova, E.S.; Markov, A.G.; Kasper, M.; Rodionov, R.N.; Spieth, P.M. Scarred Lung. An Update on Radiation-Induced Pulmonary Fibrosis. Front. Med. 2021, 7, 585756. [Google Scholar] [CrossRef] [PubMed]
- Bellou, V.; Belbasis, L.; Evangelou, E. Tobacco Smoking and Risk for Pulmonary Fibrosis. Chest 2021, 160, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Bae, W.; Lee, C.-H.; Lee, J.; Kim, Y.W.; Han, K.; Choi, S.M. Impact of Smoking on the Development of Idiopathic Pulmonary Fibrosis: Results from a Nationwide Population-Based Cohort Study. Thorax 2022, 77, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.-Y.; Kim, H.; Bae, Y.; Song, J.W. Smoking Status and Clinical Outcome in Idiopathic Pulmonary Fibrosis: A Nationwide Study. Respir. Res. 2024, 25, 191. [Google Scholar] [CrossRef] [PubMed]
- Sgalla, G.; Iovene, B.; Calvello, M.; Ori, M.; Varone, F.; Richeldi, L. Idiopathic Pulmonary Fibrosis: Pathogenesis and Management. Respir. Res. 2018, 19, 32. [Google Scholar] [CrossRef] [PubMed]
- Michalski, J.E.; Schwartz, D.A. Genetic Risk Factors for Idiopathic Pulmonary Fibrosis: Insights into Immunopathogenesis. JIR 2021, 13, 1305–1318. [Google Scholar] [CrossRef] [PubMed]
- McElroy, A.N.; Invernizzi, R.; Laskowska, J.W.; O’Neill, A.; Doroudian, M.; Moghoofei, M.; Mostafaei, S.; Li, F.; Przybylski, A.A.; O’Dwyer, D.N.; et al. Candidate Role for Toll-like Receptor 3 L412F Polymorphism and Infection in Acute Exacerbation of Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2022, 205, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Molyneaux, P.L.; Maher, T.M. The Role of Infection in the Pathogenesis of Idiopathic Pulmonary Fibrosis. European Respiratory Review 2013, 22, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Thannickal, V.J. The Aging Lung and Idiopathic Pulmonary Fibrosis. The American Journal of the Medical Sciences 2019, 357, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.V.; Schwartz, D.A. Epigenetics of Idiopathic Pulmonary Fibrosis. Translational Research 2015, 165, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Perera, U.E.; Derseh, H.B.; Dewage, S.N.V.; Stent, A.; Wijayarathna, R.; Snibson, K.J. Evaluation of microRNA Expression in a Sheep Model for Lung Fibrosis. BMC Genomics 2021, 22, 827. [Google Scholar] [CrossRef] [PubMed]
- Cadena-Suárez, A.R.; Hernández-Hernández, H.A.; Alvarado-Vásquez, N.; Rangel-Escareño, C.; Sommer, B.; Negrete-García, M.C. Role of MicroRNAs in Signaling Pathways Associated with the Pathogenesis of Idiopathic Pulmonary Fibrosis: A Focus on Epithelial-Mesenchymal Transition. IJMS 2022, 23, 6613. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Friggeri, A.; Yang, Y.; Milosevic, J.; Ding, Q.; Thannickal, V.J.; Kaminski, N.; Abraham, E. miR-21 Mediates Fibrogenic Activation of Pulmonary Fibroblasts and Lung Fibrosis. Journal of Experimental Medicine 2010, 207, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Moimas, S.; Salton, F.; Kosmider, B.; Ring, N.; Volpe, M.C.; Bahmed, K.; Braga, L.; Rehman, M.; Vodret, S.; Graziani, M.L.; et al. miR-200 Family Members Reduce Senescence and Restore Idiopathic Pulmonary Fibrosis Type II Alveolar Epithelial Cell Transdifferentiation. ERJ Open Res. 2019, 5, 00138–02019. [Google Scholar] [CrossRef] [PubMed]
- Guiot, J.; Henket, M.; Remacle, C.; Cambier, M.; Struman, I.; Winandy, M.; Moermans, C.; Louis, E.; Malaise, M.; Ribbens, C.; et al. Systematic Review of Overlapping microRNA Patterns in COVID-19 and Idiopathic Pulmonary Fibrosis. Respir. Res. 2023, 24, 112. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Su, Y.; Hsia, I.; Xu, Y.; Vincent-Chong, V.K.; Mojica, W.; Seshadri, M.; Zhao, R.; Wu, Y. Delivery of Anti-microRNA-21 by Lung-Targeted Liposomes for Pulmonary Fibrosis Treatment. Molecular Therapy - Nucleic Acids 2023, 32, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Parimon, T.; Yao, C.; Stripp, B.R.; Noble, P.W.; Chen, P. Alveolar Epithelial Type II Cells as Drivers of Lung Fibrosis in Idiopathic Pulmonary Fibrosis. IJMS 2020, 21, 2269. [Google Scholar] [CrossRef] [PubMed]
- Selman, M. Role of Epithelial Cells in Idiopathic Pulmonary Fibrosis: From Innocent Targets to Serial Killers. Proceedings of the American Thoracic Society 2006, 3, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Adams, T.S.; Schupp, J.C.; Poli, S.; Ayaub, E.A.; Neumark, N.; Ahangari, F.; Chu, S.G.; Raby, B.A.; DeIuliis, G.; Januszyk, M.; et al. Single-Cell RNA-Seq Reveals Ectopic and Aberrant Lung-Resident Cell Populations in Idiopathic Pulmonary Fibrosis. Sci. Adv. 2020, 6, eaba1983. [Google Scholar] [CrossRef] [PubMed]
- Kathiriya, J.J.; Wang, C.; Zhou, M.; Brumwell, A.; Cassandras, M.; Le Saux, C.J.; Cohen, M.; Alysandratos, K.-D.; Wang, B.; Wolters, P.; et al. Human Alveolar Type 2 Epithelium Transdifferentiates into Metaplastic KRT5+ Basal Cells. Nat. Cell. Biol. 2022, 24, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, T.; Zissel, G.; Watz, H.; Drömann, D.; Reck, M.; Kugler, C.; Rabe, K.F.; Marwitz, S. Human Alveolar Epithelial Cells Type II Are Capable of TGFβ-Dependent Epithelial-Mesenchymal-Transition and Collagen-Synthesis. Respir. Res. 2018, 19, 138. [Google Scholar] [CrossRef] [PubMed]
- Homps-Legrand, M.; Crestani, B.; Mailleux, A.A. Origins of Pathological Myofibroblasts in Lung Fibrosis: Insights from Lineage Tracing Mouse Models in the Single-Cell RNA Sequencing Era. American Journal of Physiology-Lung Cellular and Molecular Physiology 2023, 324, L737–L746. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Hu, Y. TGF β1: Gentlemanly Orchestrator in Idiopathic Pulmonary Fibrosis (Review). Int. J. Mol. Med. 2021, 48, 132. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.W.; Herzog, E.L. Regulation and Relevance of Myofibroblast Responses in Idiopathic Pulmonary Fibrosis. Curr. Pathobiol. Rep. 2013, 1, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Decaris, M.L.; Schaub, J.R.; Chen, C.; Cha, J.; Lee, G.G.; Rexhepaj, M.; Ho, S.S.; Rao, V.; Marlow, M.M.; Kotak, P.; et al. Dual Inhibition of Avβ6 and Avβ1 Reduces Fibrogenesis in Lung Tissue Explants from Patients with IPF. Respir. Res. 2021, 22, 265. [Google Scholar] [CrossRef] [PubMed]
- Bahram Yazdroudi, F.; Malek, A. Optimal Controlling of Anti-TGF-β and Anti-PDGF Medicines for Preventing Pulmonary Fibrosis. Sci. Rep. 2023, 13, 15073. [Google Scholar] [CrossRef]
- Kim, K.K.; Kugler, M.C.; Wolters, P.J.; Robillard, L.; Galvez, M.G.; Brumwell, A.N.; Sheppard, D.; Chapman, H.A. Alveolar Epithelial Cell Mesenchymal Transition Develops in Vivo during Pulmonary Fibrosis and Is Regulated by the Extracellular Matrix. Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 13180–13185. [Google Scholar] [CrossRef] [PubMed]
- Chambers, R.C. Procoagulant Signalling Mechanisms in Lung Inflammation and Fibrosis: Novel Opportunities for Pharmacological Intervention? Pharmacology 2008, 153. [Google Scholar] [CrossRef] [PubMed]
- May, J.; Mitchell, J.A.; Jenkins, R.G. Beyond Epithelial Damage: Vascular and Endothelial Contributions to Idiopathic Pulmonary Fibrosis. Journal of Clinical Investigation 2023, 133, e172058. [Google Scholar] [CrossRef] [PubMed]
- Murray, L.A.; Habiel, D.M.; Hohmann, M.; Camelo, A.; Shang, H.; Zhou, Y.; Coelho, A.L.; Peng, X.; Gulati, M.; Crestani, B.; et al. Antifibrotic Role of Vascular Endothelial Growth Factor in Pulmonary Fibrosis. JCI Insight 2017, 2, e92192. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, N.; Phan, S.H.; Imaizumi, K.; Matsuo, M.; Nakashima, H.; Kawabe, T.; Shimokata, K.; Hasegawa, Y. Endothelial–Mesenchymal Transition in Bleomycin-Induced Pulmonary Fibrosis. Am. J. Respir. Cell. Mol. Biol. 2010, 43, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Xaubet, A.; Agustí, C.; Luburich, P.; Barberá, J.A.; Carrión, M.; Ayuso, M.C.; Roca, J.; Rodriguez-Roisin, R. Interleukin-8 Expression in Bronchoalveolar Lavage Cells in the Evaluation of Alveolitis in Idiopathic Pulmonary Fibrosis. Respiratory Medicine 1998, 92, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Yang, J.; Zhang, C.; Zhang, X.; Gao, P. Neutrophils Modulate Fibrogenesis in Chronic Pulmonary Diseases. Front. Med. 2021, 8, 616200. [Google Scholar] [CrossRef] [PubMed]
- Chrysanthopoulou, A.; Mitroulis, I.; Apostolidou, E.; Arelaki, S.; Mikroulis, D.; Konstantinidis, T.; Sivridis, E.; Koffa, M.; Giatromanolaki, A.; Boumpas, D.T.; et al. Neutrophil Extracellular Traps Promote Differentiation and Function of Fibroblasts. The Journal of Pathology 2014, 233, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Pokhreal, D.; Crestani, B.; Helou, D.G. Macrophage Implication in IPF: Updates on Immune, Epigenetic, and Metabolic Pathways. Cells 2023, 12, 2193. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Tarique, A.A.; Logan, J.; Thomas, E.; Holt, P.G.; Sly, P.D.; Fantino, E. Phenotypic, Functional, and Plasticity Features of Classical and Alternatively Activated Human Macrophages. Am. J. Respir. Cell. Mol. Biol. 2015, 53, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Wu, G.; Xiong, W.; Gu, W.; Wang, C.-Y. Macrophages: Friend or Foe in Idiopathic Pulmonary Fibrosis? Respir. Res. 2018, 19, 170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tian, H.; Liu, H.; Xie, R. The Role of Macrophage-Derived TGF-Β1 on SiO2-Induced Pulmonary Fibrosis: A Review. Toxicol. Ind. Health 2021, 37, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Valencia, S.; Zhou, B. Roles of Macrophages and Endothelial Cells and Their Crosstalk in Acute Lung Injury. Biomedicines 2024, 12, 632. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Jian, Z.; Xu, T.; Li, F.; Deng, H.; Zhou, Y.; Lai, S.; Xu, Z.; Zhu, L. Macrophage Polarization: An Important Candidate Regulator for Lung Diseases. Molecules 2023, 28, 2379. [Google Scholar] [CrossRef] [PubMed]
- Kass, D.J.; Yu, G.; Loh, K.S.; Savir, A.; Borczuk, A.; Kahloon, R.; Juan-Guardela, B.; Deiuliis, G.; Tedrow, J.; Choi, J.; et al. Cytokine-Like Factor 1 Gene Expression Is Enriched in Idiopathic Pulmonary Fibrosis and Drives the Accumulation of CD4+ T Cells in Murine Lungs. The American Journal of Pathology 2012, 180, 1963–1978. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.A.; McKenzie, A.N.J. TH2 Cell Development and Function. Nat. Rev. Immunol. 2018, 18, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Huang, T.; Zhang, L. Correction to: T Cells in Idiopathic Pulmonary Fibrosis: Crucial but Controversial. Cell Death Discov. 2023, 9, 74. [Google Scholar] [CrossRef]
- Qu, X.; Yi, X.; Zhong, H.; Ruan, W.; Huang, D. Effect and Mechanism of Imbalance via Th9 Cells and Th17/Treg Cells in Inflammatory and Fibrotic Phases of Pulmonary Fibrosis in Mice. Biotechnology and Genetic Engineering Reviews 2023, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Segawa, S.; Goto, D.; Iizuka, A.; Kaneko, S.; Yokosawa, M.; Kondo, Y.; Matsumoto, I.; Sumida, T. The Regulatory Role of Interferon-γ Producing Gamma Delta T Cells via the Suppression of T Helper 17 Cell Activity in Bleomycin-Induced Pulmonary Fibrosis. Clinical and Experimental Immunology 2016, 185, 348–360. [Google Scholar] [CrossRef]
- Nie, Y.-J.; Wu, S.-H.; Xuan, Y.-H.; Yan, G. Role of IL-17 Family Cytokines in the Progression of IPF from Inflammation to Fibrosis. Military Med. Res. 2022, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Ramani, K.; Biswas, P.S. Interleukin-17: Friend or Foe in Organ Fibrosis. Cytokine 2019, 120, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Mi, S.; Li, Z.; Yang, H.-Z.; Liu, H.; Wang, J.-P.; Ma, Y.-G.; Wang, X.-X.; Liu, H.-Z.; Sun, W.; Hu, Z.-W. Blocking IL-17A Promotes the Resolution of Pulmonary Inflammation and Fibrosis Via TGF-Β1–Dependent and –Independent Mechanisms. The Journal of Immunology 2011, 187, 3003–3014. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Huang, M.; Yao, Y. Biology of Interleukin-17 and Its Pathophysiological Significance in Sepsis. Front. Immunol. 2020, 11, 1558. [Google Scholar] [CrossRef] [PubMed]
- Cipolla, E.; Fisher, A.J.; Gu, H.; Mickler, E.A.; Agarwal, M.; Wilke, C.A.; Kim, K.K.; Moore, B.B.; Vittal, R. IL-17A Deficiency Mitigates Bleomycin-induced Complement Activation during Lung Fibrosis. The FASEB Journal 2017, 31, 5543–5556. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.S.; Madala, S.K.; Ramalingam, T.R.; Gochuico, B.R.; Rosas, I.O.; Cheever, A.W.; Wynn, T.A. Bleomycin and IL-1β–Mediated Pulmonary Fibrosis Is IL-17A Dependent. Journal of Experimental Medicine 2010, 207, 535–552. [Google Scholar] [CrossRef] [PubMed]
- Martinu, T.; McManigle, W.C.; Kelly, F.L.; Nelson, M.E.; Sun, J.; Zhang, H.L.; Kolls, J.K.; Gowdy, K.M.; Palmer, S.M. IL-17A Contributes to Lung Fibrosis in a Model of Chronic Pulmonary Graft-versus-Host Disease. Transplantation 2019, 103, 2264–2274. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, R.J.J.; Knight, D.A.; Richards, C.D.; Prêle, C.M.; Lau, H.L.; Jarnicki, A.G.; Jones, J.; Bozinovski, S.; Vlahos, R.; Thiem, S.; et al. Genetic Partitioning of Interleukin-6 Signalling in Mice Dissociates Stat3 from Smad3-mediated Lung Fibrosis. EMBO Mol. Med. 2012, 4, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Roman, J.; Chiba, H. B Cells in Idiopathic Pulmonary Fibrosis: Targeting Immune Cells with Antifibrotic Agents. Am. J. Respir. Cell. Mol. Biol. 2021, 64, 652–654. [Google Scholar] [CrossRef] [PubMed]
- Prêle, C.M.; Miles, T.; Pearce, D.R.; O’Donoghue, R.J.; Grainge, C.; Barrett, L.; Birnie, K.; Lucas, A.D.; Baltic, S.; Ernst, M.; et al. Plasma Cell but Not CD20-Mediated B-Cell Depletion Protects from Bleomycin-Induced Lung Fibrosis. Eur. Respir. J. 2022, 60, 2101469. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Gonzalez, F.; Faner, R.; Rojas, M.; Agustí, A.; Serrano, M.; Sellarés, J. Cellular Senescence in Lung Fibrosis. IJMS 2021, 22, 7012. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Ding, Q.; Lin, Z.; Chen, X.; Chen, S.; Zhu, Y. New Insights of Epigenetics in Vascular and Cellular Senescence. Journal of Translational Internal Medicine 2021, 9, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Shreeya, T.; Ansari, M.S.; Kumar, P.; Saifi, M.; Shati, A.A.; Alfaifi, M.Y.; Elbehairi, S.E.I. Senescence: A DNA Damage Response and Its Role in Aging and Neurodegenerative Diseases. Front. Aging 2024, 4, 1292053. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Lu, Q.; Liu, X. Advances in Cellular Senescence in Idiopathic Pulmonary Fibrosis (Review). Exp. Ther. Med. 2023, 25, 145. [Google Scholar] [CrossRef] [PubMed]
- Moodley, Y.P.; Misso, N.L.A.; Scaffidi, A.K.; Fogel-Petrovic, M.; McAnulty, R.J.; Laurent, G.J.; Thompson, P.J.; Knight, D.A. Inverse Effects of Interleukin-6 on Apoptosis of Fibroblasts from Pulmonary Fibrosis and Normal Lungs. Am. J. Respir. Cell. Mol. Biol. 2003, 29, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Ng, B.; Cook, S.A.; Schafer, S. Interleukin-11 Signaling Underlies Fibrosis, Parenchymal Dysfunction, and Chronic Inflammation of the Airway. Exp. Mol. Med. 2020, 52, 1871–1878. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Guan, X.; Carraro, G.; Parimon, T.; Liu, X.; Huang, G.; Mulay, A.; Soukiasian, H.J.; David, G.; Weigt, S.S.; et al. Senescence of Alveolar Type 2 Cells Drives Progressive Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2021, 203, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Rana, T.; Jiang, C.; Banerjee, S.; Yi, N.; Zmijewski, J.W.; Liu, G.; Liu, R.-M. PAI-1 Regulation of P53 Expression and Senescence in Type II Alveolar Epithelial Cells. Cells 2023, 12, 2008. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xu, Z. Fibroblast Senescence in Idiopathic Pulmonary Fibrosis. Front. Cell Dev. Biol. 2020, 8, 593283. [Google Scholar] [CrossRef]
- Yanai, H.; Shteinberg, A.; Porat, Z.; Budovsky, A.; Braiman, A.; Zeische, R.; Fraifeld, V.E. Cellular Senescence-like Features of Lung Fibroblasts Derived from Idiopathic Pulmonary Fibrosis Patients. Aging 2015, 7, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Weycker, D.; Edelsberg, J.; Bradford, W.Z.; Oster, G. Incidence and Prevalence of Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2006, 174, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Asai, A.; Kawagishi, H.; Mikawa, R.; Iwashita, Y.; Kanayama, K.; Sugimoto, K.; Sato, T.; Maruyama, M.; Sugimoto, M. Elimination of p19ARF-Expressing Cells Enhances Pulmonary Function in Mice. JCI Insight 2016, 1. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.; Korfei, M.; Mutze, K.; Klee, S.; Skronska-Wasek, W.; Alsafadi, H.N.; Ota, C.; Costa, R.; Schiller, H.B.; Lindner, M.; et al. Senolytic Drugs Target Alveolar Epithelial Cell Function and Attenuate Experimental Lung Fibrosis Ex Vivo. Eur. Respir. J. 2017, 50, 1602367. [Google Scholar] [CrossRef] [PubMed]
- Justice, J.N.; Nambiar, A.M.; Tchkonia, T.; LeBrasseur, N.K.; Pascual, R.; Hashmi, S.K.; Prata, L.; Masternak, M.M.; Kritchevsky, S.B.; Musi, N.; et al. Senolytics in Idiopathic Pulmonary Fibrosis: Results from a First-in-Human, Open-Label, Pilot Study. EBioMedicine 2019, 40, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, A.; Kellogg, D.; Justice, J.; Goros, M.; Gelfond, J.; Pascual, R.; Hashmi, S.; Masternak, M.; Prata, L.; LeBrasseur, N.; et al. Senolytics Dasatinib and Quercetin in Idiopathic Pulmonary Fibrosis: Results of a Phase I, Single-Blind, Single-Center, Randomized, Placebo-Controlled Pilot Trial on Feasibility and Tolerability. eBioMedicine 2023, 90, 104481. [Google Scholar] [CrossRef] [PubMed]
- Thannickal, V.J. Evolving Concepts of Apoptosis in Idiopathic Pulmonary Fibrosis. Proceedings of the American Thoracic Society 2006, 3, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Hanson, K.M.; Hernady, E.B.; Reed, C.K.; Johnston, C.J.; Groves, A.M.; Finkelstein, J.N. Apoptosis Resistance in Fibroblasts Precedes Progressive Scarring in Pulmonary Fibrosis and Is Partially Mediated by Toll-Like Receptor 4 Activation. Toxicological Sciences 2019, 170, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhang, K.; Jiang, S.; Fu, L.; Shi, Y.; Tan, R.; Cui, J.; Zhou, Y. P53: A Key Protein That Regulates Pulmonary Fibrosis. Oxidative Medicine and Cellular Longevity 2020, 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
- The Role of Apoptosis in Pulmonary Fibrosis. Histology and Histopathology 2004, 867–881. [CrossRef]
- Tzouvelekis, A.; Harokopos, V.; Paparountas, T.; Oikonomou, N.; Chatziioannou, A.; Vilaras, G.; Tsiambas, E.; Karameris, A.; Bouros, D.; Aidinis, V. Comparative Expression Profiling in Pulmonary Fibrosis Suggests a Role of Hypoxia-Inducible Factor-1α in Disease Pathogenesis. Am. J. Respir. Crit. Care Med. 2007, 176, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, M.S.; Habiel, D.M.; Coelho, A.L.; Verri, W.A.; Hogaboam, C.M. Quercetin Enhances Ligand-Induced Apoptosis in Senescent Idiopathic Pulmonary Fibrosis Fibroblasts and Reduces Lung Fibrosis In Vivo. Am. J. Respir. Cell Mol. Biol. 2019, 60, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Liu, G.; Cai, L.; Deshane, J.; Antony, V.; Thannickal, V.J.; Liu, R.-M. Divergent Regulation of Alveolar Type 2 Cell and Fibroblast Apoptosis by Plasminogen Activator Inhibitor 1 in Lung Fibrosis. The American Journal of Pathology 2021, 191, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Disayabutr, S.; Kim, E.K.; Cha, S.-I.; Green, G.; Naikawadi, R.P.; Jones, K.D.; Golden, J.A.; Schroeder, A.; Matthay, M.A.; Kukreja, J.; et al. miR-34 miRNAs Regulate Cellular Senescence in Type II Alveolar Epithelial Cells of Patients with Idiopathic Pulmonary Fibrosis. PLoS ONE 2016, 11, e0158367. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Ge, J.; Xie, N.; Banerjee, S.; Zhou, Y.; Liu, R.-M.; Thannickal, V.J.; Liu, G. miR-34a Promotes Fibrosis in Aged Lungs by Inducing Alveolarepithelial Dysfunctions. American Journal of Physiology-Lung Cellular and Molecular Physiology 2017, 312, L415–L424. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P.; Grant, W.B.; Konstantynowicz, J.; Holick, M.F. Editorial: Classic and Pleiotropic Actions of Vitamin D. Front. Endocrinol. 2019, 10, 341. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.-G.; Kerlan, V.; Desailloud, R. Non-Classical Effects of Vitamin D: Non-Bone Effects of Vitamin D. Annales d’Endocrinologie 2021, 82, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C. Vitamin D and Its Target Genes. Nutrients 2022, 14, 1354. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Singer, F.R.; Gacad, M.A.; Sharma, O.P.; Hayes, M.J.; Vouros, P.; Holick, M.F. Isolation and Structural Identification of 1,25- Dihydroxyvitamin D3 Produced by Cultured Alveolar Macrophages in Sarcoidosis. The Journal of Clinical Endocrinology & Metabolism 1985, 60, 960–966. [Google Scholar] [CrossRef]
- Menezes, R.J.; Cheney, R.T.; Husain, A.; Tretiakova, M.; Loewen, G.; Johnson, C.S.; Jayaprakash, V.; Moysich, K.B.; Salgia, R.; Reid, M.E. Vitamin D Receptor Expression in Normal, Premalignant, and Malignant Human Lung Tissue. Cancer Epidemiology, Biomarkers & Prevention 2008, 17, 1104–1110. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Arora, J. Two Lineages of Immune Cells That Differentially Express the Vitamin D Receptor. The Journal of Steroid Biochemistry and Molecular Biology 2023, 228, 106253. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.-K.; Li, W.; Postlethwaite, A.; Tieu, E.W.; Tang, E.K.Y.; Tuckey, R.C. Detection of Novel CYP11A1-Derived Secosteroids in the Human Epidermis and Serum and Pig Adrenal Gland. Sci. Rep. 2015, 5, 14875. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Marepally, S.R.; Goh, E.S.Y.; Cheng, C.Y.S.; Janjetovic, Z.; Kim, T.-K.; Miller, D.D.; Postlethwaite, A.E.; Slominski, A.T.; Tuckey, R.C.; et al. Investigation of 20S-Hydroxyvitamin D3 Analogs and Their 1α-OH Derivatives as Potent Vitamin D Receptor Agonists with Anti-Inflammatory Activities. Sci. Rep. 2018, 8, 1478. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ramirez, J.; Han, J.; Jia, Y.; Domenico, J.; Seibold, M.A.; Hagman, J.R.; Gelfand, E.W. The Steroidogenic Enzyme Cyp11a1 Is Essential for Development of Peanut-Induced Intestinal Anaphylaxis. Journal of Allergy and Clinical Immunology 2013, 132, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.-K.; Hobrath, J.V.; Oak, A.S.W.; Tang, E.K.Y.; Tieu, E.W.; Li, W.; Tuckey, R.C.; Jetten, A.M. Endogenously Produced Nonclassical Vitamin D Hydroxy-Metabolites Act as “Biased” Agonists on VDR and Inverse Agonists on RORα and RORγ. The Journal of Steroid Biochemistry and Molecular Biology 2017, 173, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.-K.; Qayyum, S.; Song, Y.; Janjetovic, Z.; Oak, A.S.W.; Slominski, R.M.; Raman, C.; Stefan, J.; Mier-Aguilar, C.A.; et al. Vitamin D and Lumisterol Derivatives Can Act on Liver X Receptors (LXRs). Sci. Rep. 2021, 11, 8002. [Google Scholar] [CrossRef] [PubMed]
- Ito, I.; Waku, T.; Aoki, M.; Abe, R.; Nagai, Y.; Watanabe, T.; Nakajima, Y.; Ohkido, I.; Yokoyama, K.; Miyachi, H.; et al. A Nonclassical Vitamin D Receptor Pathway Suppresses Renal Fibrosis. J. Clin. Invest. 2013, 123, 4579–4594. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Ding, Q.; Wang, L.; Xu, G.; Diao, Y.; Qu, S.; Chen, S.; Shi, Y. Vitamin D3 Alleviates Pulmonary Fibrosis by Regulating the MAPK Pathway via Targeting PSAT1 Expression in Vivo and in Vitro. International Immunopharmacology 2021, 101, 108212. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, J.; Ge, X.; Du, J.; Deb, D.K.; Li, Y.C. Vitamin D Receptor Inhibits Nuclear Factor κB Activation by Interacting with IκB Kinase β Protein. Journal of Biological Chemistry 2013, 288, 19450–19458. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.M.; Gouttenoire, J.; Duong, F.H.T.; Morikawa, K.; Heim, M.H.; Moradpour, D. Vitamin D Receptor and Jak–STAT Signaling Crosstalk Results in Calcitriol-Mediated Increase of Hepatocellular Response to IFN-α. The Journal of Immunology 2014, 192, 6037–6044. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Kitagishi, Y. Peroxisome Proliferator-Activated Receptor and Vitamin D Receptor Signaling Pathways in Cancer Cells. Cancers 2013, 5, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Hu, X.; Qi, X.; Zhu, R.; Li, C.; Zhu, Y.; Yin, S.; Cheng, L.; Zhu, R. Vitamin D Ameliorates Angiotensin II-Induced Human Endothelial Progenitor Cell Injury via the PPAR-γ/HO-1 Pathway. J. Vasc. Res. 2019, 56, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Nieuwland, A.J.; Kokje, V.B.; Koning, O.H.; Hamming, J.F.; Szuhai, K.; Claas, F.H.J.; Lindeman, J.H.N. Activation of the Vitamin D Receptor Selectively Interferes with Calcineurin-Mediated Inflammation: A Clinical Evaluation in the Abdominal Aortic Aneurysm. Laboratory Investigation 2016, 96, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Deng, W.; Yang, Y.; Wei, S.; Xue, L.; Tao, S. Pharmaceutic Application of Vitamin D3 on Particle-Induced Fibrotic Effects through Induction of Nrf2 Signals. Toxicology Research 2020, 9, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Nie, H.; Ge, X.; Du, J.; Liu, W.; Li, X.; Sun, Y.; Wei, X.; Xun, Z.; Li, Y.C. Vitamin D Suppresses Bleomycin-Induced Pulmonary Fibrosis by Targeting the Local Renin–Angiotensin System in the Lung. Sci. Rep. 2021, 11, 16525. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, T.; Yao, L.; Xing, Y.; Zhao, X.; Fu, J.; Xue, X. Chronic Vitamin D Deficiency Induces Lung Fibrosis through Activation of the Renin-Angiotensin System. Sci. Rep. 2017, 7, 3312. [Google Scholar] [CrossRef] [PubMed]
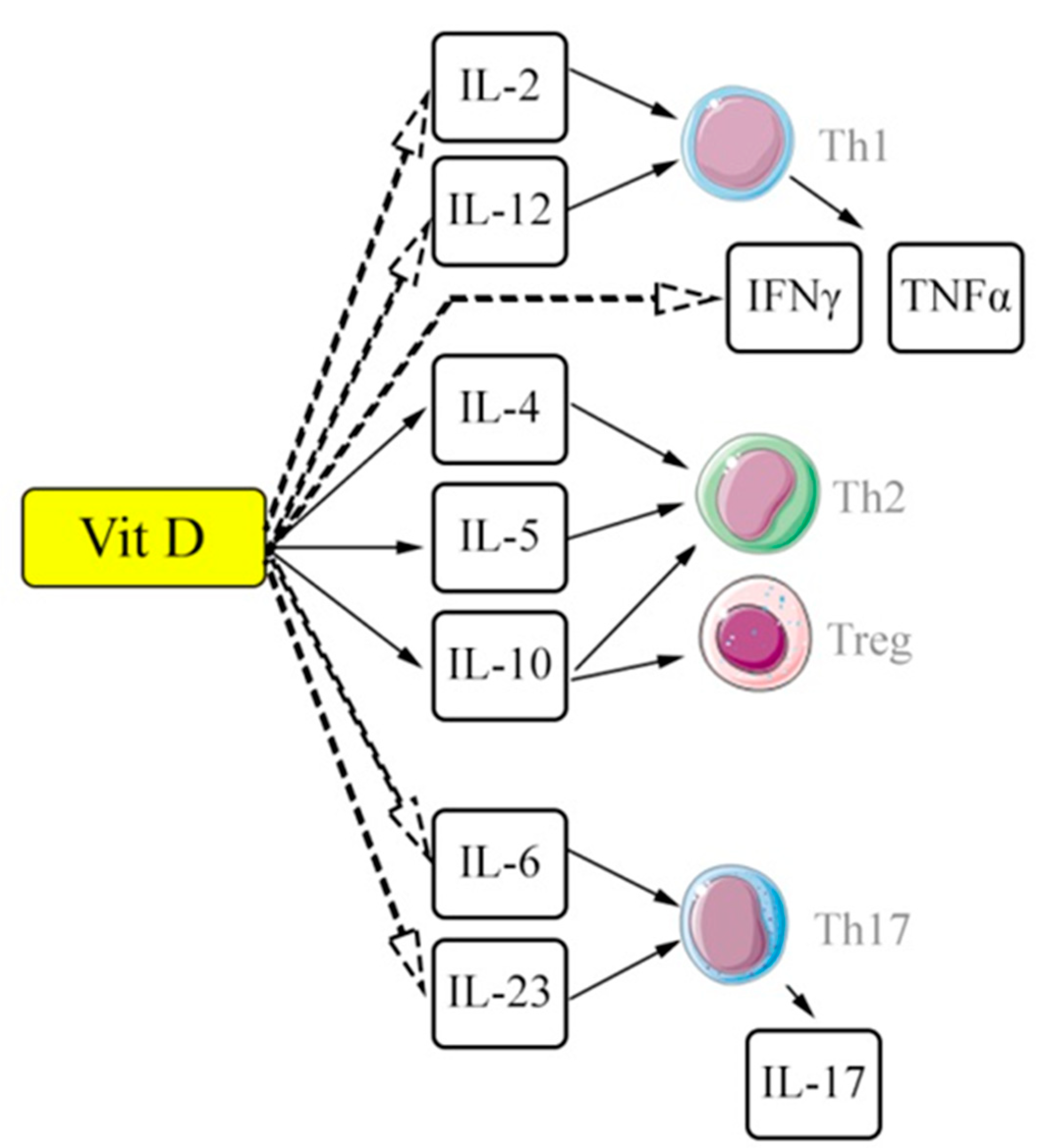
- Boonstra, A.; Barrat, F.J.; Crain, C.; Heath, V.L.; Savelkoul, H.F.J.; O’Garra, A. 1α,25-Dihydroxyvitamin D3 Has a Direct Effect on Naive CD4+ T Cells to Enhance the Development of Th2 Cells. The Journal of Immunology 2001, 167, 4974–4980. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.A.; Rahimzadeh, M.; Brierley, C.; Gration, B.; Doree, C.; Kimber, C.E.; Plaza Cajide, A.; Lamikanra, A.A.; Roberts, D.J. The Role of Vitamin D in Increasing Circulating T Regulatory Cell Numbers and Modulating T Regulatory Cell Phenotypes in Patients with Inflammatory Disease or in Healthy Volunteers: A Systematic Review. PLoS ONE 2019, 14, e0222313. [Google Scholar] [CrossRef] [PubMed]
- Barron, L.; Wynn, T.A. Fibrosis Is Regulated by Th2 and Th17 Responses and by Dynamic Interactions between Fibroblasts and Macrophages. American Journal of Physiology-Gastrointestinal and Liver Physiology 2011, 300, G723–G728. [Google Scholar] [CrossRef]
- Joshi, S.; Pantalena, L.-C.; Liu, X.K.; Gaffen, S.L.; Liu, H.; Rohowsky-Kochan, C.; Ichiyama, K.; Yoshimura, A.; Steinman, L.; Christakos, S.; et al. 1,25-Dihydroxyvitamin D 3 Ameliorates Th17 Autoimmunity via Transcriptional Modulation of Interleukin-17A. Molecular and Cellular Biology 2011, 31, 3653–3669. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Chung, S.I.; Park, H.J.; Oh, E.Y.; Kim, S.-R.; Park, K.H.; Lee, J.-H.; Park, J.-W. Obesity-Induced Vitamin D Deficiency Contributes to Lung Fibrosis and Airway Hyperresponsiveness. Am. J. Respir. Cell. Mol. Biol. 2021, 64, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.M.; Wongtrakool, C.; Welch, T.; Steinmeyer, A.; Zügel, U.; Roman, J. Vitamin D Inhibition of Pro-Fibrotic Effects of Transforming Growth Factor Β1 in Lung Fibroblasts and Epithelial Cells. The Journal of Steroid Biochemistry and Molecular Biology 2010, 118, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhan, J.; Ji, H.; Xu, Y.; Xu, Q.; Zhu, X.; Liu, Y. Fibroblast Upregulation of Vitamin D Receptor Represents a Self-Protective Response to Limit Fibroblast Proliferation and Activation during Pulmonary Fibrosis. Antioxidants 2023, 12, 1634. [Google Scholar] [CrossRef] [PubMed]
- Guijarro, T.; Magro-Lopez, E.; Manso, J.; Garcia-Martinez, R.; Fernandez-Aceñero, M.J.; Liste, I.; Zambrano, A. Detrimental Pro-Senescence Effects of Vitamin D on Lung Fibrosis. Mol. Med. 2018, 24, 64. [Google Scholar] [CrossRef] [PubMed]
- Magro-Lopez, E.; Chamorro-Herrero, I.; Zambrano, A. Effects of Hypocalcemic Vitamin D Analogs in the Expression of DNA Damage Induced in Minilungs from hESCs: Implications for Lung Fibrosis. IJMS 2022, 23, 4921. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Yang, Y.; Xue, L.; Li, B.; Zhang, Z. 1α,25-Dihydroxyvitamin D3 Attenuates TGF-β-Induced Pro-Fibrotic Effects in Human Lung Epithelial Cells through Inhibition of Epithelial–Mesenchymal Transition. Nutrients 2017, 9, 980. [Google Scholar] [CrossRef] [PubMed]
- Sari, E.; Oztay, F.; Tasci, A.E. Vitamin D Modulates E-Cadherin Turnover by Regulating TGF-β and Wnt Signalings during EMT-Mediated Myofibroblast Differentiation in A459 Cells. The Journal of Steroid Biochemistry and Molecular Biology 2020, 202, 105723. [Google Scholar] [CrossRef] [PubMed]
- Izbicki, G.; Segel, M.J.; Christensen, T.G.; Conner, M.W.; Breuer, R. Time Course of Bleomycin-induced Lung Fibrosis. Int. J. Experimental Path. 2002, 83, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Gul, A.; Yang, F.; Xie, C.; Du, W.; Mohammadtursun, N.; Wang, B.; Le, J.; Dong, J. Pulmonary Fibrosis Model of Mice Induced by Different Administration Methods of Bleomycin. BMC Pulm. Med. 2023, 23, 91. [Google Scholar] [CrossRef] [PubMed]
- Robbe, A.; Tassin, A.; Carpentier, J.; Declèves, A.-E.; Mekinda Ngono, Z.L.; Nonclercq, D.; Legrand, A. Intratracheal Bleomycin Aerosolization: The Best Route of Administration for a Scalable and Homogeneous Pulmonary Fibrosis Rat Model? BioMed Research International 2015, 2015, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Abidi, A.; Bahri, S.; Ben Khamsa, S.; Legrand, A. A Comparative Study of Intratracheal and Aerosolization Instillations of Bleomycin Inducing Experimental Lung Fibrosis in Rat. Toxicology Mechanisms and Methods 2019, 29, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yu, X.; Fang, X.; Liang, A.; Yu, Z.; Gu, P.; Zeng, Y.; He, J.; Zhu, H.; Li, S.; et al. Preventive Effects of Vitamin D Treatment on Bleomycin-Induced Pulmonary Fibrosis. Sci. Rep. 2015, 5, 17638. [Google Scholar] [CrossRef] [PubMed]
- Tzilas, V.; Bouros, E.; Barbayianni, I.; Karampitsakos, T.; Kourtidou, S.; Ntassiou, M.; Ninou, I.; Aidinis, V.; Bouros, D.; Tzouvelekis, A. Vitamin D Prevents Experimental Lung Fibrosis and Predicts Survival in Patients with Idiopathic Pulmonary Fibrosis. Pulmonary Pharmacology & Therapeutics 2019, 55, 17–24. [Google Scholar] [CrossRef]
- Lin, T.; Zhou, F.; Mao, H.; Xie, Z.; Jin, Y. Vitamin D and Idiopathic Pulmonary Fibrosis: A Two-Sample Mendelian Randomization Study. BMC Pulm. Med. 2023, 23, 309. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D Supplementation to Prevent Acute Respiratory Tract Infections: Systematic Review and Meta-Analysis of Individual Participant Data. BMJ 2017, i6583. [Google Scholar] [CrossRef] [PubMed]
- Janssens, W.; Bouillon, R.; Claes, B.; Carremans, C.; Lehouck, A.; Buysschaert, I.; Coolen, J.; Mathieu, C.; Decramer, M.; Lambrechts, D. Vitamin D Deficiency Is Highly Prevalent in COPD and Correlates with Variants in the Vitamin D-Binding Gene. Thorax 2010, 65, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Yavari, M.; Mousavi, S.A.J.; Janani, L.; Feizy, Z.; Vafa, M. Effects of Supplementation of Vitamins D, C and E on Idiopathic Pulmonary Fibrosis (IPF): A Clinical Trial. Clinical Nutrition ESPEN 2022, 49, 295–300. [Google Scholar] [CrossRef] [PubMed]
| Signaling pathway | Signaling pathway inductor | The implemented cascade of reactions | Effect of Vit D complex/VDR |
|---|---|---|---|
| SMAD | TGF-β / receptors TGF-β; angiotensin II |
Regulation of cellular cycle, differentiation (in particular of myofibroblasts), immune reactions | Suppression by reducing TGF-β expression and nuclear translocation of SMAD components |
| MARK | TGF-β; growth factors; lipopolysaccharides etc. | Regulation of cellular proliferation (↑) and differentiation, inflammatory response and elimination of cells by apoptosis (↓) | Suppression by enhancing the expression of proteinase (MAPK phosphatase-1) followed by inhibition of p38 MAPK |
| NF-κβ | Proinflammatory cytokines (IL-1. TNF α); lipopolysaccharides, growth factors | Regulation of non-specific and adaptive immunity and inflammatory response |
Suppression by inhibiting Ikkß kinase and preventing activation of NF-kß, as well as by suppressing nuclear translocation of signaling pathway components |
| JAK/STAT | Cytokines (IL-2, IL-6, IL-10, IL-12, IFN- α etc.), growth factors (EGF) | Regulation of cellular proliferation, differentiation and inflammatory response | Suppression of binding of transcription factor STAT3 to the promoter (ATF6) |
| PPAR- α/γ | Unsaturated fatty acids, eicosanoids, etc. | Regulation of metabolism of fatty acids and energy balance, regulation of immune response and cellular cycle | Competitive interaction due to binding to RXR, suppression of PPAR-α/γ promoter |
| NFAT | Ca2+ | Regulation of cellular cycle and inflammatory response | Blocking of NFAT components of Runx1 transcription factor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





