1. Introduction
Colorectal cancer (CRC) is the fourth most frequently diagnosed cancer and second most common cause of cancer death in Australia [
1,
2]. The estimated risk of being diagnosed with colorectal cancer by the age of 85 is 5.2% [
1]. The survival rate of CRC varies significantly between stages. For instance, patients with stage III CRC have a 5-year survival rate of 72%, while those with stage IV disease have a much lower survival rate of 13% [
3]. The current standard of care for management of stage III colon cancer consists of surgery followed by adjuvant (post-operative) chemotherapy to eradicate micrometastases. However, the absolute magnitude of benefit for adjuvant chemotherapy in resected stage II disease is not as clear [
4,
5]. The decision for adjuvant chemotherapy is informed in part by the presence or absence of high-risk clinicopathologic features, therefore identification and validation of biomarkers for CRC is essential for predicting high-risk patients who may benefit from additional chemotherapy.
Several biomarkers are currently in use to provide a more comprehensive assessment of the tumour in colorectal cancer, such as carcinoembryonic antigen (CEA), mismatch repair deficiency (MMR), microsatellite instability (MSI), v-raf murine sarcoma viral oncogene homolog B1 (BRAF), reticular activating system (RAS) mutations, and caudal-type homeobox transcription factor 2 (CDX2) [
6,
7]. CDX2 is a transcription factor that is essential for intestinal development and has been proposed as a potential CRC biomarker [
8]. Immunohistochemical (IHC) detection of CDX2 expression is clinically useful as a relatively specific marker for intestinal epithelial differentiation [
9]. Strong diffuse CDX2 staining is typically observed in the lining of normal intestinal epithelium, including duodenum, ileum, appendix, colon and rectum but can also be found in malignant tissue [
10]. The highest frequency of extensive CDX2 staining is in colorectal adenocarcinomas (90%), followed by ampullary (33%) and gastroesophageal adenocarcinomas (20%) [
10]. Therefore, changes in CDX2 expression is a valuable tool for understanding disease progression and prognosis. In fact, CDX2 has been found to be downregulated or lost in a proportion of CRC [
11]. Lack of CDX2 expression is associated with more aggressive features such as advanced stage, poor differentiation, vascular invasion, and BRAF mutation which can contribute to poorer survival [
12,
13,
14]. Therefore, evaluation of CDX2 expression can provide important prognostic information to allow risk stratification and personalisation of therapy. Moreover, it is a readily available and economical test, which can be readily applied to clinical practice.
Ideally, biomarkers that can be easily measured on surgical specimens would be beneficial in providing information on the biological characteristics of tumours beyond the clinical TNM classification system. This is because TNM staging assumes that all tumours within the same stage behave similarly and does not account for differences in tumour biology. There is some evidence to suggest that (i) patients with CDX2 negative tumours have lower survival rates than CDX2-positive tumours, and (ii) the loss of CDX2 expression can identify a subset of patients with stage II colon cancer that are at high risk for disease recurrence and may benefit from adjuvant chemotherapy [
15]. These findings highlight the potential clinical utility of CDX2 as a prognostic (provides information about likelihood of disease progression or outcome regardless of treatment) and predictive (provide information about likelihood of response to a particular therapy) biomarker in CRC and emphasises the importance of incorporating biomarker testing in routine practice [
16].
In this retrospective study, we assessed a cohort of patients with stage I to IV colorectal cancer for CDX2 staining and correlated it with patient demographic, clinicopathologic features, and cancer outcomes including overall survival (OS), disease free survival (DFS) and recurrence free survival (RFS). Most studies to date evaluate colon cancer or colorectal as a combined entity, with minimal focus on the rectal cohort specifically. This study investigated its prognostic and predictive value, its potential role in clinical practice, and whether this differs between colonic and rectal tumours.
2. Materials and Methods
2.1. Patients and Surgical Specimens
A database of surgical specimens collected from patients with stage I to IV colorectal cancer during the period 2000 to 2011 was retrospectively analysed and stained for CDX2. The search yielded 668 specimens, including 406 of colonic origin (from 2006 to 2011) and 262 of rectal origin (from 2000 to 2011). Right sided tumours (32.5%) were taken to include proximal colon up to splenic flexure; and left sided tumours (67.5%) consisted of splenic flexure to rectum. Biological characteristics of patient tumours was correlated with pathological stage, patient demographic, and treatment details. This study was conducted with approval of the South Western Sydney Local Health District Human Research Ethics Committee (HREC Reference: HREC/12/LPOOL/102).
2.2. Tissue Microarrays and Immunohistochemical Analysis
In this retrospective analysis, tissue microarray (TMA) slides from archival tissue samples of patients who underwent surgical resection of their primary colorectal cancer were assessed. Tissue samples were mapped according to the following locations: normal tissue close to tumour, normal tissue away from tumour, tumour centre (average score of 2 cores), tumour periphery (average score of 2 cores), lymph node metastasis (average score of 2 cores), polyp and adenoma. The tumour periphery was selected as the primary marker for analysis. The choice of tumour periphery was due to its biological relevance as an area of active invasion and interaction with the microenvironment, reflecting tumour aggressiveness and potential clinical behaviour.
CDX2 protein expression was evaluated using formalin-fixed paraffin-embedded (FFPE) tissue sections which were stained with a validated assay of mouse CDX2 monoclonal antibody (clone CDX2-88, BioGenex) at a concentration of 4mg per milliletre, using an automated staining platform. All tissue microarrays were scored for CDX2 expression by two independent pathologists who were blinded to the patients’ details. The scorers underwent a period of training with a multiheaded microscope to ensure consistent and reliable interpretation. With a test series of at least 36 tissue core sections, intraobserver and interobserver agreement were estimated by use of kappa (j) and Spearman rho (q). Training was ended when the desired level of agreement, consistent over time, was achieved (j > 0.6 and q > 0.8).
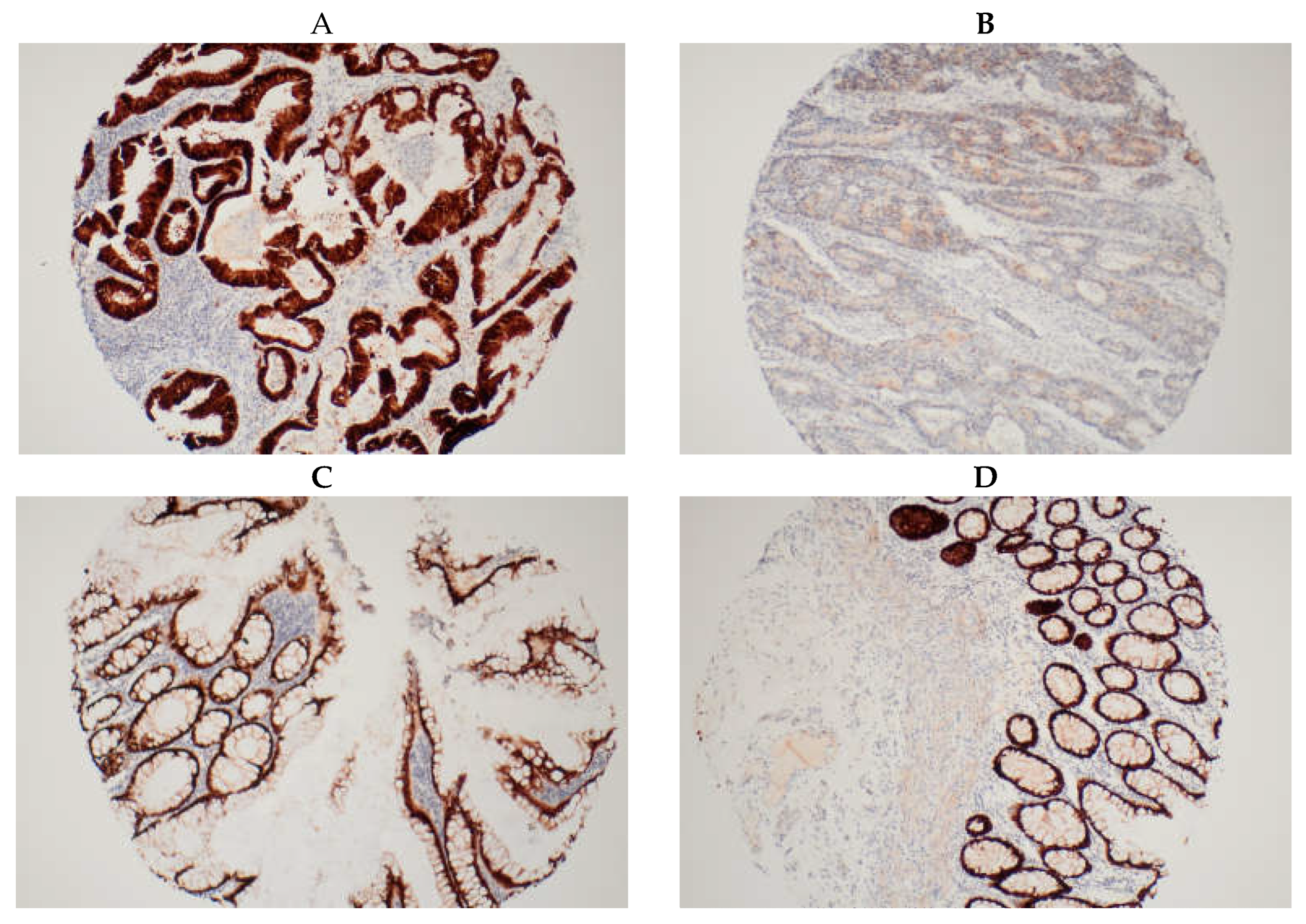
An average score was obtained from the duplicate cores of each tissue sample. A score of 1 to 5 was made according to proportion of deeply stained nuclei (
Table 1). Only deep nuclear staining and not cytoplasmic staining was considered for scoring. This selection was made with the understanding that the active state of the protein, being a transcription factor, primarily resides in the nucleus [
17]. Tissue cores from areas of the same tumour were paired at the end and if discordant, the final score for the tumour was upgraded to the higher score. Patients were stratified into two subgroups based on CDX2 expression: high (for a score of 5 i.e., 75-100% deeply stained nuclei) and low (for scores 1 to 4 i.e., less than 75% deeply stained nuclei) (
Figure 1). Staining of normal tissue adjacent to and distant from the tumour was also conducted for our internal control group. Our findings revealed that over 95% of normal colonic and rectal tissues exhibited high CDX2 expression, as expected.
2.3. Statistical Analysis
Statistical analysis was conducted using SAS Enterprise Guide version 8.2 and R statistical software version 4.2.2. Descriptive statistics was used to characterise the patient population. To evaluate the association between CDX2 staining and survival outcomes, Kaplan-Meier method and log-rank test were used to compare the two subgroups. Overall survival (OS) was defined as time from diagnosis to death from any cause or date of last follow up, disease-free survival (DFS) as time from diagnosis until first evidence of disease progression or death, recurrence-free survival (RFS) as time from diagnosis to recurrence, and cancer-specific survival (CSS) as time from diagnosis to death from disease [
18].
Univariate analysis was performed using one-sample t-test and p<0.05 was considered statistically significant. Multivariable Cox regression was performed to determine if the association between CDX2 low cancers and poorer overall survival remained significant after adjusting for confounding variables. Covariates included were pathological tumour stage, sex, tumour side, site of disease, and CDX2 expression. In the subset of patients with stage II and III disease, we conducted a multivariable model. This analysis included confounding variables from the previous model and added adjuvant chemotherapy as a covariate. Additionally, we explored interactions between CDX2 expression level, disease stage, tumour location (colon or rectum) and adjuvant chemotherapy to determine the impact of adjuvant therapy on overall survival.
3. Results
3.1. Study Population
A total of 668 patients were included in this study. A description of patient characteristics is detailed in
Table 2. The cohort was well balanced between males (58.7%) and females (41.3%). Mean age at diagnosis was 68 years (ranging from 23 to 96 years). Almost half the study population was aged above 70 years (49.1%). In relation to ethnicity, Caucasians were the majority at 86.4%, followed by Asians at 11.4%. Of note, our study cohort has a significant culturally and linguistically diverse population, particularly a substantial proportion of Asian individuals [
19].
Although all cancer stages were included, stage II and stage III were the most prominent cohort in the study, contributing 31% and 37.4% respectively. Adenocarcinoma was the predominant histological subtype (98.4%). With regard to tumour location, 60.8% of patient tumours originated in the colon and 39.2% originated in the rectum. 595 patients (92.1%) in the studied cohort had high CDX2 expression whereas only 51 patients (7.9%) had low CDX2 expression. 22 patients (3.3%) had no tissue or missing data on CDX2 expression, hence a total of 646 patients were analysed for detected CDX2 expression.
A lower proportion of stage II colon cancer patients received adjuvant chemotherapy at 17.3% compared to 63% of stage III colon cancer patients (26% single agent chemotherapy, 37% doublet). Among the stage III colon cancer cohort, notably those who received adjuvant chemotherapy were younger (mean age 63 years) compared to those who did not (mean age 77). Importantly, there was no significant difference observed in key pathological features including CDX2 expression, pathological subtype, tumour differentiation, and invasiveness between the two groups. Of the rectal cohort, most proceeded to upfront surgery (76.3%) with only 23.7% having any neoadjuvant therapy, including short course radiotherapy and long course chemoradiotherapy.
3.2. Association of CDX2 Staining with Clinicopathological Features
The patient subgroups were examined to determine if there was an association with CDX2 staining and clinicopathological features of the study population.
Table 3 presents descriptive statistics by CDX2 expression for 595 patients with CDX2 high tumours and 51 patients with CDX2 low tumours. Two clinicopathological features that were significantly associated with CDX2 expression was tumour differentiation (p<0.001) and presence of lymphovascular or perineural invasion (p=0.002). In terms of sidedness, the rate of CDX2 low expression was numerically higher in the right sided tumours (11.7%) than the left (6.0%). Similarly, there was a higher number of CDX2 low expression in colon (9.2%) versus rectum (5.7%); however, this relationship with CDX2 was not statistically significant. CDX2 expression did not differ by ethnicity.
3.3. CDX2 Low Expression Correlates with Poorer Prognosis.
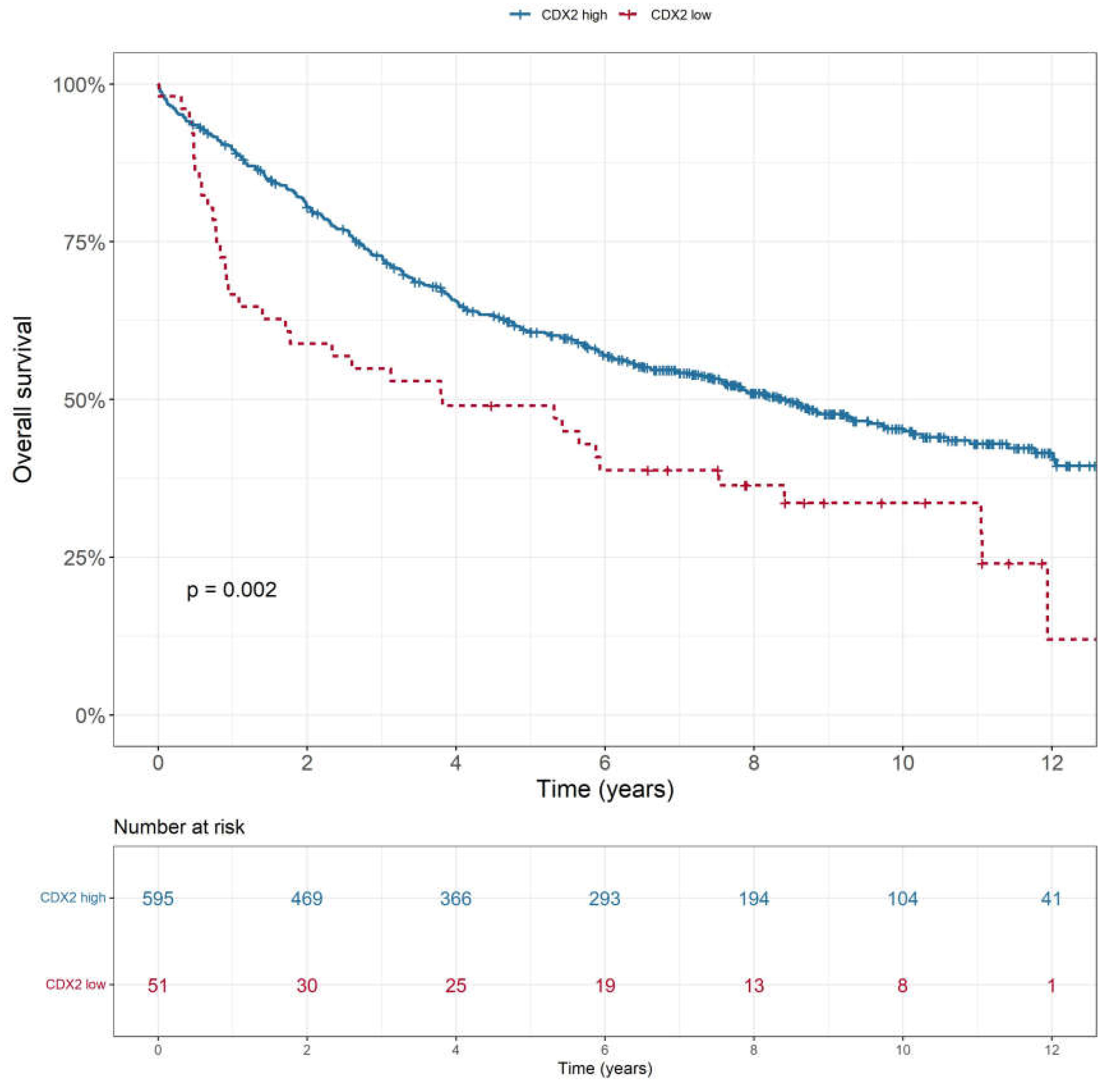
Low CDX2 expression was associated with poorer OS (p = 0.002), as shown in
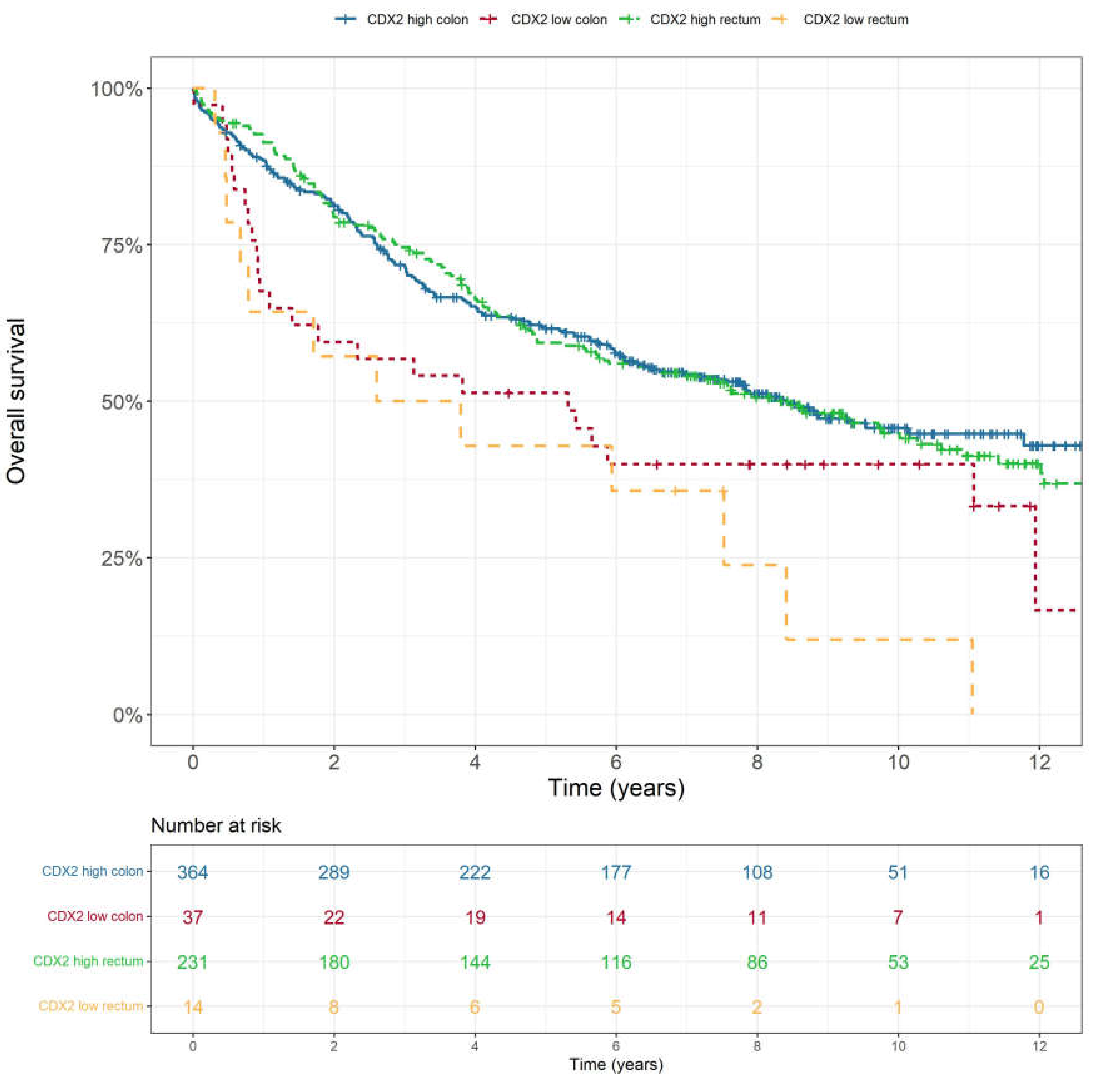
Figure 2. This was consistent across all stages (p<0.001). CDX2 low expression tumours also showed lower rates of disease-free survival (p=0.009), cancer-specific survival (p<0.001) and recurrence-free survival (p=0.004). There was no statistically significant difference in overall survival (p=0.522) or disease-free survival (p=0.126) based on tumour location i.e., colon or rectum (
Figure 3). In a multivariable analysis that adjusted for tumour stage, sex and location as confounding variables, the association between CDX2 low expressing colorectal tumours and lower rate of overall survival remained significant (
Table 4).
3.4. CDX2 as a Predictive Biomarker for Adjuvant Chemotherapy in Stage II and III Colon Cancer
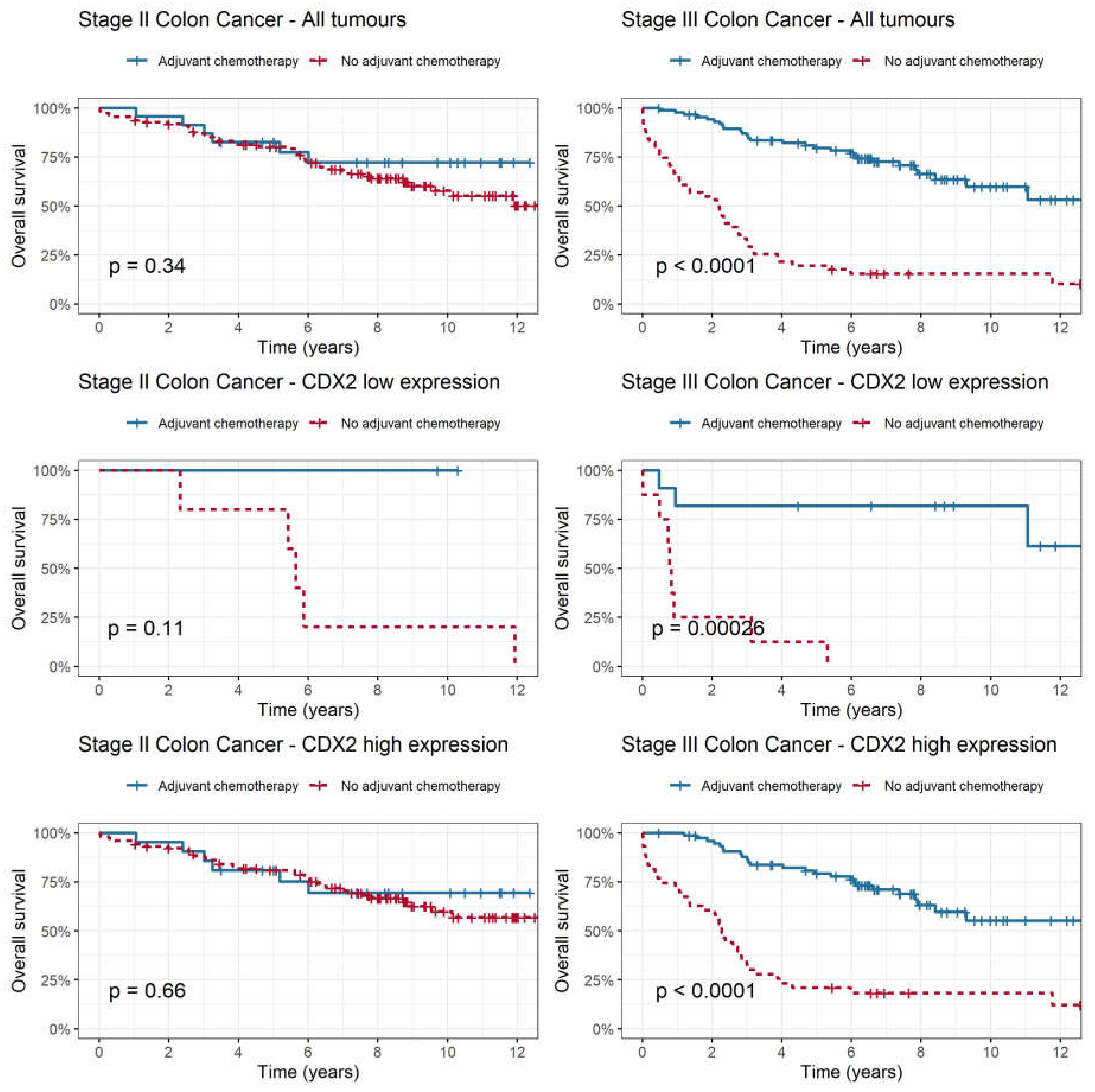
In addition to investigating CDX2 expression as a prognostic biomarker, we examined its relationship with adjuvant chemotherapy to determine if we could predict patients that might benefit from adjuvant chemotherapy (
Figure 4). Among all patients with stage II colon cancer (p=0.339) and CDX2 high subgroup (p=0.656), adjuvant chemotherapy was not associated with improvement in overall survival. In the CDX2 low subgroup, there was a trend towards improved overall survival with adjuvant chemotherapy compared to no chemotherapy; however, this was not statistically significant (p=0.113). A test for interaction between CDX2 level and adjuvant chemotherapy in stage II colon cancer was not statistically significant (p=0.98). Among patients with stage III colon cancer, there was a statistically significant improvement in rate of overall survival for the entire cohort (p<0.001) even after adjusting for disease stage, sex, and sidedness. This improvement was evident in both the CDX2 low subgroup (HR 0.05, [CI 0.01-0.23], p<0.001) and CDX2 high subgroup (HR 0.19 [CI 0.11-0.32]), p<0.0001) when adjuvant chemotherapy was administered.
3.5. CDX2 as a Predictive Biomarker in Rectal Cancer
In the rectal cohort, neoadjuvant therapy consisted of short course radiotherapy and long course chemoradiotherapy. There was no statistically significant association between the level of CDX2 expression and overall survival (p=0.825) in rectal patients who received neoadjuvant therapy. There was an improvement in overall survival in rectal cancer patients who were treated with adjuvant chemotherapy regardless of CDX2 high (p=0.017) or CDX2 low expression (p<0.0001).
4. Discussion
Precise risk stratification based on reliable prognostic markers is critical for guiding treatment decisions and accurately predicting outcomes in patients with CRC. In this study, we identified the pattern of CDX2 expression in stage I-IV CRC and correlated this with demographic, clinicopathologic features, response to adjuvant chemotherapy and survival outcomes. In addition, we examined these parameters in colonic and rectal patients separately. We showed that low CDX2 expression was significantly correlated with poor prognostic features including lack of tumour differentiation, presence of lymphovascular or perineural invasion, and poorer outcomes of OS and DFS in both colon and rectal cancers. These findings are consistent with the literature and support CDX2 expression status as a potentially valuable prognostic marker for identifying patients with more aggressive disease and poorer clinical outcomes [
20].
In addition to evaluating the prognostic value of CDX2 expression, we also investigated its potential predictive role. In stage II colon cancer, the benefit of adjuvant chemotherapy is small at approximately 5% [
21,
22]; hence, having a predictive biomarker may assist clinicians in determining which patients are most likely to benefit. In stage II colon cancer, we observed a trend towards improved survival with addition of adjuvant chemotherapy in CDX2 low patients; however, this association did not reach statistical significance. Notably, our findings differ from those of a previous study by Dalerba et al., who reported a significant benefit of adjuvant chemotherapy in this cohort [
15]. However, we acknowledge that the number of patients with stage II colon cancer and CDX2 low expression that had adjuvant chemotherapy in our study was small (less than 10), compared to Dalerba et al. where there were 25 patients. Although this study was not powered to demonstrate predictive value due to small numbers, it was indicative of a trend that tumours with low CDX2 expression may select out stage II patients that are more likely to benefit from adjuvant chemotherapy. Nevertheless, larger studies are needed to confirm CDX2 predictive utility.
In stage III colon cancer, there was a statistically significant overall survival benefit with adjuvant chemotherapy in both CDX2 high and low expression tumours. Bruun et al. suggests that in stage III patients, the 5-year RFS was higher amongst patients with loss of CDX2 expression who received adjuvant chemotherapy (61%) versus those who did not (44%) which supports our results [
23]. However, this is less clinically meaningful as most stage III colon cancer patients would be recommended adjuvant chemotherapy regardless of CDX2 expression.
This study also focused on risk prediction with CDX2 in rectal cancer as there is less available literature on this, with most grouping colon and rectal cancers into the one cohort. The lack of significant differences in survival outcomes between patients with high and low CDX2 expression who received adjuvant chemotherapy in rectal cancer suggests that CDX2 expression likely does not predict response to chemotherapy in this population. Similarly, there was no correlation between CDX2 expression and treatment outcomes in the rectal cohort that received neoadjuvant therapy. This is an important finding as CDX2 cannot be recommended as a reliable predictor of neoadjuvant treatment and adjuvant chemotherapy efficacy in rectal cancer. Adjuvant chemotherapy is an effective treatment option for rectal patients, regardless of CDX2 expression status. Based on our findings, CDX2 is not a useful predictive biomarker for adjuvant chemotherapy in rectal cancer. In addition, it is unclear to what extent adjuvant therapy is contributing to the observed outcomes given some patients had treatment pre-operatively. Nevertheless, the interpretation of these findings in the current clinical context is challenging due to significant changes in the treatment landscape of rectal cancer over the last few years, with a shift towards total neoadjuvant therapy [
24].
Heterogeneity within colorectal cancer has resulted in variable patient responses to classic systemic therapies. One approach to defining the heterogeneity is to use a framework such as the consensus molecular subtypes (CMS) to provide a systematic way of classifying colorectal cancer based on genetic and molecular characteristics [
25]. Through more nuanced insight into tumour biology, we can better predict responses to therapeutic alternatives to select the most appropriate therapy. CDX2 has been linked to the CMS system; the lack of CDX2 expression can assist in differentiating between subtypes as it is only present in the mesenchymal subgroup (CMS4) [
26]. Thus far, the implementation of CMS in trials and clinical practice has been limited by the requirement for comprehensive genomic analysis. To overcome this limitation, IHC detection can be used as a surrogate to enable more practical integration of CMS into clinical research and patient care [
25,
27].
One limitation of our retrospective study pertains to its data source, specifically its dependence on a surgical database. Consequently, our study exclusively included patients who underwent surgical interventions and had corresponding pathology slides available, thereby excluding those who did not undergo surgical procedures. Additionally, it is essential to acknowledge the challenge posed by the lack of standardisation of CDX2 staining, which makes data interpretation between studies and the clinical use of this biomarker in colorectal cancer inherently complex. There is significant variability in methods used for staining, including type of antibody, the staining protocol and interpretation of staining results. Different antibodies (e.g., clone CDX2-88 BioGenex, CellMarque, DAKO M3636, A1629 ABclonal) may have different binding specificities and sensitivities, which can affect the accuracy of the staining result [
28,
29]. The scoring system used to interpret CDX2 staining results can also vary between labs and pathologists, which can lead to differences in assignment of CDX2 expression. For our study, staining interpretation of CDX2 was given a score based on percentage of deeply stained nuclei and then classified into high or low, whereas other studies used positive or negative, normal or absent, none or weak or moderate or strong [
30]. We opted for this scoring to account for active CDX2, as nuclear localisation is a prerequisite for transcription factor activity. To address scoring variations, the International Collaboration on Cancer Reporting (ICCR) has developed guidelines for standardised reporting of colorectal cancer biomarkers, however CDX2 is yet to be included [
31].
The inconsistencies in methodologies across studies has also led to a wide range of reported loss of CDX2 expression. Our results are comparable with 7.6% showing CDX2 loss. This is consistent with literature where the rate of CDX2 loss/absence/low ranged from 5-30% [
17]. In our study, there was an increase in percentage of cases with loss of CDX2 expression as cancer stage advances, i.e., stage I (3.5%), II (5.0%), III (10.7%), IV (12.1%), which was statistically significant (p=0.015). Existing literature shows similar pattern of expression with loss of CDX2 associated with advanced stage [
32]. Some studies also suggest that CDX2 expression is higher in left-sided colorectal cancer compared to right-sided cancer, which is concordant with our results showing CDX2 high expression was higher in the left (94%) than right (88%) [
33]. The reason for this is unclear but may be related to differences in embryonic origin and molecular characteristics of left and right colon. In our study, low CDX2 expression was observed more commonly in right-sided tumours (11.7%) than left (6.0%) which was statistically significant. More research is needed to fully understand the implications of this difference for clinical practice; nonetheless, it is established that right-sided colon cancers generally have a worse prognosis. As such, loss of CDX2 expression may simply be linked to unfavourable prognostic features.
5. Conclusions
Overall, our study findings align with existing literature, providing further evidence for the correlation between CDX2 low expression and poorer overall survival in both colon and rectal cancers. However, we cannot draw definitive conclusions regarding the predictive value of CDX2 as a biomarker for colorectal cancer, especially in the rectal or left sided tumours. CDX2 low expression is associated with aggressive features such as lack of tumour differentiation, presence of lymphovascular invasion, increased frequency in advanced stage and right-sided tumours. The promising data, but lack of standardisation in CDX2 staining and interpretation supports further efforts to standardise international reporting which need to be in place before this promising biomarker can be used routinely in clinical practice.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, Wei Yen Chan, Wei Chua, Kate Wilkinson, Hiren Mandaliya, Tara Roberts, Therese Becker, Weng Ng, Cheok Soon Lee and Stephanie Lim; Data curation, Wei Yen Chan, Kate Wilkinson, Chandika Epitakaduwa, Hiren Mandaliya, Joseph Descallar, Therese Becker and Stephanie Lim; Formal analysis, Wei Yen Chan, Kate Wilkinson, Chandika Epitakaduwa, Joseph Descallar and Stephanie Lim; Funding acquisition, Cheok Soon Lee; Investigation, Joseph Descallar, Tara Roberts, Therese Becker, Cheok Soon Lee and Stephanie Lim; Methodology, Wei Chua, Kate Wilkinson, Hiren Mandaliya, Weng Ng and Stephanie Lim; Project administration, Cheok Soon Lee; Resources, Wei Chua, Weng Ng, Cheok Soon Lee and Stephanie Lim; Software, Stephanie Lim; Supervision, Therese Becker and Weng Ng; Writing – original draft, Wei Yen Chan, Wei Chua and Stephanie Lim; Writing – review & editing, Wei Yen Chan, Wei Chua, Kate Wilkinson, Hiren Mandaliya, Joseph Descallar, Tara Roberts, Therese Becker, Weng Ng, Cheok Soon Lee and Stephanie Lim.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of the South Western Sydney Local Health District Human Research Ethics Committee (HREC Reference: HREC/12/LPOOL/102, date of approval 22nd June 2012).
Informed Consent Statement
Informed consent was waived due to the study being of low or negligible risk, and all patient data was deidentified.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Welfare AIoHa. Cancer data in Australia 2022 [Available from: https://www.aihw.gov.au/reports/cancer/cancer-data-in-australia].
- Indicators NCC. Cancer Incidence 8 Aug 2022 [Available from: https://ncci.canceraustralia.gov.au/diagnosis/cancer-incidence/cancer-incidence].
- Cancer.Net A. Colorectal Cancer: Statistics 2023 [Available from: https://www.cancer.net/cancer-types/colorectal-cancer/statistics].
- Argilés, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveson, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J.; et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1291–1305. [CrossRef]
- Rebuzzi, S.E.; Pesola, G.; Martelli, V.; Sobrero, A.F. Adjuvant Chemotherapy for Stage II Colon Cancer. Cancers 2020, 12, 2584. [CrossRef]
- Koncina, E.; Haan, S.; Rauh, S.; Letellier, E. Prognostic and Predictive Molecular Biomarkers for Colorectal Cancer: Updates and Challenges. Cancers 2020, 12, 319. [CrossRef]
- Alves Martins BA, de Bulhões GF, Cavalcanti IN, Martins MM, de Oliveira PG, Martins AMA. Biomarkers in Colorectal Cancer: The Role of Translational Proteomics Research. Frontiers in Oncology. 2019;9.
- Asgari-Karchekani, S.; Karimian, M.; Mazoochi, T.; Taheri, M.A.; Khamehchian, T. CDX2 Protein Expression in Colorectal Cancer and ItsCorrelation with Clinical and Pathological Characteristics, Prognosis, and Survival Rate of Patients. J. Gastrointest. Cancer 2019, 51, 844–849. [CrossRef]
- A Moskaluk, C.; Zhang, H.; Powell, S.M.; A Cerilli, L.; Hampton, G.M.; Frierson, H.F. Cdx2 Protein Expression in Normal and Malignant Human Tissues: An Immunohistochemical Survey Using Tissue Microarrays. Mod. Pathol. 2003, 16, 913–919. [CrossRef]
- A Moskaluk, C.; Zhang, H.; Powell, S.M.; A Cerilli, L.; Hampton, G.M.; Frierson, H.F. Cdx2 Protein Expression in Normal and Malignant Human Tissues: An Immunohistochemical Survey Using Tissue Microarrays. Mod. Pathol. 2003, 16, 913–919. [CrossRef]
- Werling RW, Yaziji H, Bacchi CE, Gown AM. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27(3):303-10.
- Tomasello, G.; Barni, S.; Turati, L.; Ghidini, M.; Pezzica, E.; Passalacqua, R.; Petrelli, F. Association of CDX2 Expression With Survival in Early Colorectal Cancer: A Systematic Review and Meta-analysis. Clin. Color. Cancer 2018, 17, 97–103. [CrossRef]
- Baba, Y.; Nosho, K.; Shima, K.; Freed, E.; Irahara, N.; Philips, J.; Meyerhardt, J.A.; Hornick, J.L.; Shivdasani, R.A.; Fuchs, C.S.; et al. Relationship of CDX2 Loss with Molecular Features and Prognosis in Colorectal Cancer. Clin. Cancer Res. 2009, 15, 4665–4673. [CrossRef]
- Zhang, B.Y.; Jones, J.C.; Briggler, A.M.; Hubbard, J.M.; Kipp, B.R.; Sargent, D.J.; Dixon, J.G.; Grothey, A. Lack of Caudal-Type Homeobox Transcription Factor 2 Expression as a Prognostic Biomarker in Metastatic Colorectal Cancer. Clin. Color. Cancer 2016, 16, 124–128. [CrossRef]
- Dalerba, P.; Sahoo, D.; Paik, S.; Guo, X.; Yothers, G.; Song, N.; Wilcox-Fogel, N.; Forgó, E.; Rajendran, P.S.; Miranda, S.P.; et al. CDX2 as a Prognostic Biomarker in Stage II and Stage III Colon Cancer. New Engl. J. Med. 2016, 374, 211–222. [CrossRef]
- Ballman KV. Biomarker: Predictive or Prognostic? Journal of Clinical Oncology. 2015;33(33):3968-71.
- Saad RS, Ghorab Z, Khalifa MA, Xu M. CDX2 as a marker for intestinal differentiation: Its utility and limitations. World J Gastrointest Surg. 2011;3(11):159-66.
- Delgado A, Guddati AK. Clinical endpoints in oncology - a primer. Am J Cancer Res. 2021;11(4):1121-31.
- Statisticsc ABo. Sydney South West 2021 Census All persons QuickStats 2021 [Available from: https://abs.gov.au/census/find-census-data/quickstats/2021/127].
- Bae, J.M.; Lee, T.H.; Cho, N.-Y.; Kim, T.-Y.; Kang, G.H. Loss of CDX2 expression is associated with poor prognosis in colorectal cancer patients. World J. Gastroenterol. 2015, 21, 1457–67. [CrossRef]
- André T, de Gramont A, Vernerey D, Chibaudel B, Bonnetain F, Tijeras-Raballand A, Scriva A, Hickish T, Tabernero J, Van Laethem JL, et al. Adjuvant Fluorouracil, Leucovorin, and Oxaliplatin in Stage II to III Colon Cancer: Updated 10-Year Survival and Outcomes According to BRAF Mutation and Mismatch Repair Status of the MOSAIC Study. J Clin Oncol. 2015;33(35):4176-87.
- Varghese, A. Chemotherapy for Stage II Colon Cancer. Clin. Colon Rectal Surg. 2015, 28, 256–261. [CrossRef]
- Bruun, J.; Sveen, A.; Barros, R.; Eide, P.W.; Eilertsen, I.; Kolberg, M.; Pellinen, T.; David, L.; Svindland, A.; Kallioniemi, O.; et al. Prognostic, predictive, and pharmacogenomic assessments of CDX2 refine stratification of colorectal cancer. Mol. Oncol. 2018, 12, 1639–1655. [CrossRef]
- Liu, S.; Jiang, T.; Xiao, L.; Yang, S.; Liu, Q.; Gao, Y.; Chen, G.; Xiao, W. Total Neoadjuvant Therapy (TNT) versus Standard Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer: A Systematic Review and Meta-Analysis. Oncol. 2021, 26, e1555–e1566. [CrossRef]
- Valenzuela, G.; Canepa, J.; Simonetti, C.; de Zaldívar, L.S.; Marcelain, K.; González-Montero, J. Consensus molecular subtypes of colorectal cancer in clinical practice: A translational approach. World J. Clin. Oncol. 2021, 12, 1000–1008. [CrossRef]
- Pilati, C.; Taieb, J.; Balogoun, R.; Marisa, L.; de Reyniès, A.; Laurent-Puig, P. CDX2 prognostic value in stage II/III resected colon cancer is related to CMS classification. Ann. Oncol. 2017, 28, 1032–1035. [CrossRef]
- Li Y, Yao Q, Zhang L, Mo S, Cai S, Huang D, Peng J. IHC-Based Consensus Molecular Subtypes as A Prognostic and Predictive Biomarker for Adjuvant Chemotherapy in Patients with Stage II Colorectal Cancer. The Oncologist. 2020.
- Xu, W.; Zhu, Y.; Shen, W.; Ding, W.; Wu, T.; Guo, Y.; Chen, X.; Zhou, M.; Chen, Y.; Cui, L.; et al. Combination of CDX2 expression and T stage improves prognostic prediction of colorectal cancer. J. Int. Med Res. 2019, 47, 1829–1842. [CrossRef]
- Hestetun, K.E.; Aasebø, K.; Rosenlund, N.B.; Müller, Y.; Dahl, O.; Myklebust, M.P. Mismatch repair phenotype determines the implications of tumor grade and CDX2 expression in stage II–III colon cancer. Mod. Pathol. 2020, 34, 161–170. [CrossRef]
- Hansen, T.F.; Kjær-Frifeldt, S.; Eriksen, A.C.; Lindebjerg, J.; Jensen, L.H.; Sørensen, F.B.; Jakobsen, A. Prognostic impact of CDX2 in stage II colon cancer: results from two nationwide cohorts. Br. J. Cancer 2018, 119, 1367–1373. [CrossRef]
- Loughrey MB, Webster F, Arends MJ, Brown I, Burgart LJ, Cunningham C, Flejou JF, Kakar S, Kirsch R, Kojima M, et al. Dataset for Pathology Reporting of Colorectal Cancer: Recommendations From the International Collaboration on Cancer Reporting (ICCR). Ann Surg. 2022;275(3):e549-e61.
- Singh J, Rajesh N, Dubashi B, Maroju NK, Ganesan P, Matta KK, Charles I, Kayal S. Pattern of expression of CDX2 in colorectal cancer and its role in prognosis. Journal of Cancer Research and Therapeutics. 2022;18(Suppl 2):S420-S7.
- Ben-Aharon, I.; Goshen-Lago, T.; Sternschuss, M.; Morgenstern, S.; Geva, R.; Beny, A.; Dror, Y.; Steiner, M.; Hubert, A.; Idelevich, E.; et al. Sidedness Matters: Surrogate Biomarkers Prognosticate Colorectal Cancer upon Anatomic Location. Oncol. 2019, 24, e696–e701. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).