Submitted:
20 July 2024
Posted:
22 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Methodology
2.2.a. Water and Sediment Sampling Methodology
2.2.b. Methodology for Statistical Analysis
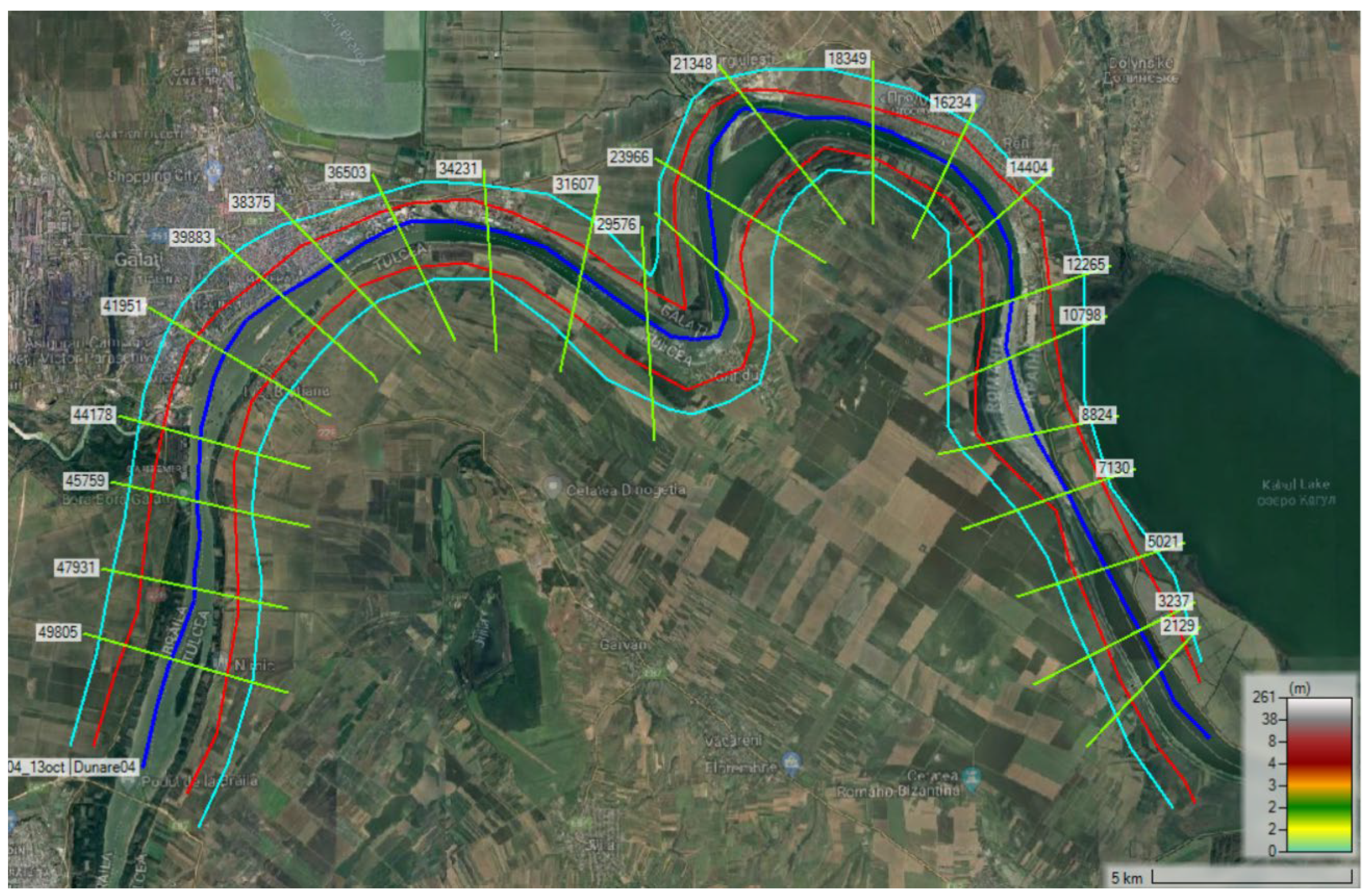
2.2.c. Methodology for Numerical Analysis
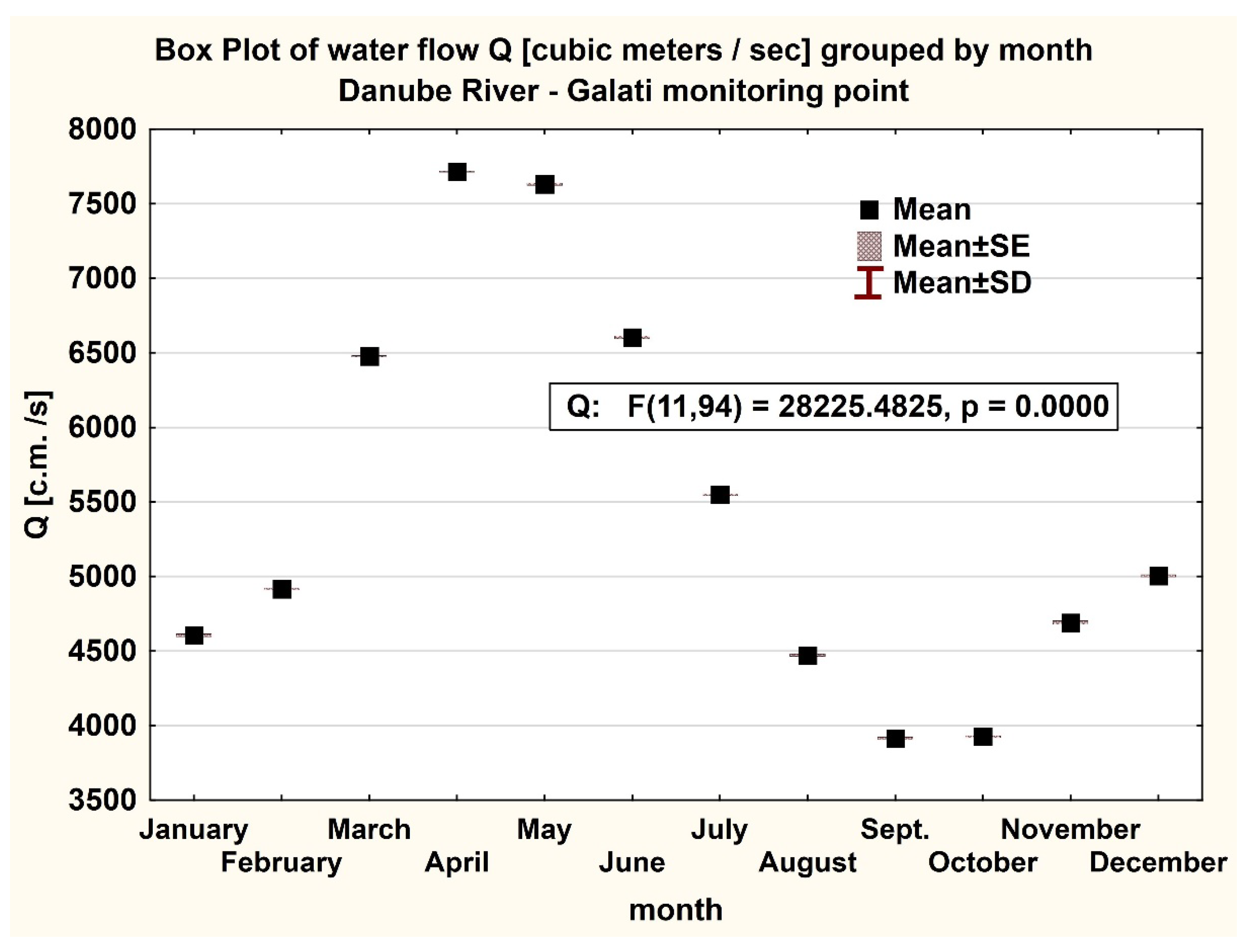
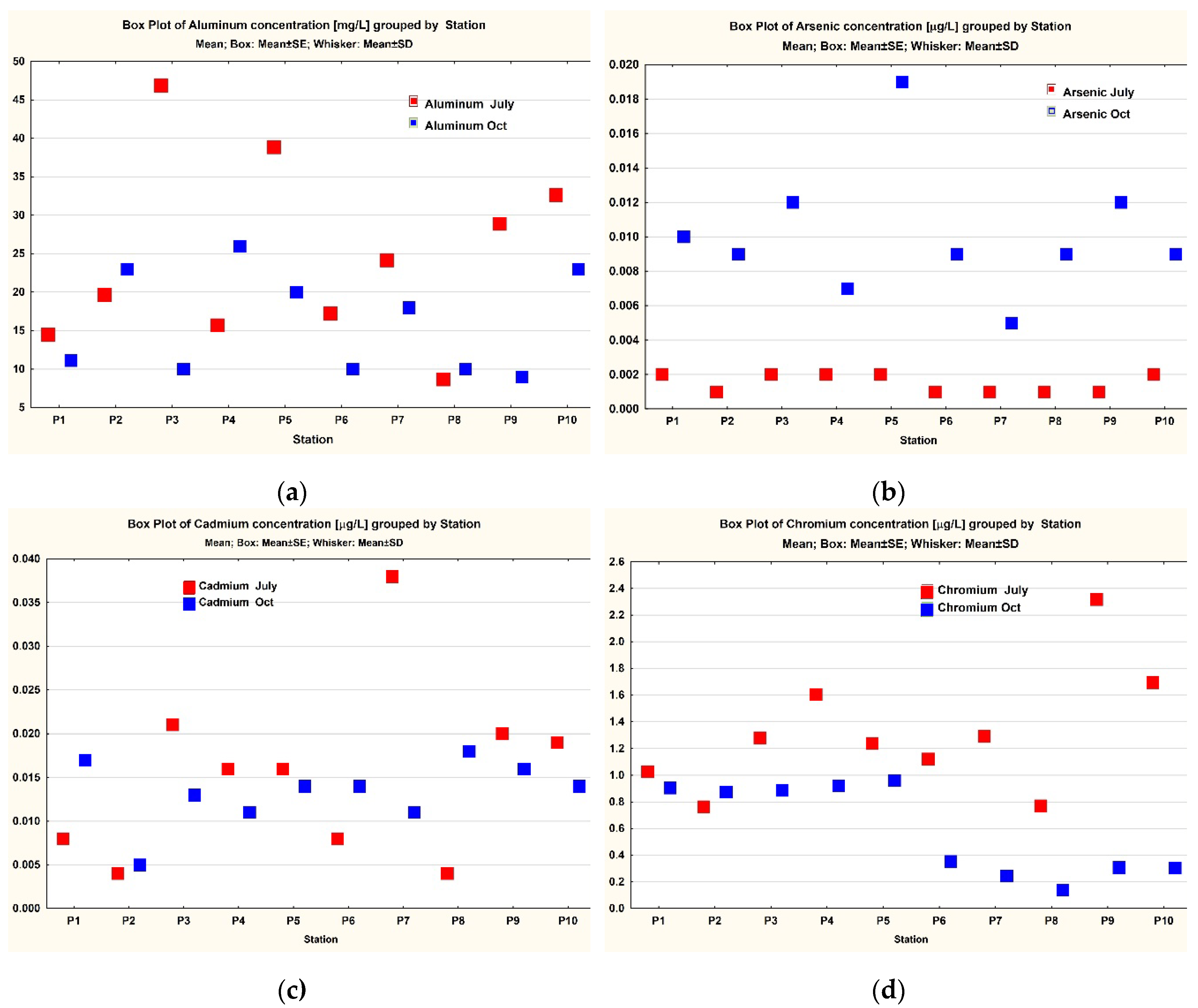
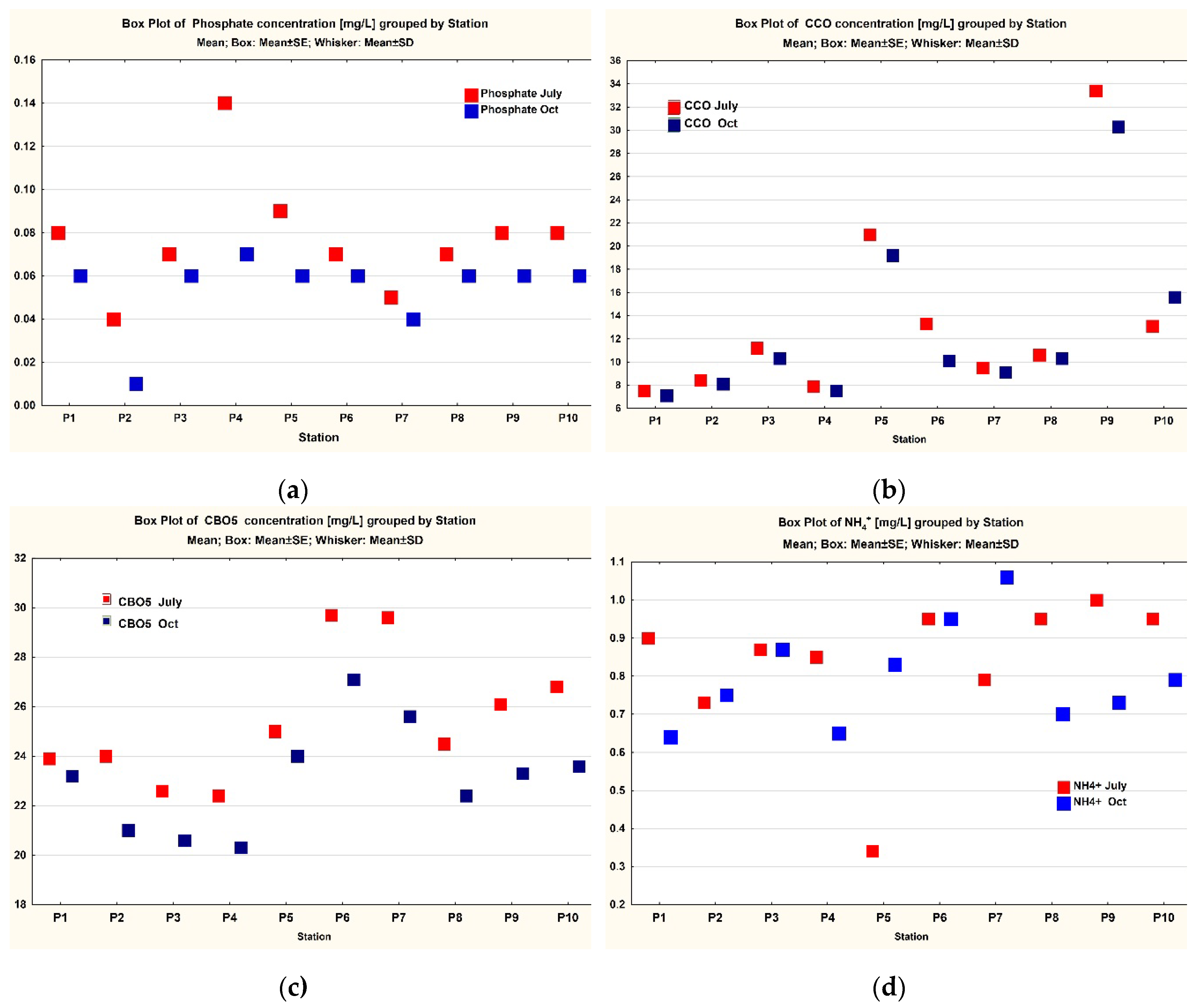
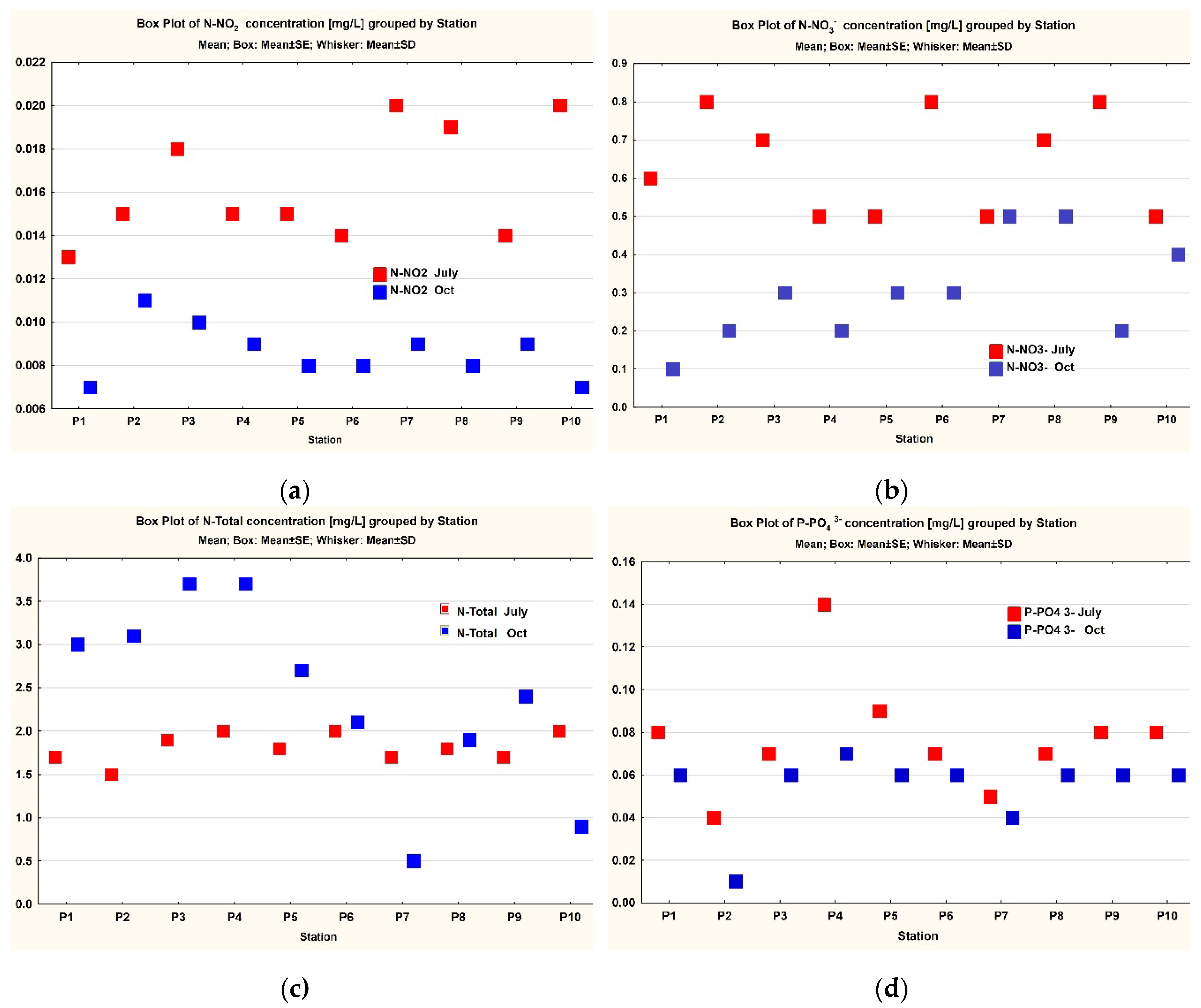
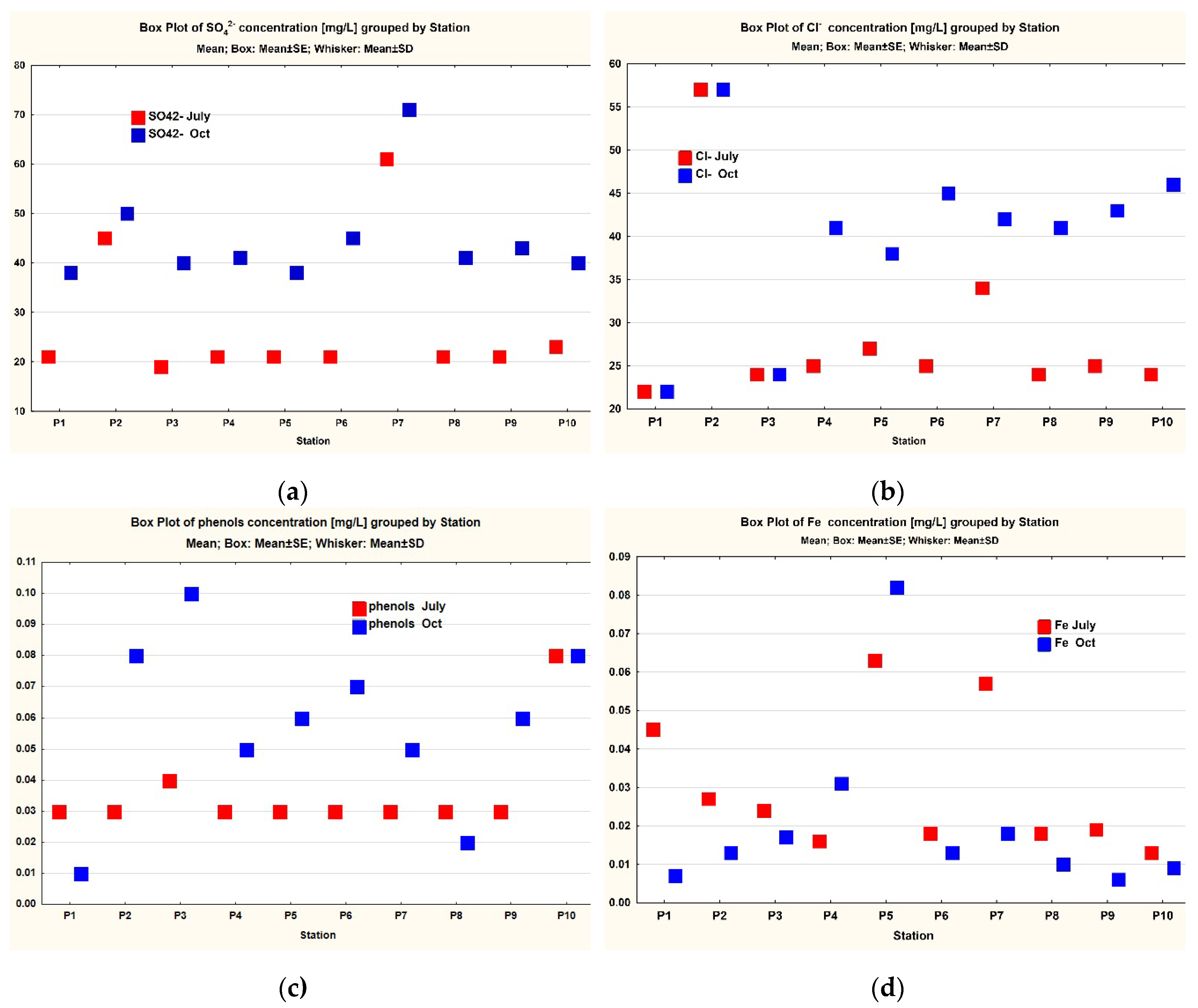
3. Results
3.1. Data Analysis
| Station | Aluminium (µg/L) | Arsenic (µg/L) | Beryllium (µg/L) |
Cadmium (µg/L) | Chromium (µg/L) | Phosphate - P-PO4 (mg/L) |
|---|---|---|---|---|---|---|
| P1 | 14.468 | 0.002 | <LOD (< 0.5 ng/L) | 0.008 | 1.027 | 0.080 |
| P2 | 19.670 | 0.001 | <LOD (< 0.5 ng/L) | 0.004 | 0.764 | 0.040 |
| P3 | 46.890 | 0.002 | <LOD (< 0.5 ng/L) | 0.021 | 1.280 | 0.070 |
| P4 | 15.704 | 0.002 | <LOD (< 0.5 ng/L) | 0.016 | 1.605 | 0.140 |
| P5 | 38.868 | 0.002 | <LOD (< 0.5 ng/L) | 0.016 | 1.239 | 0.090 |
| P6 | 17.214 | 0.001 | <LOD (< 0.5 ng/L) | 0.008 | 1.123 | 0.070 |
| P7 | 24.168 | 0.001 | <LOD (< 0.5 ng/L) | 0.038 | 1.291 | 0.050 |
| P8 | 8.665 | 0.001 | <LOD (< 0.5 ng/L) | 0.004 | 0.771 | 0.070 |
| P9 | 28.889 | 0.001 | <LOD (< 0.5 ng/L) | 0.020 | 2.318 | 0.080 |
| P10 | 32.650 | 0.002 | <LOD (< 0.5 ng/L) | 0.019 | 1.694 | 0.080 |
3.1. Statistical Approach
3.1.1. Descriptive Statistics
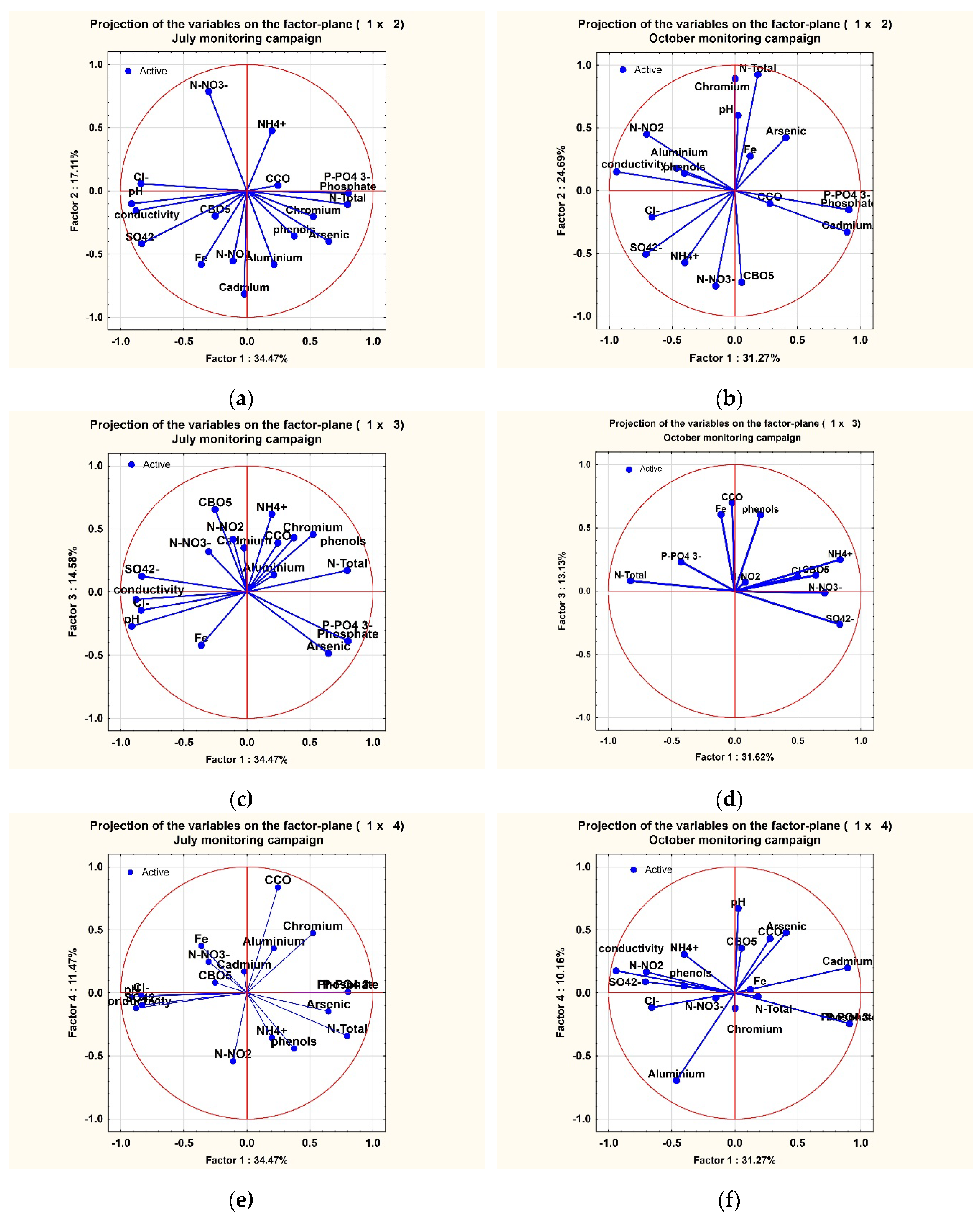
3.3. PCA Method Analysis
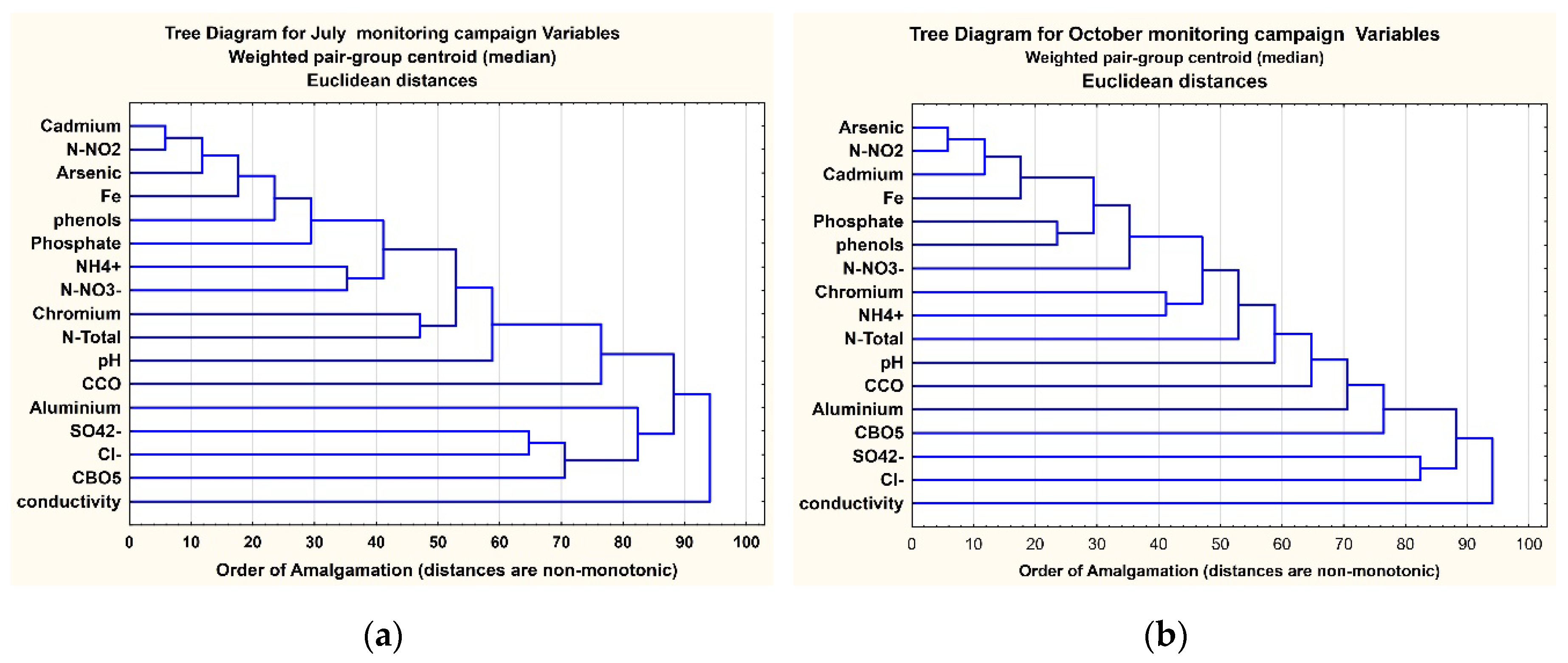
3.4. Cluster Method Analysis
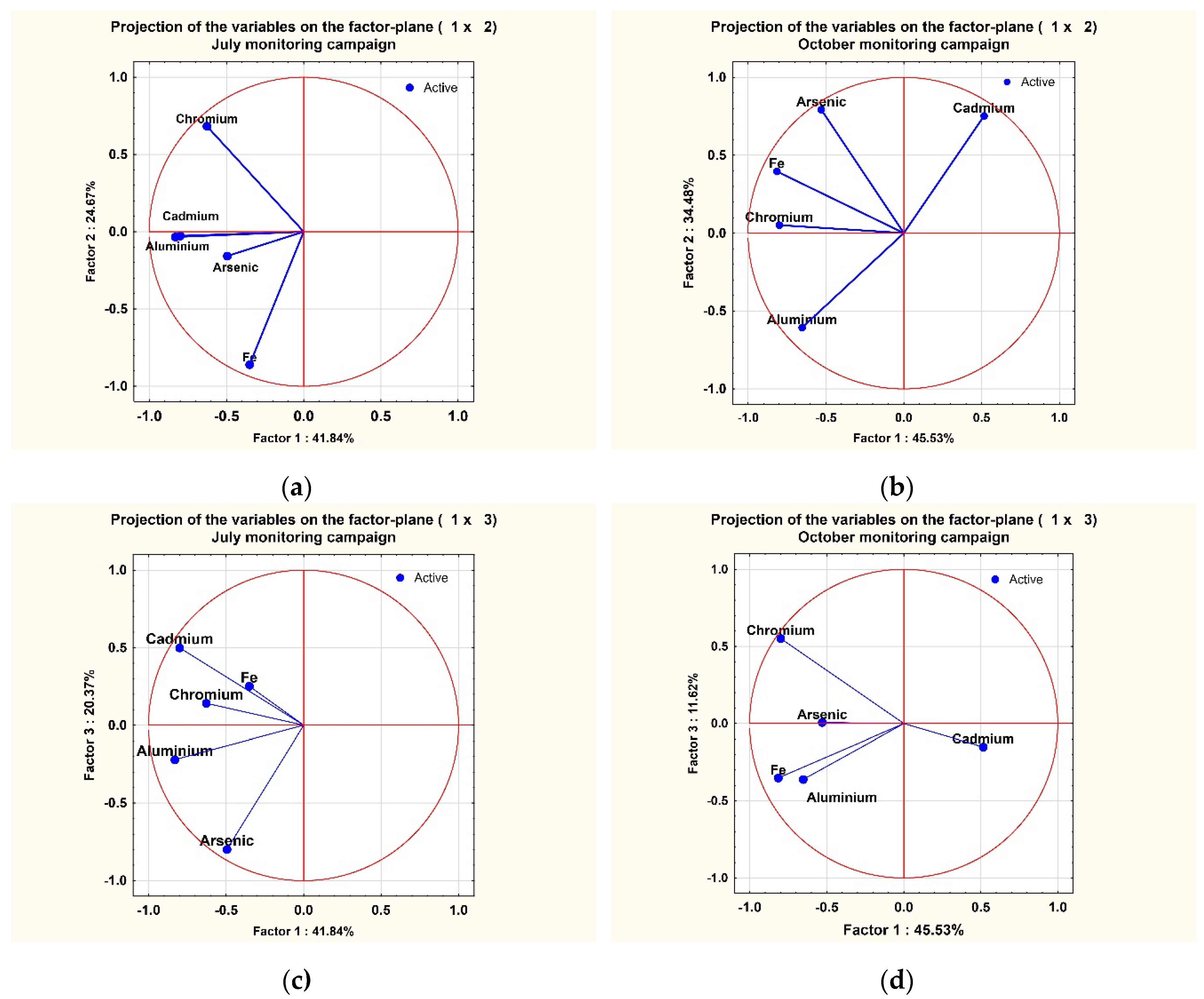
3.4. PCA Method Analysis for Metals Samples
3.4. Numerical Analysis
4. Discussion
PCA Method Analysis
Numerical Approach
- -
- A further study on heavy metals on the Danube Course should include the following elements:
- -
- A complex analysis of the bioavailability of heavy metals in sediments using high-performance analytical methods such as inductively coupled plasma mass spectrometry (ICP-MS) or atomic absorption spectroscopy (AAS) from our laboratory.
- -
- An assessment of factors influencing the sorption and mobility of heavy metals in soils and their mechanisms of accumulation.
- -
- A comparison of different technologies for the remediation of soils polluted with heavy metals, such as in-situ or ex-situ physico-chemical processes, or bioremediation using microorganisms or plants.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Popescu, F.; Trumić, M.; Cioabla, A.E.; Vujić, B.; Stoica, V.; Trumić, M.; Opris, C.; Bogdanović, G.; Trif-Tordai, G. Analysis of Surface Water Quality and Sediments Content on Danube Basin in Djerdap-Iron Gate Protected Areas. Water 2022, 14, 2991. [Google Scholar] [CrossRef]
- Popa, P.; Murariu, G.; Timofti, M.; Georgescu, L.P. MULTIVARIATE STATISTICAL ANALYSES OF DANUBE RIVER WATER QUALITY AT GALATI, ROMANIA. Environ. Eng. Manag. J. 2018, 17, 1249–1266. [Google Scholar] [CrossRef]
- Fang, T.; Lu, W.; Cui, K.; Li, J.; Yang, K.; Zhao, X.; Liang, Y.; Li, H. Distribution, Bioaccumulation and Trophic Transfer of Trace Metals in the Food Web of Chaohu Lake, Anhui, China. Chemosphere 2019, 218, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Hakanson, L. An Ecological Risk Index for Aquatic Pollution Control.a Sedimentological Approach. Water Research 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Simionov, I.-A.; Călmuc, M.; Iticescu, C.; Călmuc, V.; Georgescu, P.-L.; Faggio, C.; Petrea, Ş.-M. Human Health Risk Assessment of Potentially Toxic Elements and Microplastics Accumulation in Products from the Danube River Basin Fish Market. Environmental Toxicology and Pharmacology 2023, 104, 104307. [Google Scholar] [CrossRef] [PubMed]
- Teodorof, L.; Ene, A.; Burada, A.; Despina, C.; Seceleanu-Odor, D.; Trifanov, C.; Ibram, O.; Bratfanof, E.; Tudor, M.-I.; Tudor, M.; et al. Integrated Assessment of Surface Water Quality in Danube River Chilia Branch. Applied Sciences 2021, 11, 9172. [Google Scholar] [CrossRef]
- Fishes-08-00387.Pdf.
- Matache, M.L.; Marin, C.; Rozylowicz, L.; Tudorache, A. Plants Accumulating Heavy Metals in the Danube River Wetlands. J Environ Health Sci Engineer 2013, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, P.-L.; Moldovanu, S.; Iticescu, C.; Calmuc, M.; Calmuc, V.; Topa, C.; Moraru, L. Assessing and Forecasting Water Quality in the Danube River by Using Neural Network Approaches. Science of The Total Environment 2023, 879, 162998. [Google Scholar] [CrossRef] [PubMed]
- Bąk, Ł.; Szeląg, B.; Sałata, A.; Studziński, J. Modeling of Heavy Metal (Ni, Mn, Co, Zn, Cu, Pb, and Fe) and PAH Content in Stormwater Sediments Based on Weather and Physico-Geographical Characteristics of the Catchment-Data-Mining Approach. Water 2019, 11, 626. [Google Scholar] [CrossRef]
- Iticescu, C.; Georgescu, P.-L.; Arseni, M.; Rosu, A.; Timofti, M.; Carp, G.; Cioca, L.-I. Optimal Solutions for the Use of Sewage Sludge on Agricultural Lands. Water 2021, 13, 585. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, Z.; Cao, H.; Xu, M.; Xu, L. Concentrations, Speciation, and Ecological Risk of Heavy Metals in the Sediment of the Songhua River in an Urban Area with Petrochemical Industries. Chemosphere 2019, 219, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Simionov, I.-A.; Cristea, D.S.; Petrea, Ștefan-M. ; Mogodan, A.; Jijie, R.; Ciornea, E.; Nicoară, M.; Turek Rahoveanu, M.M.; Cristea, V. Predictive Innovative Methods for Aquatic Heavy Metals Pollution Based on Bioindicators in Support of Blue Economy in the Danube River Basin. Sustainability 2021, 13, 8936. [Google Scholar] [CrossRef]
- Calmuc, M.; Calmuc, V.; Arseni, M.; Topa, C.; Timofti, M.; Georgescu, L.P.; Iticescu, C. A Comparative Approach to a Series of Physico-Chemical Quality Indices Used in Assessing Water Quality in the Lower Danube. Water 2020, 12, 3239. [Google Scholar] [CrossRef]
- Dong, D.; Zhao, X.; Hua, X.; Liu, J.; Gao, M. Investigation of the Potential Mobility of Pb, Cd and Cr(VI) from Moderately Contaminated Farmland Soil to Groundwater in Northeast, China. Journal of Hazardous Materials 2009, 162, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, Z.; Hu, X.; Zhang, N.; Chen, T.; Ding, Z. Sorption of Pb(II) and Cu(II) on the Colloid of Black Soil, Red Soil and Fine Powder Kaolinite: Effects of pH, Ionic Strength and Organic Matter. Environmental Pollutants and Bioavailability 2019, 31, 85–93. [Google Scholar] [CrossRef]
- Resz, M.-A.; Roman, C.; Senila, M.; Török, A.I.; Kovacs, E. A Comprehensive Approach to the Chemistry, Pollution Impact and Risk Assessment of Drinking Water Sources in a Former Industrialized Area of Romania. Water 2023, 15, 1180. [Google Scholar] [CrossRef]
- Takić, L.; Mladenović-Ranisavljević, I.; Vasović, D.; Đorđević, L. The Assessment of the Danube River Water Pollution in Serbia. Water Air Soil Pollut 2017, 228, 380. [Google Scholar] [CrossRef]
- Banescu, A.; Arseni, M.; Georgescu, L.P.; Rusu, E.; Iticescu, C. Evaluation of Different Simulation Methods for Analyzing Flood Scenarios in the Danube Delta. Applied Sciences 2020, 10, 8327. [Google Scholar] [CrossRef]
- Zhang, W.; Long, J.; Zhang, X.; Shen, W.; Wei, Z. Pollution and Ecological Risk Evaluation of Heavy Metals in the Soil and Sediment around the HTM Tailings Pond, Northeastern China. IJERPH 2020, 17, 7072. [Google Scholar] [CrossRef]
- Da Silva Júnior, R.O.; Almeida, H.P.; Da Silva, M.S.; França, A.C.; Balleroni, E.; Dos Santos, N.; Vilela, P.H.; De Melo, A.M.Q.; Guimarães, J.T.F. Methodological Approach for an Online Water Quality Monitoring System in an Iron Ore Tailing Dam. Water 2023, 15, 3663. [Google Scholar] [CrossRef]
- Pham Van, C.; Chua, V. Numerical Simulation of Hydrodynamic Characteristics and Bedload Transport in Cross Sections of Two Gravel-Bed Rivers Based on One-Dimensional Lateral Distribution Method. International Journal of Sediment Research 2020, 35, 203–216. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, W.; Zeng, Y.; Liang, D.; Deng, Y.; Zhang, X.; Li, Y. How Does Three Gorges Dam Regulate Heavy Metal Footprints in the Largest Freshwater Lake of China. Environmental Pollution 2022, 292, 118313. [Google Scholar] [CrossRef] [PubMed]
- Calmuc, V.A.; Calmuc, M.; Arseni, M.; Topa, C.M.; Timofti, M.; Burada, A.; Iticescu, C.; Georgescu, L.P. Assessment of Heavy Metal Pollution Levels in Sediments and of Ecological Risk by Quality Indices, Applying a Case Study: The Lower Danube River, Romania. Water 2021, 13, 1801. [Google Scholar] [CrossRef]
- Iticescu, C.; Georgescu, L.P.; Murariu, G.; Topa, C.; Timofti, M.; Pintilie, V.; Arseni, M. Lower Danube Water Quality Quantified through WQI and Multivariate Analysis. Water 2019, 11, 1305. [Google Scholar] [CrossRef]
- Saeed, O.; Székács, A.; Jordán, G.; Mörtl, M.; Abukhadra, M.R.; Eid, M.H. Investigating the Impacts of Heavy Metal(Loid)s on Ecology and Human Health in the Lower Basin of Hungary’s Danube River: A Python and Monte Carlo Simulation-Based Study. Environ Geochem Health 2023, 45, 9757–9784. [Google Scholar] [CrossRef] [PubMed]
- Cordeli, A.N. Bioaccumulation of Metals in Some Fish Species from the Romanian Danube River: A Review. 2023.
- Simionov, I.-A.; Călmuc, M.; Iticescu, C.; Călmuc, V.; Georgescu, P.-L.; Faggio, C.; Petrea, Ş.-M. Human Health Risk Assessment of Potentially Toxic Elements and Microplastics Accumulation in Products from the Danube River Basin Fish Market. Environmental Toxicology and Pharmacology 2023, 104, 104307. [Google Scholar] [CrossRef]
- "; Dunarea de Jos"; University of Galati, Romania; Arseni, M. ; Roșu, A.; “Dunarea de Jos” University of Galati, Romania; Murariu, G.; “Dunarea de Jos” University of Galati, Romania; Georgescu, L.P.; “Dunarea de Jos” University of Galati, Romania; et al. The Role of River Channel Roughness for Water Level Modeling during the 2005 Year Flood on Siret River Using HEC-RAS Model. Ann_UGAL_Math_Phys_Mec 2019, 42, 68–76. [Google Scholar] [CrossRef]
- Water Directive.
- Barrero-Moreno, M.C.; Diaz-Vargas, C.A.; Restrepo-Parra, E. Computational Simulation of Filters Used in the Removal of Heavy Metals Using Rice Husks. Agriculture 2021, 11, 146. [Google Scholar] [CrossRef]
- Zolghadr, M.; Rafiee, M.R.; Esmaeilmanesh, F.; Fathi, A.; Tripathi, R.P.; Rathnayake, U.; Gunakala, S.R.; Azamathulla, H.M. Computation of Time of Concentration Based on Two-Dimensional Hydraulic Simulation. Water 2022, 14, 3155. [Google Scholar] [CrossRef]
- Maxim, C.; Filote, C.; Cojocaru, D.C. COMPARATIVE STUDY OF PHYSICAL INDICATORS AND THOSE OF THEIR REGIME OF OXYGEN, ON WATER QUALITY OF THE GREAT ùOMUZU RIVER, IN THE YEAR 2009. 2011.
- Iticescu, C.; Georgescu, L.P.; Murariu, G.; Topa, C.; Timofti, M.; Pintilie, V.; Arseni, M. Lower Danube Water Quality Quantified through WQI and Multivariate Analysis. Water 2019, 11, 1305. [Google Scholar] [CrossRef]
- Crişan, V.-E.; Dincă, L.; Bragă, C.; Murariu, G.; Tupu, E.; Mocanu, G.D.; Drasovean, R. The Configuration of Romanian Carpathians Landscape Controls the Volume Diversity of Picea Abies (L.) Stands. Land 2023, 12, 406. [Google Scholar] [CrossRef]
- Crişan, V.-E.; Dincă, L.; Bragă, C.; Murariu, G.; Tupu, E.; Mocanu, G.D.; Drasovean, R. The Configuration of Romanian Carpathians Landscape Controls the Volume Diversity of Picea Abies (L.) Stands. Land 2023, 12, 406. [Google Scholar] [CrossRef]
- Murariu, G.; Dinca, L.; Tudose, N.; Crisan, V.; Georgescu, L.; Munteanu, D.; Dragu, M.D.; Rosu, B.; Mocanu, G.D. Structural Characteristics of the Main Resinous Stands from Southern Carpathians, Romania. Forests 2021, 12, 1029. [Google Scholar] [CrossRef]
- Jin, Y.; Zhou, Q.; Wang, X.; Zhang, H.; Yang, G.; Lei, T.; Mei, S.; Yang, H.; Liu, L.; Yang, H.; et al. Heavy Metals in the Mainstream Water of the Yangtze River Downstream: Distribution, Sources and Health Risk Assessment. IJERPH 2022, 19, 6204. [Google Scholar] [CrossRef] [PubMed]
- Costabile, P.; Costanzo, C.; Ferraro, D.; Macchione, F.; Petaccia, G. Performances of the New HEC-RAS Version 5 for 2-D Hydrodynamic-Based Rainfall-Runoff Simulations at Basin Scale: Comparison with a State-of-the Art Model. Water 2020, 12, 2326. [Google Scholar] [CrossRef]
- Luo, K.; Liu, H.; Yu, E.; Tu, Y.; Gu, X.; Xu, M. Distribution and Release Mechanism of Heavy Metals in Sediments of Yelang Lake by DGT. Stoch Environ Res Risk Assess 2020, 34, 793–805. [Google Scholar] [CrossRef]
- Ha, M.; Schleiger, R. Environmental Science.
- Githaiga, K.B.; Njuguna, S.M.; Gituru, R.W.; Yan, X. Water Quality Assessment, Multivariate Analysis and Human Health Risks of Heavy Metals in Eight Major Lakes in Kenya. Journal of Environmental Management 2021, 297, 113410. [Google Scholar] [CrossRef]
- Feng, W.; Wang, T.; Zhu, Y.; Sun, F.; Giesy, J.P.; Wu, F. Chemical Composition, Sources, and Ecological Effect of Organic Phosphorus in Water Ecosystems: A Review. Carbon Res. 2023, 2, 12. [Google Scholar] [CrossRef]
- Xu, M.; Wang, R.; Yang, X.; Yang, H. Spatial Distribution and Ecological Risk Assessment of Heavy Metal Pollution in Surface Sediments from Shallow Lakes in East China. Journal of Geochemical Exploration 2020, 213, 106490. [Google Scholar] [CrossRef]
- Bąk, Ł.; Szeląg, B.; Sałata, A.; Studziński, J. Modeling of Heavy Metal (Ni, Mn, Co, Zn, Cu, Pb, and Fe) and PAH Content in Stormwater Sediments Based on Weather and Physico-Geographical Characteristics of the Catchment-Data-Mining Approach. Water 2019, 11, 626. [Google Scholar] [CrossRef]
- Pavel, A.; Durisch-Kaiser, E.; Balan, S.; Radan, S.; Sobek, S.; Wehrli, B. Sources and Emission of Greenhouse Gases in Danube Delta Lakes. Environ Sci Pollut Res 2009, 16, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Wang, T.; Zhu, Y.; Sun, F.; Giesy, J.P.; Wu, F. Chemical Composition, Sources, and Ecological Effect of Organic Phosphorus in Water Ecosystems: A Review. Carbon Res. 2023, 2, 12. [Google Scholar] [CrossRef]
- Bărbulescu, A.; Barbeş, L.; Dani, A. Statistical Analysis of the Quality Indicators of the Danube River Water (in Romania). In Frontiers in Water-Energy-Nexus—Nature-Based Solutions, Advanced Technologies and Best Practices for Environmental Sustainability; Naddeo, V., Balakrishnan, M., Choo, K.-H., Eds.; Advances in Science, Technology & Innovation; Springer International Publishing: Cham, 2020; pp. 277–279. ISBN 978-3-030-13067-1. [Google Scholar]
- Hakanson, L. An Ecological Risk Index for Aquatic Pollution Control.a Sedimentological Approach. Water Research 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Ultsch, A.; Lötsch, J. Euclidean Distance-Optimized Data Transformation for Cluster Analysis in Biomedical Data (EDOtrans). BMC Bioinformatics 2022, 23, 233. [Google Scholar] [CrossRef] [PubMed]
- Hemming, K.; Eldridge, S.; Forbes, G.; Weijer, C.; Taljaard, M. How to Design Efficient Cluster Randomised Trials. BMJ 2017, j3064. [Google Scholar] [CrossRef] [PubMed]
- Staples, L.; Ring, J.; Fontana, S.; Stradwick, C.; DeMaio, J.; Ray, H.; Zhang, Y.; Zhang, X. Reproducible Clustering with Non-Euclidean Distances: A Simulation and Case Study. Int J Data Sci Anal 2023. [Google Scholar] [CrossRef]
- Feio, M.J.; Hughes, R.M.; Callisto, M.; Nichols, S.J.; Odume, O.N.; Quintella, B.R.; Kuemmerlen, M.; Aguiar, F.C.; Almeida, S.F.P.; Alonso-EguíaLis, P.; et al. The Biological Assessment and Rehabilitation of the World’s Rivers: An Overview. Water 2021, 13, 371. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, Z.; Hu, X.; Zhang, N.; Chen, T.; Ding, Z. Sorption of Pb(II) and Cu(II) on the Colloid of Black Soil, Red Soil and Fine Powder Kaolinite: Effects of pH, Ionic Strength and Organic Matter. Environmental Pollutants and Bioavailability 2019, 31, 85–93. [Google Scholar] [CrossRef]
- Brunner, G. HEC-RAS Verification and Validation Tests.
- Calmuc, M.; Calmuc, V.; Arseni, M.; Topa, C.; Timofti, M.; Georgescu, L.P.; Iticescu, C. A Comparative Approach to a Series of Physico-Chemical Quality Indices Used in Assessing Water Quality in the Lower Danube. Water 2020, 12, 3239. [Google Scholar] [CrossRef]
- Antohi, V.M.; Ionescu, R.V.; Zlati, M.L.; Iticescu, C.; Georgescu, P.L.; Calmuc, M. Regional Regression Correlation Model of Microplastic Water Pollution Control Using Circular Economy Tools. IJERPH 2023, 20, 4014. [Google Scholar] [CrossRef]
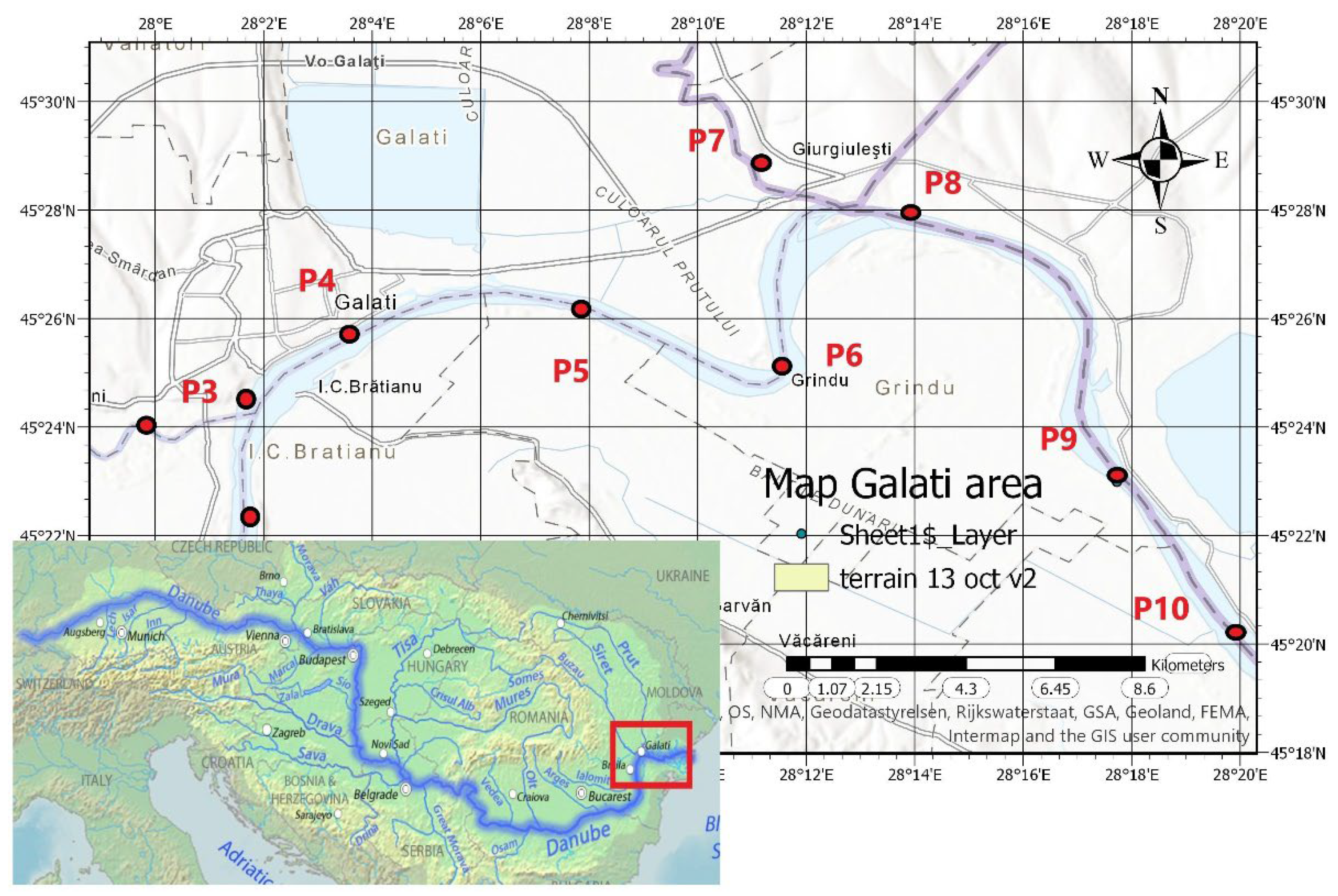
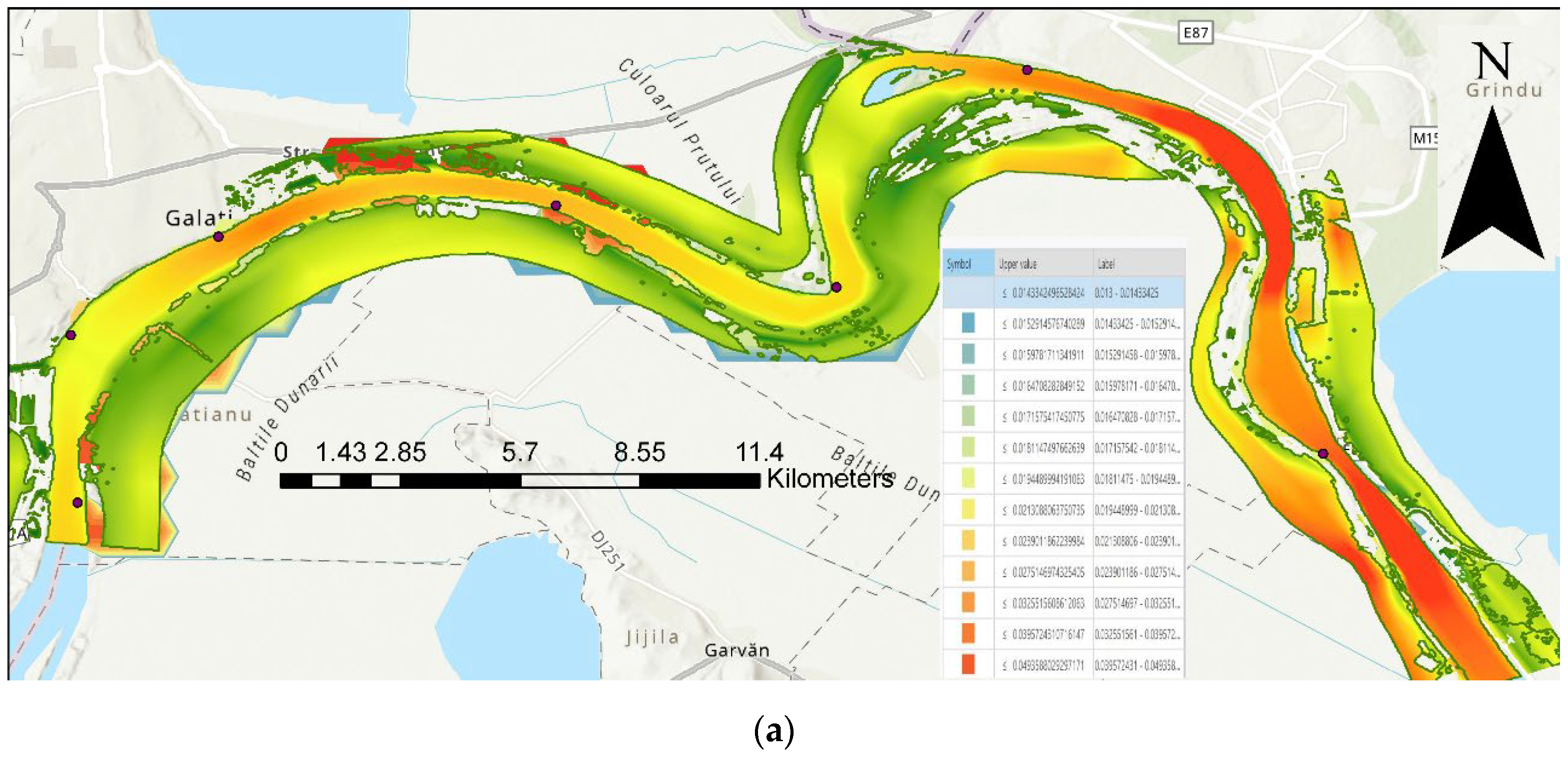
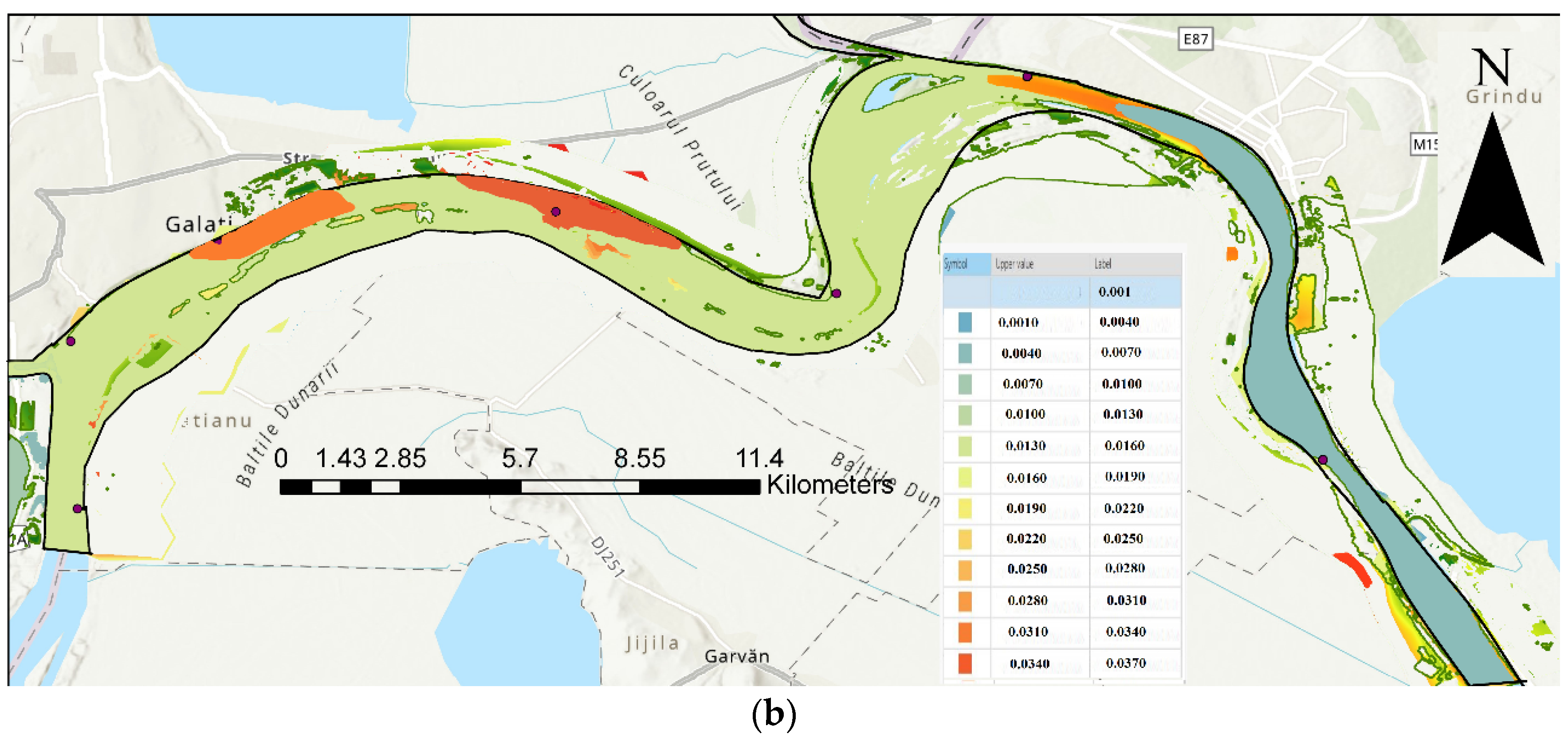
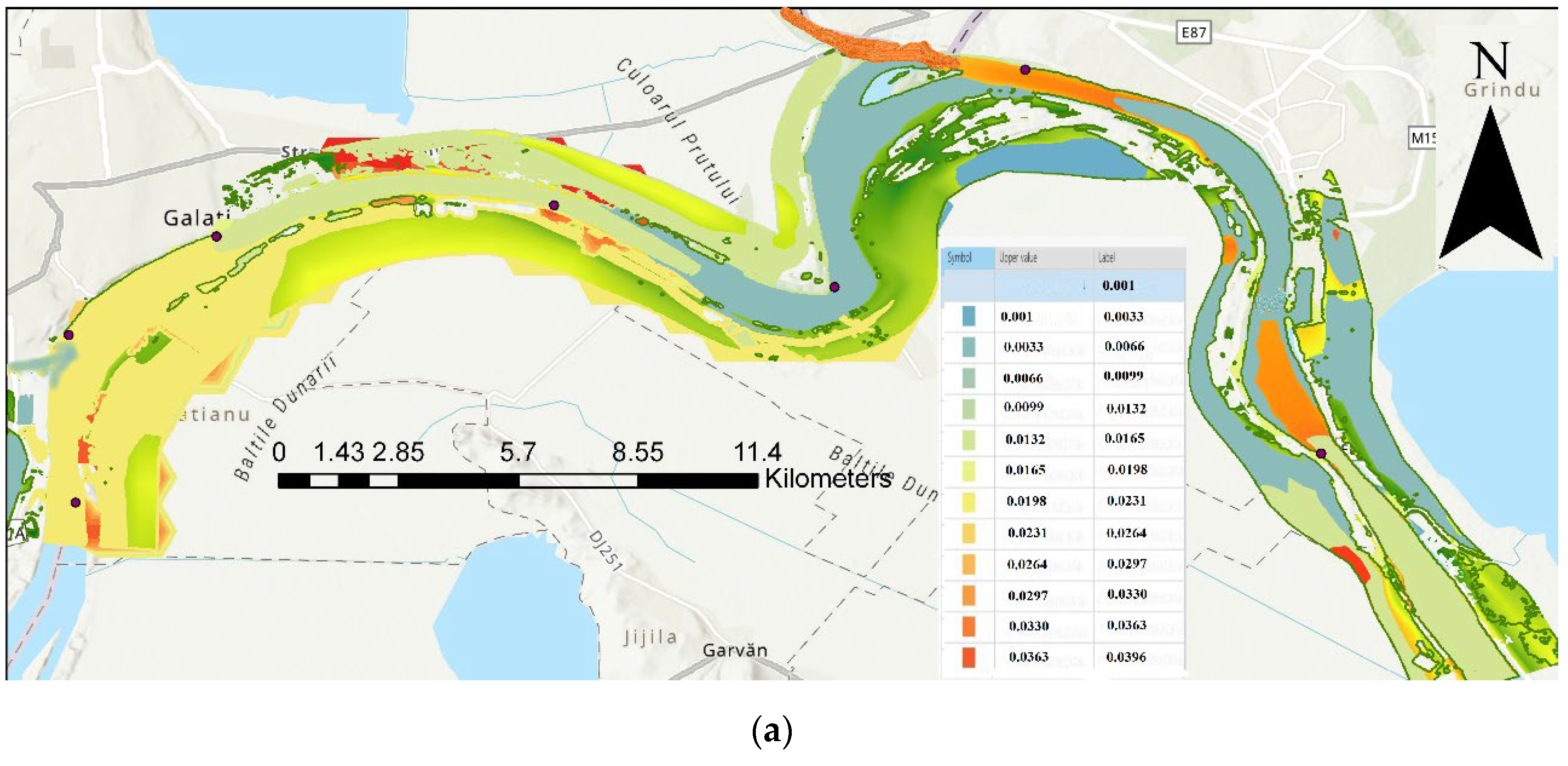
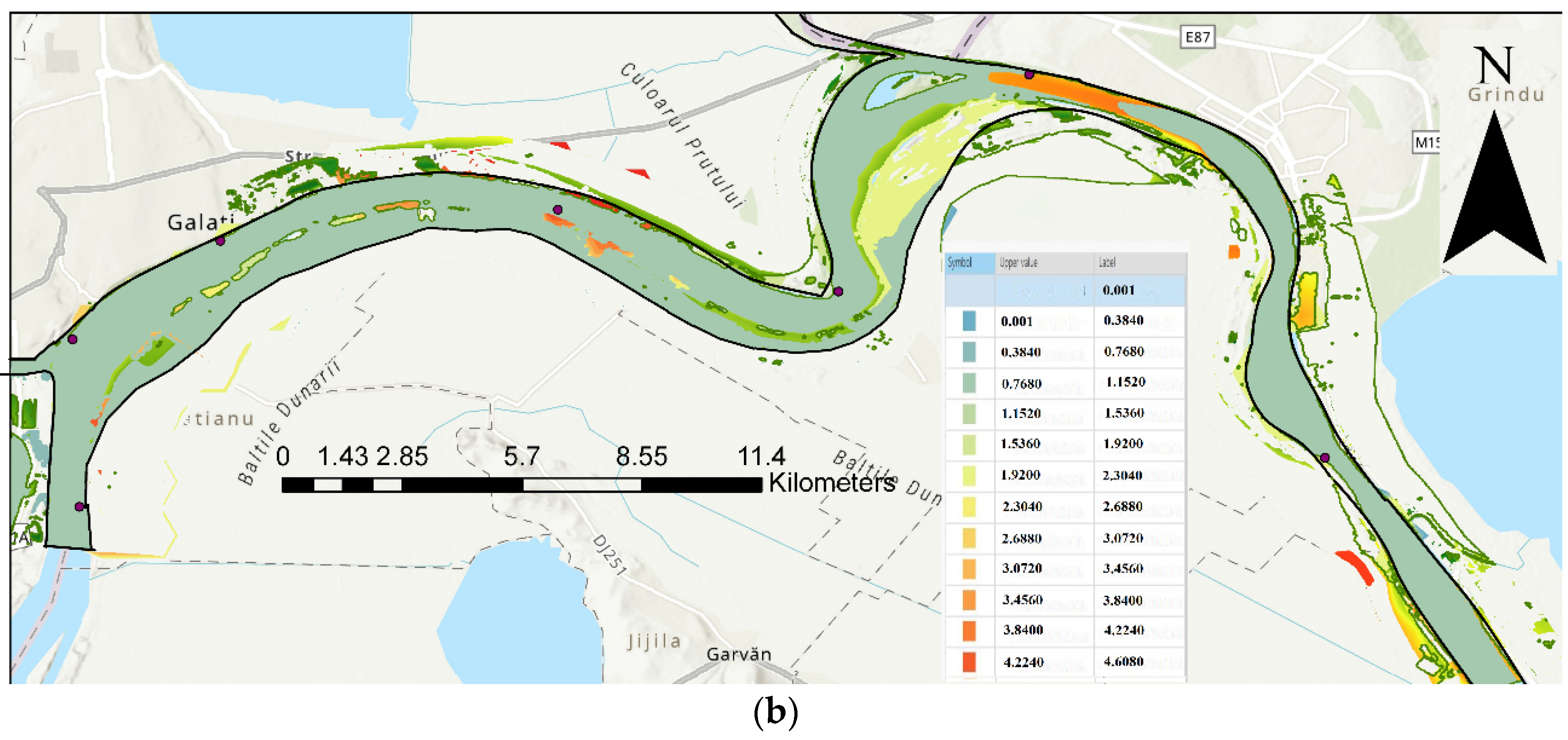
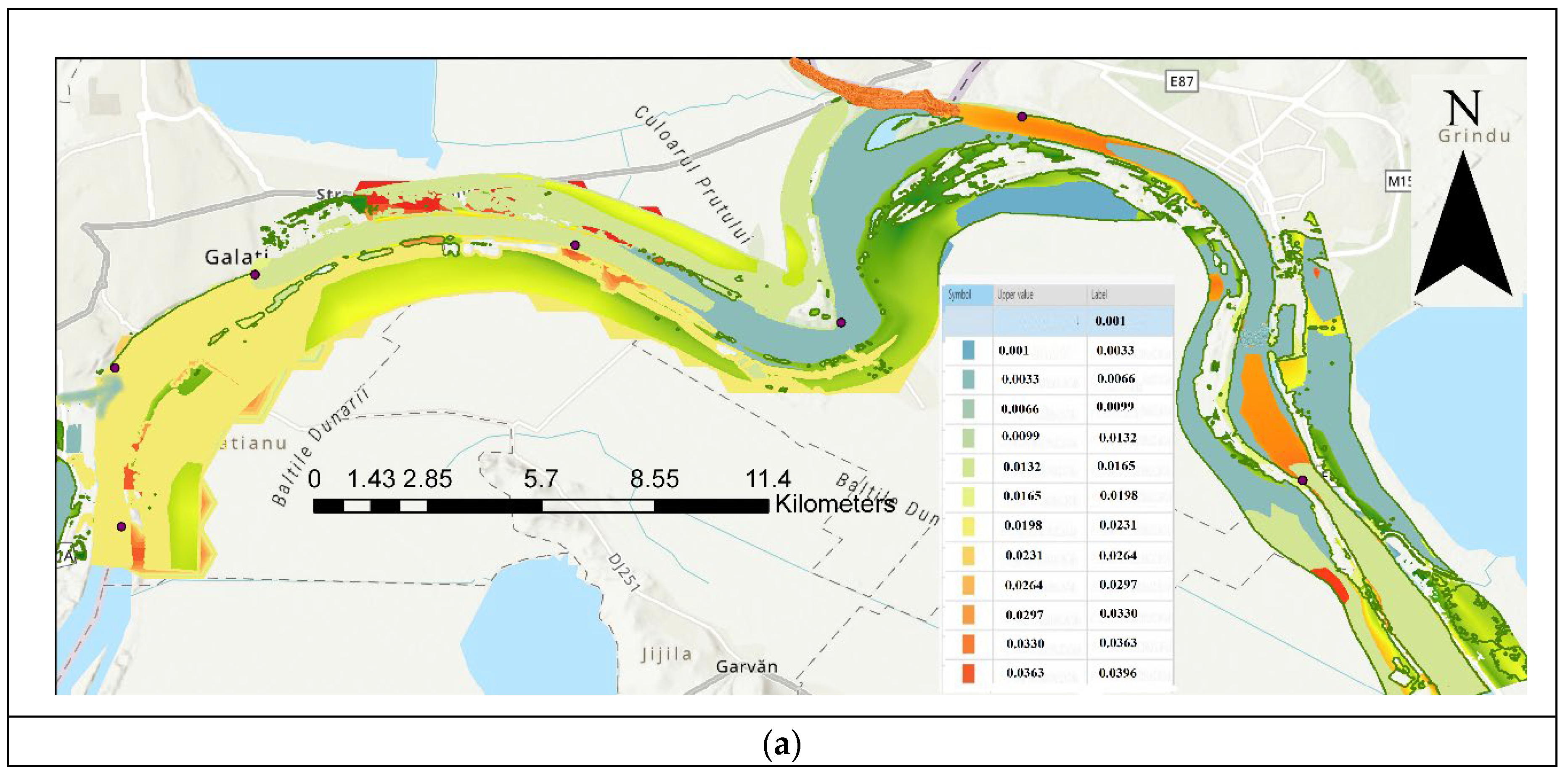

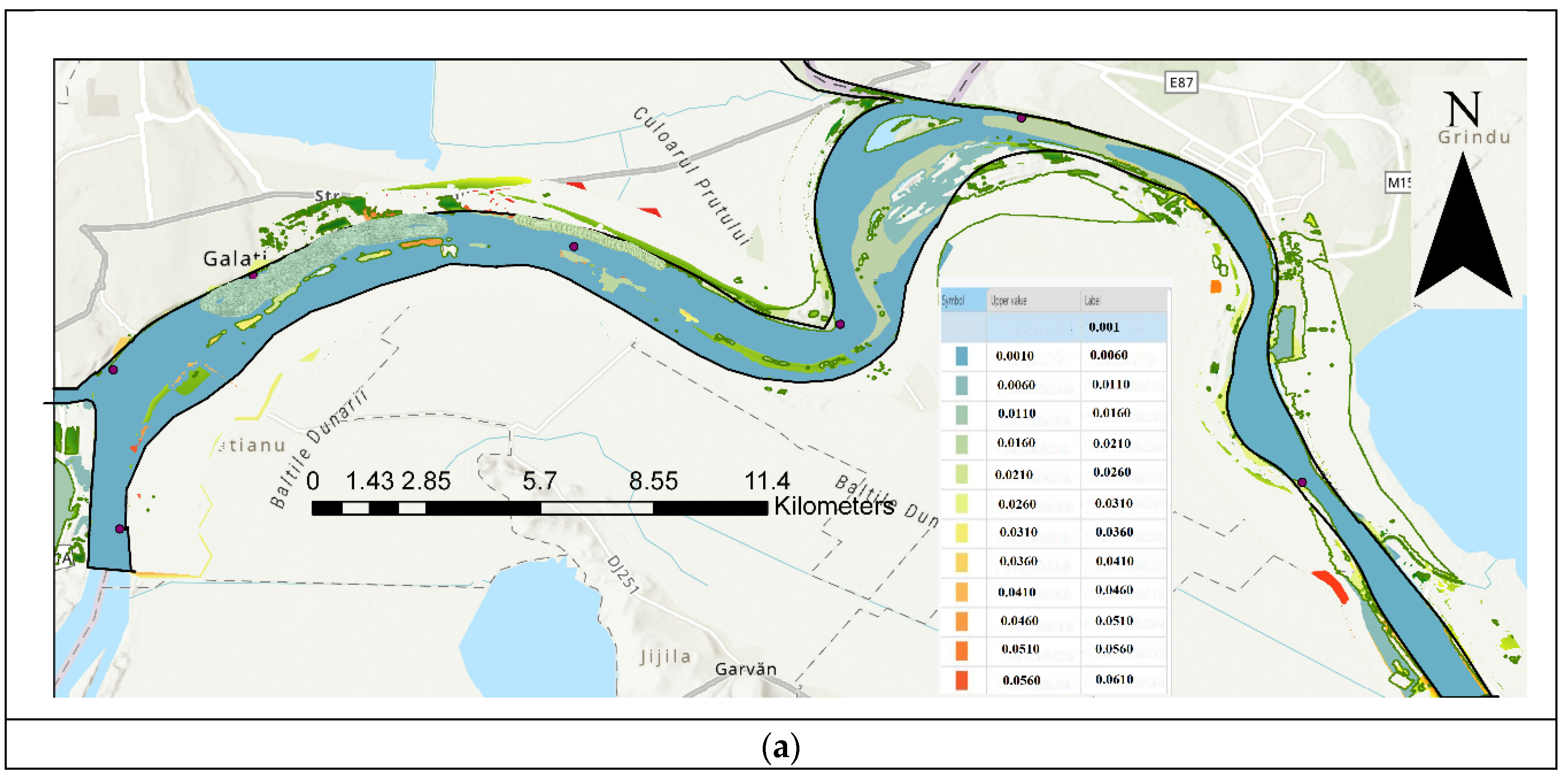
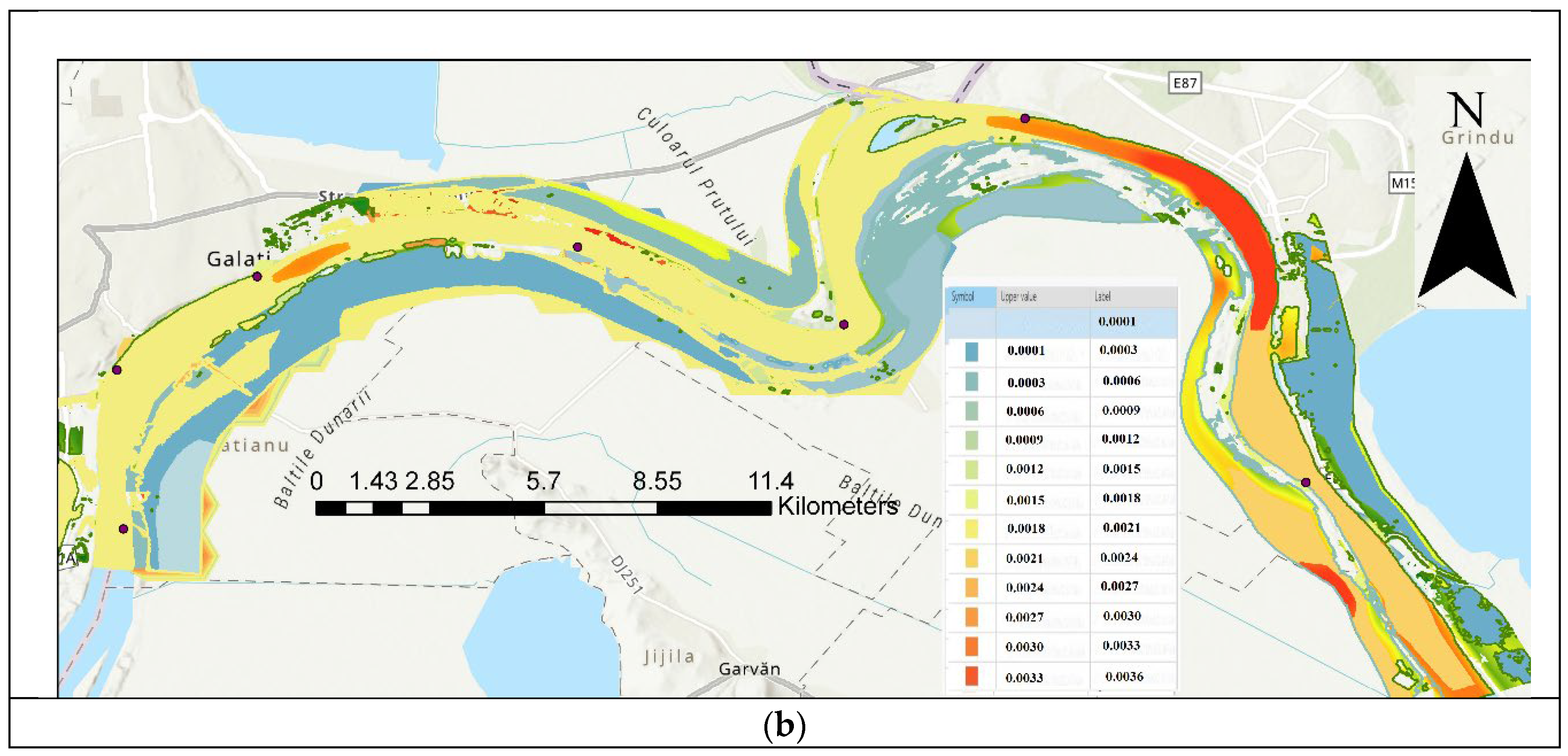
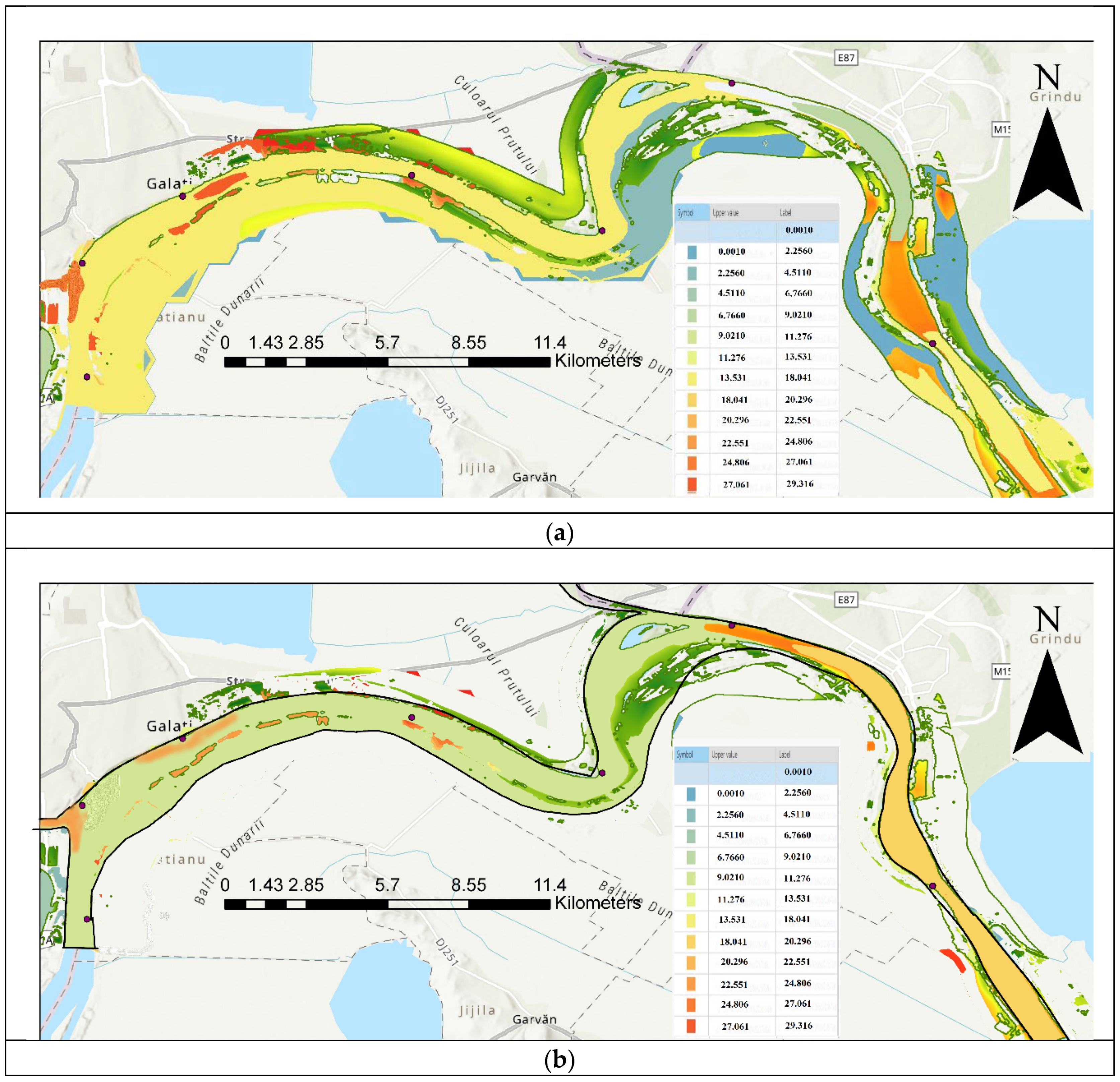
| Code/name | Station | GPS Coordinates [d.d.] |
|---|---|---|
| P1 | City water Danube Pump Station –on the Danube course | 45.37261173; 28.02879381 |
| P2 | Siret River monitoring point - on the Siret River upstream course | 45.40016436; 27.9973168 |
| P3 | Siret River confluence with Danube River monitoring point | 45.40848419; 28.027358 |
| P4 | Libertatea restaurant monitoring point –on the Danube course | 45.42957954; 28.05900221 |
| P5 | Damen Ship Yard Downstream monitoring point –on the Danube course | 45.43628888; 28.13124235 |
| P6 | Cotul Pisicii recreation area monitoring point –on the Danube course | 45.41873878; 28.19135779 |
| P7 | Prut River– Giurgiulesti monitoring point – on the Prut River upstream course | 45.48016; 28.185536 |
| P8 | Prut River confluence with Danube River monitoring point | 45.46528806; 28.23220795 |
| P9 | Ucrainean ship yard Reni monitoring point–on the Danube course | 45.38315546; 28.29554717 |
| P10 | Ucrainean passing border Isaccea monitoring point–on the Danube course | 45.28405785; 28.45693996 |
| July database | October database | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Valid N | Mean | Median | Min. | Max. | Std. Dev. |
Std. Error |
Mean |
Median |
Min. |
Max. |
Std. Dev. |
Std. Error |
| Phosphate | 10 | 0.077 | 0.075 | 0.04 | 0.14 | 0.02669 | 0.008439 | 0.054 | 0.06 | 0.01 | 0.07 | 0.01713 | 0.005416 |
| CCO | 10 | 13.59 | 10.9 | 7.5 | 33.4 | 7.99617 | 2.528612 | 12.76 | 10.2 | 7.1 | 30.3 | 7.23559 | 2.288095 |
| CBO5 | 10 | 25.46 | 24.75 | 22.4 | 29.7 | 2.5941 | 0.820325 | 23.11 | 23.25 | 20.3 | 27.1 | 2.1692 | 0.685962 |
| NH4+ | 10 | 0.833 | 0.885 | 0.34 | 1 | 0.19166 | 0.060609 | 0.797 | 0.77 | 0.64 | 1.06 | 0.13392 | 0.042349 |
| N-NO2 | 10 | 0.0163 | 0.015 | 0.013 | 0.02 | 0.00267 | 0.000844 | 0.0086 | 0.0085 | 0.007 | 0.011 | 0.00126 | 0.0004 |
| N-NO3- | 10 | 0.64 | 0.65 | 0.5 | 0.8 | 0.13499 | 0.042687 | 0.3 | 0.3 | 0.1 | 0.5 | 0.13333 | 0.042164 |
| N-Total | 10 | 1.81 | 1.8 | 1.5 | 2 | 0.16633 | 0.052599 | 2.4 | 2.55 | 0.5 | 3.7 | 1.08115 | 0.34189 |
| P-PO4 3- | 10 | 0.077 | 0.075 | 0.04 | 0.14 | 0.02669 | 0.008439 | 0.054 | 0.06 | 0.01 | 0.07 | 0.01713 | 0.005416 |
| SO42- | 10 | 27.4 | 21 | 19 | 61 | 14.04121 | 4.44022 | 44.7 | 41 | 38 | 71 | 9.91127 | 3.13422 |
| Cl- | 10 | 28.7 | 25 | 22 | 57 | 10.45679 | 3.306727 | 39.9 | 41.5 | 22 | 57 | 10.26807 | 3.24705 |
| phenols | 10 | 0.036 | 0.03 | 0.03 | 0.08 | 0.01578 | 0.004989 | 0.058 | 0.06 | 0.01 | 0.1 | 0.02741 | 0.008667 |
| Group 1 -July vs. Group 2 - Oct | Mean Group 1 |
Mean Group 2 |
t-value | df | p | t separ. var.est. | df | P 2-sided | Std. Dev. Group 1 | Std. Dev. Group 2 | F-ratio Variances |
p Variances |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phosphate July - Phosphate Oct | 0.07700 | 0.05400 | 2.2936 | 18 | 0.034064 | 2.2936 | 15.33828 | 0.036325 | 0.02669 | 0.01713 | 2 | 0.202485 |
| CCO July vs. CCO Oct | 13.59000 | 12.76000 | 0.2434 | 18 | 0.810456 | 0.2434 | 17.82312 | 0.810483 | 7.99617 | 7.23559 | 1 | 0.770730 |
| CBO5 July vs. CBO5 Oct | 25.46000 | 23.11000 | 2.1976 | 18 | 0.041303 | 2.1976 | 17.45324 | 0.041738 | 2.59410 | 2.16920 | 1 | 0.602631 |
| NH4+ July vs. NH4+ Oct | 0.83300 | 0.79700 | 0.4869 | 18 | 0.632211 | 0.4869 | 16.09645 | 0.632899 | 0.19166 | 0.13392 | 2 | 0.300431 |
| N-NO2 July vs. N-NO2 Oct | 0.01630 | 0.00860 | 8.2447 | 18 | 0.000000 | 8.2447 | 12.84941 | 0.000002 | 0.00267 | 0.00126 | 4 | 0.036529 |
| N-NO3- July vs. N-NO3- Oct | 0.64000 | 0.30000 | 5.6667 | 18 | 0.000022 | 5.6667 | 17.99726 | 0.000022 | 0.13499 | 0.13333 | 1 | 0.971262 |
| N-Total July vs. N-Total Oct | 1.81000 | 2.40000 | -1.7056 | 18 | 0.105272 | -1.7056 | 9.42581 | 0.120742 | 0.16633 | 1.08115 | 42 | 0.000005 |
| P-PO4 3- July vs. P-PO4 3- Oct | 0.07700 | 0.05400 | 2.2936 | 18 | 0.034064 | 2.2936 | 15.33828 | 0.036325 | 0.02669 | 0.01713 | 2 | 0.202485 |
| SO42- July vs. SO42- Oct | 27.40000 | 44.70000 | -3.1831 | 18 | 0.005150 | -3.1831 | 16.18487 | 0.005713 | 14.04121 | 9.91127 | 2 | 0.314085 |
| Cl- July vs. Cl- Oct | 28.70000 | 39.90000 | -2.4167 | 18 | 0.026502 | -2.4167 | 17.99403 | 0.026505 | 10.45679 | 10.26807 | 1 | 0.957620 |
| phenols July vs. phenols Oct | 0.03600 | 0.05800 | -2.2000 | 18 | 0.041109 | -2.2000 | 14.37439 | 0.044629 | 0.01578 | 0.02741 | 3 | 0.115426 |
| July database | October database | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Valid N | Mean | Median | Minim. | Maxim. | Std. Dev. |
Std. Error |
Mean | Median | Minim. | Maxim. | Std. Dev. |
Std. Error |
| Al | 10 | 24.71860 | 21.91900 | 8.665000 | 46.89000 | 12.01038 | 3.798014 | 16.01210 | 14.56050 | 9.000000 | 26.00000 | 6.656686 | 2.105029 |
| As | 10 | 0.00150 | 0.00150 | 0.001000 | 0.00200 | 0.00053 | 0.000167 | 0.01010 | 0.00900 | 0.005000 | 0.01900 | 0.003755 | 0.001187 |
| Cd | 10 | 0.01540 | 0.01600 | 0.004000 | 0.03800 | 0.01025 | 0.003243 | 0.01330 | 0.01400 | 0.005000 | 0.01800 | 0.003713 | 0.001174 |
| Cr | 10 | 1.31120 | 1.25950 | 0.764000 | 2.31800 | 0.46673 | 0.147593 | 0.58920 | 0.61350 | 0.137000 | 0.96200 | 0.343044 | 0.108480 |
| Fe | 10 | 0.030000 | 0.021500 | 0.013000 | 0.063000 | 0.018203 | 0.020600 | 0.013000 | 0.006000 | 0.082000 | 0.022751 | 0.007194 | 0.020600 |
| Group 1 vs. Group 2 |
Mean Group 1 |
MeanGroup 2 | t-value | df | p | t separ. var.est. |
df | p2-sided | Std. Dev.Group 1 | Std. Dev.Group 2 | F-ratioVariances | pVariances |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al. Jul vs. Al. Oct |
24.71860 | 16.01210 | 2.005017 | 18 | 0.060237 | 2.005017 | 14.05258 | 0.064618 | 12.01038 | 6.656686 | 3.255346 | 0.093547 |
| As Jul vs. As Oct |
0.001500 | 0.010100 | -7.17220 | 18 | 0.000001 | -7.17220 | 9.354472 | 0.000043 | 0.000527 | 0.003755 | 50.76000 | 0.000002 |
| Cd. Jul vs. Cd Oct |
0.015400 | 0.013300 | 0.608902 | 18 | 0.550198 | 0.608902 | 11.32041 | 0.554612 | 0.010255 | 0.003713 | 7.626108 | 0.005756 |
| Cr Jul. vs. Cr oct |
1.311200 | 0.589200 | 3.941673 | 18 | 0.000956 | 3.941673 | 16.52723 | 0.001104 | 0.466729 | 0.343044 | 1.851099 | 0.372571 |
| Fe Jul vs. Fe Oct |
0.030000 | 0.020600 | 1.020213 | 18 | 0.321144 | 1.020213 | 17.17325 | 0.321785 | 10 | 10 | 0.018203 | 0.022751 |
| Phosphate Jul vs. Phosphate Oct |
0.07700 | 0.05400 | 2.2936 | 18 | 0.034064 | 2.2936 | 15.33828 | 0.036325 | 0.02669 | 0.01713 | 2 | 0.202485 |
| Variable | Al | As | Cd | Cr | Fe | CCO | CBO5 | NH4+ | N-NO2 | N-NO3- | N-Total | P-PO4 3- | SO42- | Cl- | phenols |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | 1 | 0.4386 | 0.4822 | 0.3696 | 0.2077 | 0.3736 | -0.0869 | -0.366 | 0.2185 | -0.1587 | 0.1489 | -0.0579 | -0.1131 | -0.1075 | 0.3626 |
| p= --- | p=.205 | p=.158 | p=.293 | p=.565 | p=.288 | p=.811 | p=.298 | p=.544 | p=.661 | p=.681 | p=.874 | p=.756 | p=.768 | p=.303 | |
| As | 0.4386 | 1 | 0.0617 | 0.1305 | 0.1274 | -0.1911 | -0.5364 | -0.2805 | -0.0395 | -0.6247 | 0.4436 | 0.5925 | -0.4805 | -0.4335 | 0.4009 |
| p=.205 | p= --- | p=.866 | p=.719 | p=.726 | p=.597 | p=.110 | p=.432 | p=.914 | p=.053 | p=.199 | p=.071 | p=.160 | p=.211 | p=.251 | |
| Cd | 0.4822 | 0.0617 | 1 | 0.4969 | 0.372 | 0.1694 | 0.3799 | -0.0781 | 0.4864 | -0.4944 | 0.0886 | -0.0195 | 0.4834 | -0.1407 | 0.1621 |
| p=.158 | p=.866 | p= --- | p=.144 | p=.290 | p=.640 | p=.279 | p=.830 | p=.154 | p=.146 | p=.808 | p=.957 | p=.157 | p=.698 | p=.655 | |
| Cr | 0.3696 | 0.1305 | 0.4969 | 1 | -0.2396 | 0.713 | 0.1156 | 0.255 | -0.0617 | -0.1081 | 0.2698 | 0.4192 | -0.2223 | -0.3824 | 0.2841 |
| p=.293 | p=.719 | p=.144 | p= --- | p=.505 | p=.021 | p=.750 | p=.477 | p=.866 | p=.766 | p=.451 | p=.228 | p=.537 | p=.276 | p=.426 | |
| Fe | 0.2077 | 0.1274 | 0.372 | -0.2396 | 1 | -0.0137 | 0.161 | -0.7392 | -0.0526 | -0.4477 | -0.4184 | -0.2059 | 0.4286 | 0.1185 | -0.3521 |
| p=.565 | p=.726 | p=.290 | p=.505 | p= --- | p=.970 | p=.657 | p=.015 | p=.885 | p=.195 | p=.229 | p=.568 | p=.216 | p=.744 | p=.318 | |
| CCO | 0.3736 | -0.1911 | 0.1694 | 0.713 | -0.0137 | 1 | 0.1856 | -0.0485 | -0.2571 | 0.2691 | -0.0784 | 0.0623 | -0.2814 | -0.2178 | -0.0426 |
| p=.288 | p=.597 | p=.640 | p=.021 | p=.970 | p= --- | p=.608 | p=.894 | p=.473 | p=.452 | p=.829 | p=.864 | p=.431 | p=.545 | p=.907 | |
| CBO5 | -0.0869 | -0.5364 | 0.3799 | 0.1156 | 0.161 | 0.1856 | 1 | 0.1306 | 0.2122 | 0.0336 | 0.086 | -0.4144 | 0.4239 | -0.0132 | 0.1043 |
| p=.811 | p=.110 | p=.279 | p=.750 | p=.657 | p=.608 | p= --- | p=.719 | p=.556 | p=.927 | p=.813 | p=.234 | p=.222 | p=.971 | p=.774 | |
| NH4+ | -0.366 | -0.2805 | -0.0781 | 0.255 | -0.7392 | -0.0485 | 0.1306 | 1 | 0.1153 | 0.3942 | 0.229 | -0.0263 | -0.1665 | -0.285 | 0.2286 |
| p=.298 | p=.432 | p=.830 | p=.477 | p=.015 | p=.894 | p=.719 | p= --- | p=.751 | p=.260 | p=.525 | p=.943 | p=.646 | p=.425 | p=.525 | |
| N-NO2 | 0.2185 | -0.0395 | 0.4864 | -0.0617 | -0.0526 | -0.2571 | 0.2122 | 0.1153 | 1 | -0.3763 | 0.1927 | -0.2668 | 0.3582 | -0.0362 | 0.5331 |
| p=.544 | p=.914 | p=.154 | p=.866 | p=.885 | p=.473 | p=.556 | p=.751 | p= --- | p=.284 | p=.594 | p=.456 | p=.309 | p=.921 | p=.113 | |
| N-NO3- | -0.1587 | -0.6247 | -0.4944 | -0.1081 | -0.4477 | 0.2691 | 0.0336 | 0.3942 | -0.3763 | 1 | -0.3167 | -0.4565 | -0.1266 | 0.2928 | -0.3339 |
| p=.661 | p=.053 | p=.146 | p=.766 | p=.195 | p=.452 | p=.927 | p=.260 | p=.284 | p= --- | p=.373 | p=.185 | p=.727 | p=.412 | p=.346 | |
| N-Total | 0.1489 | 0.4436 | 0.0886 | 0.2698 | -0.4184 | -0.0784 | 0.086 | 0.229 | 0.1927 | -0.3167 | 1 | 0.6082 | -0.5538 | -0.6944 | 0.4404 |
| p=.681 | p=.199 | p=.808 | p=.451 | p=.229 | p=.829 | p=.813 | p=.525 | p=.594 | p=.373 | p= --- | p=.062 | p=.097 | p=.026 | p=.203 | |
| P-PO4 3- | -0.0579 | 0.5925 | -0.0195 | 0.4192 | -0.2059 | 0.0623 | -0.4144 | -0.0263 | -0.2668 | -0.4565 | 0.6082 | 1 | -0.5776 | -0.557 | 0.0211 |
| p=.874 | p=.071 | p=.957 | p=.228 | p=.568 | p=.864 | p=.234 | p=.943 | p=.456 | p=.185 | p=.062 | p= --- | p=.080 | p=.094 | p=.954 | |
| SO42- | -0.1131 | -0.4805 | 0.4834 | -0.2223 | 0.4286 | -0.2814 | 0.4239 | -0.1665 | 0.3582 | -0.1266 | -0.5538 | -0.5776 | 1 | 0.6744 | -0.1525 |
| p=.756 | p=.160 | p=.157 | p=.537 | p=.216 | p=.431 | p=.222 | p=.646 | p=.309 | p=.727 | p=.097 | p=.080 | p= --- | p=.032 | p=.674 | |
| Cl- | -0.1075 | -0.4335 | -0.1407 | -0.3824 | 0.1185 | -0.2178 | -0.0132 | -0.285 | -0.0362 | 0.2928 | -0.6944 | -0.557 | 0.6744 | 1 | -0.1899 |
| p=.768 | p=.211 | p=.698 | p=.276 | p=.744 | p=.545 | p=.971 | p=.425 | p=.921 | p=.412 | p=.026 | p=.094 | p=.032 | p= --- | p=.599 | |
| phenols | 0.3626 | 0.4009 | 0.1621 | 0.2841 | -0.3521 | -0.0426 | 0.1043 | 0.2286 | 0.5331 | -0.3339 | 0.4404 | 0.0211 | -0.1525 | -0.1899 | 1 |
| p=.303 | p=.251 | p=.655 | p=.426 | p=.318 | p=.907 | p=.774 | p=.525 | p=.113 | p=.346 | p=.203 | p=.954 | p=.674 | p=.599 | p= --- |
| Variable | Al | As | Cd | Cr | Fe | CCO | CBO5 | NH4+ | N-NO2 | N-NO3- | N-Total | P-PO4 3- | SO42- | Cl- | phenols |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | 1 | -0.1423 | -0.6543 | 0.335 | 0.3737 | -0.2228 | -0.2869 | -0.0909 | 0.1558 | -0.0155 | -0.0174 | -0.2819 | 0.1452 | 0.4777 | 0.2096 |
| p= --- | p=.695 | p=.040 | p=.344 | p=.287 | p=.536 | p=.422 | p=.803 | p=.667 | p=.966 | p=.962 | p=.430 | p=.689 | p=.163 | p=.561 | |
| As | -0.1423 | 1 | 0.2526 | 0.4151 | 0.6964 | 0.5281 | -0.0315 | -0.1253 | -0.1076 | -0.2441 | 0.3421 | 0.2177 | -0.5693 | -0.2475 | 0.1641 |
| p=.695 | p= --- | p=.481 | p=.233 | p=.025 | p=.117 | p=.931 | p=.730 | p=.767 | p=.497 | p=.333 | p=.546 | p=.086 | p=.490 | p=.651 | |
| Cd | -0.6543 | 0.2526 | 1 | -0.3699 | -0.0839 | 0.3284 | 0.2796 | -0.2214 | -0.7522 | 0.1346 | -0.1661 | 0.7652 | -0.4501 | -0.5557 | -0.5284 |
| p=.040 | p=.481 | p= --- | p=.293 | p=.818 | p=.354 | p=.434 | p=.539 | p=.012 | p=.711 | p=.647 | p=.010 | p=.192 | p=.095 | p=.116 | |
| Cr | 0.335 | 0.4151 | -0.3699 | 1 | 0.4802 | -0.2653 | -0.5368 | -0.3392 | 0.2875 | -0.6923 | 0.8094 | -0.0756 | -0.3728 | -0.3436 | 0.1409 |
| p=.344 | p=.233 | p=.293 | p= --- | p=.160 | p=.459 | p=.110 | p=.338 | p=.421 | p=.027 | p=.005 | p=.836 | p=.289 | p=.331 | p=.698 | |
| Fe | 0.3737 | 0.6964 | -0.0839 | 0.4802 | 1 | 0.1544 | 0.026 | 0.1082 | -0.0448 | 0.0037 | 0.1974 | 0.1528 | -0.1647 | -0.0506 | 0.0752 |
| p=.287 | p=.025 | p=.818 | p=.160 | p= --- | p=.670 | p=.943 | p=.766 | p=.902 | p=.992 | p=.585 | p=.673 | p=.649 | p=.890 | p=.836 | |
| CCO | -0.2228 | 0.5281 | 0.3284 | -0.2653 | 0.1544 | 1 | 0.1611 | -0.0473 | -0.0906 | -0.0656 | -0.1426 | 0.2274 | -0.2014 | 0.1353 | 0.1732 |
| p=.536 | p=.117 | p=.354 | p=.459 | p=.670 | p= --- | p=.657 | p=.897 | p=.804 | p=.857 | p=.694 | p=.528 | p=.577 | p=.709 | p=.632 | |
| CBO5 | -0.2869 | -0.0315 | 0.2796 | -0.5368 | 0.026 | 0.1611 | 1 | 0.6358 | -0.4884 | 0.3304 | -0.6813 | 0.0825 | 0.3676 | 0.1288 | -0.123 |
| p=.422 | p=.931 | p=.434 | p=.110 | p=.943 | p=.657 | p= --- | p=.048 | p=.152 | p=.351 | p=.030 | p=.821 | p=.296 | p=.723 | p=.735 | |
| NH4+ | -0.0909 | -0.1253 | -0.2214 | -0.3392 | 0.1082 | -0.0473 | 0.6358 | 1 | 0.143 | 0.56 | -0.5372 | -0.2122 | 0.6907 | 0.129 | 0.422 |
| p=.803 | p=.730 | p=.539 | p=.338 | p=.766 | p=.897 | p=.048 | p= --- | p=.694 | p=.092 | p=.109 | p=.556 | p=.027 | p=.722 | p=.224 | |
| N-NO2 | 0.1558 | -0.1076 | -0.7522 | 0.2875 | -0.0448 | -0.0906 | -0.4884 | 0.143 | 1 | -0.1318 | 0.4062 | -0.636 | 0.3439 | 0.3217 | 0.5192 |
| p=.667 | p=.767 | p=.012 | p=.421 | p=.902 | p=.804 | p=.152 | p=.694 | p= --- | p=.717 | p=.244 | p=.048 | p=.331 | p=.365 | p=.124 | |
| N-NO3- | -0.0155 | -0.2441 | 0.1346 | -0.6923 | 0.0037 | -0.0656 | 0.3304 | 0.56 | -0.1318 | 1 | -0.7322 | 0 | 0.454 | 0.2191 | 0.0304 |
| p=.966 | p=.497 | p=.711 | p=.027 | p=.992 | p=.857 | p=.351 | p=.092 | p=.717 | p= --- | p=.016 | p=1.00 | p=.187 | p=.543 | p=.934 | |
| N-Total | -0.0174 | 0.3421 | -0.1661 | 0.8094 | 0.1974 | -0.1426 | -0.6813 | -0.5372 | 0.4062 | -0.7322 | 1 | 0.096 | -0.5641 | -0.3383 | 0.1087 |
| p=.962 | p=.333 | p=.647 | p=.005 | p=.585 | p=.694 | p=.030 | p=.109 | p=.244 | p=.016 | p= --- | p=.792 | p=.089 | p=.339 | p=.765 | |
| P-PO4 3- | -0.2819 | 0.2177 | 0.7652 | -0.0756 | 0.1528 | 0.2274 | 0.0825 | -0.2122 | -0.636 | 0 | 0.096 | 1 | -0.542 | -0.5598 | -0.2414 |
| p=.430 | p=.546 | p=.010 | p=.836 | p=.673 | p=.528 | p=.821 | p=.556 | p=.048 | p=1.00 | p=.792 | p= --- | p=.106 | p=.092 | p=.502 | |
| SO42- | 0.1452 | -0.5693 | -0.4501 | -0.3728 | -0.1647 | -0.2014 | 0.3676 | 0.6907 | 0.3439 | 0.454 | -0.5641 | -0.542 | 1 | 0.3414 | 0.0344 |
| p=.689 | p=.086 | p=.192 | p=.289 | p=.649 | p=.577 | p=.296 | p=.027 | p=.331 | p=.187 | p=.089 | p=.106 | p= --- | p=.334 | p=.925 | |
| Cl- | 0.4777 | -0.2475 | -0.5557 | -0.3436 | -0.0506 | 0.1353 | 0.1288 | 0.129 | 0.3217 | 0.2191 | -0.3383 | -0.5598 | 0.3414 | 1 | 0.2756 |
| p=.163 | p=.490 | p=.095 | p=.331 | p=.890 | p=.709 | p=.723 | p=.722 | p=.365 | p=.543 | p=.339 | p=.092 | p=.334 | p= --- | p=.441 | |
| phenols | 0.2096 | 0.1641 | -0.5284 | 0.1409 | 0.0752 | 0.1732 | -0.123 | 0.422 | 0.5192 | 0.0304 | 0.1087 | -0.2414 | 0.0344 | 0.2756 | 1 |
| p=.561 | p=.651 | p=.116 | p=.698 | p=.836 | p=.632 | p=.735 | p=.224 | p=.124 | p=.934 | p=.765 | p=.502 | p=.925 | p=.441 | p= --- |
| July monitoring campaign | October monitoring campaign | |||||||
|---|---|---|---|---|---|---|---|---|
| Value number | Eigenvalue | % Total variance |
Cumulative Eigenvalue |
Cumulative % |
Eigenvalue | % Total variance |
Cumulative Eigenvalue |
Cumulative % |
| 1 | 6.204681* | 34.47045 | 6.20468 | 34.4705 | 5.628626* | 31.27015 | 5.62863 | 31.2701 |
| 2 | 3.07987* | 17.11041 | 9.28455 | 51.5809 | 4.444705* | 24.69281 | 10.07333 | 55.963 |
| 3 | 2.625296* | 14.58498 | 11.90985 | 66.1658 | 2.093713* | 11.63174 | 12.16705 | 67.5947 |
| 4 | 2.063760* | 11.46534 | 13.97361 | 77.6312 | 1.828940* | 10.16078 | 13.99599 | 77.7555 |
| 5 | 1.401129* | 7.78405 | 15.37474 | 85.4152 | 1.312745* | 7.29303 | 15.30873 | 85.0485 |
| 6 | 1.153074* | 6.40597 | 16.52781 | 91.8212 | 1.198617* | 6.65898 | 16.50735 | 91.7075 |
| 7 | 0.603383 | 3.35213 | 17.1312 | 95.1733 | 0.687419 | 3.81899 | 17.19477 | 95.5265 |
| 8 | 0.460789 | 2.55994 | 17.59199 | 97.7333 | 0.469 | 2.60556 | 17.66377 | 98.132 |
| 9 | 0.408013 | 2.26674 | 18 | 100 | 0.336233 | 1.86796 | 18 | 100 |
| July monitoring campaign | October monitoring campaign | |||||||
|---|---|---|---|---|---|---|---|---|
| Value number | Eigenvalue | % Total variance |
Cumulative Eigenvalue |
Cumulative % |
Eigenvalue | % Total variance |
Cumulative Eigenvalue |
Cumulative % |
| 1 | 2.091981* | 41.83961 | 2.091981 | 41.8396 | 2.276505* | 45.53009 | 2.276505 | 45.5301 |
| 2 | 1.233531* | 24.67062 | 3.325512 | 66.5102 | 1.724148* | 34.48295 | 4.000652 | 80.013 |
| 3 | 1.018576* | 20.37151 | 4.344087 | 86.8817 | 0.581033 | 11.62066 | 4.581685 | 91.6337 |
| 4 | 0.423145 | 8.46291 | 4.767233 | 95.3447 | 0.282902 | 5.65804 | 4.864587 | 97.2917 |
| 5 | 0.232767 | 4.65535 | 5 | 100 | 0.135413 | 2.70825 | 5 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





